Abstract

The demand of lactulose production is increasing tremendously because of its bifidogenic (prebiotic) functionality. Therefore, the isomerization of lactose to synthesize lactulose through electroactivation (EA) technology is of great interest nowadays. However, lactulose production through electroisomerization is affected by several operational and experimental conditions, and the process needs to be optimized. In this context, the EA technique was applied to isomerize lactose into lactulose in an EA reactor modulated by anion and cation exchange membranes. The effect of lactose concentrations (5, 10, 15, and 20%), applied electric fields (300, 600, and 900 mA), and processing time (0–60 min) on lactose electroisomerization rate (lactulose formation) and coproduct (glucose, galactose, and fructose) formation has been investigated. The effect of different physicochemical parameters such as pH, alkalinity, temperature, ion migration, and oxidation–reduction potential (ORP) on the conversion of lactose into lactulose was correlated with the lactulose formation to understand the involved process mechanism of action. The conversion of lactose into lactulose was lactose-concentration-, electric-current-, and EA-time-dependent and reached the highest lactulose yield of 38% at 40 min using a 900 mA current intensity in a 10% lactose solution. The results were then compared to conventional chemical isomerization maintaining similar alkaline conditions at ambient temperature (22 ± 2 °C). A higher yield of lactulose was achieved in the EA process within a short reaction time compared to that of the chemical isomerization. The outcome of this study suggests that EA is a promising technique for the enhanced production of lactulose from lactose.
1. Introduction
Nowadays, the demand of lactulose production is increasing tremendously because of its bifidogenic (prebiotic) functionality with many applications in food, nutraceuticals, and pharmaceutical industries. Lactulose (4-O-β-d-galactopyranosyl-d-fructose) is a synthetic disaccharide composed of a galactose moiety linked to a fructose moiety by a 1–4 β-glycosidic linkage.1,2 In pharmaceutical industries, lactulose is widely used as an effective drug against different diseases like acute and chronic constipation, hepatic encephalopathy, inflammatory bowel disease, and liver disease.1,3 Furthermore, it lowers blood glucose and insulin levels (antidiabetic), increases mineral absorption, and has been reported as antiendotoxin, effective in tumor prevention, as well as in hypocholesterolemia.4 In food industries, lactulose is used as a bifidus factor and has purported high stability under thermal-acidic conditions and thus can be used as an excellent ingredient for acidic foods, such as fruit juices.4 Therefore, the isomerization of lactose for large-scale production of lactulose has attracted extensive research interests in recent times.
Currently, the commercial lactulose is produced through the chemical synthesis from lactose by following an isomerization reaction in an alkaline medium according to the Lobry de Bruyn–Alberda van Ekenstein (LA) transformation.1,3 Most of these processes are generally characterized by a huge challenge for the low yield of lactulose and subsequent byproduct formation such as epilactose, galactose, glucose, and isosaccharinic acid due to the high level of lactose degradation. However, the presence of side products is undesirable, especially for food, pharmaceutical, and medical applications.1 Furthermore, a substantial amount of catalysts, such as calcium hydroxide, sodium and potassium hydroxides, sodium carbonate, magnesium oxide, tertiary amines, borates, sodium aluminates, zeolites, and eggshell powders, have been used (both in homogeneous and heterogeneous catalysis) to improve the reaction yield, which led to the extensive separation and purification steps, and subsequent increment in the production cost.5 Although the uses of several complexing reagents such as aluminates and borates could accelerate the reaction with a minimum of secondary reactions and result in a high yield of lactulose by eliminating lactulose from the reaction mixture in the form of a complex, however, they are considered to be unsatisfactory from the industrial viewpoint because of the toxicity and complexity of eliminating the aluminate and borate.6 In addition, the lack of reaction selectivity of chemical isomerization has limited its application on a large scale.7 On the other hand, the lactulose synthesis by the enzymatic process could be a suitable alternative to overcome the limitations associated with the chemical synthesis since it is usually carried out under mild conditions, which could limit the formation of side products. Thus, it would provide a high-purity final product and, consequently, could simplify the purification steps.8 However, the major problems are the low yield, extended reaction time, and high production cost depending on the microbial source of enzyme catalysts.7
Recently, an emerging technology called electroactivation (EA) has been introduced and attracted particular attention for the isomerization of lactose into lactulose without adding any alkalinizing chemicals.1,5 EA is a science devoted to studying the physicochemical and reactive properties of aqueous solutions excited by an external electric field in a reactor that is modulated by the appropriate disposition of electrodes and ion-exchange membranes to modify the activation energy required for the targeted chemical reactions.9 Fundamentally, the charged species migrate toward the electrode of opposite charge, when an aqueous solution is subjected to an external electric field.5 In fact, water splitting occurs at the interfaces of electrodes with a simultaneous generation of protons (H+) and hydroxyl (OH–) ions.10 Thus, the EA process is able to self-generate acid and alkaline conditions following the electrolysis of water molecules at the solution/anode and solution/cathode interfaces, respectively.7 In the case of the lactose isomerization reaction, the alkaline solution (catholyte) can be employed either in pure lactose or directly in situ of whey to produce lactulose by following lactose isomerization.11 Indeed, a high alkaline condition is a prerequisite to creating enough proton acceptors, required for the isomerization reaction to occur and to neutralize the acids contained in the medium that can inhibit the isomerization reaction.2,12 The formed OH– ions at the cathode interface of the EA reactor were capable of creating required alkaline conditions and act as proton acceptors in the isomerization of lactose into lactulose.2,11,13,14
Generally, the conventional isomerization of lactose into lactulose was operated at a higher temperature and required longer reaction time. In contrast, the EA process can be performed under relatively low temperature (0–30 °C) and approximately 35–45% lactulose yield with a purity of 95% can be achieved in short reaction time.15 Kareb et al.16 achieved a maximum yield of 35% lactulose after 40 min of reaction at a temperature of 10 °C under a 400 mA electric current and using 100 mL of 7% sweet whey as feed solution. In a recent study, Djouab and Aïder11 obtained a yield of 38.66% lactulose at a current intensity of 330 mA for 14 min in a 5% lactose solution. The optimization of several parameters such as current intensity, reaction time, electrolyte concentration, and reactor configuration resulted in an increased lactulose yield of up to 45%.17 These results were quite higher than those obtained by chemical synthesis (∼16–25% lactulose yield), which were operated at a higher temperature (∼70–130 °C) and prolonged reaction time (∼60–150 min) in the presence of strong bases.18−20 Lately, the effect of several process parameters of EA on the reaction yield, product purity, and process efficiency such as current intensity, electric tension, concentration, and the volume of feed solution, temperature, and reactor configuration were studied by several researchers;5,11,16,17 however, the chemical mechanisms behind this process are still not understood completely. Moreover, the effect of several physicochemical parameters such as solution alkalinity, ion migration, oxidation–reduction potential (ORP), etc. on the process performance is not studied to date. Furthermore, no study was devoted to evaluating the EA process efficiency in comparison with the conventional chemical isomerization at equivalent solution alkalinity. In this context, a detailed study of the physicochemical principles involved in the EA process to produce lactulose using the lactose aqueous solution is imperative to understand the process mechanism.
