Abstract
Sensory cells that detect mechanical forces usually have one or more specialized cilia. These mechanosensory cells underlie hearing, proprioception or gravity sensation. To date, it is unclear how cilia contribute to detecting mechanical forces and what is the relationship between mechanosensory ciliated cells in different animal groups and sensory systems. Here, we review examples of ciliated sensory cells with a focus on marine invertebrate animals. We discuss how various ciliated cells mediate mechanosensory responses during feeding, tactic responses or predator–prey interactions. We also highlight some of these systems as interesting and accessible models for future in-depth behavioural, functional and molecular studies. We envisage that embracing a broader diversity of organisms could lead to a more complete view of cilia-based mechanosensation.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: cilia, mechanosensation, marine invertebrates, sensory systems
1. Introduction
Mechanosensory cells or mechanoreceptors are specialized cells detecting mechanical forces such as shear, compression or tension. Animals have evolved mechanosensory cell types with a large morphological diversity, such as the hair cells in the inner ear of vertebrates, the Merkel cells in the vertebrate skin or chordotonal-organ sensory cells in insects. The sensory morphology of mechanoreceptors and their association with supporting structures contribute to their sensory tuning [1]. In addition, mechanosensory cells express transmembrane channels that convert the force into a cellular signal, a process known as mechanotransduction. Members from unrelated protein families including Transient Receptor Potential (TRP) channels, Piezo, Transmembrane Channel-like proteins (TMCs) and DEG/ENaC channels have been implicated in this process [2,3].
One widespread structural feature of mechanoreceptors is the presence of one or more cilia in their sensory apparatus. These cilia—defined here as microtubule-containing organelles supported by a basal body—are ultrastructurally diverse and can be either motile or non-motile. The cilia are often surrounded by microvilli in various numbers and with a range of morphologies. It is not yet clear whether cilia are directly involved in mediating mechanosensation or have other functions such as maintaining the structure of the microvillar sensory apparatus. The biophysical details of how cilia may sense forces are also unclear and several possible models have been proposed [4,5].
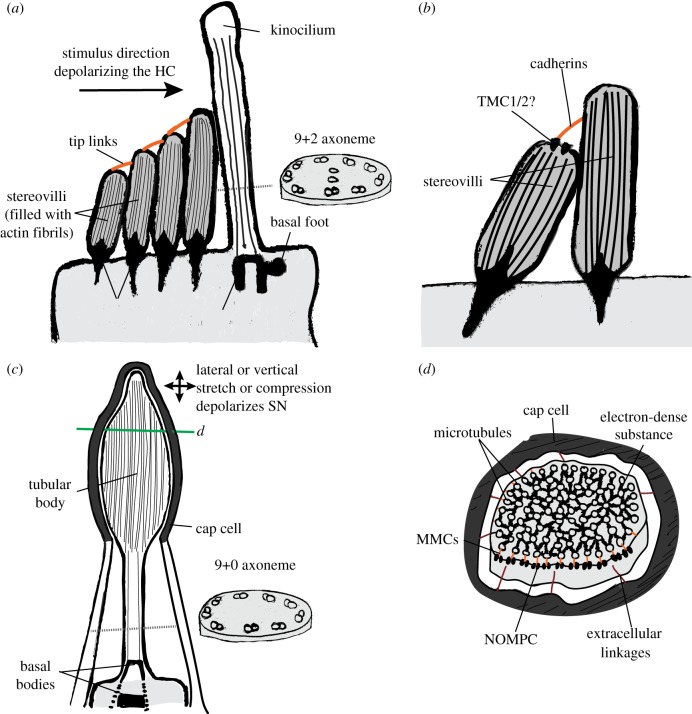
The canonical ciliated mechanosensory cell is the hair cell of vertebrates (figure 1a). Hair cells occur in the ear and in the lateral-line neuromasts [11]. Hair cells typically have a single cilium (called the kinocilium; this is absent from the hair cells of the adult organ of Corti [12]) with a 9+2 axoneme. The central microtubule pair in hair cells is oriented perpendicular to the ciliary basal foot [13]. Opposite to the basal foot, there is a group of modified microvilli called stereovilli or stereocilia (for clarity we will use the term stereovilli [14]). The stereovilli have graded lengths, with the tallest stereovilli closest to the kinocilium (figure 1a). The hair cell is depolarized when the bundle is deflected towards the kinocilium and hyperpolarized when the stereovilli are pulled in the opposite direction [15]. In a classic study, Hudspeth and Jacobs demonstrated that the stereovilli, and not the kinocilium, are the site of mechanotransduction [16]. Fibrous connections called tip links were later found connecting the distal ends of shorter stereovilli to their taller neighbours [17]. Tip links are necessary for hair cell mechanosensitivity [18], probably by gating the mechanotransduction channel sitting at the tip of the stereovilli (figure 1b). Recent studies have pointed to TMC-1/2 channels as the likely molecule transducing the mechanical stimulus [19–21]. In agreement with this, TMCs are localized to the tip of the stereovilli and interact with cadherins and other components of the tip link [22–24].
Figure 1.
The ‘model’ ciliated mechanosensory cells. (a,b) The vertebrate hair cell (HC). (a) Sketch of the apical side of a hair cell showing stereovilli and kinocilium. Axoneme is shown in cross-section (along dotted line) to reveal the 9+2 axoneme. The central pair of microtubules is perpendicular to the basal foot. Depolarization of the hair cell occurs upon mechanical stimulation of the bundle towards the basal foot. (b) Mechanotransduction in the hair cells occurs at the stereovilli rather than at the kinocilium. A mechanotransduction channel (possibly composed of TMC1/2 channels) sits at the tip of a stereovillus and is indirectly opened by stretching the tip links attached to it. Cadherins are components of the tip links. (c,d) The arthropod type 1 organ sensory cell. (c) Sketch of the cilium of a type 1 organ sensory cell. Axoneme is shown in cross-section (along dotted line) to reveal a 9+0 axoneme at its proximal side. The axoneme increases in the number of microtubules at its distal side. Stretching or compressing at the tip of the cilium depolarizes the sensory neuron (SN). This region is known as the tubular body. The apical side is closely associated to a cap cell. (d) Cross-section of the tubular body (along solid line in panel c). Microtubules at the periphery show connections to the ciliary membrane (MMCs). The mechanotransduction channel NOMPC is localized to the ciliary membrane and is mechanically coupled to the microtubules via the MMCs. Extracellular linkages from the cap cell to the cilium are also shown. Sketches in a and b based on [6,7]. Sketches in c and d based on [8–10]. (Online version in colour.)
Another model ciliated mechanosensory cell is the bipolar sensory neuron embedded in the type 1 organs of arthropods. These organs include sensory bristles, chaetae, campaniform sensilla and chordotonal organs [8]. In contrast to hair cells, the cilium in type 1 organ cells may play a more central role in mechanotransduction. The basic structure of the different type 1 organ mechanosensory cell types is similar [9], and thus the transduction apparatus likely follows similar working principles [8] (figure 1c). The cilium usually has a modified architecture with two basal bodies arranged in tandem leading to a 9+0 axoneme at the proximal side. The number of microtubules may either increase towards the distal part of the cilium (12–1000 tubules) forming a highly ordered array structure known in some cells as the tubular body [9], or remain as a normal axoneme (e.g. some chordotonal organs). The tubules at the distal side of the cilium are connected to the plasma membrane via compliant structures known as membrane integrated cones [25] (recently renamed as microtubule-membrane connections (MMCs) [26]) (figure 1d). The ciliary membrane forms extracellular bridges with the surrounding dendritic sheath. By deforming, stretching or deflecting the organ, the force is transmitted via the extracellular bridges to the ciliary tip where the mechanotransduction apparatus likely sits. The TRPN channel NOMPC is expressed at the tip of the cilium [27–29], where it forms part of the MMCs [26,30] (figure 1d). NOMPC is a mechanotransduction channel with a long chain of ankirin repeats (ARs) [31,32]. Through the binding of the ARs to microtubules, the channel in NOMPC may be opened upon deformation of the ciliary tip [33,34].
The vertebrate hair cell and the type 1 organ cells of arthropods are perhaps the best understood of all animal cells in terms of their physiology and molecular composition. However, it is not clear how these cells relate to each other in mechanistic and evolutionary terms, or which of them, if any, is more representative of how ciliated mechanosensory cells work in other animals. To tackle these questions, we need to know more about the molecular, structural and functional diversity of mechanosensory cells in the remaining major animal groups, most of which have an aquatic lifestyle. Putative mechanosensory cells in a wide range of aquatic organisms are structurally similar to the canonical vertebrate hair cell. This suggests a common origin that happened at the dawn of animal evolution [35], but a more nuanced view will likely emerge from mechanistic studies in underrepresented groups.
Here we review examples of putative and confirmed mechanosensory ciliated cells across marine invertebrates. We argue that a wider appreciation of the structural and functional diversity of mechanosensory ciliated cells in a larger range of organisms would help elucidate the mechanistic details and general principles of ciliary mechanosensation. The aquatic lifestyle of marine invertebrates relies heavily on motor and sensory cilia to generate feeding currents, propel the body or sense water movement and pressure. This makes marine organisms particularly relevant for the study of ciliary sensors and motor systems. The advent of genome editing and live imaging techniques now enable detailed mechanistic studies in some of these organisms. A broader sampling across phyla and more in-depth functional studies across diversity will also be needed to understand the evolutionary relationships of mechanosensory cells.
2. Ciliated mechanosensory cells in filter feeding
Filter feeding is widespread in both larval and adult forms of aquatic animals with motile cilia commonly generating the feeding currents [36]. While the flow pattern of the cilia-driven currents can account for the sorting and rejection of particles in some cases [37], filter feeders also use cilia to actively detect, sort or reject particles based on mechano- and chemosensation.
(a). Systems for particle retention
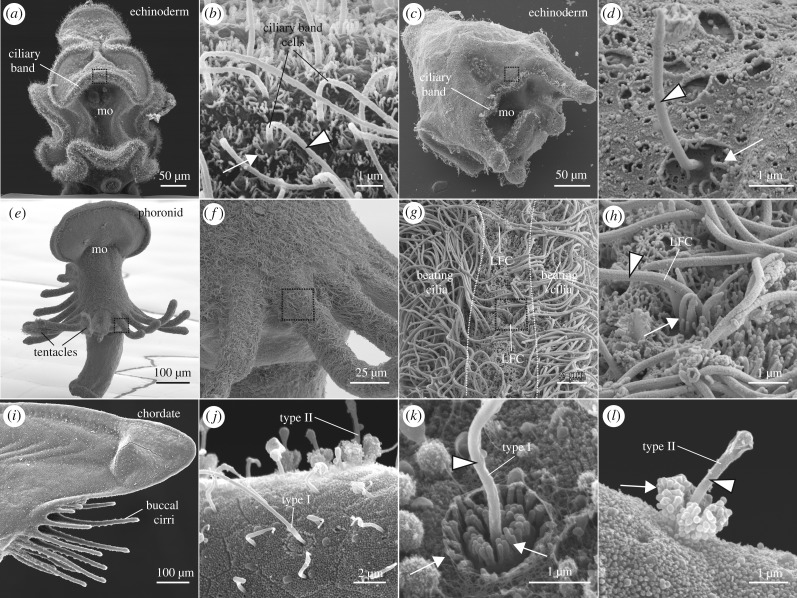
Ciliated sensory and motor systems for particle retention have primarily been studied in echinoderms (e.g. sea urchins, sea stars) and lophophorates (bryozoans, phoronids and brachiopods). The planktonic filter-feeding larvae of sea urchins use local ciliary reversals to redirect particles towards the oral field [38]. Food particles can be detected by the locomotor cilia in the oral field and can induce a local reversal in the beat direction to deflect the particle towards the mouth. Ciliary reversals occur rapidly as they are electrically controlled [39]. They are triggered by particles of a certain size and are not influenced by the chemical composition of the particle [40]. The ciliary-band cells at the anterior tip of the larva are sensitive to mechanical cues and may themselves be the particle detectors [38,41]. In echinoderms and in some hemichordate larvae, the ciliary-band cells have a hair-cell like morphology with a single cilium surrounded by microvilli [42–48] (figure 2a,b; table 1). In fact, particle-induced reversals may occur in other echinoderm and hemichordate larvae [51,81]. There are additional uniciliated sensory cells in close association with the ciliary band [49,50,82,83] (figure 2c,d), but it is still unclear whether these cells contribute to the detection of food particles.
Figure 2.
Cilia in invertebrate larvae involved in particle detection or rejection. (a,c,e,i) SEM images of planktonic larvae. (a,b) Sea star bipinnaria larva (a) has bands of uniciliated cells, each with a collar of microvilli (b). Square in (a) shows approximate region of cells shown in (b). (c,d) Sea urchin pluteus larva (c) shows uniciliated cells associated with the ciliary band (d). Square in (c) indicates approximate position of cilium shown in (d). (e,f) A phoronid larva has multiple ciliated tentacles that generate currents directed towards the mouth (mo). (g) Each tentacle has rows of laterofrontal cilia (LFC) between the fields of beating cilia. (h) LFC have collars of thick microvilli (stereovilli) that may sense particles and guide them to the mouth. Squares in (e–g) indicate blown up regions in the subsequent panel. (i,j) Adult amphioxus has cirri at the mouth (i); each is covered with epithelial and type I and II uniciliated sensory cells (j). (k) Type I sensory cells have two concentric collars of microvilli. (l) Type II cells have layered microvilli reaching half the length of the cilium. White arrows and black and white arrowheads in b, d, h, k and l point to microvilli/stereovilli and cilia, respectively.
Table 1.
Ciliary mechanosensory cells in filter feeding.
| animal group | species | stage | cell name (s) | evidence of mechanosensory modality | molecular dataa | sensory neuron (SN) or secondary sensory cell (SSC)b | cilium features | actin or other structures | location of cells |
|---|---|---|---|---|---|---|---|---|---|
| Echinodermata Hemichordata |
Echinodermata
Asterias rubens [44], Pisaster ochraceus [45,49], Lytechinus pictus [45], Psammechinus miliaris [46], Hemicentrotus pulcherrimus [50] Hemichordatata Balanoglossus biminiensis [45], Tornaria ancoratae [43], Rhabdopleura compacta [48] |
brachiolaria larva, pluteus larva, bipinnaria larva, tornaria larva, zooid stage |
prismatic cells, epaulette cells, ciliary band cells, oral field cells (Hemichordata) |
fast local ciliary reversals induced by particle approach (in echinoderms) [51,52] | — | SSC |
|
|
|
| Echinodermata |
Pisaster ochraceus [49,53], Hemicentrotus pulcherrimus [50] |
pluteus larva, bipinnaria larva |
|
— | — | SN |
|
— |
|
| Nemertea | unidentified [54] | pilidium larva | uniciliated cell | — | — | SN [55] | — uniciliated | — collar of microvilli | — ciliary band |
| lophophorates (Phoronida, Bryozoa, Brachiopoda) |
Phoronida Phoronis spp. [53,56–59], Phoronopsis harmeri [60] Bryozoa Crisia eburnea [61,62], Membranipora membranacea [63] Brachiopoda Glottidia pyramidata [64], Laqueus californianus [60] |
actinotroch larva, cyphonautes larva, adult | laterofrontal cell | flick motion of cilia and induction of ciliary reversal (Brachiopoda and Bryozoa) [65–67] | SSC |
|
— collar of 8 stereovilli |
|
|
| Cephalochordata | Branchiostoma floridae [68] | larva | oral spine cell | direct touch of cilia induces a cough response [68] | AmphiNaC [69] | SSC | — uniciliated | — no microvilli | — edge of mouth |
| Cephalochordata | Branchiostoma spp. [70,71] | adult | type I sensory cell [71] | — | — | SN |
|
|
|
| Cephalochordata | Branchiostoma spp. [70,71] | adult | type II sensory cell | mechanical stimulation of velar tentacles induces cough response [70] | — | SSC |
|
|
|
| Tunicata |
Ascidiacea Botryllus schlosseri [72], Styela plicata [73], Ciona intestinalis [74], Phallusia mammillata [74], Chelyosoma productum [74], Corella inflata [75], Clavelina lepadiformis [74], Diplosoma listerianum [74], Molgula socialis [76] Thaliacea Pyrosoma atlanticum [77], Doliolum nationalis [77] |
adult | coronal sensory cell | removal of tentacles abolishes particle rejection response [75] | TRPA [78] | SSC |
|
|
|
| Tunicata (Larvacea) | Oikopleura spp. [79] | adult? | mouth sensory cell | — | — | SSC |
|
— no microvilli | — dorsal and ventral lip |
| Porifera | Ephydatia muelleri [80] | adult |
|
removal of cilia or osculum abolishes response [80] | — | — |
|
— no microvilli | — osculum (exhalant siphon) |
aOnly molecular data related to the expression of mechanotransduction-related components is included.
bSecondary sensory cells lack a neurite projection (e.g. vertebrate hair cells).
Lophophorates use a different method of filter feeding called ciliary sieving [36,84]. These animals have stiff cilia on their tentacles (called laterofrontal cilia, LFC) that sense the drag created by food particles (figure 2e–g). Upon detecting the particle, LFC bend and start a ‘flick’ motion that pushes the particle into the feeding current towards the mouth [65–67]. They may also change the beating direction of adjacent cilia. Each laterofrontal cilium arises from a single sensory cell and is surrounded by thick microvilli [53,56,60–64,85,86] (figure 2h; table 1). The pilidium larva of nemerteans (ribbon worms) also has non-motile uniciliated cells along the ciliary band [54,55,87] (table 1). These cells may be involved in sensing particles and regulate active capture by controlling ciliary band activity or the movement of the larval lappets.
(b). Systems for particle rejection
Ciliated mechanosensory cells can also detect large particles that could clog the filtering system. In the larval cephalochordate amphioxus (lancelet), bundles of cilia called oral spines emanating from uniciliated sensory cells surround the mouth and initiate a cough response upon contact with debris [68] (table 1). Adult amphioxus also displays a cough response when mechanically stimulated from the anterior [88]. This response may be mediated by type I and type II ciliated mechanosensory cells located in a range of structures along the filtering system such as buccal cirri at the entrance of the channel [70,71] (figure 2i,j), or velar tentacles at the entrance of the digestive system (table 1). Velar tentacles are sensitive to touch [89,90] and harbour a group of cells with stiff cilia [70,71,91] that have 20 to 90 microtubules [70,71] (table 1). The cilium of a velar tentacle cell is surrounded by bulbous branched microvilli that reach half its length (type II cell in figure 2l).
Many ascidians or sea squirts (Tunicata, the sister group of vertebrates) are filter feeders. The inner side of the oral (inhalant) siphon has a ring of tentacles involved in detecting unwanted particles and triggering a rejection response [75,92,93]. Each tentacle has rows of mechanosensitive ciliated cells called the coronal cells. These cells are associated with non-ciliated supporting cells [72–74]. Coronal cells have one or more cilia (depending on the species) and either no or numerous stereovilli [72,74–76,94] (table 1). Similar to mammalian hair cells, the stereovilli of some ascidian species are graded in length. There are also fibrous structures connecting the cilium to the stereovilli and the stereovilli to each other. Similar cells can be found in the oral field of larvaceans and thaliaceans, the other two major groups of tunicates [77,79,95,96] (table 1). Members in these taxa also display particle rejection behaviours [97,98].
