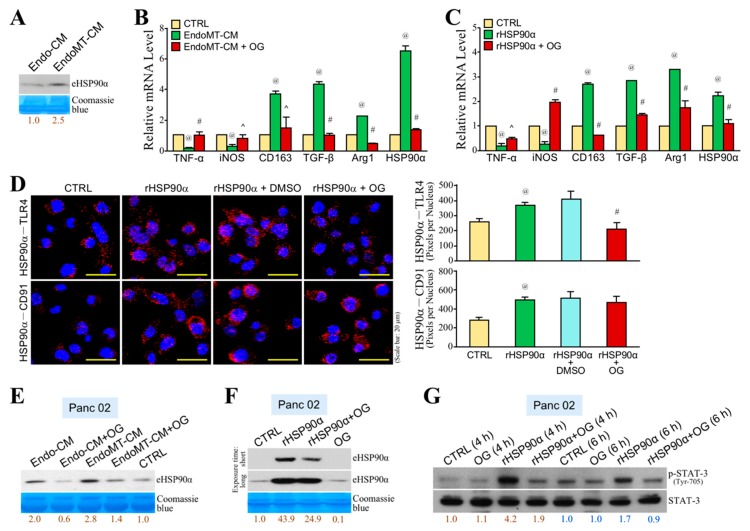
Figure 6.
OG inhibits EndoMT-induced macrophage M2-polarization and a feedforward loop of HSP90α secretion. (A) Immunoblot analysis of the eHSP90α levels from Endo-CM and EndoMT-CM. EndoMT-derived cells secreted more HSP90α when compared with Endo cells. Relative eHSP90α levels were presented after quantification of protein band intensities using ImageJ software. (B) mRNA levels of TNF-α, iNOS, CD163, TGF-β, Arg1, and HSP90α in the RAW264.7 cells treated 24 h with control medium (CTRL), EndoMT-CM, or EndoMT-CM plus 10 μM of OG. EndoMT-CM repressed mRNA levels of M1-associated TNF-α and iNOS, whereas those of M2-associated CD163, TGF-β, Arg1, and HSP90α were significantly up-regulated. The effects were abrogated by the presence of OG. @ p < 0.01 when compared with “CTRL” group. ^ p < 0.05 and # p < 0.01 when compared with “EndoMT-CM” group. (C) mRNA levels of TNF-α, iNOS, CD163, TGF-β, Arg1, and HSP90α in the RAW264.7 cells treated 24 h with PBS (CTRL) or 15 μg/mL of rHSP90α in the absence or presence of 10 μM of OG. rHSP90α treatment inhibited TNF-α and iNOS mRNA expressions but up-regulated CD163, TGF-β, Arg1, and HSP90α mRNA expression levels. The effects were antagonized by OG. @ p < 0.01 when compared with “CTRL” group. ^ p < 0.05 and # p < 0.01 when compared with “rHSP90α” group. (D) PLA showing that the physical association of eHSP90α with TLR4 but not with CD91 was prevented by OG on macrophages. Nuclei were stained with DAPI. @ p < 0.05 when compared with “CTRL” group. # p < 0.01 when compared with “rHSP90α + DMSO” group. (E) Immunoblot analysis of the eHSP90α levels from the conditioned media of the Panc 02 cells pretreated 24 h with control medium (CTRL), Endo-CM, or EndoMT-CM in the absence or presence of 5 μM of OG. The conditioned media were collected as described in Materials and Methods. The result revealed that EndoMT-CM was more than Endo-CM to enhance HSP90α secretion from Panc 02 cells, and this enhancement was inhibited by the presence of OG. (F) Immunoblot analysis of the eHSP90α levels from the conditioned media of the Panc 02 cells pretreated 24 h with 15 μg/mL of rHSP90α in the absence or presence of 5 μM of OG. rHSP90α treatment was able to stimulate HSP90α secretion from Panc 02 cells, which was inhibited by the presence of OG. (G) Immunoblot analysis of the phosphorylated and total STAT-3 levels from the Panc 02 cells treated 4 or 6 h with rHSP90α in the absence or presence of OG. Quantification of protein band intensities was performed using ImageJ software, and the relative p-STAT-3 levels were presented after normalization to total STAT-3. rHSP90α-induced STAT-3 phosphorylation (activation) was significantly suppressed by OG.