Abstract
Background
Community‐acquired pneumonia (CAP) is caused by various pathogens, traditionally divided into 'typical' and 'atypical'. Initial antibiotic treatment of CAP is usually empirical, customarily covering both typical and atypical pathogens. To date, no sufficient evidence exists to support this broad coverage, while limiting coverage is bound to reduce toxicity, resistance and expense.
Objectives
The main objective was to estimate the mortality and proportion with treatment failure using regimens containing atypical antibiotic coverage compared to those that had typical coverage only. Secondary objectives included the assessment of adverse events.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) Issue 3, 2012 which includes the Acute Respiratory Infection Group's Specialized Register, MEDLINE (January 1966 to April week 1, 2012) and EMBASE (January 1980 to April 2012).
Selection criteria
Randomized controlled trials (RCTs) of adult patients hospitalized due to CAP, comparing antibiotic regimens with atypical coverage (quinolones, macrolides, tetracyclines, chloramphenicol, streptogramins or ketolides) to a regimen without atypical antibiotic coverage.
Data collection and analysis
Two review authors independently assessed the risk of bias and extracted data from included trials. We estimated risk ratios (RRs) with 95% confidence intervals (CIs). We assessed heterogeneity using a Chi2 test.
Main results
We included 28 trials, encompassing 5939 randomized patients. The atypical antibiotic was administered as monotherapy in all but three studies. Only one study assessed a beta‐lactam combined with a macrolide compared to the same beta‐lactam. There was no difference in mortality between the atypical arm and the non‐atypical arm (RR 1.14; 95% CI 0.84 to 1.55), RR < 1 favors the atypical arm. The atypical arm showed an insignificant trend toward clinical success and a significant advantage to bacteriological eradication, which disappeared when evaluating methodologically high quality studies alone. Clinical success for the atypical arm was significantly higher for Legionella pneumophilae (L. pneumophilae) and non‐significantly lower for pneumococcal pneumonia. There was no significant difference between the groups in the frequency of (total) adverse events, or those requiring discontinuation of treatment. However, gastrointestinal events were less common in the atypical arm (RR 0.70; 95% CI 0.53 to 0.92). Although the trials assessed different antibiotics, no significant heterogeneity was detected in the analyses.
Authors' conclusions
No benefit of survival or clinical efficacy was shown with empirical atypical coverage in hospitalized patients with CAP. This conclusion relates mostly to the comparison of quinolone monotherapy to beta‐lactams. Further trials, comparing beta‐lactam monotherapy to the same combined with a macrolide, should be performed.
Plain language summary
Initial antibiotic treatment for coverage of 'atypical' pathogens for community‐acquired pneumonia in hospitalized adults
Pneumonia is a serious lung infection and is usually treated with antibiotics. Bacteria which cause community‐acquired pneumonia (CAP, pneumonia contracted outside healthcare settings) are traditionally divided into 'typical' and 'atypical', each dictating a different antibiotic treatment. Atypical bacteria include, Legionella pneumophila (L. pneumophila), Mycoplasma pneumoniae (M. pneumoniae) and Chlamydia pneumoniae (C. pneumoniae). The main 'typical' agent causing CAP is Streptococcus pneumoniae (S. pneumoniae). It is usually not possible to determine which of the many potential agents is the cause of CAP, so that antibiotic treatment is empirical, customarily covering both typical and atypical bacteria. While typical coverage is essential, the necessity of the atypical coverage has not been proven. In the previous version of this review we showed that there was no advantage to the atypical arm. Given the persisting inconsistency between current guidelines for treatment of pneumonia and the available evidence, we undertook to update this systematic review.
This Cochrane review looked at trials comparing antibiotic regimens with atypical coverage to those without, limited to hospitalized adults with CAP. We included 28 trials, involving 5939 patients. For the regimens tested, no advantage was found for regimens covering atypical bacteria in the major outcomes tested ‐ mortality and clinical efficacy. There was no significant difference between the groups in the frequency of total adverse events, or those requiring discontinuation of treatment. However, gastrointestinal events were less common in the atypical arm.
There are limitations to this review in that a single study compared the addition of the atypical antibiotic to a typical antibiotic, the major question in clinical practice; most compared a single atypical antibiotic to a single typical antibiotic. Seventeen of the 27 trials were open label, 21 of the 27 studies were sponsored by pharmaceutical companies of which all but one was conducted by the manufacturer of the atypical antibiotic.
Background
Description of the condition
Community acquired pneumonia (CAP) is a common clinical disease with considerable mortality and overwhelming economic burden. The Pneumonia Patient Outcomes Research Team (PORT) has developed a prediction scheme utilizing clinical variables to stratify CAP patients into five mortality risk classes, by which 30‐day mortality has been estimated up to 9.3% and 31.1% for patients stratified to risk class IV and V, respectively (Fine 1997). The CURB‐65 score is based on five easily measurable factors (confusion, urea, respiratory rate, blood pressure and age), by which 30‐day mortality has been estimated up to 17%, 41.5% and 57% for patients with three, four or five risk factors, respectively (Lim 2003). Although Streptococcus pneumoniae (S. pneumoniae) is the leading cause of CAP, frequency of other agents, especially those designated 'atypical pathogens' (Legionella pneumophilae (L. pneumophilae), Mycoplasma pneumoniae (M. pneumoniae) and Chlamydia pneumoniae (C. pneumoniae)), varies with geographical area, age groups and diagnostic means available. Distinctions between typical and atypical organisms is significant, as the latter share intracellularity and are treated with specific antibiotic drugs. The causing agent cannot be clearly sorted to either group by any clinical, radiological or laboratory parameter (Donowitz 2000; Levison 2001). As the microbiological diagnosis is often unknown, initial antibiotic treatment of CAP is largely empirical.
Description of the intervention
All major guidelines for treatment of CAP divide patients into three subgroups: outpatients, hospitalized patients on a general ward and hospitalized patients in intensive care units (ICUs). Major guidelines recommend in general, an antibiotic regimen covering both typical and atypical pathogens in hospitalized CAP patients (BTS 2001; Hedlund 2005; Lim 2009; Mandell 2000; Mandell 2007; Niederman 2001; Woodhead 2005; Woodhead 2011). Suggested regimens include extended spectrum cephalosporins, beta‐lactams (BLs) or beta‐lactam/beta‐lactamase inhibitors (BL/BLIs), all combined with macrolides, or a single agent with broad coverage, i.e. fluoroquinolones. The British Thoracic Society (BTS) guidelines support this recommendation in view of a high percentage of atypical pathogens, particularly L. pneumophilae, in hospitalized patients in the UK (higher, incidentally, than in North America or Europe). The American Thoracic Society (ATS) reserves recommendation of atypical coverage to clinical circumstances, although conceding elsewhere that clinical presentation cannot establish etiology. All guidelines support extensive antibiotic coverage for ICU patients, noting the prevalence as well as high mortality rates of pneumonia caused by L. pneumophilae in that population. Admittedly, these guidelines are based on insufficient evidence, as there have been only few randomized controlled trials (RCTs) assessing CAP treatment protocols.
How the intervention might work
Most trials comparing different treatment arms were performed to evaluate a specific drug, frequently a macrolide or a fluoroquinolone. Several of these trials (incidentally) compared regimens with and without atypical antibiotic coverage and found results to be generally equal (Aubier 1998; Tremolieres 1998). A meta‐analysis by Mills 2005 compared typical antibiotic coverage with BL to atypical coverage in non‐severe pneumonia and found no advantage of antibiotics with atypical coverage over BLs (risk ratio (RR) 0.97; 95% CI 0.87 to 1.07), a lower failure rate in L. pneumophilae (RR 0.4; 0.19 to 0.85), and equivalence for M. pneumoniae and C. pneumoniae. Furthermore, a large percentage of atypical pneumonia cases have been shown to be dual infections, usually in combination with a typical pathogen (Donowitz 2000). In view of these data, one must wonder what the true role of atypical agents in the treatment of pneumonia really is. If patients improve regardless of atypical coverage, should this coverage be routinely included? Furthermore, a RCT by Malhotra‐Kumar 2007 showed by obtaining pharyngeal swabs from patients before and after treatment with macrolides versus placebo, that macrolide use is the single most important driver of the emergence of macrolide resistance, and therefore the effect of prescribing macrolides is of ecological importance.
In the previous version of this systematic review (Robenshtok 2008), 25 RCTs were found comparing a treatment regimen with atypical antibiotic coverage to one without such coverage. No trials comparing an atypical antibiotic in combination with a non‐atypical antibiotic (the regimen currently recommended in all guidelines) versus the non‐atypical antibiotic alone were found. There was no difference in mortality between the atypical arm and the non‐atypical arm. The atypical arm showed an insignificant trend toward clinical success and a significant advantage to bacteriological eradication, which disappeared when evaluating methodologically high quality studies alone. A large systematic review that included observational studies, evaluated the superiority of atypical coverage to non‐atypical coverage regimens (Oosterheert 2003). Significant reduction in mortality with atypical coverage was found in six of the eight selected studies. However, these studies were non‐experimental cohort studies and outcomes showed several inconsistencies. Using propensity analysis, we showed that the major pitfall of such observational studies is the marked difference between patients prescribed non‐atypical coverage and those prescribed a regimen including atypical coverage, a difference that precludes the comparison of these treatment regimens in observational studies (Paul 2007). Expanding coverage to atypical pathogens is bound to increase toxicity, resistance and cost. Moreover, this broad coverage might be at the expense of efficacy of pneumococcal coverage (Johansen 2000).
Why it is important to do this review
Given the persisting inconsistency between current guidelines and the available evidence, we undertook to update this systematic review of hospitalized adults with CAP. The review encompassed all trials which compared treatment regimens with versus without atypical antibiotic coverage, in order to evaluate the efficacy and need of atypical coverage in this subset of patients.
Objectives
To evaluate mortality and proportion with treatment failure using regimens containing atypical antibiotic coverage compared to those without (typical coverage only).
Secondary objectives
To evaluate treatment failure with regimens containing atypical antibiotic coverage compared to those without (typical coverage only).
To evaluate the frequency of adverse effects associated with different types of antibiotic treatment.
To evaluate mortality and treatment failure of regimens containing atypical antibiotic coverage for patients with identified atypical pathogens.
To evaluate mortality and treatment failure of regimens containing atypical antibiotic coverage for patients with identified S. pneumoniae.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or quasi‐RCTs. We excluded trials with a dropout rate of over 30%.
Types of participants
Adult patients hospitalized due to suspected CAP. We did not consider patients with major immunosuppressive state(s) (i.e. malignancy, cystic fibrosis, HIV) for inclusion on this review.
Types of interventions
Studies comparing antibiotic therapy with atypical coverage to antibiotic therapy without atypical coverage. Regimens containing the following drugs, were considered atypical coverage.
Macrolides: including erythromycin, roxithromycin, azithromycin and clarithromycin.
Fluoroquinolone: including ciprofloxacin, ofloxacin, levofloxacin, trovafloxacin, moxifloxacin and grepafloxacin.
Tetracyclines: including tetracycline, doxycycline, tigecycline and minocycline.
Chloramphenicol.
Streptogramins: including pristinamycin and quinupristin‐dalfopristin.
Ketolides: including telithromycin (ketek, recently withdrawn from Food and Drug Administration (FDA) approval) (Ross 2007; Soreth 2007).
We included other drugs within the antibiotic classes listed above, if identified by our searches. We considered regimens lacking the above drugs as non‐atypical coverage. we reviewed both oral and intravenous (IV) therapies (whether comparing oral to oral, IV to oral or IV to IV).
Types of outcome measures
Primary outcomes
Overall mortality at the end of the study and up to 30 days following the end of treatment.
Secondary outcomes
Number of patients with treatment failure, as defined in the study.
Number of patients in whom bacteriological eradication was achieved.
Number of patients who needed mechanical ventilation during hospital stay.
Mean duration of hospital stay.
Number of patients excluded from outcome assessment after randomizations.
Number of patients who developed superinfection or resistance to the studied drug, defined as isolation of a pathogen or a colonizing micro‐organism resistant to the study drug, during or after antibiotic treatment.
Mean duration to regaining previous functional capacity.
Adverse events
Any serious adverse events that were fatal, life‐threatening, or requiring prolongation of existing hospitalization.
Any adverse events that required discontinuation of medication.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 3, part of The Cochrane Library, www.thecochranelibrary.com (accessed 16 April 2012), which includes the Acute Respiratory Infection Group's Specialized Register, MEDLINE (June 2007 to April Week 1, 2012) and EMBASE (June 2007 to April 2012).
We used the search strategy in Appendix 1 to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) Ovid format (Lefebvre 2011). We adapted the search for EMBASE (Appendix 2).
We inspected the references of all identified studies for more trials. In addition to this, we contacted the first or corresponding author of each included trial and researchers active in the field for information regarding unpublished trials or complementary information on their own trial. We imposed no language or publication restrictions.
See Appendix 3 for details of previous searches.
Searching other resources
In addition, we searched ClinicalTrials.gov (www.clinicaltrials.gov/) and FDA new drug approval documents for ongoing or unpublished trials (last searched 1 May, 2012).
Data collection and analysis
Selection of studies
Two review authors (DS, ER in the original review (Shefet 2005); ER, AG in the 2008 update (Robenshtok 2008); NER, ER in this current update) independently inspected each reference identified by the search and applied the inclusion criteria. For possible relevant articles, or in cases of disagreement between the two review authors, we obtained the full article and inspected it independently. A third review author (MP) checked the article in cases where resolving disagreement by discussion was not possible. When required, we contacted the trial authors of the study for clarification. We documented justifications for excluding studies from the review.
