Abstract
Background
Prenatal alcohol exposure (PAE) causes distinctive craniofacial anomalies that arise, in part, from the apoptotic elimination of neural crest (NC) progenitors that form the face. This vulnerability of NC to alcohol is puzzling as they normally express the transcriptional repressor Snail1/2 (in chick Snai2), which suppresses apoptosis and promotes their migration. Here, we investigate alcohol’s impact upon Snai2 function.
Methods
Chick cranial NC cells were treated with acute alcohol (52mM, 2hr). We evaluated NC migration, gene expression, proliferation, and apoptosis thereafter.
Results
Transient alcohol exposure induced Snai2 (191% ± 23%; p=0.003) and stimulated NC migration (p=0.0092). An alcohol-induced calcium transient mediated this Snai2 induction, and BAPTA-AM blocked whereas ionomycin mimicked these pro-migratory effects. Alcohol suppressed CyclinD1 protein content (59.1% ± 12%, p=0.007) and NC proliferation (19.7% ± 5.8%, p<0.001), but these Snai2-enriched cells still apoptosed in response to alcohol. This was explained because alcohol induced p53 (198% ± 29%, p=0.023), and the p53 antagonist pifithrin-α prevented their apoptosis. Moreover, alcohol counteracted Snai2’s pro-survival signals, and Bcl2 was repressed (68.5% ± 6.0% of controls, p=0.016) and PUMA was not induced, while ATM (1.32-fold, p=0.01) and PTEN (1.30-fold, p=0.028) were elevated.
Conclusions
Alcohol’s calcium transient uncouples the Snai2/p53 regulatory loop that normally prevents apoptosis during EMT. This represents a novel pathway in alcohol’s neurotoxicity, and complements demonstrations that alcohol suppresses PUMA in mouse NC. We propose that the neural crest’s migratory behavior, and their requirement for Snai2/p53 co-expression, makes them vulnerable to stressors that dysregulate Snai2/p53 interactions, such as alcohol.
Keywords: fetal alcohol spectrum disorder, neural crest, Snai2, p53, apoptosis, cell migration
Introduction
Prenatal alcohol exposure (PAE) is a leading cause of neurodevelopmental disability and affects 2–5% of school-age children (May et al. 2018). PAE during early cranial morphogenesis causes characteristic facial deficits that facilitate diagnosis (Klingenberg et al. 2010). These deficits reflect reduced brain growth (Lipinski et al. 2012) and impaired development of neural crest (Smith et al. 2014), a stem cell population that contributes to facial bones, certain cranial nerves, and the sympathetic /parasympathetic nervous systems. These cells originate within the neural fold lateral margins, and during neural tube closure, they delaminate and migrate to occupy the developing facial anlage. Alcohol disrupts multiple events during their development including prechordal plate extension, specification, and cell migration; it also promotes their extensive apoptosis (Smith et al. 2014). Using a chick embryo model of PAE, we showed this apoptosis originates from pharmacologically-relevant alcohol concentrations (EC50 = 52mM), which invoke a IP3-mediated intracellular calcium transient that originates from a pertussis toxin-sensitive G protein-coupled receptor and activates CaMKII (Garic et al. 2011; Garic-Stankovic et al. 2005). CaMKII loss-of-function promotes neural crest survival in alcohol’s presence, whereas targeted CaMKII activation initiates neural crest death in alcohol’s absence (Flentke et al. 2014; Garic et al. 2011).
Accompanying these cellular losses is altered neural crest migration. In alcohol’s direct presence, fewer cells exit the neural folds, travel distance is reduced, and migration is less directional (Boric et al. 2013; Czarnobaj et al. 2014; Hassler and Moran 1986; Oyedele and Kramer 2013; Rovasio and Battiato, 2002). Alcohol’s impact upon the signals that govern epithelial-mesenchymal transformation (EMT) in neural crest is unknown. Neural crest migration is initiated by the transcriptional repressor snail1/2 (del Barrio and Nieto, 2004; Thiery et al. 2009), which recruits HDAC1/SIN3A repressive complexes to the GC-rich E-box sequence –CANNTG–. Snai1/2 promotes delamination and cell cycle withdrawal through repression of the cell-adhesion protein E-cadherin (Thiery et al. 2009) and CyclinD1/D2 (Vega et al. 2004), respectively. Because delamination normally promotes p53-mediated apoptosis in non-migratory populations, Snai1/2 promotes cell survival during EMT by controlling proteins that govern p53 activity and Bcl2 stability, such as PUMA, ATM, and PTEN (Kim et al. 2011; Kurrey et al. 2009; Wu et al. 2005), several of these are dysregulated by alcohol (Chen et al. 2015; Derdak et al. 2011; Yuan et al. 2017). For neural crest, targeted Snai1/2 overexpression promotes their specification and cellular expansion (Aybar et al., 2003; Del Barrio and Nieto, 2002; LaBonne and Bronner-Fraser 1998), whereas Snai2 loss-of-function reduces both neural crest numbers and migration (Aybar et al., 2003; Carl et al., 1999; LaBonne and Bronner-Fraser 1998). In non-migratory lineages, Snai1/2 gain-of-function is oncogenic through its promotion of tumor cell invasion, cell senescence, and resistance to p53-mediated apoptosis (Thiery et al. 2009). Because the premigratory neural crest must suppress apoptosis during EMT and delamination, balanced Snai1/2 activity is essential for their survival and its imbalance would stimulate apoptosis and alter migration.
The Snai1/2 homolog Snai2 (also known as slug) promotes EMT in chick neural crest. Snai2 induction at the 6–8 somite stage initiates EMT within premigratory neural crest (del Barrio and Nieto 2002). Although alcohol causes neural crest apoptosis and alters their migration, its effects on Snai1/2 and EMT remain unknown. Here, we investigate alcohol’s impact on these processes, using a transient alcohol exposure that models binge drinking. We find that alcohol causes a calcium-mediated increase in Snai2 expression and Snai2-dependent activities, but Snai’s anti-apoptotic protection is overridden by the parallel activation of p53.
Methods
Alcohol Treatment
In ovo chick embryos (strain ‘Special Black’, Sunnyside, Beaver Dam WI; strain Rhode Island Red, North Carolina State University) at the 3–5 somite stage were randomly assigned to receive 250 μl of 0.9% saline (control) or 0.43 mmol ethanol (USP grade, Pharmco-Aaper, Brookfield CT) in isotonic saline, injected into the yolk center, then were reincubated to the desired developmental stage. Embryos experience peak alcohol levels of 50–60 mM for 90–120 min (Flentke et al. 2011), and this models a single acute binge exposure. Chick embryos of these stages are exempt from ACUC review.
Neural Crest Culture
Neural crest cultures were prepared as described (Bronner-Fraser and Garcia-Castro, 2008). Headfolds of embryos having 10–13 somites, exposed 10hr earlier to saline or alcohol as above, were isolated by dissection and transferred, dorsal side down, onto cover slips coated with 25 μg/ml bovine plasma fibronectin (Invitrogen). Explants were incubated 18hr at 37°C in F12 medium containing 10% heat inactivated fetal bovine serum, 1x penicillin / streptomycin, and 7.5% (v/v) chick embryo extract (prepared from day-10 embryos as per Bronner-Fraser and Garcia-Castro, 2008); alcohol was absent from the cultures. Some headfolds were pretreated with Bapta-AM (20 μM plus 0.02% Pluronic F127; 45min) prior to alcohol exposure, or were treated with ionomycin-only (50 nM for 2min); headfolds were washed and then explanted as above. After an 18hr culture period, cranial tissue was removed using fine forceps and migrated cells were fixed in 4% paraformaldehyde in phosphate buffered saline (1hr) for subsequent immunostaining and cell quantitation. Experiments analyzed at least 8 crania per treatment group.
To enumerate the number of DAPI+ cells within the anterior-most migratory wave, centered on the headfold’s rostral limit, we developed an image segmentation program using a watershed algorithm implemented in Matlab. First, contrast-limited adaptive histogram equalization was used to increase the contrast of imaged cells against background areas without any cells. Application of a threshold function to the contrast-enhanced image defined the cell perimeters. The number of closed perimeters provided an estimate of cell number. However, when cells are closely adjacent to or overlap, this procedure underestimates the true cell number. To reduce this source of error, a marker-based watershed segmentation algorithm was also applied. DAPI-stained nuclei served as anchors to denote individual cells as they appear significantly brighter than their immediate surroundings. This increased the average cell counts by a few percent over the simpler threshold function. We measured the maximal radial distance, drawn at a 90-degree angle from the explant, to determine the cell migration distance.
