Abstract
We review the history of the tyrosine kinase 2 (TYK2) as the founding member of the Janus kinase (JAK) family and outline its structure-function relation. Gene-targeted mice and hereditary defects of TYK2 in men have established the biological and pathological functions of TYK2 in innate and adaptive immune responses to infection and cancer and in (auto-)inflammation. We describe the architecture of the main cytokine receptor families associated with TYK2, which activate signal transducers and activators of transcription (STATs). We summarize the cytokine receptor activities with well characterized dependency on TYK2, the types of cells that respond to cytokines and TYK2 signaling-induced cytokine production. TYK2 may drive beneficial or detrimental activities, which we explain based on the concepts of tumor immunoediting and the cancer-immunity cycle in the tumor microenvironment. Finally, we summarize current knowledge of TYK2 functions in mouse models of tumor surveillance. The biology and biochemistry of JAKs, TYK2-dependent cytokines and cytokine signaling in tumor surveillance are well covered in recent reviews and the oncogenic properties of TYK2 are reviewed in the recent Special Issue ‘Targeting STAT3 and STAT5 in Cancer’ of Cancers.
Keywords: tyrosine kinase 2, Janus kinase (JAK) family, STATs, immunoediting, cancer-immunity cycle, interferons, interleukins
1. Tumor Immunosurveillance and Tumor Microenvironment
The tumor immunosurveillance hypothesis, its history and its validation by elegant experiments in cancer mouse models have been reviewed recently [1,2,3]. Tumor surveillance is the means by which the immune system identifies and eliminates (pro-)tumorous cells based on their expression of stress-induced molecules or tumor-specific antigens. The ability of cancers to develop despite an intact host immune system has long puzzled biologists. A recent explanation relies on the concept of tumor immunoediting, which takes place in three phases: elimination, equilibrium and escape [4,5,6]. The elimination phase encompasses the processes originally classified as immunosurveillance. If only a proportion of transformed cells are eliminated, there will be a temporary equilibrium between the developing tumor and the protective function of the immune system. However, most tumors eventually escape: continuous changes in chromatin activities and/or the accumulation of DNA mutations in tumor cells lead to the appearance of tumor cell variants able to suppress, avoid or resist the antitumor immune responses [7,8]. The immunoselection of tumor variants lacking strong tumor-specific antigens by CD8+ and CD4+ T cells [9,10] represents another mechanism by which cancer cells escape tumor immunity.
Clinical outcome studies in human cancers have confirmed the validity of the immunoediting concept and have shown that the failure of immune protection in many patients is caused by negative regulators of T-cell responses in lymphoid organs (checkpoints) and in the tumor microenvironment (TME). This finding has led to a complementary concept, the cancer-immunity cycle, which recognizes that the immune response in cancer is stepwise and regulated by feedforward and feedback loops, ultimately leading to the elimination of cancer cells or the perpetuation of carcinogenesis by the induction of factors that impair tumor immunity [11,12].
Inflammation in the TME is a hallmark of cancer and counteracts immune surveillance and responses to therapy [13]. The tissues, cells and noncellular components that are involved in immunity to cancer and that constitute the TMEs of solid and hematopoietic cancers have been reviewed recently [14,15,16,17,18,19]. The balance between tumor-promoting inflammation and anticancer immunity in the TME is governed by pro-inflammatory and effector cytokines. Depending on the context and duration of activity, a single cytokine may exert anti- or pro-tumorigenic activities [20,21].
Tyrosine kinase 2 (TYK2) propagates the signals of cytokines that shape the TME. Type I and type II interferons (IFNs) are pivotal in anticancer immunity [22,23]. TYK2 is central for the response to type I IFN and for the production of type II IFN as well as for the response to the IL-12 and IL-10 family of cytokines, which control immunity and inflammation (see below). The pleiotropic and context-dependent activities of these cytokines show that TYK2 is responsible for tipping the balance between tumor-restrictive and tumor-promoting functions.
2. Identification and Structure-Function Relations of TYK2
TYK2 was the first member of the family of Janus kinases (JAK1-3, TYK2) to be cloned [24,25,26]. It was also the first JAK for which a biological function was demonstrated by genetic complementation of an IFN-unresponsive human cell line generated by saturation mutagenesis and selection against type I IFN responsiveness [27]. The basic biological functions of the three other JAKs have since been elucidated by work with IFN-unresponsive cell lines, gene-targeted JAK-deficient mice and human patients with inherited JAK deficiencies [28,29,30,31,32].
The structural features of TYK2 have been reviewed elsewhere [33,34,35,36,37]. Together with JAK1 or JAK2, TYK2 is associated with heterodimeric cytokine receptor chains (see Table 1 and below). Association of JAKs with their receptor generally stabilizes the anchoring at the cell surface and regulates the endocytic trafficking of cytokine receptors [38,39]. The mechanism is best understood for TYK2 at the type I IFN receptor (IFNAR) in human cells [40,41] and is similar for other cytokine/receptor combinations [42,43]. The conformational changes of ligand-bound cytokine receptors release the autoinhibitory intramolecular fold of the kinase domain [44,45,46]. In the case of TYK2, this leads to enzymatic activation by phosphorylation of two conserved tyrosine residues in the activation loop (Y1054/Y1055 in men and Y1047/Y1048 in mice) by transactivation of the adjacent JAK1/2 or by autophosphorylation. Cytokine-activated JAKs phosphorylate several tyrosine residues on the cytoplasmic receptor chains, creating docking sites for the SH2 domains of members of the signal transducer and activator of transcription (STAT) family. STATs are phosphorylated on a critical C-terminal tyrosine, undergo conformational changes, translocate as homo- or heterodimers to the nucleus and regulate transcriptional activity [32,47,48]. Some STATs also exhibit transcriptional activity when non-phosphorylated; these have been covered in recent reviews [49,50]. TYK2 activity is negatively regulated by intrinsic mechanisms, such as post-translational modifications and conformational inhibition [33,37], and by extrinsic effectors, such as protein tyrosine phosphatases (PTPs, e.g., PTB1B, SHP1 [51,52]) and suppressor of cytokine signaling (SOCS) proteins (e.g., SOCS1, SOCS3 [53]). In addition, chaperones, in particular heat shock protein (HSP) 90 [54], and noncoding RNAs [55,56] influence the stability and production of TYK2.
Table 1.
TYK2-dependent cytokine receptor signaling validated by loss or inhibition of TYK2.
| Cytokine Family | Receptor (R) Chain (⇔ TYK2) | Cytokine & Specific R Chain (⇔ JAK1 or 2) |
STATs Activated 1 | Producing Cells | Responding Cells |
|---|---|---|---|---|---|
| Type I IFNs | IFNAR1 |
IFNα/β2 IFNAR2 ⇔ JAK1 |
STAT1, STAT23 STAT3-6 |
all cells, pDCs (professional type I IFN producers) | all cells |
| IL-12/23 family | IL-12Rβ1 |
IL-12 IL-12Rβ2 ⇔ JAK2 |
STAT4 STAT1/3/5 |
monocytes, macrophages, DCs | activated NK and T cells |
|
IL-23 IL-23R ⇔ JAK2 |
STAT3, STAT4 STAT1/5 |
monocytes, macrophages, DCs | activated T cells, ILCs, NK cells, monocytes, macrophages, DCs | ||
| IL-10 family | IL-10R2 |
IL-10 IL-10R1 ⇔ JAK1 |
STAT3 STAT1/5 |
Monocytes 4, macrophages 4, DCs NK cells 5, T cells, B cells |
all subsets of leukocytes |
|
IL-22 IL-22R1 ⇔ JAK1 |
STAT3 STAT1/5 |
T cells, ILCs, NK/T cells, macrophages, neutrophils, DC, fibroblasts | epithelial cells, keratinocytes, hepatocytes | ||
|
IL-266 IL-20R1 ⇔ JAK1 |
STAT3 STAT1 |
Th17 cells, NK cells, macrophages, fibroblasts | non-hematopoietic and hematopoietic cell subsets 7 | ||
|
type III IFNs/IFNλ IL-28R ⇔ JAK1 |
STAT1, STAT23 STAT3-6 |
all cells 8 | epithelial cells, DCs, neutrophils a.o. 9 |
⇔, associated with; a.o., and others; 1 bold font, main STATs activated by respective cytokine receptor engagement; normal font, STAT activation is dependent on cell type or of less clear biological relevance; 2 see text for complete nomenclature of subtypes; 3 trimer of STAT1-STAT2-IRF9 (ISGF3); 4 anti-inflammatory activity; 5 immune stimulatory activity; 6 does not exist in rodents; 7 for details see [57,58]; 8 overlap with IFN type I production, for differences in IFN type III and I induction see text and [59]; 9 for cell type and species-specificity see text and [60]. Gp130-utilizing cytokines (IL-6, IL-11, IL-27, IL-35, CNTF, CT-1, LIF, OSM) can activate TYK2, although there is no evidence that TYK2 is required for the downstream activation of STATs [61]. The dependency of IL-4/IL-13 signaling on TYK2 is understudied in LOF patients and mice; see other reviews [62,63].
3. Loss-of-Function Mutations of TYK2 in Men and Mice
TYK2/Tyk2 is located on chromosome 19 in men and on chromosome 9 in mice. The gene is expressed in all tissues. The first comprehensive studies of the biology of TYK2 relied on genetically engineered mice with a Tyk2 loss-of-function (LOF) [64,65,66] or on the tissue-specific ablation of TYK2 [67]. They established that TYK2 is required for the immune response to infections and cancer and for the development of inflammation. Additionally, spontaneously mutated mouse strains have been discovered that carry mutations causing TYK2 deficiency: the B10.D1-H2q/SgJ mouse strain harbors an amino acid exchange (TYK2E775K) that destabilizes the protein [68], while the SWR/J or SJL/J strains harbor Tyk2 promoter mutations that reduce TYK2 levels to below the limit of biochemical detection [69]. TYK2 promoter variants in men are associated with an increased risk of virus-induced diabetes [70,71]. The first report of an inborn missense mutation leading to loss of TYK2 in a human patient stems from 2006; since then a further nine patients with complete loss of TYK2 have been reported [42,43,72,73]. Despite the fundamental differences in environmental conditions between men and mice and the extensive medical interventions in human patients, the effects of TYK2 deficiency in the two species are highly similar in terms of cytokine responses (see below and Table 2). In addition to its enzymatic activity, TYK2 has scaffolding functions, e.g., in endocytic cytokine receptor trafficking, PI3K signaling crosstalk and basal mitochondrial respiration [49,52]. To study the kinase-independent functions of TYK2 in vivo and to evaluate the efficacy and side effects of pharmaceutical inhibitors, we and others have generated mice expressing TYK2 with a point mutation in the ATP-binding pocket of the kinase domain (Tyk2K923E and Tyk2K923R-TG-Cre) [74,75]. Tyk2K923E mice phenocopy mice lacking TYK2 with respect to cytokine responses and susceptibility to viral infections [74,76]. In men, a common and a less frequent missense allele lead to expression of TYK2P1104A or TYK2I684S, which lack catalytic activity and impair cytokine responses [77,78,79,80,81,82,83,84]. Tyk2P1124A mice carrying the corresponding amino acid substitutions recapitulate the phenotypes in men [79,82].
Table 2.
