Abstract
Motile cilia are miniature, whip-like organelles whose beating generates a directional fluid flow. The flow generated by ciliated epithelia is a subject of great interest, as defective ciliary motility results in severe human diseases called motile ciliopathies. Despite the abundance of motile cilia in diverse organs including the nervous system, their role in organ development and homeostasis remains poorly understood. Recently, much progress has been made regarding the identity of motile ciliated cells and the role of motile-cilia-mediated flow in the development and physiology of the nervous system. In this review, we will discuss these recent advances from sensory organs, specifically the nose and the ear, to the spinal cord and brain ventricles.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: cilia, Foxj1, cerebrospinal fluid, olfaction, nervous system, ependymal cell
1. Introduction
The motile cilium is an evolutionarily conserved organelle. Even unicellular organisms harbour multiple motile cilia (Paramecium and Tetrahymena) or a single pair of flagella (Chlamydomonas) that are structurally similar to vertebrate cilia [1]. The conserved ciliary core, known as the axoneme, consists of nine microtubule doublets that surround a central microtubule pair and is referred to as a 9 + 2 structure. Ciliary motility is driven by axonemal dyneins, which create sliding interactions between outer microtubules, while other motor proteins, intraflagellar transport proteins, carry cargo into and out of the cilium [1]. Ciliates can interact with their environment in complex ways [2]. Hence, it is not surprising that occasionally sensory components, such as insulin-like receptors [3], are found on the cilium together with other components of signal transduction cascades [4]. In metazoans, besides the motile cilium, another type of sensory cilium exists: the immotile primary cilium. This cilium lacks dynein arms, accounting for its immotility [5], and often the central microtubule pair [6]. Hence, they are referred to as 9 + 0 cilia. Primary cilia are major signalling hubs [7], exhibiting receptors for serotonin [8], Hedgehog [9] and various odours [10]. The sensory role, however, is not limited to primary cilia, as motile cilia express signalling components too, such as bitter taste-like receptors in respiratory cilia [11] or progesterone receptors in oviductal cilia [12]. Whether cilia harness dynein arms or not is largely determined by specific expression of the Foxj1 transcription factor. This transcription factor alone is sufficient to generate motile cilia [13–15] and is therefore regarded as a marker for motile ciliated cells. Cells, harbouring either a single, two or multiple motile cilia [16], exist in various parts of the nervous system where they generate specific flow patterns. We will here describe the identity and function of motile ciliated cells in the nose, the ear, the spinal cord and the brain primarily in animal models used in research and in humans.
2. Cilia in the nose
Chemosensation in vertebrates occurs in dedicated olfactory and gustatory organs. In the nose, bipolar olfactory sensory neurons (OSNs) [17] protrude several olfactory cilia from their dendritic knobs into the nasal cavity and are indispensable to the nasal epithelium across animal species. Indeed, mutations affecting ciliogenesis or intraflagellar transport of transduction components into the olfactory cilia [10,18] result in anosmia in humans, mice [19] and zebrafish [20]. In addition, the olfactory epithelium contains microvillous odorant receptor cells, glia-like support cells (sustentacular cells) and basal cells which replenish the OSNs. The anatomically separated respiratory epithelium consists of mucus-producing goblet cells and multiciliated support cells [21–24]. This arrangement is observed in all vertebrates and has been documented for humans [23], mice [24], clawed frogs [21] and zebrafish [22] (figures 1 and 2a). Nevertheless, vertebrate olfactory organs are highly variable. Fish exhibit an aquatic nose, mammals an airborne nose and amphibians a combination of the two [17]. Despite vast differences in their environment, odorant receptors are conserved between aquatic and terrestrial animals [17] and localize specifically to the cilia of the OSNs [10]. Olfactory cilia lack dynein arms in many species including humans [23], rodents [30] and zebrafish [22], and are therefore considered immotile. Yet, OSNs express the motile ciliary marker Foxj1 in mice [31,32] and olfactory placodes express foxj1b in zebrafish [33,34]. Strikingly, olfactory cilia were observed to be motile in frog [35] and trout [36]. The motile nature of those cilia, however, remains puzzling.
Figure 1.
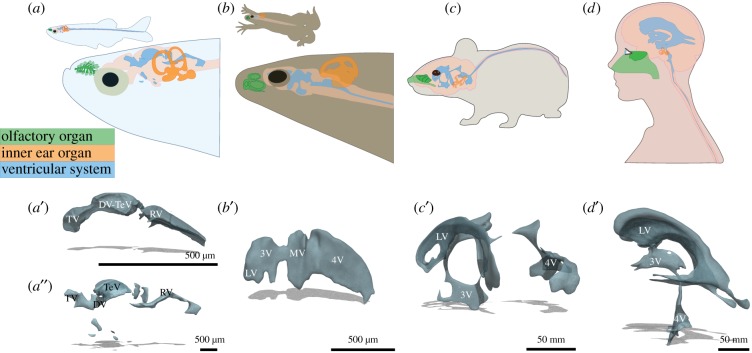
Schematic depiction of sensory and ventricular systems across vertebrates. (a–d) Olfactory organs (green), inner ears (orange), ventricular systems (blue) and central nervous systems (pink) in zebrafish (a), frog (b), mouse (c) and human (d) are shown. Sensory regions in olfactory organs are coloured dark green, while non-sensory regions are light green. Three-dimensional renderings of brain ventricular systems of (a′) a 2-day old zebrafish [25], (a″) a three-month-old zebrafish [26], (b′) a stage 45 Xenopus tropicalis [27], (c′) an average adult mouse [28] and (d′) an adult human [29] are shown. TV, telencephalic; DV, diencephalic ventricle; TeV, tectal ventricle; RV, rhombencephalic ventricle; LV, lateral ventricle; 3V, 3rd ventricle; MV, mesencephalic ventricle; 4V, 4th ventricle.
Figure 2.
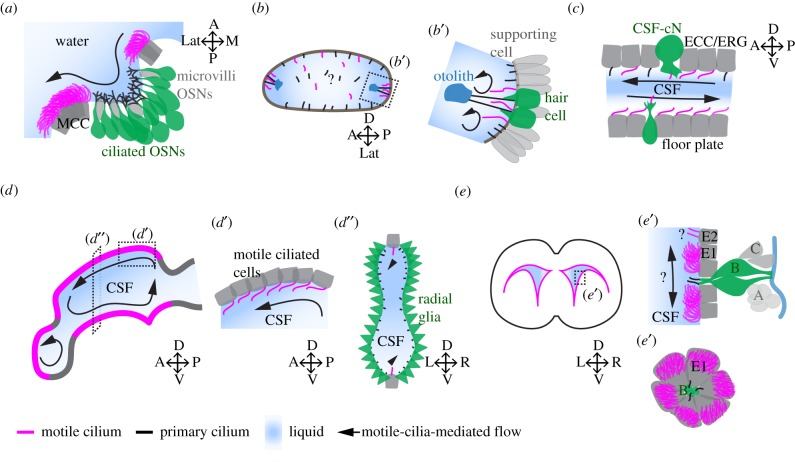
Schematic depiction of various cavities of the nervous system lined with motile cilia. (a) The olfactory organ of a zebrafish larva is composed of multiciliated cells (MCC) located at the outer rim of the nasal cavity. MCC bear multiple motile cilia (magenta), which generate a directional fluid flow of water. Ciliated OSNs (green), which bear multiple primary cilia (black) and microvilli OSN (grey) are located at the bottom of the nasal cavity. (b,b′) The otic vesicle of a zebrafish embryo at 18–24 hpf contains hair cells (green), or tether cells, that bear primary cilia capable of tethering the otolith (blue). Next to hair cells, there are motile cilia on supporting cells that generate a rotational flow near the otolith. (c) The central canal of the spinal cord is composed of cerebrospinal fluid-contacting neurons (CSF-cNs; green), which bear a microvilli tuft and a motile cilium in zebrafish. ECC (grey), also known as ERG in zebrafish, are located on the floor plate or the dorsal wall of the central canal and bear a cilium. Note that there are more motile cilia in the ventral part of the central canal than the dorsal plane at early developmental stage, and that the CSF flow is bidirectional. (d) The brain ventricular system of the zebrafish larva is decorated by motile cilia (magenta) at very specific locations along the midline. Motile-cilia-mediated flow is complex and compartmentalized to individual ventricles. (d′) Sagittal view of the inset in (d) showing that cells bear a single cilium oriented anteriorly in the same direction as fluid flow. (d″) Transverse view shows that motile cilia are located in the ventral and dorsal wall of the diencephalic-tectal ventricle. Elsewhere, radial glia (RG, green) project their primary cilium into the CSF-filled cavity. (e) ECs, which bear motile cilia, are located along the medial and lateral wall of the mouse lateral ventricle. (e′) Transverse section through the inset in (e) reveals that NSCs of the SVZ are located directly under the ependyma layer made of multiciliated E1 cells and bi-ciliated E2 cells. NSCs also known as B cells (green) project their primary cilium towards the CSF-filled ventricle in addition to contacting the blood vessel (blue), while transient amplifying cells (C cells, grey) and migrating neuroblasts (A cells, grey) lose their direct interaction with the CSF. En face representation shows the pinwheel structure composed of E1 and B cells. Note the translational polarity of the motile cilia of E1 cells. A, anterior; P, posterior; L, left; R, right; D, dorsal; V, ventral; M, medial; Lat, lateral. Motile cilia are in magenta, primary cilia are in black.
