Abstract
Dual-tasking charges the sensorimotor system with performing two tasks simultaneously. Center of pressure (COP) analysis reveals the postural control that is altered during dual-tasking, but may not reveal the underlying neural mechanisms. In the current study, we hypothesized that the minimal intervention principle (MIP) provides a concept by which dual-tasking effects on the organization and prioritization of postural control can be predicted. Postural movements of 23 adolescents (age 12.7 ± 1.3; 8 females) and 15 adults (26.9 ± 2.3) were measured in a bipedal stance with eyes open, eyes closed and eyes open while performing a dual-task using a force plate and 39 reflective markers. COP data was analyzed by calculating the mean velocity, standard deviation and amplitude of displacement. Kinematic data was examined by performing a principal component analysis (PCA) and extracting postural movement components. Two variables were determined to investigate changes in amplitude (aVark) and in control (Nk) of the principal movement components. Results in aVark and in Nk agreed well with the predicted dual-tasking effects. Thus, the current study corroborates the notion that the MIP should be considered when investigating postural control under dual-tasking conditions.
Keywords: motor control, automatization, optimal feedback control, minimal intervention principle, principal component analysis, postural control, adolescents and adults, attentional focus
1. Introduction
Human motor control is largely automatized in many every-day situations, which means that sensorimotor processing requires little or no attention [1]. The so-called dual-task paradigm has been used by researchers to investigate this connection between postural control and attentional focus [2,3]. Interestingly, results are not always consistent when evaluating the effect of performing a concurrent cognitive task on the neural control of stabilizing one’s posture. Some researchers argue that postural control is controlled in a more effective way during dual-tasking [4,5,6,7,8], suggesting that automatic processes regulate postural control [1,3,4,9]. However, others have found traditional variables like center of pressure (COP) sway variability, velocity, frequency, or area to be increased and interpret this as less effective postural control [10,11,12,13,14,15] resulting from cognitive resource competition [10]. With regard to different sensory situations, closed eyes in a bipedal stance usually produce an increase in the sway [16,17,18], arguably due to missing sensory information provided by vision. There is much debate about the differences in postural control between adolescents and adults. Some researchers report that postural control is well developed around the age of 12 [19,20] whereas others report differences in equilibrium scores until the age of 15–16 [21,22].
An often-cited model for motor control is the optimal feedback control (OFC) theory, which predicts the minimal intervention principle (MIP) [23]. The MIP suggests that the controller focuses more on the task-relevant dimensions in the control of different movement dimensions, while more variability is tolerated in movement dimensions that are not task-relevant [23,24,25]. “Movement dimensions” in this context relate to the degrees of freedom in the human body, enabled by joints between the segments [26,27] and to the coordinative patterns that emerge in the control of these degrees of freedom. A suitable approach to evaluate human postural control is to analyze kinematic data using principal component analysis (PCA), which reveals different movement strategies [28,29,30,31]. In an unconstrained bipedal stance with eyes open, the most dominant movement component is the anterior-posterior ankle sway (ankle strategy) with an approximately 70% overall share of the postural variance [29,31,32,33]. Research investigating postural control by measuring muscle activity using electromyography [34] or joint torques [35] support the ankle strategy as the dominant coordinative pattern in the bipedal stance.
Trying to stand as still as possible is an unusual task, since performing several tasks simultaneously while standing occurs often in daily life situations [10,36]. Focusing on standing as still as possible directs a large proportion of attention to the motor control task. In this sense, a single-task “standing as quiet as possible” exercise leads to much less automatized postural control [3,10]. We hypothesized that focusing one’s attention on postural control with an explicit instruction to “move as little as possible” can override neural mechanisms such as the MIP. If, however, attention is drawn away from the task of balancing, e.g., towards a concurrent cognitive task, then we expect that the control of postural movements will be more automatized, and that the MIP will play a more important role in the automatized control.
The main purpose of the current study was to better understand the effect of dual-tasking on postural control. We hypothesized that the MIP needs to be considered when assessing the effects of dual-tasking on postural control, which would imply that divergent effects should be expected between different movements’ dimensions (coordinative patterns within the postural movements). More specifically, we hypothesized that a more automatized neural control would prioritize specific movement components that threaten postural stability, such as the anterior-posterior ankle strategy in a normal bipedal stance. Such an upregulation in control may lead to reduced movement amplitude and increased frequency of control interventions (changes in acceleration). In contrast, the more automatized neural controller might de-prioritize higher order movement components, which are less critical for maintaining stability. This de-prioritization may lead to an increase in the movement amplitude and a decrease in the frequency of control interventions. In movement dimensions that are largely irrelevant for maintaining stability, such as lateral sway in bipedal standing (where stability is provided mainly by the two legs), we did not expect to observe an effect of dual-tasking, since stability in these movement components does not fully rely on a permanent neuromuscular control. We distinguished between adult participants and adolescents, since automatization of postural control might not yet be fully developed in the latter. Finally, as a comparison, we also evaluated COP movements.
2. Materials and Methods
2.1. Ethics
All participants were informed about the measurement procedures, about any possible risks involved and provided written informed consent regarding their participation. Participants were free to withdraw from the experiment at any time without reason. Prior to any measurements, the study had been approved by the Board for Ethical Questions in Science of the University of Innsbruck (Certificate 09/2018). All measurements and the use of equipment and procedures were performed in accordance with the Declaration of Helsinki (1964).
2.2. Participants
A total of 38 volunteers (23 adolescents, 15 adults) were included in the current study (Table 1). Participants were recruited from local sport clubs and represented a healthy and above-average physically active population. Diagnosed injuries, such as concussion or neurological disorders within the last six months, as well as known problems in joints, tendons or muscles were set as exclusion criteria.
Table 1.