Inherited and gene-targeted mutations of TYK2 in mice and men.
| Mutation | Disease | IL-10 | IL-12 | IL-22 | IL-23 | IFN-I | IFN-III | ||
|---|---|---|---|---|---|---|---|---|---|
| mouse | LOF of TYK2 | Tyk2 −/− | impaired tumor surveillance; susceptible to microbial infections; resistance/protection during inflammatory challenges |
normal [64,66]; impaired [85] |
impaired 1 [76,86,87] 2; [64,66,85,88,89,90,91,92,93,94] |
impaired [95,96] | impaired [91,94,96,97] | impaired [98] 2; [64,66,74,99,100,101] |
n.d. |
| Tyk2mut | susceptible to parasite infection; protection during inflammatory challenge |
n.d. | impaired [68,102] | n.d. | impaired [68,102] | impaired [68,99] | n.d. | ||
| Tyk2Pmut | virus-induced diabetes | n.d. | n.d. | n.d. | n.d. | impaired [69] | n.d. | ||
| KI-TYK2 | Tyk2K923E; Tyk2K923R | impaired tumor surveillance; susceptible to virus infection; restoration of obesity |
n.d. | impaired [76] 2 | n.d. | n.d. | impaired [74,75] | n.d. | |
| Tyk2P1124A | protection during inflammatory challenge | n.d. | impaired [79,82] | n.d. | impaired [79,82] | impaired [79,82] | n.d. | ||
| human | LOF of TYK2 | patient 1 | bacterial, viral and fungal infections, HIES |
impaired [72,103] | impaired [72,103] | n.d. | impaired [72] | impaired [72] | n.d. |
| patients 2–10 | bacterial and viral infections, no HIES 3 |
impaired [42,43,103] | impaired [42,43,73,103] | n.d. | impaired [43] | impaired [42,43] | impaired [43]; normal [42] |
||
| promoter variants | virus-induced type I diabetes | n.d. | n.d. | n.d. | n.d. | impaired [70,71] | n.d. | ||
| KI-TYK2 | TYK2P1104A | mycobacterial infection; autoimmune protected/susceptible |
normal [77] 4 [79]/[78] |
normal [77,78,81] 5; impaired [79] |
n.d. | impaired [77,79,82] 6; normal [78] 5 |
normal [77]; impaired [78,79,82] |
n.d. | |
| TYK2I684S | healthy; autoimmune protected |
normal [77] |
normal [77]; impaired [81] |
n.d. | normal [77] |
normal [77] |
n.d. |
LOF of TYK2, deficient of protein or substantially lowered protein level; KI-TYK2, kinase-inactive TYK2; Tyk2mut, natural TYK2 deficient mouse strain; Tyk2Pmut, Tyk2 promoter mutation; n.d., not determined; 1 note that in [86,87] IFN-II production is attributed to impaired IL-12 responses; 2 references related to tumor surveillance; 3 note that the patient in [42] shows IL-6-independent HIES-like symptoms; 4 note that cytokine signaling was analyzed in EBV-transformed patient and healthy donor cells; 5 normal Th1 and Th17 polarization indicative of no gross impairment of IL-12 and IL-23 signaling [78]; 6 not reaching statistical significance in [82]. IL-6 signaling is unaffected in LOF of TYK2 and KI-TYK2 patients and mice [42,43,64,66,77,79]; IL-13 signaling was not assessed in LOF of TYK2 men and mice and found unaffected in KI-TYK2 patients [79]
4. TYK2-Dependent Cytokine Responses and Their Involvement in Immunity to Cancer
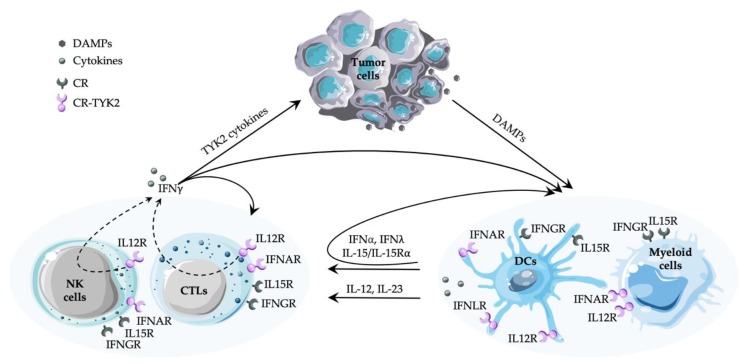
TYK2 is associated with heterodimeric/multimeric cytokine receptors, where it acts in concert with JAK1 or JAK2. Comprehensive reviews list the cytokines and growth factors able to activate TYK2 by phosphorylation of the critical tyrosine residues in the activation loop [35,104,105,106]. Here we focus on the cytokine-TYK2 signaling that transduces TYK2 phosphorylation into physiological changes validated by TYK2 deficiency in patients or mouse models (see Table 2) and/or by pharmacological inhibition of TYK2 [107,108,109,110]. Signaling molecules include the interleukin (IL)-12 family (IL-12, IL-23), the type I IFN subfamily (including IFNα subtypes, IFNβ), and the IL-10 family (IL-10, IL-22, IL-26, type III IFNs) [37,111,112]. Some TYK2-dependent cytokines have a central role in tumor immunosurveillance (type I IFN, IL-12, see Figure 1), some are thought to be important based on assigned functions in immunity or inflammation (e.g., IL-23, IL-10, IL-22) and some are attracting attention for evolutionary reasons (e.g., IL-26, type III IFN; [113,114]). Depletion of TYK2 does not completely abrogate tyrosine phosphorylation of downstream STATs by all three families of cytokine receptor (Table 1 and see below), suggesting that TYK2 is the subordinate JAK and mainly amplifies/sustains responses [61]. The partial functionality of the receptors in the absence of TYK2 is consistent with reports that high doses of cytokines (e.g., type I IFN, IL-12, IL-23, IL-10, IL-22) overcome the phenotypic consequences of TYK2 deficiency in primary cells and in vivo (e.g., [66,85,95,96,97]).
Figure 1.
Simplified scheme of the cancer-immunity cycle and the involvement of TYK2, focusing on innate and adaptive immune cells in tumor surveillance and the feedforward loops in the TYK2-dependent cytokine responses and production. Type II IFN and IL-15 production largely depends on TYK2, while the cytokine receptors signal independently of TYK2. Type I and III IFNs, IL-12 and IL-23 act through TYK2-dependent receptors, with an amplification of type I and III IFN production by TYK2 signaling. Effects of the cytokines on shaping the TME and the stroma cells are reviewed elsewhere [19,115,116]. CR, cytokine receptor; CR-TYK2, TYK2-dependent cytokine receptor; DAMPs, danger-associated molecular patterns; IFNα, type I IFN; IFNγ, type II IFN; IFNλ, type III IFN; IFNLR, receptor for type III IFN, IFNλ; IL12R, receptor for family members IL-12 or IL-23; IL15R, simplified for IL-15 responses through IL-15/IL-15 Rα trans-presentation or soluble IL-15 binding to IL-15Rβ/γC/IL-15Rα (see text); TYK2 cytokines, cytokines depending on TYK2 with respect to signaling and/or production. The figure was compiled with Servier Medical Art (https://smart.servier.com).
4.1. Type I IFNs
The type I IFN cascade is one of the most extensively studied signaling pathways. In men and mice, type I IFNs belong to a multigene family containing numerous IFNα subtypes and one IFNβ, IFNε, IFNκ, IFNω (absent in mice) and IFNζ (also called limitin, absent in men) [117,118,119]. As subtype-specific functions in antiviral and antiproliferative activities are well described [120,121,122], we focus here on the activity of the type I IFN family in the context of anticancer immunity.
Under homeostatic conditions, most cells produce moderate amounts of type I IFN, but specialized cells of the immune system, mainly plasmacytoid dendritic cells (pDCs), produce high amounts when activated [123,124,125]. All type I IFNs signal through a ubiquitously expressed receptor composed of the IFNAR1 and the IFNAR2 chain (Table 1). When the corresponding cytokine binds to the high-affinity IFNAR2, the TYK2-associated IFNAR1 is recruited to form a ternary signaling-competent complex that activates JAK1 and TYK2 [122,126]. The major signal is through STAT1/STAT2 dimers that, together with interferon regulatory factor 9 (IRF9), form transcription factor IFN-stimulated gene factor 3 (ISGF3) and bind to IFN-stimulated response elements (ISRE) in regulatory regions of IFN-stimulated genes (ISGs). STAT1 homodimers are less strongly activated and bind to IFNγ-activated sequences (GAS) [47]. In macrophages, type I IFN converts the ISGF3 subunits from a STAT2-IRF9 complex that drives homeostatic expression to the complete STAT1-STAT2-IRF9 complex [127], thereby inducing the transcription of an extended set of genes. In addition, non-canonical STAT-containing complexes, such as unphosphorylated ISGF3, ISGF3-II (which consists of STAT1, unphosphorylated STAT2 and IRF9) and STAT2/IRF9, contribute to type I IFN responses at distinct stages after signal initiation [128,129,130,131,132]. The response to type I IFN is made even more plastic by cell-type specificities at multiple steps of the signaling cascade, including the activation of STAT3, STAT4, STAT5 and STAT6 [122,133].
Over a thousand ISGs have been described [134]. Most of them encode proteins with antiviral, immunomodulatory and/or (auto-)inflammatory functions [135,136,137,138]. Immunomodulatory and anticancer functions include the direct induction of cytokines (e.g., IFNγ, IL-12, IL-15) and chemokines or the expansion of cytokine-producing cells (e.g., IFNγ-producing T cells), the maturation and activation of DCs and natural killer (NK) cells, proliferative and anti-apoptotic effects on effector T cells and the inhibition of T-regulatory (Treg) or myeloid-derived suppressor cells (MDSC) [139,140,141,142]. The reduced number of Treg cells and their functional suppression has been demonstrated with Ifnar1−/− mice or by delivery of type I IFN into the TME. It is context-dependent and is attributed to crosstalk with antigen-presenting cells (APCs), enhanced IL-6 activity and a suppressive Treg gene signature [143,144,145]. Pattern recognition receptor (PRR) signaling and type I IFN induction counteracts MDSC expansion and function [146,147]. Tumor cell-intrinsic type I IFN functions include the induction of tumor cell death, enhanced MHC class I expression and tumor antigen presentation [139,140,141,142]. However, not all IFN functions are anti-tumorigenic: type I IFN targets the genes encoding various immune checkpoint proteins (e.g., PD-1, PD-L1) [148,149] and sustained exposure to type I IFN leads to T cell exhaustion or resistance to immune checkpoint therapy [150,151].
The full activation of STAT1-3 upon type I IFN stimulation absolutely requires TYK2, as proven in human and murine LOF mutants (see above and Table 2), while the contribution of TYK2 to the activation of STAT4-6 and to the formation of non-canonical STAT complexes remains to be elucidated.
4.2. IL-12 and IL-23
The heteromeric IL-12 and IL-23 cytokines contain a common cytokine subunit (p40) and signal through receptor complexes that share the TYK2-associated IL-12Rβ1 receptor chain (Table 1). The specific receptor chains transduce the signal and are associated with JAK2. IL-12Rβ1 is expressed on T cells, NK cells and DCs [152,153,154]. Although similar signaling pathways are activated by IL-12 and IL-23, the cellular responses differ markedly [155,156,157].
4.2.1. IL-12
IL-12 is largely produced by myeloid cells and DCs in response to activation of PRRs by microbial or damage-associated stimuli [158,159]. IL-12 is composed of p35 and p40 and signals through IL-12Rβ1 and IL-12Rβ2. The IL-12-specifc IL-12Rβ2 chain is expressed mainly on activated T cells and NK cells but also on other cell types, such as DCs and, at a low level, on non-activated NK cells [160,161]. It is upregulated in the presence of cytokines, such as IFNα/β, IFNγ, IL-18 and IL-27 [162,163,164,165], and is downregulated when IL-4 is induced [166]. IL-12 activates STAT4, leading to high levels of IFNγ. To a lesser extent it also activates STAT1 and STAT3-5, which explains its pleiotropic functions that include the regulation of many other cytokines, chemokines and receptor chains [161]. IL-12 is central to the coordination of innate and adaptive immunity and has critical roles in Th1 differentiation [153,154,161]. Its anticancer and anti-metastatic properties have been extensively examined in a variety of murine tumor models. They include the sustained production of IFNγ from NK and T cells as well as the stimulation of proliferation and cytotoxicity of activated NK cells and CD8+ and CD4+ T cells [167,168]. IL-12 appears to be more potent in tumor surveillance when it is in the TME than when it is systemic [169].
The dependence of in vivo IL-12 responses on TYK2 is clear in Tyk2−/− mouse models of infection, inflammation and cancer, in Tyk2P1124A mouse models of inflammation ([61,79,82] and see below) and in patients with inherited LOF TYK2, who suffer from increased microbial infections and skin inflammation ([43,81] and see Table 2).
4.2.2. IL-23
IL-23 is largely produced by APCs upon infectious and inflammatory stimuli [170,171,172]. It is composed of p40 and p19 and signals through IL-12Rβ1 and IL-23R. IL-23R is present on activated and memory T cells, on innate lymphoid cells (ILCs), including NK cells, and, at lower levels, on monocytes/macrophages and DCs [173,174]. Many of these immune cells carry IL-23R constitutively, while others only express the gene at particular stages of differentiation [170]. IL-23 signaling causes the formation of STAT3/STAT3, STAT3/STAT4 and STAT4/STAT4 dimers and, like IL-12, IL-23 also induces the phosphorylation of STAT1 and STAT5. The contributions of the distinct STAT-containing complexes to the IL-23 response are not fully elucidated [175,176]. Although IL-23 does not directly promote Th cell differentiation, it is a key cytokine for the maintenance and activity of Th17 cells. IL-23 induces the expression of pro-inflammatory cytokines, such as IL-17A, IL-17F and IL-22, of the Th17 lineage-defining transcription factor RORγc and of its own specific receptor chain IL-23R [157]. IL-23 also acts on other cell types and, like IL-12, induces the transcription of IFNγ [153,161].
