Abstract
The inner ear, which mediates the senses of hearing and balance, derives from a simple ectodermal vesicle in the vertebrate embryo. In the zebrafish, the otic placode and vesicle express a whole suite of genes required for ciliogenesis and ciliary motility. Every cell of the otic epithelium is ciliated at early stages; at least three different ciliary subtypes can be distinguished on the basis of length, motility, genetic requirements and function. In the early otic vesicle, most cilia are short and immotile. Long, immotile kinocilia on the first sensory hair cells tether the otoliths, biomineralized aggregates of calcium carbonate and protein. Small numbers of motile cilia at the poles of the otic vesicle contribute to the accuracy of otolith tethering, but neither the presence of cilia nor ciliary motility is absolutely required for this process. Instead, otolith tethering is dependent on the presence of hair cells and the function of the glycoprotein Otogelin. Otic cilia or ciliary proteins also mediate sensitivity to ototoxins and coordinate responses to extracellular signals. Other studies are beginning to unravel the role of ciliary proteins in cellular compartments other than the kinocilium, where they are important for the integrity and survival of the sensory hair cell.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: zebrafish, otic vesicle, kinocilia, motile cilia, otolith, sensory hair cell
1. Introduction
The cilia of vertebrate sensory organs are extraordinarily diverse in morphology and function, forming an integral part of cellular receptors for light, odorants, sound, gravity and motion. The zebrafish, Danio rerio, is a widely used vertebrate model system for development and disease, with particular advantages for combining imaging in the live animal with genetic or pharmacological manipulation of protein function. Cilia in the zebrafish retina, nose,1 inner ear and lateral line have all been the subject of intense study; in this review, I focus on cilia in the inner ear, with occasional reference to the lateral line. From the earliest stages of its development, the inner ear is a major site of expression for genes coding for ciliary proteins. Analysis of the different ciliary types in the early otic vesicle has revealed some fascinating biology linking both immotile kinocilia and motile cilia-driven fluid flow with the formation of the otoliths. In the lateral line, a sensory system closely related to the inner ear, kinocilia of the sensory hair cells in superficial neuromasts are uniquely accessible for study. Both the ear and lateral line in zebrafish form important models for our understanding of hearing, balance and deafness in humans.
2. Three distinct types of cilia in the zebrafish otic vesicle
There are at least three distinct types of cilia in the zebrafish ear at early stages. From the onset of hollowing of the otic placode to form a vesicle at 18 h post fertilization (hpf), most cells of the otic epithelium bear a single immotile cilium on their apical (luminal) side [2]. Each sensory hair cell also bears a single cilium, the kinocilium, located on one side of the stereociliary bundle, an apical collection of microvillar-like projections that respond to mechanical deflection. In the early otic vesicle (20–25-somite stage), hair cells (tether cells) form in two pairs, one at each pole of the otic vesicle [3,4], and thus initially there are only four kinocilia among the 200 or so cilia in total in each ear (figure 1a,b). Despite the prefix kino-, indicating movement, zebrafish hair cell kinocilia are not thought to generate active motility. However, motile cilia (distinct from the kinocilia and the immotile cilia) are also found in the ear, with most concentrated at the poles in close proximity to the hair cells [2,8,9]. Although some reports have suggested otherwise, motile cilia only appear to make up a small fraction of the total number of cilia in the ear, with the vast majority (92–98%) being immotile [2].
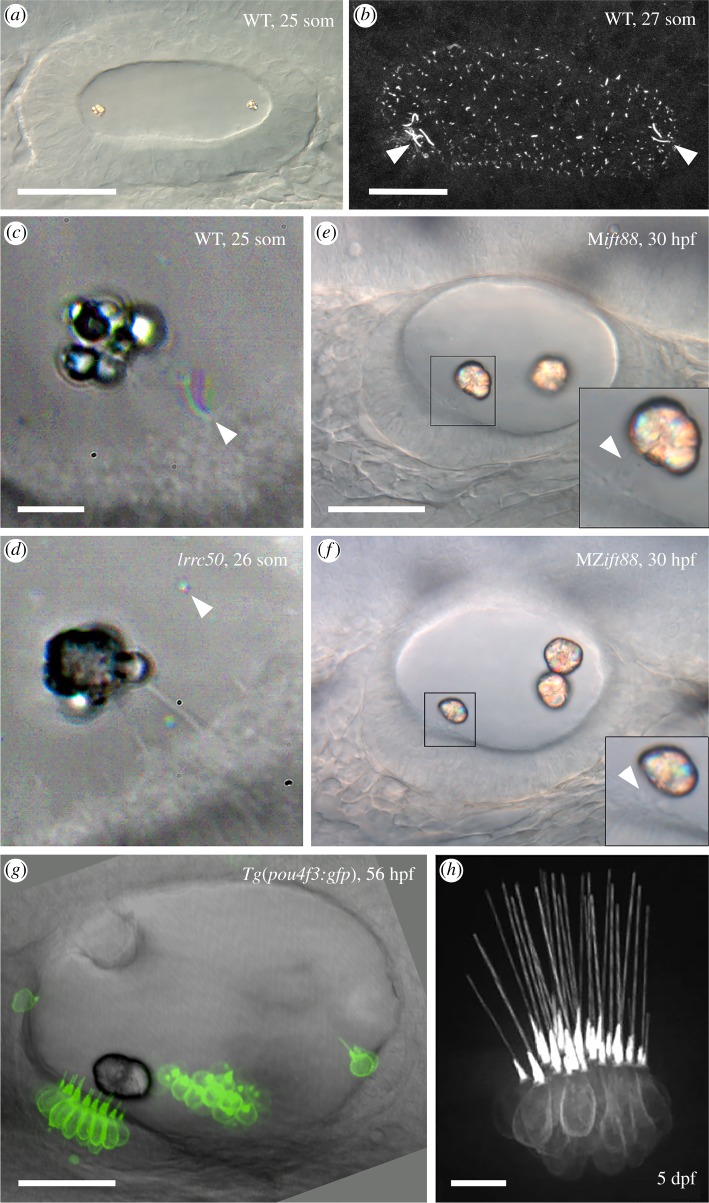
Figure 1.
Cilia and otolith formation in the zebrafish ear. (a) Differential interference contrast image of the wild-type otic vesicle at the 25-somite stage, showing the two birefringent otoliths. Scale bar, 40 µm. (b) Lumen of the otic vesicle at the 27-somite stage, stained with an anti-acetylated Tubulin antibody. The arrowheads mark longer tether kinocilia and motile cilia at the anterior and posterior poles of the otic lumen. Scale bar, 20 µm. (c) Time-to-colour merged image of the nascent otolith in a phenotypically wild-type embryo at the 25-somite stage (sibling of the embryo in d). The colour (arrowhead) indicates a motile cilium near the otolith. Scale bar, 10 µm (also applies to d). (d) Time-to-colour merged image of the nascent otolith in a homozygous lrrc50 (dnaaf1) mutant. The otolith has adhered to the tips of two kinocilia (clearly visible in this image), despite the lack of ciliary motility. The arrowhead marks an otolith precursor particle. (e) Phenotypically wild-type embryo (sibling of the embryo in f) lacking maternal ift88 contribution but with normal zygotic ift88 function at 30 hpf. Two otoliths are present, attached to kinocilia (arrowhead, inset). Scale bar, 30 µm (also applies to f). (f) Typical otolith phenotype for a ciliary mutant, in this case an embryo lacking both maternal and zygotic ift88 function. Three otoliths are present, but still localize to the anterior and posterior poles of the ear. The anterior otolith sits directly on the hair cell stereociliary bundles (arrowhead, inset); all cilia are absent. (g) Transgenic zebrafish otic vesicle at 56 hpf expressing GFP in hair cells. Note that the kinocilia of hair cells in the utricular macula do not all contact the anterior otolith (bottom left). (h) Image of a crista in the same transgenic line at 5 dpf. Note the long straight kinocilia. Scale bar, 10 µm. a, b, e, f and g are lateral views with anterior to the left. Abbreviations: dpf, days post fertilization; hpf, hours post fertilization; M, maternal; MZ, maternal-zygotic; som, somite stage; WT, phenotypically wild-type. a, c, d and e are reproduced from [5]; b and f are reproduced from [2]; g is reproduced from [6]; h is reproduced from [7].