Anthropometric information about the participants; mean ± SD; BMI = Body Mass Index.
| Participants | Age (years) | Body Height (cm) | Body Weight (kg) | BMI |
|---|---|---|---|---|
| All (n = 38) | 18.3 ± 7.2 | 171.7 ± 14.3 | 63.8 ± 16.5 | 21.1 ± 2.8 |
| Adolescents (n = 23) | 12.7 ± 1.3 | 163.6 ± 12.1 | 54.0 ± 13.1 | 19.8 ± 2.8 |
| Female (n = 8) | 11.5 ± 1.3 | 152.7 ± 9.4 | 41.9 ± 9.9 | 17.7 ± 2.4 |
| Male (n = 15) | 13.3 ± 0.8 | 169.5 ± 9.0 | 60.3 ± 10.3 | 20.9 ± 2.4 |
| Adults Male (n = 15) | 26.9 ± 2.3 | 183.9 ± 7.3 | 78.9 ± 7.3 | 23.3 ± 1.1 |
2.3. Measurement Procedures
The participants were instructed to stand as steady as possible on a marked area on the ground, in a hip-wide stance, with their hands on the hips. Three different balance trials were analyzed. The first trial, standing in a bipedal stance with eyes open, lasted 60 s and participants were asked to focus their gaze on a cross placed 5.5m in front of them at a height of 1.75 m. The second trial, standing in a bipedal stance with eyes closed, lasted 30 s. The third trial lasted 60 s and was again a hip-wide, bipedal, eyes-open trial, in which participants were asked to count backwards in steps of two starting from a random three-digits number (e.g., 374, 372, 370, …). Counting backwards in steps of two was chosen for the dual-task condition since it is a continuous task that is difficult enough to permanently require cognitive resources, provoking an automatized postural control, but it is not so difficult that it might disturb postural control [10]. Participants were asked to count as fast and correctly as possible. The number of answers and of mistakes were recorded to encourage participants to try their best, however, for the purpose of the current study the actual cognitive performance was not further analyzed. Between trials, participants had breaks of at least 90 s in which they could move freely around the room. The order of the trials was not randomized since, on the one hand, we did not anticipate fatigue effects, and, on the other hand, we could not rule out that participants might silently repeat a similar cognitive task (silently counting) if a trial without instructions followed a dual-tasking trial. By performing the single-task balancing trials first, we hoped to avoid preconditioning the participants.
2.4. Instrumentation
The motion of the COP was recorded at 3 kHz using a ground-embedded AMTI force plate (AMTI, Watertown, USA). Synchronized with the force plate, kinematic data quantifying postural movements of the body were collected at 250 Hz using an 8-camera Vicon motion tracking system (Vicon Motion Systems Ltd., Oxford, UK) and 39 retro-reflective markers attached to the volunteers at anatomical landmarks on the skin or on tight sport-clothing using double sided tape. Markers on the head and wrists were attached using modified sweatbands. The marker model was based on the full-body Plug-In Gait marker model provided by Vicon. Force plate and kinematic data were synchronously collected using the Vicon software (Vicon Nexus, Version 2.2.3; Vicon Motion Systems Ltd., Oxford, UK).
2.5. Data Processing
MatLab R2019b (The Mathworks Inc. Natick, MA, USA) was used to process the data from the force plate, as well as the kinematic motion tracking data. In all trials, the first five 5 s were omitted to avoid “settling-in” effects and the following 25 s were selected for the analysis.
2.6. Processing of COP Data
The center of pressure (COP) data was down sampled to 250 Hz to have equal frequencies for further analysis. The force plate recorded COP data, which was processed in the anterior-posterior and medio-lateral direction. The standard deviation of COP displacement, the COP mean velocity and the amplitude of COP displacement were calculated [37]. To normalize and enable comparisons between participants of different size, COP data was normalized using the statokinesigram method [38]. The used code for calculating the variables using MatLab can be found in the Supplementary Material.
2.7. Processing of Kinematic Data: Calculation of Principal Movements (PMs)
The kinematic data was analyzed using a principal component analysis (PCA) to evaluate postural movements, i.e., changes in the postural configuration [29]. All of the following PCA-based calculations were computed using a software package called “PManalyzer” [39], which is provided through open access. As a first analysis step, gaps in the marker trajectories were filled [40,41], then the data from each trial was normalized by subtracting the mean posture and dividing by the trial’s mean Euclidean distance [29,32], finally, the marker coordinates were weighed according to the relative body mass, which they represent [29,42]. This normalization was designed to remove anthropometric differences while preserving the relative amplitude of the marker movements and to ensure that each participant equally affected the PCA output [29,32]. Hence, after the normalization, the data from all trials and all subjects could be concatenated into one single input matrix for the PCA [28,30,43,44]. Therefore, the PCA resulted in one set of eigenvalues EVk and one set of eigenvectors PCk (k represents the order index), which were common to all participants and all trials. These PCk form an orthonormal basis in which postural movements can be quantitatively compared between subjects. Then, the normalized data from each trial were projected onto the PCk-basis vectors, which yield subject- and trial-specific scores PPk(t), where PP stands for principal (postural) position and each value of the PP-time series indicates how much the posture at time t deviates from the mean posture according to the movement pattern defined by the associated PCk vector [28,32,45].
Together, the PCk vector and the PPk time series can be called a principal movement (PMk), which is an analogy for other variables that are often used to describe human movement, for instance, the “knee movement” is also defined through an algorithm that encodes how the “knee angle” is calculated from the available information (in the PMk the PCk vectors provide this algorithm) and the actual evolution of the variable during the trial (knee angle(t); PPk(t)). This analogy can be further expanded: by (double) differentiation, one obtains the angular velocity (angular acceleration) of the knee angle, and similarly, one can calculate the principal velocity PVk and principal acceleration PAk of each PMk [29], thus quantifying the rate and acceleration at which postural changes occur. The PMk thus provide a set of variables (whole-body movement patterns) that quantify the specific task/movement for which they were calculated. From a (bio-)mechanical point of view, the PMk were validated by demonstrating that an independently measured kinematic variable, the center of pressure movement COP(t), can be predicted from the PMk with very high precision [29].