Transient Transfection
Electroporation of in ovo embryos was performed as described (Flentke et al. 2011). cDNA encoding Snai2 was from Addgene (#34584; Cambridge, MA); cDNA encoding enhanced green fluorescent protein (eGFP) was a kind gift of T. Suzuki. Constructs were used at a 3:1 ratio (1.2:0.4 μg/μl) of experimental:eGFP cDNA. DNA (2 μl) was electroporated into the hindbrain lumen at the 5–8 somite stage and treated with saline or alcohol 3hr later as above. Hindbrains lacking eGFP expression were excluded from analysis. We evaluated 10–12 embryos per treatment and experiments were performed in triplicate.
Western Blot
This was performed as described (Flentke et al. 2011). Antibodies were directed against Snail2 (#27568, 1:1000; Abcam), CyclinD1 (#100–1939 1:1000; Novus), Bcl2 (#610538, 1:1000; BD Biosciences), PUMA (#SPC-166, 1:2000; StressMarq Biosciences, Victoria, BC), p53 (#SC-99, 1:2000; Santa Cruz), E-cadherin (#07–697, 1:1000, EMD Millipore), and GAPDH (#G8795, 1:50,000, Sigma, St. Louis MO). Vendors confirmed the immunizing peptide sequence is conserved in chick. For some western blots, bands were normalized against GAPDH and visualized using infrared dye-conjugated secondary antibodies and the Odyssey system (LiCor); GAPDH protein content is unaffected by alcohol in this model (Flentke et al. 2011). For other western blots, bands were instead normalized against total protein content per lane (Revert™ Total Protein Stain; LiCor, # 926–11021), visualized using chemiluminescence with horseradish peroxidase secondary antibodies, and imaged with the Azure Biosystems C600 system (Dublin, CA). Experiments were performed in triplicate and analyzed pools of 8–10 dissected headfolds per lane.
Quantitative PCR
qPCR followed MIQE standards and as previously described (Flentke et al. 2011). Pooled RNA was isolated from 25 stage-matched headfolds (13–16 somite stage) at 18hr following in ovo alcohol or saline treatment, using the Ambion Melt Total Nucleic Acid Isolation kit. cDNA synthesis used the Improm2 reverse transcriptase (Promega, Madison WI). qPCR used the SYBR Select Master Mix (ABI, # 4472913) on the BioRad CFX96, and with the following protocol: preincubation 50°C 2 minutes, 95°C 2 minutes, 40–55 cycles of 95°C 15 seconds, 55°C 15 seconds, 72°C 45 seconds. Expression was normalized to GAPDH content, which is unaffected by alcohol in this model. Mean relative expression was calculated using the 2-ΔΔCT method. Samples were run in triplicate and each gene was analyzed in at least three separate experiments. The Snail2 primers (XM_419196.6) generated a 137bp product and were: Forward 5’-CAGCGGTTCAGAAAGTCCCA; Reverse 5’-TGGCCAACCCAGAGAAAGTG. The GAPDH primers (M11213) generated a 189bp product and were: Forward 5’- CGTGTTGTGGACTTGATGGT; Reverse 5’- TGGAGGAAGAAATTGGAGGA. Primers were from IDT (Coralville, IA) and were chosen based on having an efficiency greater than 95%. Test and housekeeping genes were diluted to run within 3 Cqs of each other.
Immunohistochemistry
This was performed on paraffin-embedded tissue sections or neural crest explants as described (Flentke et al. 2011), evaluating 3–5 embryos per treatment and 5–7 hindbrain sections per embryo. Antibodies were directed against Snail2 (#27568, 1:1000, AbCam), E-cadherin (#07–697, 1:1000, EMD Millipore), and p53 (#SC-99, 1:2000, Santa Cruz). Isotype-directed secondary antibodies were conjugated with Alexa488. Nuclei were visualized using DAPI stain. All images were photographed using the same exposure.
Quantitation of Proliferation and Apoptosis
To quantify proliferation, 0.5 μM BrdU was applied directly to in ovo embryo and embryos were fixed 2hr later. Incorporated BrdU was unmasked by pretreatment in 4N HCl (25 min) and detected using biotin-conjugated antibody (#2284, 1:1000, AbCam) and streptavidin-Alexa488 conjugate. To quantify apoptosis, we performed DNA-end labeling on cranial sections or fixed explant cultures using the TUNEL detection kit (Promega, Madison WI). To test for p53 involvement, an AG-X50 anion exchange bead (Bio-Rad) soaked in 100 μM pifithrin-α (Sigma-Aldrich), a p53 inhibitor (Komarova and Gudkov, 2000), was applied to the in ovo presumptive hindbrain 2hr after alcohol administration. Beads were removed 3hr thereafter and cell death visualized 15hr later using Lyso-Tracker Red (Flentke et al. 2011). Experiments utilized 10–12 embryos/treatment and were performed in triplicate.
Data Analysis
All studies compared embryos of equivalent developmental stage; the individual embryo was the unit of analysis except for western blots and qPCR, which used pooled tissue. Experimenters were blinded as to treatment. Data were first tested for normality using the Shapiro-Wilk test using SigmaPlot v13.0 (Systat Software, Point Richmond CA). Normally-distributed data were analyzed using either unpaired t-test or one-way analysis of variance and the appropriate post hoc test. Data not normally distributed were analyzed using Kruskal-Wallis one-way analysis of variance on ranks, followed by pairwise multiple comparisons (Dunn’s Method) for post hoc analysis. P<0.05 was the criterion for significance. Results are presented as mean ± SEM unless indicated otherwise.
Results
Transient alcohol exposure enhances neural crest migration
When placed in explant culture, neural crest exhibited robust emigration from the dorsal neural folds. As described by others (Druckenbrod and Epstein, 2005; Simpson et al. 2014), we observed two migrating populations, the highly dense “followers” that comprise a majority of emigrants (Figure 1A, asterisk within dashed line) and a small subset of “pioneers” whose population was less dense and comprised a migratory front that was physically separated from the followers by a small gap (Figure 1A, arrow above dashed line). Alcohol exposure prior to explant significantly increased the total number of migrating neural crest cells (Mean ± S.D.: Alc 4721 ± 758, Con 3261 ± 1368, p=0.0092; Figure 1C) and expanded both the “pioneer” subpopulation at the advancing migration front (Alc 901 ± 256, Con 616 ± 333, p=0.0375; Figure 1D, compare arrows in 1A vs. 1B) and the dense “follower” pool (Alc 3820 ± 793, Con 2645 ± 325, p=0.0242; Figure 1E). The relative area encompassed by the migratory wave was unaffected by alcohol exposure (relative area: Control 187,379 ± 113,062 pixels, Ethanol 167,230 ± 79,097 pixels).
Figure 1. Transient alcohol exposure enhances neural crest migration.
Chick embryos were exposed in ovo to 52 mM alcohol at stage 8/9 (4–7 somites) and the dissected crania were explanted 10hr later to assess cell migration. (A, B) Representative images of explanted crania show increased migrating cell numbers following alcohol exposure 10 hours earlier (B), as compared with controls (A); cells above dashed line (arrow) represent pioneer cells at migratory front; cells beneath the dashed line (*) represent followers. (C-E) Quantification of cell migration in response to alcohol or control treatment. Transient alcohol exposure significantly increased the total number of migrating cells (C), and increased the number of both pioneer cells (D; arrow in A and B) and the follower cells (E; asterisk in A). Each × indicates the value obtained from an individual crania; N=10 for control, N=14 for alcohol. Data were analyzed using two-tailed Student’s t-test with unequal variance.
