Abstract
Traditional anti-cancer treatments are inefficient against glioblastoma, which remains one of the deadliest and most aggressive cancers. Nano-drugs could help to improve this situation by enabling: (i) an increase of anti-glioblastoma multiforme (GBM) activity of chemo/gene therapeutic drugs, notably by an improved diffusion of these drugs through the blood brain barrier (BBB), (ii) the sensibilization of radio-resistant GBM tumor cells to radiotherapy, (iii) the removal by surgery of infiltrating GBM tumor cells, (iv) the restoration of an apoptotic mechanism of GBM cellular death, (v) the destruction of angiogenic blood vessels, (vi) the stimulation of anti-tumor immune cells, e.g., T cells, NK cells, and the neutralization of pro-tumoral immune cells, e.g., Treg cells, (vii) the local production of heat or radical oxygen species (ROS), and (viii) the controlled release/activation of anti-GBM drugs following the application of a stimulus. This review covers these different aspects.
Keywords: nanoparticle, nanomedicine, glioblastoma, GBM, oncology, nanotechnology, tumor targeting, enhanced permeability and retention (EPR), blood brain barrier
1. Introduction
Glioblastoma is one of the most aggressive and difficult to treat cancers. It is characterized by a life expectancy following diagnosis of only 12–18 months [1]. Standard treatments are ineffective for a number of reasons, such as the incapacity of surgery to remove all glioblastoma multiforme (GBM) tumor cells, notably the infiltrative ones, the difficulty for chemo-therapeutic drugs to reach the tumor, due to the blood brain barrier (BBB) that prevents them from diffusing towards the tumor, and the limitations of radiotherapy, which cannot easily eradicate radio-resistant GBM cells, notably stem cell ones. To these difficulties, the very particular location of GBM should be added, which makes it difficult to eradicate GBM cells while avoiding damaging healthy brain cells.
To overcome these hurdles, the use of nanoparticulate anti-GBM drugs has been suggested. The interest of nano-formulated drugs for cancer treatment has been reviewed elsewhere [2,3,4]. These drugs first improve the targeting of tumor cells by: (i) promoting the drug diffusion through the blood brain barrier, (ii) specific tumor targeting mechanisms relying on an enhanced permeability and retention (EPR) effect, with molecules attached to nano-drugs that bind tumor cell receptors, and diffusion of these nano-drugs towards the tumor by application of a magnetic field gradient, and (iii) a homogeneous distribution of anti-GBM drugs within the tumor, notably using convection enhanced delivery. Nano-formulations also increase the efficacy of anti-GBM drugs through multiple mechanisms of antitumor activities, such as: (i) improved efficacy of chemo/gene therapeutic drugs, in particular by promoting cellular internalization of these drugs, (ii) a radio-sensitizing effect, which increases the efficacy of radio-therapy against GBM tumor, (iii) immune mechanisms relying on activation of anti-tumor immune cells, e.g., T cells, NK cells, and/or deactivation of pro-tumor immune cells, e.g., Treg cells, (iv) destruction of angiogenic blood vessels, (v) local production of heat or radical oxygen species (ROS), (vi) illumination of GBM tumor border to ease GBM removal by surgery, (vii) a Trojan horse method in which anti-tumor drugs enter GBM by escaping the monitoring/defense system of the tumor, and (viii) restoration of the GBM cell death apoptotic pathway. The purpose of this review is to describe these different anti-GBM nano-drugs and their mechanisms of action, and to highlight their advantages compared with non-nanoparticulate systems. This review is broader in scope than previous ones [4,5,6], which focus on nanoparticle (NP) BBB penetration and specific types of nanomaterials (NM). It describes more types of NM, in particular metallic ones, which can play an essential role in fighting against GBM disease.
2. Different Types of Nano-Systems for GBM Treatment
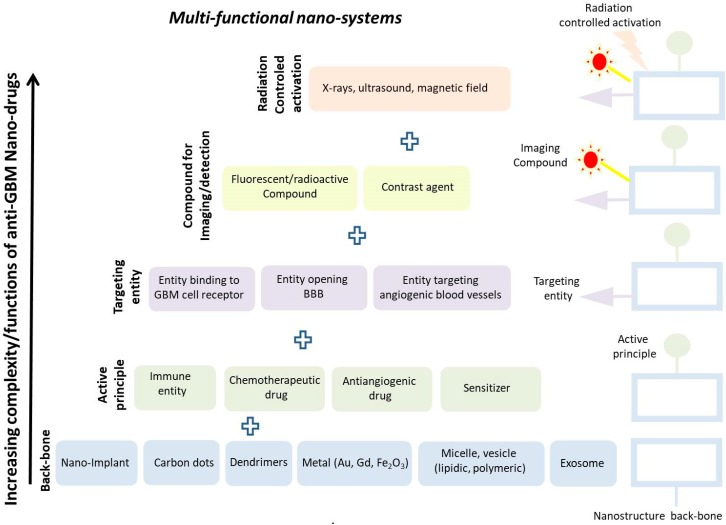
Nano-systems are often used or presented as nanometric platforms with a backbone made of various elements such as vesicles (lipidic, micellar, polymeric or exosomes), linear polymers, metals (Au, Gd, graphene), carbon dots, nano-implants, dendrimers [7], inside or at the surface of which are inserted both active anti-GBM principles such as immune cells, chemotherapeutic/anti-angiogenic drugs or sensitizers, as well as moieties, which either target GBM cellular receptors/angiogenic blood vessels or open the BBB, and therefore help the active principles to reach GBM tumor cells [8]. These nano-systems can also be associated with fluorescent/radioactive compounds to enable their localization in the organism [9]. On top of that, it may in some cases be possible to activate these nano-systems on demand by deciding to apply (or not) an external source of energy such as X-rays, ultrasounds, or alternating magnetic field [10]. Figure 1 summarizes how such structures are built up, while Table 1 presents various nano-systems used for GBM treatment, with their backbone composition, functionalization/coating, mechanism of action, and efficacy demonstrated in vitro and/or in vivo. To the knowledge of the author, nano-systems are the only structures that combine so many different functionalities within a single drug unit, explaining the surge of interest that they have triggered. As an example, a nano-complex comprising a nanoparticulate backbone (PAMAM) associated with a targeting agent (RGD) and a chemotherapeutic drug (ATO), designated as RGDyC-mPEG-PAMAM/ATO, led to a decrease in GBM tumor volume, which was about two and four times larger than that reached with PAM associated with ATO (mPEG-PAMAM/ATO) and free ATO, respectively [11]. However, the above presentation does not take into consideration the following considerations. First, the nanoparticle backbone can have in itself the various functionalities described above without needing additional compounds. This can be the case when the backbone produces anti-tumor activity through the generation of an immune response [12] or ROS [13] (e.g., following its dissolution in the organism), or when it targets the tumor by being directly administered in the tumor or by passively diffusing towards the tumor via the EPR effect [14], or when it acts as contrast agent due to its composition/size/crystallinity, as is the case for iron oxide nanoparticles [15]. Second, the multiple functions of nano-systems are interdependent with each other (e.g., anti-GBM activity directly depends on the targeting efficacy of these drugs). Third, the binding/interacting properties of the compounds with the nanoparticle backbone are often not examined, and in many cases it is possible that the observed anti-GBM activity is due to the nanoparticle backbone and not to the compound, which has either detached from the backbone or has been destroyed in vivo. Therefore, although nano-system multi-functionality is an enormous advantage that will certainly help improve anti-GBM drug efficacy, it is difficult to determine with certainty its origin and to associate it with the various activities of the multiple components that it comprises. It may therefore be advisable to explore the multiple functions of the nanoparticle backbone and foresee its use as a single component before adding multiple compounds to it, whose functions are difficult to establish with certainty and that cannot easily be integrated in a nanoparticulate formulation using a good manufacturing practice (GMP) pharmaceutical fabrication process. Such reasoning is supported by the results obtained with simple nanoparticulate systems lacking therapeutic/targeting agents, which yielded strong anti-GBM activity, as shown for purified magnetosomes introduced in mouse GBM tumors and exposed to several alternating magnetic field (AMF) applications, leading to full tumor disappearance in treated GBM-bearing mice [16].
Figure 1.
A schematic showing how a nanoparticulate system can be built up to include various functionalities to fight against GBM disease, such as: (i) a backbone, (ii) an active principle, (iii) a targeting moiety, and (iv) a compound used for imaging/detection.
Table 1.
