Abstract
Background
Oxygen has long been implicated in the pathogenesis of retinopathy of prematurity (ROP) and is rigorously monitored in today's neonatal intensive care units. Recent research using a feline model has shown an improvement in ROP outcome of kittens treated with supplemental oxygen. Current treatment for ROP by retinal ablation is not without complications so a non‐invasive method of treatment is preferred. The possible effects of long term oxygen supplementation on chronic lung disease, length of hospital stay and growth and development are, however, unknown.
Objectives
To determine whether, in preterm or low birth weight infants with prethreshold ROP, targeting higher as compared to normal transcutaneous oxygen levels or pulse oximetry levels when using supplemental oxygen reduces the progression of ROP to threshold disease and improves visual outcome without any adverse effects.
Search methods
The standard search strategy of the Cochrane Neonatal Review Group was used. This included searches of the Oxford Database of Perinatal Trials, MEDLINE, previous reviews including cross references, abstracts, conferences and symposia proceedings, expert informants and journal handsearching. An additional literature search of the MEDLINE (1966 to June 2002), EMBASE (1980 to April 2002), and CINAHL (1982 to April 2002) databases was conducted in order to locate any trials in addition to those provided by the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 2, 2002).
Selection criteria
All randomised or quasi randomised studies comparing higher versus normal target oxygen levels in preterm or low birthweight infants with prethreshold ROP were eligible for inclusion.
Data collection and analysis
The methodological quality of the one eligible trial was assessed independently by two authors for the degree of selection, performance, attrition and detection bias. Data regarding clinical outcomes including progression to threshold ROP, blindness or severe visual impairment, mortality, respiratory morbidities and long term growth were extracted and reviewed independently by two authors. Results were compared and differences resolved as required. Data analysis was conducted according to the standards of the Cochrane Neonatal Review Group.
Main results
The one trial included in this review enrolled 649 infants. There was a trend for supplemental oxygen to reduce the progression to threshold ROP, however this did not reach statistical significance (RR 0.84, 95% CI 0.70, 1.02). A subgroup analysis of those infants without plus disease showed significantly fewer infants progressing to threshold ROP in infants treated with supplemental oxygen. However this analysis was not pre‐specified so these results should be interpreted with caution. No significant effects were detected on blindness or severe visual function at three months corrected age, mortality, pneumonia, chronic lung disease or weight gain. Adverse pulmonary events were more common in the higher oxygen saturation group and these infants were in hospital and on supplemental oxygen for longer. Longer term visual outcomes were not reported.
Authors' conclusions
The results of this systematic review do not show a statistically significant reduction in the rate of progression to threshold ROP with supplemental oxygen treatment, but reveal increased adverse pulmonary sequelae with higher oxygen targeting in this group of preterm infants. Future research needs to be directed towards the question of whether infants without plus disease are more likely to respond to supplemental oxygen therapy than those with plus disease.
Plain language summary
Supplemental oxygen for the treatment of prethreshold retinopathy of prematurity
Increased oxygen supplementation for babies with signs of worsening retinopathy of prematurity (ROP) may not prevent development of this eye disease, and may lead to lung complications. Very preterm babies are at risk of damage to their sight from ROP (retinopathy of prematurity). Oxygen plays a part in the development of ROP. The amount of oxygen babies receive in neonatal intensive care is very carefully monitored to try to lower the risk of ROP and limit the possibility of lung damage. One option is increasing the oxygen level to babies who are showing signs of worsening ROP. However, the review of the one available trial found that increased supplemental oxygen did not reduce the chances of ROP progressing, but may harm the lungs of babies showing signs of worsening ROP.
Background
Retinopathy of prematurity (ROP) is a common retinal neovascular disorder occurring almost exclusively in infants born at less than 30 weeks gestation (Donoghue 2002). In most of these infants the abnormal retinal vasculature regresses and the ROP resolves. However in a small percentage of infants the abnormal vessels continue to grow leading to hemorrhagic and eventually fibrotic retinal scarring and detachment (Watts 1992). Severe ROP may result in unfavourable visual outcomes in 40 to 50 percent of cases at one year follow up compared to less than one percent of infants with no or less severe ROP (CRYO‐ROP 1994). Even with treatment, severe ROP is associated with unfavourable visual outcomes in approximately 11 percent of cases at three months of age (Laser ROP 1994).
The International Classification of ROP describes the disorder by location (zones 1 to 3), severity (stages 1 to 4), amount of disease (clock hours) and presence or absence of venous dilatation and arteriolar tortuosity ("plus" disease) (ICROP 1984). In addition, infants have been categorised as either having pre‐threshold disease (zone 1 disease of any stage or zone 2, stage 2 with "plus" disease or zone 2, stage 3) or threshold disease (five contiguous or eight cumulative clock hours of stage 3 ROP in zone 1 or 2 with "plus" disease) (CRYO‐ROP 1988). Infants with threshold disease, prior to the introduction of surgery to halt the progression of ROP, were predicted to have an almost 50% risk of blindness (CRYO‐ROP 1988).
