Abstract
The brain ventricles are interconnected, elaborate cavities that traverse the brain. They are filled with cerebrospinal fluid (CSF) that is, to a large part, produced by the choroid plexus, a secretory epithelium that reaches into the ventricles. CSF is rich in cytokines, growth factors and extracellular vesicles that glide along the walls of ventricles, powered by bundles of motile cilia that coat the ventricular wall. We review the cellular and biochemical properties of the ventral part of the third ventricle that is surrounded by the hypothalamus. In particular, we consider the recently discovered intricate network of cilia-driven flows that characterize this ventricle and discuss the potential physiological significance of this flow for the directional transport of CSF signals to cellular targets located either within the third ventricle or in the adjacent hypothalamic brain parenchyma. Cilia-driven streams of signalling molecules offer an exciting perspective on how fluid-borne signals are dynamically transmitted in the brain.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: cerebrospinal fluid, extracellular vesicles, fluid dynamics, translational polarity, hypothalamus, tanycyte
1. Introduction
Greek physicians already knew about the liquor-filled delicate cavities (the ‘ventricles’) inside the human brain and considered them as the seat of mental capacity, including cognition, memory, awareness and imagination. The physician philosophers thought that the ‘Common’ and the ‘Imaginative’ senses reside in the two large lateral ventricles located mirror-symmetrically in the front of the brain. The bipartite middle ventricle would encompass the ‘Estimative’ and ‘Phantastic’ senses. The last ventricle in the back of the brain would be devoted to the ‘Memorative’ sense [1]. Modern neurobiology places mental functions right into the dense tissue of the brain composed of neurons, nerve fibres and glia cells.
Despite their ‘downgrading’, brain ventricles continue to attract attention, perhaps more now than in the past when physiologists viewed them primarily as toxic waste dumps for metabolites that the brain eliminated. Contemporary neurobiologists view the cerebrospinal fluid (CSF)-filled ventricles as dynamic reservoirs of signalling substances and nutrients that can reach neurons, glia cells and stem cells (figure 1). This way of looking at ventricles leads to a host of questions. Which signalling molecules are present in the CSF? How do they interact with the various ependymal and subependymal cell types? How are signals taken up by cells? Which physiological processes does uptake trigger? Are CSF components delivered uniformly to all nearby brain tissues or is substance delivery targeted to a specific location? With regard to the last question, Faubel et al. [2] have identified a cilia-propelled network of CSF streams that run along the walls of the ventricle. These streams may transport factors in CSF to particular ependymal or subependymal tissue regions. It was known that motile cilia propel CSF along the ventricular surface [3,4] but not that flows form a system of interwoven streams.
Figure 1.
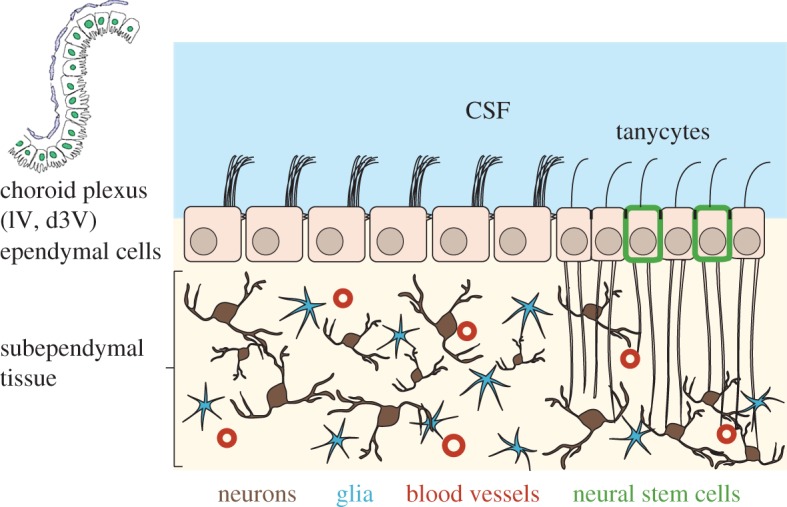
Scheme of the anatomy of the ventral part of the third ventricle (v3V). The lateral ventricles (lV), the dorsal part of the third ventricle (d3V) and the fourth ventricle (4V) contain a choroid plexus (CP) that secretes CSF. Propelled by beating cilia bundles located at the apical side of ependymal cells, CSF partitions above the ependymal cell layer in a complex manner (figure 3a). Subependymal neurons may be scattered or form clusters (nuclei) that carry out specific functions such as control of circadian timing or control of energy metabolism. In the v3v, the ependymal layer contains tanycytes, which are specialized glia cells that are bi-ciliated and send long processes that contact neurons, glia and blood vessels in the subependymal brain tissue. Some of the tanycytes have stem cell properties. CSF and interstitial fluid can pass between ependymal cells, while tanycytes have occluding junctions that form a seal preventing passage of solutes and water.
The aim of this brief review is to provide background knowledge for selected components of the ventricular system (figure 1), and information that should be helpful for designing experiments that address some of the above raised questions.