In this study, several EA process parameters such as lactose concentration, electric current intensity, and EA duration were studied to understand their effect on solution pH, alkalinity, temperature, ion migration, ORP, and sugar profile. Furthermore, the conversion rate of lactose into lactulose was compared with that of the conventional chemical isomerization under equivalent solution alkalinity.
2. Results and Discussion
2.1. Evolution of pH
The evolution of pH in the cathodic compartment during 60 min of EA for different lactose solutions (5, 10, 15, and 20%) at three different current intensities (300, 600, and 900 mA) is presented in Figure 1. It can be seen that the current intensity, EA time, and lactose concentration have a significant effect on the pH of the medium in the cathodic compartment. The obtained data showed that the pH increased drastically within the first 5 min for all current intensities and concentrations. Thereafter, the pH was differently increasing during the remaining 55 min EA time and was dependent on the current intensity and lactose concentration. During this period, the pH was increasing slowly until 60 min of EA and reached a plateau for 300 and 600 mA current intensities. However, the pH evolution reached a maximum value after 30 min of treatment under a 900 mA current intensity, followed by a slight decreasing pattern after 30 min of treatment whatever the lactose concentration used. The rate of increment was observed to be higher for greater current intensities while it was slightly lower for the higher lactose concentrations.
Figure 1.
Evolution of pH as a function of the EA time for (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions at different current intensities (300, 600, and 900 mA).
Two phenomena of reduction and oxidation have occurred during the electrolysis of water or any aqueous solution. A reduction reaction occurs at the negatively charged electrode (cathode),5,9 and electrons (e–) from the cathode are donated to the positively charged ions, like hydrogen cations to form hydrogen gas (H2) and OH– (eqs 1 and 2). The formation of OH– ions was responsible for the pH increase in the cathodic compartment.5,21 Indeed, pH is the most important parameter in lactose isomerization for lactulose synthesis, and it should be as high as possible (>pH 9) for isomerization to occur.5
Cathode (reduction)
| 1 |
| 2 |
The rate of pH increment that was higher for greater current intensities might be due to the more intensive formation of OH– ions from the water electrolysis (eq 2). Consequently, an increase in the concentration of the OH– ions was obtained in the cathodic compartment, which leads to an increase of the solution pH.11 This phenomenon was expected because the rate of water electrolysis is directly proportional to the electric current applied,15,16 because the flow of electrons migrating through the electrochemical reactor might be increased by amplifying the electric current intensity, and subsequently, a higher dissociation of water molecules has occurred at the electrode/solution interface.11,22 On the other hand, an oxidation reaction has occurred at the positively charged electrode (anode), and free electrons (e–) migrated to the anode.5,9 This migration produces oxygen gas (O2) by transferring electrons to the anode (eq 3) and, consequently, lowering the pH of the anodic compartment by increasing the H+ ions (eq 4). However, the inference of this acidic pH in the anodic compartment and alkaline pH in the cathodic compartment has been avoided using an anion exchange membrane (AEM) between the anodic and the central compartments, and a cation exchange membrane (CEM) between the cathodic and the central compartments (Figure 14).
Figure 14.

Schematic diagram of the electroactivation reactor used for the isomerization of lactose to lactulose.
Anode (oxidation)
| 3 |
| 4 |
A drastic increase of the pH within the first 5 min of EA can be explained by the generation of a higher amount of OH– ions due to the intensive water electrolysis at the beginning of the reaction to allow the electric current transfer in the cathode–solution interface. Thereafter, a lower rate of pH increase was observed because the solution became saturated with OH– ions.11,15 Similar observations to this study were reported by Djouab and Aïder,11 and they found that the evolution of pH followed a drastic increase (0–21 min) and a slower increase (21–63 min) during the EA of whey permeate (WP) with different current intensities (110, 220, and 330 mA). They obtained a pH of 11.59 after 63 min EA at a 330 mA current intensity for a 5% lactose solution. However, the geometrical parameters in their study were different from those used in the present one, mainly the cathodic compartment volume and the distance between the cathode and the cation exchange membrane. These parameters seem to be highly significant in terms of the overall process performance. In the present study, the highest pH values of 11.64 ± 0.05 (60 min), 11.86 ± 0.07 (60 min), and 11.77 ± 0.07 (30 min) were achieved for a 5% lactose solution at 300, 600, and 900 mA current intensities, respectively. The highest pH values of 11.26 ± 0.04 (60 min), 11.48 ± 0.05 (60 min), and 11.37 ± 0.09 (30 min) were achieved for a 10% lactose solution, whereas they were 10.95 ± 0.07 (60 min), 11.10 ± 0.02 (60 min), 11.05 ± 0.03 (30 min) for a 20% lactose solution at 300, 600, and 900 mA current intensities, respectively. These results were in good agreement with those previously reported.5,11 It was observed that the rate of pH increment was slightly lower for the higher lactose concentrations. Similar to this observation, Aider and de Halleux3 reported that the pH of the concentrated lactose solutions was more difficult to increase. Likewise, Aider and Gimenez-Vidal5 reported that the pH was instantly raised to the mean values of 11.46 ± 0.09 and 11.16 ± 0.11 during the first 10 min of EA at a 100 mA current intensity for 5 and 10% lactose solutions, respectively. The rate of pH increment was slightly lower for the solutions with higher lactose concentration because the rate of water electrolysis was lower for a higher concentration of lactose.
In the present study, the phenomenon consisting of a decline of pH due to the rapid degradation of lactulose into galactose and acidic compounds, which was previously reported for chemical lactulose synthesis,12,20 was not observed to have occurred for 300 and 600 mA current intensities. However, the pH was observed to be decreased after 30 min of treatment for 900 mA. The pH decrease could be attributed to the formation of reaction byproducts with an acid character during this stage3 or to water splitting at the cation exchange membrane interface facing the central compartment. The latter hypothesis is more realistic because it has already been reported in a recent study by Djouab and Aïder11 that only a low level of galactose can be formed as a reaction byproduct during the lactose electroisomerization in situ of whey permeate (WP).