We showed previously that alcohol induces a calcium transient within the neural folds including neural crest, and this calcium transient mediates these cells’ apoptosis (Garic et al. 2011; Garic-Stankovic et al. 2005). We hypothesized this calcium transient might also underlie their enhanced migration. Blockade of this calcium transient during the alcohol exposure period (stage 8, 3–5 somites) and not during migration itself, normalized neural crest migratory numbers (Alc 47954 ± 7864, Con 20549 ± 2507, Alc + Bapta 20507 ± 2235; Figure 2A) and the distance migrated (Alc 475 ± 30 μm, Con 330 ± 19 μm, Alc + Bapta 317 ± 34 μm; Figure 2B) in alcohol-exposed neural crest. In control neural crest, Bapta-AM pretreatment did not affect the migrating cell numbers (Con 20549 ± 2507; Con + Bapta 21897 ± 3688) or the distance traveled (Con 330 ± 19 μm, Con + Bapta 342 ± 27 μm). Independent elicitation of a calcium transient, using brief exposure to the calcium ionophore ionomycin prior to migration, was sufficient to increase the number of migrating cells in alcohol’s absence (Con 20549 ± 2507, Ionomycin 51503 ± 11606; Figure 2C) and enhance their migratory distance (Con 371 ± 124 μm, Ionomycin 592 ± 62 μm, Figure 2D). Thus, the alcohol-stimulated EMT observed here was mechanistically linked to the well-characterized intracellular calcium transient that alcohol provokes in this cell population.
Figure 2. An alcohol-dependent intracellular calcium transient mediates the increased cell migration in response to alcohol.
Chick embryos were pretreated with the intracellular calcium chelator Bapta-AM for 2hr prior to in ovo exposure to 52 mM alcohol at stage 8/9 (4–7 somites); the dissected crania were explanted 10hr later and cell migration was quantified thereafter. (A, B) Transient alcohol exposure significantly increased the total number of migrating cells (A) and the distance traveled (B). Bapta-AM pretreatment normalized both migratory numbers and migratory distance in alcohol-exposed cells, and did not affect control cells. (C, D) Brief exposure to the calcium ionophore ionomycin was sufficient to increase the migratory cell numbers (C) and maximum distance traveled (D) in neural crest cells from otherwise untreated crania. Data were analyzed using one-way analysis of variance and Holm-Sidak post-hoc analysis. Values are mean ± S.D. of 8–12 crania per treatment group and were performed in triplicate.
Alcohol induces Snai2 in cranial neural crest
In the chick embryo, EMT is mediated by the transcriptional repressor Snai2 and, consistent with their increased migratory capacity, alcohol increased Snai2 protein content within isolated crania by 224% ± 28% (p = 0.002; Figure 3A). Immunohistology revealed that the majority of Snai2+ cells were present in the cranial mesenchyme and ectoderm, suggesting this increase reflected increased Snai2+ neural crest cell numbers rather than ectopic expression by a different cell population (Figure 3B). Alcohol increased the expression of transcripts encoding Snai2 by 191% ± 23% (Figure 3C, p=0.003), and did not affect Snai1 expression, suggesting Snai2 induction was transcriptional and independent of Snai1. Signals that specifically govern Snai2 induction in neural crest are linked to neural crest specification and induction and are incompletely understood. Alcohol did not alter the expression of numerous genes that promote neural crest formation including Cdh6, Pax7, Zic1, FoxD3, Ets1, Sox9, Sox10, Twist, and AP-2, as quantified in our RNA-Seq dataset from these cells (Berres et al. 2017). Other neural crest inducers had reduced expression, including Id1 (0.615-fold, p = 0.0121, adjusted Bonferroni), bmp4 (0.566-fold, p=0.00159), shh (0.608, p=7.22 × 10−6), hoxA1 (0.629, p=0.0027), and fgf8 (0.645-fold, p=0.00354). Snail1/2 enhances EMT through its direct repression of E-cadherin (Thiery et al. 2009), and we observed no diminution of E-cadherin (CDH1) abundance within the cranial epithelium 10 hours after alcohol exposure, as visualized by immunohistology (Supplemental Figure 1A, B), and by western blot its cranial content was increased (189% ± 50%, p<0.05) in response to alcohol (Supplemental Figure 1C). Taken together, these results were inconsistent with a hypothesis that alcohol induced ectopic Snai2 populations within the neuroepithelium or ectoderm.
Figure 3. Alcohol induces Snai2 within cranial neural crest in a calcium-dependent manner.
(A) Snai2 protein content in 10–12 somite-stage dissected crania is significantly increased 10hr after exposure to 52 mM alcohol, as quantified by western blot analysis. Content is normalized against Gapdh. (B) Immunostain for Snai2 protein (green) in hindbrain sections shows this increase does not represent an ectopic Snai2 induction, but is confined to Snai2+ mesenchymal and ectodermal cells consistent with neural crest (compare green signal at arrows). (C) Snai2 mRNA expression, but not Snai1 mRNA, is significantly increased 10hr after exposure to 52 mM alcohol, as quantified by qPCR. Values are normalized against Gapdh. (D) Pretreatment with Bapta-AM prevented the induction of Snai2 by alcohol and did not affect its expression in controls, as measured by qPCR. Ionomycin treatment of otherwise normal cells was sufficient to induce Snai2. Values are mean ± SEM of three independent experiments having 7–10 crania per treatment. Data analysis used two-tailed Student’s t-test for (A, C), and one-way analysis of variance and Holm-Sidak post-hoc analysis for (D). C, control; C+B, control pretreated with Bapta-AM; C+Io, control treated with ionomycin; Et, ethanol-treated; Et+B, ethanol pretreated with Bapta-AM.
We then considered whether the afore-mentioned alcohol-induced calcium transient was responsible for the elevated Snai2. Pretreatment of these cells with Bapta-AM attenuated alcohol’s induction of Snai2 transcription (p=0.020), whereas brief exposure to ionomycin was sufficient to induce Snai2 in alcohol’s absence (p=0.05; Figure 3D). Taken together, these data suggested that the alcohol-induced calcium transient was responsible for the increased migratory capacity and Snai2 expression in these cells, and it did not represent increased neural crest induction per se.
Alcohol suppresses proliferation in Snai2+ cells
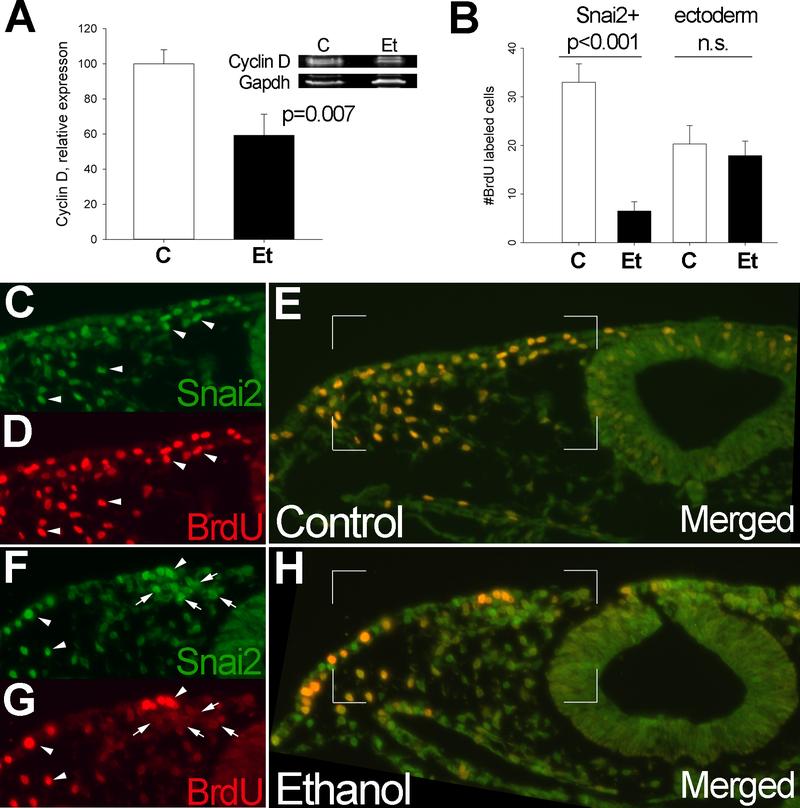
During EMT, Snai2 regulates the cell cycle through its repression of Cyclin D (CCND1/D2) transcription via its –CANNTG– binding site in the Cyclin D promoter (Vega et al. 2004). Alcohol exposure significantly reduced cellular CyclinD1 protein content to 59.3% ± 12% of control levels (p=0.007; Figure 4A), an outcome consistent with the elevated Snai2. Enumeration of incorporated BrdU and Snai2 protein using immunohistology revealed that 10hr after brief alcohol exposure, there was a lasting reduction in neural crest proliferation within cranial regions. Controls had 33.0 ± 3.8 BrdU+Snai2+ cells/section, whereas alcohol populations had 6.5 ± 1.9 BrdU+Snai2+ cells/section, a level that was just 19.7% of control values (p<0.001) (Figure 4B). In controls, the preponderance of Snai2+ cells had incorporated BrdU (arrowheads, Figure 4C–E), whereas alcohol-exposed hindbrain contained fewer double-labeled Snai2+ cells (arrowheads) and more Snai2+ cells that contained little BrdU signal (arrows, Figure 4F–H) This repression of proliferation was selective for neural crest, because alcohol did not affect the proliferative index within the ectoderm (control, 20.3 ± 3.8 BrdU+ cells/ectoderm; alcohol, 17.9 ± 3.0 BrdU+ cells/ectoderm, p>0.05; Figure 4B).
