Abstract
Background
In both adult rheumatoid arthritis (RA) and juvenile arthritis, the focus has shifted from 'inflammation parameters' to more patient centered disability outcomes. In RA this resulted in the development of the Outcome Measures in Arthritis Clinical Trials (OMERACT), and in juvenile arthritis the Pediatric Rheumatology International Trials Organization (PRINTO) core set. This PRINTO‐core set was established using a combination of statistical and consensus formation techniques. This core set contains a number of patient centered disability measures. This review systematically searched the available literature and reports the available evidence of efficacy of MTX, with special focus on patient centered disability measures in Juvenile Idiopathic Arthritis (JIA).
Objectives
To perform a systematic review on the effects of MTX on functional ability, range of motion, quality of life, overall well‐being and pain for patients with JIA.
Search methods
The Cochrane Controlled Trials Register (CCTR) and MEDLINE were searched up to March 2001, using the search strategy sensitive for randomised controlled trials, used by the Cochrane Collaboration.
Selection criteria
Randomized controlled trials and controlled clinical trials comparing MTX against placebo or standard care in patients with Juvenile Idiopathic Arthritis (JIA) were selected.
Data collection and analysis
Two reviewers (TT, JN) determined the studies to be included in this review and extracted the data of patient centered disability measures. The data were pooled using standardized mean differences (SMD) for limited joint range score, number of joints with swelling. The number of joints with pain on motion were evaluated using weighted mean differences (WMD). Physicians global assessment, parents global assessment and withdrawals due to efficacy and side effects were evaluated with pooled odds ratios (OR).
Main results
Only two studies with a total 165 JIA patients under 18 years of age were included in this review. For JIA patients, MTX therapy had small to moderate effects on patient centered disability outcomes. The effect on joint range of motion, number of joints with pain and swelling and physician's and parent's assessment of disease activity showed a relative percentage improvement from 3 to 23% greater with MTX than with placebo.
Authors' conclusions
Current evidence suggests that MTX does have minimal clinically significant effects (>20%) on patient centered disability measures in JIA patients.
Plain language summary
Methotrexate for juvenile idiopathic arthritis
Methotrexate (MTX) is a commonly used immuno modifying drug for children with juvenile arthritis. It is believed that MTX is an effective medication for children with juvenile arthritis. We reviewed the existing literature on the efficacy of MTX on patient centered disability measures. Two trials were found and the results were pooled. We found small to moderate effects of MTX. These effects ranged from 3 to 23% greater improvement in the MTX group than the placebo group. However, most of these effects were too small to be clinically significant.
Background
Juvenile Idiopathic Arthritis (JIA) is the currently proposed international name for the classification of chronic childhood arthritis (Petty 1998). Diagnosis is confirmed when the onset of the arthritis is before the age of 16, the duration of the symptoms exceeds 6 weeks and other known causes are excluded. In JIA seven distinct subtypes are known (Petty 1998).
Methotrexate is a folic acid antagonist, widely used for the treatment of neoplastic disorders. Its mechanism of action in childhood arthritis is not yet known, but the immuno modifying actions might be mediated by adenosine. Currently, MTX is among the most commonly used immuno modifying drugs for the treatment of adult RA. MTX is also considered effective for JIA, and is a commonly used second‐line agent for the treatment of juvenile arthritis (Onel 2000).
In both adult RA and JIA the focus has shifted from 'inflammation parameters' to more patient centered outcomes. For the RA this resulted in the development of the OMERACTs core set for RA (OMERACT 1997), in JIA the PRINTO‐core set (Giannini 1997). The OMERACT‐core set consists of patient and physician global assessment, pain, disability, and an acute‐phase reactant The PRINTO‐core set was established using a combination of statistical and consensus formation techniques. This core set contains a number of patient centered disability measures; physician global assessment of disease activity; parent or patient (if appropriate in age) global assessment of overall well being; functional ability; number of joints with active arthritis; number of joints with limited range of motion; erythrocyte sedimentation rate.
