Studies have shown an association between female infertility and exposure to endocrine-disrupting chemicals (EDCs), yet strategies for neutralizing such effects are lacking. Bisphenol A (BPA) is a prevalent EDC that affects...
Keywords: meiosis, germline, C. elegans, Bisphenol A, Coenzyme Q10
Abstract
Endocrine-disrupting chemicals are ubiquitously present in our environment, but the mechanisms by which they adversely affect human reproductive health and strategies to circumvent their effects remain largely unknown. Here, we show in Caenorhabditis elegans that supplementation with the antioxidant Coenzyme Q10 (CoQ10) rescues the reprotoxicity induced by the widely used plasticizer and endocrine disruptor bisphenol A (BPA), in part by neutralizing DNA damage resulting from oxidative stress. CoQ10 significantly reduces BPA-induced elevated levels of germ cell apoptosis, phosphorylated checkpoint kinase 1 (CHK-1), double-strand breaks (DSBs), and chromosome defects in diakinesis oocytes. BPA-induced oxidative stress, mitochondrial dysfunction, and increased gene expression of antioxidant enzymes in the germline are counteracted by CoQ10. Finally, CoQ10 treatment also reduced the levels of aneuploid embryos and BPA-induced defects observed in early embryonic divisions. We propose that CoQ10 may counteract BPA-induced reprotoxicity through the scavenging of reactive oxygen species and free radicals, and that this natural antioxidant could constitute a low-risk and low-cost strategy to attenuate the impact on fertility by BPA.
FERTILITY decay related to aging in women, also referred to as the “maternal age effect,” is not attributable to a single factor but to an interplay of both endogenous and exogenous features (Nagaoka et al. 2012). Recent studies have shown an association between female infertility and exposure to endocrine-disrupting chemicals (EDCs) (Messerlian et al. 2016; Bloom et al. 2017; Wang et al. 2017a). Bisphenol A (BPA) is a widespread EDC employed in the manufacture of plastics and epoxy resins used in several consumer goods, such as polycarbonate water bottles, the inside lining of cans, food packaging, toys, and cash register receipts (Rocha et al. 2018). Studies in mice and chimps have shown that BPA exposure adversely affects reproduction, resulting in aneuploidy and infertility (Hunt et al. 2003; Susiarjo et al. 2007), whereas in humans it is associated with an increased risk of miscarriages, delayed menarche in girls, and late pubertal progression in boys (Lawson et al. 2011; Hunt et al. 2012; Lathi et al. 2014; Shen et al. 2015; Wang et al. 2017b; Watkins et al. 2017). We have previously demonstrated that exposure to this EDC leads to decreased brood size and embryonic lethality as a result of disruption of DNA double-strand break repair (DSBR) progression, producing genomic instability during meiosis in the nematode Caenorhabditis elegans (Allard and Colaiacovo 2010). Given the widespread prevalence of EDCs in our environment, it is critically important to understand their impact on reproductive health, as well as to identify ways to mitigate their effects and improve fertility and early embryogenesis outcomes.
Coenzyme Q10 (CoQ10) is a potent antioxidant that has been reported to attenuate the damage promoted by a number of toxicants (Binukumar et al. 2011; Kandhare et al. 2013; Arany et al. 2017) and to have a beneficial effect on fertility (Ben-Meir et al. 2015). For instance, the age-related decline in oocyte quality and quantity (Ben-Meir et al. 2015) and the obesity-induced oocyte mitochondrial defects observed in female mice (Boots et al. 2016) were reversed by the administration of CoQ10. However, while some studies on women undergoing in vitro fertilization (IVF) treatment suggest a correlation between elevated levels of CoQ10 and improved fertility (Gat et al. 2016; Xu et al. 2018), others have failed to detect an effect (Bentov et al. 2014). To test the rescuing potential of CoQ10 against BPA-induced reprotoxic effects, we employed the nematode C. elegans, which is a powerful multicellular model system for studies on meiosis because: (1) its germline is well characterized and amenable to study using genetic, biochemical, and molecular biology tools combined with powerful cytological approaches (Colaiácovo 2006; Lui and Colaiacovo 2013); (2) C. elegans is transparent and nuclei are ordered in a spatiotemporal gradient within the germline, allowing for visualization of qualitative and quantitative changes in oogenesis and embryogenesis; (3) it has a short life cycle, developing from eggs to egg-laying adults via four larval stages (L1–L4) in 3 days at 20° vs. several months and years in rodents and humans, respectively; (4) there is a large number of offspring laid by each individual (200–300 eggs in 3–4 days); and (5) it is a model highly predictive of mammalian reproductive toxicities that provides a cost-effective and easy to control alternative over conventional toxicological studies on mammals (Allard et al. 2013). In addition, genes of the CoQ biosynthetic pathway are conserved between C. elegans and humans, and analysis of genetic mutants has shown a role for CoQ in supporting embryonic development and fertility in the worm (Asencio et al. 2009).
Here, we demonstrate that BPA leads to increased meiotic DSBs and oxidative stress in the germline, and show for the first time that CoQ10 can rescue decreased fecundity and reproductive toxicity effects induced by BPA in this system. After supplementation with CoQ10, BPA-treated worms showed a reduction in the levels of chromosome defects in oocytes at diakinesis. CoQ10 treatment also decreased the elevated levels of germ cell apoptosis and pCHK-1 signal seen in BPA-treated worms. In addition, we found elevated DSB levels and impaired DSB repair, as revealed by elevated levels of RAD-51 foci following BPA exposure that were significantly decreased following CoQ10 supplementation. Using an oxidative stress reporter strain, where GFP expression is driven by the glutathione S-transferase 4 (gst-4) promoter (Detienne et al. 2016), we observed a significant decrease in GFP signal in gonads of CoQ10-treated worms compared to BPA-only exposed worms, suggesting that BPA exposure generates oxidative stress in the germ cells that is neutralized by CoQ10. Our observation of rescued Mitotracker signal in CoQ10-treated worms suggests that the increased oxidative stress reported by the gst-4::GFP transgene in the gonads may be due, in part, to increased mitochondrial dysfunction following BPA exposure. Moreover, CoQ10 supplementation significantly decreased the elevated expression of oxidative stress response genes (sod-1 and gpx-4) observed in BPA-exposed germlines. Finally, live imaging and high-throughput analysis indicate that CoQ10 supplementation can reduce the elevated levels of BPA-induced defects and chromosome nondisjunction observed in early embryos. These findings reveal that BPA may induce DNA damage in the germline, due, in part, to elevated oxidative stress, and suggest that CoQ10 rescues BPA-induced reprotoxicity through the scavenging of reactive oxygen species and free radicals. We propose that supplementation with this natural antioxidant may constitute a low-risk and low-cost strategy to attenuate the impact on fertility from BPA exposure.
Materials and Methods
C. elegans strains and experimental design
C. elegans strains were cultured according to Brenner (1974) at 20° on nematode growth medium (NGM) plates without cholesterol, given that cholesterol masks the effects of endocrine-disrupting chemicals in C. elegans (Goldstein 1986; Hoshi et al. 2003; Allard and Colaiacovo 2010). All experiments were done in a col-121(nx3) mutant background (Watanabe et al. 2005), which increases chemical sensitivity, allowing for analysis of effects of lower doses of BPA (Allard et al. 2013). The following mutations and chromosome rearrangements were used in this study: LGI: rad-54(ok615); LGIII: glp-1(bn18); LGIV: col-121(nx3); LGV: yIs34[Pxol-1::GFP, rol-6]; LGX: sek-1(km4), transgenes: H2B::mCherry; γ-tubulin::GFP; dvIs19 [(pAF15)gst-4p::GFP::NLS] III (Leiers et al. 2003; Watanabe et al. 2005; Allard and Colaiacovo 2010; Nottke et al. 2011). All lines used for this study are listed in Supplemental Material, Table S1.
BPA (≥99%) (Sigma-Aldrich) and CoQ10 (Kaneka, ubiquinol ≥96%, ubiquinone ≤2%) were dissolved in dimethylsulfoxide (DMSO). BPA and DMSO (control) were added to the medium before pouring plates for a final DMSO concentration of 0.1%. Exposure was carried out by placing synchronized L1 stage worms [synchronized by sodium hypochlorite treatment as in Stiernagle (2006)] onto DMSO/BPA-containing plates seeded with Escherichia coli strain OP50. Plates were then incubated for ∼53 hr at 20° until worms reached the L4 stage. At the L4 stage, worms were washed three times with M9, and exposed to DMSO, DMSO + CoQ10, BPA or BPA + CoQ10 in liquid culture (M9 buffer containing OP50 grown to OD600 = 24) in 24-well plates. Between 200 and 300 worms were placed in each well. After 24 hr, worms were washed five times in M9 and collected for evaluation.