Figure 4. Alcohol substantially reduces CyclinD expression and proliferation in neural crest.
(A) CyclinD1/ protein content is significantly reduced 10hr after 52 mM alcohol exposure, as shown by western blot analysis and normalized against Gapdh; The CyclinD1 antibody detects a doublet in chick (Clark et al. 2000). Mean ± SD of three replicates using independent protein extracts, analysis using two-tailed Student’s t-test. (B) Enumeration of BrdU+ cells within control and alcohol-exposed Snai2+ populations and within the overlying ectoderm. Alcohol exposure significantly reduced the number of BrdU+Snai2+ cells, but did not alter BrdU+ cell numbers within the adjacent ectoderm. Mean ± SEM of triplicate experiments having 8–10 embryos per treatment. Analysis using two-tailed Student’s t-test. (C-H) Representative histochemical sections visualize Snai2 protein (green, C, F) and BrdU (red, D, G) in control (C-E) and alcohol-exposed (F-H) hindbrains in transverse cross-section. Alcohol-exposed crania contain fewer Snai2+BrdU+ cells (arrowheads) and more Snai2+ BrdU- cells (arrows). The boxed region in E and H indicates the merged region depicted in single channels.
Ectopic Snai2 Enhances Cell Migration but does not Prevent Alcohol-Induced Apoptosis
Although the increased EMT and reduced proliferation in these alcohol-exposed cells was consistent with their increased Snai2 content, it was at odds with their well-described apoptotic fate (Smith et al., 2014). This led us to question whether Snai2 was capable of inhibiting apoptosis in alcohol-treated neural crest. To test this, we electroporated neural crest populations in ovo with ectopic Snai2, and asked if it prevented the apoptosis of alcohol-exposed cells. Co-electroporation with eGFP was used to visualize the electroporated cells. As assessed using either LysoTracker Red (LTR) or TUNEL, controls exhibited little apoptosis in neural crest-enriched dorsal cranial regions (Figure 5A, 5B, 5E), and transient transfection with the parent eGFP vector did not worsen cell death (compare TUNEL or LTR signal in unelectroporated (left) and electroporated (right, *) hindbrain halves, Figure 5B, 5E), and as confirmed by enumeration of TUNEL+ cells (Control left 6.5 ± 3.5 cells/section, Control right 4.3 ± 2.5 cells/section; Figure 5M). As expected, alcohol exposure significantly increased the incidence of TUNEL+ and LTR+ cells in neural crest-enriched populations (Figure 5G, 5H, 5K), and this was not worsened by eGFP (Figure 5K). Overexpression of exogenous Snai2, in otherwise normal embryos, did not reduce the number of apoptotic cells in neural crest and adjacent neural tube (compare electroporated (*) and non-electroporated hindbrain halves, Figure 5C, 5D, 5F). However, ectopic Snai2 failed to attenuate apoptosis in alcohol-exposed cranial regions (Figure 5I, 5J, 5L), and this was confirmed through quantification of TUNEL+ signal in the unelectroporated (left) and electroporated (right) hindbrain halves (Figure 5M). The TUNEL signal was more lateral on the electroporated side, and this likely reflected the Snai2-enhanced migratory capacity of these cells as per below.
Figure 5. Ectopic Snai2 does not prevent neural crest apoptosis.
Neural folds were electroporated with eGFP-only or Snai2 plus eGFP at stage 9 (7–8 somites), exposed to alcohol or saline control 3hr later, and apoptosis was assessed 10hr thereafter using LysoTracker Red (LTR; A-D, G-J) or TUNEL (E, F, K, L). In all images, the transfected side is on the embryo’s and viewers’ right side, as indicated by the eGFP signal in intact embryos (A-D, G-J), and by an asterisk (*) on all images (E, F, K, L). The white line demarcates the transfected and non-transfected sides in the TUNEL-stained transverse sections, taken at the level of the hindbrain. (A-F) In controls, eGFP-only (A) does not increase apoptosis levels as assessed using LTR (B, white dots at arrows) or TUNEL (E, green signal at arrows). Ectopic Snai2 plus eGFP (C) does not increase cell death, as assessed by LTR (D) or TUNEL (F). (G-L) In contrast, alcohol causes significantly more apoptosis in neural crest and neural progenitors, revealed by LTR (H) or TUNEL (K). Ectopic Snai2 did not reduce the incidence of apoptosis following alcohol exposure, as compared with the apoptosis levels in the hindbrain’s untransfected left side, as assessed using LTR (H versus J, compare white signal between left and right sides at arrows) or using TUNEL (K versus L, compare green signal on right sides with asterisk). (M) Quantitation of TUNEL+ neural crest in sections of alcohol-treated (Et) and control (C) right hindbrain halves transfected with eGFP-only or Snai2 + eGFP. Values are mean ± SEM of triplicate experiments having N=7–9 embryos per treatment. * indicates p<0.001 compared with its electroporation control, analyzed using Kruskal-Wallis one-way analysis of variance on ranks, followed by pairwise multiple comparison procedures using Dunn’s Method for post-hoc analysis.
In contrast to its inability to prevent alcohol-induced apoptosis, ectopic Snai2 enhanced cell migration in these cell populations. Directed overexpression of Snai2 increased the total number of migrating neural crest cells in control cultures (Con 3251 ± 1386, Con + Snai2 4325 ± 985, p=0.041) to levels indistinguishable from alcohol treatment (Alc 4721 ± 758, p=0.407; Supplemental Figure 2A). In alcohol-exposed cells, Snai2 overexpression further increased the number of “pioneer” cells (Alc 901 ± 256, Alc + Snai2 2281 ± 1138, p=0.028; Supplemental Figure 2B), but did not additionally increase the number of “follower” cells (Alc 3820 ± 793, Alc + Snai2 3140 ± 1140, p=0.141), suggesting that alcohol did not increase the total number of migrating cells (i.e. did not induce ectopic Snai2) but instead enhanced the migratory activity of existing cells (Supplemental Figure 2C).
Snai2 is produced from a spliced transcript, and we considered whether alcohol induced an alternative Snai2 form that might have altered activity. Exome analysis of these cells (Berres et al. 2017) revealed no significant changes in Snai2 splicing patterns or exon representation (Supplemental Figure 2). Moreover, western blot analysis detected only a single band (Figure 3A). Taken together, these data showed that ectopic Snai2 was functional in alcohol-exposed neural crest and could enhance their migration, but it was incapable of preventing their apoptosis.
Alcohol Activates p53 in Snai2+ Cells
The failure of Snai2 to prevent these cells’ apoptosis in response to alcohol suggested the presence of additional factors that overrode its anti-apoptotic effects, but not its migratory effects. Snai1/2 confers resistance to apoptosis through its suppression of the TP50 cell death pathway (Tribulo et al. 2004; Vega et al. 2004; Wu et al. 2005). We investigated the consequences of alcohol exposure to p53 activity in this cell population. Premigratory neural crest cells are normally enriched in stabilized p53 protein, and their p53 content is down-regulated at the onset of migration (Rinon et al. 2011). We replicated that observation and p53 levels were low to undetectable in these control crania(Figure 6A), and in explants of migrating neural crest (Figure 6B, upper panel). In contrast, alcohol treatment increased p53 protein content in these neural folds to levels that were 198% ± 29% of controls (p=0.023, Figure 6A). This increase was confirmed by immunohistology, and the migrating, alcohol-treated neural crest cells were enriched in nuclear p53 protein, whereas migrating, control populations contained few p53+ cells (compare arrows, Figure 6B), suggesting that alcohol-treated neural crest failed to down-regulate p53 prior to their emigration.