Retrolental fibroplasia (stage 3 ROP with plus disease(Reese 1953))has been associated with supplemental oxygen administration since the 1950's when it was shown that unrestricted oxygen exposure for premature infants regardless of clinical requirement resulted in an increase in retrolental fibroplasia (Askie 2002). A meta‐analysis has shown that the relative restriction of oxygen resulted in a greater than 70% decrease in ROP (Watts 1992; Askie 2002). Following this discovery there was a period where supplemental oxygen was severely restricted and ROP rates fell. In retrospect, however, many of the infants at greatest risk of developing ROP did not survive (Cross 1973) . From retrospective studies in England and Wales and the United States, Cross concluded that for every case of blindness prevented there was an excess of 16 deaths from hypoxia. Analysis of arterial oxygen tension became available in the 1960's and, later, continuous estimations of arterial oxygen levels could be measured with transcutaneous oxygen monitoring and pulse oximetry. Despite rigorous monitoring of oxygen, preterm infants who are at risk still develop ROP today. There have been reports of infants who developed ROP who never received supplemental oxygen (Adamkin 1977) and of infants who did not develop ROP despite very high oxygen levels (Aranda 1974). It is currently thought that retinopathy of prematurity is a multifactorial disease.
Current treatment for severe retinopathy is invasive and involves ablation of the avascular retina by cryotherapy or laser photocoagulation (Chan‐Ling 1995). The CRYO‐ROP trial showed that cryotherapy achieved an initial reduction in unfavourable outcome of 50% (CRYO‐ROP 1988). However, even after invasive treatment of the avascular portion of the retina next to the abnormal vessels, retinal detachment and blindness still occur in some infants. Also retinal ablation while beneficial is not without complication. There have been reports of iris atrophy, cataracts and hypotony following this procedure (Kaiser 2001).
Non‐invasive treatments of ROP have been postulated. One of these is supplemental oxygen therapy aimed at targeting higher oxygen levels in the blood. However there is little consensus as to the appropriate normal levels of oxygen for maximising short or long term benefits whilst minimising harmful effects. Uncertainty exists as to the range of blood oxygen levels that should be targeted in preterm and low birthweight infants (Askie 2002). Different methods are used to monitor oxygen levels such as arterial blood gas sampling (intermittent), transcutaneous oxygen monitoring (intermittent or continuous) or pulse oximetry (intermittent or continuous). Pulse oximeters can be further divided into two types, those that include fractional or those that include functional saturation in their algorithms. These differences in algorithms can lead to differences in saturation of at least 2 to 3 percent (Grieve 1997), with functional oximeters reading higher than oximeters using a fractional oxygen saturation algorithm.
The physiology behind the postulation that supplemental oxygen can halt and reverse the progression of ROP is as follows. In the first phase of ROP exposure of the extremely preterm infant to the relatively hyperoxic extra‐uterine environment after birth leads to down regulation of vascular endothelial growth factor (VEGF) production and the cessation of normal blood vessel growth (Pierce 1996). The density of blood vessels in the retina is then insufficient once the metabolic demand from the avascular retina increases. A rebound overproduction of VEGF to compensate for the tissue metabolic imbalance leads to the abnormal vascularization typical of ROP (Chan‐Ling 1995). Kittens with hyperoxia‐induced ROP that recovered in 28% oxygen had less severe retinopathy than those recovered in room air (Phelps 1988). Unfortunately the animal models of ROP do not progress to full detachment and blindness as ROP does in some infants and therefore may not completely reflect the pathophysiology in humans (Phelps 1988). Supplemental oxygen for the treatment of prethreshold ROP is hypothesised to reduce the retinal neovascularization that causes the ROP by controlling the rate of revascularization. However increasing the oxygen given to these infants may result in other problems such as increased length of stay in the hospital and chronic lung disease (Frank 1985).
Objectives
To determine whether, in preterm or low birth weight infants with prethreshold ROP, targeting higher as compared to normal transcutaneous oxygen levels or pulse oximetry levels when using supplemental oxygen reduces the progression of ROP to threshold disease and improves visual outcome without any adverse effects. A priori sub‐group analyses: ‐ Infants of different gestational age and birth weight subgroups, as there are different baseline risks of the outcome measures in these sub‐groups. ‐ Type of monitoring used in the study i.e. transcutaneous oxygen monitoring or pulse oximetry ‐ Different target ranges for oxygenation
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi randomised studies comparing higher versus normal target oxygen levels in preterm or low birthweight infants with prethreshold ROP were eligible for inclusion.
Types of participants
Preterm (< 37 weeks gestation at birth) or low birthweight infants (< 2500 g at birth) with prethreshold retinopathy of prematurity. There were no restrictions on postnatal age at entry.
Types of interventions
Higher target transcutaneous oxygen levels or target pulse oximetry levels using supplemental oxygen, versus normal target levels. Eligible trials must have used a measure of oxygenation, either continuously or intermittently, using pulse oximeters (fractional or functional haemoglobin algorithms) and/or transcutaneous monitors.
Types of outcome measures
Ten outcome measures were chosen as being representative of the clinically important measures of effectiveness. They were:‐
Primary: Progression to threshold ROP and/or retinal ablation surgery Blindness or severe visual impairment (visual acuity 20/200 or worse)
Secondary: Visual function (visual acuity, refraction and structural outcome) ‐ short and long term Length of hospital stay (days) Number of days of supplemental oxygen Mortality before hospital discharge Chronic lung disease (oxygen requirement at 36 weeks postmenstrual age [PMA]) /bronchopulmonary dysplasia (chest x‐ray changes at 36 weeks PMA), pneumonia and other significant respiratory morbidities Growth/weight gain ‐ short and long term
Search methods for identification of studies
Relevant trials were identified from searches of the Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library Issue 2, 2002), Oxford Database of Perinatal trials, MEDLINE (1966 to June 2002), EMBASE (1980 to April 2002), and CINAHL (1982 to April 2002). We also searched specialised registers such as Australian Perinatal Trial Register (May 2002), several Internet based registers of ongoing and recently published trials, reference lists of relevant reviews and studies, and contacted experts in the field. There were no language restrictions imposed on the search strategy.
