Abstract
Introduction: Despite improvements in radiation therapy, chemotherapy and surgical procedures over the last 30 years, pancreatic cancer 5-year survival rate remains at 9%. Reduced stroma permeability and heterogeneous blood supply to the tumour prevent chemoradiation from making a meaningful impact on overall survival. Hypoxia-activated prodrugs are the latest strategy to reintroduce oxygenation to radioresistant cells harbouring in pancreatic cancer. This paper reviews the current status of photon and particle radiation therapy for pancreatic cancer in combination with systemic therapies and hypoxia activators. Methods: The current effectiveness of management of pancreatic cancer was systematically evaluated from MEDLINE® database search in April 2019. Results: Limited published data suggest pancreatic cancer patients undergoing carbon ion therapy and proton therapy achieve a comparable median survival time (25.1 months and 25.6 months, respectively) and 1-year overall survival rate (84% and 77.8%). Inconsistencies in methodology, recording parameters and protocols have prevented the safety and technical aspects of particle therapy to be fully defined yet. Conclusion: There is an increasing requirement to tackle unmet clinical demands of pancreatic cancer, particularly the lack of synergistic therapies in the advancing space of radiation oncology.
Keywords: pancreatic cancer, proton therapy, carbon ion therapy, stereotactic body radiation therapy, hypoxia activated prodrug, radiosensitizer
1. Introduction
Pancreatic cancer is the seventh most lethal solid tumour worldwide. Independent of the disease stage, the 5-year survival rate remains at 9% [1]. Optimal treatment typically involves a multimodality approach of surgical resection combined with chemotherapy and/or radiation therapy [2]. The local control (LC) and overall survival (OS) rates remain low for these patients despite 40–50% presenting metastasis-free locally advanced pancreatic cancer (LAPC) [3]. Currently, complete resection provides the only cure with uncertainty surrounding pre- or post-surgical chemoradiation [4].
Current therapeutic approaches are limited by hypoxia and blood barrier-like reductions in efficiency of pre- or post-surgical chemoradiation. Solid LAPC tumours with hypoxic cells not only reduce the effectiveness of photon radiation therapy (XRT) but also limit the intake of chemotherapeutic agents. Conventionally, 3D conformal radiation therapy (3DCRT) has previously proven insufficient in terms of achieving satisfactory tumour control probability (TCP) and normal tissue complication probability (NTCP) [5]. Modulated XRT techniques such as intensity modulated radiation therapy (IMRT) and volume modulated arc radiation therapy (VMAT) are now well established standards of care at reducing NTCP in clinical practice. However, the role of XRT for LAPC remains controversial as chemoradiation has not proven to significantly impact TCP for LAPC patients, irrespective of the technique used [6,7,8,9].
Recent advancements in delivery, image guidance and planning have guided novel techniques of radiation dose escalation. Stereotactic body radiation therapy (SBRT) allows larger doses of up to 25 Gy per fraction to be delivered (as opposed to 2 Gy per fraction), increasing the radiobiological effectiveness of XRT. However, due to the anatomical location of the pancreas and proximity to critical normal structures, doses required to provide sufficient LC are still prohibitive [10].
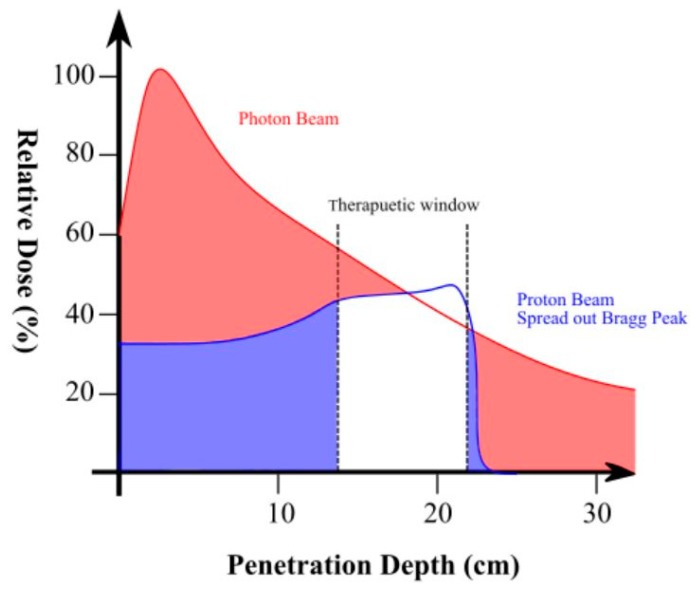
Advancements in particle therapy (e.g., proton and carbon ion) provide a different solution to delivering enhanced biological damage compared to XRT due to more advantageous dose deposition based on the Bragg Peak (Figure 1) [11]. The physical properties of charged particle dose deposition in tissue result in sharper dose distribution enabling tolerable full-dose radiation delivery whilst minimising NTCP to surrounding organs-at-risk (OAR). Therapeutic resistance in the tumour microenvironment, however, may remain as the relative biological effectiveness (RBE) of proton therapy (PT) is only 10% higher than XRT [12]. As a result, investigations into heavier particles which induce more severe DNA damage continue (compared to both PT and XRT). Carbon-ion therapy (C-ion) utilises charge carbon-12 ions of larger mass to increase direct DNA damage effectiveness by a factor of approximately two to four [13,14,15,16]. Recently established at a handful of institutions, the improved dose conformation and potential for overcoming effects of hypoxia is currently being investigated as a potential solution for LAPC [11,17].
Figure 1.
Spread out Bragg Peak of proton therapy (red) compared to photon radiation therapy (blue). The shaded areas represent relative dose to normal tissue.
Alternate pathways to combat LAPC radiation resistance are also being investigated, including the implementation of synergistic agents. Hypoxia activated drugs (HAPs) are synergistic agents introduced to overcome this longstanding radiobiological challenge of hypoxia. The enhanced anti-tumour activity of HAPs combined with conventional and new chemoradiation treatments has long been hypothesised across several cancer sites; however, the clinical implementation has been vastly under-explored [18].
The aim of this work was to review the current status of combined knowledge for XRT, PT and C-ion therapy. This paper will look at the reported results of chemoradiation trials using PT, C-ion and XRT to determine if either modality has impacted OS, with the emphasis on radiation modality only. Similarly, the current pre-clinical and clinical status of hypoxia-targeted treatment combined with or without XRT will be assessed for effectiveness in the treatment of pancreatic cancer.
2. Methods
A search strategy was conducted using MEDLINE® database in April 2019 with the relevant key terms encompassing the themes of: proton therapy, pancreatic cancer and hypoxia targeting adjuvant therapy (Appendix A). Limitations were applied to the search, including: English language, published from 2000 to present. The search identified 87 papers. Conference abstracts, duplicates across the searches and papers which involved 3DCRT treatments were excluded (as per the exclusion criteria in Appendix B). A total of 38 papers were exported for the purpose of this review. The reference lists were pearled for other literature (including C-ion trials), resulting in 82 papers in total.
This systematic review was performed according to the PRISMA statement (http://www.prisma-statement.org/). All methods for exclusion criteria, data extraction and quality assessment were specified in advance. However, due to the evaluated number of participants, variation in reporting and data collection a systematic analysis was not possible due to the lack of consistency. Data were extracted and tabulated to provide a clear integrative overview of the results to date for chemoradiation for pancreatic cancer. The review protocol was not registered with any organisation.
Most the trials discussed have used chemotherapy, however, we have not compared the individual chemotherapy schedules. The chemoradiation combinations are tabulated and where appropriate, we commented on the side effects of the chemoradiation.
3. Results and Discussion
3.1. Combating Hypoxia in Pancreatic Cancer
Historically, some of the largest prospective phase III chemoradiation trials for pancreatic cancer, including the European Study Group for Pancreatic Cancer-1 (ESPAC-1) and RTOG 97-04 trials, found that neither chemotherapy nor XRT alone could provide tumour control [19,20]. The results extended into single- and multi-agent gemcitabine chemoradiation trials such as LAP07, showing no statistical impact on OS [8]. Despite these trials utilising 3DCRT, now inadequate compared to IMRT and VMAT, they all demonstrated the radiation resistance of pancreatic cancer. Hypoxia has long been recognised as the main cause of radiation resistance in pancreatic cancer [21]. Failure of studies to account for the presence of hypoxia has prevented them reaching meaningful survival endpoints [22]. As such, there is a clinical need to address hypoxia in pancreatic cancer and develop novel therapies that specifically target and exploit these oxygen-deficient regions in order to improve systemic and local therapies. Oxygenating these hypoxic regions can affect the lethality of photon radiation therapy by threefold [23].
To date, even the most clinically effective chemoradiation regimen had little impact on TCP due to the reduced permeability of the stroma and heterogeneous blood supply to the tumour [24]. Several strategies have demonstrated limited clinical benefit at reintroducing oxygen to regions of the tumour which harbour hypoxic cells prior to XRT. Some strategies include hyperbaric chambers, high oxygen-content gas breathing, invasive needle insertions, and blood transfusions [25].
Hypoxia targeting drugs have been developed to synergistically enhance fractionated XRT by targeting a variation of physiological characteristics and molecular pathways of tumours [18]. Of particular interest are the successful clinical trials investigating the concept of hypoxia-activated prodrugs (HAPs); a bioreductive agent which is metabolised into an active vasodilating drug in hypoxic tumour tissue [26].
3.2. Hypoxia Activated Prodrug
Extensive preclinical evaluations of Evofosfamide (TH-302) Threshold Pharmaceuticals (South San Francisco, CA, USA) have demonstrated promising antineoplastic properties in the treatment of pancreatic cancer, improving the clinical efficacy of chemotherapy and/or XRT [18,27,28]. TH-302 is a prevalent “dual-function” radiosensitising agent: producing a potent DNA-alkylating species which damage DNA in both proliferating and quiescent cells and modify free radical damage [23,29]. TH-302 unique biochemical properties allow it to be relatively non-toxic to normoxic tissues through activation of cytotoxic products in hypoxic conditions only [23].
The possibility of TH-302 working concurrently with chemoradiation for pancreatic cancer have long been tested in pre-clinical and in vitro studies [23,30,31]. The addition of TH-302 improved the outcome of chemoradiation for pancreatic xenograft models, overcoming the biological impact of hypoxia. Lohse, et al. [23] combined fractionated XRT with TH-302 to significantly delay growth and reduce tumour volume in patient-derived xenograft models; a result seen in neither XRT or TH-302 treatments alone. A result was more dominant in rapidly growing patient-derived pancreatic xenograft models compared to slow growing hypoxic models. This impacts on tumour growth rates, which is a strong predictor of OS for clinical application and efficacy of the treatment combination.
Borad, et al. [27] conducted the first randomised phase II clinical trial to demonstrate the potential outcomes of combining TH-302 with gemcitabine alone. The dual-drug combination extended to the global placebo-controlled randomised phase III “MAESTRO” trial (NCT01746979) with contrasting results. The results demonstrated no OS benefit from combining TH-302 with gemcitabine (median of 8.7 months compared to 7.6 months for gemcitabine alone) [32]. However, the treatment combination demonstrated favourable signs of antitumour activity regarding patient PFS (median of 5.5 months compared to 3.7 months for gemcitabine alone) and higher objective response rate [33]. Ideally, TH-302 and gemcitabine should not be used alone but in combination with XRT to enhance the biological damage. These contradictory results leave a gap for further research examining TH-302 combined with chemoradiation for pancreatic cancer.
The use of TH-302 with chemotherapy was ineffective at providing a statistically significant impact on patient outcome; however, as previously mentioned, the combination with XRT in vivo and in vitro studies showed potential. The first in-human clinical trial testing TH-302 with chemoradiation was planned to be a phase I non-randomised, single-arm, trial in Dutch oesophageal adenocarcinoma patients (NCT02598687) [34]. Although not pancreatic, the similar anatomical location of the oesophageal adenocarcinoma (at the oesophago-gastric junction) had potential to give insight demonstrating the NTCP effects of the trimodality therapy on radiosensitive gastric OAR. However, due to the previous failure of TH-302 to reach its primary endpoint when combined with chemotherapy alone in trials for soft tissue sarcoma (NCT01440088) and pancreatic cancer (MAESTRO), the study was eventually withdrawn [33,35].
3.3. HAPs Limitations and Future Work
Major restrictions in HAPs development are from functional introduction by the unpredictable tumour vasculature which prevents regular implementation [25]. Dose dependence and fibrosis at the site of prolonged injection were limitations experienced during TH-302 testing. Other classes of drugs have been limited by functional requirements such as local administration, speed of breakdown and tolerability [18]. Investigations continue into alternate HAPs in the clinical context of pancreatic cancer, see Table 1 for an overview of past and current studies.
Table 1.