IL-23 acts antagonistically to its close relative IL-12 [177]. It is a central molecular link between the Th17-driven pro-inflammatory processes that promote tumor growth and the tumor-inhibiting reduction of the Th1 signature and cytotoxic T lymphocyte (CTL) activity in the TME [178,179]. Its dependence on TYK2 has only been studied in inflammatory conditions and bacterial infections in mice [96,97,180] and in infectious diseases in men [77,181] (and see Table 2).
4.3. IL-10 Family Cytokines
The TYK2-associated IL-10R2 (also IL-10Rβ, Table 1) is shared by a diverse set of cytokines, namely IL-10, IL-22, IL-26, and the more distantly related IFNλ (type III IFN, also IL-28A, IL-28B, IL-29). The second and specific receptor chains bind with high affinity and transduce the signal, and all are associated with JAK1. IL-10R2 is relatively widely expressed, and its cell-type specificity is mainly determined by the second receptor chain [182].
4.3.1. IL-10
T cells are the major source of IL-10, but other immune cells, including APCs, NK cells, and B cells, also produce it [183,184,185]. IL-10 production in myeloid cells is largely driven by signals downstream of various PRRs, while IL-10 production in T cells is driven by lineage-specifying cytokines (e.g., IL-12, IL-4, and TGFβ), although it is enhanced by a variety of other cytokines [186]. Expression of IL-10R1 (also IL-10Rα) is restricted to leukocytes and is particularly high in monocytes and macrophages. Binding of IL-10 to its receptor leads to the JAK1/TYK2-dependent phosphorylation of STAT3, which is the major signal transducer for IL-10. STAT1 and STAT5 are also activated, but their contribution to the IL-10 response remains unclear. IL-10 is an irreplaceable anti-inflammatory cytokine that acts mainly on macrophages and DCs to limit excessive inflammation and to inhibit cytokine production by Th1 cells [187,188,189]. IL-10 also exerts immunostimulatory and pro-inflammatory effects, for example, enhancing B-cell, granulocyte and mast-cell differentiation, and NK-/T-cell cytotoxicity [190,191]. It is becoming increasingly evident that IL-10 has a dual role in tumorigenesis. It contributes to the initial immune response to cancer by substantially boosting the proliferation and cytolytic activity of CD8+ T cells in combination with IL-2 and in combination with IL-18 by increasing the frequency and cytolytic activity of NK cells [192,193]. However, prolonged production of IL-10 in the TME is a potent promoter of cancer as it contributes to the constitutive activation of STAT3 in immune cells and tumor cells, thereby restraining the anti-cancer immune responses and driving oncogenic signaling [194].
The role of murine TYK2 in IL-10 signaling is unclear. It may depend on the cell type or the cellular activation state [61,195]. Human TYK2-deficient patients show impaired IL-10 responses [43], while TYK2P1104 patients show unperturbed IL-10 signaling [77]. That the enzymatic activity of TYK2 in IL-10 signaling in primary human (as well as murine) cells is at least partially redundant is evident from experiments with TYK2-selective inhibitors: JAK1 inhibitors strongly blunt IL-10 responses [109].
4.3.2. IL-22
IL-22 is produced by many types of immune cells, including αβ and γδ T cells and ILCs in lymphoid tissues; and macrophages, neutrophils, DCs, and fibroblasts in non-lymphoid tissues. The non-lymphoid sources produce less IL-22 than the lymphoid sources [196,197]. IL-22 acts through high affinity binding to the JAK1-associated IL-22R1, which is only present on epithelial cells, keratinocytes, and hepatocytes. IL-22 mainly signals through STAT3 but also activates low levels of STAT1 and STAT5 [198,199]. IL-22 has important functions in tissue remodeling and repair during inflammation and in antimicrobial defense [196,200,201,202]. It confers pro-survival, cell migratory, and angiogenic capacity that—at least initially—protects against tumor formation, although the same functions support tumor growth and metastasis at later stages of cancer. IL-22 promotes tumors of epithelial origin, such as lung, liver, pancreatic, and gastrointestinal cancers [203,204,205].
Experiments in LOF mice have shown that TYK2 is required for IL-22 responses during skin and intestinal inflammation [95,96]. The only evidence for the role of the IL-22-TYK2 axis in men comes from the pharmacological inhibition of TYK2, which blocks IL-22 responses in various human cells [108,109].
4.3.3. IL-26
Most vertebrates have IL-26, although it is not found in rats or mice. It is secreted by immune cells, including Th17 cells, NK cells, and macrophages, as well as by fibroblasts [206]. It binds to IL-20R1 with high affinity and requires IL-10R2 to form a receptor complex capable of transducing a signal [207,208]. IL-20R1 is present on a variety of non-hematopoietic tissues and cells and on some hematopoietic cells, such as neutrophils and primary blood monocytes [58,209]. IL-26 activates STAT3 and to a lesser extent STAT1 [207,209]. It is not yet been shown to activate JAK1 and TYK2 in human cells but can be assumed to do so as these factors are associated with IL-20R1 and IL-10Rβ2, respectively. In addition to binding to the conventional cytokine receptor, IL-26 is a carrier molecule for DNA and activates PRRs [210]. IL-26 was discovered relatively recently, and its cellular responses are still poorly known. Its major biological functions seem to be linking T-cell functions to epithelial cells and in antimicrobial and inflammatory activities [206,210,211]. As it is not found in rodents, most of its (patho-)physiological properties have been described ex vivo using human or chicken cells. There is only one report of its involvement in tumorigenesis, which shows that it activates STAT3 in gastric cancer [212]. It is likely that the extracellular DNA carrier capacity of IL-26 and/or its aberrant expression may drive the chronicity of inflammation and hence tumor progression. The first report of the IL-26-triggered phosphorylation of TYK2 stems from chicken cells, in which TYK2 seems to interact with JAK2 rather than with JAK1 [213].
4.3.4. Type III IFNs
Four type III IFN (IFNλ) members are known in men (IFNλ1-3, formerly IL-29, IL-28A, and IL-28B, and the recently identified IFNλ4) and two in mice (IFNλ2 and IFNλ3) [59]. As is the case for tape I IFN, type III IFN can be produced by all cells upon activation of a variety of PRRs. The mode of induction and regulation is different in myeloid and epithelial cells [214,215]. All IFNλs signal through the TYK2-associated IL-10R2 and the JAK1-associated IL-28R (also called IFNλR1). IL-28R is mainly restricted to epithelial cells and human, but not murine, hepatocytes. Some subsets of macrophages and DCs in men and mice and NK cells and neutrophils in mice (controversially for men) have also been reported to respond to IFNλ [60,216,217]. Similar to type I IFNs, IFNλs mainly induce the phosphorylation of STAT1/STAT2 heterodimers, which interact with IRF9 to form the ISGF3 complex, although STAT3-6 is also activated [217]. In common with type I IFN, although in fewer cell types, IFNλ shape immunity to infection and cancer and can elicit autoimmune reactions. The current model is that type III IFN acts as the initial line of defense against infection and/or damage to epithelial barriers, while the more potent type I IFN response is activated when local responses are insufficient [60,117,217,218,219].
As they are not active in all cell types, type III IFNs are less involved in the systemic over-stimulation of the immune response or exacerbation of inflammation, suggesting that they might usefully be applied in cancer therapy [220,221]. Particular attention is being paid to IFNλ-activated pDCs that produce type I IFNs and chemokines, thereby recruiting activated T lymphocytes and orchestrating complex immune cell interactions [222]. Analogous to type I IFN, type III IFN targets genes that promote tumorigenesis, depending on context and tumor stage [220].
Unperturbed or suboptimal IFNλ signal transduction is found in TYK2-deficient patient cells [42,43], and pharmacological inhibition of TYK2 reduces the IFNλ responses [107,108].
5. Cytokines Produced in Major Dependence of TYK2
Cytokine signaling is highly integrated and depends on crosstalk as well as feedforward and feedback mechanisms. Cytokine responses generally lead to altered patterns of cytokine production. We shall confine ourselves to a brief review of the cytokines that are produced in TYK2 responses (Figure 1).
5.1. Type I IFN
Type I (and III) IFN expression is upregulated by IRF3, IRF5, IRF7, and IRF8 downstream of PRRs. As IRF7 is a type I IFN response gene, its expression results in a feedforward loop that drives maximum expression of type I IFN [223,224]. We and others have shown that the tonic and the type I IFN-inducible level of IRF7 transcript drops dramatically in the absence of TYK2 in human and murine cells [225,226]. In addition, systemic IFNβ production in an endotoxemia model is severely reduced in Tyk2-deficient mice [99].
5.2. IL-15
IL-15 transcription is highly induced by type I IFN and PRR signals in a variety of tissues and cell types. Complex post-transcriptional regulation largely limits IL-15 production to monocytes, macrophages, and DCs. The IL15 receptor belongs to the common gamma chain (γC, CD132) receptor family. The interaction of IL-15 with cells is unique. Not only does it signal in a soluble form, it also binds to IL-15Rα on the cell surface and is trans-presented to cells that express the IL-2/IL-15Rβ and γC receptors. The IL-15/IL-15Rα complex can also be cleaved into the extracellular space [227,228]. IL-15 is central to the development, survival, and activation of APCs, mast cells, neutrophils, NK cells, T cells, and B cells and enhances NK and CD8+ T-cell cytotoxicity. IL-15 is dynamically regulated in the TME and enhances anti-tumor immunity [227,229,230]. We have shown that the absence of TYK2 in DCs results in reduced levels of IL-15Rα on DCs and macrophages in naïve mice ([93] and see below). However, the nature of the TYK2-dependent signals that drive IL-15/IL-15Rα expression under homeostatic conditions is unclear.
5.3. Type II IFN
Type II IFN (IFNγ) is primarily produced by cells of the innate and adaptive immune system, including NK cells and innate lymphoid cells (ILCs), T helper 1 (Th1) cells, and CD8+ CTLs. IFNγ is the most potent mediator of IL-12, but its production is also induced by other cytokines (primarily IL-18 and IL-23) or through the activation of PRRs or reactive antigen receptors. The IFNGR complex is widely expressed and, similar to IFNγ, is subject to a positive feedback loop during T cell differentiation. IFNγ shapes the TME by enhancing the activity of macrophages, CTLs, and Th1 cells, as well as by suppressing Treg function, angiogenesis, and metastasis. It also acts directly on tumor cells, increasing their antigenicity and promoting cell death. However, like most other cytokines, IFNγ induces inhibitory feedback mechanisms (e.g., upregulating SOCS proteins [53,231]), inhibitory receptor signaling (e.g., PD-L1, [232]) and other genes involved in immunosuppression or immune-evasion [233,234,235]. Mutations in IFNγ pathway genes underlie resistance to immune checkpoint therapies, i.e., block of PD1/PD-L1 or CTL4-A [236,237,238]. We and others have established that IL-12- or IL-23-activated TYK2 is central to the production of IFNγ during immunity to tumors and to infections as well as during inflammation ([52,61,93] and see Table 2).
5.4. IL-17
The IL-17 family consists of IL-17A-F and is crucial for the host defense against microbial infections and the development of inflammatory diseases. Although IL-17A is the signature cytokine produced by T helper 17 (Th17) cells, IL-17A, and other IL-17 family cytokines are produced by many immune and non-immune cells [239,240]. The IL-23-TYK2 axis is central to the maintenance of Th17 cells and sustained IL-17 production. In many human malignancies, a Th17 cell signature (RORC, IL17, IL23, STAT3) is associated with poor outcome, whereas Th1 cell signatures (IFNG, STAT1, TBX21) and CD8+ CTLs (PRF1, GZMB) are linked to better overall survival [240,241,242,243]. Th17 cells and associated cytokines in the TME can promote tumorigenesis by perpetuating inflammation and/or aggravating the aggressiveness of the tumor cells. The importance of TYK2 in the regulation of IL-17A production in men and mice is well documented [43,77,91,96,97,103,244,245,246].
6. TYK2 and Immunity to Cancer
Human TYK2 LOF patients have not been reported to be more prone to tumors, although this surprising non-finding probably relates largely to the patients’ ages and treatment histories. In cancer databases, LOF or lowered TYK2 levels are generally correlated with poor prognosis, while high TYK2 levels are associated with tumorigenesis. The discrepancy may relate to the lack of information on whether TYK2 is in the tumor or the TME and/or to the lack of distinction between phosphorylated and unphosphorylated TYK2 [247].