A summary of in vitro/in vivo anti-glioblastoma multiforme (GBM) activities reported in the literature for various nano-drugs with different compositions, abilities to bypass (or not) the blood brain barrier (BBB), and mechanisms of actions. NA: not available. Iv: intravenous. It: intratumoral. In: intranasal; IONP: Iron oxide nanoparticle
| Nanoparticle Backbone Composition |
Size (nm) |
Functionalization Coating |
Bypass BBB | In Vitro Efficacy | In Vivo Efficacy | Mech. of Action | Admin. | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mesoporous silica | 220 | Curcumin + chrysin | NA | NA | NA | pH dependant release of curcumin/chrysin + cellular internalization |
In | [17] |
| Mesoporous Silica | 100–200 | PDA+Asn-Gly-Arg (targ: CD13) |
Yes | Cytotoxicity demonstrated on C6 cells | Increased survival in glioma bearing rates treated with NP | Increase accumulation in tumor tissue | Iv | [18] |
| Carbon nanotubes (CNT) | 100–200 | PEG | NA | Demonstrated on several cell lines (U87, U373, D54 NHA) | NA | Heat produced by CNT exposed to lIR aser | It | [19] |
| Carbon dots | 6–8 | NA | NA | Cytotoxicity on U87 cells | Increased survival of mice bearing U87 GBM tumors treated by CD exposed to IR laser | Heat produced by CD exposed to lIR aser | Iv | [20] |
| Carbon dots | 2–4 | transferrin (targeted ligand) + Epirubicin, temozolomide (anti-cancer drugs) |
NA | Cytoxicity demonstrated on SJGBM2, CHLA266, CHLA200 and U87 GBM cells | NA | Target specifically GBM cells Enhances the effect of chemotherapy |
NA | [21] |
| Iron oxide nanoparticle (IONP) | NA | PEI | NA | More cytotoxicity using MHT than exogenous heating | NA | Heat produced by IONP exposed to AMF | NA | [22] |
| IONP | 30–50 | Polyplex + BCNU | Yes | Cytotoxicity towards GBM cells | NA | Release of BCNU + internalization (intranasal admin) |
NA | [23] |
| Fe3O4/Gd2O3 | 7 | Cisplatin + lactoferrin + RGD | Yes | Cytotoxicity towards GBM cells | Increased survival of U87-Luc bearing mice treated with NP | Internalization in cancer cells and release of Fe2+, Fe3+ (Fenton reaction favored by cisplatin) | Iv | [24] |
| IONP | 55 | Chitosan | NA | Cytotoxoicity towards GBM cells (C6 and U87) | NA | Accumulation of NP in tumor following iv adminiistration | Iv | [25] |
| Magnetosome (IONP) | 40 | PEI, chitosan, neridronate | NA | Cytotoxicity demonstrated on GL261 and RG2 cells | NA | Heat produced by magnetosomes exposed to AMF | NA | [26] |
| Magnetosome (IONP) | 40 | Poly-L-lysine, citric acid, oleic acid, CMD | NA | Cytotoxicity demonstrated on GL261 and RG2 cells | NA | Heat produced by magnetosomes exposed to AMF | NA | [27] |
| Nanoparticle backbone composition |
Size (nm) |
Functionalization Coating |
Bypass BBB | In vitro efficacy | In vivo efficacy | Mech. of action | Admin. | Ref. |
| Au NP | 40 | Fe3O4 + DNA | NA | Cytoxicity demonstrated on C6 cells | Decreased tumor growth in mice bearing C6 tumor treated by NP exposed to laser | Heat produced by NP exposed to laser + gene therapy | Iv | [28] |
| Au NP | 20–35 | Ala-Ala-Asn- Cys-Lys or 2-cyano-6-aminobenzothiazole modified AuNPs + DOX |
NA | Cytotoxicity on C6 cells | Increased survivavl for mice treated with NP | Nanoparticle aggregation that blocks exocytosis and nanoparticle backflow in blood stream | Iv | [29] |
| Au NP AuNRs@SiO2 |
75 | RVG29; PEG | yes | Cytotoxicity towards N2a cells | Tumor growth delay in mice bearing Na2 tumors | Photothermal therapy (NP mimicking virus) Bypass BBB through interaction between RVG29 and AchR |
It | [30] |
| Au | 35 | RGD | NA | NA | Enhanced accumulation in brain due to RGD | NA | Iv | [31] |
| Au | 5 | DNA | NA | Cytotoxicity towards CSC-like U251MG-P1 cells and GBM U251MG cells in the presence of radiation | NA | Radiosensitize GBM stem cells (enhancement of abnormal nuclei) |
NA | [32] |
| Au | 37 | Silica coated | yes | In vitro uptake of NP in U87-MG | In vivo delineation of glioblastoma | Endocytosis by tumor associated macrophages. Enables delineation of GBM tumor margin by fluorescence | Iv | [33] |
| Au | 30 | Irridium inserted inside NP | NA | Cytotoxicity towards U87 Luc cells at very low NP concentration (< 0.5 µM) due to combined PTT/PDT | NA | Combination of cell imaging/ PTT/ PDT | NA | [34] |
| Au | 130 | Albumin | NA | NA | Decrease in tumor growth in mice bearing N2a tumor | Combination of: - albumin for biocompatibility - Gold for photothermy (passive targeting) |
Iv | [35] |
| Au + IONP | 30 | Chitosan+miRNA + TMZ+PEG-T7 peptide | Yes (in admin) |
NA | NP treatment leads to: (i) accumulation of miRNAs in GBM tumor; (ii) increased survival of mice bearing GM tumor | Increased activity of miRNA + TMZ | In | [36] |
| Gd (AGuIX) |
3 | polysiloxane | NA | NA | Improved survivakl of rats bearing 9L GBM treated with AGUIX + RT | Improves delineation of GBM tumor; | iv | [37] |
| Nanoparticle backbone composition |
Size (nm) |
Functionalization Coating |
Bypass BBB | In vitro efficacy | In vivo efficacy | Mech. of action | Admin. | Ref. |
| Liposome (nano) | <50 | Docetaxel | NA | Increased cytotoxicity on C6 glioma cells compared with free drug | NA | Increased DTX accumulation in brain compared with free drug | NA | [38] |
| Liposome (nano) | 100–135 | DOPA + DNA |
NA | Cellular uptake in GBM cells via receptor LAT1 | Increaed survival of mice treated with liposomes | Chemotherapy + immunotherapy | Iv | [39] |
| Liposome (thermo-responsive) | < 270 | PCTX | Yes | Cytoxicity towards GBM cells increases between 37 and 39°C | NA | Release of drugs with increasing temperature | NA | [40] |
| Liposome | 50 | cyclic peptide iRGD + siRNA-EGFR + siRNA-PD-L1 | Yes | Cytoxicity towards U87 and GL-261 cells | Increased survival of mice bearing GL-261 glioblastoma tumors. | Increased targeting (radiotherapy + RGD) + chemotherapy + immunotherapy | Iv | [41] |
| Liposome + IONP |
50–100 | Temozolomide | Yes | Cytotoxicity towards U87-Luc cella | NA | Heat under AMF application + drug release | NA | [42] |
| Liposome | 100–150 | RGD + TMZ + Vincristine | NA | Cytotoxicity towards U87 and T98G GBM cells | Tumor growth deay in mice tbearing U87 GBM tumors reated with NP | Specific targerting of GBM cells + drug release | Iv | [43] |
| Liposome | 100–150 | Ursolic acid + EGCG + MAN | Yes | Cytotoxicity towards C6 GBM cells | Inhibition of GBM C6 tumor growth | EGCG induce apoptosis of GBM cells. MAN for targeting. UA, anti-cancer drug. | Iv | [44] |
| Liposome | 121 | TMZ | NA | Cytotoxicity towards CNS-1 GBM cells | Increase survival of GBM bearing rats (lipsomal formulation more efficient than free TMZ) | Increases the anti-tumor efficacy of TMZ | it | [45] |
| Nanoparticle backbone composition |
Size (nm) |
Functionalization Coating |
Bypass BBB | In vitro efficacy | In vivo efficacy | Mech. of action | Admin. | Ref. |
| Aptamer (ssDNA) | NA | DOX | NA | Aptamer causes more inhibition on targeted cells A-172 than non-targeted cells MCF-7. |
NA | Selective targeting of GBM cells | NA | [46] |
| Dendrimer (3G3) | NA | Curcumin | NA | NP internalized inside tumor cells selectively within nuclei. | NA | Selective cytotoxicity towards GBM cells | Iv | [47] |
| Dendrimer | NA | Arg-Gly-Asp (RGDyC) + αvβ3 integrin targeting ligand + PEG + ATO | yes | More cytotoxic than free ATO on C6 cell lines | RGDyC-mPEG-PAMAM could enhance the antitumor of ATO to glioma, it provides a desirable strategy for targeted therapy of glioma. | selective release of ATO at acidic pH | Iv | [11] |
| Polymer (PLGA) | NA | Chlorotoxin + Morusin | NA | Cytotoxicity towards GI-1 and U87 cell lines. | NA | Specifically target chloride channels (CIC-3) and matrix metalloproteinase (MMP-2), present in GBM cells/environment. Cytoxicity through ROS production | NA | [48] |
| Polymer (PLGA) | 100 | Nano-graphene + 5-iodo-2-deoxyuridine (IUdR) |
NA | NP cause damage towards U87MG cell line in the presence of Xray (6 MV) and NIR laser. |
NA | Reduced the plating efficiency of the cells Specific targeting of GBM cells |
NA | [49] |
| Polymer (albumin) |
150 | paclitaxel and fenretinide | yes | Cytotoxicity towards U87 cells | Tumor growth delay and increased survival in mice bearing U87 GBM tumors. | Croosing of BBB Release of PTX |
Iv | [50] |
| Polymer (poly(amine-co-ester) terpolymer) |
NA | BBB modulator Lexiscan, NECA, minoxidil) |
yes | NA | Increased survival of mice bearing intracranial GL-261 GBM | Accumulation in brain tumor and trigger gene therapy/chemotherapy | Iv | [51] |
| Polymer (PLGA-PLA-PCL) (nano-implant) |
NA | TMZ | NA | NA | Increased survival of rats bearing GBM | Release of TMZ under laser excitation | NA | [52] |
| Polymer (Methylene Blue Oleate Salt-Loaded Polymeric NP) |
170 | Methylene blue | Yes | NPs inhibit U87 and T98G cells | NPs bypass BBB more efficientlt than free drugs. | Drug release in GBM tumor | iv | [53] |
| Nanoparticle composition |
Size (nm) |
Functionalization Coating |
Bypass BBB | In vitro efficacy | In vivo efficacy | Mech. of action | Admin. | Ref. |
| Micelle (PEtOz-SS-PCL) |
100–150 | DOX | yes | Cytotoxicity towards C6 cells | Efficacy shown on orthotopic C6-Luci cells-bearing mice | Overcomes BBB and enhances DOX effect (release of DOX inside cells) | Iv | [54] |
| Micelle | 80 | BCNU + T7 peptide | yes | Cytotoxicity towards U87 GBM cells | Increased survival of mice bearing U87 GBM tumors | Accumulation of NP in tumor Increase in drug efficacy BCNU |
Iv | [55] |
| Micelle (polymeric) |
25 | RI-VAP + D-VAP (targeting); paclitaxel (drug) | na | Cytotoxicity towards U87 and HUVEC cells | Delays tumor growth of mice bearing U87 GBM tumors | Targets tumors + release drug | Iv | [56] |
| Micelle | NA | panobinostat | Yes | Cytotoxicity towards F98, MO59K and U87-MG GBM cells | Increased survival of rats bearing GBM tumors | Inhibition of pan-histone deacetylase enhanced by NP | it | [57] |
| Micelle (FA-PEG-PCL) |
20 | luteolin | yes | Enhanced growth inhibition and more apoptosis of GL261 cells with NP | Increases survival of mice bearing GL261 GBM tumor | Increases the effect of luteolin | NA | [58] |
| Micelle (MPEG-PCL) |
50 | luteolin | NA | Luteolin/MPEG-PCL micelles had stronger cytotoxicity and induced a higher percentage of apoptosis in C6 and U87 cells than free luteolin Apotosis induced through mitochondrial pathway |
Tumor growth delay in mice bearing C6 GBM tumors. | release of luteolin Luteolin/MPEG-PCL micelles induced more glioma cell apoptosis than free luteolin and inhibited neovascularization in tumor tissues |
Iv | [59] |
| Micelle (Au+IONP) |
PEG | NA | Increase in cell DNA damage when GBM cells are incubated with NP and irradiated at 4 Gy | Possibility to image tumor border by MRI (T2 contrast) | Radiosensitization: increase in DNA breaks when NP irradiated. | iv | [60] | |
| RNA (+ MNP) |
10 | PEI | NA | No cytotoxicity | NA | Immune response against the tumor | NA | [61] |
| RNA (+ lipoprotein) |
20–40 | none | yes | Cytotoxicity towards C6 cells | Improved survival on mice bearing patient derived GICs glioblastoma | RNA-interfering efficiency, increases glioblastoma cell apoptosis |
Iv | [62] |
| miRNAs (+ polymer NP) |
100 | none | NA | Cytotoxicity towards GBM cells | Treated mice bearing GBM have long term survival | Increase the efficacy of radiotherapy | It | [63] |
| miRNA (+ nanogel) |
200 | polyglycerol | NA | Cytotoxicity towards U87-Luc cells | Tumor growth delay observed in mice bearing xenograft U87 GBM tumors. | Gene targeting responsible for tumor cell suppression | It | [64] |
| RNAi (+ liposome) |
NA | NA | NA | Reduction of GBM tumor sphere formation by NP | Prolonges survival of mice bearing GBM tumors | Target brain tumor-initiating cells | it | [62] |
The activity of such nano-drugs can also in some cases be controlled by the application of an external source of energy (X-rays, ultrasound, magnetic field).