Overview of studies investigating hypoxia-activated drugs (pHAPs) in pancreatic cancer (2000-Current).
| HAP | Author | Chemotherapy | XRT | Study | Sample Size | Limitations | Summary of Applicable Findings |
|---|---|---|---|---|---|---|---|
| TH-302 | Weiss, et al. [28] | No | No | Phase I clinical trial | 57 | Only 2 pancreatic patients in the study | TH-302 was well tolerated during the first phases of monotherapy investigations with only mild concern regarding high-grade skin and mucosal toxicities above 240 mg/m2. |
| Borad, et al. [77] | Yes | No | Phase I/II clinical study | 46 | No full publication of results. No XRT | Overall response rate of 21% and median PFS time of 5.9 months across advanced pancreatic cancer patients. | |
| Sun, et al. [78] | No | No | In vitro | Monotherapy study | TH-302 antitumour activity was reported as dose-dependent. | ||
| Meng, et al. [79] | No | No | In vivo | Monotherapy study | TH-302 requires more severe hypoxia for to produce higher rates of anti-tumour activity. | ||
| Borad, et al. [27] | Yes | No | Phase II clinical trial (NCT01144455) |
229 | No XRT | First randomised Phase II clinical trial to demonstrate the potential outcomes of combining TH-302 with gemcitabine. Demonstrated improved tumour response and PFS (median 5.6 vs 3.6 months) compared to gemcitabine alone. | |
| Sun, et al. [80] | Yes | No | In vitro | No XRT | TH-302, gemcitabine and nab-paclitaxel were assessed as tolerable and providing favourable anti-tumour activity. | ||
| Wojtkowiak, et al. [81] | No | No | In vitro and in vivo | Monotherapy study | Identified biomarkers which may predict a significant decrease in tumour growth with TH-302. | ||
| Lohse, et al. [23] | No | Yes | In vitro | No chemotherapy | Reduced tumour growth rates demonstrate a strong predictor of OS for clinical application and efficacy of the treatment combination. | ||
| Van Cutsem, et al. [33] | Yes | No | Phase III ‘MAESTRO’ clinical trial (NCT01746979) | 693 | No full publication of results. No overall survival benefit with the treatment combination of TH-302 with gemcitabine (median of 8.7 months compared to 7.6 months for gemcitabine alone) | Treatment combination demonstrated favourable signs of antitumour activity regarding patient PFS (median of 5.5 months compared to 3.7 months for gemcitabine alone) and higher objective response rate. | |
| Hajj, et al. [30] | No | Yes | In vitro | Single-fraction 15 Gy | Combination produced significant growth delay compared to either TH-302 or XRT treatments alone. | ||
| Clofibrate | Xue, et al. [82] | No | Yes | In vivo | No chemotherapy Single-fraction 4 Gy |
Reduction in the affinity of haemoglobin for oxygen and thus acting as a radiosensitiser for pancreatic xenografts. | |
| Papaverine | Benej, et al. [25] | No | Yes | In vivo | No chemotherapy. | Significantly enhances tumour response to XRT in terms of LC and OS. | |
| PR-350 | Shibamoto, et al. [83] | No | Yes | In vitro and in vivo | Large amount required, therefore not predicted to have a high radiosensitising effect in clinical studies | Effective radiosensitiser in pancreatic cancer cell lines and xenografts. | |
| Sunamura, et al. [84] | No | Yes | Phase III clinical trial | 48 | Intraoperative XRT | PR-350 group showed higher survival rates and more effective control than the group that did not receive the radiosensitizer. | |
| Karasawa, et al. [85] | No | Yes | Phase III clinical trial | 47 | Intraoperative XRT No chemotherapy |
No difference in short-term survival. | |
| Metformin | Lipner, et al. [86] | No | No | In vitro | Monotherapy study. | All tested pancreatic cell lines were resistant to metformin. | |
| Benej, et al. [25] | No | Yes | In vivo | Metformin required 24 hours to reach full mitochondrial inhibition and clinical effectiveness | Papaverine was more suitable radiosensitiser, taking only 30 min to reach clinical effectiveness (similar to Atovaquone). | ||
| OXY111A | Limani, et al. [87] | No | No | Ib/IIa clinical trial (NCT02528526) |
69 | Study last updated at recruiting in 2015 | Pending results. Study aims to assess the safety, tolerability, and efficacy of the HAP. |
| PR-104 | Patterson, et al. [88] | Yes | Yes | In vitro | Single-fraction 10 Gy | Clinical benefit adding PR-104 to standard gemcitabine and XRT care. | |
| McKeage, et al. [89] | Yes | Phase Ib clinical trial (NCT00459836) |
42 | 4 patients with pancreatic cancer, remaining 3 had other diseases | PR-104 combined with docetaxel results in dose-limiting toxicities. |
HAP: hypoxia activated prodrug, PFS: progression-free survival, LC: local control, OS: overall survival, XRT: photon radiation therapy, Gy: Gray.
3.4. Stereotactic Body Radiation Therapy
The introduction of advanced XRT techniques and procedures such as breath-hold, real-time tumour tracking, respiratory motion reconstructions and soft tissue matching have allowed dose escalation to be safely administered. SBRT further refines the target conformality of XRT to feasibly deliver a higher Biologically Effective Dose (BED) in a shorter period in order to improve LC for LAPC patients from 79% to 94%, translating to an increased OS [3,36,37,38,39,40]. The SBRT chemoradiation trial results summarised in Table 2 are difficult to compare and interpret between studies due to the diversity of fractionation schemes, recording of statistics and inhomogeneity of recruited patients (e.g., respectability and disease status).
Table 2.
Review of stereotactic body radiation therapy (SBRT) pancreatic cancer clinical studies (2000–current).
| Author | Disease | Sample Size | Study Design | Chemotherapy | Total Dose (Gy) and Fractionation | Acute Side Effects Criteria for Adverse Events Version |
Late Side Effects | MST Months (Range) |
1-year OS Rate | 2-year OS Rate | PFS | FFLP | Median FU Period Months (Range) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang, et al. [22] Φ * | Unresectable LAPC | 77 | Retrospective, single institute, combination of phase I and phase II studies. | Variety of gemcitabine-based regimens | 25 in 1 | Grade ≥ 2 = 5% NR |
Grade ≥ 2 = 4% Grade ≥ 3 = 9% |
11.8 | 21% | NR | 1-year = 9% | 1-year = 84% | 6 (3–31) |
| Schellenberg, et al. [48] * | LAPC | 20 | Prospective, phase II trial, single institute. | Gemcitabine 1000 mg/m2 weekly (days 1, 8, and 15) | 25 in 1 | Grade ≥ 2 = 15% Grade 3 = 0% V3 [90] |
Grade ≥ 2 = 15% Grade ≥ 3 = 5% |
11.8 | 50% | 20% | Median time to progression was 9.2 months | 1-year = 94% | 2 patients remaining alive = 25.1–36.4 months |
| Schellenberg, et al. [41] * | LAPC | 16 | Prospective, phase II, single institute. | Gemcitabine 1000 mg/m2 weekly (days 1, 8, and 15) | 25 in 1 | Grade 2 = 13% Grade 3 = 6% V3 [90] |
Grade ≥ 2 = 33% Grade ≥ 3 = 13% |
11.4 | 50% | Estimate 18% | Median time to progression was 9.7 months. | 1-year = 100% | 9.1 (22.3 for living patients) |
| Hoyer, et al. [47] * | Unresectable LAPC | 22 | Prospective, phase II, single institute. | NR | 45 in 3 | Grade ≥ 2 = 100% NR |
Grade ≥ 2 = 94% | 5.4 | 5% | NR | 1-year = 9% Median time to progression was 4.8 months. |
Local control rate = 57% | 14 days–18 months |
| Wild, et al. [91] * | Recurrent | 18 | Reirradiation, retrospective, single institute. | 5-fluorouracil-based regimen for 10 patients Gemcitabine- based regimen for 7 patients |
25, 20 or 27 in 5 After chemoradiation of 50.4 Gy in 27 |
Grade 2 = 28% Grade 3 = 0% NR |
Grade 3 = 6% | 8.8 | NR | NR | Median = 3.7 months | 1-year = 62% | 34.3 (6.4–61.6) |
| Macchia, et al. [92] * | Unresectable disease or recurrent | 16 | Prospective, phase I, single institute. | Variety of chemotherapy regimens | 20–35 in 4–7 | Grade 1 = 50% Grade 2 = 0% NR |
Grade 3 = 6.3% | NR Overall response rate =56.2% |
NR | 50% | 2-year distant progression free = 58.7% | 2-year local progression free = 85.7% | 24 (10–85) |
| Didolkar, et al. [39] * | Unresectable LAPC | 85 | Retrospective, single institute. | Variety of gemcitabine-based regimens post-SBRT | 15–30 in 1–4 | NR acute compared to late Grade ≥ 3 = 22.3% V2 [93] |
18.6 | 50% Median 1-year = 13.4 months |
NR | NR | Local control = 91.7% | NR (25.8 months at last follow up) |
|
| Mahadevan, et al. [54] † | LAPC | 36 | Retrospective, single institute. | Gemcitabine 1000 mg/m2 weekly (for 6 months) | 24, 30 or 36 in 3 | Grade 1 = 42% Grade 2 = 25% Grade 3 = 8% NR |
Grade ≥ 3 = 6% | 14.3 | NR | NR | Median = 9.6 months | Local control = 78% | 24 (12–33) |
| Mahadevan, et al. [3] † | LAPC | 39 | Retrospective, single institute. | Gemcitabine 1000 mg/m2 weekly (for 6 months) | 24 or 30 in 3 | Grade 1 = 41% Grade 2 = 23% Grade 3 = 0% V3 [90] |
Grade 3 = 9% | 20 | NR | NR | Median = 15 months | Local control = 85% FFLP= 31% |
21 (6–33) |
| Lominska, et al. [94] † | LAPC | 28 | Reirradiation, retrospective, single institute. | Variety of chemotherapy regimens | 20–30 in 3–5 After 50.4 Gy XRT |
Grade 2 = 4% V3 [90] |
Grade 3 = 7% | 5.9 | 18% | NR | NR | 1-year = 70% | 5.9 (1–27) |
| Dagoglu, et al. [95] † | Recurrent | 30 | Reirradiation, retrospective, single institute. | Variety of chemotherapy regimens Gemcitabine for 14 patients FOLFOX for 6 patients Erlotinib for 12 patients None for 5 patients |
24–36 in 3–5 | Grade 3 = 11% NR |
Grade 3 = 7% | 14 | 50% | 5% | 78% | NR | 11 (4–24) |
| Tozzi, et al. [44] † | Unresectable LAPC = 21 Locally recurrent = 9 |
30 | Prospective, single institute (consecutive enrolment). | Variety of gemcitabine-based regimens | 36–45 in 6 | Grade 1 = 43% Grade 2 = 10% Grade 3= 0% V3 [90] |
Grade 3 = 0% | 11 | Median OS at 1-year = 47% | NR | Median PFS = 8 months | 1-year = 96% (for 45 Gy group) and 85% for others | 11.0 (2–28) |
| Gurka, et al. [96] † | LAPC (with elective nodes) | 10 | Prospective, single institute, pilot trial. | Concurrent gemcitabine with 1000 mg/m2 for 6 cycles | 25 in 5 | Grade 1 = 60% Grade 3 = 0% V3 [90] |
Grade 2 = 0% | 12.2 | NR | NR | 6.8 months | 1-year = 40% | Until death |
| Koong, et al. [42] * | LAPC | 15 | Prospective, single institute, phase I. | Prior to enrolment 2 patients received conventional 5-FU–based chemoradiation to a dose of 50 Gy and 1 patient received chemotherapy alone. | 15 (3 patients), 20 (5 patients), 25 (7 patients) in 1 | Grade 1 = 13% Grade 2 = 20% Grade 3 = 0% GI toxicities were scored according to the Radiation Therapy Oncology Group acute radiation morbidity criteria. |
NR | 11 | NR | NR | Median time to progression = 2 months | Local control = 75% | 5 |
| Koong, et al. [38] * | LAPC | 16 | Prospective, single institute, phase II. | Concurrent 5-fluorouracil | 45 in 25 (IMRT) and 25 in 1 (SBRT) | Grade 0= 18.7% Grade 1= 43.7% Grade 2= 25% Grade 3= 12.5% GI toxicities were scored according to the Radiation Therapy Oncology Group acute radiation morbidity criteria. |
NR | 8.3 | 15% | NR | Median time to progression = 4.38 months | 1-year = 8% Local control = 94% |
5.75 |
| Polistina, et al. [97] * | Unresectable LAPC | 33 | Prospective, single institute. | Gemcitabine 1000 mg/m2 weekly (for 6 weeks) | 30 in 3 | Grade 1 = 21.7% Grade 2 = 0% V3 [90] |
