Abstract
Background
Traditional Chinese medicine (TCM) was the main form of treatment in China for psychiatric illnesses until the development of antipsychotic drugs in the 1950's. Antipsychotic drugs have become the primary intervention for schizophrenia, although herbal medicines can still form part of the treatment.
Objectives
To review Chinese herbal medicine, used alone or as part of a TCM approach, for people with schizophrenia and related psychoses.
Search methods
We undertook electronic searches of the Cochrane Schizophrenia Group's register (December 2003), the Traditional Chinese Medical Literature Analysis and Retrieval Database (TCMLARS) (October 2003), Chinese Biomedical Database (CBM) (December 2003), China National Knowledge Infrastructure Database (May 2004), Complementary Medicine Database (AMED) (December 2003). We contacted the Chinese Cochrane Centre, the Cochrane Complementary Medicine Field and first authors of included studies and inspected reference lists for additional studies.
Selection criteria
We included all relevant randomised controlled trials involving people with schizophrenia‐like illnesses, allocated to Chinese herbal medicine, including any Chinese herbs (single or mixture), compared with placebo/no treatment or antipsychotic drugs.
We updated this search July 2012 and added 45 new trials to the awaiting classification section.
Data collection and analysis
We independently extracted data and calculated fixed effects relative risk (RR), the 95% confidence intervals (CI) for homogeneous dichotomous data, and, where appropriate, the number needed to treat (NNT) on an intention‐to‐treat basis. For continuous data, we calculated weighted mean differences (WMD).
Main results
Only one small trial of the seven included studies truly evaluated TCM for schizophrenia. The other trials evaluated Chinese herbs for schizophrenia. We found one study comparing Chinese herbal medicine with antipsychotic drugs. Data for the global state outcome 'no change/worse' favoured people allocated to antipsychotic medication (n=90, RR 1.88 CI 1.2 to 2.9, NNH 4 CI 2 to 12). Six trials compared Chinese herbal medicine in combination with antipsychotic with antipsychotic drugs alone. One trial found global state 'not improved/worse' favoured the herbal medicine/antipsychotic combination (n=123, RR 0.19 CI 0.1 to 0.6, NNT 6 CI 5 to 11). Two studies (n=103) also found short‐term data from the Clinical Global Impression scale favoured the herbal medicine plus antipsychotic group (WMD ‐0.46 CI ‐0.9 to ‐0.1) compared with those given only antipsychotics. Significantly fewer people in the experimental group left the study early compared with those given antipsychotics alone (n=1004, 6 RCTs, RR 0.30 CI 0.16 to 0.58, NNT 21 CI 18 to 35). Reports of constipation were significantly lower in the treatment group compared to those receiving antipsychotics (n=67, 1 RCT, RR 0.03 CI 0.0 to 0.5, NNH 2 CI 2 to 4).
Authors' conclusions
Chinese herbal medicines, given in a Western biomedical context, may be beneficial for people with schizophrenia when combined with antipsychotics. Traditional Chinese medicine is also under‐evaluated, but results from one pioneering study that attempted to evaluate TCM should encourage further trials.
Note: the 45 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.
Keywords: Humans; Antipsychotic Agents; Antipsychotic Agents/therapeutic use; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Medicine, Chinese Traditional; Randomized Controlled Trials as Topic; Schizophrenia; Schizophrenia/drug therapy
Plain language summary
Chinese herbal medicine for schizophrenia
Antipsychotic medication is the mainstay of treatment for people with schizophrenia, and although effective, still leaves some people with distressing symptoms and/or disabling adverse effects. Safer and more effective health care interventions are needed.
Traditional Chinese medicine (TCM) has been used to treat mental health disorders, including schizophrenia, for more than 2000 years. Chinese herbs may also have antipsychotic properties when used in a Western biomedical context. In this review we sought and found trials relevant to the effects of both approaches for schizophrenia. Traditional Chinese medicine methodology has been evaluated for schizophrenia, but the one included study was too limited in terms of sample size and study length to guide good practice. However, this pioneering study does show that TCM can be evaluated for its efficacy for people with schizophrenia , and should encourage trialists to undertake further, more comprehensive trials in this area.
The use of Chinese herbs in a Western medicine context, without incorporating TCM methodology, has been evaluated in six trials, although again these are limited by their sample size and study length. The results of these six trials suggest that using Chinese herbs alone for psychotic symptoms may not be indicated, but if used in conjunction with Western antipsychotic drugs, they may be beneficial in terms of mental state, global functioning and decrease of adverse effects. However, further trials are needed before the effects of TCM for people with schizophrenia can be evaluated with any real confidence.
Background
Antipsychotic drugs have been the mainstay of treatment for schizophrenia since the early 1950s. While effective for some, these treatments still leave many with disabling symptoms or adverse effects. Perhaps because of the obvious shortcomings of manufactured drugs, herbal medicines are commonly used for psychiatric purposes in both developing and developed countries (Walter 1999). It has been reported that some Chinese herbal medicines are effective for psychosis and that combination treatments (drugs plus herbs) are useful to enhance antipsychotic efficacy, or reduce the period of recovery and adverse effects (Saku 1991, Luo 1997, Wang 1998). In addition Chinese herbal medicines may be more accessible, socially acceptable, tolerable and inexpensive than the more conventional drugs available from the pharmaceutical industry.
Chinese medicine (now commonly referred to as traditional Chinese medicine or TCM) has been used to treat schizophrenia‐like illnesses (i.e. Dian Kuang/withdrawal mania) for over 2000 years (Ming 2001). Traditional Chinese medicine includes several health interventions: Chinese herbal medicine, acupuncture, Tui Na, exercise and dietary therapy. Its theories for the aetiology, pathology and treatment of schizophrenia are different to those of Western medicine. Schizophrenia is diagnosed, primarily by operationalised criteria such as the Diagnostic and Statistical Manual (DSM), the International Classification of Diseases (ICD) or their equivalents, and it is diagnosed in China by the Chinese Classification of Mental Disorders (CCMD). Traditional Chinese medicine uses different methodology to diagnose mental health disorders such as schizophrenia by pattern differentiation. The four diagnostic methods (inspection, listening/smelling, inquiry and palpation) are the means to obtain patient information (Xu 1991). This information is then analysed using one or several diagnostic models (Zang Fu theory, Eight Principles i.e. yin/yang, interior/exterior, excess/deficiency, hot/cold, also Four Levels, Six Divisions, 5 phases, Qi and Blood, Fluids and humours, Three burners) to differentiate the pattern and form a diagnosis. Thus, two people diagnosed with schizophrenia could nevertheless, from a TCM perspective, have different clinical features and require different medicines. There are five main patterns which fall within the disease category of Dian Kuang/withdrawal mania which may also include the Western diagnosis of schizophrenia. The five types are: 1. Phlegm‐fire; 2. Phlegm‐damp; 3. Qi stagnation with blood stasis; 4. Hyperactivity of fire due to yin deficiency; 5. other miscellaneous types (Zhang 1996).
Chinese herbal medicine is a generic term that encompasses plants, fungi, resins, animal and mineral substances. Its materia medica principally consists of medicinal plants, although there are approximately 30 animal and 15 mineral substances listed (Li 2002). These medicinal substances are usually given within a formula which typically consists of four to 12 herbs, but herbs can be prescribed individually or within a formula containing substantially more than 12. Administration is in the form of decoctions, pills, powders, tablets, phials, and more recently, as standardised plant extracts. Standardised extracts using the whole plant will be included in this review, but the use of isolated 'active' phytochemicals, i.e. single chemical constituents, is not representative of Chinese herbal medicine and those studies will be excluded.
Objectives
To review the effects of Chinese herbal medicine for people with schizophrenia and related psychosis.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. Where a trial was described as 'double‐blind' but it was implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see 'Types of outcome measures') when these 'implied randomisation' studies were added, then we included these in the final analysis. If there was a substantive difference, we only used clearly randomised trials and the results of the sensitivity analysis were described in the text. We excluded Quasi‐randomised studies, such as those allocated by using alternate days of the week. Where studies used standardised extracts of the whole plant, we included these types of studies in the review, but excluded studies using isolated 'active' phytochemical ingredients.
Types of participants
We included people with schizophrenia, schizophreniform psychosis and schizophrenia‐like illnesses, diagnosed by any criteria.
Types of interventions
1. Chinese herbal medicines (plant, animal or mineral) in any dose or combination given either on their own or in combination with the full approach of traditional Chinese medicine.
2. Chinese herbal medicines (plant, animal or mineral) in any dose or combination given either on their own or in combination with the full approach of traditional Chinese medicine combined with antipsychotic drugs.
3. Placebo or no treatment.
4. Antipsychotic drugs produced by pharmaceutical companies: any compound, dose, pattern or means of administration.
Types of outcome measures
We identified the following outcomes of interest:
1. Clinical response 1.1 No clinically significant response in global state ‐ as defined by each of the studies* 1.2 Average score/change in global state 1.3 No clinically significant response on psychotic symptoms ‐ as defined by each of the studies 1.4 Average score/change on psychotic symptoms 1.5 No clinically significant response on positive symptoms ‐ as defined by each of the studies 1.6 Average score/change in positive symptoms 1.7 No clinically significant response on negative symptoms ‐ as defined by each of the studies 1.8 Average score/change in negative symptoms 1.9 Use of additional medication for psychiatric symptoms
2. Extrapyramidal adverse effects 2.1 Movement disorders 2.2 Incidence of use of antiparkinson drugs 2.3 Average score/change in extrapyramidal adverse effects ‐ including extrapyramidal effects
3. Other adverse effects, general and specific*
4. Service utilisation outcomes 4.1 Hospital admission* 4.2 Days in hospital 4.3 Times of relapse
5. Quality of life/satisfaction with care for either recipients of care or careers 5.1 Significant change in quality of life/satisfaction* 5.2 Average score/change in quality of life/satisfaction
6. Leaving the study early
7. Death, suicide or natural causes
8. Economic outcomes
We divided outcomes into primary (*) and secondary, and into short‐term (less than three months), medium term (three to 12 months) and long term (more than one year).