Data extraction and management
Two review authors (DS, ER in the original review (Shefet 2005); ER, AG in the 2008 update (Robenshtok 2008); NER, ER in this current update) independently extracted data from the included trials. A third review author (MP) extracted the data in case of disagreement between the two review authors. We discussed the data extraction, documented decisions and, where necessary, we contacted the trial authors for clarification.
We identified trials by the name of the first author and year in which the trial was first published and ordered chronologically. We extracted, checked and recorded the following data.
Trial characteristics
1. Year(s) and country/countries of study. 2. Trial sponsor. 3. Publication status: published in journal; abstract/proceeding; unpublished. 4. Intention‐to‐treat (ITT) analysis: performed; possible to extract; efficacy analysis. 5. Randomization methods: allocation generation; concealment; blinding. 6. Failure definition: including time of failure assessment. 7. Study follow‐up duration.
Patient characteristics
8. Number of patients randomized and evaluated, per group. 9. Age: mean or median.
Infection characteristics
10. Number of patients with documented typical pathogen. 11. Number of patients with documented atypical pathogen. 12. Number of patients with infections caused by bacteria resistant to the administered antibiotic regimen. 13. Patients with S. pneumococcus, L. pneumophilae and Mycoplasma infections. 14. Severity of the disease (as classified by PORT).
Intervention characteristics
15. Antibiotics type, dose and route of administration. 16. Treatment duration. 17. Treatment modifications.
Measures of outcome, extracted as number of patients per group
18. Overall mortality at end of study follow‐up. 19. Bacteriological eradication. 20. Treatment failure: as defined in study, with and without treatment modifications. 21. Mechanical ventilation. 22. Adverse events: life‐threatening events, events necessitating specific medical treatment, events requiring cessation of treatment.
Assessment of risk of bias in included studies
Two review authors (DS, ER in the original review (Shefet 2005); ER, AG in the 2008 update (Robenshtok 2008); NER, ER in this current update) assessed the risk of bias in trials fulfilling the review inclusion criteria. We used the 'Risk of bias' tool (Higgins 2011) to assess methodological quality according to: sequence generation, allocation concealment, blinding, incomplete outcome data addressed, free of selective reporting and free of other bias. A third review author (MP) was responsible for resolving disagreements.
We assessed risk of bias by allocation concealment (low risk of bias ‐ adequate allocation concealment; unclear allocation concealment; high risk of bias ‐ inadequate allocation concealment, i.e. quasi‐randomized studies). We graded randomization similarly (low risk ‐ table of random numbers, computer generated or coin tossing; unclear; high risk ‐ anything else) and did the same for blinding (low risk ‐ triple‐blinded or double‐blinded; high risk ‐ patient‐blinded or no blinding; and unclear when there was not enough data).
Measures of treatment effect
We analyzed dichotomous data by calculating the RR for each trial with the uncertainty in each result being expressed using 95% CIs.
Unit of analysis issues
We analyzed studies with non‐standard designs as follows.
Cluster‐randomized trials: this design would not be appropriate to evaluate antibiotic treatment for CAP.
Cross‐over trials: this design would not be appropriate to evaluate antibiotic treatment for CAP.
Studies with multiple treatment groups: we assessed intervention groups for relevance for our review. If more than two groups were relevant, we combined groups to create a single pair‐wise comparison.
Dealing with missing data
We contacted the original trial authors for missing data and requested additional information. We assumed the data to be missing at random if additional data were unavailable. We noted missing data in the 'Risk of bias' table and performed a sensitivity analysis.
We did not include the data in the meta‐analysis in cases of missing standard deviations.
Assessment of heterogeneity
We initially assessed heterogeneity in the results of the trials by inspection of graphical presentations and by calculating a test of heterogeneity (Chi2 test). We had anticipated between‐trial variation in estimation of morbidity and mortality for those patients who were hospitalized on a general ward or in ICU, if trials were performed in different geographical areas and among different age groups or if per different drug regimens were assessed. We performed subgroup analyses in order to assess the impact of these possible sources of heterogeneity on the main results.
Assessment of reporting biases
We examined a funnel plot estimating the precision of trials (plots of logarithm of the RR for efficacy against the sample size) in order to estimate potential asymmetry.
Data synthesis
We used a fixed‐effect model throughout the review. We compared the fixed‐effect model to a random‐effects model when we observed significant heterogeneity between the trials (P < 0.10). Continuous data were not available for analysis in this review.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis to assess the impact of the various treatment regimens, to analyze treatment effects on particular patient groups, and to investigate for heterogeneity. We planned to analyze mortality and clinical failure only on the following subgroups.
Type of antimicrobial agent in the atypical arm: quinolones, macrolides, combination of quinolones and macrolides, and others.
Age: under or over 65 years.
Geographical area: Europe, North America, and others.
Methodology: allocation generation, allocation concealment, blinding, and ITT analysis.
Type of bacteria: S. pneumoniae, atypical pathogens, and legionella.
Sponsoship: sponsored, non‐sponsored, and unclear sponsorship.
Sensitivity analysis
We performed sensitivity analyses in order to assess the robustness of the findings to different aspects of the trials' methodology: allocation concealment (adequate or unclear), randomizations, blinding, exclusions after randomizations (reported or not reported), sample size (up to 100 or more than 100 patients), and length of follow‐up (up to one month or one to 12 months).
Results
Description of studies
Results of the search
In this 2012 update we retrieved 1301 records from the electronic database searches. The CENTRAL search identified 304 records, MEDLINE identified 594 records and the EMBASE search identified 823 records. We evaluated all records for this update. We did not evaluate further any studies in which the abstract suggested comparator antibiotic regimens incompatible with the inclusion criteria. We similarly excluded studies of outpatients, nosocomial (hospital‐acquired) infections, respiratory tract infections other than pneumonia and non‐randomized studies.
We retrieved 66 publications for full‐text inspection, of which we excluded 38.
Included studies
Thirty publications fulfilled our inclusion criteria. Two were substudies of a parallel publication (Allegra 1995 of Rizzato 1997; Jardim 2003 of Petitpretz 2001). Thus, we included 28 trials in the review. We identified three new studies in the 2012 update (Hatipoglu 2010; Hong‐yun 2007; Kohno 2011). Two were published as abstracts only. We requested additional information from nearly all the trial authors, seven of whom responded.
Study details can be found in the Characteristics of included studies table. The studies were performed between 1982 and 2011. Sixteen were multicentered. Fifteen were performed in Europe, three in North America and ten in other countries (Japan, Argentina and South Africa) or multicontinental. Twenty‐one of the included trials were in English (including two abstracts, one of which was probably originally in Chinese), three in Japanese, two in French, one in Spanish and one in German. The studies encompassed 5939 randomized patients, of whom 5444 patients could be evaluated for our primary outcome analysis (overall mortality). The number of participants was 100 or lower in nine trials and over 100 in 19 trials (range 40 to 808 participants). All trials were restricted to adults (defined as aged above 18 years, and in one case above 16 years (Tremolieres 1998). The mean age in 14 studies was under 65 and in nine studies over 65, two of which were preformed in nursing homes (Hirata‐Dulas 1991; Peterson 1988) and one was restricted per definition to patients over 70 (Romanelli 2002). Five studies did not report the mean age.
The inclusion criteria in most studies were adults hospitalized with CAP. Three trials included outpatients (Chuard 1989; Petitpretz 2001; Tremolieres 1998), who comprised less than 25% of patients. A fourth study (Lode 1995) stated that most patients were hospitalized, without further clarification. Nine of 24 studies included patients with either nosocomial pneumonia (three studies) or bronchitis (six studies), in addition to patients with CAP. In six studies (Carbon 1992; Feldman 2001; Hirata‐Dulas 1991; Miki 1984; Norrby 1998; Peterson 1988), CAP patients consisted of over 60% (and in three of which, over 90%) of the patients. In two studies (Gleadhill 1986; Kobayashi 1984) results were given and analyzed separately for the CAP population. In the ninth study (Khan 1989), the distribution of nosocomial pneumonia versus CAP was unclear. However, the excellent results in a senior population suggest a low rate of nosocomial pneumonia. Two studies were carried out in nursing homes (Hirata‐Dulas 1991; Peterson 1988).
Pneumonia was defined by a combination of clinical signs, chest X‐ray (sole criteria in two studies ‐ Bohte 1995; Vanderdonckt 1990 ‐ and absent in five), laboratory (white blood count, 12 studies) and/or bacteriological evidence (six studies, in one as a necessary condition) (Zeluff 1988). The adjusted mean rate of bacteriological documentation was 47% in 20 studies that reported on the number of patients with bacteriological confirmation. Four studies were restricted to severe pneumonia (Fourrier 1986; Khan 1989; Rizzato 1997; Vanderdonckt 1990). In contrast, three studies excluded severe infections (Carbon 1992; Kalbermatter 2000; Lephonte 2004). Four studies included suspected pneumococcal infection (Bohte 1995; Lephonte 2004; Petitpretz 2001; Zeluff 1988), the latter was done exclusively in gold‐miners and subsequently evaluated only the proven cases; a fifth study excluded suspected cases of atypical pneumonia (Tremolieres 1998). Eleven studies provided the percentage of patients with chronic obstructive pulmonary disease (COPD) (most others included the data in combination with other major co morbidities). In eight studies, up to a quarter of the patients were previously diagnosed with COPD. In three, the number of COPD patients exceeded 30% (up to 52.5%, Rizzato 1997). None of the studies included a comparison of COPD patients and/or smokers to non‐COPD patients and/or non‐smokers.
The specific antibiotic regimens used are detailed in the Characteristics of included studies table. The atypical arm consisted of a quinolone in 21 studies, a macrolide in five and pristinamycine in one study. In the remaining study, patients were randomized to one of three arms ‐ quinolone, macrolide and a non‐atypical regimen, and the two former arms were evaluated together (Lode 1995). In all but three studies, the atypical arm was given as a monotherapy. In one case quinolone was combined with teicoplanin (Rizzato 1997) and in the second, macrolide was combined with either cephalosporin or an aminoglycoside (AG) (the two arms evaluated together, Romanelli 2002), the third is an abstract assessing the addition of an atypical antibiotic to a BL (Hatipoglu 2010). The drugs were administered orally in all but eight studies, of which most switched to oral administration within a few days.
The non‐atypical arm consisted of BLs or cephalosporins: BL in nine cases, BL/BLI in three cases, cephalosporin in 11 cases, carbapenems in two cases and penicillin in one. One study had two non‐atypical arms, cephalosporin or a BL/BLI, which were evaluated together (Kalbermatter 2000). Another study compared the atypical arm to 'customary antibiotic therapy', including BL, anti‐staphylococcal drugs, cephalosporin and AG (Fourrier 1986). All BLs, one cephalosporin and two BL/BLI (12 studies) were administered orally, one cephalosporin was given intra‐muscularly and the remaining drugs (15 studies) were administered intravenously.
Comparisons included: quinolone to BL (eight), BL/BLI (one), cephalosporin (nine, eight as quinolone monotherapy), carbapenem (one) or various non‐atypical drugs (two); macrolide to penicillin (one), BL/BLI (one), cephalosporin (one) or to carbapenems (two, one as macrolide monotherapy); quinolone and macrolide to BL/BLI (one). One study compared pristinamycine with amoxycillin. One comparison of combination cephalosporin‐macrolide to cephalosporin monotherapy was found.
The main outcome measure in all studies was clinical failure. Six studies mandated radiological resolution for success definition and one asked for bacteriological eradication. In four studies definition of success was unclear. None of the studies named mortality as a primary outcome.
Twenty‐one trials assessed bacteriological failure, defined by eradication. Some reported results per patients, whereas others by pathogens (occasionally more than one pathogen per patient). Most took follow‐up bacteriological specimens only from those patients found initially positive. Only 10 studies performed serology tests for atypical pathogens, one of which found all tests negative (Vogel 1991). Four studies were evaluated for atypical pathogens eradication rate while the other six did not fully report bacteriological success rates.
Adverse events were addressed in all studies but the two abstracts. Two studies failed to report the number of events per treatment arm (Hirata‐Dulas 1991; Lode 1995), the latter reporting the number of events in the comparator arms alone. Data relating specifically to antibiotic‐associated diarrhoea were scarce and did not permit reliable evaluation.
Seven studies assessed duration of hospital stay, none reported standard deviations. Two of these studies reported mean deviations. Therefore, continuous data was not available for analysis in this review.
Excluded studies
We retrieved 66 publications for full‐text inspection, of which we excluded 38 (Characteristics of excluded studies table). Reasons for exclusion (occasionally more than one per study) included: studies addressing outpatients (11), allowance of atypical antibiotics in the non‐atypical arm (12), nosocomial infections (5), double publication or pooled data (5), studies addressing only patients with bronchitis (2) and a dropout rate of over 30% (1). Six excluded studies investigated general severe infections, including but not exclusively, pneumonia. The percentage of pneumonia cases was usually small, some may have been nosocomial and the data were not always given separately per site of infection. One was a retrospective observational cohort study.
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarized in Figure 2.
1.