Nonetheless, multiciliated cells are found near the olfactory epithelium in many species (figure 2a). Their function, however, remains poorly understood. Motile cilia may remove pathogens entrapped in mucus away from the olfactory epithelium [37], in a process similar to the mucociliary clearance of the lung epithelium [38]. Olfactory cilia are the single direct entry point into the central nervous system from the outside [39] and are specifically targeted by pathogens [40]. Thus, mucociliary clearance [38] may complement other defence mechanisms including enzymatic activity targeting pathogens [41] and sneeze reflexes [42].
Additionally, motile-cilia-mediated flow may contribute to odour sampling in the nose. In the aquatic environment, odour molecules flow through the olfactory organ, either by diffusion or by active mechanisms including motile cilia [43–45]. Motile cilia not only attract odorants into the nasal cavity to aid odour detection, but simultaneously repel odours to enhance detection of rapidly succeeding odour plumes in the zebrafish nose pit (figure 2a) [44]. Cilia-mediated flow may also support the sequential enzymatic conversion and delivery of odorants, such as ATP, to the nose [45]. For mammals and other terrestrials, such as insects, volatile odours first need to reach OSNs through diffusion or active transport into the mucus or fluid surrounding OSNs [17]. In mammals, sniffing not only aids odorant transport to the olfactory epithelium, but also induces sniff-cycle-related temporal dynamics in the olfactory bulb that facilitate odour coding [46,47]. Such a process may be mediated by mechanosensitivity of olfactory cilia [48]. To further aid phase transition of odorants, both mammals and insects express odorant-binding proteins, which travel freely in the mucus and help capture volatile odours into the mucus layer close to the sensory cilia [41,49]. It remains unclear whether motile-cilia-mediated mucus flow in the nasal cavity of mammals contributes to the clearance of odours, and thereby plays similar roles as in fish.
3. Cilia in the inner ear
Whereas OSNs are key to olfaction, hair cells are essential to auditory and vestibular processing [50]. The hair cell bundle consists of multiple tapering stereocilia and sometimes a single kinocilium, the only true cilium. Both vestibular and cochlear kinocilia do not directly transduce sensory information (for a review of cilia in the development of the inner ear, see [51]). Even more so, hair cells in the cochlea of mammalians lose their cilium during the maturation process [50]. Instead, gated ion channels on stereocilia open upon deflection of the hair bundle and initiate a cellular response. Hair cells mediate stimulus detection of head rotation in the semicircular canals, linear acceleration in the otolithic organs, and sound in the otolithic organs of fish or amphibians, and in the cochlea in mammals [52].
In mammals, otolithic kinocilia lack inner dynein arms [53] and are thus considered immotile. Similarly, kinocilia in the zebrafish inner ear are considered to be immotile [51,54–57], despite an initial report stating otherwise [58]. Surprisingly, oscillating kinocilia have been observed in the otolith organ of eels [59]. Despite the immotility of the kinocilium, hair cells express the motile cilia marker foxj1b in zebrafish [33,54], foxj1.2 in the clawed frog otic vesicle, [60,61], and Foxj1 in mice in the cochlea prenatally [62], and in the utricle both pre- and postnatally [62,63]. Interestingly, motile cilia in auditory organs have been found elsewhere. For instance, the chordotonal sensory neurons of Drosophila bear cilia whose motility is shown to amplify environmental sounds [64], such that mutants lacking ciliary motility are deaf [65]. By contrast, mammalian hair cells do not rely on kinocilia to amplify environmental sounds. Instead, this role is attributed to an active piezo-element in the hair cell membrane [52]. Beating cilia have also been observed in the zebrafish otic vesicle during early development, specifically on cells neighbouring hair cells [54–58] (figure 2b). Besides clear evidence of ciliary motility, there is no consensus on the timely location [54–58], the ciliary beat frequency [55–58] or the consequences of ciliary immotility on otolith formation. Even though ablation of ciliary motility in zebrafish affects the otolithic number [54,58] and shape [54,55], these phenotypes disappear at later developmental stages [56,57]. It is possible that other mechanisms regulate the later development of the ear. Bodily movements may be involved in this process since restraining larval movements perturbs otolith development [56,66] and even aggravates the motile-cilia-mediated ear phenotype [57]. Even though the otolith phenotype disappears over time, the young zebrafish larva may depend on motile ciliated flow for its inner ear function at early age. Since otolith size affects auditory perception [67] and vestibular processing [68], defects in otolith formation may result in an imbalance that compromises larval zebrafish survival [68]. In fish, otoliths are directly tethered to kinocilia, while in amniotes, multiple hair cells are covered by an otoconia-covered membrane. Little is known, however, about the presence or absence of motile cilia in amniotes. Although otolith formation in fish may not directly translate to mammals, understanding cilia-mediated control, as well as the importance of Foxj1 expression, could provide important mechanistic insights into ear development.
4. Motile cilia in the spinal cord
Motile cilia are observed on several cell types in the spinal cord, including floor plate cells, ependymal cells (ECs) and cerebrospinal fluid-contacting neurons (CSF-cNs) (figure 2c). These cells are adjacent to the central canal, which elongates from the brain ventricles throughout the entire spinal cord and is filled with cerebrospinal fluid (CSF) [69,70].
(a). Identity of motile ciliated cells in the spinal cord
The floor plate is present during early development in all vertebrates and consists of cells populating the ventral midline of the neural tube. Through the secretion of Sonic hedgehog, floor plate cells play key roles in the patterning of the neural tube [71]. Work in various animal models including zebrafish [13], mice and chick [72], revealed that the floor plate cells harbour cilia and express typical markers of cilia motility including Foxj1. Moreover, ciliary motility was observed in zebrafish floor plate cells [73–75], but remains to be investigated in other species.
Ependymal cells of the central canal (ECCs), which commonly refer to the cells directly contacting the central canal [76], also harbour motile cilia (figure 2c). ECCs primarily originate from the ventral progenitor domains of the neural tube during spine development [77–79] and retain the ability to proliferate at postnatal stages. Most proliferation occurs either during spinal cord growth [80,81] or upon spinal cord injury [76,82,83]. ECCs have been observed in all analysed vertebrate central canals and possess fewer cilia than ECs of the brain. In fish and amphibians, ECCs have commonly been referred to as ependymo-radial glial cells (ERG) [84]. In fish, birds, amphibians and reptilians, ECCs harbour one and sometimes two cilia [83,85], while in mice, rat and guinea pig, ECCs are bi-ciliated [80,85,86], or occasionally bear up to three to four cilia in multinucleated cells [80,82]. In larger mammals, such as rabbits [85], macaques [81] and humans [81], two populations of ECCs with either 1–2 or 20–30 cilia coexist and are spatially organized; multiciliated cells are located laterally, while mono- and bi-ciliated cells are situated ventrally and dorsally [81]. This suggests that the number of cilia on ECCs correlates with the size of the spinal cord and central canal [85]. Interestingly, only bi-ciliated cells were shown to proliferate [81].
CSF-cNs, also known as Kolmer–Agdhur cells, are the third motile ciliated cell type in the spinal cord. They primarily constitute GABAergic and PKD2L1-positive neurons located at the interface between the nervous system and the CSF [69,87–90]. Two populations of CSF-cNs coexist: the dorsal CSF-cN′ and the ventral CSF-cN″, which emerge from different progenitor domains during early spinal cord development [69,87,91–94]. The morphology of CSF-cNs is peculiar. They display an apical dendritic extension directed towards the central canal, protruding a tuft of microvilli [69,90,95]. CSF-cNs possess a cilium in clawed frog [96,97], lamprey [88,98,99], zebrafish [100], chick [101] and turtle [102], and the motility of this cilium was confirmed in lamprey and zebrafish [88,99,100] (figure 2c). However, there is no consensus on whether a cilium exists on CSF-cNs in mammals [69]. Considering their particular morphology and resemblance to hair cells, CSF-cNs were suggested to be sensory neurons integrating mechanosensory and chemosensory cues from the CSF [69]. Recent evidence in zebrafish and lamprey confirmed that CSF-cNs are mechanosensory [74,99,100]. CSF-cNs respond to both the continuous CSF flow present in the central canal and bending of the tail through the specific expression of PKD2L1 [74,100], a channel previously implicated in flow sensation [103,104]. They also detect pH changes in the CSF through acid-sensing ion channels [99] and PKD2L1 [89] in mice and lamprey. CSF-cNs were shown to maintain spine morphology [74] and modulate locomotion [100] in zebrafish. Yet, the importance of motile cilia in CSF-cNs physiology remains poorly understood. Sternberg et al. [74] observed that the response of CSF-cNs to muscle contractions was reduced in the absence of ciliary motility, although PKD2L1 correctly localizes to the apical extensions of CSF-cNs. These results suggest that the motile cilium of CSF-cNs may contribute to the sensory function, but the precise mechanisms remain to be discovered.