3. Ultrastructure of cilia in the zebrafish ear
Transmission electron microscopy of kinocilia from zebrafish inner ear hair cells reveals a 9 + 2 axoneme (nine doublet microtubules surrounding a central pair of single microtubules) [10], as described for the supernumerary ear kinocilia in homozygous mibta52b mutants [9]. Although based on very few examples in zebrafish, these studies corroborate findings for utricular and lateral line kinocilia in another teleost fish, the burbot (Lota lota) [11–13]. Fish sensory hair cell kinocilia thus make up a class of cilium (9 + 2, but immotile) often ignored in overviews of ciliary ultrastructure. Hair cell kinocilia are thought to have outer dynein arms and radial spokes, but to lack inner dynein arms and nexin links, as summarized by McHenry & van Netten [14], although this has not been shown definitively for the zebrafish. It will be exciting to see whether the relatively new technique of cryo-electron tomography, already so successful in revealing spectacular ultrastructural detail in the motile 9 + 2 flagellum of the unicellular alga Chlamydomonas [15,16], and of the actin filaments in mammalian vestibular hair cell stereocilia [17,18], can be applied to zebrafish hair cell kinocilia.
Kinocilia of amphibian vestibular hair cells also have the 9 + 2 pattern (for example, those of the marsh frog, Pelophylax ridibundus [19]). However, kinocilia of mammalian (rat and guinea pig) vestibular hair cells have been described to have a 9 + 0 axoneme, with electron-dense material (but no clear microtubules) at the centre [20,21]. In amniote auditory hair cells, there are further variations: the transient kinocilia of immature hair cells in cochleae of the chick and mouse, for example, have a 9 + 0 arrangement near the base, but a pattern of eight doublet microtubules surrounding one central doublet (presumed to be one of the nine outer doublets displaced inward) further up the ciliary shaft [22,23]. The axonemal configuration of the short immotile cilia and the motile cilia present at early stages in the zebrafish otic vesicle has not yet been described. Given the similarity of the immotile short cilia to primary cilia, and the rotational movement of the motile cilia (see below), it might be expected that both types have a 9 + 0 axoneme.
4. Ciliary motility and cilia-driven fluid flow in the zebrafish ear
Motile cilia in the zebrafish ear move in a rotational fashion, with an estimated beat frequency of 28–44 Hz, depending on stage (and possibly other factors such as strain and temperature). Beating cilia can be imaged using high-speed video microscopy at 300 frames per second and above, and are found to be concentrated at the anterior and posterior poles of the early otic vesicle, close to, but distinct from, the immotile tether kinocilia [2,8,9]. In the early otic vesicle, fluid flow driven by ciliary movement can be inferred from observations of the free otolith precursor particles within the vesicle lumen, which move in vortices near the anterior and posterior poles [3]. The flow has been mapped using particle image velocimetry, by collecting otolith precursor particles in an optical trap and releasing them into the flow near motile cilia [8]. These measurements, together with a physical model simulating cilia-driven flow in a spherical cavity, indicated that the flow field near the tether kinocilia (where most motile cilia are concentrated) is advective, whereas the flow field further from the otolith is dominated by diffusion [8].
In contrast to the motile cilia in the ear, the kinocilia of zebrafish ear and lateral line hair cells are immotile and rigid in the living animal [2,14]. This is beautifully illustrated by live imaging of the Tg(pou4f3 : gfp) line, which expresses a membrane-tethered GFP specifically in hair cells, revealing the long straight kinocilium, bright stereociliary bundle and hair cell soma of crista hair cells [7] (figure 1h). Tether cell kinocilia at the poles of the early otic vesicle show some passive displacement as a result of the beating of nearby motile cilia, but do not display active movement [2].
5. Expression of genes required for ciliary motility in the zebrafish ear
The transcription factor Foxj1 is required for the expression of a suite of genes coding for proteins expressed in motile cilia [9,24,25]. There are two foxj1 genes in the zebrafish genome: the ear is a major site of expression for foxj1b, but not foxj1a [24–26]. foxj1b is initially expressed throughout the otic placode at 10 hpf, some 8 h before a lumen forms in the vesicle and motile cilia are apparent. In the otic vesicle, foxj1b expression becomes restricted to the poles at 22 hpf, apparently marking the first hair cells (the tether cells) developing there [9]. (Expression in hair cells is surprising, as the kinocilia, as discussed above, are immotile.) Otic expression of foxj1b is dependent on Wnt signalling: it is strongly downregulated after heat shock-induced mis-expression of the Wnt inhibitor Dkk [27].
In a screen for genes regulated by Foxj1 transcription factors, over 600 differentially expressed genes were uncovered in zebrafish embryos overexpressing foxj1a [28]. In this study, the authors assayed for otolith abnormalities (see below) as one of five tissue-specific phenotypic criteria to indicate a successful disruption of motile cilia. Of 50 randomly selected genes tested for function by morpholino-mediated knockdown, two genes appeared to be required for the function of motile cilia in all tissues except the otic vesicle, whereas knockdown of another two (arhgef18b and ect2l, coding for guanine nucleotide exchange factors) gave defects that were specific to the ear and Kupffer's vesicle [28]. These results highlight potential commonalities between the motile cilia in the ear and those in Kupffer's vesicle, both of which have rotational movement.
As expected for tissues expressing foxj1b, the zebrafish otic placode and vesicle are prominent sites of expression for many genes known to be required for ciliogenesis and ciliary motility (see [2] and references therein). These include genes coding for Dyneins and Dynein Axonemal Assembly Factors (DNAAFs) [24,29–34]. A comprehensive expression analysis of dynein axonemal heavy chain (dnah) genes in the zebrafish revealed several expressed in the ear, including one (dnah9l) that was ear-specific [29]. The tissue specificity of the dnah expression patterns appears to correlate with the type of ciliary movement present (oscillatory or rotational): notably, the subset of dnah genes expressed in the ear largely seems to overlap with that expressed in the floorplate and Kupffer's vesicle, again highlighting tissues with rotational ciliary movement [29]. All four zebrafish PIH (Protein Interacting with Hsp90) genes are also expressed in the otic placode or vesicle, some very strongly [29]. PIH gene products are DNAAFs implicated in the pre-assembly of dynein arms prior to their transport into cilia. Transcripts for components of the radial spokes of the axoneme (leucine-rich repeat-containing protein 23, Lrrc23) and radial spoke head proteins (Rsph9 and Rsph4a) are also expressed in the zebrafish otic placode during somite stages [35,36].
