Abstract
Background
Meconium aspiration syndrome may cause severe respiratory distress in the newborn infant, with an associated high morbidity and mortality. A chemical pneumonitis is believed to occur secondary to bile, bile acids and pancreatic secretions contained in meconium. It has therefore been hypothesised that corticosteroids may be of benefit in the management of this condition through their anti‐inflammatory properties.
Objectives
The objective of this review was to determine whether steroid therapy for meconium aspiration syndrome decreases the morbidity and mortality associated with this condition without adverse effects.
Search methods
Searches were made of PREMEDLINE and MEDLINE from 1966 to April 2003, CINAHL back to 1982, Current Contents back to 1998, The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2003) and Oxford Database of Perinatal Trials. The search included cross‐referencing of previous reviews, and a review of abstracts, conference and symposia proceedings published in Pediatric Research from 1993 to 2003.
Selection criteria
Randomised controlled trials and quasi‐randomised trials comparing steroid treatment to no steroid treatment for neonates with meconium aspiration syndrome were considered for this review.
Data collection and analysis
The methodological quality of each trial was assessed independently by each author. Data were extracted, analysed and results reviewed independently by each author. Meta‐analysis was performed with RevMan 4.2, using the fixed effects model. Mean difference (MD) and weighted mean differences (WMD) with 95% confidence intervals in brackets for continuous variables and Relative Risk (RR) with 95% confidence intervals for categorical data were reported.
Main results
Three randomised controlled trials were identified. Two trials, by Wu 1999 (50 participants) and Yeh 1977 (35 participants), were included in the review. The trial by Davey 1995, as yet unpublished, was excluded from this review as insufficient information about methodology and results were available. On meta‐analysis, there was no significant reduction in mortality [typical RR 0.95 (0.20, 4.58)]. A small but significant increase in duration of oxygen therapy was seen with the use of steroids [WMD 30.0 hours (8.4, 51.6)]. There was no significant difference in duration of hospital stay in the study by Wu 1999 [MD 0.00 days (‐3.09, 3.09)]. Duration of mechanical ventilation was reported by Wu 1999 with no significant difference seen [MD ‐1.10 days (‐2.79, 0.59)]. Incidence of air leak was reported by Yeh 1977 with no significant difference detected [RR 0.64 (0.18, 2.26)]. Long‐term outcome was not reported in either of the two studies.
Authors' conclusions
At present, there is insufficient evidence to assess the effects of steroid therapy in the management of meconium aspiration syndrome. A further large randomised controlled trial assessing potential benefits and harm would be required to determine its role.
Plain language summary
Steroid therapy for meconium aspiration syndrome in newborn infants
More research needed to show whether corticosteroids could reduce complications and mortality in newborn babies with meconium aspiration syndrome.
A bowel movement (meconium) from an unborn baby in stress during labour can enter the lungs when the baby starts to breathe after birth. Suction and/or intubation are used to try and remove the meconium from the baby's breathing passages, but some babies will still develop meconium aspiration syndrome. Those babies will have breathing difficulties which can lead to breathing failure and death. Corticosteroids are anti‐inflammatory drugs that have been tried for babies with meconium aspiration syndrome. However, the review of trials found that there is not enough evidence to assess the potential benefits and harms of this treatment.
Background
Meconium is a viscous green substance found in the fetal gastrointestinal tract from approximately the 10th week of gestation. It is an heterogenous substance composed of a mixture of bile, bile acids, mucous, pancreatic secretions and cellular debris (Wiswell 1993). Approximately 10 to 15 % of pregnancies will be complicated by the passage of meconium around the time of delivery (Wiswell 1993). It is estimated that despite current interventions such as intubation with tracheal suction, five to 12% of infants born through meconium stained amniotic fluid will still develop meconium aspiration syndrome (Wiswell 2000).
Meconium aspiration syndrome is defined as respiratory distress associated with the passage of meconium around the time of birth, with characteristic radiological changes and without an alternative etiology for the respiratory symptoms. The radiological features consist of areas of atelectasis and consolidation, along with regions of hyperexpansion. Histologically, a significant inflammatory reaction is seen, with a diffuse pulmonary infiltrate of polymorphonuclear leukocytes. Release of cytokines from these leukocytes, along with the direct toxicity of bile salts, has been postulated to result in a chemical pneumonitis (Oelberg 1990). Hypoxemia and hypercapnia may occur, requiring respiratory support. Secondary pulmonary vasoconstriction may follow, resulting in persistent pulmonary hypertension of the newborn (Wiswell 1993).