(b). Functions of ciliary beating in the development and maintenance of the spine
In agreement with the observations of ciliary motility in the spinal cord, movement of CSF occurs along the central canal. This is well described in zebrafish from 24 h of development, when most of the motile cilia are located on the ventral part of the central canal and generate a bidirectional flow, moving caudally along the ventral wall and rostrally along the dorsal wall [74,75,105] (figure 2c).
Work in zebrafish has provided many insights regarding the function of motile cilia in spine development. Ciliary motility is essential for the straightening of the body axis at early developmental stages in zebrafish. N-ethyl-N-nitrosourea mutagenesis screens were the first to describe zebrafish mutants with a curly tail phenotype [106], which has since been ascribed to motile ciliary defects [107]. This phenotype is only recently being understood. First, ciliary motility and CSF flow are crucial to form the Reissner's fibre [75], which is an extracellular thread primarily composed of the glycoprotein SCO-spondin secreted by the floor plate and the subcommissural organ [108]. In turn, the Reissner's fibre is needed for the straightening of the body axis, in a process independent from CSF flow or cilia motility, which is poorly understood [75]. Second, motile-cilia-mediated transport of molecules from the brain to the spinal cord [73,74] controls spine development. Brain-derived adrenaline induces the release of the urotensin peptides URP1 and URP2 by spinal CSF-cNs, which act on the muscles of the developing embryos to straighten the body [109]. Surprisingly, no other animal model but the zebrafish shows such striking developmental defects upon loss of ciliary motility in the early neural tube [72].
Later in development, CSF flow and ciliary beating help maintain a straight body axis in zebrafish. The inhibition of motile cilia function at post-embryonic stages reveals a high incidence of scoliosis [26,110,111]. In addition, a zebrafish model of scoliosis carrying a mutation in the ptk7 gene shows defects in cilia and ciliary flow [110,112] even before the appearance of spinal curvature [113]. The mechanisms linking scoliosis, ciliary motility and CSF flow are still poorly understood. Neuroinflammation may be responsible, as proinflammatory signals are sufficient to induce scoliosis-like spinal curvature, and treatment with immunomodulating therapies reduces the severity of scoliosis [113]. Next, the abovementioned adrenaline–urotensin signalling may be involved in this phenotype, since urotensin receptor uts2ra mutant zebrafish develop scoliosis [109]. Interestingly, rescuing the expression of the scoliosis-associated gene ptk7 solely in the motile ciliated cells of the brain ventricles is sufficient to rescue the scoliosis phenotype [110,113]. This suggests that brain-released factors travel to the central canal and maintain the straight body axis, but the precise molecular mechanisms remain unknown. Most studies on the importance of cilia-mediated flow have been performed on zebrafish, and some studies support a conserved role for CSF flow in spine development in mammals. For instance, developmental scoliosis is observed in human conditions associated with perturbations of CSF flow, including neural tube closure, spinal canal cyst and Chiari malformation [114]. Nonetheless, stenosis of the human central canal has been observed in the healthy population after the age of 10 years, yet it remains a subject of debate and raises questions to the function of the central canal in adult human physiology [81,115,116].
5. Motile cilia in the brain ventricular system
Motile cilia are also found within the brain ventricular system, which is the conserved complex of CSF-filled cavities in the brain. Here, CSF is circulated throughout the brain ventricular system to nourish the brain, maintain brain homeostasis and support neurogenesis [117–121]. One major contributor to such CSF flow is the motile cilia of the ECs lining the ventricles [122,123].
(a). Development and cellular composition of the brain ventricular system
The embryonic brain vesicles, which later develop into the brain ventricular system, are remarkably conserved across vertebrates. Initially, the hollow neural tube bends to generate three fluid-filled cavities, one in the telencephalon, one in the diencephalon and one in the rhombencephalon, akin to the three ventricular cavities of the larval zebrafish (figure 1) [124,125]. As the brain further develops to its adult anatomy, the telencephalic ventricle transforms into two lateral ventricles in amphibians and mammals, but not in zebrafish, such that the mature ventricular system constitutes four cavities (figure 1) [26,124,125]. Furthermore, the telencephalic ventricle in teleost fish is located dorsally above the brain parenchyma, in contrast with the deeply embedded ventricles of other vertebrates [126]. This is likely due to the unique telencephalic morphogenesis of teleosts, wherein the tissue everts and folds outwards [126], contrasting the telencephalic evagination of other vertebrates.
Already in 1836, the neuroanatomist Purkinje described ciliary beating on cells along the sheep ventricles [127]. Since then, these cells, referred to as ECs, have been described in both fish [25,110,128–130], amphibians [131–133] and mammals [16,123]. Traditionally, the ECs of the brain are defined as Foxj1-positive, motile ciliated, cuboidal cells generating near-wall CSF flow [16,25,29,133–135]. In mammals, the multiciliated ependymal lining appears during late prenatal and early postnatal stages [136–140], even though ECs are already committed during embryonic development [137,140,141]. ECs derive from embryonic radial glial cells, which are neural stem cells (NSCs) generating neurons [142], glia [137,141,143], as well as the NSCs (termed B cells) of the adult neurogenic subventricular zone (SVZ) [140,141]. Furthermore, the ECs and adult NSCs share a subpopulation of radial glia as their common progenitors [140,141]. In contrast with ECs of the spinal cord that can proliferate postnatally, ECs in the mammalian brain are considered to be post-mitotic [135,141]. Yet, this is still debated, as some studies suggest ECs may dedifferentiate and proliferate [144–146]. Like mammals, both zebrafish and the clawed frog have a multiciliated ventricular lining in adult stages, despite the presence of motile monociliated cells in larvae [25,129,130,133,147] (figure 2d). Interestingly, although most of the adult ventricular lining of rodents consists of multiciliated cells, even in the mouse, mono- and bi-ciliated cells do exist [148,149] (figure 2e). Since these cells contact the ventricular lumen and extend long radial processes into the neuropil, they are thought to be tanycytes relaying chemical and mechanical information from the CSF to the underlying neurons [149].
In addition to the important role of ECs in circulating CSF, studies also suggest that ECs secrete molecules into the fluid [150], and thus relay signals from the neural tissue to the CSF. Nevertheless, the main contributors to the CSF contents in adult vertebrates are the choroid plexuses [151,152]. These structures, which exist in each ventricle in mammals, consist of specialized epithelial cells, transporting ions and water from blood capillaries to the ventricular lumen. Furthermore, the choroid plexus cells themselves produce and secrete many proteins into the CSF [153]. As such, the choroid plexuses make up a barrier between the blood and the CSF, tightly controlling the CSF content. Interestingly, the choroid plexus cells exhibit cilia, which are motile in zebrafish [154], but mostly immotile in mice [155]. The function of these cilia is not fully understood, yet in zebrafish they may contribute to CSF flow [154], while in mice they are suggested to serve a chemosensory function [155].
(b). Regulation of the cerebrospinal fluid flow
The flow of CSF within the ventricular system, which is contributed to by multiple factors in addition to cilia, is complex. Moreover, the properties of CSF flow vary with the proximity to the ventricular walls, following a principle known as boundary layers [29]. Therefore, the description of CSF flow is commonly separated into two major levels: the macrofluidic, bulk flow amid the ventricular cavities, and the microfluidic, near-wall flow contributed by the ECs [29]. In mammals, the bulk CSF flow emerges at the secretion sites (the choroid plexuses) and moves through the third and fourth ventricles into the subarachnoid space, wherein it escapes the brain ventricular system [156–158]. This overall bulk, unidirectional flow is suggested to arise from several sources, like the pressure gradient caused by CSF secretion and exchange of CSF for interstitial fluid across the ependymal lining [137]. Pressure changes may also be contributed by the cardiac [29,159] and respiratory cycles [159,160]. Interestingly, bodily movement temporally changes the direction of CSF flow in humans [161] and in the zebrafish brain ventricular system [25]. Since many physiological parameters impact the bulk flow, and are difficult to measure with high spatial and temporal resolution, most studies focus on the cilia-mediated flow along the ventricular walls. The contribution of motile cilia in CSF flow is clearly demonstrated in zebrafish [25,110], clawed frog [27,133], rodents (e.g. [123,136,138,162,163]), pigs [162] and humans [123]. Notably, the cilia-mediated, near-wall flow is complex, wherein local domains of cilia-generated currents may serve to target certain molecules to specific areas [25,162] (figure 2d). It is well documented that such cilia-mediated flow is crucial to maintain a properly functioning brain ventricular system, as zebrafish, clawed frog and mouse ciliary mutants display ventricular defects eventually causing hydrocephalus (e.g. [25–27,110,111,133,136,138,163]). Surprisingly, human patients with primary ciliary dyskinesia rarely develop hydrocephalus [164]. This observation poses the question as to whether the relative importance of near-wall cilia-mediated flow and bulk flow differs across species. The ventricular sizes may also influence the importance of bulk compared to near-wall flow, and advocates the continued use of several animal models to disentangle the CSF flow patterns. Such animal models will also be pivotal to understand how the CSF flow patterns are regulated. A few studies revealed that neural states may impact the ciliary beating of ECs. For instance, the CSF flow patterns change in the third ventricle of mice during the day versus the night [162]. Furthermore, neuropeptides like melanin-concentrating hormone may increase the ciliary beating frequency of the ECs [165]. Yet, the influence of such local changes in ciliary beating on the global CSF flow remains poorly understood.
