Abstract
Living creatures exhibit a remarkable diversity of locomotion mechanisms, evolving structures specialized for interacting with their environment. In the vast majority of cases, locomotor behaviours such as flying, crawling and running are orchestrated by nervous systems. Surprisingly, microorganisms can enact analogous movement gaits for swimming using multiple, fast-moving cellular protrusions called cilia and flagella. Here, I demonstrate intermittency, reversible rhythmogenesis and gait mechanosensitivity in algal flagella, to reveal the active nature of locomotor patterning. In addition to maintaining free-swimming gaits, I show that the algal flagellar apparatus functions as a central pattern generator that encodes the beating of each flagellum in a network in a distinguishable manner. The latter provides a novel symmetry-breaking mechanism for cell reorientation. These findings imply that the capacity to generate and coordinate complex locomotor patterns does not require neural circuitry but rather the minimal ingredients are present in simple unicellular organisms.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: cilia coordination, basal coupling, locomotion, central pattern generator oscillations, mechanosensitivity
1. The locomotor gaits of organisms
Since the invention of photography, the natural habits of organisms have come under increasing scrutiny. Modern optical technologies have enabled resolution of ever-finer detail, so that we can visualize and track behaviour across scales [1,2]. Consider for example the gallop of a horse: how are its four feet coordinated? This fast action cannot be resolved by the human eye, so the question remained unanswered until 1878, when (reputedly to settle a bet) the photographer Eadweard Muybridge successfully imaged the gait sequences of a horse [3]. Control of rhythmic limb movements in both vertebrates and invertebrates is determined by neuronal networks termed central pattern generators (CPG), where motor feedback is not required [4]. Indeed, CPGs in in vitro preparations can produce activity patterns that are identifiable with the in vivo behaviour. For instance, in the pteropod Clione limacine (sea butterfly), which swims with two dorsal wings, the neuronal pattern for basic synchronous movement (fictive swimming) can be reproduced in isolated pedal ganglia [5]. A striking example of the genetic basis of gait-control and CPGs pertains to the Icelandic horse, where the ability to pace (legs on the same side of the body moving synchronously) was shown to be associated with a point mutation [6].
At the microscale, organisms interact with their world following very different physical principles. Here, inertia is negligible and many species developed motile appendages that are slender and suited to drag-based propulsion through fluids. From these simplest designs, myriad locomotion strategies have evolved. The model microswimmer Escherichia coli is peritrichously flagellated—bundles of rigid (prokaryotic) flagella rotate synchronously in the same sense to elicit forward swimming (a ‘run’), but lose synchrony and unbundle (a ‘tumble’) when one or more of the motors reverse direction (see [7] and references therein). The latter results in rapid changes in reorientation. In this way, a bacterium controls its propensity for reorientation to bias its swimming towards or away from chemo-attractants or repellents [8]. It has been shown that bundling is largely owing to hydrodynamic interactions between the rotating filaments [9], but unbundling is stochastic. By contrast, eukaryotes exhibit more deterministic responses to vectorial cues [10]. Phototactic reorientation by the biflagellate alga Chlamydomonas reinhardtii requires bilateral symmetry-breaking in a pair of apparently identical flagella [11], though differences in signal transduction must be present [12–14]. Eukaryotic flagella and cilia possess a flexible and distributed molecular architecture allowing for many more degrees of freedom than prokaryotic flagella. This versatility, particularly when multiple appendages are attached to the same cell, allows algal flagellates to orchestrate diverse swimming gaits such as the breaststroke, trot or gallop [15]. Extensive research has already shown that distinct waveforms and beat frequencies can be produced by the same ciliary structure [16–18], depending on intrinsic and extrinsic forcing [19–21]. This ability to modulate dynein motor cooperativity to produce distinct beating modes on a single cilium does not however explain the higher-level coordination over a network of such oscillating structures. To achieve the latter, intracellular control mechanisms are implicated (reviewed in [22]). In this article, I present new findings on gait patterning in microalgae, which reveal that single-celled multiflagellates can actively dictate the dynamics and activity of each individual flagellum. I further propose that symmetry-breaking processes in the flagellar apparatus are causal to this distinguishability between flagella. The attainment of control specificity of locomotor appendages may be a key innovation in the evolution toward increasingly deterministic movement in eukaryotes.
2. Gait species-specificity
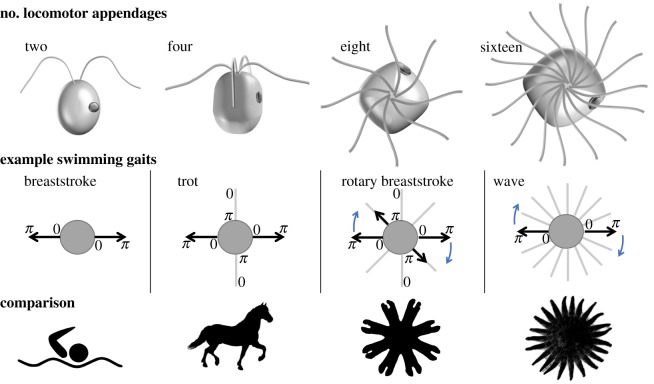
We focus on unicellular algae bearing 2, 4, 8 or 16 flagella and study the patterns of flagellar actuation and movement. Representative gaits are compared to animals with analogous limb positioning (figure 1), for which the neuronal basis of movement control has been clearly demonstrated (except in the sea star, which is yet to be subject to systematic investigation). In all cases, the movement operates in a very different physical and size regime [23,24] from that encountered by the microeukaryotes from this study. Species-specificity of locomotor behaviours also applies to flagellates: organisms with an identical number and arrangement of flagella can still assume different locomotor gaits [15]. Concurrently, a single species can be capable of multiple gaits associated with significant changes in the flagellar beat pattern. For instance, sudden environmental perturbations elicit so-called shock responses in multiple species, in which flagella reverse their beating direction (but not the direction of wave propagation) to produce transient backward movement or even cessation of swimming [25,26]. Some species have a stop state [27], in which all flagella become reversibly quiescent. We organize this section by characterizing first the gaits used for forward swimming, and then separately, introducing the phenomenon of selective beat activation in free-swimming cells.