2. Brain ventricles
The central nervous system of vertebrate animals develops from the neural tube. The wall of the tube consists of neuronal progenitor cells and encloses the CSF-filled ventricle. Through a combination of proliferation, migration and differentiation of the progenitor cells, fore-, mid- and hindbrain form at the front end of the neural tube, while the back end of the neural tube gives rise to the spinal cord. Brain ventricles co-develop with the nervous system so that around the time of birth, four distinct brain ventricles have formed. In the postnatal brain, ventricles are enclosed by the ependyma that consists mostly of E1 cells (ependymal cells, ependymocytes, figure 1; [5,6]). E1 cells derive from embryonic radial glia cells [7,8] and carry a characteristic apical bundle of motile cilia that coordinately beat and in this way propel CSF along the ventricular wall (figure 1).
The shape of the ventricular cavity in brains from different species varies enormously to match the diversity of brain anatomy [9]. Most vertebrates have four ventricles except for teleost fishes, which have three. Ventricles are serially docked and interconnect through interventricular foramina. In rodents (figure 2), the two crescent-shaped lateral ventricles (lV) are each embedded in one of the forebrain hemispheres and connect, via a pair of interventricular passages, to the third ventricle (3V). The narrow 3V is squeezed in between the left and right halves of the diencephalon. In rodents, 3V is separated into a ventral (v3v) and a dorsal (d3V) part. The ventral part resides between the right and the left halves of the hypothalamus. Posteriorly, 3V connects through the aqueduct to the fourth ventricle (4V) that is surrounded by the mid- and hindbrain tissue. 4V connects to both the central canal of the spinal cord and to the subarachnoid space. The latter surrounds the brain and spinal cord. CSF exits the subarachnoid space through arachnoid granulations and in this way flows into the vascular system [10]. An additional exit path for CSF is the perineural flux across the cribriform plate and from there on to the nasal mucosa [11].
Figure 2.
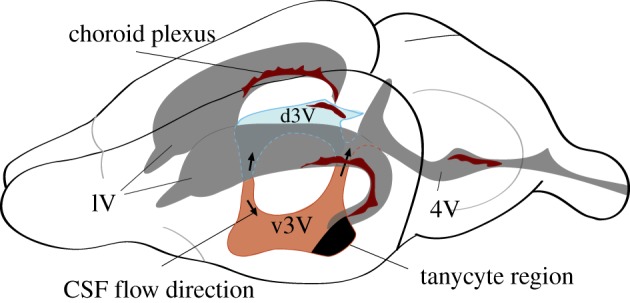
The four CSF-filled ventricles in the adult mouse brain. Highlighted in colour are the dorsal (d3V, blue) and ventral (v3v, light brown) parts of the third ventricle. The two lateral ventricles feed via a canal into the mid-plane located 3V. At the site of junction, CSF flows either dorsally (up arrow) into the d3V or ventrally (down arrow) into the v3v. At their back end, d3V and v3v connect via the aqueduct into 4V. Most of the lining of the ventricles consists of ependymal cells (E1 cells). The dark-shaded area in v3v consists primarily of α- and β-tanycytes. Dark brown features represent the secretory epithelium of the choroid plexi that releases CSF and secretes a great variety of small and macromolecular solutes. Dorsal is on the top and anterior to the left.
3. Choroid plexus, a source of signalling factors and extracellular vesicles
The intensely vascularized structure of the plexus choroïdes or choroid plexus (CP) inside the lateral ventricles of the human brain is shown in the detailed anatomical drawings by Vesalius [12]. This particular CP had already been identified in antiquity as a worm-like structure. There is a CP in each of the four ventricles that continuously produces, in humans, about 500 ml of CSF per day [13]. The continuous production of CSF contributes to a steady ventricular CSF flow. Heartbeat, breathing and body motion also contribute to CSF flow [10,14–17]. In rodents, the v3v has narrow entry and exit ducts and the wall-to-wall distance measures only 100–200 µm. Therefore, the major driving force for the flow is likely to be the beating of cilia bundles.
In the brain of lampreys, a primitive vertebrate animal, the CP distributes throughout the ventricles [18]. In mammals [19], CP is present in each of the four ventricles at specific locations (figure 2). Histologically, the CP consists of a secretory epithelium that encloses a web of fenestrated blood vessels. CP produces and releases CSF and electrolytes into the ventricles [20]. In addition, CP also provides various micronutrients, hormones, neurotransmitters, neurotrophins, peptide hormones, such as melanin-concentrating hormone, and growth factors that occur in CSF and in some cases are secreted in an age-dependent manner [21–25]. These agents are either directly synthesized by CP epithelial cells or enter the CP as components of the blood that pass through the fenestrated capillaries. Tight junctions connect the CP epithelial cells that form the blood–CSF barrier. Thus, a passage of substances into the ventricles requires selective trans-epithelial transporters. Such transporters are abundantly expressed in CP epithelial cells [26]. CSF constituents also enter into the ventricular space from the interstitial fluid-containing parenchyma. Lastly, there is release of neuropeptides and neurotransmitters from CSF-contacting neurons or neurons whose axonal terminals contact the CSF [27]. Some of the CSF hormones modulate cilia beating frequency and thus influence CSF flow [22].