(c). Functions of the cerebrospinal fluid flow
The differential nature of the bulk flow and the near-wall flow suggests these different levels of CSF flow may serve different means of relaying signalling and developmental cues. It is likely that the bulk mid-ventricular flow supports brain homeostasis and volume transmission, which is the long-range, intercellular communication [166,167]. Transfer of neuropeptides across brain ventricles was shown to not only promote basic physiological needs like hunger [168], but also increase neuronal excitability to enhance cortical alertness in response to acute stress [150].
Considering the apposition of the embryonic and adult neurogenic zone to the ventricles (figure 2d,e), it is highly probable that CSF flow supports the neurogenic capacity of NSCs through the specific delivery of chemical or mechanical cues. Interestingly, the CSF proteome is regionalized due to the differential transcriptome of the choroid plexuses in the various brain regions in mouse embryos [153] and also changes substantially from early to late embryonic stages [169] and during ageing [152]. These regional and temporal changes in the CSF composition have a direct impact on the cell fate and proliferation rate of the neurogenic tissue apposing the CSF [152,153,169]. Furthermore, these secreted signals can be rather specific. For instance, WNT5A, secreted by the hindbrain choroid plexuses, travels to distinct neural progenitors within the developing hindbrain to support cerebellar development [170]. Altogether, these findings indicate that CSF flow promotes regionalization of the CSF contents as proposed in larval zebrafish [25] (figure 2d). Other developmentally important CSF-borne signals, like Igf-2 [117], may be localized to specific areas by the near-wall flow patterns. Whether this supports the distinctive differentiation of brain regions remains to be investigated.
While the neurogenic capacity is largely retained in teleosts and amphibians throughout adulthood [84,147,171], mammalian neurogenesis is confined to two brain regions, namely the SVZ [172,173], located just beneath the ependyma of the lateral ventricle, and the subgranular zone of the hippocampal dentate gyrus [174]. In mice, NSCs located in the SVZ extend a primary, immotile cilium into the ventricles (figure 2e). This apical extension is surrounded by ECs to form the so-called pinwheels [148]. Since primary cilia display a multitude of mechano- and chemosensory receptors [6,7,175], cilia of the neural progenitors may integrate cues from the CSF flow and regulate neurogenesis. In the embryonic and early postnatal stages in mice, the primary cilia of radial glia express the mechanosensors PKD1 and PKD2 [176], which not only regulate the polarity of multiciliated ECs [176], but also promote the differentiation of radial glial cells to neurons [121]. The primary cilia of NSCs may play similar functions in adults. Indeed, ablation of primary cilia in a subpopulation of SVZ NSCs resulted in reduced neurogenesis [118]. Furthermore, a study in mice demonstrated that applying mechanical forces onto the ventricle-contacting, apical domain of adult NSCs in the SVZ promoted neuronal proliferation through the flow-sensing epithelial sodium channel [119] in a cilia-independent manner. However, the nature of the incoming signal to the NSCs remains poorly understood and may also be chemical. Studies have demonstrated that the binding of developmental signalling cues to receptors on the NSC primary cilia may promote proliferation [117] or maintain cellular quiescence [120]. While these studies suggest that CSF flow transports molecules to the SVZ in the lateral ventricle, CSF flow may also support a more fine-tuned distribution of molecules into gradients. For instance, Sawamoto et al. [177] showed that the distribution of the chemorepellent Slit2 may drive proper neuroblast migration from the SVZ towards the olfactory bulb.
6. Conclusion
Motile cilia serve a variety of functions within the nervous system. There is now clear evidence for the role of these organelles in circulating fluid, in sensory systems like the nose and ear, in the spinal cord and in the brain ventricles during organ development and homeostasis. It remains, however, less understood how motile cilia allow the nervous system to sample its environment, whether it is achieved through the establishment of chemical gradients, delivery of molecules to precise targets or mechanical forces. Moreover, it is remarkable that the nervous system may manipulate ciliary beating, yet the extent and impact of such regulation on the development and physiology of the brain remain to be studied. Investigations across systems, species and developmental stages will now be pivotal to further disentangle this mutual dependence of the nervous system on proper motile cilia functioning. Ultimately, such studies will be essential to understand and develop treatments for disorders associated with ciliary defects, like scoliosis and hydrocephalus.
Acknowledgements
We thank V. Kurcuoglu (interfacegroup.ch, University of Zurich), E. Deniz (Yale University) and R Gray (University of Texas, Austin), for sharing brain ventricular data, E. Yaksi and A. Utz for critical comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
All authors wrote and edited the manuscript. C.R. and N.J.Y. assembled the figures, and all authors provided feedback.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NTNU, Helse Midt-Norge (N.J.-Y.) and Boehringer Ingelheim Fonds (C.R.).
References
- 1.Mitchell DR. 2007. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv. Exp. Med. Biol. 607, 130–140. ( 10.1007/978-0-387-74021-8_11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston RR, Usherwood PNR. 1988. L-Glutamate-induced membrane hyperpolarization and behavioural responses in Paramecium tetraurelia. J. Comp. Physiol. A 164, 75–82. ( 10.1007/BF00612720) [DOI] [PubMed] [Google Scholar]
- 3.Christensen ST, Guerra CF, Awan A, Wheatley DN, Satir P. 2003. Insulin receptor-like proteins in Tetrahymena thermophila ciliary membranes. Curr. Biol. 13, R50–R52. ( 10.1016/S0960-9822(02)01425-2) [DOI] [PubMed] [Google Scholar]
- 4.Valentine MS, Rajendran A, Yano J, Weeraratne SD, Beisson J, Cohen J, Koll F, Van Houten J. 2012. Paramecium BBS genes are key to presence of channels in cilia. Cilia 1, 16 ( 10.1186/2046-2530-1-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satir P, Heuser T, Sale WS. 2014. A structural basis for how motile cilia beat. Bioscience 64, 1073–1083. ( 10.1093/biosci/biu180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fliegauf M, Benzing T, Omran H. 2007. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880–893. ( 10.1038/nrm2278) [DOI] [PubMed] [Google Scholar]
- 7.Nachury MV, Mick DU. 2019. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 20, 389–405. ( 10.1038/s41580-019-0116-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brailov I, Bancila M, Brisorgueil M-J, Miquel M-C, Hamon M, Vergé D. 2000. Localization of 5-HT6 receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 872, 271–275. ( 10.1016/S0006-8993(00)02519-1) [DOI] [PubMed] [Google Scholar]
- 9.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. 2005. Vertebrate smoothened functions at the primary cilium. Nature 437, 1018–1021. ( 10.1038/nature04117) [DOI] [PubMed] [Google Scholar]
- 10.Williams CL, McIntyre JC, Norris SR, Jenkins PM, Zhang L, Pei Q, Verhey K, Martens JR. 2014. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat. Commun. 5, 5813 ( 10.1038/ncomms6813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. 2009. Motile cilia of human airway epithelia are chemosensory. Science 325, 1131–1134. ( 10.1126/science.1173869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bylander A, Lind K, Goksor M, Billig H, Larsson DG. 2013. The classical progesterone receptor mediates the rapid reduction of fallopian tube ciliary beat frequency by progesterone. Reprod. Biol. Endocrinol. 11, 33 ( 10.1186/1477-7827-11-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X, Ng CP, Habacher H, Roy S. 2008. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat. Genet. 40, 1445–1453. ( 10.1038/ng.263) [DOI] [PubMed] [Google Scholar]
- 14.Stubbs JL, Oishi I, Izpisua Belmonte JC, Kintner C. 2008. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat. Genet. 40, 1454–1460. ( 10.1038/ng.267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choksi SP, Babu D, Lau D, Yu X, Roy S. 2014. Systematic discovery of novel ciliary genes through functional genomics in the zebrafish. Development 141, 3410–3419. ( 10.1242/dev.108209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spassky N, Meunier A. 2017. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423–436. ( 10.1038/nrm.2017.21) [DOI] [PubMed] [Google Scholar]
- 17.Ache BW, Young JM. 2005. Olfaction: diverse species, conserved principles. Neuron 48, 417–430. ( 10.1016/j.neuron.2005.10.022) [DOI] [PubMed] [Google Scholar]
- 18.Jenkins PM, McEwen DP, Martens JR. 2009. Olfactory cilia: linking sensory cilia function and human disease. Chem. Senses 34, 451–464. ( 10.1093/chemse/bjp020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulaga HM, et al. 2004. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat. Genet. 36, 994 ( 10.1038/ng1418) [DOI] [PubMed] [Google Scholar]
- 20.Bergboer JGM, Wyatt C, Austin-Tse C, Yaksi E, Drummond IA. 2018. Assaying sensory ciliopathies using calcium biosensor expression in zebrafish ciliated olfactory neurons. Cilia 7, 2 ( 10.1186/s13630-018-0056-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen A, Reiss JO, Gentry CL, Burd GD. 1998. Ultrastructure of the olfactory organ in the clawed frog, Xenopus laevis, during larval development and metamorphosis. J. Comp. Neurol. 398, 273–288. () [DOI] [PubMed] [Google Scholar]
- 22.Hansen A, Zeiske E. 1998. The peripheral olfactory organ of the zebrafish, Danio rerio: an ultrastructural study. Chem. Senses 23, 39–48. ( 10.1093/chemse/23.1.39) [DOI] [PubMed] [Google Scholar]
- 23.Jafek BW. 1983. Ultrastructure of human nasal mucosa. Laryngoscope 93, 1576–1599. ( 10.1288/00005537-198312000-00011) [DOI] [PubMed] [Google Scholar]
- 24.Frisch D. 1967. Ultrastructure of mouse olfactory mucosa. Am. J. Anat. 121, 87–119. ( 10.1002/aja.1001210107) [DOI] [PubMed] [Google Scholar]
- 25.Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, Yaksi E, Jurisch-Yaksi N. 2019. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr. Biol. 29, 229–241. ( 10.1016/j.cub.2018.11.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konjikusic MJ, et al. 2018. Mutations in Kinesin family member 6 reveal specific role in ependymal cell ciliogenesis and human neurological development. PLoS Genet. 14, e1007817 ( 10.1371/journal.pgen.1007817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Date P, Ackermann P, Furey C, Fink IB, Jonas S, Khokha MK, Kahle KT, Deniz E. 2019. Visualizing flow in an intact CSF network using optical coherence tomography: implications for human congenital hydrocephalus. Sci. Rep. 9, 6196 ( 10.1038/s41598-019-42549-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen Mouse Common Coordinate Framework. 2017. (Allen Institute for Brain Science), 7 April 2019 See http://download.alleninstitute.org/informatics-archive/current-release/mouse_ccf/annotation/ccf_2017/structure_meshes/73.obj.