NR | 10.6 | 39.1% | 0% | Median time to progression = 7.3 months | 1-year = 82.6% | 9 |
| Rwigema, et al. [98] * | LAPC (mix of metastatic (11%), unresectable (56%) and recurrent disease (16%)) | 71 | Retrospective, single institute. | Variety of chemotherapy regimens | 18–25 in 1–3 | Grade 1 = 24% Grade 2 = 11.3% Grade 3 = 4.2% NR |
Grade 1 = 4.2% | 10.3 months overall median OS | 41% | NR | NR | Overall 1-year = 48.5% 1-year = 38% for unresectable 1-year = 18.8% for recurrent group 1-year = 40% for metastatic group |
12.7 (4–26) |
| Herman, et al. [55] * | Unresectable LAPC | 49 | Prospective single-arm, multi-institutional, phase II. | Gemcitabine 1000 mg/m2 (3 doses) followed by a week break prior to SBRT | 33 in 5 | Grade ≥ 2 = 2% V4 [99] |
Grade ≥ 2 = 11% | 13.9 (10.2–16.7) | 59% | 18% | Median PFS = 7.8 months 1-year = 32% 2-year = 10% |
1-year = 78% | 13.9 (3.9–45.2) |
| Chuong, et al. [37] † (and *) | Nonmetastatic LAPC (16 patients) and borderline resectable pancreatic cancer (57)) | 73 | Retrospective, single institute. | Induction gemcitabine-based regimens delivered over 3 cycles followed by SBRT |
35–50 in 5 | Grade ≥ 3 = 0 V4 [99] |
Grade ≥ 3 = 5.3% | 15 (LAPC) 16.4 (borderline) |
68.1% (LAPC) 72.2% borderline |
NR | Median PFS = 9.8 months 1-year PFS LAPC = 41% 1-year PFS borderline = 42.8% |
1-year LC for non-surgical patients= 81% | 10.5 (2.2–25.9) |
| Comito, et al. [40] * | Unresectable LAPC | 45 | Prospective, observational, single-arm, single institute, phase II. | 71% completed regimens 2 weeks prior to SBRT 19% received gemcitabine-based regimens |
45 in 6 | Grade 1–2 = 49% Grade ≥ 3 = 0% V3 [90] |
Grade 2 = 4% Grade ≥ 3 = 0% |
19 | 85% | 33% | Median PFS = 8 months | Median FFLP = 26 months 1-year = 87% 2-year = 87% |
13.5 months (6–48) |
| Gurka, et al. [36] * (and †) | Borderline resectable and inoperable LAPC | 38 | Retrospective, single institute. | Variety of gemcitabine-based regimens | 25–30 (one patient received 15) in 5 | Grade 2 = NR Grade 3 = 5.2% V3 [90] |
Grade 3 = 5.2% Grade 4 = 5.2% Grade 5 = 5.2% |
14.3 | NR | NR | 9.2 months | Local control rate = 79% | NR |
| Mellon, et al. [46] * | Borderline resectable and LAPC | 159 (110 BRPC and 49 LAPC) |
Retrospective, single institute. | Variety of induction chemotherapy regimens | 28–30 in 5 | Grade1-2 = 52% Grade 3 = 11% V4 [99] |
Grade 3 = 11% | 19.2 (borderline) 15 (LAPC) |
NR | NR | Event free survival = 11.9 months in borderline and 13.2 in LAPC |
1-year locoregional control = 78% | 5.6 (2.1–15.4) |
| Pollom, et al. [43] * | Unresectable (133), borderline resectable (11) pancreatic adenocarcinoma | 167 | Retrospective, single institute. | Variety of induction chemotherapy regimens (82% were gemcitabine-based) | 25 in 1 (76 patients) 25–45 in 5 (91 patients) |
Single-fraction: Grade ≥ 2 = 25% Multi-fraction: Grade ≥ 2 = 8.7% V4 [99] |
Single-fraction: Grade ≥ 3 = 12.3% Multi-fraction: Grade ≥ 3 = 5.6% |
13.6 | Single-fraction= 30.8% Multi- fraction= 34.9% |
NR | NR | NR | 7.9 (0.1–63.6) |
GI: gastrointestinal, MST: median survival time, OS: overall survival, XRT: photon radiation therapy, FU: follow up, NR: not reported, LAPC: locally advanced pancreatic cancer, IMRT: intensity modulated radiation therapy. Φ: Includes 40 patients from Schellenberg, et al. [41], Koong, et al. [42] and Koong, et al. [38]. *: OS measured from diagnosis †: OS measured from start of SBRT.
Single-fraction SBRT was originally investigated as advantageous based on convenience, reduction in interference of systemic therapy and intensification of dose in order to improve TCP. Chang et al. [22] is the largest retrospective study of the published single-fraction SBRT trials (including 40 participants from previous studies by Schellenberg et al. [41], Koong et al. [42] and Koong et al. [38]. Single-fraction SBRT for unresectable pancreatic cancer patients at Stanford University demonstrated promising LC rates between 75% and 100% but relatively unaffected MST (11–11.8 months). However, this large reported series was limited by a shorter median follow up time (5–9.1 months). Additionally, the inability for a single-fraction to exploit the reoxygenation of hypoxic tumour cells could have also potentially prevented the treatment modality from making a meaningful impact on survival rates.
Pollom, et al. [43] retrospectively analysed the outcomes of 167 patients who underwent either single- vs multi-fraction SBRT in a bid to determine the optimal radiation treatment schedule. Minimal difference existed for the survival rates between the single- and multi-fraction groups, with no compromise on LC. There were, however, significantly fewer acute grade > 2 and late grade ≥ 3 GI toxicities in multi-fraction SBRT, despite the multi-fraction group having a larger median PTV.
Acute GI toxicity for SBRT is not substantially different from that of conventionally fractionated XRT; however, the incidence of late toxicity remains a concern especially in the context of pre-surgical downstaging. Combined with concurrent chemotherapy several SBRT studies experienced a trade-off of LC for late grade ≥ 2 GI toxicities [44]. Though the quick course is favourable for LAPC clinical management, surgery is still the only curative treatment [5,45]. SBRT studies, by Mellon, et al. [46] and Chuong et al. [37] demonstrated 51% and 56% of borderline resectable patients, respectively, were able to undergo post-SBRT resection with high complete resection rates. Mellon et al.’s downstaging of initially unresectable disease successfully resulted in an increased median OS (14.0 months for unresected patients vs. 34.2 months for surgically resected patients) [46]. In addition, Chuong et al. [37] demonstrated that a further 10% of LAPC patients underwent post-SBRT resection with curative intent.
Previously, larger target margins (median planning target volume of 136 cm3) encompassed more of the duodenal mucosa and increased number of fields resulted in higher incidence of late grade ≥ 2 GI toxicities (up to 94% in Hoyer, et al.’s [47] multi-fraction SBRT study) [41]. More recently Chuong, et al.’s [37] and Comito, et al.’s [40] fractionated SBRT studies demonstrated a lower incidence of acute toxicities (grade 3 ≥ 0) for median planning target volumes of (111.01 and 64.7 cm3, respectively) paired with a high 1-year LC (≥ 81%) and MST (15 and 19 months, respectively) for LAPC patients.
Even with advancements in precision-guided protocols of XRT current limitations still exist in radiation resistance of the tumour microenvironment. SBRT remains controversial due to the decreased time for tumour reoxygenation [23,48,49]. In addition to reducing interruptions to systemic therapy and improving patient quality of life, the clinical impact of SBRT still remains modest at best. Therefore, future studies are required to better integrate systemic therapy with SBRT in order to improve OS whilst lowering integral dose to OAR. A gap which may possibly be exploited by particle therapy and the further development of systemic therapies such as HAPs.
3.5. Proton Therapy
Summary of PT clinical studies in Table 3 demonstrates an improved 2-year OS and MST ranging from 31–50.8% and 18.4–25.6 months [45,50]. The enhanced biological damage of PT radiation dose delivery compared to XRT (i.e., dose reporting) across the studies varies in terms of: RBE, cobalt Gray equivalents (CGE refers to absorbed dose × 1.1 (RBE) to express the biologic effective proton dose), Gray equivalents (GyE refers to proton physical dose (in Gray) × 1.1 (RBE)). We have used the individual publications’ definitions in our Table 3 of PT chemoradiation studies (Table 3).
Table 3.
Review of proton therapy pancreatic cancer clinical studies (2000–current).
| Author | Disease | Sample Size | Study Design | Chemotherapy | Total Dose and Fractionation | Acute Side Effects Criteria for Adverse Events Version |
Late Side Effects | MST Months (Range) |
1-year OS Rate | 2-year OS Rate | PFS | FFLP | Median FU Period Months (Range) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hiroshima, et al. [45] † | Unresectable LAPC | 42 | Retrospective, single institute. | Concurrent chemotherapy (gemcitabine (38 patients) or S-1 (3 patients)). | 50 GyE (12 patients) and 54–67.5 GyE (30 patients) in 25-33 |
Grade 1 = 9.5% Grade 2 = 36% Grade 3 = 40% Grade 4 = 4.8% V4 [99] |
Grade 1 = 7% Grade 2 = 4.8% Grade 3 = 0% |
25.6 | 77.8% | 50.8% | Median time to local recurrence = 36 months | 1-year LC rate = 83.3% 2-year LC rate = 78.9% |
14 (2.4–47.6) |
| Murphy, et al. [100] † | Borderline resectable PDAC | 48 | Prospective, single institute, phase II trial. | Neoadjuvant FOLFIRINOX (8 cycles). Vascular involvement resolution determined whether patients received short-course capecitabine or long-course fluorouracil or capecitabine chemoradiation. |
25 GyE in 5 | Grade 3 GI = 10% V4 [99] |
NR | 37.7 | NR | 56% | 14.7 months 2-year PFS = 43 |
NR | 18 |
| Sachsman, et al. [50] Φ | Unresectable LAPC |
11 | Prospective, single institute, phase II trial. | Concomitant capecitabine (1000 mg orally twice daily) 5 days/week |
59.4 Gy (RBE) in 33 | Grade 2 = 9% Grade 3 = 0% NR |
Grade 2 = 0% Grade 3 = 0% |
18.4 | 61% | 31% | 1-year =55% 2-year =14% |
1-year = 86% 2-year = 69% |
14 (5–25) For surviving patients 23 (8–25) |
| Terashima, et al. [52] § | LAPC (T3-T4) regardless of adjacency | 40 | Prospective, single institute, phase I/II trial. | Concurrent gemcitabine 800 mg/m2 weekly, for 30 min for the initial 3 weeks (days 1, 8, and 15) during 5 weeks of PT |
67.5 GyE in 25 | Grade 3 = 95% Grade 4 = 7.5% V3 [90] |
Grade 3 = 8% Grade 5 = 2% |
NR | 78.8% | NR | 1-year = 60.8% | 1-year = 79.9% | 12.1 (3.2–22.3) |
| Terashima, et al. [52] § | LAPC (T3-T4) adjacent to the GI |
5 | Prospective, single institute, phase I/II trial. | Concurrent gemcitabine 800 mg/m2 weekly, for 30 min for the initial 3 weeks (days 1, 8, and 15) during 5 weeks of PT |
50 GyE in 25 | Grade 3 = 100% V3 [90] |
NR | NR | NR | NR | NR | NR | 12.3 (8.2–18.6) |
| Terashima, et al. [52] § | LAPC (T3-T4) non-adjacent to the GI |
5 | Prospective, single institute, phase I/II trial. | Concurrent gemcitabine 800 mg/m2 weekly, for 30 min for the initial 3 weeks (days 1, 8, and 15) during 5 weeks of PT |
70.2 GyE in 26 | Grade 3 = 100% V3 [90] |
Grade 3 = 100% | NR | NR | NR | NR | NR | 19.6 (17.7–21.5) |
| Terashima, et al. [52] | Combined group | 50 | Prospective, single institute, phase I/II trial. | Concurrent gemcitabine 800 mg/m2 weekly, for 30 min for the initial 3 weeks (days 1, 8, and 15) during 5 weeks of PT |
50–70.2 GyE in 25–26 | NR | 76.8% | NR | 64.3% | 1-year = 81.7% | 12.5 | ||
| Nichols, et al. [59] | Pancreatic and ampullary adenocarcinoma Resected = 5, marginally resectable = 5, and unresectable = 12. |
22 | Prospective, single institute. | Concomitant capecitabine (1000 mg orally twice daily) |
50.4–59.4 CGE in 28–33 |
Grade 2 = 13.6% Grade 3 = 0% V4 [99] |
NR | 11= resected 14 = for marginally resectable 8.8 = unresectable |
NR | NR | NR | NR | 11 (5–36) |