Search methods for identification of studies
1. Electronic searching We identified relevant randomised trials by searching the following electronic databases:
1.1 We searched the Cochrane Schizophrenia Group's Register (December 2003) was searched using the phrase:
[(*herb* or *Chinese* or *plant* or *complementary* or *alternative* or *yang* or *yin* or *traditional* or *oriental* or * TCM* or *TCD*) in REFERENCE abstract, title, original title or indexing or (*herb* or *Chinese* or *plant* or *complementary* or *alternative* or *yang* or *yin* or *traditional* or *oriental*) in STUDY interventions]
1.2 We searched TCMLARS database (October 2003) using the phrase:
[(schizophrenia, random, herbal,schizo) in abstract, subject heading, title]
1.3 We searched the Chinese Biomedical Database (1981 ‐December 2003) using the Cochrane Schizophrenia Group's search phrase:
schizophrenia or paranoid disorders or schizo* or hebephren* or oligophreni* or psychotic* or psychosis or psychoses or (((chronic* or sever*) and mental*) and (ill* or disorder*))
and using the Chinese Cochrane Centre's search phrase (Figure 1)
1.

1.4 We searched the Allied and Complementary Medicine Database (AMED) (December 2003) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials and schizophrenia (see Group search strategy) combined with the phrase:
[(*herb* or *Chinese* or *plant* or *complementary* or *alternative* or *yang* or *yin* or *traditional* or *oriental* or * TCM* or *TCD*) in REFERENCE abstract, title, original title or indexing or (*herb* or *Chinese* or *plant* or *complementary* or *alternative* or *yang* or *yin* or *traditional* or *oriental*) in STUDY interventions]
1.5 We contacted The Chinese Cochrane Centre and the Cochrane Complementary Medicine Field for knowledge of any additional studies.
1.6 Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group’s Trials Register (July 2012) using the phrase:
[(*herb* or *Chinese* or * plant* or *complementary* or *alternative* or * yang* or * yin* or *traditional* or *oriental* or * TCM* or * TCD*) in REFERENCE abstract, title, original title or indexing or (*herb* or *Chinese* or *plant* or *complementary* or *alternative* or *yang* or *yin* or *traditional* or *oriental* *(TCM)*) in STUDY interventions]
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches of relevant journals and conference proceedings (see Group Module). Incoming trials are assigned to existing or new review titles.
2. Reference lists We inspected references of all identified studies (included and excluded) for further relevant trials.
3. Personal contact We contacted the first author of each included study for information regarding unpublished trials and extra data on the published trials.
Data collection and analysis
1. Selection of studies We (JR and MZ) inspected all reports of studies identified by the search. LZ re‐inspected a randomly selected sample of 10% of all reports in order to allow selection to be reliable. Where disagreement occurred, we sought to resolve this by discussion, or where there was still doubt, we acquired the full article for further inspection. Once we had obtained the full articles we independently decided whether they met the review criteria. We resolved any disagreements that occurred by discussion, and when this was not possible sought further information and added these trials to the list of those awaiting assessment.
2. Assessment of methodological quality We allocated trials to three quality categories, as described in the Cochrane Collaboration Handbook (Alderson 2004). When disputes arose as to which category a trial should be allocated, we attempted to resolve these disputes by discussion. When this was not possible and further information was needed to clarify which category to allocate the trial, we did not enter the data and the trial was allocated to those awaiting assessment. We only included trials that met methodological quality criteria A or B.
3. Data management 3.1 Data extraction JR, LZ and JX independently extracted data from selected trials. When disputes arose, we attempted to resolve these by discussion. When this was not possible we did not enter the data, but added the outcome of the trial to the list of those awaiting assessment.
3.2 Intention to treat analysis We excluded data from studies where more than 50% of participants in any group were lost to follow up (this does not include the outcome of 'leaving the study early'). In studies with less than 50% dropout rate, we considered people leaving the study early to have had the negative outcome, except for the event of adverse effects and death.
The impact of including studies with high attrition rates (25‐50%) will be analysed in a sensitivity analysis. If inclusion of data from this group did result in a substantive change in the estimate of effect, we did not add this data to trials with less attrition, but presented it separately.
4. Data analysis 4.1 Binary data For binary outcomes we calculated an estimate of the relative risk (RR) and its 95% (Fixed effect) confidence intervals (CI). Where possible, we also calculated the number needed to treat (NNT) statistic. If heterogeneity was found (see section 5) we used a random effects model.
4.2 Continuous data 4.2.1 Skewed data: Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution, Altman 1996); (c) if a scale started from a positive value (such as PANSS which can have values from 30‐210) the calculation described above in (b) was modified to take the scale starting point into account. In these cases skewness is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score.
4.2.2 Summary statistic: For continuous outcomes we estimated a weighted mean difference (WMD) between groups. Again, if heterogeneity was found (see section 5) we used a random effects model.
4.2.3 Rating scales: A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and many are not valid, or are ad hoc. Unpublished instruments are more likely to represent statistically significant findings than those that have been put into print (Marshall 2000). Therefore, we included only continuous data from rating scales if the measuring instrument had been described in a peer‐reviewed journal and the instrument was either a self report or completed by an independent rater or relative (not the therapist).
4.2.4 Endpoint versus change data: When continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), there is no way of knowing whether data are non‐normally distributed (skewed) or not. If both endpoint and change date were available for the same outcomes then we only reported the former in this review.
4.2.5 Cluster trials Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
5. Test for heterogeneity Firstly, we considered all of the included studies within any comparison to judge clinical heterogeneity. Then we visually inspected graphs used to investigate the possibility of statistical heterogeneity and supplemented this by using, primarily, the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 75%, we interpreted this as indicating the presence of high levels of heterogeneity (Higgins 2003). If inconsistency was high, we did not summate the data, but presented it separately and reasons for heterogeneity were investigated.
6. Addressing publication bias We entered all data from the included studies into a funnel graph (trial effect against trial size) in an attempt to investigate the likelihood of overt publication bias (Egger 1997).
7. General Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for Chinese herbal medicine.
Results
Description of studies
1. Excluded studies We excluded seven studies. Three were not randomised (Cao 2000, Gong 2000, Rong 2001). One, Han 2002, did not report if randomisation was used and although it was double blind, the report did not contain any usable data and despite contacting the authors we received no reply. We had to exclude two studies, Wang 1998 a and Zhao 1997, because we could not extract any usable data from either paper. Again we contacted the authors but received no reply. We excluded Zhen 1992 because it compared haloperidol to pipothiazine.
2. Awaiting assessment Forty five studies are awaiting assessment.
3. Ongoing studies We are not aware of any studies that are ongoing.
4. Included studies We were able to include seven studies. All were randomised. Three were stated to be double blind although no description was provided; all three used Ginkgo biloba standardised extract (EGb761) as the herbal intervention (Luo 1997 a, Meng 1996, Zhang 2001). Chen 1997 was a single blind study. Zhang 1987 did not report whether blinding was used. Zhang 1997 and Zhu 1996 were open label studies. The four studies which were not double blind all used herbal decoctions (liquid form).
4.1 Length of trials The shortest study was 20 days (Zhang 1987) and the longest lasted for six months (Chen 1997). Five trials were between one month and four months.
4.2 Participants All participants were diagnosed with schizophrenia. Operationalised criteria were used by Chen 1997 (CCMD‐2), Luo 1997 a (CCMD‐2‐R, ICD‐10) Meng 1996 (CCMD‐2), Zhang 2001(DSM‐III‐R), Zhang 1997and Zhu 1996 (CCMD‐2). Zhang 1987 did not report using predefined diagnostic criteria. Luo 1997 a, Meng 1996, Zhang 1997, Zhang 2001 randomised both men and women. Zhu 1996 involved only women and Chen 1997and Zhang 1987 did not report on gender. Ages ranged between 16 and 61 years.
4.3 Setting Five trials were undertaken in a hospital setting (Chen 1997, Luo 1997 a, Meng 1996, Zhang 2001 and Zhu 1996). Zhang 1997 used both hospital and community settings and Zhang 1987 did not report where the study was undertaken.
4.4 Study size Luo 1997 a was the largest study with 545 participants. The smallest studies randomised only 40 (Meng 1996) and 67 people (Zhu 1996). The remaining studies randomised around 100 people, Zhang 1987 (n=90), Chen 1997, (n=120), Zhang 1997(n=123) and Zhang 2001 (n=109).
4.5 Interventions The treatment groups were given herbal medicines alone or in combination with antipsychotics. All the comparator groups received antipsychotics alone.
Chen 1997 used an empirical herbal medicine formula, Xingshen (30 ml/bid) combined with antipsychotics. Luo 1997 a employed Ginkgo biloba extract (EGb 761, 120 mg/tds) plus maintenance antipsychotics. Meng 1996 used Ginkgo biloba extract (EGb 761, 120‐240 mg/day) plus continuation of antipsychotics. Zhang 2001 also employed Ginkgo biloba extract (EGb 761, 360 mg/day) combined with haloperidol. Zhang 1987 used the herbal formula Dang gui cheng qi tang (100‐200 ml/day). Zhu 1996 employed a two herb combination of Radix Rhei palmatum and Hirudo seu Whitmania plus chlorpromazine. Zhang 1997 was the only study administering two different herbal formulas for the treatment group, based on TCM pattern differentiation (Dang gui cheng qi tang plus antipsychotics or Xiao yao san plus antipsychotics) and reported these as a single group. The included studies table provides a detailed description of those formulas used.
The comparator drug chlorpromazine was used by Zhu 1996 with a maximum of 400 mg per day; Zhang 1987 also used doses of chlorpromazine between 300‐600 mg per day. In Zhang 2001 the comparator was haloperidol given as per body weight (0.25 mg/kg/day). Chen 1997 employed three different antipsychotics comparison groups (chlorpromazine n=17, clozapine n=36, sulpiride n=7) with ranges reported between 300 and 800 mg/day. Luo 1997 a, Meng 1996 and Zhang 1997 did not report the type of antipsychotics used in the comparator group.
