Abstract
Mucociliary clearance (MCC) is one of the most important defence mechanisms of the human respiratory system. Its failure is implicated in many chronic and debilitating airway diseases. However, due to the complexity of lung organization, we currently lack full understanding on the relationship between these regional differences in anatomy and biology and MCC functioning. For example, it is unknown whether the regional variability of airway geometry, cell biology and ciliary mechanics play a functional role in MCC. It therefore remains unclear whether the regional preference seen in some airway diseases could originate from local MCC dysfunction. Though great insights have been gained into the genetic basis of cilia ultrastructural defects in airway ciliopathies, the scaling to regional MCC function and subsequent clinical phenotype remains unpredictable. Understanding the multiscale mechanics of MCC would help elucidate genotype–phenotype relationships and enable better diagnostic tools and treatment options. Here, we review the hierarchical and variable organization of ciliated airway epithelium in human lungs and discuss how this organization relates to MCC function. We then discuss the relevancy of these structure–function relationships to current topics in lung disease research. Finally, we examine how state-of-the-art computational approaches can help address existing open questions.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: mucociliary clearance, cilia biomechanics, lung disease, genotype–phenotype relationships, structure–function relationships, multiscale mechanics
1. Introduction
The adult human lung has a surface area of roughly 80–200 m2 and it processes 10 000 l of air daily for gas exchange [1,2]. The continuous passage of air exposes the lungs to inhaled microbes, particulates and harmful gaseous compounds. Defence mechanisms include coughing, anatomical barriers, adjustable flow dynamics, airway epithelial host defence and the immune system; however, the foremost defence system is mucociliary clearance (MCC) [3,4]. In MCC, the motile cilia of the airway epithelium continuously transport a thin layer of mucus out of the lungs, taking with it all trapped matter (mucus escalator) and thereby cleansing the epithelial surface. The biomechanics of MCC have been studied extensively in both experimental and theoretical models of ciliated epithelia, mostly without taking into account the context of organ function or specific lung microenvironment [5,6]. Focusing on this reduced model has been pivotal for revealing universal mechanisms of ciliary function and mucus transport, including the origins of beat polarity [7] and metachronal beat [8], as well as their disruptions in airway disease [9]. However, many current challenges in human respiratory research cannot be adequately addressed unless we understand how MCC biomechanics scale from the individual cilia to the clinical phenotype. That is to say, we need to link cellular properties via tissue mechanics to mucus transport throughout the lungs. Here, we discuss the important biological role of (i) the variable structural and kinematic properties associated with different spatial scales, lung regions and disease states, and (ii) the functional consequences of this diversity on ciliary mucus transport in health and disease. Given that direct experimental studies in the human lungs remain impractical, we also discuss new powerful computational modelling tools that have the capacity to integrate and investigate the spectrum and scaling of ciliary structure, function and fluid flow in silico.
2. The multiscale mechanics of mucociliary transport in the human airways
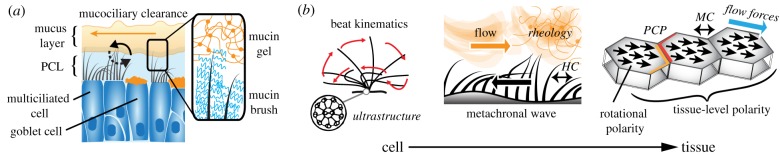
MCC is powered by an estimated 2 × 1012 motile cilia that propel a layer of mucus towards the larynx [3,4]. Healthy airway epithelia contain mostly multiciliated cells and are covered with an airway surface liquid (ASL) consisting of two components, a mucus layer near the tip of the cilia that is propelled by the ciliary power stroke and entraps inhaled particles and foreign pathogens, and a low-viscosity periciliary layer (PCL) in between the cilia that facilitates the ciliary return stroke (Gel-on-Liquid model) [3,10]. According to recent studies, the PCL additionally contains a fine matrix mesh that prevents mucus from seeping into the PCL (Gel-on-Brush model) [10] (figure 1a). Despite the relative simplicity of MCC, experimental and computational studies have revealed a sophisticated hierarchical tissue organization underlying the ciliary-powered transport mechanism (figure 1b). On the organelle level, this includes the well-defined ultrastructure, orientation and beat kinematics of individual cilia, all of which are thought to depend on genetically pre-programmed processes, including planar cell polarity signalling [11]. Indeed, many pathologies affecting MCC are rooted in gene mutations that cause ultrastructural defects of the ciliary apparatus [12]. A striking level of organization arising from the properties of individual cilia is their metachronal beat synchronization, which is thought to reflect passive hydrodynamic coupling rather than active coordination [8]. Two important organizational features are the rotational polarity of all cilia in a given cell, and the collective polarity of multiple ciliated cells in a plane [13]. This multi-level organization may also depend on collective ciliary beat rather than being genetically hard-coded. Specifically, tissue-wide beat polarity can be fine-tuned through sensing of, and alignment to, the dominant cilia-powered flow direction [14]. Mechanical interaction of ciliated cells via cytoskeletal links also appears to contribute to both rotational and tissue-wide polarity [15]. In addition to the role of ciliated cells, ASL rheology is a major factor that determines the mechanics and efficiency of MCC, and altered fluid properties contribute to the severe disease symptoms of asthma and cystic fibrosis [9,16].
Figure 1.
Elements and mechanics of MCC. (a) Brush-on-gel model of MCC: goblet cells produce gel-forming mucins that constitute the mucus layer as well as brush forming mucins in the PCL that tether to cell membranes. Multiciliated cells propel the mucus layer during the power stroke (black solid arrow) and move through the PCL during the recovery stroke (black dashed arrow) to achieve directional mucociliary clearance (orange solid arrow). (b) Important structure–function relationships of MCC at cellular and tissue level, including ciliary beat kinematics which depend on ciliary ultrastructure; metachronal beat facilitated by hydrodynamic coupling (HC) and, in interaction with the ASL rheology, leading to fluid flow; and rotational and tissue-level polarity of ciliary beat which is modulated by planar cell polarity (PCP) signalling, mechanical coupling (MC) of cytoskeletal elements and potentially the feedback from directional fluid flow forces. (Online version in colour.)