The purpose of this review is to systematically search the available literature and report the available evidence of efficacy of MTX, with special focus on patient centered disability measures in JIA.
Objectives
To evaluate the effects of MTX therapy on patient centered disability measures such as functional ability, range of motion, quality of life, overall well‐being and pain in patients with JIA. Safety and side effects of MTX therapy will also be addressed in this review.
Methods
Criteria for considering studies for this review
Types of studies
1. Randomized controlled trials 2. Controlled clinical trials
Types of participants
Juvenile Idiopathic Arthritis (JRA/ JCA) patients under 18 years of age.
Oligo (Pauci) articular JIA
Poly articular JIA
Systemic onset JIA
Diagnosed by rheumatologist on established criteria from an (inter)national organization, such as the International League Against Rheumatism (ILAR), American College of Rheumatology (ACR) or European League Against Rheumatism (EULAR).
Types of interventions
MTX vs placebo
MTX vs no treatment
MTX vs Therapy Y
MTX vs standard care
Types of outcome measures
This review focuses on patient centered disability measures as suggested by Giannini 1997:
Functional Ability;
Range of motion measures;
Number of Joints with swelling;
Number of Joints with pain;
Quality of Life;
Parent/patient/physicians global assessment of overall well‐ being.
Safety and side effects.
Number of withdrawals overall and withdrawals due to lack of efficacy and side effects.
Search methods for identification of studies
We searched MEDLINE using the strategy developed by Dickersin et al (Dickersin 1994) up to and including March 2001 and the Cochrane Controlled Trials Register Issue 1, 2001. Reference lists of all trials selected through the electronic search were manually searched to identify additional trials. Search strategy: Identifying trials: 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized controlled trials.sh. 4. random allocation.sh. 5. double blind method.sh. 6. single blind method.sh. 7. 1 or 2 or 3 or 4 or 5 or 6 8. (animal not (human and animal)).sh. 9. 7 not 8 10. clinical trial.pt. 11. exp clinical trials/ 12. (clin$ adj25 trial$).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 14. placebos.sh. 15. placebo$.ti,ab. 16. random$.ti,ab. 17. research design.sh. 18. volunteer$.ti,ab. 19. 10 or 11 or 12 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20. 19 not 8 21. 20 not 9 22. 9 or 21 Identifying juvenile arthritis patients: 23. Arthritis, Juvenile Rheumatoid.sh 24. Arthritis, Juvenile Chronic.tw 25. Arthrit$, Juvenile.tw 26. Arthrit$.tw 27. Child$.tw 28. Adolescen$.tw 29. Adult 30. or/23‐26 31. 30 not 29 32. or/27‐29 33. 31 and 32 Identifying therapy: 34. MTX.tw 35. Methotrexate.tw 36. 34 or 35 37. 22 and 33 and 36 There were no language restrictions.
Three publications were identified: Woo 2000, Giannini 1992 and Pham 1999. Pham 1999 was excluded because it was not a clinical trial.
Data collection and analysis
Data extracted from the identified studies included study characteristics, methodological quality, and functional outcome measures of efficacy of the MTX therapy.
The efficacy of MTX was analysed for the available study outcome measures at the end of the studies. End‐of‐trial data were extracted from the available studies and entered in RevMan 4.1. The data were pooled using standardized mean differences (SMD) for limited joint range score and number of joints with swelling. Number of joints with pain on motion were evaluated with weighted mean difference (WMD) because only data from one study was available. Physicians global assessment, parents global assessment, and number of withdrawals due to efficacy and side effects were evaluated with pooled odds ratios (OR). Negative values of SMD and WMD indicate a benefit of the active drug over placebo.
Fixed effects models were used throughout. Random effects models were used for outcomes showing statistically significant heterogeneity.
Results
Description of studies
Two controlled clinical trials were identified in the literature (Giannini 1992, Woo 2000). No trials were excluded from this review.