There has been considerable interest in the therapeutic potential of steroids in the management of meconium aspiration syndrome, due to their anti‐inflammatory properties. In a randomised controlled trial of cortisol in a rabbit model of meconium aspiration syndrome, a slight decrease in both respiratory rate and the severity of pulmonary histopathological changes was seen in the cortisol treated rabbits, but there was also increased mortality in the treatment group (Frantz 1975). A more recent animal study on a newborn piglet model of meconium aspiration found improvement in lung function following treatment with dexamethasone (Khan 1999).
Objectives
The objective of this review was to determine whether steroid therapy for meconium aspiration syndrome decreases the incidence of chronic lung disease and mortality associated with this disease, without an associated increase in adverse effects such as neurodevelopmental disability.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised trials were considered for this review.
Types of participants
Newborn infants with a history of meconium stained amniotic fluid and clinical and radiological findings consistent with a diagnosis of meconium aspiration syndrome (areas of atelectasis and consolidation along with regions of hyperexpansion).
Types of interventions
Systemic or inhaled steroid compared to no steroid treatment.
Types of outcome measures
Primary outcome measures: 1. Chronic lung disease (oxygen dependency at 28 days of age) 2. Mortality (before hospital discharge, before age 1 year) 3. Long term growth, and neurodevelopmental outcome assessed at age 1, 2 and 5 years with validated assessment tools
Secondary outcome measures: 1. Duration of mechanical ventilation 2. Duration of oxygen therapy 3. Pulmonary air leak syndromes (pneumothorax, pulmonary interstitial emphysema) 4. Intraventricular haemorrhage and/or periventricular leukomalacia on head ultrasound studies 5. Duration of hospital stay
Post‐hoc outcome measures: 1. Acute adverse steroid effects (weight loss, hyperglycaemia, hypertension and gastrointestinal haemorrhage) 2. Treatment with extra‐corporeal membrane oxygenation (ECMO)
Search methods for identification of studies
See: Cochrane Neonatal Review Group search strategy
Using MeSH search terms ' meconium aspiration syndrome ' and 'exp infant, newborn' searches were made of PREMEDLINE and MEDLINE from 1966 to April 2003, CINAHL from 1992 to April 2003, Current Contents from 1998 to April 2003, the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2003) and the Oxford Database of Perinatal Trials. The search included cross‐referencing of previous reviews, and a review of abstracts, conference and symposia proceedings published in Pediatric Research from 1993 to April 2003.
Data collection and analysis
The reviewers independently searched for and assessed trials for inclusion and methodological quality. Studies were assessed using the following key criteria: blinding of randomisation, blinding of intervention, completeness of follow up and blinding of outcome measurement. Data were extracted independently by the reviewers. Differences were resolved by discussion and consensus of the reviewers. Meta‐analysis was performed using RevMan 4.2. Mean difference (MD) and weighted mean differences (WMD) were reported for continuous variables. For categorical outcomes the Relative Risk (RR) was reported. For significant findings, number needed to treat (NNT) would be reported. 95% confidence intervals were reported in brackets. Heterogeneity tests were used to help decide whether the trials merited pooling. The fixed effects model was used for meta‐analysis.
We sought clarification of the data from the authors of both studies but did not receive additional information. As the variation around the mean duration of oxygen therapy reported by Yeh 1977 was so small, and because standard error was used elsewhere in the paper to describe variance, this was assumed to be the standard error and was converted to standard deviation for our analysis.
Results
Description of studies
See: Tables, Characteristics of included studies.
Three randomised controlled trials were identified with the Search Strategy. Two randomised controlled studies were included in this systematic review, Yeh 1977 and Wu 1999. The third study, Davey 1995 which examined dexamethasone versus placebo therapy in the treatment of meconium aspiration syndrome was excluded as it was published in abstract form only. We contacted the authors who were unable to provide sufficient data for inclusion in this review.