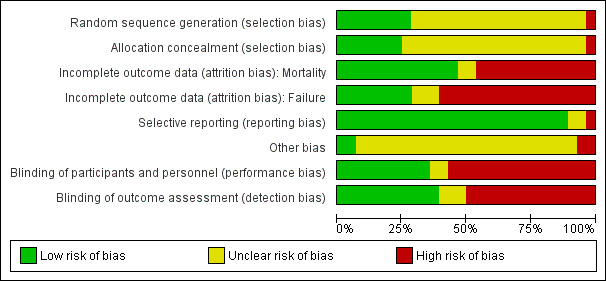
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
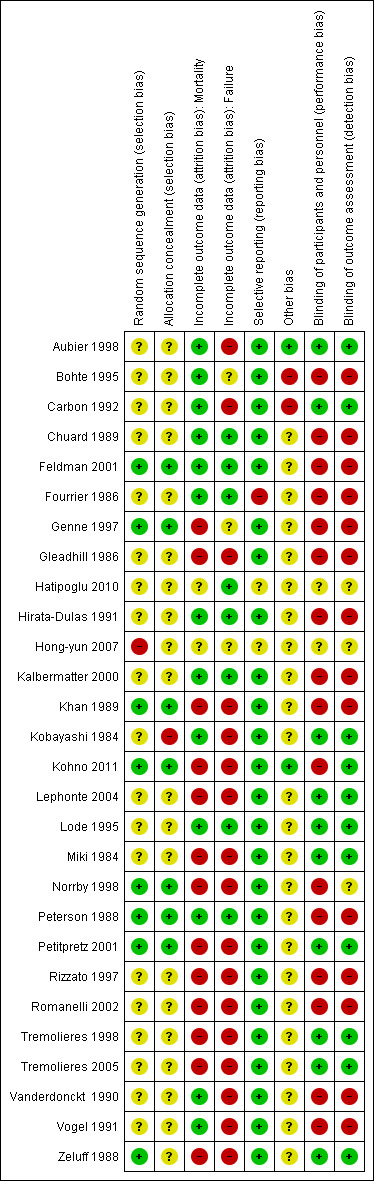
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequate allocation concealment was reported in 7 out of 28 studies. Insufficient information was available for the other studies. Allocation generation was adequate in 10 out of 28 studies. No information was available for 18 studies. All studies of adequate allocation concealment were also of adequate allocation generation.
Blinding
Ten studies were double‐blind, one was single‐blinded and the remaining (17 out of 28) were open label.
Incomplete outcome data
We separated between three levels of possible bias as follows.
Studies performed by ITT.
Per‐protocol studies, in which the number of dropouts was given per study arm.
Per‐protocol studies, in which the number of dropouts was reported or could be calculated but not given per study arm.
As mentioned above, the primary outcome in all studies was clinical success. Seven studies reported results by ITT (type 1). Fifteen additional studies reported the number of dropouts per study arm (type 2), permitting re‐analysis by ITT assuming failure for all dropouts. Six studies did not refer to dropouts (type 3) and were analyzed by evaluated patients only in the sensitivity analysis. Mortality was not a primary outcome and was usually reported in the safety analysis. Thirteen studies recounted information regarding overall mortality by ITT (type 1), while 11 provided data per‐protocol. One study does not specifically mention deaths (Kobayashi 1984), as is the case for the two abstracts (Hatipoglu 2010; Hong‐yun 2007). Twenty‐five of the 27 studies could be evaluated for mortality, encompassing 5444 of 5939 randomized patients (91.6%).
Twenty studies reported bacteriological eradication rates, encompassing 2310 patients/isolates. All but five trials reported on adverse events per treatment arm, including 4918 patients. None of the trials mentioned duration to regaining previous functional capacity, nor were there sufficient data regarding the number of patients who required mechanical ventilation.
Five studies assessed duration of hospital stay, two of these reported mean duration stay (Hirata‐Dulas 1991; Rizzato 1997); one reported median duration (Norrby 1998); and the rest reported the total number of days in hospital (Chuard 1989; Romanelli 2002). Only one study (Rizzato 1997) reported standard deviation. Therefore, continuous data were not available for analysis in this review.
Follow‐up duration was specified in 22 studies (81%), of which 16 studies (59%) defined a specific time for outcome measurement. Follow‐up ranged from immediately after completion of treatment to three months after.
Selective reporting
We did not find any specific concerns over selective. All trials defined clinical success or failure as their primary outcome and reported on it. Mortality was not reported as a separate outcome in methods but was reported in results as a safety measure or otherwise. Specific data are reported for each study in the Characteristics of included studies tables.
Other potential sources of bias
At least 21 of the 27 studies were sponsored by pharmaceutical companies; all but one of these manufactured the atypical drug.
Two of the studies included in this review were abstracts, with data extracted only from the abstracts and no further data were available. Randomization and allocation concealment procedures in these trials are unknown. One of the abstracts was published in the Chinese journal Chinese Journal of Antibiotics (Hong‐yun 2007) but we could not obtain its full‐text. We failed to contact the authors of this abstract, and therefore we have no detailed information about randomizations and allocation concealment in this study.
Effects of interventions
Overall mortality (Analyses 1.1 to 1.7)
Twenty‐five of the 27 studies could be evaluated for mortality, encompassing 5444 of 5939 randomized patients (91.6%). Six studies reported no deaths, 12 reported a mortality rate between 0.4% to 5%, six reported a rate of 5% to 8%, and one study reported a 25% mortality rate (Fourrier 1986). In three studies, two of which were abstracts, mortality was not mentioned (Hatipoglu 2010; Hong‐yun 2007; Kobayashi 1984). The adjusted mean mortality rate was 3.5%. None of the deaths according to the authors were related to the drug treatment.
There was no significant difference between the atypical arm and the non‐atypical arm in the overall mortality rate (RR 1.14; 95% CI 0.84 to 1.55, Analysis 1.1). The difference remained non‐significant when evaluating quinolone therapy (RR 0.98; 95% CI 0.69 to 1.39) and macrolide therapy (RR 1.25; 95% CI 0.52 to 3.01) alone. No heterogeneity was seen for the overall comparison (Chi2 test = 8.34, df =17, P = 0.96, I2 statistic = 0%).
1.1. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 1 Mortality per antibiotic (ABX) treatment.
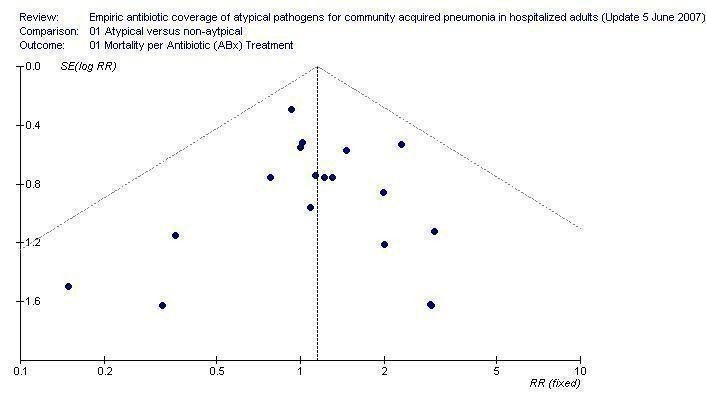
Mortality was further analyzed by patient characteristics: age (mean age older or younger than 65 years); and geographical area (Europe, North America or other). Neither comparison disclosed a significant difference (Analysis 1.2 and Analysis 1.3, respectively). Overall mortality in both arms was similar when analyzing studies by randomizations generation, allocation concealment and blinding (Analysis 1.4, Analysis 1.5 and Analysis 1.6, respectively), as well as in the ITT analysis (Analysis 1.7). The effect of sponsorship by pharmaceutical companies could not be evaluated, as in the non‐sponsored trials there was no mortality. In the funnel plot for overall mortality, which included 19 trials in which mortality was more than zero, results are symmetrically centered around the combined RR (Figure 3).
1.2. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 2 Mortality per age.
1.3. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 3 Mortality ‐ per geographical area.
1.4. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 4 Mortality per allocation generation.
1.5. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 5 Mortality per allocation concealment.
1.6. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 6 Mortality per blinding.
1.7. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 7 Mortality ‐ ITT analysis.
3.
Clinical failure (Analyses 1.8 to 1.18)
Clinical failure was the primary outcome in all 28 studies, comprising 5419 patients (of 5939 randomized). A non‐significant trend toward an advantage to the atypical arm was observed combining all studies (RR 0.93; 95% CI 0.84 to 1.04) (Analysis 1.8). No heterogeneity for the total results was seen (Chi2 test = 29.53, df = 24, P = 0.2, I2 statistic = 18.7%). When evaluating the different atypical regimens, quinolone monotherapy showed non‐significant advantage (RR 0.89; 95% CI 0.79 to 1.02) while macrolide monotherapy showed non‐significant disadvantage (RR 1.11; 95% CI 0.76 to 1.62). The non‐significant advantage of the atypical arm was seen in young patients (mean age < 65 years: RR 0.93; 95% CI 0.81 to 1.06) as well as older patients (mean age older than 65 years: RR 0.91; 95% CI 0.75 to 1.10) (Analysis 1.9). The advantage was statistically significant in the European studies (RR 0.85; 95% CI 0.74 to 0.98) but not in studies carried out elsewhere (Analysis 1.10).
1.8. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 8 Clinical failure per antibiotic (ABx) treatment.
1.9. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 9 Clinical failure per age.
1.10. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 10 Clinical failure per geographical area.
There was no advantage to atypical treatment in studies of high methodological quality. There was no difference in clinical efficacy when combining studies that scored A in randomizations generation (RR 0.99; 95% CI 0.82 to 1.19) or allocation concealment (RR 0.99; 95% CI 0.82 to 1.19). Studies of B score accentuated an advantage toward the atypical arm (Analysis 1.11; Analysis 1.12). Rates of clinical failure were similar in trials sponsored by pharmaceutical companies (RR 0.97; 95% CI 0.86 to 1.08, 21 trials) and non‐sponsored trials (RR 1.02; 95% CI 0.64 to 1.62, 3 trials) (Analysis 1.18).
1.11. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 11 Clinical failure per allocation generation.
1.12. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 12 Clinical failure per allocation concealment.
1.18. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 18 Clinical failure per sponsorship.
We performed an ITT versus per‐protocol design sensitivity analysis, counting all dropouts as failures in the ITT group (Analysis 1.14). No significant differences between type 1 (RR 0.92; 95% CI 0.76 to 1.12), type 2 (RR 0.94; 95% CI 0.84 to 1.05) or type 3 (RR 0.85; 95% CI 0.63 to 1.15) studies, although type 3 studies showed a higher effect estimate for atypical treatment.
1.14. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 14 Clinical failure ‐ ITT analysis.
Clinical failure with macrolide antibiotic treatment was the only comparison in which heterogeneity was detected (Chi2 test = 6.68, df = 3, P = 0.08, I2 statistic = 55.1%). Re‐analysis done by the random‐effects model did not alter results.
Clinical failure rates were evaluated per pathogens isolated. Of the 1021 pneumococcal pneumonia cases reported, there was a non‐significant advantage to the non‐atypical arm (RR 1.22; 95% CI 0.88 to 1.7, Analysis 1.15). Data were insufficient to distinctively analyze cases of pneumococcal bacteraemia. In contrast to S. pneumoniae, there was a non‐significant advantage to the atypical arm in the treatment of atypical pathogens (RR 0.52; 95% CI 0.24 to 1.10, Analysis 1.16). Eradication of L. pneumophilae in particular was highly significant, with a RR of 0.17 and a narrow CI (0.05 to 0.63), although only 43 cases were available (Analysis 1.17).
1.15. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 15 Clinical failure ‐ pneumococcal pneumonia.
1.16. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 16 Clinical failure ‐ atypical pathogens.
1.17. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 17 Clinical failure ‐ Legionella pneumophilae.
Bacteriological failure (Analyses 1.19 to 1.20)
Twenty studies reported bacteriological eradication rates, encompassing 2310 patients/isolates (mixed results, as each study used different measures, see above). There was a statistically significant advantage to bacteriological eradication in the atypical treatment arm (RR 0.80; 95% CI 0.65 to 0.98, Analysis 1.19). No heterogeneity was seen (Chi2 test = 12.4, df = 18, P = 0.0.83, I2 statistic = 0%). When considering only the high quality studies ('A' score in allocation generation), no significant difference was detected in bacteriological eradication between the two treatment arms (RR 0.90; 95% CI 0.61 to 1.32, Analysis 1.20). Again, no heterogeneity was seen (Chi2 test = 1.35, df = 6, P = 0.97, I2 statistic = 0%). Only five studies reported super‐infections, with numbers too small for statistical assessment.
1.19. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 19 Bacteriological failure.
1.20. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 20 Bacteriological failure per allocation generation.
Adverse events (Analyses 1.21 to 1.23)
All but five trials reported on adverse events per treatment arm, including 4918 patients. Total adverse events (Analysis 1.21) were similar in both treatment arms (RR 1.02; 95% CI 0.93 to 1.13). No heterogeneity was seen (Chi2 test = 39.93, df = 21, P = 0.009, I2 statistic = 29.8%). Gastrointestinal events (Analysis 1.22) were reported in 16 studies and were significantly less common in the atypical arm (RR 0.7; 95% CI 0.53 to 0.92). However, definition of gastrointestinal events differed, some including abdominal pain and some diarrhoea alone, thereby excluding an accurate comparison of the frequency of antibiotic‐associated diarrhoea. Both arms were nearly equal with regard to adverse events requiring discontinuation (RR 1.01; 95% CI 0.72 to 1.41, Analysis 1.23).
1.21. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 21 Adverse events ‐ total.
1.22. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 22 Adverse events ‐ gastrointestinal events.
1.23. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 23 Adverse events ‐ requiring discontinuation of treatment.
Other outcomes
Treatment duration was conveyed in 14 studies and was almost uniformly 10 days, with no difference between the arms. None of the trials mentioned duration to regaining previous functional capacity, nor were there sufficient data regarding the number of patients who required mechanical ventilation.
Discussion
Summary of main results
The primary outcome we assessed was overall mortality. There was no difference between the atypical arm and the non‐atypical arm with respect to this outcome. This did not change when analyzing results per population characteristics or methodology. There was a non‐significant trend toward clinical success in the atypical arm, accentuated with quinolone monotherapy. The advantage disappeared when evaluating high‐quality methodological studies alone (i.e. studies that had a low risk of selection bias). A significant advantage in bacteriological eradication was detected in the atypical arm, especially in reference to L. pneumophilae eradication. This advantage was not demonstrated in an analysis restricted to studies with adequate allocation generation. There was no statistically significant difference in the frequency of total adverse events between the two groups, although less gastrointestinal events were noted in the atypical arm.