Figure 1.
Locomotor patterning in unicellular algae. Algae with 2, 4, 8 and 16 flagella are shown with representative swimming gaits. These are characterized by specific phase relationships between the flagella (0 to 2π for a complete beat cycle). (Different species exhibit different gaits and swimming behaviours even for an identical arrangement of flagella.) Animal models with an equivalent number of locomotor appendages are shown for comparison. These are respectively, a human swimmer, a horse, a jellyfish ephyra (juvenile jellyfish) and the predatory sunflower sea star Pycnopodia helianthoides which walks with 16–24 limbs. (Online version in colour.)
(a). Run gaits for forward swimming
The biflagellate C. reinhardtii is favoured for biophysical models of swimmers [28]. Cells execute a forward breaststroke gait in which the two flagella are synchronized, interrupted by phase slips. Stochastic switching between this characteristic in-phase breaststroke and a perfectly antiphase (or freestyle) gait was also discovered in a phototaxis mutant [29]. Experimental and theoretical evidence overwhelmingly implicates intracellular coupling as the dominant mechanism for flagellar coupling [15,30–32]. However, phase synchrony is not a given: heterotrophic biflagellates such as Polytoma uvella have two flagella of slightly unequal lengths and different beat frequencies, which remain largely asynchronous. With more than two flagella, new gaits become accessible. Depending on flagella configuration and ultrastructure, different interflagellar phase patterns are sustained during steady, forward swimming. Comparison with quadrupedal locomotion is instructive here [33]. Among species with four flagella, a robust trotting gait comprising two alternating pairs of synchronous breaststrokes is assumed by two marine Pyramimonas species: P. parkeae and P. tychotreta, whereas galloping gaits are consistently displayed by Tetraselmis sp. and Carteria crucifera (see electronic supplementary material, videos S1–S5). Only three extant species are known to have eight flagella [34]. Of these, Pyramimonas octopus coordinates its eight flagella into a unique forward propulsion gait that we term the rotary breaststroke (see §4). Identifying equivalence between this and octopedal animal movement is more challenging. The spider possesses obvious bilateral symmetry not present in the algae, though alternating patterns of leg movements are observed [35]. Jellyfish ephyrae use cycles of contraction and relaxation involving the simultaneous movement of their eight soft appendages, but move in an inertial regime [36]. Finally, the Arctic species Pyramimonas cyrtoptera has 16 flagella [37] that can be actuated metachronously rather than in discrete patterns. This may be the critical number at which the flagella begin to interact hydrodynamically [15]. In this limiting scenario, the wave-like coordination of many closely separated appendages may help to avoid collisions and mirrors the locomotion mechanisms adopted by arthropods [38,39].
(b). Gait-switching and partial activation
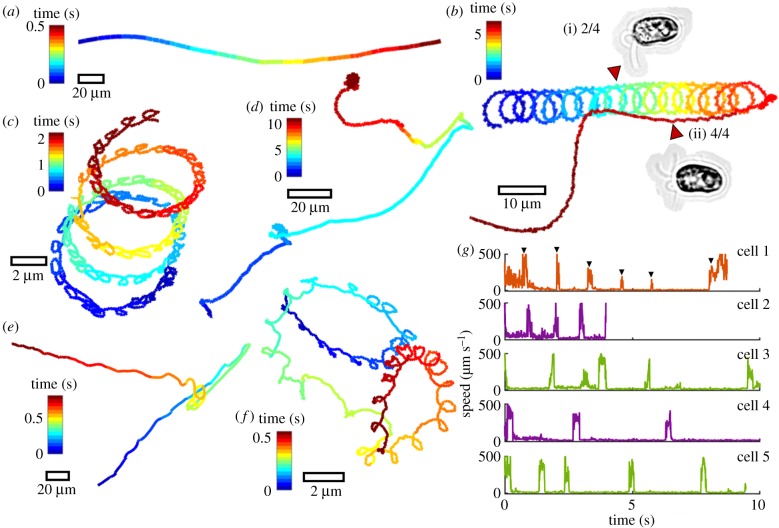
In animals, the capacity to produce multiple locomotor modes is critical for survival. Gait often depends on the speed of the desired movement. For example, the ghost crab (Ocypode ceratophthalma) becomes bipedal at the highest speeds [40]. Likewise, we find that unicellular flagellates dynamically reconfigure their motor apparatus to produce different gaits that are coupled to photo- or mechano-sensory pathways. Next, we compare the motion repertoire of different multiflagellate species. Freely swimming cells were imaged at kilohertz and sub-micrometre resolution, so that flagella motion could be discerned at the same time as the cell body movement. Strong behavioural stereotypy was observed within each species, but heterogeneity across species (figure 2). Consistently, tracks presenting high curvature loops were found to result from a previously uncharacterized motility mechanism—namely, the partial activation of flagella. For the same cell, beating can be restricted to a subset of flagella with the remaining flagella quiescent. Asymmetries arising from the actively beating flagellar subgroup produce large-angle turns in the free-swimming trajectory. A previously quiescent flagellum can subsequently become active again, and vice versa (figure 2b and electronic supplementary material, video S1). Four different quadriflagellate species showed selective activation of flagella (P. parkeae, Tetraselmis suecica, T. subcordiformis and P. tychotreta). In cells exhibiting motion intermittency such as P. parkeae (figure 2d), flagella alternate between periods of bursting activity and total quiescence (figure 2g; electronic supplementary material), analogous to the physiological behaviour of motor neurons.
Figure 2.