Of note is the time difference of 22 years between the studies by Yeh 1977 and Wu 1999. There were 35 infants in the study by Yeh 1977, and 50 infants in the study by Wu 1999, giving a total of 85 infants in the two studies. Similar diagnostic criteria were used in each study, with the definition of meconium aspiration syndrome based on the presence of meconium staining of the skin, with associated respiratory distress and radiological findings consistent with meconium aspiration syndrome. The type of intervention and duration of treatment varied between the two studies. Yeh 1977 compared a two day course of intravenous hydrocortisone with lactose placebo, whereas Wu 1999 compared a seven day reducing course of intravenous dexamethasone to saline placebo. Both studies examined the outcomes of mortality, need for mechanical ventilation and duration of oxygen therapy. Yeh 1977 also assessed the incidence of air leak syndromes. Assisted ventilation was reported as an outcome, but the study did not define whether this included or excluded continuous positive airways pressure. The study did not report the incidence of chronic lung disease, hyperglycaemia, hypertension, weight loss, duration of mechanical ventilation, length of hospital stay or long term outcome. Wu 1999 reported the duration of mechanical ventilation, length of hospital stay, weight loss, hypertension and hyperglycaemia, but did not report the incidence of air leak syndromes. Neither study reported outcome data following hospital discharge.
Risk of bias in included studies
See: Tables, Characteristics of included studies
In Yeh 1977 allocation concealment was adequate, using a random number table with master code in a sealed envelope. There were no exclusions after randomisation. Blinding of intervention was achieved using identical treatment and placebo vials. While the trial was described as double blind, the methods of blinding of outcome assessment were not described.
The study by Wu 1999 was described as a double blind randomised trial, but the methods of allocation concealment and blinding of intervention and outcome assessment after randomisation were not clearly outlined by the authors. There were no exclusions after randomisation.
Effects of interventions
Primary outcome measures: 1. Chronic lung disease (oxygen dependency at 28 days of age) This outcome was reported only by Wu 1999. No infants in either steroid‐treated or control groups developed chronic lung disease in this study.
2. Mortality Mortality before hospital discharge was assessed by Yeh 1977 and Wu 1999 (85 infants). Neither study reported a significant difference between the steroid‐treated compared to control groups. On meta‐analysis there was no significant difference in the mortality rate before hospital discharge between the steroid‐treated and control groups [typical RR 0.95 (0.2‐4.58)]. Mortality at one year follow up was not reported in either study.
3. Long‐term growth and neurodevelopmental outcome assessed at age 1, 2 and 5 years with validated assessment tools. These outcomes were not reported in either of the included studies.
Secondary outcome measures: 1. Duration of mechanical ventilation Only Wu 1999 reported this outcome (50 infants). In this trial the duration of mechanical ventilation was reported as significantly shorter in the steroid treated group (mean 3.5 days) compared to control group (4.6 days). Our analysis, however, found this difference not to be statistically significant.
2. Duration of oxygen therapy Both Yeh 1977 and Wu 1999 reported this outcome (85 infants). Yeh 1977 reported that infants in the steroid‐treated group took longer to wean to room air (68.9 +/‐ 38.6 hours) than infants in the control group (36.6 +/‐ 29.3 hours). Wu 1999 reported no significant difference in duration of oxygen therapy. On meta‐analysis there was a significant increase in hours on oxygen therapy in the steroid‐treated group compared to the control group [WMD 30.0 hours (8.4‐51.6)].
3. Pulmonary air leak syndromes (pneumothorax, pulmonary interstitial emphysema) The incidence of air leak was only reported by Yeh 1977 (35 infants) who found no significant difference between the steroid‐treated and control groups [RR 0.64 (0.18‐2.26)].
4. Intraventricular haemorrhage and/or periventricular leukomalacia on head ultrasound studies Neither of the included studies reported data on this outcome.
5. Duration of hospital stay Duration of hospital stay was only reported by Wu 1999 (50 infants) who found no significant difference in the number of days in hospital between with those treated with steroids and those receiving placebo [MD 0.0 days (‐3.09‐3.09)].
Post‐hoc outcome measures: 1. Acute adverse steroid effects (weight loss, hyperglycaemia, hypertension and gastrointestinal haemorrhage) Only Wu 1999 reported weight loss. While more weight loss was seen on days 5 to 10 in the steroid‐treated group, this weight loss was not quantified, and the statistical and clinical significance were not described. Wu 1999 reported that transient elevations of blood glucose and blood pressure were noted on days 2, 3, 5 and 7 in the steroid‐treated group; data were not available. Yeh 1977 did not report blood glucose levels or blood pressure measurements. Neither Wu 1999 nor Yeh 1977 reported the incidence of gastrointestinal haemorrhage.
