Abstract
How is sensing carried out by cilia in the mouse node, zebrafish Kupffer's vesicle and similar left–right (LR) organizer organs in other species? Two possibilities have been put forward. In the former, cilia would detect some chemical species in the fluid; in the latter, they would detect fluid flow. In either case, the hypothesis is that an imbalance would be detected between this signalling coming from cilia on the left and right sides of the organizer, which would initiate a cascade of signals leading ultimately to the breaking of LR symmetry in the developing body plan of the organism. We review the evidence for both hypotheses.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: primary cilia, chemosensing, mechanosensing, node, Kupffer's vesicle, left–right organizer
1. Nodal flow and the left–right organizer
Vertebrate organisms are generally externally close to being left–right (LR) symmetric. However, they need to have broken LR symmetry in their internal body plan in order to fit their organs within the body cavity. Thus, the heart is to the left, the liver to the right and so on, in humans as well as in many other vertebrate species. This is not random symmetry breaking, which would produce 50% of a species with the heart on one side and 50% on the other; instead, one version (situs solitus) is the norm, and its mirror image, termed situs inversus, is very rare (circa 1 in 10 000 in humans). The mechanism of symmetry breaking during development chosen by nature for this system has recourse to fluid flow [1–7]. A specialized organ existing only transiently during development—the node in the mouse, Kupffer's vesicle (KV) in the zebrafish, and termed in general the LR organizer—in many vertebrate species contains motile cilia that stir a liquid. The cilia whirl in a given sense that is determined by the structure of the proteins that form the molecular motor that powers each cilium. The given sense of rotation, clockwise when viewed from above, together with a tilt towards an axis of symmetry that has already been determined are together sufficient to produce a flow that breaks the LR symmetry. This symmetry-breaking mechanism is a beautiful example of biology making use of physics [3,8]. But, how is the flow sensed by the biology in order to translate this physical symmetry breaking into biological symmetry breaking? This aspect of the mechanism is still not clear and is what we discuss here.
How is sensing of the broken symmetry carried out? It is probable that as well as creating the fluid flow in the mouse node, zebrafish KV and similar organizer organs, cilia are also involved in its sensing. Two possibilities have been put forward, which appear to be the only basic viable ciliary sensing mechanisms: chemosensing and mechanosensing. In the former, cilia would detect the presence of some chemical species in the fluid; in the latter, they would detect fluid flow. In either case, the hypothesis is that an imbalance would be detected between this signalling coming from cilia on the left and right sides of the organizer, which would initiate a cascade of signals leading ultimately to the breaking of LR symmetry in the body plan of the developing organism. Let us review the evidence for both hypotheses.
2. Molecular chemosensing
Chemosensory cilia are well known from other organs, for example, in the olfactory apparatus, in the airways, etc. These are also examples where this function is linked to fluid flows transporting specific substances. In the case of the mouse node, the hypothesis is that morphogen molecules, entering the node symmetrically from both sides, may be dissolved in the liquid and the fluid flow driven by the cilia may induce an asymmetry in the concentration of a morphogen across the node, an imbalance that might be chemosensed by cilia at either side of the node (figure 1). Simulations by Cartwright et al. [8] showed that for this idea to be feasible, the molecules should have a short active lifetime; otherwise, if they would remain active for too long and a uniform concentration would build up across the node, so in that case, they could not perform a symmetry-breaking function. Figure 1 shows a schematic display of this mechanism for both the mouse node (a) and the zebrafish vesicle (b). In the case of the node in the mouse (figure 1a), the direct flow, much stronger than the return, together with diffusion and the finite lifetime of the advected morphogen, induces an asymmetric distribution in the bulk, shown colour-coded. In KV of the zebrafish, instead, a vortical flow around a centre displaced towards the anterior direction has the analogous effect of LR symmetry breaking in the distribution of a finite-lifetime advected molecule.
Figure 1.
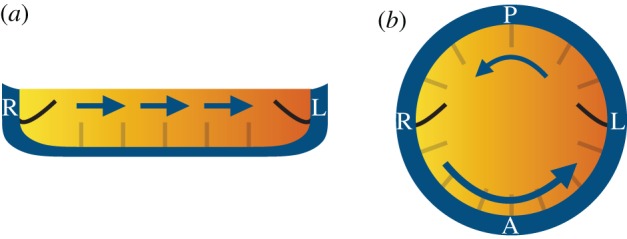
Molecular chemosensing in (a) the mouse node and (b) the zebrafish KV. A directional cilia-driven flow (arrows) could potentially generate a LR asymmetric morphogen gradient (colourmap) to be sensed differentially by symmetrically distributed cilia from the left and right sides. (L, left of the embryo; R, right; A, anterior; P, posterior). (Online version in colour.)