Active gait reconfiguration and heterogeneity in single-cell swimming trajectories. Persistent directional swimming (a) is contrasted with asymmetric and chiral trajectories (b–f). Free-swimming cells of the quadriflagellate Tetraselmis subcordiformis are shown in (b,c). In (b), a cell transitions from a spinning gait of two flagella (i), to a galloping gait of all four flagella (ii). In (c), beating is restricted to only one of four flagella, resulting in a strongly helical trajectory. A different quadriflagellate Pyramimonas parkeae exhibits intermittency: in trajectory (d) a cell alternates between a trot gait of four flagella (active state) and a stop state with no flagella activity (inactive state). In (e), the octoflagellate Pyramimonas octopus undergoes reorientation following a shock response (see also [27]). (f) A P. parkeae cell presenting a single actively beating flagellum. (g) The swimming speed of P. parkeae was measured in a population of individuals, which shows a characteristic activity timescale for ‘bursting’ (electronic supplementary material, videos S1–S3).
Next, we resolve gait transients and interflagellar phase dynamics while keeping track of flagellum identity. Automated methods could not follow the flagella movement reliably. Owing to the three-dimensional movement and continuous cell axial rotation, features of interest do not always remain in focus. Instead, flagella tip positions were manually annotated in TrackMate [41]. The distance between flagellum tip and cell centroid undergoes oscillations for an actively beating flagellum but remains constant for a quiescent flagellum. We use this distance as proxy for flagellar phase (figure 3). The quadriflagellate C. crucifera displays stochastic gait-switching between forward swimming and transient shocks, as does P. octopus [27]. Shocks involve simultaneous conversion of all four flagella to a hyperactivated undulatory state (figure 3a). After approximately 0.3 s, the canonical ciliary beat pattern is recovered. A rhythmic galloping gait resumes after only a few asynchronous beat cycles. The rapidity of phase-resetting responses in these organisms further implies that gait-control is active [42]. In figure 3b, we demonstrate partial activation in P. tychotreta [43]. Here, full beat cycles are sustained in flagella f 1,3, while beating in the diametrically opposite pair f 2,4 is suppressed throughout. Small fluctuations detected in the inactive flagella are owing to passive movement induced by the beating of nearby flagella. Further examples of quadriflagellates in which beating is dynamically constrained to one, two or three out of a total possible four flagella are provided in the electronic supplementary material.
Figure 3.
Quadriflagellate gaits resolved at the single-flagellum level in free-swimming cells. Displacement from tip to centre of the cell body was used as proxy for flagellar phase for each flagellum, labelled (f1–4). Frames corresponding to specific timepoints are highlighted. Scalebars for cell size reference. (a) Carteria crucifera has a galloping gait during forward runs, but exhibits noise-induced shocks involving reorientation of the flagella (time 3). Recovery of the original rhythm (phase-resetting), completed within four beat cycles. (b) Pyramimonas tychotreta has a canonical trotting gait during forward runs but exhibits partial activation of flagella. Beating is restricted to the pair f 1,3, but suppressed in f 2,4. Synchrony of f 1,3 drifts from antiphase (times 3,4) to in-phase (time 5) over the course of this recording (electronic supplementary material, videos S4 and S5).
3. Intermittency and temporal ordering in a flagellar network
Free-swimming individuals are not amenable to long-time imaging. In order to investigate the long-time statistics of locomotor patterning, experiments were also conducted on micropipette-held (body-fixed) organisms. Single or double micropipette configurations were used to position, manipulate single cells, and to apply localized mechanical perturbations to single flagella. Not all species were suitable for the micropipette technique; however in some cases, captured cells prematurely shed their flagella. This is likely related to a stress-induced, calcium-dependent deflagellation response [44,45]. We identified two quadriflagellate species that were minimally affected by pipette-capture. These are P. parkeae and T. suecica, which are representative of the two known quadriflagellar arrangements: type I and II in [15] (see also figure 4). In P. parkeae, the flagella form a cruciate arrangement, while in T. suecica they align into two anti-parallel pairs. Both flagellates show partial activation and beat intermittency.
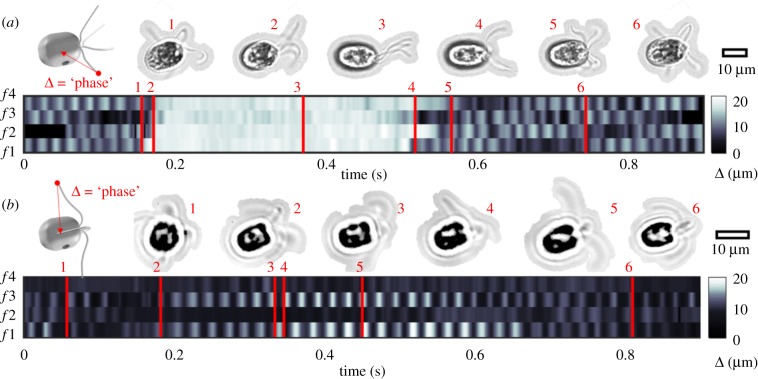
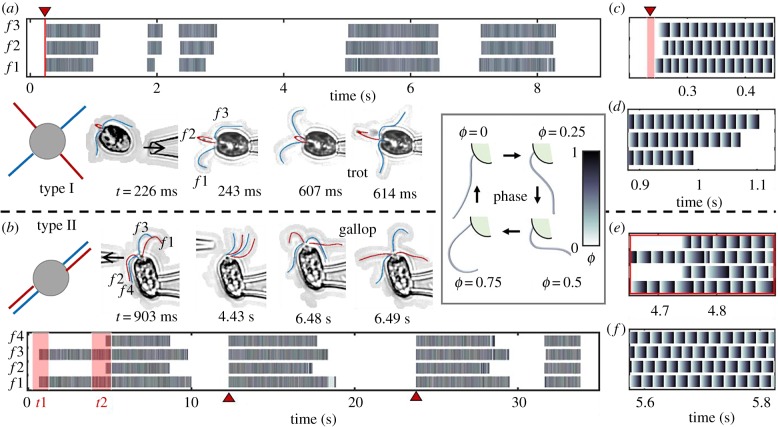
Figure 4.