Failure was commonly defined as lack of clinical improvement, deterioration, relapse and/or modifications of the antibiotic treatment. These endpoints are subjective and should therefore be studied with care, especially as many are non‐blinded. Although pharmaceutical companies producing the atypical antibiotic sponsored most studies, the point estimate for sponsored and non‐sponsored trials for clinical failure is similar. The similar response of the young and old is somewhat surprising, as one might anticipate that younger people would benefit more from atypical coverage, given a higher prevalence of atypical pneumonia in this age group. Perhaps this prevalence diminishes in the hospitalized population. A higher L. pneumophilae prevalence in the elder population may also explain the similarity.
Overall completeness and applicability of evidence
Mortality data were obtained for 91.6% of randomized patients. The overall mortality in included studies (adjusted mean 3.5%) was lower than reported in the literature (MedisGroups overall mortality of 10.6% (Iezzoni 1988); PORT validation cohort inpatient mortality of 8.0% (Fine 1997)). This is surprising in view of the fact that 12 of the 27 studies target relatively severe pneumonia cases (two studies conducted in nursing homes, one excluding patients under 70 years of age, three including suspected or proven pneumococcal pneumonia (the fourth excluded severe cases), one excluding suspected atypical cases, four restricted to clinically severe pneumonia and one restricted to moderate‐severe pneumonia (see above). The low mortality rate is not a result of under‐reporting, as mortality data were almost complete. Mortality was not a primary outcome in any of the studies, although it is obviously the most significant outcome in a potentially lethal infectious episode.
Quality of the evidence
The atypical arm's advantage in bacteriological eradication could not be explained by the coverage of atypical pathogens alone: results of clinical success in those cases were equivocal and their proven share of events was small. As demonstrated above, its reliability is questionable, since it became insignificant in the evaluation of high quality studies. One should note that the atypical arm was usually a new drug (tested by the manufacturer) and therefore expected resistance rates were small, compared to the typical arm which comprised of BL or cephalosporins. Furthermore, most publications date to the early 1990s, while new resistant trends have emerged since then (for example increasing pneumococcal resistance to quinolones). The clinical advantage of L. pneumophilae coverage is not surprising. It is interesting to note that cases of atypical pneumonia (including L. pneumophilae) often resolved even in the treatment arm lacking atypical coverage. Only a minority were co‐infections with typical pathogens.
The total of adverse events did not differ significantly between treatment arms. Gastrointestinal side effects were studied separately in order to try and assess frequency of antibiotic‐associated diarrhoea after BL or cephalosporin treatment and were found to be significantly more prevalent in the non‐atypical arm. However, the trials differed in definition of events to a point that precludes this assessment.
We had set off to investigate the contribution of atypical coverage to empiric treatment of CAP in hospitalized patients. The most suitable study for our purpose is one comparing a BL or cephalosporin with the same BL or cephalosporin combined with a macrolide or a quinolone. The last update found no such trials. In this update we found only a single abstract assessing directly the addition of an atypical antibiotic to a BL, which found no positive effect on treatment success rates with the addition of atypical coverage (Hatipoglu 2010). The need to add a macrolide to a BL therapy is a common dilemma manifested within the guidelines themselves. Our meta‐analysis is therefore chiefly based on comparison of BL or cephalosporin regimens to atypical monotherapy, mainly quinolone monotherapy. Regarding this comparison, we found no advantage to atypical coverage in terms of mortality or clinical success. The relatively low mortality rates in the existing hospitalized CAP trials included in the mortality analysis, further limits our conclusion.
Potential biases in the review process
Inclusion of abstracts and studies with missing data might have introduced bias.
Agreements and disagreements with other studies or reviews
The objective of our study was to assess the efficacy and need of adding antibiotic coverage for atypical pathogens in hospitalized patients with CAP, in terms of mortality and successful treatment.
All major guidelines for treatment of CAP recommend in general, an antibiotic regimen covering both typical and atypical pathogens in hospitalized CAP patients (BTS 2001; Hedlund 2005; Lim 2009; Mandell 2000; Mandell 2007; Niederman 2001; Woodhead 2005). Admittedly, these guidelines are based on insufficient evidence, as there have been only few randomized controlled trials assessing CAP treatment protocols. Most trials comparing different treatment arms were performed to evaluate a specific drug, frequently a macrolide or a fluoroquinolone. Several of these trials (incidentally) compared regimens with and without atypical antibiotic coverage and found results to be generally equal (Aubier 1998; Tremolieres 1998).
A large systematic review that included observational studies, evaluated the superiority of atypical coverage to non‐atypical coverage regimens (Oosterheert 2003). Significant reduction in mortality with atypical coverage was found in six of the eight selected studies. However, these studies were non‐randomized cohort studies and outcomes showed several inconsistencies. Using propensity analysis, we showed that the major pitfall of such observational studies is the marked difference between patients prescribed non‐atypical coverage and those prescribed a regimen including atypical coverage, a difference that precludes the comparison of these treatment regimens in observational studies (Paul 2007). Expanding coverage to atypical pathogens is bound to increase toxicity, resistance and cost. Moreover, this broad coverage might be at the expense of efficacy of pneumococcal coverage (Johansen 2000).
We conclude that no benefit of survival or clinical efficacy was shown to empirical atypical coverage in hospitalized patients with CAP. Further randomized controlled trials, comparing BL or cephalosporins therapy to BL or cephalosporins combined with a macrolide in this population, using mortality as its primary outcome, should be performed.
Authors' conclusions
Implications for practice.
No benefit of survival or clinical efficacy was shown to empirical atypical coverage in hospitalized patients with CAP. This conclusion relates mostly to the comparison of quinolone monotherapy with BL monotherapy.
Implications for research.
A RCT of a BL monotherapy compared to the same BL combined with a macrolide or a quinolone in hospitalized patients with CAP should be performed. Until then, the major regimen recommended in current guidelines remains unsubstantiated by evidence. The trial should have adequate randomizations generation and concealment and should report patient relevant outcomes (FDA 2009) and mortality. The trial should include patients with severe pneumonia, compatible (as regards severity) to those observed in sound observational studies.
What's new
| Date | Event | Description |
|---|---|---|
| 16 April 2012 | New search has been performed | Searches updated. Three new trials were included (Hatipoglu 2010; Hong‐yun 2007; Kohno 2011) and three new trials were excluded (Chokshi 2007; Ott 2008; Sujata 2008). After integrating the data from the new included studies, no significant changes were noted in any of the comparisons evaluated. Our conclusions and recommendations for further research remain unchanged |
| 14 September 2011 | New citation required but conclusions have not changed | A new author joined the team to lead the update of this review |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 22 June 2008 | Amended | Converted to new review format. |
| 12 November 2007 | New search has been performed | In the 2007 update, one new study was included and six new studies were excluded. After integrating the data from the new included study, no significant changes were noted in all comparisons evaluated. Our conclusions and recommendations for further research remain unchanged. |
| 20 January 2005 | New search has been performed | Searches conducted. |
Acknowledgements
We thank the trial authors who responded to our requests: Dr. Claude Carbon, Dr. Francois Fourrier, Dr. Daniel Genne, Dr. Hartmut Lode, Dr. Phillip Peterson, Dr. Patrick Petitpretz and Dr. Jose Sifuentes Osornio. We are indebted to Dr. Karla Soares‐Weiser for her guidance. We would like to thank Dr. Gabriel Izbicki for his translation from German and to Ms. Rika Fujia for obtaining and translating the Japanese articles. Warm thanks to the Cochrane Acute Respiratory Infections Group editorial team for their guidance throughout the review (Liz Dooley, Managing Editor) and the help devising and conducting the search (Sarah Thorning, Trials Search Co‐ordinator). We wish to thank the following people for commenting on this latest updated draft: Linda Hornbeek, Richard Taggart, Theo Verheij, Nelcy Rodriguez and Allen Cheng.
Appendices
Appendix 1. CENTRAL and MEDLINE search strategy
MEDLINE (PUBMED) 1 exp Pneumonia/ 2 (pneumon* or cap).tw. 3 1 or 2 4 exp Anti‐Bacterial Agents/ 5 antibiotic*.tw. 6 exp Quinolones/ 7 exp Macrolides/ 8 exp Tetracyclines/ 9 exp Chloramphenicol/ 10 exp Streptogramins/ 11 Ketolides/ 12 (quinolon* or fluoroquinolon* or macrolid* or doxycyclin* or tetracyclin* or chloramphenicol* or streptogramin* or ketolid* or erythromycin* or roxithromycin* or azithromycin* or clarithromycin* or ciprofloxacin* or ofloxacin* or levofloxacin* or trovafloxacin* or moxifloxacin* or grepafloxacin* or tigecyclin* or minocyclin* or pristinamycin* or quinupristin* or telithromycin*).tw,nm. 13 or/4‐12 14 3 and 13
Appendix 2. EMBASE search strategy
#13. #9 AND #12 3,993 1 Jun 2011 #12. #10 OR #11 866,353 1 Jun 2011 #11. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 826,523 1 Jun 2011 #10. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 242,926 1 Jun 2011 #9. #3 AND #8 54,097 1 Jun 2011 #8. #4 OR #5 OR #6 OR #7 788,217 1 Jun 2011 #7. quinolon*:ab,ti OR fluoroquinolon*:ab,ti OR macrolid*:ab,ti OR doxycyclin*:ab,ti OR tetracyclin*:ab,ti OR chloramphenicol*:ab,ti OR streptogramin*:ab,ti OR ketolid*:ab,ti OR erythromycin*:ab,ti OR roxithromycin*:ab,ti OR azithromycin*:ab,ti OR clarithromycin*:ab,ti OR ciprofloxacin*:ab,ti OR ofloxacin*:ab,ti OR levofloxacin*:ab,ti OR trovafloxacin*:ab,ti OR moxifloxacin*:ab,ti OR grepafloxacin*:ab,ti OR tigecyclin*:ab,ti OR minocyclin*:ab,ti OR pristinamycin*:ab,ti OR quinupristin*:ab,ti OR telithromycin*:ab,ti AND [embase]/lim 95,680 1 Jun 2011 #6. 'quinolone derivative'/exp OR 'macrolide'/exp OR 'tetracycline derivative'/exp OR 'chloramphenicol'/exp OR 'streptogramin derivative'/exp OR 'ketolide'/exp AND [embase]/lim 230,698 1 Jun 2011 #5. antibiotic*:ab,ti AND [embase]/lim 176,808 1 Jun 2011 #4. 'antibiotic agent'/exp AND [embase]/lim 706,809 1 Jun 2011 #3. #1 OR #2 169,385 1 Jun 2011 #2. pneumon*:ab,ti OR cap:ab,ti AND [embase]/lim 122,834 1 Jun 2011 #1. 'pneumonia'/de OR 'infectious pneumonia'/exp AND [embase]/lim 92,744 1 Jun 2011
Appendix 3. Previous search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 2) which includes the Acute Respiratory Infection Group's specialized register, MEDLINE (January 1966 to June 2007), and EMBASE (January 1980 to June 2007).
We ran the following search strategy over MEDLINE and CENTRAL and adapted it for EMBASE.
MEDLINE (PUBMED) 1 exp Anti‐Bacterial Agents/ 2 exp Quinolones/ 3 exp Fluoroquinolones/ 4 exp Macrolides/ 5 exp Doxycycline/ 6 exp Tetracycline/ 7 exp Chloramphenicol/ 8 exp Streptogramins/ 9 exp Ketolides/ 10 (Quinolon$ or Fluoroquinolon$ or Macrolide$ or Doxycycline$ or Tetracycline$ or Chloramphenicol$).ti,ab. 11 empirical antibiotic$.ti,ab. 12 empiric antibiotic$.ti,ab. 13 or/1‐12 14 exp Community‐Acquired Infections/ 15 exp Pneumonia/ 16 and/14‐15 17 (community acquired pneumonia or CAP).mp. 18 16 or 17 19 exp Inpatients/ 20 inpatient$.ti,ab. 21 exp Hospitalization/ 22 ((hospitaliz$ or hospitalis$) adj patient$).mp. 23 or/17‐20 24 13 and 18 and 23
We inspected the references of all identified studies for more trials. In addition to this, we contacted the first or corresponding author of each included trial and researchers active in the field for information regarding unpublished trials or complementary information on their own trial. We imposed no language or publication restrictions.