2.8. Dependent PM-Based Variables
In addition to the eigenvalues EVk, which were introduced above and which allow comparison to many other studies [28,30,31,43,44,45], we also calculated the absolute variance (aVark) of each PPk(t), which quantifies the subject- and trial-specific variance represented by each postural movement component. The variable aVark provides a measure for the amplitude of the associated movement component observed during each balancing trial.
In order to investigate alterations in neuromuscular control, we determined the number of zero crossings (Nk) in the PAk time series [45]. Each postural acceleration PAk is the result of the interplay between agonistic and antagonistic muscle groups. A large number of zero crossings suggests a tightly controlled movement that is frequently adjusted by the sensorimotor system. A decline in Nk could be an indication that the processing time for adjustments increases, or it could be an indication that a movement component is not controlled as tightly, e.g., because the system attributes less priority to the movement component. A number of previous studies have already used this variable and found that it is sensitive, for example, to age differences or to leg dominance [45,46,47].
2.9. Statistics
Shapiro Wilk Tests were used to test for normal distribution in the dependent variables. Three different trials were measured for each participant. Therefore, to asses for overall differences between the trials, a repeated, measured analysis of variance (rANOVA) was applied. Group (adolescents/adult) was considered as a between-subject factor. Since a separate rANOVA was calculated for each of the first 10 PMs, the Holm-Bonferroni correction [48] was applied to correct for a familywise accumulation of alpha errors. Then, p-values were ranked from lowest to highest and compared to a Holm-Bonferroni adjusted alpha level, which were calculated using the following equation:
If the Holm-Bonferroni corrected rANOVA indicated a significant difference, then a post-hoc test was performed to determine which trial(s) differed from the other(s). Here a normal Bonferroni-correction was applied.
If data were not normally distributed, then Friedmann tests with Dunn-Bonferroni post-hoc tests were performed. All statistical testing was conducted using SPSS (IBM SPSS Statistics, Version 24, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Center of Pressure Motion
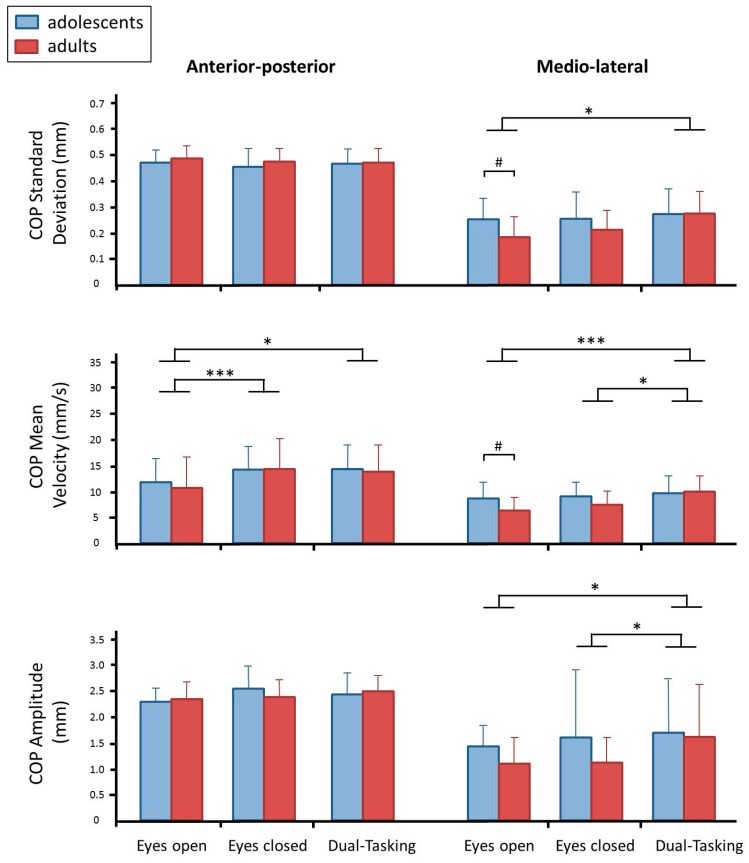
In the anterior-posterior direction (Figure 1, column 1), differences were only observed in the mean velocity of the COP motion (F(2, 72) = 11.107, p < 0.001, η2 = 0.236). Post-hoc tests showed that the COP mean velocity was smaller in the eyes-open trial compared to the eyes-closed trial (p < 0.001) and to the dual-tasking trials (p = 0.003).
Figure 1.
Characterization of the center of pressure (COP) motion: normalized standard deviation (SD), mean velocity, and amplitude. The anterior-posterior motion is shown on the left, the medio-lateral on the right. Blue bars represent the adolescents, red bars represent the adults (mean ± standard error). Asterisks indicate significance between trials (p < 0.001 ***, p < 0.05 *) and hashtags between groups (p < 0.05 #).
Also, in the medio-lateral direction (Figure 1, column 2), differences were found in the mean velocity (F(2, 72) = 13.796, p < 0.001, η2 = 0.277). The mean velocities of COP were faster during dual-tasking compared to the eyes-open situation (p < 0.001) and compared to standing with eyes-closed (p < 0.008). Moreover, the standard deviation of the COP (quantifying medio-lateral sway) differed between trials F(2, 72) = 5.809, p = 0.005, η2 = 0.139. In the eyes-open trial, the standard deviation was smaller than during dual-tasking (p = 0.004). The amplitude of COP presented similar results, showing larger amplitude in dual-task trials than in eyes-open (p = 0.048) and eyes-closed trials (p = 0.048).
Group effects appeared in two variables: the adolescents had larger standard deviations and faster mean velocities in the medio-lateral COP motion in the eyes-open trials (t(36) = 2.592, p = 0.014 and t(36) = 2.714, p = 0.010, respectively).