Giannini 1992: Randomized, controlled, double‐blind, multicenter study in centres in the United States and the Soviet Union. This study was designed to investigate the effectiveness and safety of orally administered MTX. The duration of the study was six months and included those patients that had three or more joints with active arthritis that were not controlled by non steroidal anti‐inflammatory drugs (NSAIDs) or second‐line agents. There were three groups, (1) 10 mg MTX per m2 body surface area; (2) 5 mg MTX per m2 body surface area; (3) placebo. In total, 127 patients were included in the study. Only the data of groups 1 and 3 were used in this review, because 5 mg was considered too low to be effective.
Woo 2000: Randomized, controlled, double‐blind, crossover, multicentre study in centres in the United Kingdom and France. The study was designed to investigate the effectiveness and safety of orally administered MTX in extended oligoarticular and systemic arthritis. The duration of the study was 12 months, the treatment schedule consisted of an initial 4‐month active/placebo treatment period followed by a 2‐month washout period, and then a second 4‐month placebo/active treatment period followed by another 2 month washout. There were two groups: (1) 15 mg MTX per m2 body surface area (which could be increased to 20 mg MTX per m2 body surface area); (2) placebo. In total 88 patients were included in the study.
Both trials included 'lab parameters', as well as a few patient centered outcomes. Woo et al (Woo 2000) reported that there were no validated functional assessment tools at the time they started the trial. However, the Juvenile Arthritis Functional Assessment Scale (JAFAS) (Lovell 1989) was already available in 1989. Neither trial included functional outcomes such as walking time, as is common in adult RA trials (Suarez‐Almazor 2000).
Risk of bias in included studies
The methodological quality of the two trials was assessed using a validated checklist (Jadad 1996). This instrument assesses the quality of the randomisation (2 points), and the double blinding procedure (2 points). It also checks for a description of withdrawals and dropouts (1 point). The range of scores is 0 (worst) to 5 (best). Both included studies scored the maximum 5 points on the Jadad checklist.
Effects of interventions
Two controlled trials were included in the analysis (Giannini 1992, Woo 2000). In total, 165 patients were included in this review. In the Giannini study 38 patients received MTX and 39 received placebo. The Woo study was a cross‐over trial, so 88 patients received MTX and functioned as a control. The MTX treatment period of the trials ranged from 4 (Woo 2000) to 6 months (Giannini 1992). The dosage of the MTX in these trials ranged from 10 mg MTX per m2 body surface area (Giannini 1992), to 20 mg MTX per m2 body surface area (Woo 2000).
Not all the functional outcome measures of interest (see methods section) were reported in the two trials. Functional assessment and quality of life were not reported as a study outcome in either trial. We used the final results of both trials for the analysis. For one study (Giannini 1992), we only used one arm for the analysis. In the analysis 4 of 5 outcome measures (limited joint range score, number of joints with swelling, physicians global assessment, number of joints with pain on motion) showed a significant effect (p<0.05), favouring MTX over placebo. Parents assessment (only reported in Woo 2000), did not show a significant effect (p=0.12). The standardized weighted mean differences for the various outcome measures were as follows: 1) Improvement in joint range score ‐1.51 (95% CI, ‐2.97, ‐0.06)and 2) number of joints with swelling ‐1.71 (95% CI,‐2.0, ‐1.41). Weighted mean difference for number of joints with pain on motion was ‐3.90 (95 % CI, ‐4.66, ‐3.14). Odds ratios for the physicians' global assessment was 2.66 (95% CI, 1.54, 4.58), and for parents' assessment of disease activity 1.69 (95% CI, 0.87, 3.27), indicating a small to moderate effect, favouring MTX.
The OR for overall withdrawals from MTX therapy was 1.68 (95% CI, 0.63, 4.44) compared to placebo, suggesting there is no difference between MTX and placebo in terms of safety and side effects.