Data and analyses
Comparison 1. Atypical versus non‐aytpical.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality per antibiotic (ABX) treatment | 25 | 5444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.55] |
| 1.1 Quinolone (atypical arm) | 19 | 3698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.39] |
| 1.2 Macrolide (atypical arm) | 4 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.52, 3.01] |
| 1.3 Combined quinolone and macrolide (atypical arm) | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.81, 6.44] |
| 1.4 Pristinamycine (atypical arm) | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.37, 10.69] |
| 2 Mortality per age | 25 | 5444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.55] |
| 2.1 Mean age under 65 years old | 15 | 3820 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.75, 1.94] |
| 2.2 Mean age over 65 years old | 8 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.72, 1.69] |
| 2.3 Data unavailable | 2 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.93] |
| 3 Mortality ‐ per geographical area | 25 | 5444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.55] |
| 3.1 Europe | 14 | 3209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.79, 1.89] |
| 3.2 North America | 3 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.52, 3.86] |
| 3.3 Other | 8 | 2003 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.62, 1.60] |
| 4 Mortality per allocation generation | 25 | 5444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.55] |
| 4.1 A | 10 | 1953 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.68, 1.68] |
| 4.2 B | 15 | 3491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.79, 1.81] |
| 4.3 C | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Mortality per allocation concealment | 25 | 5444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.55] |
| 5.1 A | 7 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.65] |
| 5.2 B | 18 | 3854 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.81, 1.83] |
| 5.3 C | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mortality per blinding | 25 | 5444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.55] |
| 6.1 Non‐blinded | 16 | 2290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.72, 1.50] |
| 6.2 Single‐blind | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Double or triple‐blind | 9 | 3154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.79, 2.38] |
| 7 Mortality ‐ ITT analysis | 12 | 2143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.70, 2.15] |
| 7.1 ITT (type 1) | 12 | 2143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.70, 2.15] |
| 8 Clinical failure per antibiotic (ABx) treatment | 28 | 5419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 8.1 Quinolone (atypical arm) | 21 | 3704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.02] |
| 8.2 Macrolide (atypical arm) | 5 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.62] |
| 8.3 Combined quinolone and macrolide (atypical arm) | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.17] |
| 8.4 Pristinamycine (atypical arm) | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.77, 1.81] |
| 9 Clinical failure per age | 28 | 5419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 9.1 Mean age under 65 years old | 15 | 3554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| 9.2 Mean age over 65 years old | 8 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.10] |
| 9.3 Data unavailable | 5 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.50] |
| 10 Clinical failure per geographical area | 28 | 5419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 10.1 Europe | 15 | 3084 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 10.2 North America | 3 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.59, 1.45] |
| 10.3 Other | 10 | 2103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.24] |
| 11 Clinical failure per allocation generation | 28 | 5419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 11.1 A | 10 | 1878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.19] |
| 11.2 B | 17 | 3467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 11.3 C | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.29 [0.41, 130.64] |
| 12 Clinical failure per allocation concealment | 28 | 5419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 12.1 A | 7 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.19] |
| 12.2 B | 20 | 3762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.02] |
| 12.3 C | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.29 [0.41, 130.64] |
| 13 Clinical failure per blinding | 28 | 5419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 13.1 Non‐blinded | 18 | 2415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 13.2 Single‐blind | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Double or triple‐blind | 10 | 3004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.11] |
| 14 Clinical failure ‐ ITT analysis | 28 | 5682 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 14.1 ITT studies (type 1) | 7 | 1232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.76, 1.12] |
| 14.2 Dropouts assumed as failure (type 2) | 15 | 3849 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.05] |
| 14.3 Non‐ITT, dropouts cannot be calculated (type 3) | 6 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 15 Clinical failure ‐ pneumococcal pneumonia | 18 | 1021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.88, 1.70] |
| 16 Clinical failure ‐ atypical pathogens | 4 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.24, 1.10] |
| 17 Clinical failure ‐ Legionella pneumophilae | 5 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.63] |
| 18 Clinical failure per sponsorship | 28 | 5419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 18.1 Sponsored trials | 21 | 4540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.08] |
| 18.2 Non‐sponsored trials | 3 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.64, 1.62] |
| 18.3 Unclear sponsorship | 4 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.49, 0.93] |
| 19 Bacteriological failure | 21 | 2310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 19.1 Overall | 21 | 2310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 20 Bacteriological failure per allocation generation | 21 | 2310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 20.1 A | 8 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.32] |
| 20.2 B | 13 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.59, 0.96] |
| 21 Adverse events ‐ total | 24 | 4918 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |
| 22 Adverse events ‐ gastrointestinal events | 16 | 4129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.53, 0.92] |
| 23 Adverse events ‐ requiring discontinuation of treatment | 12 | 3806 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.72, 1.41] |
1.13. Analysis.
Comparison 1 Atypical versus non‐aytpical, Outcome 13 Clinical failure per blinding.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aubier 1998.
| Methods | Randomized Multicenter | |
| Participants | Adults (mean age: 42) Hospitalized CAP | |
| Interventions | Atypical: PO sparfloxacin 400 mg X 1/d followed by 200 mg X 1/d Non‐atypical: PO amoxacillin 1 G X /d | |
| Outcomes | Success: resolution of signs and symptoms Modification = failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 266/329 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Bohte 1995.
| Methods | Randomized Multicenter | |
| Participants | Adults (18 to 75, age ˜ 53) CAP ‐ clinically suspected pneumococcal pneumonia (suspected Legionella pneumophilae (L. pneumophilae) Staphylococcus aureus (S. aureus) and Klebsiella pneumonia (K. pneumonia) excluded) Hospitalized | |
| Interventions | Atypical: PO azithromycin 500 mg X 2/d loading dose, followed by PO azithromycin 500 mg X 1/d Non‐atypical: IV benzylpenicillin 1,000,000 IU X 4/d | |
| Outcomes | Failure = clinical, radiological, change of antibiotics | |
| Notes | Study included a second comparison between erythromycin and azithromycin, which was excluded from analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Unclear risk | 64/66 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | High risk | Not ITT (4/108 lost) Sponsored: grant from Pfizer Inc. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None (open label) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Carbon 1992.
| Methods | Randomized Multicenter | |
| Participants | Adults (mean age: 55) Hospitalized CAP (severe pneumonia excluded) | |
| Interventions | Atypical: PO temafloxacin 600 mg X 2/d Non‐atypical: PO amoxicillin 500 mg X 3/d | |
| Outcomes | Failure = persistence of symptoms or signs, treatment modification Modification = failure | |
| Notes | Severe pneumonia excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 243/246 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | High risk | Not ITT Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Chuard 1989.
| Methods | Randomized Multicenter | |
| Participants | Adults (mean ˜ 66) 95% hospitalized 58% CAP (42% bronchitis or COPD exacerbation) | |
| Interventions | Atypical: PO ofloxacin 200 mg X 2/d (several 400 mg X 2/d) Non‐atypical: PO amoxicillin 750 mg X 3/d (several 375 mg X 4/d) | |
| Outcomes | Clinical | |
| Notes | Another trial reported in same study of non‐randomized CAP suspected of atypical infection; relationship to this trial unclear Results given are for all patients, including non‐pneumonia Study in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None (open label) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Feldman 2001.
| Methods | Randomized Multicentered | |
| Participants | Adults 18 to 70 (mean age: 43) Hospitalized ˜ 90% CAP; ˜ 10% nosocomial | |
| Interventions | Atypical: IV sitafloxacin 400 mg X 1/d Non‐atypical: IV imipenem/cilastatin 500 mg X 3/d | |
| Outcomes | Clinical success (as defined by investigator) | |
| Notes | Phase II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization code designed to allocate equal numbers of patients to the 2 treatment groups using the method of random‐permutated blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Sealed, serially‐numbered envelopes, each containing details of the treatment allocation for a single patient |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None (open label) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None (open label) |
Fourrier 1986.
| Methods | Randomized Allocation A/B | |
| Participants | Adults (no age mentioned) Hospitalized CAP; severe (per clinical) | |
| Interventions | Atypical: PO pefloxacine 1200 mg/d Non‐atypical: customary antibiotics:
|
|
| Outcomes | Failure = opposite of complete recovery | |
| Notes | A lot of data missing (letter), even after correspondence with author Study in French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Number of patients with bacteriologically documented pathogen and pneumococcal pneumonia was lost (correspondence with author) |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Maybe single‐blinded ‐ simple randomization |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Genne 1997.
| Methods | Randomized | |
| Participants | Adults (mean age: 70) Hospitalized CAP | |
| Interventions | Atypical: IV clarythromycin 500 mg X 2/d 3 to 5/d , followed by PO clarythromycin 500 mg X 2/d Non‐atypical: IV amoxicillin‐clavulanate 1.2 G X 4/d 3‐5/d, followed by PO amoxicillin‐clavulanate 625 mg X 3/d | |
| Outcomes | Failure = change of ABX, persistence or progression of sx/radiological finding, death, s/e enabling completion of trial | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using sealed envelopes with random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes with numbers |
| Incomplete outcome data (attrition bias) Mortality | High risk | 112/127 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Unclear risk | 112/127 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Gleadhill 1986.
| Methods | Randomized | |
| Participants | Adults (mean 71) 46% CAP; 50% COPD exacerbation; 4% acute bronchitis Hospitalized | |
| Interventions | Atypical: PO ciprofloxacin 500 mg X 2/d Non‐atypical: PO amoxicillin 250 mg X 3/d | |
| Outcomes | Clinical and bacteriological:
|
|
| Notes | Less than 50% CAP Data analyzed only for pneumonia patients (given separately) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | High risk | 48/52 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 48/52 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsor? |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Hatipoglu 2010.
| Methods | Randomized | |
| Participants | Adults (no age mentioned) Hospitalized CAP, moderately‐severe | |
| Interventions | Atypical: ceftriaxone + clarithromycin Non‐atypical: ceftriaxone | |
| Outcomes | Success rate Incremental cost‐effectiveness analysis |
|
| Notes | Data extracted from abstract, no correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not enough data |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not enough data |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough data |
Hirata‐Dulas 1991.
| Methods | Randomized | |
| Participants | Adults (mean age: 79) Nursing home residents Hospitalized Nursing home‐acquired lower respiratory tract infection (54 pneumonia, 6 acute bronchitis) | |
| Interventions | Atypical: IV ciprofloxacin 200 mg/400 mg* X 2/d, followed by PO ciprofloxacin 750 mg X 2/d (* protocol changed from 200 mg to 400 mg on 4/1989, 5/24 P in new protocol) Non‐atypical: IV ceftriaxone 2 G X 1/d, followed by IM ceftriaxone 1 G X 1/d | |
| Outcomes | Success = resolution or marked improvement in clinical signs and symptoms of lower RTI and completion of 14 d treatment course Failure = all cause death, early termination due to lack of improvement, adverse event or superinfection Indeterminate = withdrawal due to protocol specification or to other factors unrelated to study drug administration Moderation = failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None (unknown) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None (unknown) |
Hong‐yun 2007.
| Methods | Randomized | |
| Participants | Adults (no age mentioned) Hospitalized CAP | |
| Interventions | Atypical: iv gatifloxacin (400 mg) Non‐atypical: iv cefuroxime (2 G) | |
| Outcomes | Success: clinical and bacteriological Adverse reactions |
|
| Notes | Data extracted from abstract, no correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Failure | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not enough data |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not enough data |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough data |
Kalbermatter 2000.
| Methods | Randomized | |
| Participants | Adults (mean age: 69 years) Hospitalized CAP; severe pneumonia was excluded | |
| Interventions | Atypical: PO levofloxacin 500 mg X 2/d Non‐atypical: IV amoxicillin/clavulanate 1 G X 3/d IV ceftriaxone 1 G X 2/d | |
| Outcomes | Success = clinical and radiological Modification = failure | |
| Notes | 3 arms, amoxicillin/clavulanate and ceftriaxone calculated together as non‐atypical arm Study in Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Not identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Khan 1989.
| Methods | Randomized | |
| Participants | Adults (mean age: 58) Severe bacterial respiratory tract infection (34/122 presumed bacterial, no isolation) CAP + nursing home‐acquired + nosocomial | |
| Interventions | Atypical: IV ciprofloxacin 200 mg X 2/d (5 patients received 300 mg X 2/d) Non‐atypical: IV ceftazidime 1 to 2 G X 2/d to 3/d | |
| Outcomes | Failure = continuation/worsening of signs and symptoms | |
| Notes | Distribution of nosocomial pneumonia versus CAP unclear; good results suggest low rate of nosocomial pneumonia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Antibiotics were administered with a computer‐generated, randomized code |
| Allocation concealment (selection bias) | Low risk | Antibiotics were administered in a sequential manner |
| Incomplete outcome data (attrition bias) Mortality | High risk | 122/140 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 122/140 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Manufacturer/Pharmaceutical company sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Kobayashi 1984.
| Methods | Randomized | |
| Participants | Adults CAP + COPD (results given separately) | |
| Interventions | Atypical: PO levofloxacin 200 mg X 3/d Non‐atypical: PO amoxicillin 250 mg X 4/d | |
| Outcomes | ||
| Notes | Study in Japanese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 74/76 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Kohno 2011.
| Methods | Randomized, open‐label, non‐inferiority study Multicenter |
|
| Participants | Adults (mean age: 58.5) CAP mild to severe (70% moderate) | |
| Interventions | Atypical: IV levofloxacin 500 mg X 1/d for 7 to 14 days Non‐atypical: IV ceftriaxone 1 G X 2/d for 7 to 14 days | |
| Outcomes | Success: clinical, radiological and laboratory | |
| Notes | Study in Japanese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central, registration control system |
| Allocation concealment (selection bias) | Low risk | Evaluator center, randomly allocated patients |
| Incomplete outcome data (attrition bias) Mortality | High risk | 200/260 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 200/260 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Low risk | Not identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A separate party committee evaluated the effects of the drugs, not knowing which drug had been used |
Lephonte 2004.
| Methods | Randomized Multicenter | |
| Participants | Adults (mean age: 54) CAP Suspected pneumococcal pneumonia > 90% hospitalized | |
| Interventions | Atypical: PO gemifloxacin 320 mg X 1/d for 1 week Non‐atypical: amoxicillin/clavulanate 1 G/125 mg X 3/d for 10 days | |
| Outcomes | Failure = clinical, bacteriological and radiological | |
| Notes | Phase III Suspected pneumococcal pneumonia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | High risk | 249/324 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 249/324 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Manufacturer/Pharmaceutical company sponsored No ITT analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Lode 1995.