Furthermore, simulations can predict the range of molecular weight that such a morphogen could have [9]. Let us perform a simple calculation to indicate how this mechanism would work. We take the example of the mouse node. We need (i) a quick deactivation compared to diffusion (τdeactivation ≤ τdiff) and (ii) a fast enough flow U, i.e. longitudinal transport (along the length L of the node) faster than diffusion over its depth, H; i.e. τadv = L/U ≤ H2/D = τdiff. With L ≈ 50 µm, H ≈ 10–20 µm and U ≈ 10–20 µm s−1 for the mouse node, this leads to an upper bound for the diffusion coefficient of D ≤ D* = H2 U/L = 10−10 m2 s−1 or, equivalently, a lower bound for the hydrodynamic (Stokes) radius of a putative morphogen of . This estimate seems reasonable for a morphogen molecule and moreover fits quite well with a 2–10 nm estimate from Ferreira et al. [10] for the case of KV (see their fig. 7F). However, molecular chemosensing appears to be incompatible with the results of Shinohara et al. [11], where very slow leftward flow (U ≤ 1 µm s−1) still results in situs solitus. The more general problem with the chemosensing hypothesis in this form is that, as yet, no such dissolved morphogen has been detected.
3. Vesicular chemosensing
A variant of the chemosensing hypothesis emerged in 2005: instead of a dissolved morphogen, experimental data appeared to show bodies, termed nodal vesicular parcels (NVPs), in the nodal liquid [12]. The fluid mechanics of this vesicular chemosensing was analysed by Cartwright et al. [9]. These NVPs moving in the nodal fluid, if they contained a morphogen, might act as described for a dissolved morphogen above, except that owing to their far greater mass, they would not undergo molecular diffusion, but only dispersion. This would effectively allow for their asymmetric LR accumulation, and possibly even for slow flows [13]. NVPs might still be chemosensed by cilia at either side of the node and there might still be an LR imbalance owing to the direction of fluid flow (figure 2). The nature of the fluid flow in the node—its low Reynolds number—implies that fluid inertia is negligible [14,15], so that there is no possibility of an NVP ‘splatting’ like a ripe tomato against a cilium or a wall. Thus, in this case, the chemosensing would need to involve an active biochemical rupture process to break an NVP and sense the morphogen within [9]. Such a rupture mechanism would provide an analogue of the finite activity lifetime of the morphogen required in the former version [2]. Recent theoretical work has hypothesized a link between fluid flow and shear-induced exocytosis of extracellular vesicles in the node and the KV [16]. The proposal is compatible with viable shear values measured in other cases but remains to be tested in this context. The general problem with the NVP mechanism is that, after the initial work of Tanaka et al. [12], no further experimental evidence has definitely shown the presence of these bodies.
Figure 2.
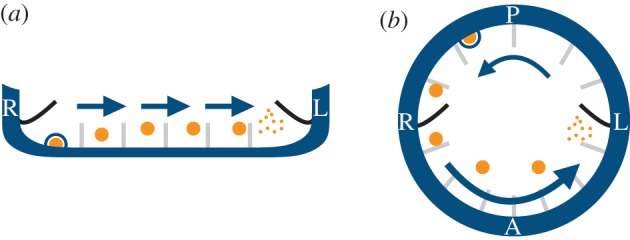
Vesicular chemosensing in (a) the mouse node and (b) the zebrafish KV. A directional cilia-driven flow (arrows) could potentially transport morphogen-filled vesicular parcels (NVPs, orange circles) towards the left where an as-yet unknown active mechanism would break the parcels asymmetrically, releasing morphogen molecules to be sensed by symmetrically distributed cilia. Moreover, the release of NVPs (KVVPs) into the node (KV) might be mediated by shear-induced exocytosis at the walls (blue circle). (L, left of the embryo; R, right; A, anterior; P, posterior). (Online version in colour.)
4. Mechanosensing
The alternative to either form of chemosensing is mechanosensing. Mechanosensory cilia are known to sense fluid flow in, for example, the kidneys, where a breakdown of this sensing mechanism in polycystic kidney disease leads to abnormal kidney development [3,17]. In kidney cilia, the mechanosensory signal is proportional to the amount of bending of the cilium [18]. Can this sensory mechanism be acting in the node? In earlier work, we argued that it could not [8]. Our argument then was that the symmetry of flow across the node, allied to the fact that the flow operates at very low Reynolds number, implies that at the equivalent places on the left and the right, the flow magnitude will be exactly the same, and hence, a mechanosensory mechanism would give the same signal on left and right and could not function as required. The former point is correct: the flow magnitudes are indeed necessarily symmetric between left and right across the node owing to basic fluid mechanics; however, the latter point is not correct.