Clinical importance: In absolute values the MTX treatment improved the limited joint range score by 6.3 'points'. The number of joints with swelling decreased by 3.2 joints, and there were on average 2.7 joints with less pain on motion. As both trials did not report how many joints they assessed in their trial, and only one trial (Woo 2000) reported baseline values, it was not possible to express this change as a percentage. For two outcomes it was possible to relate the changes to baseline scores, as Woo (Woo 2000) reported baseline scores. The limited joint range score improved 2.7 %, and the number of joints with swelling improved almost 18 %. In the limited joint range score, both studies showed statistically significant heterogeneity. Therefore a random effects model was used for studying the effects of this outcome measure.
For overall assessment of disease activity, the relative percentage difference was 13% for parent's assessment and 23% for physician assessment, both in favour of MTX.
Discussion
Methotrexate has already been used for half a century in the treatment of adult RA patients (Suarez‐Almazor 2000). Currently, MTX is also considered effective for JIA, and therefore commonly used in the treatment of JIA. The majority of the evidence for this belief however, originates from non‐controlled studies.
The purpose of this systematic review was to evaluate the effects of MTX on patient centered outcome measures for JIA patients. Only 2 studies met the inclusion criteria for this review. The MTX treatment period of the trials ranged from 4 (Woo 2000) to 6 months (Giannini 1992), the dosage of the MTX in these trials ranged from 10 mg MTX per m2 body surface area (Giannini 1992), to 20 mg MTX per m2 body surface area (Woo 2000).
Not all the functional outcome measures of interest (see methods section) were reported in the two trials. Functional assessment and quality of life were not reported as a study outcome in either trial. One of the trials (Woo 2000) used a cross‐over design. We used the final results of both trials for the analysis. For one study, we only used one arm for the analysis.
The heterogeneity between the two trials concerning the effects of MTX on limited joint range score could be due to non random selection of missing observations. Woo (Woo 2000) reports only data of 53 patients, however they included 88 children at entry of the trial. Substantial differences were found between MTX and placebo for swelling and number of joints with pain, all favouring MTX treatment.
All differences between placebo and MTX were statistically significantly, except for parents global assessment in the Woo trial (Woo 2000). However the clinical importance of these effects are not defined. In Juvenile Arthritis clinically important improvement is defined as 30 percent improvement in three out of 6 variables in the core set (Giannini 1992). For adult RA a clinically significant effect is defined when the effect is at least greater than 20 percent in 4 out of 6 ACR core set measures: patient and physician global assessment, pain, disability, and an acute‐phase reactant (Felson 1995). We defined a 20 percent improvement in one of the outcome measures as clinical important. In the patient centered disability measures used in the current review, only clinically significant effects of MTX were found for physician assessment in favour of MTX. A possible explanation for this effect might be the difference in focus of the physician from the scope of the parents. Physicians assessment might be more dependent on inflammation parameters, whereas the parents assessment is more dependent from the patients functional performance.
Although functional outcome measures were available in the literature, the emphasis of both trials was still on outcome measures related to the inflammatory process (joint pain, joint swelling, range of motion). Due to insufficient data, we were unable to evaluate the effects of MTX on functional outcomes.
Authors' conclusions
Implications for practice.
Current evidence of efficacy of MTX in JIA is scarce. Most of the evidence are from uncontrolled clinical trials suggesting MTX is an effective agent for treating active JIA. Data from controlled clinical trials suggests that MTX does have statistically significant effects on patient centered disability measures in JIA patients with active arthritis, however the clinical relevance of these effects are questionable.
Implications for research.
We recommend performing randomised trials using sufficient numbers of patients, including functional assessment and quality of life measures, for determining the efficacy of MTX on patient centered disability measures in JIA. A clear description of baseline characteristics, drop‐outs, blinding procedures, co‐interventions, outcome measures and clinical importance of the effects should be reported. Randomised trials of high methodological quality results in reliable evidence for the effectiveness of MTX for JIA patients.
What's new
| Date | Event | Description |
|---|---|---|
| 23 June 2008 | Amended | Converted to new review format. C051‐R |
History
Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 29 August 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors want to thank Vivian Welch for her helpful comments on the protocol and manuscript.