4.6 Outcomes Three studies report short term data (less than three months) (Meng 1996, Zhang 1987, Zhu 1996). Four trials report medium term data (Chen 1997, Luo 1997 a, Zhang 2001, Zhang 1997). We were unable to use data from some studies because raw scores were not presented, instead outcomes were reported as p‐values, without means and standard deviations being provided, or, the scale used was not identified. Zhang 2001 was the only study to predefine clinical improvement for the BPRS, SAPS and SANS scales as a 30% reduction as being clinically significant.
4.6.1 Outcome scales: details of scales that provided usable data are shown below. Reasons for exclusion of data from instruments are given under 'Outcomes' in the Excluded studies' section.
4.6.1.1 Global state scales 4.6.1.1.1 Clinical Global Impression Scale ‐ CGI (Guy 1970) The CGI is a three‐item scale commonly used in studies on schizophrenia that enables clinicians to quantify severity of illness and overall clinical improvement. The items are: severity of illness; global improvement and efficacy index. A seven‐point scoring system is usually used with low scores indicating decreased severity and/or greater recovery. Meng 1996 and Zhu 1996 reported CGI data.
4.6.1.2 Mental state 4.6.1.2.1 Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) The BPRS is an 18‐item scale measuring positive symptoms, general psychopathology and affective symptoms. The original scale has sixteen items, but a revised eighteen‐item scale is commonly used. Scores can range from 0‐126. Each item is rated on a seven‐point scale, with high scores indicating more severe symptoms. Luo 1997 a, Meng 1996, Zhang 1997, Zhang 2001 and Zhu 1996 reported BPRS data.
4.6.1.2.2 Scale for the Assessment of Negative Symptoms ‐ SANS (Andreasen 1983) This scale allows a global rating of the following negative symptoms: alogia (impoverished thinking), affective blunting, avolition‐apathy, anhedonia‐asociality and attention impairment. Assessments are made on a six‐point scale (0=not at all to 5=severe). Higher scores indicate more symptoms. Data for this scale were reported by Chen 1997, Luo 1997 a, Meng 1996 and Zhang 2001.
4.6.1.2.3 Scale for the Assessment of Positive Symptoms ‐ SAPS (Andreasen 1983) This six‐point scale gives a global rating of positive symptoms such as delusions, hallucinations and disordered thinking. Higher scores indicate more symptoms. Zhang 2001 was the only study to report SAPS data.
4.6.1.3 Adverse events 4.6.1.3.1 Treatment Emergent Symptom Scale/Form ‐ TESS/F (Guy 1976) This checklist assesses a variety of characteristics for each adverse event, including severity, relationship to the drug, temporal characteristics (timing after a dose, duration and pattern during the day), contributing factors, course, and action taken to counteract the effect. Symptoms can be listed a priori or can be recorded as observed by the investigator. Zhang 2001 reported data for this scale.
Risk of bias in included studies
1. Randomisation All included studies were stated to be randomised but no study described how randomisation was undertaken. Trials had evenly balanced numbers of participants in each arm, except for Luo 1997 a (315 ‐ herbal medicine group, 230 ‐ control group). We therefore classified all studies as category B (unclear allocation concealment) with a moderate risk of overestimating the estimate of effect.
2. Blindness No included study reported exactly how blinding was undertaken. The three studies that were described as double blind all used Ginkgo biloba EGb761. The trials using liquid herbal decoctions were either open label, single blind or blinding was not reported. No study reported if they tested blinding.
3. Loss to follow up Follow up was good with all studies reporting few or no participants being lost to follow up.
4. Data reporting Overall, data reporting was good with most outcomes derived from scales providing usable data. Only a few studies reported unusable data.
Effects of interventions
1. The Search The CSG register search resulted in over 500 citations. An additional 37 citations were obtained from TCMLARS database and an addtional 106 were found from the search performed by the Chinese Cochrane Center (see figure 1 (Figure 1) for the Chinese characters used in the search phrase). We were only able to include seven trials in the review and added seven more trials to the list of excluded studies.
2. COMPARISON 1. HERBAL MEDICINE versus ANTIPSYCHOTICS
2.1 Global state Only Zhang 1987 included a direct comparison and reported global state data as 'not improved/worse' at 20 days. Results significantly favoured the chlorpromazine group compared to those receiving Dang gui cheng qi tang (n=90, RR 1.88 CI 1.2 to 2.9, NNH 4 CI 2 to 14).
2.2 Leaving the study early There was no loss to follow up in either group by 20 days.
3. COMPARISON 2. HERBAL MEDICINE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS
3.1 Global state Zhang 1997 reported three out of 66 people having a response of 'no change or worse' whilst receiving Dang gui cheng qi tang or Xiao yao san in combination with antipsychotics. For those receiving only antipsychotics, the outcome was higher with 14 out of 57 having no change or worse. This result significantly favours the combination group receiving both the herbal medicine and antipsychotics (n=123, RR 0.19 CI 0.1 to 0.6, NNT 6 CI 5 to 11).
Meng 1996 and Zhu 1996 report data for short term clinical global impression scores. Results again significantly favoured people receiving herbal medicine plus antipsychotics (n=103, WMD ‐0.46 CI ‐0.9 to ‐0.1) compared with those given only antipsychotics.
3.2 Mental state Zhang 2001 reported data for mental state using the BPRS, SANS and SAPS scales. BPRS data for the outcome 'not improved/worse' were equivocal (n=109, RR 0.78 CI 0.52 to 1.16). Again Zhang 2001 found no significant differences in mental state between groups on the SANS scale (n=109, RR 0.87 CI 0.66 to 1.15). Using the SAPS scale for positive symptoms, the study found a slight difference between groups, favouring those receiving herbal medicine and antipyschotics, (n=109, RR 0.69 CI 0.5 to 1.0 NNT 6 CI 4 to 162).
BPRS endpoint scores (short term) reported by Meng 1996 and Zhu 1996 favoured the treatment group receiving herbal medicine and antipsychotics (n=103, WMD ‐2.41 CI ‐3.9 to ‐1.0) compared with those given antipsychotics alone. Data, however, were heterogeneous (I‐square 82%). Medium term BRPS data by Luo 1997 a and Zhang 2001 also significantly favoured the treatment group (n=621, WMD ‐4.17 CI ‐5.5 to ‐2.8). Medium term SANS scores reported by Chen 1997, Luo 1997 a and Zhang 2001 significantly favoured the treatment group (n=741, WMD ‐9.15 CI ‐12.1 to ‐6.2), although these data were also quite heterogeneous (I‐squared 70%). Additional skewed data reported by Meng 1996 for the SANS, Zhang 1997 for the BPRS and Zhang 2001 for the SAPS tended to favour the combination groups and are presented in tables.
3.3 Adverse events Extrapyramidal symptoms were equivocal from the study by Zhu 1996. Reports of constipation by Zhu 1996 were significantly lower in the treatment group 0/32 compared to those receiving antipsychotics 19/35 (n=67, RR 0.03 CI 0.0 to 0.5, NNH 2 CI 2 to 4). Average TESS data ‐ a general symptom checklist ‐ were reported by Zhang 2001, however data are skewed but do seem to favour the combination group. These data are reported only as tables.
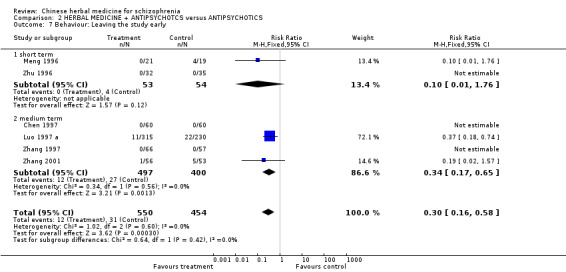
3.4 Behaviour Leaving the study early (short term ‐by eight weeks) reported by Meng 1996 and Zhu 1996 found no significant differences between groups. Medium term data show that significantly fewer people in the treatment group left the study early compared to those given antipsychotics (n=1004, 6 RCTs, RR 0.30 CI 0.16 to 0.58, NNT 21 CI 18 to 35).
4. COMPARISON 3. SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS
4.1 Global state The Ginkgo biloba/antipsychotic combination did have more of an effect than the result with all herbal data combined and the combination data were quite heterogeneous suggesting that adding herbs, simply as one intervention may not be advisable. The ginkgo biloba result (total n=36) was not statistically significantly different from the combined result (n=103). 4.2 Mental state
BPRS scores, significantly favoured the ginkgo biloba plus antipsychotic group (n=36, 1 RCT, WMD ‐4.00 CI ‐6.0 to ‐2.0) and this result was more significant than the combined herbal results (n=103, 2 RCTs, WMD ‐2.41 CI ‐3.85 to ‐0.97) and the latter finding did have high I‐squared percentage (81%) indicating heterogeneity. To confuse matters, the negative symptom scores, as measured on the SANS, were heterogeneous within the ginkgo biloba alone group, but were homogeneous for the all herbs combined group
4.3 Behaviour For this outcome we found no suggestion in any of the studies that administering ginkgo biloba alone was different in effect to administering a combination of Chinese herbs.
Discussion
1. General This review extracted data from seven trials, with six of these involving people diagnosed with schizophrenia without further differentiation according to TCM theory. Only Zhang 1997 involved people diagnosed with schizophrenia and then categorised the participants according to TCM pattern differentiation. Therefore the applicability of the results of this review is limited by the methodology used in these six studies which do not reflect usual TCM clinical diagnostic practice. Also, only Zhang 1997, after having randomised, gave those in the treatment group the herbal medicines indicated for that TCM pattern. Other studies simply gave a pre‐designated herbal medication based solely on having schizophrenia. This would not necessarily reflect standard clinical TCM practice.
We found that most of these studies investigate the effects of Chinese herbs and not the complex approach of traditional Chinese medicine characterised by pattern differentiation and tailored treatment to the individual.