6. The role of zebrafish otic cilia in otolith formation
Many organisms, both invertebrate and vertebrate, sense gravity and other linear acceleration through an inertial mass coupled to a mechanosensitive sensory cell type, which is often ciliated. In ctenophores (comb jellies), for example, lithocytes—entire cells whose cytoplasm harbours a membrane-bound concretion—are actively transported from the base to the tip of sensory cilia in a truly astonishing example of ciliary surface transport (see [37] and references therein). In teleost fish, the inertial mass is the otolith, a dense crystalline aggregate of calcium carbonate and protein that sits over hair cells in the sensory maculae, embedded within an extracellular matrix, the otolithic membrane (for a recent review, see [38]). Otoliths grow by daily incremental deposition of otolithic material: sectioning through an otolith thus reveals rings that can be used to determine the age and life history of a fish [39], just as in the trunk of a tree.
Both kinocilia and motile cilia are implicated in the earliest stages of otolith formation in the zebrafish, as summarized in figure 2. Otolith precursor particles are initially found distributed throughout the otic vesicle lumen, but adhere specifically to the tips of kinocilia on the first hair cells to differentiate in the ear (named tether cells for this reason) [2,3,9]. The composition of the particles has not been fully described, but they are likely to contain Cadherin11 [40], Otoconin90 [41,42] and Starmaker [43,44]. At the kinociliary tip, the bound otolith precursor particles serve as a nucleation site for deposition of further layers of protein and mineral (calcium carbonate), and thus the tether kinocilia become partially embedded within the structure of the otolith. Efficient production or nucleation of the precursor particles, in both zebrafish and medaka, appears to require the activity of the enzyme polyketide synthase [41,45,46]. Growing zebrafish otoliths incorporate a number of proteins, including Otolith Matrix Protein-1, Sparc, Starmaker and others [47–50]. Exciting progress is being made in elucidating the mechanism of otolith biomineralization, which is dependent on the activity of the calcium-binding protein Starmaker [43,50,51] and the conserved proton-selective ion channel Otopetrin 1 [44,52–54].
Figure 2.
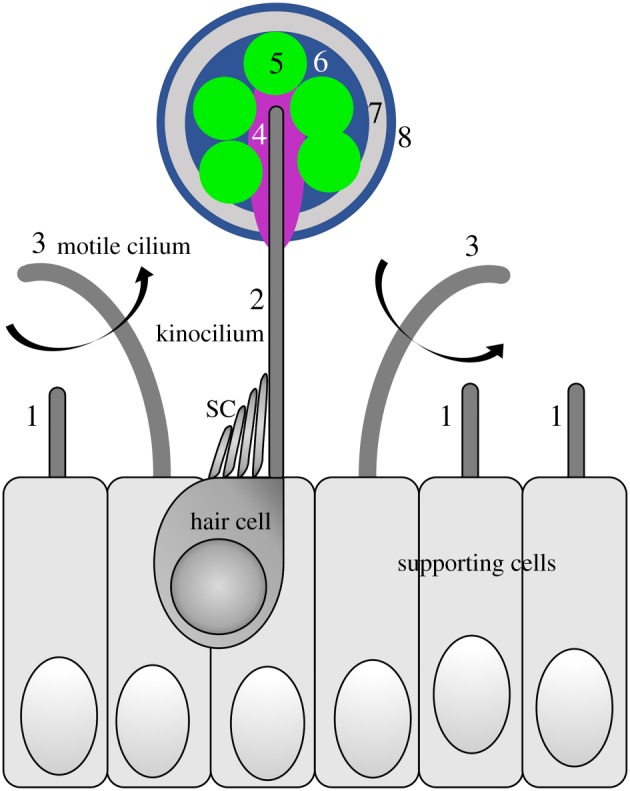
Schematic diagram summarizing the main early steps in zebrafish otolith formation discussed in this review. A single sensory hair cell is shown for simplicity, but tether cells normally form in pairs (figure 1d). Not to scale. SC, stereocilia. For details and supporting references, see the text. Key: 1, short, immotile cilia, present on most otic epithelial cells; 2, tether kinocilium, present on each of the first sensory hair cells to develop in the ear; 3, motile cilia—these contribute to the accuracy of otolith precursor particle tethering; 4, extracellular proteins, possibly bound to the kinociliary tip, required for otolith precursor particle tethering: Otogelin (and others?); 5, otolith precursor particles, thought to contain Cadherin11, Otoconin90 and Starmaker; 6, otolith proteins incorporated after initial nucleation, including Otolith Matrix Protein-1, Sparc, Starmaker and others; 7, biomineralization: deposition of crystalline calcium carbonate, requiring the activity of Starmaker and Otopetrin 1; 8, further layers added daily throughout life.
A defect in otolith formation is a signature phenotype of almost all zebrafish ciliary mutants and morphants described to date, including those with a loss of ciliary motility [2,9,36,55–61]. This observation led to the idea that ciliary motility must somehow be critical for otolith formation. As discussed above, ciliary flow can readily be demonstrated in the zebrafish ear, and various interpretations have been proposed for its function: that it agitates and distributes the otolith precursor particles, preventing their premature aggregation [3,8,9]; that it creates an advection zone to draw free precursor particles towards the nucleating otolith [8]; or that it contributes to otolith shape [8]. Nevertheless, the otolith phenotype of ciliary mutants is often mild, variable and incompletely penetrant: a typical defect is the presence of three otoliths rather than two, which may resolve over time to give the normal pattern.
A breakthrough in understanding came from the unexpected observation that homozygous maternal-zygotic (MZ) mutants for an intraflagellar transport (IFT) gene, ift88, which lack cilia altogether [62], have a similarly mild otolith phenotype [2] (figure 1e,f). On close inspection, the otoliths in MZift88tz288b mutants formed close to the surface of the sensory epithelium, but still nucleated on time at the poles of the ear, in close proximity to the tether cells, which are present but lack kinocilia in this mutant. This suggested that both the immotile tether kinocilia and ciliary motility are, in fact, dispensable for otolith formation. Notably, premature aggregation of otolith precursor particles was not observed in either MZift88tz288b mutants (lacking cilia altogether) or in the lrrc50 (dnaaf1) mutant, which lacks ciliary motility (figure 1c,d) [2,31,32], and so the proposition that ciliary flow acts to prevent premature aggregation of otolith precursor particles seems unlikely.
A much more striking otolith phenotype—quite distinct from that of the ciliary or biomineralization mutants—results from the lack of tether cells in the zebrafish ear. Injection of an atoh1b morpholino into wild-type embryos, which prevents the formation of tether cells, but does not compromise ciliogenesis or ciliary motility, disrupts the early stages of otolith formation completely: otolith precursor particles remain distributed throughout the ear. Here, they become larger than normal and appear birefringent, demonstrating that the process of otolith biomineralization does not require attachment to kinocilia. Eventually, the crystalline particles coalesce to form a single untethered otolith [2]. This observation strongly suggested that it is the presence of tether cells, rather than cilia or cilia motility per se, that is essential for otolith development, implicating the function of a hair cell-specific factor or factors in the initial tethering step. Such a factor, perhaps produced by the tether cells, might become transported to the kinociliary tip.