3.2. Amplitude of Postural Movement Components
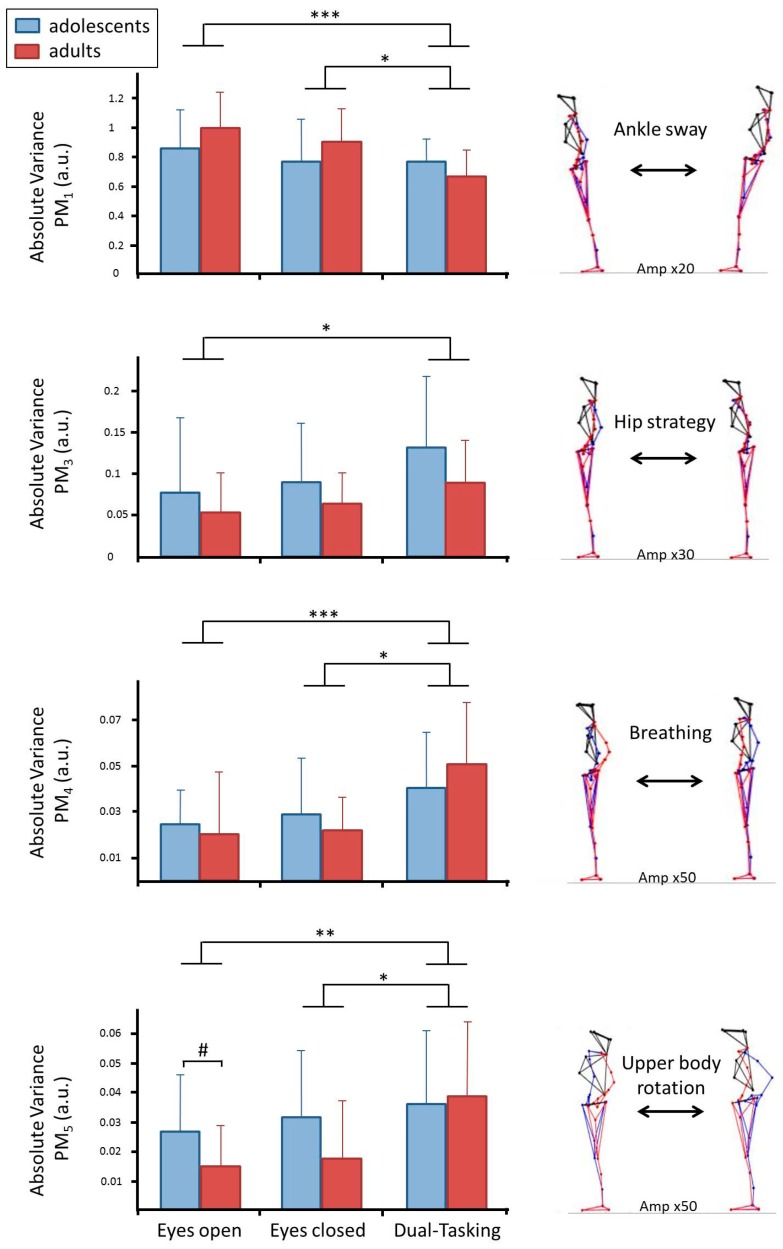
The first ten principal components explained 97.67% of the total postural variance. Video files provided in the Supplementary Materials visualize the movement components. Table 2 presents the eigenvalues EVk of each component and offers a qualitative description of the main aspects that each PMk represented. The table also includes the p-values obtained from rANOVAS (F) or Friedmann tests (X2) calculated on the absolute variances aVark for each PMk, and lists the rank and Holm-Bonferroni adjusted alpha-level. Table 3 lists the results of the post-hoc analysis. In summary, we observed that dual-tasking caused a decrease in movement amplitude in PM1, but an increase (or no changes) in the higher order movement components (Figure 2).
Table 2.
Eigenvalues EVk, a qualitative description of each principal movement (PM), the applied statistics (X2 for Friedmann and F for rANOVA), the calculated p-value to determine significant differences in aVark between trials, the resulting rank, and the adjusted alpha-level resulting from the Holm-Bonferroni correction are displayed. Significant p-values are printed in bold.
| PMk | EVk (%) | Qualitative Description of PMk | Statistics | p-Value | Rank | Holm-Correction of Alpha-Level |
|---|---|---|---|---|---|---|
| PM1 | 63.75 | Ankle sway anterior-posterior | F | 0.000010 | 2 | 0.0056 |
| PM2 | 14.77 | Hip sway medio-lateral | X 2 | 0.035362 | 9 | 0.0250 |
| PM3 | 7.29 | Hip flexion anterior-posterior | X 2 | 0.002479 | 7 | 0.0125 |
| PM4 | 2.67 | Frontal plane trunk rotation | X 2 | 0.000120 | 3 | 0.0063 |
| PM5 | 2.40 | Breathing | X 2 | 0.000683 | 6 | 0.0100 |
| PM6 | 2.09 | Breathing and head movement | X 2 | 0.030197 | 8 | 0.0167 |
| PM7 | 1.07 | Knee flexion anterior-posterior | X 2 | 0.056789 | 10 | 0.0500 |
| PM8 | 0.77 | Hip tilting anterior-posterior | X 2 | 0.000294 | 5 | 0.0083 |
| PM9 | 0.69 | Bringing shoulders back | X 2 | 0.000156 | 4 | 0.0071 |
| PM10 | 0.58 | Shifting legs anterior-posterior | X 2 | 0.000004 | 1 | 0.0050 |
Table 3.
Statistical results for the differences between trials in the aVark of PMk based on rANOVA (F) and Friedmann test (X2). The degrees of freedom (DoF) and p-values are reported, as well as the post-hoc results with p-values and Rosenthal’s r as effect size (r). Asterisks indicate significant difference (p < 0.001 ***; p = 0.001 **; p < 0.05 *). Abbreviations: EO—eyes-open; EC—eyes-closed; DT—dual-task.
| Overall Trial Effect on Variance | Post-Hoc | ||||||
|---|---|---|---|---|---|---|---|
| PMk | F|X2 | DoF | p-Value | EO–DT | r | EC–DT | r |
| PM1 | 13.623 | 2, 72 | 0.001 *** | 0.001 *** | - | 0.016 * | - |
| PM3 | 12.0 | 2 | 0.002 * | 0.002 * | 0.128 | 0.117 | 0.077 |
| PM4 | 18.053 | 2 | 0.001 *** | 0.001 *** | 0.149 | 0.004 * | 0.120 |
| PM5 | 14.579 | 2 | 0.001 * | 0.001 ** | 0.132 | 0.009 * | 0.111 |
| PM8 | 16.262 | 2 | 0.001 *** | 0.001 *** | 0.141 | 0.002 * | 0.115 |
| PM9 | 17.526 | 2 | 0.001 *** | 0.001 *** | 0.141 | 0.002 * | 0.128 |
| PM10 | 24.789 | 2 | 0.001 *** | 0.001 *** | 0.166 | 0.001 *** | 0.153 |
Figure 2.