Reversible rhythmogenesis and gait mechanosensitivity in quadriflagellates. (a) The four flagella of P. parkeae have a cruciate arrangement (type I, top view). The captured cell has two flagella in the focal plane (f 1,3) and two others moving transversely—these are labelled as f 2 and are tracked as one (see electronic supplementary material, video S6). (b) The four flagella of T. suecica have an anti-parallel arrangement (type II, top view). Mechanical perturbations are introduced at times t1,2, and spontaneous shocks are detected at times indicated by red pointers. (c–f) are zoomed-in plots of (a,b), at time points of interest. At the moment of capture, the flagella begin to beat, but not simultaneously; a regular trot gait emerges within three beat cycles (c). Spontaneous termination of beating does not occur at the same time in all flagella (d). Induced changes in locomotor patterning (e) are compared with the regular phase patterning (f) observed during the trotting gait. (See electronic supplementary material, video S7.) Inset: flagellar phase is defined according to progression through the beat cycle.
For pipette-fixed individuals, flagellar phases could be extracted automatically from imaging data to characterize the emergence and decay of coordination in the flagellar network. Phase patterns are reminiscent of classical studies of footfall patterns and limbed locomotion in animals [46,47]. In long-time, high-speed recordings, reversible transitions in locomotor patterning were found in both species. These occurred spontaneously, but could also be induced by mechanical forcing. This touch-dependent gait-sensitivity is a novel manifestation of a mechanoresponse in motile cilia and flagella. At the moment of capture, contact with the pipette can induce rhythmogenesis in a cell with initially quiescent flagella (figure 4a; electronic supplementary material, video S6). Similarly, changes in locomotor patterning were induced when fluid was injected manually (approx. 1 s pulses) via a second pipette in the vicinity of a quiescent cell (figure 4b). The first pulse administered at time t1 induced beating in f1,3 only. This biflagellate state continued until the second pulse (time t2), which then resulted in beating in all four flagella (electronic supplementary material, video S7). In both cases, even after cessation of external perturbations, the cells continued to exhibit intermittent activity over tens of seconds. We conclude that spontaneous transitions in behaviour measured during free-swimming (figure 2) are replicated in pipette-fixed individuals.
Next, we emphasize two aspects of gait reconfigurability in multiflagellates. First, neither activation (e.g. figure 4c) nor deactivation of beating (e.g. figure 4d) necessarily occurs simultaneously in all four flagella. However, the shock response (which is an all-or-none response depending on threshold perturbation magnitude [27,48]) is associated with a strong process asymmetry, the forward reaction (activation) is simultaneous but the reverse (deactivation) is sequential—different flagella can cease beating at different times (figure 4b). Second, after any gait transition, the reestablishment of an expected run gait (whether the trot or gallop) is not instantaneous, but rapid. The canonical temporal ordering of the flagella is recovered after only a few beat cycles.
4. Symmetry-breaking in the flagellar apparatus
In this section, we elaborate on the non-identity of flagella in multiflagellate algae. Adapting terminology from gait analyses of limbed animal locomotion, we distinguish between symmetric gaits (e.g. the trot, pronk) and asymmetric gaits (e.g. the gallop, bound). A quadrupedal gait is referred to as being symmetric if footfall patterns of both pairs of feet are evenly spaced in time and as asymmetric if the activity of at least one pair is unevenly spaced in time. Different gaits can therefore arise from the same underlying locomotor circuit. The presence of more than two flagella also allows for diverse possibilities for symmetry-breaking even during regular gait patterning (not transitional gaits).
We focus now on the rotary breaststroke of P. octopus, which has not been described in any other organism. Gait ordering in multiflagellates is particularly difficult to visualize, owing to the highly three-dimensional swimming. Additionally, P. octopus rotates slowly clockwise about its own axis when viewed from the anterior end (electronic supplementary material, video S8). All eight flagella are distinguishable simultaneously only when the cell is swimming into or out of the focal plane (figure 5; electronic supplementary material, video S9). Recordings of such transients were made, from which the extremal positions of each flagellum could be followed in time and tracked manually in ImageJ [41]. As before, a normalized flagellar phase was computed for each flagellum. We find that phase ordering propagates directionally, in the same sense as the about-axis rotation (figure 5c). (Gaits are similarly directional in the related Pyramimonas cyrtoptera, which swims with 16 flagella.) A pairwise Pearson-correlation matrix was computed for each tracked cell to quantify the degree of correspondence between the eight flagella (figure 5d). A strong positive correlation was measured between diametrically opposite flagella (dashed white lines are guides), which tend to move synchronously to produce a noisy sequence of intercalated breaststrokes (first 1 : 5, then 2 : 6 and so on).
Figure 5.