Figure 6. Alcohol induces p53 and p53-mediated apoptosis in neural crest.
(A) Western blot analysis reveals significantly more p53 protein content in alcohol-treated (Et) neural folds compared with controls (C). * p=0.023 using two-tailed unpaired Student’s t-test. (B) Explant culture shows elevated p53 protein content (arrows) within alcohol-exposed migrating neural crest populations, as compared with control populations. (C, D) The p53 antagonist pifithrin-α prevents alcohol-induced cell death within hindbrain populations including the presumptive neural crest within rhombomere 4 (R4, compare white dots at arrow), but it does not prevent the endogenous cell death of presumptive neural crest in rhombomeres 3 and 5 (asterisks). Values in (D) are mean ± SD, analyzed using one-way analysis of variance and Holm-Sidak post-hoc analysis. N=9–11 embryos per treatment.
To functionally test for p53 involvement in this alcohol-induced apoptosis, we treated cells with pifithrin-α, a well-characterized reversible inhibitor of p53 (Komarova and Gudkov 2000; Rinon et al. 2011). In the normal hindbrain, neural crest progenitors underwent programmed cell death within rhombomeres 3 and 5 (asterisks in left upper panel, Figure 6C), and they exhibited little cell death within adjacent rhombomeres 4 and 6 and the neural crest populations that emerged from those rhombomeres (arrow, upper left panel in Figure 6C), as we and others have shown (Ellis et al. 2002; Flentke et al. 2011, 2014). As expected, alcohol exposure expanded cell death within rhombomeres 4 and 6, as well as within migrating neural crest populations (compare signal at asterisks and arrow, right upper panel in Figure 6C; Flentke et al. 2011, 2014). In controls, pifithrin-α treatment attenuated the level of apoptosis within the rhombomeres 3 and 5 that normally experience significant programmed cell death (lower left panel, Figure 6C). Alcohol-exposed cells treated with pifithrin-α had significantly fewer apoptotic cells within rhombomere 4 and the neural crest emerging from it (lower right panel, Figure 6C). Enumeration of cell death confirmed these observations (Figure 6D), and alcohol treatment caused significantly more apoptosis than controls (p<0.001). Pifithrin-α significantly reduced cell death in response to alcohol (p<0.001), and the mean number of apoptotic cells no longer differed between control and alcohol-exposed cells (p>0.05). This indicated that the alcohol-induced cell death within neural crest progenitors was p53-dependent, and it is consistent with the p53-mediated apoptosis that was previously described for alcohol-exposed mouse neural crest (Chen et al. 2015).
p53 and Snai2 normally work in direct opposition, so their joint enrichment in these cells was puzzling. Snai2 counters the pro-apoptotic effects of p53 through its transcriptional repression of the p53-upregulated modulator of apoptosis (PUMA/BBC3; Wu et al., 2005). In mouse neural crest, alcohol induces PUMA through suppression of miR-125b (Chen et al. 2015). We hypothesized that the co-expression of Snai2 and p53 in our model might be due to PUMA misexpression. Unfortunately, the identity of PUMA in avian species is unresolved and a PUMA orthologue is not annotated in the Galgal5 genome assembly. This precluded its transcriptional quantitation. Two protein isoforms, PUMAα (23kDa) and PUMAβ (16.5kDa), are described in mammals (Nakano and Vousden 2001). PUMA-directed antibodies failed to detect a 25kDa band in chick, but did detect a predicted 16kDa form that may represent PUMAβ, and this latter was not significantly reduced in alcohol-exposed cells (78% ± 14%, p=0.422; Supplemental Figure 4A) despite their Snai2 expression. Snai2 targets other apoptotic effectors in addition to PUMA, and it represses ATM and PTEN while stabilizing Bcl-2 (Kurrey et al. 2009; Vega et al. 2004; Wu et al. 2005). When we reevaluated our published RNA-Seq data for these cells (Berres et al. 2017), we found that alcohol significantly up-regulated the expression of both ATM (1.383-fold relative to control; p=0.0110, adjusted Bonferroni) and PTEN (1.296-fold, p=0.0279), and there was a trend to reduced expression of Bcl2-family members (BCL2L1, 0.7799-fold, p=0.051; BCL7B, 0.7599-fold, p=0.0529). Alcohol significantly (p=0.016) reduced Bcl2 protein content to 68.5% ± 6.0% of control values (Supplemental Figure 4B). Attempts to quantify Bax and Bad were unsuccessful as the identity of these genes is also unresolved in Galgal5. MicroRNAs miR-34a and miR-125b are also implicated in the Snail/p53 regulatory loop (Chen et al. 2015; Kim et al. 2011), and although we identified 23 miRNAs having differential representation in these cells, miR-34 and miR-125b were not among these (Al-Shaer, Flentke, Berres, Garic, and Smith, submitted). Although limitations of the Galgal5 annotation prevented a full exploration of the Snai2-PUMA relationship in these cells, the overall expression of those effectors we could quantify suggests that alcohol overrides the anti-apoptotic protections normally conferred by Snai2 up-regulation.
Discussion
The most important finding from this study is that alcohol disconnects the regulatory relationship between p53 and Snai2 that normally controls cell fate and survival, and this dysregulation contributes to these cells’ apoptosis and the facial dysmorphology of PAE. A brief, acute exposure to pharmacologically relevant alcohol concentration induced Snai2 transcriptionally and enhanced the capacity of neural crest progenitors to undergo EMT and emigrate from the dorsal neural tube region, and these actions were calcium-mediated. Normally, Snai2 suppresses PUMA to repress p53 activity, but in this model alcohol overrode any such protections and instead activated p53 to induce these cells’ apoptosis. In mouse, alcohol induces p53 and its downstream target PUMA to induce neural crest apoptosis (Chen et al. 2015; Yuan et al. 2017). Although we could not independently validate a role for PUMA, the p53-mediated apoptosis and dysregulated Snai2/p53 interactions described here are consistent with and extend that work. That this same pathway independently emerged from both chick and mouse models of alcohol-exposure suggests these responses may have a broader evolutionary relevance for humans.
The neural crest lineage is somewhat unique in that it undergoes a Snai1/2-mediated EMT that is necessary for their delamination from the dorsal neuroectoderm and migration into ventral regions where they differentiate into diverse fates (Nieto, 2002). The enhanced migration in these alcohol-treated cells is consistent with their increased Snai2 content, as Snai2 mediates their delamination and transformation into a migratory mesenchymal lineage (Nieto 2002). Insight into the mechanism underlying this Snai2 elevation comes from demonstrations that EMT in neural crest is stimulated, in part, by signals originating from the non-canonical PCP/Wnt-calcium pathway (De Calisto et al. 2005) and the subsequent loss of nuclear-localized, transcriptionally active β-catenin (de Melker et al. 2004). We have shown elsewhere that alcohol initiates events that strongly parallel these endogenous Wnt-calcium signals, including the G-protein-mediated mobilization of intracellular calcium stores and downstream activation of CaMKII (Garic et al. 2011; Garic-Stankovic et al. 2005), followed by the CaMKII-mediated destabilization and loss of transcriptional β-catenin (Flentke et al. 2011, 2014). Our demonstration that an intracellular calcium transient is necessary and sufficient to induce Snai2 links it mechanistically to these previously-described calcium signals, and it also implicates calcium – perhaps through Wnt-calcium – as an endogenous effector of Snai2 induction and EMT. Calcium signals regulate EMT and metastasis (Prevarskaya et al. 2011), but to our knowledge have yet to be implicated in the initial events of neural crest EMT. Although neural crest is a uniquely migratory lineage, these findings have broader implications because Snai-dependent cell deadhesion and delamination is an essential step in tumor metastasis. Alcohol is a recognized tumor promoter and has been shown to induce Snai in both the primary colonic epithelium and in transformed epithelial cell lines, and it activates EMT-like processes that include the loss of intracellular tight junctions and adherens junctions (Elamin et al. 2014; Forsyth et al. 2010; Ward et al. 2011). Its promotion of Snai2 expression and EMT herein is consistent with that oncogenic potential.