In a quest to identify the otolith precursor-binding factor, we and others turned to mutants that phenocopied the atoh1b morphant. Isolated in forward mutagenesis screens on the basis of their phenotype—a single otolith, rather than two, in each ear—there were several promising candidates, initially given the charming names of einstein (German: one stone) [63], rock solo [64] and monolith [65]. Both the einstein [66] and rock solo [64] mutants were found to disrupt the gene otogelin, which codes for a large glycoprotein. Otogelin remains the best candidate to date for an otolith precursor-binding factor. Although expression of otogelin mRNA is not restricted to hair cells [66], staining with an anti-Otogelin antibody suggests that the protein itself becomes concentrated at the distal half of the tether kinocilia [41]. How Otogelin gets there is not currently understood. It is possible that one or more adhesion proteins, trafficked to the kinociliary tip, help to localize it there. In this context, it is interesting to note that the integrin Itga8 and protocadherin Pcdh15 localize in a complex at the kinociliary tip of zebrafish lateral line hair cells [67]. In chick auditory hair cells, transport of Pcdh15 to the kinociliary tip is dependent on the IFT machinery and FGFR1 activity [22]. Zebrafish lateral line and amniote auditory kinocilia, of course, do not bind otoliths, but it is possible that a tether cell-specific factor contributes to the localization of both Otogelin protein and otolith precursor particles at the tips of tether kinocilia via a similar mechanism.
Note that ear and otolith morphology vary enormously between different teleosts, and it is not known whether otoliths are nucleated in the same way in other species. Otolith formation was reported to be normal in the medaka mutant kintoun, which lacks ciliary motility as a result of mutation in a gene coding for a dynein arm pre-assembly factor [45]. As in zebrafish, this argues against an essential requirement for ciliary motility in otolith formation in this species. In the zebrafish, after the initial nucleation step, it is unlikely that cilia play a further role in otolith growth. Additional hair cell kinocilia do not all contact the otolith directly as the tether kinocilia do (figure 1g), and total otolith growth is unaffected by focal laser ablation of motile cilia after otolith nucleation [8]. As the sensory patch and otolith develop further, a layer of extracellular matrix, the otolithic membrane, is deposited on the apical surface of the sensory patch, and it is this structure that now tethers the otolith to its cognate sensory epithelium [66]. Several protein components of the otolith and otolithic membrane in zebrafish are conserved with those of otoconia and the otoconial membrane, respectively, in vestibular end organs of the mammalian inner ear (reviewed in [68]). Here, multiple small otoconia are present rather than the large individual otoliths found in bony fish. A role for cilia in the initial formation of mammalian otoconia has not been described, to my knowledge.
7. Length control of cilia in the zebrafish ear and lateral line
The length of cilia in the zebrafish ear is both dynamic and sensitive to perturbation. All cilia in the ear are initially of similar length at the very earliest otic vesicle stages (19 somites), but rapidly become differentiated from one another [2]. By the 21-somite stage, cilia at the poles of the ear (tether cell kinocilia and motile cilia) are longer than other cilia throughout the vesicle lumen. Within a few hours, the motile cilia have regressed in length, and by 24 hpf are about half the length of the kinocilia [2]. Kinocilia of different sensory hair cell types can also be distinguished on the basis of length. At 24–25 hpf, the tether kinocilia in the presumptive sensory maculae are about 8 µm long, with the distal 3 µm embedded in the otolith [2]. Kinocilia of the crista hair cells are the longest, projecting up to 30 µm into the lumen of the ear at 4 days post fertilization (dpf) [7,69] (figure 1h). Kinocilia on lateral line hair cells are 14–20 µm long in the zebrafish larva, occupying about half the length of the gelatinous cupula that covers them [14,69,70]. In adult teleost fish, kinociliary length can also vary in different regions of the sensory maculae, as shown for the blue gourami, Trichogaster trichopterus [71].
The length of a cilium depends on a balance between kinesin-driven anterograde (IFT-B) and dynein-driven retrograde (IFT-A) transport along the axoneme. Mutations in genes coding for proteins involved in ciliogenesis or IFT can thus disrupt cilia length, resulting in either shorter or longer cilia than normal. Disruption of foxj1b results in shorter motile cilia and kinocilia in the ear, whereas transgenic mis-expression results in the development of long motile cilia throughout the otic vesicle [9]. Ubiquitous transgenic expression of a GFP-tagged murine Arl13b protein in the zebrafish, while labelling most cilia very effectively [72], also increases their length, and is reported to disrupt both ciliary motility and otolith formation in the ear [73]. Mutation of arl13b results in shorter lateral line kinocilia; cilia in the ear were not examined [74]. Cilia in all tissues, including the ear, are missing in MZ mutants for the IFT-B gene ift88 [62] (see above) and the ciliogenesis gene ta3 [75], while disruption of another IFT-B gene, ift46 [76], or the IFT-A gene ift122 [77] results in fewer or abnormal cilia in many tissues, including the ear and lateral line.
Different ciliary types in the zebrafish ear also have different genetic requirements for some gene products. Mutation of the abcc4 gene, which codes for a transporter involved in prostaglandin signalling, appears to disrupt short cilia, but not tether kinocilia, in the early otic vesicle [60]. The long kinocilia of the cristae seem particularly resistant to genetic perturbation of kinesin subunit genes, which may indicate the existence of additional crista-specific IFT-B components. Crista cilia were unaffected by mutation of kif3b, which codes for a heterotrimeric kinesin II subunit, whereas cilia in maculae were lost altogether [78]. Short crista kinocilia were also able to develop in a strong loss-of-function kif3a mutant, which otherwise eliminates cilia in maculae of the ear and in several other tissues, including the lateral line [79]. Cilia length in the zebrafish ear is also governed by the activity of Crumbs genes [80,81]. mRNA for crb3a is strongly and almost exclusively expressed in the ear; the Crb3a protein localizes to the base of hair cell kinocilia at the otic vesicle stage, and to the basal body of kinocilia in adult hair cells. Morpholino-mediated knockdown of crb3a results in shorter kinocilia in maculae [80], but interestingly, in loss-of-function mutants for crb3a or crb2b, cilia in the cristae get longer [81].
8. The role of kinocilia and ciliary proteins in hair cell planar polarity, stereociliary bundle assembly and mechanosensitivity
The eccentric position of the kinocilium on the hair cell apical surface defines the directional sensitivity of the cell: deflection of the stereociliary bundle in the direction of the kinocilium depolarizes the cell, whereas deflection in the opposite direction hyperpolarizes the cell (see [70] and references therein). This can be demonstrated in the zebrafish larva by imaging of lateral line hair cells labelled with the calcium indicator GCaMP6s after deflection of kinocilia in the neuromast cupula with a glass fibre or fluid jet [70,82]. The kinociliary axoneme is arranged in a polar fashion with respect to the hair bundle, with the first doublet microtubule (number 1) located on the side facing the stereociliary bundle [11].