Results for PM1, PM3, PM4, PM5 in variable aVark. Lines with asterisks (p < 0.001 ***; p = 0.001 **; p < 0.05 *) indicate significant differences between trials and hashtags between groups (p < 0.05 #). Blue bars represent adolescents, red bars adults (mean ± standard error). A visualization of the movement component through their extreme positions is included on the right. For better clarity, the deviation from the mean posture is amplified by the specified factor Amp.
3.3. Control of Postural Movement Components
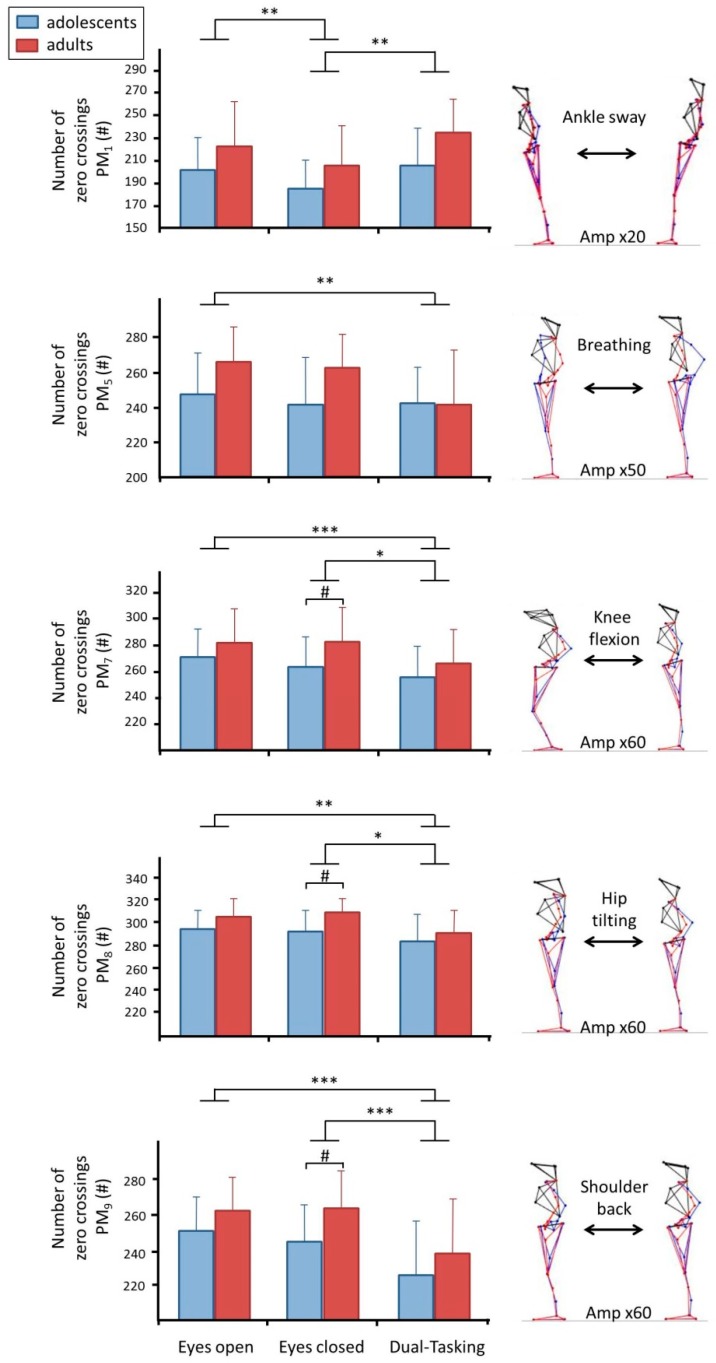
Within the first 10 PMk, significant trial effects in Nk were found in PM1, PM5, PM7, PM8 and PM9. (Table 4 and Figure 3). In PM1, N1 was smaller in the eyes-closed trials compared to eyes-open (p = 0.001) and dual-tasking trials (p = 0.001). In all higher order components, we observed either no differences (PM2, PM3, PM4, PM6, PM10,) or that Nk decreased in the dual-tasking trial compared to the eyes-open trial (PM5) or compared to both the eyes-open and the eyes-closed trials (PM7, PM8, PM9). Group differences were (after Holm-Bonferroni correction) found in PM7 (t(36) = 2.568, p = 0.015), PM8 (t(36) = 3.114, p = 0.004) and PM9 (t(36) = 2.871, p = 0.007) with adults showing greater Nk than adolescents, however, these differences occurred only in the eyes-closed trial.
Table 4.
Results of the statistical analysis evaluating trial differences in the variable Nk within the first 10 PMs. All data were normally distributed and thus the results are based on rANOVAs. The table lists the F-values, degrees of freedom (DoF), p-values, partial eta-squared η2, and where applicable the significance of pairwise post-hoc comparisons. Asterisks indicate significant results after applying the Holm-Bonferroni correction (p < 0.001 ***; p = 0.001 **; p < 0.05 *). Post-hoc tests revealed differences between eyes-open (EO), eyes-closed (EC) and dual-tasking (DT) trials.