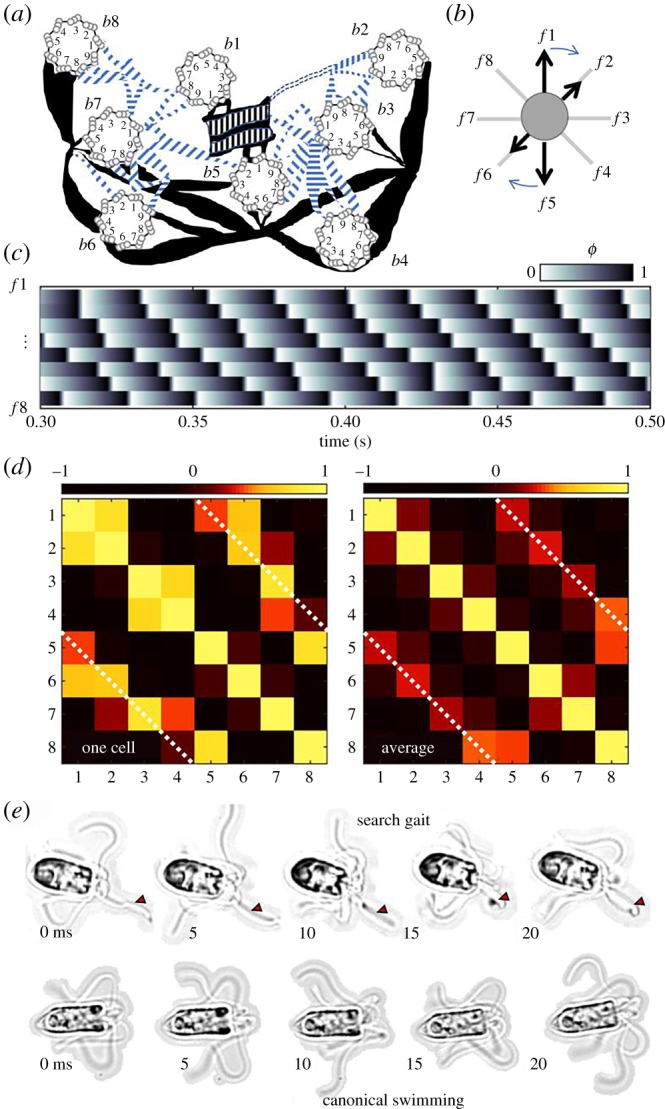
Pyramimonas octopus exhibits symmetry-breaking in both structure and movement. (a) Schematic (redrawn from [49]) of the basal apparatus comprising eight basal bodies (b1–b8) whence emanate the flagella (f1–f8). Thick and thin filaments couple the flagella at specific locations to produce the octoflagellate's unique rotary breaststroke (b). Stereotypical phase dynamics for the numbered flagella are displayed in (c). Cross-correlation matrices for one cell and group average (n = 8) both show strong synchrony between diametrically opposite flagella (d). (e) A small number of cells adopt a ‘search’ gait, in which a single flagellum (marked by the red arrow) is held extended in front of the cell (electronic supplementary material, video S10). Canonical swimming is also shown for comparison.
Given the directional nature of this swimming gait, asymmetries in developmental patterning must be present. This is corroborated by the only ultrastructural study available on this species [49]. The flagella emerge from separate basal bodies (centrioles during cell division), which are localized to the anterior of the organism (figure 5a). Electron microscopy sections reveal a complex basal architecture lacking in rotational symmetry. Individual basal bodies are positioned uniquely according to generational age [49]. A contractile network of thick and thin fibres (some are striated) connect specific numbered microtubule triplets to provide intracellular coupling between flagella [15]. No obvious structural or morphological differences have been reported in the flagellar axonemes. Consequently, it is not possible from light microscopy data to prescribe the identity of the individual flagella in accordance with the numbering system devised from TEM images. Flagella numbering is thus distinguishable only up to circular permutation. Nonetheless, robust in-phase synchrony during free-swimming pertains to diametrically opposite flagella (positive phase correlation, figure 5d). This is in good agreement with the presence of direct physical coupling between distinguished pairs of flagella. Particularly, the principal pair b1 and b5 is connected by a thick striated structure known as the synistosome, which is functionally related to the distal fibre in C. reinhardtii [50]. The lack of correlation between certain basal body pairs (figure 4d) will be investigated further in future studies to determine if this is related to the detailed topology of the fibre network (e.g. b2 and 8 appear unconnected).
Finally, we find that in approximately 5% of cases, free-swimming cells exhibit a distinguished flagellum that is held extended in front of the cell body (electronic supplementary material, video S10). Figure 5 compares this ‘search gait’ with the canonical forward swimming gait of this organism. The beat pattern in this single flagellum is undulatory (sperm-like), in contrast to the lateral (ciliary) stroke used by the other seven flagella. These observations further highlight the capacity for producing heterodynamic behaviour in a network of outwardly identical flagella.
5. Discussion and outlook
The present article is devoted to the gait dynamics of free-living unicellular algal flagellates, advancing the state-of-the-art from single-cell to single-flagellum resolution. Performing a comparative study, I showed that gait coordination in algal flagellates is not only intracellular but also active. The same network of flagella can undergo dynamic reconfiguration over fast timescales to generate different locomotor patterns. Multiple species share an ability to reversibly activate beating in a subset of flagella, while suppressing the activity of the remainder. This is an extreme instantiation of symmetry-breaking in the algal flagella apparatus. Individually, a eukaryotic flagellum or cilium can be in an oscillatory or non-oscillatory state, while beating can be modulated by extrinsic factors including mechanosensitivity [27,51,52], but how can drastically distinct beat patterns be produced in different flagella of the same cell? We suggest two sources of this apparent control specificity in coupled flagella: (i) physical asymmetries in the basal apparatus and (ii) differences in signal transduction. The two contributions are not mutually exclusive.
In some species, physical asymmetries are obvious. Heterokont biflagellates can have one long flagellum and one short, or one smooth and one hairy (bearing mastigonemes). In our case, control specificity is all the more surprising as we only considered species whose flagella exhibit no exterior morphological differences. During flagellar assembly and lengthening, tubulin subunits are shuttled from an intracellular pool by molecular motors and added to the tips of growing flagella by a process known as intraflagellar transport [53]. This process is unlikely to result in structural heterogeneities between the individual flagella axonemes. Instead, asymmetries arising from physiological differences in basal body age [54] and associated contractile structures may be more significant. In flagellate green algae, basal body duplication is semi-conservative. The two flagella of C. reinhardtii are termed cis or trans according to their proximity to a single eyespot: the trans basal body is inherited from the parental cell but the cis is formed anew. This distinction likely underlies their differential calcium response, important during phototaxis [11]. The octoflagellate exhibits an extreme form of this asymmetric ultrastructural patterning, where the eight flagella are again distinguishable by the age of the attached basal body and organization of accessory structures (figure 5a).