In mammalian auditory hair cells, the kinocilium plays a role in orientation of the hair cell stereociliary bundle, together with planar cell polarity genes (see [83] and references therein). However, there is currently no strong evidence to suggest a role for kinocilia in establishing hair bundle polarity in zebrafish hair cells, which are thought to be more closely related to mammalian vestibular hair cells. Normal stereocilia polarity was observed in both ear and lateral line hair cells in the zebrafish ift88tz288b mutant, which has missing or stunted kinocilia [5,70]. This contrasts with the obvious disruption of planar cell polarity in mammalian auditory hair cells in the murine Ift88 mutant, where mis-oriented and even circular stereociliary bundles were observed in the organ of Corti [83]. Although hair bundle polarity is not affected in zebrafish zygotic ift88tz288b mutants, hair bundle morphology is abnormal and fewer hair cells form stereocilia [5,84]. Analysis of individual mutant phenotypes and assays for protein colocalization revealed that the Ift88 protein helps to stabilize a complex of the Usher syndrome 1 (USH1) proteins Cdh23, Harmonin and MyoVIIa in the endoplasmic reticulum (ER), ensuring their correct trafficking for assembly of the stereociliary bundle. Disruption of any one component of the complex can result in ER stress and eventual hair cell death [84]. Thus, Ift88 and possibly other ciliary proteins have additional roles in protein or vesicular traffic within hair cells.
Kinocilia of immature zebrafish lateral line hair cells also have an early role in mediating hair cell mechanosensitivity, a property restricted to the stereociliary bundle in mature hair cells [70]. Small but robust responses were measured in hair cells with morphologically immature stereociliary bundles, but with an opposite polarity to responses found in mature hair cells. By using the ift88tz288b mutant, in which cilia are either absent or stunted, the authors were able to show that these early responses were mediated by kinocilia. The loss of responses in mutants for the cadherin genes cdh23 and pcdh15, together with pharmacological disruption, demonstrated that the early polarity-reversed responses were dependent on the integrity of kinocilial links between the kinocilium and adjacent stereocilia [70].
9. The role of kinocilia and ciliary proteins in sensitivity to aminoglycoside ototoxicity
Aminoglycoside antibiotics are potent ototoxins, killing sensory hair cells in both zebrafish and mammals (see [85] and references therein). As a result, off-target damage to the auditory and vestibular systems can be a considerable clinical problem in patients treated with aminoglycosides for serious acute or chronic bacterial infections. Work in the zebrafish has revealed that cilia and ciliary proteins appear to play a role in the uptake and/or toxicity of these compounds. Mutations in anterograde IFT-B, retrograde IFT-A and ciliary transition zone genes in the zebrafish all confer protection against neomycin ototoxicity [74,86,87]. In most IFT mutants, where ciliary morphology and hair cell mechanotransduction are compromised, neomycin uptake into the cell was disrupted [74,87]. However, mutations in transition zone genes (cc2d2a, mks1, cep290)—where ciliogenesis, mechanotransduction and neomycin uptake were unaffected—still conferred partial protection, suggesting that transition zone proteins may modulate trafficking or metabolism of neomycin once it has entered the hair cell [74]. These studies are important for our understanding of aminoglycoside uptake and toxicity in hair cells, with implications for the search for and design of new otoprotective compounds or alternative antibiotic agents.
10. Role of cilia in signalling in the zebrafish ear
Cilia are well known to act as cellular antennae for the reception of various extracellular signals (reviewed in [88]). As the zebrafish ear is a prominent site of expression for many ciliary genes, it is perhaps surprising that few studies to date have addressed the contribution of cilia to cell signalling in this tissue. In zebrafish, the complete loss of cilia in MZift88tz288b mutants, or depletion of cilia in igu (dzip1) mutants, reduces levels but expands the reach of Hedgehog pathway activity, rather than eliminating it [62]. This finding corroborates preliminary observations of the later ear phenotype of MZift88 mutants, which have morphological abnormalities that resemble those of igu (dzip1) mutants [5], but are not a close phenocopy of the mirror-symmetric double-anterior ears generated by a strong loss of Hh signalling (see [89] and references therein). Signalling via cilia or cilia-driven flow, therefore, does not appear to play a major role in otic axial patterning in the otic vesicle. This contrasts with the similarly sized Kupffer's vesicle, where ciliary-driven flow is essential for left–right patterning of the embryo (see [90] and references therein). However, given the mix of motile and non-motile ciliary types in both the early otic vesicle and Kupffer's vesicle [90], their shared dependence on Wnt signalling [27], and the overlap in their ciliary transcriptome profile [28,29], this remains an interesting area to explore further.
The active phosphorylated form of type II calcium/calmodulin kinase CaMK-II is expressed in the zebrafish ear in hair cell kinocilia, most strongly at their base, where it may localize to the ciliary transition zone and/or stereociliary bundle [91]. Interestingly, disruption of CaMK-II activity—either pharmacologically or via morpholino-mediated knockdown of camk2g1—resulted in a disruption of Delta–Notch signalling, and the development of supernumerary tether cells. Morphants and treated embryos had otolith defects that resembled those of mib1ta52b (loss of Notch signalling) mutants at 24 hpf, where multiple tether kinocilia make contact with otolithic material [2,91]. At later stages (30–72 hpf), camk2g1 morphants or treated embryos showed a phenotype characteristic of ciliary mutants, with ectopic fused or third otoliths in the ear [91]. Thus, signalling via calcium, CaMK-II, Delta and cilia appears to be closely linked in the zebrafish ear.
11. Conclusion
Our understanding of ciliary structure and function has come a long way in the last 50 years, although many of the stunning electron micrographs of kinocilia from the 1960s [11,12,20] are still among the clearest in the literature, and repay careful attention. There remains enormous interest in cilia, not least for their important link to rare genetic disease: mutations in the human orthologues of many of the genes discussed here result in ciliopathies that affect the quality of life and are challenging to diagnose and treat. Sensorineural deafness is a feature of several of these syndromes, underlining the need to understand cilia function in the inner ear. Here, the zebrafish holds promise as a model system, partly because the otoliths are a highly visible proxy readout of ciliary integrity. As technologies to achieve tissue-specific disruption of zebrafish gene function improve (e.g. [92–94]), new ways of working to analyse ciliary function in the ear will become possible, where—unlike in the brain or kidney—loss of function should not compromise adult viability. Cilia in the zebrafish ear are also interesting in their own right; key among the problems remaining to be solved is an understanding of the unique protein or lipid composition at the tip of the tether kinocilium that distinguishes it from other cilia, allowing otolith precursor particles to bind there so precisely and robustly. Comparison of the rotational motile cilia in the otic vesicle to those in Kupffer's vesicle or the floorplate may also lead to general insights into mechanisms of cilia-driven flow and its consequences for developmental patterning.
Acknowledgements
Thanks are due to Raymond Goldstein, Gáspár Jékely and Kirsty Wan for organizing a hugely enjoyable and thought-provoking meeting, which provided the springboard for this review. I am grateful to Georgina Stooke-Vaughan for comments on the manuscript.
Endnote
For a review of motile cilia in the olfactory system, see reference [1].
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
Work in the Whitfield lab is funded by the BBSRC (grant no. BB/S007008/1).