| Overall Trial Effect on NoZC | Post-Hoc | ||||||
|---|---|---|---|---|---|---|---|
| PMk | F | DoF | p-Value | η 2 | EO–EC | EO–DT | EC–DT |
| PM1 | 10.813 | 1.707, 61.437 | 0.001 *** | 0.231 | 0.001 ** | 0.490 | 0.001 * |
| PM2 | 1.079 | 2, 72 | 0.345 | 0.029 | |||
| PM3 | 0.501 | 2, 72 | 0.608 | 0.014 | |||
| PM4 | 4.267 | 2, 72 | 0.018 * | 0.106 | |||
| PM5 | 8.360 | 1.695, 61.016 | 0.001 * | 0.188 | 0.342 | 0.001 ** | 0.083 |
| PM6 | 0.797 | 1.633, 58.779 | 0.433 | 0.022 | |||
| PM7 | 9.444 | 2, 72 | 0.001 *** | 0.208 | 1.000 | 0.001 *** | 0.010 * |
| PM8 | 11.176 | 1.711, 61,607 | 0.001 *** | 0.237 | 1.000 | 0.005 * | 0.001 * |
| PM9 | 19.233 | 1.349, 48.580 | 0.001 *** | 0.348 | 1.000 | 0.001 *** | 0.001 *** |
| PM10 | 2.248 | 2, 72 | 0.113 | 0.059 | |||
Figure 3.
Results for PM1, PM5, PM7, PM8, PM9 in variable Nk. Lines with asterisks (p < 0.001 ***; p = 0.001 **; p < 0.05 *) indicate significant differences between trials and hashtags between groups (p < 0.05 #). Blue bars represent adolescents, red bars represent adults (mean ± standard error). A visualization of the movement component through their extreme positions is included on the right. For better clarity, the deviation from the mean posture is amplified by the specified factor Amp.
4. Discussion
4.1. Main Results
The current study investigated dual-tasking effects, and hypothesized that the MIP needs to be considered. We argued that this implies divergent effects in different movement dimensions/components, depending on the relevance of the movement component for the task of maintaining stability. Specifically, we predicted for dual-tasking that anterior-posterior ankle sway, which is claimed to be of high importance for postural control [29,31,32] and was represented in PM1, would decrease in amplitude (aVark) while the frequency of control interventions (Nk) would increase [45,46,47]. Our results supported the first prediction (aVar1 decreased), but did not confirm the second prediction (N1 was not significantly different from eyes-open standing and the difference to N1 in the eyes-closed trial is most likely an effect of the system coping with reduced sensory information in the eyes-closed trial [17]). For higher-order, anterior-posterior movement components, e.g., PM3, PM7, PM8, PM9, and PM10, we had predicted a de-prioritization in the dual-tasking condition, which would manifest as an increase in movement amplitude and a decrease in Nk. Our results corroborated the amplitude prediction in PM3, PM8, PM9, and PM10 and the Nk prediction in PM7, PM8, and PM9. Finally, we expected that lateral ankle sway, which was represented in PM2, would not be affected by dual-tasking. Our results also corroborated this assumption, as no significant differences were found in aVar2 or N2. In summary, the results of the current study supported many of the various predictions. In contrast, no result was observed that directly contradicts the hypothesis of the minimum intervention principle [23] influencing how neural control of posture changes as attention is drawn away from the balancing task to a concurrent cognitive task.
In the current study we also hypothesized that adults would show clearer effects of dual-tasking since automatized postural control might be more refined compared to the still developing adolescents [19,20,21,22]. At first glance, several of the graphs in Figure 2 and Figure 3 seem to support this supposition—the dual-tasking effect seems to be driven more by the adult group than by the adolescent group, which is in line with previous research [9,49]. However, overall the statistical results could not support this assumption—most likely due to insufficient power. Differences between adults and adolescents in neural control of postural movements, and how this control may be affected by dual-tasking need to be investigated in future studies.
A curious result was observed in the COP data: our hypothesis predicted effects in the anterior-posterior movement components whereas we expected no effects on the medio-lateral sway—predictions that were largely corroborated when assessing the actual kinematic movement patterns. The COP motion, however, showed no effect on anterior-posterior movement amplitude, only on the anterior-posterior component of the COP velocity. In contrast, significant increases in medio-lateral COP movement amplitude and COP velocity were found for dual-tasking. The results for the anterior-posterior direction might be explained by a cancelation effect: the movement amplitude of PM1 decreased, but the movement amplitude of higher order movement components increased. It appears that these opposing effects canceled out their effect on the anterior-posterior COP motion. The increase in medio-lateral COP amplitude might be explained by the fact that the more complex higher-order movement components are less pure-plane motions than the lower-order ankle sway movement components. The increases that were observed in the amplitude of higher-order movement components may therefore affect medio-lateral COP motion. However, in the medio-lateral direction there is no opposing effect of a lower-order ankle sway that would diminish the effect on COP motion. Hence, an increase in medio-lateral COP motion amplitude was detectable.
According to the literature, COP presents larger sway in dual-tasking in both directions, the anterior-posterior [4,5] and medio-lateral axis [6]. These results and the observations of the COP motion presented here thus support the notion that postural movement patterns should be investigated in postural control research. Analyzing COP motion may not be sufficient to fully understand the changes that take place in the neural control of postural stability.
The current pilot study used the MIP as a theoretical framework to explain the effects of dual-tasking on postural control, and to our knowledge, presented the first application where the MIP was used to predict differences between single- and dual-tasking. Focusing on postural control in a bipedal stance confirmed the existing results concerning human movement strategies, but also helped to gather new insights into how movement control appears to be organized by the postural control system. Future studies should consider the MIP as an underlying mechanism and help to deepen the understanding of motor control hierarchies.
4.2. Limitations
The relatively small sample size is a limitation of the current study, especially after dividing into two groups. On the one hand, we could observe highly significant differences in aVark variance and Nk in the principal components, however, with small effect sizes (Table 3 and Table 4), resulting in small statistical power. On the other hand, the trend in differences seen between adolescents and adults might be significant if the study was repeated with a larger sample.
A third limitation is that the analysis was restricted to the first ten PMs. This restriction implies an approximation of the “real” postural movements, which can only be fully reconstructed from the whole set of PMs.
The non-randomized order of the trials might be seen as limitation; however, we did not expect the participants to fatigue, as we provided them at least 90 s of rest after every trial. Additionally, we hoped to prevent a preconditioning of the participants as mentioned in the Methods section.