| Methods | Randomized Multicenter | |
| Participants | Adults (mean age: 54) Hospitalized and outpatients CAP | |
| Interventions | Atypical: PO sparfloxacin 400 mg loading dose, followed by PO sparfloxacin 200 mg X 1/d PO erythromycin 1 G X 2/d Non‐atypical: PO amoxicillin‐clavulanic acid 500/125 mg X 3/d | |
| Outcomes | Failure = clinical, radiological, change of antibiotics Modification = failure | |
| Notes | 3 arms in 2:1:1 randomizations (sparfloxacin: amoxicillin‐clavulanic acid: erythromycin); the two atypical arms were calculated as one, in comparison to the non‐atypical arm Note: also outpatients ‐ no further data, short of stating the majority of patients were hospitalized | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Miki 1984.
| Methods | Randomized | |
| Participants | Adults Hospitalized Suspected bacterial pneumonia (CAP), small percentage of upper RTI | |
| Interventions | Atypical: PO enoxacin 600 mg/d Non‐atypical: PO amoxicillin 1 G/d | |
| Outcomes | ||
| Notes | Study in Japanese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | High risk | 145\147 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 139/147 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Norrby 1998.
| Methods | Randomized Multicenter |
|
| Participants | Adults (mean 65) Hospitalized Pneumonia (CAP 94%; nosocomial 6%) | |
| Interventions | Atypical: IV levofloxacin 500 mg X 2/d, followed by PO levofloxacin 500 mg X 2/d Non‐atypical: IV ceftriaxone 4 G X 1/d | |
| Outcomes | Cure = all infection related signs and symptoms disappeared or returned to pre‐infection state and chest X‐ray findings at least improved Failure = all infection related signs and symptoms unchanged or worsened; patients developed new clinical findings consistent with active infection; patient died due to the infection; drug study discontinued; one or more antibiotics added to drug due to treatment failure | |
| Notes | CAP and nosocomial infection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central, computerized telephone system |
| Allocation concealment (selection bias) | Low risk | Evaluator‐blinded for selected patients |
| Incomplete outcome data (attrition bias) Mortality | High risk | 619/625 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 619/625 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored No ITT |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Three types of assessments were performed according to the study protocol: protocol, conducted by computer; investigator, conducted by the investigator and evaluator‐blinded, conducted by an external evaluator for selected patients using blinded data |
Peterson 1988.
| Methods | Randomized | |
| Participants | Adults > 60 years old (mean: 81 years) Nursing home residents Hospitalized CAP/RTI | |
| Interventions | Atypical: PO ciprofloxacin 750 mg X 2/d Non‐atypical: IM cefamandole 1 G X 4/d | |
| Outcomes | Success = improvement in signs and symptoms and patient not given another course of drug treatment for RTI within 6 weeks of discharge | |
| Notes | 2/3 CAP, 1/3 RTI, most data combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment code |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Petitpretz 2001.
| Methods | Randomized Multinational, multicenter | |
| Participants | Adults (mean age: 51) CAP: suspected pneumococcal pneumonia 79% hospitalized | |
| Interventions | Atypical: PO moxifloxacin 400 mg X 1/d Non‐atypical: PO Amoxicillin 1 G X 3/d Both for 10 days | |
| Outcomes | Clinical response Failure = insufficient resolution of symptoms/death | |
| Notes | Presumed pneumococcal pneumonia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author confirmed in correspondence adequate methods |
| Allocation concealment (selection bias) | Low risk | Author confirmed in correspondence adequate methods |
| Incomplete outcome data (attrition bias) Mortality | High risk | 408/411 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 408/411 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Rizzato 1997.
| Methods | Randomized Multicenter | |
| Participants | Age > 65 or 18 to 65 years + comorbidities (mean age: 68.5) Severe CAP: exclusion: "risk of death within 48h of study start" Hospitalized | |
| Interventions | Atypical: IV teicoplanin 400 mg LD, followed by 400/200 mg X 1/d (per investigator's discretion) + PO ciprofloxacin 500 mg X 2/d Non‐atypical: IV ceftriaxone 2 G X 1/d | |
| Outcomes | Clinical response: success (cure, improvement), failure and data could not be evaluated | |
| Notes | Severe CAP; elderly or comorbidities Allegra 1995 is a double publication of this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | High risk | 225/240 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 225/240 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Romanelli 2002.
| Methods | Randomized Multicenter | |
| Participants | Elderly > 70 (mean age: 76) CAP Hospitalized | |
| Interventions | Atypical: IV clarythromycin 500 mg + IV ceftriaxone 1 G X 2/d IV clarythromycin 500 mg + IV amikacin 250 mg X 2/d versus Non‐atypical: IV meropenem 500 mg X 3/d IV imipenem/cilastatin 500 mg X 3/d | |
| Outcomes | Clinical response Failure = no improvement or deterioration of signs and symptoms OR relapse | |
| Notes | Elderly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | High risk | 204/226 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 204/226 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Tremolieres 1998.
| Methods | Randomized Multicenter | |
| Participants | Adults > 16 years old (mean age: 52) 75% hospitalized CAP: exclusion: high probability of atypical pneumonia | |
| Interventions | Atypical: PO trovafloxacin 200 mg X 1/d Non‐atypical: PO amoxicillin 1 G X 3/d | |
| Outcomes | Primary = clinical response; Secondary = bacteriological response Failure = lack of resolution of signs and symptoms/radiological failure/additional ABX given to patient | |
| Notes | Adult defined as age > 16 years old (rather than 18 years old, as in other studies) Only 75% hospitalized | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Mortality | High risk | 335/344 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 285/344 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Tremolieres 2005.
| Methods | Randomized Multicenter | |
| Participants | Adults > 18 years old (mean age: 48) CAP | |
| Interventions | Atypical: PO pristinamycin 1 G x 3 Non‐atypical: amoxacillin 1 G x 3 | |
| Outcomes | Clinical failure, bacteriological failure, pneumococcal treatment failure, mortality, s/e | |
| Notes | 20% with COPD; 6.5% asthma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not identified |
| Allocation concealment (selection bias) | Unclear risk | Not identified |
| Incomplete outcome data (attrition bias) Mortality | High risk | 285/342 patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 285/342 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | sponsored by Pfizer Center Research |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
Vanderdonckt 1990.
| Methods | Randomized Multicentre | |
| Participants | Adults (mean 60) Severe bronchopulmonary infections Hospitalized | |
| Interventions | Atypical: PO pefloxacin 400 mg X 2/d Non‐atypical: IV ceftazidime 2 G X 2/d | |
| Outcomes | Success not defined in study Bacteriological outcome | |
| Notes | Severe infection = failure on previous treatment, multi‐resistant pathogens, severe underlying disease, poor general condition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not identified |
| Allocation concealment (selection bias) | Unclear risk | Not identified |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 156/170 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Unknown sponsorship |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Vogel 1991.
| Methods | Randomized Multicenter | |
| Participants | Adults (mean age: 62) Hospitalized CAP | |
| Interventions | Atypical: PO temafloxacin 600 mg X 2/d Non‐atypical: IV cefotaxime 2 G X 3/d | |
| Outcomes | Cure: all signs and symptoms resolved Improvement: reduction of severity or numbers of signs and symptoms Failure: evidence of fever or worsening of signs, symptoms or chest X‐ray after 3 treatment days Modification = ? | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not identified |
| Allocation concealment (selection bias) | Unclear risk | Not identified |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All patients evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 75/100 patients evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | Sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None |
Zeluff 1988.
| Methods | Randomized | |
| Participants | Adults (mean age: 31; all South‐African black gold miners) Hospitalized Pneumococcal pneumonia per chest X‐ray and sputum (analyzed only culture positive) | |
| Interventions | Atypical: PO roxithromycin 150 mg X 2/d Non‐atypical: PO cephradine 1 G X 2/d | |
| Outcomes | Clinical response (= improvement of signs and symptoms and resolution of lung infiltrate) | |
| Notes | Pneumococcal pneumonia in a young homogenous group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An allocation schedule of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not identified |
| Incomplete outcome data (attrition bias) Mortality | High risk | 158/160 participants evaluated |
| Incomplete outcome data (attrition bias) Failure | High risk | 90/160 participants evaluated |
| Selective reporting (reporting bias) | Low risk | Not identified |
| Other bias | Unclear risk | ? sponsored |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
ABX: antibiotics CAP: community‐acquired pneumonia COPD: chronic obstructive pulmonary disease IM: intramuscular ITT: intention‐to‐treat IV: intravenous LD: loading dose PO: per oral RTI: respiratory tract infection s/e: side effects 1/d: once a day 2/d: two times a day 3/d: three times a day 4/d: four times a day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ailani 1999 | Doxycycline versus standard therapy ‐ latter arm not differentiated to regimens with atypical coverage and those without such coverage |
| Aubier 1996 | Pooled analysis of 2 studies (1 = this paper) Sparfloxacin versus comparator arm, including erythromycin The results of studies are combined, thus no differentiation between atypical and non‐atypical coverage Note: very large study (N = 1137 |
| Chokshi 2007 | Retrospective observational cohort study |
| Donowitz 1997 | Outpatients |
| Fass 1989 | Study of general serious infections Of 98 participants, 29 were non‐randomized but included with randomized participants in the analysis Only 21/98 participants with pneumonia, some may be nosocomial |
| File 1997 | Atypical coverage (macrolide or doxycycline) could be added to non‐atypical arm at investigator's discretion (if atypical pathogens were suspected or proven) |
| Fink 1994 | Of 402 participants randomized, 78% were diagnosed with nosocomial pneumonia |
| Fong 1995 | Outpatients |
| Geijo Martinez 2002 | Non‐atypical group were given a macrolide optionally |
| Hagberg 2002 | Outpatients |
| Hoepelman 1993 |
|
| Katz 2004 | In the typical arm, patients could receive azithromycin or/and flagyl |
| Khajotia 1990 | Over 80% lower respiratory tract infection without parenchymal involvement per chest X‐ray. No separate information given for the (15 + 7) patients with CAP |
| Kinasewitz 1991 | Outpatients |
| Krumpe 1999 | Study of treatment of severe infections: of 540 patients enrolled, 310 were diagnosed with pneumonia, of whom more than 50% (57% of original patients) were diagnosed with nosocomial pneumonia |
| Kuzman 2005 | In the typical arm 30% received doxycycline |
| Leophonte 1999 | Meta‐analysis of 5 trials, out of which 2 are included and 2 are excluded in this study; the fifth is yet to be located |
| Levine 1989 | > 30% dropout rate (45/113 patients) |
| Lode 1987 | Study of general severe clinical infections. Pneumonia patients 25/66, some may be nosocomial (no response from trial author) |
| Lode 1990 | Reports 4 trials. The first is included in our study and the second compares two quinolones. The third and the forth have insufficient data regarding the study populations and outcomes. Not published elsewhere |
| Lode 1998 | Retroactive analysis of 4 randomized clinical trials, concentrating on CAP patients with pneumococcal bacteraemia. Relevant studies were extracted and analyzed separately |
| Lode 2004 | Both ambulatory and hospitalized patients could be included in the study but that information was not recorded. Therefore, the proportion of hospitalized patients is unknown |
| Mendoca 2004 | In the typical arm 11% received a macrolide antibiotic |
| Mouton 1991 | Non‐atypical group were given amoxicillin and/or erythromycin |
| O'Doherty 1997 | Outpatients |
| Ott 2008 | Study of aspiration pneumonia and lung abscess |
| Peacock 1987 | Study of general serious infections. Small number of pneumonia patients (7 and 4) |
| Plouffe 1996 | Ofloxacin versus standard therapy ‐ latter arm not differentiated to regimens with atypical coverage and those without such coverage |
| Rahav 2004 | Outpatients |
| Siami 1995 | Of 54 randomized patients, 89% were diagnosed with nosocomial pneumonia and only 11% diagnosed with CAP |
| Sifuentes 1989 | Study of general severe infections. Small number of pneumonia patients. Data of mortality not given separately |
| Snydman 1995 | Previously published (Fink 1994, excluded due to a high percentage of nosocomial pneumonia in enrolled patients) |
| Stocks 1989 | Outpatients |
| Sujata 2008 | In the typical arm, patients could receive macrolide |
| Torres 2003 | Outpatients |
| Trenholme 1989 |
|
| Welte 2005 | In the typical arm, patients could receive erythromycin |
| Wollschlager 1987 |
|
CAP: community‐acquired pneumonia
Contributions of authors
Noa Eliakim Raz (NER) co‐ordinated this updated review, guided by Leonard Leibovic (LL). NER and Eyal Robenshtok (ER) were responsible for data collection, writing to trial authors for additional information and organizing retrieval of papers. NER and ER were responsible for undertaking searches. NER, ER, Daphna Shefet (DS), Anat Gafter‐Gvili (AG) and Mical Paul (MP) were responsible for screening search results, abstracting data from papers, screening retrieved papers against inclusion criteria and appraising quality of papers (the latter review author was in‐charge in case of disagreement). NER was responsible for entering data into Review Manager 5 (RevMan). All review authors participated in analysis and interpretation of data. NER and ER were responsible for writing the review.
Sources of support
Internal sources
Rabin Medical Center ‐ Beilinson Campus, Israel.
External sources
EU 5th Framework: TREAT project (grant number: 1999‐11459), Not specified.