References
- 1.Ringers C, Olstad EW, Jurisch-Yaksi N. 2020. The role of motile cilia in the development and physiology of the nervous system. Phil. Trans. R. Soc. B 375, 20190156 ( 10.1098/rstb.2019.0156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stooke-Vaughan GA, Huang P, Hammond KL, Schier AF, Whitfield TT. 2012. The role of hair cells, cilia and ciliary motility in otolith formation in the zebrafish otic vesicle. Development 139, 1777–1787. ( 10.1242/dev.079947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. 1997. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev. Biol. 191, 191–201. ( 10.1006/dbio.1997.8736) [DOI] [PubMed] [Google Scholar]
- 4.Haddon C, Lewis J. 1996. Early ear development in the embryo of the zebrafish, Danio rerio. J. Comp. Neurol. 365, 113–123. () [DOI] [PubMed] [Google Scholar]
- 5.Stooke-Vaughan GA. 2013. The role of cilia in inner ear development and otolith formation in the zebrafish otic vesicle. PhD thesis, University of Sheffield; See http://etheses.whiterose.ac.uk/id/eprint/4992. [Google Scholar]
- 6.Maier EC, Saxena A, Alsina B, Bronner ME, Whitfield TT. 2014. Sensational placodes: neurogenesis in the otic and olfactory systems. Dev. Biol. 389, 50–67. ( 10.1016/j.ydbio.2014.01.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stawicki TM, Owens KN, Linbo T, Reinhart KE, Rubel EW, Raible DW. 2014. The zebrafish merovingian mutant reveals a role for pH regulation in hair cell toxicity and function. Dis. Models Mech. 7, 847–856. ( 10.1242/dmm.016576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu D, Freund JB, Fraser SE, Vermot J. 2011. Mechanistic basis of otolith formation during teleost inner ear development. Dev. Cell 20, 271–278. ( 10.1016/j.devcel.2010.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Lau D, Ng CP, Roy S. 2011. Cilia-driven fluid flow as an epigenetic cue for otolith biomineralization on sensory hair cells of the inner ear. Development 138, 487–494. ( 10.1242/dev.057752) [DOI] [PubMed] [Google Scholar]
- 10.Leventea E, Hazime K, Zhao C, Malicki J. 2016. Analysis of cilia structure and function in zebrafish. Methods Cell Biol. 133, 179–227. ( 10.1016/bs.mcb.2016.04.016) [DOI] [PubMed] [Google Scholar]
- 11.Flock Å, Duvall AJ. 1965. The ultrastructure of the kinocilium of the sensory cells in the inner ear and lateral line organs. J. Cell Biol. 25, 1–8. ( 10.1083/jcb.25.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flock Å, Wersäll J. 1962. A study of the orientation of the sensory hairs of the receptor cells in the lateral line organ of fish, with special reference to the function of the receptors. J. Cell Biol. 15, 19–27. ( 10.1083/jcb.15.1.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flock Å. 1964. Structure of the macula utriculi with special reference to directional interplay of sensory responses as revealed by morphological polarization. J. Cell Biol. 22, 413–431. ( 10.1083/jcb.22.2.413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHenry MJ, van Netten SM.. 2007. The flexural stiffness of superficial neuromasts in the zebrafish (Danio rerio) lateral line. J. Exp. Biol. 210, 4244–4253. ( 10.1242/jeb.009290) [DOI] [PubMed] [Google Scholar]
- 15.Nicastro D. 2009. Cryo-electron microscope tomography to study axonemal organization. Methods Cell Biol. 91, 1–39. ( 10.1016/S0091-679X(08)91001-3) [DOI] [PubMed] [Google Scholar]
- 16.Bui KH, Pigino G, Ishikawa T. 2011. Three-dimensional structural analysis of eukaryotic flagella/cilia by electron cryo-tomography. J. Synchrotron Radiat. 18, 2–5. ( 10.1107/S0909049510036812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metlagel Z, et al. 2019. Electron cryo-tomography of vestibular hair-cell stereocilia. J. Struct. Biol. 206, 149–155. ( 10.1016/j.jsb.2019.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sazzed S, Song J, Kovacs JA, Wriggers W, Auer M, He J. 2018. Tracing actin filament bundles in three-dimensional electron tomography density maps of hair cell stereocilia. Molecules 23, 882 ( 10.3390/molecules23040882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagel G, Neugebauer D-C, Schmidt B, Thurm U. 1991. Structures transmitting stimulatory force to the sensory hairs of vestibular ampullae of fishes and frog. Cell Tiss. Res. 265, 567–578. ( 10.1007/BF00340881) [DOI] [Google Scholar]
- 20.Hamilton DW. 1969. The cilium on mammalian vestibular hair cells. Anat. Rec. 164, 253–258. ( 10.1002/ar.1091640301) [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi T, Takasaka T, Tonosaki A, Watanabe H. 1989. Fine structure of guinea pig vestibular kinocilium. Acta Otolaryngol. 108, 26–30. ( 10.3109/00016488909107388) [DOI] [PubMed] [Google Scholar]
- 22.Honda A, Kita T, Seshadri SV, Misaki K, Ahmed Z, Ladbury JE, Richardson GP, Yonemura S, Ladher RK. 2018. FGFR1-mediated protocadherin-15 loading mediates cargo specificity during intraflagellar transport in inner ear hair-cell kinocilia. Proc. Natl Acad. Sci. USA 115, 8388–8393. ( 10.1073/pnas.1719861115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobkowicz HM, Slapnick SM, August BK. 1995. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J. Neurocytol. 24, 633–653. ( 10.1007/BF01179815) [DOI] [PubMed] [Google Scholar]
- 24.Yu X, Ng CP, Habacher H, Roy S. 2008. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat. Genet. 40, 1445–1453. ( 10.1038/ng.263) [DOI] [PubMed] [Google Scholar]
- 25.Hellman NE, et al. 2010. The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch. Proc. Natl Acad. Sci. USA 107, 18 499–18 504. ( 10.1073/pnas.1005998107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian T, Zhao L, Zhao X, Zhang M, Meng A. 2009. A zebrafish gene trap line expresses GFP recapturing expression pattern of foxj1b. J. Genet. Genomics 36, 581–589. ( 10.1016/S1673-8527(08)60150-2) [DOI] [PubMed] [Google Scholar]
- 27.Caron A, Xu X, Lin X. 2012. Wnt/β-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer's vesicle. Development 139, 514–524. ( 10.1242/dev.071746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choksi SP, Babu D, Lau D, Yu X, Roy S. 2014. Systematic discovery of novel ciliary genes through functional genomics in the zebrafish. Development 141, 3410–3419. ( 10.1242/dev.108209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi H, Oda T, Kikkawa M, Takeda H. 2018. Systematic studies of all PIH proteins in zebrafish reveal their distinct roles in axonemal dynein assembly. eLife 7, e36979 ( 10.7554/eLife.36979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe KM, et al. 2016. c21orf59/kurly controls both cilia motility and polarization. Cell Rep. 14, 1841–1849. ( 10.1016/j.celrep.2016.01.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rooijen E, Giles RH, Voest EE, van Rooijen C, Schulte-Merker S, van Eeden FJ.. 2008. LRRC50, a conserved ciliary protein implicated in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1128–1138. ( 10.1681/ASN.2007080917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan-Brown J, Schottenfeld J, Okabe N, Hostetter CL, Serluca FC, Thiberge SY, Burdine RD. 2008. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev. Biol. 314, 261–275. ( 10.1016/j.ydbio.2007.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hjeij R, et al. 2014. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am. J. Hum. Genet. 95, 257–274. ( 10.1016/j.ajhg.2014.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jerber J, Baas D, Soulavie F, Chhin B, Cortier E, Vesque C, Thomas J, Durand B. 2014. The coiled-coil domain containing protein CCDC151 is required for the function of IFT-dependent motile cilia in animals. Hum. Mol. Genet. 23, 563–577. ( 10.1093/hmg/ddt445) [DOI] [PubMed] [Google Scholar]