Coronal cells in ascidians share a regulatory gene-expression signature with vertebrate hair cells and express the TRP channel TRPA, but not TRPN [78]. Whether TRPA is important for mechanosensation in the tunicate coronal cells is not known. Hair cells in the zebrafish lateral line express both TRPA and TRPN [99,100] but neither of these molecules is critical for mechanotransduction at the stereovilli [101,102].
In the examples above, particle rejection is mediated by the nervous system. Ciliated mechanosensory cells have also been described in the neuron-less sponges, animals that are also mostly filter-feeders. These animals also display behaviours triggered by particle accumulation. Some sponges have a slow ‘sneezing response’ that clears their system of debris [103,104]. This response may be mediated by ciliated pinacocytes in the exhalant siphon (osculum) (table 1). Removal of either cilia or the osculum leads to the loss of this response [80]. Commonly used blockers of mechanotransduction in hair cells also reversibly inhibit the sneezing response. The ciliated cells may detect a reduction in water flow due to clogging to initiate the response [80,105–107].
3. Ciliated mechanosensory cells in prey and predator detection
Aquatic organisms generate a hydrodynamic signal as they swim. These signals can be used to locate potential prey or detect predators [108]. Mechanosensation through direct touch can also be involved in predator–prey interactions. Ciliated mechanosensory cells that likely mediate such behaviours have been described on the body surface of a large diversity of aquatic invertebrates [109]. These cells are tuned to different types of mechanical stimuli and can mediate prey location and approach, prey capture or defensive startle and escape responses.
(a). Prey location
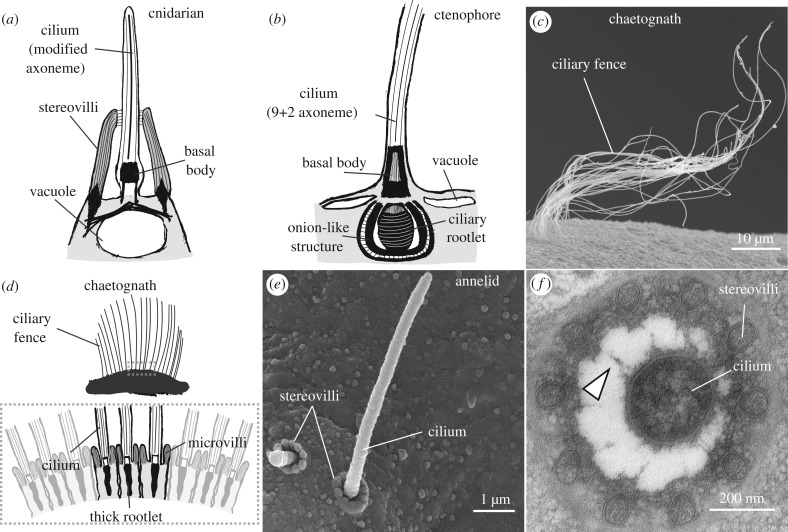
Cnidarians, the sister group of bilaterians, have multiple ciliated sensory cell types and show varied behaviours in response to mechanical cues produced by their prey. Cnidarian polyps are attached to the substrate and capture their prey with tentacles around the mouth. Polyps respond to low-frequency vibrations or to touch by bending towards the source of the signal [110–112]. This behaviour could allow the polyp to catch passing prey and may be induced by collared uniciliated sensory cells in the tentacles and other parts of the body [112–117] (figure 3a; table 2). In anthozoan polyps, sensory structures called hair bundles have stereovilli with radially decreasing length linked by fibrils at their tips in a similar fashion to vertebrate hair cell stereovilli [113,117] (table 2). Loose-patch recordings combined with flow stimulation induce negative or positive graded currents as a function of the intensity and direction of the stimulus [124], which may be due to the asymmetric arrangement of stereovilli. The stereovilli label with antibodies against a TRPA homologue [127] and a cadherin-23 homologue [125,126]. The latter molecule specifically localizes to the tip link-like structures [125]. Masking the cadherin with antibodies affected the structure and mechanosensitivity of the sensory bundle [126].
Figure 3.
Structural diversity of vibration receptors. (a) Sketch of a cnidarian vibration receptor. The cilium has a modified axoneme, stereovilli and a vacuole at the base. (b) Sketch of a putative ctenophore vibration receptor. Note the absence of microvilli and the onion-like structure at the base. (c) SEM micrograph of a chaetognath ciliary fence. (d) (Top) Sketch of a ciliary fence in a chaetognath. Dashed rectangle highlights the region expanded below. (Bottom) Sketch of the ciliary fence receptors showing their collared cilia and their tight arrangement along the fence. (e) SEM micrograph of a collar receptor in the annelid P. dumerilii. (f) TEM micrograph of a collar receptor. Arrowhead points to fibres connecting the cilium and the stereovilli. Sketches in a, b and d are based on images/schematics in [112,116], [118–120] and [121,122]. Image in f is taken from [123].
Table 2.
Ciliary mechanosensory cells in prey–predator interactions.
| animal group | species | stage | cell name (s) | evidence of mechanosensory modality | molecular dataa | sensory neuron (SN) or secondary sensory cell (SSC)b | cilium features | actin or other structures | location of cells |
|---|---|---|---|---|---|---|---|---|---|
| Cnidaria (Hydrozoa and Cubozoa) |
Coryne pintneri [112], Stauridiosarsia producta [114], Carybdea marsupialis [115], Coryne tubulosa [116] |
polyp | mechanosensitive ciliated cell type type-2 cell [115], concentric hair cells [116] |
vibration or touch of tentacles bearing these cells induces behaviour [112] | — | SSC (e.g. in [116]) |
|
|
|
| Cnidaria (Anthozoa) |
Diadumene lineata [117], Ceriantheopis americanus [113], Stomphia coccinea [113] |
polyp | anemone hair bundle (HB) sensory neuron, ciliary cone sensory cell |
mechano-induced currents in HBs [124] | cadherin-23 [125,126], TRPA [127] |
SN |
|
|
|
| Cnidaria (Hydrozoa) | Aglantha digitale [128,129] | medusa | ciliated comb pad (C) cell, T cell |
responsiveness to short-range vibration (C cell) or to long- range vibration (T cell) [129] | — | SN |
|
|
|
| Cnidaria (Hydrozoa and Cubozoa) |
Hydra spp. [130–132], Stauridiosarsia producta [114], Tubularia larynx [131], Carybdea marsupialis [115], Gonionemus vertens [133], Physalia physalis [134], Craspedacusta sowerbii [135] |
polyp, medusa, siphonophore |
nematocyte | generator potentials upon mechanical stimulation [136,137] | NOMPC [138] | SSC |
|
|
— tentacles |
| Ctenophora |
Eucharis multicornis [139], Lampetia pancerina [140], Callianira bialata [140], Pleurobrachia rhodopis [140], Beroe cucumis [119], Pleurobrachia pileus [141], Euplokamis sp. [118] |
adult | sensory cell, Tastborsten |
touch of the cilium and vibration induces prey-seeking response [139] | — | SN |
|
|
|
| Chaetognatha | Spadella spp. [121,122,142] | adult | ciliary fence receptors (CFRs groups of >100), ciliary tuft receptors (CTRs groups of <100) | touch of the fence leads to escape response [143] | — | SN/SSC |
|
|
|
| Tunicata (Larvacea) | Oikopleura spp. [144] | — | Langerhans receptor |
|
— | SSC |
|
— no microvilli | — tail |
| Annelida |
Platynereis dumerilii [123], Lumbricus terrestris [145], Hirudo medicinalis [146], Eurythoe complanata [147], Ophryotrocha puerilis [148], Dinophilus gyrociliatus [149] |
nectochaete larva [123], adult [145–148] |
CR [123], S hair [146], type I collar receptor [147], type 3 collar receptor [148], type Ia collar receptor [149] |
PKD1 and PKD2 [123] | SN |
|
|
— sensory organs and epidermis across the body |
aOnly molecular data related to the expression of mechanotransduction-related components is included.
bSecondary sensory cells lack a neurite projection (e.g. vertebrate hair cells).
In addition to hair-cell like cells, cnidarians have evolved a highly specialized ciliated mechanosensory cell called the nematocyte. These cells are sensitive to vibrations and respond cell-autonomously by an explosive discharge of a stinging capsule that injects toxins into their prey [151,152]. Mechanical stimulation drives action potentials in the cell [136,137], while coincident chemical stimuli can increase the probability of activation [153,154]. Nematocytes have a sensory part called the cnidocil apparatus, which is responsible for both sensory modalities. The cnidocil is a modified cilium associated with a bundle of microvilli that is connected by fibres to the cilium [115,130–133,135,155] (table 2).
Evidence points to a major role of the cilium in mechanotransduction at the nematocyte. First, deflection of the cnidocil induces mechanoelectrical potentials independent of stimulus direction [137]. Second, removal of the cnidocil drastically reduces the receptor potential upon contact [154]. This process may involve the mechanotransduction channel NOMPC since at least one of the four NOMPC homologues in Hydra localizes to the cnidocil [138]. Moreover, MMCs such as those in the campaniform receptors of Drosophila (figure 1d) are also present in the cnidocil [131]. This suggests that MMCs may also form a gating spring to open the NOMPC channel upon cnidocil stimulation [138]. Cnidarians thus seem to have different mechanosensory cell types that rely more on stereovilli (hair bundles) or on cilia (nematocytes) for sensory transduction.
Ctenophores (comb jellies) have also evolved efficient mechanisms for prey detection [156–159]. Ctenophore prey detection likely depends on flow or contact sensitivity conferred by ciliated sensory structures. The lobate ctenophore Eucharis has multiple finger-like protrusions carrying ciliated neurons at their tips with a single long non-motile cilium and a highly modified root and basal apparatus [139] (figure 3b; table 2). The protrusions are sensitive to vibrations and this may underlie the ability of the animals to detect prey. Similar cells line the tentacles of cydippid ctenophores [118,140] and the lips of beroid ctenophores [119]. In the latter, the cilium is flanked by actin-microfilament-supported pegs.
Chaetognaths (arrow worms) are planktonic predators that can detect minute short-range vibrations generated by their prey [143,160,161]. This ability is likely conferred by numerous putative mechanosensitive organs called ‘ciliary fences’ [121,122,142,162] (figure 3c). These organs are mechanosensitive and are composed of parallel arrays of uniciliated neurons each with regularly arranged collars of slender microvilli (figure 3d; table 2). The sensory arrays contain cells with cilia of different diameters and lengths in a defined arrangement within the array [122]. These structural features and their location along the body may account for the increased ability of these animals to locate the source of vibrations and respond in a defined frequency range [143,160].
(b). Predator detection
Planktonic organisms often rely on mechanical stimuli in order to detect approaching predators and display a defence response or escape.
The planktonic larvacean Oikopleura shows responses to touch or vibrations [163]. In its tail, there are mechanosensory cells called Langerhans receptors [144,164] that may mediate the escape response to touch in these animals [144,163]. The Langerhans receptors have a long process supported by multiple parallel microtubules—a modification also seen in the tubular body of insect type 1 mechanosensory organs and in the amphioxus type II cells (see above). Langerhans receptors have no associated microvilli and a highly modified basal apparatus that lacks a typical basal body (table 2). Ultrastructural studies of these receptors across development are needed to track how its unconventional sensory dendrite is formed.
The planktonic larva of the annelid Platynereis also shows a startle response upon water-borne vibrations [123]. The vibration receptors have a non-motile cilium and a collar of stereovilli (figure 3e,f). Collar receptors (CRs) with similar ultrastructure are present in other annelids (table 2; [145–149]). CRs in Platynereis express homologues of the Polycystin 1 and 2 gene families, which are important for the function and structure of ciliated mechanosensory cells in both vertebrates and invertebrates [165–168]. Polycystin-deficient Platynereis larvae generated by CRISPR/Cas9 mutagenesis are not sensitive to vibrations and are more easily captured by planktonic predators [123].
The larger planktonic jellyfish Aglantha digitale (phylum Cnidaria) also shows an escape response upon direct contact with a predator [169]. This animal has at least three types of ciliated mechanosensory cells implicated in responses to touch and vibrations. Collar cells or C cells are found in the velum and have a single cilium and a polarized collar of microvilli [128,129,170] (table 2). These cells connect to the giant axon system that triggers the predator escape response [170]. Laser ablation of the C cells eliminated neuronal activity in the giant axon induced by vibrational stimulation [129]. T cells and F cells are other ciliated mechanoreceptors found in the velum [129] (table 2), but their exact sensory range and contribution to the response are still to be defined.
The detection of vibrations in the environment may have been among the first sensory functions of cilia in the last eukaryotic common ancestor. Ciliated unicellular organisms such as Paramecium and Chlamydomonas show fast escape responses triggered by contact and vibration [171,172]. These experimentally tractable systems are valuable to address the role of cilia in mechanosensation and to trace the evolution of this sensory modality. In Paramecium, frontal contact of the cell with an obstacle leads to ciliary arrests followed by reversal of the ciliary stroke. Although cilia are in principle stimulated in this response, deciliated cells still retain depolarizing receptor potentials, thus indicating that the site of mechanotransduction is at the cell rather than at the ciliary membrane [173]. The long Chlamydomonas cilia (commonly referred to as flagella) are mechanosensitive [174] and suppression of a TRPV channel located in the proximal side of the cilium leads to a reduction in avoidance responses [175]. Another unicellular green alga, Pyramimonas octopus shows high sensitivity to contacts to its locomotory cilia, upon which a fast escape response is enacted [176]. Such enhanced sensitivity may be achieved by micrometer-scale movements of its cilia during non-swimming periods. Thus, at least in algae, cilia may have an active role in sensing mechanical cues.
4. Ciliated mechanosensory cells in orientation and navigation
Orientation or navigation guided by mechanical signals is common in aquatic organisms. In this section, we review examples where ciliated mechanoreceptors have been implicated in various forms of orientation and navigation responses, including gravitaxis, flow and pressure sensation.
(a). Gravitaxis
Many aquatic organisms can orient relative to the gravity vector. In some cases, orientation is passive due to the shape and density distribution of the animal. In other cases, orientation is active and depends on sensors in the body. Statocysts are the most common type of gravisensory organs, and they often contain ciliated mechanosensory cells and a small concretion or statolith.
In cnidarian medusae, the statocysts are located at the edge of the velum and contain a concretion that likely stimulates mechanosensory cilia when the animal turns upside down [128,177–183]. Different types of ciliated cells either with or without stereovilli can be found in the cnidarian statocyst, even in a single species (table 3). The individual contributions of these cells to mechanosensation has not been elucidated [179] and may also include sound perception [210].
Table 3.
Ciliary mechanosensory cells in posture and orientation.
| animal group | species | stage | cell name (s) | evidence of mechanosensory modality | molecular dataa | sensory neuron (SN) or secondary sensory cell (SSC)b | cilium features | actin or other structures | location of cells |
|---|---|---|---|---|---|---|---|---|---|
| Cnidaria |
Halistaura cellularia [179], Aegina citrea [179], Solmissus marshalli [179], Cunina lativentris [182], Rhopalonema velatum [182], Geryonia proboscidalis [182], Aurelia aurita [183], Aglantha digitale [128] |
medusa | type A–C sensory cells [179], type I receptor [183], ciliated sensory cell |
removal of statocyst leads to loss of righting behaviour [180] | — | SN |
|
|
— statocyst |
| Ctenophora |
Pleurobrachia pileus [184,185], Bolinopsis sp. [184] |
adult | balancer ciliated cell | deflection of a balancer increases or decreases beating frequency [184,186] | — | — |
|
— no microvilli | — statocyst (aboral sense organ) |
| Mollusca |
Bivalves Pecten sp. [187,188] Gastropods Pomacea paludosa [189], Aplysia californica [190,191], Pterotrachea sp. [187], Lymnaea stagnalis [192], Biomphalaria glabrata [193] Nudibranchs Rostanga pulchra [194], Hermissenda crassicornis [195] Cephalopods Octopus vulgaris [196], Sepia officinalis, Loligo vulgaris [197] |
adult | hair cell | generator potentials recorded in statocyst cells upon mechanical displacement or statocyst rotation (nudibranchs [198]; gastropods [199,200] cephalopods [201]) | — | SN/SSC |
|
||
| Tunicata |
Ascidiacea Diplosoma macdonaldi [204], Ciona spp. [205], Botryllus schlosseri [206] Larvacea Oikopleura dioica [207] |
swimming larva (ascidian) | statocyst-associated cells, antenna cells, S1 cells (B. sch.) |
statolith-ablated larva (Ciona) fails to show gravitaxis [208] | — | SN |
|
|
— sensory vesicle |
| Cephalochordata | Branchiostoma floridae [209] | 12.5-day larva | ciliary bulb cell | — | — | SN |
|
— | — infundibular region of cerebral vesicle |
aOnly molecular data related to the expression of mechanotransduction-related components is included.
bSecondary sensory cells lack a neurite projection (e.g. vertebrate hair cells).
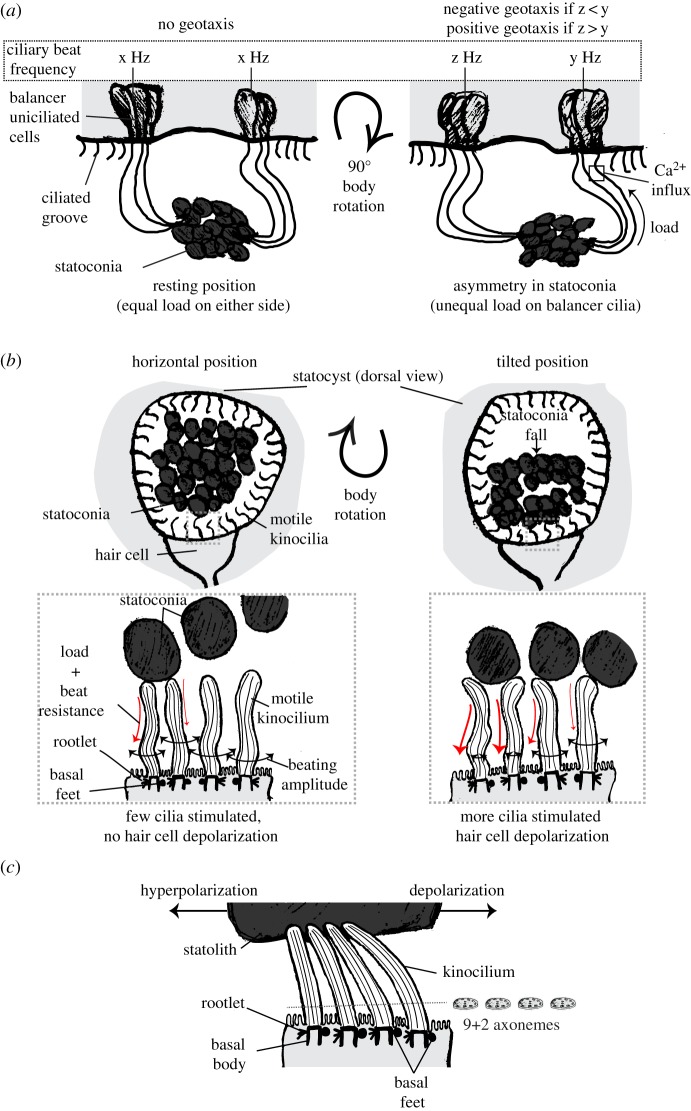
The aboral sense organ of ctenophores contains a statocyst involved in controlling body posture [211]. The statocyst contains one statolith in the middle that is attached to four groups of ciliated cells (called the balancers) [184,185], one in each quadrant of the animal (table 3). Each balancer has several cilia that sporadically beat as a unit. When the statolith presses the cilia upon a change in posture, their beating frequency changes as a function of statolith load (figure 4a) [184,186]. The balancer cilia are hydrodynamically coupled to the locomotor ciliary comb plates. Changes in ciliary beat frequency have been suggested to propagate through this coupling and cause a turning response [184]. The deflection-induced ciliary beating in the balancer cells is dependent on influx of Ca2+ at the base of the cilium [212]. Ca2+ levels are probably modulated by mechanically induced electrical changes in the membrane of balancer cells [184].