We later realized that we were wrong: that it is in theory possible for a mechanosensory cilium to detect a difference between the left and right sides of the node, because such a cilium is not a point object, but an extended object. At any given point in the flow, there is symmetry between the left- and the right-hand sides of the node, but two cilia placed in equivalent positions on the left and right would not have equivalent experiences in an imposed right–left flow: that on one side will bend outwards into the node, while that on the other will bend towards the wall. This difference allows for the two cilia at left and right to bend by different amounts, as in general the flow closer to the wall will be slower and that further from the wall, faster. So, a cilium bending towards the wall will bend less than one bending out into the interior of the flow (figure 3). Thus, mechanosensing is, at least in theory, an alternative to chemosensing for LR symmetry breaking [19]. A similar idea could in principle also work in the KV. The predominantly anti-clockwise vortical flow, circulating around the dorsoventral axis when viewed from the dorsal pole of the KV, can differentially deflect cilia positioned closer to the left or the right pole, respectively. In both cases, the proposed mechanism effectively transduces an LR symmetric scalar flow magnitude signal into a vectorial one via a finite-size sensor.
Figure 3.
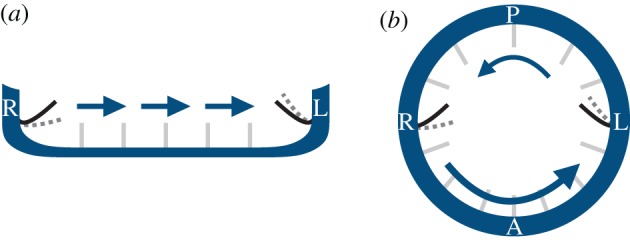
Mechanosensing in (a) the mouse node and (b) the zebrafish KV. Owing to their finite size, cilia from the left/right side of the node (KV) could potentially bend differentially (grey dotted lines) when exposed to a directional cilia-driven flow (arrows). (L, left of the embryo; R, right; A, anterior; P, posterior). (Online version in colour.)
Shinohara et al. [11] reported on mutant mice embryos with only two motile cilia that nevertheless still produce consistently elevated gene expression on the right, despite very weak and localized flow. Moreover, even in normal embryos, asymmetric expression of the same gene occurs in the early stages of cilia-driven flow, before a global flow is established. These observations have been interpreted as supporting mechanosensing [20] rather than a transport-based process, i.e. while mechanical forces are transmitted almost instantly, the timescales for morphogen or vesicle transport from one side of the node to the other are extended greatly by the reduced flow field.
On the other hand, while theoretically feasible, mechanosensing has been found to be unreliable as a robust LR determinant in the KV. The slow nature of the vortical flow, combined with the short length of the KV cilia compared to other systems, induces only an extremely weak deflection, necessitating an extreme ciliary mechanosensing sensitivity for it to be functional. Moreover, the flow-induced torques on the KV cilia are of the same order as their variability (10−19 N m) owing both to the natural temporal and spatial variability in cilia positioning and beating, and to thermal fluctuations, producing overall an extremely poor signal to noise ratio [10].
Moreover, Delling et al. [21] reported that nodal cilia, in common with other primary cilia, are not calcium-sensitive mechanosensors. Forcing cilia deflection by physiological or even highly supraphysiological levels of fluid flow does not induce calcium increases, which implies that mechanosensation, if it originates in primary cilia, does not operate via calcium signalling. These experimental results, although not necessarily ruling out mechanosensing as an LR determinant, question established ideas on how it might operate and pose new dilemmas as to the details of the mechanism.
5. Ciliary multitasking
Need there be two types of cilia in the organizer, those that are motile and those that perform sensing, or might motile cilia themselves perform the sensing?
There is certainly no physics that says you have to either chemosense or move, but not both. So, it seems perfectly feasible, at least from the point of view of the physics, that cilia could both stir the flow and simultaneously chemosense it. That is indeed the case, for instance, for the motile cilia in human airway epithelia [22]. Either molecular or the vesicular chemosensing might function in this fashion.
On the other hand, if a cilium is mechanosensing using the amount of bending of the cilium, cilia on the left and on the right, stirring the fluid with the same force, would have to detect the slight difference in amplitude in opposite directions in their flow-induced trajectories. There are plenty of examples where a strong external flow induces a mechanoresponse in motile cilia/flagella, but achieving this while being motile requires substantial extra bending to do so. Thus, flagellated microswimmers are able to mechanosense approaching predators while swimming. But for the slow cilia-driven flows within LR organizers, the corresponding maximum amplitude difference between one side and the other will be slight. This brings with it the same question raised earlier of whether this effect might be below the threshold of mechanosensory sensitivity.