2.2. Migration Pattern of Potassium Ions
The variation of potassium concentration in the central compartment was studied during the EA process of different lactose solutions (5, 10, 15, and 20%) at different current intensities (300, 600, and 900 mA), and the obtained results are presented in Figure 2. It appears that the concentrations of K+ were decreasing in the central compartment over the running time for all current intensities and solution concentrations. The decreasing rate was appeared to be higher at the beginning of the reaction whatever the current intensities. However, the decreasing rate was relatively higher for greater current intensities and higher solution concentrations. It was observed that the K+ concentrations were decreasing gradually following a quasi-linear behavior for 300 and 600 mA during the 60 min of EA process, whereas they were decreasing more drastically during the first 30 min for a 900 mA current intensity. Thereafter, it has steadily declined until the end of the EA process. This observation regarding the migration of potassium ions from the central compartment toward the cathodic one was correlated with the evolution of pH when 900 mA was applied to the EA reactor. Indeed, under this electric current intensity, the pH and solution alkalinity decreased approximately after 30 min of EA. This may be caused by water splitting at the cation exchange membrane (CEM)–solution interface to compensate for the lack of the current carriers toward the cathode. Thus, this water splitting created enough H+ ions, which competed for electromigration with the K+ ions.
Figure 2.
Variation of potassium concentration in the central compartment for (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions during the EA process at different current intensities (300, 600, and 900 mA).
The concentration of K+ was decreasing in the central compartment because the cations (K+) of the used electrolyte (K2SO4) migrated toward the cathode through the CEM by the attraction of the negatively charged cathode,11 where there were OH– ions and some other ions such as K+ and Cl– ions, which came from 0.01 M KCl added at the beginning of the process to ensure the conductivity of the lactose solution. Similarly, the anions (SO42–) might be migrated toward the anodic compartment (where there were H+ ions) through the AEM by the attraction of the positively charged anode (Figure 14). The rate of K+ ion migration was slowed down at the end of the EA process. This could be explained by the concentration polarization phenomenon, which was created by a difference between the ion transfer numbers in the solution and in the membranes, leading to a variation of the electrolyte concentration near the membrane surface and a considerable potential drop in the polarized region known as the Nernst layer, thus decreasing ion migration and solution demineralization.23 However, it is obvious that the migration of K+ ions was more intensive during the first 30 min for a 900 mA current intensity because of higher current intensity, and thereafter, it would have been reached to a limiting current density.23 As a result, the resistance of the system was significantly increased (Figure S1). At this stage, water dissociation might be occurred at the interface between the CEM and solution in the central compartment due to a continuous current regime.23 Consequently, more H+ and OH– ions would have been produced by water splitting at the interface of the CEM to avoid the ion depletion in the central compartment (eq 5). The newly generated H+ ions might be migrated to the cathodic compartment through the CEM by the attraction of the negatively charged cathode.11 The H+ ions might be competing with K+ ions to travel toward the cathodic compartment due to the higher electrical mobility of the H+ ions than K+ ions. Thus, the rate of K+ ion migration has been slowed down more apparently after 30 min of EA at a current intensity of 900 mA. This fact of H+ ion migration from the central compartment could also be correlated to the decrease in pH after 30 min of EA for that causing acidification of the solution of the cathodic compartment11
| 5 |
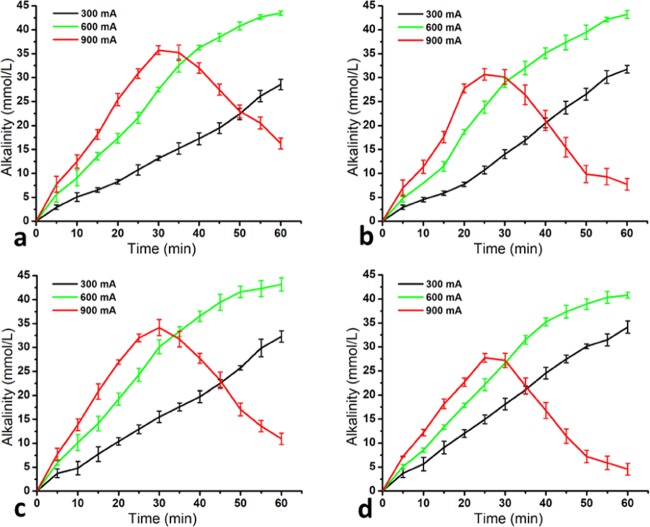
2.3. Evolution of Alkalinity
The variation of alkalinity in the cathodic compartment was determined for different lactose solutions (5, 10, 15, and 20%) during the EA process at different current intensities of 300, 600, and 900 mA, as shown in Figure 3. It appeared that the current intensity and running time have a highly significant effect (p < 0.001) on solution alkalinity. It was linearly increasing during the 60 min of EA for a 300 mA current intensity and reached 28.53, 31.73, 32.27, and 34.13 mmol/L of alkalinity for 5, 10, 15, and 20% lactose solutions, respectively. For a 600 mA current intensity, it has been increased during the 60 min of EA but showed some incurving behavior that could be interpreted as a tendency to reach a plateau. A maximum alkalinity of 43.47, 43.20, 43.20, and 40.80 mmol/L was achieved at the end of the reaction for 5, 10, 15, and 20% lactose solutions, respectively. Contrary to 300 and 600 mA, the alkalinity of the catholyte (electroactivated lactose solution) reached a maximum value of 35.73, 30.13, 34.13, and 27.20 mmol/L after 30 min of treatment, and thereafter, it was decreasing gradually down to 16.27, 7.73, 10.93, and 4.53 mmol/L at the end of the EA treatment for 5, 10, 15, and 20% lactose solutions, respectively.
Figure 3.
Variation of alkalinity in (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions during the EA process at different current intensities (300, 600, and 900 mA).