To be usable on humans, nanoparticles should be stable and release their active principle in a controlled fashion, two aspects that necessitate some optimization.
3. Nano-Drugs Improve GBM Drug Delivery
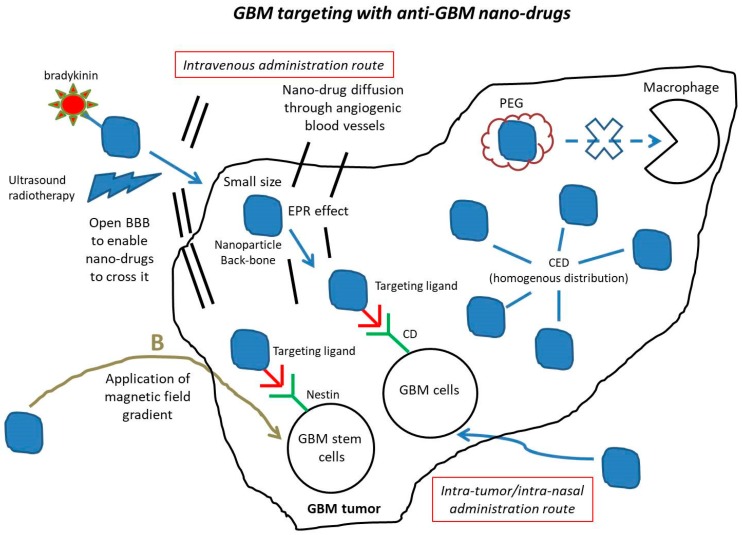
It is believed that anti-GBM drugs are not fully effective due to the existence of a series of barriers that prevent them from reaching these tumors. Such barriers include the BBB that blocks the entrance of drugs into GBM tumors, macrophages that can capture anti-GBM drugs, and anti-GBM drugs lacking a specific targeting mechanism to reach/bind GBM tumor cells, in particular GBM infiltrating/stem cells that play a major role in the development of this disease. In order to overcome these barriers, a series of strategies have been proposed, which are summarized below and in schematic Figure 2.
Figure 2.
A schematic figure showing the various mechanisms by which anti-GBM nano-drugs can target the GBM tumor.
First, an intratumor administration route could be used to enable anti-GBM drugs to directly reach GBM tumors without having to cross the BBB. On the one hand, this can be achieved by injecting these drugs with a syringe or catheter at tumor location. It has been shown that nano-drugs administered in this way can remain in the tumor for a sufficiently long time to efficiently destroy the tumor (i.e., about one month) [16,65]. By contrast, intratumor administration of non-nano-formulated drugs is largely inefficient, resulting in drugs being either metabolized, eliminated [66], or diffusing towards several locations surrounding GBM tumors such as ventricles, subarachnoid space, cerebrospinal fluid or blood [67]. On the other hand, intratumor administration can be carried out with the help of convection enhanced delivery (CED), a technique following which anti-GBM drugs could diffuse at precisely controlled infusion rates towards GBM tumors under a hydrostatic pressure gradient using microcatheters implanted inside the tumor. While this method was reported to be largely inefficient to administer non-nano-formulated anti-GBM drugs in GBM tumors, it yielded for nano-formulated ones a homogenous distribution in the tumor and a half-life and diffusion volume larger than those of free drugs. These properties resulted in an enhanced anti-tumor efficacy, through sustained release of an active principle such as carboplatin from a nano-structure such as poly(lactic-co-glycolic acid) (PLGA) copolymer [68]. Furthermore, it has been shown that CED could result in antitumor efficacy at a much smaller dose of nanoparticles than both non-CED intratumor injection [69], and intravenous administration [70], an aspect that should receive full consideration given the high toxicity profile of chemotherapeutic drugs used for GBM treatments [71].
Second, another method to enable anti-GBM drugs reaching GBM tumors without being stuck by the BBB, consists of injecting these drugs by an intranasal route as carried out with Polyplex coated magnetite (Nano-co-Plex) loaded with BCNU or T7-targeted polyGIONs, enabling the active principles, miRNAs or BCNU, to efficiently reach GBM tumors [23,36].
Third, by applying MRI-guided focused ultrasounds [72] on the BBB, or by administering bradykinin [73,74], it has been possible to open or weaken this barrier and let anti-GBM drugs diffuse through it.
Fourth, passive targeting, also designated as EPR effect, can let the anti-GBM drugs diffuse through the holes of the blood vessels irrigating GBM tumors. To optimize the efficacy of this targeting, nano-drugs can either be coated with a substance such as polyethylene glycol (PEG) to prevent their capture by macrophages [75], or be of sufficiently small size (i.e., typically below 5 nm) to enable their diffusion through the holes of the angiogenic blood vessels [76].
Fifth, active targeting can be achieved by attaching substances to nanoparticles that specifically target a part of GBM tumor cells [6], such as an antigen (i.e., A2B5), differentiation clusters (i.e., CD15, CD33, CD44, or CD133), receptors of cytokines (i.e., interleukin13 receptor), and several proteins (i.e., Integrin-a6, α5β3, ανβ3 or L1CAM), which are expressed in GBM cells and can promote tumorigenesis. Examples of such substances include CTX, Pep-1, CBP4 and RGD, targeting CIC-13 chloride channel/matrix metalloproteinase (MMP-2), Interleukin13 receptor, CD33 and α5β3/ανβ3, respectively [48,71,77].
Sixth, magnetic targeting is an interesting concept, which relies on the application of a magnetic field directly on magnetic anti-GBM drugs, to trigger the diffusion of these drugs towards the tumor. Although this approach has shown some efficacy on small animals (e.g., magnetic anti-GBM drugs accumulated in rat glioblastoma following the application of a 0.5 T magnetic field [78]), its translation for human applications faces certain difficulties. On the one hand, equipment that generates a sufficiently strong and precise magnetic field gradient to trigger the diffusion of anti-GBM drugs towards tumors is currently lacking. On the other hand, the existence of many biological mechanisms, such as those of the immune system, blood flow, solid tissue resistance and cellular internalization, makes it difficult to model the diffusion of nano-drugs in vivo by only taking into consideration the properties of the applied magnetic field.
Seventh, one of the most important aspects to consider in the treatment of GBM is the targeting of GBM stem/infiltrating cells. Indeed, the failure of conventional therapies against this disease largely comes from their inability to target such cells. By functionalizing Gold nanorods with Nestin, which has been identified as a marker of these cells, it has been possible to selectively internalize gold nanorods in these cells [79,80].
Regarding the delivery of drugs with the help of nanoparticles, one of its most delicate aspects, which requiring further studies, concerns the preservation of the drug efficacy during its transport to the tumor.
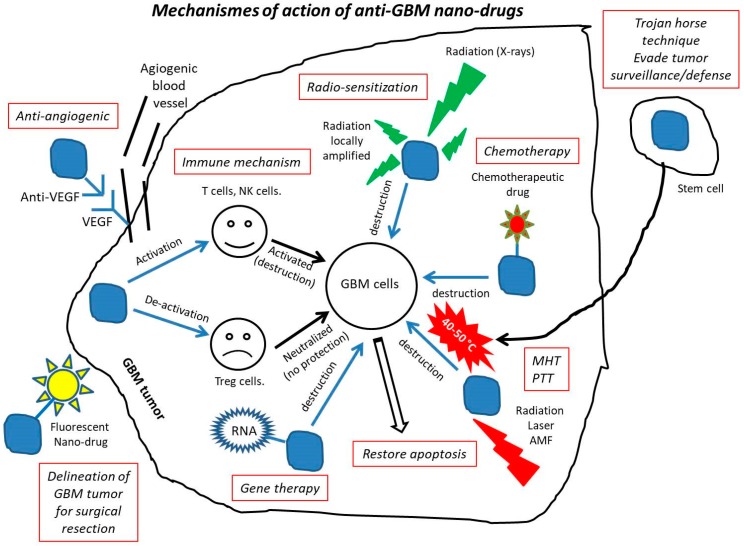
4. Mechanisms of Action of Anti-GBM Nano-Drugs
The different mechanisms of action of anti-GBM nano-drugs are summarized in Figure 3. Surgery, which is the first line of treatment recommended for removing operable GBM, can be improved by nano-therapy. Although this treatment enables removing a large part of the tumor, complete tumor surgical resection is almost impossible due to the infiltrative nature of GBM cells into surrounding normal brain tissues. Among the different methods suggested to improve the efficacy of surgery, the specific imaging of infiltrating GBM cells seems promising, since it enables visualizing them, which is a prerequisite for being able to remove them entirely. A series of different types of nano-systems have been developed to make this imaging method efficient. They work through: (i) the imaging of tumor-associated macrophages using near-infrared fluorescent silica coated iron oxide nanoparticles leading to the delineation of GBM tumor [33], (ii) a combined MR/fluorescent imaging method using targeting nanoparticles containing both MRI contrast (Gd-DTPA) and fluorescent dye (5-ALA) molecules [81], (iii) a nanostructure comprising indocyanine green (ICG) for NIR fluorescence and the trileucine peptide as fluorescence enhancer resulting in an intensive and long-lasting tumor fluorescence of human U87MG glioblastoma in mice and in an improved removal of these tumors [82].