- 35.Sedykh I, TeSlaa JJ, Tatarsky RL, Keller AN, Toops KA, Lakkaraju A, Nyholm MK, Wolman MA, Grinblat Y. 2016. Novel roles for the radial spoke head protein 9 in neural and neurosensory cilia. Scient. Rep. 6, 34437 ( 10.1038/srep34437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Xie H, Wang Y, Zhao C. 2018. Radial spoke proteins regulate otolith formation during early zebrafish development. FASEB J. 32, 3984–3992. ( 10.1096/fj.201701359R) [DOI] [PubMed] [Google Scholar]
- 37.Noda N, Tamm SL. 2014. Lithocytes are transported along the ciliary surface to build the statolith of ctenophores. Curr. Biol. 24, R951–R952. ( 10.1016/j.cub.2014.08.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz-Mirbach T, Ladich F, Plath M, Heß M. 2019. Enigmatic ear stones: what we know about the functional role and evolution of fish otoliths. Biol. Rev. Camb. Philos. Soc. 94, 457–482. ( 10.1111/brv.12463) [DOI] [PubMed] [Google Scholar]
- 39.Campana SE, Neilson JD. 1985. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 42, 1014–1032. ( 10.1139/f85-127) [DOI] [Google Scholar]
- 40.Clendenon SG, Shah B, Miller CA, Schmeisser G, Walter A, Gattone VH 2nd, Barald KF, Liu Q, Marrs JA. 2009. Cadherin-11 controls otolith assembly: evidence for extracellular cadherin activity. Dev. Dyn. 238, 1909–1922. ( 10.1002/dvdy.22015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiessen KD, Grzegorski SJ, Chin Y, Higuchi L, Wilkinson CJ, Shavit JA, Kramer KL. 2019. Zebrafish otolith biomineralization requires polyketide synthase. Mech. Dev. 157, 1–9. ( 10.1016/j.mod.2019.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petko JA, Kabbani N, Frey C, Woll M, Hickey K, Craig M, Canfield VA, Levenson R. 2009. Proteomic and functional analysis of NCS-1 binding proteins reveals novel signaling pathways required for inner ear development in zebrafish. BMC Neurosci. 10, 27 ( 10.1186/1471-2202-10-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Söllner C, Burghammer M, Busch-Nentwich E, Berger J, Schwartz H, Riekel C, Nicolson T. 2003. Control of crystal size and lattice formation by Starmaker in otolith biomineralization. Science 302, 282–286. ( 10.1126/science.1088443) [DOI] [PubMed] [Google Scholar]
- 44.Söllner C, Schwarz H, Geisler R, Nicolson T. 2004. Mutated otopetrin 1 affects the genesis of otoliths and the localization of Starmaker in zebrafish. Dev. Genes Evol. 214, 582–590. ( 10.1007/s00427-004-0440-2) [DOI] [PubMed] [Google Scholar]
- 45.Hojo M, et al. 2015. Unexpected link between polyketide synthase and calcium carbonate biomineralization. Zool. Lett. 1, 3 ( 10.1186/s40851-014-0001-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MS, Philippe J, Katsanis N, Zhou W. 2019. Polyketide synthase plays a conserved role in otolith formation. Zebrafish 16, 363–369. ( 10.1089/zeb.2019.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang YJ, Stevenson AK, Yau PM, Kollmar R. 2008. Sparc protein is required for normal growth of zebrafish otoliths. J. Ass. Res. Otolaryngol. 9, 436–451. ( 10.1007/s10162-008-0137-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rotllant J, Liu D, Yan YL, Postlethwait JH, Westerfield M, Du SJ.. 2008. Sparc (Osteonectin) functions in morphogenesis of the pharyngeal skeleton and inner ear. Matrix Biol. 27, 561–572. ( 10.1016/j.matbio.2008.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murayama E, Herbomel P, Kawakami A, Takeda H, Nagasawa H. 2005. Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae. Mech. Dev. 122, 791–803. ( 10.1016/j.mod.2005.03.002) [DOI] [PubMed] [Google Scholar]
- 50.Kalka M, Markiewicz N, Ptak M, Sone ED, Ożyhar A, Dobryszycki P, Wojtas M. 2019. In vivo and in vitro analysis of starmaker activity in zebrafish otolith biomineralization. FASEB J. 33, 6877–6886. ( 10.1096/fj.201802268R) [DOI] [PubMed] [Google Scholar]
- 51.Wojtas M, Hołubowicz R, Poznar M, Maciejewska M, Ożyhar A, Dobryszycki P. 2015. Calcium ion binding properties and the effect of phosphorylation on the intrinsically disordered Starmaker protein. Biochemistry 54, 6525–6534. ( 10.1021/acs.biochem.5b00933) [DOI] [PubMed] [Google Scholar]
- 52.Saotome K, Teng B, Tsui CCA, Lee WH, Tu YH, Kaplan JP, Sansom MSP, Liman ER, Ward AB. 2019. Structures of the otopetrin proton channels Otop1 and Otop3. Nat. Struct. Mol. Biol. 26, 518–525. ( 10.1038/s41594-019-0235-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu YH, et al. 2018. An evolutionarily conserved gene family encodes proton-selective ion channels. Science 359, 1047–1050. ( 10.1126/science.aao3264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes I, et al. 2004. Otopetrin 1 is required for otolith formation in the zebrafish Danio rerio. Dev. Biol. 276, 391–402. ( 10.1016/j.ydbio.2004.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao C, Wang G, Amack JD, Mitchell DR. 2010. Oda16/Wdr69 is essential for axonemal dynein assembly and ciliary motility during zebrafish embryogenesis. Dev. Dyn. 239, 2190–2197. ( 10.1002/dvdy.22355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panizzi JR, Jessen JR, Drummond IA, Solnica-Krezel L. 2007. New functions for a vertebrate Rho guanine nucleotide exchange factor in ciliated epithelia. Development 134, 921–931. ( 10.1242/dev.02776) [DOI] [PubMed] [Google Scholar]
- 57.Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. 2009. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458, 651–654. ( 10.1038/nature07753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson CJ, Carl M, Harris WA. 2009. Cep70 and Cep131 contribute to ciliogenesis in zebrafish embryos. BMC Cell Biol. 10, 17 ( 10.1186/1471-2121-10-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pathak N, Austin CA, Drummond IA. 2011. Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J. Biol. Chem. 286, 11 685–11 695. ( 10.1074/jbc.M110.209817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin D, et al. 2014. Prostaglandin signalling regulates ciliogenesis by modulating intraflagellar transport. Nat. Cell Biol. 16, 841–851. ( 10.1038/ncb3029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jurisch-Yaksi N, et al. 2013. Rer1p maintains ciliary length and signaling by regulating γ-secretase activity and Foxj1a levels. J. Cell Biol. 200, 709–720. ( 10.1083/jcb.201208175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang P, Schier AF. 2009. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136, 3089–3098. ( 10.1242/dev.041343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitfield TT, et al. 1996. Mutations affecting development of the zebrafish inner ear and lateral line. Development 123, 241–254. [DOI] [PubMed] [Google Scholar]