The first link between TYK2 and immunity to cancer came from studies in Tyk2-deficient mice, which develop hematopoietic malignancies upon oncogenic induction or systemic transplantation of tumors, for example, Abelson-induced B-lymphoid leukemia/lymphoma, Tel-JAK2-induced T-lymphoid leukemia, and EL4 lymphoma [86,98]. The high susceptibility of Tyk2−/− mice to lymphoid tumors is the result of an impaired tumor surveillance, in particular, a decreased cytotoxic capacity of NK/NKT and CTL cells [86,98]. The importance of TYK2 for NK cell- and CD8+ T cell-targeted tumor growth restriction was confirmed by studies with local tumor transplant models, using lymphoma (RMA-S and RMA-Rae1) and adenocarcinoma (MC38) cells [67,76,93]. Reduced CTL-dependent tumor cell killing is linked to impaired type I IFN signaling, although the mechanisms are elusive [98]. Defective NK cell-dependent tumor growth control in the absence of TYK2 correlates with defects in splenic NK-cell maturation; NK-cell development in the bone marrow is independent of TYK2 [76]. Studies with conditional knockout mice have shown that TYK2 in DCs (CD11c+) and other non-NK cells, rather than in NK cells themselves, drives terminal NK cell maturation in the spleen [93]. The work also implicates TYK2 in the regulation of IL-15Rα on CD11c+ cells but not NK cells or T cells. Administration of exogenous IL-15/IL-15Rα to Tyk2-deficient mice restores NK-cell maturation and NK cell-dependent control of tumor growth [93]. In contrast to the situation in NK cells, the CTL-dependent restriction of locally transplanted tumors is independent of TYK2 in myeloid cells and DCs [67]. TYK2 has a kinase-independent function in NK cell-mediated tumor surveillance, as evidenced by the partial restoration of NK-cell maturation and cytotoxicity in Tyk2K923E mice [76].
Work in a murine model of breast cancer has shown that TYK2 deficiency enhances tumor growth and metastasis by stimulating MDSCs rather than by inhibiting the activity of T cells or NK cells [87]. TYK2 signaling has also been implicated in tumor-cell infiltration and metastasis in human prostate cancer patients [248], a finding recapitulated in Eμ-Myc transgenic mice, in which TYK2 deficiency reduces tumor-cell invasiveness into the liver [249]. For further details on the role of TYK2 in tumor-cell invasiveness, we refer the reader to a recent review [247].
The data from mouse models indicate that the functions of TYK2 in tumor surveillance are context-dependent. Although TYK2-dependent cytokines have been implicated in a plethora of processes in anti-tumor immunity (Figure 1), the consequences of TYK2 deficiency on tumor immunity are only beginning to emerge. The fascinating challenge to deciphering the impact of TYK2 in inhibiting or promoting cancer growth stems from its amplifier function, which exacerbates the general complexity of the spatial-temporal activities and networked hierarchies in signaling circuits. The contribution of TYK2 to the TME is hard to predict as cell-type specific and local high concentrations of cytokines might overcome or mask the effects of TYK2 deficiency.
7. Conclusions and Outlook
The pivotal role of TYK2 in (auto-)immune and -inflammatory diseases was established in gene-targeted mice and human patients with mutated Tyk2/TYK2 alleles. Studies of TYK2 in anticancer immunity in mice have revealed a pivotal role of TYK2 in the NK- and T-cell-mediated elimination of tumor cells. The conditional ablation of TYK2 uncovered the cell-intrinsic and -extrinsic requirements for TYK2 in the crosstalk between DCs, macrophages, and cytolytic effector cells. As the other JAK family members, TYK2 also has oncogenic properties: these are reviewed elsewhere [52,247,250].
The pharmacological inhibition of TYK2 (TYK2inibs) is a recognized approach to the treatment of chronic inflammatory and autoimmune diseases and is under consideration for cancer treatment. Several TYK2inibs are in clinical trials [108,251,252,253]. A CTLA-4-TYK2-STAT3 axis has been reported in B cell lymphoma cells and tumor-associated B cells [254] and will be relevant to immune checkpoint therapy. A TYK2inib has promising immunotherapeutic effects in several tumor transplant mouse models. The TME had reduced levels of PD-1, indicative of less immune cell exhaustion and reduced Treg infiltration [255]. The translation of these findings into the clinic would represent an important extension of the use of TYK2inibs in cancer treatment.
We are applying advanced sequencing methods to obtain detailed mechanistic insights into the crosstalk and the kinase-dependent functions of TYK2. We are creating an integrated map of the chromatin landscape and the activities of splenic myeloid cells, NK cells, DCs, and T cells in homeostatic conditions and intend to apply the same strategy to the TME and/or splenocytes under tumor burden. This will enable us to define the molecular/functional intersection of TYK2 as a tumor surveyor and/or as a promoter of carcinogenesis by shaping immune and oncogenic pathways. Complementary to analyzing the chromatin architecture, it would be highly informative to determine the cellular interactomes of TYK2 in the TME by quantitative proteomics [256], single cell proteomics [257,258], or multidimensional imaging [259]. This would also pave the way for determining the (patho-)physiological relevance of the subcellular distributions of JAKs and STATs, for example, revealing the nuclear functions of TYK2 [260] and other JAKs [261] and the impact of TYK2 [262] and STATs on mitochondrial function [263,264]. Our ultimate vision is to use the ‘relative’ molecular simplicity of the JAK-STAT axis as a template for the development of computational pathway modeling and prediction [265,266,267].
Acknowledgments
We thank Caroline Lassnig for critical reading of the manuscript.
Author Contributions
S.M.-M., M.M., N.S. and B.S. designed the draft of the review and performed the literature search. A.K., M.K., S.S. performed additional literature search. A.K., S.M.-M. and S.S. compiled the tables and the figure. M.K., S.M.-M., M.M. B.S. and G.T. provided the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Austrian Science Fund FWF DK W1212, SFB F6101 and F6106, and DocFund DOC32-B28.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Finn O.J. A Believer’s overview of cancer immunosurveillance and immunotherapy. J. Immunol. 2018;200:385–391. doi: 10.4049/jimmunol.1701302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridman W.H. From cancer immune surveillance to cancer immunoediting: Birth of modern immuno-oncology. J. Immunol. 2018;201:825–826. doi: 10.4049/jimmunol.1800827. [DOI] [PubMed] [Google Scholar]
- 3.Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8:7175–7180. doi: 10.18632/oncotarget.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 5.Dunn G.P., Old L.J., Schreiber R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 7.Mittal D., Gubin M.M., Schreiber R.D., Smyth M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr. Opin. Genet. Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesely M.D., Schreiber R.D. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alspach E., Lussier D.M., Miceli A.P., Kizhvatov I., DuPage M., Luoma A.M., Meng W., Lichti C.F., Esaulova E., Vomund A.N., et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Pio R., Ajona D., Ortiz-Espinosa S., Mantovani A., Lambris J.D. Complementing the cancer-immunity cycle. Front. Immunol. 2019;10:774. doi: 10.3389/fimmu.2019.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritter B., Greten F.R. Modulating inflammation for cancer therapy. J. Exp. Med. 2019;216:1234–1243. doi: 10.1084/jem.20181739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaria O., Cornen S., Daeron M., Morel Y., Medzhitov R., Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. doi: 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopken U.E., Rehm A. Targeting the tumor microenvironment of leukemia and lymphoma. Trends Cancer. 2019;5:351–364. doi: 10.1016/j.trecan.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Salmon H., Remark R., Gnjatic S., Merad M. Host tissue determinants of tumour immunity. Nat. Rev. Cancer. 2019;19:215–227. doi: 10.1038/s41568-019-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott D.W., Gascoyne R.D. The tumour microenvironment in B cell lymphomas. Nat. Rev. Cancer. 2014;14:517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 19.Valkenburg K.C., de Groot A.E., Pienta K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018;15:366–381. doi: 10.1038/s41571-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagerling C., Casbon A.J., Werb Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015;25:214–220. doi: 10.1016/j.tcb.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn G.P., Bruce A.T., Sheehan K.C., Shankaran V., Uppaluri R., Bui J.D., Diamond M.S., Koebel C.M., Arthur C., White J.M., et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 23.Shankaran V., Ikeda H., Bruce A.T., White J.M., Swanson P.E., Old L.J., Schreiber R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 24.Firmbach-Kraft I., Byers M., Shows T., Dalla-Favera R., Krolewski J.J. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990;5:1329–1336. [PubMed] [Google Scholar]
- 25.Krolewski J.J., Lee R., Eddy R., Shows T.B., Dalla-Favera R. Identification and chromosomal mapping of new human tyrosine kinase genes. Oncogene. 1990;5:277–282. [PubMed] [Google Scholar]
- 26.Wilks A.F. The JAK kinases: not just another kinase drug discovery target. Semin. Cell Dev. Biol. 2008;19:319–328. doi: 10.1016/j.semcdb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Velazquez L., Fellous M., Stark G.R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-L. [DOI] [PubMed] [Google Scholar]
- 28.Darnell J.E., Jr., Kerr I.M., Stark G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 29.Ihle J.N., Witthuhn B.A., Quelle F.W., Yamamoto K., Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- 30.O’Shea J.J., Schwartz D.M., Villarino A.V., Gadina M., McInnes I.B., Laurence A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard C., Bobby Gaspar H., Al-Herz W., Bousfiha A., Casanova J.L., Chatila T., Crow Y.J., Cunningham-Rundles C., Etzioni A., Franco J.L., et al. International union of immunological societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J. Clin. Immunol. 2018;38:96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark G.R., Darnell J.E. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babon J.J., Lucet I.S., Murphy J.M., Nicola N.A., Varghese L.N. The molecular regulation of janus kinase (JAK) activation. Biochem. J. 2014;462:1–13. doi: 10.1042/BJ20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrao R., Lupardus P.J. The janus kinase (JAK) FERM and SH2 domains: Bringing specificity to JAK-receptor interactions. Front. Endocrinol. 2017;8:71. doi: 10.3389/fendo.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammaren H.M., Virtanen A.T., Raivola J., Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019;118:48–63. doi: 10.1016/j.cyto.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 36.Hubbard S.R. Mechanistic insights into regulation of JAK2 tyrosine kinase. Front. Endocrinol. 2017;8:361. doi: 10.3389/fendo.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris R., Kershaw N.J., Babon J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27:1984–2009. doi: 10.1002/pro.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cendrowski J., Maminska A., Miaczynska M. Endocytic regulation of cytokine receptor signaling. Cytokine Growth Factor Rev. 2016;32:63–73. doi: 10.1016/j.cytogfr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Moraga I., Spangler J., Mendoza J.L., Garcia K.C. Multifarious determinants of cytokine receptor signaling specificity. Adv. Immunol. 2014;121:1–39. doi: 10.1016/b978-0-12-800100-4.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uze G., Schreiber G., Piehler J., Pellegrini S. The receptor of the type I interferon family. Curr. Top. Microbiol. Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- 41.Zheng H., Qian J., Baker D.P., Fuchs S.Y. Tyrosine phosphorylation of protein kinase D2 mediates ligand-inducible elimination of the Type 1 interferon receptor. J. Biol. Chem. 2011;286:35733–35741. doi: 10.1074/jbc.M111.263608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs S., Kaiser-Labusch P., Bank J., Ammann S., Kolb-Kokocinski A., Edelbusch C., Omran H., Ehl S. Tyrosine kinase 2 is not limiting human antiviral type III interferon responses. Eur. J. Immunol. 2016;46:2639–2649. doi: 10.1002/eji.201646519. [DOI] [PubMed] [Google Scholar]