Scoring embryonic lethality, larval lethality, and brood size
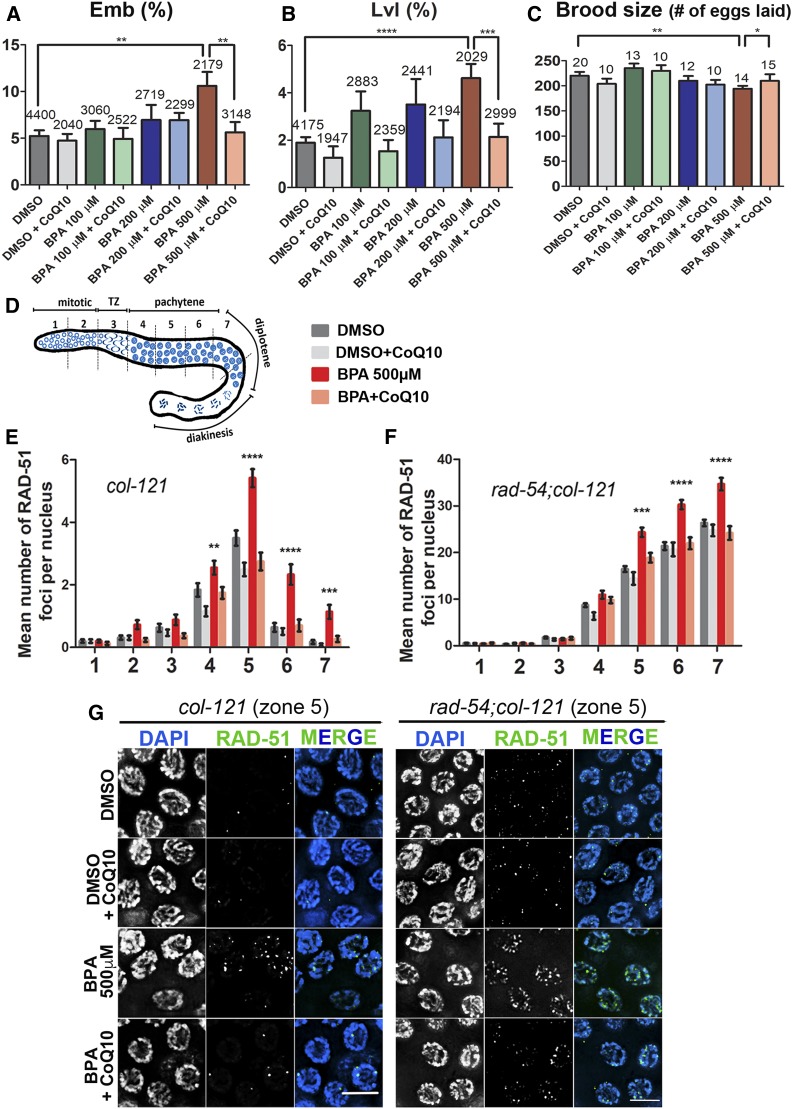
Age-matched worms exposed as described above (from L1 to 24 hr post-L4) were washed five times in M9 and transferred to NGM plates to score their embryonic lethality, larval lethality, and brood size. Worms were transferred to new NGM plates every 24 hr for four consecutive days. The total number of fertilized eggs laid, hatched, and the number of progeny that reached adulthood were scored. At least two independent biological replicates for each condition were assessed. P-values for col-121 worms (Figure 1) for (A) Emb: BPA 500 µM vs. DMSO, P = 0.0031; BPA 500 µM vs. BPA 500 µM + CoQ10, P = 0.0049; (B) Lvl: BPA 500 µM vs. DMSO, P < 0.0001; BPA 500 µM vs. BPA 500 µM + CoQ10, P = 0.0015; (C) Brood size: BPA 500 µM vs. DMSO, P = 0.0074; BPA 500 µM vs. BPA 500 µM CoQ10, P = 0.0173.
Figure 1.
CoQ10 rescues BPA-induced embryonic lethality (Emb), larval lethality (Lvl), and decreased brood size, and it protects from BPA-induced defects in DNA double-strand break formation and repair. (A) Embryonic lethality observed among the progeny of worms exposed to vehicle alone (0.1% DMSO) or the indicated doses of BPA, either with or without subsequent supplementation with 100 µg/ml CoQ10. Indicated above the error bars are the total numbers of eggs laid by the exposed hermaphrodites. (B) Larval lethality observed among the hatched progeny of exposed worms. Indicated above the error bars are the total numbers of larvae hatched from the eggs laid by the exposed hermaphrodites. (C) Mean number of eggs laid by worms exposed to the indicated conditions. Indicated above the error bars are the total numbers of hermaphrodites exposed from L1 to 24 hr post-L4, for which entire brood sizes were scored. Error bars represent SEM. P values calculated by the two-tailed Mann-Whitney test, 95% C.I. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. (D) Diagram of the C. elegans germline indicating the position of the seven zones scored for RAD-51 foci. TZ, transition zone, corresponds to leptotene/zygotene stages of meiosis. (E and F) Mean number of RAD-51 foci scored per nucleus (y-axis) for each zone along the germline axis (x-axis) for the indicated genotypes. (E) Histogram indicates a significant increase in levels of RAD-51 foci in col-121 worms exposed to BPA-only compared to other cases. (F) Histogram indicates that the mean number of RAD-51 foci detected per nucleus is increased starting in midpachytene (zone 5) in rad-54;col-121 worms exposed to BPA-only compared to other cases. (G) Representative images of the immunostaining for RAD-51 on midpachytene nuclei (zone 5) from dissected gonads of col-121 (left) and rad-54;col-121 (right) treated worms. Error bars in (E and F) represent SEM. Two-tailed Mann-Whitney test, 95% C.I. Levels of RAD-51 foci in worms exposed to BPA only are statistically different from other cases with the following P values: **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. DMSO was used at 0.1%; CoQ10 at 100 µg/ml, and BPA at 500 µM. Bar, 5 μm. N = 6 gonads.
DAPI analysis and immunostaining
Whole-mount preparation of dissected gonads, DAPI staining, immunostaining, and analysis of stained germline nuclei were performed as previously reported (Colaiácovo et al. 2003). Primary antibodies were used at the following dilutions: rabbit α-RAD-51 at 1:10,000 [Novus Biological (SDI)], and rabbit α-pCHK-1 at 1:100 (Santa Cruz). The secondary antibody used was Alexa-488 α-rabbit (1:500) (Jackson Immunochemicals). At least two independent biological replicates for each condition were assessed with each primary antibody.
Imaging and microscopy
Immunofluorescence images were collected at 0.2-μm intervals with an IX-70 microscope (Olympus) and a cooled CCD camera (model CH350; Roper Scientific) controlled by the DeltaVision system (Applied Precision). The representative images presented correspond to projections approximately halfway through three-dimensional (3D) data stacks of whole nuclei, except for the −1 oocytes at diakinesis, in which entire nuclei were captured. Images were subjected to deconvolution analysis using the SoftWorx 3.3.6 program (Applied Precision) as described in de Carvalho et al. (2008).
Live imaging was performed as described in Allard and Colaiacovo (2010). Briefly, worms were immobilized with 0.01% levamisole on 3% agarose pads. Images were captured with a 60X objective every 10 sec on the IX-70 microscope described above. Nine independent biological replicates were done for the live imaging assay.
Quantitative spatiotemporal analysis for RAD-51 foci
Quantitation of RAD-51 foci for all seven zones composing the germline of both col-121 and rad-54;col-121 worms was performed as previously described (Colaiácovo et al. 2003). The average number of nuclei scored per zone (n) from six gonads for each group was as follows: for the col-121 line: zone 1 (n = 91 ± 11), zone 2 (n = 97 ± 11), zone 3 (n = 79 ± 6), zone 4 (n = 80 ± 5), zone 5 (n = 78 ± 8), zone 6 (n = 69 ± 8), and zone 7 (n = 56 ± 4). For the rad-54;col-121 line: zone 1 (n = 92 ± 18), zone 2 (n =106 ± 23), zone 3 (n = 97 ± 18), zone 4 (n =57 ± 4), zone 5 (n = 44 ± 6), zone 6 (n = 37 ± 1) and zone 7 (n = 32 ± 6). Statistical comparisons between treatments were performed using the two-tailed Mann-Whitney test, 95% confidence interval (C.I). P-values for col-121 worms (Figure 1E) in zone 4: BPA 500 µM vs. DMSO, P = 0.0076; BPA 500 µM vs. DMSO + CoQ10, P < 0.0001; BPA 500 µM vs. BPA 500 µM + CoQ10, P = 0.006; in zones 5 and 6: BPA 500 µM vs. DMSO; BPA 500 µM vs. DMSO + CoQ10; BPA 500 µM vs. BPA 500 µM + CoQ10, P < 0.0001 (all pairs); in zone 7: BPA 500 µM vs. DMSO, P = 0.0002; BPA 500 µM vs. DMSO + CoQ10, P < 0.0001; BPA 500 µM vs. BPA 500 µM + CoQ10, P = 0.0004. P-values for rad-54;col-121 worms (Figure 1F) in zone 5: BPA 500 µM vs. DMSO, P < 0.0001; BPA 500 µM vs. DMSO + CoQ10, P < 0.0001; BPA 500 µM vs. BPA 500 µM + CoQ10, P = 0.001; in zones 6 and 7: BPA 500 µM vs. DMSO; BPA 500 µM vs. DMSO + CoQ10; BPA 500 µM vs. BPA 500 µM + CoQ10, P < 0.0001 (all pairs).
Quantitative analysis for pCHK-1 foci and mitotic nuclear diameter
Quantitation of pCHK-1 foci in nuclei at the premeiotic tip (zones 1 and 2) and pachytene (zones 5 and 6) regions in the germlines of col-121 worms was performed as previously described (Kim and Colaiacovo 2014). The average number of nuclei scored per zone (n) from six gonads for each group was as follows: Premeiotic tip: zone 1 (n = 87 ± 8) and zone 2 (n = 76 ± 21); Pachytene: zone 5 (n = 75 ± 7) and zone 6 (n = 62 ± 11). Nuclear diameters of nuclei in the premeiotic tip were measured using the ruler tool available in the SoftWorx 3.3.6 program (Applied Precision). Statistical comparisons between treatments were performed using the two-tailed Mann-Whitney test, 95% C.I.