Figure 4.
Diversity in function and structure of ciliated statocysts. (a) (Left) Sketch of a ctenophore statocyst in its resting position (i.e. animal in vertical position). Water surface is to the top. Balancer cilia on opposite sides have the same beating frequency (x Hz). (Right) Upon body rotation the statoconia attached to the balancer cilia exert more load on one group of balancers (arrow). For clarity, the statocyst is not rotated in the sketch. This leads to an asymmetry in stimulation, and thus a consequent difference in beat frequency (z and y). The sign of geotaxis depends on the relative beating frequencies of each balancer. Beating only occurs at the base of the cilia (square) and it depends on Ca2+. (b) (Upper left) Sketch of a non-cephalopod mollusc statocyst in its resting position (i.e. animal in horizontal position). View from the dorsal side. (Lower left) Close-up view of a multiciliated hair cell in resting position. Basal feet are not oriented in the same direction. Some of the motile kinocilia sporadically contact statoconia, thus affecting their beating amplitude. This leads to an increase in voltage noise in the cell, but still below the firing threshold. (Upper right) Upon tilting, statoconia redistribute to one side, thus increasing the chance of cilia colliding with them. (Lower right) More cilia make contact with statoconia, causing depolarization of the cell. (c) Sketch of a cephalopod multiciliated hair cell. Kinocilia are not motile and are attached to the overlying statolith. All basal feet are oriented in the same direction, which is perpendicular to the central pair of the 9+2 axoneme. The cell is depolarized when the cilia are deflected in the direction towards the basal feet and hyperpolarized when bent in the opposite direction. Sketches in a, b and c are based on [184,212], [195,213,214] and [197,215], respectively. (Online version in colour.)
Molluscs also have statocysts and show gravitactic responses [192,216]. The statocysts have mechanosensory cells with multiple cilia (e.g. 30–40 in Pomacea paludosa) and small microvilli arranged in rings or irregularly among the cilia [187–194] (table 3). In non-cephalopod molluscs, cilia are often motile and statoliths or statoconia can move freely upon changes in the animal's posture (figure 4b, top). Direct contact of the motile cilia with the statoconia depolarizes the mechanosensory cells [198–200] (figure 4b, bottom). The responses are graded according to the magnitude of stimulus [217,218] and are not dependent on stimulus direction [219]. The motility of the cilium in these mollusc hair cells leads to a constant fluctuation in voltage due to random contacts with the statoconia [220–222]. A change in the orientation of the animal leads to more cilia contacting the statoconia, which in turn leads to a summed and concerted change in voltage of the cell by the activation of the mechanotransduction apparatus in each cilium [213,214,223] (figure 4b, bottom right). Cilium motility may serve two purposes in these cells. First, it may amplify the signal by the combined effect of statoconia loading and the resistance to beating. Second, it may be a mechanism to actively monitor statoconia load by sustained depolarization events due to the constant interactions caused by the beating cilia [214]. Cilium-motility-driven modulation of mechanotransduction (or active mechanosensing) is also known from chordotonal organs in insects [224,225].
In cephalopods, the statocyst is embedded in a complex equilibrium organ with analogous functions to the vertebrate ear [226–230]. Like other molluscs, mechanosensory hair cells in the cephalopod statocyst have several kinocilia (up to 200 in decapod cephalopods) and short microvilli [196] (table 3). The kinocilia in cephalopods are not motile and connect to each other at their tips by membrane junctions [231]. The basal feet of the cilia in a single cell are oriented in the same direction [196,197]. Recordings from the statocyst afferent nerves have shown maximal directional sensitivity to vibrations that are parallel to the axis of the morphological polarization of the hair cells [201,232,233] (figure 4c). The polarization of cilia in cephalopod hair cells thus has similar functional consequences to the polarization of stereovilli in vertebrate hair cells.
Some planktonic tunicates are geotactic and also have a statocyst with ciliated sensory cells [206,207,234–236]. The sensory vesicle of the ascidian larva hosts a pigmented statocyst composed of a cellular otolith and associated ciliated sensory cells [204] (table 3). When the otolith is ablated, the larva loses the ability to swim towards the surface [208]. Gravitaxis may rely on the stimulation of the ciliated cells by the otolith [204,237,238]. In Ciona larvae, the ciliated sensory cells (called antenna cells) are glutamatergic [239]. Their neuronal circuitry down to the muscle effectors was recently reconstructed by serial electron microscopy [205].
A less conventional ciliated statocyst was found in the amphioxus larva [209,240]. Although no geotactic response has been reported in this animal, a balance organ may be responsible for its hovering swimming behaviour [241]. A putative balance organ was found at the posterior side of the cerebral vesicle. This organ is composed of a group of ‘ciliary bulb’ cells, each with a single cilium filled at its distal end with a dense bulky matrix (table 3). Adjacent to these highly modified cilia there are slender cilia emanating from accessory cells. In theory, the dense bulbous cilia would be moved by gravity, thereby activating the accessory cells by contacting their cilia. This may allow the larva to monitor its body orientation [209].
Several other invertebrate planktonic animals, including bryozoan larvae and chaetognaths, show geotactic responses albeit these animals lack a statocyst [242–244]. In at least some cases, these are active responses, as shown by centrifugation experiments [242]. The gravisensory organ in these animals has not yet been found, but as suggested for amphioxus, it may lie in the different modified ciliated structures reported in these animals [86,121,245].
(b). Detection of flow
Detection of flow (rheoreception) is common among aquatic animals and it is often used for orientation [246]. In fish, lamprey and tadpoles rheoreception is mediated by hair cells in the lateral line [247]. Aquatic invertebrates such as nudibranchs, brittle stars and flatworms also respond to the effects of turbulence and flow [248–255]. Ciliated sensory cells have been proposed as potential flow receptors in these and other animals [256,257] but, to our knowledge, direct physiological evidence is still lacking. Some of these animals also respond to chemicals delivered by the flow [258], which highlights the need of carefully controlling for such confounding effects in rheotaxis experiments.
Cephalopods have an organ with functional similarities to the vertebrate lateral line. These ‘epidermal’ lines are composed of multiciliated sensory cells similar to the cells lining the statocyst [203] (see above). Electrophysiological recordings from the epidermal line nerve indicate that the organ is sensitive to small water movements [215,259]. Chemical ablation of the epidermal line in squid reduces its ability to detect a fish predator [260,261]. Whether the organ is also involved in navigation and orientation like the lateral line in fish has not been studied.
Sponges also have ciliated sensory cells implicated in flow sensation. The cone cells or apopylar cells are uniciliated cells in the excurrent pore (apopyle) of the choanocyte chamber [105,262–264]. These cells have a ‘fringe’ of microvilli oriented towards the choanocyte chamber [263]. Cone cells may detect flow and help control its direction.
Small planktonic larvae are exposed to local flow variations caused by waves, filter feeders or turbulence [265–268] and can respond by changing their swimming behaviour [269–272]. Such responses usually involve vertical movements in the water column to enter or exit particular flows [273]. For example, different mollusc larvae can either sink, or actively swim downwards or upwards in response to different flow environments [270,274–279]. Arthropod larvae also swim upwards in response to increased turbulence [280]. In oyster larvae, the internal ciliated statocyst is thought to mediate responses to waves and turbulence [276,281]. Sea urchin larvae lack a statocyst but are able to adjust their swimming speed according to turbulence levels [282].
(c). Pressure sensation
The locomotory response to changes in hydrostatic pressure is termed barokinesis [283,284]. Barokinesis has been documented in various marine animals, including ctenophores [285], annelids [286,287], cnidarians [286,288], molluscs [289–291] and arthropods [286,289,292–294]. The response usually involves an increase in swimming activity with increasing pressure. Pressure is an indicator of depth and responding to pressure can enable an animal to counter downwelling and upwelling currents [295]. Pressure changes may also entrain tidal clocks [296–298].
Fish sense hydrostatic pressure and this ability is likely mediated by receptors lining the swimming bladder [299]. Animals without a swimming bladder may use ciliated sensory cells to detect changes in pressure. Dogfish hair cells increase their firing frequency under increased hydrostatic pressure [300]. Crabs use instead a modified type 1 organ in their statocysts to detect pressure changes. It consists of a long cuticular hair attached to ciliated sensory cells at its base. Upon changes in pressure the thread hair serves as a piston pressing against the sensory cilia, thereby causing cell depolarization [301]. Putative ciliated pressure receptors have been described in ctenophores [120,285], tunicates [206,235], amphioxus [302–304] and barnacles [305]. These cells usually have branched cilia with a 9+0 axoneme and bulbous tips protruding into an internal liquid-filled cavity. In support of the involvement of such a type of ciliated cell in pressure detection, we recently found in Platynereis a branched ciliated neuron showing a correlated increase in activity with increases in pressure (LA Bezares-Calderón and G Jékely 2016, unpublished). One hypothetical mechanism to explain how cilia sense small pressure changes is basal body movement by fluid displaced from the tip of the bulbous cilium [306].
5. Ciliated mechanosensory cells in larval settlement
The majority of marine invertebrates have a biphasic life cycle with a larval planktonic stage and an adult benthic stage. During settlement, planktonic larvae need to find a suitable substrate.
They achieve this by integrating multiple cues, primarily chemical but also mechanical, about the substrate and the flow environment. In this section, we highlight a few cases where there is potential involvement of ciliated mechanosensory cells in regulating settlement.
(a). Topography-based settlement
The mechanical detection of the substrate may allow larvae to detect appropriate surfaces. Echinoderm larvae that already contain the adult rudiment use their tube feet to scan the substratum [307]. This direct contact together with chemical cues is required to induce settlement. At the end of the tube feet, the larvae have uniciliated sensory receptors with a collar of microvilli that may work as substrate sensors [308].
Substrate topography can also influence settlement. Some coral and sponge larvae prefer to settle on surfaces with holes of a particular size [309]. Both sponge and coral larvae have diverse ciliated sensory cells including cells with a long cilium and a collar of microvilli [310–317]. Ascidian larvae also have a preference for particular substrates [318,319]. These larvae use adhesive papillae at their anterior end to find a suitable substrate for settlement and metamorphosis [234,320]. Ciliated sensory cells in the papillae may be used to detect the mechanical properties of a particular substrate [321–328]. That said, the settlement preference for a particular surface topography may in some cases be an indirect effect of the flow environment created by it and detected by the larva (see below) [329].
(b). Flow-based settlement
Flow sensation has been shown to influence larval settlement in several animal groups [266,267,330]. Cnidarian, barnacle and annelid larvae are able to settle under different flow regimes by integrating flow and settlement cues [331–335]. In some larvae, flow detection alone can recruit larvae to the substrate where they are more likely to encounter chemical cues [336]. For example, oyster larvae stop swimming and sink in response to the turbulent layer near the substrate (ca 1 cm from the surface) [278]. In some echinoderm larvae, turbulence may induce competence to settle [273,337,338].
The flow-sensory mechanisms have not been identified in these larvae. Sensory cells regulating settlement are often found at the anterior tip of larvae in a structure known as the apical organ. Some of these cells have long cilia exposed to the flow environment [42,245,339–341]. Chemical disruption of the apical organ cilia in sea urchin larvae leads to swimming defects [342]. The Platynereis larva has sensory cells in the apical region with a motile cilium and a collar of microvilli that respond to changes in flow ([343]; LA Bezares-Calderón and G Jékely 2015, unpublished). Apical organ cells express different ion channels that are associated with mechanosensation in vertebrates. For example, cells in the apical organ of sea urchin, Platynereis and cnidarian larvae express ASIC, and/or TRP channels such as NOMPC or TRPV [343–346]. Whether and how these cells contribute to substrate or flow sensation is yet to be explored.
6. Ciliated mechanosensory cells in proprioception
Proprioception is a sensory ability to monitor mechanical stress, load and tension on different parts of the body. Proprioceptive systems have mostly been studied in arthropods and mammals [347], but they also occur in other animals to control active locomotion and body posture (e.g. [348]). While mammalian proprioceptors are non-ciliated sensory cells, some arthropod proprioceptors have a cilium, such as the sensory cells in the chordotonal organs or campaniform sensilla.
There are only a few known examples of ciliated proprioceptors outside the arthropods and vertebrates, mostly in cnidarians. For instance, cerianthid cnidarians have sensory cells with both cilium and stereovilli embedded in a muscle cell [349] (figure 5, table 4). This cell has afferent and efferent connections with fibres controlling muscle activity, perhaps forming a basic proprioceptive circuit. Hydra has in its tentacles a putative proprioceptive ciliated neuron enclosed by epitheliomuscular cells and with its cilium and stereovilli parallel to the mesoglea [350–352] (table 4). Cubozoan medusae also have cilia associated with circular muscle in the epidermis [354] (table 4). Finally, a species of marine nematode has sensory cells with the cilium tightly associated with muscle fibres [353] (table 4).
Figure 5.
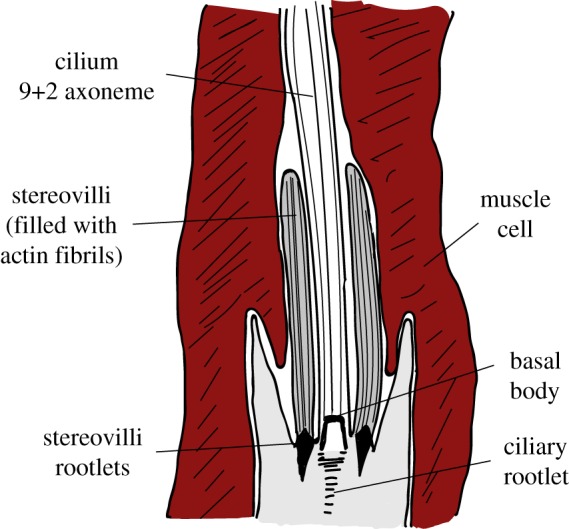
Sketch of a cnidarian proprioceptor. The sensory dendrite including the cilium and the stereovilli are embedded in a muscle cell. Muscle contraction may stimulate the cilium and/or the stereovilli and depolarize the cell. Sketch based on [349]. (Online version in colour.)
Table 4.
Ciliary mechanosensory cells in proprioception.
| animal group | species | stage | cell name (s) | evidence of mechanosensory modality | molecular dataa | sensory neuron (SN) or secondary sensory cell (SSC)b | cilium features | actin or other structures | location of cells |
|---|---|---|---|---|---|---|---|---|---|
| Cnidaria | Hydra spp. [350–352] | polyp | sensorimotor interneuron | — | — | SN |
|
|
|
| Cnidaria | Ceriantheopsis americanus [349] | polyp | bipolar mechanoreceptor | — | — | SN |
|
|
— body wall |
| Nematoda | Deontostoma californicum [353] | — | proprioceptor metaneme [353] | — | — | SSC |
|
|
— hypodermal lateral nerve cords |
aOnly molecular data related to the expression of mechanotransduction-related components is included.
bSecondary sensory cells lack a neurite projection (e.g. vertebrate hair cells).
A highly sophisticated ciliated proprioceptor system was reported in squids. These animals are able to move their head independent of the rest of the body. To achieve a fine control of head movements, squids have a series of ciliated proprioceptors in the ‘neck’ region with similar morphology to those receptors in the statocyst [202]. These cells are oriented at different angles on the neck surface. Depending on the angle of movement of the head a defined subset of cells is stimulated.
Proprioceptive neurons, neuronal units and behaviours in other marine animals such as sea stars, molluscs or annelids have been reported [252,355–357]. Most of the identified or proposed proprioceptors have a non-ciliated (i.e. multidendritic) morphology similar to spindle receptors in vertebrates [358]. Although more comprehensive ultrastructural descriptions would be needed to rule out the presence of cilia in some of these cases, ciliary mechanoreception does not seem to be generally required for proprioceptive functions.
7. Promising avenues to study ciliary mechanosensation
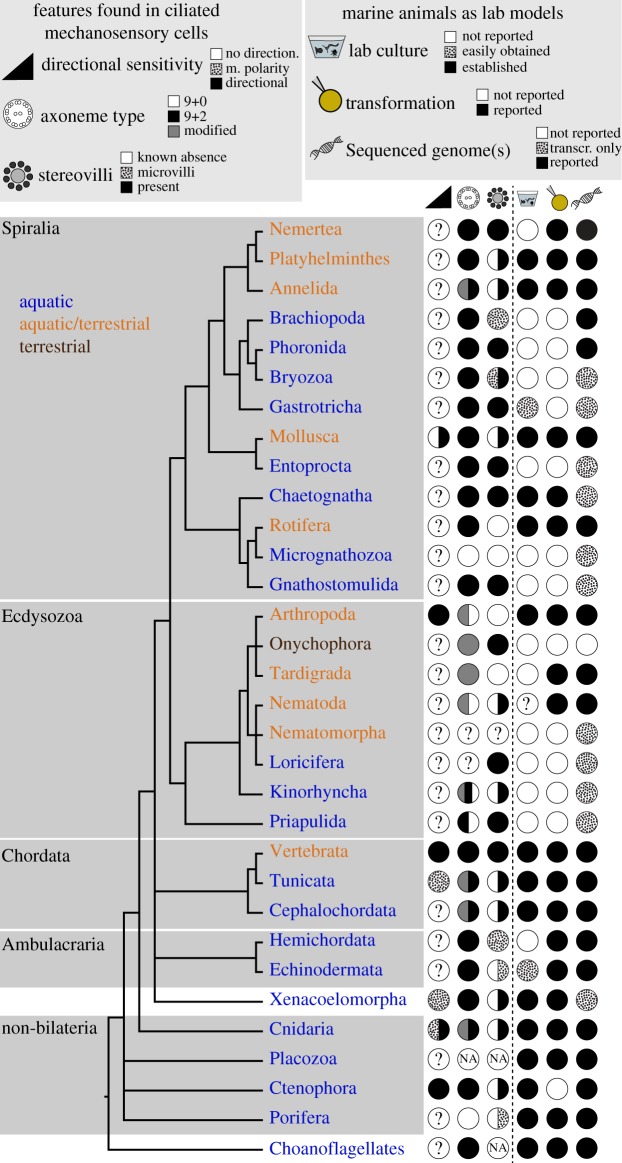
The examples reviewed here illustrate the diversity in structure and function of ciliated mechanosensory cells across animals (figure 6). Our knowledge of the physiology and function of most of the examples discussed here is fragmentary. Exploring some of these systems further could open up new perspectives in understanding the mechanisms of ciliary mechanosensation and the evolution of mechanoreception.