- 43.Kreins A.Y., Ciancanelli M.J., Okada S., Kong X.F., Ramirez-Alejo N., Kilic S.S., El Baghdadi J., Nonoyama S., Mahdaviani S.A., Ailal F., et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J. Exp. Med. 2015;212:1641–1662. doi: 10.1084/jem.20140280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lupardus P.J., Ultsch M., Wallweber H., Bir Kohli P., Johnson A.R., Eigenbrot C. Structure of the pseudokinase-kinase domains from protein kinase TYK2 reveals a mechanism for Janus kinase (JAK) autoinhibition. Proc. Natl. Acad. Sci. USA. 2014;111:8025–8030. doi: 10.1073/pnas.1401180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Min X., Ungureanu D., Maxwell S., Hammaren H., Thibault S., Hillert E.K., Ayres M., Greenfield B., Eksterowicz J., Gabel C., et al. Structural and functional characterization of the JH2 pseudokinase domain of JAK family tyrosine kinase 2 (TYK2) J. Biol. Chem. 2015;290:27261–27270. doi: 10.1074/jbc.M115.672048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallweber H.J., Tam C., Franke Y., Starovasnik M.A., Lupardus P.J. Structural basis of recognition of interferon-alpha receptor by tyrosine kinase 2. Nat. Struct. Mol. Biol. 2014;21:443–448. doi: 10.1038/nsmb.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy D.E., Darnell J.E., Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 48.Schindler C., Plumlee C. Inteferons pen the JAK-STAT pathway. Semin. Cell Dev. Biol. 2008;19:311–318. doi: 10.1016/j.semcdb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majoros A., Platanitis E., Kernbauer-Holzl E., Rosebrock F., Muller M., Decker T. Canonical and non-canonical aspects of JAK-STAT signaling: Lessons from interferons for cytokine responses. Front. Immunol. 2017;8:29. doi: 10.3389/fimmu.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J., Stark G.R. Roles of unphosphorylated STATs in signaling. Cell Res. 2008;18:443–451. doi: 10.1038/cr.2008.41. [DOI] [PubMed] [Google Scholar]
- 51.Bohmer F.D., Friedrich K. Protein tyrosine phosphatases as wardens of STAT signaling. JAKSTAT. 2014;3:e28087. doi: 10.4161/jkst.28087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leitner N.R., Witalisz-Siepracka A., Strobl B., Muller M. Tyrosine kinase 2-Surveillant of tumours and bona fide oncogene. Cytokine. 2017;89:209–218. doi: 10.1016/j.cyto.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Kershaw N.J., Murphy J.M., Lucet I.S., Nicola N.A., Babon J.J. Regulation of janus kinases by SOCS proteins. Biochem. Soc. Trans. 2013;41:1042–1047. doi: 10.1042/BST20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bocchini C.E., Kasembeli M.M., Roh S.H., Tweardy D.J. Contribution of chaperones to STAT pathway signaling. JAKSTAT. 2014;3:e970459. doi: 10.4161/21623988.2014.970459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z.Y., Yang L., Liu X.J., Wang X.Z., Pan Y.X., Luo J.M. The long noncoding RNA MEG3 and its target miR-147 regulate JAK/STAT pathway in advanced chronic myeloid leukemia. EBioMedicine. 2018;34:61–75. doi: 10.1016/j.ebiom.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witte S., Muljo S.A. Integrating non-coding RNAs in JAK-STAT regulatory networks. JAKSTAT. 2014;3:e28055. doi: 10.4161/jkst.28055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephen-Victor E., Fickenscher H., Bayry J. IL-26: An emerging proinflammatory member of the IL-10 cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:e1005624. doi: 10.1371/journal.ppat.1005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tengvall S., Che K.F., Linden A. Interleukin-26: An emerging player in host defense and inflammation. J. Innate Immun. 2016;8:15–22. doi: 10.1159/000434646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hemann E.A., Gale M., Jr., Savan R. Interferon lambda genetics and biology in regulation of viral control. Front. Immunol. 2017;8:1707. doi: 10.3389/fimmu.2017.01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye L., Schnepf D., Staeheli P. Interferon-lambda orchestrates innate and adaptive mucosal immune responses. Nat. Rev. Immunol. 2019;19:614–625. doi: 10.1038/s41577-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 61.Strobl B., Stoiber D., Sexl V., Mueller M. Tyrosine kinase 2 (TYK2) in cytokine signalling and host immunity. Front. Biosci. 2011;16:3214–3232. doi: 10.2741/3908. [DOI] [PubMed] [Google Scholar]
- 62.Bhattacharjee A., Shukla M., Yakubenko V.P., Mulya A., Kundu S., Cathcart M.K. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic. Biol. Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Junttila I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018;9:888. doi: 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karaghiosoff M., Neubauer H., Lassnig C., Kovarik P., Schindler H., Pircher H., McCoy B., Bogdan C., Decker T., Brem G., et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. doi: 10.1016/S1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 65.Sheehan K.C., Lai K.S., Dunn G.P., Bruce A.T., Diamond M.S., Heutel J.D., Dungo-Arthur C., Carrero J.A., White J.M., Hertzog P.J., et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J. Interferon Cytokine Res. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- 66.Shimoda K., Kato K., Aoki K., Matsuda T., Miyamoto A., Shibamori M., Yamashita M., Numata A., Takase K., Kobayashi S., et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561–571. doi: 10.1016/S1074-7613(00)00055-8. [DOI] [PubMed] [Google Scholar]
- 67.Vielnascher R.M., Hainzl E., Leitner N.R., Rammerstorfer M., Popp D., Witalisz A., Rom R., Karaghiosoff M., Kolbe T., Muller S., et al. Conditional ablation of TYK2 in immunity to viral infection and tumor surveillance. Transgenic Res. 2014;23:519–529. doi: 10.1007/s11248-014-9795-y. [DOI] [PubMed] [Google Scholar]
- 68.Shaw M.H., Boyartchuk V., Wong S., Karaghiosoff M., Ragimbeau J., Pellegrini S., Muller M., Dietrich W.F., Yap G.S. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc. Natl. Acad. Sci. USA. 2003;100:11594–11599. doi: 10.1073/pnas.1930781100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Izumi K., Mine K., Inoue Y., Teshima M., Ogawa S., Kai Y., Kurafuji T., Hirakawa K., Miyakawa D., Ikeda H., et al. Reduced Tyk2 gene expression in beta-cells due to natural mutation determines susceptibility to virus-induced diabetes. Nat. Commun. 2015;6:6748. doi: 10.1038/ncomms7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mine K., Hirakawa K., Kondo S., Minami M., Okada A., Tsutsu N., Yokogawa Y., Hibio Y., Kojima F., Fujimoto S., et al. Subtyping of type 1 diabetes as classified by anti-GAD antibody, IgE levels, and tyrosine kinase 2 (TYK2) promoter variant in the japanese. EBioMedicine. 2017;23:46–51. doi: 10.1016/j.ebiom.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagafuchi S., Kamada-Hibio Y., Hirakawa K., Tsutsu N., Minami M., Okada A., Kai K., Teshima M., Moroishi A., Murakami Y., et al. TYK2 promoter variant and diabetes mellitus in the japanese. EBioMedicine. 2015;2:744–749. doi: 10.1016/j.ebiom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minegishi Y., Saito M., Morio T., Watanabe K., Agematsu K., Tsuchiya S., Takada H., Hara T., Kawamura N., Ariga T., et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Sarrafzadeh S.A., Mahloojirad M., Casanova J.L., Badalzadeh M., Bustamante J., Boisson-Dupuis S., Pourpak Z., Nourizadeh M., Moin M. A new patient with inherited TYK2 deficiency. J. Clin. Immunol. 2019 doi: 10.1007/s10875-019-00713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prchal-Murphy M., Semper C., Lassnig C., Wallner B., Gausterer C., Teppner-Klymiuk I., Kobolak J., Muller S., Kolbe T., Karaghiosoff M., et al. TYK2 kinase activity is required for functional type I interferon responses in vivo. PLoS ONE. 2012;7:e39141. doi: 10.1371/journal.pone.0039141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raje V., Derecka M., Cantwell M., Meier J., Szczepanek K., Sisler J.D., Strobl B., Gamero A., Harris T.E., Larner A.C. Kinase inactive tyrosine kinase (Tyk2) supports differentiation of brown fat cells. Endocrinology. 2017;158:148–157. doi: 10.1210/en.2015-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prchal-Murphy M., Witalisz-Siepracka A., Bednarik K.T., Putz E.M., Gotthardt D., Meissl K., Sexl V., Müller M., Strobl B. In vivo tumor surveillance by NK cells requires TYK2 but not TYK2 kinase activity. Oncoimmunology. 2015;4:e1047579. doi: 10.1080/2162402X.2015.1047579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boisson-Dupuis S., Ramirez-Alejo N., Li Z., Patin E., Rao G., Kerner G., Lim C.K., Krementsov D.N., Hernandez N., Ma C.S., et al. Tuberculosis and impaired IL-23-dependent IFN-gamma immunity in humans homozygous for a common TYK2 missense variant. Sci. Immunol. 2018;3:eaau8714. doi: 10.1126/sciimmunol.aau8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Couturier N., Bucciarelli F., Nurtdinov R.N., Debouverie M., Lebrun-Frenay C., Defer G., Moreau T., Confavreux C., Vukusic S., Cournu-Rebeix I., et al. Tyrosine kinase 2 variant influences T lymphocyte polarization and multiple sclerosis susceptibility. Brain. 2011;134:693–703. doi: 10.1093/brain/awr010. [DOI] [PubMed] [Google Scholar]
- 79.Dendrou C.A., Cortes A., Shipman L., Evans H.G., Attfield K.E., Jostins L., Barber T., Kaur G., Kuttikkatte S.B., Leach O.A., et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci. Transl. Med. 2016;8:363ra149. doi: 10.1126/scitranslmed.aag1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diogo D., Bastarache L., Liao K.P., Graham R.R., Fulton R.S., Greenberg J.D., Eyre S., Bowes J., Cui J., Lee A., et al. TYK2 protein-coding variants protect against rheumatoid arthritis and autoimmunity, with no evidence of major pleiotropic effects on non-autoimmune complex traits. PLoS ONE. 2015;10:e0122271. doi: 10.1371/journal.pone.0122271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Enerback C., Sandin C., Lambert S., Zawistowski M., Stuart P.E., Verma D., Tsoi L.C., Nair R.P., Johnston A., Elder J.T. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci. Rep. 2018;8:7043. doi: 10.1038/s41598-018-25282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorman J.A., Hundhausen C., Kinsman M., Arkatkar T., Allenspach E.J., Clough C., West S.E., Thomas K., Eken A., Khim S., et al. The TYK2-P1104A Autoimmune Protective Variant Limits Coordinate Signals Required to Generate Specialized T Cell Subsets. Front. Immunol. 2019;10:44. doi: 10.3389/fimmu.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerner G., Ramirez-Alejo N., Seeleuthner Y., Yang R., Ogishi M., Cobat A., Patin E., Quintana-Murci L., Boisson-Dupuis S., Casanova J.L., et al. Homozygosity for TYK2 P1104A underlies tuberculosis in about 1% of patients in a cohort of European ancestry. Proc. Natl. Acad. Sci. USA. 2019;116:10430–10434. doi: 10.1073/pnas.1903561116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z., Gakovic M., Ragimbeau J., Eloranta M.L., Ronnblom L., Michel F., Pellegrini S. Two rare disease-associated Tyk2 variants are catalytically impaired but signaling competent. J. Immunol. 2013;190:2335–2344. doi: 10.4049/jimmunol.1203118. [DOI] [PubMed] [Google Scholar]
- 85.Shaw M.H., Freeman G.J., Scott M.F., Fox B.A., Bzik D.J., Belkaid Y., Yap G.S. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J. Immunol. 2006;176:7263–7271. doi: 10.4049/jimmunol.176.12.7263. [DOI] [PubMed] [Google Scholar]
- 86.Stoiber D., Kovacic B., Schuster C., Schellack C., Karaghiosoff M., Kreibich R., Weisz E., Artwohl M., Kleine O.C., Muller M., et al. TYK2 is a key regulator of the surveillance of B lymphoid tumors. J. Clin. Investig. 2004;114:1650–1658. doi: 10.1172/JCI200422315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Q., Sturgill J.L., Kmieciak M., Szczepanek K., Derecka M., Koebel C., Graham L.J., Dai Y., Chen S., Grant S., et al. The role of Tyk2 in regulation of breast cancer growth. J. Interferon Cytokine Res. 2011;31:671–677. doi: 10.1089/jir.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aizu K., Li W., Yajima T., Arai T., Shimoda K., Nimura Y., Yoshikai Y. An important role of Tyk2 in APC function of dendritic cells for priming CD8+ T cells producing IFN-gamma. Eur. J. Immunol. 2006;36:3060–3070. doi: 10.1002/eji.200636173. [DOI] [PubMed] [Google Scholar]
- 89.Hashiguchi T., Oyamada A., Sakuraba K., Shimoda K., Nakayama K.I., Iwamoto Y., Yoshikai Y., Yamada H. Tyk2-dependent bystander activation of conventional and nonconventional Th1 cell subsets contributes to innate host defense against Listeria monocytogenes infection. J. Immunol. 2014;192:4739–4747. doi: 10.4049/jimmunol.1303067. [DOI] [PubMed] [Google Scholar]
- 90.Hosogi M., Tonogaito H., Aioi A., Hamada K., Shimoda K., Muromoto R., Matsuda T., Miyachi Y. Hapten-induced contact hypersensitivity is enhanced in Tyk2-deficient mice. J. Dermatol. Sci. 2004;36:51–56. doi: 10.1016/j.jdermsci.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Oyamada A., Ikebe H., Itsumi M., Saiwai H., Okada S., Shimoda K., Iwakura Y., Nakayama K.I., Iwamoto Y., Yoshikai Y., et al. Tyrosine kinase 2 plays critical roles in the pathogenic CD4 T cell responses for the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009;183:7539–7546. doi: 10.4049/jimmunol.0902740. [DOI] [PubMed] [Google Scholar]