The following search strategy was used to search MEDLINE and was modified as necessary for EMBASE and CINAHL 1 exp Retinopathy of Prematurity/ 2 (retinopathy adj5 prematur$).mp. 3 retrolental fibroplasia.mp. 4 or/1‐3 5 exp Oxygen Inhalation Therapy/ 6 supplemental oxygen.mp 7 oxygen saturation.mp. 8 exp Monitoring, Physiologic/ 9 exp Blood Gas Analysis/ 10 or/5‐9 11 clinical trial.pt. 12 (random$ or trial$ or double blind$ or placebo$).mp. 13 or/11‐12 14 4 and 10 and 13
Data collection and analysis
Selection of trials: The standard methods set out by The Cochrane Neonatal Review Group were used to select trials. Two reviewers looked at titles and abstracts to identify potentially relevant trials using the selection criteria. Trials that clearly failed to meet the inclusion criteria were not reviewed. Those that could not be excluded were reviewed in full text. In all instances, differences of opinion were resolved by discussion.
Quality of trials: The methodological quality of the included trial was assessed independently by two reviewers with discrepancies resolved by discussion. Quality was based on concealment of randomisation and allocation, blinding of intervention and outcome assessment, follow up rates and power calculation. Additional information was sought from the authors when necessary. Studies having greater than 20% attrition for the primary outcome were to be excluded from analysis.
Data extraction: Data were extracted by two reviewers using a standardised data form. Levels of agreement between the reviewers were greater than 90%.
Statistical analysis: The standard method of the Cochrane Neonatal Review Group was used. Categorical data were expressed as relative risk and risk difference with 95% confidence interval and number needed to treat or harm as appropriate. Continuous data were analysed using weighted mean difference with 95% confidence intervals. A fixed effects model was to be used for meta‐analysis. In order to assess the effect of missing data on the primary outcome, progression to threshold ROP, analyses of best case/worst case scenarios were undertaken.
In this review the analyses were performed on all enrollees according to the assigned treatment arm (sensitivity analysis on failure to complete the primary endpoint assessment).
Sub‐group analysis: A priori sub‐group analysis was to be performed for infants with birthweights less than 1500 g or less than 1000 g or infants with a gestational age at birth less than 32 weeks or less than 28 weeks. Other secondary analyses to be performed were those using different monitors to measure the oxygen levels, for example pulse oximeters (including brands of pulse oximeters) and transcutaneous monitors, and intermittent versus continuous monitoring.
Results
Description of studies
One trial, STOP‐ROP 2000, assessing the use of supplemental oxygen in the treatment of prethreshold retinopathy of prematurity (ROP), met the inclusion criteria . Between February 1994 and March 1999, this trial enrolled 649 preterm infants with prethreshold ROP (see Additional Table 2 for definitions) and randomly assigned them to target a fractional oxygen saturation range of either 96 to 99% (experimental, supplemental or higher oxygen saturation range group) or 89 to 94% (control, conventional or normal oxygen saturation range group) for a minimum of two weeks or until the primary ophthalmic outcomes were reached. The mean birthweight and gestational age of the enrolled infants were 726 grams and 25.4 weeks respectively. The mean age at randomisation was 35.6 weeks PMA. Randomisation was stratified by severity of eye disease (severe versus less severe ROP, see Additional Table 3 for definitions). Infants were not considered for randomisation if their median pulse oximetry saturations were greater than 94% in room air after four hours continuous monitoring or they had lethal anomalies or congenital anomalies of the eye. The primary outcome measure in this trial was progression to threshold ROP by three months corrected age as assessed by study accredited ophthalmologists who were masked to the infants' treatment allocation. Several other clinically important secondary outcomes were also assessed. A more detailed description of this study can be found in the Characteristics of Included Studies table.
1. Definitions of ROP Severity Categories in STOP‐ROP.
| Threshold ROP | |
| Zone II | Presence of posterior pole dilation/tortuosity in at least 2 posterior pole quadrants (plus disease), and stage 3 ROP for at least 5 contiguous clock hours or 8 non‐contiguous clock hours |
| Zone I | ROP (any stage) with posterior pole dilation/tortuosity in at least 2 posterior pole quadrants (plus disease), or stage 3 ROP with or without plus disease |
| Beyond Threshold | Stage 4 ROP, stage 5 ROP, or massive vitreal haemorrhage obscuring the view of the fundus |
| Prethreshold ROP | |
| Zone II | Any number of clock hours of stage 3 ROP less than threshold severity, or any stage 2 ROP with at least 2 quadrants of posterior pole dilation/tortuosity disease (plus disease |
| Zone I | Any ROP less than threshold severity. |
2. Stratification of ROP disease severity (definition) in STOP‐ROP.
| Stratum A (severe ROP) | Either study eye having 1 or more clock hours of any stage ROP in zone I, or when the fellow eye was already at threshold or worse |
| Stratum B (less severe ROP) | Zone II prethreshold ROP in both eyes or in the second eye at less than prethreshold |
Risk of bias in included studies
The single eligible trial, STOP‐ROP 2000, used random patient allocation with concealment of allocation. Telephone randomisation by a central coordinating centre, using the Wei‐Lachin Urn scheme, occurred in 64% of eligible patients. When the coordinating centre was unavailable, study centres used sequentially numbered, sealed envelopes provided in advance by the coordinating centre.
There was no blinding of the intervention, with bedside nurses, attending clinicians and parents aware of treatment allocation. Due to the nature of the intervention it would have been difficult to blind these caregivers, therefore it was deemed to be appropriate. There was, however, blinding of outcome assessment.