Figure 6.
Animal phylogeny shown to indicate main features of ciliary mechanosensory cells and the degree of current experimental accessibility of each major taxon. First column: evidence of directional sensitivity, morphological polarity (m. polarity) or of no directionality. Second column: type of axoneme found in mechanosensory cells. Third column: presence/absence of cells with stereovilli. Fourth column: marine species that can be reared in the laboratory (dotted circle: a species cannot be breed in the laboratory but can be regularly obtained from the wild). Fifth column: transformation techniques available (e.g. microinjection or electroporation). Sixth column: genome sequence reported (dotted circle: only transcriptome assembly reported). Note that other groups of marine organisms not covered in this review (e.g. Rotifers, Platyhelminthes) also offer good opportunities for experimental analyses. Phylogeny is based on [359–361]. See electronic supplementary material, table S1 for references and further details. (Online version in colour.)
There are many interesting unanswered questions regarding the function and physiology of ciliary mechanoreceptors. These questions could now be revisited with state-of-the-art techniques in the emerging marine experimental systems. For example, one could test whether the Langerhans receptors or the coronal organ cells in tunicates are mechanosensitive, as in these and many other cases physiological and genetic evidence is still lacking (tables 1–4). The ultrastructural diversity of putative mechanoreceptors—sometimes even within the same animal—suggests functional and sensory specializations.
Recent developments in genomics, imaging, serial electron microscopy and genome editing have enabled functional studies in several marine organisms. Genetic transformation and the generation of gene knockouts is now feasible in many of the animals discussed above (figure 6) [362]. This is complemented by an ever-increasing number of marine invertebrates that can be reared in the laboratory and that have a genome sequence available. Further progress in understanding the function of ciliated mechanosensory cells will nonetheless require more detailed and quantitative behavioural studies. These approaches could be combined with high-resolution serial electron microscopy reconstruction of sensory structures and neural circuits, as has been possible in C. elegans [363] and more recently in marine larvae [123,205].
The structural diversity of mechanosensory cilia in marine invertebrates has long been recognized [364], as well as the rich treasure trove of behavioural mechanisms to be found in marine organisms [365]. We believe the time is ripe to functionally address these aspects in order to have a more unified view of cilia-based mechanosensation.
Supplementary Material
Acknowledgement
We thank Elizabeth A. Williams for comments on the manuscript. We also thank Èlia Benito-Gutiérrez for providing the amphioxus samples.
Data accessibility
This article does not contain any additional data.
Authors' contributions
G.J. collected plankton samples; J.B. prepared and acquired the SEM data; L.A.B.-C. compiled the literature, drew the sketches and assembled the figures; L.A.B.-C. and G.J. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
L.A.B.-C. was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) (JE 777/3-1).
References
- 1.Katta S, Krieg M, Goodman MB. 2015. Feeling force: physical and physiological principles enabling sensory mechanotransduction. Annu. Rev. Cell Dev. Biol. 31, 347–371. ( 10.1146/annurev-cellbio-100913-013426) [DOI] [PubMed] [Google Scholar]
- 2.Delmas P, Coste B. 2013. Mechano-gated ion channels in sensory systems. Cell 155, 278–284. ( 10.1016/j.cell.2013.09.026) [DOI] [PubMed] [Google Scholar]
- 3.Ranade SS, Syeda R, Patapoutian A. 2015. Mechanically activated ion channels. Neuron 87, 1162–1179. ( 10.1016/j.neuron.2015.08.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nag S, Resnick A. 2017. Biophysics and biofluid dynamics of primary cilia: evidence for and against the flow-sensing function. Am. J. Physiol. Renal Physiol. 313, F706–F720. ( 10.1152/ajprenal.00172.2017) [DOI] [PubMed] [Google Scholar]
- 5.Ferreira RR, Fukui H, Chow R, Vilfan A, Vermot J. 2019. The cilium as a force sensor—myth versus reality. J. Cell Sci. 132, jcs213496 ( 10.1242/jcs.213496) [DOI] [PubMed] [Google Scholar]
- 6.Hudspeth AJ. 2014. Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci. 15, 600–614. ( 10.1038/nrn3786) [DOI] [PubMed] [Google Scholar]
- 7.Fettiplace R. 2009. Defining features of the hair cell mechanoelectrical transducer channel. Pflugers Arch. 458, 1115–1123. ( 10.1007/s00424-009-0683-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kernan MJ. 2007. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 454, 703–720. ( 10.1007/s00424-007-0263-x) [DOI] [PubMed] [Google Scholar]
- 9.Keil TA. 1997. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 39, 506–531. () [DOI] [PubMed] [Google Scholar]
- 10.Moulins M. 1976. Ultrastructure of chordotonal organs. In Structure and function of proprioceptors in the invertebrates (ed. Mill PJ.), pp. 387–426. New York, NY: Chapman and Hall. [Google Scholar]
- 11.Jande SS. 1966. Fine structure of lateral-line organs of frog tadpoles. J. Ultrastruct. Res. 15, 496–509. ( 10.1016/S0022-5320(66)80122-3) [DOI] [PubMed] [Google Scholar]
- 12.Flock Å, Kimura R, Lundquist P-G, Wersäll J. 1962. Morphological basis of directional sensitivity of the outer hair cells in the organ of Corti. J. Acoust. Soc. Am. 34, 1351–1355. ( 10.1121/1.1918345) [DOI] [Google Scholar]
- 13.Flock A, Duvall AJ. 1965. The ultrastructure of the kinocilium of the sensory cells in the inner ear and lateral line organs. J. Cell Biol. 25, 1–8. ( 10.1083/jcb.25.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neugebauer DC, Thurm U. 1984. Chemical dissection of stereovilli from fish inner ear reveals differences from intestinal microvilli. J. Neurocytol. 13, 797–808. ( 10.1007/BF01148494) [DOI] [PubMed] [Google Scholar]
- 15.Hudspeth AJ, Corey DP. 1977. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl Acad. Sci. USA 74, 2407–2411. ( 10.1073/pnas.74.6.2407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudspeth AJ, Jacobs R. 1979. Stereocilia mediate transduction in vertebrate hair cells (auditory system/cilium/vestibular system). Proc. Natl Acad. Sci. USA 76, 1506–1509. ( 10.1073/pnas.76.3.1506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickles JO, Comis SD, Osborne MP. 1984. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15, 103–112. ( 10.1016/0378-5955(84)90041-8) [DOI] [PubMed] [Google Scholar]
- 18.Assad JA, Shepherd GM, Corey DP. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994. ( 10.1016/0896-6273(91)90343-X) [DOI] [PubMed] [Google Scholar]
- 19.Pan B, et al. 2018. TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99, 736–753.e6. ( 10.1016/j.neuron.2018.07.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. 2013. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79, 504–515. ( 10.1016/j.neuron.2013.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawashima Y, et al. 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J. Clin. Invest. 121, 4796–4809. ( 10.1172/JCI60405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahendrasingam S, Furness DN. 2019. Ultrastructural localization of the likely mechanoelectrical transduction channel protein, transmembrane-like channel 1 (TMC1) during development of cochlear hair cells. Sci. Rep. 9, 1274 ( 10.1038/s41598-018-37563-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepermans E, Petit C. 2015. The tip-link molecular complex of the auditory mechano-electrical transduction machinery. Hear. Res. 330, 10–17. ( 10.1016/j.heares.2015.05.005) [DOI] [PubMed] [Google Scholar]
- 24.Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, Nicolson T. 2014. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc. Natl Acad. Sci. USA 111, 12 907–12 912. ( 10.1073/pnas.1402152111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurm U, Erler G, Gödde J, Kastrup H, Keil T, Völker W, Vohwinkel B. 1983. Cilia specialized for mechanoreception. J. Submicrosc. Cytol. 15, 151–155. [Google Scholar]
- 26.Liang X, et al. 2013. A NOMPC-dependent membrane-microtubule connector is a candidate for the gating spring in fly mechanoreceptors. Curr. Biol. 23, 755–763. ( 10.1016/j.cub.2013.03.065) [DOI] [PubMed] [Google Scholar]
- 27.Liang X, Madrid J, Saleh HS, Howard J. 2011. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton 68, 1–7. ( 10.1002/cm.20493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng LE, Song W, Looger LL, Jan LY, Jan YN. 2010. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67, 373–380. ( 10.1016/j.neuron.2010.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Moon S, Cha Y, Chung YD. 2010. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE 5, e11012 ( 10.1371/journal.pone.0011012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Gao Y, He J, Cui L, Meissner J, Verbavatz JM, Li B, Feng X, Liang X. 2019. Ultrastructural organization of NompC in the mechanoreceptive organelle of Drosophila campaniform mechanoreceptors. Proc. Natl Acad. Sci. USA 116, 7343–7352. ( 10.1073/pnas.1819371116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225. ( 10.1038/nature11685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker RG, Willingham AT, Zuker CS. 2000. A Drosophila mechanosensory transduction channel. Science 287, 2229–2234. ( 10.1126/science.287.5461.2229) [DOI] [PubMed] [Google Scholar]
- 33.Jin P, et al. 2017. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547, 118–122. ( 10.1038/nature22981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, et al. 2015. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell 162, 1391–1403. ( 10.1016/j.cell.2015.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arendt D, Benito-Gutierrez E, Brunet T, Marlow H. 2015. Gastric pouches and the mucociliary sole: setting the stage for nervous system evolution. Phil. Trans. R. Soc. B 370, 20150286 ( 10.1098/rstb.2015.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riisgård HU, Larsen PS. 2010. Particle capture mechanisms in suspension-feeding invertebrates. Mar. Ecol. Prog. Ser. 418, 255–293. ( 10.3354/meps08755) [DOI] [Google Scholar]
- 37.Nawroth JC, Guo H, Koch E, Heath-Heckman EAC, Hermanson JC, Ruby EG, Dabiri JO, Kanso E, McFall-Ngai M. 2017. Motile cilia create fluid-mechanical microhabitats for the active recruitment of the host microbiome. Proc. Natl Acad. Sci. USA 114, 9510–9516. ( 10.1073/pnas.1706926114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strathmann RR, Jahn TL, Fonseca JRC. 1972. Suspension feeding by marine invertebrate larvae: clearance of particles by ciliated bands of a rotifer, pluteus, and trochophore. Biol. Bull. 142, 505–519. ( 10.2307/1540326) [DOI] [Google Scholar]
- 39.Mackie GO, Spencer AN, Strathmann R. 1969. Electrical activity associated with ciliary reversal in an echinoderm larva. Nature 223, 1384–1385. ( 10.1038/2231384a0) [DOI] [Google Scholar]
- 40.Appelmans N. 1994. Sites of particle selection determined from observations of individual feeding larvae of the sand dollar Dendraster excentricus. Limnol. Oceanogr. Lett. 39, 404–411. ( 10.4319/lo.1994.39.2.0404) [DOI] [Google Scholar]
- 41.Gustafson T, Lundgren B, Treufeldt R. 1972. Serotonin and contractile activity in the echinopluteus. Exp. Cell Res. 72, 115–139. ( 10.1016/0014-4827(72)90573-3) [DOI] [PubMed] [Google Scholar]
- 42.Chia F-S, Burke RD, Koss R, Mladenov PV, Rumrill SS. 1986. Fine structure of the doliolaria larva of the feather star Florometra serratissima (Echinodermata: Crinoidea), with special emphasis on the nervous system. J. Morphol. 189, 99–120. ( 10.1002/jmor.1051890202) [DOI] [PubMed] [Google Scholar]
- 43.Dautov SS, Nezlin LP. 1992. Nervous system of the tornaria larva (Hemichordata: Enteropneusta). A histochemical and ultrastructural study. Biol. Bull. 183, 463–475. ( 10.2307/1542023) [DOI] [PubMed] [Google Scholar]
- 44.Nerrevang A, Wingstrand KG. 1970. On the occurrence and structure of choanocyte-like cells in some echinoderms. Acta Zool. 51, 249–270. ( 10.1111/j.1463-6395.1970.tb00436.x) [DOI] [Google Scholar]
- 45.Lacalli TC, West JE. 1993. A distinctive nerve cell type common to diverse deuterostome larvae: comparative data from echinoderms, hemichordates and amphioxus. Acta Zool. 74, 1–8. ( 10.1111/j.1463-6395.1993.tb01215.x) [DOI] [Google Scholar]
- 46.Ryberg E, Lundgren B. 1977. Extra-ectodermal strands in the ciliated bands of the echinopluteus. Dev. Growth Differ. 19, 299–308. ( 10.1111/j.1440-169X.1977.00299.x) [DOI] [PubMed] [Google Scholar]
- 47.Nakajima Y. 1986. Development of the nervous system of sea urchin embryos: formation of ciliary bands and the appearance of two types of ectoneural cells in the pluteus (ectoneural cell/sea urchin pluteus/ciliary band/axoneme). Dev. Growth Differ. 28, 531–542. ( 10.1111/j.1440-169X.1986.00531.x) [DOI] [PubMed] [Google Scholar]
- 48.Dilly PN. 1972. The structures of the tentacles of Rhabdopleura compacta (Hemichordata) with special reference to neurociliary control. Z. Zellforsch. Mikrosk. Anat. 129, 20–39. ( 10.1007/BF00307107) [DOI] [PubMed] [Google Scholar]
- 49.Burke RD. 1983. The structure of the larval nervous system of Pisaster ochraceus (Echinodermata: Asteroidea). J. Morphol. 178, 23–35. ( 10.1002/jmor.1051780103) [DOI] [PubMed] [Google Scholar]
- 50.Nakajima Y. 1986. Presence of a ciliary patch in preoral epithelium of sea urchin plutei. Dev. Growth Differ. 28, 243–249. ( 10.1111/j.1440-169X.1986.00243.x) [DOI] [PubMed] [Google Scholar]
- 51.Strathmann RR. 2007. Time and extent of ciliary response to particles in a non-filtering feeding mechanism. Biol. Bull. 212, 93–103. ( 10.2307/25066587) [DOI] [PubMed] [Google Scholar]
- 52.Strathmann RR. 1971. The feeding behavior of planktotrophic echinoderm larvae: mechanisms, regulation, and rates of suspension feeding. J. Exp. Mar. Biol. Ecol. 6, 109–160. ( 10.1016/0022-0981(71)90054-2) [DOI] [Google Scholar]
- 53.Lacalli TC. 1990. Structure and organization of the nervous system in the actinotroch larva of Phoronis vancouverensis. Phil. Trans. R. Soc. Lond. B 327, 655–685. ( 10.1098/rstb.1990.0104) [DOI] [Google Scholar]
- 54.Lacalli TC, West JE. 1985. The nervous system of a pilidium larva: evidence from electron microscope reconstructions. Can. J. Zool. 63, 1909–1916. ( 10.1139/z85-284) [DOI] [Google Scholar]
- 55.Hindinger S, Schwaha T, Wanninger A. 2013. Immunocytochemical studies reveal novel neural structures in nemertean pilidium larvae and provide evidence for incorporation of larval components into the juvenile nervous system. Front. Zool. 10, 31 ( 10.1186/1742-9994-10-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hay-Schmidt A. 1989. The nervous system of the actinotroch larva of Phoronis muelleri (Phoronida). Zoomorphology 108, 333–351. ( 10.1007/BF00312274) [DOI] [Google Scholar]
- 57.Pardos F, Roldán C, Benito J, Emig CC. 1991. Fine structure of the tentacles of Phoronis australis Haswell (Phoronida, Lophophorata). Acta Zool. 72, 81–90. ( 10.1111/j.1463-6395.1991.tb00320.x) [DOI] [Google Scholar]
- 58.Fernández I, Pardos F, Benito J, Roldán C. 1996. Ultrastructural observations on the phoronid nervous system. J. Morphol. 230, 265–281. () [DOI] [PubMed] [Google Scholar]
- 59.Pardos F, Roldán C, Benito J, Aguirre A, Fernández I. 1993. Ultrastructure of the lophophoral tentacles in the genus Phoronis (Phoronida, Lophophorata). Can. J. Zool. 71, 1861–1868. ( 10.1139/z93-265) [DOI] [Google Scholar]
- 60.Gilmour THJ. 1978. Ciliation and function of the food-collecting and waste-rejecting organs of lophophorates. Can. J. Zool. 56, 2142–2155. ( 10.1139/z78-290) [DOI] [Google Scholar]