These well-known relationships suggest that establishment and maintenance of MCC depends on a highly conserved structural organization of the ciliary epithelium. A recent study suggests the importance of structural heterogeneity in tracheal MCC [17]. However, the geometry of the airways as well as the proportion of ciliated and mucus-producing cell types also vary greatly by lung regions and it is currently not clear if these changes affect local MCC.
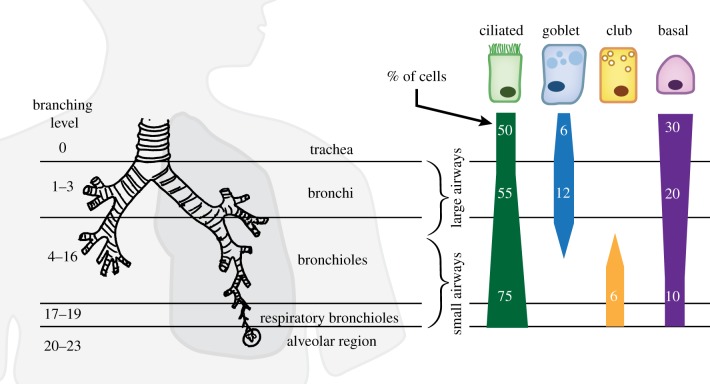
Briefly, the lung tracheobronchial tree is functionally divided into a conducting zone formed by bronchi and bronchioles, and a respiratory zone formed by respiratory bronchioles, alveolar ducts and finally the alveoli, where gas exchange occurs (figure 2). Though quantitative data from human lungs are sparse, it is known that throughout the respiratory tree, duct diameter and cellular composition of the airway epithelium change as a function of branching generation. This review focuses on the range from trachea to respiratory bronchioles, as this is where ciliated cells are present (figure 2). The trachea and bronchi are lined by a pseudostratified epithelium formed by a variety of cells including approximately 50% of ciliated cells, approximately 12% of secretory (mostly goblet) cells and approximately 20–30% of basal cells, in addition to undifferentiated cells [18,20]. As the airways continue to branch into bronchioles and decrease in diameter, a decrease in density of basal and mucus-producing goblet cells is observed, whereas club cells, which are thought to produce watery secretions, become more prevalent in the small airways (diameter < 2 mm) compared to the large airways (diameter > 2 mm). By contrast, the density of ciliated cells remains relatively unchanged [19]; however, total cell density is lower in the small airways, possibly reflecting larger cell bodies as the epithelium becomes more cuboidal in the small airways [18], resulting in a higher relative proportion of ciliated cells [21,22]. The effect of these compositional changes on MCC remains unclear, partly because we are lacking quantitative data on the surface area and spatial distribution of cell types (e.g. random or clustered). Also, as should be pointed out, the cell type proportions listed above might be updated in the future since these numbers are based on relatively few studies which differ in species, tissue preparation methods and quantification metrics and often contradict each other. For example, it was found in both human and rat lungs that the percentage of ciliated surface area increases in the distal airways [19], which contradicts the findings in mice [23]; however, currently, it is impossible to attribute the conflict to either species–species differences or differences in methods. In addition to these common and well-described cell populations, there are likely other, rarer cell types in the human airways that thus far have mainly been characterized in rodent airways, including (chemosensory) brush or tuft cells [24–26], solitary chemosensory cells (SSC) [27] and ion-gating ionocytes [28]. There is also evidence for functional subtypes of club cells in rodents [28] and location- and disease-associated subtypes of ciliated cells in humans [26]. Their functional roles remain to be discovered; however, it is already evident that even sparse cell types may greatly affect mucus clearance from the lung, as in the case of ionocytes, which make up only 1% of all cells in the mouse airway epithelium yet greatly modulate airway fluid and mucus physiology [28].
Figure 2.
Variability of lung epithelial composition in the tracheobronchial tree. The relative abundance of ciliated, secretory goblet and club cells, as well as basal cells changes as a function of airway branching level and diameter. Cell percentage values estimated from Crystal et al. [18] and Mercer et al. [19]. (Online version in colour.)
Additionally, the mucus environment also depends on the lung region. From the trachea until the termination of the cartilaginous bronchioles, secretions of mixed submucosal glands [29] and goblet-cell-derived mucus comprise the bulk of ASL in the large airway region, whereas in the smaller airways, the mucus produced by goblet cells is likely gradually replaced by watery club-cell secretions [20]. Accompanying this anatomical diversity between lung regions, there is also evidence for a generally higher ciliary beat frequency (CBF) in the large airways compared to the small airways [30], and considerable variance of at least some cellular parameters within the same region, such as ciliary beat angle [31].
3. Relevancy of multiscale mucociliary clearance for human health
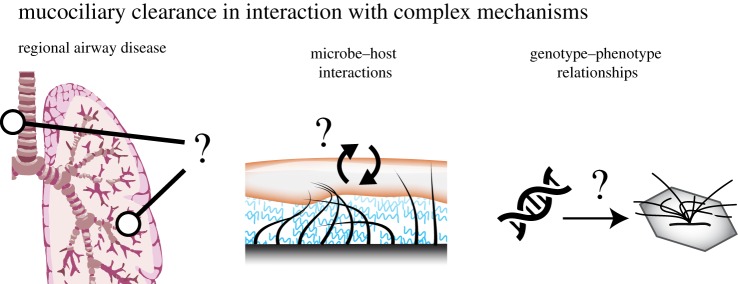
The regional and local variability of airway morphology suggests a potential link between lung anatomy and MCC function, as well as susceptibility to disease. Here, we examine three areas of research that could particularly benefit from further insights into this relationship: regional airway diseases, microbe–host interactions and genotype–phenotype relationships (figure 3).
Figure 3.
Three research areas that involve the intimate relationship between MCC and local lung environment. (Online version in colour.)