The infants in the control and experimental arms were similar at baseline with respect to birthweight, gestational age, pulmonary status, gender, race, medications and socioeconomic status.
Loss to follow‐up with respect to the primary eye endpoint (no data for primary study outcome of progression to threshold ROP) occurred in twenty six (8%) infants in the control group (nine withdrew from the study, two had early ablative surgery and 15 missed eye exams) and twenty six (8%) infants in the experimental group (two died, nine withdrew from the study, three had early ablative surgery and 12 missed eye exams). Ophthalmic outcome data were incomplete at three months corrected age for twenty four (7%) infants in the control group (seven died, 16 withdrew and one was lost to follow‐up) and twenty five (8%) infants in the experimental group (nine died, 15 withdrew and one was lost to follow‐up).
Effects of interventions
Ophthalmic outcomes The results of the one trial included in this review (STOP‐ROP 2000) indicate that there was a trend for supplemental oxygen to reduce the progression to threshold ROP but this did not reach statistical significance (RR 0.84, 95%CI 0.70, 1.02). The number needed to treat for this outcome was 14.5. There was no significant difference in blindness or severe visual impairment between the infants having higher oxygen saturation targets compared with the control infants (RR 1.08, 95%CI 0.52, 2.26). Blindness or severe visual impairment in this study refers to partial or total retinal detachment, retinal folds or obstruction of the visual axis. There was also no significant effect on the anatomic finding of macular ectopia at three months corrected age (RR 1.00, 95%CI 0.47, 2.13).
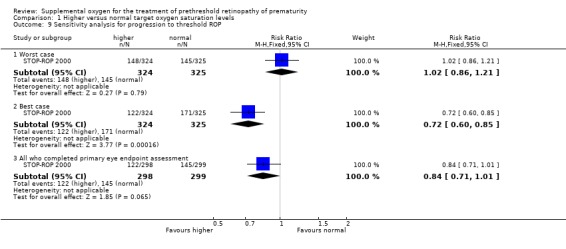
A best case/worst case analysis, accounting for the possible effects of missing outcome data, shows that the relative risk for progression to threshold ROP must be between 0.72 (0.60, 0.85) and 1.02 (0.86, 1.21). See Table 4. So assuming all missing patients in the supplemental arm did not progress to threshold ROP and all the missing infants in the control arm progressed to threshold ROP (best case), then treatment of prethreshold ROP with higher oxygen saturations would significantly reduce the number of infants progressing to threshold disease.
3. Best case/worst case analysis for progression to threshold ROP in STOP‐ROP.
| Experimental group | Control group | RR (95%CI) | |
| Number of infants enrolled | 324 | 325 | |
| Number of missing infants | 26 | 26 | |
| Reported | 122/298 | 145/299 | 0.84 (0.71,1.01) |
| Worst case | 148/324 | 145/325 | 1.02 (0.86,1.21) |
| Best case | 122/324 | 171/325 | 0.72 (0.60,0.85) |
A subgroup analysis of progression to threshold ROP by presence or absence of plus disease was not a prespecified outcome of the reviewers or the study authors. However, the reviewers felt that the result was of clinical importance and had biological plausibility so the results have been included in this review. Caution, however, should be taken when interpreting results from post hoc analyses as the results could be due to random error. In infants with plus disease, there was no significant difference in progression to threshold ROP between the treatment groups (RR 1.09, 95%CI 0.85, 1.40). However, significantly fewer infants with no plus disease at randomisation progressed to threshold ROP in the experimental group (RR 0.70, 95%CI 0.54, 0.90).
Mortality Mortality was an infrequent outcome in the STOP‐ROP 2000 study and hence the power was insufficient to assess clinically important differences. No evidence of effect was found, either in all cause mortality (RR 1.30, 95%CI 0.48, 3.53) or deaths from pulmonary causes (RR 1.68, 95%CI 0.40, 7.10) by three months corrected age.
Pulmonary sequelae Our pre‐specified outcome of chronic lung disease (CLD) and/or bronchopulmonary dysplasia (BPD) was not specifically reported in the one included trial. As the mean age at randomisation was 35.6 weeks PMA and the intervention involved targeting either oxygen saturation range for a minimum of two weeks, it is likely that virtually all enrolled infants would have been oxygen‐dependent at 36 weeks PMA, rendering the reporting of this outcome in the included trial meaningless.
Another pre‐specified outcome, pneumonia, was also not reported as a single outcome in the included STOP‐ROP 2000 trial. However, the composite and clinically meaningful outcome of pneumonia/CLD events was reported. This was defined as probable or definite pneumonia, an acute exacerbation of CLD, or some combination of these two events such that the study neonatologist could not distinguish between them. There was no significant difference in the number of infants with pneumonia/CLD events (RR 1.52, 95%CI 0.94, 2.47) between the two treatment groups in the one included trial.
The final pre‐specified pulmonary outcome was other significant respiratory morbidities. The included trial reported this outcome as adverse pulmonary events by three months corrected age. An adverse pulmonary event was defined in the STOP‐ROP 2000 trial as any one or more of the following by three months corrected age: remaining hospitalised; remaining on study equipment, oxygen, steroids, methylxanthines, or diuretics. There was a significant increase in the number of adverse pulmonary events in the supplemental oxygen group (RR 1.24, 95%CI 1.06, 1.44). Using number to harm this equates to one extra infant with an adverse pulmonary event for every nine infants treated with higher target oxygen saturations. However, there was no significant difference in another important respiratory morbidity, the number of infants rehospitalized for pulmonary reasons (RR 0.89, 95%CI 0.60, 1.32). The individual components of the composite adverse pulmonary events outcome were also reported in the included trial. Significantly more of the infants in the supplemental group were still in hospital (RR 1.86 95%CI 1.12, 3.10), were receiving diuretics (RR 1.47, 95% CI 1.16, 1.87) and remained on supplemental oxygen (RR 1.26, 95%CI 1.05, 1.51) at three months corrected age.