Asymmetries in the control architecture itself are sufficient but not necessary to template asymmetric dynamics. A radially symmetric nerve ring innervates the 5-legged starfish [55], yet the organism routinely performs a breaststroke gait differentiating between two pairs of side arms and a single leading arm. It would therefore be interesting to explore whether the ‘special’ flagellum in P. octopus is always the same flagellum (as uniquely identified by basal body numbering), or whether different flagella can take turns to assume the ‘exploratory’ position (electronic supplementary material, video S10). Given the dual sensory and motor capabilities of cilia and flagella, this could signify an early evolution of division of labour. We suggest that symmetry-breaking in excitatory or inhibitory signalling in the algal flagella apparatus specifies the local state of contractility of intracellular fibres to control the activation state of individual flagella. This may be likened to the case of vertebrate limbs, where antagonistic control of flexor and extensor muscles is provided by motor neurons or to marine invertebrate larvae, where specialized ciliomotor neurons induce coordinated ciliary arrest [56]. We showed that in multiflagellates the ability to activate subsets of flagella provides a novel mechanism for trajectory reorientations that is distinct from steering in sperm [57] and in other uniflagellates that rely upon a change in the beat pattern of a single flagellum. A quadriflagellate can turn left, right, up or down, depending on which of its four flagella is active. However, the extent to which organisms make use of these capabilities warrants further study.
The diverse gaits of multiflagellates may have arisen from different evolutionary pressures associated with a need to occupy different ecological niches. Even small genetic changes could cause sufficient genetic rewiring of the basal apparatus and differences in locomotor pattern. With increasing flagella number, we suggest that new gaits arose from superpositions of gaits from flagellates with fewer flagella. In this perspective of gait modularity, the octoflagellate rotary breaststroke may have originated from intercalation of two quadriflagellate trotting gaits, and the trot or gallop from intercalcation of two pairs of biflagellate gaits. Indeed, it is thought that P. octopus branched from a related quadriflagellate Pyramimonas species via incomplete cell division [49].
In conclusion, the capacity to generate and coordinate complex behaviour is by no means exclusive to organisms possessing neural circuitry. In diverse unicellular flagellates, phase-resetting processes and transitions in locomotor patterning occurred much faster than is possible by any passive means, implicating excitable signalling. In future work, we will seek to identify structures capable of functioning as pacemakers for locomotor patterning in flagellates. By analogy with CPGs, we suggest that parts of the algal cytoskeleton may be independently capable of generating coupled oscillations. This has enabled these organisms to dynamically reconfigure their gaits in response to environmental changes and uncertainty, without the need for a central controller. The latter feat harkens to the embodiment perspective applicable to designing bioinspired robots, in which ‘control of the whole’ is ‘outsourced to the parts’ [58]. As modern technology strives towards greater automation, engineering adaptability into artificial systems has remained a formidable challenge. In this sense, cell motility is in fact a form of physical embodiment, wherein the compliant cytoskeleton and appendages enact morphological computation. In the light of these findings, we may wish to extend the scope of gait research beyond model vertebrates and invertebrates [24,59] to include aneural organisms. Much can be gained from exploring how simple unicellular organisms sculpt motor output and achieve sensorimotor integration—for herein lie the evolutionary origins of decentralized motion control.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
I thank Ray Goldstein and Gáspár Jékely for discussions and an anonymous referee for suggesting the relevance of embodiment concepts from control theory.
Data accessibility
Please refer to the electronic supplementary material for videos S1-10, and supplementary text file for more information. Additional datasets are available at https://doi.org/10.5281/zenodo.3551942.
Competing interests
We declare we have no competing interests.
Funding
Financial support is gratefully acknowledged from the University of Exeter.
References
- 1.Taute KM, Gude S, Tans SJ, Shimizu TS. 2015. High-throughput 3D tracking of bacteria on a standard phase contrast microscope. Nat. Commun. 6, 8776 ( 10.1038/ncomms9776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catavitello G, Ivanenko Y, Lacquaniti F, Calabrese RL, Chowdhury R. 2018. A kinematic synergy for terrestrial locomotion shared by mammals and birds. eLife 7, e38190 ( 10.7554/eLife.38190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muybridge E. 1979. Muybridge's complete human and animal locomotion, vol. 1 New York, NY: Dover Publications. [Google Scholar]
- 4.Ijspeert AJ. 2008. Central pattern generators for locomotion control in animals and robots: a review. Neural Netw. 21, 642–653. ( 10.1016/j.neunet.2008.03.014) [DOI] [PubMed] [Google Scholar]
- 5.Arshavsky YI, Deliagina TG, Orlovsky GN, Panchin YV, Popova LB, Sadreyev RI. 1998. Analysis of the central pattern generator for swimming in the mollusk Clione. Ann. NY Acad. Sci. 860, 51–69. ( 10.1111/j.1749-6632.1998.tb09038.x) [DOI] [PubMed] [Google Scholar]