2. Methodological Quality Overall, the quality of reporting on randomisation and blinding was poor, with no studies describing how either randomisation and/or blinding was conducted. We therefore classified all studies in category B (unclear allocation concealment) with a moderate risk of overestimating the estimate of effect. Ensuring participants are blind to their treatment is especially difficult when herbal medicines are given in a liquid form (decoction) which can be readily seen, smelt and tasted. There is a view that this method of prescribing herbal medicines is more efficacious than pills or concentrated powders. However, this obviously raises questions of the placebo effect on these results.
3. COMPARISON 1. HERBAL MEDICINE versus ANTIPSYCHOTICS
3.1. Global state For the outcome 'not improved/worse' the results significantly favoured the control group receiving chlorpromazine when compared to the treatment group receiving Dang gui cheng qi tang. This suggests antipsychotics are superior to herbal medicine when assessed against global state outcomes (NNH 4 CI 2 to 14). However, this outcome is based on a single study (Zhang 1987, n=90) lasting 20 days with participants given Chinese herbs according to a diagnosis of schizophrenia without further differentiation according to TCM pattern differentiation. Results should therefore be interpreted with caution given the limitations of the study design, but nevertheless they do not support Dang gui cheng qi tang as a sole treatment for schizophrenia.
3.2 Behaviour The fact that no one left this study by 20 days could be a reflection on study design, the interventions, or the regimen of care offered.
4. COMPARISON 2. HERBAL MEDICINE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS
4.1. Global state The treatment group receiving either Dang gui cheng qi tang or Xiao yao san plus antipsychotics reported significantly less people having an outcome of 'no change or worse' compared to participants receiving only antipsychotics, when measured using the Clinical Global Impression scale (NNT 6 CI 5 to 11). This suggests that the combined effect of herbal medicines and antipsychotics is better than antipsychotics alone. Results are from a single study (Zhang 1997, n=123) of 12 weeks duration and again caution is needed when interpreting the results. However, this study did treat participants according to TCM pattern differentiation. Continuous scores from the CGI scale (short term) also significantly favoured those given herbal medicine and antipsychotics. Although these results are difficult to interpret from a clinical perspective, they are broadly encouraging and suggest combining herbal medicines with antipsychotics is beneficial, although results are only based on two small studies (total n=103).
4.2. Mental state Zhang 2001 (n=109) reported dichotomised mental state scores (BPRS and SANS), equivocal for both the treatment and control groups. SAPS scores however, showed borderline significance in favour of the herbal medicine/antipsychotic combination. Again, drawing firm conclusions from data from one small study would be imprudent. Continuous data, however, provided more robust results with three studies reporting negative symptom scores (Chen 1997, Luo 1997 a, Zhang 2001: n=741). Medium term data was significant and favoured the treatment group. Meng 1996 and Zhu 1996 (n=103) found short‐term mental state scores on the BPRS to be significantly better for those receiving herbal medicines plus antipsychotics (although the data for this outcome were heterogeneous) and similar positive results were found for medium term data on the same scale (Luo 1997 a, Zhang 1987: n=621). The changes are all difficult to interpret from the clinical perspective and we are not sure that they have a considerable clinical meaning, but they do suggest that the combination treatment may have something to offer. The results do suggest a modest beneficial effect on mental state for those taking antipsychotics with herbal medicine, although care needs to be taken as none of the studies presenting mental state data had differentiated and treated participants according to TCM pattern differentiation.
4.3. Adverse events Only one study (Zhu 1996) reported on extrapyramidal symptoms, which were not significantly different between groups. TESS scores were reported but data were skewed, although the authors reported no differences in mean scores between treatment and control group. Constipation was significantly more frequent in the control group with NNT of 2. This probably reflects that the treatment group, who were given the same constipating antipsychotic (chlorpromazine) as the control group, had this effect offset by also receiving the purgative herb Rhizoma Rhei palmatum (rhubarb rhizome).
4.4. Behaviour All studies report data for leaving early but almost everyone completed according to protocol. The short term data were not statistically significant but heterogeneous (I² 76%) medium term data showed significantly less people in the herbal medicine plus antipsychotic group leaving the study early compared to the comparator group receiving antipsychotics alone (n=1004, 2% vs 7%). In the context of these studies, the addition of herbal medicine did not worsen treatment compliance, There is even the suggestion that the addition of the herbal medicine made it easier for participants to take standard antipsychotics.
5. COMPARISON 3. SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS This review combined outcomes in accordance with the protocol and therefore studies using different herbal medicines were pooled where appropriate. The authors are aware that in doing so size estimates may potentially be over or underestimated. Therefore a sensitivity analysis was performed comparing studies using only Ginkgo biloba (EGb761) combined with antipsychotics to those combining all types of herbal formulations. The sensitivity analysis did not have much power. Studies were all small, so attempting to investigate for significant differences between them when confidence intervals were expected to be wide, was probably inadvisable. In addition, the single herb ginkgo biloba was not used as it would be in TCM i.e. multi‐herb combination given according to pattern differentiation. Nevertheless, when evaluating this one Chinese herbal medicine within a Western biomedical context, there were some suggestions that it may have specific effects. For global and mental state outcomes, the ginkgo biloba/antipsychotic combination did have more of an effect than the result with all herbal data combined. These findings are from very small trials and further large scale studies are needed.
Authors' conclusions
Implications for practice.
1. People with schizophrenia There is no evidence from this review to suggest that herbal medicine, when given alone, offers benefits equal to or beyond those obtained with antipsychotic drugs. Chinese herbal medicine, when used together with antipsychotic medication, may well offer benefits in terms of improving symptoms and lessening adverse events, but evidence is too limited to say anything with confidence. In terms of trial‐based evidence, both Chinese herbs and the full traditional Chinese medicine approach must be considered experimental.
2. Clinicians How clinicians should incorporate these findings into everyday practice is unclear until further evidence can be ascertained. However, those practicing TCM or using Chinese herbs should be encouraged to see that the data from these trials, though few, are not discouraging. Western psychiatrists not trained in TCM or the use of Chinese herbs should not dismiss these approaches. More trial‐based evidence exists for their use than for many routine practices in Western models of care.
3. Funders and policy makers Traditional Chinese Medicine and Chinese herbs are widely used and the trials seem to suggest there may be some effect. This intervention seems to have enough supporting evidence both from ancient practice and trials to be worth further investigation. This review does not contain enough evidence to either support or refute the policy of use of traditional Chinese medicine or Chinese herbs.
4. Note: the 45 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.
Implications for research.
1. General This review of Chinese herbal medicine for schizophrenia would have had some more data if the guidelines in the CONSORT (Moher 2001) statement had been followed. Clear descriptions of randomisation would have reassured users of these trials that selection bias had been minimised. Well‐described and tested blinding could have encouraged confidence in the control of performance and detection bias. Attempts should also be made to blind control groups when liquid herbal medicines are given to reassure the reader that outcomes are as free from bias as possible.
2. Specific This small review raises many questions. There are some suggestions that the use of Chinese herbs, even within the context of Western traditions of diagnosis and practice, have some properties that could be beneficial and these should be evaluated within a large randomised study. Furthermore we found that the holistic and tailored approach of traditional Chinese medicine was only once formally evaluated in a trial. This fascinating ancient tradition and package of care has stood a test of time and has been administered to millions of people suffering from various manifestations of schizophrenia. One study has shown that it is possible to evaluate TCM within a randomised study and, we suggest, that further more comprehensive studies would represent a beneficial amalgamation of the two traditions: Western scientific objectivity with Chinese personal and tailored care.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2012 | Amended | Update search of Cochrane Schizophrenia Group's Trial Register (see Search methods for identification of studies), 45 studies added to awaiting classification. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 26 April 2008 | Amended | Converted to new review format. |
| 3 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Mark Fenton for the trial search, Tessa Grant and Gill Rizzello for their editorial assistance and Clive Adams for his advice and Chris Cates for his statistical advice.
Data and analyses
Comparison 1. HERBAL MEDICINE versus ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: Not improved/worse ‐ short term | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.24, 2.86] |
| 2 Behaviour: Leaving the study early ‐ short term | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 HERBAL MEDICINE versus ANTIPSYCHOTICS, Outcome 1 Global state: Not improved/worse ‐ short term.
1.2. Analysis.

Comparison 1 HERBAL MEDICINE versus ANTIPSYCHOTICS, Outcome 2 Behaviour: Leaving the study early ‐ short term.
Comparison 2. HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Not improved/worse ‐ medium term | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.61] |
| 2 Global state: 2. Average score ‐ short term (CGI endpoint , high score=worse) | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.86, ‐0.06] |
| 3 Mental state: 1. Not improved ‐ various measures ‐ medium term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 overall ‐ BPRS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.16] |
| 3.2 negative symptoms ‐ SANS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.66, 1.15] |
| 3.3 positive symptoms ‐ SAPS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] |
| 4 Mental state: 2. Average score (BPRS endpoint, high score=worse) | 4 | 724 | Mean Difference (IV, Fixed, 95% CI) | ‐3.33 [‐4.32, ‐2.34] |
| 4.1 short term | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐3.85, ‐0.97] |
| 4.2 medium term | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | ‐4.17 [‐5.54, ‐2.79] |
| 5 Mental state: 3. Average scores ‐ various scales (endpoint, high score=worse, skewed data) | Other data | No numeric data | ||
| 5.1 overall ‐ medium term ‐ BPRS | Other data | No numeric data | ||
| 5.2 negative symptoms ‐ short term ‐ SANS | Other data | No numeric data | ||
| 5.3 positive symptoms ‐ medium term ‐ SAPS | Other data | No numeric data | ||
| 6 Mental state: 4. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse) | 3 | 741 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐12.10, ‐6.20] |
| 7 Behaviour: Leaving the study early | 6 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.58] |
| 7.1 short term | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 7.2 medium term | 4 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |
| 8 Adverse effects: 1. Various symptoms ‐ short term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 extrapyramidal | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.18, 1.64] |
| 8.2 gastrointestinal ‐ constipation | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.45] |
| 9 Adverse effects: 2. Average score ‐ short term (TESS endpoint, high score=worse, skewed data) | Other data | No numeric data |
2.1. Analysis.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 1 Global state: 1. Not improved/worse ‐ medium term.
2.2. Analysis.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 2 Global state: 2. Average score ‐ short term (CGI endpoint , high score=worse).