(a). Region-specific airway function and disease
The variable organization of the airway epithelium raises the question of whether MCC differs between lung regions, and what the functional consequences could be for health and disease. As detailed above, the density of mucus-producing goblet cells in the respiratory epithelium gradually reduces with the branching of the airways until near zero [19]; this relationship might reduce the risk of clogging narrow airways with mucus and may affect mucus composition in different regions of the lungs. Indeed, regional differences in ASL composition were already demonstrated in canines [32] and it would be interesting to determine if this also holds true in humans, whether it correlates with secretory cell abundance and what the local effects are on CBF and MCC. This is especially interesting since chronic inflammatory lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) are characterized by goblet-cell hyperplasia and metaplasia, resulting in mucus hypersecretion [33]. Understanding the consequences of this remodelling on local ASL composition and MCC (e.g. in the large airway versus the small airway environment) would be highly informative for therapeutic strategies.
In addition to local changes in ASL composition, there are multiple other factors that can modulate CBF and MCC and could thereby contribute to lung disease. For example, CBF is sensitive to temperature, ATP availability, pH and mucus viscosity [34–36]. Especially in disease, these factors may be altered locally [34], thereby changing CBF and contributing to impaired MCC. One intriguing hypothesis is that MCC is not the only transport function accomplished by airway cilia and that some lung regions have a ciliary organization tuned towards facilitating other fluid interactions attributed to motile cilia, such as fluid mixing [37] or mechanochemical sensing [38]. For example, it has been proposed that surfactants are mixed into the ASL in order to reduce surface tension and prevent mucus bridging, especially in the small airways [39]; hence, it would be very interesting to investigate whether the small airway ciliary mechanics were found to enhance fluid mixing, which could facilitate the homogeneous distribution of surfactant in the ASL. Alternatively, the regional organization of the airway epithelium could reflect an allometric scaling law by which homogeneous MCC is achieved across the complex branching geometry of the lung.
Understanding region-specific airway function in combination with lung-wide flow mechanics will also provide insights into why, in lung disease, regions of the lungs are differentially affected. For example, patient-specific inhalation patterns of cigarette smoke, an established risk factor for COPD, correlate with disease phenotype. Specifically, the degree by which smoke is inhaled into the alveolar space positively correlates with the risk of developing emphysema, which destroys the alveolar space, and negatively correlates with developing bronchitis and productive cough, which indicates interception of smoke by ciliated airway surfaces [40]. Currently, it cannot be discriminated whether altered smoke inhalation is causal or a consequence of patient-specific disease phenotypes. Probing the interaction between airflow, particle deposition and (locally) defective MCC could help us understand patient-specific penetration and residence time of noxious particles in the alveolar and airway spaces. For example, in some patients, the small airways may accumulate noxious particles more readily and hence be more susceptible to damage [41]. Indeed, certain patterns of small-airway branching can cause increased susceptibility to COPD [42]. Further, in asthmatic and COPD subjects, altered expiratory flow patterns were associated with lung regional impairment of MCC [43]. Both examples further indicate that airflow dynamics and associated patterns of particle deposition also matter.
In general, in airway disease, MCC is often negatively affected [44], either due to changes in mucus composition and production (due to detrimental effects on ciliary function or cell number) or due to altered patterns of airflow and particle deposition in obstructed airways. Indeed, radioactive particle tracing has revealed that the combination of these mechanisms can lead to disease-specific impairments of MCC; for example, in cystic fibrosis, reduced MCC of the small airways leads to prolonged particle retention in these regions [45]. In addition, MCC of the small airways have been shown to decline with age [46]. Furthermore, cigarette smoke exposure results in reduced cilia length [47], ciliophagy [48] and airway epithelial remodelling with reduced levels of ciliated cells [49]. In addition, flavouring chemicals in electronic cigarettes were demonstrated to negatively affect ciliated cell number [50] and impair ciliary beat [51]. Since ciliary density is directly related to the distance mucus can be transported [52], altered ciliary densities through damage or in airway disease could directly lead to mucus congestion. Cigarette smoke may also impair MCC by negatively affecting the expression of cystic fibrosis transmembrane regulator (CFTR), an important ion channel for maintaining proper mucus osmolarity [53,54]. Clearly, an important role of impaired MCC in disease symptoms and development has been established. However, to our knowledge, the impairment, sensitivity and contributions of different lung regions remain mostly unknown, and our understanding of airway disease development would greatly benefit from exploring the role of regionally specific and locally impaired MCC.
(b). Microbe–host interactions
In healthy individuals, the presence of a low-abundant microbiota in the airways is a result of a constant turnover of microbes entering by inhalation and microaspiration and exiting by MCC and cough [55]. MCC normally dominates this process but when it is impaired, for example, in patients with (chronic) airway diseases, a third factor contributes to the composition of the microbiota: local bacterial growth conditions. These conditions are shaped by, for example, host–microbe interactions, epithelial host defence, pH, temperature and others, and if they are favourable to growth, they can support the unwanted outgrowth of bacteria into these niches and cause the altered microbiota composition in patients with airway disease [56].
Although little is known about the direct effects of bacteria or bacteria-derived mediators on MCC, toxins derived from the lung-disease-associated pathogens Pseudomonas aeruginosa and non-typeable Haemophilus influenzae were demonstrated to slow down CBF, most likely by affecting intracellular adenosine nucleotide levels [57]. Similarly, the whooping-cough-causing pathogen Bordetella bronchiseptica produces a ciliostatic toxin that is key to early colonization events [58]. Such bacteria-mediated damage to MCC could contribute to the local outgrowth of specific bacteria as seen in patients with COPD or cystic fibrosis. Conversely, host defence mechanisms can also respond to the increased presence of bacteria or noxious compounds by enhancing MCC. Specifically, motile cilia express specific taste receptors that sense bitter compounds, such as bacterial products or nicotine, and sweet taste receptors that can detect the local reduction in sugar compounds due to microbial activity. Taste receptor activation causes nitric oxide (NO)-mediated increases in ciliary beat frequency and hence MCC [59,60]. A combination of factors such as bacterial phenotype, infection stage, released virulence factors and other stressors therefore contribute to whether MCC defences are increased or weakened by bacteria. Changes in mucus composition can further promote bacterial outgrowth; for example, changes in mucus pH can impair bacterial clearance from the mucus [61] or mucus plugging could favour the outgrowth of anaerobic bacteria [62]. Strikingly, it was demonstrated that these anaerobic bacteria subsequently break down mucus, thereby releasing amino acids and short-chain fatty acids that serve as nutrients for the outgrowth of other bacteria that would normally not be able to survive in the lungs [62]. These local bacterial niches are especially evident in patients with (end-stage) chronic lung disease [56] and could therefore locally affect MCC, further aggravating disease or contribute to development of disease exacerbations. A better understanding of the extent to which microbiota shifts affect MCC, and by which mechanisms, could contribute to the development of therapies that aim at normalizing MCC.