| Hitchcock, et al. [60] | Unresectable LAPC | 15 | Retrospective, single institute. | Concomitant capecitabine (1000 mg orally twice daily) |
59.40 Gy (RBE) in 33 50.40 Gy (RBE) for 1 patient |
NR | NR | 24 (10–30) for 5 resected patients | NR | NR | NR | NR | NR |
| Takatori, et al. [53] * | Unresectable LAPC | 91 | Prospective, single institute. | Concurrent gemcitabine (800 mg/m2 on days 1, 8, and 15) for the initial 3 weeks during 5 weeks of PT |
67.5 GyE in 25 | NR Gastric/duodenal ulcers incidence = 49.4% V3 [90] |
Grade 4 GI = 1% Grade 5 GI = 2% |
NR | NR | NR | NR | NR | 10 |
| Hong, et al. [62] ^ | Resectable PDAC |
3 | Prospective, single institute, phase I trial. | Concurrent capecitabine at 825 mg/m2 orally twice daily | 25 Gy (RBE) in 5 | Grade 3 = 67% NR |
NR | NR | NR | NR | NR | NR | 12 |
| Hong, et al. [62] ^ | Resectable PDAC |
12 | Prospective, single institute, phase I trial. | Concurrent capecitabine at 825 mg/m2 orally twice daily | 30 Gy (RBE) in 10 | Grade 3 = 16% NR |
NR | NR | NR | NR | NR | NR | 12 |
| Combined Hong, et al. [62] |
Resectable PDAC |
15 | Prospective, single institute, phase I trial. | Concurrent capecitabine at 825 mg/m2 orally twice daily | 25–30 Gy (RBE) in 5–10 |
Grade 3 = 27% NR |
NR | NR | 75% | NR | Median relapse free survival was 10 months |
NR | 12 |
| Hong, et al. [63] | Resectable PDAC | 50 | Prospective, single institute phase I/II study. 15 patients from Hong, et al. [62] phase I study. |
Concurrent capecitabine at 825 mg/m2 orally twice daily. Gemcitabine for 6 months starting post-operative 4 to 10 weeks |
25 GyE in 5 | Grade 2 = 31.4% Grade 3 = 4.1% (phase II patients only) Grade 4 = 0% NR |
NR | 17.3 (11.2–29.2) months for non-resected For the 37 eligible resected patient = 27.0 (16.2–32.3) |
NR | 42% | 10.4 (7.5–17.1) months for non-resected For the 37 Eligible resected patient = 14.5 (10.2–21.8) Whole group = 10 |
Locoregional failure occurred = 16.2% Distant recurrence occurred = 72.9% |
12 patients alive at 38 months |
| Boimel, et al. [101] | Locally recurrent LAPC | 15 | Reirradiation study, retrospective, single institute. | Variety of chemotherapy regimens. 67% of patients received concurrent chemotherapy | 37.5–59.4 Gy (RBE) Prior radiation dose 30–59.4 Gy |
Grade ≥ 3 = 13% V4 [99] |
NR | 16.7 | 67% | NR | Distant metastasis free survival 1-year = 64% |
72% | 15.7 (2–48) |
| Jethwa, et al. [58] £ | LAPC | 13 | Retrospective, non-randomised, single institute. | Concurrent capecitabine 825mg/m2 twice daily. 2 patients received concurrent 5-fluorouracil 225mg/m2 |
50 Gy (RBE) in 25 | Grade 1 = 46% Grade 2 = 15% Grade ≥ 3 = 0% V4 [99] |
NR | NR | 62% | 40% | 1-year local control rate = 66% | 1- and 2- year freedom from distant metastasis rate = 53% and 28% | 16 (9–24) |
| Kim, et al. [57] | LAPC (4 recurrent, 1 metastatic) | 37 | Retrospective, non-randomised, single institute. | Variety of chemotherapy regimens. 21.6% patients received induction chemotherapy | 45 and 30 GyE in 10 | Anaemia: Grade 1 = 32.4% Grade 2 = 8.1% Leukopenia: Grade 1 = 21.4% Grade 2 = 2.7% Grade 1 thrombocytopenia= 2.7% Grade 1 abdominal pain = 16.2% Anorexia: Grade 1 = 10.8% Grade 2 = 8.1% Stomatitis: Grade 1 = 2.7% Grade 2 = 2.7% Vomiting: Grade 1 = 8.1% Grade 2 = 5.4% Grade ≥ 3 = 0% V4 [99] |
Grade 1-2 = NR Grade ≥ 3 = 0% |
19.3 | OS rates = 75.7% | NR | Relapse free survival = 33.2% | 64.8% | 16.7 months (2.3–32.1 months) |
| Maemura, et al. [71] | Unresectable LAPC | 25 | Prospective, non-randomised, single institute. | Induction and concurrent chemotherapy (gemcitabine or S-1) | 50 Gy (XRT) or 67.5 GyE (PT) in 25 | XRT = higher incidence of haematological toxicity, grade 3 = 3 patients PT = grade 2 or 3 gastric ulcer = 2 patients V4 [99] |
NR | XRT = 23.4 PT = 22.3 |
XRT = 86.7% PT = 80% |
XRT = 33.3% PT = 45% |
Median time (15.4 months) to progression was the same across both PT and XRT. | Local progression: XRT = 40% PT = 60% Disease control rates: XRT = 93% PT = 80% |
NR |
| Tseng, et al. [64] ‡ * | Resectable LAPC | 47 | Retrospective, single institute. | Concurrent neoadjuvant capecitabine (825 mg/m2 twice daily over 1 week (41 patients) or 2 weeks (6 patients) for 5 days a week |
25 GyE in 5 | Grade 1 = 51% Grade 2 = 4% Grade ≥ 3 = 0% V3 [90] |
NR | NR | NR | NR | NR | NR | 8.5 (7 days–18.6 months) |
RBE: relative biological effectiveness, GI: gastrointestinal, MST: median survival time, OS: overall survival, XRT: photon radiation therapy, PT: proton therapy, FU: follow up, NR: not reported, LAPC: locally advanced pancreatic cancer, PFS: progression-free survival, PDAC: pancreatic ductal adenocarcinoma. Φ: OS measured from start of treatment. *: follow up from the end of radiation. †: OS measured from start of chemoradiation. §: Terashima, et al. [52] reported on 3 dosing protocols based on disease. Separate results are reported where available, ‡: Patient overlap with Hong, et al. [62]. ^: Hong, et al. [62] reported on 2 dosing levels. Separate results are reported where available. £: Jethwa, et al. [58] reported on 2 chemotherapy regimens. Separate results are reported where available. Dose to target volumes: RBE: Relative biological effective dose. CGE: Cobalt Gray equivalents (absorbed dose × 1.1 (RBE) to express the biologic effective proton dose). GyE: Gray equivalents (proton physical dose (in Gray) × 1.1 (RBE)).
Several current PT studies compare their work to 3DCRT trial data which delivered a significantly higher integral dose than newer treatment modalities. The rate of upper GI toxicity was compared with 3DCRT studies which inherently use larger radiation field sizes. Studies as late as 2012 still compared dose to the irradiated small bowel and side effects from PT with patients undergoing 3DCRT, demonstrating a gap between conventional XRT and particle therapy [51]. As such further studies are required to compare the latest techniques (IMRT) in order to compare the clinical efficiency of current particle therapy treatment.
Studies previously linked aggressive radiation therapy dose (67.5–70.2 GyE), concomitant delivery of full-dose gemcitabine and size and/or orientation of radiation therapy fields (inclusion of prophylactic nodal regions) to an increase in high grade GI toxicities. As seen in the Table 3, incidence of grade 3 GI toxicity largely varies across PT studies from 0% and 50%.
First attempts at dose escalation performed by Terashima, et al. [52] and Takatori, et al. [53] observed a particularly high incidence (50%) of GI radiation-induced ulcers of 50 and 126 enrolled patients, respectively. The two studies from Hyogo Ion Beam Medical Center employed a significantly a higher prescription of 2.5 to 2.7 GyE per fraction compared to other PT studies in Table 3. Late grade ≥ 3 GI effects (10%) incidence was significantly higher than SBRT such as Mahadevan, et al.’s [54] and Herman, et al.’s [55] SBRT studies (6%). Speculation surrounded contributing factors such as radiation field size (including regional lymph nodes) and orientation increased the rate of upper GI toxicities [56]. These factors combined with the higher proton RBE reported for the distal end of the Bragg peak may have also influenced higher occurrence rates of late Grade 3 GI toxicities [12].
Ongoing developments in PT have demonstrated favourable dosimetry in order to facilitate hypofractionation and dose escalation. Kim, et al.’s [57] and Jethwa, et al.’s [58] recent retrospective studies observed no late or acute grade ≥ 3 GI toxicities. Kim, et al.’s [57] multivariate analysis identified that induction chemotherapy was a significant factor for overall survival (21.6 months vs. 16.7 months). MST remained mildly improved (19.3 months) for patients with localized inoperable disease until Hiroshima et al.’s latest PT study. Hiroshima, et al. [45] finessed higher dose delivery (50 GyE with a 17.5 GyE boost) through opposed anterior-posterior beam arrangement reporting an increased MST of 25.6 months and 1-year OS of 77.8%. Altering Terashima et al.’s additional boost field by 10% of the dose prescription, Hiroshima, et al. [45] demonstrated no grade ≥ 2 GI acute or grade ≥ 3 GI late adverse events. 17 patients who received the concomitant boost up to 67.5 GyE demonstrated an improved median OS of 42.5 months and median time to local recurrence of >36 months. Concluding a PT dose of up to 67.5 GyE predicts a significant improvement in OS and LC of pancreatic cancer patients.
To date, while PT clinical results have had a positive impact on MST and OS for pancreatic cancer treatment, no chemoradiation combination has resulted in a statistically significant increase in survival. Investigations into concomitant capecitabine by Sachsman et al. [50] and Nichols, et al.’s [59] reported no acute grade 3 GI toxicities and minimal acute grade 2 GI toxicities (9% and 13.6%, respectively). All 3 patients who received grade 2 acute GI toxicities in Nichols, et al. [59] prospective PT trial received anterior and lateral beams. Based on evidence that a more heavily weighted posterior-anterior field eliminated grade 2 GI toxicity and improved medians weight lost, this arrangement was then modified for the successive 19 patients.
Concern regarding the increased risk of surgical complications and late effects of combined therapy on the GI tract tissue prior to treatment have guided investigations into post-operative PT. Suggesting that post-operative PT may fail due to the extended time period required for the surgery to heal, Hitchcock et al.’s [60] pre-operative study demonstrated five initially unresectable patients becoming resectable and resultant improved median OS of 24 months (range, 10–30).
Pre-operative chemoradiation reduces resource (and cost) demand and required appointments for a patient to attend compared to long fractionation schedules. Tolerability of short course pre-operative radiation therapy has proven feasible in combination with capecitabine [61]. In particular, PT studies by Hong, et al. [62], Hong, et al. [63] and Tseng, et al. [64] demonstrated improved surgical resection and tolerability (acute grade ≥ 3 GI toxicity ≤ 27%).
PT treatment schemes for LAPC patients currently being investigated include the feasibility proton reirradiation after SBRT, combination therapy or simultaneous integrated boost in PT [65]. Reviews have emphasised the requirement for multimodality treatment exploration in controlled clinical trials in order to make a meaningful impact on LAPC outcome [4]. However, this does not fix the fundamental issue of hypoxia and/or improve the outcomes of OS. More investigations into the effectiveness and long-term outcomes of PT for LAPC are therefore required. Pairing dose escalation and concomitant boost technique and the physical advantages of PT with improved systemic drugs could further improve treatment outcomes.
3.6. Carbon Ion
At the time of this review, only four C-ion chemoradiation studies had published the outcomes for LAPC patients, all from Japan. A summary of these studies along with published recruiting and withdrawn studies are in Table 4.
Table 4.