Generally, the isomerization reaction requires proton acceptors (OH– ions), and this can be achieved in a high alkaline medium through water electrolysis at the cathode/solution interface under the influence of an external electrical field.15 As seen from Figure 3, the evolution of alkalinity was significantly higher for greater current intensities, except when the conditions leading to water splitting were reached such as the case, after 30 min of EA when 900 mA was used. This is attributed to the fact that the higher current intensity resulted in faster decomposition of water.16 The alkalinity was gradually increased during the 60 min of EA for 300 and 600 mA because the dissociation of water at both electrode interfaces produces H+ and OH– ions, as discussed in Section 2.1. The OH– ions in the cathodic side might be attracted by the positively charged anode, but the ions transported by the electric current repulsed by the negatively charged CEM. Thus, the high concentration of OH– ions at the cathode interface was able to create an alkaline condition and could act as proton acceptors, which is a key condition for the occurrence of the isomerization reaction of lactose into lactulose.5,11 Moreover, the K+ ions were continuously migrating to the cathodic compartment, which reacted with the OH– ions to make high alkalinity of the catholyte (eq 6). However, they reached a plateau after 30 min of EA for a 900 mA current intensity, which might be attributed to the saturation of the catholyte with the OH– ions. Thereafter, the alkalinity was gradually decreasing because the migrated H+ ions from the central compartment caused acidification of the solution, as discussed in the previous Section 2.2. This result is in concordance with the pH decline after 30 min of EA for a 900 mA current intensity
| 6 |
2.4. Evolution of Oxidation–Reduction Potential (ORP)
The evolution of ORP in the cathodic compartment during the EA of different lactose solutions (5, 10, 15, and 20%) at different current intensities (300, 600, and 900 mA) is shown in Figure 4. The ORP values in the cathodic compartment were decreased drastically to a value of around −800 mV within the first 5 min of EA for all current intensities and concentrations used and then reached a quasi-steady state. Thereafter, it remained almost constant during the 60 min of treatment for 300 and 600 mA. However, a minor increasing tendency was observed after 30 min for 900 mA. No significant changes were observed for the solution concentrations.
Figure 4.
Evolution of ORP in (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions during the EA process at different current intensities (300, 600, and 900 mA).
The ORP is one of the most important parameters of water or any aqueous solution that can be modified by means of EA. In fact, EA is the process of transferring any solution into a nonequilibrium thermodynamic state, which is accompanied by a change in the internal energy of the system.24 The observed reducing ORP of the EA solution in the cathodic compartment is also due, to a high extent, to its saturation by hydrogen gas that is formed following water electrolysis at the cathode–solution interface. Indeed, it is well known that hydrogen gas is a reducing agent when it reacts with nonmetals. The results obtained in the present study were in good agreement with those previously reported by Nabok and Plutahin.25 They obtained the ORP values of −767 and +905 mV by means of the electroactivation of aqueous solutions in the cathodic and anodic chambers, respectively, while the control aqueous solution exhibited an ORP of +220 mV. This phenomenon can be explained by the formation of unstable complexes, such as (OO), (OO)+, and (HH)+ due to the dissociation of water molecules and their vibrational modes.9,26 Shironosov and Shironosov27 also explained the anomalies in the pH and ORP of EA water by the stable, high-energy resonant water microclusters due to covibrating dipoles of water molecules and charged species near-electrode interfaces. In another study by Hricova et al.,28 an acidic electrolyzed water (pH: 2–3, ORP > 1100 mV) and a basic electrolyzed water (pH: 10–13, ORP: −800 to −900 mV) were obtained by the electrolysis of dilute NaCl solution. The electroactivated solutions in the cathodic chamber were characterized by a negative ORP, which probably related to a training effect of excess electrons (e–) formed after electrochemical activation.29 Moreover, EA is an electrochemical process that can cause the formation of different ionic species and radicals, e.g., the formation of highly active reducers such as OH–, H–, •H, •HO, •O–, •O2–, •HO2, H2O2–, and H3O2–, which may lead to a high reduction potential.5
In the present study, the ORP values in the cathodic compartment were decreased sharply to a value of around −800 mV within the first 5 min of the EA process. The drastic change in ORP within the first 5 min was attributed to the generation of excessive electrons and the formation of other highly active reducers because of the rigorous electrolysis of the solution. Besides the formation of diverse compounds, the EA induced dynamic water electrolysis in the cathodic compartment, resulting in an enhancement of the negative charge concentration through the accumulation of the hydroxyl groups.30 Thereafter, it reached a quasi-steady state when the system became saturated with the charged species. The slight increase in ORP (i.e., reactivity decreased) after 30 min of EA for 900 mA might be due to the migration of H+ H3O+ H3SO4+ toward the cathodic compartment from the central compartment because the newly migrated ions might reduce the number of electrons (e.g., 2H+ + 2e– → H2(g)) in the cathodic compartment.
2.5. Evolution of Temperature
The changes in temperature in the cathodic compartment were observed during the EA process of different lactose solutions (5, 10, 15, and 20%) at different current intensities (300, 600, and 900 mA), as presented in Figure 5. The temperature was gradually increased during the 60 min of the EA process for all current intensities and solution concentrations. The rate of increment was significantly higher for the greater current intensities. As can be seen for a 5% lactose solution (Figure 5a), the temperature was significantly increased to 29.30, 37.97, and 48.70 °C for 300, 600, and 900 mA current intensities, respectively. Some increase in temperature was also observed for the higher concentrations of lactose solutions; however, the difference was not too significant even if some tendency was noticed. For instance, the temperature was slightly increased to 37.97, 38.80, 38.99, and 40.87 °C when a 600 mA current intensity was used for 5, 10, 15, and 20% lactose solution concentrations, respectively.
Figure 5.
Variation of temperature in (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions during the EA process at different current intensities (300, 600, and 900 mA).
The observed temperature rise is mainly the result of the Joule effect in the electrodes, and the generated heat was dissipated in the solution. It was not due to the system electrical resistance because the solutions used in the EA reactor were conductive enough and allowed easy current transfer through the system, which led to a greater water dissociation to create high alkalinity in the cathodic side of the reactor. Some initial resistance of the ion-exchange membranes used could also at some extent contribute to the temperature rise. Indeed, Cifuentes-Araya et al.23 reported that the initial system global resistance in a membrane process can be due to the intrinsic resistance of the membranes and the resistance of the feed solutions, whereas the system global resistance during and at the end of the process was correlated with an evolutionary demineralization and the presence of fouling. Nevertheless, Djouab and Aïder11 did not reveal any fouling of membrane in the EA of pure lactose and WP. Therefore, the global electrical resistance (Figure S1) occurred in the present study, which might be due to the intrinsic resistance of the membranes and the resistance of the feed solutions. The demineralization due to the ion migration could be another possible reason for decreasing conductivity in the central compartment, as discussed in Section 2.2. As a result, the resistance of the system was increased significantly. Thus, the variation of temperature during the isomerization time might be a consequence of the decreased conductivity of the lactose solution during the passage of the electric current.15 The rate of temperature increment was significantly higher for the greater current intensities because the heated energy dissipation (i.e., the increase in temperature) is proportional to the increase in the electric current and electric tension, as described by Joule’s law.15 An increase in temperature was also observed for the higher lactose concentrations, which might be due to the higher resistance for greater concentrations of the feed solution.