- 29.Siyahhan B, Knobloch V, de Zélicourt D, Asgari M, Schmid Daners M, Poulikakos D, Kurtcuoglu V. 2014. Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J. R. Soc. Interface 11, 20131189 ( 10.1098/rsif.2013.1189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menco BPM. 1984. Ciliated and microvillous structures of rat olfactory and nasal respiratory epithelia. Cell Tissue Res. 235, 225–241. [DOI] [PubMed] [Google Scholar]
- 31.Sammeta N, Yu TT, Bose SC, McClintock TS. 2007. Mouse olfactory sensory neurons express 10 000 genes. J. Comp. Neurol. 502, 1138–1156. ( 10.1002/cne.21365) [DOI] [PubMed] [Google Scholar]
- 32.Larson ED, Pathak S, Ramakrishnan VR, Finger TE. 2019. A subset of olfactory sensory neurons express Forkhead Box J1-driven eGFP. Chem. Senses 44, 663–671. ( 10.1093/chemse/bjz060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian T, Zhao L, Zhao X, Zhang M, Meng A. 2009. A zebrafish gene trap line expresses GFP recapturing expression pattern of foxj1b. J. Genet. Genom. 36, 581–589. ( 10.1016/S1673-8527(08)60150-2) [DOI] [PubMed] [Google Scholar]
- 34.Aamar E, Dawid IB. 2008. Isolation and expression analysis of foxj1 and foxj1.2 in zebrafish embryos. Int. J. Dev. Biol. 52, 985–991. ( 10.1387/ijdb.072477ea) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reese T. 1965. Olfactory cilia in the frog. J. Cell Biol. 25, 209–230. ( 10.1083/jcb.25.2.209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhein LD, Cagan RH, Orkand PM, Dolack MK. 1981. Surface specializations of the olfactory epithelium of rainbow trout, Salmo gairdneri. Tissue Cell 13, 577–587. ( 10.1016/0040-8166(81)90028-8) [DOI] [PubMed] [Google Scholar]
- 37.Shang Y, Inthavong K, Tu J. 2019. Development of a computational fluid dynamics model for mucociliary clearance in the nasal cavity. J. Biomech. 85, 74–83. ( 10.1016/j.jbiomech.2019.01.015) [DOI] [PubMed] [Google Scholar]
- 38.Bustamante-Marin XM, Ostrowski LE. 2017. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 9, a028241 ( 10.1101/cshperspect.a028241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doty RL. 2008. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann. Neurol. 63, 7–15. ( 10.1002/ana.21327) [DOI] [PubMed] [Google Scholar]
- 40.Milho R, Frederico B, Efstathiou S, Stevenson PG. 2012. A heparan-dependent herpesvirus targets the olfactory neuroepithelium for host entry. PLoS Pathog. 8, e1002986 ( 10.1371/journal.ppat.1002986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heydel JM, et al. 2013. Odorant-binding proteins and xenobiotic metabolizing enzymes: implications in olfactory perireceptor events. Anat. Rec. 296, 1333–1345. ( 10.1002/ar.22735) [DOI] [PubMed] [Google Scholar]
- 42.Baraniuk JN, Kim D. 2007. Nasonasal reflexes, the nasal cycle, and sneeze. Curr. Allergy Asthma Rep. 7, 105–111. ( 10.1007/s11882-007-0007-1) [DOI] [PubMed] [Google Scholar]
- 43.Cox JPL. 2008. Hydrodynamic aspects of fish olfaction. J. R. Soc. Interface 5, 575–593. ( 10.1098/rsif.2007.1281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiten I, et al. 2017. Motile-cilia-mediated flow improves sensitivity and temporal resolution of olfactory computations. Curr. Biol. 27, 166–174. ( 10.1016/j.cub.2016.11.036) [DOI] [PubMed] [Google Scholar]
- 45.Wakisaka N, Miyasaka N, Koide T, Masuda M, Hiraki-Kajiyama T, Yoshihara Y. 2017. An adenosine receptor for olfaction in fish. Curr. Biol. 27, 1437–1447. ( 10.1016/j.cub.2017.04.014) [DOI] [PubMed] [Google Scholar]
- 46.Spors H, Wachowiak M, Cohen LB, Friedrich RW. 2006. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J. Neurosci. 26, 1247–1259. ( 10.1523/JNEUROSCI.3100-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shusterman R, Smear MC, Koulakov AA, Rinberg D. 2011. Precise olfactory responses tile the sniff cycle. Nat. Neurosci. 14, 1039 ( 10.1038/nn.2877) [DOI] [PubMed] [Google Scholar]
- 48.Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. 2007. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat. Neurosci. 10, 348–354. ( 10.1038/nn1856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briand L, Eloit C, Nespoulous C, Bézirard V, Huet J-C, Henry C, Blon F, Trotier D, Pernollet J-C. 2002. Evidence of an odorant-binding protein in the human olfactory mucus: location, structural characterization, and odorant-binding properties. Biochemistry 41, 7241–7252. ( 10.1021/bi015916c) [DOI] [PubMed] [Google Scholar]
- 50.Hudspeth AJ. 1989. How the ear's works work. Nature 341, 397–404. ( 10.1038/341397a0) [DOI] [PubMed] [Google Scholar]
- 51.Whitfield TT. 2019. Cilia in the developing zebrafish ear. Phil. Trans. R. Soc. B 375, 20190163 ( 10.1098/rstb.2019.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudspeth AJ. 2014. Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci. 15, 600–614. ( 10.1038/nrn3786) [DOI] [PubMed] [Google Scholar]
- 53.Kikuchi T, Takasaka T, Tonosaki A, Watanabe H. 1989. Fine structure of guinea pig vestibular kinocilium. Acta Otolaryngol. 108, 26–30. ( 10.3109/00016488909107388) [DOI] [PubMed] [Google Scholar]
- 54.Yu X, Lau D, Ng CP, Roy S. 2011. Cilia-driven fluid flow as an epigenetic cue for otolith biomineralization on sensory hair cells of the inner ear. Development 138, 487–494. ( 10.1242/dev.057752) [DOI] [PubMed] [Google Scholar]
- 55.Wu D, Freund JB, Fraser SE, Vermot J. 2011. Mechanistic basis of otolith formation during teleost inner ear development. Dev. Cell 20, 271–278. ( 10.1016/j.devcel.2010.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stooke-Vaughan GA, Huang P, Hammond KL, Schier AF, Whitfield TT. 2012. The role of hair cells, cilia and ciliary motility in otolith formation in the zebrafish otic vesicle. Development 139, 1777–1787. ( 10.1242/dev.079947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han X, Xie H, Wang Y, Zhao C. 2018. Radial spoke proteins regulate otolith formation during early zebrafish development. FASEB J. 32, 3984–3992. ( 10.1096/fj.201701359R) [DOI] [PubMed] [Google Scholar]
- 58.Colantonio JR, Vermot J, Wu D, Langenbacher AD, Fraser S, Chen J-N, Hill KL. 2009. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature 457, 205– 209 ( 10.1038/nature07520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rüsch A, Thurm U. 1990. Spontaneous and electrically induced movements of ampullary kinocilia and stereovilli. Hear. Res. 48, 247–263. ( 10.1016/0378-5955(90)90065-W) [DOI] [PubMed] [Google Scholar]
- 60.Choi VM, Harland RM, Khokha MK. 2006. Developmental expression of FoxJ1.2, FoxJ2, and FoxQ1 in Xenopus tropicalis. Gene Expr. Patterns 6, 443–447. ( 10.1016/j.modgep.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 61.Chung HA, Medina-Ruiz S, Harland RM. 2014. Sp8 regulates inner ear development. Proc. Natl Acad. Sci. USA 111, 6329–6334. ( 10.1073/pnas.1319301111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elkon R, et al. 2015. RFX transcription factors are essential for hearing in mice. Nat. Commun. 6, 8549 ( 10.1038/ncomms9549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheffer DI, Shen J, Corey DP, Chen Z-Y. 2015. Gene expression by mouse inner ear hair cells during development. J. Neurosci. 35, 6366–6380. ( 10.1523/JNEUROSCI.5126-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Göpfert M, Humphris A, Albert J, Robert D, Hendrich O. 2005. Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc. Natl Acad. Sci. USA 102, 325–330. ( 10.1073/pnas.0405741102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore DJ, et al. 2013. Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am. J. Hum. Genet. 93, 346–356. ( 10.1016/j.ajhg.2013.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. 1997. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev. Biol. 191, 191–201. ( 10.1006/dbio.1997.8736) [DOI] [PubMed] [Google Scholar]