2. Treatment with extra‐corporeal membrane oxygenation (ECMO) Neither Wu 1999 nor Yeh 1977 reported ECMO as an outcome in their studies.
Discussion
Only two randomised controlled trials, Yeh 1977 and Wu 1999, were identified for inclusion in this review, with insufficient data to include a third study by Davey 1995. Mortality and duration of oxygen therapy were the only common outcomes reported in the two studies, limiting the scope of the meta‐analysis .There was also a time lag of 22 years between the two studies, and variation in both the type of steroid chosen and duration of therapy. The sample size of both studies was small, giving a total of only 85 patients in the meta‐analysis. With these limitations, caution is advised in the interpretation of the results of this systematic review.
No significant difference was seen in mortality with steroid treatment. In fact a surprising small but significant increase in days of oxygen therapy was found. Limited data were available regarding acute adverse effects of steroid therapy such as hyperglycaemia and hypertension with Wu 1999 suggesting there was no effect, while neither study provided information regarding long term respiratory and neurodevelopmental outcome.
In contrast to the statistically significant result reported in the paper by Wu 1999, our analysis of the difference in duration of mechanical ventilation using the data reported showed that the difference between the two groups was not significant. Data published in the paper suggest that the mean duration of mechanical ventilation reported for the steroid treated group may be incorrect, as there were no infants on mechanical ventilation at four days. The authors were contacted but were unable to provide additional information.
It has previously been noted that survivors of meconium aspiration may develop symptoms of obstructive airways disease in later childhood (MacFarlane 1988, Swaminathan 1989). An increased incidence of cerebral palsy has also been noted in infants with a history of meconium staining at delivery (Nelson 1989) compared to the general population. These outcomes are therefore important when planning studies involving infants with meconium aspiration syndrome. A recently published case series of 14 patients treated with intravenous dexamethasone for ventilated infants with meconium aspiration syndrome intends to follow up longer term developmental outcome (da Costa 2001). Endeavours to assess long term respiratory and neuro‐developmental sequelae of meconium aspiration syndrome within the context of randomised controlled trials are also desirable. There remains considerable interest in the possible benefits of steroid therapy for meconium aspiration syndrome. Previous animal studies and a prospective case series have shown encouraging results (Khan 1999, da Costa 2001). Given current concerns regarding the possible role of postnatal dexamethasone in the aetiology of cerebral palsy in preterm infants, caution is required in the use of steroids during the neonatal period. However, in view of the significant mortality and morbidity associated with meconium aspiration syndrome, including respiratory failure requiring ECMO therapy (Davey 1995), steroids continue to be prescribed in the clinical setting for infants with this condition (Davey 1995, da Costa 2001). At present there is insufficient evidence available to fully evaluate the risks and benefits of steroid treatment. A larger randomised controlled trial including careful assessment of short and long term adverse effects would be required to further delineate the role of steroid therapy in the management of meconium aspiration syndrome.
Authors' conclusions
Implications for practice.
At present, there is insufficient evidence to assess the effects of steroid therapy in the management of meconium aspiration syndrome.
Implications for research.
Given the small number and sample sizes of randomised controlled trials undertaken to date, and the mortality and morbidity associated with meconium aspiration syndrome itself, a large randomised controlled trial including assessment of acute and long‐term adverse effects would be required to assess effectiveness. The limited information available to date suggests early parenteral dexamethasone would be an appropriate intervention for further study (da Costa 2001).
What's new
| Date | Event | Description |
|---|---|---|
| 22 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 14 July 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Professor David Henderson‐Smart and Jane Bell from the Australasian Co‐ordinating Network for the Cochrane Neonatal Review Group for their support in the preparation of this review.
Data and analyses
Comparison 1. Steroid vs placebo in the treatment of meconium aspiration syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chronic lung disease (oxygen dependency at 28 days) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Mortality (before hospital discharge) | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.20, 4.58] |
| 3 Duration of mechanical ventilation (days) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.79, 0.59] |
| 4 Duration of oxygen therapy (hours) | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 30.00 [8.40, 51.61] |
| 5 Pulmonary air leak syndrome | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.18, 2.26] |
| 6 Duration of hospital stay (days) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.09, 3.09] |
1.1. Analysis.
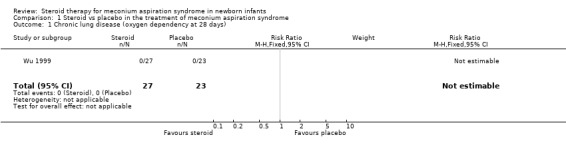
Comparison 1 Steroid vs placebo in the treatment of meconium aspiration syndrome, Outcome 1 Chronic lung disease (oxygen dependency at 28 days).