2.3. Analysis.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 3 Mental state: 1. Not improved ‐ various measures ‐ medium term.
2.4. Analysis.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 4 Mental state: 2. Average score (BPRS endpoint, high score=worse).
2.5. Analysis.
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 5 Mental state: 3. Average scores ‐ various scales (endpoint, high score=worse, skewed data).
| Mental state: 3. Average scores ‐ various scales (endpoint, high score=worse, skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| overall ‐ medium term ‐ BPRS | ||||
| Zhang 1997 | Herbal medicine + antipsychotics | 13.4 | 6.7 | 66 |
| Zhang 1997 | Antipsychotics | 26.6 | 15.2 | 57 |
| negative symptoms ‐ short term ‐ SANS | ||||
| Meng 1996 | Ginkgo (EGb61) | 37.1 | 16.0 | 21 |
| Meng 1996 | Antipsychotics | 46.6 | 30.0 | 15 |
| positive symptoms ‐ medium term ‐ SAPS | ||||
| Zhang 2001 | Ginkgo biloba (EGb) + haloperidol | 7.1 | 8.4 | 56 |
| Zhang 2001 | Haloperidol | 11 | 10.5 | 53 |
2.6. Analysis.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 6 Mental state: 4. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse).
2.7. Analysis.
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 7 Behaviour: Leaving the study early.
2.8. Analysis.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 8 Adverse effects: 1. Various symptoms ‐ short term.
2.9. Analysis.
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 9 Adverse effects: 2. Average score ‐ short term (TESS endpoint, high score=worse, skewed data).
| Adverse effects: 2. Average score ‐ short term (TESS endpoint, high score=worse, skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| Zhang 2001 | Ginkgo biloba (EGb) + haloperidol | 1.8 | 2.8 | 56 |
| Zhang 2001 | Haloperidol | 2.7 | 4.6 | 53 |
Comparison 3. SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: Average score ‐ short term (CGI endpoint , high score=worse) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 ginkgo biloba alone | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.29, ‐0.31] |
| 1.2 other herbs combined | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.86, ‐0.06] |
| 2 Mental state: 1. Average score (BPRS endpoint, high score=worse) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 ginkgo biloba alone | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐5.97, ‐2.03] |
| 2.2 other herbs combined | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐3.85, ‐0.97] |
| 3 Mental state: 2. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 ginkgo biloba alone | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐12.52, ‐5.79] |
| 3.2 other herbs combined | 3 | 741 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐12.10, ‐6.20] |
| 4 Behaviour: Leaving the study early | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 short term ‐ ginkgo biloba alone | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 4.2 short term ‐ other herbs combined | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 4.3 medium term ‐ ginkgo biloba alone | 2 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |
| 4.4 medium term ‐ other herbs combined | 4 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |
3.1. Analysis.

Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 1 Global state: Average score ‐ short term (CGI endpoint , high score=worse).
3.2. Analysis.

Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 2 Mental state: 1. Average score (BPRS endpoint, high score=worse).
3.3. Analysis.

Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 3 Mental state: 2. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse).
3.4. Analysis.

Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 4 Behaviour: Leaving the study early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 1997.
| Methods | Allocation: randomised. Blindness: single (assessors blinded). Duration: 6 months. Design: parallel group (without pattern differentiation). | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). N=120. Age: ˜27‐58 years. Sex: not reported. Setting: hospital. History: chronic illness, mean 17 years. | |
| Interventions | 1. Xingshen + antipsychotic: dose Xingshen 30ml/bid + antipsychotics (chlorpromazine n=16, clozapine n=38, sulpiride n=6) dosage ˜300‐700 mg/day. N=60. 2. Antipsychotics: dose chlorpromazine n=17, clozapine n=36, sulpiride n=7, dosage ˜250‐800 mg/day (no further details). N=60. Herbal formula ‐ Xingshen: Semen Cuscutae chinensis (Tu Si Zi) Cortex Cinnamomi cassiae (Rou Gui) Herba Epimedii (Yin Yang Huo) Rhizoma Curculiginis orchioidis (Xian mao) Radix Polygoni multiflori (He shou wu) Radix et Caulis jixueteng (Ji xue tang) Fructus Broussonetiae papyrifera (Chu shi zi) Grams not specified. |
|
| Outcomes | Leaving the study early. Mental state: SANS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Luo 1997 a.
| Methods | Allocation: randomised. Blindness: double. Duration: 16 weeks. Design: parallel groups (without pattern differentiation). | |
| Participants | Diagnosis: schizophrenia (ICD 10 and CCMD‐2‐R). N=545. Age: 18‐60 years. Sex: 418M, 127F. Setting: hospital. History: not reported. | |
| Interventions | 1. Ginkgo biloba (EGb761) + maintenance antispychotics: dose EGb761, 120 mg/tds, antipsychotics. N=315. 2. Placebo + Antipsychotics: dose maintenance antipsychotics. N=230. Maintenance antipsychotics included clozapine, chlorpromazine, sulpiride, perphenazine and haloperidol. Doses and frequency not reported. |
|
| Outcomes | Leaving the study early.
Mental state: BPRS, SANS. Unable to use ‐ Adverse effects: TESS (no usable data), SAS (data reported from subgroup), extrapyramidal symptoms (scale not stated). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Meng 1996.
| Methods | Allocation: randomised. Blindness: double. Duration: 8 weeks. Design: parallel groups (without pattern differentiation). | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). N=40. Age: 18‐60 years. Sex: male and female. Setting: hospital. History: not reported. | |
| Interventions | 1. Ginkgo biloba (EGb761) + antipsychotics: dose range EGb761, 120 mg/day first week, then 240 mg/day + continuation of antipsychotics (dosage not reported). N=21. 2. Placebo + Antipsychotics: dose (no further details). N=19. |
|
| Outcomes | Leaving the study early. Global state: CGI. Mental state: BPRS, SANS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zhang 1987.
| Methods | Allocation: 'divided into groups randomly'. Blindness: not reported. Duration: 20 days. Design: parallel groups (without pattern differentiation). | |
| Participants | Diagnosis: schizophrenia. N=90. Age: 16‐51 years. Sex: not reported. Setting: hospital. History: mostly first admissions (n=82). Excluded: those who showed remission of symptoms on admission. | |
| Interventions | 1. Dang gui cheng qi tang: dose ˜ mean 50 ml/bid, max. 200 ml/day. N=45. 2. Chlorpromazine: any dose as required. N=45. Herbal formula ‐ Dang gui cheng qi tang: Radix Angelica sinensis (Dang Gui) 30g Radix et Rhizoma rhei (Da Huang) 30g Natrii sulfas (Mang xiao) 15g Fructus Poncirus trifoliate (Zhi shi) 12g Fructus trichosanthis (Gua Lou) 15g Additional herbs added as required for blood stagnation: Semen persicae (Tao ren) Radix Curcumae (Yu Jin) Radix Paeonia rubrae (Chi Shao Yao) Radix Bupleuri (Chai hu) Radix Scutellariae (Huang qin) Flos Carthami (Hong hua) Rhizoma Ligustici chuanxiong (Chuan xiong) Additional herbs added as required for hallucinations, restlessness and insomnia: Radix Stephaniae tetrandrae (Fang ji) Radix Ledebouriellae (Fang feng) Sclerotium Poriae cocos (Fu ling) Radix Polygalae (Yuan zhi) Fructus Ziziphi jujubae (Da zao) Radix rehmanniae (Sheng di huang) Rhizoma Acori graminei (Shi chang pu) Os draconis (Long gu) Concha ostreae (Mu li) Haematitum (Zhe shi) grams not specified. |
|
| Outcomes | Leaving the study early. Global state: not improved/worse. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zhang 1997.
| Methods | Allocation: randomised. Blindness: open label. Duration: 12 weeks. Design: randomised into 2 treatment groups, herbal medicine and control; herbal medicine group further divided, non‐randomly, into 2 groups based on pattern differentiation. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2) . N=123. Age: mean ˜ 32 years. Sex: male and female. Setting: hospital and community. History: mean length of illness 11 years. | |
| Interventions | 1. Herbal medicine: Dang gui cheng qi tang (those with yang syndrome), 100 ml 2‐3/day + antipsychotics (unclear which type or dosage, n=32); Xiao yao san (those with yin syndrome) 100ml 2‐3/day + antipsychotics (unclear which type or dosage, n=34). N=66. 2. Antipsychotics: dose and type not reported. N=57. Herbal formula ‐Dang gui cheng qi tang: Radix Angelica sinensis (Dang gui) 15g Fructus Immaturus (Zhi shi) 15g Radix et Rhizoma rhei (Da huang) 15g Fructus Lycii (Gou qi zi) 15g Natrii sulfas (Mang xiao) 15g Sclerotium Poriae cocos (Fu ling) 15g Rhizoma Atractylodis macrocephalae (Bai Zhu) 15g Herbal formula ‐ Xiao Yao san: Radix Angelica sinensis (Dang gui) 15g Radix Bupleuri (Chai hu) 15g Sclerotium Poriae cocos (Fu ling) 15g Radix Paeoniae lactiflorae (Bai Shao Yao) 15g Rhizoma Atractylodis macrocephalae (Bai Zhu) 15g Radix polygalae (Yuan zhi) 15g Aconiti Carmichaeli praeparata (Fu zi) 15g Radix Glycyrrhizae uralensis (Gan Cao) 15g Rhizoma Rheum palmatum (Da huang) 15g Radix Astragali membranacei (Huang Qi) 15g |
|
| Outcomes | Leaving the study early. Global state: not improved/worse. Mental state: BPRS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zhang 2001.