- 64.Roberts R, Elsner J, Bagnall MW. 2017. Delayed otolith development does not impair vestibular circuit formation in zebrafish. J. Ass. Res. Otolaryngol. 18, 415–425. ( 10.1007/s10162-017-0617-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riley BB, Grunwald DJ. 1996. A mutation in zebrafish affecting a localized cellular function required for normal ear development. Dev. Biol. 179, 427–435. ( 10.1006/dbio.1996.0272) [DOI] [PubMed] [Google Scholar]
- 66.Stooke-Vaughan GA, Obholzer ND, Baxendale S, Megason SG, Whitfield TT. 2015. Otolith tethering in the zebrafish otic vesicle requires Otogelin and α-Tectorin. Development 142, 1137–1145. ( 10.1242/dev.116632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodman L, Zallocchi M. 2017. Integrin α8 and Pcdh15 act as a complex to regulate cilia biogenesis in sensory cells. J. Cell Sci. 130, 3698–3712. ( 10.1242/jcs.206201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lundberg YW, Xu Y, Thiessen KD, Kramer KL. 2014. Mechanisms of otoconia and otolith development. Dev. Dyn. 244, 239–253. ( 10.1002/dvdy.24195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Imtiaz A, et al. 2018. CDC14A phosphatase is essential for hearing and male fertility in mouse and human. Hum. Mol. Genet. 27, 780–798. ( 10.1093/hmg/ddx440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kindt KS, Finch G, Nicolson T. 2012. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev. Cell 23, 329–341. ( 10.1016/j.devcel.2012.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Popper AN, Hoxter B. 1981. The fine structure of the sacculus and lagena of a teleost fish. Hear. Res. 5, 245–263. ( 10.1016/0378-5955(81)90049-6) [DOI] [PubMed] [Google Scholar]
- 72.Borovina A, Superina S, Voskas D, Ciruna B. 2010. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat. Cell Biol. 12, 407–412. ( 10.1038/ncb2042) [DOI] [PubMed] [Google Scholar]
- 73.Lu H, Toh MT, Narasimhan V, Thamilselvam SK, Choksi SP, Roy S. 2015. A function for the Joubert syndrome protein Arl13b in ciliary membrane extension and ciliary length regulation. Dev. Biol. 397, 225–236. ( 10.1016/j.ydbio.2014.11.009) [DOI] [PubMed] [Google Scholar]
- 74.Stawicki TM, et al. 2016. Cilia-associated genes play differing roles in aminoglycoside-induced hair cell death in zebrafish. G3 6, 2225–2235. ( 10.1534/g3.116.030080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ben J, Elworthy S, Ng AS, van Eeden F, Ingham PW.. 2011. Targeted mutation of the talpid3 gene in zebrafish reveals its conserved requirement for ciliogenesis and Hedgehog signalling across the vertebrates. Development 138, 4969–4978. ( 10.1242/dev.070862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee MS, et al. 2015. IFT46 plays an essential role in cilia development. Dev. Biol. 400, 248–257. ( 10.1016/j.ydbio.2015.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boubakri M, Chaya T, Hirata H, Kajimura N, Kuwahara R, Ueno A, Malicki J, Furukawa T, Omori Y. 2016. Loss of ift122, a retrograde intraflagellar transport (IFT) complex component, leads to slow, progressive photoreceptor degeneration due to inefficient opsin transport. J. Biol. Chem. 291, 24 465–24 474. ( 10.1074/jbc.M116.738658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao C, Omori Y, Brodowska K, Kovach P, Malicki J. 2012. Kinesin-2 family in vertebrate ciliogenesis. Proc. Natl Acad. Sci. USA 109, 2388–2393. ( 10.1073/pnas.1116035109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pooranachandran N, Malicki JJ. 2016. Unexpected roles for ciliary kinesins and intraflagellar transport proteins. Genetics 203, 771–785. ( 10.1534/genetics.115.180943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omori Y, Malicki J. 2006. oko meduzy and related crumbs genes are determinants of apical cell features in the vertebrate embryo. Curr. Biol. 16, 945–957. ( 10.1016/j.cub.2006.03.058) [DOI] [PubMed] [Google Scholar]
- 81.Hazime K, Malicki JJ. 2017. Apico-basal polarity determinants encoded by crumbs genes affect ciliary shaft protein composition, IFT movement dynamics, and cilia length. Genetics 207, 1041–1051. ( 10.1534/genetics.117.300260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lukasz D, Kindt KS. 2018. In vivo calcium imaging of lateral-line hair cells in larval zebrafish. J. Vis. Exp. 141, e58794 ( 10.3791/58794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. 2008. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 40, 69–77. ( 10.1038/ng.2007.54) [DOI] [PubMed] [Google Scholar]
- 84.Blanco-Sánchez B, Clément A, Fierro J Jr, Washbourne P, Westerfield M. 2014. Complexes of Usher proteins preassemble at the endoplasmic reticulum and are required for trafficking and ER homeostasis. Dis. Models Mech. 7, 547–559. ( 10.1242/dmm.014068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. 2003. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J. Ass. Res. Otolaryngol. 4, 219–234. ( 10.1007/s10162-002-3022-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW. 2008. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 4, e1000020 ( 10.1371/journal.pgen.1000020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stawicki TM, Linbo T, Hernandez L, Parkinson L, Bellefeuille D, Rubel EW, Raible DW. 2019. The role of retrograde intraflagellar transport genes in aminoglycoside-induced hair cell death. Biol. Open 8, bio038745 ( 10.1242/bio.038745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nachury MV, Mick DU. 2019. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 20, 389–405. ( 10.1038/s41580-019-0116-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartwell RD, England SJ, Monk NAM, van Hateren NJ, Baxendale S, Marzo M, Lewis KE, Whitfield TT.. 2019. Anteroposterior patterning of the zebrafish ear through Fgf- and Hh-dependent regulation of hmx3a expression. PLoS Genet. 15, e1008051 ( 10.1371/journal.pgen.1008051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sampaio P, et al. 2014. Left-right organizer flow dynamics: how much cilia activity reliably yields laterality? Dev. Cell. 29, 716–728. ( 10.1016/j.devcel.2014.04.030) [DOI] [PubMed] [Google Scholar]
- 91.Rothschild SC, Lahvic J, Francescatto L, McLeod JJ, Burgess SM, Tombes RM. 2013. CaMK-II activation is essential for zebrafish inner ear development and acts through Delta–Notch signaling. Dev. Biol. 381, 179–188. ( 10.1016/j.ydbio.2013.05.028) [DOI] [PubMed] [Google Scholar]
- 92.Yamaguchi N, Colak-Champollion T, Knaut H. 2019. zGrad is a nanobody-based degron system that inactivates proteins in zebrafish. eLife 8, e43125 ( 10.7554/eLife.43125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirchgeorg L, Felker A, van Oostrom M, Chiavacci E, Mosimann C.. 2018. Cre/lox-controlled spatiotemporal perturbation of FGF signaling in zebrafish. Dev. Dyn. 247, 1146–1159. ( 10.1002/dvdy.24668) [DOI] [PubMed] [Google Scholar]
- 94.Savage AM, et al. 2019. tmem33 is essential for VEGF-mediated endothelial calcium oscillations and angiogenesis. Nat. Commun. 10, 732 ( 10.1038/s41467-019-08590-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.