1.2. Analysis.
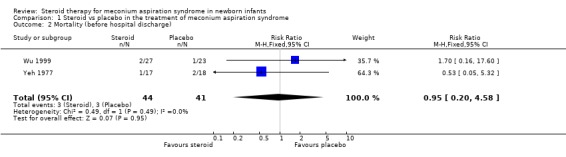
Comparison 1 Steroid vs placebo in the treatment of meconium aspiration syndrome, Outcome 2 Mortality (before hospital discharge).
1.3. Analysis.
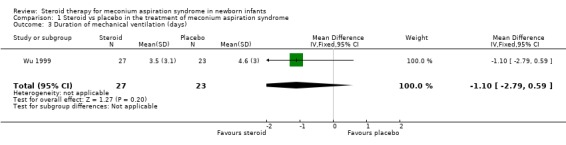
Comparison 1 Steroid vs placebo in the treatment of meconium aspiration syndrome, Outcome 3 Duration of mechanical ventilation (days).
1.4. Analysis.
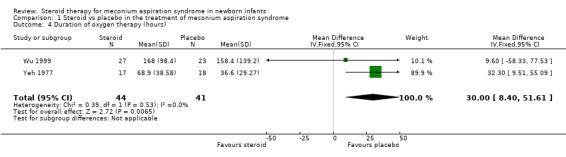
Comparison 1 Steroid vs placebo in the treatment of meconium aspiration syndrome, Outcome 4 Duration of oxygen therapy (hours).
1.5. Analysis.
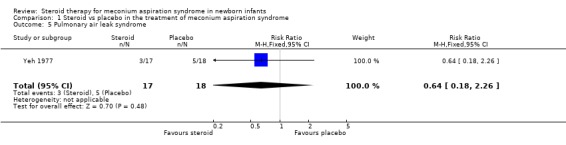
Comparison 1 Steroid vs placebo in the treatment of meconium aspiration syndrome, Outcome 5 Pulmonary air leak syndrome.
1.6. Analysis.
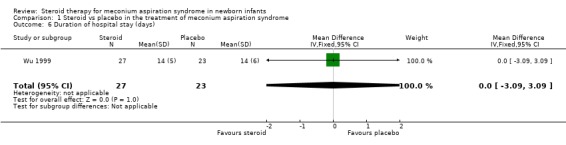
Comparison 1 Steroid vs placebo in the treatment of meconium aspiration syndrome, Outcome 6 Duration of hospital stay (days).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Wu 1999.
| Methods | Randomised controlled trial‐ double blind. Blinding of randomisation ‐ can't tell. Randomised assigment list used for randomisation, but concealment unclear. Intervention adequately blinded, with equal volumes of treatment and saline placebo. Follow up complete for all enrolled subjects. Method of blinding outcome assessment not clearly defined. | |
| Participants | 50 newborn infants with meconium aspiration syndrome and severe respiratory distress. | |
| Interventions | Intervention group n=27; Control group n=23. Intravenous dexamethasone or saline placebo of equivalent volume given immediately after diagnosis of meconium aspiration syndrome, then q12h for 7 days Initial dose: 1mg/kg/dose; day 1‐3: 0.5mg/kg/dose; day 4‐7: 0.25mg/kg/dose. | |
| Outcomes | Pulmonary hypertension, mortality, intubation, duration of mechanical ventilation, duration of oxygen therapy, length of hospitalisation, acid base status, weight loss, blood glucose, blood pressure. | |
| Notes | Study assumed to have been conducted in Taiwan, dates unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yeh 1977.