Besides bacteria, viruses also actively target ciliated cells or ciliary function. For example, human immunodeficiency virus (HIV) suppresses ciliogenesis and tracheobronchial MCC and decreases expression of CFTR via transforming growth factor β (TGF-β) signalling [63]. Human parainfluenza virus type 3 (PIV) preferentially infects ciliated cells [64] as does human respiratory syncytial virus (RSV) [65]. Ciliary dyskinesia is therefore an early sign of RSV infection [66]. Within 24 h of infection in nasal epithelial cells, virus was observed on the apical surface of ciliated cells, but not on the ciliary axoneme [66], and ciliary dyskinesia was present. Little is known about how these virus infections affect MCC and how widely and quickly the damage manifests in the airways. By better understanding the local conditions, possibly therapies could be developed that do not need to target the virus itself but could stimulate MCC to prevent local mucus stagnation and accumulation of noxious or inflammatory stimuli.
(c). Genotype–phenotype relationships
Genetic mutations of either motile or chemosensory ciliary function can lead to airway disease. Inheritable diseases that impair ciliary motility are collectively known as primary ciliary dyskinesia (PCD) [67]. In the airways, PCD is broadly associated with chronic bronchitis, sinusitis and atelectasis, and these symptoms have been attributed to defective clearance of lung mucus. PCD is the result of recessive mutations in one or many genes, with at least mutations in 37 separate loci identified and linked to specific ultrastructural abnormities of the ciliary axoneme [12]. These recessive mutations account for around 70% of the diagnosed cases of PCD and can present as a spectrum of ciliary defects. A typical multiciliated respiratory epithelial cell contains 200–300 cilia [68]. In the past, severely reduced ciliation, now known as reduced generation of motile cilia (RGMC), was often attributed to infection, poor sample preparation for analysis or more generically as ‘ciliary aplasia’. Through genome sequencing, mutations in genes such as Cyclin O (CCNO) and multicilin (MCIDAS), found in a highly conserved region of chromosome 5, were discovered to result in decreased basal body replication and migration to the airways apical surface, resulting in reduced ciliation [69–71]. These mutations likely occur more frequently than previously diagnosed and lead to severe clinical manifestations. Attributing a genetic basis for ciliary aplasia reconfirms the gamut of genotypes and phenotypes that could lead to defective motile cilia in the airways.
In most instances of PCD, cilia with different PCD genotypes are associated with characteristic defects in the ciliary length, waveform and beat kinematics [72]. However, there has not been a clear relationship between genotypes, motility defects, MCC and respiratory phenotypes. Subsequently, many genotype–phenotype relationships remain unexplained and surprising. For example, CCDC39 and CCDC40 mutations (causing microtubular disorganization) lead to ciliary dysmotility or immotility that appears similar to the effects of other gene mutations; however, subjects with CCDC39 and CCDC40 are more likely to develop more severe pulmonary disease [73], suggesting that small differences in ciliary defects can manifest in substantially different clinical outcomes. Interestingly, CCDC39 and CCDC40 mutations result in absent inner dynein arm, misplaced radial spokes and microtubular disorganization in only a subpopulation of cilia [74]. Also, the resulting ciliary motility defects are variable, ranging from altered waveform and reduced ciliary beat frequency to immobility. Further studies are needed to understand whether this mechanical heterogeneity is sufficient to increase the detrimental effects of the mutation on MCC compared to a more homogeneous impairment of ciliary function. In general, research is needed to address the question of how ultrastructural defects and their combinations manifest in MCC and at the clinical level.
As prospective longitudinal studies are being carried out evaluating genotype with clinical features, correlations with lung function are starting to emerge. Subjects with absent inner dynein arm, central apparatus defects and microtubular disorganization have worse lung function and a greater decline in lung function over time than those with the more common outer dynein arm mutations DNAH5 [75]. Although yet to be identified, it also remains plausible that complex heterozygosity with allelic mutations in two different PCD genes could lead to mild symptomatic disease. In-depth understanding of how specific ultrastructural defects change the lungs capacity for MCC will be critical to understanding the heterogeneity and complete range of ciliopathy-related diseases.
As mentioned earlier, motile cilia of the airways also have sensory capabilities, such as sensing bitter compounds. Sensory activation leads to increased beat frequency [38] and enhanced MCC [76] which is thought to more effectively clear pathogens and toxins. Indeed, genetic variants of the chemosensory receptors that are linked to reduced sensitivity increase the susceptibility to respiratory infection [60]. It would be very interesting to map chemosensitivity and beat frequency response as a function of airway region and to study how this distribution might affect airway defence.
In conclusion, systematically integrating research on MCC with genetic, cellular and clinical studies could help identify (regional) genotype–phenotype relationships and ultimately link the onset and progression of respiratory disease to specific gene mutations, yielding enormous opportunities for improving diagnostics and treatment options. As discussed in the final section, one way to advance this integration of biological data is to develop and employ computational models of MCC.