Data and analyses
Comparison 1. MTX vs. Placebo ‐ Efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in limited joint range score | 2 | 181 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐2.97, ‐0.06] |
1.1. Analysis.
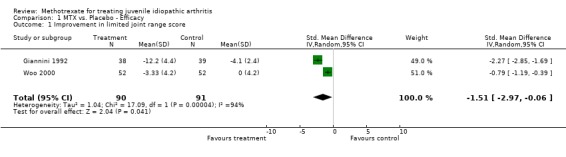
Comparison 1 MTX vs. Placebo ‐ Efficacy, Outcome 1 Improvement in limited joint range score.
Comparison 2. MTX vs Placebo ‐ Efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in Number of Joints with Swelling (synovitis) | 2 | 243 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.71 [‐2.00, ‐1.41] |
2.1. Analysis.
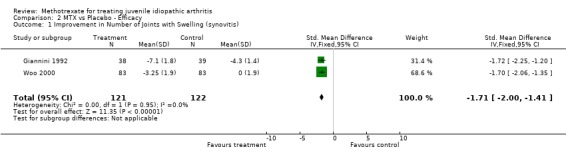
Comparison 2 MTX vs Placebo ‐ Efficacy, Outcome 1 Improvement in Number of Joints with Swelling (synovitis).
Comparison 3. MTX vs Placebo ‐ Efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physicians' Global Assessment of Patients' Response toTherapy (Improvement) | 2 | 228 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.54, 4.58] |
3.1. Analysis.
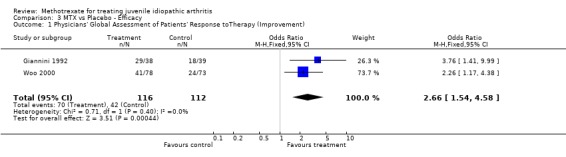
Comparison 3 MTX vs Placebo ‐ Efficacy, Outcome 1 Physicians' Global Assessment of Patients' Response toTherapy (Improvement).
Comparison 4. MTX vs Placebo ‐ Efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parents assessment of disease activitiy | 2 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.87, 3.27] |
4.1. Analysis.
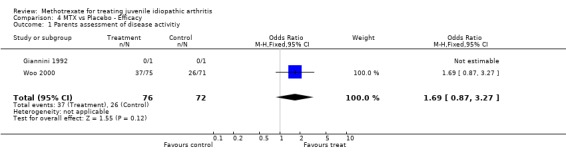
Comparison 4 MTX vs Placebo ‐ Efficacy, Outcome 1 Parents assessment of disease activitiy.
Comparison 5. MTX vs Placebo ‐ Efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in Number of Joints with pain on motion | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐4.66, ‐3.14] |
5.1. Analysis.
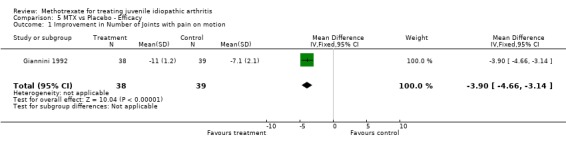
Comparison 5 MTX vs Placebo ‐ Efficacy, Outcome 1 Improvement in Number of Joints with pain on motion.
Comparison 6. MTX: safety and toxicity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 withdrawals | 2 | 263 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.63, 4.44] |
6.1. Analysis.
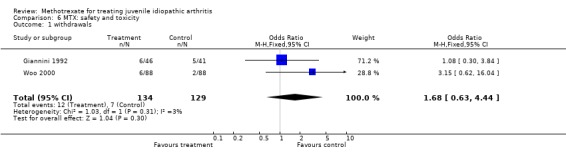
Comparison 6 MTX: safety and toxicity, Outcome 1 withdrawals.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Giannini 1992.