| Methods | Randomised controlled trial‐double blind. Blinding or randomization ‐ yes. Random number sequence with master code in sealed envelope. Blinding of intervention achieved with identical vials of treatment and placebo. Outcome data available for all enrolled subjects. Methods of blinding of outcome assessment not clearly defined. | |
| Participants | 35 newborn infants with meconium staining of the skin and positive tracheal aspiration of meconium who had one of two criteria: 1: clinical signs of respiratory distress in the first 4 hours and/or 2: radiographic findings of aspiration syndrome. | |
| Interventions | Intervention group n=17; Control group n=18. Identical vials of hydrocortisone or placebo (lactose hydrous) in similar diluent administered at same intervals. Hydrocortisone 20mg/kg initially then q12h for 4 doses given immediately after diagnosis. | |
| Outcomes | Need for mechanical ventilation, incidence of air leak, mortality, alveolar‐arterial oxygen gradient, duration of oxygen therapy, length of respiratory distress. | |
| Notes | Study conducted between October 1974 and September 1975 in hospital setting in Illinois, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davey 1995 | Abstract contained insufficient information about methodology and results. Two authors contacted but could not provide additional data. |
Contributions of authors
Dr Meredith Ward is the primary author of the review, and has determined objectives, types of studies, interventions and outcomes for inclusion. Dr John Sinn assisted in writing the review. Both authors have independently performed searches of the current literature, assessed study methodology and extracted the available data.
Sources of support
Internal sources
Westmead Hospital, Westmead, Australia.
Royal Hospital for Women, Randwick, Australia.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Wu 1999 {published data only}
- Wu JM, Yeh TF, Wang JY, Wang JN, Lin YJ, Hsieh WS, Lin CH. The role of pulmonary inflammation in the development of pulmonary hypertension in newborn with meconium aspiration syndrome. Pediatric Pulmonology ‐ Supplement 1999;18:205‐208. [PubMed] [Google Scholar]
Yeh 1977 {published data only}
- Yeh TF, Srinivasan G, Harris V, Pildes RS. Hydrocortisone therapy in meconium aspiration syndrome: A controlled study. Journal of Pediatrics 1977;90:140‐143. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Davey 1995 {published data only}
- Davey AM, Kueser TJ, Turner HF. Randomised controlled trial of early dexamethasone therapy in the treatment of meconium aspiration syndrome. Pediatric Research 1995;37:329A. [1958] [Google Scholar]
Additional references
Burke 1973
- Burke‐Strickland M. Tracheobronchial lavage in small infants. Minnesota Medicine 1973;56:287‐89. [PubMed] [Google Scholar]
da Costa 2001
- Costa DE, Nair AK, Pai MG, Al Khusaiby SM. Steroids in full term infants with respiratory failure and pulmonary hypertension due to meconium aspiration syndrome. European Journal of Pediatrics 2001;160:150‐53. [DOI] [PubMed] [Google Scholar]
Frantz 1975
- Frantz ID, Wang NS, Thach BT. Experimental meconium aspiration: effect of glucocorticoid treatment. Journal of Pediatrics 1975;86:438‐41. [DOI] [PubMed] [Google Scholar]
Khan 1999
- Khan AM, Shabarek FM, Kutchback JW, Lally KP. Effects of dexamethasone on meconium aspiration syndrome in newborn piglets. Pediatric Research 1999;46:179‐83. [DOI] [PubMed] [Google Scholar]
MacFarlane 1988
- MacFarlane PI, Heaf DP. Pulmonary function in children after neonatal meconium aspiration syndrome. Archives of Disease in Childhood 1988;63:368‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 1989
- Nelson KB. Relationship of intrapartum and delivery room events to long‐term neurologic outcome. Clinics in Perinatology 1989;16:995‐1007. [PubMed] [Google Scholar]
Oelberg 1990
- Oelberg DG, Downey SA, Flynn MM. Bile salt‐induced intracellular calcium accumulation in type II pneumocytes. Lung 1990;168:297‐308. [DOI] [PubMed] [Google Scholar]
Swaminathan 1989
- Swaminathan S, Quinn J, Stabile MW, et al. Long‐term pulmonary sequelae of meconium aspiration syndrome. Journal of Pediatrics 1989;114:356‐61. [DOI] [PubMed] [Google Scholar]
Wiswell 1993
- Wiswell TE, Bent RC. Meconium staining and the meconium aspiration syndrome. Unresolved issues. Pediatric Clinics of North America 1993;40:955‐81. [DOI] [PubMed] [Google Scholar]
Wiswell 2000
- Wiswell TE, Gannon CM, Jacob J, Goldsmith L, Szlyd E, et al. Delivery room management of the apparently vigorous meconium‐stained neonate: Results of the multicenter, international collaborative trial. Pediatrics 2000;105:1‐7. [DOI] [PubMed] [Google Scholar]