The CSF also contains extracellular vesicles (EVs) [28,29]. EVs are membrane vesicles of a diameter between 30 and 150 nm and are of endocytic origin. Most cell types secrete EVs. EVs contain cellular proteins, small molecules and nucleic acids such as miRNA and non-coding RNAs [30–32]. EVs are likely to play a role in intercellular communication, in pathogenesis and are a reservoir of biomarkers and may also be in a pathway by which cells pass on unwanted materials. EVs play a role in different stem cell niches such as the mesenchymal stem cell niche, cancer stem cell niche and the pre-metastatic niche [33–35]. A community compendium for EVs is found in databases such as Vesiclepedia [36,37] and ExoCarta [38–40].
Adult neurogenic stem cell niches in which neurogenesis persists after birth are found in the subventricular zone of the lateral ventricle, in the subgranular zone of the dentate gyrus and along and beneath the v3v wall [8,24,41,42]. It has been hypothesized [43] that EVs that contain signalling factors required for neuronal development are carried along in the embryonic and adult CSF, thereby reaching stem cell niches [24]. EV-borne components are insulin-like growth factor [44], folate receptor-α and pro-inflammatory miRNA such as miRNA-146a and miRNA-155. These CP-derived EVs are not only transported by CSF but can also cross the ependymal cell layer and reach brain parenchyma cells [45,46]. One significant advantage of EV-borne signalling factors is that they can be specifically labelled with fluorescent antibodies directed against EV transmembrane proteins and be traced by live fluorescence microscopy in space and time. Despite much research activity, EV research results are at times ambiguous owing to differences in purification methods, EV heterogeneity, heterogeneous sources and influence of growth conditions on the cargo of EVs [35,47–49]. Accordingly, it is of great importance to use reproducible and thorough methods for isolation, purification and characterization of EVs.
In summary, the CP is a rich source of signalling compounds, some of which are dissolved single molecules, while others are packaged into EVs or other types of lipid particles [49]. Because EVs can be labelled with fluorescent antibodies against the extracellular domains of EV transmembrane proteins, and because EVs can be visualized by fluorescence microscopy and nanoscopy, they are a CSF component amenable to the study of cilia-mediated transport in the ventricular system.
4. Cellular constituents of ependyma
Knowing the biology and biochemistry of the ependymal cells is crucial since these cells form the boundary between CSF and brain parenchyma [5,6]. Through their apical cilia bundles, they are capable of moving CSF, and they represent the first point of contact between CSF constituents and the brain. The ependyma of the v3v is predominantly made of E1 cells (ependymal cells) that are apically covered with microvilli, but also includes E2 and E3 cells [50]. E2 cells carry apically two (potentially motile) 9 + 2 cilia and are likely to be identical with α-tanycytes [50–53]. E3 cells are uni- or bi-ciliated and are better known as β-tanycytes. Cilia of E3 cells are thought to be sensory cilia. E3 cells are most abundant in an approximately rectangular patch of ependyma in the posterior most part of the v3v (black area in figure 2). E2 cells are also found in this patch and additionally are scattered throughout the posterior-most third of the v3v and in a stripe extending along the ventral aspect of the outflow tract [50]. Like E1 cells, E2 and E3 cells have their apical side directly exposed to CSF. Basally, tanycytes extend one or two long processes that reach deep into the brain parenchyma (figure 1; [51,53]) where they contact several hypothalamic nuclei as well as blood vessels.
The current literature discusses four types of tanycytes [42,52], but the tanycyte population may be even more diverse. α1-Tanycytes populate the upper half of the tanycyte patch and their processes project into the dorsomedial and ventromedial hypothalamic nuclei. α2-Tanycytes are located ventral to their α1-siblings and send processes to ventromedial and arcuate hypothalamic nuclei. When exposed to growth factors by intraventricular infusion, α1- and α2-tanycytes will proliferate and, depending on the nature of the infused factor, give rise to neurons, α1- and α2-tanycytes, astrocytes and oligodendrocytes [54,55]. Thus, factors delivered to the v3v through CSF are capable of triggering cell proliferation. Ventral to the α2-tanycyte region are β1-tanycytes that send processes to the arcuate nucleus and the median eminence. The CSF-contacting face of the median eminence is made of the apical face of type β2-tanycytes that carry sensory cilia. In juvenile mice, β-tanycytes proliferate and yield astrocytes and neurons targeted to the median eminence. β2-Tanycytes also proliferate to sustain a size expansion of the median eminence typically seen in juvenile animals [42,56]. β2-Tanycytes extend their basal protrusions towards the plexus of fenestrated blood capillaries that are part of the hypophyseal portal microcirculation. This contact of tanycyte protrusions with the capillaries could allow the transfer of blood-borne substances into the tanycyte and then into the CSF and vice versa. Tanycytes of the E3 type also populate the organum vasculosum of the laminae terminalis (OVLT) located at the anterior wall of the v3v. These tanycytes are in direct contact with v3v through the apical area of the tanycytes that sprouts sensory cilia [50,57].