- 67.Inoue M, Tanimoto M, Oda Y. 2013. The role of ear stone size in hair cell acoustic sensory transduction. Sci. Rep. 3, 2114 ( 10.1038/srep02114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riley BB, Moorman SJ. 2000. Development of utricular otoliths, but not saccular otoliths, is necessary for vestibular function and survival in zebrafish. J. Neurobiol. 43, 329–337. () [DOI] [PubMed] [Google Scholar]
- 69.Djenoune L, Wyart C. 2017. Light on a sensory interface linking the cerebrospinal fluid to motor circuits in vertebrates. J. Neurogenet. 31, 113–127. ( 10.1080/01677063.2017.1359833) [DOI] [PubMed] [Google Scholar]
- 70.Orts-Del'Immagine A, Wyart C. 2017. Cerebrospinal-fluid-contacting neurons. Curr. Biol. 27, R1198–R1200. ( 10.1016/j.cub.2017.09.017) [DOI] [PubMed] [Google Scholar]
- 71.Placzek M, Briscoe J. 2005. The floor plate: multiple cells, multiple signals. Nat. Rev. Neurosci. 6, 230–240. ( 10.1038/nrn1628) [DOI] [PubMed] [Google Scholar]
- 72.Cruz C, et al. 2010. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development 137, 4271–4282. ( 10.1242/dev.051714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. 2005. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 132, 1907–1921. ( 10.1242/dev.01772) [DOI] [PubMed] [Google Scholar]
- 74.Sternberg JR, et al. 2018. Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat. Commun. 9, 3804 ( 10.1038/s41467-018-06225-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cantaut-Belarif Y, Sternberg JR, Thouvenin O, Wyart C, Bardet PL. 2018. The Reissner fiber in the cerebrospinal fluid controls morphogenesis of the body axis. Curr. Biol. 28, 2479–2486. ( 10.1016/j.cub.2018.05.079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Becker CG, Becker T, Hugnot JP. 2018. The spinal ependymal zone as a source of endogenous repair cells across vertebrates. Prog. Neurobiol. 170, 67–80. ( 10.1016/j.pneurobio.2018.04.002) [DOI] [PubMed] [Google Scholar]
- 77.Briscoe J, Ericson J. 2001. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 11, 43–49. ( 10.1016/S0959-4388(00)00172-0) [DOI] [PubMed] [Google Scholar]
- 78.Masahira N, et al. 2006. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev. Biol. 293, 358–369. ( 10.1016/j.ydbio.2006.02.029) [DOI] [PubMed] [Google Scholar]
- 79.Fu H, Qi Y, Tan M, Cai J, Hu X, Liu Z, Jensen J, Qiu M. 2003. Molecular mapping of the origin of postnatal spinal cord ependymal cells: evidence that adult ependymal cells are derived from Nkx6.1+ ventral neural progenitor cells. J. Comp. Neurol. 456, 237–244. ( 10.1002/cne.10481) [DOI] [PubMed] [Google Scholar]
- 80.Alfaro-Cervello C, Soriano-Navarro M, Mirzadeh Z, Alvarez-Buylla A, Garcia-Verdugo JM. 2012. Biciliated ependymal cell proliferation contributes to spinal cord growth. J. Comp. Neurol. 520, 3528–3552. ( 10.1002/cne.23104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alfaro-Cervello C, et al. 2014. The adult macaque spinal cord central canal zone contains proliferative cells and closely resembles the human. J. Comp. Neurol. 522, 1800–1817. ( 10.1002/cne.23501) [DOI] [PubMed] [Google Scholar]
- 82.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. 2008. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 6, e182 ( 10.1371/journal.pbio.0060182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribeiro A, Monteiro JF, Certal AC, Cristovao AM, Saude L. 2017. Foxj1a is expressed in ependymal precursors, controls central canal position and is activated in new ependymal cells during regeneration in zebrafish. Open Biol. 7, 170139 ( 10.1098/rsob.170139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker CG, Becker T. 2015. Neuronal regeneration from ependymo-radial glial cells: cook, little pot, cook! Dev. Cell 32, 516–527. ( 10.1016/j.devcel.2015.01.001) [DOI] [PubMed] [Google Scholar]
- 85.Nakayama Y, Kohno K. 1974. Number and polarity of the ependymal cilia in the central canal of some vertebrates. J. Neurocytol. 3, 449–458. ( 10.1007/BF01098732) [DOI] [PubMed] [Google Scholar]
- 86.Kohno K. 1969. Electron microscopic studies on Reissner's fiber and the ependymal cells in the spinal cord of the rat. Z. Zellforsch. Mikrosk. Anat. 94, 565–573. ( 10.1007/BF00936062) [DOI] [PubMed] [Google Scholar]
- 87.Djenoune L, et al. 2014. Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front. Neuroanat. 8, 26 ( 10.3389/fnana.2014.00026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jalalvand E, Robertson B, Wallen P, Hill RH, Grillner S. 2014. Laterally projecting cerebrospinal fluid-contacting cells in the lamprey spinal cord are of two distinct types. J. Comp. Neurol. 522, 1753–1768. ( 10.1002/cne.23542) [DOI] [PubMed] [Google Scholar]
- 89.Orts-Del'Immagine A, Seddik R, Tell F, Airault C, Er-Raoui G, Najimi M, Trouslard J, Wanaverbecq N. 2016. A single polycystic kidney disease 2-like 1 channel opening acts as a spike generator in cerebrospinal fluid-contacting neurons of adult mouse brainstem. Neuropharmacology 101, 549–565. ( 10.1016/j.neuropharm.2015.07.030) [DOI] [PubMed] [Google Scholar]
- 90.Vigh B, Vigh-Teichmann I. 1998. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc. Res. Tech. 41, 57–83. () [DOI] [PubMed] [Google Scholar]
- 91.Djenoune L, et al. 2017. The dual developmental origin of spinal cerebrospinal fluid-contacting neurons gives rise to distinct functional subtypes. Sci. Rep. 7, 719 ( 10.1038/s41598-017-00350-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petracca YL, Sartoretti MM, Di Bella DJ, Marin-Burgin A, Carcagno AL, Schinder AF, Lanuza GM. 2016. The late and dual origin of cerebrospinal fluid-contacting neurons in the mouse spinal cord. Development 143, 880–891. ( 10.1242/dev.129254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park HC, Shin J, Appel B. 2004. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development 131, 5959–5969. ( 10.1242/dev.01456) [DOI] [PubMed] [Google Scholar]
- 94.Yeo SY, Chitnis AB. 2007. Jagged-mediated Notch signaling maintains proliferating neural progenitors and regulates cell diversity in the ventral spinal cord. Proc. Natl Acad. Sci. USA 104, 5913–5918. ( 10.1073/pnas.0607062104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desban L, Prendergast A, Roussel J, Rosello M, Geny D, Wyart C, Bardet P-L. 2019. Regulation of the apical extension morphogenesis tunes the mechanosensory response of microvilliated neurons. PLoS Biol. 17, e3000235 ( 10.1371/journal.pbio.3000235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dale N, Roberts A, Ottersen OP, Storm-Mathisen J. 1987. The morphology and distribution of ‘Kolmer-Agduhr cells', a class of cerebrospinal-fluid-contacting neurons revealed in the frog embryo spinal cord by GABA immunocytochemistry. Proc. R. Soc. Lond. B 232, 193–203. ( 10.1098/rspb.1987.0068) [DOI] [PubMed] [Google Scholar]
- 97.Alibardi L. 1990. Cerebrospinal-fluid contacting neurons inside the regenerating caudal spinal-cord of Xenopus tadpoles. Boll. Zool. 57, 309–315. ( 10.1080/11250009009355713) [DOI] [Google Scholar]
- 98.Schotland JL, Shupliakov O, Grillner S, Brodin L. 1996. Synaptic and nonsynaptic monoaminergic neuron systems in the lamprey spinal cord. J. Comp. Neurol. 372, 229–244. () [DOI] [PubMed] [Google Scholar]
- 99.Jalalvand E, Robertson B, Wallen P, Grillner S. 2016. Ciliated neurons lining the central canal sense both fluid movement and pH through ASIC3. Nat. Commun. 7, 10002 ( 10.1038/ncomms10002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohm UL, et al. 2016. CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nat. Commun. 7, 10866 ( 10.1038/ncomms10866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schueren AM, DeSantis M. 1985. Cellular heterogeneity in the ependymal layer of the chicken's lumbosacral spinal cord. Exp. Neurol. 87, 387–391. ( 10.1016/0014-4886(85)90230-4) [DOI] [PubMed] [Google Scholar]