Figure 3.
A schematic figure presenting the various mechanisms of action of anti-GBM drugs.
Nano-formulated anti-GBM drugs can also improve the efficacy of chemotherapy by increasing the activity of various anti-tumor drugs, such as: (i) secondary metabolites of algae, which induced cytotoxicity towards A-172 glioblastoma cells when loaded in nano–microparticles [83], (ii) arsenic trioxide, which enabled treating temozolomide (TMZ)-resistant GBM cells following their encapsulation in liposomes in the presence of Mn [84], (iii) carboplatin, which led to higher tumor cytotoxicity, reduced neuronal toxicity and prolonged tissue half-life on rat and porcine GBM model when they were associated with PLGA copolymer [68], (iv) chlorotoxin, whose efficacy was increased compared with free drugs when they were conjugated with iron oxide nanoparticle, a behavior attributed to nanoparticles leading to a longer blood half-life, a better ability to cross the BBB and a faculty to be internalized in cells without losing its therapeutic activity [85], (v) cisplatin, which could move within the porous extracellular matrix between GBM cells when loaded with PEG-coated nanoparticles, yielding deeper brain penetration than non-pegylated cisplatin, and resulting in an increased survival of rats bearing GBM by more than two weeks compared with Cisplatin alone [66], (vi) curcumin, whose bioavailability and water solubility were increased when they were encapsulated in a dendrosome, suppressing U87MG cells growth without affecting healthy cells [86], (vii) doxorubicin, which led to remission among 20% of rats bearing GBM treated with DOX bound to polysorbate-coated nanoparticles [87], (viii) paclitaxel, which was more efficiently transported through the BBB and led to an improved anti-proliferative efficacy when it was associated with NPs and specific peptides Pep-1 designed to cross the BBB than when it was free [88], (ix) temozolomide (TMZ), which did not denature and could be specifically delivered to GBM cells with the help of the targeting peptide chlorotoxin (CTX), leading to enhanced cytotoxicity towards GBM cells compared with free TMZ [89].
Together with surgery and chemotherapy, radiotherapy is the third leg of GBM treatments. It has been shown that anti-GBM nano-formulations could enhance the effect of radiotherapy by: (i) increasing the downregulation of PD-L1 and EGFR using solid/lipid nanoparticles, resulting in a decrease of glioblastoma growth and prolonged mouse survival [41], (ii) enhancing the EPR effect, leading to better diffusion of nanoparticles to GBM tumors [90], (iii) sensitizing GBM cells to radiation by making GBM stem cells enter the radiation sensitive G2/M phase using the Sonic hedgehog ligand [91], by increasing DNA double-strand breaks using BSA–Au nanoparticles [92], or by exposing iodine nanoparticles to radiations [93], and (iv) enhancing the expression of the targets of CTX (i.e., MMP-2 and ClC-3), as well as BBB permeability and cellular internalization, leading to GBM tumor growth inhibition in vivo, using PLGA nanoparticles conjugated to chlorotoxin (CTX) [94]. The radio-sensitizing effect, which is often sought for when exposing nanoparticles to X-rays, is usually described as being optimal for nanoparticles of high atomic number Z, due to certain physical effects such as photoelectric ones, which are enhanced at high Z values. Such reasoning was used to justify the choice of nanoparticles composed of chemical elements with high Z values such as hafnium oxide. To be more complete, it should also probably take into account a whole set of other mechanisms that may occur during these nanoparticle/radiation interactions, such as other physical effects that could occur for low Z values (Compton effect), radiation amplification beyond nanoparticle surface, the formation of free radicals, nanoparticle distribution inside cells or in the extracellular matrix, nanoparticle interaction with biological material such as proteins, cell organelles, cell membranes and lipids, nanoparticle diffusion in liquids such as blood/cytoplasm or solids such as tissues, capture of nanoparticles by macrophages, stimulation of the immune system, and in vivo degradation, dissolution, disassembly or reassembly of nanoparticles.
Another commonly described mechanism of activation of anti-GBM nano-drugs relies on the release of such drugs under the application of various stimuli, such as:
Redox variations, which are due to a larger glutathione concentration inside than outside cells, where glutathione is responsible for cleavage of disulfide bonds [95], resulting in selective intracellular release of various chemotherapeutics from nanostructures (e.g., PTX from self-assembled nanoparticles [68] or DOX from polymeric micelles [54]).
pH, which is usually more acidic inside than outside the tumor, enabling the release of anti-GBM drugs from nanostructures when the linkers between them is acid-sensitive (e.g., DOX could be selectively released in tumors from polymeric micelles using poly(histidine) as linker [96]).
High intensity focused ultrasound, which can yield a higher accumulation of DOX in tumor tissue than in healthy tissue when applied on a nanostructure consisting of DOX attached to polymer [97].
Alternating magnetic field, which can trigger TMZ release from a lipid-based magnetic NM, and whose therapeutic activity against U87MG cells is enhanced by mild heating at 43 °C under these conditions of excitation [42].
Although these stimuli have been shown to increase anti-tumor efficacy in many studies, the mechanism by which the latter occurs is largely unknown, and drug release from the nanoparticle backbone, which is the most commonly brought forward explanation to account for such efficacy, has not been firmly demonstrated in vivo to the knowledge of the author.
When they are prepared in specific conditions (i.e., usually with a metal composition), nano-drugs can produce heat locally, essentially through the following two pathways:
Magnetic hyperthermia (MHT), in which magnetic nanoparticles are excited by an alternating magnetic field, producing localized nanoparticle heating, leading to anti-GBM efficacy in various GBM models (i.e., cellular, pre-clinical, and clinical ones) [98,99].
Photothermal therapy (PTT), where nanoparticles such as gold or iron oxide are exposed to near infra-red light (e.g., at 808 nm), absorbing this light and transforming it into localized heat through plasmonic effect, leading to the selective photothermal destruction of GBM cells at typical temperatures of 50–60 °C [35,100,101,102,103].
To foresee a widespread use of these techniques, MHT would first require installation of medically compatible induction coils in various hospitals, while PTT would need to overcome the drawbacks of low penetration depth and small exposed surface areas reached with the commonly used optical fibers to carry laser light.
In addition to heat, local perturbation can also be due to radical oxygen species (ROS) produced by anti-GBM nano-drugs. Most often, this effect is achieved using the photodynamic therapy (PDT) technique in which a photosensitizer such as porphyrin is exposed to laser light to generate ROS. The advantage of using the PDT technique in the presence of a nanoparticulate system comes from the control of ROS production that it enables (i.e., in theory ROS generation occurs at the nanoparticle location). As an example, mitochondrial-targeted photosensitizer-loaded albumin nanoparticles showed an enhanced cellular uptake and greater phototoxicity towards GBM cells than healthy cells in vitro, as well as a faculty to accumulate in GBM tumor and yield significant tumor suppression in a mouse GBM tumor model [104].
Another important type of anti-GBM treatment is gene therapy (RNA), which uses different types of RNA associated to nanoparticulate systems such as double-stranded RNA, siRNA, miR-101, resulting in an enhanced apoptosis of GBM cells as well as an inhibition of growth and migration of these cells through the targeting of specific genes (miR-34) or proteins (SOX9 or RAS) involved in the regulation/arrest of cellular pathways/cycles [61,64,105,106].
It has also been suggested to use the immune system to fight against GBM. Following this approach, nanotechnologies can yield certain improvements compared with non-nanoparticular treatments such as: (i) a better delivery in the tumor of immune entities such as checkpoint inhibitor antibodies to cytotoxic T-lymphocyte associated antigen 4 (a-CTLA-4) or programmed cell death-1 (a-PD-1), notably by enabling them to cross the BBB, resulting in an increase of T lymphocytes and NK cells, a decrease of regulatory T cells (Tregs), and an increased survival of GBM-bearing mice [107], (ii) the delivery of different immune entities either simultaneously [108] or within the immune-suppressive tumor microenvironment (TME), leading to an increase of CD8+ T cells when sHDL nano-discs associated with CpG were delivered in GBM animal tumors [109], and (iii) the switching of tumor-associated macrophages (TAM) from a pro-GBM to an anti-GBM activity, a behavior that was observed when TAM were loaded with nano-diamonds bearing doxorubicin [54].
Furthermore, nanotechnologies can help fighting against angiogenesis, a phenomenon in which blood vessels that irrigate GBM tumors are more numerous and abnormal compared with normal blood vessels, supporting the progression, infiltration and migration of GBM cells. This can be achieved by nano-drugs promoting the growth of new non-angiogenetic vessels [110], or neutralizing certain angiogenetic growth factors including members of the vascular endothelium growth factor (VEGF) family [111], Angiogenin [112], or Tetrac [113], in particular, to improve the results obtained with non-nanoparticulate formation containing certain anti-angiogenic compounds such as anti-VEGF that failed to show a therapeutic benefit in clinical trials (phase III) [114]. More specifically, it has been demonstrated that complexes formed by Angiogenin (ANG) and gold nanoparticles (AuNPs) resulted in anti-angiogenic behavior towards GBM tumor cells [112]. Nano-formulated Tetrac led to devascularization of GBM tumor vessels (i.e., a 95% loss of tumoral vascularity in mice bearing GBM xenografts) [103], while graphite/graphene nanoparticles yielded a decreased concentration of intracellular ROS and RNS, hence affecting a series of mechanisms involved in the promotion of angiogenesis [115].
Tumor microenvironment (TME) is known to prevent most anti-GBM therapies from being fully efficient, through very complex and yet partly unknown properties. Indeed, TME is not only characterized by the presence of angiogenesis, but also by hypoxia, mild acidity, specific redox reactions, high interstitial pressure and dense stromal structure. When a nanoparticle based anti-GBM therapeutic approach is considered, such properties should be fully taken into consideration, in particular for reaching a homogenous NP distribution, which can be strongly affected by TME properties [96,116,117]. Methods to control nanoparticle distribution/activity within TME should be developed to achieve efficient anti-GBM treatment with nano-drugs.