The Rothschild‐Caesarea Foundation “Optimal antibiotic treatment of moderate to severe infections: Integration of PCR technology and medical informatics/artificial intelligence”, Not specified.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aubier 1998 {published data only}
- Aubier M, Verster R, Regamey C, Geslin P, Vercken JB, Sparfloxacin European Study Group. Once‐daily sparfloxacin versus high‐dosage amoxicillin in the treatment of community‐acquired, suspected pneumococcal pneumonia in adults. Clinical Infectious Diseases 1998;26(6):1312‐20. [DOI] [PubMed] [Google Scholar]
Bohte 1995 {published data only}
- Bohte R, van't Wout JW, Lobatto S, Blusse van Oud Alblas A, Boekhout M, Nauta EH, et al. Efficacy and safety of azithromycin versus benzylpenicillin or erythromycin in community‐acquired pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1995;14(3):182‐7. [DOI] [PubMed] [Google Scholar]
Carbon 1992 {published data only}
- Carbon C, Lephonte P, Petitpretz P, Chauvin JP, Hazebroucq J. Efficacy and safety of temafloxacin versus those of amoxicillin in hospitalized adults with community‐acquired pneumonia. Antimicrobial Agents and Chemotherapy 1992;36(4):833‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuard 1989 {published data only}
- Chuard C, Regamey C. Effect and tolerance of ofloxacin in bronchopulmonary infections in comparison with amoxicillin. Schweizerische Medizinische Wochenschrift 1989;119(52):1913‐6. [PubMed] [Google Scholar]
Feldman 2001 {published data only}
- Feldman C, White H, O'Grady J, Flitcroft A, Briggs A, Richards G. An open, randomised, multi‐centre study comparing the safety and efficacy of sitafloxacin and imipenem/cilastatin in the intravenous treatment of hospitalised patients with pneumonia. International Journal of Antimicrobial Agents 2001;17(3):177‐88. [DOI] [PubMed] [Google Scholar]
Fourrier 1986 {published data only}
- Fourrier F, Chopin C, Lestavel P, Savage C. Initial antibiotherapy in severe bacterial bronchopneumopathies. Randomized study of a new quinolone: pefloxacin. La Presse Medicale 1986;15(26):1240. [PubMed] [Google Scholar]
Genne 1997 {published data only}
- Genné D, Siegrist HH, Humair L, Janin‐Jaquat B, Torrenté A. Clarithromycin versus amoxicillin‐clavulanic acid in the treatment of community‐acquired pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1997;16(11):783‐8. [DOI] [PubMed] [Google Scholar]
Gleadhill 1986 {published data only}
- Gleadhill IC, Ferguson WP, Lowry RC. Efficacy and safety of ciprofloxacin in patients with respiratory infections in comparison with amoxycillin. Journal of Antimicrobial Chemotherapy 1986;18(Supp D):133‐8. [DOI] [PubMed] [Google Scholar]
Hatipoglu 2010 {unpublished data only}
- Hatipoglu ON, Uysal S, Altiay G, Sut N, Tabakoglu E. The effect of addition of macrolide to a beta‐lactam antibiotic on treatment success in patients with moderately severe community acquired pneumonia (Abstract). Proceedings of the European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010.
Hirata‐Dulas 1991 {published data only}
- Hirata‐Dulas CA, Stein DJ, Guay DR, Gruninger RP, Peterson PK. A randomized study of ciprofloxacin versus ceftriaxone in the treatment of nursing home‐acquired lower respiratory tract infections. Journal of the American Geriatrics Society 1991;39(10):979‐85. [DOI] [PubMed] [Google Scholar]
Hong‐yun 2007 {published data only}
- Hong‐yun L, Chao Q, Qian L, Qun F. Observation of the therapeutic effects of gatifloxacin on community acquired pneumonia. Chinese Journal of Antibiotics 2007, (5). [Google Scholar]
Kalbermatter 2000 {published data only}
- Kalbermatter V, Bagilet D, Diab M, Javkin E. Oral levofloxacin versus intravenous ceftriaxone and amoxicillin/clavulanic acid in the treatment of community‐acquired pneumonia that requires hospitalization. Medicina Clinica 2000;115(15):561‐3. [DOI] [PubMed] [Google Scholar]
Khan 1989 {published data only}
- Khan FA, Basir R. Sequential intravenous‐oral administration of ciprofloxacin vs ceftazidime in serious bacterial respiratory tract infections. Chest 1989;96(3):453‐6. [DOI] [PubMed] [Google Scholar]
Kobayashi 1984 {published data only}
- Kobayashi H, Takamura K, Kono K, Onodera S, Sasaki N, Nagahama F, et al. Comparison of DL‐8280 and amoxicillin in the treatment of respiratory tract infections. Kansenshogaku Zasshi 1984;58(6):525‐55. [DOI] [PubMed] [Google Scholar]
Kohno 2011 {published data only}
- Kohno S, Watanabe A, Aoki N, Niki Y, Kadota J, Fujita J, et al. Clinical phase III comparative study of intravenous levofloxacin and ceftriaxone in community‐acquired pneumonia treatment. Japanese Journal of Chemotherapy 2011;59:32‐45. [Google Scholar]
Lephonte 2004 {published data only}
- Leophonte P, File T, Feldman C. Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community‐acquired pneumonia of suspected pneumococcal origin. Respiratory Medicine 2004;98(8):708‐20. [DOI] [PubMed] [Google Scholar]
Lode 1995 {published data only}
- Lode H, Garau J, Grassi C, Hosie J, Huchon G, Legakis N, et al. Treatment of community‐acquired pneumonia: a randomized comparison of sparfloxacin, amoxycillin‐clavulanic acid and erythromycin. European Respiratory Journal 1995;8(12):1999‐2007. [DOI] [PubMed] [Google Scholar]
Miki 1984 {published data only}
- Miki F, Saito A, Tomizawa M, Nakayama I, Takebe K, Takashima T, et al. Comparative study of the effectiveness of enoxacin and amoxicillin in bacterial pneumonia by a double blind method. Kansenshogaku Zasshi 1984;58(10):1083‐113. [DOI] [PubMed] [Google Scholar]
Norrby 1998 {published data only}
- Norrby SR, Petermann W, Willcox PA, Vetter N, Salewski E. A comparative study of levofloxacin and ceftriaxone in the treatment of hospitalized patients with pneumonia. Scandinavian Journal of Infectious Diseases 1998;30(4):397‐404. [DOI] [PubMed] [Google Scholar]
Peterson 1988 {published data only}
- Peterson PK, Stein D, Guay DR, Logan G, Obaid S, Gruninger R, et al. Prospective study of lower respiratory tract infections in an extended‐care nursing home program: potential role of oral ciprofloxacin. American Journal of Medicine 1988;85(2):164‐71. [DOI] [PubMed] [Google Scholar]
Petitpretz 2001 {published data only}
- Jardim JR, Rico G, Roza C, Obispo E, Urueta J, Wolff M, et al. A comparison of moxifloxacin and amoxicillin in the treatment of community‐acquired pneumonia in Latin America: results of a multicenter clinical trial. Archives de Bronchoneumologia 2003;39(9):387‐93. [DOI] [PubMed] [Google Scholar]
- Petitpretz P, Arvis P, Marel M, Moita J, Urueta J, CAP5 Moxifloxacin Study Group. Oral moxifloxacin vs high‐dosage amoxicillin in the treatment of mild‐to‐moderate, community‐acquired, suspected pneumococcal pneumonia in adults. Chest 2001;119(1):185‐95. [DOI] [PubMed] [Google Scholar]
Rizzato 1997 {published data only}
- Allegra L, Berni F, Boisisio E, Clini V, Dal Negro R, Damato S, et al. Efficacy of the teicoplanin‐ciprofloxacin combination in high risk community acquired pneumonia: Comparison to ceftriaxone in a multicenter study. Internista 1995;31:39‐43. [Google Scholar]
- Montemurro L, Rizzato G, Multicenter Study Group. The teicoplanin‐ciprofloxacin combination versus ceftriaxone in community acquired pneumonia of elderly and patients with underlying diseases. European Respiratory Journal 1993;7(Supp 18):170. [Google Scholar]
- Rizzato G, Allegra L: Multicentre Italian Study Group (co‐ordinated by Centro Thorax). Efficacy and tolerability of teicoplanin‐ciprofloxacin combination in severe community‐acquired pneumonia. Comparison with ceftriaxone in a multicentre Italian study. Clinical Drug Investigation 1997;14(5):337‐45. [Google Scholar]
Romanelli 2002 {published data only}
- Romanelli G, Cravarezza P, Pozzi A, Franchino L, Ravizzola G, Zulli R, et al. Carbapenems in the treatment of severe community‐acquired pneumonia in hospitalized elderly patients: a comparative study against standard therapy. Journal of Chemotherapy 2002;14(6):609‐17. [DOI] [PubMed] [Google Scholar]
Tremolieres 1998 {published data only}
- Tremolieres F, Kock F, Pluck N, Daniel R. Trovafloxacin versus high‐dose amoxicillin (1g three times daily) in the treatment of community‐acquired bacterial pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1998;17(6):447‐53. [DOI] [PubMed] [Google Scholar]
Tremolieres 2005 {published data only}
- Tremolieres F, Mayaud C, Mouton Y, Weber P, Dellatolas F, Caulin E. Efficacy and safety of pristinamycin vs amoxicillin in community acquired pneumonia in adults. Pathologie‐biologie (Paris) 2005;53(8‐9):503‐10. [DOI] [PubMed] [Google Scholar]
Vanderdonckt 1990 {published data only}
- Vanderdonckt J. Comparison of pefloxacin with ceftazidime in severe bronchopulmonary infections. Journal of Antimicrobial Chemotherapy 1990;26(Suppl B):111‐6. [DOI] [PubMed] [Google Scholar]
Vogel 1991 {published data only}
- Vogel F, Lode H. The use of oral temafloxacin compared with a parenteral cephalosporin in hospitalized patients with pneumonia. Journal of Antimicrobial Chemotherapy 1991;28(Supp C):81‐6. [DOI] [PubMed] [Google Scholar]
Zeluff 1988 {published data only}
- Zeluff BJ, Lowe P, Koorhof HJ, Gentry LO. Evaluation of roxithromycin (RU‐965) versus cephradine in pneumococcal pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1988;7(1):69‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ailani 1999 {published data only}
- Ailani RK, Agastya G, Ailani RK, Mukunda BN, Shekar R. Doxycycline is a cost‐effective therapy for hospitalized patients with community‐acquired pneumonia. Archives of Internal Medicine 1999;159(3):266‐70. [DOI] [PubMed] [Google Scholar]
Aubier 1996 {published data only}
- Aubier M, Lode H, Gialdroni‐Grassi G, Huchon G, Hosie J, Legakis N, et al. Sparfloxacin for the treatment of community‐acquired pneumonia: a pooled data analysis of two studies. Journal of Antimicrobial Chemotherapy 1996;37(Suppl A):73‐82. [DOI] [PubMed] [Google Scholar]
Chokshi 2007 {published data only}
- Chokshi R, Restrepo MI, Weeratunge N, Frei CR, Anzueto A, Mortensen EM. Monotherapy versus combination antibiotic therapy for patients with bacteremic Streptococcus pneumoniae community‐acquired pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 2007;26(7):447‐51. [DOI] [PubMed] [Google Scholar]
Donowitz 1997 {published data only}
- Donowitz GR, Brandon ML, Salisbury JP, Harman CP, Tipping DM, Urick AE, et al. Sparfloxacin versus cefaclor in the treatment of patients with community‐acquired pneumonia: a randomized, double‐masked, comparative, multicenter study. Clinical Therapeutics 1997;19(5):936‐53. [DOI] [PubMed] [Google Scholar]
Fass 1989 {published data only}
- Fass RJ, Plouffe JF, Russell JA. IV/oral ciprofloxacin vs ceftazidime in the treatment of serious infections. American Journal of Medicine 1989;87(5A):164‐8. [DOI] [PubMed] [Google Scholar]
File 1997 {published data only}
- File TM, Segreti J, Dunbar L, Player R, Kohler R, Williams RR, et al. A multicenter, randomized study comparing the efficacy and safety of intravenous and/or oral levofloxacin versus ceftriaxone and/or cefuroxime axetil in treatment of adults with community‐acquired pneumonia. Antimicrobial Agents and Chemotherapy 1997;41(9):1965‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fink 1994 {published data only}
- Fink MP, Snydman DR, Niederman MS, Leeper KV Jr, Johnson RH, Heard SO, et al. The Severe Pneumonia Study Group. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double‐blind trial comparing intravenous ciprofloxacin with imipenem‐cilastatin. Antimicrobial Agents and Chemotherapy 1994;38(3):547‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fong 1995 {published data only}
- Fong IW, Laforge J, Dubois J, Small D, Grossman R, Zakhari R, The Canadian Bronchitis Study Group. Clarithromycin versus cefaclor in lower respiratory tract infections. Clinical and Investigative Medicine. Médecine Clinique et Experimentale 1995;18(2):131‐8. [PubMed] [Google Scholar]
Geijo Martinez 2002 {published data only}
- Geijo Martinez MP, Diaz de Tuesta, Chow‐Quan AM, Herranz CR, Gomez Criado C, Dimas Nunez JF, et al. Levofloxacin versus beta‐lactamic therapy in community acquired pneumonia that requires hospitalisation [Levofloxacino frente a betalactamicos en el tratamiento de la neumonia adquirida en la comunidad con ingreso hospitalario]. Anales de Medicina Interna 2002;19(12):609‐11. [PubMed] [Google Scholar]
Hagberg 2002 {published data only}
- Hagberg L, Torres A, Rensburg D, Leroy B, Rangaraju M, Ruuth E. Efficacy and tolerability of once‐daily telithromycin compared with high‐dose amoxicillin for treatment of community‐acquired pneumonia. Infection 2002;30(6):378‐86. [DOI] [PubMed] [Google Scholar]
Hoepelman 1993 {published data only}
- Hoepelman AI, Sips AP, Helmond JL, Barneveld PW, Neve AJ, Zwinkels M, et al. A single‐blind comparison of three‐day azithromycin and ten‐day co‐amoxiclav treatment of acute lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):147‐52. [DOI] [PubMed] [Google Scholar]
Katz 2004 {published data only}