Growth There was no significant difference in weight gain either during the first two weeks of the study intervention (MD ‐13.00 grams, 95%CI ‐34.55, 8.55) or by three months corrected age (MD ‐80.00 grams, 95%CI ‐237.77, 77.77).
A priori sub‐group analysis A priori sub‐group analysis could not be performed for infants with birthweights less than 1500g or less than 1000g, or infants with a gestational age at birth less than 32 weeks or less than 28 weeks in the one eligible trial because these data were not available. Therefore the secondary goals of the sub‐group analysis, to determine whether the effectiveness of the treatment and its safety were different in infants of differing birthweight and gestational age, could not be achieved. Other secondary analyses to be performed were those using different monitors to measure the oxygen levels, for example pulse oximeters (including brands of pulse oximeters) and transcutaneous monitors, and intermittent versus continuous monitoring. The only trial eligible for this review targeted higher versus normal oxygen saturation ranges using continuous pulse oximetry. No studies meeting the entry criteria were found that used alternative methods for oxygen monitoring and/or different target ranges for oxygen levels.
Discussion
The STOP‐ROP 2000 study concluded, as did the authors of this review, that there was a trend to a reduced rate of progression from prethreshold to threshold ROP in infants with higher targeted oxygen saturation. This only reached statistical significance in infants who did not have plus disease at randomisation (post hoc analysis). As stated in the results, this was not a prespecified sub‐group analysis of this review and the results must be interpreted with caution. However, a possible explanation is that hyperoxia causes down regulation of VEGF production by the avascular retina leading to the reduction in blood vessel growth. In plus disease (dilation and tortuosity of the retinal blood vessels) the rate and density of vascularization may not be susceptible to this process. Plus disease therefore may be an indicator that ROP is more advanced and less likely to respond to treatment. However, data from an excluded non‐randomised retrospective study by Gaynon 1997 showed a benefit of increased oxygen saturation on ROP progression despite all enrolled infants having at least two quadrants of plus disease (part of the eligibility criteria).
The two excluded studies in this review (Gaynon 1997; Seiberth 1998) looked at increased oxygen saturation levels for infants with prethreshold ROP. They both showed a decrease in progression to threshold ROP in infants with increased oxygen saturation levels compared to infants who had normal oxygen saturation levels. There are many reasons, in addition to differences in study design, why these two small observational studies, using historical controls, may have had different results from that of the randomised trial included in this review, including lower disease severity, heavier infants and improvements in care and outcomes over time. These studies used higher minimum oxygen saturation levels than STOP‐ROP 2000 and did not have an upper oxygen saturation limit. However, they used a different type of pulse oximeter (saturation readings approximately 1.6 saturation points higher than the STOP‐ROP 2000 oximeter), and adding this difference to the STOP‐ROP 2000 supplemental range results in very similar target saturation levels making this an unlikely reason for any difference. The Gaynon 1997 study examined their infants weekly compared to every two weeks in the STOP‐ROP 2000 study centres prior to the diagnosis of prethreshold disease, so infants were probably placed in increased oxygen earlier than the STOP‐ROP 2000 trial. Also, in the STOP‐ROP 2000 trial there was some delay in starting the treatment due to confirming the diagnosis with a second ophthalmologist and obtaining informed consent. Fifty percent of infants in the trial were not randomised in the first 24 hours as the authors had initially intended. Increasing oxygen saturation levels earlier, by starting oxygen immediately after diagnosis, examining the infants more often to diagnose significant disease earlier or changing the definition of prethreshold ROP, may improve the outcome and reduce the need for peripheral retinal ablation. There is a study currently enrolling infants (ETROP) that is comparing ablation of the unvascularized retina prior to the eye reaching threshold disease with the current practice of treatment once threshold is reached with the hypothesis that fewer infants in the early treatment arm will have adverse eye outcomes. The same hypothesis could also be relevant re the timing of higher oxygen levels for the treatment of ROP.
The only randomised controlled trial (STOP‐ROP 2000) looking at the use of higher oxygen levels to treat prethreshold ROP failed to enroll the prespecified number of infants. Therefore, there is the possibility of a type II error, that is, the study may have missed a significant effect because of reduced enrollment and a subsequent decrease in statistical power (from 90% to approximately 80%).
This study did not demonstrate that increased oxygen saturation levels are deleterious as far as established ROP is concerned. However, these data do not support the use of higher saturation levels for more immature infants where ROP is not already established. To date, the only randomised trial that has attempted to assess the effect of higher oxygen saturation target ranges on longer term growth and development (BOOST 2002) found no significant difference in growth, development or adverse eye outcomes for those targeting a higher oxygen saturation range. A secondary hypothesis of the STOP‐ROP 2000 study was that higher oxygen saturations would benefit infants with CLD resulting in increased growth and lung function. However, the results show that increasing the fractional oxygen saturation range from 89% to 94% to 96% to 99% had no significant effect on short term growth, neurodevelopment, rehospitalisation for pulmonary reasons, pneumonia/CLD exacerbations or mortality. More infants in the higher oxygen saturation group, however, were still on supplemental oxygen and in hospital at three months corrected age and had more adverse pulmonary events. Any benefit in the trend to reduce the number of infants with threshold disease and therefore reduce the number needing ablative surgery needs to be weighed against the number of infants still needing oxygen and hospital care at three months corrected age and the effect that this may have on family and cost of care. If the number needing ablative surgery can be reduced this will also benefit those infants with CLD by avoiding a general anaesthesia.