4. Computational models of structure–function relationships in mucociliary clearance
Mathematical modelling has been mainly concerned with describing, deciphering and predicting three aspects of cilia mechanics: (i) the molecular underpinnings of ciliary oscillations [77–81], (ii) the emergence of cilia coordination and metachronism [82–87], and (iii) cilia-driven flows. Cilia-driven flows are typically of the order of 100 µm s−1, implying that inertia is negligible and viscous forces are dominant, even at low fluid viscosities (e.g. in water). Fluid transport in this drag-dominated regime is not intuitive. It depends on both the cilia asymmetric beating pattern and the metachronal coordination between neighbouring cilia. A great deal of research effort focuses on transport by planar and spatially homogeneous arrays of cilia with prescribed kinematics and coordination, as reviewed in Smith et al. [5]. These models typically consider single- or two-layer Newtonian fluids, with particular interest in the effect of fluid viscosity or the viscoelastic properties of the mucus layer on the overall mucociliary transport [88]. The outcome is to quantify beat-averaged flow profiles across the ASL depth, and the effects of altered fluid rheology, CBF, cilia–mucus penetration and other parameters on these flow profiles, as well as the relation between transport and mixing of passive tracers [37]. However, rigorous assessment of the merits of these models in comparison to experimental findings remains sparse [5]. At present, there is no experimental benchmark under agreed upon ‘nominal’ and ‘altered’ cilia conditions that can be used as a testbed for theoretical and computational models [89]. To evaluate mathematical models, we need to compare their behaviour to experimental results, but, more importantly, we need to test their predictive ability under altered conditions. A major difficulty arises from the fact that the MCC system is composed of multiple scales that are coupled together via mechanical and chemical feedback and feed-forward loops from mucus mechanics, to cilia beat kinematics, to molecular motor activity. Many of the loops in this hierarchical organization are not well understood, as highlighted earlier. Mathematical modelling could play an important role in formulating and testing hypotheses regarding the interdependence of these distinct hierarchies. To succeed in this mission, we need, in addition to new models with increasingly more accurate rheology and fluid mechanics, (i) models that connect the molecular machinery underlying cilia oscillations to cilia beating and cilia coordination, taking into account chemical signalling to and from the epithelial cells; (ii) multiscale models that account for spatial heterogeneity in cilia distribution and parameters and that link them to cilia coordination and to mucus secretion and transport; and (iii) the integration of more accurate biological data with the new generation of computational models. A particularly exciting research direction would be to employ high-content imaging [72,90] to connect these theoretical models to experimental observations [91].
5. Conclusion
MCC is a major defence mechanism of the lung whose impairment can cause tissue damage and respiratory stress contributing to the development of a variety of lung diseases. As discussed, it is a significant challenge to understand how (regional) differences in MCC contribute to the onset and progression of airway disease because of the many variables involved. For once, the structure–function relationships of MCC in the human lung span multiple spatial scales, ranging from the beat of nanoscale organelles to clinically observable ‘productive cough’. The establishment of functional MCC depends on both hard-wired genetic and dynamic tuning mechanisms. Adding to this complexity are the regional variability of the cellular and extracellular elements of MCC as well as their dynamic interactions with environmental factors, such as particles and bacteria. Moreover, MCC function is sensitive to noxious effects of harmful agents it is designed to clear. Recently, however, promising advances have been made towards disentangling these complex interactions: human tissue engineering can be applied to reverse-engineer select defects of MCC using patient-specific induced pluripotent stem cells and to thereby verify and investigate disease mechanisms [92]. We are proposing that computational tools could be very helpful in integrating these and other experimental data and predicting the relative role individual biological factors may play in stabilizing or disrupting MCC, and to what effect. Combining these computational and empirical models would greatly help in extrapolating genetic and cellular properties to clinical phenotypes and aid the development of new diagnostic tools and treatment options.
Data accessibility
This article has no additional data.
Authors' contributions
J.C.N. and E.K. designed the outline and scope of the review. J.C.N. drafted the manuscript. All authors helped to write and critically review the work and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
A.R. is supported by the Hastings Foundation and grant no. 5R01HL139828, 2018-22. A.M.v.d.D. is supported by an EU Marie Curie Global Fellowship (no. 748569; 2018-2020). E.K. is supported by NSF INSPIRE Award no. 1608744.
References
- 1.McGeown JG. 2007. Master medicine: physiology E-book: a core text of human physiology with self assessment. London, UK: Churchill Livingstone. [Google Scholar]
- 2.Fröhlich E, Mercuri A, Wu S, Salar-Behzadi S. 2016. Measurements of deposition, lung surface area and lung fluid for simulation of inhaled compounds. Front. Pharmacol. 7, 181 ( 10.3389/fphar.2016.00181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanner A, Salathé M, O'Riordan TG. 1996. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 154, 1868–1902. ( 10.1164/ajrccm.154.6.8970383) [DOI] [PubMed] [Google Scholar]
- 4.Bustamante-Marin XM, Ostrowski LE. 2017. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 9, a028241 ( 10.1101/cshperspect.a028241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DJ, Gaffney EA, Blake JR. 2008. Modelling mucociliary clearance. Respir. Physiol. Neurobiol. 163, 178–188. ( 10.1016/j.resp.2008.03.006) [DOI] [PubMed] [Google Scholar]