| Methods | Randomized allocation Double blind allocation and assessment Duration 6 months Sample size at entry: MTX 10 mg: 46 Placebo: 41 | |
| Participants | Patients with resistant JIA Mean age ‐ 10.4 Females ‐ 77% Concomitant use of steroids ‐ 33% | |
| Interventions | Oral MTX 10 mg/m2/wk Study comparing 2 dosages (5 and 10 mg/m2/wk) ‐ only higher dose included | |
| Outcomes | Joint range of motion Swollen joints Joints with pain on motion Physicians global | |
| Notes | Quality score ‐ 5 Medication provided by Lederle Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Woo 2000.
| Methods | Randomized allocation Double blind allocation and assessment Cross‐over design Duration 4 months Sample size at entry: 88 | |
| Participants | Patients with resistant JIA Mean age ‐ 8.3 Females ‐ 70% Concomitant use of steroids ‐ 51% | |
| Interventions | Oral MTX 15 mg/m2/wk | |
| Outcomes | Joint range of motion Swollen joints Physicians global Parents global | |
| Notes | Quality score ‐ 5 Medication provided by Lederle Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Contributions of authors
TT: writing and designing protocol for review, performed literature search, data extraction, statistical analysis, writing manuscript
JN: writing and designing protocol for review, data extraction, writing and reviewing manuscript
PH: writing and designing protocol for review, writing and reviewing manuscript
Sources of support
Internal sources
Wilhelmina Children's Hospital Utrecht, Netherlands.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Giannini 1992 {published data only}
- Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.‐U.S.S.R. double‐blind, placebo‐controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children's Study Group. N Engl J Med 1992;326(16):1043‐9. [DOI] [PubMed] [Google Scholar]
Woo 2000 {published data only}
- Woo P, Southwood TR, Prieur AM, Dore CJ, Grainger J, David J, et al. Randomized, placebo‐controlled, crossover trial of low‐dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis and Rheumatism 2000;43(8):1849‐57. [DOI] [PubMed] [Google Scholar]
Additional references
Dickersin 1994
- Dickersin, K, Scherer, R, Lefebvre, C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R Jr, Paulus H, Strand V, et al. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38(6):727‐735. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giannini 1997
- Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40(7):1202‐9. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Lovell 1989
- Lovell DJ, Howe S, Shear E, Hartner S, McGirr G, Schulte M, et al. Development of a disability measurement tool for juvenile rheumatoid arthritis. The Juvenile Arthritis Functional Assessment Scale. Arthritis Rheum 1989;32(11):1390‐5. [DOI] [PubMed] [Google Scholar]
OMERACT 1997
- OMERACT III. Outcome Measures in Arthritis Clinical Trials. Cairns, Australia, April 16‐19, 1996. Proceedings. Journal Of Rheumatology 1997;24(4):763‐802. [PubMed] [Google Scholar]
Onel 2000
- Onel, KB. Advanced in the medical treatment of juvenile rheumatiod arthritis. Curr Opin Ped 2000;12(72):75. [DOI] [PubMed] [Google Scholar]
Petty 1998
- Petty RE, Southwood TR, Baum J, Bhettat E, Glass DN, Manners P, Maldonado‐Cocco J, Suarez‐Almazor M, Orozco‐Alcala J, Prieur AM. Revision of the proposed classification criteria for Juvenile Idiopathic Arthritis: Durban 1997. J Rheumatol 1998;25(10):1991‐1994. [PubMed] [Google Scholar]
Pham 1999
- Pham B, Cranney A, Boers M, Verhoeven AC, Wells G, Tugwell P. Validity of area‐under‐the‐curve analysis to summarize effect in rheumatoid arthritis clinical trials. J. Rheumatol. 1999;26(3):712‐716. [PubMed] [Google Scholar]
Suarez‐Almazor 2000
- Suarez‐Almazor ME, Belseck E, Shea B, Wells G, Tugwell P. Methotrexate for rheumatoid arthritis (Cochrane Review). Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI] [PubMed] [Google Scholar]