Review of carbon ion therapy pancreatic cancer clinical studies (2000–current).
| Author | Disease | Sample Size | Study Design | Chemotherapy | Total Dose and Fractionation | Acute Side Effects Criteria for Adverse Events Version |
Late Side Effects | MST Months | 1-year OS Rate | 2-year OS Rate | PFS | FFLP | Median FU Period Months (Range) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shinoto, et al. [68] Φ | Unresectable LAPC | 64 | Retrospective, single institute. | Gemcitabine or S-1 | 55.2 Gy (RBE) in 12 | Grade 2 = 26% Grade 3 = 6% V4 [99] |
Grade 2 = 6% Grade ≥3 = 0% |
25.1 | 84% | 53% | 2-year = 23% | 2-year LC = 82% | 24.4 (5.1–46.1) |
| Kawashiro, et al. [17] Φ | Unresectable LAPC | 72 | Retrospective, non-randomized, multi- institutional study. | 68% received concurrent gemcitabine (1000 mg/m2 weekly) | 52.8 Gy (RBE) or 55.2 Gy (RBE) in 12 | Grade 2 = 44% Grade 3 = 28.1% Grade 4 = 1% V4 [99] |
Grade 1 = 99% Grade 2 = 0% Grade 3 = 1% |
21.5 | 73% | 46% | Local recurrence incidence at 1- year and 2- year = 16% and 24% | Distant metastasis-free survival at 1-year and 2-year = 41% and 28%. Median distant metastasis-free survival = 8.3 months |
13.6 (2.8–37.9) For surviving patients 14.7 (3.2–37.5) |
| Combs, et al. [102] | LAPC | 33 | Prospective, phase I, single institute. | Concurrent gemcitabine (300 mg/m2) | 45–53 GyE in 3 | PHOENIX-01 trial withdrawn (before enrolment). | |||||||
| Shinoto, et al. [67] Φ | Unresectable LAPC | 72 | Prospective, single institute. | Concurrent gemcitabine (400–100 mg/m2) on days 1,8 and 15. | 43.2–55.2 GyE in 12 | Grade 1 ≥ GI ulcer = 15% Grade ≥ 3 haematologic toxicities = 53% V3 [90] |
Grade 3 = 1.4% | 19.6 | 73% | 35% overall For ≥ 45.6 GyE group 2-year OS = 48% |
The median time to progression was 5.9 months. 86% experienced distant metastases |
1-year = 92% 2-year = 83% |
≥ 2 years |
| Shinoto, et al. [70] Φ | Potentially resectable LAPC | 26 | Phase 1, single institute. | NR | 30–36.8 GyE in 8 | Grade 1 = 3.8% Grade 3 = 3.8% V2 [93] |
Grade 4 = 3.8% | 18.6 | 69% For patient who underwent surgical resection = 81% |
NR | No patients experienced local recurrence. distant metastasis in 65% of patients |
81% of patients underwent surgery. 5-year survival rates for all 26 patients and for those who underwent surgery were 42% and 52% |
33.8 |
| Shinoto, et al. [103] | LAPC | 45 | Phase II, single institute. | Concurrent S-1 administered orally twice a day (80 mg/m2) for 28 days every 6 weeks. | 55.2 GyE in 12 | Currently recruiting | |||||||
RBE: relative biological effectiveness, GI: gastrointestinal, MST: median survival time, OS: overall survival, RT: radiation therapy, FU: follow up, NR: not reported, LAPC: locally advanced pancreatic cancer, PFS: Progression-free survival. Φ: OS measured from start of treatment. Dose to target volumes: RBE: relative biological effective dose. GyE: Gray equivalents (carbon physical dose (in Gray) × (RBE)).
The National Institute of Radiological Sciences, is responsible for most of the published particle therapy reports in pancreatic cancer patients, demonstrating higher LC using the heavy C-ion therapy. Despite these promising preliminary results, the use of C-ion for these radioresistant tumours is vastly under-explored; contributing to 5.4% of their workload [66]. C-ion studies available for comparison (Table 4) demonstrate similar survival rates across Table 2 and Table 3 in terms of median OS, 1- and 2-year FFLP/LC. However, C-ion provided similarly high rates of GI toxicities as PT.
As demonstrated in Table 4, current C-ion studies successfully delivered up to 55.2 GyE in 12 fractions. Shinoto, et al. [67] performed the first observational trial to combine full-dose gemcitabine with an escalated C-ion dose (55.2 GyE) demonstrating similar survival (MST of 19.6 months and 2-year FFLP of 83%) to Hiroshima et al.’s PT study (MST of 25.6 months and 2-year LC of 78.9%) for LAPC patients. Current recommendations for C-ion LAPC treatment are therefore full-dose gemcitabine (1000 mg/m2) with 55.2 GyE in 12 fractions.
Shinoto, et al. [68], more recently, validated the efficacy and safety of 55.2 GyE, demonstrating an increase in OS rates (2-year OS rose from 48% in 2016 to 53% in 2018) with acceptable late (3%) and acute (0%) grade 3 GI toxicities. The maximum tolerated dose was evaluated as safe, under the conditions of respiratory-gating and stringent selection criteria. Although the enrolled participants demonstrated a range of target volume to GI tract distances (range: 0 mm to ≥ 10 mm) not all patients were selected for C-ion therapy. Patient eligibility for C-ion remains based on the relationship between tumour to GI tract distance and achievability of dose constraints, potentially skewing the incidence and severity of GI toxicities as only patients with favourable GI tract distances were selected for these trials.
3.7. Gaps in Particle Therapy for Pancreatic Cancer
Similar complications are of concern with C-ion as with PT, including radiation-induced ulcers in the stomach and duodenum and intraoperative fibrosis. Fukumitsu, et al.’s [65] PT simulation study correlated an increase in GI toxicities to proximity of target volume to GI tract, causing it to receive increased high radiation dose. Kawashiro, et al. [69] tested the feasibility of C-ion finding it non-ideal for tumours located in close proximity (≥ 5 mm) to the GI tract (unrelated to the anatomical location within the pancreas head, body or tail).
Another contributing factor to increased radiation-induced ulcers is the determination/uncertainties of RBE and biological optimization using planning algorithms for PT and C-ion. RBE for particle therapy is a complex function and estimated planned dose may vary when clinically translated, contributing to unforeseen acute and/or late toxicities. Feasibility and tolerability of the physical properties of C-ion chemoradiation such as a higher concentration of particles and concern of fibrosis in a pre-operative setting was initially tested by Shinoto, et al. [70]. A 5-year survival rate of 52% was estimated for the 21 patients who underwent surgical resection. The reduction in penumbra of C-ion reduced the damage to surrounding normal tissue, resulting in minimal change to the tissue during surgical resection and negligible fibrosis (with a similar time delay between RT and surgery between both studies).
As previously discussed in PT; the high rates of toxicity have been attributed to several confounding factors including aggressive radiation therapy dose (67.5–70.2 GyE), high dose per fraction (2.7 GyE), concomitant delivery of ≥800 mg/m2 gemcitabine and size and/or orientation of radiation therapy fields (inclusion of prophylactic nodal regions).
Shinoto, et al.’s [70] C-ion study resulted in patterns of initial disease progression (65% of the patients experiencing distant metastasis and 8% regional recurrence) in the absence of chemotherapy. Established as a broad ranging anti-tumour treatment gemcitabine is the most widely recommended chemotherapy agent to reduce this risk of distant metastasis and regional recurrence, the major mode of LAPC treatment failure. However, administration of gemcitabine has been linked to a higher incidence of grade ≥ 3 haematological toxicities across chemoradiation trials (Table 2, Table 3 and Table 4). Maemura, et al.’s [71] comparative PT study of 25 patients (10 undergoing PT and 15 undergoing hyper-fractionated XRT) only had two patients develop grade ≥ 2 gastric ulcers, still appearing advantageous compared to XRT and C-ion regarding high grade haematological toxicity when gemcitabine was employed. In fact, Hiroshima, et al.’s [45] PT trial reported all grade ≥ 3 and 4 events were haematologic and correlated with full-dose gemcitabine and/or speculated as high doses to the spleen (as previously described in XRT studies [72,73]).
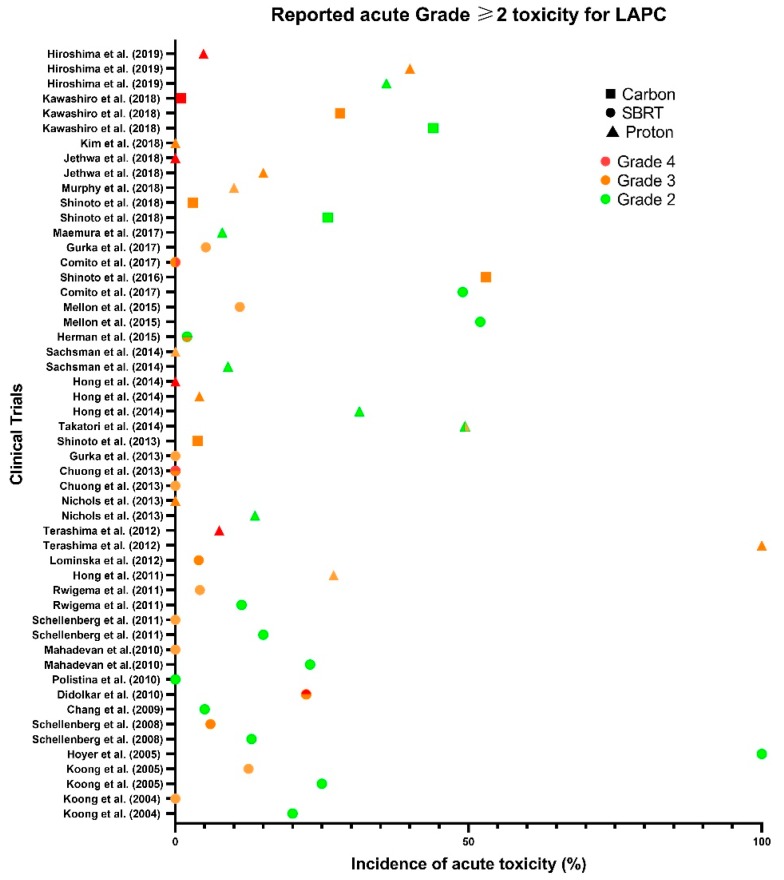
Developments in particle therapy have marginally improved MST, LC and OS in recent years, whilst marginally reducing the incidence and grade of GI toxicities compared to XRT (Figure 2 and Figure 3). However, high-grade haematological toxicities experienced seem to be irrespective of modality type, and with a requirement for effective systematic therapies to prevent metastases, there seems to be no solution yet (Figure 4) [17,67]. As many patients in Table 4 experienced multiple toxicities, it is difficult to interpret the individual and compounded impact of C-ion chemoradiation.
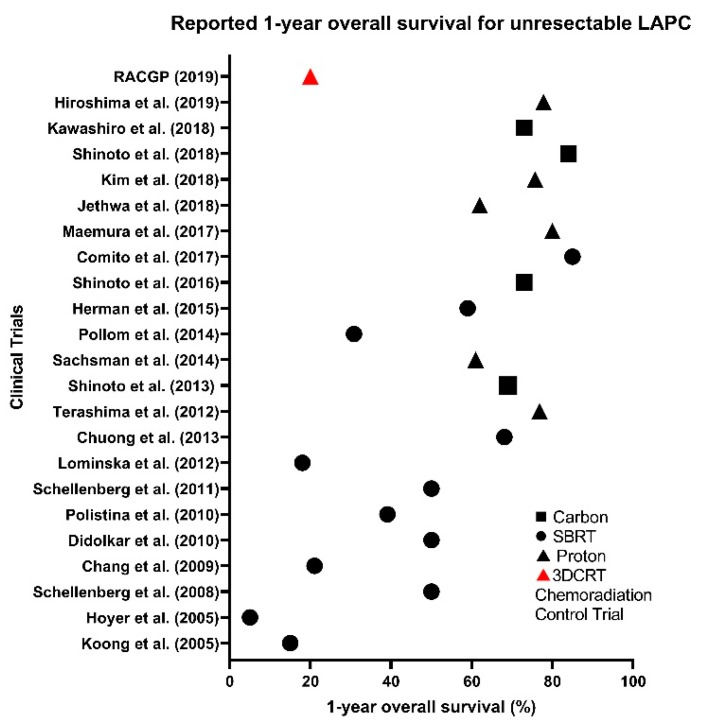
Figure 2.
Reported 1-year overall survival (OS) rate for unresectable LAPC across SBRT (●), proton (▲) and carbon (∎) clinical trials compared to 3D conformal chemoradiation (▲) control trial.
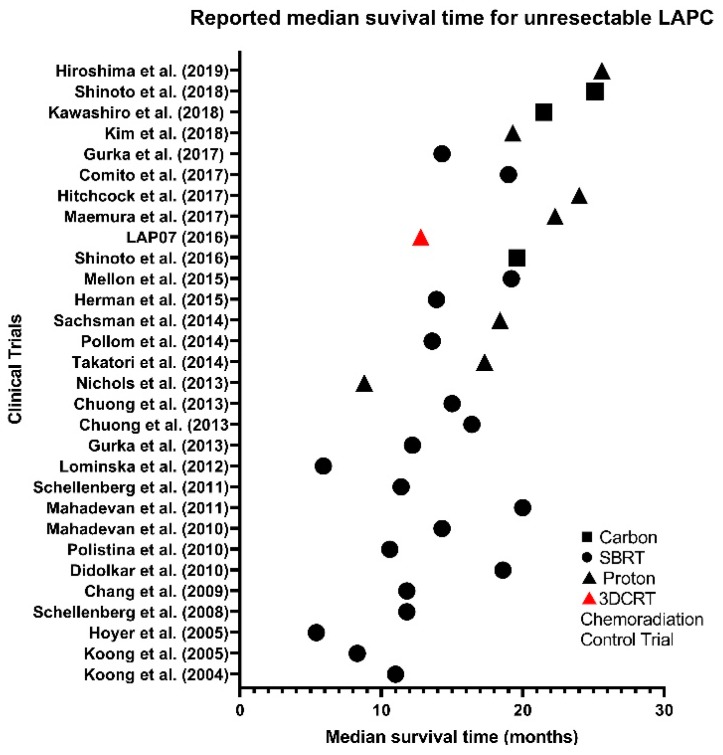
Figure 3.