- 6.Sears PR, Yin W-N, Ostrowski LE. 2015. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, 99–108. ( 10.1152/ajplung.00024.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vladar EK, Nayak JV, Milla CE, Axelrod JD. 2016. Airway epithelial homeostasis and planar cell polarity signaling depend on multiciliated cell differentiation. JCI Insight 1, e88027 ( 10.1172/jci.insight.88027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitran SM. 2007. Metachronal wave formation in a model of pulmonary cilia. Comput. Struct. 85, 763–774. ( 10.1016/j.compstruc.2007.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonser LR, Zlock L, Finkbeiner W, Erle DJ. 2016. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J. Clin. Invest. 126, 2367–2371. ( 10.1172/JCI84910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. 2012. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941. ( 10.1126/science.1223012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutin C, et al. 2014. A dual role for planar cell polarity genes in ciliated cells. Proc. Natl Acad. Sci. USA 111, E3129–E3138. ( 10.1073/pnas.1404988111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horani A, Ferkol TW, Dutcher SK, Brody SL. 2016. Genetics and biology of primary ciliary dyskinesia. Paediatr. Respir. Rev. 18, 18–24. ( 10.1016/j.prrv.2015.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks ER, Wallingford JB. 2014. Multiciliated cells. Curr. Biol. 24, R973–R982. ( 10.1016/j.cub.2014.08.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guirao B, et al. 2010. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341–350. ( 10.1038/ncb2040) [DOI] [PubMed] [Google Scholar]
- 15.Werner ME, Hwang P, Huisman F, Taborek P, Clare CY, Mitchell BJ. 2011. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 195, 19–26. ( 10.1083/jcb.201106110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin BK. 2007. Mucus structure and properties in cystic fibrosis. Paediatr. Respir. Rev. 8, 4–7. ( 10.1016/j.prrv.2007.02.004) [DOI] [PubMed] [Google Scholar]
- 17.Juan GRR-S, Mathijssen AJTM, He M, Jan L, Marshall W, Prakash M. 2019. Multi-scale spatial heterogeneity enhances particle clearance in airway ciliary arrays. bioRxiv ( 10.1101/665125) [DOI] [PMC free article] [PubMed]
- 18.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. 2008. Airway epithelial cells: current concepts and challenges. Proc. Am. Thorac. Soc. 5, 772–777. ( 10.1513/pats.200805-041HR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer RR, Russell ML, Roggli VL, Crapo JD. 1994. Cell number and distribution in human and rat airways. Am. J. Respir. Cell Mol. Biol. 10, 613–624. ( 10.1165/ajrcmb.10.6.8003339) [DOI] [PubMed] [Google Scholar]
- 20.Rokicki W, Rokicki M, Wojtacha J, Dżeljijli A. 2016. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir. Torakochir. Pol. 13, 26–30. ( 10.5114/kitp.2016.58961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raman T, O'Connor TP, Hackett NR, Wang W, Harvey B-G, Attiyeh MA, Dang DT, Teater M, Crystal RG. 2009. Quality control in microarray assessment of gene expression in human airway epithelium. BMC Genomics 10, 493 ( 10.1186/1471-2164-10-493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, et al. 2017. Smoking-dependent distal-to-proximal repatterning of the adult human small airway epithelium. Am. J. Respir. Crit. Care Med. 196, 340–352. ( 10.1164/rccm.201608-1672OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toskala E, Smiley-Jewell SM, Wong VJ, King D, Plopper CG. 2005. The temporal and spatial distribution of ciliogenesis in the tracheobronchial airways of mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L454–L459. ( 10.1152/ajplung.00036.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid L, Meyrick B, Antony VB, Chang L-Y, Crapo JD, Reynolds HY. 2005. The mysterious pulmonary brush cell. Am. J. Respir. Crit. Care Med. 172, 136–139. ( 10.1164/rccm.200502-203WS) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krasteva G, et al. 2011. Cholinergic chemosensory cells in the trachea regulate breathing. Proc. Natl Acad. Sci. USA 108, 9478–9483. ( 10.1073/pnas.1019418108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira BFA, et al. 2019. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 25, 1153–1163. ( 10.1038/s41591-019-0468-5) [DOI] [PubMed] [Google Scholar]
- 27.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. 2011. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm. Med. 11, 3 ( 10.1186/1471-2466-11-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montoro DT, et al. 2018. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319 ( 10.1038/s41586-018-0393-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggi CA, Giachetti A, Dey RD, Said SI. 1995. Neuropeptides as regulators of airway function: vasoactive intestinal peptide and the tachykinins. Physiol. Rev. 75, 277–322. ( 10.1152/physrev.1995.75.2.277) [DOI] [PubMed] [Google Scholar]
- 30.Joki S, Saano V. 1994. Ciliary beat frequency at six levels of the rabbit and rat respiratory tract in cow, dog, guinea-pig, pig. Clin. Exp. Pharmacol. Physiol. 21, 427–434. ( 10.1111/j.1440-1681.1994.tb02537.x) [DOI] [PubMed] [Google Scholar]
- 31.Rautiainen M, Collan Y, Nuutinen J. 1986. A method for measuring the orientation (beat direction) of respiratory cilia. Arch. Oto-rhino-laryngol. 243, 265–268. ( 10.1007/BF00464443) [DOI] [PubMed] [Google Scholar]
- 32.Boucher RC, Stutts MJ, Bromberg PA, Gatzy JT. 1981. Regional differences in airway surface liquid composition. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 50, 613–620. ( 10.1152/jappl.1981.50.3.613) [DOI] [PubMed] [Google Scholar]
- 33.Curran DR, Cohn L. 2010. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am. J. Respir. Cell Mol. Biol. 42, 268–275. ( 10.1165/rcmb.2009-0151TR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luk CK, Dulfano MJ. 1983. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin. Sci. 64, 449–451. ( 10.1042/cs0640449) [DOI] [PubMed] [Google Scholar]