- 6.Andersson LS, et al. 2012. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 488, 642–646. ( 10.1038/nature11399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauga E. 2019. Bacterial hydrodynamics. Annu. Rev. Fluid Mech . 48, 105–130. ( 10.1146/annurev-fluid-122414-034606) [DOI] [Google Scholar]
- 8.Blair DF. 1995. How bacteria sense and swim. Annu. Rev. Microbiol. 49, 489–522. ( 10.1146/annurev.mi.49.100195.002421) [DOI] [PubMed] [Google Scholar]
- 9.Man Y, Page W, Poole RJ, Lauga E. 2017. Bundling of elastic filaments induced by hydrodynamic interactions. Phys. Rev. Fluids 2, 123101 ( 10.1103/PhysRevFluids.2.123101) [DOI] [Google Scholar]
- 10.Jikeli JF, et al. 2015. Sperm navigation along helical paths in 3D chemoattractant landscapes. Nat. Commun. 6, 7985 ( 10.1038/ncomms8985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruffer U, Nultsch W. 1987. Comparison of the beating of cis- and trans-flagella of Chlamydomonas cells held on micropipettes. Cell Motil. Cytoskeleton. 7, 87–93. ( 10.1002/cm.970070111) [DOI] [PubMed] [Google Scholar]
- 12.Hegemann P. 1997. Vision in microalgae. Planta . 203, 265–274. ( 10.1007/s004250050191) [DOI] [PubMed] [Google Scholar]
- 13.Ruffer U, Nultsch W. 1991. Flagellar photoresponses of Chlamydomonas cells held on micropipettes II. Change in flagellar beat pattern. Cell Motil. Cytoskeleton. 18, 269–278. ( 10.1002/cm.970180404) [DOI] [Google Scholar]
- 14.Wan KY, Leptos KC, Goldstein RE. 2014. Lag, lock, sync, slip: the many ‘phases’ of coupled flagella. J. R. Soc. Interface. 11, 20131160 ( 10.1098/rsif.2013.1160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan KY, Goldstein RE. 2016. Coordinated beating of algal flagella is mediated by basal coupling. Proc. Natl Acad. Sci. USA 113, E2784–E2793. ( 10.1073/pnas.1518527113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolley DM, Vernon GG. 2001. A study of helical and planar waves on sea urchin sperm flagella, with a theory of how they are generated. J. Exp. Biol. 204, 1333–1345. [DOI] [PubMed] [Google Scholar]
- 17.Sartori P, Geyer VF, Scholich A, Julicher F, Howard J. 2016. Dynamic curvature regulation accounts for the symmetric and asymmetric beats of Chlamydomonas flagella. Elife 5, e13258 ( 10.7554/eLife.13258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man Y, Ling F, Kanso E. 2019. Cilia oscillations. Phil. Trans. R. Soc. B 375, 20190157 ( 10.1098/2019.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang ACH, Lam AT, Riedel-Kruse IH. 2018. Polygonal motion and adaptable phototaxis via flagellar beat switching in the microswimmer Euglena gracilis. Nat. Phys. 14, 1216–1222. ( 10.1038/s41567-018-0277-7) [DOI] [Google Scholar]
- 20.Qin B, Gopinath A, Yang J, Gollub JP, Arratia PE. 2015. Flagellar kinematics and swimming of algal cells in viscoelastic fluids. Sci. Rep. 5, 9190 ( 10.1038/srep09190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun SY, et al. 2018. Flagellum couples cell shape to motility in Trypanosoma brucei. Proc. Natl Acad. Sci. USA. 115, E5916–E5925. ( 10.1073/pnas.1722618115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan KY. 2018. Coordination of eukaryotic cilia and flagella. Essays Biochem. 62, 829–838. ( 10.1042/EBC20180029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nawroth JC, Feitl KE, Colin SP, Costello JH, Dabiri JO. 2010. Phenotypic plasticity in juvenile jellyfish medusae facilitates effective animal–fluid interaction. Biol. Lett. 6, 389–393. ( 10.1098/rsbl.2010.0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz PS. 2015. Evolution of central pattern generators and rhythmic behaviours. Phil. Trans. R. Soc. B. 371, 20150057 ( 10.1098/rstb.2015.0057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt JA, Eckert R. 1976. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature 262, 713–715. ( 10.1038/262713a0) [DOI] [PubMed] [Google Scholar]
- 26.Kunita I, Kuroda S, Ohki K, Nakagaki T. 2014. Attempts to retreat from a dead-ended long capillary by backward swimming in Paramecium. Front. Microbiol. 5, 270 ( 10.3389/fmicb.2014.00270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan KY, Goldstein RE. 2018. Time irreversibility and criticality in the motility of a flagellate microorganism. Phys. Rev. Lett. 121, 058103 ( 10.1103/PhysRevLett.121.058103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeanneret R, Contino M, Polin M. 2016. A brief introduction to the model microswimmer Chlamydomonas reinhardtii. Eur. Phys. J. Spec. Top. 225, 2141–2156. ( 10.1140/epjst/e2016-60065-3) [DOI] [Google Scholar]
- 29.Leptos KC, Wan KY, Polin M, Tuval I, Pesci AI, Goldstein RE. 2013. Antiphase synchronization in a flagellar-dominance mutant of Chlamydomonas. Phys. Rev. Lett. 111, 158101 ( 10.1103/PhysRevLett.111.158101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klindt GS, Ruloff C, Wagner C, Friedrich BM. 2017. In-phase and anti-phase flagellar synchronization by waveform compliance and basal coupling. New J. Phys. 19, 113052 ( 10.1088/1367-2630/aa9031) [DOI] [Google Scholar]
- 31.Quaranta G, Aubin-Tam ME, Tam D. 2015. Hydrodynamics versus intracellular coupling in the synchronization of eukaryotic flagella. Phys. Rev. Lett. 115, 238101 ( 10.1103/PhysRevLett.115.238101) [DOI] [PubMed] [Google Scholar]
- 32.Liu YJ, Claydon R, Polin M, Brumley DR. 2018. Transitions in synchronization states of model cilia through basal-connection coupling. J. R. Soc. Interface. 15, 20180450 ( 10.1098/rsif.2018.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golubitsky M, Stewart I, Buono L, Collins JJ. 1998. A modular network for legged locomotion. Physica D 115, 56–72. ( 10.1016/S0167-2789(97)00222-4) [DOI] [Google Scholar]
- 34.Moestrup O, Hill DRA. 1991. Studies on the Genus Pyramimonas (Prasinophyceae) from Australian and European waters: P. propulsa sp. nov and P. mitra sp. nov. Phycologia 30, 534–546. ( 10.2216/i0031-8884-30-6-534.1) [DOI] [Google Scholar]