- 92.Schleicher U., Mattner J., Blos M., Schindler H., Rollinghoff M., Karaghiosoff M., Muller M., Werner-Felmayer G., Bogdan C. Control of Leishmania major in the absence of Tyk2 kinase. Eur. J. Immunol. 2004;34:519–529. doi: 10.1002/eji.200324465. [DOI] [PubMed] [Google Scholar]
- 93.Simonovic N., Witalisz-Siepracka A., Meissl K., Lassnig C., Reichart U., Kolbe T., Farlik M., Bock C., Sexl V., Müller M., et al. NK Cells Require Cell-Extrinsic and -Intrinsic TYK2 for Full Functionality in Tumor Surveillance and Antibacterial Immunity. J. Immunol. 2019;202:1724–1734. doi: 10.4049/jimmunol.1701649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tokumasa N., Suto A., Kagami S., Furuta S., Hirose K., Watanabe N., Saito Y., Shimoda K., Iwamoto I., Nakajima H. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood. 2007;110:553–560. doi: 10.1182/blood-2006-11-059246. [DOI] [PubMed] [Google Scholar]
- 95.Hainzl E., Stockinger S., Rauch I., Heider S., Berry D., Lassnig C., Schwab C., Rosebrock F., Milinovich G., Schlederer M., et al. Intestinal epithelial cell tyrosine kinase 2 transduces IL-22 signals to protect from acute colitis. J. Immunol. 2015;195:5011–5024. doi: 10.4049/jimmunol.1402565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishizaki M., Muromoto R., Akimoto T., Sekine Y., Kon S., Diwan M., Maeda H., Togi S., Shimoda K., Oritani K., et al. Tyk2 is a therapeutic target for psoriasis-like skin inflammation. Int. Immunol. 2014;26:257–267. doi: 10.1093/intimm/dxt062. [DOI] [PubMed] [Google Scholar]
- 97.Nakamura R., Shibata K., Yamada H., Shimoda K., Nakayama K., Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J. Immunol. 2008;181:2071–2075. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]
- 98.Simma O., Zebedin E., Neugebauer N., Schellack C., Pilz A., Chang-Rodriguez S., Lingnau K., Weisz E., Putz E.M., Pickl W.F., et al. Identification of an indispensable role for tyrosine kinase 2 in CTL-mediated tumor surveillance. Cancer Res. 2009;69:203–211. doi: 10.1158/0008-5472.CAN-08-1705. [DOI] [PubMed] [Google Scholar]
- 99.Bosmann M., Strobl B., Kichler N., Rigler D., Grailer J.J., Pache F., Murray P.J., Muller M., Ward P.A. Tyrosine kinase 2 promotes sepsis-associated lethality by facilitating production of interleukin-27. J. Leukoc. Biol. 2014;96:123–131. doi: 10.1189/jlb.3A1013-541R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karaghiosoff M., Steinborn R., Kovarik P., Kriegshauser G., Baccarini M., Donabauer B., Reichart U., Kolbe T., Bogdan C., Leanderson T., et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat. Immunol. 2003;4:471–477. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 101.Strobl B., Bubic I., Bruns U., Steinborn R., Lajko R., Kolbe T., Karaghiosoff M., Kalinke U., Jonjic S., Muller M. Novel functions of tyrosine kinase 2 in the antiviral defense against murine cytomegalovirus. J. Immunol. 2005;175:4000–4008. doi: 10.4049/jimmunol.175.6.4000. [DOI] [PubMed] [Google Scholar]
- 102.Ishizaki M., Muromoto R., Akimoto T., Ohshiro Y., Takahashi M., Sekine Y., Maeda H., Shimoda K., Oritani K., Matsuda T. Tyk2 deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Int. Immunol. 2011;23:575–582. doi: 10.1093/intimm/dxr057. [DOI] [PubMed] [Google Scholar]
- 103.Ma C.S., Wong N., Rao G., Nguyen A., Avery D.T., Payne K., Torpy J., O’Young P., Deenick E., Bustamante J., et al. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J. Exp. Med. 2016;213:1589–1608. doi: 10.1084/jem.20151467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Banerjee S., Biehl A., Gadina M., Hasni S., Schwartz D.M. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: Current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bousoik E., Montazeri Aliabadi H. “Do we know jack” about JAK? A closer look at JAK/STAT signaling pathway. Front. Oncol. 2018;8:287. doi: 10.3389/fonc.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roskoski R., Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 2016;111:784–803. doi: 10.1016/j.phrs.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 107.Berg J., Zscheppang K., Fatykhova D., Tonnies M., Bauer T.T., Schneider P., Neudecker J., Ruckert J.C., Eggeling S., Schimek M., et al. Tyk2 as a target for immune regulation in human viral/bacterial pneumonia. Eur. Respir. J. 2017;50:1601953. doi: 10.1183/13993003.01953-2016. [DOI] [PubMed] [Google Scholar]
- 108.Burke J.R., Cheng L., Gillooly K.M., Strnad J., Zupa-Fernandez A., Catlett I.M., Zhang Y., Heimrich E.M., McIntyre K.W., Cunningham M.D., et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci. Transl. Med. 2019;11:eaaw1736. doi: 10.1126/scitranslmed.aaw1736. [DOI] [PubMed] [Google Scholar]
- 109.Sohn S.J., Barrett K., Van Abbema A., Chang C., Kohli P.B., Kanda H., Smith J., Lai Y., Zhou A., Zhang B., et al. A restricted role for TYK2 catalytic activity in human cytokine responses revealed by novel TYK2-selective inhibitors. J. Immunol. 2013;191:2205–2216. doi: 10.4049/jimmunol.1202859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Works M.G., Yin F., Yin C.C., Yiu Y., Shew K., Tran T.T., Dunlap N., Lam J., Mitchell T., Reader J., et al. Inhibition of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J. Immunol. 2014;193:3278–3287. doi: 10.4049/jimmunol.1400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liongue C., Ward A.C. Evolution of Class I cytokine receptors. BMC Evol. Biol. 2007;7:120. doi: 10.1186/1471-2148-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Renauld J.C. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat. Rev. Immunol. 2003;3:667–676. doi: 10.1038/nri1153. [DOI] [PubMed] [Google Scholar]
- 113.Liongue C., Sertori R., Ward A.C. Evolution of cytokine receptor signaling. J. Immunol. 2016;197:11–18. doi: 10.4049/jimmunol.1600372. [DOI] [PubMed] [Google Scholar]
- 114.Liongue C., Taznin T., Ward A.C. Signaling via the CytoR/JAK/STAT/SOCS pathway: Emergence during evolution. Mol. Immunol. 2016;71:166–175. doi: 10.1016/j.molimm.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Bu L., Baba H., Yoshida N., Miyake K., Yasuda T., Uchihara T., Tan P., Ishimoto T. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 2019;38:4887–4901. doi: 10.1038/s41388-019-0765-y. [DOI] [PubMed] [Google Scholar]
- 116.Vilgelm A.E., Richmond A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front. Immunol. 2019;10:333. doi: 10.3389/fimmu.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lazear H.M., Schoggins J.W., Diamond M.S. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Pesch V., Lanaya H., Renauld J.C., Michiels T. Characterization of the murine alpha interferon gene family. J. Virol. 2004;78:8219–8228. doi: 10.1128/JVI.78.15.8219-8228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu L., Yang L., Liu W. Distinct evolution process among type I interferon in mammals. Protein Cell. 2013;4:383–392. doi: 10.1007/s13238-013-3021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gibbert K., Schlaak J.F., Yang D., Dittmer U. IFN-alpha subtypes: distinct biological activities in anti-viral therapy. Br. J. Pharmacol. 2013;168:1048–1058. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ng C.T., Mendoza J.L., Garcia K.C., Oldstone M.B. Alpha and beta type 1 interferon SIgnaling: Passage for diverse biologic outcomes. Cell. 2016;164:349–352. doi: 10.1016/j.cell.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schreiber G. The molecular basis for differential type I interferon signaling. J. Biol. Chem. 2017;292:7285–7294. doi: 10.1074/jbc.R116.774562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ali S., Mann-Nuttel R., Schulze A., Richter L., Alferink J., Scheu S. Sources of type I interferons in infectious immunity: Plasmacytoid dendritic cells not always in the driver’s seat. Front. Immunol. 2019;10:778. doi: 10.3389/fimmu.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swiecki M., Colonna M. Type I interferons: Diversity of sources, production pathways and effects on immune responses. Curr. Opin. Virol. 2011;1:463–475. doi: 10.1016/j.coviro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schreiber G., Piehler J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 2015;36:139–149. doi: 10.1016/j.it.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 127.Platanitis E., Demiroz D., Schneller A., Fischer K., Capelle C., Hartl M., Gossenreiter T., Muller M., Novatchkova M., Decker T. A molecular switch from STAT2-IRF9 to ISGF3 underlies interferon-induced gene transcription. Nat. Commun. 2019;10:2921. doi: 10.1038/s41467-019-10970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abdul-Sater A.A., Majoros A., Plumlee C.R., Perry S., Gu A.D., Lee C., Shresta S., Decker T., Schindler C. Different STAT transcription complexes drive early and delayed responses to type I IFNs. J. Immunol. 2015;195:210–216. doi: 10.4049/jimmunol.1401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Au-Yeung N., Mandhana R., Horvath C.M. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. JAKSTAT. 2013;2:e23931. doi: 10.4161/jkst.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blaszczyk K., Nowicka H., Kostyrko K., Antonczyk A., Wesoly J., Bluyssen H.A. The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses. Cytokine Growth Factor Rev. 2016;29:71–81. doi: 10.1016/j.cytogfr.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 131.Fink K., Grandvaux N. STAT2 and IRF9: Beyond ISGF3. JAKSTAT. 2013;2:e27521. doi: 10.4161/jkst.27521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W., Yin Y., Xu L., Su J., Huang F., Wang Y., Boor P.P.C., Chen K., Wang W., Cao W., et al. Unphosphorylated ISGF3 drives constitutive expression of interferon-stimulated genes to protect against viral infections. Sci. Signal. 2017;10:eaah4248. doi: 10.1126/scisignal.aah4248. [DOI] [PubMed] [Google Scholar]
- 133.van Boxel-Dezaire A.H., Rani M.R., Stark G.R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 134.Rusinova I., Forster S., Yu S., Kannan A., Masse M., Cumming H., Chapman R., Hertzog P.J. Interferome v2.0: An updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41:D1040–D1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barrat F.J., Crow M.K., Ivashkiv L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crow M.K., Olferiev M., Kirou K.A. Type I interferons in autoimmune disease. Annu. Rev. Pathol. 2019;14:369–393. doi: 10.1146/annurev-pathol-020117-043952. [DOI] [PubMed] [Google Scholar]
- 137.Kretschmer S., Lee-Kirsch M.A. Type I interferon-mediated autoinflammation and autoimmunity. Curr. Opin. Immunol. 2017;49:96–102. doi: 10.1016/j.coi.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 138.McNab F., Mayer-Barber K., Sher A., Wack A., O’Garra A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dunn G.P., Koebel C.M., Schreiber R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 140.Musella M., Manic G., De Maria R., Vitale I., Sistigu A. Type-I-interferons in infection and cancer: Unanticipated dynamics with therapeutic implications. Oncoimmunology. 2017;6:e1314424. doi: 10.1080/2162402X.2017.1314424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Owen K.L., Brockwell N.K., Parker B.S. JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression. Cancers. 2019;11:2002. doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parker B.S., Rautela J., Hertzog P.J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer. 2016;16:131–144. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 143.Gangaplara A., Martens C., Dahlstrom E., Metidji A., Gokhale A.S., Glass D.D., Lopez-Ocasio M., Baur R., Kanakabandi K., Porcella S.F., et al. Type I interferon signaling attenuates regulatory T cell function in viral infection and in the tumor microenvironment. PLoS Pathog. 2018;14:e1006985. doi: 10.1371/journal.ppat.1006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hashimoto H., Ueda R., Narumi K., Heike Y., Yoshida T., Aoki K. Type I IFN gene delivery suppresses regulatory T cells within tumors. Cancer Gene Ther. 2014;21:532–541. doi: 10.1038/cgt.2014.60. [DOI] [PubMed] [Google Scholar]
- 145.Pace L., Vitale S., Dettori B., Palombi C., La Sorsa V., Belardelli F., Proietti E., Doria G. APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. J. Immunol. 2010;184:5969–5979. doi: 10.4049/jimmunol.0900526. [DOI] [PubMed] [Google Scholar]