Taken together, the wall of the third ventricle is histologically complex and is made of several cell types. The dominant cells are the ependymocytes that sustain a lifelong complex CSF flow. Tanycytes provide a conduit between ventricular CSF and the brain parenchyma. Tanycytes also form bona fide stem cell niches in the adult brain, and these niches respond to CSF-infused growth factors by proliferation and differentiation.
5. v3v exhibits complex cilia-driven flows
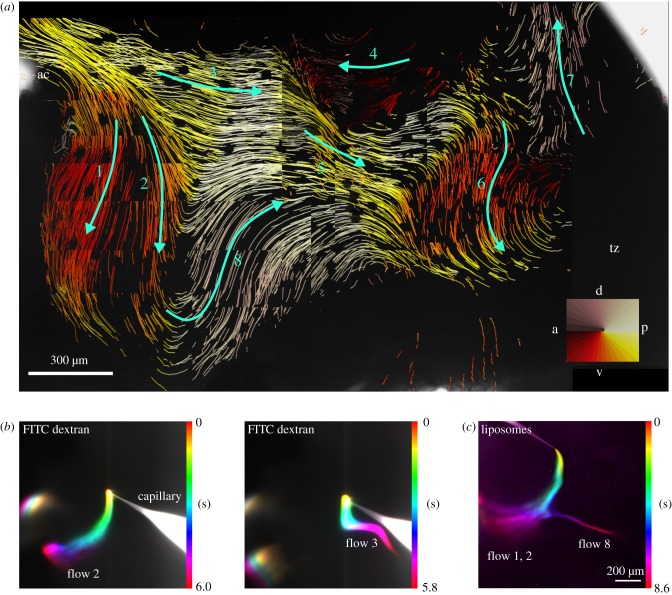
Does CSF flow through the ventricles like water would flow in a system of interconnected rivers and lakes? This is definitely not the case in the v3v. Explants of the ventricular walls show complex flow patterns that consist of eight distinct streams that are interconnected like roads in a map (figure 3a). To generate this flow map, 1 µm diameter fluorescent beads are added in bulk to the medium in which the v3v explants are kept. The movement of beads evoked by beating cilia bundles is captured and a particle tracking software is used to compute individual bead tracks [2]. These tracks are plotted on top of the v3v explant, resulting in a map of continuous, interwoven flows that represent fluid movement across the entire v3v surface. Alternatively, fluid flow can readily be visualized by locally applying fluorescein isothiocyanate (FITC)-dextran (figure 3b) or fluorescent liposomes (figure 3c) to the ependyma of the explant. Rates of transport of beads and liposomes across the ependyma are in the range of several hundred micrometres per second. Since in the mouse, the v3v is about 2 mm long, moving of CSF from the inflow to the outflow occurs within a few seconds. Either of these methods reveals a complex flow pattern in the v3v that consists of straight flows, bent flows and whirls capable of directional transport and of subdividing the ventricular volume [2]. These flows are entirely regulated by properly oriented cilia bundles. Cilia bundle orientation is genetically determined by planar cell polarity proteins [4,58–60].
Figure 3.
Flows in the v3v. (a) Flow map generated by particle tracking shows eight streams represented by green arrows. See colour compass for the flow direction colour code. Inflow and outflow ducts are on the top left and top right, respectively. The anterior commissure (ac) is a nerve fibre located below the inflow duct. Tanycytes (tz) do not carry cilia bundles and thus do not generate flow. Dorsal (d) is on the top and anterior (a) to the left. (b) Propagation of FITC-dextran and (c) fluorescently labelled liposomes in cilia generated near-wall flow. A capillary was used to inject small amounts of either FITC–dextran (b) or fluorescently labelled liposomes (c) into the inflow region of the v3v. The propagation was recorded over time and a temporal colour code applied using Fiji. The temporal colour code shows the change of the fluorescence intensity over time. The applied droplets of FITC-dextran and liposomes followed the cilia-generated streamlines. Raw data for (b) are from [2]. Dorsal is on the top and anterior to the left.
6. Fluid dynamics
CSF flow in the ventricular system of mammalian brains is driven by CSF secretion into the ventricles and by motile cilia beating, and plays an important role in the delivery of CSF components and washout of waste [2]. CSF is not stagnant but pulsating in vivo owing to heart beating [15,17,61], respiration [14] and even head movement [62]. Different approaches have been used to investigate the CSF flow experimentally or numerically (see [63] and the references therein). Non-invasive MRI offers a way to evaluate the flow in vivo [14,61,62] but cannot provide detailed flow information owing to its limited spatial resolution, especially for the mouse brain. Particle tracking with microscopy is capable of recording cilia-induced flow networks along the wall in opened ventricles [2] but does not give the volumetric flow.