- 35.Wilson D. 1967. Stepping patterns in tarantula spiders. J. Exp. Biol. 47, 133–151. [Google Scholar]
- 36.Ren Z, Hu W, Dong X, Sitti M. 2019. Multi-functional soft-bodied jellyfish-like swimming. Nat. Commun. 10, 2703 ( 10.1038/s41467-019-10549-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daugbjerg N, Moestrup O. 1992. Ultrastructure of Pyramimonas cyrtoptera sp nov (prasinophyceae), a species with 16 flagella from Northern Foxe Basin, Arctic Canada, including observations on growth-rates. Can. J. Bot. 70, 1259–1273. ( 10.1139/b92-159) [DOI] [Google Scholar]
- 38.Anderson B, Shultz J, Jayne B. 1995. Axial kinematics and muscle activity during terrestrial locomotion of the centipede Scolopendra heros. J. Exp. Biol. 198, 1185. [DOI] [PubMed] [Google Scholar]
- 39.Kano T, Sakai K, Yasui K, Owaki D, Ishiguro A. 2017. Decentralized control mechanism underlying interlimb coordination of millipedes. Bioinspir. Biomim. 12, 036007 ( 10.1088/1748-3190/aa64a5) [DOI] [PubMed] [Google Scholar]
- 40.Burrows M, Hoyle G. 1973. The mechanism of rapid running in the ghost crab, Ocypode ceratophthalma. J. Exp. Biol. 58, 327–349. [Google Scholar]
- 41.Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, Bednarek SY, Shorte SL, Eliceiri KW. 2017. TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80–90. ( 10.1016/j.ymeth.2016.09.016) [DOI] [PubMed] [Google Scholar]
- 42.Guo H, Wan KY, Nawroth J, Kanso E. 2018. Transitions in the synchronization modes of elastic filaments through basal-coupling. Bull. Am. Phys. Soc. 63, 13. [Google Scholar]
- 43.Daugbjerg N. 2000. Pyramimonas tychotreta, sp. nov. (Prasinophyceae), a new marine species from Antarctica: light and electron microscopy of the motile stage and notes on growth rates. J. Phycol. 36, 160–171. ( 10.1046/j.1529-8817.2000.99157.x) [DOI] [Google Scholar]
- 44.Quarmby LM, Hartzell HC. 1994. Two distinct, calcium-mediated, signal-transduction pathways can trigger deflagellation in Chlamydomonas reinhardtii. J. Cell Biol. 124, 807–815. ( 10.1083/jcb.124.5.807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheeler GL, Joint I, Brownlee C. 2008. Rapid spatiotemporal patterning of cytosolic Ca2+ underlies flagellar excision in Chlamydomonas reinhardtii. Plant J. 53, 401–413. ( 10.1111/j.1365-313X.2007.03349.x) [DOI] [PubMed] [Google Scholar]
- 46.Aguilar J, et al. 2016. A review on locomotion robophysics: the study of movement at the intersection of robotics, soft matter and dynamical systems. Rep. Prog. Phys. 79, 110001 ( 10.1088/0034-4885/79/11/110001) [DOI] [PubMed] [Google Scholar]
- 47.Grabowska M, Godlewska E, Schmidt J, Daun-Gruhn S. 2012. Quadrupedal gaits in hexapod animals—inter-leg coordination in free-walking adult stick insects. J. Exp. Biol. 215, 4255–4266. ( 10.1242/jeb.073643) [DOI] [PubMed] [Google Scholar]
- 48.Harz H, Nonnengasser C, Hegemann P. 1992. The photoreceptor current of the green-alga Chlamydomonas. Phil. Trans. R. Soc. Lond. B 338, 39–52. ( 10.1098/rstb.1992.0127) [DOI] [Google Scholar]
- 49.Moestrup O, Hori T. 1989. Ultrastructure of the flagellar apparatus in Pyramimona octopus (prasinophyceae) II: flagellar roots, connecting fibers, and numbering of individual flagella in green-algae. Protoplasma 148, 41–56. ( 10.1007/BF01403990) [DOI] [Google Scholar]
- 50.Ringo DL. 1967. Flagellar motion and fine structure of flagellar apparatus in Chlamydomonas. J. Cell Biol. 33, 543–571. ( 10.1083/jcb.33.3.543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathijssen A, Culver J, Bhamla MS, Prakash M. 2019. Collective intercellular communication through ultra-fast hydrodynamic trigger waves. Nature 10, 560–564. ( 10.1038/s41586-019-1387-9) [DOI] [PubMed] [Google Scholar]
- 52.Bezares-Calderón LA, Berger J, Jékely G. 2019. Diversity of cilia-based mechanosensory systems and their functions in marine animal behaviour. Phil. Trans. R. Soc. B 375, 20190376 ( 10.1098/rstb.2019.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbaum JL, Witman GB. 2002. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813–825. ( 10.1038/nrm952) [DOI] [PubMed] [Google Scholar]
- 54.Bornens M. 2018. Cell polarity: having and making sense of direction—on the evolutionary significance of the primary cilium/centrosome organ in Metazoa. Open Biol. 8, 180052 ( 10.1098/rsob.180052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe W, Kano T, Suzuki S, Ishiguro A. 2011. A decentralized control scheme for orchestrating versatile arm movements in ophiuroid omnidirectional locomotion. J. R. Soc. Interface. 9, 102–109. ( 10.1098/rsif.2011.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veraszto C, Ueda N, Bezares-Calderon LA, Panzera A, Williams EA, Shahidi R, Jekely G. 2017. Ciliomotor circuitry underlying whole-body coordination of ciliary activity in the Platynereis larva. Elife 6, e26000 ( 10.7554/eLife.26000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong A, Rode S, Kaupp UB, Gompper G, Elgeti J, Friedrich BM, Alvarez L. 2019. The steering gaits of sperm. Phil. Trans. R. Soc. B 375, 20190149 ( 10.1098/rstb.2019.0149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeifer R, Lungarella M, Iida F. 2007. Self-organization, embodiment, and biologically inspired robotics. Science 318, 1088–1093. ( 10.1126/science.1145803) [DOI] [PubMed] [Google Scholar]
- 59.Pearson KG. 1993. Common principles of motor control in vertebrates and invertebrates. Annu. Rev. Neurosci. 16, 265–297. ( 10.1146/annurev.ne.16.030193.001405) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please refer to the electronic supplementary material for videos S1-10, and supplementary text file for more information. Additional datasets are available at https://doi.org/10.5281/zenodo.3551942.