Due to their self-renewability, high tumorigenicity, infiltrative behavior and radio/chemo resistance, GBM stem cells are thought to be responsible for the recurrence/persistence of GBM disease. Several nanoparticle-based approaches have hence been proposed to eradicate these cells. They are based on: (i) an enhanced response to irradiation due to a faculty of internalization of certain nanoparticulate systems such as chitosan-capped gold nanoparticles, enabling concentrating anti-GBM drugs inside cells [118], or due to the inhibition of CSC specific pathways/receptors [119], (ii) reducing the tumor-propagating human cancer stem cells through intracellular delivery of anti-GBM drugs such as miRNA, specifically inside GBM CSC, using nanoparticle-drug complex such as bioreducible poly(β-amino ester) nanoparticles associated with miRNA [63], (iii) inhibiting autophagy, resulting in a decrease of stemness-associating genes (SOX2, POU5F1 and NANOG) using nanoparticles linked to PTX and chloroquine (CQ) [120], or (iv) preventing hemolysis induced by certain anti-GBM drugs and hence enhancing cytotoxicity towards GBM stem cells through the use of a nanoplatform consisting of chitosan nanoparticles associated with anti-hemolytic 1,3β-Glucan and paclitaxel as chemotherapeutic drug [121].
The Trojan horse technique consists of inserting foreign entities in tumors to destroy them while minimizing identification, detection or destruction of these entities by the tumor surveillance/defense system, where such system is most often described as being immunologic [107]. It can consist of immune-conjugates [107] or stem cells containing anti-GBM drugs [122], with the aim of improving the specific release of anti-GBM drugs in tumors.
A final consideration concerns apoptosis, a mechanism of cellular death that could be weakened among GBM tumor cells, possibly making these cells resistant to standard treatments [123]. With the help of nanoparticulate systems, it has been shown that apoptosis could be restored, for example by heating magnetic nanoparticles in the presence of GBM cancer cells under the application of an alternating magnetic field at mild temperatures of 40–50 °C [1,16,42].
Regarding the mechanisms of action, it is of common practice to classify them in different categories and to identify a dominant one, which is brought forward to explain the origin of NP anti-tumor activity. However, the reality is probably different, being a superposition of different mechanisms coexisting together.
To conclude, I have highlighted various mechanisms of nano-drugs against GBM. While the diversity of these mechanisms is a considerable advantage to promote the emergence of an efficient anti-GBM treatment, it also requires identifying the most efficient one. Indeed, such identification could enable the use of a single drug unit triggering an optimal anti-GBM mechanism, which would be easier to develop than a drug comprising multiple compounds designed to neutralize various GBM tumorigenic functions simultaneously. However, this necessitates standardized pre-clinical tests to be able to compare the efficacy of the different anti-GBM drugs under development worldwide.
Furthermore, I have explained that nano-therapeutics present a number of appealing features that could help fighting GBM disease such as: (i) the faculty of these nano-drugs to diffuse through the BBB, targeting GBM tumors by active or magnetic targeting and hence efficiently reaching GBM tumors, or (ii) an enhanced anti-tumor activity through various local mechanisms (e.g., stimulation of the immune system, the generation of ROS, or the excitation of these drugs by an external source of energy). However, some of the barriers to reach an efficient GBM treatment do not depend on the type of tested therapy. Indeed, they are due to: (i) a lack of preclinical models that are close enough to human GBM, (ii) the difficulty in carrying out clinical trials on a sufficiently large number of patients to reach statistical significance of the clinical data, (iii) the design of clinical trials that plan to treat GBM patients at a too advanced stage of the disease, and (iv) the too late detection of GBM disease. To develop an efficient treatment against GBM requires overcoming all these various hurdles. This is the reason why this task is so difficult.
Abbreviations
| AMF | Alternating magnetic field |
| ATO | Arsenic trioxide |
| Au | Gold |
| BBB | Blood brain barrier |
| BCNU | Carmustine |
| CED | Convection enhanced delivery |
| CQ | Chloroquine |
| CTX | Chlorotoxin |
| CSC | Cancer stem cells |
| DOX | Doxorubicin |
| EPR | Enhanced permeability and retention effect |
| Gd | Gadolinium |
| GBM | Glioblastoma multiforme |
| GMP | Good manufacturing practice |
| MHT | Magnetic hyperthermia, the application of an alternating magnetic field on magnetic nanoparticles |
| NK | Natural killer |
| PTX | Paclitaxel |
| PAM | Polyacrylamide |
| PAMAM | Poly(amidoamine) |
| PDT | Photodynamic therapy, the application of a laser light on a photosensitizer to produce ROS |
| PLGA | Poly(lactic-co-glycolic acid) |
| PEG | Polyethylene glycol |
| PTT | Photothermal therapy, the application of laser light on nanoparticles, producing heat through plasmonic effects |
| RGD | Arginylglycylaspartic acid peptide |
| RNS | Radical nitrogen species |
| ROS | Radical oxygen species |
| T cells | T lymphocytes |
| TMZ | Temozolomide |
| TME | Tumor microenvironment |
| VEGF | Vascular endothelium growth factor |
Funding
This research was funded by private investors of the start-up Nanobacterie, the BPI (“banque publique d’investissement”), the region of Paris (“Paris Région Entreprise”), the French Research Tax Credit program (“crédit d’impôt recherche”), the incubator Paris Biotech Santé, the ANRT (CIFRE 2014/0359, CIFRE 2016/0747, CIFRE 2013/0364, CIFRE 2015/976), the Eurostars programs (Nanoneck-2 E9309 and Nanoglioma E11778), the AIR program (“aide à l’innovation responsable”) from the region of Paris (A1401025Q), the ANR (“Agence Nationale de la Recherche”) Méfisto, the Nomis Foundation.
Conflicts of Interest
Edouard Alphandéry has been working in the start-up Nanobacterie.
References
- 1.Alphandéry E. Glioblastoma Treatments: An Account of Recent Industrial Developments. Front. Pharmacol. 2018;9:879. doi: 10.3389/fphar.2018.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahanban-Esfahlan A., Seidi K., Jaymand M., Schmidt T.L., Majdi H., Javaheri T., Jahanban-Esfahlang M., Zarei P. Dynamic DNA nanostructures in biomedicine: Beauty, utility and limits. J. Control. Release. 2019;315:166–185. doi: 10.1016/j.jconrel.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahanban-Esfahlan R., Seidi K., Jahanban-Esfahlan A., Jaymand M., Alizadeh E., Majdi H., Najjar R., Javaheri T., Zare P. Static DNA Nanostructures for Cancer Theranostics: Recent Progress in Design and Applications. Nanotechnol. Sci. Appl. 2019;12:25. doi: 10.2147/NSA.S227193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhoury M. Drug delivery approaches for the treatment of glioblastoma multiforme. Artif. Cells Nanomed. Biotechnol. 2016;44:1365–1373. doi: 10.3109/21691401.2015.1052467. [DOI] [PubMed] [Google Scholar]
- 6.Glaser T., Han I., Wu L., Zeng X. Targeted Nanotechnology in Glioblastoma Multiforme. Front. Pharmacol. 2017;8:166. doi: 10.3389/fphar.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad M., Lambe U.P., Brar B., Shah I., Manimegalai J., Ranjan K., Rao R., Kumar S., Mahant S., Khurana S.K., et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018;97:1521–1537. doi: 10.1016/j.biopha.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Wadajkar A.S., Dancy J.G., Hersha D.S., Anastasiadis P., Tran N.L., Woodworth G.F., Winkles J.A., Kim A.J. Tumor-targeted Nanotherapeutics: Overcoming Treatment Barriers for Glioblastoma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1439. doi: 10.1002/wnan.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonawala1 S., Ali M.M. Application of Dendrimer-based Nanoparticles in Glioma Imaging. J. Nanomed Nanotechnol. 2017;8:3. doi: 10.4172/2157-7439.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sneider A., VanDyke D., Paliwal S., Rai P. Remotely Triggered Nano-Theranostics for Cancer Applications. Nanotheranostics. 2017;1:1–22. doi: 10.7150/ntno.17109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y., Han S., Zheng H., Ma R., Ping Y., Zou J., Tang H., Zhang Y., Xu X., Li F. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int. J. Nanomed. 2018;13:5937–5952. doi: 10.2147/IJN.S175418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yong S.B., Chung J.Y., Song Y., Kim J., Ra S., Kim Y.H. Non-viral nano-immunotherapeutics targeting tumor microenvironmental immune cells. Biomaterials. 2019;219:119401. doi: 10.1016/j.biomaterials.2019.119401. [DOI] [PubMed] [Google Scholar]
- 13.Yang B., Chen Y., Shi J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 14.Nel A., Ruoslahti E. New Insights into “Permeability” as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics. ACS Nano. 2017;11:9567–9569. doi: 10.1021/acsnano.7b07214. [DOI] [PubMed] [Google Scholar]