- 61.Nielsen C. 1987. Structure and function of metazoan ciliary bands and their phylogenetic significance. Acta Zool. 68, 205–262. ( 10.1111/j.1463-6395.1987.tb00892.x) [DOI] [Google Scholar]
- 62.Nielsen C, Riisgård HU. 1998. Tentacle structure and filter-feeding in Crisia eburnea and other cyclostomatous bryozoans, with a review of upstream-collecting mechanisms. Mar. Ecol. Prog. Ser. 168, 163–186. ( 10.3354/meps168163) [DOI] [Google Scholar]
- 63.Atkins D. 1955. The ciliary feeding mechanism of the cyphonautes larva [Polyzoa Ectoprocta]. J. Mar. Biol. Assoc. U.K. 34, 451–466. ( 10.1017/S0025315400008754) [DOI] [Google Scholar]
- 64.Gilmour THJ. 1981. Food-collecting and waste-rejecting mechanisms in Glottidia pyramidata and the persistence of lingulacean inarticulate brachiopods in the fossil record. Can. J. Zool. 59, 1539–1547. ( 10.1139/z81-209) [DOI] [Google Scholar]
- 65.Strathmann RR. 2006. Versatile ciliary behaviour in capture of particles by the bryozoan cyphonautes larva. Acta Zool. 87, 83–89. ( 10.1111/j.1463-6395.2006.00224.x) [DOI] [Google Scholar]
- 66.Riisgård HU, Nielsen KK, Fuchs J, Rasmussen BF, Obst M, Funch P. 2005. Ciliary feeding structures and particle capture mechanism in the freshwater bryozoan Plumatella repens (Phylactolaemata). Invertebr. Biol. 123, 156–167. ( 10.1111/j.1744-7410.2004.tb00151.x) [DOI] [Google Scholar]
- 67.Strathmann RR. 2005. Ciliary sieving and active ciliary response in capture of particles by suspension-feeding brachiopod larvae. Acta Zool. 86, 41–54. ( 10.1111/j.0001-7272.2005.00185.x) [DOI] [Google Scholar]
- 68.Lacalli TC, Gilmour THJ, Kelly SJ. 1999. The oral nerve plexus in amphioxus larvae: function, cell types and phylogenetic significance. Proc. R. Soc. Lond. B 266, 1461–1470. ( 10.1098/rspb.1999.0801) [DOI] [Google Scholar]
- 69.Candiani S, Oliveri D, Parodi M, Pestarino M. 2006. Expression of AmphiNaC, a new member of the amiloride-sensitive sodium channel related to degenerins and epithelial sodium channels in amphioxus. Int. J. Biol. Sci. 2, 79–86. ( 10.7150/ijbs.2.79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bone Q, Best ACG. 1978. Ciliated sensory cells in amphioxus (Branchiostoma). J. Mar. Biol. Assoc. U.K. 58, 479 ( 10.1017/S0025315400028137) [DOI] [Google Scholar]
- 71.Lacalli TC, Hou S. 1999. A reexamination of the epithelial sensory cells of amphioxus (Branchiostoma). Acta Zool. 80, 125–134. ( 10.1046/j.1463-6395.1999.80220005.x) [DOI] [Google Scholar]
- 72.Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MDC, Manni L. 2003. Novel, secondary sensory cell organ in ascidians: in search of the ancestor of the vertebrate lateral line. J. Comp. Neurol. 461, 236–249. ( 10.1002/cne.10666) [DOI] [PubMed] [Google Scholar]
- 73.Manni L, Caicci F, Gasparini F, Zaniolo G, Burighel P. 2004. Hair cells in ascidians and the evolution of lateral line placodes. Evol. Dev. 6, 379–381. ( 10.1111/j.1525-142X.2004.04046.x) [DOI] [PubMed] [Google Scholar]
- 74.Manni L, Mackie GO, Caicci F, Zaniolo G, Burighel P. 2006. Coronal organ of ascidians and the evolutionary significance of secondary sensory cells in chordates. J. Comp. Neurol. 495, 363–373. ( 10.1002/cne.20867) [DOI] [PubMed] [Google Scholar]
- 75.Mackie GO, Burighel P, Caicci F, Manni L. 2006. Innervation of ascidian siphons and their responses to stimulation. Can. J. Zool. 84, 1146–1162. ( 10.1139/z06-106) [DOI] [Google Scholar]
- 76.Caicci F, Burighel P, Manni L. 2007. Hair cells in an ascidian (Tunicata) and their evolution in chordates. Hear. Res. 231, 63–72. ( 10.1016/j.heares.2007.05.007) [DOI] [PubMed] [Google Scholar]
- 77.Caicci F, Gasparini F, Rigon F, Zaniolo G, Burighel P, Manni L. 2013. The oral sensory structures of Thaliacea (Tunicata) and consideration of the evolution of hair cells in Chordata. J. Comp. Neurol. 521, 2756–2771. ( 10.1002/cne.23313) [DOI] [PubMed] [Google Scholar]
- 78.Rigon F, Gasparini F, Shimeld SM, Candiani S, Manni L. 2018. Developmental signature, synaptic connectivity and neurotransmission are conserved between vertebrate hair cells and tunicate coronal cells. J. Comp. Neurol. 526, 957–971. ( 10.1002/cne.24382) [DOI] [PubMed] [Google Scholar]
- 79.Rigon F, Stach T, Caicci F, Gasparini F, Burighel P, Manni L. 2013. Evolutionary diversification of secondary mechanoreceptor cells in Tunicata. BMC Evol. Biol. 13, 112 ( 10.1186/1471-2148-13-112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ludeman DA, Farrar N, Riesgo A, Paps J, Leys SP. 2014. Evolutionary origins of sensation in metazoans: functional evidence for a new sensory organ in sponges. BMC Evol. Biol. 14, 3 ( 10.1186/1471-2148-14-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hart MW, Miller RL, Madin LP. 1994. Form and feeding mechanism of a living Planctosphaera pelagica (phylum Hemichordata). Mar. Biol. 120, 521–533. ( 10.1007/BF00350072) [DOI] [Google Scholar]
- 82.Lacalli TC, Kelly SJ. 2002. Anterior neural centres in echinoderm bipinnaria and auricularia larvae: cell types and organization. Acta Zool. 83, 99–110. ( 10.1046/j.1463-6395.2002.00103.x) [DOI] [Google Scholar]
- 83.Lacalli TC, Gilmour THJ, West JE. 1990. Ciliary band innervation in the bipinnaria larva of Piaster ochraceus. Phil. Trans. R. Soc. Lond. B 330, 371–390. ( 10.1098/rstb.1990.0206) [DOI] [Google Scholar]
- 84.Larsen PS, Riisgård HU. 2002. On ciliary sieving and pumping in bryozoans. J. Sea Res. 48, 181–195. ( 10.1016/S1385-1101(02)00136-3) [DOI] [Google Scholar]
- 85.Nielsen C. 2005. Ciliary filter-feeding structures in adult and larval gymnolaemate bryozoans. Invertebr. Biol. 121, 255–261. ( 10.1111/j.1744-7410.2002.tb00065.x) [DOI] [Google Scholar]
- 86.Gruhl A, Schwaha T. 2015. Bryozoa (Ectoprocta). In Structure and evolution of invertebrate nervous systems (eds Schmidt-Rhaesa A, Harzsch S, Purschke G), pp. 325–340. New York, NY: Oxford University Press. [Google Scholar]
- 87.von Dassow G, Emlet RB, Maslakova SA. 2013. How the pilidium larva feeds. Front. Zool. 10, 47 ( 10.1186/1742-9994-10-47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrington EJW. 1965. The Biology of Hemichordata & Protochordata. San Francisco, CA: W.H. Freeman. [Google Scholar]
- 89.Parker GH. 1908. The sensory reactions of amphioxus. Proc. Am. Acad. Arts Sci. 43, 415 ( 10.2307/20022358) [DOI] [Google Scholar]
- 90.Bone Q. 1961. The organization of the atrial nervous system of amphioxus (Branchiostoma lanceolatum (Pallas)). Phil. Trans. R. Soc. Lond. B 243, 241–269. ( 10.1098/rstb.1961.0002) [DOI] [Google Scholar]
- 91.Dale Stokes M, Holland ND. 1995. Embryos and larvae of a lancelet, Branchiostoma floridae, from hatching through metamorphosis: growth in the laboratory and external morphology. Acta Zool. 76, 105–120. ( 10.1111/j.1463-6395.1995.tb00986.x) [DOI] [Google Scholar]
- 92.Day EC. 1919. The physiology of the nervous system of the tunicate. I. The relation of the nerve ganglion to sensory responses. J. Exp. Zool. 28, 307–335. ( 10.1002/jez.1400280206) [DOI] [Google Scholar]
- 93.Hecht S. 1918. The physiology of Ascidia atra Lesueur. II. Sensory physiology. J. Exp. Zool. 25, 261–299. [Google Scholar]
- 94.Caicci F, Degasperi V, Gasparini F, Zaniolo G, Del Favero M, Burighel P, Manni L. 2010. Variability of hair cells in the coronal organ of ascidians (Chordata, Tunicata). Can. J. Zool. 88, 567–578. ( 10.1139/Z10-036) [DOI] [Google Scholar]
- 95.Bone Q, Gorski G, Pulsford AL. 1979. On the structure and behaviour of Fritillaria (Tunicata: Larvacea). J. Mar. Biol. Assoc. U.K. 59, 399 ( 10.1017/S0025315400042715) [DOI] [Google Scholar]
- 96.Olsson R, Holmberg K, Lilliemarck Y. 1990. Fine structure of the brain and brain nerves of Oikopleura dioica (Urochordata, Appendicularia). Zoomorphology 110, 1–7. ( 10.1007/BF01632806) [DOI] [Google Scholar]
- 97.Deibel D, Paffenhöfer G-A. 1988. Cinematographic analysis of the feeding mechanism of the pelagic tunicate Doliolum nationalis. Bull. Mar. Sci. 43, 404–412. [Google Scholar]
- 98.Galt CP, Mackie GO. 1971. Electrical correlates of ciliary reversal in Oikopleura. J. Exp. Biol. 55, 205–212. [Google Scholar]
- 99.Sidi S, Friedrich RW, Nicolson T. 2003. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301, 96–99. ( 10.1126/science.1084370) [DOI] [PubMed] [Google Scholar]
- 100.Corey DP, et al. 2004. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432, 723–730. ( 10.1038/nature03066) [DOI] [PubMed] [Google Scholar]
- 101.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. 2006. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289. ( 10.1016/j.neuron.2006.03.042) [DOI] [PubMed] [Google Scholar]
- 102.Shin J-B, Adams D, Paukert M, Siba M, Sidi S, Levin M, Gillespie PG, Gründer S. 2005. Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc. Natl Acad. Sci. USA 102, 12 572–12 577. ( 10.1073/pnas.0502403102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elliott GRD, Leys SP. 2007. Coordinated contractions effectively expel water from the aquiferous system of a freshwater sponge. J. Exp. Biol. 210, 3736–3748. ( 10.1242/jeb.003392) [DOI] [PubMed] [Google Scholar]
- 104.Prosser CL, Nagai T, Nystrom RA. 1962. Oscular contractions in sponges. Comp. Biochem. Physiol. 6, 69–74. ( 10.1016/0010-406X(62)90044-0) [DOI] [PubMed] [Google Scholar]
- 105.Hammel JU, Nickel M. 2014. A new flow-regulating cell type in the demosponge Tethya wilhelma—functional cellular anatomy of a leuconoid canal system. PLoS ONE 9, e113153 ( 10.1371/journal.pone.0113153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parker GH. 1910. The reactions of sponges, with a consideration of the origin of the nervous system. J. Exp. Zool. 8, 1–41. ( 10.1002/jez.1400080102) [DOI] [Google Scholar]
- 107.Ludeman DA, Reidenbach MA, Leys SP. 2017. The energetic cost of filtration by demosponges and their behavioural response to ambient currents. J. Exp. Biol. 220, 995–1007. ( 10.1242/jeb.146076) [DOI] [PubMed] [Google Scholar]
- 108.Kiørboe T, Jiang H, Gonçalves RJ, Nielsen LT, Wadhwa N. 2014. Flow disturbances generated by feeding and swimming zooplankton. Proc. Natl Acad. Sci. USA 111, 11 738–11 743. ( 10.1073/pnas.1405260111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt-Rhaesa A. 2007. Sensory organs. In The evolution of organ systems (ed. Schmidt-Rhaesa A.), pp. 118–147. New York, NY: Oxford University Press. [Google Scholar]
- 110.Josephson RK. 1961. The response of a hydroid to weak water-borne disturbances. J. Exp. Biol. 38, 17–27. [Google Scholar]
- 111.Passano LM, Pantin FA. 1955. Mechanical stimulation in the sea-anemone Calliactis parasitica. Proc. R. Soc. Lond. B 143, 226–238. ( 10.1098/rspb.1955.0007) [DOI] [Google Scholar]
- 112.Tardent P, Schmid V. 1972. Ultrastructure of mechanoreceptors of the polyp Coryne pintneri (Hydrozoa, Athecata). Exp. Cell Res. 72, 265–275. ( 10.1016/0014-4827(72)90589-7) [DOI] [PubMed] [Google Scholar]
- 113.Peteya DJ. 1975. The ciliary-cone sensory cell of anemones and cerianthids. Tissue Cell 7, 243–252. ( 10.1016/0040-8166(75)90003-8) [DOI] [PubMed] [Google Scholar]
- 114.Golz R, Thurm U. 1994. The ciliated sensory cell of Stauridiosarsia producta (Cnidaria, Hydrozoa)—a nematocyst-free nematocyte? Zoomorphology 114, 185–194. ( 10.1007/BF00403266) [DOI] [Google Scholar]
- 115.Golz R, Thurm U. 1993. Ultrastructural evidence for the occurrence of three types of mechanosensitive cells in the tentacles of the cubozoan polyp Carybdea marsupialis. Protoplasma 173, 13–22. ( 10.1007/BF01378858) [DOI] [Google Scholar]
- 116.Holtmann M, Thurm U. 2001. Variations of concentric hair cells in a cnidarian sensory epithelium (Coryne tubulosa). J. Comp. Neurol. 432, 550–563. ( 10.1002/cne.1119) [DOI] [PubMed] [Google Scholar]
- 117.Watson GM, Mire P, Hudson RR. 1997. Hair bundles of sea anemones as a model system for vertebrate hair bundles. Hear. Res. 107, 53–66. ( 10.1016/S0378-5955(97)00022-1) [DOI] [PubMed] [Google Scholar]
- 118.Mackie GO, Mills CE, Singla CL. 1988. Structure and function of the prehensile tentilla of Euplokamis (Ctenophora, Cydippida). Zoomorphology 107, 319–337. ( 10.1007/BF00312216) [DOI] [Google Scholar]
- 119.Tamm S, Tamm S. 1991. Actin pegs and ultrastructure of presumed sensory receptors of Beroë (Ctenophora). Cell Tissue Res. 264, 151–159. ( 10.1007/BF00305733) [DOI] [PubMed] [Google Scholar]
- 120.Hernandez-Nicaise ML. 1984. Ctenophora. In Biology of the integument: 1 Invertebrates (eds Bereiter-Hahn J, Matoltsy AG, Richards KS), pp. 96–111. Berlin, Germany: Springer. [Google Scholar]
- 121.Bone Q, Pulsford A. 1978. The arrangement of ciliated sensory cells in Spadella (Chaetognatha). J. Mar. Biol. Assoc. U.K. 58, 565 ( 10.1017/S0025315400041229) [DOI] [Google Scholar]
- 122.Müller CHG, Rieger V, Perez Y, Harzsch S. 2014. Immunohistochemical and ultrastructural studies on ciliary sense organs of arrow worms (Chaetognatha). Zoomorphology 133, 167–189. ( 10.1007/s00435-013-0211-6) [DOI] [Google Scholar]
- 123.Bezares-Calderón LA, Berger J, Jasek S, Verasztó C, Mendes S, Gühmann M, Almeda R, Shahidi R, Jékely G. 2018. Neural circuitry of a polycystin-mediated hydrodynamic startle response for predator avoidance. Elife 7, e36262 ( 10.7554/eLife.36262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mire P, Watson GM. 1997. Mechanotransduction of hair bundles arising from multicellular complexes in anemones. Hear. Res. 113, 224–234. ( 10.1016/S0378-5955(97)00145-7) [DOI] [PubMed] [Google Scholar]
- 125.Watson GM, Pham L, Graugnard EM, Mire P. 2008. Cadherin 23-like polypeptide in hair bundle mechanoreceptors of sea anemones. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 194, 811–820. ( 10.1007/s00359-008-0352-0) [DOI] [PubMed] [Google Scholar]
- 126.Tang P-C, Watson GM. 2014. Cadherin-23 may be dynamic in hair bundles of the model sea anemone Nematostella vectensis. PLoS ONE 9, e86084 ( 10.1371/journal.pone.0086084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mahoney JL, Graugnard EM, Mire P, Watson GM. 2011. Evidence for involvement of TRPA1 in the detection of vibrations by hair bundle mechanoreceptors in sea anemones. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 197, 729–742. ( 10.1007/s00359-011-0636-7) [DOI] [PubMed] [Google Scholar]
- 128.Singla CL. 1983. Fine structure of the sensory receptors of Aglantha digitale (Hydromedusae: Trachylina). Cell Tissue Res. 231, 415–425. ( 10.1007/BF00222191) [DOI] [PubMed] [Google Scholar]
- 129.Arkett SA, Mackie GO, Meech RW. 1988. Hair cell mechanoreception in the jellyfish Aglantha digitale. J. Exp. Biol. 135, 329–342. [Google Scholar]
- 130.Slautterback DB. 1967. The cnidoblast-musculoepithelial cell complex in the tentacles of Hydra. Z. Zellforsch. Mikrosk. Anat. 79, 296–318. ( 10.1007/BF00369292) [DOI] [PubMed] [Google Scholar]
- 131.Golz R, Thurm U. 1991. Cytoskeleton-membrane interactions in the cnidocil complex of hydrozoan nematocytes. Cell Tissue Res. 263, 573–583. ( 10.1007/BF00327291) [DOI] [Google Scholar]
- 132.Golz R. 1994. Apical surface of hydrozoan nematocytes: structural adaptations to mechanosensory and exocytotic functions. J. Morphol. 222, 49–59. ( 10.1002/jmor.1052220106) [DOI] [PubMed] [Google Scholar]
- 133.Westfall JA. 1970. The nematocyte complex in a hydromedusan, Gonionemus vertens. Z. Zellforsch. Mikrosk. Anat. 110, 457–470. ( 10.1007/BF00330098) [DOI] [PubMed] [Google Scholar]
- 134.Westfall JA. 1965. Nematocysts of the sea anemone Metridium. Am. Zool. 5, 377–393. ( 10.1093/icb/5.3.377) [DOI] [PubMed] [Google Scholar]
- 135.Hausmann K, Holstein T. 1985. Bilateral symmetry in the cnidocil-nematocyst complex of the freshwater medusa Craspedacusta sowerbii Lankester (Hydrozoa, Limnomedusae). J. Ultrastruct. Res. 90, 89–104. ( 10.1016/0889-1605(85)90119-3) [DOI] [Google Scholar]
- 136.Thurm U, Lawonn P. 1990. The sensory properties of the cnidocil-apparatus as a basis for prey capture in Hydra attenuata. Verhandlung. Deutsch. Zool. Gesellschaft 83, 431–432. [Google Scholar]