Alternatively, detailed CSF flow in the 3D ventricles can be investigated with computational fluid dynamics. Previous numerical studies focused on the human brain and investigated the CSF flow in idealized geometry or subject-specific geometry of ventricles reconstructed from medical imaging [64–67]. In most investigations, the effect of cilia on the flow was not studied. Recently, Siyahhan et al. [68] took into account the effect of the cilia beating pattern in the lateral ventricles of the human brain by applying a force density on the flow and studied the near-wall CSF flow dynamics. However, the cilia or beating directions were assumed to be aligned with the CSF net flow, which may not be true when there is a complex cilia pattern in the ventricles as we detect in our experimental investigation in the mouse (figure 3a) [2]. Interestingly, the Siyahhan et al. simulations [68] show that flow in the appendix of the human 3V had a very small oscillatory component, suggesting a prominent role of cilia there.
Compared with the human brain ventricles, the two parts (dorsal and ventral) of the 3V of the mouse brain are much more separated. The ventral part is also much narrower than its dorsal counterpart. As a result, most oscillatory CSF net flow will take the dorsal route owing to its smaller resistance and, therefore, the flow in the ventral part will be dominated by cilia beating.
7. Comparing flow directions in explants with the intrinsic translational polarity of the v3v
Media-immersed v3v explants exhibit a complex flow pattern. What is the evidence that this pattern reflects the CSF flow in vivo? There are two lines of evidence that suggest that flow directions in explants and in the live animal are very similar. First, it was observed that in the lateral ventricle, cilia effective stroke and flow directions, as detected by particle tracking (figure 3a), are correlated [4]. In the v3v, flows 3 and 4 are directly opposing each other (figure 3a). The corresponding movies of cilia beating in the ependyma underneath flows 3 and 4 show that cilia beat in the opposite direction as well (Movie S4 in [2]). One could still argue that in explants cilia bundles have reoriented relative to their position in the brain. This implies that translational polarity would change upon dissection and/or transfer of the v3v tissue into the culture medium. Immunohistochemical staining of ependymal cells and of cilia bundles in native v3v ependyma allows one to determine translational polarity. Figure 4 shows translational polarity for E1 cells driving flow 3. The arrows represent translational polarity cell-by-cell point in their majority towards the posterior end of the v3v. Figure 3a shows that flow 3 is also pointed towards the posterior end of the v3v. The example discussed here suggests that there is a good match between flow direction in the explant and translational polarity in the corresponding region in the intact brain.
Figure 4.
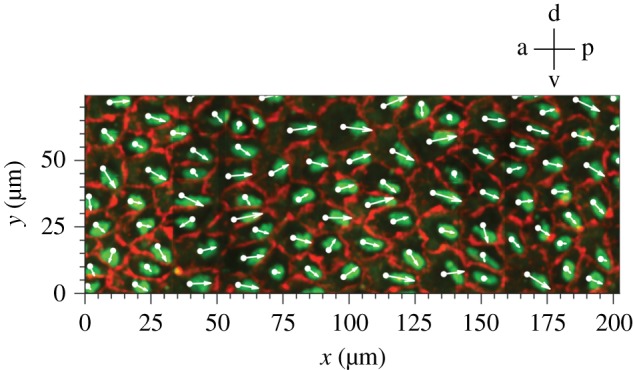
Translational polarity of ependymal cells in flow module 3. White arrows show the direction of translational polarization, which is determined by drawing a vector from the cell centroid to the centroid of the cilia bundle. Cell boundaries were detected with an anti-β-catenin antibody and basal bodies were stained with anti-γ-tubulin antibody.
8. Outlook
Why is there a ventricle in the hypothalamus and what would be the purpose of a complex directional flow pattern in the v3v? The answer could be that CSF delivers factors to specific hypothalamic nuclei, especially those that are located at or near the basal side of ependyma. Ependymal flow 8 passes above the suprachiasmatic nucleus, which regulates the circadian clock. Flow 6 passes by the arcuate nucleus, which controls various metabolic functions. Tanycytes provide a direct exchange between CSF and neuronal or vascular targets. Flow 6 streams to the tanycyte region, while flow 7 leads away from the tanycytes. In the explant, there is no flow above the tanycytes, but in the closed ventricle in the brain, flows 6 and 7 would provide coordinate transport of CSF solutes to and then away from the tanycyte region.
This view of the ventricular system as a site of targeted flow provides a number of challenges. What are the substances that are being delivered? There are many factors in CSF, and they not only need to be identified but assays for a function in the hypothalamus need to be developed. These functions could relate to metabolism, circadian timing or the control of stem cell growth and differentiation. How can CSF constituents transit from CSF to their subependymal targets? For solutes such as growth factors, there may be specific receptors on E1, E2 or E3 cells. The uptake of EVs may involve endocytosis [69]. Where do signals come from? One likely source is the secretory epithelium of the CP, but it is unclear how signals coming from the lateral ventricles or the d3V are funnelled into the v3v. Could the divergent flows at the inflow act as a sorting device? Do the observed flows change rhythmically and do they change during the lifespan of an animal? That there is directional movement of solutes, signalling molecules and EVs in the fluid phases of the central nervous system (extracellular fluid and CSF) has long been known and has led to the concept of ‘volume transmission’ [70,71]. The case can be made that cilia-driven directional flows are a manifestation of volume transmission in that the ciliary conveyor belts provide the means of transportation.