- 102.Vigh B, Vigh-Teichmann I, Aros B. 1977. Special dendritic and axonal endings formed by the cerebrospinal fluid contacting neurons of the spinal cord. Cell Tissue Res. 183, 541–552. ( 10.1007/BF00225666) [DOI] [PubMed] [Google Scholar]
- 103.Bezares-Calderon LA, Berger J, Jasek S, Veraszto C, Mendes S, Guhmann M, Almeda R, Shahidi R, Jekely G. 2018. Neural circuitry of a polycystin-mediated hydrodynamic startle response for predator avoidance. eLife 7, e36262 ( 10.7554/eLife.36262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delmas P. 2004. Polycystins: from mechanosensation to gene regulation. Cell 118, 145–148. ( 10.1016/j.cell.2004.07.007) [DOI] [PubMed] [Google Scholar]
- 105.Thouvenin O, et al. 2019. Origin of the bidirectionality of cerebrospinal fluid flow and impact on long-range transport between brain and spinal cord. bioRxiv, 627166. (doi:10.1101/627166)
- 106.Brand M, et al. 1996. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development 123, 129–142. [DOI] [PubMed] [Google Scholar]
- 107.Grimes DT. 2019. Developmental biology: go with the flow to keep the body straight. Curr. Biol. 29, R101–R103. ( 10.1016/j.cub.2018.12.011) [DOI] [PubMed] [Google Scholar]
- 108.Munoz RI, Kahne T, Herrera H, Rodriguez S, Guerra MM, Vio K, Hennig R, Rapp E, Rodriguez E. 2019. The subcommissural organ and the Reissner fiber: old friends revisited. Cell Tissue Res. 375, 507–529. ( 10.1007/s00441-018-2917-8) [DOI] [PubMed] [Google Scholar]
- 109.Zhang X, et al. 2018. Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat. Genet. 50, 1666–1673. ( 10.1038/s41588-018-0260-3) [DOI] [PubMed] [Google Scholar]
- 110.Grimes DT, Boswell CW, Morante NF, Henkelman RM, Burdine RD, Ciruna B. 2016. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 352, 1341–1344. ( 10.1126/science.aaf6419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buchan JG, Gray RS, Gansner JM, Alvarado DM, Burgert L, Gitlin JD, Gurnett CA, Goldsmith MI. 2014. Kinesin family member 6 (kif6) is necessary for spine development in zebrafish. Dev. Dyn. 243, 1646–1657. ( 10.1002/dvdy.24208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayes M, Gao X, Yu LX, Paria N, Henkelman RM, Wise CA, Ciruna B. 2014. ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nat. Commun. 5, 4777 ( 10.1038/ncomms5777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Gennip JLM, Boswell CW, Ciruna B. 2018. Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis. Sci. Adv. 4, eaav1781 ( 10.1126/sciadv.aav1781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boswell CW, Ciruna B. 2017. Understanding idiopathic scoliosis: a new zebrafish school of thought. Trends Genet. 33, 183–196. ( 10.1016/j.tig.2017.01.001) [DOI] [PubMed] [Google Scholar]
- 115.Yasui K, Hashizume Y, Yoshida M, Kameyama T, Sobue G. 1999. Age-related morphologic changes of the central canal of the human spinal cord. Acta Neuropathol. 97, 253–259. ( 10.1007/s004010050982) [DOI] [PubMed] [Google Scholar]
- 116.Milhorat TH, Kotzen RM, Anzil AP. 1994. Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J. Neurosurg. 80, 716–722. ( 10.3171/jns.1994.80.4.0716) [DOI] [PubMed] [Google Scholar]
- 117.Lehtinen MK, et al. 2011. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893–905. ( 10.1016/j.neuron.2011.01.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A. 2014. Primary cilia are required in a unique subpopulation of neural progenitors. Proc. Natl Acad. Sci. USA 111, 12 438–12 443. ( 10.1073/pnas.1321425111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petrik D, Myoga MH, Grade S, Gerkau NJ, Pusch M, Rose CR, Grothe B, Götz M. 2018. Epithelial sodium channel regulates adult neural stem cell proliferation in a flow-dependent manner. Cell Stem Cell 22, 865–878. ( 10.1016/j.stem.2018.04.016) [DOI] [PubMed] [Google Scholar]
- 120.Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D'Ocon P, Farinas I. 2014. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 83, 572–585. ( 10.1016/j.neuron.2014.06.015) [DOI] [PubMed] [Google Scholar]
- 121.Winokurow N, Schumacher S. 2019. A role for polycystin-1 and polycystin-2 in neural progenitor cell differentiation. Cell. Mol. Life Sci. 76, 2851–2869. ( 10.1007/s00018-019-03072-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Worthington WC, Cathcart RS. 1963. Ependymal cilia: distribution and activity in the adult human brain. Science 139, 221–222. ( 10.1126/science.139.3551.221) [DOI] [PubMed] [Google Scholar]
- 123.Worthington WC Jr, Cathcart RS III. 1966. Ciliary currents on ependymal surfaces. Ann. NY Acad. Sci. 130, 944–950. ( 10.1111/j.1749-6632.1966.tb12638.x) [DOI] [PubMed] [Google Scholar]
- 124.Lowery LA, Sive H. 2009. Totally tubular: the mystery behind function and origin of the brain ventricular system. Bioessays 31, 446–458. ( 10.1002/bies.200800207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Korzh V. 2018. Development of brain ventricular system. Cell. Mol. Life Sci. 75, 375–383. ( 10.1007/s00018-017-2605-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Butler AB. 2000. Topography and topology of the teleost telencephalon: a paradox resolved. Neurosci. Lett. 293, 95–98. ( 10.1016/S0304-3940(00)01497-X) [DOI] [PubMed] [Google Scholar]
- 127.Brightman MW, Palay SL. 1963. The fine structure of ependyma in the brain of the rat. J. Cell Biol. 19, 415–439. ( 10.1083/jcb.19.2.415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fame RM, Chang JT, Hong A, Aponte-Santiago NA, Sive H. 2016. Directional cerebrospinal fluid movement between brain ventricles in larval zebrafish. Fluids Barriers CNS 13, 11 ( 10.1186/s12987-016-0036-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ogino T, Sawada M, Takase H, Nakai C, Herranz-Perez V, Cebrian-Silla A, Kaneko N, Garcia-Verdugo JM, Sawamoto K. 2016. Characterization of multiciliated ependymal cells that emerge in the neurogenic niche of the aged zebrafish brain. J. Comp. Neurol. 524, 2982–2992. ( 10.1002/cne.24001) [DOI] [PubMed] [Google Scholar]
- 130.Lindsey BW, Darabie A, Tropepe V. 2012. The cellular composition of neurogenic periventricular zones in the adult zebrafish forebrain. J. Comp. Neurol. 520, 2275–2316. ( 10.1002/cne.23065) [DOI] [PubMed] [Google Scholar]
- 131.Jones HC. 1979. Fenestration of the epithelium lining the roof of the fourth cerebral ventricle in amphibia. Cell Tissue Res. 198, 129–136. ( 10.1007/BF00234840) [DOI] [PubMed] [Google Scholar]
- 132.Jones HC, Jopling CA. 1983. The development of interependymal pores in the rhombencephalic posterior tela in late embryonic, larval and metamorphosing stages of Rana pipiens. Brain Res. 283, 121–130. ( 10.1016/0165-3806(83)90168-2) [DOI] [PubMed] [Google Scholar]
- 133.Hagenlocher C, Walentek P, Müller C, Thumberger T, Feistel K. 2013. Ciliogenesis and cerebrospinal fluid flow in the developing Xenopus brain are regulated by foxj1. Cilia 2, 12 ( 10.1186/2046-2530-2-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Del Bigio MR. 2010. Ependymal cells: biology and pathology. Acta Neuropathol. 119, 55–73. ( 10.1007/s00401-009-0624-y) [DOI] [PubMed] [Google Scholar]
- 135.Jacquet BV, et al. 2009. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development 136, 4021–4031. ( 10.1242/dev.041129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. 2005. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132, 5329–5339. ( 10.1242/dev.02153) [DOI] [PubMed] [Google Scholar]