2.6. Evolution of Lactulose Formation
The isomerization yield of lactose into lactulose during the EA process at different current intensities (300, 600, and 900 mA) was studied over time (60 min) for different lactose concentrations (5, 10, 15, and 20%), as presented in Figure 6. As can be observed, the current intensity, running time, and solution concentration had a significant effect (p < 0.001) on the conversion rate of lactose into lactulose.
Figure 6.
Evolution of lactulose yield as a function of the EA time for (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions at different current intensities (300, 600, and 900 mA).
From Figure 6a for a 5% feed solution, it can be seen that the lactulose was gradually increased until 50 min for 600 mA and thereafter reached a plateau (∼37% lactulose); however, it reached the plateau at 30 min for 900 mA (∼37% lactulose) and then slightly decreased at the end of the EA process (∼36% lactulose). Whereas, the formation of lactulose was sharply increased to ∼30% until 35 min for 300 mA, and no significant enhancement was observed afterward (∼33% at 60 min). For 10% lactose (Figures 6b and 7), the lactulose yield was increased until the end for 600 mA and thereafter reached a plateau (∼36% lactulose), but it reached the plateau at 40 min for 900 mA (∼38% lactulose) and later slightly decreased (∼36% lactulose). However, the formation of lactulose was started at 15 min for 300 mA (∼12% lactulose) and then gradually increased until the end (∼29% lactulose). As can be seen from Figure 6c (for a 15% lactose solution), the lactulose began to form at different times for different current intensities such as 25, 35, and 60 min for 900, 600, and 300 mA, respectively. It can be observed that ∼36% lactulose was produced for 900 mA at 60 min, while it was only ∼13% for 300 mA and ∼25% for 600 mA. On the other hand, no lactulose was formed for 300 mA during the 60 min EA process when a 20% lactose solution was used (Figure 6d). However, ∼31% lactulose was produced at 60 min for 900 mA, while it was only ∼21% for 600 mA.
Figure 7.
High-performance liquid chromatography (HPLC) chromatograms of lactose electroisomerization into lactulose in EA for a 10% lactose solution; (a) initial feed solution, (b) at 300 mA after 60 min (29%), (c) at 600 mA after 60 min (36%), and (d) at 900 mA after 40 min (38%).
2.6.1. Effect of Solution pH
The pH of the lactose solution had a significant (p < 0.001) influence on lactulose formation. However, different phenomena were observed depending on the lactose solution concentration. For a 5% lactose solution, lactulose was started to form while the pH was above 10 (pH > 10.00). It can be seen from Figure 1a that the pH reached 10 within 5 min of EA for all three current intensities, and lactulose was started to form at the same time (Figure 6a). Whereas, for 10% lactose, the lactulose was found to be created while the pH achieved a value higher than 10.30 (pH > 10.30). Although the pH reached 10.30 within 5 min of EA for 600 mA and 900 mA current intensities (Figure 1b), however, it reached beyond 10.30 at 15 min for 300 mA and the lactulose began to produce at the same time (Figure 6b). On the other hand, no lactulose was found until the pH reached above 11 (pH ≈ 11.10) for 15 and 20% lactose solutions. Consequently, the formation of lactulose was observed to begin at different EA times (Figure 6c), and even no lactulose was found for 300 mA in a 20% lactose solution (Figure 6d) because the pH never reached 11. It can be seen (Figure 1) that the pH was observed to be decreased after 30 min of EA at 900 mA for all lactose concentrations. It is interesting to mention that the formation of lactulose was almost stable at that condition (after 30 min of EA at 900 mA) in 5 and 10% lactose solutions even though the pH decreased slightly. In contrast, the formation of lactulose was found to increase in the same condition (after 30 min of EA at 900 mA) for 15 and 20% lactose solutions. It is worth noting that the rate of lactulose formation was always higher for greater current intensities, and this phenomenon was correlated to the pH evolution. Furthermore, the formation of galactose increased linearly with lactulose until the end of the EA process (Figure 8). The yield of galactose was increased with isomerization time because the lactulose formed by the isomerization reaction was later hydrolyzed into galactose (Figure S2).15 Nevertheless, the results showed that only galactose was generated as a side product; no other impurities (such as tagatose, epilactose, etc.) were found in the reaction medium (Figure 7). However, trace amounts of glucose and fructose were found in some cases depending on the experimental conditions (Figure 7 and Table S1). Here, it can be mentioned that the glucose and fructose could be isomerized into galactose during the EA process of lactose, whey, or WP (Figure S2).11,16
Figure 8.
Formation of galactose as a function of the EA time for (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions at different current intensities (300, 600, and 900 mA).
As can be seen from Figure 8, the formation of galactose followed a similar trend for all concentrations. However, the greater current intensity and running time led to the increased formation of galactose (Figure 9). For instance, the formation of galactose was higher at a 900 mA current intensity, particularly, it increased drastically after 30 min of EA (Figures 8 and 9), while the pH was slightly decreased (Figure 1). In this case, the longer treatment time was immaterial but energy consuming, since no significant increase in lactulose formation was observed after 30 min but increased the galactose formation to an unacceptable level. This finding of the present study was in good agreement with those previously reported for lactose and WP.11,17 Here, it can be noted that the commercial lactulose (as syrup) must not contain more than 12% lactose, 16% galactose, 8% epilactose, and 1% fructose according to United States Pharmacopeia.31
Figure 9.
HPLC chromatograms of galactose formation as a function of the current intensity and running in EA for a 5% lactose solution; (a) at 300 mA after 60 min (2.65%), (b) at 600 mA after 60 min (7.49%), (c) at 900 mA after 30 min (6.54%), and (d) at 900 mA after 60 min (20%).
2.6.2. Effect of Lactose Solution Alkalinity
The isomerization of lactose into lactulose is feasible only under a high alkaline condition because the high alkalinity of the reaction medium is a sine qua non condition for the successful conversion of lactose into lactulose.5 Indeed, the molecular rearrangement of lactose into lactulose requires proton acceptors, which was ensured by achieving a high alkaline medium in the cathodic compartment of the EA reactor.5,16 However, different amounts of alkalinity were observed to be required for producing lactulose in different lactose solutions. Lactulose began to form while the alkalinity obtained a value of ∼3 and ∼5 mmol/L for 5 and 10% lactose solutions, respectively; thereafter, it was increasing with time. It is worth noting that the rate of lactulose formation was hindered but not reduced while the alkalinity was decreasing after 30 min at 900 mA (Figure 3a,b); rather, it reached a plateau and remained stable (Figure 6a,b). On the other hand, no lactulose was found to be produced before the alkalinity reached beyond ∼30 mmol/L for 15 and 20% lactose solutions. Consequently, the formation of lactulose was noticed to start at different times of EA (Figure 6c,d), and even no lactulose was found for 300 mA in a 20% lactose solution (Figure 6d). Unlike 5 and 10% lactose solutions, a different scenario was observed for 15 and 20% lactose solutions at 900 mA, where the lactulose formation was not impeded, even though the alkalinity was decreasing after 30 min (Figure 3c,d). However, the formation of galactose was seen to be drastically increased at this stage (i.e., after 30 min at 900 mA) of EA for all lactose concentrations (Figure 8).