Reported median survival time (MST) for unresectable LAPC across SBRT (●), proton (▲) and carbon (∎) clinical trials compared to 3D conformal chemoradiation (▲) control trial.
Figure 4.
Reported acute Grade ≥ 2 toxicity for resectable and unresectable LAPC across SBRT (●), proton (▲) and carbon (∎) clinical trials. Prescribed dose and fractionation varied across studies.
In the context of tolerability, 53% of Shinoto, et al.’s [67] C-ion patients experienced grade ≥ 3 acute haematologic toxicities, which related to the type and dose of gemcitabine as learnt in Terashima, et al.’s [52] and Takatori, et al.’s [53] PT studies. Seven of Shinoto, et al.’s [67] 11 enrolled patients treated with 55.2 GyE developed grade 1 or 2 ulcers (and 1 patient a grade 3) which may be due to the exclusion of patients who had direct invasion of tumour into the mucosal GI tract surface reducing GI toxicities, as the treatment field was not in close proximity as it was in PT. The retrospective nature of this study, therefore, had the potential for selection bias; patients could be excluded if not consecutively enrolled. The lower rate of grade 3 ulcers in Kawashiro, et al.’s [17] and Shinoto, et al.’s [68] C-ion studies could also be attributed to selection bias of patients as candidates were selected depending on the lesions contact with the GI tract. Selection bias of these results in Table 4 is difficult to translate to a wider population; improved eligibility criteria is therefore required for C-ion studies.
3.8. Limitations and Future Work
Throughout this review, several recurring methodological issues were present, which reduces the ability to directly compare and deduce the current status of studies across Table 2, Table 3 and Table 4. Limited details existed in the reporting guidelines of studies such as the date from which OS was measured and the Common Terminology Criteria for Adverse Events used (if there was one recorded), hindering their reproducibility. Studies in this review measure OS statistics either from diagnosis or from the start date of treatment (or both), which reduces the ability to compare the results and, if incorrectly interpreted by researchers, significantly affect expectations of clinical trials. The Common Toxicity Criteria ranged across studies; which may have resulted in different grading of adverse effects.
Optimal therapeutic strategies need to be investigated now that PT has been confirmed as safe and effective. PT studies focussed on escalating dose whilst minimising grade 3 GI side effects (which hinder surgical resection in pre-operative setting and reduce quality of life). Many studies were difficult to directly compare as the methodology, dose, timing and type of chemotherapy, RT technique, patient position, contrast, daily filling of stomach, beam delivery technique (scattering or scanning) and disease were vastly different.
The experience with heavy particles is limited to a few institutions, and no conclusion can yet be drawn about their effectiveness or toxicity [74]. Institutions have determined the efficacy of particle therapy compared to XRT, with several prospective and retrospective studies indicating an improvement in outcomes using PT for LAPC. It is difficult to conclude in lieu of randomized control studies with only eight groups having published preliminary clinical data on the treatment of pancreatic cancer patients with PT. Furthermore, PT is often prescribed on the basis of insurance coverage and insufficient evidence and lack of cost-benefit effectiveness to support the funding of PT clinical trials have left a gap in our understanding of the role of PT in LAPC [58]. Potentially pancreatic cancer patients without access to PT could be enrolled in multi-national trials to overcome the ethical equipoise of prescribing XRT modality.
As evident in Table 3, the clinical progress of combined particle therapy is vastly underexplored, and further studies are necessary to obtain more robust data on its effectiveness and toxicity. There needs to be a more detailed examination of the relationship between irradiation dose and outcome [45]. Patients often experienced more than one toxicity throughout their course of treatment (e.g., haematological and GI). Not only are toxicities often recorded only by incidence of grade, they are often not distinguished by type (haematological or GI). It is increasingly hard to decipher the impact of radiation versus systemic therapy, resulting in gaps of knowledge for patient outcomes. More attention needs to be placed on the systemic and consolidative therapies now that the effectiveness of particle therapy has improved, which could reduce the haematological toxicities. Additionally, QUANTEC data [75] is currently based on dose-volume analyses of rectal and cervical cancer patients [72,76]. Finally, there is an increasing requirement to evaluate and estimate the true RBE value of particle therapy treatment.
Inconsistencies in methodology, recording parameters and guidelines have prevented the safety and technical aspects of particle therapy to be fully defined. Investigations are beginning to perform longer follow up times and employ more transparent enrolment criteria (i.e., image staging of LAPC). As evident in this evaluation further research is required worldwide in reporting guidelines.
Future trials should therefore focus on alternate systemic therapies which reduces the risk of distant failures whilst minimising toxicity when combined with particle therapy. Before pairing them with rapidly advancing targeted therapeutic agents, more stringent consistency in reporting is required to deduce the accumulative effect of systemic therapies.
4. Conclusions
There is a clear requirement for aggressive multimodality therapy to tackle the unmet clinical demands of pancreatic cancer. Particle therapy is clearly associated with better LC; however, translating this into improved OS will require ongoing investigations into systemic therapies. Phase II trials are required to prospectively validate the results presented in this review. For comparable conclusions to be drawn these international trials would require a consensus on the prescription and reporting guidelines.
Improvements in imaging and medical biomarkers have recently allowed us to identify hypoxic regions. Despite this, a limited number of studies address the biological and clinical challenges of pancreatic cancer suggestive of why attempts at current RT have proven unremarkable. More research and clinical investigations are required which consider the tumour biology and systemic combined therapy to improve patient survival and NTCP. This paper demonstrates that the targeting of hypoxic regions within pancreatic tumours using HAPs compliments already established chemoradiation regimens. Particle therapy still requires improvements in systemic therapy as minor progress has been noted in chemoradiation alone, even with advancing modalities and techniques.
Appendix A
| Search Term | Hits | |
|---|---|---|
| 1 | (proton therap * or proton beam * or proton pencil beam *).mp. | 6280 |
| 2 | (carbon ion therap * or carbon beam *).mp. | 425 |
| 3 | 1 or 2 | 6594 |
| 4 | (Radiol * or radiotherapy * or radiation therap * or chemoradio * or Radiosurger * or stereotactic * or SBRT or SABR or ablative * or dose escalat * or dose-escalat *).mp. | 719,296 |
| 5 | exp Radiotherapy | 177,645 |
| 6 | 4 or 5 | 732,871 |
| 7 | 3 and 6 | 5310 |
| 8 | ((vasodilator * or oxygenat * or vessel * or dilat * or oxygen * or vasostimula * or hypox * or oxygen-mimetic or hypoxia-activated) adj3 (radiosensiti* or activated? or prodrug * or agent * or stimulated * or therapy? or drug * or pharmaceutical? or drug * or HAP)).mp. | 80,168 |
| 9 | exp Pancreatic Neoplasms | 72,324 |
| 10 | ((Pancreas or pancreatic or LAPC or PDAC or ductal) adj3 (cancer * or neoplasm * or malignan * or tumor? * or carcinoma ? or adenocarcinoma ?)).mp. | 117,214 |
| 11 | 9 or 10 | 119,161 |
| 12 | 6 and 8 and 11 | 32 |
| 13 | 7 and 8 and 11 | 1 |
| 14 | 12 or 13 | 32 |
| 15 | 7 or 11 | 70 |
| 16 | 14 or 15 | 101 |
| 17 | Limit 16 to English language | 95 |
| 18 | Limit 17 to yr = “2000–Current” | 87 |
*: symbol used to include a variation of word endings.
Appendix B
Exclusion criteria:
Conference abstracts
Case studies
Dosimetric studies
3D conformal radiation therapy treatment
Author Contributions
M.D., M.S., P.W. and E.B. designed the review; M.D., M.S. and E.B. screened the abstracts (if a consensus was not met a fourth opinion was sought from P.W.); M.D. interpreted the data and drafted the article; M.S., P.W. and E.B. helped revise and draft the manuscript; M.D., M.S., P.W. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported through the Australian Government Research Training Program Scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
Availability of Data and Materials
Data supporting the results reported in the article can be found on the University of South Australia database and are available upon request.
References
- 1.Rawla P., Sunkara T., Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sereti E., Karagianellou T., Kotsoni I., Magouliotis D., Kamposioras K., Ulukaya E., Sakellaridis N., Zacharoulis D., Dimas K. Patient Derived Xenografts (PDX) for personalized treatment of pancreatic cancer: Emerging allies in the war on a devastating cancer? J. Proteom. 2018;188:107–118. doi: 10.1016/j.jprot.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Mahadevan A., Miksad R., Goldstein M., Sullivan R., Bullock A., Buchbinder E., Pleskow D., Sawhney M., Kent T., Vollmer C., et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:e615–e622. doi: 10.1016/j.ijrobp.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 4.Nichols R., Huh S., Li Z., Rutenberg M. Proton therapy for pancreatic cancer. World J. Gastrointest. Oncol. 2015;7:141–147. doi: 10.4251/wjgo.v7.i9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohopolski M., Glaser S., Vargo J., Balasubramani G., Beriwal S. Stereotactic body radiotherapy for locally-advanced unresectable pancreatic cancer-patterns of care and overall survival. J. Gastrointest. Oncol. 2017;8:766–777. doi: 10.21037/jgo.2017.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauffert B., Mornex F., Bonnetain F., Rougier P., Mariette C., Bouché O., Bosset J., Aparicio T., Mineur L., Azzedine A., et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann. Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 7.Loehrer P., Feng Y., Cardenes H., Wagner L., Brell J., Cella D., Flynn P., Ramanathan R., Crane C., Alberts S., et al. Gemcitabine Alone Versus Gemcitabine Plus Radiotherapy in Patients With Locally Advanced Pancreatic Cancer: An Eastern Cooperative Oncology Group Trial. J. Clin. Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammel P., Huguet F., van Laethem J., Goldstein D., Glimelius B., Artru P., Borbath I., Bouche O., Shannon J., Andre T., et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Josef E., Shields A., Vaishampayan U., Vaitkevicius V., El-Rayes B., McDermott P., Burmeister J., Bossenberger T., Philip P. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Josef E., Schipper M., Francis I., Hadley S., Ten-Haken R., Lawrence T., Normolle D., Simeone D., Sonnenday C., Abrams R., et al. A Phase I/II Trial of Intensity Modulated Radiation (IMRT) Dose Escalation with Concurrent Fixed-dose Rate Gemcitabine (FDR-G) in Patients With Unresectable Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall E., Giaccia A. Radiobiology for the Radiologist. 7th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2012. [Google Scholar]
- 12.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014;59:R419. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 13.Choi J., Kang J. Basics of particle therapy II: Relative biological effectiveness. Radiat. Oncol. 2012;30:1–13. doi: 10.3857/roj.2012.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fossati P., Matsufuji N., Kamada T., Karger C. Radiobiological issues in prospective carbon ion therapy trials. Med. Phys. 2018;45:e1096–e1110. doi: 10.1002/mp.12506. [DOI] [PubMed] [Google Scholar]
- 15.Habermehl D., Ilicic K., Dehne S., Rieken S., Orschiedt L., Brons S., Haberer T., Weber K.-J., Debus J., Combs S. The relative biological effectiveness for carbon and oxygen ion beams using the raster-scanning technique in hepatocellular carcinoma cell lines. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0113591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weyrather W., Kraft G. RBE of carbon ions: Experimental data and the strategy of RBE calculation for treatment planning. Radiother. Oncol. 2004;73:S161–S169. doi: 10.1016/S0167-8140(04)80041-0. [DOI] [PubMed] [Google Scholar]