A potential confound is that alcohol’s stimulation of Snai2 expression and cell migration in these neural crest cells is inconsistent with its well-known ability to inhibit neural crest migration. However, we note this inhibitory action is typically studied in alcohol’s presence and often uses suprapharmacological exposure (Czarnobaj et al. 2014; Hassler and Moran 1986; Oyedele and Kramer 2013; Rovasio and Battiato, 2002). In contrast, the phenotype studied here was a consequence of prior alcohol exposure, and alcohol was no longer present in the embryo or explant media. We suggest that alcohol has distinct effects upon neural crest depending upon these cells’ developmental stage. Exposure at the onset of EMT alters their cellular trajectory to induce Snai2 and enhance their migratory capacity. In contrast, exposure during migration dismantles focal adhesion formation and causes cytoskeletal derangements that reduce migratory distance and direction (Hassler and Moran 1986; Rovasio and Battiato, 2002). These latter actions might be governed by alcohol’s direct interaction with lipid rafts and its physical disruption of the internal hydrogen bonds that modulate protein structure and function, as exemplified for the L1 adhesion protein (Ramanathan et al., 1996; Tang et al., 2011). Furthermore, endogenous calcium transients are implicated in the transition of already-migrating NC into their post-migratory or aggregation state (McKinney and Kulesa 2011); a similar calcium transient elicited by alcohol might trigger a parallel transition of migrating NC into a post-migratory phenotype. Of obvious interest is whether binge alcohol similarly up-regulates Snai2 in transformed cells, akin to observations in colorectal tissue (Elamin et al. 2014; Forsyth et al. 2010; Ward et al. 2011); such an oncogenic priming could help explain why alcohol enhances metastatic tumor risk (Maeda et al. 1998; Meadows et al. 2015).
It was surprising that these cells were vulnerable to alcohol-induced apoptosis, given their elevated Snai2 content, and this could be explained because p53 remained elevated and was not down-regulated when migration commenced (Rinon et al. 2011). P53 is a transcriptional effector that promotes apoptosis in response to cellular stress, and alcohol induces p53 in diverse cell lineages including developing neurons (Anthony et al. 2008; Derdak et al. 2011; Pani et al. 2004) and mouse neural crest (Chen et al. 2015; Yuan et al. 2017). The elevated p53 protein documented here is consistent with those reports. P53 is essential for normal neural crest development and is held under tight regulatory control; p53 loss-of-function enhances neural crest proliferation, whereas gain-of-function suppresses their expansion and directly mediates their apoptotic depletion (Armstrong et al. 1995; Rinon et al. 2011). Imbalanced p53 activity causes craniofacial anomalies and plays a mechanistic role in multiple neurocristopathies including CHARGE, Treacher Collins, and Diamond Blackfan anemia (Chakraborty et al. 2011; Yelick and Trainor, 2015). Demonstration that p53 contributes to the neural crest depletion and facial dysmorphology in both mouse and chick models of FASD adds this disorder to the family of p53-mediated neurocristopathies. We note this mechanism of alcohol targets the premigratory period of neural crest, and it is distinct from alcohol’s disruption of midline structures during head process formation to produce cranial ciliopathies (Lipinski et al. 2012).
Under normal conditions, p53 and Snai1/2 regulate cell survival through complex and opposing interactions. Snai1/2 suppresses p53 activity through its regulation of Bcl family members. Specifically, Snai2 represses PUMA, which is a Bcl-2-related protein induced by p53 to promote apoptosis via its modulation of Bax family members (Wu et al. 2005). Conversely, wild-type p53 destabilizes Snai2 protein through enhancement of MDM2-mediated ubiquitination of Snail (Kurrey et al. 2009; Lim et al. 2010; Wang et al 2009). Insight into how alcohol uncouples this checkpoint relationship between Snai2 and p53 comes from the recent demonstration that alcohol stimulates PUMA and Bak1 expression in mouse neural crest through its repression of miR-125b (Chen et al. 2015). In our cells, a 16kDa protein that might represent PUMA-β was not induced by alcohol, and this might be explained by the elevated Snai2. However, we are left with the puzzle as to why p53 failed to induce PUMA, and it is possible that this 16kDa protein does not represent PUMA. Unfortunately, attempts to further interrogate this network were stymied because many components of this pathway are not annotated in Galgal5. However, although co-expression of Snai2 and p53 was unexpected, it is not without precedent and was recently described for aortic valve interstitial cells in response to warfarin-induced stress, and it may be noteworthy that these cells are endothelial in origin and similarly emerged from an EMT event (Gao et al. 2018).
Why does p53 activity dominate over Snai2 in these cells? Factors in addition to Snai2 regulate p53 and our preliminary data suggest an alternate mechanism by which alcohol activates p53. In normal cells, p53 protein is inactivated through interaction with the E3 ubiquitin ligase MDM2, which targets p53 for proteolytic destruction (Vassilev et al., 2004). In response to cellular stress, MDM2 is silenced and the stabilized p53 protein becomes a major effector of apoptosis and cell cycle withdrawal. One such stressor is nucleolar stress, and cells monitor ribosome biosynthesis as a surrogate for that stress (Chakraborty et al. 2011). Neural crest cells are especially vulnerable to nucleolar stress, and loss-of-function in ribosome biogenesis initiates their p53-mediated apoptosis and causes significant craniofacial deficits (Chakraborty et al. 2011; Yelick and Trainor, 2015). We recently reported that alcohol significantly reduces ribosomal protein expression in these neural crest cells (Berres et al., 2017), and this same gene cluster has altered representation in alcohol-exposed mouse neural folds (Downing et al. 2012), and in chick strains with heightened alcohol vulnerability (Garic et al. 2014). Alcohol is well known to suppress ribosome biogenesis (Cunningham et al., 2001), and ribosomal protein knockdown sensitizes neural crest to alcohol-mediated apoptosis and facial deficits (Berres et al. 2017). While it is unknown why neural crest are so vulnerable to nucleolar stress, we speculate its origins may lie with their unique migratory capacity and the need to suppress apoptosis during EMT (Rinon et al. 2011). Premigratory neural crest is enriched in MDM2 (Daujat et al. 2001) and nuclear p53 (Krinka 2001) and, at the onset of migration, upregulation of Snai2 reduces p53 while promoting cell delamination (Rinon et al. 2011). Alcohol disrupted this Snai2-p53 relationship and p53 was instead sustained, leading to their apoptosis. A similar situation occurs in ribosomopathies, wherein endogenous Snai2 fails to prevent p53-mediated neural crest death. Moreover, p53 has been shown to bind and directly stimulate Snai2 transcription (Wu et al. 2005; Gao et al. 2018), and it is possible the elevated Snai2 represents a failed attempt to counteract this p53 increase.
In summary, we report here that alcohol disrupts the regulatory loop between Snai2 and p53 that normally provides tight control on neural crest expansion, survival, and migration. Alcohol’s effects on neural crest are complex, and in addition to its suppression of sonic hedgehog signaling during head process formation (Lipinski et al. 2012), this dysregulation contributes to the facial deficits caused by PAE through the activation of p53 (Chen et al. 2015; Yuan et al. 2017). As Snai2, p53, and MDM2 are also oncogenes with crucial roles in transformation and metastasis, alcohol’s dysregulation of these signals across multiple cell lineages (Elamin et al. 2014; Forsythe et al., 2010; Ward et al. 2011, and herein) have larger implications for the mechanisms underlying alcohol-related pathologies and tumor progression.
Supplementary Material
Supplemental Figure 1. Alcohol does not reduce E-cadherin content. (A, B) E-cadherin protein (red) was visualized using immunohistology 10hr after alcohol exposure. Representative sections indicate that its content did not decrease within epithelium in response to alcohol. (C) Western blot analysis of isolated crania indicated that alcohol significantly increased total E-cadherin content 10hr after alcohol exposure. Mean ± SD of three replicate western blots, *p<0.05 by Student’s two-tailed t-test, and sampling 3–5 crania per treatment per experiment.