5. Conclusions
The current study hypothesized that the MIP provides a concept by which dual-tasking effects on the organization and prioritization of postural control can be predicted. The experimental results were in good agreement with these predictions. Therefore, we suggest that the MIP should be considered in future investigations of postural control under dual-tasking conditions.
Acknowledgments
The authors would like to thank all volunteers for their participation; Tanja Csaszar, Manuel Eder, Nicole Voithofer and Michael Wurzer for their effort to make the measurements with the adolescents possible. The help of Stefanie Niedermeier, Lisa Thaler, Florian Hofstätter and Daniel Jochum during the measurements was highly appreciated.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/10/1/1/s1, Video S1: A video visualizing the analyzed PMs 1–5, Video S2 visualizes PMs 6–10, Code S3 is the code used for calculation of the COP variables.
Author Contributions
F.W. and P.F. initially conceived the experiment. F.W. designed and conducted the experiment. F.T. was involved in the measurement process and gathered parts of the data. A.P. helped implement the analysis procedures and gave valuable input in writing the manuscript; F.W. produced and analyzed the results and wrote the first draft of the paper. F.W. and P.F. contributed equally in interpreting the results and to revising and finalizing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Open access publishing fees were covered by a University of Innsbruck grant. The corresponding author F.W. currently holds a scholarship by the University of Innsbruck (funding-code: 2017/PSY/SPORT-23). Co-author A.P. holds a scholarship by the University of Phayao, Phayao, Thailand (grant number 28082015).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Lajoie Y., Richer N., Jehu D.A., Tran Y. Continuous Cognitive Tasks Improve Postural Control Compared to Discrete Cognitive Tasks. J. Mot. Behav. 2016;48:264–269. doi: 10.1080/00222895.2015.1089833. [DOI] [PubMed] [Google Scholar]
- 2.Woollacott M., Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/S0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 3.Wulf G., McNevin N., Shea C.H. The automaticity of complex motor skill learning as a function of attentional focus. Q. J. Exp. Psychol. 2001;54:1143–1154. doi: 10.1080/713756012. [DOI] [PubMed] [Google Scholar]
- 4.Polskaia N., Richer N., Dionne E., Lajoie Y. Continuous cognitive task promotes greater postural stability than an internal or external focus of attention. Gait Posture. 2015;41:454–458. doi: 10.1016/j.gaitpost.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Prado J.M., Stoffregen T.A., Duarte M. Postural Sway during Dual Tasks in Young and Elderly Adults. Gerontology. 2007;53:274–281. doi: 10.1159/000102938. [DOI] [PubMed] [Google Scholar]
- 6.Riley M.A., Baker A.A., Schmit J.M., Weaver E. Effects of visual and auditory short-term memory tasks on the spatiotemporal dynamics and variability of postural sway. J. Mot. Behav. 2005;37:311–324. doi: 10.3200/JMBR.37.4.311-324. [DOI] [PubMed] [Google Scholar]
- 7.Stins J.F., Roerdink M., Beek P.J. To freeze or not to freeze? Affective and cognitive perturbations have markedly different effects on postural control. Hum. Mov. Sci. 2011;30:190–202. doi: 10.1016/j.humov.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Swan L., Otani H., Loubert P.V., Sheffert S.M., Dunbar G.L. Improving balance by performing a secondary cognitive task. Br. J. Psychol. 2004;95:31–40. doi: 10.1348/000712604322779442. [DOI] [PubMed] [Google Scholar]
- 9.Olivier I., Palluel E., Nougier V. Effects of attentional focus on postural sway in children and adults. Exp. Brain Res. 2008;185:341–345. doi: 10.1007/s00221-008-1271-6. [DOI] [PubMed] [Google Scholar]
- 10.Huxhold O., Li S.-C., Schmiedek F., Lindenberger U. Dual-tasking postural control: Aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res. Bull. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Jamet M., Deviterne D., Gauchard G.C., Vançon G., Perrin P.P. Higher visual dependency increases balance control perturbation during cognitive task fulfilment in elderly people. Neurosci. Lett. 2004;359:61–64. doi: 10.1016/j.neulet.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Jamet M., Deviterne D., Gauchard G.C., Vançon G., Perrin P.P. Age-related part taken by attentional cognitive processes in standing postural control in a dual-task context. Gait Posture. 2007;25:179–184. doi: 10.1016/j.gaitpost.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Melzer I., Benjuya N., Kaplanski J. Age-related changes of postural control: Effect of cognitive tasks. Gerontology. 2001;47:189–194. doi: 10.1159/000052797. [DOI] [PubMed] [Google Scholar]
- 14.Pellecchia G.L. Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture. 2003;18:29–34. doi: 10.1016/S0966-6362(02)00138-8. [DOI] [PubMed] [Google Scholar]
- 15.Woollacott M., Vander Velde T. Non-visual spatial tasks reveal increased interactions with stance postural control. Brain Res. 2008;1208:95–102. doi: 10.1016/j.brainres.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Stins J.F., Michielsen M.E., Roerdink M., Beek P.J. Sway regularity reflects attentional involvement in postural control: Effects of expertise, vision and cognition. Gait Posture. 2009;30:106–109. doi: 10.1016/j.gaitpost.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Hansson E.E., Beckman A., Håkansson A. Effect of vision, proprioception, and the position of the vestibular organ on postural sway. Acta Otolaryngol. 2010;130:1358–1363. doi: 10.3109/00016489.2010.498024. [DOI] [PubMed] [Google Scholar]
- 18.Cote K.P., Brunet M.E., Gansneder B.M., Shultz S.J. Effects of Pronated and Supinated Foot Postures on Static and Dynamic Postural Stability. J. Athl. Train. 2005;40:41–46. [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson M.L., Christou E., Rosengren K.S. Children achieve adult-like sensory integration during stance at 12-years-old. Gait Posture. 2006;23:455–463. doi: 10.1016/j.gaitpost.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldi N.M., Polastri P.F., Barela J.A. Age-related changes in postural control sensory reweighting. Neurosci. Lett. 2009;467:225–229. doi: 10.1016/j.neulet.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Hirabayashi S., Iwasaki Y. Developmental perspective of sensory organization on postural control. Brain Dev. 1995;17:111–113. doi: 10.1016/0387-7604(95)00009-Z. [DOI] [PubMed] [Google Scholar]