- 17.Kawashiro S., Yamada S., Okamoto M., Ohno T., Nakano T., Shinoto M., Shioyama Y., Nemoto K., Isozaki Y., Tsuji H., et al. Multi-institutional Study of Carbon-ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int. J. Radiat. Oncol. Biol. Phys. 2018;101:1212–1221. doi: 10.1016/j.ijrobp.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 18.Mistry I., Thomas M., Calder E., Conway S., Hammond E. Clinical Advances of Hypoxia-Activated Prodrugs in Combination with Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017;98:1183–1196. doi: 10.1016/j.ijrobp.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Koshy M., Landry J., Cavanaugh S., Fuller C., Willett C., Abrams R., Hoffman J., Thomas C. A challenge to the therapeutic nihilism of ESPAC-1. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:965–966. doi: 10.1016/j.ijrobp.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Regine W., Winter K., Abrams R., Safran H., Hoffman J., Konski A., Benson A., Macdonald J., Kudrimoti M., Fromm M., et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 21.Koong A., Mehta V., Le Q., Fisher G., Terris D., Brown J., Bastidas A., Vierra M. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:919–922. doi: 10.1016/S0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 22.Chang D., Schellenberg D., Shen J., Kim J., Goodman K., Fisher G., Ford J., Desser T., Quon A., Koong A. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 23.Lohse I., Rasowski J., Cao P., Pintilie M., Do T., Tsao M., Hill R., Hedley D. Targeting hypoxic microenvironment of pancreatic xenografts with the hypoxia-activated prodrug TH-302. Oncotarget. 2016;7:33571–33580. doi: 10.18632/oncotarget.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu J., Saiyin H., Fu D., Li J. Stroma—A Double-Edged Sword in Pancreatic Cancer: A Lesson from Targeting Stroma in Pancreatic Cancer With Hedgehog Signaling Inhibitors. Pancreas. 2018;47:382–389. doi: 10.1097/MPA.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 25.Benej M., Hong X., Vibhute S., Scott S., Wu J., Graves E., Le Q., Koong A., Giaccia A., Yu B., et al. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc. Natl. Acad. Sci. USA. 2018;115:10756–10761. doi: 10.1073/pnas.1808945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng Y., Ma J., Zhan Y., Xu X., Zeng Q., Liang J., Chen X. Hypoxia-activated prodrugs and redox-responsive nanocarriers. Int. J. Nanomed. 2018;13:6551–6574. doi: 10.2147/IJN.S173431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borad M., Reddy S., Bahary N., Uronis H., Sigal D., Cohn A., Schelman W., Stephenson J., Chiorean G., Rosen P., et al. Randomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients with Advanced Pancreatic Cancer. J. Clin. Oncol. 2015;33:1475–1481. doi: 10.1200/JCO.2014.55.7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss G., Infante J., Chiorean G., Borad M., Bendell J., Molina J., Tibes R., Ramanathan R., Lewandowski K., Jones S., et al. Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of TH-302, a Hypoxia-Activated Prodrug, in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 29.Wardman P. Chemical Radiosensitizers for Use in Radiotherapy. J. Clin. Oncol. 2007;19:397–417. doi: 10.1016/j.clon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Hajj C., Russell J., Hart C., Goodman K., Lowery M., Haimovitz-Friedman A., Deasy J., Humm J. A Combination of Radiation and the Hypoxia-Activated Prodrug Evofosfamide (TH-302) is Efficacious against a Human Orthotopic Pancreatic Tumor Model. Transl. Oncol. 2017;10:760–765. doi: 10.1016/j.tranon.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nytko K., Grgic I., Bender S., Ott J., Guckenberger M., Riesterer O., Pruschy M. The hypoxia-activated prodrug evofosfamide in combination with multiple regimens of radiotherapy. Oncotarget. 2017;8:23702–23712. doi: 10.18632/oncotarget.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindner L. Hypoxia-activated prodrug: An appealing preclinical concept yet lost in clinical translation. Lancet Oncol. 2017;18:991–993. doi: 10.1016/S1470-2045(17)30401-1. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E., Lenz H., Furuse J., Tabernero J., Heinemann V., Ioka T., Bazin I., Ueno M., Csõszi T., Wasan H., et al. MAESTRO: A randomized, double-blind phase III study of evofosfamide (Evo) in combination with gemcitabine (Gem) in previously untreated patients (pts) with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma (PDAC) J. Clin. Oncol. 2016;34:4007. doi: 10.1200/JCO.2016.34.15_suppl.4007. [DOI] [Google Scholar]
- 34.Larue R., Van De Voorde L., Berbée M., van Elmpt W., Dubois L., Panth K., Peeters S., Claessens A., Schreurs W., Nap M., et al. A phase 1 ‘window-of-opportunity’ trial testing evofosfamide (TH-302), a tumour-selective hypoxia-activated cytotoxic prodrug, with preoperative chemoradiotherapy in oesophageal adenocarcinoma patients. BMC Cancer. 2016;16:644. doi: 10.1186/s12885-016-2709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chawla S.P., Cranmer L.D., Van Tine B.A., Reed D.R., Okuno S.H., Butrynski J.E., Adkins D.R., Hendifar A.E., Kroll S., Ganjoo K.N. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 2014;32:3299–3306. doi: 10.1200/JCO.2013.54.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurka M., Kim C., He A., Charabaty A., Haddad N., Turocy J., Johnson L., Jackson P., Weiner L., Marshall J., et al. Stereotactic Body Radiation Therapy (SBRT) Combined With Chemotherapy for Unresected Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. 2017;40:152–157. doi: 10.1097/COC.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuong M., Springett G., Freilich J., Park C., Weber J., Mellon E., Hodul P., Malafa M., Meredith K., Hoffe S., et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:516–522. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Koong A., Christofferson E., Le Q., Goodman K., Ho A., Kuo T., Ford J., Fisher G., Greco R., Norton J., et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Didolkar M., Coleman C., Brenner M., Chu K., Olexa N., Stanwyck E., Yu A., Neerchal N., Rabinowitz S. Image-Guided Stereotactic Radiosurgery for Locally Advanced Pancreatic Adenocarcinoma Results of First 85 Patients. J. Gastrointest. Surg. 2010;14:1547–1559. doi: 10.1007/s11605-010-1323-7. [DOI] [PubMed] [Google Scholar]
- 40.Comito T., Cozzi L., Clerici E., Franzese C., Tozzi A., Iftode C., Navarria P., D’Agostino G., Rimassa L., Carnaghi C., et al. Can Stereotactic Body Radiation Therapy Be a Viable and Efficient Therapeutic Option for Unresectable Locally Advanced Pancreatic Adenocarcinoma? Results of a Phase 2 Study. Technol. Cancer Res. Treat. 2017;16:295–301. doi: 10.1177/1533034616650778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schellenberg D., Goodman K., Lee F., Chang S., Kuo T., Ford J., Fisher G., Quon A., Desser T., Norton J., et al. Gemcitabine Chemotherapy and Single-Fraction Stereotactic Body Radiotherapy for Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 42.Koong A., Le Q., Ho A., Fong B., Fisher G., Cho C., Ford J., Poen J., Gibbs I., Mehta V., et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Pollom E., Alagappan M., von Eyben R., Kunz P., Fisher G., Ford J., Poultsides G., Visser B., Norton J., Kamaya A., et al. Single-versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: Outcomes and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2014;90:918–925. doi: 10.1016/j.ijrobp.2014.06.066. [DOI] [PubMed] [Google Scholar]
- 44.Tozzi A., Comito T., Alongi F., Navarria P., Iftode C., Mancosu P., Reggiori G., Clerici E., Rimassa L., Zerbi A., et al. SBRT in unresectable advanced pancreatic cancer: Preliminary results of a mono-institutional experience. Radiat. Oncol. 2013;8:1–8. doi: 10.1186/1748-717X-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiroshima Y., Fukumitsu N., Saito T., Numajiri H., Murofushi K., Ohnishi K., Nonaka T., Ishikawa H., Okumura T., Sakurai H. Concurrent chemoradiotherapy using proton beams for unresectable locally advanced pancreatic cancer. Radiother. Oncol. 2019;136:37–43. doi: 10.1016/j.radonc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Mellon E., Hoffe S., Springett G., Frakes J., Strom T., Hodul P., Malafa M., Chuong M., Shridhar R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54:979–985. doi: 10.3109/0284186X.2015.1004367. [DOI] [PubMed] [Google Scholar]
- 47.Hoyer M., Roed H., Sengelov L., Traberg A., Ohlhuis L., Pedersen J., Nellemann H., Kiil Berthelsen A., Eberholst F., Engelholm S., et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother. Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Schellenberg D., Kim J., Christman-Skieller C., Chun C., Columbo L., Ford J.M., Fisher G.A., Kunz P.L., Van Dam J., Quon A., et al. Single-Fraction Stereotactic Body Radiation Therapy and Sequential Gemcitabine for the Treatment of Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 49.De Bari B., Porta L., Mazzola R., Alongi F., Wagner A., Schäfer M., Bourhis J., Ozsahin M. Hypofractionated radiotherapy in pancreatic cancer: Lessons from the past in the era of stereotactic body radiation therapy. Crit. Rev. Oncol. Hematol. 2016;103:49–61. doi: 10.1016/j.critrevonc.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Sachsman S., Nichols R., Morris C., Zaiden R., Johnson E., Awad Z., Bose D., Ho M., Huh S., Li Z., et al. Proton Therapy and Concomitant Capecitabine for Non-Metastatic Unresectable Pancreatic Adenocarcinoma. Int. J. Part. Ther. 2014;1:692–701. doi: 10.14338/IJPT.14-00006.1. [DOI] [Google Scholar]
- 51.Huang J., Robertson J., Ye H., Margolis J., Nadeau L., Yan D. Dose–Volume Analysis of Predictors for Gastrointestinal Toxicity After Concurrent Full-Dose Gemcitabine and Radiotherapy for Locally Advanced Pancreatic Adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:1120–1125. doi: 10.1016/j.ijrobp.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Terashima K., Demizu Y., Hashimoto N., Jin D., Mima M., Fujii O., Niwa Y., Takatori K., Kitajima N., Sirakawa S., et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother. Oncol. 2012;103:25–31. doi: 10.1016/j.radonc.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 53.Takatori K., Terashima K., Yoshida R., Horai A., Satake S., Ose T., Kitajima N., Kinoshita Y., Demizu Y., Fuwa N. Upper gastrointestinal complications associated with gemcitabine-concurrent proton radiotherapy for inoperable pancreatic cancer. J. Gastroenterol. 2014;49:1074–1080. doi: 10.1007/s00535-013-0857-3. [DOI] [PubMed] [Google Scholar]
- 54.Mahadevan A., Jain S., Goldstein M., Miksad R., Pleskow D., Sawhney M., Brennan D., Callery M., Vollmer C. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:735–742. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 55.Herman J., Chang D., Goodman K., Dholakia A., Raman S., Hacker-Prietz A., Iacobuzio-Donahue C., Griffith M., Pawlik T., Pai J., et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nichols R., Hoppe B. RE: Takatori K, Terashima K, Yoshida R, Horai A, Satake S, Ose T, Kitajima N, Kinoshita, Y., Demizu, Y., Fuwa, N. Upper gastrointestinal complications associated with gemcitabine-concurrent proton radiotherapy for inoperable pancreatic cancer. J Gastroenterol. 2013; (E-pub only) J. Gastrointest. Oncol. 2013;4:E33–E34. doi: 10.3978/j.issn.2078-6891.2013.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim T., Lee W., Woo S., Kim H., Oh E., Lee J., Han S., Park S., Suh Y., Moon S., et al. Effectiveness and Safety of Simultaneous Integrated Boost-Proton Beam Therapy for Localized Pancreatic Cancer. Technol. Cancer Res. Treat. 2018;17:1–9. doi: 10.1177/1533033818783879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jethwa K., Tryggestad E., Whitaker T., Giffey B., Kazemba B., Neben-Wittich M., Merrell K., Haddock M., Hallemeier C. Initial experience with intensity modulated proton therapy for intact, clinically localized pancreas cancer: Clinical implementation, dosimetric analysis, acute treatment-related adverse events, and patient-reported outcomes. Adv. Radiat. Oncol. 2018;3:314–321. doi: 10.1016/j.adro.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols R., George T., Zaiden R., Awad Z., Asbun H., Huh S., Ho M., Mendenhall N., Morris C., Hoppe B. Proton therapy with concomitant capecitabine for pancreatic and ampullary cancers is associated with a low incidence of gastrointestinal toxicity. Acta Oncol. 2013;52:498–505. doi: 10.3109/0284186X.2012.762997. [DOI] [PubMed] [Google Scholar]