- 137.Brinkmann M, Oliver D, Thurm U. 1996. Mechanoelectric transduction in nematocytes of a hydropolyp (Corynidae). J. Comp. Physiol. A 178, 125–138. ( 10.1007/BF00189597) [DOI] [Google Scholar]
- 138.Schüler A, Schmitz G, Reft A, Özbek S, Thurm U, Bornberg-Bauer E. 2015. The rise and fall of TRP-n, an ancient family of mechanogated ion channels, in Metazoa. Genome Biol. Evol. 7, 1713–1727. ( 10.1093/gbe/evv091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Horridge GA. 1965. Non-motile sensory cilia and neuromuscular junctions in a ctenophore independent effector organ. Proc. R. Soc. Lond. B 162, 333–350. ( 10.1098/rspb.1965.0042) [DOI] [Google Scholar]
- 140.Hernandez-Nicaise ML. 1974. Ultrastructural evidence for a sensory-motor neuron in Ctenophora. Tissue Cell 6, 43–47. ( 10.1016/0040-8166(74)90021-4) [DOI] [PubMed] [Google Scholar]
- 141.Krisch B. 1973. Über das Apikalorgan (Statocyste) der Ctenophore Pleurobrachia pileus. Z. Zellforsch. Mikrosk. Anat. 142, 241–262. ( 10.1007/BF00307035) [DOI] [PubMed] [Google Scholar]
- 142.Reisinger E. 1970. Zur Problematik der Evolution der Coelomaten. J. Zoolog. Syst. Evol. Res. 8, 81–109. ( 10.1111/j.1439-0469.1970.tb00867.x) [DOI] [Google Scholar]
- 143.Horridge GA, Boulton PS. 1967. Prey detection by Chaetognatha via a vibration sense. Proc. R. Soc. Lond. B 168, 413–419. ( 10.1098/rspb.1967.0072) [DOI] [Google Scholar]
- 144.Bone Q, Ryan KP. 1979. The Langerhans receptor of Oikopleura (Tunicata: Larvacea). J. Mar. Biol. Assoc. U.K. 59, 69 ( 10.1017/S002531540004618X) [DOI] [Google Scholar]
- 145.Knapp MF, Mill PJ. 1971. The fine structure of ciliated sensory cells in the epidermis of the earthworm Lumbricus terrestris. Tissue Cell 3, 623–636. ( 10.1016/S0040-8166(71)80009-5) [DOI] [PubMed] [Google Scholar]
- 146.Phillips CE, Friesen WO. 1982. Ultrastructure of the water-movement-sensitive sensilla in the medicinal leech. J. Neurobiol. 13, 473–486. ( 10.1002/neu.480130603) [DOI] [PubMed] [Google Scholar]
- 147.Purschke G, Hugenschütt M, Ohlmeyer L, Meyer H, Weihrauch D. 2017. Structural analysis of the branchiae and dorsal cirri in Eurythoe complanata (Annelida, Amphinomida). Zoomorphology 136, 1–18. ( 10.1007/s00435-016-0336-5) [DOI] [Google Scholar]
- 148.Schlawny A, Grünig C, Pfannenstiel HD. 1991. Sensory and secretory cells of Ophryotrocha puerilis (Polychaeta). Zoomorphology 110, 209–215. ( 10.1007/BF01633005) [DOI] [Google Scholar]
- 149.Windoffer R, Westheide W. 1988. The nervous system of the male Dinophilus gyrociliatus (Annelida: Polychaeta). I. Number, types and distribution pattern of sensory cells. Acta Zool. 69, 55–64. ( 10.1111/j.1463-6395.1988.tb00901.x) [DOI] [Google Scholar]
- 150.Friesen WO. 1981. Physiology of water motion detection in the medicinal leech. J. Exp. Biol. 92, 255–275. [DOI] [PubMed] [Google Scholar]
- 151.Holstein T, Tardent P. 1984. An ultrahigh-speed analysis of exocytosis: nematocyst discharge. Science 223, 830–833. ( 10.1126/science.6695186) [DOI] [PubMed] [Google Scholar]
- 152.Pantin CFA. 1942. The excitation of nematocysts. J. Exp. Biol. 19, 294–310. [Google Scholar]
- 153.Oliver D, Brinkmann M, Sieger T, Thurm U. 2008. Hydrozoan nematocytes send and receive synaptic signals induced by mechano-chemical stimuli. J. Exp. Biol. 211, 2876–2888. ( 10.1242/jeb.018515) [DOI] [PubMed] [Google Scholar]
- 154.Thurm U, Brinkmann M, Golz R, Holtmann M, Oliver D, Sieger T. 2004. Mechanoreception and synaptic transmission of hydrozoan nematocytes. Hydrobiologia 530–531, 97–105. ( 10.1007/s10750-004-2679-z) [DOI] [Google Scholar]
- 155.Cormier SM, Hessinger DA. 1980. Cnidocil apparatus: sensory receptor of Physalia nematocytes. J. Ultrastruct. Res. 72, 13–19. ( 10.1016/S0022-5320(80)90130-6) [DOI] [PubMed] [Google Scholar]
- 156.Colin SP, Costello JH, Hansson LJ, Titelman J, Dabiri JO. 2010. Stealth predation and the predatory success of the invasive ctenophore Mnemiopsis leidyi. Proc. Natl Acad. Sci. USA 107, 17 223–17 227. ( 10.1073/pnas.1003170107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Waggett R. 1999. Capture mechanisms used by the lobate ctenophore, Mnemiopsis leidyi, preying on the copepod Acartia tonsa. J. Plankton Res. 21, 2037–2052. ( 10.1093/plankt/21.11.2037) [DOI] [Google Scholar]
- 158.Colin SP, MacPherson R, Gemmell B, Costello JH, Sutherland K, Jaspers C. 2015. Elevating the predatory effect: sensory-scanning foraging strategy by the lobate ctenophore Mnemiopsis leidyi. Limnol. Oceanogr. Lett. 60, 100–109. ( 10.1002/lno.10007) [DOI] [Google Scholar]
- 159.Costello JH, Loftus R, Waggett R. 1999. Influence of prey detection on capture success for the ctenophore Mnemiopsis leidyi feeding upon adult Acartia tonsa and Oithona colcarva copepods. Mar. Ecol. Prog. Ser. 191, 207–216. ( 10.3354/meps191207) [DOI] [Google Scholar]
- 160.Newbury TK. 1972. Vibration perception by chaetognaths. Nature 236, 459–460. ( 10.1038/236459a0) [DOI] [Google Scholar]
- 161.Feigenbaum D, Reeve MR. 1977. Prey detection in the Chaetognatha: response to a vibrating probe and experimental determination of attack distance in large aquaria. Limnol. Oceanogr. Lett. 22, 1052–1058. ( 10.4319/lo.1977.22.6.1052) [DOI] [Google Scholar]
- 162.Feigenbaum DL. 1978. Hair-fan patterns in the Chaetognatha. Can. J. Zool. 56, 536–546. ( 10.1139/z78-077) [DOI] [Google Scholar]
- 163.Bone Q, Mackie GO. 1975. Skin impulses and locomotion in Oikopleura (Tunicata: Larvacea). Biol. Bull. 149, 267–286. ( 10.2307/1540527) [DOI] [PubMed] [Google Scholar]
- 164.Langerhans P. 1877. Zur anatomie der appendicularien. Monatsberichte der Koniglischen Preussichen Akademie Wissenschaften zu Berlin, 556–561. [Google Scholar]
- 165.Nauli SM, et al. 2003. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137. ( 10.1038/ng1076) [DOI] [PubMed] [Google Scholar]
- 166.Steigelman KA, et al. 2011. Polycystin-1 is required for stereocilia structure but not for mechanotransduction in inner ear hair cells. J. Neurosci. 31, 12 241–12 250. ( 10.1523/JNEUROSCI.6531-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barr MM, Sternberg PW. 1999. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401, 386–389. ( 10.1038/43913) [DOI] [PubMed] [Google Scholar]
- 168.Sternberg JR, et al. 3804. Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat. Commun. 9, 2018 ( 10.1038/s41467-018-06225-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Donaldson S, Mackie GO, Roberts A. 1980. Preliminary observations on escape swimming and giant neurons in Aglantha digitale (Hydromedusae: Trachylina). Can. J. Zool. 58, 549–552. ( 10.1139/z80-076) [DOI] [Google Scholar]
- 170.Roberts A, Mackie GO. 1980. The giant axon escape system of a hydrozoan medusa, Aglantha digitale. J. Exp. Biol. 84, 303–318. [DOI] [PubMed] [Google Scholar]
- 171.Jennings HS. 1900. Studies on reactions to stimuli in unicellular organisms. V. On the movements and motor reflexes of the Flagellata and Ciliata. Am. J. Physiol. 3, 229–260. ( 10.1152/ajplegacy.1900.3.6.229) [DOI] [Google Scholar]
- 172.Fujiu K, Nakayama Y, Yanagisawa A, Sokabe M, Yoshimura K. 2009. Chlamydomonas CAV2 encodes a voltage-dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 19, 133–139. ( 10.1016/j.cub.2008.11.068) [DOI] [PubMed] [Google Scholar]
- 173.Machemer H, Ogura A. 1979. Ionic conductances of membranes in ciliated and deciliated Paramecium. J. Physiol. 296, 49–60. ( 10.1113/jphysiol.1979.sp012990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yoshimura K. 1996. A novel type of mechanoreception by the flagella of Chlamydomonas. J. Exp. Biol. 199, 295–302. [DOI] [PubMed] [Google Scholar]
- 175.Fujiu K, Nakayama Y, Iida H, Sokabe M, Yoshimura K. 2011. Mechanoreception in motile flagella of Chlamydomonas. Nat. Cell Biol. 13, 630–632. ( 10.1038/ncb2214) [DOI] [PubMed] [Google Scholar]
- 176.Wan KY, Goldstein RE. 2018. Time irreversibility and criticality in the motility of a flagellate microorganism. Phys. Rev. Lett. 121, 058103 ( 10.1103/PhysRevLett.121.058103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fränkel G. 1925. Der statische sinn der medusen. Z. Vgl. Physiol. 2, 658–690. [Google Scholar]
- 178.Bozler E. 1926. Sinnes- und nervenphysiologische Untersuchungen an Scyphomedusen. Z. Vgl. Physiol. 4, 37–80. ( 10.1007/BF00341786) [DOI] [Google Scholar]
- 179.Singla CL. 1975. Statocysts of Hydromedusae. Cell Tissue Res. 158, 391–407. ( 10.1007/BF00223835) [DOI] [PubMed] [Google Scholar]
- 180.Mackie GO. 1980. Slow swimming and cyclical ‘fishing’ behavior in Aglantha digitale (Hydromedusae: Trachylina). Can. J. Fish. Aquat. Sci. 37, 1550–1556. ( 10.1139/f80-200) [DOI] [Google Scholar]
- 181.Albert DJ. 2011. What's on the mind of a jellyfish? A review of behavioural observations on Aurelia sp. jellyfish. Neurosci. Biobehav. Rev. 35, 474–482. ( 10.1016/j.neubiorev.2010.06.001) [DOI] [PubMed] [Google Scholar]
- 182.Horridge GA. 1969. Statocysts of medusae and evolution of stereocilia. Tissue Cell 1, 341–353. ( 10.1016/S0040-8166(69)80029-7) [DOI] [PubMed] [Google Scholar]
- 183.Hündgen M, Biela C. 1982. Fine structure of touch-plates in the scyphomedusan Aurelia aurita. J. Ultrastruct. Res. 80, 178–184. ( 10.1016/S0022-5320(82)90016-8) [DOI] [PubMed] [Google Scholar]
- 184.Tamm SL. 1982. Ctenophora. In Electrical conduction and behaviour in ‘simple’ invertebrates (ed. Shelton GAB.), pp. 266–358. Oxford, UK: Clarendon Press. [Google Scholar]
- 185.Tamm SL, Tamm S. 2002. Novel bridge of axon-like processes of epithelial cells in the aboral sense organ of ctenophores. J. Morphol. 254, 99–120. ( 10.1002/jmor.10019) [DOI] [PubMed] [Google Scholar]
- 186.Horridge GA. 1965. Relations between nerves and cilia in ctenophores. Am. Zool. 5, 357–375. ( 10.1093/icb/5.3.357) [DOI] [PubMed] [Google Scholar]
- 187.Barber VC, Dilly PN. 1969. Some aspects of the fine structure of the statocysts of the molluscs Pecten and Pterotrachea. Z. Zellforsch. Mikrosk. Anat. 94, 462–478. ( 10.1007/BF00936053) [DOI] [PubMed] [Google Scholar]
- 188.Cragg SM, Nott JA. 1977. The ultrastructure of the statocysts in the pediveliger larvae of Pecten maximus (L.) (Bivalvia). J. Exp. Mar. Biol. Ecol. 27, 23–36. ( 10.1016/0022-0981(77)90051-X) [DOI] [Google Scholar]
- 189.Stahlschmidt V, Wolff HG. 1972. The fine structure of the statocyst of the prosobranch mollusc Pomacea paludosa. Z. Zellforsch. Mikrosk. Anat. 133, 529–537. ( 10.1007/BF00307133) [DOI] [PubMed] [Google Scholar]
- 190.McKee AE, Wiederhold ML. 1974. Aplysia statocyst receptor cells: fine structure. Brain Res. 81, 310–313. ( 10.1016/0006-8993(74)90944-5) [DOI] [PubMed] [Google Scholar]
- 191.Coggeshall RE. 1969. A fine structural analysis of the statocyst in Aplysia californica. J. Morphol. 127, 113–131. ( 10.1002/jmor.1051270107) [DOI] [Google Scholar]
- 192.Geuze JJ. 1967. Observations on the function and the structure of the statocysts of Lymnaea stagnalis (L.). Neth. J. Zool. 18, 155–204. ( 10.1163/002829668X00090) [DOI] [Google Scholar]
- 193.Gao W, Wiederhold ML. 1997. The structure of the statocyst of the freshwater snail Biomphalaria glabrata (Pulmonata, Basommatophora). Hear. Res. 109, 109–124. ( 10.1016/S0378-5955(97)00058-0) [DOI] [PubMed] [Google Scholar]
- 194.Chia FS, Koss R, Bickell LR. 1981. Fine structural study of the statocysts in the veliger larva of the nudibranch, Rostanga pulchra. Cell Tissue Res. 214, 67–80. [DOI] [PubMed] [Google Scholar]
- 195.Kuzirian AM, Alkon DL, Harris LG. 1981. An infraciliary network in statocyst hair cells. J. Neurocytol. 10, 497–514. ( 10.1007/BF01262418) [DOI] [PubMed] [Google Scholar]
- 196.Barber VC. 1966. The fine structure of the statocyst of Octopus vulgaris. Z. Zellforsch. Mikrosk. Anat. 70, 91–107. ( 10.1007/BF00345067) [DOI] [Google Scholar]
- 197.Budelmann BU. 1979. Hair cell polarization in the gravity receptor systems of the statocysts of the cephalopods Sepia officinalis and Loligo vulgaris. Brain Res. 160, 261–270. ( 10.1016/0006-8993(79)90423-2) [DOI] [PubMed] [Google Scholar]
- 198.Alkon DL. 1975. Responses of hair cells to statocyst rotation. J. Gen. Physiol. 66, 507–530. ( 10.1085/jgp.66.4.507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wiederhold ML. 1974. Aplysia statocyst receptor cells: intracellular responses to physiological stimuli. Brain Res. 78, 490–494. ( 10.1016/0006-8993(74)90931-7) [DOI] [PubMed] [Google Scholar]
- 200.Gallin EK, Wiederhold ML. 1977. Response of Aplysia statocyst receptor cells to physiologic stimulation. J. Physiol. 266, 123–137. ( 10.1113/jphysiol.1977.sp011759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Williamson R. 1990. The responses of primary and secondary sensory hair cells in the squid statocyst to mechanical stimulation. J. Comp. Physiol. A 167, 655–664. [Google Scholar]
- 202.Preuss T, Budelmann BU. 1995. Proprioceptive hair cells on the neck of the squid Lolliguncula brevis: a sense organ in cephalopods for the control of head-to-body position. Phil. Trans. R. Soc. Lond. B 349, 153–178. ( 10.1098/rstb.1995.0101) [DOI] [PubMed] [Google Scholar]
- 203.Sundermann G. 1983. The fine structure of epidermal lines on arms and head of postembryonic Sepia officinalis and Loligo vulgaris (Mollusca, Cephalopoda). Cell Tissue Res. 232, 669–677. ( 10.1007/BF00216437) [DOI] [PubMed] [Google Scholar]
- 204.Torrence SA. 1986. Sensory endings of the ascidian static organ (Chordata, Ascidiacea). Zoomorphology 106, 61–66. ( 10.1007/BF00312108) [DOI] [Google Scholar]
- 205.Ryan K, Lu Z, Meinertzhagen IA. 2016. The CNS connectome of a tadpole larva of Ciona intestinalis (L.) highlights sidedness in the brain of a chordate sibling. Elife 5, e16962 ( 10.7554/eLife.16962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sorrentino M, Manni L, Lane NJ, Burighel P. 2001. Evolution of cerebral vesicles and their sensory organs in an ascidian larva. Acta Zool. 81, 243–258. ( 10.1046/j.1463-6395.2000.00054.x) [DOI] [Google Scholar]
- 207.Holmberg K. 1984. A transmission electron microscopic investigation of the sensory vesicle in the brain of Oikopleura dioica (Appendicularia). Zoomorphology 104, 298–303. ( 10.1007/BF00312011) [DOI] [Google Scholar]
- 208.Tsuda M, Sakurai D, Goda M. 2003. Direct evidence for the role of pigment cells in the brain of ascidian larvae by laser ablation. J. Exp. Biol. 206, 1409–1417. ( 10.1242/jeb.00235) [DOI] [PubMed] [Google Scholar]
- 209.Lacalli TC, Kelly SJ. 2001. The infundibular balance organ in amphioxus larvae and related aspects of cerebral vesicle organization. Acta Zool. 81, 37–47. ( 10.1046/j.1463-6395.2000.00036.x) [DOI] [Google Scholar]
- 210.Solé M, Lenoir M, Fortuño JM, Durfort M, van der Schaar M, André M. 2016. Evidence of cnidarians sensitivity to sound after exposure to low frequency underwater sources. Sci. Rep. 6, 37979 ( 10.1038/srep37979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tamm SL. 2014. Cilia and the life of ctenophores. Invertebr. Biol. 133, 1–46. [Google Scholar]
- 212.Lowe B. 1997. The role of Ca2+ in deflection-induced excitation of motile, mechanoresponsive balancer cilia in the ctenophore statocyst. J. Exp. Biol. 200, 1593–1606. [DOI] [PubMed] [Google Scholar]
- 213.Grossman Y, Alkon DL, Heldman E. 1979. A common origin of voltage noise and generator potentials in statocyst hair cells. J. Gen. Physiol. 73, 23–48. ( 10.1085/jgp.73.1.23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stommel EW, Stephens RE, Alkon DL. 1980. Motile statocyst cilia transmit rather than directly transduce mechanical stimuli. J. Cell Biol. 87, 652–662. ( 10.1083/jcb.87.3.652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Budelmann B-U. 1988. Morphological diversity of equilibrium receptor systems in aquatic invertebrates. In Sensory biology of aquatic animals (eds Atema J, Fay RR, Popper AN, Tavolga WN), pp. 757–782. New York, NY: Springer. [Google Scholar]