Within this multitasking framework, another mechanosensing proposal suggests that motile ciliated cells might self-sense their ciliary beating. In principle, a motile cilium capable of mechanosensing should as it moves be able to detect inhomogeneities in its fluid environment caused by the proximity of walls. A given sense of rotation plus a nearby wall in a given direction indeed provides the possibility of symmetry breaking, as required. The torque components caused by the motion of an active cilium through the viscous fluid at its base largely surpass those caused by the directional flow. In particular, the meridional component shows a temporal average of opposite sign at the left and right hemispheres of the KV of order 10−17 N m that could potentially allow LR discrimination [10]. However, results from flow reversal [23] and halting [24] experiments seem difficult to reconcile with this hypothesis: artificially induced reversal of the directional flow should enhance, rather than reverse, LR asymmetry, but making the fluid viscoelastic brings the directional flow to a halt and disturbs the laterality mechanism, even though the cilia are still motile.
The idea that motile cilia might multitask using either chemosensing or mechanosensing has not been much favoured in the mouse. There, the two-population idea arose that some cilia are motile and produce flow; others immotile and sensory [25]. However, in fish, Kamura et al. [26] showed in medaka KV that cilia move and sense at the same time. It seems probable that the zebrafish KV works in a similar way. But a few cilia are seen that are immotile [27]. Do these play a chemo- or mechano-sensory role for LR determination?
6. So, which is it, chemosensing or mechanosensing?
Chemosensing and mechanosensing have both been put forward as the mechanisms by which flow within the mouse node and similar organizers is translated into a signal that leads to LR symmetry breaking. They appear to be the only viable mechanisms by which cilia might sense the fluid mechanics. The question of which is acting is still open. Although these physical arguments that we have surveyed are quite general, it is, of course, possible that one mechanism might be involved in one species; the other in another.
Chemosensing of a dissolved morphogen seems eminently physically plausible, but there is no experimental evidence for such a morphogen, and we would have expected to see some by now; chemosensing of a vesicle-contained morphogen likewise seems possible, but after some first promising evidence for vesicles in the flow, the lack of replication means that this observation now seems dubitable; mechanosensing is physically possible, but comparison with its threshold in systems where it is known to act implies that it would be scarcely sensitive enough to be viable in LR organizer flows.
As we survey these possible mechanisms, we recall a saying attributed to Alfonso X of Castile, ‘el sabio’: ‘If the Lord Almighty had consulted me before embarking on creation thus, I should have recommended something simpler’.
Data accessibility
This article has no additional data.
Authors' contributions
All have contributed to the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We acknowledge the financial support of the Spanish MINCINN project FIS2016-77692-C2.
References
- 1.Cartwright JHE, Piro N, Piro O, Tuval I. 2008. Fluid dynamics of establishing left–right patterning in development. Birth Defects Res. C: Embryo Today: Rev. 84, 95–101. ( 10.1002/bdrc.20127) [DOI] [PubMed] [Google Scholar]
- 2.Cartwright JHE, Piro N, Piro O, Tuval I. 2008. Fluid dynamics of nodal flow and left–right patterning in development. Dev. Dyn. 237, 3477–3490. ( 10.1002/dvdy.21672) [DOI] [PubMed] [Google Scholar]
- 3.Cartwright JHE, Piro O, Tuval I. 2009. Fluid dynamics in developmental biology: moving fluids that shape ontogeny. HFSP J. 3, 77–93. ( 10.2976/1.3043738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DJ, Montenegro-Johnson TD, Lopes SS. 2019. Symmetry-breaking cilia-driven flow in embryogenesis. Annu. Rev. Fluid Mech. 51, 105–128. ( 10.1146/annurev-fluid-010518-040231) [DOI] [Google Scholar]
- 5.Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. 2005. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left–right development of the brain, heart and gut. Development 132, 1247–1260. ( 10.1242/dev.01663) [DOI] [PubMed] [Google Scholar]