Quantitative analysis of germ cell apoptosis
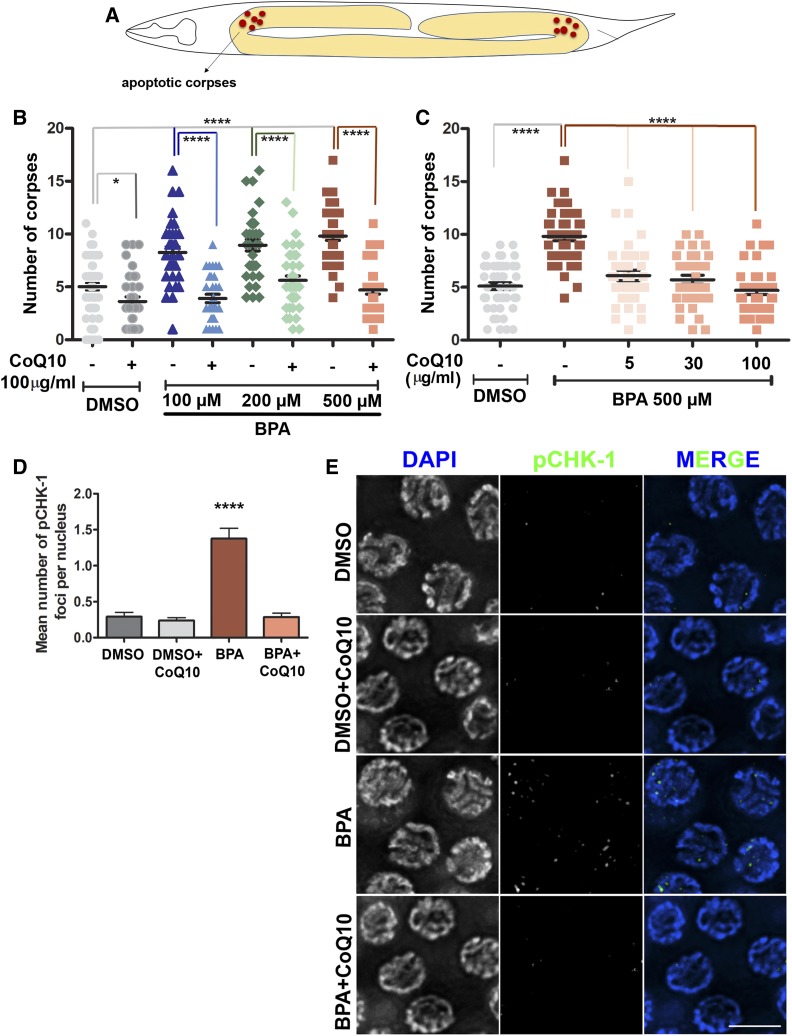
Germ cell corpses were scored in adult (24 hr post L4) hermaphrodites (col-121 worms), using acridine orange as described in Kelly et al. (2000), utilizing a Leica DM5000B fluorescence microscope. Between 32 and 67 gonads were scored, from at least three independent biological replicates for each chemical treatment. Statistical comparisons between treatments were performed using the two-tailed Mann-Whitney test, 95% C.I. P-values for Figure 2: (B): DMSO vs. DMSO + CoQ10, P = 0.0156; DMSO vs. BPA 100 µM +CoQ10, P = 0.0435; DMSO vs. BPA 100, 200, and 500 µM, P < 0.0001 (all pairs); BPA 100 µM vs. BPA 100 µM +CoQ10, BPA 200 µM vs. BPA 200 µM + CoQ10, BPA 500 µM vs. BPA 500 µM + CoQ10, P < 0.0001 (all pairs); in (C): BPA 500 µM vs. BPA 500 µM + CoQ10 at 5 or 30 or 100 µg/ml, P < 0.0001 (all pairs); in (D): BPA 500 µM vs. DMSO, BPA 500 µM vs. DMSO + CoQ10, BPA 500 µM vs. BPA 500 µM + CoQ10, P < 0.0001 (all pairs).
Figure 2.
CoQ10 rescues BPA-induced elevated germ cell apoptosis and pCHK-1 foci levels detected in pachytene nuclei. (A) Schematic representation of the U-shaped gonad arms of C. elegans indicating where the acridine orange stained corpses are scored. (B) Dose-response curve showing the quantification of apoptotic corpses for the indicated doses of BPA with and without CoQ10 (100 µg/ml) supplementation. (C) Lower doses of CoQ10 were also able to counteract the elevated BPA-induced (500 µM) germ cell apoptosis. Two-tailed Mann-Whitney test, 95% C.I. *P ≤ 0.05; ***P ≤ 0.001; ****P ≤ 0.0001. DMSO was used at 0.1%. N ≥ 32, number of gonads scored per condition. (D) Mean number of pCHK-1 foci per nucleus ± SEM scored in mid to late pachytene for the indicated conditions. (E) Representative images of the immunostaining for pCHK-1 on midpachytene (zone 5) nuclei from dissected gonads. Two-tailed Mann-Whitney test, 95% C.I. ****P ≤ 0.0001 compared to all other conditions. DMSO was used at 0.1%; CoQ10 at 100 µg/ml and BPA at 500 µM. Bar, 5 µm. N = 6, number of gonads scored per condition.
Oxidative stress analysis
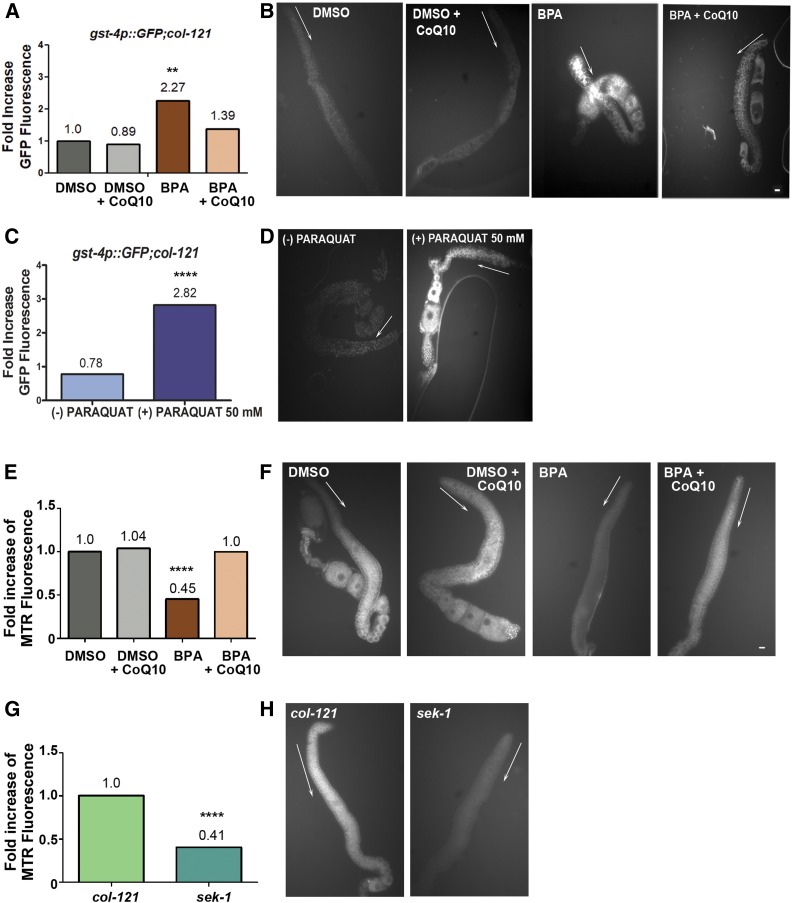
For evaluation of oxidative stress, we used a reporter strain carrying GFP expressed under the control of the gst-4 promoter {dvIs19 [(pAF15)gst-4p::GFP::NLS] III; col-121(nx3) IV}. After treatment, worms were washed in M9 five times, and their gonads were dissected and examined utilizing a Leica DM5000B fluorescence microscope. As controls, we also compared 24 hr post-L4 gst4p::GFP worms that were either treated (+) or not (−) with paraquat 50 mM for 1 hr, given that paraquat is a compound that leads to elevated reactive oxygen species in the worm by increasing superoxide levels primarily in mitochondria (Castello et al. 2007; Keith et al. 2014). The fluorescence intensities of the gonads and background were quantified using ImageJ software. Four independent biological replicates were performed for the oxidative stress assay. Between 26 and 29 gonads were scored for each chemical treatment. Statistical comparisons between treatments were performed using the two-tailed Mann-Whitney test, 95% C.I. P-values (Figure 5A): BPA 500 µM vs. DMSO, P = 0.0002; BPA 500 µM vs. DMSO + CoQ10, P < 0.0001; BPA 500 µM vs. BPA 500 µM + CoQ10, P = 0.0157.
Figure 5.
BPA-induced oxidative stress and mitochondrial dysfunction in the germline are rescued by CoQ10 supplementation. (A) Fold increase of gonadal GFP fluorescence showing CoQ10 rescues the elevated signal in gonads from worms exposed to BPA. gst-4p::GFP;col-121 transgenic worms were examined. (B) Dissected gonads from transgenic gst-4p::GFP; col-121 worms exposed to the indicated chemicals. GFP signal is elevated in the germlines of BPA-exposed worms, and that signal is decreased in the gonads of BPA-exposed worms supplemented with CoQ10. Gonads from worms exposed to either DMSO-only or DMSO + CoQ10 are shown as controls. (C) Fold increase of gonadal GFP fluorescence showing paraquat treatment (50 mM) significantly augments the signal in gst-4p::GFP;col-121 worms. (D) Dissected gonads from transgenic gst-4p::GFP; col-121 worms either exposed (+) or not (−) to paraquat 50 mM. (E–H) Staining of mitochondria using Mitotracker Red was examined in the gonads. (E) Fold increase of gonadal Mitotracker Red (MTR) fluorescence showing CoQ10 rescues mitochondrial function in BPA-exposed worms. (F) Dissected gonads from col-121 worms exposed to the indicated chemicals. Mitotracker Red signal is significantly decreased in the germlines of BPA-exposed worms and increased in the gonads of BPA-exposed worms supplemented with CoQ10. Gonads from worms exposed to either DMSO-only or DMSO + CoQ10 are shown as controls. (G) Quantification of gonadal Mitotracker Red (MTR) signal detected in untreated sek-1 mutants showing a significant decrease compared to untreated col-121 (control). (H) Dissected gonads from col-121 and sek-1 worms were examined after Mitotracker staining. White arrows are positioned adjacent to the premeiotic region indicating the direction of germline progression. Dissected gonads are shown to facilitate visualization and quantitation of signal specifically in the germline, which otherwise is confounded by signal in other regions of the worms’ bodies. Statistically different from DMSO and DMSO + CoQ10 at P ≤ 0.0001, statistically different from BPA + CoQ10 at **P ≤ 0.01 (A); statistically different from (−) paraquat at ****P ≤ 0.0001 (C); statistically different from DMSO and BPA at ****P < 0.0001, statistically different from BPA + CoQ10 at ****P < 0.0001 (E); statistically different from col-121 and sek-1 at ****P < 0.0001 (G) by the two-tailed Mann-Whitney test, 95% C.I. DMSO used at 0.1%; CoQ10 at 100 µg/ml and BPA at 500 µM. Bar, 5 µm. N ≥ 18, number of gonads scored per treatment.
