Abstract
Noonan syndrome with multiple lentigines (NSML), formerly known as LEOPARD Syndrome, is a rare autosomal dominant disorder. Approximately 90% of NSML cases are caused by missense mutations in the PTPN11 gene which encodes the protein tyrosine phosphatase SHP2. A human induced pluripotent stem cell (iPSC) line was generated using peripheral blood mononuclear cells (PBMCs) from a patient with NSML that carries a gene mutation of p.Q510P on the PTPN11 gene using non-integrating Sendai virus technique. This iPSC line offers a useful resource to study the disease pathophysiology and a cell-based model for drug development to treat NSML.
Resource utility
This hiPSC line is a useful tool for studing disease phenotype and disease pathophysiology as well as serving as a cell-based disease model for drug development to treat patients with Noonan syndrome with multiple lentigines (NSML).
Resource details
Noonan syndrome with multiple lentigines (NSML), formerly known as LEOPARD Syndrome, is a rare autosomal dominant disorder whose major characteristic features include lentigines, craniofacial dysmorphism, myocardium or valve abnormalities, electrocardiographic conduction defects and sensorineural deafness. Approximately 90% of NSML cases are caused by missense mutations in the PTPN11 gene, which encodes the protein tyrosine phosphatase SHP2 [1–3].
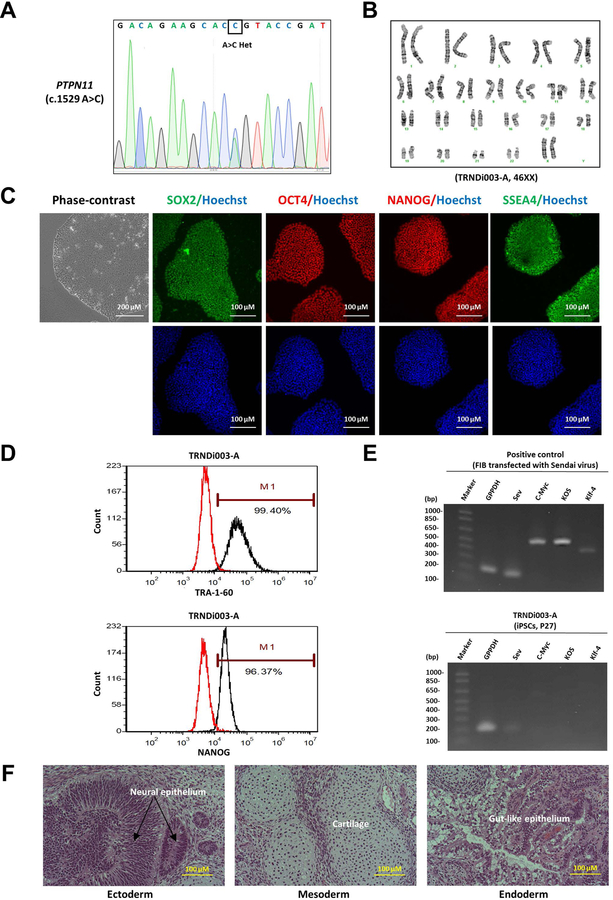
In this study, a human induced pluripotent stem cell (iPSC) line was established using peripheral blood mononuclear cells (PBMCs) from a 13 year old female patient with atrial septal defect Secundum and pulmonary stenosis carrying a heterozygous gene mutation of a p.Q510P in the PTPN11 gene (see Table 1). To generate the iPSCs, the non-integrating CytoTune-Sendai viral vector kit (A16517, Thermo Fisher Scientific) containing OCT3/4, KLF4, SOX2 and C-MYC pluripotency transcription factors was employed to transduce the patient cells using a previously described method [4, 5]. The iPS cell line named TRNDi003-A was generated and the mutation of PTPN11 gene in the TRNDi003-A iPSC line was confirmed by Sanger sequencing of the PCR product harboring the single nucleotide variation (SNV) (Fig. 1A). The patient iPS cells exhibited a classical embryonic stem cell morphology (Fig. 1C) and expressed the major pluripotent protein markers of NANOG, SOX2, OCT4, SSEA4 and TRA-1–60 (Fig. 1C). They also had a normal karyotype (46, XX), as confirmed by the G-banded karyotyping (Fig. 1B). In addition, flow cytometric analysis showed that the expression levels of NANOG and cell surface marker TRA-1–60 were over 96%. (Fig. 1D). The Sendai virus vector (SeV) clearance was detected with reverse transcription polymerase chain reaction (RT-PCR) using SeV-specific primers; the vector disappeared by passage 27 (Fig. 1E). This iPSC line was not contaminated with mycoplasma (Supplementary Fig. S1) and the short tandem repeat (STR) DNA profile of 16 loci was established from iPSC TRNDi003-A (information available from the authors). Furthermore, the pluripotency of this iPS cell line was confirmed by a teratoma formation experiment that exhibited its ability to differentiate into cells of all three germ layers (Ectoderm, neural epithelium; Mesoderm, cartilage; Endoderm, gut-like epithelium) in vivo (Fig. 1F).