- 15.Li L., Jiang W., Luo K., Song H., Lan F., Wu Y., Gu Z. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics. 2013;3:595–615. doi: 10.7150/thno.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alphandéry E., Idbaih A., Adam C., Delattre J.-Y., Schmitt C., Guyot F., Chebbi I. Development of non-pyrogenic magnetosome minerals coated with poly-l-lysine leading to full disappearance of intracranial U87-Luc tumors in 100% of treated mice using magnetic hyperthermia. Biomaterials. 2019;141:210–222. doi: 10.1016/j.biomaterials.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Lungarea S., Hallam K., Badhana R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016;513:280–293. doi: 10.1016/j.ijpharm.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Hu J., Zhang X., Wen Z., Tan Y., Huang N., Cheng S., Zheng H., Cheng Y. Asn-Gly-Arg-modified polydopamine-coated nanoparticles for dual-targeting therapy of brain glioma in rats. Oncotarget. 2016;7:73681–73696. doi: 10.18632/oncotarget.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eldridge B.N., Bernish B.W., Fahrenholtz C.D., Singh R. Photothermal therapy of glioblastoma multiforme using multiwalled carbon nanotubes optimized for diffusion in extracellular space. ACS Biomater. Sci. Eng. 2016;2:963–976. doi: 10.1021/acsbiomaterials.6b00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian M., Du Y., Wang S., Li C., Jiang H., Shi W., Chen J., Wang Y., Wagner E., Huang R. Highly Crystalline Multicolor Carbon Nanodots for Dual-Modal Imaging-Guided Photothermal Therapy of Glioma. ACS Appl. Mater. Interfaces. 2018;10:4031–4040. doi: 10.1021/acsami.7b19716. [DOI] [PubMed] [Google Scholar]
- 21.Hettiarachchi S.D., Graham R.M., Mintz K.J., Zhou Y., Vanni S., Penga Z., Leblanc R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale. 2019;11:6192–6205. doi: 10.1039/C8NR08970A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanz B., Calatayud M.P., Torres T.E., Fanarrag M.L., Ibarra M.R., Goya G.F. Magnetic hyperthermia enhances cell toxicity with respect to exogenous heating. Biomaterials. 2017;114:62–70. doi: 10.1016/j.biomaterials.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Akiloa O.D., Choonara Y.E., Strydom A.M., du Toita L.C., Kumara P., Modic G., Pillaya V. An in vitro evaluation of a carmustine-loaded Nano-co-Plex for potential magnetic-targeted intranasal delivery to the brain. Int. J. Pharm. 2016;500:196–209. doi: 10.1016/j.ijpharm.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Shen Z., Liu T., Li Y., Lau J., Yang Z., Fan W., Zhou Z., Shi C., Ke C., Bregadze V.E., et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano. 2018;12:11355–11365. doi: 10.1021/acsnano.8b06201. [DOI] [PubMed] [Google Scholar]
- 25.Shevtsov M., Nikolaev B., Marchenko Y., Yakovleva L., Skvortsov N., Mazur A., Tolstoy P., Ryzhov V., Multhoff G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS -DX-SPIONs) Int. J. Nanomed. 2018;13:1471–1482. doi: 10.2147/IJN.S152461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamdous Y., Chebbi I., Mandawala C., Le Fèvre R., Guyot F., Seksek O., Alphandéry E. Biocompatible coated magnetosome minerals with various organization and cellular interaction properties induce cytotoxicity towards RG-2 and GL-261 glioma cells in the presence of an alternating magnetic field. J. Nanobiotechnol. 2017;15:74. doi: 10.1186/s12951-017-0293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandawala C., Chebbi I., Durand-Dubief M., Le Fèvre R., Hamdous Y., Guyot F., Alphandéry E. Biocompatible and stable magnetosome minerals coated with poly-l-lysine, citric acid, oleic acid, and carboxy-methyl-dextran for application in the magnetic hyperthermia treatment of tumors. J. Mater. Chem. B. 2017;5:7644–7660. doi: 10.1039/C6TB03248F. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y., Zhou Y., Zhao N., Liu F., Xu F.-J. Multifunctional pDNA-Conjugated Polycationic Au Nanorod-Coated Fe 3 O 4 Hierarchical Nanocomposites for Trimodal Imaging and Combined Photothermal/Gene Therapy. Small. 2016;18:2459–2468. doi: 10.1002/smll.201600271. [DOI] [PubMed] [Google Scholar]
- 29.Ruan S., Hu C., Tang X., Cun X., Xiao W., Shi K., He Q., Gao H. Increased Gold Nanoparticle Retention in Brain Tumors by in Situ Enzyme-Induced Aggregation. ACS Nano. 2016;10:10086–10098. doi: 10.1021/acsnano.6b05070. [DOI] [PubMed] [Google Scholar]
- 30.Lee C., Hwang H.S., Lee S., Kim B., Kim J.O., Oh K.T., Lee E.S., Choi H.-G., Youn Y.S. Rabies Virus-Inspired Silica-Coated Gold Nanorods as a Photothermal Therapeutic Platform for Treating Brain Tumors. Adv. Mater. 2017;29:1605563. doi: 10.1002/adma.201605563. [DOI] [PubMed] [Google Scholar]
- 31.Albertini B., Mathieu V., Iraci N., Van Woensel M., Schoubben A., Donnadio A., Greco S.M.L., Ricci M., Temperini A., Blasi P., et al. Tumor Targeting by Peptide-Decorated Gold Nanoparticles. Mol. Pharm. 2019;16:2430–2444. doi: 10.1021/acs.molpharmaceut.9b00047. [DOI] [PubMed] [Google Scholar]
- 32.Kunoh T., Shimura T., Kasai T., Matsumoto S., Mahmud H., Khayrani A.C., Seno M., Kunoh H., Takada J. Use of DNA-generated gold nanoparticles to radiosensitize and eradicate radioresistant glioma stem cells. Nanotechnology. 2019;30:055101. doi: 10.1088/1361-6528/aaedd5. [DOI] [PubMed] [Google Scholar]
- 33.Lee C., Kim G.R., Yoon J., Kim S.E., Yoo A.S., Piao X. In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance. Sci. Rep. 2018;8:11122. doi: 10.1038/s41598-018-29424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricciardi L., Sancey L., Palermo G., Termine R., De Luca A., Szerb E.I., Aiello I. Plasmon-mediated cancer phototherapy: The combined effect of thermal and photodynamic processes. Nanoscale. 2017;9:19279–19289. doi: 10.1039/C7NR05522F. [DOI] [PubMed] [Google Scholar]
- 35.Seo B., Lima K., Kim S.S., Oh K.T., Lee E.S., Choi H.-G., Shin B.S., Youn Y.S. Small gold nanorods-loaded hybrid albumin nanoparticles with high photothermal efficacy for tumor ablation. Colloids Surf. B Biointerfaces. 2019;179:340–351. doi: 10.1016/j.colsurfb.2019.03.068. [DOI] [PubMed] [Google Scholar]
- 36.Sukumar U.K., Bose RJ C., Malhotra M., Babikir H.A., Afjei R., Robinson E., Zeng Y., Chang E., Habte F., Sinclair R., et al. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials. 2019;218:119342. doi: 10.1016/j.biomaterials.2019.119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dufort S., Appelboom G., Verry C., Barbier E.L., Lux F., Bräuer-Krisch E., Sancey L., Chang S.D., Zhang M., Roux S., et al. Ultrasmall theranostic gadolinium-based nanoparticles improve high-grade rat glioma survival. J. Clin. Neurosci. 2019;67:215–219. doi: 10.1016/j.jocn.2019.05.065. [DOI] [PubMed] [Google Scholar]
- 38.Shaw T.K., Dey D.M.G., Pal M.M., Paul P., Chakraborty S., Ali K.A., Mukherjee B., Bandyopadhyay A.K., Mandal M. Successful delivery of docetaxel to rat brain using experimentally developed nanoliposome: A treatment strategy for brain tumor. Drug Deliv. 2017;24:346–357. doi: 10.1080/10717544.2016.1253798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhunia S., Vangala V., Bhattacharya D., Ravuri H.G., Kuncha M., Chakravarty S., Sistla R., Chaudhuri A. Large Amino Acid Transporter 1 Selective Liposomes of L-DOPA Functionalized Amphiphile for Combating Glioblastoma. Mol. Pharm. 2017;14:3834–3847. doi: 10.1021/acs.molpharmaceut.7b00569. [DOI] [PubMed] [Google Scholar]
- 40.Rehman M., Madni A., Shi D., Ihsan A., Tahir N., Chang K.R., Javed I., Webster T.J. Enhanced blood brain barrier permeability and glioblastoma cell targeting via thermoresponsive lipid nanoparticles. Nanoscale. 2017;9:15434–15440. doi: 10.1039/C7NR05216B. [DOI] [PubMed] [Google Scholar]
- 41.Erel-Akbaba G., Carvalho L.A., Tian T., Zinter M., Akbaba H., Obeid P.J., Chiocca E.A., Weissleder R., Kantarci A.G., Tannous B.A. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano. 2019;13:4028–4040. doi: 10.1021/acsnano.8b08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tapeinos C., Marino A., Battaglini M., Migliorin S., Brescia R., Scarpellini A., Fernández C.D.J., Prato M., Dragog F., Ciofani G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale. 2019;11:1–368. doi: 10.1039/C8NR05520C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Zhai M., Chen Z., Han X., Yu F., Li Z., Xie X., Han C., Yu L., Yang Y., et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017;24:1045–1055. doi: 10.1080/10717544.2017.1344334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying X., Wang Y., Xu H., Li X., Yan H., Tang H., Wen C., Li Y. The construction of the multifunctional targeting ursolic acids liposomes and its apoptosis effects to C6 glioma stem cells. Oncotarget. 2017;8:64129–64142. doi: 10.18632/oncotarget.19784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordling-David M.M., Yaffe R., Guez D., Meirow H., Last D., Grad A., Salomon S., Sharabi S., Levi-Kalisman Y., Golomb G., et al. Liposomal temozolomide drug delivery using convection enhanced delivery. J. Control. Release. 2017;261:138–146. doi: 10.1016/j.jconrel.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Bayrac A.T., Akca O.E., Eyidogan F.I., Oktem H.A. Target-specific delivery of doxorubicin to human glioblastoma cell line via ssDNA aptamer. J. Biosci. 2018;43:97–104. doi: 10.1007/s12038-018-9733-x. [DOI] [PubMed] [Google Scholar]
- 47.Gamage N.H., Jing L., Worsham M.J., Ali M.M. Targeted Theranostic Approach for Glioma Using Dendrimer-Based Curcumin Nanoparticle. J. Nanomed Nanotechnol. 2016;7:393. doi: 10.4172/2157-7439.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal S., Muniyandi P., Maekawa T., Kumar D.S. Vesicular systems employing natural substances as promising drug candidates for MMP inhibition in glioblastoma: A nanotechnological approach. Int. J. Pharm. 2018;551:339–361. doi: 10.1016/j.ijpharm.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 49.Kargar S., Khoei S., Khoee S., Shirvalilou S., Mahdavi S.R. Evaluation of the combined effect of NIR laser and ionizing radiation on cellular damages induced by IUdR-loaded PLGA-coated Nano-graphene oxide. Photodiagn. Photodyn. Ther. 2018;21:91–97. doi: 10.1016/j.pdpdt.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Lin T., Zhao P., Jiang Y., Tang Y., Jin H., Pan Z., He H., Yang V.C., Huang Y. Blood−Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano. 2016;10:9999–10012. doi: 10.1021/acsnano.6b04268. [DOI] [PubMed] [Google Scholar]