Protein targeting mechanisms in cells may serve as a useful paradigm for thinking about the ventricular transport. A key insight for understanding protein targeting within cells was the realization that proper sorting and delivery relies on information encoded in the protein itself. In analogy, it is possible that EVs, potential carriers of CSF signals in the ventricular system, expose on their surface protein sequences that are used as a postal code for sorting into specific streams and for proper delivery to the apical face of specific E1, E2 and E3 cells. Addressing all these points experimentally is a daunting task. The reward of such an effort could be the discovery of a cilia motility-based signalling system in an important and ancient part of the brain.
Data accessibility
This article has no additional data.
Authors' contributions
All co-authors have contributed to the text and figures of this review.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Max Planck Society.
References
- 1.Cunningham A, Kiusukawa S. 2010. Natural philosophy epitomised: a translation of books 8–11 of Gregor Reisch's Philosophical pearl (1503). London, UK: Ashgate. [Google Scholar]
- 2.Faubel R, Westendorf C, Bodenschatz E, Eichele G. 2016. Cilia-based flow network in the brain ventricles. Science 353, 176–178. ( 10.1126/science.aae0450) [DOI] [PubMed] [Google Scholar]
- 3.Sawamoto K, et al. 2006. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629–632. ( 10.1126/science.1119133) [DOI] [PubMed] [Google Scholar]
- 4.Mirzadeh Z, Han YG, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. 2010. Cilia organize ependymal planar polarity. J. Neurosci. 30, 2600–2610. ( 10.1523/JNEUROSCI.3744-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Bigio MR. 1995. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia 14, 1–13. ( 10.1002/glia.440140102) [DOI] [PubMed] [Google Scholar]
- 6.Del Bigio MR. 2010. Ependymal cells: biology and pathology. Acta Neuropathol. 119, 55–73. ( 10.1007/s00401-009-0624-y) [DOI] [PubMed] [Google Scholar]
- 7.Redmond SA, Figueres-Onate M, Obernier K, Nascimento MA, Parraguez JI, Lopez-Mascaraque L, Fuentealba LC, Alvarez-Buylla A. 2019. Development of ependymal and postnatal neural stem cells and their origin from a common embryonic progenitor. Cell Rep. 27, 429–441.e3. ( 10.1016/j.celrep.2019.01.088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortiz-Alvarez G, et al. 2019. Adult neural stem cells and multiciliated ependymal cells share a common lineage regulated by the geminin family members. Neuron 102, 159–172.e7. ( 10.1016/j.neuron.2019.01.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voogd J, Nieuwenhuys R, Dongen PAMv, Donkelaar HJt. 1998. Mammals. In The central nervous system of vertebrates (eds Nieuwenhuys R, Donkelaar HJt, Nicholson C), pp. 1637–2097. Berlin, Germany: Springer; ( 10.1007/978-3-642-18262-4) [DOI] [Google Scholar]
- 10.Hladky SB, Barrand MA. 2014. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26 ( 10.1186/2045-8118-11-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagra G, Koh L, Zakharov A, Armstrong D, Johnston M. 2006. Quantification of cerebrospinal fluid transport across the cribriform plate into lymphatics in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1383–R1389. ( 10.1152/ajpregu.00235.2006) [DOI] [PubMed] [Google Scholar]
- 12.Vesalius A. 1543. De Humani corporis fabrica. Basilea, Switzerland: Johannes Oporinus. [Google Scholar]
- 13.Cutler RW, Page L, Galicich J, Watters GV. 1968. Formation and absorption of cerebrospinal fluid in man. Brain 91, 707–720. ( 10.1093/brain/91.4.707) [DOI] [PubMed] [Google Scholar]
- 14.Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. 2015. Inspiration is the major regulator of human CSF flow. J. Neurosci. 35, 2485–2491. ( 10.1523/JNEUROSCI.3246-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, Yaksi E, Jurisch-Yaksi N. 2019. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr. Biol. 29, 229–241.e6. ( 10.1016/j.cub.2018.11.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Date P, Ackermann P, Furey C, Fink IB, Jonas S, Khokha MK, Kahle KT, Deniz E. 2019. Visualizing flow in an intact CSF network using optical coherence tomography: implications for human congenital hydrocephalus. Sci. Rep. 9, 6196 ( 10.1038/s41598-019-42549-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fame RM, Chang JT, Hong A, Aponte-Santiago NA, Sive H. 2016. Directional cerebrospinal fluid movement between brain ventricles in larval zebrafish. Fluids Barriers CNS 13, 11 ( 10.1186/s12987-016-0036-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieuwenhuys R, Nicholson C. 1998. Lampreys, Petromyzontoidea. In The central nervous system of vertebrates (eds Nieuwenhuys R, Donkelaar HJt, Nicholson C), pp. 413–415. Berlin, Germany: Springer; ( 10.1007/978-3-642-18262-4) [DOI] [Google Scholar]
- 19.Lun MP, Monuki ES, Lehtinen MK. 2015. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445–457. ( 10.1038/nrn3921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damkier HH, Brown PD, Praetorius J. 2010. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 25, 239–249. ( 10.1152/physiol.00011.2010) [DOI] [PubMed] [Google Scholar]