Staining of mitochondria
To evaluate effects on mitochondrial morphology or function stemming from BPA exposure, we used Mitotracker Red CMXRos (Invitrogen)—a mitochondrial dye that accumulates in a manner dependent on mitochondrial membrane potential—in worms exposed to DMSO, DMSO + CoQ10, BPA, and BPA + CoQ10 as described above. Mitotracker Red CMXRos was dissolved in M9 to a stock concentration of 1 µg/µl and added to late L4 stage col-121 worms in liquid culture at a final concentration of 1 µg/ml for 24 hr. After treatment, worms were washed in M9 five times, and placed on regular NGM plates containing only OP50 E. coli for 1 hr to clear any residual dye present on their intestinal tracts. Gonads were dissected and examined utilizing a Leica DM5000B fluorescence microscope. As controls, we also compared untreated col-121 and sek-1 mutants, given that sek-1 encodes for a MAPKK that phosphorylates and activates p38 MAPK under oxidative stress conditions (Inoue et al. 2005; Hourihan et al. 2016). The fluorescence intensities of the cytoplasmic area of the gonads and background were quantified using ImageJ software. Three independent biological replicates were performed for the Mitotracker Red staining assay (DMSO: n = 31, DMSO + CoQ10: n = 32, BPA 500 µM: n = 31, BPA 500 µM + CoQ10: n = 27, col-121, untreated: n = 21, sek-1, untreated: n = 18). Statistical comparisons between treatments were performed using the two-tailed Mann-Whitney test, 95% C.I. P-values (Figure 5E): BPA 500 µM vs. DMSO, P < 0.0001; BPA 500 µM vs. BPA 500 µM + CoQ10, P < 0.0001; (Figure 5G): col-121 vs. sek-1, P < 0.0001.
Worm lysis and liquid chromatography–tandem mass spectrometry analysis of BPA
After exposure, worms were washed 10 times in M9 buffer, and the worm pellet was frozen in liquid nitrogen. The pellet was then resuspended in lysis buffer [25 mM Hepes (pH 7.6), 5 mM EDTA, 0.5 M sucrose, 0.5% CHAPS, and 0.5% deoxycholic acid]. This solution was sonicated at 4° for 10 cycles (1 min on and 1 min off per cycle) using a Diagenode Bioruptor Plus 300 (Diagenode, Belgium), and centrifuged at 10,000 rpm for 15 min to remove residual nonsonicated/lysed fragments. Total BPA was quantified in the lysates by LC-MS/MS as described in Zhang et al. (2016). Briefly, 150 µl of lysate was transferred into a polypropylene (PP) tube, spiked with 40 ng of 13C12-BPA (as an internal standard) and β-glucuronidase for deconjugation of BPA. BPA was extracted from the digested lysate using liquid–liquid extraction with ethyl acetate. The solvent was evaporated to dryness, and reconstituted with methanol for LC-MS/MS analysis. The chromatographic separation and quantification of BPA were achieved by a Shimadzu Prominence Modular HPLC system (LC-20 AD UFLC; Shimadzu Corporation, Kyoto, Japan) connected with a Betasil C18 column (2.1 mm–100 mm, 5 mm; Thermo Electron Corp., Waltham, MA) coupled with API 3200 triple quadrupole mass spectrometer (ABSciex, Foster City, CA). An isotope dilution method was used for quantification. A nine-point calibration curve (1–400 ng/ml) with a regression coefficient (R) of 0.997 was constructed for the quantification of BPA. Quality control protocols include procedural blank, matrix blank, matrix spike (fortified at 300 ng BPA), and duplicate analyses were performed along with treated worm lysates. A trace level of BPA was found in procedural blanks (1.2 ng) and matrix blanks (2.8 ng), and these values were subtracted from sample concentrations. The mean recovery (%) of BPA from the matrix spike experiment was 83%, and the duplicate analysis of samples yielded a coefficient of variation <10%. Two independent biological replicates were done for the analysis of BPA levels. Representative LC-MS/MS chromatograms are shown in Figure S1.
Quantitative RT-PCR analysis
Four independent biological replicates of ∼50 worms each were collected in TRIzol (Invitrogen). The RNA was extracted using Direct-zol RNA Kit (Zymo). cDNA was synthesized using iScript gDNA Clear cDNA Synthesis Kit (Bio-Rad) and reverse transcription/real-time quantitative PCR was carried out using SsoFast EvaGreen Supermix (Bio-Rad), according to the manufacturer’s protocol. Primer amplification efficiencies were calculated from the standard curve. Each sample was run in triplicate. Sample values were normalized to tba-1 (tubulin), and the relative fold change to DMSO was calculated. Statistical comparisons between treatments were performed using the unpaired two-tailed t-test, 95% C.I. Primer sequences are listed in Table S2.
Analysis with the COPAS biosort
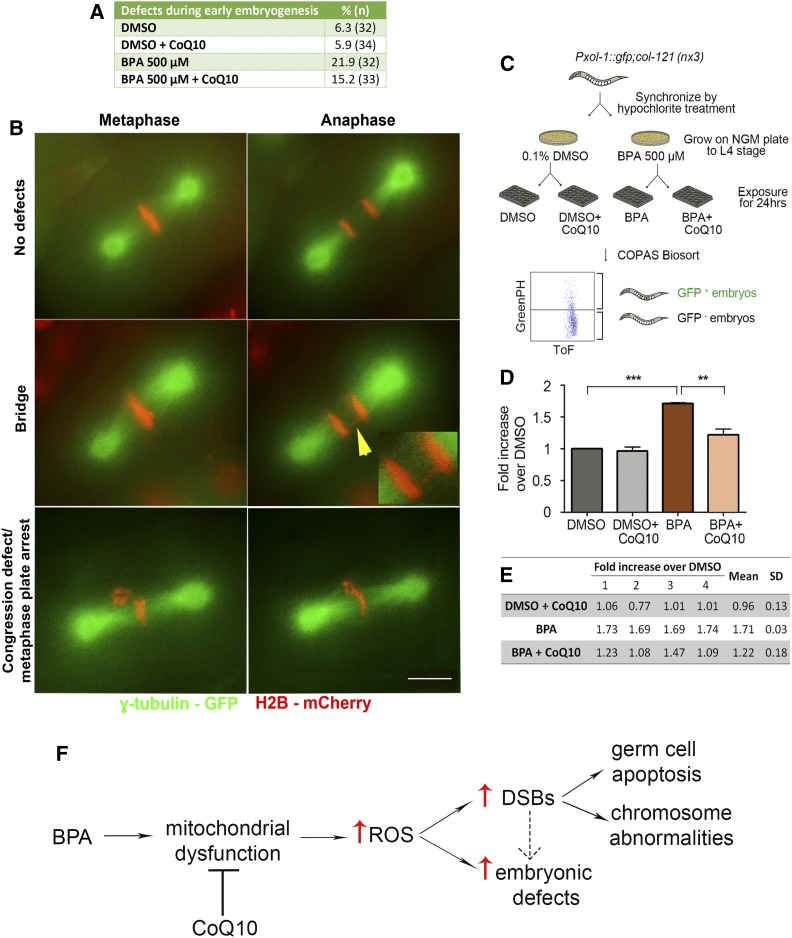
Strain CV369 {col-121(nx3)IV; yIs34[Pxol-1::GFP;rol-6]V} was used for analysis. The exposed worms were washed five times with M9 and analyzed through the COPAS Biosort (Union Biometrica, Holliston, MA) as described in Shin et al. (2019). This analysis takes advantage of the transparency of C. elegans and the low levels of males (X0 < 0.2% of offspring of self-fertilizing XX hermaphrodites) to identify chemical exposures resulting in increased chromosome nondisjunction. Specifically, chemicals resulting in impaired X chromosome segregation lead to a higher incidence of males detected as elevated levels of GFP+ embryos (destined to become males) in the uterus of exposed worms. Time-of-flight (Tof) and GFP peak height were used as reading parameters in the COPAS Biosort. Four independent biological repeats, encompassing a total of >5600 worms for each condition, were run through the COPAS Biosort. Fold-increase GFP+ signal over DMSO alone was calculated for each biological repeat, and then an average of the fold increase was calculated (Figure 6, D and E).
Figure 6.
CoQ10 supplementation rescues BPA-induced defects during the first embryonic division. (A and B) A higher frequency of chromosome segregation defects during the first embryonic cell division is seen in H2B::mCherry; γ-tubulin::GFP; col-121 embryos after treatment with BPA compared to vehicle (DMSO). CoQ10 partially rescues this effect. (A) Quantification of defects detected during the first embryonic cell division. (B) Time-lapse images of the first embryonic division. Shown are examples of normal chromosome alignment at metaphase and segregation at anaphase (top row), with chromosomes in red and spindle microtubules in green. Center and bottom rows show examples of chromatin bridges (yellow arrowhead; insert shows a magnified image of the chromatin bridge), congression defects, and metaphase arrest observed. A Pearson Chi-Square test of independence was calculated for a pairwise comparison of defective and nondefective first embryonic divisions. DMSO used at 0.1%; CoQ10 at 100 µg/ml. Bar, 5 µm. N ≥ 32 per condition. (C–E) High-throughput analysis with the COPAS Biosort shows CoQ10 rescues the elevated chromosome nondisjunction detected during embryogenesis following BPA exposure. (C) Flowchart for high-throughput analysis with the COPAS Biosort. Age-matched L1 stage animals were grown on either DMSO- or BPA-containing NGM plates until reaching the L4 stage. L4 stage animals from DMSO plates were dispensed into 24-well plates (300 worms/well) containing OP50 E. coli (OD600 = 24) in M9 buffer and either DMSO alone or DMSO with CoQ10, and L4 stage animals from BPA plates were dispensed into 24-well plates with either BPA or BPA with CoQ10. After a 24-hr incubation at 20°, worms were thoroughly washed with M9 and sorted with the COPAS Biosort based on fluorescence intensity. Time-of-flight (Tof) and GFP peak height were used as reading parameters. (D) Levels of GFP+ embryos (resulting from X chromosome nondisjunction) detected in the uterus of BPA-treated worms are significantly higher relative to DMSO (***P < 0.0001) and CoQ10 treatment results in a significant reduction in the elevation induced by BPA (**P = 0.0021). DMSO used at 0.1%; BPA at 500 µM; CoQ10 at 100 µg/ml. Statistical comparisons were performed using the unpaired two-tailed t-test, 95% C.I. Error bars represent SEM. (E) Readouts obtained with the COPAS Biosort for the indicated chemicals. Shown is the fold increase over DMSO alone calculated for each biological repeat (labeled as 1 through 4). SD, standard deviation. (F) Model for how CoQ10 restores fertility by rescuing BPA-induced oxidative DNA damage in the C. elegans germline. BPA exposure may lead to mitochondrial dysfunction in the germline resulting in increased reactive oxygen species (ROS) that in turn may account, at least in part, for the increased levels of DNA double-strand breaks (DSBs) and the embryonic defects observed. Unrepaired or aberrantly repaired meiotic DSBs lead to the activation of a late pachytene DNA damage checkpoint resulting in increased germ cell apoptosis, the increased chromosome abnormalities detected in oocytes at late diakinesis, and some of the observed embryonic defects (the latter is depicted by a dashed arrow, given that a direct effect on embryos may also be possible). Only the pathway/mode of action directly tested in this study is depicted, but other studies have shown additional effects such as alterations in gene expression (Allard and Colaiacovo 2010; Chen et al. 2016).