- 137.Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. 2005. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25, 10–18. ( 10.1523/JNEUROSCI.1108-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abdelhamed Z, et al. 2018. A mutation in Ccdc39 causes neonatal hydrocephalus with abnormal motile cilia development in mice. Development 145, dev154500 ( 10.1242/dev.154500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coletti AM, et al. 2018. Characterization of the ventricular–subventricular stem cell niche during human brain development. Development 145, dev170100 ( 10.1242/dev.170100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Redmond SA, Figueres-Onate M, Obernier K, Nascimento MA, Parraguez JI, Lopez-Mascaraque L, Fuentealba LC, Alvarez-Buylla A. 2019. Development of ependymal and postnatal neural stem cells and their origin from a common embryonic progenitor. Cell Rep. 27, 429–441. ( 10.1016/j.celrep.2019.01.088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ortiz-Alvarez G, et al. 2019. Adult neural stem cells and multiciliated ependymal cells share a common lineage regulated by the geminin family members. Neuron 102, 159–172. ( 10.1016/j.neuron.2019.01.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. 2001. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720. ( 10.1038/35055553) [DOI] [PubMed] [Google Scholar]
- 143.Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. 2001. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2, 287 ( 10.1038/35067582) [DOI] [PubMed] [Google Scholar]
- 144.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. 1999. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96, 25–34. ( 10.1016/S0092-8674(00)80956-3) [DOI] [PubMed] [Google Scholar]
- 145.Carlen M, et al. 2009. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 12, 259–267. ( 10.1038/nn.2268) [DOI] [PubMed] [Google Scholar]
- 146.Shah PT, Stratton JA, Stykel MG, Abbasi S, Sharma S, Mayr KA, Koblinger K, Whelan PJ, Biernaskie J. 2018. Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell 173, 1045–1057. ( 10.1016/j.cell.2018.03.063) [DOI] [PubMed] [Google Scholar]
- 147.Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. 2011. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 138, 4831–4841. ( 10.1242/dev.072587) [DOI] [PubMed] [Google Scholar]
- 148.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. 2008. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265–278. ( 10.1016/j.stem.2008.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mirzadeh Z, et al. 2017. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat. Commun. 8, 13759 ( 10.1038/ncomms13759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alpar A, et al. 2018. Hypothalamic CNTF volume transmission shapes cortical noradrenergic excitability upon acute stress. EMBO J. 37, e100087 ( 10.15252/embj.2018100087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lun MP, Monuki ES, Lehtinen MK. 2015. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445 ( 10.1038/nrn3921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F. 2016. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19, 643–652. ( 10.1016/j.stem.2016.06.013) [DOI] [PubMed] [Google Scholar]
- 153.Lun MP, et al. 2015. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci. 35, 4903–4916. ( 10.1523/jneurosci.3081-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Leeuwen LM, Evans RJ, Jim KK, Verboom T, Fang X, Bojarczuk A, Malicki J, Johnston SA, van der Sar AM. 2018. A transgenic zebrafish model for the in vivo study of the blood and choroid plexus brain barriers using claudin 5. Biol. Open 7, bio030494 ( 10.1242/bio.030494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Narita K, Kawate T, Kakinuma N, Takeda S. 2010. Multiple primary cilia modulate the fluid transcytosis in choroid plexus epithelium. Traffic 11, 287–301. ( 10.1111/j.1600-0854.2009.01016.x) [DOI] [PubMed] [Google Scholar]
- 156.Cushing H. 1925. The third circulation and its channels. Lancet 2, 851–857. [Google Scholar]
- 157.Laterra JJ, Goldstein GW. 2013. The blood-brain barrier, choroid plexus, and cerebrospinal fluid. In Principles of neural science (eds Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ), pp. 1565–1580, 5th edn New York, NY: The McGraw-Hill Companies. [Google Scholar]
- 158.Mogi K, Adachi T, Izumi S, Toyoizumi R. 2012. Visualisation of cerebrospinal fluid flow patterns in albino Xenopus larvae in vivo. Fluids Barriers CNS 9, 9 ( 10.1186/2045-8118-9-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Simon MJ, Iliff JJ. 2016. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta Mol. Basis Dis. 1862, 442–451. ( 10.1016/j.bbadis.2015.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dreha-Kulaczewski S, Joseph AA, Merboldt K-D, Ludwig H-C, Gärtner J, Frahm J. 2015. Inspiration is the major regulator of human CSF flow. J. Neurosci. 35, 2485–2491. ( 10.1523/JNEUROSCI.3246-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu Q, Yu S-B, Zheng N, Yuan X-Y, Chi Y-Y, Liu C, Wang X-M, Lin X-T, Sui H-J. 2016. Head movement, an important contributor to human cerebrospinal fluid circulation. Sci. Rep. 6, 31787 ( 10.1038/srep31787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Faubel R, Westendorf C, Bodenschatz E, Eichele G. 2016. Cilia-based flow network in the brain ventricles. Science 353, 176–178. ( 10.1126/science.aae0450) [DOI] [PubMed] [Google Scholar]
- 163.Ibañez-Tallon I, Pagenstecher A, Fliegauf M, Olbrich H, Kispert A, Ketelsen U-P, North A, Heintz N, Omran H. 2004. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13, 2133–2141. ( 10.1093/hmg/ddh219) [DOI] [PubMed] [Google Scholar]
- 164.Lee L. 2013. Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J. Neurosci. Res. 91, 1117–1132. ( 10.1002/jnr.23238) [DOI] [PubMed] [Google Scholar]
- 165.Conductier G, et al. 2013. Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat. Neurosci. 16, 845–847. ( 10.1038/nn.3401) [DOI] [PubMed] [Google Scholar]
- 166.Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. 1986. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol. Scand. 128, 201–207. ( 10.1111/j.1748-1716.1986.tb07967.x) [DOI] [PubMed] [Google Scholar]
- 167.Bjorefeldt A, Illes S, Zetterberg H, Hanse E. 2018. Neuromodulation via the cerebrospinal fluid: insights from recent in vitro studies. Front. Neural Circuits 12, 5 ( 10.3389/fncir.2018.00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Noble EE, et al. 2018. Control of feeding behavior by cerebral ventricular volume transmission of melanin-concentrating hormone. Cell Metab. 28, 55–68. ( 10.1016/j.cmet.2018.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chau KF, et al. 2015. Progressive differentiation and instructive capacities of amniotic fluid and cerebrospinal fluid proteomes following neural tube closure. Dev. Cell 35, 789–802. ( 10.1016/j.devcel.2015.11.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaiser K, et al. 2019. WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis. Nat. Commun. 10, 1498 ( 10.1038/s41467-019-09298-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kizil C, Kaslin J, Kroehne V, Brand M. 2012. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 72, 429–461. ( 10.1002/dneu.20918) [DOI] [PubMed] [Google Scholar]
- 172.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. 1997. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061. ( 10.1523/JNEUROSCI.17-13-05046.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716. ( 10.1016/S0092-8674(00)80783-7) [DOI] [PubMed] [Google Scholar]
- 174.Alvarez-Buylla A, Lim DA. 2004. For the long run: maintaining germinal niches in the adult brain. Neuron 41, 683–686. ( 10.1016/S0896-6273(04)00111-4) [DOI] [PubMed] [Google Scholar]
- 175.Ferreira RR, Fukui H, Chow R, Vilfan A, Vermot J. 2019. The cilium as a force sensor—myth versus reality. J. Cell Sci. 132, jcs213496 ( 10.1242/jcs.213496) [DOI] [PubMed] [Google Scholar]
- 176.Ohata S, Herranz-Perez V, Nakatani J, Boletta A, Garcia-Verdugo JM, Alvarez-Buylla A. 2015. Mechanosensory genes Pkd1 and Pkd2 contribute to the planar polarization of brain ventricular epithelium. J. Neurosci. 35, 11 153–11 168. ( 10.1523/JNEUROSCI.0686-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sawamoto K, et al. 2006. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629–632. ( 10.1126/science.1119133) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.