- 35.Yoshitsugu M, Rautiainen M, Matsune S, Nuutinen J, Ohyama M. 1993. Effect of exogenous ATP on ciliary beat of human ciliated cells studied with differential interference microscope equipped with high speed video. Acta Otolaryngol. 113, 655–659. ( 10.3109/00016489309135880) [DOI] [PubMed] [Google Scholar]
- 36.Green A, Smallman LA, Logan AC, Drake-Lee AB. 1995. The effect of temperature on nasal ciliary beat frequency. Clin. Otolaryngol. Allied Sci. 20, 178–180. ( 10.1111/j.1365-2273.1995.tb00040.x) [DOI] [PubMed] [Google Scholar]
- 37.Ding Y, Nawroth J, McFall-Ngai M, Kanso E. 2014. Mixing and transport by ciliary carpets: a numerical study. J. Fluid Mech. 743, 124–140. ( 10.1017/jfm.2014.36) [DOI] [Google Scholar]
- 38.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. 2009. Motile cilia of human airway epithelia are chemosensory. Science 325, 1131–1134. ( 10.1126/science.1173869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hohlfeld JM. 2002. The role of surfactant in asthma. Respir. Res. 3, 4 ( 10.1186/rr176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart JI, Criner GJ. 2013. The small airways in chronic obstructive pulmonary disease: pathology and effects on disease progression and survival. Curr. Opin. Pulm. Med. 19, 109–115. ( 10.1097/MCP.0b013e32835ceefc) [DOI] [PubMed] [Google Scholar]
- 41.Heyder J. 2004. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc. 1, 315–320. ( 10.1513/pats.200409-046TA) [DOI] [PubMed] [Google Scholar]
- 42.Smith BM, et al. 2018. Human airway branch variation and chronic obstructive pulmonary disease. Proc. Natl Acad. Sci. USA 115, E974–E981. ( 10.1073/pnas.1715564115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smaldone GC, Foster WM, O'Riordan TG, Messina MS, Perry RJ, Langenback EG. 1993. Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest 103, 1390–1396. ( 10.1378/chest.103.5.1390) [DOI] [PubMed] [Google Scholar]
- 44.Tilley AE, Walters MS, Shaykhiev R, Crystal RG. 2015. Cilia dysfunction in lung disease. Annu. Rev. Physiol. 77, 379–406. ( 10.1146/annurev-physiol-021014-071931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regnis JA, Zeman KL, Noone PG, Knowles MR, Bennett WD. 2000. Prolonged airway retention of insoluble particles in cystic fibrosis versus primary ciliary dyskinesia. Exp. Lung Res. 26, 149–162. ( 10.1080/019021400269844) [DOI] [PubMed] [Google Scholar]
- 46.Svartengren M, Falk R, Philipson K. 2005. Long-term clearance from small airways decreases with age. Eur. Respir. J. 26, 609–615. ( 10.1183/09031936.05.00002105) [DOI] [PubMed] [Google Scholar]
- 47.Leopold PL, O'Mahony MJ, Lian XJ, Tilley AE, Harvey B-G, Crystal RG. 2009. Smoking is associated with shortened airway cilia. PLoS ONE 4, e8157 ( 10.1371/journal.pone.0008157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cloonan SM, Lam HC, Ryter SW, Choi AM. 2014. ‘Ciliophagy’: the consumption of cilia components by autophagy. Autophagy 10, 532–534. ( 10.4161/auto.27641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crystal RG. 2014. Airway basal cells. The ‘smoking gun’ of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 190, 1355–1362. ( 10.1164/rccm.201408-1492PP) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park H-R, O'Sullivan M, Vallarino J, Shumyatcher M, Himes BE, Park J-A, Christiani DC, Allen J, Lu Q. 2019. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci. Rep. 9, 1400 ( 10.1038/s41598-018-37913-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, Jaspers I. 2019. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung Cell. Mol. Physiol. 316, L470–L486. ( 10.1152/ajplung.00304.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khelloufi M-K, Loiseau E, Jaeger M, Molinari N, Chanez P, Gras D, Viallat A. 2018. Spatiotemporal organization of cilia drives multiscale mucus swirls in model human bronchial epithelium. Sci. Rep. 8, 2447 ( 10.1038/s41598-018-20882-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dransfield MT, et al. 2013. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest 144, 498–506. ( 10.1378/chest.13-0274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. 2006. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir. Crit. Care Med. 173, 1139–1144. ( 10.1164/rccm.200508-1330OC) [DOI] [PubMed] [Google Scholar]
- 55.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, Curtis JL. 2015. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann. Am. Thorac. Soc. 12, 821–830. ( 10.1513/AnnalsATS.201501-029OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erb-Downward JR, et al. 2011. Analysis of the lung microbiome in the ‘healthy’ smoker and in COPD. PLoS ONE 6, e16384 ( 10.1371/journal.pone.0016384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanthakumar K, Taylor GW, Cundell DR, Dowling RB, Johnson M, Cole PJ, Wilson R. 1996. The effect of bacterial toxins on levels of intracellular adenosine nucleotides and human ciliary beat frequency. Pulm. Pharmacol. 9, 223–230. ( 10.1006/pulp.1996.0028) [DOI] [PubMed] [Google Scholar]
- 58.Anderton TL, Maskell DJ, Preston A. 2004. Ciliostasis is a key early event during colonization of canine tracheal tissue by Bordetella bronchiseptica. Microbiology 150, 2843–2855. ( 10.1099/mic.0.27283-0) [DOI] [PubMed] [Google Scholar]
- 59.Jain R, Javidan-Nejad C, Alexander-Brett J, Horani A, Cabellon MC, Walter MJ, Brody SL. 2012. Sensory functions of motile cilia and implication for bronchiectasis. Front. Biosci. (Schol. Ed.) 4, 1088–1098. ( 10.2741/s320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Workman AD, Palmer JN, Adappa ND, Cohen NA. 2015. The role of bitter and sweet taste receptors in upper airway immunity. Curr. Allergy Asthma Rep. 15, 72 ( 10.1007/s11882-015-0571-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pezzulo AA, et al. 2012. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113. ( 10.1038/nature11130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flynn JM, Niccum D, Dunitz JM, Hunter RC. 2016. Evidence and role for bacterial mucin degradation in cystic fibrosis airway disease. PLoS Pathog. 12, e1005846 ( 10.1371/journal.ppat.1005846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chinnapaiyan S, Parira T, Dutta R, Agudelo M, Morris A, Nair M, Unwalla HJ. 2017. HIV infects bronchial epithelium and suppresses components of the mucociliary clearance apparatus. PLoS ONE 12, e0169161 ( 10.1371/journal.pone.0169161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 79, 1113–1124. ( 10.1128/JVI.79.2.1113-1124.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 76, 5654–5666. ( 10.1128/jvi.76.11.5654-5666.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith CM, et al. 2014. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur. Respir. J. 43, 485–496. ( 10.1183/09031936.00205312) [DOI] [PubMed] [Google Scholar]