Table 1:
Characterization and validation
| Classification | Test | Result | Data |
|---|---|---|---|
| Morphology | Photography | Normal | Fig. 1 Panel C |
| Phenotype | Immunocytochemistry | SOX2, OCT4, NANOG, SSEA-4 | Fig. 1 Panel C |
| Flow cytometry | TRA-1-60 (99.40%); NANOG (96.37%) | Fig. 1 Panel D | |
| Genotype | Karyotype (G-banding) and resolution | 46XY Resolution: 425–475 | Fig. 1 Panel B |
| Identity | Not performed | N/A | |
| STR analysis | 16 sites tested, all sites matched | Available from the authors | |
| Mutation analysis (IF APPLICABLE) | Sequencing | PTPN11, pQ510P | Fig. 1 Panel A |
| Southern Blot OR WGS | N/A | N/A | |
| Microbiology and virology | Mycoplasma | Mycoplasma testing by luminescence. Negative | Supplementary Fig. S1 |
| Differentiation potential | Teratoma formation | Teratoma with three germlayers formation. Ectoderm (neural epithelium); Mesoderm (cartilage); Endoderm (gut-like epithelium) | Fig. 1 Panel F |
| Donor screening (OPTIONAL) | HIV 1 + 2 Hepatitis B, Hepatitis C | N/A | N/A |
| Genotype additional info (OPTIONAL) | Blood group genotyping | N/A | N/A |
| HLA tissue typing | N/A | N/A |
Fig. 1.
Characterization of TRNDi003-A iPSC line. A) Detection of a heterozygous variant of p.Q510P (c.1529 A>C) in exon 12 of the PTPN11 gene. B) Cytogenetic analysis showing a normal karyotype (46, XX). C) Left: Phase contrast imaging of TRNDi003-A colonies grown on Matrigel at passage 10. Right: Representative immunofluorescent micrographs of iPSCs positive for stem cell markers: SOX2, OCT4, NANOG, and SSEA4. Nucleus is labelled with Hoechst (in blue). D) Flow cytometry analysis of pluripotency protein markers: TRA-1-60 and NANOG. E) RT-PCR verification of the clearance of Sendai virus from the reprogrammed cells. Sendai virus vector transduced fibroblasts was used as positive control. F) Pathological analysis of a teratoma from TRNDi003-A iPSC, showing a normal ectodermal, endodermal and mesodermal differentiation.
Materials and Methods
Cell culture
TRNDi003-A iPSCs were cultured in StemFlex™ Medium (Thermo Fisher Scientific) on Matrigel (Corning, Cat# 354277)-coated plates at 37 °C in humidified air with 5% CO2 ad 5% O2. The cells were passaged with 0.5 mM Ethylenediaminetetraacetic acid (EDTA) at a general 1:6 ratio when they reached 80% confluency.
Reprogramming of human PBMCs
Patient PBMCs (LS PTPN11 Q510P, #9915) were obtained from Dr. Amy Roberts at Boston Children’s Hospital. One million patient PBMCs were cultured in one well of 12-well tissue culture plate with 1ml StemSpan™ SFEM II medium supplemented with StemSpan™ Erythroid Expansion Supplement (StemCell Technologies) for expansion of human erythroid cells for 10 days and then reprogrammed into iPSCs using the non-integrating CytoTune–Sendai viral vector kit (A16517, Thermo Fisher Scientific) following the method described previously [4, 5]. On day 4, cells were re-plated onto a Matrigel-coated dish in E8-media based reprogramming media, and fed every other day until day 20 when individual colonies were passaged by the EDTA dissociation method into separate wells in E8 medium. The selected iPSC colonies were further cultured for more than 15 passages before downstream application.