- 146.Metzger P., Kirchleitner S.V., Kluge M., Koenig L.M., Horth C., Rambuscheck C.A., Bohmer D., Ahlfeld J., Kobold S., Friedel C.C., et al. Immunostimulatory RNA leads to functional reprogramming of myeloid-derived suppressor cells in pancreatic cancer. J. Immunother. Cancer. 2019;7:288. doi: 10.1186/s40425-019-0778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang C.X., Ye S.B., Ni J.J., Cai T.T., Liu Y.N., Huang D.J., Mai H.Q., Chen Q.Y., He J., Zhang X.S., et al. STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion. Cell. Death Differ. 2019;26:2314–2328. doi: 10.1038/s41418-019-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jacquelot N., Yamazaki T., Roberti M.P., Duong C.P.M., Andrews M.C., Verlingue L., Ferrere G., Becharef S., Vetizou M., Daillere R., et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 2019;29:846–861. doi: 10.1038/s41422-019-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xiao W., Klement J.D., Lu C., Ibrahim M.L., Liu K. IFNAR1 controls autocrine type I IFN regulation of PD-L1 expression in myeloid-derived suppressor cells. J. Immunol. 2018;201:264–277. doi: 10.4049/jimmunol.1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brockwell N.K., Parker B.S. Tumor inherent interferons: Impact on immune reactivity and immunotherapy. Cytokine. 2019;118:42–47. doi: 10.1016/j.cyto.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 151.McLane L.M., Abdel-Hakeem M.S., Wherry E.J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 152.Robinson R.T. IL12Rbeta1: the cytokine receptor that we used to know. Cytokine. 2015;71:348–359. doi: 10.1016/j.cyto.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tait Wojno E.D., Hunter C.A., Stumhofer J.S. The immunobiology of the interleukin-12 family: Room for discovery. Immunity. 2019;50:851–870. doi: 10.1016/j.immuni.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vignali D.A., Kuchroo V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chognard G., Bellemare L., Pelletier A.N., Dominguez-Punaro M.C., Beauchamp C., Guyon M.J., Charron G., Morin N., Sivanesan D., Kuchroo V., et al. The dichotomous pattern of IL-12r and IL-23R expression elucidates the role of IL-12 and IL-23 in inflammation. PLoS ONE. 2014;9:e89092. doi: 10.1371/journal.pone.0089092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Croxford A.L., Kulig P., Becher B. IL-12-and IL-23 in health and disease. Cytokine Growth Factor Rev. 2014;25:415–421. doi: 10.1016/j.cytogfr.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 157.Teng M.W., Bowman E.P., McElwee J.J., Smyth M.J., Casanova J.L., Cooper A.M., Cua D.J. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 158.Liu J., Cao S., Kim S., Chung E.Y., Homma Y., Guan X., Jimenez V., Ma X. Interleukin-12: an update on its immunological activities, signaling and regulation of gene expression. Curr. Immunol. Rev. 2005;1:119–137. doi: 10.2174/1573395054065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 160.Trinchieri G., Pflanz S., Kastelein R.A. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/S1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 161.Watford W.T., Hissong B.D., Bream J.H., Kanno Y., Muul L., O’Shea J.J. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 162.Chang J.T., Segal B.M., Nakanishi K., Okamura H., Shevach E.M. The costimulatory effect of IL-18 on the induction of antigen-specific IFN-gamma production by resting T cells is IL-12 dependent and is mediated by up-regulation of the IL-12 receptor beta2 subunit. Eur. J. Immunol. 2000;30:1113–1119. doi: 10.1002/(SICI)1521-4141(200004)30:4<1113::AID-IMMU1113>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 163.Smeltz R.B., Chen J., Ehrhardt R., Shevach E.M. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J. Immunol. 2002;168:6165–6172. doi: 10.4049/jimmunol.168.12.6165. [DOI] [PubMed] [Google Scholar]
- 164.Wandinger K.P., Sturzebecher C.S., Bielekova B., Detore G., Rosenwald A., Staudt L.M., McFarland H.F., Martin R. Complex immunomodulatory effects of interferon-beta in multiple sclerosis include the upregulation of T helper 1-associated marker genes. Ann. Neurol. 2001;50:349–357. doi: 10.1002/ana.1096. [DOI] [PubMed] [Google Scholar]
- 165.Yoshida H., Hunter C.A. The immunobiology of interleukin-27. Annu. Rev. Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 166.Szabo S.J., Dighe A.S., Gubler U., Murphy K.M. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tugues S., Burkhard S.H., Ohs I., Vrohlings M., Nussbaum K., Vom Berg J., Kulig P., Becher B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–246. doi: 10.1038/cdd.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu M., Mizoguchi I., Morishima N., Chiba Y., Mizuguchi J., Yoshimoto T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin. Dev. Immunol. 2010;2010:832454. doi: 10.1155/2010/832454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Berraondo P., Etxeberria I., Ponz-Sarvise M., Melero I. Revisiting interleukin-12 as a cancer immunotherapy agent. Clin. Cancer Res. 2018;24:2716–2718. doi: 10.1158/1078-0432.CCR-18-0381. [DOI] [PubMed] [Google Scholar]
- 170.Croxford A.L., Mair F., Becher B. IL-23: One cytokine in control of autoimmunity. Eur. J. Immunol. 2012;42:2263–2273. doi: 10.1002/eji.201242598. [DOI] [PubMed] [Google Scholar]
- 171.Hawkes J.E., Yan B.Y., Chan T.C., Krueger J.G. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J. Immunol. 2018;201:1605–1613. doi: 10.4049/jimmunol.1800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schon M.P., Erpenbeck L. The interleukin-23/interleukin-17 axis links adaptive and innate immunity in psoriasis. Front. Immunol. 2018;9:1323. doi: 10.3389/fimmu.2018.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Belladonna M.L., Renauld J.C., Bianchi R., Vacca C., Fallarino F., Orabona C., Fioretti M.C., Grohmann U., Puccetti P. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 2002;168:5448–5454. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 174.Parham C., Chirica M., Timans J., Vaisberg E., Travis M., Cheung J., Pflanz S., Zhang R., Singh K.P., Vega F., et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 175.Mathur A.N., Chang H.C., Zisoulis D.G., Stritesky G.L., Yu Q., O’Malley J.T., Kapur R., Levy D.E., Kansas G.S., Kaplan M.H. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 176.Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 177.Kortylewski M., Xin H., Kujawski M., Lee H., Liu Y., Harris T., Drake C., Pardoll D., Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ngiow S.F., Teng M.W., Smyth M.J. A balance of interleukin-12 and -23 in cancer. Trends Immunol. 2013;34:548–555. doi: 10.1016/j.it.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 179.Yan J., Smyth M.J., Teng M.W.L. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb. Perspect. Biol. 2018;10:a028530. doi: 10.1101/cshperspect.a028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ishizaki M., Akimoto T., Muromoto R., Yokoyama M., Ohshiro Y., Sekine Y., Maeda H., Shimoda K., Oritani K., Matsuda T. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J. Immunol. 2011;187:181–189. doi: 10.4049/jimmunol.1003244. [DOI] [PubMed] [Google Scholar]
- 181.Nemoto M., Hattori H., Maeda N., Akita N., Muramatsu H., Moritani S., Kawasaki T., Maejima M., Ode H., Hachiya A., et al. Compound heterozygous TYK2 mutations underlie primary immunodeficiency with T-cell lymphopenia. Sci. Rep. 2018;8:6956. doi: 10.1038/s41598-018-25260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ouyang W., O’Garra A. IL-10 family cytokines IL-10 and IL-22: From basic science to clinical translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 183.Couper K.N., Blount D.G., Riley E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 184.Hedrich C.M., Bream J.H. Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol. Res. 2010;47:185–206. doi: 10.1007/s12026-009-8150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saraiva M., O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 186.Gabrysova L., Howes A., Saraiva M., O’Garra A. The regulation of IL-10 expression. Curr. Top. Microbiol. Immunol. 2014;380:157–190. doi: 10.1007/978-3-662-43492-5_8. [DOI] [PubMed] [Google Scholar]
- 187.Hutchins A.P., Diez D., Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief. Funct Genom. 2013;12:489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Murray P.J. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 189.Walter M.R. The molecular basis of IL-10 function: from receptor structure to the onset of signaling. Curr. Top. Microbiol. Immunol. 2014;380:191–212. doi: 10.1007/978-3-662-43492-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mosser D.M., Zhang X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muhl H. Pro-inflammatory signaling by IL-10 and IL-22: Bad habit stirred up by interferons? Front. Immunol. 2013;4:18. doi: 10.3389/fimmu.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dennis K.L., Blatner N.R., Gounari F., Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 2013;25:637–645. doi: 10.1097/CCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mannino M.H., Zhu Z., Xiao H., Bai Q., Wakefield M.R., Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367:103–107. doi: 10.1016/j.canlet.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 194.Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 195.Wilbers R.H.P., van Raaij D.R., Westerhof L.B., Bakker J., Smant G., Schots A. Re-evaluation of IL-10 signaling reveals novel insights on the contribution of the intracellular domain of the IL-10R2 chain. PLoS ONE. 2017;12:e0186317. doi: 10.1371/journal.pone.0186317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Parks O.B., Pociask D.A., Hodzic Z., Kolls J.K., Good M. Interleukin-22 signaling in the regulation of intestinal health and disease. Front. Cell Dev. Biol. 2015;3:85. doi: 10.3389/fcell.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zenewicz L.A. IL-22: There is a gap in our knowledge. Immunohorizons. 2018;2:198–207. doi: 10.4049/immunohorizons.1800006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wolk K., Witte E., Witte K., Warszawska K., Sabat R. Biol.ogy of interleukin-22. Semin. Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 199.Zenewicz L.A., Flavell R.A. Recent advances in IL-22 biology. Int. Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 200.Dudakov J.A., Hanash A.M., van den Brink M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Eidenschenk C., Rutz S., Liesenfeld O., Ouyang W. Role of IL-22 in microbial host defense. Curr. Top. Microbiol. Immunol. 2014;380:213–236. doi: 10.1007/978-3-662-43492-5_10. [DOI] [PubMed] [Google Scholar]
- 202.Sabat R., Ouyang W., Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat. Rev. Drug Discov. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 203.Hernandez P., Gronke K., Diefenbach A. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 2018;48:15–31. doi: 10.1002/eji.201747183. [DOI] [PubMed] [Google Scholar]
- 204.Lim C., Savan R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014;25:257–271. doi: 10.1016/j.cytogfr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 205.Markota A., Endres S., Kobold S. Targeting interleukin-22 for cancer therapy. Hum. Vaccin Immunother. 2018;14:2012–2015. doi: 10.1080/21645515.2018.1461300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Niess J.H., Hruz P., Kaymak T. The interleukin-20 cytokines in intestinal diseases. Front. Immunol. 2018;9:1373. doi: 10.3389/fimmu.2018.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Donnelly R.P., Sheikh F., Kotenko S.V., Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukoc. Biol. 2004;76:314–321. doi: 10.1189/jlb.0204117. [DOI] [PubMed] [Google Scholar]
- 208.Sheikh F., Baurin V.V., Lewis-Antes A., Shah N.K., Smirnov S.V., Anantha S., Dickensheets H., Dumoutier L., Renauld J.C., Zdanov A., et al. Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J. Immunol. 2004;172:2006–2010. doi: 10.4049/jimmunol.172.4.2006. [DOI] [PubMed] [Google Scholar]
- 209.Donnelly R.P., Sheikh F., Dickensheets H., Savan R., Young H.A., Walter M.R. Interleukin-26: An IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev. 2010;21:393–401. doi: 10.1016/j.cytogfr.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Larochette V., Miot C., Poli C., Beaumont E., Roingeard P., Fickenscher H., Jeannin P., Delneste Y. IL-26, a cytokine with roles in extracellular DNA-induced inflammation and microbial defense. Front. Immunol. 2019;10:204. doi: 10.3389/fimmu.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Braum O., Pirzer H., Fickenscher H. Interleukin-26, a highly cationic T-cell cytokine targeting epithelial cells. Antiinflamm. Antiallergy Agents Med. Chem. 2012;11:221–229. doi: 10.2174/1871523011202030221. [DOI] [PubMed] [Google Scholar]