Statistical analysis
Statistical analysis and figures were prepared using GraphPad Prism version 5.0 (GraphPad Software). Detailed descriptions are indicated in the figure legends.
Data availability
Strains and reagents are available upon request. The authors affirm that all data necessary for confirming the conclusions of this article are represented fully within the article and its tables and figures. All supplemental information has been uploaded to figshare. Supplemental Information: The representative LC-MS/MS chromatograms for the analysis of BPA levels in worm lysates and additional information on pCHK-1 foci scored along the germline are shown in Figures S1 and S2, respectively. Table S1 indicates the C. elegans lines used for this study and Table S2 shows the primers utilized for quantitative RT-qPCR analysis. Supplemental material available at figshare: https://doi.org/10.25386/genetics.10782575.
Results and Discussion
CoQ10 supplementation rescues the increased embryonic lethality, larval lethality, and decreased brood size stemming from BPA exposure in C. elegans
BPA exposure impairs mammalian reproductive health, as evidenced by decreased quality and production of both sperm and oocytes (Hunt et al. 2003; Kato et al. 2006; Susiarjo et al. 2007; Mok-Lin et al. 2010), increased embryonic lethality, and increased recurrence of miscarriages in humans (Sugiura-Ogasawara et al. 2005; Salian et al. 2009). Our previous study in C. elegans has shown that BPA exposure results in decreased brood size and increased embryonic lethality (Allard and Colaiacovo 2010). To address whether CoQ10 can counteract the deleterious effects of BPA on the germline, we continuously exposed worms starting at the L1 larval stage (early developmental stage) to three different concentrations of BPA: 100, 200, and 500 µM (or 22.83, 45.66, and 114.15 µg/ml, respectively). CoQ10 (5, 30, and 100 µg/ml) was then given for 24 hr starting at late L4 when the germline is fully formed, and all meiotic nuclei are undergoing oogenesis. This exposure protocol was used in an attempt to approximate human exposures, which take place throughout the life span of an individual, and the potential use of CoQ10 as an intervention prior to or during pregnancy in humans. We used col-121(nx3) worms for this and all subsequent studies herein since this mutation in a cuticle collagen gene, isolated in a screen for hypersensitivity to BPA (Watanabe et al. 2005), increases cuticle permeability, allowing for analysis of BPA effects at lower doses without perturbing worm development or meiosis (Allard et al. 2013). Although we had previously employed ethanol as vehicle for our meiotic studies, we were unable to dissolve CoQ10 in the dose range tested using ethanol. For this reason, we used DMSO as vehicle instead, which is known to produce baseline toxicity (Katiki et al. 2013; Parodi et al. 2015). Although not statistically significant, a tendency of protective effects for CoQ10 was observed in DMSO + CoQ10 worms when considering the baseline effects produced by the inherent toxicity of DMSO (Figure 1, A–C). A beneficial role for CoQ10 in promoting fertility became further apparent when assessing the BPA-treated worms (Figure 1, A–C). BPA exposure at the highest dose tested (500 µM) resulted in statistically increased embryonic lethality (Emb), larval lethality (Lvl), and decreased brood size (sterility). CoQ10 supplementation significantly rescued the decreased brood size, embryonic lethality, and larval lethality produced by exposure to BPA. Taken together, these results support a beneficial role for this natural supplement in rescuing the C. elegans germline from the effects of BPA exposure.
BPA uptake by C. elegans and CoQ10 supplementation doses
To determine the internal levels of BPA, we measured the concentration of BPA in worm lysates by liquid chromatography–tandem mass spectrometry analysis (Figure S1). This analysis revealed that an exposure to 500 μM BPA through the protocol described above resulted in 45 μg of total BPA per gram of worm (adults, 24 hr post-L4) pellet, or 0.045 ng of BPA per each worm, since an individual worm weighs ∼1 μg (Muschiol et al. 2009). Therefore, we are assessing the reproductive effects of BPA in worms at internal levels consistent with low-dose exposure in the general human population, as suggested by the amounts found in human specimens such as placenta (∼6014 ng BPA), amniotic fluid (∼3320 ng), follicular fluid (∼0.12 ng per follicle), and umbilical cord tissue (∼422 ng) (calculated considering the estimation of the total mass of the organ/volume of fluid) (Vandenberg et al. 2010). In addition, the doses of CoQ10 (5, 30, and 100 µg/ml) used to test if this coenzyme can reduce the germline defects resulting from BPA exposure are comparable to those used in prior C. elegans studies, and sixfold lower than those used in mouse studies (Fischer et al. 2014; Ben-Meir et al. 2015).
CoQ10 supplementation protects from BPA-induced meiotic DSBs, resulting in reduced germ cell apoptosis and DNA damage checkpoint activation and improved chromosome morphology at diakinesis
Progression of meiotic recombination in the C. elegans germline can be assessed by using an antibody recognizing the RAD-51 protein, which is the ortholog of Saccharomyces cerevisiae protein Rad51 involved in strand invasion/exchange during DSB repair (Sung 1994; Colaiácovo et al. 2003). RAD-51 foci can be quantified in nuclei throughout the germline, with levels increasing in a SPO-11-dependent manner upon entrance into meiosis (zone 3, Figure 1D), peaking by midpachytene (zone 5) and then decreasing by late pachytene (zone 7) as meiotic DSB repair is completed (Alpi et al. 2003; Colaiácovo et al. 2003). As we previously reported, BPA exposure results in altered meiotic DSBR progression as evidenced by significantly elevated levels of RAD-51 foci detected specifically during meiosis (not in mitotic nuclei in the premeiotic tip or in premeiotic S phase, zones 1 and 2) in BPA- compared to vehicle-treated worms (Figure 1, D and E and Allard and Colaiacovo 2010).
Here, we show that CoQ10 supplementation significantly decreased the BPA-induced increment in RAD-51 foci seen during pachytene (zones 4–7) (Figure 1, E and G). To distinguish whether the elevated levels of RAD-51 foci reflect elevated DSB formation and/or impaired DSBR progression, we examined rad-54;col-121 double mutants. In a rad-54 mutant background, DSBR is blocked and DSB-bound RAD-51 is not removed, thus allowing for the quantification of RAD-51 foci to serve as a readout of the total number of DSBs formed (Mets and Meyer 2009). We observed a significant increase in RAD-51 levels in rad-54;col-121 double mutant worms exposed to BPA 500 µM compared to all the other groups from midpachytene (zone 5) onwards, suggesting elevated DSB formation upon BPA exposure (Figure 1, F and G), and providing new insights into our previous analysis (Allard and Colaiacovo 2010). Importantly, the BPA-induced elevated DSB levels were significantly decreased with CoQ10 supplementation.
After exposure to BPA, late pachytene nuclei carrying unrepaired DSBs and/or aberrant recombination intermediates will be removed by apoptosis via activation of the CEP-1/p53-mediated DNA damage checkpoint (Allard and Colaiacovo 2010). Consistent with these findings, the higher number of RAD-51 foci seen in BPA (500 µM)-exposed gonads was accompanied by a significant increase in the number of apoptotic nuclei in the germline (Figure 2, A and B). Importantly, supplementation with CoQ10 (100 µg/ml) was able to significantly reduce the elevated apoptosis detected at various doses of BPA exposure (Figure 2B). Moreover, we observed that CoQ10, even at lower doses (5 and 30 µg/ml), protected from BPA-induced (500 µM) increased levels of germ cell apoptosis (Figure 2C).
The ability of CoQ10 supplementation to rescue elevated BPA-induced germ cell apoptosis is further supported by the observed decrease in signal for phosphorylated CHK-1 (pCHK-1), which is a checkpoint kinase that is normally elevated upon DNA damage checkpoint activation (Kalogeropoulos et al. 2004), and which we have previously shown is elevated upon BPA exposure (Figure 2, D and E; and see Allard and Colaiacovo 2010). In addition, we did not detect either elevated pCHK-1 signal in mitotic germline nuclei (Figure S2, A and B) or an increased nuclear diameter for nuclei in the premeiotic tip (mitotic nuclei), which is characteristic of S-phase arrest induced by DNA damage (Garcia-Muse and Boulton 2005; Kim and Colaiacovo 2014) (Figure S2C), suggesting that BPA exposure specifically activates the meiotic DNA damage checkpoint in late pachytene. We also corroborated previous data pointing to BPA-dependent specific stress during meiosis within the germline, since we did not observe elevated levels of RAD-51 foci in zones 1 and 2 of the gonad (premeiotic tip) (Allard and Colaiacovo 2010) (Figure 1, E and F).