2.6.3. Effect of Temperature
The temperature is one of the most important parameters in the isomerization of lactose into lactulose. During the EA process, the temperature increased throughout the isomerization time, which has already been explained in the previous Section 2.5. As can be seen from Figure 6, the formation of lactulose was proportional to the temperature increment, i.e., increasing the temperature increases the production of lactulose. However, the formation of byproduct, i.e., galactose, was also increased with increasing the temperature. Similar findings to the present study have been reported in several studies.15,32 This might be due to the increased conductivity of lactose with the increase of temperature because the ions that were present in the solution (i.e., K+, SO42–) moved quickly at the high temperature and at the crossing of the electrical current.7 The amount of galactose increased with the elevated temperature because greater activation energy formed and which pushed the reaction on the other side pathways.16,20 It seems to be important to point out that the synthesis of lactulose without heat is possibly contrary to that already been reported in the literature. The alkaline isomerization of lactose to lactulose via the LA rearrangement was usually carried out at a high temperature in the range of 50–130 °C combined with different reaction times.18−20,33,34 Contrary to these studies, the temperature has never been exceeded 50 °C in the present study using EA. Similar to this study, Aissa and Aïder7 demonstrated that lactulose could be obtained at a low temperature such as 0, 5, and 10 °C in the EA reactor. They achieved a lactulose yield of 25 ± 1.34% (with a purity of 95 ± 1.34%) at a temperature of 0 °C and a pH of 10–10.50 at a short duration of 2 min using the EA process.7
2.7. Conventional Chemical Lactose Isomerization
The conventional isomerization reactions were carried out at ambient temperature using similar lactose concentrations (5, 10, 15, and 20%) and generating equivalent alkalinity as those generated using 300, 600, and 900 mA in the EA treatment. The alkalinity-equivalent tests were conducted to observe the lactulose yield compared to the results obtained by EA under similar solution alkalinity. The obtained results are presented in Figure 10. As can be observed, the pH, running time, and solution concentration had a significant effect on the conversion rate of lactose into lactulose. For 5 and 10% lactose (Figure 10a,b), the lactulose was gradually increased until the end of the reactions, although they started to form at a different reaction time. On the other hand, no lactulose was found to be formed for 15 and 20% lactose solutions during the isomerization process, except for the alkalinity equivalent to 900 mA in a 15% lactose feed solution (Figure 10c,d).
Figure 10.
Formation of lactulose as a function of isomerization time for (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions.
It can be seen for 5% lactose that approximately ∼12, ∼23, and ∼20% lactulose were formed at the end of the reactions for the alkalinity of equivalent to that of the EA treatments at 300, 600, and 900 mA, respectively (Figure 10a). These results were correlated to the pH rise of 11.31, 11.65, and 11.58 for the equivalent alkalinity to the EA treatments at 300, 600, and 900 mA, respectively (Figure 11a). Although the increment of pH in a 10% lactose solution (Figure 11b) was less than that of a 5% lactose solution, however, the lactulose formation was comparatively higher for a 10% lactose solution (Figure 10b). The yields of lactulose for a 10% lactose solution were ∼13, ∼27, and ∼26% for the alkalinity of equivalent to 300, 600, and 900 mA, respectively (Figures 10b and 12), and the pH values were 11.06, 11.33, and 11.08 for the alkalinity of equivalent to 300, 600, and 900 mA, respectively (Figure 11b). As can be seen from Figure 10c (for a 15% lactose solution), ∼25% lactulose was produced only for equivalent to 900 mA at a pH of 10.93, but no lactulose was found for equivalent to 300 and 600 mA, even though the pH reached 10.87 and 11.11 at the end of the reaction, respectively (Figure 11c). On the other hand, no lactulose was formed when a 20% lactose solution was used (Figure 10d), which might be due to the inadequate pH values of 10.72, 10.85, and 10.57 for an equivalent to 300, 600, and 900 mA, respectively (Figure 11d).
Figure 11.
Evolution of pH as a function of isomerization time in (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions.
Figure 12.
HPLC chromatograms of lactose isomerization into lactulose using the chemical method for a 10% lactose solution; (a) initial feed solution, (b) equivalent to 300 mA after 60 min (13%), (c) equivalent to 600 mA after 60 min (27%), and (d) equivalent to 900 mA after 30 min (26%).
It has been previously reported that the highest isomerization yield in an alkaline medium could be achieved with a pH of 1120,34 and at temperatures higher than 70 °C.17,35,36 In a recent study, Seo et al.37 observed that the lactulose yield could be increased from 4 to 29.6% in a 20 min reaction time by increasing the temperature from 60 to 90 °C using cheese whey as lactose source and sodium carbonate as catalyst. Hashemi and Ashtiani20 achieved an optimum conversion of 25.40%% (with 5.58% galactose as byproduct) at 70 °C and a pH of 11 for 60 min using 10% lactose in the feed solution. In our study, the same amount of lactulose (i.e., 25.85%) was obtained at ambient temperature and pH 11.08 (alkalinity equivalent to EA at 900 mA in 10% lactose) in 30 min. Moreover, only 0.84% galactose was produced as the byproduct at this condition due to the reduced temperature. It is worth noting from this finding that the lactulose can be produced at ambient temperature using adequate solution alkalinity. This new finding from the present work is fundamentally essential because it highlighted the fact that not only the solution pH but also its alkalinity are very important to achieve the isomerization reaction of lactose into lactulose. Besides, it has been reported that the conversion of lactose into lactulose was typically followed by a rapid degradation of lactulose into galactose, tagatose, epilactose, and many other byproducts with an acidic character such as isosaccharinic acids and formic acids in the chemical-based processes,11,20,38−40 causing the lowering of pH in a medium.12,20 Basically, heating lactose in an alkaline solution causes isomerization and degradation of lactose and lactulose; epilactose and galactose were produced.20,32 However, this phenomenon did not occur in the present study, and consequently, only glucose was found as the reaction byproduct other than that of galactose (Tables S2 and S3).