- Katz E, Larsen LS, Fogarty CM, Hamed K, Song J, Choudhri S. Safety and efficacy of sequential i.v. to p.o. moxifloxacin versus conventional combination therapies for the treatment of community‐acquired pneumonia in patients requiring initial i.v. therapy. Journal of Emergency Medicine 2004;27(4):395‐405. [DOI] [PubMed] [Google Scholar]
Khajotia 1990 {published data only}
- Khajotia R, Drlicek M, Vetter N. A comparative study of ofloxacin and amoxycillin/clavulanate in hospitalized patients with lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1990;26(Suppl D):83‐91. [DOI] [PubMed] [Google Scholar]
Kinasewitz 1991 {published data only}
- Kinasewitz G, Wood RG. Azithromycin versus cefaclor in the treatment of acute bacterial pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1991;10(10):872‐7. [DOI] [PubMed] [Google Scholar]
Krumpe 1999 {published data only}
- Krumpe PE, Cohn S, Garreltes J, Ramirez J, Coulter H, Haverstock D, et al. Ciprofloxacin Study Group. Intravenous and oral mono‐ or combination‐therapy in the treatment of severe infections: ciprofloxacin versus standard antibiotic therapy. Journal of Antimicrobial Chemotherapy 1999;43(Supp A):117‐28. [DOI] [PubMed] [Google Scholar]
Kuzman 2005 {published data only}
- Kuzman I, Dakovic‐Rode O, Oremus M, Banaszak AM. Clinical efficacy and safety of a short regimen of azithromycin sequential therapy vs standard cefuroxime sequential therapy in the treatment of community‐acquired pneumonia: an international, randomized, open‐label study. Journal of Chemotherapy 2005;17(6):636‐42. [DOI] [PubMed] [Google Scholar]
Leophonte 1999 {published data only}
- Leophonte P, Veyssier P. Levofloxacin in the treatment of community‐acquired pneumococcal pneumonia. La Presse Medicale 1999;28(36):1975‐9. [PubMed] [Google Scholar]
Levine 1989 {published data only}
- Levine D, McNeil P, Lerner SA. Randomized, double‐blind comparative study of intravenous ciprofloxacin versus ceftazidime in the treatment of serious infections. American Journal of Medicine 1989;87(5A):160‐3. [DOI] [PubMed] [Google Scholar]
Lode 1987 {published data only}
- Lode H, Wiley R, Hoffken G, Wagner J, Borner K, Lode H, et al. Prospective randomized controlled study of ciprofloxacin versus imipenem‐cilastatin in severe clinical infections. Antimicrobial Agents and Chemotherapy 1987;31(10):1491‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lode 1990 {published data only}
- Lode H, Wiley E, Olschewski P, Sievers H, Wintermantel M, Baetz R, et al. Prospective randomized clinical trials of new quinolones versus beta‐lactam antibiotics in lower respiratory tract infections. Scandinavian Journal of Infectious Diseases 1990;68(Suppl):50‐5. [PubMed] [Google Scholar]
Lode 1998 {published data only}
- Lode H, Aubier M, Portier H, Ortquist A, Sparfloxacin Study Group. Sparfloxacin as alternative treatment to standard therapy for community‐acquired bacteremic pneumococcal pneumonia. Clinical Microbiology and Infection 1998;4(3):135‐43. [DOI] [PubMed] [Google Scholar]
Lode 2004 {published data only}
- Lode H, Magyar P, Muir JF, Loos U, Kleutgens K, International Gatifloxacin Study Group. Once‐daily oral gatifloxacin vs three‐times‐daily co‐amoxiclav in the treatment of patients with community‐acquired pneumonia. Clinical Microbiology and Infection 2004;10(6):512‐20. [DOI] [PubMed] [Google Scholar]
Mendoca 2004 {published data only}
- Mendonca JS, Yamaguti A, Correa JC, Badaro R. Gatifloxacin in the treatment of community‐acquired pneumonias: a comparative trial of ceftriaxone, with or without macrolides, in hospitalized adult patients with mild to moderately severe pneumonia. Brazilian Journal of Infectious Diseases 2004;8(1):90‐100. [DOI] [PubMed] [Google Scholar]
Mouton 1991 {published data only}
- Mouton Y, Beuscart C, Leroy O, Ajana F, Charrel J, Groupe Multicentrique. Evaluation of ciprofloxacin versus amoxicillin + clavulanic acid or erythromycin for the empiric treatment of community‐acquired pneumonia. Pathologie‐Biologie 1991;39(1):34‐7. [PubMed] [Google Scholar]
O'Doherty 1997 {published data only}
- O'Doherty BO, Dutchman DA, Pettit R, Maroli A. Randomized, double blind, comparative study of grepafloxacin and amoxycillin in the treatment of patients with community‐acquired pneumonia. Journal of Antimicrobial Chemotherapy 1997;40(Supp A):73‐81. [DOI] [PubMed] [Google Scholar]
Ott 2008 {published data only}
- Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection 2008;36(1):23‐30. [DOI] [PubMed] [Google Scholar]
Peacock 1987 {published data only}
- Peacock J Jr, Pegram SP, Weber SF, Leone PA. Prospective, randomized comparison of sequential intravenous followed by oral ciprofloxacin with intravenous ceftazidime in the treatment of serious infections. American Journal of Medicine 1989;87(5A):185S‐90S. [DOI] [PubMed] [Google Scholar]
Plouffe 1996 {published data only}
- Plouffe JF, Herbert MT, File TM Jr, Baird I, Parsons JN, Kahn JB, et al. Ofloxacin versus standard therapy in treatment of community‐acquired pneumonia requiring hospitalization. Antimicrobial Agents and Chemotherapy 1996;40(5):1175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahav 2004 {published data only}
- Rahav G, Fidel J, Gibor Y, Shapiro M. Azithromycin versus comparative therapy for the treatment of community acquired pneumonia. International Journal of Antimicrobial Agents 2004;24(2):181‐4. [DOI] [PubMed] [Google Scholar]
Siami 1995 {published data only}
- Siami GA, Wilkins WT, Bess DT, Christman JW. Comparison of ciprofloxacin with imipenem in the treatment of severe pneumonia in hospitalised geriatric patients. Drugs 1995;49(Supp 2):436‐8. [DOI] [PubMed] [Google Scholar]
Sifuentes 1989 {published data only}
- Sifuentes‐Osornio J, Macias A, Amieva RI, Ramos A, Ruiz‐Palacios GM. Intravenous ciprofloxacin and ceftazidime in serious infections. A prospective, controlled clinical trial with third‐party blinding. American Journal of Medicine 1989;87(5A):202S‐5S. [DOI] [PubMed] [Google Scholar]
Snydman 1995 {published data only}
- Snydman D, Fink F, Niederman M, Reinhart H. Treatment of severe pneumonia in hospitalised patients. Results of a multicentre trial comparing i.v. ciprofloxacin with imipenem/cilastatin. Drugs 1995;49(Supp 2):439‐41. [DOI] [PubMed] [Google Scholar]
Stocks 1989 {published data only}
- Stocks JM, Wallace RJ Jr, Griffith DE, Garcia JG, Kohler RB. Ofloxacin in community‐acquired lower respiratory infections. A comparison with amoxicillin or erythromycin. American Journal of Medicine 1989;87(6C):52‐6. [PubMed] [Google Scholar]
Sujata 2008 {published data only}
- Bhavnani SM, Ambrose PG. Cost‐effectiveness of oral gemifloxacin versus intravenous ceftriaxone followed by oral cefuroxime with/without a macrolide for the treatment of hospitalized patients with community‐acquired pneumonia. Diagnostic Microbiology and Infectious Disease 2008;60(1):59‐64. [DOI] [PubMed] [Google Scholar]
Torres 2003 {published data only}
- Torres A, Muir JF, Corris P, Kubin R, Duprat‐Lomon I, Sagnier PP, et al. Effectiveness of oral moxifloxacin in standard first‐line therapy in community‐acquired pneumonia. European Respiratory Journal 2003;21(1):135‐43. [DOI] [PubMed] [Google Scholar]
Trenholme 1989 {published data only}
- Trenholme GM, Schmitt BA, Spear J, Gvazdinskas LC. Randomized study of intravenous/oral ciprofloxacin versus ceftazidime in the treatment of hospital and nursing home patients with lower respiratory tract infections. American Journal of Medicine 1989;87(Supp 5A):116S‐8S. [DOI] [PubMed] [Google Scholar]
Welte 2005 {published data only}
- Welte T, Petermann W, Schurmann D, Bauer TT, Reimnitz P. Treatment with sequential intravenous or oral moxifloxacin was associated with faster clinical improvement than was standard therapy for hospitalized patients with community‐acquired pneumonia who received initial parenteral therapy. Clinical Infectious Diseases 2005;41(12):1697‐705. [DOI] [PubMed] [Google Scholar]
Wollschlager 1987 {published data only}
- Wollschlager CM, Raoof S, Khan FA, Guarneri JJ, LaBombardi V, Afzal Q. Controlled, comparative study of ciprofloxacin versus ampicillin in treatment of bacterial respiratory tract infections. American Journal of Medicine 1987;82(4A):164‐8. [PubMed] [Google Scholar]
Additional references
BTS 2001
- British Thoracic Society. BTS guidelines for the management of community acquired pneumonia in adults. Thorax 2001;56(Suppl 4:IV):1‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donowitz 2000
- Donowitz G, Mandell G. Acute Pneumonia. In: Mandell GL, Bennet JE, Dolin R editor(s). Principles and Practice of Infectious Diseases. 5th Edition. Vol. 1, Philadelphia: Churchill Livingstone, 2000:717‐43. [Google Scholar]
FDA 2009
- FDA. Guidance for industry. Patient‐reported outcome measures: use in medical product development to support labeling claims. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf (accessed May 2010) (accessed May 2010). [DOI] [PMC free article] [PubMed]
Fine 1997
- Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. New England Journal of Medicine 1997;336(4):243‐50. [DOI] [PubMed] [Google Scholar]
Hedlund 2005
- Hedlund J, Stralin K, Ortqvist A, Holmberg H. Swedish guidelines for the management of community‐acquired pneumonia in immunocompetent adults. Scandinavian Journal of Infectious Diseases 2005;37:791‐805. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Iezzoni 1988
- Iezzoni LI, Moskowitz MA. A clinical assessment of MedisGroups. JAMA 1988;260(21):3159‐63. [DOI] [PubMed] [Google Scholar]
Johansen 2000
- Johansen HK, Jensen TG, Dessau RB, Lundgren B, Frimodt‐Moller N. Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo. Journal of Antimicrobial Chemotherapy 2000;46:973‐80. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Levison 2001
- Levison M. Pneumonia, including necrotizing pulmonary infections (lung abscess). In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL editor(s). Harrison's Principles of Internal Medicine. 15th Edition. Vol. 2, New York: McGraw‐Hill, 2001:1475‐85. [Google Scholar]
Lim 2003
- Lim WS, Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58(5):377‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2009
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults. Thorax 2009;64(Suppl 3):1‐55. [DOI] [PubMed] [Google Scholar]
Malhotra‐Kumar 2007
- Malhotra‐Kumar S, Lammens C, Coenen S, Herck K, Goossens H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide‐resistant streptococci in healthy volunteers: a randomised, double‐blind, placebo‐controlled study. Lancet 2007;369(9560):482‐90. [DOI] [PubMed] [Google Scholar]
Mandell 2000
- Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH, The Canadian Community‐Acquired Pneumonia Working Group. Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clinical Infectious Diseases 2000;31:383‐421. [DOI] [PubMed] [Google Scholar]
Mandell 2007
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clinical Infectious Diseases 2007;44(Suppl 2):27‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2005
- Mills DG, Oehley MR, Arrol B. Effectiveness of beta‐lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis. BMJ 2005;330(7489):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niederman 2001
- Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. American Thoracic Society. Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1730‐54. [DOI] [PubMed] [Google Scholar]
Oosterheert 2003
- Oosterheert JJ, Bonten MJM, Hak E, Schneider MM, Hoepelman IM. How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community‐acquired pneumonia? A systematic review. Journal of Antimicrobial Chemotherapy 2003;52(4):555‐63. [DOI] [PubMed] [Google Scholar]
Paul 2007
Ross 2007
- Ross DB. The FDA and the case of Ketek. New England Journal of Medicine 2007;356(16):1601‐4. [DOI] [PubMed] [Google Scholar]
Soreth 2007
- Soreth J, Cox E, Kweder S, Jenkins J, Galson S. Ketek ‐ the FDA perspective. New England Journal of Medicine 2007;356(16):1675‐6. [DOI] [PubMed] [Google Scholar]
Woodhead 2005
- Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, Ortqvist A, et al. Guidelines for the management of adult lower respiratory tract infections. European Respiratory Journal 2005;26:1138‐80. [DOI] [PubMed] [Google Scholar]
Woodhead 2011
- Woodhead M, Blasi F, Ewig S, Huchon G, Leven M, Ortqvist A, et al. Guidelines for the management of adult lower respiratory tract infections. Clinical Microbiology infections 2011;17(6):1‐24. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Robenshtok 2008
- Robenshtok E, Shefet D, Gafter‐Gvili A, Paul M, Vidal L, Leibovici L. Empiric antibiotic coverage of atypical pathogens for community‐acquired pneumonia in hospitalized adults. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004418.pub3] [DOI] [PubMed] [Google Scholar]
Shefet 2005
- Shefet D, Robenshtok E, Paul M, Leibovici L. Empiric antibiotic coverage of atypical pathogens for community‐acquired pneumonia in hospitalized adults. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004418.pub3] [DOI] [PubMed] [Google Scholar]