- 67.Zariwala MA, Knowles MR, Leigh MW. 2015. Primary ciliary dyskinesia. In Genereviews (eds Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, Amemiya A), online. Seattle, WA: University of Washington. [PubMed] [Google Scholar]
- 68.Nanjundappa R, Kong D, Shim K, Stearns T, Brody SL, Loncarek J, Mahjoub MR. 2019. Regulation of cilia abundance in multiciliated cells. eLife 8, e44039 ( 10.7554/eLife.44039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amirav I, et al. 2016. Systematic analysis of CCNO variants in a defined population: implications for clinical phenotype and differential diagnosis. Hum. Mutat. 37, 396–405. ( 10.1002/humu.22957) [DOI] [PubMed] [Google Scholar]
- 70.Boon M, et al. 2014. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 5, 4418 ( 10.1038/ncomms5418) [DOI] [PubMed] [Google Scholar]
- 71.Van Eys J, Patterson JH.. 1973. The effects of metabolic inhibitors on the autohaemolysis test and its relevance to the mechanism of the red cell lysis. Br. J. Haematol. 24, 37–47. ( 10.1111/j.1365-2141.1973.tb05725.x) [DOI] [PubMed] [Google Scholar]
- 72.Chioccioli M, Feriani L, Nguyen Q, Kotar J, Dell SD, Mennella V, Amirav I, Cicuta P. 2019. Quantitative high-speed video profiling discriminates between DNAH11 and HYDIN variants of primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 199, 1436–1438. ( 10.1164/rccm.201812-2256LE) [DOI] [PubMed] [Google Scholar]
- 73.Davis SD, et al. 2015. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am. J. Respir. Crit. Care Med. 191, 316–324. ( 10.1164/rccm.201409-1672OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antony D, et al. 2013. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganisation and absent inner dynein arms. Hum. Mutat. 34, 462–472. ( 10.1002/humu.22261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis SD, et al. 2019. Primary ciliary dyskinesia: longitudinal study of lung disease by ultrastructure defect and genotype. Am. J. Respir. Crit. Care Med. 199, 190–198. ( 10.1164/rccm.201803-0548OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ortiz JL, Ortiz A, Milara J, Armengot M, Sanz C, Compañ D, Morcillo E, Cortijo J. 2016. Evaluation of mucociliary clearance by three dimension Micro-CT-SPECT in guinea pig: role of bitter taste agonists. PLoS ONE 11, e0164399 ( 10.1371/journal.pone.0164399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brokaw CJ. 2009. Thinking about flagellar oscillation. Cell Motil. 66, 425–436. ( 10.1002/cm.20313) [DOI] [PubMed] [Google Scholar]
- 78.Camalet S, Jülicher F. 2000. Generic aspects of axonemal beating. New J. Phys. 2, 24 ( 10.1088/1367-2630/2/1/324) [DOI] [Google Scholar]
- 79.Bayly PV, Dutcher SK. 2016. Steady dynein forces induce flutter instability and propagating waves in mathematical models of flagella. J. R. Soc. Interface 13, 20160523 ( 10.1098/rsif.2016.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ling F, Guo H, Kanso E. 2018. Instability-driven oscillations of elastic microfilaments. J. R. Soc. Interface 15, 20180594 ( 10.1098/rsif.2018.0594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han J, Peskin CS. 2018. Spontaneous oscillation and fluid–structure interaction of cilia. Proc. Natl Acad. Sci. USA 115, 4417–4422. ( 10.1073/pnas.1712042115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uchida N, Golestanian R. 2011. Generic conditions for hydrodynamic synchronization. Phys. Rev. Lett. 106, 058104 ( 10.1103/PhysRevLett.106.058104) [DOI] [PubMed] [Google Scholar]
- 83.Wan KY, Goldstein RE. 2016. Coordinated beating of algal flagella is mediated by basal coupling. Proc. Natl Acad. Sci. USA 113, E2784–E2793. ( 10.1073/pnas.1518527113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo H, Fauci L, Shelley M, Kanso E. 2018. Bistability in the synchronization of actuated microfilaments. J. Fluid Mech. 836, 304–323. ( 10.1017/jfm.2017.816) [DOI] [Google Scholar]
- 85.Vilfan A, Jülicher F. 2006. Hydrodynamic flow patterns and synchronization of beating cilia. Phys. Rev. Lett. 96, 058102 ( 10.1103/PhysRevLett.96.058102) [DOI] [PubMed] [Google Scholar]
- 86.Bruot N, Cicuta P. 2016. Realizing the physics of motile cilia synchronization with driven colloids. Annu. Rev. Condens. Matter Phys. 7, 323–348. ( 10.1146/annurev-conmatphys-031115-011451) [DOI] [Google Scholar]
- 87.Pellicciotta N, Hamilton E, Kotar J, Faucourt M, Degehyr N, Spassky N, Cicuta P. 2019. Synchronization of mammalian motile cilia in the brain with hydrodynamic forces. bioRxiv ( 10.1101/668459) [DOI] [PMC free article] [PubMed]
- 88.Guo H, Kanso E. 2017. A computational study of mucociliary transport in healthy and diseased environments. Eur. J. Comput. Mech. 26, 4–30. ( 10.1080/17797179.2017.1321206) [DOI] [Google Scholar]
- 89.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. 1998. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J. Clin. Invest. 102, 1125–1131. ( 10.1172/JCI2687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feriani L, Juenet M, Fowler CJ, Bruot N, Chioccioli M, Holland SM, Bryant CE, Cicuta P. 2017. Assessing the collective dynamics of motile cilia in cultures of human airway cells by multiscale DDM. Biophys. J. 113, 109–119. ( 10.1016/j.bpj.2017.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin J, Nicastro D. 2018. Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360, aar1968 ( 10.1126/science.aar1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, Verma IM. 2014. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 111, E1723–E1730. ( 10.1073/pnas.1403470111) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.