Genome analysis of variant in PTPN11 gene
The genome analysis of variants in PTPN11 was conducted through Applied StemCell (Milpitas, California, USA). Briefly, genomic DNA was extracted from hiPSC line TRNDi003-A using QuickExtract™ DNA Extraction Solution (Lucigen) followed by PCR amplification using MyTaq™ Red Mix (Bioline, Taunton, MA). Amplifications were carried out using standard protocol. Genotyping of the p. Q510P variant (p. Gln510Pro, c.1529 A>C) in exon 12 of the PTPN11 gene was performed using Sanger sequencing analysis. The specific primers for gene amplification and sequencing are listed in Table 2.
Table 2:
Reagents details
| Antibodies used for immunocytochemistry/flow-cytometry | |||
|---|---|---|---|
| Antibody | Dilution | Company Cat # and RRID | |
| Pluripotency Markers | Mouse anti-SOX2 | 1:50 | R & D systems, Cat# MAB2018, RRID: AB_358009 |
| Pluripotency Markers | Rabbit anti-NANOG | 1:400 | Cell signaling, Cat# 4903, RRID: AB_10559205 |
| Pluripotency Markers | Rabbit anti-OCT4 | 1:400 | Thermo Fisher, Cat# A13998, RRID: AB_2534182 |
| Pluripotency Markers | Mouse anti-SSEA4 | 1:1000 | Cell signaling, Cat# 4755, RRID: AB_1264259 |
| Secondary Antibodies | Donkey anti-Mouse IgG (Alexa Fluor 488) | 1:400 | Thermo Fisher, Cat# A21202, RRID: AB_141607 |
| Secondary Antibodies | Donkey anti-Rabbit IgG (Alexa Fluor 594) | 1:400 | Thermo Fisher, Cat# A21207, RRID: AB_141637 |
| Flow Cytometry Antibodies | Anti-Tra-1-60-DyLight 488 | 1:50 | Thermo Fisher, Cat# MA1-023-D488X, RRID: AB_2536700 |
| Flow Cytometry Antibodies | Anti-Nanog-Alexa Fluor 488 | 1:50 | Millipore, Cat# FCABS352A4, RRID: AB_10807973 |
| Flow Cytometry Antibodies | anti-SSEA-4-Alexa Fluor 488 | 1:50 | Thermo Fisher, Cat# 53-8843-41, RRID: AB_10597752 |
| Flow Cytometry Antibodies | Mouse-IgM-DyLight 488 | 1:50 | Thermo Fisher, Cat# MA1-194-D488, RRID: AB_2536969 |
| Flow Cytometry Antibodies | Rabbit IgG-Alexa Fluor 488 | 1:50 | Cell Signaling, Cat#4340S, RRID: AB_10694568 |
| Flow Cytometry Antibodies | Mouse IgG3-FITC | 1:50 | Thermo Fisher, Cat# 11-4742-42, RRID: AB_2043894 |
| Primers | |||
| Target | Forward/Reverse primer (5′–3′) | ||
| Sev specific primers (RT-PCR) | Sev/181 bp | GGA TCA CTA GGT GAT ATC GAG C/ ACC AGA CAA GAG TTT AAG AGA TAT GTA TC | |
| Sev specific primers (RT-PCR) | KOS/528 bp | ATG CAC CGC TAC GAC GTG AGC GC/ ACC TTG AC A ATC CTG ATG TGG | |
| Sev specific primers (RT-PCR) | KIf4/410 bp | TTC CTG CAT GCC AGA GGA GCC C/ AAT GTA TCG AAG GTG CTC AA | |
| Sev specific primers (RT-PCR) | C-Myc/523 bp | TAA CTG ACT AGC AGG CTT GTC G/ TCC ACA TAC AGT CCT GGA TGA TGA TG | |
| House-Keeping gene (RT-PCR) | GAPDH/197 bp | GGA GCG AGA TCC CTC CAA AAT/ GGC TGT TGT CAT ACT TCT CAT GG | |
| Targeted mutation analysis (PCR) | PTPN11(c.1529 A>C)/530 bp | GCC ATG GCC TTT TGT TGC AT/ CCT GCT CAA AAG GAG AGC GT | |
Immunocytochemistry staining