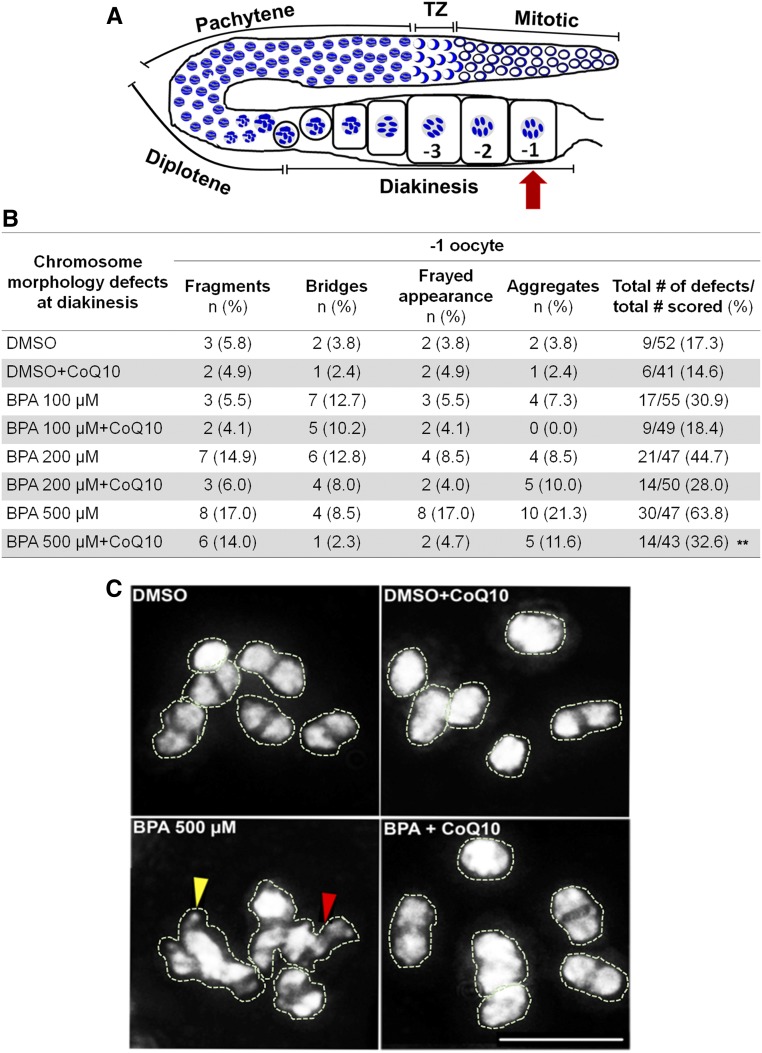
Finally, we assessed whether CoQ10 rescues the defects in chromosome morphology observed in oocytes at diakinesis as a result of altered DSB formation and repair following BPA exposure. We examined the diakinesis oocyte that is most proximal to the spermatheca (−1 oocyte; Figure 3A), and the defects scored included chromosome aggregation, frayed appearance, the presence of chromosome fragments and chromatin bridges, which can stem from elevated DSB formation and/or impaired DSB repair. High-resolution microscopy revealed a direct correlation between the dose of BPA and the percentage of −1 oocytes exhibiting chromosome morphology defects, which was reduced by CoQ10 supplementation (Figure 3, B and C). However, only BPA 200 µM and 500 µM produced a significant increase in the percentage of chromosome morphology defects at diakinesis in comparison to DMSO (44.7% and 63.8% compared to 17.3%, respectively, P = 0.0043 and P < 0.0001 by Fisher’s exact test). After supplementation with CoQ10, the frequency of defects detected for all three doses of BPA were reduced compared to worms exposed only to BPA. However, only BPA 500 µM-treated worms supplemented with CoQ10 exhibited a statistically significant decrease in the levels of defects compared to BPA-only treated worms (P = 0.0035). Taken together, these results reveal that BPA exposure results in increased levels of DSB formation specifically during meiosis and support an improvement in the maintenance of genomic integrity exerted by CoQ10 against the effects of BPA during meiosis.
Figure 3.
CoQ10 ameliorates the deleterious effects of BPA on chromosome morphology observed at diakinesis (−1 oocyte). (A) Schematic representation of one gonad arm of C. elegans indicating the location of the −1 oocyte (red arrow). (B) Quantification of the chromosome morphology defects observed in bivalents at diakinesis. n = number of −1 oocytes in which each indicated type of defect was observed. DMSO was used at 0.1%; CoQ10 at 100 µg/ml. Fisher’s exact test pairwise comparisons for the total numbers of defects/total number of oocytes scored revealed **P = 0.0035 for BPA 500 µM vs. BPA 500 µM + CoQ10, P = 0.0043 for DMSO vs. BPA 200 µM and P < 0.0001 for DMSO vs. BPA 500 µM. (C) Representative images of the DAPI-stained bodies in the −1 oocyte. Six intact bivalents are shown for control and either control or BPA 500 µM supplemented with CoQ10. In contrast, higher frequencies of oocytes carrying chromosomes with a frayed appearance, aggregates, chromatin bridges (red arrowhead), and fragments (yellow arrowhead) are observed upon BPA-treatment. Bivalents and aggregates have been traced to facilitate visualization of the DAPI-stained bodies being scored, given that, once the 3D data stacks encompassing whole nuclei are “flattened,” some of the bivalents look superimposed onto each other in the final projections. Bar, 5 µm.
CoQ10 supplementation counteracts BPA-induced oxidative stress and altered gene expression of antioxidant enzymes in the germline
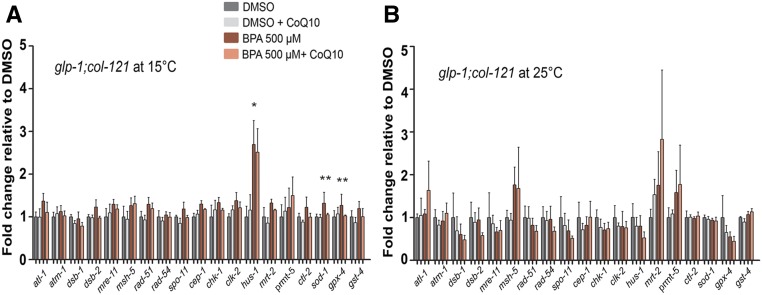
We had previously shown that exposure to BPA resulted in germline-specific altered expression of DSBR genes in C. elegans (Allard and Colaiacovo 2010). To examine gene expression upon supplementation with CoQ10, and distinguish changes taking place in the soma from those in the germline, we performed quantitative RT-PCR using a glp-1(bn18) temperature-sensitive mutant (Austin and Kimble 1987), which lacks a germline at 25°, but is essentially wild type at 15° (Figure 4, A and B). We focused this analysis on a set of 15 genes involved in meiotic DSB formation (dsb-1, dsb-2, spo-11), DSB repair (mre-11, msh-5, rad-51, rad-54), and DNA damage response (atl-1, atm-1, cep-1, chk-1, clk-2, hus-1, mrt-2, prmt-5). However, we also examined the expression of four additional genes (ctl-2, sod-1, gpx-4, and gst-4) involved in response to oxidative stress given that BPA has been associated with oxidative DNA damage (Rocha et al. 2017, 2018), and we observe that the antioxidant CoQ10 rescues many of the germline defects caused by BPA. Three genes were significantly upregulated specifically in the germline of BPA-treated worms: hus-1, which encodes for a checkpoint protein required for DNA damage-induced CEP-1/p53-dependent apoptosis (Hofmann et al. 2002), and sod-1 and gpx-4, which encode for a copper/zinc superoxide dismustase and a selenoprotein glutathione peroxidase, respectively, both known to protect cells from oxidative damage (Giglio et al. 1994; Halliwell and Gutteridge 2007). CoQ10 supplementation was not able to alleviate the upregulation of hus-1 in the germline of BPA-treated worms, although it was able to reduce germ cell apoptosis even at low concentrations (Figure 2, B and C). While changes in expression for other genes not included in our analysis might explain this outcome, it is also possible that the beneficial effects on germ cell apoptosis are due, at least in part, to changes in HUS-1 protein stability or localization that were not assessed in our current study. However, supplementation with the antioxidant resulted in a significant reduction in the expression levels of the antioxidant enzymes seen elevated in germlines exposed to BPA. We did not observe the downregulation of the DSBR genes rad-54, mre-11, and mrt-2 reported previously following exposure to BPA (Allard and Colaiacovo 2010). Potential explanations for that include differences in the worm background (we used the cuticle sensitized col-121 background vs. N2), dose (we used 500 µM vs. 1 mM) and exposure time (in the current study BPA exposures extended from L1 until 24 hr post L4 vs. eggs until 24 hr post L4) between the studies.
Figure 4.
CoQ10 counteracts BPA-induced altered gene expression of antioxidant enzymes in the germline. (A and B) Expression levels of a panel of genes implicated in DSBR, DNA damage response, and oxidative stress scavenging were assayed by quantitative RT-PCR. Analysis of glp-1;col-121 worms at 15°C (A) reports expression in both the germline and the soma, while only expression in the soma can be scored at 25°C (B). Error bars represent SEM. BPA is statistically different from DMSO at *P = 0.0286 (hus-1); BPA is statistically different from BPA + CoQ10 at **P = 0.006 and **P = 0.0017 for sod-1 and gpx-4, respectively, by the unpaired two-tailed t-test, 95% C.I. DMSO was used at 0.1%; CoQ10 at 100 µg/ml.
Given the altered expression for oxidative stress response genes and the elevated DSB levels we observed upon BPA exposure, we next assessed the redox state in the gonads after exposure to BPA, as well as whether rescuing oxidative imbalance is one of the main mechanisms accounting for the protective role of CoQ10 against BPA-induced reprotoxicity. Using a reporter strain carrying GFP expressed under the control of the gst-4 promoter, we observed that exposure to BPA resulted in elevated GFP fluorescence signal in the gonads compared to vehicle alone (Figure 5, A and B). In contrast, gonads of CoQ10-supplemented worms showed a significant reduction in GFP signal, suggesting that the reactive oxygen species generated by BPA in the germ cells are efficiently neutralized by CoQ10 treatment. Further evidence that increased GFP fluorescence signal in the gonads is a readout of oxidative stress stems from the increased signal observed upon treatment with the reactive oxygen species-generating compound paraquat (50 mM) compared to untreated [(−) paraquat] gonads (Figure 5, C and D). Given that mitochondria are involved in reactive oxygen species metabolism (Brookes et al. 2004), we examined mitochondrial function using a mitochondrial-targeted dye (MitoTracker Red CMXRos) that accumulates within mitochondria dependent on mitochondrial membrane potential. Gonads from BPA-exposed worms exhibited reduced Mitotracker signal, indicative of mitochondrial dysfunction, whereas Mitotracker signal was rescued upon supplementation with CoQ10 (Figure 5, E and F). Supporting specificity of this analysis, gonads from untreated col-121 worms, where mitochondrial function is supposedly normal, showed Mitotracker red signal (Figure 5, G and H) comparable to that observed in col-121 worms treated either with DMSO or DMSO + CoQ10 (Figure 5, E and F). In comparison, untreated sek-1 worms, which lack the ortholog of mammalian MKK3/6 in the p38 MAPK pathway and therefore exhibit mitochondrial dysfunction and increased oxidative stress, show a significant decrease in gonadal Mitotracker signal (Figure 5, G and H) (Chen et al. 2018). Oxidative stress is known to produce DNA DSBs (O’Driscoll and Jeggo 2006), and, therefore, these results also imply that BPA-induced oxidative stress, due, at least in part, to mitochondrial dysfunction, is a potential mechanism by which exposure to this plasticizer leads to the elevated levels of DSBs we have observed in the worm germlines.