- 216.Alkon DL. 1974. Associative training of Hermissenda. J. Gen. Physiol. 64, 70–84. ( 10.1085/jgp.64.1.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alkon DL, Bak A. 1973. Hair cell generator potentials. J. Gen. Physiol. 61, 619–637. ( 10.1085/jgp.61.5.619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wood J, Von Baumgarten RJ. 1972. Activity recorded from the statocyst nerve of Pleurobranchaea californica during rotation and at different tilts. Comp. Biochem. Physiol. A Physiol. 43, 495–502. ( 10.1016/0300-9629(72)90236-8) [DOI] [PubMed] [Google Scholar]
- 219.Wolff HG. 1972. Multi-directional sensitivity of statocyst receptor cells of the opisthobranch gastropod Aplysia limacina. Mar. Behav. Physiol. 1, 361–373. ( 10.1080/10236247209386910) [DOI] [Google Scholar]
- 220.Wiederhold ML. 1976. Mechanosensory transduction in ‘sensory’ and ‘motile’ cilia. Annu. Rev. Biophys. Bioeng. 5, 39–62. ( 10.1146/annurev.bb.05.060176.000351) [DOI] [PubMed] [Google Scholar]
- 221.Wiederhold ML. 1978. Membrane voltage noise associated with ciliary beating in the Aplysia statocyst. Brain Res. 156, 369–374. ( 10.1016/0006-8993(78)90521-8) [DOI] [PubMed] [Google Scholar]
- 222.Gilula NB, Satir P. 1972. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 53, 494–509. ( 10.1083/jcb.53.2.494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.DeFelice LJ, Alkon DL. 1977. Voltage noise from hair cells during mechanical stimulation. Nature 269, 613–615. ( 10.1038/269613a0) [DOI] [PubMed] [Google Scholar]
- 224.Moran DT, Varela FJ, Rowley JC. 1977. Evidence for active role of cilia in sensory transduction. Proc. Natl Acad. Sci. USA 74, 793–797. ( 10.1073/pnas.74.2.793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Göpfert MC, Robert D. 2003. Motion generation by Drosophila mechanosensory neurons. Proc. Natl Acad. Sci. USA 100, 5514–5519. ( 10.1073/pnas.0737564100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Boycott BB. 1960. The functioning of the statocysts of Octopus vulgaris. Proc. R. Soc. Lond. B 152, 78–87. ( 10.1098/rspb.1960.0025) [DOI] [PubMed] [Google Scholar]
- 227.Mooney TA, Hanlon RT, Christensen-Dalsgaard J, Madsen PT, Ketten DR, Nachtigall PE. 2010. Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to low-frequency particle motion and not pressure. J. Exp. Biol. 213, 3748–3759. ( 10.1242/jeb.048348) [DOI] [PubMed] [Google Scholar]
- 228.Budelmann B-U, Wolff HG. 1973. Gravity response from angular acceleration receptors in Octopus vulgaris. J. Comp. Physiol. 85, 283–290. ( 10.1007/BF00694234) [DOI] [Google Scholar]
- 229.Dijkgraaf S. 1961. The statocyst of Octopus vulgaris as a rotation receptor. Pubblicaz. Staz. Zool. Napoli 32, 64–87. [Google Scholar]
- 230.Young JZ. 1960. The statocysts of Octopus vulgaris. Proc. R. Soc. Lond. B 152, 3–29. ( 10.1098/rspb.1960.0019) [DOI] [PubMed] [Google Scholar]
- 231.Budelmann BU. 2000. Kinociliary mechanoreceptors in the equilibrium receptor organs of cephalopods. In Cell and molecular biology of the ear (ed. Lim DJ.), pp. 3–17. Boston, MA: Springer. [Google Scholar]
- 232.Budelmann BU, Williamson R. 1994. Directional sensitivity of hair cell afferents in the Octopus statocyst. J. Exp. Biol. 187, 245–259. [DOI] [PubMed] [Google Scholar]
- 233.Maturana HR, Sperling S. 1963. Unidirectional response to angular acceleration recorded from the middle cristal nerve in the statocyst of Octopus vulgaris. Nature 197, 815–816. ( 10.1038/197815b0) [DOI] [Google Scholar]
- 234.Svane IB, Young CM. 1989. The ecology and behavior of ascidian larvae. Oceanogr. Mar. Biol. Ann. Rev. 27, 45–90. [Google Scholar]
- 235.Eakin RM, Kuda A. 1971. Ultrastructure of sensory receptors in ascidian tadpoles. Z. Zellforsch. Mikrosk. Anat. 112, 287–312. ( 10.1007/BF02584045) [DOI] [PubMed] [Google Scholar]
- 236.Dilly PN. 1962. Studies on the receptors in the cerebral vesicle of the ascidian tadpole, I. The otolith. J. Cell Sci. 3, 13–20. [Google Scholar]
- 237.Sakurai D, Goda M, Kohmura Y, Horie T, Iwamoto H, Ohtsuki H, Tsuda M. 2004. The role of pigment cells in the brain of ascidian larva. J. Comp. Neurol. 475, 70–82. ( 10.1002/cne.20142) [DOI] [PubMed] [Google Scholar]
- 238.Ohtsuki H. 1990. Inner structures of the cerebral vesicle in the ascidian larva Styela plicata: a SEM study. Zoolog. Sci. 7, 739–746. [Google Scholar]
- 239.Horie T, Kusakabe T, Tsuda M. 2008. Glutamatergic networks in the Ciona intestinalis larva. J. Comp. Neurol. 508, 249–263. ( 10.1002/cne.21678) [DOI] [PubMed] [Google Scholar]
- 240.Lacalli TC. 1996. Frontal eye circuitry, rostral sensory pathways and brain organization in amphioxus larvae: evidence from 3D reconstructions. Phil. Trans. R. Soc. Lond. B 351, 243–263. ( 10.1098/rstb.1996.0022) [DOI] [Google Scholar]
- 241.Stokes MD, Holland ND. 1995. Ciliary hovering in larval lancelets (=amphioxus). Biol. Bull. 188, 231–233. ( 10.2307/1542300) [DOI] [PubMed] [Google Scholar]
- 242.Pires A, Woollacott RM. 1983. A direct and active influence of gravity on the behavior of a marine invertebrate larva. Science 220, 731–733. ( 10.1126/science.220.4598.731) [DOI] [PubMed] [Google Scholar]
- 243.Sweatt AJ, Forward RB. 1985. Diel vertical migration and photoresponses of the chaetognath Sagitta hispida Conant. Biol. Bull. 168, 18–31. ( 10.2307/1541170) [DOI] [Google Scholar]
- 244.McCarthy DA, Forward RB Jr, Young CM. 2002. Ontogeny of phototaxis and geotaxis during larval development of the sabellariid polychaete Phragmatopoma lapidosa. Mar. Ecol. Prog. Ser. 241, 215–220. ( 10.3354/meps241215) [DOI] [Google Scholar]
- 245.Reed CG, Ninos JM, Woollacott RM. 1988. Bryozoan larvae as mosaics of multifunctional ciliary fields: ultrastructure of the sensory organs of Bugula solonifera (Cheilostomata: Cellularioidea). J. Morphol. 197, 127–145. ( 10.1002/jmor.1051970202) [DOI] [PubMed] [Google Scholar]
- 246.Chapman JW, Klaassen RHG, Drake VA, Fossette S, Hays GC, Metcalfe JD, Reynolds AM, Reynolds DR, Alerstam T. 2011. Animal orientation strategies for movement in flows. Curr. Biol. 21, R861–R870. ( 10.1016/j.cub.2011.08.014) [DOI] [PubMed] [Google Scholar]
- 247.Montgomery JC, Baker CF, Carton AG. 1997. The lateral line can mediate rheotaxis in fish. Nature 389, 960–963. ( 10.1038/40135) [DOI] [Google Scholar]
- 248.Field LH, Macmillan DL. 1973. An electrophysiological and behavioural study of sensory responses in Tritonia (Gastropoda, Nudibranchia). Mar. Behav. Physiol. 2, 171–185. ( 10.1080/10236247309386923) [DOI] [Google Scholar]
- 249.Murray JA, Willows AOD. 1996. Function of identified nerves in orientation to water flow in Tritonia diomedea. J. Comp. Physiol. A 178, 201–209. [DOI] [PubMed] [Google Scholar]
- 250.Murray JA, Hewes RS, Willows AO. 1992. Water-flow sensitive pedal neurons in Tritonia: role in rheotaxis. J. Comp. Physiol. A 171, 373–385. [DOI] [PubMed] [Google Scholar]
- 251.Wyeth RC, Willows AOD. 2006. Odours detected by rhinophores mediate orientation to flow in the nudibranch mollusc, Tritonia diomedea. J. Exp. Biol. 209, 1441–1453. ( 10.1242/jeb.02164) [DOI] [PubMed] [Google Scholar]
- 252.Moore A, Cobb JLS. 1986. Neurophysiological studies on the detection of mechanical stimuli by Ophiura ophiura (L.). J. Exp. Mar. Biol. Ecol. 104, 125–141. ( 10.1016/0022-0981(86)90100-0) [DOI] [Google Scholar]
- 253.Castilla JC, Crisp DJ. 1973. Responses of Asterias rubens to water currents and their modification by certain environmental factors. Neth. J. Sea Res. 7, 171–190. ( 10.1016/0077-7579(73)90043-4) [DOI] [Google Scholar]
- 254.Müller H-G. 1936. Untersuchungen über spezifische organe niederer sinne bei rhabdocoelen turbellarien. Z. Vgl. Physiol. 23, 253–292. ( 10.1007/BF00344199) [DOI] [Google Scholar]
- 255.McCullagh GB, Bishop CD, Wyeth RC. 2014. One rhinophore probably provides sufficient sensory input for odour-based navigation by the nudibranch mollusc Tritonia diomedea. J. Exp. Biol. 217, 4149–4158. ( 10.1242/jeb.111153) [DOI] [PubMed] [Google Scholar]
- 256.Chia FS, Koss R. 1982. Fine structure of the larval rhinophores of the nudibranch, Rostanga pulchra, with emphasis on the sensory receptor cells. Cell Tissue Res. 225, 235–248. ( 10.1007/BF00214678) [DOI] [PubMed] [Google Scholar]
- 257.Ehlers U, Ehlers B. 1977. Monociliary receptors in interstitial Proseriata and Neorhabdocoela (Turbellaria Neoophora). Zoomorphologie 86, 197–222. ( 10.1007/BF00993666) [DOI] [Google Scholar]
- 258.Wyeth RC. 2019. Olfactory navigation in aquatic gastropods. J. Exp. Biol. 222(Suppl. 1), jeb185843 ( 10.1242/jeb.185843) [DOI] [PubMed] [Google Scholar]
- 259.Bleckmann H, Budelmann BU, Bullock TH. 1991. Peripheral and central nervous responses evoked by small water movements in a cephalopod. J. Comp. Physiol. A 168, 247–257. ( 10.1007/BF00218417) [DOI] [PubMed] [Google Scholar]
- 260.York CA, Bartol IK, Krueger PS. 2016. Multiple sensory modalities used by squid in successful predator evasion throughout ontogeny. J. Exp. Biol. 219, 2870–2879. ( 10.1242/jeb.140780) [DOI] [PubMed] [Google Scholar]
- 261.York CA, Bartol IK. 2014. Lateral line analogue aids vision in successful predator evasion for the brief squid, Lolliguncula brevis. J. Exp. Biol. 217, 2437–2439. ( 10.1242/jeb.102871) [DOI] [PubMed] [Google Scholar]
- 262.Langenbruch P-F, Weissenfels N. 1987. Canal systems and choanocyte chambers in freshwater sponges (Porifera, Spongillidae). Zoomorphology 107, 11–16. ( 10.1007/BF00312124) [DOI] [Google Scholar]
- 263.Boury-Esnault N, De Vos L, Donadey C, Vacelet J. 1984. Comparative study of the choanosome of Porifera: 1. The Homoscleromorpha. J. Morphol. 180, 3–17. ( 10.1002/jmor.1051800103) [DOI] [PubMed] [Google Scholar]
- 264.Weissenfels N. 1980. Bau und Funktion des Süßwasserschwamms Ephydatia fluviatilis L. (Porifera). Zoomorphologie 95, 27–40. ( 10.1007/BF01342232) [DOI] [Google Scholar]
- 265.Metaxas A. 2001. Behaviour in flow: perspectives on the distribution and dispersion of meroplanktonic larvae in the water column. Can. J. Fish. Aquat. Sci. 58, 86–98. ( 10.1139/f00-159) [DOI] [Google Scholar]
- 266.Koehl MAR, Cooper T. 2015. Swimming in an unsteady world. Integr. Comp. Biol. 55, 683–697. ( 10.1093/icb/icv092) [DOI] [PubMed] [Google Scholar]
- 267.Koehl MAR, Hadfield MG. 2010. Hydrodynamics of larval settlement from a larva's point of view. Integr. Comp. Biol. 50, 539–551. ( 10.1093/icb/icq101) [DOI] [PubMed] [Google Scholar]
- 268.Levin LA. 2006. Recent progress in understanding larval dispersal: new directions and digressions. Integr. Comp. Biol. 46, 282–297. ( 10.1093/icb/icj024) [DOI] [PubMed] [Google Scholar]
- 269.Pepper RE, Jaffe JS, Variano E, Koehl MAR. 2015. Zooplankton in flowing water near benthic communities encounter rapidly fluctuating velocity gradients and accelerations. Mar. Biol. 162, 1939–1954. ( 10.1007/s00227-015-2713-x) [DOI] [Google Scholar]
- 270.Fuchs HL, Hunter EJ, Schmitt EL, Guazzo RA. 2013. Active downward propulsion by oyster larvae in turbulence. J. Exp. Biol. 216, 1458–1469. ( 10.1242/jeb.079855) [DOI] [PubMed] [Google Scholar]
- 271.Wheeler JD, Helfrich KR, Anderson EJ, Mullineaux LS. 2015. Isolating the hydrodynamic triggers of the dive response in eastern oyster larvae. Limnol. Oceanogr. Lett. 60, 1332–1343. ( 10.1002/lno.10098) [DOI] [Google Scholar]
- 272.Fuchs HL, Specht JA, Adams DK, Christman AJ. 2017. Turbulence induces metabolically costly behaviors and inhibits food capture in oyster larvae, causing net energy loss. J. Exp. Biol. 220, 3419–3431. ( 10.1242/jeb.161125) [DOI] [PubMed] [Google Scholar]
- 273.Hodin J, Ferner MC, Ng G, Lowe CJ, Gaylord B. 2015. Rethinking competence in marine life cycles: ontogenetic changes in the settlement response of sand dollar larvae exposed to turbulence. R. Soc. open sci. 2, 150114 ( 10.1098/rsos.150114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wheeler JD, Helfrich KR, Anderson EJ, McGann B, Staats P, Wargula AE, Wilt K, Mullineaux LS. 2013. Upward swimming of competent oyster larvae Crassostrea virginica persists in highly turbulent flow as detected by PIV flow subtraction. Mar. Ecol. Prog. Ser. 488, 171–185. ( 10.3354/meps10382) [DOI] [Google Scholar]
- 275.Fuchs HL, Mullineaux LS, Solow AR. 2004. Sinking behavior of gastropod larvae (Ilyanassa obsoleta) in turbulence. Limnol. Oceanogr. Lett. 49, 1937–1948. ( 10.4319/lo.2004.49.6.1937) [DOI] [Google Scholar]
- 276.Fuchs HL, Christman AJ, Gerbi GP, Hunter EJ, Diez FJ. 2015. Directional flow sensing by passively stable larvae. J. Exp. Biol. 218, 2782–2792. ( 10.1242/jeb.125096) [DOI] [PubMed] [Google Scholar]
- 277.Fuchs HL, Solow AR, Mullineaux LS. 2010. Larval responses to turbulence and temperature in a tidal inlet: habitat selection by dispersing gastropods? J. Mar. Res. 68, 153–188. ( 10.1357/002224010793079013) [DOI] [Google Scholar]
- 278.Finelli CM, Wethey DS. 2003. Behavior of oyster (Crassostrea virginica) larvae in flume boundary layer flows. Mar. Biol. 143, 703–711. ( 10.1007/s00227-003-1110-z) [DOI] [Google Scholar]
- 279.Fuchs HL, Gerbi GP, Hunter EJ, Christman AJ. 2018. Waves cue distinct behaviors and differentiate transport of congeneric snail larvae from sheltered versus wavy habitats. Proc. Natl Acad. Sci. USA 115, E7532–E7540. ( 10.1073/pnas.1804558115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Welch JM, Forward RB, Howd PA. 1999. Behavioral responses of blue crab Callinectes sapidus postlarvae to turbulence: implications for selective tidal stream transport. Mar. Ecol. Prog. Ser. 179, 135–143. ( 10.3354/meps179135) [DOI] [Google Scholar]
- 281.Fuchs HL, Gerbi GP. 2016. Seascape-level variation in turbulence- and wave-generated hydrodynamic signals experienced by plankton. Prog. Oceanogr. 141, 109–129. ( 10.1016/j.pocean.2015.12.010) [DOI] [Google Scholar]
- 282.Wheeler JD, Chan KY, Anderson EJ, Mullineaux LS. 2016. Ontogenetic changes in larval swimming and orientation of pre-competent sea urchin Arbacia punctulata in turbulence. J. Exp. Biol. 219, 1303–1310. ( 10.1242/jeb.129502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Blaxter JHS. 1978. Baroreception. In Sensory ecology (ed Ali MA.), pp. 375–409. Boston, MA: Springer. [Google Scholar]
- 284.Macdonald AG, Fraser PJ. 1999. The transduction of very small hydrostatic pressures. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 122, 13–36. ( 10.1016/S1095-6433(98)10173-3) [DOI] [PubMed] [Google Scholar]
- 285.Singarajah KV. 1991. Behaviour of Pleurobrachia pileus to changes in hydrostatic pressure and the possible location of baroreceptors. Mar. Behav. Physiol. 19, 45–59. ( 10.1080/10236249109378794) [DOI] [Google Scholar]
- 286.Knight-Jones EW, Qasim SZ. 1955. Responses of some marine plankton animals to changes in hydrostatic pressure. Nature 175, 941–942. ( 10.1038/175941a0) [DOI] [PubMed] [Google Scholar]
- 287.Marsden JR. 1994. Vertical movements and distribution of planktonic larvae of the serpulid polychaete Spirobranchus polycerus (Schmarda); effects of changes in hydrostatic pressure. J. Exp. Mar. Biol. Ecol. 176, 87–105. ( 10.1016/0022-0981(94)90199-6) [DOI] [Google Scholar]
- 288.Stake JL, Sammarco PW. 2003. Effects of pressure on swimming behavior in planula larvae of the coral Porites astreoides (Cnidaria, Scleractinia). J. Exp. Mar. Biol. Ecol. 288, 181–201. ( 10.1016/S0022-0981(03)00018-2) [DOI] [Google Scholar]
- 289.Rice AL. 1964. Observations on the effects of changes of hydrostatic pressure on the behaviour of some marine animals. J. Mar. Biol. Assoc. U.K. 44, 163–175. ( 10.1017/S0025315400024723) [DOI] [Google Scholar]
- 290.Scatà G, Jozet-Alves C, Thomasse C, Josef N, Shashar N. 2016. Spatial learning in the cuttlefish Sepia officinalis: preference for vertical over horizontal information. J. Exp. Biol. 219, 2928–2933. ( 10.1242/jeb.129080) [DOI] [PubMed] [Google Scholar]
- 291.Mann R, Wolf CC. 1983. Swimming behavior of larvae of the ocean quahog Arctica islandica in response to pressure and temperature. Mar. Ecol. Prog. Ser. 13, 211–218. ( 10.3354/meps013211) [DOI] [Google Scholar]