Authors' conclusions
Implications for practice.
No clear benefit from the use of increased oxygen saturation in the treatment of prethreshold ROP was shown in the one study eligible for this review. However, fewer infants without plus disease progressed to threshold ROP in the increased oxygen saturation group, but as this sub‐group analysis was not prespecified, caution needs to be taken when interpreting the results. The finding of increased adverse pulmonary sequelae in preterm infants with prethreshold ROP when a higher oxygen saturation range was targeted warrants consideration by clinicians weighing up the benefits and harms of this treatment.
Implications for research.
More trials looking at the difference in outcome of prethreshold ROP with and without plus disease are needed to determine if treatment with supplemental oxygen benefits infants who do not have plus disease. Long term visual outcome also needs to be studied in these infants because higher oxygen saturation levels may have later benefits.
What's new
| Date | Event | Description |
|---|---|---|
| 22 May 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 3 December 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank the staff at the Australasian Cochrane Centre for their assistance in compiling this review during the Cochrane review completion course.
Data and analyses
Comparison 1. Higher versus normal target oxygen saturation levels.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression to threshold ROP by 3 months corrected age | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.02] |
| 1.1 Infants with severe ROP at randomisation (stratum A) | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.13] |
| 1.2 Infants with less severe ROP at randomisation (stratum B) | 1 | 469 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.68, 1.07] |
| 2 Blindness or severe visual impairment at 3 months corrected age | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.52, 2.26] |
| 3 Macular ectopia at 3 months corrected age | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.47, 2.13] |
| 4 Mortality between randomization and 3 months corrected age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Pulmonary deaths | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.40, 6.94] |
| 4.2 Non pulmonary deaths | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.25, 3.98] |
| 4.3 All deaths | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.49, 3.42] |
| 5 Remained in hospital at 3 months corrected age | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.12, 3.10] |
| 6 Remained on supplemental oxygen at 3 months corrected age | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.05, 1.51] |
| 7 Pulmonary morbidities at or by 3 months corrected age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pneumonia / CLD events | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.94, 2.47] |
| 7.2 Rehospitalization for pulmonary reasons | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.32] |
| 7.3 Apnea/bradys triple baseline rate | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.70, 1.91] |
| 7.4 Methylxanthines | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.75, 1.60] |
| 7.5 Postnatal steroids | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.76, 1.67] |
| 7.6 Diuretics | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.16, 1.87] |
| 7.7 Any adverse pulmonary event by 3 months corrected age | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.44] |
| 8 Growth / weight gain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Weight gain in first two weeks post randomisation (grams) | 1 | 649 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐34.55, 8.55] |
| 8.2 Weight gain from randomisation to 3 months corrected age (grams) | 1 | 649 | Mean Difference (IV, Fixed, 95% CI) | ‐80.0 [‐237.77, 77.77] |
| 9 Sensitivity analysis for progression to threshold ROP | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Worst case | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.86, 1.21] |
| 9.2 Best case | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.85] |
| 9.3 All who completed primary eye endpoint assessment | 1 | 597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.71, 1.01] |
1.1. Analysis.
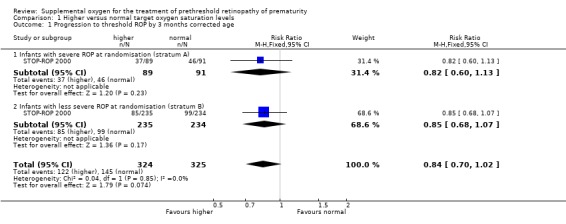
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 1 Progression to threshold ROP by 3 months corrected age.
1.2. Analysis.
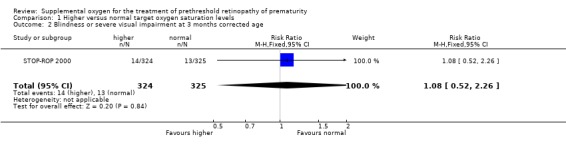
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 2 Blindness or severe visual impairment at 3 months corrected age.
1.3. Analysis.
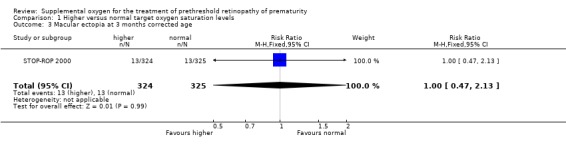
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 3 Macular ectopia at 3 months corrected age.
1.4. Analysis.
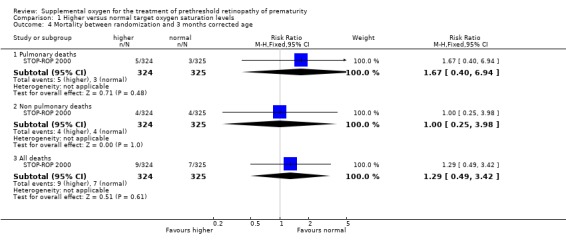
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 4 Mortality between randomization and 3 months corrected age.
1.5. Analysis.
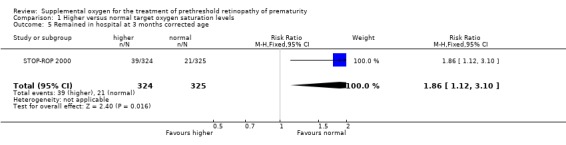
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 5 Remained in hospital at 3 months corrected age.