- 60.Hitchcock K., Nichols R., Morris C., Bose D., Hughes S., Stauffer J., Celinski S., Johnson E., Zaiden R., Mendenhall N., et al. Feasibility of pancreatectomy following high-dose proton therapy for unresectable pancreatic cancer. World J. Gastrointest. Surg. 2017;9:103–108. doi: 10.4240/wjgs.v9.i4.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chuong M., Badiyan S., Yam M., Li Z., Langen K., Regine W., Morris C., Snider J., Mehta M., Huh S., et al. Pencil beam scanning versus passively scattered proton therapy for unresectable pancreatic cancer. J. Gastrointest. Oncol. 2018;9:687–693. doi: 10.21037/jgo.2018.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong T., Ryan D., Blaszkowsky L., Mamon H., Kwak E., Mino-Kenudson M., Adams J., Yeap B., Winrich B., DeLaney T., et al. Phase I study of preoperative short-course chemoradiation with proton beam therapy and capecitabine for resectable pancreatic ductal adenocarcinoma of the head. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:151–157. doi: 10.1016/j.ijrobp.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 63.Hong T., Ryan D., Borger D., Blaszkowsky L., Yeap B., Ancukiewicz M., Deshpande V., Shinagare S., Wo J., Boucher Y., et al. A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:830–838. doi: 10.1016/j.ijrobp.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng Y., Wo J., Ancukiewicz M., Adams J., Depauw N., Mamon H., Hong T. Dosimetric predictors of nausea and vomiting: An exploratory analysis of a prospective phase I/II trial with neoadjuvant accelerated short-course radiotherapy and capecitabine for resectable pancreatic cancer. J. Radiat. Oncol. 2013;2:427–434. doi: 10.1007/s13566-013-0114-7. [DOI] [Google Scholar]
- 65.Fukumitsu N., Okumura T., Hiroshima Y., Ishida T., Numajiri H., Murofushi K., Ohnishi K., Aihara T., Ishikawa H., Tsuboi K., et al. Simulation study of dosimetric effect in proton beam therapy using concomitant boost technique for unresectable pancreatic cancers. Jpn. J. Radiol. 2018;36:456–461. doi: 10.1007/s11604-018-0743-2. [DOI] [PubMed] [Google Scholar]
- 66.Mohamad O., Makishima H., Kamada T. Evolution of Carbon Ion Radiotherapy at the National Institute of Radiological Sciences in Japan. Cancers. 2018;10:66. doi: 10.3390/cancers10030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shinoto M., Yamada S., Terashima K., Yasuda S., Shioyama Y., Honda H., Kamada T., Tsujii H., Saisho H. Carbon Ion Radiation Therapy with Concurrent Gemcitabine for Patients With Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016;95:498–504. doi: 10.1016/j.ijrobp.2015.12.362. [DOI] [PubMed] [Google Scholar]
- 68.Shinoto M., Terashima K., Suefuji H., Matsunobu A., Toyama S., Fukunishi K., Shioyama Y. A single institutional experience of combined carbon-ion radiotherapy and chemotherapy for unresectable locally advanced pancreatic cancer. Radiother. Oncol. 2018;129:333–339. doi: 10.1016/j.radonc.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 69.Kawashiro S., Mori S., Yamada S., Miki K., Nemoto K., Tsuji H., Kamada T. Dose escalation study with respiratory-gated carbon-ion scanning radiotherapy using a simultaneous integrated boost for pancreatic cancer: Simulation with four-dimensional computed tomography. Br. J. Radiol. 2017;90:1–7. doi: 10.1259/bjr.20160790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinoto M., Yamada S., Yasuda S., Imada H., Shioyama Y., Honda H., Kamada T., Tsujii H., Saisho H. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer. 2013;119:45–51. doi: 10.1002/cncr.27723. [DOI] [PubMed] [Google Scholar]
- 71.Maemura K., Mataki Y., Kurahara H., Kawasaki Y., Iino S., Sakoda M., Ueno S., Arimura T., Higashi R., Yoshiura T., et al. Comparison of proton beam radiotherapy and hyper-fractionated accelerated chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2017;17:833–838. doi: 10.1016/j.pan.2017.07.191. [DOI] [PubMed] [Google Scholar]
- 72.Zschaeck S., Blümke B., Wust P., Kaul D., Bahra M., Riess H., Klein F., Sinn M., Pelzer U., Budach V., et al. Dose-escalated radiotherapy for unresectable or locally recurrent pancreatic cancer: Dose volume analysis, toxicity and outcome of 28 consecutive patients. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0186341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu C.-P., Shi J., Chen Y.-X., Xie W.-F., Lin Y. Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: A meta-analysis. Radiother. Oncol. 2011;99:108–113. doi: 10.1016/j.radonc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Mitin T., Zietman A. Promise and pitfalls of heavy-particle therapy. J. Clin. Oncol. 2014;32:2855–2863. doi: 10.1200/JCO.2014.55.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emami B., Lyman J., Brown A., Coia L., Goitein M., Munzenrider J., Shank B., Solin L., Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-Y. [DOI] [PubMed] [Google Scholar]
- 76.Kavanagh B., Pan C., Dawson L., Das S., Li X., Ten Haken R., Miften M. Radiation Dose–Volume Effects in the Stomach and Small Bowel. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:S101–S107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 77.Borad M., Chiorean E., Molina J., Mita A., Infante J., Schelman W., Traynor A., Vlahovic G., Mendelson D., Reddy S. Clinical benefits TH-302, a tumor-selective, hypoxia-activated prodrug, and gemcitabine in first-line pancreatic cancer (PanC) J. Clin. Oncol. 2011;29:265. doi: 10.1200/jco.2011.29.4_suppl.265. [DOI] [Google Scholar]
- 78.Sun J., Liu Q., Wang J., Ahluwalia D., Ferraro D., Wang Y., Duan J., Ammons S., Curd J., Matteucci M., et al. Selective Tumor Hypoxia Targeting by Hypoxia-Activated Prodrug TH-302 Inhibits Tumor Growth in Preclinical Models of Cancer. Clin. Cancer Res. 2012;18:758–770. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- 79.Meng F., Evans J., Bhupathi D., Banica M., Lan L., Lorente G., Duan J., Cai X., Mowday A., Guise C., et al. Molecular and Cellular Pharmacology of the Hypoxia-Activated Prodrug TH-302. Mol. Cancer Ther. 2012;11:740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- 80.Sun J., Liu Q., Ahluwalia D., Li W., Meng F., Wang Y., Bhupathi D., Ruprell A., Hart C. Efficacy and safety of the hypoxia-activated prodrug TH-302 in combination with gemcitabine and nab-paclitaxel in human tumor xenograft models of pancreatic cancer. Cancer Biol. Ther. 2015;16:438–449. doi: 10.1080/15384047.2014.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wojtkowiak J., Cornnell H., Matsumoto S., Saito K., Takakusagi Y., Dutta P., Kim M., Zhang X., Leos R., Bailey K., et al. Pyruvate sensitizes pancreatic tumors to hypoxia-activated prodrug TH-302. Cancer Metab. 2015;3:2. doi: 10.1186/s40170-014-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue J., Zhu W., Song J., Jiao Y., Luo J., Yu C., Zhou J., Wu J., Chen M., Ding W.Q., et al. Activation of PPARα by clofibrate sensitizes pancreatic cancer cells to radiation through the Wnt/β-catenin pathway. Oncogene. 2017;37:953–962. doi: 10.1038/onc.2017.401. [DOI] [PubMed] [Google Scholar]
- 83.Shibamoto Y., Kubota T., Kishii K., Tsujitani M. Radiosensitivity of human pancreatic cancer cells in vitro and in vivo, and the effect of a new hypoxic cell sensitizer, doranidazole. Radiother. Oncol. 2000;56:265–270. doi: 10.1016/S0167-8140(00)00181-X. [DOI] [PubMed] [Google Scholar]
- 84.Sunamura M., Karasawa K., Okamoto A., Ogata Y., Nemoto K., Hosotani R., Nishimura Y., Matsui K., Matsuno S. Phase III Trial of Radiosensitizer PR-350 Combined with Intraoperative Radiotherapy for the Treatment of Locally Advanced Pancreatic Cancer. Pancreas. 2004;28:330–334. doi: 10.1097/00006676-200404000-00023. [DOI] [PubMed] [Google Scholar]
- 85.Karasawa K., Sunamura M., Okamoto A., Nemoto K., Matsuno S., Nishimura Y., Shibamoto Y. Efficacy of novel hypoxic cell sensitiser doranidazole in the treatment of locally advanced pancreatic cancer: Long-term results of a placebo-controlled randomised study. Radiother. Oncol. 2008;87:326–330. doi: 10.1016/j.radonc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 86.Lipner M., Marayati R., Deng Y., Wang X., Raftery L., O’Neil B., Yeh J. Metformin Treatment Does Not Inhibit Growth of Pancreatic Cancer Patient-Derived Xenografts. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Limani P., Linecker M., Kron P., Samaras P., Pestalozzi B., Stupp R., Jetter A., Dutkowski P., Mullhaupt B., Schlegel A., et al. Development of OXY111A, a novel hypoxia-modifier as a potential antitumor agent in patients with hepato-pancreato-biliary neoplasms-Protocol of a first Ib/IIa clinical trial. BMC Cancer. 2016;16:812. doi: 10.1186/s12885-016-2855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patterson A., Ferry D., Edmunds S., Gu Y., Singleton R., Patel K., Pullen S., Hicks K., Syddall S., Atwell G., et al. Mechanism of Action and Preclinical Antitumor Activity of the Novel Hypoxia-Activated DNA Cross-Linking Agent PR-104. Clin. Cancer Res. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 89.McKeage M., Jameson M., Ramanathan R., Rajendran J., Gu Y., Wilson W., Melink T., Tchekmedyian N.S. PR-104 a bioreductive pre-prodrug combined with gemcitabine or docetaxel in a phase Ib study of patients with advanced solid tumours. BMC Cancer. 2012;12:496–505. doi: 10.1186/1471-2407-12-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events (version 3) [(accessed on 29 January 2019)]; Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf.
- 91.Wild A., Hiniker S., Chang D., Tran P., Khashab M., Limaye M., Laheru D., Le D., Kumar R., Pai J., et al. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: Experience from two institutions. J. Gastrointest. Oncol. 2013;4:343–351. doi: 10.3978/j.issn.2078-6891.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macchia G., Morganti A., Cilla S., Ippolito E., Massaccesi M., Picardi V., Mattiucci G., Bonomo P., Tambaro R., Pacelli F., et al. Quality of life and toxicity of stereotactic radiotherapy in pancreatic tumors: A case series. Cancer Investig. 2012;30:149–155. doi: 10.3109/07357907.2011.640649. [DOI] [PubMed] [Google Scholar]
- 93.Cancer Therapy Evaluation Program Common Toxicity Criteria (version 2) [(accessed on 6 June 2019)]; Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
- 94.Lominska C., Unger K., Nasr N., Haddad N., Gagnon G. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat. Oncol. 2012;7:74. doi: 10.1186/1748-717X-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dagoglu N., Callery M., Moser J., Tseng J., Kent T., Bullock A., Miksad R., Mancias J., Mahadevan A. Stereotactic Body Radiotherapy (SBRT) Reirradiation for Recurrent Pancreas Cancer. J. Cancer. 2016;7:283–288. doi: 10.7150/jca.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gurka M., Collins S., Slack R., Tse G., Charabaty A., Ley L., Berzcel L., Lei S., Suy S., Haddad N., et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat. Oncol. 2013;8:1–9. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Polistina F., Costantin G., Casamassima F., Francescon P., Guglielmi R., Panizzoni G., Febbraro A., Ambrosino G. Unresectable locally advanced pancreatic cancer: A multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann. Surg. Oncol. 2010;17:2092–2101. doi: 10.1245/s10434-010-1019-y. [DOI] [PubMed] [Google Scholar]
- 98.Rwigema J., Parikh S., Heron D., Howell M., Zeh H., Moser A., Bahary N., Quinn A., Burton S. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am. J. Clin. Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 99.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events (version 4) [(accessed on 29 January 2019)]; Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf.
- 100.Murphy J., Wo J., Ryan D., Jiang W., Yeap B., Drapek L., Blaszkowsky L., Kwak E., Allen J., Clark J., et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:963–969. doi: 10.1001/jamaoncol.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boimel P., Berman A., Li J., Apisarnthanarax S., Both S., Lelionis K., Larson G., Teitelbaum U., Lukens J., Ben-Josef E., et al. Proton beam reirradiation for locally recurrent pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2017;8:665–674. doi: 10.21037/jgo.2017.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Combs S., Habermehl D., Kieser M., Dreher C., Werner J., Haselmann R., Jakel O., Jager D., Buchler M., Debus J. Phase I study evaluating the treatment of patients with locally advanced pancreatic cancer with carbon ion radiotherapy: The PHOENIX-01 trial. BMC Cancer. 2013;13:1–8. doi: 10.1186/1471-2407-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shinoto M., Shioyama Y., Suefuji H., Matsunobu A., Toyama S., Kudo S. A phase II clinical trial of carbon-ion radiotherapy and concurrent S-1 chemotherapy for locally advanced pancreatic cancer. J. Clin. Oncol. 2015;33 doi: 10.1200/jco.2015.33.3_suppl.tps504. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the results reported in the article can be found on the University of South Australia database and are available upon request.