- 292.Wheeler DE, Epifanio CE. 1978. Behavioral response to hydrostatic pressure in larvae of two species of xanthid crabs. Mar. Biol. 46, 167–174. ( 10.1007/BF00391533) [DOI] [Google Scholar]
- 293.Forward RB, Wellins CA, Buswell CU. 1989. Behavioral responses of larvae of the crab Neopanope sayi to hydrostatic pressure. Mar. Ecol. Prog. Ser. 57, 267–277. ( 10.3354/meps057267) [DOI] [Google Scholar]
- 294.Forward RB, Wellins CA. 1989. Behavioral responses of a larval crustacean to hydrostatic pressure: Rhithropanopeus harrisii (Brachyura: Xanthidae). Mar. Biol. 101, 159–172. ( 10.1007/BF00391455) [DOI] [Google Scholar]
- 295.Genin A, Jaffe JS, Reef R, Richter C, Franks PJS. 2005. Swimming against the flow: a mechanism of zooplankton aggregation. Science 308, 860–862. ( 10.1126/science.1107834) [DOI] [PubMed] [Google Scholar]
- 296.Enright JT. 1965. Entrainment of a tidal rhythm. Science 147, 864–867. ( 10.1126/science.147.3660.864) [DOI] [PubMed] [Google Scholar]
- 297.O'Neill JS, Lee KD, Zhang L, Feeney K, Webster SG, Blades MJ, Kyriacou CP, Hastings MH, Wilcockson DC. 2015. Metabolic molecular markers of the tidal clock in the marine crustacean Eurydice pulchra. Curr. Biol. 25, R326–R327. ( 10.1016/j.cub.2015.02.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Schnytzer Y, Simon-Blecher N, Li J, Waldman Ben-Asher H, Salmon-Divon M, Achituv Y, Hughes ME, Levy O. 4917. Tidal and diel orchestration of behaviour and gene expression in an intertidal mollusc. Sci. Rep. 8, 2018 ( 10.1038/s41598-018-23167-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Holbrook RI, de Perera TB. 2011. Fish navigation in the vertical dimension: can fish use hydrostatic pressure to determine depth? Fish Fish. 12, 370–379. ( 10.1111/j.1467-2979.2010.00399.x) [DOI] [Google Scholar]
- 300.Fraser PJ, Shelmerdine RL. 2002. Dogfish hair cells sense hydrostatic pressure. Nature 415, 495–496. ( 10.1038/415495a) [DOI] [PubMed] [Google Scholar]
- 301.Fraser PJ, Macdonald AG. 1994. Crab hydrostatic pressure sensors. Nature 371, 383–384. ( 10.1038/371383b0) [DOI] [Google Scholar]
- 302.Bone Q. 1959. Observations upon the nervous systems of pelagic tunicates. J. Cell Sci. 3, 167–181. [Google Scholar]
- 303.Bone Q. 1960. A note on the innervation of the integument in amphioxus, and its bearing on the mechanism of cutaneous sensibility. J. Cell Sci. 3, 371–379. [Google Scholar]
- 304.Baatrup E. 1982. On the structure of the corpuscles of de Quatrefages (Branchiostoma lanceolatum (P)). Acta Zool. 63, 39–44. ( 10.1111/j.1463-6395.1982.tb00757.x) [DOI] [Google Scholar]
- 305.Walker G. 1974. The fine structure of the frontal filament complex of barnacle larvae (Crustacea: Cirripedia). Cell Tissue Res. 152, 449–465. ( 10.1007/BF00218931) [DOI] [PubMed] [Google Scholar]
- 306.Bell A. 2008. The pipe and the pinwheel: is pressure an effective stimulus for the 9+0 primary cilium? Cell Biol. Int. 32, 462–468. ( 10.1016/j.cellbi.2008.03.001) [DOI] [PubMed] [Google Scholar]
- 307.Cameron RA, Hinegardner RT. 1974. Initiation of metamorphosis in laboratory cultured sea urchins. Biol. Bull. 146, 335–342. ( 10.2307/1540409) [DOI] [PubMed] [Google Scholar]
- 308.Burke RD. 1980. Podial sensory receptors and the induction of metamorphosis in echinoids. J. Exp. Mar. Biol. Ecol. 47, 223–234. ( 10.1016/0022-0981(80)90040-4) [DOI] [Google Scholar]
- 309.Whalan S, Wahab MA, Sprungala S, Poole AJ, de Nys R. 2015. Larval settlement: the role of surface topography for sessile coral reef invertebrates. PLoS ONE 10, e0117675 ( 10.1371/journal.pone.0117675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Leys SP, Degnan BM. 2001. Cytological basis of photoresponsive behavior in a sponge larva. Biol. Bull. 201, 323–338. ( 10.2307/1543611) [DOI] [PubMed] [Google Scholar]
- 311.Mah JL, Leys SP. 2017. Think like a sponge: the genetic signal of sensory cells in sponges. Dev. Biol. 431, 93–100. ( 10.1016/j.ydbio.2017.06.012) [DOI] [PubMed] [Google Scholar]
- 312.Richards GS, Simionato E, Perron M, Adamska M, Vervoort M, Degnan BM. 2008. Sponge genes provide new insight into the evolutionary origin of the neurogenic circuit. Curr. Biol. 18, 1156–1161. ( 10.1016/j.cub.2008.06.074) [DOI] [PubMed] [Google Scholar]
- 313.Chia F-S, Koss R. 1979. Fine structural studies of the nervous system and the apical organ in the planula larva of the sea anemone Anthopleura elegantissima. J. Morphol. 160, 275–297. ( 10.1002/jmor.1051600303) [DOI] [PubMed] [Google Scholar]
- 314.Lyons KM. 1973. Collar cells in planula and adult tentacle ectoderm of the solitary coral Balanophyllia regia (Anthozoa Eupsammiidae). Z. Zellforsch. Mikrosk. Anat. 145, 57–74. ( 10.1007/BF00307189) [DOI] [PubMed] [Google Scholar]
- 315.Vandermeulen JH. 1975. Studies on reef corals. III. Fine structural changes of calicoblast cells in Pocillopora damicornis during settling and calcification. Mar. Biol. 31, 69–77. [Google Scholar]
- 316.Chia F-S, Crawford B. 1977. Comparative fine structural studies of planulae and primary polyps of identical age of the sea pen, Ptilosarcus gurneyl. J. Morphol. 151, 131–157. ( 10.1002/jmor.1051510108) [DOI] [PubMed] [Google Scholar]
- 317.Marlow HQ, Srivastava M, Matus DQ, Rokhsar D, Martindale MQ. 2009. Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 69, 235–254. ( 10.1002/dneu.20698) [DOI] [PubMed] [Google Scholar]
- 318.Chase AL, Dijkstra JA, Harris LG. 2016. The influence of substrate material on ascidian larval settlement. Mar. Pollut. Bull. 106, 35–42. ( 10.1016/j.marpolbul.2016.03.049) [DOI] [PubMed] [Google Scholar]
- 319.Flores AR, Faulkes Z. 2008. Texture preferences of ascidian tadpole larvae during settlement. Mar. Freshw. Behav. Physiol. 41, 155–159. ( 10.1080/10236240802360914) [DOI] [Google Scholar]
- 320.Millar RH. 1971. The biology of ascidians. In Advances in marine biology (eds Russell FS, Yonge M), vol. 9, pp. 1–100. London, UK: Elsevier. [Google Scholar]
- 321.Caicci F, Zaniolo G, Burighel P, Degasperi V, Gasparini F, Manni L. 2010. Differentiation of papillae and rostral sensory neurons in the larva of the ascidian Botryllus schlosseri (Tunicata). J. Comp. Neurol. 518, 547–566. ( 10.1002/cne.22222) [DOI] [PubMed] [Google Scholar]
- 322.Zeng F, Wunderer J, Salvenmoser W, Hess MW, Ladurner P, Rothbächer U. 2019. Papillae revisited and the nature of the adhesive secreting collocytes. Dev. Biol. 448, 183–198. ( 10.1016/j.ydbio.2018.11.012) [DOI] [PubMed] [Google Scholar]
- 323.Gianguzza M, Dolcemascolo G, Fascio U, De Bernardi F. 1999. Adhesive papillae of Ascidia malaca swimming larvae: investigations on their sensory function. Invertebr. Reprod. Dev. 35, 239–250. ( 10.1080/07924259.1999.9652390) [DOI] [Google Scholar]
- 324.Torrence SA. 1983. Ascidian larval nervous system: anatomy, ultrastructure and metamorphosis. PhD thesis, University of Washington.
- 325.Sotgia C, Fascio U, Melone G, De Bernardi F. 1998. Adhesive papillae of Phallusia mamillata larvae: morphology and innervation. Zoolog. Sci. 15, 363–370. ( 10.2108/zsj.15.363) [DOI] [PubMed] [Google Scholar]
- 326.Dolcemascolo G, Pennati R, De Bernardi F, Damiani F, Gianguzza M. 2009. Ultrastructural comparative analysis on the adhesive papillae of the swimming larvae of three ascidian species. Invertebrate Surviv. J. 6, S77–S86. [Google Scholar]
- 327.Pennati R, Groppelli S, De Bernardi F, Mastrototaro F, Zega G. 2009. Immunohistochemical analysis of adhesive papillae of Clavelina lepadiformis (Müller, 1776) and Clavelina phlegraea (Salfi, 1929) (Tunicata, Ascidiacea). Eur. J. Histochem. 53, e4 ( 10.4081/ejh.2009.e4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Pennati R, Zega G, Groppelli S, De Bernardi F. 2007. Immunohistochemical analysis of the adhesive papillae of Botrylloides leachi (Chordata, Tunicata, Ascidiacea): implications for their sensory function. Ital. J. Zool. 74, 325–329. ( 10.1080/11250000701562229) [DOI] [Google Scholar]
- 329.Johnson KB. 2017. Laboratory settlement of the eastern oyster Crassostrea virginica influenced by substratum concavity, orientation, and tertiary arrangement. J. Shellfish Res. 36, 315–324. ( 10.2983/035.036.0203) [DOI] [Google Scholar]
- 330.Vogel S. 1996. Life in moving fluids: the physical biology of flow, 2nd edn Princeton, NJ: Princeton University Press. [Google Scholar]
- 331.Butman CA, Grassle JP, Webb CM. 1988. Substrate choices made by marine larvae settling in still water and in a flume flow. Nature 333, 771–773. ( 10.1038/333771a0) [DOI] [Google Scholar]
- 332.Mullineaux LS, Butman CA. 1991. Initial contact, exploration and attachment of barnacle (Balanus amphitrite) cyprids settling in flow. Mar. Biol. 110, 93–103. ( 10.1007/BF01313096) [DOI] [Google Scholar]
- 333.Pawlik JR, Butman CA. 1993. Settlement of a marine tube worm as a function of current velocity: interacting effects of hydrodynamics and behavior. Limnol. Oceanogr. Lett. 38, 1730–1740. ( 10.4319/lo.1993.38.8.1730) [DOI] [Google Scholar]
- 334.Harii S, Kayanne H. 2002. Larval settlement of corals in flowing water using a racetrack flume. Mar. Technol. Soc. J. 36, 76–79. ( 10.4031/002533202787914188) [DOI] [Google Scholar]
- 335.Altieri AH. 2003. Settlement cues in the locally dispersing temperate cup coral Balanophyllia elegans. Biol. Bull. 204, 241–245. ( 10.2307/1543595) [DOI] [PubMed] [Google Scholar]
- 336.Pawlik JR, Butman CA, Starczak VR. 1991. Hydrodynamic facilitation of gregarious settlement of a reef-building tube worm. Science 251, 421–424. ( 10.1126/science.251.4992.421) [DOI] [PubMed] [Google Scholar]
- 337.Gaylord B, Hodin J, Ferner MC. 2013. Turbulent shear spurs settlement in larval sea urchins. Proc. Natl Acad. Sci. USA 110, 6901–6906. ( 10.1073/pnas.1220680110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ferner MC, Hodin J, Ng G, Gaylord B. 2019. Brief exposure to intense turbulence induces a sustained life-history shift in echinoids. J. Exp. Biol. 222, jeb187351 ( 10.1242/jeb.187351) [DOI] [PubMed] [Google Scholar]
- 339.Bonar DB. 1978. Ultrastructure of a cephalic sensory organ in larvae of the gastropod Phestilla sibogae (Aeolidacea, Nudibranchia). Tissue Cell 10, 153–165. ( 10.1016/0040-8166(78)90014-9) [DOI] [PubMed] [Google Scholar]
- 340.Cantell C-E, Franzén Å, Sensenbaugh T. 1982. Ultrastructure of multiciliated collar cells in the pilidium larva of Lineus bilineatus (Nemertini). Zoomorphology 101, 1–15. ( 10.1007/BF00312027) [DOI] [Google Scholar]
- 341.Nezlin LP, Yushin VV. 2004. Structure of the nervous system in the tornaria larva of Balanoglossus proterogonius (Hemichordata: Enteropneusta) and its phylogenetic implications. Zoomorphology 123, 1–13. ( 10.1007/s00435-003-0086-z) [DOI] [Google Scholar]
- 342.Jin Y, Yaguchi S, Shiba K, Yamada L, Yaguchi J, Shibata D, Sawada H, Inaba K. 2013. Glutathione transferase theta in apical ciliary tuft regulates mechanical reception and swimming behavior of sea urchin embryos. Cytoskeleton 70, 453–470. ( 10.1002/cm.21127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Calderón LAB. 2019. The function of mechanosensory systems in the startle behavior of planktonic larvae. PhD thesis, Universität Tübingen ( 10.15496/publikation-30979) [DOI]
- 344.Slota LA, Miranda EM, McClay DR. 2019. Spatial and temporal patterns of gene expression during neurogenesis in the sea urchin Lytechinus variegatus. Evodevo 10, 2 ( 10.1186/s13227-019-0115-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Marlow H, Tosches MA, Tomer R, Steinmetz PR, Lauri A, Larsson T, Arendt D. 2014. Larval body patterning and apical organs are conserved in animal evolution. BMC Biol. 12, 7 ( 10.1186/1741-7007-12-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sinigaglia C, Busengdal H, Lerner A, Oliveri P, Rentzsch F. 2015. Molecular characterization of the apical organ of the anthozoan Nematostella vectensis. Dev. Biol. 398, 120–133. ( 10.1016/j.ydbio.2014.11.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tuthill JC, Azim E. 2018. Proprioception. Curr. Biol. 28, R194–R203. ( 10.1016/j.cub.2018.01.064) [DOI] [PubMed] [Google Scholar]
- 348.Li W, Feng Z, Sternberg PW, Xu XZS. 2006. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440, 684–687. ( 10.1038/nature04538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Peteya DJ. 1973. A possible proprioceptor in Ceriantheopsis americanus (Cnidaria, Ceriantharia). Z. Zellforsch. Mikrosk. Anat. 144, 1–10. ( 10.1007/BF00306682) [DOI] [PubMed] [Google Scholar]
- 350.Golz R, Thurm U. 1991. Cytoskeletal modifications of the sensorimotor interneurons of Hydra vulgaris (Cnidaria, Hydrozoa), indicating a sensory function similar to chordotonal receptors of insects. Zoomorphology 111, 113–118. ( 10.1007/BF01632877) [DOI] [Google Scholar]
- 351.Westfall JA. 1973. Ultrastructural evidence for a granule-containing sensory-motor-interneuron in Hydra littoralis. J. Ultrastruct. Res. 42, 268–282. ( 10.1016/S0022-5320(73)90055-5) [DOI] [PubMed] [Google Scholar]
- 352.Kinnamon JC, Westfall JA. 1982. Types of neurons and synaptic connections at hypostome-tentacle junctions in Hydra. J. Morphol. 173, 119–128. ( 10.1002/jmor.1051730110) [DOI] [PubMed] [Google Scholar]
- 353.Hope WD, Gardiner SL. 1982. Fine structure of a proprioceptor in the body wall of the marine nematode Deontostoma californicum Steiner and Albin, 1933 (Enoplida: Leptosomatidae). Cell Tissue Res. 225, 1–10. ( 10.1007/BF00216213) [DOI] [PubMed] [Google Scholar]
- 354.Simmons SL, Satterlie RA. 2018. Tentacle musculature in the cubozoan jellyfish Carybdea marsupialis. Biol. Bull. 235, 91–101. ( 10.1086/699325) [DOI] [PubMed] [Google Scholar]
- 355.Horridge GA. 1963. Proprioceptors, bristle receptors, efferent sensory impulses, neurofibrils and number of axons in the parapodial nerve of the polychaete Harmothoe. Proc. R. Soc. Lond. B 157, 199–222. ( 10.1098/rspb.1963.0005) [DOI] [Google Scholar]
- 356.Evans CG, Cropper EC. 1998. Proprioceptive input to feeding motor programs in Aplysia. J. Neurosci. 18, 8016–8031. ( 10.1523/JNEUROSCI.18-19-08016.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Dorsett DA. 1976. The structure and function of proprioceptors in soft-bodied invertebrates. In Structure and function of proprioceptors in the invertebrates (ed. Mill PJ.), pp. 443–479. London, UK: Chapman and Hall. [Google Scholar]
- 358.Kennedy WR, Webster HF, Yoon KS. 1975. Human muscle spindles: fine structure of the primary sensory ending. J. Neurocytol. 4, 675–695. ( 10.1007/BF01181630) [DOI] [PubMed] [Google Scholar]
- 359.Marlétaz F, Peijnenburg KTCA, Goto T, Satoh N, Rokhsar DS. 2019. A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr. Biol. 29, 312–318.e3. ( 10.1016/j.cub.2018.11.042) [DOI] [PubMed] [Google Scholar]
- 360.Philippe H, et al. 2019. Mitigating anticipated effects of systematic errors supports sister-group relationship between Xenacoelomorpha and Ambulacraria. Curr. Biol. 29, 1818–1826.e6. ( 10.1016/j.cub.2019.04.009) [DOI] [PubMed] [Google Scholar]
- 361.Laumer CE, et al. 2019. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. R. Soc. B 286, 20190831 ( 10.1098/rspb.2019.0831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Momose T, Concordet J-P. 2016. Diving into marine genomics with CRISPR/cas9 systems. Mar. Genomics 30, 55–65. ( 10.1016/j.margen.2016.10.003) [DOI] [PubMed] [Google Scholar]
- 363.Doroquez DB, Berciu C, Anderson JR, Sengupta P, Nicastro D. 2014. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. Elife 3, e01948 ( 10.7554/eLife.01948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Barber VC. 1974. Cilia in sense organs. In Cilia and flagella (ed. Sleigh MA.), pp. 403–433. New York, NY: Academic Press Inc. [Google Scholar]
- 365.Bullock TH. 1997. Neuroethology of zooplankton. In Zooplankton: sensory ecology and physiology (eds Lenz P, Hartline DK, Purcell J, Macmillan D), pp. 1–16. Amsterdam, The Netherlands: CRC Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article does not contain any additional data.