1.6. Analysis.
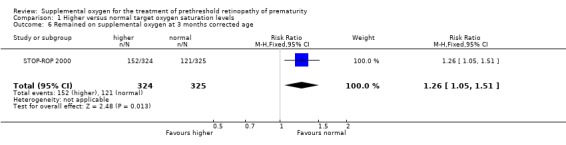
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 6 Remained on supplemental oxygen at 3 months corrected age.
1.7. Analysis.
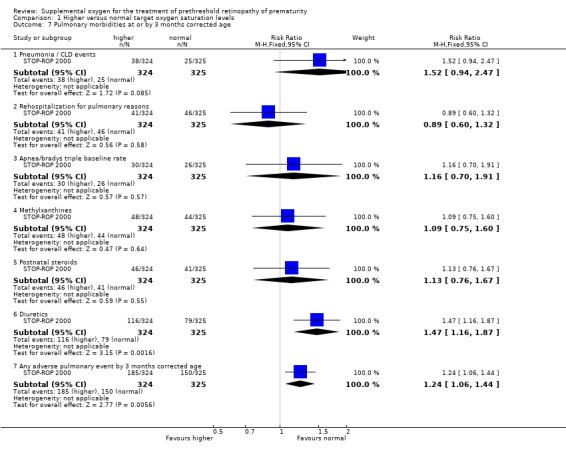
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 7 Pulmonary morbidities at or by 3 months corrected age.
1.8. Analysis.
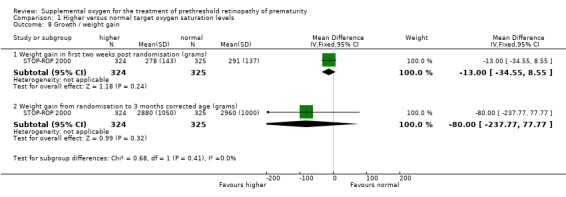
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 8 Growth / weight gain.
1.9. Analysis.
Comparison 1 Higher versus normal target oxygen saturation levels, Outcome 9 Sensitivity analysis for progression to threshold ROP.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
STOP‐ROP 2000.
| Methods | Random allocation ‐ yes, stratified by severity of disease and participating center. See Table 02. Concealed allocation ‐ yes Baseline comparablity ‐ yes Blind assessors ‐ yes, study‐certified ophthalmologists who assessed eligibility, progression of ROP and study endpoints were blinded to treatment allocation by covering the pulse oximeter and computer during examination. Blind parents/guardians ‐ no Blind bedside nurses and attending neonatologists ‐ no Adequate follow‐up ‐ yes (> 90%) Length of follow‐up ‐ 3 months corrected age (52 weeks post menstral age ‐ target of 50 ‐ 54 weeks PMA). Some infants (number not stated) were examined after 54 weeks PMA. Intention to treat analysis ‐ no for eye endpoints (corrected in the review), yes for other outcomes Between group comparisons ‐ ?no Point estimates and variablility ‐ yes Eligibility criteria ‐ yes | |
| Participants | Study enrollment occurred between February 1994 and March 1999. 1213 Premature infants (definition not stated) with prethreshold ROP in at least one eye confirmed by a second ophthalmologist were potentially eligible for the study. For definitions of threshold and prethreshold ROP used in the STOP‐ROP study see Additional Table 01. Infants were excluded if after 4 hours continuous monitoring their median pulse oximetry saturations were greater than 94% in room air or they had lethal anomalies or congenital anomalies of the eye. 649 infants were enrolled in the trial and randomised to the control or experimental group. The mean BW was 721g and 731g in the control and experimental groups respectively. The mean gestational age was 25.4 weeks in both groups. The ranges for gestational age or birthweight were not stated. The mean PMA at randomisation was 35.6 weeks. | |
| Interventions | Infants were placed on continuous pulse oximetry monitors (Ohmeda 3740) to maintain saturations between 89% and 94% (n = 325) in the control group and 96% to 99% (n =324) in the experimental group. Laptop computers were connected to the pulse oximeters to monitor, record and report trends in oxygen saturation for each infant. It was the intention of the study authors that the intervention start within 24 hours of the diagnosis of prethreshold ROP. However only 33% of infants were randomised less than 24 hours after diagnosis and 27% were not randomised until more than 48 hours after diagnosis. The enrolled infants were examined weekly until both eyes reached ophthalmic endpoints and again at 3 months PMA. Target saturation levels were maintained for 2 weeks even if primary ophthalmic outcomes were reached sooner. After 2 weeks assigned treatment ceased after both eyes reached primary ophthalmic outcomes. Parents were permitted to take infants home on study equipment. At least one of the 2 study ophthalmologists for study entry in each centre had to be certified by the STOP‐ROP study. For adverse eye endpoint, both examiners had to be certified. | |
| Outcomes | Progression to threshold ROP Weight gain Length gain Head circumference increase PMA at discharge home PMA to achieve oral feeding Number of infants with CLD/pneumonia, sepsis and apnoea or bradycardia. Outcomes at 3 months PMA:‐ number of infants hospitalised, rehospitalised, rehospitalised for pulmonary reasons,on study equipment, on oxygen, on steroids, on diuretics, on methylxanthines, all deaths, room saturations too low to test, room air oxygen saturations, developmental assessment | |
| Notes | Intially a sample size of 880 infants was required to provide 90% power with an overall type 1 error rate of 0.025 to detect a one third reduction in pregression to threshold disease or a 10% absolute reduction based on a predicted rate of progression in the control arm of 30%. Power calculations were revised due to poor enrollment after 3.3 years. It was calculated that an enrollment of 633 infants completing the study would provide 83% power to detect a fall in progression rate from 30% to 20%. The final number of 597 infants with ophthalmic endpoints resulted in a power of approximately 80% to detect a fall in rate of progression of 10%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gaynon 1997 | This was a non‐randomised retrospective cohort study. Oxygen saturation levels for infants with prethreshold ROP were increased in a stepwise fashion (target minimum 92%‐99%) over a number of years (1985‐1993). Patient allocation was not randomised and thus the study was excluded from the review. |