- 51.Han L., Kong D.K., Zheng M.-Q., Murikinati S., Ma C., Yuan P., Li L., Tian D., Cai Q., Ye C., et al. Increased Nanoparticle Delivery to Brain Tumors by Autocatalytic Priming for Improved Treatment and Imaging. ACS Nano. 2016;10:4209–4218. doi: 10.1021/acsnano.5b07573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramachandran R., Junnuthula V.R., Gowd G.S., Ashokan A., Thomas J., Peethambaran R., Thomas A., Unni AK K., Panikar D., Nair S.V., et al. Theranostic 3-Dimensional nano brain-implant for prolonged and localized treatment of recurrent glioma. Sci. Rep. 2017;7:43271. doi: 10.1038/srep43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castañeda-Gill J.M., Ranjan A.P., Vishwanatha J.K. Development and Characterization of Methylene Blue Oleate Salt-Loaded Polymeric Nanoparticles and their Potential Application as a Treatment for Glioblastoma. J. Nanomed Nanotechnol. 2017;8:449. doi: 10.4172/2157-7439.1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y., Baiyang L., Leran B., Zhen W., Yandong X., Baixiang D., Dandan Z., Yufu Z., Jun L., Rutong Y., et al. Reduction-responsive PEtOz-SS-PCL micelle with tailored size to overcome blood–brain barrier and enhance doxorubicin antiglioma effect. Drug Deliv. 2017;24:1782–1790. doi: 10.1080/10717544.2017.1402218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bi Y., Liu L., Lu Y., Sun T., Shen C., Chen X., Chen Q., An S., He X., Ruan C., et al. T7 Peptide-Functionalized PEG-PLGA Micelles Loaded with Carmustine for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces. 2016;8:27465–27473. doi: 10.1021/acsami.6b05572. [DOI] [PubMed] [Google Scholar]
- 56.Ran D., Mao J., Shen Q., Xie C., Zhan C., Wang R., Lu W. GRP78 enabled micelle-based glioma targeted drug delivery. J. Control. Release. 2017;255:120–131. doi: 10.1016/j.jconrel.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Singleton W.G., Collins A.M., Bienemann A.S., Killick-Cole C.L., Haynes H.R., Asby D.J., Butts C.P., Wyatt M.J., Barua N.U., Gill S.S. Convection enhanced delivery of panobinostat (LBH589)-loaded pluronic nano-micelles prolongs survival in the F98 rat glioma model. Int. J. Nanomed. 2017;12:1385–1399. doi: 10.2147/IJN.S125300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C., Xu Q., Chen X., Liu J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int. J. Nanomed. 2019;14:7515–7531. doi: 10.2147/IJN.S214585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng S., Cheng Y., Teng Y., Liu X., Yu T., Wang Y., Liu J., Hu Y., Wu C., Wang X., et al. Application of luteolin nanomicelles anti-glioma effect with improvement in vitro and in vivo. Oncotarget. 2017;8:61146–61162. doi: 10.18632/oncotarget.18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun L., Chen Y., Zhou Y., Guo D., Fan Y., Guo F., Zheng Y., Chen W. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J. Pharm. Sci. 2017;12:418–423. doi: 10.1016/j.ajps.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grabowska M., Grześkowiak B.F., Szutkowski K., Wawrzyniak D., Głodowicz P., Barciszewski J., Jurga S., Rolle K., Mrowczyński R. Nano-mediated delivery of double-stranded RNA for gene therapy of glioblastoma multiforme. PLoS ONE. 2019;14:e0213852. doi: 10.1371/journal.pone.0213852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu D., Khan O.F., Suvàc M.L., Dong B., Panek W.K., Xiao T., Wu M., Han Y., Ahmed A.U., Balyasnikova I.V., et al. Multiplexed RNAi therapy against brain tumorinitiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc. Natl. Acad. Sci. USA. 2017;114:E6147–E6156. doi: 10.1073/pnas.1701911114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez-Bertoni H., Kozielski K.L., Rui Y., La R., Vaughan H., Wilson D.R., Mihelson N., Eberhart C.G., Laterra J., Green J.J. Bioreducible Polymeric Nanoparticles Containing Multiplexed Cancer Stem Cell Regulating miRNAs Inhibit Glioblastoma Growth and Prolong Survival. Nano Lett. 2018;18:4086–4094. doi: 10.1021/acs.nanolett.8b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shatsberg Z., Zhang X., Ofek P., Malhotr S., Krivitsky A., Scomparin A., Tiram G., Calderón M., Haag R., Satchi-Fainaro R. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J. Control. Release. 2016;239:159–168. doi: 10.1016/j.jconrel.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 65.Alphandéry E., Idbaih A., Adam C., Delattre J.-Y., Schmitt C., Guyot F., Chebbi I. Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J. Control. Release. 2017;262:259–272. doi: 10.1016/j.jconrel.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 66.Zhang C., Nance E.A., Mastorakos P., Chisholma P., Berry S., Eberhart C., Tyler B., Brem H., Soo J.S., Hanes J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Control. Release. 2017;263:112–119. doi: 10.1016/j.jconrel.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young J.S., Bernal G., Polster S.P., Nunez L., Larsen G.F., Mansour N., Podell M., Yamini B. Convection Enhanced Delivery of Polymeric Nanoparticles Encapsulating Chemotherapy in Canines with Spontaneous Supratentorial Tumors. World Neurosurg. 2018;117:e698–e704. doi: 10.1016/j.wneu.2018.06.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arshad A., Yang B., Bienemann A.S., Barua N.U., Wyatt M.J., Woolley M., Johnson D.E., Edler K.J., Gill S.S. Convection-Enhanced Delivery of Carboplatin PLGA Nanoparticles for the Treatment of Glioblastoma. PLoS ONE. 2015;10:e0132266. doi: 10.1371/journal.pone.0132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finbloom J.A., Aanei I.L., Bernard J.M., Klass S.H., Elledge S.K., Han K., Ozawa T., Nicolaides T.P., Berger M.S., Francis M.B. Evaluation of Three Morphologically Distinct Virus-Like Particles as Nanocarriers for Convection-Enhanced Drug Delivery to Glioblastoma. Nanomaterials. 2018;8:1007. doi: 10.3390/nano8121007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alphandéry E. Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology. 2019;13:573–596. doi: 10.1080/17435390.2019.1572809. [DOI] [PubMed] [Google Scholar]
- 71.Ganipineni P.L., Danhier F., Préat V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release. 2018;281:42–57. doi: 10.1016/j.jconrel.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 72.Coluccia D., Figueiredo C.A., Wu M.Y., Riemenschneider A.N., Diaz Luck A., Smith C., Das S., Ackerley C., O’Reilly M., Hynynen K., et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2018;14:1137–1148. doi: 10.1016/j.nano.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 73.Azad T.D., Pan J., Connolly I.D., Remington A., Wilson C.M., Grant G.A. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg Focus. 2015;38:E9. doi: 10.3171/2014.12.FOCUS14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parodi A., Rudzinska M., Deviatkin A.A., Soond S.M., Baldin A.V., Zamyatnin A.A. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics. 2019;11:245. doi: 10.3390/pharmaceutics11050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu S.S., Lau C.M., Thomas S.N., Jerome W.G., Maron D.J., Dickerson J.H., Hubbell J.A., Giorgio T.D. Size- and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages. Int. J. Nanomed. 2012;7:799–813. doi: 10.2147/IJN.S28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng C., Gao X., Xu J., Du B., Ning X., Tang S., Bachoo R.M., Yu M., Ge W.-P., Zheng J. Targeting orthotopic gliomas with renal-clearable luminescent gold nanoparticles. Nano Res. 2017;10:1366–1376. doi: 10.1007/s12274-017-1472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang Y., Wang X., Liu X., Lv W., Zhang H., Zhang M., Li X., Xin H., Xu Q. Enhanced Antiglioma Efficacy of Ultrahigh Loading Capacity Paclitaxel Prodrug Conjugate Self-Assembled Targeted Nanoparticles. ACS Appl. Mater. Interfaces. 2017;9:211–217. doi: 10.1021/acsami.6b13805. [DOI] [PubMed] [Google Scholar]
- 78.Shirvalilou S., Khoei S., Khoee S., Raoufi N.J., Karimi M.R., Shakeri-Zadeh A. Development of a magnetic nano-graphene oxide carrier for improved glioma-targeted drug delivery and imaging: In vitro and in vivo evaluations. Chem. Biol. Interact. 2018;295:97–108. doi: 10.1016/j.cbi.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 79.Gonçalves D.P.N., Rodriguez R.D., Kurth T., Bray L.J., Binner M., Jungnickel C., Gür F.N., Poser S.W., Schmidt T.L., Zahn D.R.T., et al. Enhanced targeting of invasive glioblastoma cells by peptidefunctionalized gold nanorods in hydrogel-based 3D culture. Acta Biomater. 2017;58:12–25. doi: 10.1016/j.actbio.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 80.Gonçalves D.P.N., Park D.M., Schmidt T.L., Werner C. Modular peptide-functionalized gold nanorods for effective glioblastoma multicellular tumor spheroid targeting. Biomater. Sci. 2018;6:1140–1146. doi: 10.1039/C7BM01107E. [DOI] [PubMed] [Google Scholar]
- 81.Ni D., Zhang J., Bu W., Xing H., Han F., Xiao Q., Yao Z., Chen F., He Q., Liu J., et al. Dual-Targeting Upconversion Nanoprobes across the BloodBrain Barrier for Magnetic Resonance/Fluorescence Imaging of Intracranial Glioblastoma. ACS Nano. 2014;8:1231–1242. doi: 10.1021/nn406197c. [DOI] [PubMed] [Google Scholar]