- 212.You W., Tang Q., Zhang C., Wu J., Gu C., Wu Z., Li X. IL-26 promotes the proliferation and survival of human gastric cancer cells by regulating the balance of STAT1 and STAT3 activation. PLoS ONE. 2013;8:e63588. doi: 10.1371/journal.pone.0063588. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 213.Truong A.D., Hong Y., Hoang C.T., Lee J., Hong Y.H. Chicken IL-26 regulates immune responses through the JAK/STAT and NF-kappaB signaling pathways. Dev. Comp. Immunol. 2017;73:10–20. doi: 10.1016/j.dci.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 214.Iversen M.B., Paludan S.R. Mechanisms of type III interferon expression. J. Interferon Cytokine Res. 2010;30:573–578. doi: 10.1089/jir.2010.0063. [DOI] [PubMed] [Google Scholar]
- 215.Odendall C., Kagan J.C. The unique regulation and functions of type III interferons in antiviral immunity. Curr. Opin. Virol. 2015;12:47–52. doi: 10.1016/j.coviro.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wack A., Terczynska-Dyla E., Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat. Immunol. 2015;16:802–809. doi: 10.1038/ni.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zanoni I., Granucci F., Broggi A. Interferon (IFN)-lambda Takes the Helm: Immunomodulatory roles of type III IFNs. Front. Immunol. 2017;8:1661. doi: 10.3389/fimmu.2017.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stanifer M.L., Pervolaraki K., Boulant S. Differential regulation of type I and type III interferon signaling. Int. J. Mol. Sci. 2019;20:1445. doi: 10.3390/ijms20061445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lazear H.M., Nice T.J., Diamond M.S. Interferon-lambda: Immune functions at barrier surfaces and beyond. Immunity. 2015;43:15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lasfar A., Zloza A., Silk A.W., Lee L.Y., Cohen-Solal K.A. Interferon lambda: Toward a dual role in cancer. J. Interferon Cytokine Res. 2019;39:22–29. doi: 10.1089/jir.2018.0046. [DOI] [PubMed] [Google Scholar]
- 221.Stiff A., Carson W., III Investigations of interferon-lambda for the treatment of cancer. J. Innate Immun. 2015;7:243–250. doi: 10.1159/000370113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Finotti G., Tamassia N., Cassatella M.A. Interferon-lambdas and plasmacytoid dendritic cells: A close relationship. Front. Immunol. 2017;8:1015. doi: 10.3389/fimmu.2017.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Honda K., Takaoka A., Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 224.Michalska A., Blaszczyk K., Wesoly J., Bluyssen H.A.R. A positive feedback amplifier circuit that regulates interferon (IFN)-stimulated gene expression and controls type I and type II IFN responses. Front. Immunol. 2018;9:1135. doi: 10.3389/fimmu.2018.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mostafavi S., Yoshida H., Moodley D., LeBoite H., Rothamel K., Raj T., Ye C.J., Chevrier N., Zhang S.Y., Feng T., et al. Parsing the interferon transcriptional network and its disease associations. Cell. 2016;164:564–578. doi: 10.1016/j.cell.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vogl C., Flatt T., Fuhrmann B., Hofmann E., Wallner B., Stiefvater R., Kovarik P., Strobl B., Muller M. Transcriptome analysis reveals a major impact of JAK protein tyrosine kinase 2 (Tyk2) on the expression of interferon-responsive and metabolic genes. BMC Genom. 2010;11:199. doi: 10.1186/1471-2164-11-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Leonard W.J., Lin J.X., O’Shea J.J. The gammac family of cytokines: Basic biology to therapeutic ramifications. Immunity. 2019;50:832–850. doi: 10.1016/j.immuni.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 228.Robinson T.O., Schluns K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017;190:159–168. doi: 10.1016/j.imlet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Santana Carrero R.M., Beceren-Braun F., Rivas S.C., Hegde S.M., Gangadharan A., Plote D., Pham G., Anthony S.M., Schluns K.S. IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc. Natl. Acad. Sci. USA. 2019;116:599–608. doi: 10.1073/pnas.1814642116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Waldmann T.A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol. Res. 2015;3:219–227. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yoshimura A., Naka T., Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 232.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A., Zaretsky J.M., Sun L., Hugo W., Wang X., et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell. Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Alspach E., Lussier D.M., Schreiber R.D. Interferon gamma and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb. Perspect. Biol. 2019;11:a028480. doi: 10.1101/cshperspect.a028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Castro F., Cardoso A.P., Goncalves R.M., Serre K., Oliveira M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ivashkiv L.B. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018;18:545–558. doi: 10.1038/s41577-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Budczies J., Bockmayr M., Klauschen F., Endris V., Frohling S., Schirmacher P., Denkert C., Stenzinger A. Mutation patterns in genes encoding interferon signaling and antigen presentation: A pan-cancer survey with implications for the use of immune checkpoint inhibitors. Genes Chromosomes Cancer. 2017;56:651–659. doi: 10.1002/gcc.22468. [DOI] [PubMed] [Google Scholar]
- 237.Gao J., Shi L.Z., Zhao H., Chen J., Xiong L., He Q., Chen T., Roszik J., Bernatchez C., Woodman S.E., et al. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167:397–404. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shin D.S., Zaretsky J.M., Escuin-Ordinas H., Garcia-Diaz A., Hu-Lieskovan S., Kalbasi A., Grasso C.S., Hugo W., Sandoval S., Torrejon D.Y., et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Amatya N., Garg A.V., Gaffen S.L. IL-17 signaling: The yin and the yang. Trends Immunol. 2017;38:310–322. doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McGeachy M.J., Cua D.J., Gaffen S.L. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chang S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharm Res. 2019;42:549–559. doi: 10.1007/s12272-019-01146-9. [DOI] [PubMed] [Google Scholar]
- 242.Knochelmann H.M., Dwyer C.J., Bailey S.R., Amaya S.M., Elston D.M., Mazza-McCrann J.M., Paulos C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018;15:458–469. doi: 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li X., Bechara R., Zhao J., McGeachy M.J., Gaffen S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019;20:1594–1602. doi: 10.1038/s41590-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dejima T., Shibata K., Yamada H., Hara H., Iwakura Y., Naito S., Yoshikai Y. Protective role of naturally occurring interleukin-17A-producing gammadelta T cells in the lung at the early stage of systemic candidiasis in mice. Infect. Immun. 2011;79:4503–4510. doi: 10.1128/IAI.05799-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ma C.S., Wong N., Rao G., Avery D.T., Torpy J., Hambridge T., Bustamante J., Okada S., Stoddard J.L., Deenick E.K., et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J. Allergy Clin. Immunol. 2015;136:993–1006. doi: 10.1016/j.jaci.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ubel C., Graser A., Koch S., Rieker R.J., Lehr H.A., Muller M., Finotto S. Role of Tyk-2 in Th9 and Th17 cells in allergic asthma. Sci. Rep. 2014;4:5865. doi: 10.1038/srep05865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wöss K., Simonovic N., Strobl B., Macho-Maschler S., Müller M. TYK2: An upstream kinase of STATs in cancer. Cancers. 2019;11:1728. doi: 10.3390/cancers11111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ide H., Nakagawa T., Terado Y., Kamiyama Y., Muto S., Horie S. Tyk2 expression and its signaling enhances the invasiveness of prostate cancer cells. Biochem. Biophys. Res. Commun. 2008;369:292–296. doi: 10.1016/j.bbrc.2007.08.160. [DOI] [PubMed] [Google Scholar]
- 249.Schuster C., Muller M., Freissmuth M., Sexl V., Stoiber D. Commentary on H. Ide et al., Tyk2 expression and its signaling enhances the invasiveness of prostate cancer cells. Biochem. Biophys. Res. Commun. 2008;366:869–870. doi: 10.1016/j.bbrc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 250.Ubel C., Mousset S., Trufa D., Sirbu H., Finotto S. Establishing the role of tyrosine kinase 2 in cancer. Oncoimmunology. 2013;2:e22840. doi: 10.4161/onci.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Banfield C., Scaramozza M., Zhang W., Kieras E., Page K.M., Fensome A., Vincent M., Dowty M.E., Goteti K., Winkle P.J., et al. The Safety, tolerability, pharmacokinetics, and pharmacodynamics of a TYK2/JAK1 inhibitor (PF-06700841) in healthy subjects and patients with plaque psoriasis. J. Clin. Pharmacol. 2018;58:434–447. doi: 10.1002/jcph.1046. [DOI] [PubMed] [Google Scholar]
- 252.He X., Chen X., Zhang H., Xie T., Ye X.Y. Selective Tyk2 inhibitors as potential therapeutic agents: a patent review (2015–2018) Expert Opin. Ther. Pat. 2019;29:137–149. doi: 10.1080/13543776.2019.1567713. [DOI] [PubMed] [Google Scholar]
- 253.Papp K., Gordon K., Thaci D., Morita A., Gooderham M., Foley P., Girgis I.G., Kundu S., Banerjee S. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N. Engl. J. Med. 2018;29:1313–1321. doi: 10.1056/NEJMoa1806382. [DOI] [PubMed] [Google Scholar]
- 254.Herrmann A., Lahtz C., Nagao T., Song J.Y., Chan W.C., Lee H., Yue C., Look T., Muelfarth R., Li W., et al. CTLA4 promotes Tyk2-STAT3 dependent B cell oncogenecity. Cancer Res. 2017;77:5118–5128. doi: 10.1158/0008-5472.CAN-16-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Reader J., Williams N., Bojdo J., Worthington J., Mitchell T. Immunotherapeutic effects of the TYK2 inhibitor SAR-20351 in syngeneic tumor models [abstract] Mol. Cancer Ther. 2019;18:C086. doi: 10.1158/1535-7163.TARG-19-C086. [DOI] [Google Scholar]
- 256.Howden A.J.M., Hukelmann J.L., Brenes A., Spinelli L., Sinclair L.V., Lamond A.I., Cantrell D.A. Quantitative analysis of T cell proteomes and environmental sensors during T cell differentiation. Nat. Immunol. 2019;20:1542–1554. doi: 10.1038/s41590-019-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wagner J., Rapsomaniki M.A., Chevrier S., Anzeneder T., Langwieder C., Dykgers A., Rees M., Ramaswamy A., Muenst S., Soysal S.D., et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell. 2019;177:1330–1345. doi: 10.1016/j.cell.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wu T., Wu X., Wang H.Y., Chen L. Immune contexture defined by single cell technology for prognosis prediction and immunotherapy guidance in cancer. Cancer Commun. 2019;39:21. doi: 10.1186/s40880-019-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Si Y., Merz S.F., Jansen P., Wang B., Bruderek K., Altenhoff P., Mattheis S., Lang S., Gunzer M., Klode J., et al. Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue. Sci. Immunol. 2019;4:eaaw9159. doi: 10.1126/sciimmunol.aaw9159. [DOI] [PubMed] [Google Scholar]
- 260.Johnson H.M., Noon-Song E., Ahmed C.M. Noncanonical IFN signaling, steroids, and STATs: A probable role of V-ATPase. Mediators Inflamm. 2019;2019:4143604. doi: 10.1155/2019/4143604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zouein F.A., Duhe R.J., Booz G.W. JAKs go nuclear: emerging role of nuclear JAK1 and JAK2 in gene expression and cell growth. Growth Factors. 2011;29:245–252. doi: 10.3109/08977194.2011.614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Potla R., Koeck T., Wegrzyn J., Cherukuri S., Shimoda K., Baker D.P., Wolfman J., Planchon S.M., Esposito C., Hoit B., et al. Tyk2 tyrosine kinase expression is required for the maintenance of mitochondrial respiration in primary pro-B lymphocytes. Mol. Cell. Biol. 2006;26:8562–8571. doi: 10.1128/MCB.00497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Avalle L., Poli V. Nucleus, mitochondrion, or reticulum? STAT3 a la carte. Int. J. Mol. Sci. 2018;19:2820. doi: 10.3390/ijms19092820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Meier J.A., Larner A.C. Toward a new STATe: The role of STATs in mitochondrial function. Semin. Immunol. 2014;26:20–28. doi: 10.1016/j.smim.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hoang T.H., Zhao Y., Lam Y., Piekos S., Han Y.C., Reilly C., Joshi P., Hong S.H., Sung C.O., Giardina C., et al. BioTarget: A computational framework identifying cancer type specific transcriptional targets of immune response pathways. Sci. Rep. 2019;9:9029. doi: 10.1038/s41598-019-45304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu Y. A global immune gene expression signature for human cancers. Oncotarget. 2019;10:1993–2005. doi: 10.18632/oncotarget.26773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Stoney R., Robertson D.L., Nenadic G., Schwartz J.M. Mapping biological process relationships and disease perturbations within a pathway network. NPJ Syst. Biol. Appl. 2018;4:22. doi: 10.1038/s41540-018-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]