| Seiberth 1998 | Preterm infants with stage 3 ROP between 1994 an 1996 were treated with supplemental oxygen to maintain pulse oximetry saturations above 98%. The incidence of threshold ROP was compared to a historical control group of preterm infants born between 1991 and 1993. Patients were not randomly allocated to a treatment and therefore this non‐randomised, retrospective cohort study was excluded from the review. |
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
STOP‐ROP 2000 {published data only}
- The STOP‐ROP Multicenter Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP‐ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 2000;105:295‐310. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gaynon 1997 {published data only}
- Gaynon MW, Stevenson DK, Sunshine P, Fleisher BE, Landers MB. Supplemental oxygen may decrease progression of prethreshold disease to threshold retinopathy of prematurity. Journal of Perinatology 1997;17:434‐438. [PubMed] [Google Scholar]
Seiberth 1998 {published data only}
- Seiberth V, Linderkamp O, Vardarli I, Jendritza W, Vogele C, Knorz MC. Oxygen therapy in acute retinopathy of prematurity Stage 3. Investigative Ophthalmology and Visual Science 1998;39:S820. [Google Scholar]
Additional references
Adamkin 1977
- Adamkin DH, Shott RJ, Cook LN, Andrews BF. Nonhyperoxic retrolental fibroplasia. Pediatrics 1977;60:828‐30. [PubMed] [Google Scholar]
Aranda 1974
- Aranda JV, Sweet AY. Sustained hyperoxemia without cicatricial retrolental fibroplasia. Pediatrics 1974;54:434‐7. [PubMed] [Google Scholar]
Askie 2002
- Askie LM, Henderson‐Smart DJ. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001077] [DOI] [PubMed] [Google Scholar]
BOOST 2002
- Askie LM, Henderson‐Smart DJ, Irwig L, Simpson JM. The effect of differing oxygen saturation targeting ranges on long term growth and development of extremely preterm, oxygen dependent infants: the BOOST Trial [abstract]. Pediatric Research. 2002; Vol. 51:378A.
Chan‐Ling 1995
- Chan‐Ling T, Gock B, Stone J. Supplemental oxygen therapy: basis for noninvasive treatment of retinopathy of prematurity. Investigative Ophthalmology and Visual Science 1995;36:1215‐30. [PubMed] [Google Scholar]
Cross 1973
- Cross KW. Cost of preventing retrolental fibroplasia. Lancet 1973;2:954‐6. [DOI] [PubMed] [Google Scholar]
CRYO‐ROP 1988
- CRYO‐ROP Cooperative Group. Multicentre trial of cryotherapy for retinopathy of prematurity. Archives of Ophthalmology 1988;106:471‐9. [DOI] [PubMed] [Google Scholar]
CRYO‐ROP 1994
- CRYO‐ROP Cooperative Group. The natural ocular outcome of premature birth and retinopathy: status at 1 year. Archives of Ophthalmology 1994;112:903‐12. [DOI] [PubMed] [Google Scholar]
Donoghue 2002
- Donoghue D, ANZNN. The report of the Australian and New Zealand Neonatal Network 2000. Sydney: ANZNN, 2002:34. [Google Scholar]
ETROP
- Good WV, Hardy RJ. The multicenter study of early treatment for retinopathy of prematurity (ETROP). Ophthalmology 2001;108:1013‐4. [DOI] [PubMed] [Google Scholar]
Frank 1985
- Frank L. Effects of oxygen on the newborn. Federation Proceedings 1985;44:2328‐34. [PubMed] [Google Scholar]
Grieve 1997
- Grieve SH, McIntosh N, Laing IA. Comparison of two different pulse oximeters in monitoring preterm infants. Critical Care Medicine 1997;25:2051‐4. [DOI] [PubMed] [Google Scholar]
ICROP 1984
- ICROP. An International Classification of Retinopathy of Prematurity. Pediatrics 1984;74:127‐33. [PubMed] [Google Scholar]
Kaiser 2001
- Kaiser RA, Trese MT. Iris atrophy, cataracts and hypertony following peripheral ablation for threshold retinopathy of prematurity. Archives of Ophthalmology 2001;119:615‐7. [PubMed] [Google Scholar]
Laser ROP 1994
- The Laser ROP Study Group. Laser therapy for retinopathy of prematurity. Archives of Ophthalmology 1994;112:154‐6. [DOI] [PubMed] [Google Scholar]
Phelps 1988
- Phelps DL. Reduced severity of oxygen‐induced retinopathy in kittens recovered in 28% oxygen. Pediatric Research 1988;24:106‐9. [DOI] [PubMed] [Google Scholar]
Pierce 1996
- Pierce EA, Foley ED, Smith LEH. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Archives of Ophthalmology 1996;114:1219‐54. [DOI] [PubMed] [Google Scholar]
Reese 1953
- Reese AB, King M, Owens WC. A classification of retrolental fibroplasia. American Journal of Ophthalmology 1953;36:1333‐5. [PubMed] [Google Scholar]
Watts 1992
- Watts JL. Retinopathy of prematurity. In: Sinclair JC, Bracken MB editor(s). Effective care of the newborn infant. New York: Oxford University Press, 1992:617‐38. [Google Scholar]