| Methods | Allocation: randomised. Blindness: double. Duration: 12 weeks. Design: parallel groups (without pattern differentiation). | |
| Participants | Diagnosis: schizophrenia (DSM III R). N=109. Age: 27‐61 years, mean ˜ 44 years. Sex: 63M, 46F. Setting: hospital. History: treatment resistant; average length of illness 21.5 years (minimum 5 years). Excluded: bipolar affective disorder, organic mental disorders, substance or alcohol abuse, brain damage, epilepsy, severe physical disease, pregnancy or breast feeding. | |
| Interventions | 1. Ginkgo biloba (EGb761) + antipsychotics: dose EGb761, 360 mg/day, + haloperidol 0.25 mg/kg/day. N=56. 2. Haloperidol: dose 0.25 mg/kg/day + placebo. N=53. |
|
| Outcomes | Leaving the study early. Mental state: BPRS, SAPS, SANS. Adverse effects: TESS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zhu 1996.
| Methods | Allocation: randomised. Blindness: open label. Duration: 30 days. Design: parallel groups (without pattern differentiation). | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). N=67. Age: 17‐53 years. Sex: female. Setting: hospital. History: not reported. | |
| Interventions | 1. Hirudo seu Whitmania (Shui zhi) and Rhizoma Rheum palmatum (Da huang) (doses not specified) + chlorpromazine =/<300 mg/day). N=32. 2. Chlorpromazine: dose =/<400 mg/day. N=35. |
|
| Outcomes | Leaving the study early.
Global state: CGI.
Mental state: BPRS.
Adverse effects: extrapyramidal, gastrointestinal. Unable to use ‐ Adverse effects: haematology (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
DSM ‐ Diagnostic and Statistical Manual ICD 10 ‐ International Classification of Diseases CCMD ‐2‐R ‐ Chinese Classification of Mental Disorders Second Edition Revised
Global state: CGI ‐ Clinical Global Impression
Mental state: BPRS ‐ Brief Psychiatric Rating Scale SANS ‐ Scale for the Assessment of Negative Symptoms SAPS ‐ Scale for the Assessment of Positive Symptoms
Adverse effects: TESS ‐Treatment Emergent Symptom Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cao 2000 | Allocation: not randomised. |
| Gong 2000 | Allocation: not randomised. |
| Han 2002 | Allocation: not reported, double blind (wrote to authors, no reply). Participants: people with schizophrenia. Interventions: chinese medicine versus clozapine. Outcomes: no usable data. |
| Rong 2001 | Allocation: not randomised. |
| Wang 1998 a | Allocation: randomised. Participants: peole with schizophrenia. Intervention: Chinese herbal medicine versus antispychotics. Outcomes: no time points reported (authors contacted, no reply). |
| Zhao 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: Ginkgo biloba + antipsychotics versus antipsychotics +placebo. Outcomes: no usable data. |
| Zhen 1992 | Allocation: randomised. Participants: people with schizophrenia. Interventions: haloperidol decanoate versus pipothiazine palmitate. |
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Chen 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Chiu 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Hung 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Mei 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Miyaoka 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Mundewadi 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01045720.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Sanchez 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Tang 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Wang 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Xiao 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Yan 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Zhang 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Zhou 1989.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
于瑞丽, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
孟彬, 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
尹道亮 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
左潇, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
庞铁良, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张子梅, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
彭东阳 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
易世国, 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
林升 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
林虹, 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
柳贵明, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
段武钢, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
段武钢, 2010a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
汪艳, 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
洪娜 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
王鹤秋, 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
石建喜, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
罗世芳, 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
翁深宏, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
肖世富, 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
胡海燕, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
胥德广, 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
解克平 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
解克平, 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
邓方渝 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
郝广义, 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
鄢爱平, 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
陈克彦 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
马俊国, 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
黄平, 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Contributions of authors
John Rathbone ‐ prepared the protocol, selected studies, extracted and inputed data, analysed the results, wrote the text of the review and contacted authors.
Lan Zhang ‐ prepared the protocol, translated and extracted data.
Mingming Zhang ‐ organised electronic searches of Chinese databases, selected studies and wrote to authors.
Jun Xia ‐ extracted and translated data, wrote to authors.
Prof. Xiehe Liu ‐ provided advice for the protocol.
Yanchun Yang ‐ provided advice for the protocol.
Sources of support
Internal sources
West China Hospital of Sichuan University, China.
Chinese Cochrane Centre, China.
Chinese Centre for Evidence‐Based Medicine, China.
External sources
China Medical Board of New York (grant No. 98‐680), USA.
Declarations of interest
John Rathbone ‐ licentiate in oral herbal medicine.
Lan Zhang ‐ no known conflict of interest.
Mingming Zhang ‐ no known conflict of interest.
Jun Xia ‐ no known conflict of interest.
Xiehe Liu ‐ no known conflict of interest.
Yanchun Yang ‐ no known conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Chen 1997 {published data only}
- Chen YD, He AN, Chen L. The clinical observataion on 120 cases of schizophrenic negative symptoms treated with "Xin Shen" mixure. Journal of University Traditional Chinese Medicine 1997;20(3):19‐21. [Google Scholar]
- He AG, Chen, YD, Zhang XW, Du ZJ, Guao J, Zhao GY. A controlled study of 'xingshenheji' treating schizophrenia with negative syndrome. Zhong Guo Min Zheng Yi Xue Za Zhi 1995;7(1):1‐3. [Google Scholar]
Luo 1997 a {published data only}
- *Luo HC, Shen Y, Meng FQ. Therapeutic effect of shuxuening combining neuroleptics for the treatment of chronic schizophrenia ‐ a double blind study [[data not available]]. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1997;17(3):139‐42. [EMBASE 2000308182] [PubMed] [Google Scholar]
- Jiang Yu. Clinical Study of EGb761 Combined Antipsychotics Treating Chronic Schizophrenia. Zhong Guo Min Zheng Yi Xue Za Zhi 1996;8(4):219‐21. [Google Scholar]
- Ping Liu, Hechun Luo, Yuchun Sheng. Combined use of Ginkgo biloba extracts on the efficacy and adverse reactions of various antipsychotics. Chinese Journal of Clinical Pharmacology 1997;13(4):193‐98. [Google Scholar]
Meng 1996 {published data only}
- Meng Fan Qiang, Cui Yu Hua, Wang Shan Hui. A double‐blind placebo controlled study of EGb761 in the treatment of chronic schizophrenia. Jounal of Clinical Psychological Medicine 1996;6(6):339‐41. [Google Scholar]
Zhang 1987 {published data only}
- Zhang LD, Tang YH, Zhu WB, Xu SH. Comparative study of schizophrenia treatment with electroacupuncture, herbs and chlorpromazine. Chinese Medical Journal 1987;100:152‐7. [PubMed] [Google Scholar]
- Zhang LD, Xu SH, Tang YH, Zhu WB. A comparative study of the treatment of schizophrenia with electric acupuncture, herbal decoction and chlorpromazine. American Journal of Acupuncture 1990;18(1):11‐4. [MEDLINE: ; PMID 10416732] 10416732 [Google Scholar]
Zhang 1997 {published data only}
- Zhang Liang Dong, Zhou Gang, Jin Zong Yu. Biochemical study on TCM Treatment of Schizophrenia Based on SyndromeTyping. Journal of Traditional Chinese Medicine 38;3:173‐75. [Google Scholar]
Zhang 2001 {published data only}
- *Zhang YZ, Zhou DF, Zhang PY, Wu Gui Ying, Su Jian Min, Cao Lian Yuan. A Double‐Blind, Placebo‐Controlled Trial of Extract of Ginkgo biloba Added to Haloperidol in Treatment ‐Resistant Patients With Schizophrenia. Journal of Clinical Psychiatry 2001;62:878‐83. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Su Jian Min, Zhang Pei Yan. The effects of Extracts of Ginkgo Biloba Added to Haloperidol on Superoxidase Dismutase in Inpatients With Chronic Schizophrenia. Journal of Clinical Psychopharmacology 2001;21(1):85‐88. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Zhang PY. Extract of Ginkgo biloba added to haloperidol was effective for positive symptoms in refractory schizophrenia. Evidence‐Based Mental Health 2002;5(3):90. [2003031658 NLM Unique Identifier: 22165225] [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Zhang PY, Wu GY, Su JM, Cao LY. Extract of Ginkgo biloba added to haloperidol was effective for positive symptoms in refractory schizophrenia. Journal of Clinical Psychiatry 2001;62(11):878‐83. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Zhou DF, Zhang PY. A randomized double‐blind, placebo‐controlled study on the effect of ginkgo biloba extract (EGb761) plus haloperidol chronic schizophrenia. Chinese Journal of Nervous and Mental Diseases 1997;23(6):348‐50. [Google Scholar]
- Zhou DF, Zhang YX, Su J, Nan Z, Cui Y, Liu J, Guan Z, Zhang P, Shen Y. The effects of classic antipsychotic haloperidol plus the extract of ginkgo biloba on superoxide dismutase in patients with chronic refractory schizophrenia. Chinese Medical Journal 1999;112(12):1093‐6. [MEDLINE: ; PMID 11721446] [PubMed] [Google Scholar]
Zhu 1996 {published data only}
- Zhu YZ, Kang B, Zhu QQ. Clinical study of shuizhi‐dahuang mixture in treating schizophrenics with blood stasis syndrome [[data not available]]. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1996;16(11):646‐8. [MEDLINE: ; PMID 9772611] [PubMed] [Google Scholar]
References to studies excluded from this review
Cao 2000 {published data only}
- Cao XinDon, Wang Wei. Comparison between Therapeutic Effect of Risperidone in Treating Different Traditional Chinese Medicine Syndrome Types of First‐Episode Schizophrenia. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(6):421‐23. [PubMed] [Google Scholar]
Gong 2000 {published data only}
- Gong Mei Fang, Zu Yu Mei, Jin Ping, Kang Yu Ping. Clinical investigation of Qingnao Anshen secoction on hemorrheology in the patients with schizophrenia and depression. Hebei Journal of Traditional Chinese Medicine 2000;22(8):571‐73. [Google Scholar]
Han 2002 {published data only}
- Han B, Zhang M, Liu L, Liang A, Riederer P. A double blind controlled trial of traditional chinese medicine and clozapine in schizophrenia. International journal of neuropsychopharmacology (Abstracts of the 23rd congress of the Collegium international Neuro‐Psychopharmacologicum) Jun 23‐27, Montreal, Canada. 2002, issue Suppl 1:190.