- 21.Ashkenazi R, Holman RB, Vogt M. 1973. Release of transmitters into the perfused third cerebral ventrical of the cat. J. Physiol. 233, 195–209. ( 10.1113/jphysiol.1973.sp010305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conductier G, et al. 2013. Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat. Neurosci. 16, 845–847. ( 10.1038/nn.3401) [DOI] [PubMed] [Google Scholar]
- 23.Lun MP, et al. 2015. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci. 35, 4903–4916. ( 10.1523/JNEUROSCI.3081-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F. 2016. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19, 643–652. ( 10.1016/j.stem.2016.06.013) [DOI] [PubMed] [Google Scholar]
- 25.Janssen SF, van der Spek SJ, Ten Brink JB, Essing AH, Gorgels TG, van der Spek PJ, Jansonius NM, Bergen AA. 2013. Gene expression and functional annotation of the human and mouse choroid plexus epithelium. PLoS ONE 8, e83345 ( 10.1371/journal.pone.0083345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA. 2013. Transporters of the blood–brain and blood–CSF interfaces in development and in the adult. Mol. Aspects Med. 34, 742–752. ( 10.1016/j.mam.2012.11.006) [DOI] [PubMed] [Google Scholar]
- 27.Tong CK, et al. 2014. Axonal control of the adult neural stem cell niche. Cell Stem Cell 14, 500–511. ( 10.1016/j.stem.2014.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vella LJ, Greenwood DL, Cappai R, Scheerlinck JP, Hill AF. 2008. Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet. Immunol. Immunopathol. 124, 385–393. ( 10.1016/j.vetimm.2008.04.002) [DOI] [PubMed] [Google Scholar]
- 29.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RT, Webb DJ, Dear JW. 2012. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 10, 5 ( 10.1186/1479-5876-10-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420. [PubMed] [Google Scholar]
- 31.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. 2006. Exosomes: a common pathway for a specialized function. J. Biochem. 140, 13–21. ( 10.1093/jb/mvj128) [DOI] [PubMed] [Google Scholar]
- 32.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. ( 10.1038/ncb1596) [DOI] [PubMed] [Google Scholar]
- 33.Luga V, et al. 2012. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151, 1542–1556. ( 10.1016/j.cell.2012.11.024) [DOI] [PubMed] [Google Scholar]
- 34.Nakano S, Yamamoto S, Okada A, Nakajima T, Sato M, Takagi T, Tomooka Y. 2017. Role of extracellular vesicles in the interaction between epithelial and mesenchymal cells during oviductal ciliogenesis. Biochem. Biophys. Res. Commun. 483, 245–251. ( 10.1016/j.bbrc.2016.12.158) [DOI] [PubMed] [Google Scholar]
- 35.Shurtleff MJ, Temoche-Diaz MM, Schekman R. 2018. Extracellular vesicles and cancer: caveat lector. Annu. Rev. Cancer Biol. 2, 395–411. ( 10.1146/annurev-cancerbio-030617-050519) [DOI] [Google Scholar]
- 36.Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S. 2019. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 47, D516–D519. ( 10.1093/nar/gky1029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalra H, et al. 2012. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 10, e1001450 ( 10.1371/journal.pbio.1001450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keerthikumar S, et al. 2016. ExoCarta: a web-based compendium of exosomal cargo. J. Mol. Biol. 428, 688–692. ( 10.1016/j.jmb.2015.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. 2012. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 40, D1241–D1244. ( 10.1093/nar/gkr828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathivanan S, Simpson RJ. 2009. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 9, 4997–5000. ( 10.1002/pmic.200900351) [DOI] [PubMed] [Google Scholar]
- 41.Obernier K, Alvarez-Buylla A. 2019. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 146 ( 10.1242/dev.156059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo S, Blackshaw S. 2018. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 170, 53–66. ( 10.1016/j.pneurobio.2018.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batiz LF, Castro MA, Burgos PV, Velasquez ZD, Munoz RI, Lafourcade CA, Troncoso-Escudero P, Wyneken U. 2015. Exosomes as novel regulators of adult neurogenic niches. Front. Cell Neurosci. 9, 501 ( 10.3389/fncel.2015.00501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feliciano DM, Zhang S, Nasrallah CM, Lisgo SN, Bordey A. 2014. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS ONE 9, e88810 ( 10.1371/journal.pone.0088810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grapp M, et al. 2013. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat. Commun. 4, 2123 ( 10.1038/ncomms3123) [DOI] [PubMed] [Google Scholar]