Further strengthening the link between BPA exposure, oxidative DNA damage, and fertility problems, a recent study has shown that knocking down the scavenger superoxide dismutase 1 in the Drosophila oocyte leads to less efficient maintenance of sister chromatid cohesion resulting in chromosome segregation errors (Perkins et al. 2016). The authors recognize oxidative damage as a physiological mechanism that triggers the human maternal age effect. In the same way, our findings suggest that a major mechanism underlying the toxicity of BPA on reproductive health is oxidative damage. According to our observations, the increase in the levels of reactive oxygen species following BPA exposure induces DNA damage, resulting in augmented germ cell apoptosis and altered meiotic DSBR progression. An extrapolation based on the findings by Perkins et al. (2016) would be that BPA-induced oxidative stress may also impact the stability of sister chromatid cohesion, leading to chromosome segregation errors. While we have not observed evidence of premature loss of sister chromatid cohesion based on the number of DAPI-stained bodies scored in oocytes at diakinesis, we cannot exclude the possibility that this could become more apparent at later stages of cell division or in worms exposed for more extended periods (i.e., aged worms).
CoQ10 supplementation rescues BPA-induced chromosome segregation defects during early embryogenesis
To assess whether CoQ10 may be beneficial also during early embryogenesis, we performed live-imaging of the first embryonic division in H2B::mCherry; γ-tubulin::GFP transgenic worms. Results corroborated our previous data showing that worms exposed to BPA exhibited a higher frequency of defects (21.9%, n = 32) during the first embryonic cell division, such as chromosomes failing to properly align at the metaphase plate, metaphase plate arrest, and presence of chromatin bridges during the metaphase to anaphase transition (Allard and Colaiacovo 2010), in comparison to vehicle (6.3%, n = 32) (Figure 6, A and B). In this assay, we observed that CoQ10 supplementation provided a modest decrease to the higher frequency of defects found during early embryogenesis in comparison to exposure to BPA (21.9% for BPA-only vs. 15.2%, n = 33, for BPA + CoQ10). We suggest that this partial rescue is due to the limitation of the number of events that can be assessed by live-imaging. Specifically, when we monitored embryonic lethality levels (Figure 1A), in which CoQ10 was seen to significantly overcome BPA 500 µM-induced embryonic lethality, the number of events scored was much higher (n = 2179 embryos for BPA-exposed and n = 3148 embryos for BPA + CoQ10) than those assessed by live-imaging analysis (n = 32 embryos for BPA, and n = 33 embryos for BPA + CoQ10). Therefore, the partial effect of CoQ10 in decreasing the frequency of defective early embryonic divisions may stem from technical limitations on the number of events that can be scored by the more laborious live-imaging approach. To address this, we applied a high-throughput screening platform developed recently in the laboratory to screen large numbers of animals for increased aneuploidy as a result of impaired germline function (Allard et al. 2013; Shin et al. 2019) (Figure 6C). With this strategy, worms carrying a Pxol-1::gfp transcriptional reporter are assessed based on fluorescence intensity with a large object flow cytometry system (COPAS Biosort; Union Biometrica). GFP expression is controlled by a male-specific promoter allowing for measurement of the levels of GFP+ eggs (destined to become males) in the uterus of exposed mothers as a result of X chromosome nondisjunction (see Material and Methods). More than 5600 animals were screened in four biological repeats for each condition. BPA-treated worms exhibited a significant higher fold increase in GFP+ embryos compared to DMSO, and treatment with CoQ10 (BPA + CoQ10) significantly lowered the levels of GFP+ embryos (Figure 6, D and E), indicating that CoQ10 is rescuing the elevated levels of chromosome nondisjunction detected during embryogenesis following BPA exposure.
In conclusion, due to the potential of endocrine disruptors such as BPA to cause toxicity, the identification of new potential protective agents is imperative. Here, we corroborated and extended our previous findings on the mechanisms by which BPA—a highly prevalent EDC in our environment—disturbs the germline and results in increased embryonic lethality, larval lethality, and decreased brood size. We complemented the toxicological analysis of BPA by showing that exposure to this plasticizer may generate reactive oxygen species in the gonads, which our analysis suggests can induce altered meiotic DSB levels. Elevated meiotic DSBs, coupled to impaired meiotic DSB repair, ultimately result in activation of the DNA damage checkpoint, elevated levels of germ cell apoptosis and chromosome morphology defects in late prophase I, as well as the elevated expression of genes encoding for antioxidant enzymes specifically in the germline. Taken together, our data suggest that CoQ10 counteracts BPA-induced toxicity on reproductive health through the scavenging of reactive oxygen species and free radicals (Figure 6F), raising the exciting possibility that supplementation with this natural antioxidant may constitute a low-risk and low-cost strategy to attenuate the impact on fertility exerted by BPA.
Acknowledgments
We thank Doris Lui, Laura Ivon Lascarez Lagunas, and Pedro Saavedra for technical support, Randi H. Goldman for initial discussions, and Marina Martinez-Garcia for critical reading of this manuscript. We thank Kaneka Corporation for kindly providing CoQ10 samples. The oxidative stress-inducible GFP transgenic strain CL2166 (genotype: dvIs19 [(pAF15)gst-4p::GFP::NLS] III) was provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). This work was supported by a São Paulo Research Foundation (FAPESP) Post-Doctoral fellowship (FAPESP 2016/23481-2) (to M.F.H.C.) and by support from the McKenzie Family Trust (to M.P.C.). The authors declare no competing financial interests.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.10782575.
Communicating editor: J. Engebrecht
Literature Cited
- Allard P., and Colaiacovo M. P., 2010. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA 107: 20405–20410. 10.1073/pnas.1010386107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard P., Kleinstreuer N. C., Knudsen T. B., and Colaiacovo M. P., 2013. A C. elegans screening platform for the rapid assessment of chemical disruption of germline function. Environ. Health Perspect. 121: 717–724. 10.1289/ehp.1206301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpi A., Pasierbek P., Gartner A., and Loidl J., 2003. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112: 6–16. 10.1007/s00412-003-0237-5 [DOI] [PubMed] [Google Scholar]
- Arany I., Carter A., Hall S., Fulop T., and Dixit M., 2017. Coenzyme Q10 protects renal proximal tubule cells against nicotine-induced apoptosis through induction of p66shc-dependent antioxidant responses. Apoptosis 22: 220–228. 10.1007/s10495-016-1309-3 [DOI] [PubMed] [Google Scholar]
- Asencio C., Navas P., Cabello J., Schnabel R., Cypser J. R. et al. , 2009. Coenzyme Q supports distinct developmental processes in Caenorhabditis elegans. Mech. Ageing Dev. 130: 145–153. 10.1016/j.mad.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Austin J., and Kimble J., 1987. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51: 589–599. 10.1016/0092-8674(87)90128-0 [DOI] [PubMed] [Google Scholar]
- Ben-Meir A., Burstein E., Borrego-Alvarez A., Chong J., Wong E. et al. , 2015. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 14: 887–895. 10.1111/acel.12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov Y., Hannam T., Jurisicova A., Esfandiari N., and Casper R. F., 2014. Coenzyme Q10 supplementation and oocyte aneuploidy in women undergoing IVF-ICSI treatment. Clin. Med. Insights Reprod. Health 8: 31–36. 10.4137/CMRH.S14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binukumar B. K., Gupta N., Bal A., and Gill K. D., 2011. Protection of dichlorvos induced oxidative stress and nigrostriatal neuronal death by chronic coenzyme Q10 pretreatment. Toxicol. Appl. Pharmacol. 256: 73–82. 10.1016/j.taap.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Bloom M. S., Fujimoto V. Y., Storm R., Zhang L., Butts C. D. et al. , 2017. Persistent organic pollutants (POPs) in human follicular fluid and in vitro fertilization outcomes, a pilot study. Reprod. Toxicol. 67: 165–173. 10.1016/j.reprotox.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Boots C. E., Boudoures A., Zhang W., Drury A., and Moley K. H., 2016. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum. Reprod. 31: 2090–2097. 10.1093/humrep/dew181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P. S., Yoon Y., Robotham J. L., Anders M. W., and Sheu S. S., 2004. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287: C817–C833. 10.1152/ajpcell.00139.2004 [DOI] [PubMed] [Google Scholar]
- Castello P. R., Drechsel D. A., and Patel M., 2007. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J. Biol. Chem. 282: 14186–14193. 10.1074/jbc.M700827200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Shu L., Qiu Z., Lee D. Y., Settle S. J. et al. , 2016. Exposure to the BPA-substitute Bisphenol S causes unique alterations of germline function. PLoS Genet. 12: e1006223 10.1371/journal.pgen.1006223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Cao X., Lu H., Wen P., Qi X. et al. , 2018. N-(3-oxo-acyl) homoserine lactone induced germ cell apoptosis and suppressed the over-activated RAS/MAPK tumorigenesis via mitochondrial-dependent ROS in C. elegans. Apoptosis 23: 626–640. 10.1007/s10495-018-1478-3 [DOI] [PubMed] [Google Scholar]
- Colaiácovo M. P., 2006. The many facets of SC function during C. elegans meiosis. Chromosoma 115: 195–211. 10.1007/s00412-006-0061-9 [DOI] [PubMed] [Google Scholar]
- Colaiácovo M. P., MacQueen A. J., Martinez-Perez E., McDonald K., Adamo A. et al. , 2003. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 5: 463–474. 10.1016/S1534-5807(03)00232-6 [DOI] [PubMed] [Google Scholar]
- de Carvalho C. E., Zaaijer S., Smolikov S., Gu Y., Schumacher J. M. et al. , 2008. LAB-1 antagonizes the Aurora B kinase in C. elegans. Genes Dev. 22: 2869–2885. 10.1101/gad.1691208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detienne G., Van de Walle P., De Haes W., Schoofs L., and Temmerman L., 2016. SKN-1-independent transcriptional activation of glutathione S-transferase 4 (GST-4) by EGF signaling. Worm 5: e1230585 10.1080/21624054.2016.1230585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Niklowitz P., Menke T., and Doring F., 2014. Promotion of growth by Coenzyme Q10 is linked to gene expression in C. elegans. Biochem. Biophys. Res. Commun. 452: 920–927. 10.1016/j.bbrc.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Garcia-Muse T., and Boulton S. J., 2005. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 24: 4345–4355. 10.1038/sj.emboj.7600896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat I., Blanco Mejia S., Balakier H., Librach C. L., Claessens A. et al. , 2016. The use of coenzyme Q10 and DHEA during IUI and IVF cycles in patients with decreased ovarian reserve. Gynecol. Endocrinol. 32: 534–537. 10.3109/09513590.2015.1137095 [DOI] [PubMed] [Google Scholar]