Not only the pH and temperature, but the solution alkalinity was also a key factor in lactulose production. For example, lactulose began to form at an alkalinity of 9.07 mmol/L (pH = 10.43, 10 min, alkalinity equivalent to 600 mA) in 5% lactose, whereas it was 18.67 mmol/L (pH 10.71, 20 min, alkalinity equivalent to 600 mA) in 10% lactose. Nevertheless, the pH was also varied for the same amount of alkalinity/catalyst depending on the solution concentrations and consequently influenced the lactulose yield. For instance, although the same amount of alkalinity (∼43 mmol/L at alkalinity equivalent to 600 mA for 5, 10, and 15% lactose concentrations) was produced at the end of the reactions (60 min), however, the pH values were 11.65, 11.33, and 11.11 and lactulose was 22.73, 27.13, and 0% for 5, 10, and 15% lactose concentrations, respectively. It is also important to note that the concentration of the catalyst and the rate of pH or alkalinity generation, i.e., the dosing pattern of the catalyst, might play a pivotal role in the lactulose yield. For example, the alkalinity (concentration of the catalyst) and the pH were almost the same at the end of the reaction for alkalinity equivalent to 300 mA (at 60 min) and 900 mA (at 30 min) in a 10% lactose solution. However, the formation of lactulose (i.e., 28.85%) for alkalinity equivalent to 900 mA at 30 min was higher than for the lactulose (i.e., 13.41%) of alkalinity equivalent to 300 mA at 60 min. A similar phenomenon was also found for alkalinity equivalent to 300 mA and 900 mA in a 15% lactose solution. Thus, from the obtained results of the chemical lactose isomerization into lactulose at alkalinity-equivalent conditions as those formed during EA, it can be observed that the EA technique was far more effective than the chemical method.
It is also worth noting that the ORPs in the chemical isomerization of different lactose solutions (5, 10, 15, and 20%) were reduced only to a value of around +50 to +100 mV within the first 5 min depending on the concentration and alkalinity (equivalent to different current intensities), as shown in Figure 13. Thereafter, it gradually decreased till the end of the reaction and then reached a value of around 0 mV. In contrast, the ORP values in EA were decreased drastically to a value of around −800 mV within the first 5 min for all current intensities and concentrations and remained almost steady during the EA process (Figure 4). The electric field triggers the feed solution to be transformed into a metastable state and made the solution highly activated. Therefore, the reduced ORP of the EA lactose solutions in a metastable state rendered the solutions highly reactive because the reactivity of the electroactivated solutions was significantly increased under this state than a normal state.9 Thus, the excessive internal potential energy of the activated solution possibly intensified the isomerization reaction of lactose into lactulose. Consequently, a higher yield of lactulose was achieved in the EA process compared to that of the chemical isomerization. Therefore, the modified ORP and critical pH of the EA lactose solutions in a metastable state could make the solutions highly reactive and convenient for nonconventional chemical reactions and different applications in the food industry and biotechnology, including food safety.9,41 Indeed, the ORP of the drinking water/aqueous solutions should be negative to be highly efficient for physiological activities in humans.9,42
Figure 13.
Evolution of ORP as a function of isomerization time in (a) 5%, (b) 10%, (c) 15%, and (d) 20% lactose solutions.
3. Conclusions
In this study, the electroisomerization of lactose into lactulose was successfully carried out using the EA technology. Moreover, lactose isomerization into lactulose by EA was compared with the chemical method using KOH at equivalent solution alkalinity as in the EA method. The obtained results demonstrated that in contrast to the chemical method, the EA process was found to give a higher yield of lactulose in a reduced reaction time for all conditions. The highest lactulose yield was obtained during the electroisomerization process of lactose and was ∼38% at 40 min using a 900 mA current intensity in a 10% lactose solution with a solution pH of 11.27 and the alkalinity of 21.07 mmol/L. The highest lactulose yield obtained during the conventional chemical isomerization process was ∼27% at 60 min in a 10% lactose solution, while the pH was 11.33 for the alkalinity of 43.20 mmol/L (equivalent to 600 mA in EA). The correlated lactulose yield with the process parameters suggested that the lactulose can be produced at ambient temperature without additional heating if the required alkaline condition is achieved, although a higher temperature was positively correlated to the lactulose formation but leading to a higher byproduct formation. A highly alkaline condition was required for the formation of lactulose; however, the lactulose produced in the medium did not reduce during the EA process while the alkalinity has been declined. Furthermore, EA triggers the feed solution to transfer into a metastable state characterized by unusual values of the chemical and physical parameters such as the ORP, pH, and alkalinity. Thus, it can be concluded on the basis of the compared approaches (EA vs chemical) that EA significantly reduced the activation energy required for the isomerization reaction of lactose into lactulose, and consequently, a higher yield of lactulose was achieved within a shorter duration at ambient temperature compared to that of the chemical isomerization using KOH as catalyst. In addition, the electroisomerization process was carried out under complete autocatalytic conditions, i.e., no alkali was added to the reaction medium. Finally, the findings of this study provide an insight into the feasibility of the electroisomerization of lactose into lactulose and its process mechanism of action. In contrast to the chemical method, the EA process was found to offer a higher potential for an economic and environmentally friendly approach to produce lactulose by the isomerization of lactose. However, further research is still required to understand the thermodynamics behind the EA phenomena of aqueous solutions, mainly regarding its action on the reaction activation energy.
4. Materials and Methods
4.1. Chemicals and Reagents
The high-purity chemicals and reagents (purity ≥ 95%) of analytical or high-performance liquid chromatography (HPLC) grade were obtained from different suppliers. Lactose, lactulose, fructose, glucose, and galactose (HPLC grade) were purchased from Sigma-Aldrich (Ottawa, Ontario, Canada). Phenolphthalein (C20H14O4) was procured from MAT Laboratory Inc. (Laboratoire Mat Inc., Quebec, Canada). Potassium chloride (KCl) and hydrochloric acid (HCl) were procured from Fisher Chemical (Geel, Belgium). Potassium sulfate (K2SO4) and lactose (C12H22O11·H2O) powder used in this study were obtained from Sigma-Aldrich Co. (St. Louis, MO). All solutions were prepared in deionized (DI) water. The cation exchange membrane (CEM) and anion exchange membrane (AEM) were purchased from Membrane International Inc. (Ringwood, NJ) and were used directly in the reactor without any pretreatment.