Rong 2001 {published data only}
- Rong Yu. Controlled treatment of Que‐Dian Decocotion with low dosage of Clozapine in treating schizophrenia. Zhe Jiang Zhong Xi Yi Jie He Za Zhi 2001;11(8):470‐1. [Google Scholar]
Wang 1998 a {published data only}
- Wang B. A controlled study of the efficacy of Chinese herb medicine in schizophrenic patients. Chinese Journal of Neurology and Psychiatry 1986;18(1):44‐6. [Google Scholar]
- Wang B. Traditional Chinese medical treatment to invigorate blood and relieve stasis treatment of schizophrenia: comparison with antipsychotics treatment. Psychiatry and Clinical Neurosciences 1998;52(Suppl):S329‐30. [MEDLINE: ; PMID 9895184] [DOI] [PubMed] [Google Scholar]
Zhao 1997 {published data only}
- Zhao XY, He Ming, Luo Hechun. Effects of Ginkgo biloba Special Extract Egb761 on Nailfold Microcirculation and Symptoms of Chronic Schizophrenics. Chinese Journal of Microcirculation 1997;7(1):25‐27. [Google Scholar]
Zhen 1992 {published data only}
- Zhen HB, Feng RS. Chinese drug therapy in treatment of schizophrenia. Chinese Journal of Nervous and Mental Disorders 1992;18(6):362‐3. [MEDLINE: ; PMID 10674777] 10674777 [Google Scholar]
References to studies awaiting assessment
Chen 2008 {published data only}
- Chen Z, Wang G, Wang X, Li L, Xiao D. Effects of zhuangyang capsules on the serum prolactin of schizophrenic patients treated with risperidone. Wuhan Daxue Xuebao [Yixue Ban] 2008;29(6):798‐800. [Google Scholar]
Chen 2009 {published data only}
- Chen ZH, Wang GH, Wang XP, Huo YX, Yang MH, Li L, et al. Effect of Warm‐Supplementing Kidney Yang (WSKY) added to risperidone on quality of life in patients with schizophrenia: a RANDOMized controlled trial. Clinical Rehabilitation 2009;23(11):963‐72. [DOI] [PubMed] [Google Scholar]
Chiu 2010 {published data only}
- Chiu S, Cernovsky Z, Husni M, Copen J, Campbell RJ, Carr J. Panax ginseng augmentation of subsyndromal depressive symptoms (ssd) in schizophrenia: A multi‐site rct study. Proceedings of the 163rd Annual Meeting of the American Psychiatric Association; 2010 May 22‐26; New Orleans, LA. 2010.
Hung 2011 {published data only}
- Hung CC, Fu PK, Wang HY, Chan CH, Lan TH. Treatment effects of traditional chinese medicines suoquan pill and wuling powder on clozapine‐induced hypersalivation in patients with schizophrenia: Study protocol of a RANDOMized, PLACEBO‐controlled trial. Journal of Chinese Integrative Medicine 2011;9(5):495‐502. [PubMed] [Google Scholar]
Mei 2005 {published data only}
- Mei G, Zeng L, Liu J, Liu D. Zao‐Ren‐An‐Shen‐Tang (TCM) and chlorpromazine for schizophrenia. Proceedings of the 8th Conference of Integrated Traditional Chinese Medicine and Western Medicine for Mental Health Problems. 2005:61‐3.
Miyaoka 2009 {published data only}
- Miyaoka T, Furuya M, Yasuda H, Hayashida M, Nishida A, Inagaki T, et al. Yi‐Gan San as adjunctive therapy for treatment‐resistant schizophrenia: an open‐label study. Clinical Neuropharmacology 2009;32(1):6‐9. [DOI] [PubMed] [Google Scholar]
Mundewadi 2008 {published data only}
- Mundewadi AA, Joshi DD, Arekar AS, Bakre GB, Mundewadi RA. A RANDOMized, controlled, clinical trial of a herbal combination of aqueous extracts of bacopa monnieri and nardostachys jatamansi in schizophrenia, compared to standard anti‐psychotic drug, olanzapine as an active control. Bombay Hospital Journal 2008;50(3):456‐65. [Google Scholar]
NCT01045720 {published data only}
- NCT01045720. The treatment of 2 Chinese medicines in clozapine‐induced hypersalivation in schizophrenia. http://www.clinicaltrials.gov 2010.
Sanchez 2011 {published data only}
- Sanchez V, Chiu S, Cernovsky Z, Copen J, Husni M. Panax ginseng augmentation: Effects on depressive and negative symptom clusters and neurocognition in schizophrenia. Journal of Pharmacy and Pharmaceutical Sciences 2011;3:36s. [Google Scholar]
Tang 2005 {published data only}
- Tang YY, Jia HX, Jiang ZK, Cao XD, Yuan HN, Zhang JZ. Clinical observation of Jian‐Pi‐Bu‐Shen (TCM) treating memory deterioration in patients with schizophrenia. Proceedings of the 8th Conference of Integrated Traditional Chinese Medicine and Western Medicine for Mental Health Problems. 2005:32‐4.
Wang 2005 {published data only}
- Wang HQ. Correlation of dose and effect of traditional Chinese medicine for schizophrenia. Proceedings of the 8th Conference of Integrated Traditional Chinese Medicine and Western Medicine for Mental Health Problems. 2005:133.
Xiao 2011 {published data only}
- Xiao SF, Xue HB, Li X, Chen C, Li GJ, Yuan CM, et al. A DOUBLE‐BLIND, PLACEBO‐controlled study of traditional chinese medicine sarsasapogenin added to risperidone in patients with negative symptoms dominated schizophrenia. Neuroscience Bulletin 2011;27(4):258‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2005 {published data only}
- Yan JY, Zhang JW, Li YM, Ding QZ. Jie‐Jing‐Tang for antipsychotics induced EPS. Proceedings of the 8th Conference of Integrated Traditional Chinese Medicine and Western Medicine for Mental Health Problems. 2005:74‐7.
Zhang 2011 {published data only}
- Zhang WF, Tan YL, Zhang XY, Chan RCK, Wu HR, Zhou DF. Extract of ginkgo biloba treatment for tardive dyskinesia in schizophrenia: A RANDOMized, DOUBLE‐BLIND, PLACEBO‐controlled trial. Journal of Clinical Psychiatry 2011;72(5):615‐21. [DOI] [PubMed] [Google Scholar]
Zhou 1989 {published data only}
- Zhou K. Clinical observation of Da Ying Pian for decreasing western medicine dose and toxic side effect in treating schizophrenia. Shanghai Journal of Traditional Chinese Medicine [Shang Hai Zhong Yi Yao Za Zhi][中医杂志] 1989;2:8‐9. [Google Scholar]
于瑞丽, 2010 {published data only}
- 于瑞丽, 戚元丽, 侯彩兰, 贾福军. Bupleurum reconcile soup on the clinical efficacy of antipsychotic induced hyperprolactinemia [柴胡调和汤对抗精神病药所致高催乳素血症的临床疗效]. 广东医学 2010;31(19):2587‐9. [Google Scholar]
孟彬, 2009 {published data only}
- 孟彬, 王跃升, 龚毅, 林涛, 杨建华, 何艳. Sleep Capsule and clonazepam treatment of schizophrenia ‐ Sipan controlled study of sleep disorders [舒眠胶囊与氯硝西畔治疗住院精神分裂症睡眠障碍的对照研究]. Yunan Journal of Traditional Chinese Medicine and Materia Medica 2009;30(2):14‐5. [Google Scholar]
尹道亮 2011 {published data only}
- 尹道亮. The multipurpose discussion of the ginkgo biloba [银杏叶片的多用途探讨]. 中国医药指南 2011;9(23):306‐7. [Google Scholar]
左潇, 2010 {published data only}
- 左潇, 杨杰, 于得霞. The modified guizhifuling soup merger with quetiapine fumarate. Clinical efficacy for the treatment of chronic negative symptoms of schizophrenia [加味桂枝茯苓汤合并富马酸喹硫平治疗慢性精神分裂症阴性症状的临床疗效]. 中国健康心理学杂志 2010;18(12):1417‐9. [Google Scholar]
庞铁良, 2010 {published data only}
- 庞铁良, 孙秀琪, 宋翠双. Pu Xingqing impaired treatment. A clinical observation [蒲星清障方治疗狂病痰火扰神证临床观察]. 中国中医急症 2010;19(12):2054‐5. [Google Scholar]
张子梅, 2010 {published data only}
- 张子梅, 王云, 孙富根. Yi capsule role of adjuvant therapy in schizophrenia and safety evaluation [益脑胶囊在精神分裂症中的辅助治疗作用及安全性评价]. 中国医院药学杂志 2010;30(16):1373‐5. [Google Scholar]
彭东阳 2010 {published data only}
- 彭东阳. Integrative treatment of schizophrenia, 36 cases [中西医结合治疗精神分裂症36例疗效观察]. 中医药导报 2010;16(11):50‐1. [Google Scholar]
易世国, 2009 {published data only}
- 易世国, 曾德志, 罗建武, 周桂明. Qingxin decoction and clinical observation of ziprasidone treatment of schizophrenia [清心汤联合齐拉西酮治疗精神分裂症临床观察]. Chinese Journal of Ethnomedicine and Ethnopharmacy 2009;24:116‐8. [Google Scholar]
林升 2010 {published data only}
- 林升. Wendan decoction combined risperidone in the treatment of the clinical efficacy of first‐episode schizophrenia [温胆汤加减联合利培酮治疗首发精神分裂症的临床疗效分析]. 当代医学 2010;16(20):156‐7. [Google Scholar]