For immunofluorescence staining, patient iPSCs were fixed in 4% paraformaldehyde for 15 min, rinsed with Dulbecco’s phosphate-buffered saline (DPBS), and permeabilized with 0.3% Triton X-100 in DPBS for 15 min. The cells were then incubated with the Image-iT™ FX signal enhancer (ThermoFisher Scientific) for 40 min at room temperature in a humidified environment, followed by incubation with primary antibodies including SOX2, OCT4, NANOG and SSEA4, diluted in the Image-iT™ FX signal enhancer blocking buffer, overnight at 4 °C. After washing with DPBS, a corresponding secondary antibody conjugated with Alexa Fluor 488 or Alex Fluor 594 was added to the cells and incubated for 1 h at room temperature (Antibodies used are listed in Table 2). Cells were washed and then stained with Hoechst 33342 for 15 min and imaged using an INCell Analyzer 2200 imaging system (GE Healthcare) with 20X objective lens and Texas Red, FITC and DAPI filter sets.
Flow Cytometry analysis
The iPSCs were harvested using TrypLE Express enzyme (Thermo Fisher). Cells were fixed with 4% paraformaldehyde for 10 min at room temperature and then washed with phosphate-buffered saline (PBS). Before fluorescence-activated cell sorting analysis, cells were permeabilized with 0.2% Tween-20 in PBS for 10 min at room temperature and stained with fluorophore conjugated antibodies for 1 h at 4°C on a shaker. Relative fluorophore conjugated animal nonimmune Immunoglobulin was used as the negative control. (Antibodies and nonimmune immunoglobulin used are listed in Table 2). Cells were then analyzed on a BD AccuriC6 FlowCytometry system (BD Biosciences).
G-banded Karyotyping
The G-banding karyotype analysis was conducted at WiCell Research Institute (Madison, WI, USA). Cell harvest, slide preparation and G-banded karyotyping experiments were performed using standard cytogenetic protocols. Cells were incubated with ethidium bromide and colcemid and then placed in hypotonic solution followed by fixation. Metaphase cell preparations were stained with Leishman’s stain. A total of 20 randomly selected metaphases were analyzed by G-banding for each cell line.
Short tandem repeat (STR) DNA profile analysis
Patient fibroblasts and derived iPSC lines were sent to the WiCell Institute for Short Tandem Repeat (STR) analysis performed by the Translational Research Initiatives in Pathology (TRIP) Laboratory at University of Wisconsin – Madison. Briefly, the Promega PowerPlex® 16 HS System (Promega, Madison, WI) was used in multiplex polymerase chain reaction (PCR) to amplify fifteen STR loci (D5S818, D13S317, D7S820, D16S539, vWA, TH01, TPOX, CSF1PO, D18S51, D21S11, D3S1358, D8S1179, FGA, Penta D, Penta E) plus a gender determining marker, Amelogenin (AMEL). The PCR product was capillary electrophoresed on an ABI 3500xL Genetic Analyzer (Applied Biosystems) using the Internal Lane Standard 600 (ILS 600) (Promega, Madison, WI). Data was analyzed using GeneMapper® v 4.1 software (Applied Biosystems).
Mycoplasma detection
Mycoplasma testing was performed and analyzed using the Lonza MycoAlert kit following the instructions from the company. Ratio B/A > 1.2 indicates mycoplasma positive, 0.9–1.2 result indicates mycoplasma ambiguous and Ratio B/A < 0.9 indicates mycoplasma negative.