- 6.Hirokawa N, Tanaka Y, Okada Y, Takeda S. 2006. Nodal flow and the generation of left–right asymmetry. Cell 125, 33–45. ( 10.1016/j.cell.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 7.Montenegro-Johnson TD, Baker DI, Smith DJ, Lopes SS. 2016. Three-dimensional flow in Kupffer's Vesicle. J. Math. Biol. 73, 705–725. ( 10.1007/s00285-016-0967-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartwright JHE, Piro O, Tuval I. 2004. Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates. Proc. Natl Acad. Sci. USA 101, 7234–7239. ( 10.1073/pnas.0402001101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartwright JHE, Piro N, Piro O, Tuval I. 2007. Embryonic nodal flow and the dynamics of nodal vesicular parcels. J. R. Soc. Interface 4, 49–56. ( 10.1098/rsif.2006.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira RR, Vilfan A, Jülicher F, Supatto W, Vermot J. 2017. Physical limits of flow sensing in the left-right organizer. eLife 6, e25078 ( 10.7554/eLife.25078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinohara K, et al. 2012. Two rotating cilia in the node cavity are sufficient to break left–right symmetry in the mouse embryo. Nat. Commun. 3, 622 ( 10.1038/ncomms1624) [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Okada Y, Hirokawa N. 2005. Fgf-induced vesicular release of sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature 435, 172–177. ( 10.1038/nature03494) [DOI] [PubMed] [Google Scholar]
- 13.Gallagher MT, Montenegro-Johnson TD, Smith DJ.2019. Simulations of particle tracking in the oligociliated mouse node and implications for left-right symmetry breaking mechanics. bioRxiv 624940. ( ) [DOI]
- 14.Cartwright JHE, Feudel U, Károlyi G, De Moura A, Piro O, Tél T. 2010. Dynamics of finite-size particles in chaotic fluid flows. In Nonlinear dynamics and chaos: advances and perspectives (eds M Thiel, J Kurths, MC Romano, G Károlyi, A Moura), pp. 51–87. Berlin, Germany: Springer; ( 10.1007/978-3-642-04629-2_4) [DOI] [Google Scholar]
- 15.Aref H, et al. 2017. Frontiers of chaotic advection. Rev. Mod. Phys. 89, 025007 ( 10.1103/RevModPhys.89.025007) [DOI] [Google Scholar]
- 16.Solowiej-Wedderburn J, Smith DJ, Lopes SS, Montenegro-Johnson TD. 2019. Wall stress enhanced exocytosis of extracellular vesicles as a possible mechanism of left-right symmetry-breaking in vertebrate development. J. Theor. Biol. 460, 220–226. ( 10.1016/j.jtbi.2018.10.015) [DOI] [PubMed] [Google Scholar]
- 17.Yoder BK. 2007. Role of primary cilia in the pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 18, 1381–1388. ( 10.1681/ASN.2006111215) [DOI] [PubMed] [Google Scholar]
- 18.Praetorius H, Frokiaer J, Nielsen S, Spring KR. 2003. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J. Membr. Biol. 191, 193–200. ( 10.1007/s00232-002-1055-z) [DOI] [PubMed] [Google Scholar]
- 19.Ferreira RR, Fukui H, Chow R, Vilfan A, Vermot J. 2019. The cilium as a force sensor−myth versus reality. J. Cell Sci. 132, jcs213496 ( 10.1242/jcs.213496) [DOI] [PubMed] [Google Scholar]
- 20.Omori T, Winter K, Shinohara K, Hamada H, Ishikawa T. 2018. Simulation of the nodal flow of mutant embryos with a small number of cilia: comparison of mechanosensing and vesicle transport hypotheses. R. Soc. open sci. 5, 180601 ( 10.1098/rsos.180601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delling M, Indzhykulian A, Liu X, Li Y, Xie T, Corey DP, Clapham DE. 2016. Primary cilia are not calcium-responsive mechanosensors. Nature 531, 656–660. ( 10.1038/nature17426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. 2009. Motile cilia of human airway epithelia are chemosensory. Science 325, 1131–1134. ( 10.1126/science.1173869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nonaka S, Shiratori H, Saijoh Y, Hamada H. 2002. Determination of left–right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96–99. ( 10.1038/nature00849) [DOI] [PubMed] [Google Scholar]
- 24.Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. 2007. Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 17, 60–66. ( 10.1016/j.cub.2006.10.067) [DOI] [PubMed] [Google Scholar]
- 25.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. 2003. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell 114, 61–73. ( 10.1016/S0092-8674(03)00511-7) [DOI] [PubMed] [Google Scholar]
- 26.Kamura K, Kobayashi D, Uehara Y, Koshida S, Iijima N, Kudo A, Yokoyama T, Takeda H. 2011. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left–right axis. Development 138, 1121–1129. ( 10.1242/dev.058271) [DOI] [PubMed] [Google Scholar]
- 27.Sampaio P, et al. 2014. Left–right organizer flow dynamics: how much cilia activity reliably yields laterality? Dev. Cell 29, 716–728. ( 10.1016/j.devcel.2014.04.030) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