- 46.Balusu S, et al. 2016. Identification of a novel mechanism of blood–brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol. Med. 8, 1162–1183. ( 10.15252/emmm.201606271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maas SLN, Breakefield XO, Weaver AM. 2017. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 27, 172–188. ( 10.1016/j.tcb.2016.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thery C, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 ( 10.1080/20013078.2018.1535750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser K, et al. 2019. WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis. Nat. Commun. 10, 1498 ( 10.1038/s41467-019-09298-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirzadeh Z, et al. 2017. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat. Commun. 8, 13759 ( 10.1038/ncomms13759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolborea M, Dale N. 2013. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 36, 91–100. ( 10.1016/j.tins.2012.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizzoti K, Lovell-Badge R. 2017. Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol. Cell. Endocrinol. 445, 7–13. ( 10.1016/j.mce.2016.08.020) [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Caceres C, et al. 2019. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 22, 7–14. ( 10.1038/s41593-018-0286-y) [DOI] [PubMed] [Google Scholar]
- 54.Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK. 2013. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 33, 6170–6180. ( 10.1523/JNEUROSCI.2437-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robins SC, et al. 2013. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 4, 2049 ( 10.1038/ncomms3049) [DOI] [PubMed] [Google Scholar]
- 56.Lee DA, et al. 2012. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 15, 700–702. ( 10.1038/nn.3079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prager-Khoutorsky M, Bourque CW. 2015. Anatomical organization of the rat organum vasculosum laminae terminalis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R324–R337. ( 10.1152/ajpregu.00134.2015) [DOI] [PubMed] [Google Scholar]
- 58.Spassky N, Meunier A. 2017. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423–436. ( 10.1038/nrm.2017.21) [DOI] [PubMed] [Google Scholar]
- 59.Boutin C, et al. 2014. A dual role for planar cell polarity genes in ciliated cells. Proc. Natl Acad. Sci. USA 111, E3129–E3138. ( 10.1073/pnas.1404988111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuo M, Shimada A, Koshida S, Saga Y, Takeda H. 2013. The establishment of rotational polarity in the airway and ependymal cilia: analysis with a novel cilium motility mutant mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L736–L745. ( 10.1152/ajplung.00425.2012) [DOI] [PubMed] [Google Scholar]
- 61.Enzmann DR, Pelc NJ. 1993. Cerebrospinal fluid flow measured by phase-contrast cine MR. Am. J. Neuroradiol. 14, 1301–1307, 1309–1310. [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Q, Yu SB, Zheng N, Yuan XY, Chi YY, Liu C, Wang XM, Lin XT, Sui HJ. 2016. Head movement, an important contributor to human cerebrospinal fluid circulation. Sci. Rep. 6, 31787 ( 10.1038/srep31787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linninger AA, Tangen K, Hsu C-Y, Frim D. 2016. Cerebrospinal fluid mechanics and its coupling to cerebrovascular dynamics. Annu. Rev. Fluid Mech. 48, 219–257. ( 10.1146/annurev-fluid-122414-034321) [DOI] [Google Scholar]
- 64.Linninger AA, Tsakiris C, Zhu DC, Xenos M, Roycewicz P, Danziger Z, Penn R. 2005. Pulsatile cerebrospinal fluid dynamics in the human brain. IEEE Trans. Biomed. Eng. 52, 557–565. ( 10.1109/TBME.2005.844021) [DOI] [PubMed] [Google Scholar]
- 65.Kurtcuoglu V, Poulikakos D, Ventikos Y. 2005. Computational modeling of the mechanical behavior of the cerebrospinal fluid system. J. Biomech. Eng. 127, 264–269. ( 10.1115/1.1865191) [DOI] [PubMed] [Google Scholar]
- 66.Kurtcuoglu V, Soellinger M, Summers P, Boomsma K, Poulikakos D, Boesiger P, Ventikos Y. 2007. Computational investigation of subject-specific cerebrospinal fluid flow in the third ventricle and aqueduct of Sylvius. J. Biomech. 40, 1235–1245. ( 10.1016/j.jbiomech.2006.05.031) [DOI] [PubMed] [Google Scholar]
- 67.Edi Azali H, Kahar O, Mohamed Rafiq Abdul K, Azian Abdul A. 2011. Computational investigation on CSF flow analysis in the third ventricle and aqueduct of Sylvius. IIUM Eng. J. 12 ( 10.31436/iiumej.v12i3.158) [DOI] [Google Scholar]
- 68.Siyahhan B, Knobloch V, de Zelicourt D, Asgari M, Schmid Daners M, Poulikakos D, Kurtcuoglu V. 2014. Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J. R. Soc. Interface 11, 20131189 ( 10.1098/rsif.2013.1189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cocucci E, Meldolesi J. 2015. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 25, 364–372. ( 10.1016/j.tcb.2015.01.004) [DOI] [PubMed] [Google Scholar]
- 70.Fuxe K, Agnati LF. 1991. Volume transmission in the brain: novel mechanisms for neural transmission. New York, NY: Raven Press. [Google Scholar]
- 71.Fuxe K, Borroto-Escuela DO. 2016. Volume transmission and receptor–receptor interactions in heteroreceptor complexes: understanding the role of new concepts for brain communication. Neural Regen. Res. 11, 1220–1223. ( 10.4103/1673-5374.189168) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.