- Giglio A. M., Hunter T., Bannister J. V., Bannister W. H., and Hunter G. J., 1994. The copper/zinc superoxide dismutase gene of Caenorhabditis elegans. Biochem. Mol. Biol. Int. 33: 41–44. [PubMed] [Google Scholar]
- Goldstein P., 1986. Nuclear aberrations and loss of synaptonemal complexes in response to diethylstilbestrol (DES) in Caenorhabditis elegans hermaphrodites. Mutat. Res. 174: 99–107. 10.1016/0165-7992(86)90098-9 [DOI] [PubMed] [Google Scholar]
- Halliwell B., and Gutteridge J. M. C., 2007. Free Radicals in Biology and Medicine, Oxford University Press, New York. [Google Scholar]
- Hofmann E. R., Milstein S., Boulton S. J., Ye M., Hofmann J. J. et al. , 2002. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 12: 1908–1918. 10.1016/S0960-9822(02)01262-9 [DOI] [PubMed] [Google Scholar]
- Hoshi H., Kamata Y., and Uemura T., 2003. Effects of 17beta-estradiol, bisphenol A and tributyltin chloride on germ cells of Caenorhabditis elegans. J. Vet. Med. Sci. 65: 881–885. 10.1292/jvms.65.881 [DOI] [PubMed] [Google Scholar]
- Hourihan J. M., Moronetti Mazzeo L. E., Fernandez-Cardenas L. P., and Blackwell T. K., 2016. Cysteine sulfenylation directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol. Cell 63: 553–566. 10.1016/j.molcel.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P. A., Koehler K. E., Susiarjo M., Hodges C. A., Ilagan A. et al. , 2003. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 13: 546–553. 10.1016/S0960-9822(03)00189-1 [DOI] [PubMed] [Google Scholar]
- Hunt P. A., Lawson C., Gieske M., Murdoch B., Smith H. et al. , 2012. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl. Acad. Sci. USA 109: 17525–17530. 10.1073/pnas.1207854109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Hisamoto N., An J. H., Oliveira R. P., Nishida E. et al. , 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19: 2278–2283. 10.1101/gad.1324805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeropoulos N., Christoforou C., Green A. J., Gill S., and Ashcroft N. R., 2004. chk-1 is an essential gene and is required for an S-M checkpoint during early embryogenesis. Cell Cycle 3: 1196–1200. 10.4161/cc.3.9.1116 [DOI] [PubMed] [Google Scholar]
- Kandhare A. D., Ghosh P., Ghule A. E., and Bodhankar S. L., 2013. Elucidation of molecular mechanism involved in neuroprotective effect of Coenzyme Q10 in alcohol-induced neuropathic pain. Fundam. Clin. Pharmacol. 27: 603–622. 10.1111/fcp.12003 [DOI] [PubMed] [Google Scholar]
- Katiki L. M., Ferreira J. F., Gonzalez J. M., Zajac A. M., Lindsay D. S. et al. , 2013. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet. Parasitol. 192: 218–227. 10.1016/j.vetpar.2012.09.030 [DOI] [PubMed] [Google Scholar]
- Kato H., Furuhashi T., Tanaka M., Katsu Y., Watanabe H. et al. , 2006. Effects of bisphenol A given neonatally on reproductive functions of male rats. Reprod. Toxicol. 22: 20–29. 10.1016/j.reprotox.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Keith S. A., Amrit F. R., Ratnappan R., and Ghazi A., 2014. The C. elegans healthspan and stress-resistance assay toolkit. Methods 68: 476–486. 10.1016/j.ymeth.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Kelly K. O., Dernburg A. F., Stanfield G. M., and Villeneuve A. M., 2000. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 156: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. M., and Colaiacovo M. P., 2014. ZTF-8 interacts with the 9–1-1 complex and is required for DNA damage response and double-strand break repair in the C. elegans germline. PLoS Genet. 10: e1004723 10.1371/journal.pgen.1004723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathi R. B., Liebert C. A., Brookfield K. F., Taylor J. A., vom Saal F. S. et al. , 2014. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 102: 123–128. 10.1016/j.fertnstert.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C., Gieske M., Murdoch B., Ye P., Li Y. et al. , 2011. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol. Reprod. 84: 79–86. 10.1095/biolreprod.110.084814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiers B., Kampkotter A., Grevelding C. G., Link C. D., Johnson T. E. et al. , 2003. A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic. Biol. Med. 34: 1405–1415. 10.1016/S0891-5849(03)00102-3 [DOI] [PubMed] [Google Scholar]
- Lui D. Y., and Colaiacovo M. P., 2013. Meiotic development in Caenorhabditis elegans. Adv. Exp. Med. Biol. 757: 133–170. 10.1007/978-1-4614-4015-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C., Wylie B. J., Minguez-Alarcon L., Williams P. L., Ford J. B. et al. , 2016. Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction. Epidemiology 27: 879–888. 10.1097/EDE.0000000000000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets D. G., and Meyer B. J., 2009. Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell 139: 73–86. 10.1016/j.cell.2009.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok-Lin E., Ehrlich S., Williams P. L., Petrozza J., Wright D. L. et al. , 2010. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 33: 385–393. 10.1111/j.1365-2605.2009.01014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschiol D., Schroeder F., and Traunspurger W., 2009. Life cycle and population growth rate of Caenorhabditis elegans studied by a new method. BMC Ecol. 9: 14 10.1186/1472-6785-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S. I., Hassold T. J., and Hunt P. A., 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13: 493–504. 10.1038/nrg3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottke A. C., Beese-Sims S. E., Pantalena L. F., Reinke V., Shi Y. et al. , 2011. SPR-5 is a histone H3K4 demethylase with a role in meiotic double-strand break repair. Proc. Natl. Acad. Sci. USA 108: 12805–12810. 10.1073/pnas.1102298108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll M., and Jeggo P. A., 2006. The role of double-strand break repair - insights from human genetics. Nat. Rev. Genet. 7: 45–54. 10.1038/nrg1746 [DOI] [PubMed] [Google Scholar]
- Parodi D. A., Sjarif J., Chen Y., and Allard P., 2015. Reproductive toxicity and meiotic dysfunction following exposure to the pesticides Maneb, Diazinon and Fenarimol. Toxicol. Res. (Camb.) 4: 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A. T., Das T. M., Panzera L. C., and Bickel S. E., 2016. Oxidative stress in oocytes during midprophase induces premature loss of cohesion and chromosome segregation errors. Proc. Natl. Acad. Sci. USA 113: E6823–E6830. 10.1073/pnas.1612047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B. A., Asimakopoulos A. G., Barbosa F. Jr., and Kannan K., 2017. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ. 586: 152–162. 10.1016/j.scitotenv.2017.01.193 [DOI] [PubMed] [Google Scholar]
- Rocha B. A., Asimakopoulos A. G., Honda M., da Costa N. L., Barbosa R. M. et al. , 2018. Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ. Int. 116: 269–277. 10.1016/j.envint.2018.04.023 [DOI] [PubMed] [Google Scholar]
- Salian S., Doshi T., and Vanage G., 2009. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology 265: 56–67. 10.1016/j.tox.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Shen Y., Zheng Y., Jiang J., Liu Y., Luo X. et al. , 2015. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: evidence from a case-control study in eastern China. PLoS One 10: e0127886 10.1371/journal.pone.0127886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N., Cuenca L., Karthikraj R., Kannan K., and Colaiacovo M. P., 2019. Assessing effects of germline exposure to environmental toxicants by high-throughput screening in C. elegans. PLoS Genet. 15: e1007975 10.1371/journal.pgen.1007975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T., 2006. Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.101.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura-Ogasawara M., Ozaki Y., Sonta S., Makino T., and Suzumori K., 2005. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 20: 2325–2329. 10.1093/humrep/deh888 [DOI] [PubMed] [Google Scholar]
- Sung P., 1994. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 265: 1241–1243. 10.1126/science.8066464 [DOI] [PubMed] [Google Scholar]
- Susiarjo M., Hassold T. J., Freeman E., and Hunt P. A., 2007. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 3: e5 10.1371/journal.pgen.0030005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Chahoud I., Heindel J. J., Padmanabhan V., Paumgartten F. J. et al. , 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 118: 1055–1070. 10.1289/ehp.0901716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhang R., Jin F., Lou H., Mao Y. et al. , 2017a Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ. Int. 102: 207–212. 10.1016/j.envint.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Wang Z., Li D., Miao M., Liang H., Chen J. et al. , 2017b Urine bisphenol A and pubertal development in boys. Int. J. Hyg. Environ. Health 220: 43–50. 10.1016/j.ijheh.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Mitani N., Ishii N., and Miki K., 2005. A mutation in a cuticle collagen causes hypersensitivity to the endocrine disrupting chemical, bisphenol A, in Caenorhabditis elegans. Mutat. Res. 570: 71–80. 10.1016/j.mrfmmm.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Watkins D. J., Sanchez B. N., Tellez-Rojo M. M., Lee J. M., Mercado-Garcia A. et al. , 2017. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ. Res. 159: 143–151. 10.1016/j.envres.2017.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Nisenblat V., Lu C., Li R., Qiao J. et al. , 2018. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod. Biol. Endocrinol. 16: 29 10.1186/s12958-018-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Xue J., Gao C. Z., Qiu R. L., Li Y. X. et al. , 2016. Urinary Concentrations of bisphenols and their association with biomarkers of oxidative stress in people living near e-waste recycling facilities in China. Environ. Sci. Technol. 50: 4045–4053. 10.1021/acs.est.6b00032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and reagents are available upon request. The authors affirm that all data necessary for confirming the conclusions of this article are represented fully within the article and its tables and figures. All supplemental information has been uploaded to figshare. Supplemental Information: The representative LC-MS/MS chromatograms for the analysis of BPA levels in worm lysates and additional information on pCHK-1 foci scored along the germline are shown in Figures S1 and S2, respectively. Table S1 indicates the C. elegans lines used for this study and Table S2 shows the primers utilized for quantitative RT-qPCR analysis. Supplemental material available at figshare: https://doi.org/10.25386/genetics.10782575.