Testing for Sendai reprogramming vector clearance
Total RNA was isolated from iPSC TRNDi003-A at passage 27 using RNeasy Plus Mini Kit (Qiagen). Human fibroblasts (Coriell Institute, GM05659) after transduction with Sendai virus for 4 days was used as a positive control. A total of 1 μg RNA/reaction was reverse transcribed with SuperScript™ III First-Strand Synthesis SuperMix kit, and PCR was performed using Platinum II Hot-Start PCR Master Mix (Thermo Fischer Scientific). The amplifications were carried out using the following program: 94°C, 2 mins; 30 cycles of [94°C, 15 s, 60°C, 15 s and 68°C, 15 s] on Mastercycler pro S (Eppendorf) with the primers listed in Table 2. The products were then loaded to the E-Gel® 1.2% with SYBR Safe™ gel, run at 120V electric field and then imaged by G: Box Chemi-XX6 gel doc system (Syngene, Frederick, MD)
Teratoma formation assay
Patient iPSCs cultured in 6- well plates were dissociated with 0.5mM EDTA and approximately 1 × 107 dissociated cells were resuspended in 400 μl culture medium supplied with 25mM HEPES (pH7.4) and stored on ice. Then, 50% volume (200 μl) of cold Matrigel (Corning, 354277) was added and mixed with the cells. The mixture was injected subcutaneously into NSG mice (JAX No. 005557) at 150 μl per injection site. Visible tumors were removed 6–8 weeks post injection, and were immediately fixed in 10% Buffer Balanced Formalin. The fixed tumors were embedded in paraffin and stained with hematoxylin and eosin.
Supplementary Material
Resource Table.
| Unique stem cell line identifier | TRNDi003-A |
| Alternative name(s) of stem cell line | HT215A |
| Institution | National Institutes of Health National Center for Advancing Translational Sciences Bethesda, Maryland, USA |
| Contact information of distributor | Dr. Wei Zheng Wei.Zheng@nih.gov |
| Type of cell line | iPSC |
| Origin | Human |
| Additional origin info | Age: 13-year-old Sex: Female Ethnicity: Somalian |
| Cell Source | Peripheral blood mononuclear cells (PBMCs) |
| Clonality | Clonal |
| Method of reprogramming | Integration-free Sendai viral vectors |
| Genetic Modification | NO |
| Type of Modification | N/A |
| Associated disease | Noonan syndrome with multiple lentigines (NSML) |
| Gene/locus | PTPN11Q510P |
| Method of modification | N/A |
| Name of transgene or resistance | N/A |
| Inducible/constitutive system | N/A |
| Date archived/stock date | 03-26-2017 |
| Cell line repository/bank | Human Pluripotent Stem Cell Registry https://hpscreg.eu/cell-line/TRNDi003-A |
| Ethical approval | Completed under Boston Children’s Hospital IRB Protocol 08-05-0208 |
Acknowledgement
We would like to thank Dr. Zu-xi Yu of the pathology Core of National Heart, Lung and Blood Institute, National Institutes of Health for sectioning and staining the tumors for the teratoma formation assay. We also would like to thank WiCell and the University of Wisconsin Translational Research Initiatives in Pathology laboratory for STR DNA analysis and thank Maya Gosztyla at National Center for Advancing Translational Sciences for mycoplasma testing service. This work was supported by the Therapeutics for Rare and Neglected Diseases Program, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health. Finally, we would like to thank the donors to the NSML Research Fund, Department of Cardiology, Boston Children’s Hospital.
References
- [1].Ogata T, Yoshida R , PTPN11 mutations and genotype-phenotype correlations in Noonan and LEOPARD syndromes, Pediatr Endocrinol Rev, 2 (2005) 669–674. [PubMed] [Google Scholar]
- [2].Pierpont ME, Digilio MC, Cardiovascular disease in Noonan syndrome, Curr Opin Pediatr, (2018). [DOI] [PubMed] [Google Scholar]
- [3].Sarkozy A, Digilio MC, Dallapiccola B, Leopard syndrome, Orphanet J Rare Dis, 3 (2008) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G, Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions, Nat Protoc, 7 (2012) 2029–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beers J, Linask KL, Chen JA, Siniscalchi LI, Lin Y, Zheng W, Rao M, Chen G, A costeffective and efficient reprogramming platform for large-scale production of integration-free human induced pluripotent stem cells in chemically defined culture, Sci Rep, 5 (2015) 11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.