- 82.Patil R., Galstyan A., Sun T., Shatalova E.S., Butte P., Mamelak A.N., Carico C., Kittle D.C., Grodzinski Z.B., Chiechi A., et al. Polymalic acid chlorotoxin nanoconjugate for near-infrared fluorescence guided resection of glioblastoma multiforme. Biomaterials. 2019;206:146–159. doi: 10.1016/j.biomaterials.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karakaş C.Y., Şahin H.T., İnan B., Özçimen D., Erginer Y.Ö. In vitro cytotoxic activity of microalgal extracts loaded nano–micro particles produced via electrospraying and microemulsion methods. Biotechnol. Prog. 2019;2019:e2876. doi: 10.1002/btpr.2876. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L., Zhang Z., Mason R.P., Sarkaria J.N., Zhao D. Convertible MRI contrast: Sensing the delivery and release of antiglioma nano-drugs. Sci. Rep. 2015;5:09874. doi: 10.1038/srep09874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mu Q., Lin G., Patton V.K., Wang K., Press O.W., Zhang M. Gemcitabine and Chlorotoxin Conjugated Iron Oxide Nanoparticles for Glioblastoma Therapy. J. Mater. Chem. B. 2016;7:32–36. doi: 10.1039/C5TB02123E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mirgani M.T., Isacchi B., Sadeghizadeh M., Marra F., Bilia A.R., Mowla S.J., Najafi F., Babaei E. Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int. J. Nanomed. 2014;9:403–417. doi: 10.2147/IJN.S48136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steiniger S.C.J., Kreuter J., Khalansky A.S., Skidan I.N., Bobruskin A.I., Smirnova Z.S., Severin S.E., Uhl R., Kock M., Geiger K.D., et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int. J. Cancer. 2004;109:759–767. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 88.Di Mauro P.P., Cascante A., Vilà P.B., Gómez-Vallejo V., Llop J., Borrós S. Peptide functionalized and high drug loaded novel nanoparticles as dualtargeting drug delivery system for modulated and controlled release of paclitaxel to brain glioma. Int. J. Pharm. 2018;553:169–185. doi: 10.1016/j.ijpharm.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 89.Fang C., Wang K., Stephen Z.R., Mu Q., Kievit F.M., Chiu D.T., Press O.W., Zhang M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces. 2015;7:6674–6682. doi: 10.1021/am5092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mi1 Y., Shao Z., Vang J., Kaidar-Person O., Wang A.Z. Application of nanotechnology to cancer radiotherapy. Cancer Nano. 2016;7:11. doi: 10.1186/s12645-016-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morgenroth1 A., Vogg AT J., Ermert K., Zlatopolskiy B., Mottaghy F.M. Hedgehog signaling sensitizes Glioma stem cells to endogenous nano-irradiation. Oncotarget. 2014;5:5483–5493. doi: 10.18632/oncotarget.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen N., Yang W., Bao Y., Xu H., Qin S., Tu Y. BSA capped Au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. RSC Adv. 2015;5:40514–40520. doi: 10.1039/C5RA04013B. [DOI] [Google Scholar]
- 93.Hainfeld J.F., Ridwan S.M., Stanishevskiy1 Y., Panchal R., Slatkin1 D.N., Smilowitz H.M. Iodine nanoparticles enhance radiotherapy of intracerebral human glioma in mice and increase efficacy of chemotherapy. Sci. Rep. 2019;9:4505. doi: 10.1038/s41598-019-41174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tamborini M., Locatelli E., Rasile M., Monaco I., Rodighiero S., Corradini I., Franchini M.C., Passoni L., Matteoli M. A Combined Approach Employing Chlorotoxin-Nanovectors and Low Dose Radiation To Reach Infiltrating Tumor Niches in Glioblastoma. ACS Nano. 2016;10:2509–2520. doi: 10.1021/acsnano.5b07375. [DOI] [PubMed] [Google Scholar]
- 95.Fernandes C., Suares D., Yergeri M. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018;9:1230. doi: 10.3389/fphar.2018.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uthaman S., Huh K.M., Park I.-K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018;22:22. doi: 10.1186/s40824-018-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang F.-Y., Teng M.-C., Lu M., Liang H.-F., Lee Y.-R., Yen C.-C., Liang M.-L., Wong T.-T. Treating glioblastoma multiforme with selective high-dose liposomal doxorubicin chemotherapy induced by repeated focused ultrasound. Int. J. Nanomed. 2012;7:965–974. doi: 10.2147/IJN.S29229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen L., Wu Y., Wu H., Li J., Xie J., Zang F., Maa M., Gu N., Zhang Y. Magnetic targeting combined with active targeting of dual-ligand iron oxide nanoprobes to promote the penetration depth in tumors for effective magnetic resonance imaging and hyperthermia. Acta Biomater. 2019;96:491–504. doi: 10.1016/j.actbio.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 99.Gupta R., Sharma D. Biofunctionalization of magnetite nanoparticles with stevioside: Effect on the size and thermal behaviour for use in hyperthermia applications. Int. J. Hyperth. 2019;36:302–312. doi: 10.1080/02656736.2019.1565787. [DOI] [PubMed] [Google Scholar]
- 100.Cabada T.F., de Pablo C.S.L., Serrano A.M., del Pozo Guerrero F., Olmedo J.J.S., Gomez M.R. Induction of cell death in a glioblastoma line by hyperthermic therapy based on gold nanorods. Int. J. Nanomed. 2012;7:1511–1523. doi: 10.2147/IJN.S28470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jang Y., Lee N., Kim J.H., Park Y.I., Piao Y. Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications. Nanomaterials. 2018;8:252. doi: 10.3390/nano8040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J., Zhou Z., Zhang F., Xu H., Chen W., Jiang T. A novel nanocomposite based on fluorescent turn-on gold nanostars for near-infrared photothermal therapy and self-theranostic caspase-3 imaging of glioblastoma tumor cell. Colloids Surf. B Biointerfaces. 2018;170:303–311. doi: 10.1016/j.colsurfb.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 103.Xu H.-L., ZhuGe D.-L., Chen P.-P., Tong M.-G., Lin M.-T., Jiang X., Zheng Y.-W., Chen B., Li X.-K., Zhao Y.-Z. Silk fibroin nanoparticles dyeing indocyanine green for imaging-guided photo-thermal therapy of glioblastoma. Drug Deliv. 2018;25:364–375. doi: 10.1080/10717544.2018.1428244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang J.-H., Ko Y.T. Dual-selective photodynamic therapy with a mitochondria-targeted photosensitizer and fiber optic cannula for malignant brain tumors. Biomater. Sci. 2019;7:2812–2825. doi: 10.1039/C9BM00403C. [DOI] [PubMed] [Google Scholar]
- 105.Huang J.-L., Jiang G., Song Q.-X., Gu X., Hu M., Wang X.-L., Song H.-H., Chen L.-P., Lin Y.-Y., Jiang D., et al. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis. Nat. Commun. 2017;8:15144. doi: 10.1038/ncomms15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu N., Zhang L., Wang Z., Cheng Y., Zhang P., Wang X., Wen W., Yang H., Liu H., Jin W., et al. MicroRNA-101 inhibits proliferation, migration and invasion of human glioblastoma by targeting SOX9. Oncotarget. 2017;8:19244–19254. doi: 10.18632/oncotarget.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galstyan A., Markman J.L., Shatalova E.S., Chiechi1 A., Korman A.J., Patil R., Klymyshyn D., Tourtellotte W.G., Israel1 L.L., Ljubimov BO V.A., et al. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 2019;10:3850. doi: 10.1038/s41467-019-11719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang P., Wang X., Liang X., Yang J., Zhang C., Kong D., Wang W. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 2019;85:1–26. doi: 10.1016/j.actbio.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 109.Kadiyala P., Li D., Nuñez F.M., Altshuler D., Doherty R., Kuai R., Yu M., Kamran N., Edwards M., Moon J.J., et al. High Density Lipoprotein-Mimicking Nanodiscs for Chemo-Immunotherapy against Glioblastoma Multiforme. ACS Nano. 2019;13:1365–1384. doi: 10.1021/acsnano.8b06842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abdalla AM E., Xiao L., Ullah M.W., Yu M., Ouyang C., Yang C. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics. 2018;8:533–548. doi: 10.7150/thno.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caffo M., Cardali S.M., Fazzari E., Barresi V., Caruso G. Nanoparticles drug-delivery systems and antiangiogenic approaches in the treatment of gliomas. Glioma. 2018;1:183–188. doi: 10.4103/glioma.glioma_43_18. [DOI] [Google Scholar]
- 112.Naletova I., Cucci L.M., D’Angeli F., Anfuso C.D., Magrì A., La Mendola D., Lupo G., Satriano C. A Tunable Nanoplatform of Nanogold Functionalised with Angiogenin Peptides for Anti-Angiogenic Therapy of Brain Tumours. Cancers. 2019;11:1322. doi: 10.3390/cancers11091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sudha T., Bharali D.J., Sell S., Darwish NH E., Davis P.J., Mousa S.A. Nanoparticulate Tetrac Inhibits Growth and Vascularity of Glioblastoma Xenografts. Horm. Canc. 2017;8:157–165. doi: 10.1007/s12672-017-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clavreu A., Pourbaghi-Masouleh M., Roger E., Menei P. Nanocarriers and nonviral methods for delivering antiangiogenic factors for glioblastoma therapy: The story so far. Int. J. Nanomed. 2019;14:2497–2513. doi: 10.2147/IJN.S194858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wierzbicki M., Sawosz E., Strojny B., Jaworski1 S., Grodzik M., Chwalibog A. NF-κB-related decrease of glioma angiogenic potential by graphite nanoparticles and graphene oxide nanoplatelets. Sci. Rep. 2018;8:14733. doi: 10.1038/s41598-018-33179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miao L., Huang L. Exploring the Tumor Microenvironment with Nanoparticles. Cancer Treat. Res. 2015;166:193–226. doi: 10.1007/978-3-319-16555-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu S., Gu Z., Zhao Y. Harnessing Tumor Microenvironment for Nanoparticle-Mediated Radiotherapy. Adv. Therap. 2018;1:1800050. doi: 10.1002/adtp.201800050. [DOI] [Google Scholar]
- 118.Aldea M., Potara M., Soritau O., Florian I.S., Florea A., Nagy-Simon T., Pileczki V., Brie I., Maniu D., Kacso G. Chitosan-capped gold nanoparticles impair radioresistant glioblastoma stem-like cells. JBUON. 2018;23:800–813. [PubMed] [Google Scholar]
- 119.Khan I.S., Ehtesham M. Targeting glioblastoma cancer stem cells: The next great hope? Neurosurg. Focus. 2014;37:E7. doi: 10.3171/2014.9.FOCUS14509. [DOI] [PubMed] [Google Scholar]
- 120.Lu L., Shen X., Tao B., Lin C., Li K., Luo Z., Cai K. The nanoparticle-facilitated autophagy inhibition of cancer stem cells for improved chemotherapeutic effects on glioblastomas. J. Mater. Chem. B. 2019;7:2054–2062. doi: 10.1039/C8TB03165G. [DOI] [PubMed] [Google Scholar]
- 121.Singh P.K., Srivastava A.K., Dev A., Kaunda B., Choudhury S.R., Karmakar S. 1, 3β-Glucan anchored, paclitaxel loaded chitosan nanocarrier endows enhanced hemocompatibility with efficient anti-glioblastoma stem cells therapy. Carbohydr. Polym. 2018;180:365–375. doi: 10.1016/j.carbpol.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 122.Suryaprakash S., Lao Y.-H., Cho H.-Y., Li M., Ji H.Y., Shao D., Hu H., Quek C.H., Huang D., Mintz R.L., et al. Engineered Mesenchymal Stem Cell/Nanomedicine Spheroid as an Active Drug Delivery Platform for Combinational Glioblastoma Therapy. Nano Lett. 2019;19:1701–1705. doi: 10.1021/acs.nanolett.8b04697. [DOI] [PubMed] [Google Scholar]
- 123.Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]