Supplemental Figure 2. Ectopic Snai2 enhances cell migration in control and alcohol-exposed neural crest. Cranial regions were electroporated with Snai2, exposed in ovo to saline or alcohol as described in Methods, and cell migration was quantified from dissected crania in explant culture. (A) Ectopic Snai2 increased the total number of migrating neural crest cells in non-alcohol treated embryos to levels not different from alcohol-treated explants (p=0.407). Ectopic Snai2 did not further increase total migration in the alcohol-treated explants as compared with alcohol-treated crania lacking ectopic Snai2 (p=0.144). (B) Ectopic Snai2 increased the number of migrating pioneer cells in both control and alcohol-treated explants, and further increased the number of pioneer cells in alcohol-exposed explants (p=0.028). (C) Ectopic Snai2 increased the number of follower neural crest cells in both control and alcohol-treated explants, and the mean values did not differ between them (p=0.141). Each × indicates the value obtained from an individual crania, testing 7–9 embryos per treatment. Data were analyzed using two-tailed Student’s t-test with unequal variance, and were compared against values from cells that lacked ectopic Snai2 as presented in Figure 1.
Supplemental Figure 3. Exome analysis of G. gallus Snai2 in response to alcohol exposure. Upper panel: Abundance of spliced transcripts encoding Snai2 in alcohol-exposed (red) and control (blue) cranial neural fold populations. Transcriptome information is taken from Berres et al. (2017). Lower panel: Abundance of exons encoding Snai2 in alcohol-exposed (red) and control (blue) neural crest populations, as performed using RNA-Seq and described in Berres et al. (2017). These findings are inconsistent with a hypothesis that alcohol may have induced an alternately-spliced version of Snai2 having altered protein function.
Supplemental Figure 4. Alcohol-mediated apoptosis is preceded by loss of Bcl2 and no change in PUMA. (A) Alcohol does not significantly affect the content of a 16.5 kDa protein that reacts with anti-PUMA antibody and may represent PUMAβ. Protein content is normalized against total protein per lane. (B) In contrast, alcohol significantly reduces Bcl2 protein content, as normalized against Gapdh. Each lane represents an isolated headfold. Values are mean ± SD of triplicate experiments and analyzed using two-tailed Student’s t-test.
Acknowledgements
Supported by R01 AA11085 to SMS. The authors have no conflict of interest to declare.
References
- Anthony B, Zhou FC, Ogawa T, Goodlett CR, Ruiz J. (2008) Alcohol exposure alters cell cycle and apoptotic events during early neurulation. Alc. Alcohol 43, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR. (1995) High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol 5, 931–936. [DOI] [PubMed] [Google Scholar]
- Aybar MJ, Nieto MA, Mayor R. (2003) Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 130, 483–494. [DOI] [PubMed] [Google Scholar]
- Berres ME, Garic A, Flentke GR, Smith SM. (2017) Transcriptome Profiling Identifies Ribosome Biogenesis as a Target of Alcohol Teratogenicity and Vulnerability during Early Embryogenesis. PLoS One 12, e0169351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boric K, Orio P, Vieville T, Whitlock K. (2013) Quantitative analysis of cell migration using optical flow. PLOS One. 8, e69574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M, Garcia-Castro M. (2008) Manipulations of neural crest cells or their migratory pathways, in: Methods in Cell Biology, Avian Embryology, 2nd ed. (Bronner-Fraser M, ed.) Vol. 87, pp. 76–97. Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- Carl TF, Dufton C, Hanken J, Klymkowsky MW. (1999) Inhibition of neural crest migration in Xenopus using antisense slug RNA. Dev. Biol 213, 101–115. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Uechi T, Kenmochi N. (2011) Guarding the ‘translation apparatus’: defective ribosome biogenesis and the p53 signaling pathway. RNA 2, 507–522. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu J, Feng WK, Wu X, Chen SY. (2015) MiR-125b protects against ethanol-induced apoptosis in neural crest cells and mouse embryos by targeting Bak 1 and PUMA. Exp. Neurol 271, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W, Black EJ, Maclaren A, Kruse U, Lathangue N, Vogt PK, Gillespie DAF. (2000) v-Jun Overrides the Mitogen Dependence of S-Phase Entry by Deregulating Retinoblastoma Protein Phosphorylation and E2F-Pocket Protein Interactions as a Consequence of Enhanced Cyclin E-cdk2 Catalytic Activity. Mol. Cell. Biol 20, 2529–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CC, Preedy VR, Paice AG, Hesketh JE, Peters TJ, Patel VB, Volpi E, Mawatari K, Masaki H, Mori M, Torii K. (2001) Ethanol and protein metabolism. Alcohol. Clin. Exp. Res 25, 262S–268S. [DOI] [PubMed] [Google Scholar]
- Czarnobaj J, Bagnall KM, Bamforth JS, Milos NC. (2014) The different effects on cranial and trunk neural crest cell behaviour following exposure to a low concentration of alcohol in vitro. Arch. Oral Biol 59, 500–512. [DOI] [PubMed] [Google Scholar]
- Daujat S, Neel H, Piette. (2001) Preferential expression of Mdm2 oncogene during the development of neural crest and its derivatives in mouse early embryogenesis. Mech. Dev 103, 163–165. [DOI] [PubMed] [Google Scholar]
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. (2005) Essential role of non-canonical Wnt signalling in neural crest migration. Development 132, 2587–2597. [DOI] [PubMed] [Google Scholar]
- Del Barrio MG, Nieto MA. (2004) Relative expression of Slug, RhoB, and HNK-1 in the cranial neural crest of the early chicken embryo. Dev. Dyn 229, 136–139. [DOI] [PubMed] [Google Scholar]
- de Melker AA, Desban N, Duband JL. (2004) Cellular localization and signaling activity of beta-catenin in migrating neural crest cells. Dev. Dyn 230, 708–26. [DOI] [PubMed] [Google Scholar]
- Derdak Z, Lang CH, Villegas KA, Tong M, Mark NM, de la Monte SM, Wands JR. (2011) Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. J. Hepatol 54, 164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing C, Flink S, Florez-McClure ML, Johnson TE, Tabakoff B, Kechris KJ. (2012) Gene expression changes in C57BL/6J and DBA/2J mice following prenatal alcohol exposure. Alcohol. Clin. Exp. Res 36, 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. (2005) The pattern of neural crest advance in the cecum and colon. Dev Biol 287:125–33. [DOI] [PubMed] [Google Scholar]
- Elamin E, Aasclee A, Troost F, Dekker J, Jonkers D. (2014) Activation of the epithelial-to-mesenchymal transition factor Snail mediates acetaldehyde-induced intestinal epithelial barrier disruption. Alcohol. Clin. Exp. Res 38, 344–353. [DOI] [PubMed] [Google Scholar]
- Ellies DL, Tucker AS, Lumsden A. (2002) Apoptosis of premigratory neural crest cells in rhombomeres 3 and 5: consequences for patterning of the branchial region. Dev. Biol 251, 118–128. [DOI] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Amberger E, Hernandez M, Smith SM. (2011) Calcium-mediated repression of beta-catenin and its transcriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Res. A 91, 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Hernandez M, Smith SM. (2014) CaMKII represses transcriptionally active beta-catenin to mediate acute ethanol neurodegeneration and can phosphorylate beta-catenin. J. Neurochem 128, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. (2010) Alcohol stimulates activation of Snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol. Clin. Exp. Res 34, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Ji Y, Lu Y, Qiu M, Shen Y, Wang Y, Kong X, Shao Y, Sheng Y, Sun W. (2018) Low-level overexpression of p53 promotes warfarin-induced calcification of porcine aortic valve interstitial cells by activating Slug gene transcription. J. Biol. Chem 293, 3780–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic A, Berres ME, Smith SM. (2014) High-throughput transcriptome sequencing identifies candidate genetic modifiers of vulnerability to fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res 38, 1874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic A, Flentke GR, Amberger E, Hernandez M, Smith SM. (2011) CaMKII activation is a novel effector of alcohol’s neurotoxicity in neural crest stem/progenitor cells. J. Neurochem 118, 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez MR, Chiang PJ, Debelak-Kragtorp KA, Flentke GR, Armant DR, Smith SM. (2005) Ethanol triggers neural crest apoptosis through the selective activation of a pertussis toxin-sensitive G protein and a phospholipase Cβ-dependent Ca2+ transient. Alcohol. Clin. Exp. Res 29, 1237–1246. [DOI] [PubMed] [Google Scholar]