- 22.Steindl R., Kunz K., Schrott-Fischer A., Scholtz A.W. Effect of age and sex on maturation of sensory systems and balance control. Dev. Med. Child Neurol. 2006;48:477–482. doi: 10.1017/S0012162206001022. [DOI] [PubMed] [Google Scholar]
- 23.Todorov E., Jordan M.I. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 2002;5:1226–1235. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- 24.Todorov E. Optimality principles in sensorimotor control. Nat. Neurosci. 2004;7:907–915. doi: 10.1038/nn1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott S.H. Optimal feedback control and the neural basis of volitional motor control. Nat. Rev. Neurosci. 2004;5:532–545. doi: 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein N. The Co-Ordination and Regulation of Movements. Pergamon-Press Ltd.; Headington Hill Hall, UK: 1967. [Google Scholar]
- 27.Diedrichsen J., Shadmehr R., Ivry R.B. The coordination of movement: Optimal feedback control and beyond. Trends Cogn. Sci. 2010;14:31–39. doi: 10.1016/j.tics.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daffertshofer A., Lamoth C.J.C., Meijer O.G., Beek P.J. PCA in studying coordination and variability: A tutorial. Clin. Biomech. 2004;19:415–428. doi: 10.1016/j.clinbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Federolf P.A. A novel approach to study human posture control: “Principal movements” obtained from a principal component analysis of kinematic marker data. J. Biomech. 2016;49:364–370. doi: 10.1016/j.jbiomech.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Troje N.F. Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. J. Vis. 2002;2:371–387. doi: 10.1167/2.5.2. [DOI] [PubMed] [Google Scholar]
- 31.Wachholz F., Kockum T., Haid T., Federolf P. Changed Temporal Structure of Neuromuscular Control, Rather Than Changed Intersegment Coordination, Explains Altered Stabilographic Regularity after a Moderate Perturbation of the Postural Control System. Entropy. 2019;21:614. doi: 10.3390/e21060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Federolf P., Roos L., Nigg B.M. Analysis of the multi-segmental postural movement strategies utilized in bipedal, tandem and one-leg stance as quantified by a principal component decomposition of marker coordinates. J. Biomech. 2013;46:2626–2633. doi: 10.1016/j.jbiomech.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Winter D. Human balance and posture control during standing and walking. Gait Posture. 1995;3:193–214. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- 34.Horak F.B., Nashner L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 35.Runge C.F., Shupert C.L., Horak F.B., Zajac F.E. Ankle and hip postural strategies defined by joint torques. Gait Posture. 1999;10:161–170. doi: 10.1016/S0966-6362(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 36.Massion J. Postural control system. Curr. Opin. Neurobiol. 1994;4:877–887. doi: 10.1016/0959-4388(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 37.Duarte M., Freitas S.M.S.F. Revision of posturography based on force plate for balance evaluation. Braz. J. Phys. Ther. 2010;14:183–192. doi: 10.1590/S1413-35552010000300003. [DOI] [PubMed] [Google Scholar]
- 38.De Oliveira J.M. Statokinesigram normalization method. Behav. Res. 2017;49:310–317. doi: 10.3758/s13428-016-0706-4. [DOI] [PubMed] [Google Scholar]
- 39.Haid T.H., Zago M., Promsri A., Doix A.-C.M., Federolf P.A. PManalyzer: A Software Facilitating the Study of Sensorimotor Control of Whole-Body Movements. Front. Neuroinform. 2019;13 doi: 10.3389/fninf.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Federolf P.A. A Novel Approach to Solve the “Missing Marker Problem” in Marker-Based Motion Analysis That Exploits the Segment Coordination Patterns in Multi-Limb Motion Data. PLoS ONE. 2013;8:e78689. doi: 10.1371/journal.pone.0078689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gløersen Ø., Federolf P. Predicting Missing Marker Trajectories in Human Motion Data Using Marker Intercorrelations. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0152616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gløersen Ø., Myklebust H., Hallén J., Federolf P. Technique analysis in elite athletes using principal component analysis. J. Sports Sci. 2017:1–9. doi: 10.1080/02640414.2017.1298826. [DOI] [PubMed] [Google Scholar]
- 43.Bockemühl T., Troje N.F., Dürr V. Inter-joint coupling and joint angle synergies of human catching movements. Hum. Mov. Sci. 2010;29:73–93. doi: 10.1016/j.humov.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Verrel J., Lövdén M., Schellenbach M., Schaefer S., Lindenberger U. Interacting effects of cognitive load and adult age on the regularity of whole-body motion during treadmill walking. Psychol. Aging. 2009;24:75–81. doi: 10.1037/a0014272. [DOI] [PubMed] [Google Scholar]
- 45.Haid T.H., Doix A.-C.M., Nigg B.M., Federolf P.A. Age Effects in Postural Control Analyzed via a Principal Component Analysis of Kinematic Data and Interpreted in Relation to Predictions of the Optimal Feedback Control Theory. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Promsri A., Haid T., Federolf P. How does lower limb dominance influence postural control movements during single leg stance? Hum. Mov. Sci. 2018;58:165–174. doi: 10.1016/j.humov.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Promsri A., Longo A., Haid T., Doix A.-C.M., Federolf P. Leg Dominance as a Risk Factor for Lower-Limb Injuries in Downhill Skiers—A Pilot Study into Possible Mechanisms. Int. J. Environ. Res. Public Health. 2019;16:3399. doi: 10.3390/ijerph16183399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- 49.Rival C., Ceyte H., Olivier I. Developmental changes of static standing balance in children. Neurosci. Lett. 2005;376:133–136. doi: 10.1016/j.neulet.2004.11.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.