林虹, 2011 {published data only}
- 林虹, 寻知元, 徐志刚, 李育红, 王志凌. Flavored madness wake soup of schizophrenia the drift intervention [加味癫狂梦醒汤对精神分裂症th1/th2漂移干预的研究]. 四川中医 2011;29(08):18‐20. [Google Scholar]
柳贵明, 2010 {published data only}
- 柳贵明, 张跃坤. Clozapine in combination shuxuening the treatment of long‐term schizophrenia clinical observation [氯氮平合用舒血宁治疗长期住院精神分裂症临床观察]. 中国民康医学 2010;22(20):2576‐614. [Google Scholar]
段武钢, 2010 {published data only}
- 段武钢, 曾德志, 罗建武, 华曙光, 周桂明, 罗世芳. Nerves mainly treating the negative symptoms of schizophrenia [安神汤治疗以阴性症状为主精神分裂症的研究]. Modern Research of Integrated Chinese and Western Medicine [现代中西医结合杂志] 2010;19(15):1831‐2. [Google Scholar]
段武钢, 2010a {published data only}
- 段武钢, 曾德志, 罗建武, 周桂明, 商秀珍, 罗世芳. Ning Xin Tang on the treatment of refractory schizophrenia synergies [宁心汤对难治性精神分裂症治疗的增效作用]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2010;20(3):188‐9. [Google Scholar]
汪艳, 2011 {published data only}
- 汪艳, 韩遵民, 赵铁牛, 张智龙, 吴炳忠. Transfer hub of yin and yang needle treatment of schizophrenia, depression, 35 patients with clinical studies [转枢阴阳针法治疗精神分裂症后抑郁患者35例临床研究]. 中医杂志 2011;52(17):1472‐4. [Google Scholar]
洪娜 2010 {published data only}
- 洪娜. Western combination therapy in 60 Cases of schizophrenia [中西药联合治疗精神分裂症60例疗效观察]. 中国现代药物应用 2010;4(7):124‐5. [Google Scholar]
王鹤秋, 2009 {published data only}
- 王鹤秋, 许本键, 马永春, 金枝. Shuxuening combined atypical antipsychotic treatment observation of 34 cases of chronic schizophrenia [舒血宁合用非典型抗精神病药治疗慢性精神分裂症34例疗效观察]. Zhejiang Journal of Traditional Chinese Medicine 2009;9(44):648‐9. [Google Scholar]
石建喜, 2010 {published data only}
- 石建喜, 赵建龙, 岳华, 张伟, 高明秀. Yang xin the 2nd experience of 33 cases treatment of schizophrenia [养心2号方治疗精神分裂症33例体会]. Journal of Traditional Chinese Medicine [Chung i Tsa Chih Ying Wen Pan][中醫雜志] 2010;25(8):1405‐6. [Google Scholar]
罗世芳, 2011 {published data only}
- 罗世芳, 曾德志, 樊学文, 罗建武. Shugan tang combined quetiapine treatment of 50 cases of chronic schizophrenia [疏肝汤合并喹硫平治疗慢性精神分裂症50例临床观察]. 中医药导报 2011;17(09):16‐8. [Google Scholar]
翁深宏, 2010 {published data only}
- 翁深宏, 王高华, 王晓萍, 肖卫东, 蔡红娇. Vitro cultivated the bezoar merger haloperidol in the treatment of schizophrenia, randomized double‐blind controlled study [体外培育牛黄合并氟哌啶醇治疗精神分裂症的随机双盲对照研究]. 中国中西医结合杂志 2010;30(11):1213‐5. [PubMed] [Google Scholar]
肖世富, 2011 {published data only}
- 肖世富, 薛海波, 李霞, 陈超, 李冠军, 苑成梅, et al. Risperidone combining with the ZMS treatment of negative symptoms in schizophrenia, double‐bind, randomized controlled study [利培酮合并知母皂甙元治疗阴性症状为主的精神分裂症的双盲随机对照研究(英文)]. Neuroscience Bulletin 2011;27(04):258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
胡海燕, 2010 {published data only}
- 胡海燕, 朱未名, 王文花, 郑虹. Ditan soup mengshi blood of patients with schizophrenia interleukin its clinical efficacy study [礞石涤痰汤对精神分裂症患者血液白细胞介素的影响及其临床疗效研究]. 中华中医药学刊 2010;28(3):652‐4. [Google Scholar]
胥德广, 2011 {published data only}
- 胥德广, 陈有福. The shengmai capsules collaborative clinical observation of the antipsychotic treatment of schizophrenia [生脉胶囊协同抗精神病药治疗精神分裂症的临床观察]. 现代中西医结合杂志 2011;20(27):3404‐5. [Google Scholar]
解克平, 2009 {published data only}
- 解克平, 韩莹. Effect of traditional medicine on amenorrhea caused by antipsychotics in female patients with schizophrenia [中成药治疗抗精神病药物引起女性精神分裂症患者闭经的研究]. Shandong Archives of Psychiatry [山东精神医学] 2009;22(1):25‐6. [Google Scholar]
解克平 2010 {published data only}
- 解克平. Chinese medicine treatment of risperidone‐induced amenorrhea clinical control study [中药治疗利培酮引起的闭经临床对照研究]. 精神医学杂志 2010;23(06):461‐2. [Google Scholar]
邓方渝 2010 {published data only}
- 邓方渝. Mirtazapine joint Guipi the treatment of schizophrenia clinical studies of depression [米氮平联合归脾丸治疗精神分裂症后抑郁的临床研究]. 临床和实验医学杂志 2010;9(21):1623‐4. [Google Scholar]
郝广义, 2009 {published data only}
- 郝广义, 张万红, 李春来, 文香兰. Yiqihuoxue efficacy in treating schizophrenia [益气活血法结合西药治疗精神分裂症疗效观察]. Journal of Chinese Rural Physician [中国社区医师:综合版] 2009;8:14. [Google Scholar]
鄢爱平, 2009 {published data only}
- 鄢爱平, 曾德志, 樊学文, 罗建武. Treatment of chronic schizophrenia anshen supporting role [安神汤治疗慢性精神分裂症的辅助作用]. Chinese Journal of Ethnomedicine and Ethnopharmacy 2009;23:63‐5. [Google Scholar]
陈克彦 2009 {published data only}
- 陈克彦. Chinese medicine the treatment efficacy of schizophrenia negative symptoms [中药联合治疗精神分裂症阴性症状疗效观察]. Shanghai Journal of Traditional Chinese Medicine [Shang Hai Zhong Yi Yao Za Zhi][中医杂志] 2009;50:148‐9. [Google Scholar]
马俊国, 2009 {published data only}
- 马俊国, 徐富玲, 谭余龙. Lok film treatment with antipsychotics result of bak foong pills efficacy of menstrual disorders [安乐片与乌鸡白凤丸治疗抗精神病药物所致月经紊乱的疗效观察]. Journal of Qiqihar Medical College [Qiqihar Yixueyuan xuebao][齊齊哈爾醫學院學報] 2009;30(12):1475‐6. [Google Scholar]
黄平, 2011 {published data only}
- 黄平, 廖彦浔, 叶慧, 虞晓兰. 阿立哌唑治疗抗精神病药所致闭经的精神分裂症对照研究 Aripiprazole treatment of antipsychotic induced amenorrhea in schizophrenia. 临床精神医学杂志 2011;21(03):201‐2. [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins JPT. Cochrane Reviewers' Handbook 4.2.2 [updated December 2003]. The Cochrane Library. 1. Chichester, UK: John Wiley & Sons, Ltd, 2004. [In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1983
- Andreasen NC. Negative symptoms in schizophrenia. Archives of General Psychiatry 1983;39:784‐8. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behaviour. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994.. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1970
- Guy W, Bonato RR, eds. Clinical Global Impressions. In: Manual for the ECDEU Assessment Battery 2. Rev ed. National Institute of Mental Health, 1970. [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology (DOTES: Dosage Record and Treatment Emergent Symptom Scale). Rockville: National Institute of Mental Health, 1976. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2002
- Li X, Wei W. Chinese Materia Medica combinations and applications. UK: Donica Publishing Ltd, 2002. [Google Scholar]
Luo 1997
- Luo HC, Shen YC, Meng FQ. Therapeutic effect of shuxuening combining neuroleptics for the treatment of chronic schizophrenia ‐ a double blind study. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih 1997 Mar;17(3):139‐42. [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Ming 2001
- Translated by Zhu Ming. The Medical Classics of the Yellow Emperor. Beijing: Foreign Language Press, 2001. [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Saku 1991
- Saku M. The current clinical practice of herbal medicine in psychiatry in mainland China: a review of literature. Japanese Journal of Psychiatry and Neurology 1991;45(4):825‐32. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
Walter 1999
- Walter G, Rey JM. The relevance of herbal treatments for psychiatric practice. Australian and New Zealand Journal of Psychiatry 1999;33(4):482‐9. [DOI] [PubMed] [Google Scholar]
Wang 1998
- Wang B. Traditional Chinese medical treatment to invigorate blood and relieve stasis treatment of schizophrenia: comparison with antipsychotic treatment. Psychiatry and Clinical Neuroscience 1998 Dec;52 Suppl:S329‐30. [DOI] [PubMed] [Google Scholar]
Xu 1991
- Xu X. The English‐Chinese Encyclopedia of Practical Traditional Chinese Medicine. Beijing: Higher Education Press, 1991. [Google Scholar]
Zhang 1996
- Zhang Jizhi. Current status of integrated traditional and Western medicine study on schizophrenia. Chinese Journal of Integrated traditional and Western medicine 1996;16(11):643‐45. [PubMed] [Google Scholar]