- Hassler JA, Moran DJ. (1986) Effects of ethanol on the cytoskeleton of migrating and differentiating neural crest cells: possible role in teratogenesis. J. Craniofac. Genet. Dev. Biol. Suppl 2, 129–136. [PubMed] [Google Scholar]
- Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER, Rowe RG, Lee S, Maher CA, Weiss SJ, Yook JI. (2011) A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J. Cell. Biol 195, 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP, Wetherill L, Rogers J, Moore E, Ward R, Autti-Ramo I, et al. (2010) Prenatal alcohol exposure alters the patterns of facial asymmetry. Alcohol 44, 649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova EA, Gudkov AV. (2000) Suppression of p53: a new approach to overcome side effects of antitumor therapy. Biochemistry 65, 41–48. [PubMed] [Google Scholar]
- Krinka D, Raid R, Pata I, Karner J, Maimets T. (2001) In situ hybridization of chick embryos with p53-specific probe and their immunostaining with anti-p53 antibodies. Anat. Embryol 204, 207–215. [DOI] [PubMed] [Google Scholar]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. (2009) Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 27, 2059–2068. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. (1998) Neural crest induction in Xenopus: evidence for a two-signal model. Development 12, 2403–2414. [DOI] [PubMed] [Google Scholar]
- Lim S-O, Kim H, Jung G. (2010) p53 inhibits tumor cell invasion via the degradation of snail protein in hepatocellular carcinoma. FEBS Let. 584, 2231–2236. [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Hammond P, O’Leary-Moore SK, Ament JJ, Pecevich SJ, Jiang Y, et al. (2012) Ethanol-induced face-brain dysmorphology patterns are correlative and exposure-stage dependent. PLOS One. 7, e43067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Nagawa H, Maeda T, Koike H, Kasai H. (1998) Alcohol consumption enhances liver metastasis in colorectal carcinoma patients. Cancer 83, 1483–1488. [DOI] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, et al. (2018) Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckinney MC, Kulesa PM. (2011) In vivo calcium dynamics during neural crest cell migration and patterning using GCaMP3. Dev Biol. 358, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows GG, Zhang H. (2015) Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival. Alcohol Res. 3, 311–322. [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694. [DOI] [PubMed] [Google Scholar]
- Nieto MA. (2002) The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol 3, 155–166. [DOI] [PubMed] [Google Scholar]
- Oyedele OO, Kramer B. (2013) Nuanced but significant: how ethanol perturbs avian cranial neural crest cell actin cytoskeleton, migration and proliferation. Alcohol 47, 417–426. [DOI] [PubMed] [Google Scholar]
- Pani G, Fusco S, Colavitti R, Borrello S, Maggiano N, Cravero AA, et al. (2004) Abrogation of hepatocyte apoptosis and early appearance of liver dysplasia in ethanol-fed p53-deficient mice. Biochem. Biophys. Res. Commun 325, 97–100. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Skryma R, Shuba Y. (2011) Calcium in tumour metastasis: new roles for known actors. Nat. Rev. Cancer 11, 609–618. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Wilkemeyer MF, Mittal B, Perides G, Charness ME. (1996) Alcohol inhibits cell-cell adhesion mediated by human L1. J. Cell. Biol 133, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinon A, Molchadsky A, Nathan E, Yovel G, Rotter V, Sarig R, Tzahor E. (2011) p53 coordinates cranial neural crest cell growth and epithelial-mesenchymal transition/delamination processes. Development 138, 1827–1838. [DOI] [PubMed] [Google Scholar]
- Rovasio RA, Battiato NL. (2002) Ethanol induces morphological and dynamic changes on in vivo and in vitro neural crest cells. Alcohol. Clin. Exp. Res 26, 1286–1298. [DOI] [PubMed] [Google Scholar]
- Simpson MJ, Haridas P, McElwain DLS. (2014) Do pioneer cells exist? PLOS ONE. 9:e85488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Garic A, Flentke GR, Berres ME. (2014) Neural crest development in fetal alcohol syndrome. Birth Defects Res. C 102, 210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Farah B, He M, Fox S, Malouf A, Littner Y, Bearer CF. (2011) Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. J. Neurochem 119, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–90. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Sanchez SS, Mayor R. (2004) A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of the neural crest. Dev. Biol 275, 325–342. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. (2004) In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848. [DOI] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 18, 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH, Yuan A, Lin CW Yang SC, Chan WK, Li KC, Hong TM, Yang PC. (2009) p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat. Cell Biol 11, 694–704. [DOI] [PubMed] [Google Scholar]
- Ward ST, Gangi-Garimella D, Shields MA, Collander BA, Siddiqui MAQ, Krantz SB, Munshi HG. (2011) Ethanol differentially regulates Snail family of transcription factors and invasion of premalignant and malignant pancreatic ductal cells. J. Cell. Biochem 112, 2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W-S, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. (2005) Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 123, 641–653. [DOI] [PubMed] [Google Scholar]
- Yelick PC, Trainor PA. (2015) Ribosomopathies: Global process, tissue specific defects. Rare Dis. 3, e1025185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Chen X, Liu J, Feng W, Wu X, Chen SY. (2017) Up-regulation of Siah1 by ethanol triggers apoptosis in neural crest cells through p38 MAPK-mediated activation of p53 signaling pathway. Arch. Toxicol 91, 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Alcohol does not reduce E-cadherin content. (A, B) E-cadherin protein (red) was visualized using immunohistology 10hr after alcohol exposure. Representative sections indicate that its content did not decrease within epithelium in response to alcohol. (C) Western blot analysis of isolated crania indicated that alcohol significantly increased total E-cadherin content 10hr after alcohol exposure. Mean ± SD of three replicate western blots, *p<0.05 by Student’s two-tailed t-test, and sampling 3–5 crania per treatment per experiment.
Supplemental Figure 2. Ectopic Snai2 enhances cell migration in control and alcohol-exposed neural crest. Cranial regions were electroporated with Snai2, exposed in ovo to saline or alcohol as described in Methods, and cell migration was quantified from dissected crania in explant culture. (A) Ectopic Snai2 increased the total number of migrating neural crest cells in non-alcohol treated embryos to levels not different from alcohol-treated explants (p=0.407). Ectopic Snai2 did not further increase total migration in the alcohol-treated explants as compared with alcohol-treated crania lacking ectopic Snai2 (p=0.144). (B) Ectopic Snai2 increased the number of migrating pioneer cells in both control and alcohol-treated explants, and further increased the number of pioneer cells in alcohol-exposed explants (p=0.028). (C) Ectopic Snai2 increased the number of follower neural crest cells in both control and alcohol-treated explants, and the mean values did not differ between them (p=0.141). Each × indicates the value obtained from an individual crania, testing 7–9 embryos per treatment. Data were analyzed using two-tailed Student’s t-test with unequal variance, and were compared against values from cells that lacked ectopic Snai2 as presented in Figure 1.
Supplemental Figure 3. Exome analysis of G. gallus Snai2 in response to alcohol exposure. Upper panel: Abundance of spliced transcripts encoding Snai2 in alcohol-exposed (red) and control (blue) cranial neural fold populations. Transcriptome information is taken from Berres et al. (2017). Lower panel: Abundance of exons encoding Snai2 in alcohol-exposed (red) and control (blue) neural crest populations, as performed using RNA-Seq and described in Berres et al. (2017). These findings are inconsistent with a hypothesis that alcohol may have induced an alternately-spliced version of Snai2 having altered protein function.
Supplemental Figure 4. Alcohol-mediated apoptosis is preceded by loss of Bcl2 and no change in PUMA. (A) Alcohol does not significantly affect the content of a 16.5 kDa protein that reacts with anti-PUMA antibody and may represent PUMAβ. Protein content is normalized against total protein per lane. (B) In contrast, alcohol significantly reduces Bcl2 protein content, as normalized against Gapdh. Each lane represents an isolated headfold. Values are mean ± SD of triplicate experiments and analyzed using two-tailed Student’s t-test.