4.2. Electroactivation Protocol
An EA reactor made of Plexiglas, comprising of three compartments (anodic, central, and cathodic compartments), was used in this study (Figure 14). In brief, the anodic compartment was linked to the positive side of a DC-regulated power generator (model: CSI12001X, CircuitSpecialists.com) through a titanium electrode coated with ruthenium–iridium (RuO2–IrO2–TiO2), and the cathodic compartment was connected to the negative side using a food-grade stainless steel electrode. The anodic and cathodic compartments were separated by the central compartment, and it was communicating with the anodic and cathodic compartments through an anion (AMI 7001S) and a cation (CMI 7000S) exchange membrane, respectively. The freshly prepared lactose solution (350 mL) of different concentrations (5, 10, 15, and 20%; w/v) in 0.01 M KCl was placed in the cathodic compartment, whereas the central and anodic compartments were filled with the 0.1 M K2SO4 solution. The experiments were carried out under different current intensities set at 300, 600, and 900 mA for 60 min. The samples were collected from the cathodic and central compartments in a regular interval of 5 min and were kept at 4 °C until further analysis. All experiments were conducted at ambient temperature (22 ± 2 °C). Prior to each batch, the EA reactor was properly cleaned with DI water and filled with DI water after each use to maintain high membrane hydration.
4.3. Evaluation of pH, Alkalinity, Oxidation–Reduction Potential (ORP), and Temperature
The temperature, ORP, pH, and alkalinity of the lactose solution in the cathodic compartment were measured in 5 min intervals during the 60 min of the EA process. The pH was determined using an Oakton pH 700 digital pH meter equipped with a pH probe (Oakton, Vernon Hills, IL). The temperature and ORP were measured using an ORP meter (Ultrapen, Myron L Company, Carlsbad, CA). Titratable alkalinity (catholyte) of the electroactivated lactose solutions was determined using a titration method. In brief, 5 mL of the corresponding solution was collected from the cathodic compartment in a beaker. Thereafter, two drops of phenolphthalein were added to form a pink color, and the sample was titrated from the burette filled with 0.1 M HCl. The catholyte was titrated until the pink color disappeared. The final volume of 0.1 M HCl in the burette was recorded when the end point reached. Finally, the total alkalinity was calculated using eq 7 and expressed in mmol/L
| 7 |
Vtitrant: the total volume of the titrant (0.1 M HCl) used for titration in mL; Ctitrant: the titrant concentration in mol/L; Vsample: the volume of the sample that was taken for titration in mL; total alkalinityeq: equivalent NaOH/KOH concentration in the electroactivated solutions in mmol/L(equiv).
4.4. Determination of Potassium Concentration
The concentration of potassium (K+) ions in the central compartment was determined using atomic absorption spectrometry. The samples (that were collected from the central compartment in a regular interval of 5 min during the 60 min of EA process) were analyzed according to a standard protocol for the atomic absorption spectrometer (PerkinElmer Instruments, model: AAnalyst 200).
4.5. Conventional Chemical Isomerization of Lactose
Conventional chemical isomerization was carried out using similar lactose concentrations (5, 10, 15, and 20%) in the feed solutions and adding the equivalent (to the total alkalinity in EA) amounts of potassium hydroxide (KOH) to the feed. In brief, the total alkalinity (mmol/L) of the EA lactose solutions was expressed to the equivalent amounts of KOH (mg/L). The equivalent amount of KOH was added to the feed in a regular interval of 10 min, and the mixture was stirred at ambient temperature (22 ± 2 °C). The pH and ORP were monitored in 5 min intervals during the 60 min of reaction. The samples were collected from the feed solution at a regular interval of 5 min and were kept at 4 °C until further analysis.
4.6. Determination of Sugar Composition
The sugar contents of all samples (i.e., EA lactose solutions and chemically isomerized lactose solutions) were determined using a high-performance liquid chromatography (HPLC) system (Waters, Millipore Corp., Milford, MA). The system was equipped with a refractive index detector (Hitachi, model: L-7490) and a carbohydrate analysis column (Waters Sugar Pak-I, 300 × 6.5 mm2, Waters Co.). The column temperature was maintained at 90 °C. The isocratic mobile phase consisting of a solution of 50 mg/L ethylenediaminetetraacetic acid (EDTA) was used as the mobile phase at a flow rate of 0.5 mL/min. The injection volume was 50 μL, and the running time was set at 30 min per sample. The identification and quantification of sugars were accomplished by comparing their retention times with the standard solutions of lactose, lactulose, glucose, galactose, and fructose.
4.7. Statistical Analysis
Statistical analysis was performed using a complete randomized factorial design with repeated measurements. The factors were current intensity, lactose concentration, and reaction time. The dependent variables were the pH of the catholyte, alkalinity, K+ ion migration, temperature, ORP, lactulose yield, as well as the yield of byproducts (galactose, glucose, and fructose). Each experiment was carried out in triplicate, and mean values ± standard deviation was used. Differences at p < 0.05 were considered to be significant. Analysis of variance (ANOVA) of the data was performed using SAS software (V9.3, SAS Institute Inc., Cary, NC).
Acknowledgments
The project was supported by Fonds de recherche du Québec, Nature et Technologie (FRQNT), Grant # 2019-PR-256871, and the authors would like to acknowledge the financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03705.
Global electric resistance of EA reactor as a function of time at different current intensities (Figure S1); the possible mechanism pathway of the electroisomerization of lactose into lactulose and subsequent galactose formation as a reaction byproduct using the electroactivation process (Figure S2); the formation of glucose and fructose for different lactose solutions at different current intensities of EA (Table S1); the formation of galactose and glucose for 5 and 10% lactose solutions in chemical isomerization (Table S2); the formation of galactose and glucose for 15 and 20% lactose solutions in chemical isomerization (Table S3); residual lactose (%) in different electroactivated lactose solutions (5, 10, 15, and 20%) (Table S4); and residual lactose (%) in different chemically isomerized lactose solutions (5, 10, 15, and 20%) (Table S5) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kareb O.; Aïder M. Whey and its derivatives for probiotics, prebiotics, synbiotics, and functional foods: a critical review. Probiotics Antimicrob. 2018, 11, 348–369. 10.1007/s12602-018-9427-6. [DOI] [PubMed] [Google Scholar]
- Djouab A.; Aïder M. Effect of Drying Temperature on the Antioxidant Capacity of a Cathodic Electroactivated Whey Permeate. ACS Sustainable Chem. Eng. 2019, 7, 5111–5121. 10.1021/acssuschemeng.8b05962. [DOI] [Google Scholar]
- Aider M.; de Halleux D. Isomerization of lactose and lactulose production. Trends Food Sci. Technol. 2007, 18, 356–364. 10.1016/j.tifs.2007.03.005. [DOI] [Google Scholar]
- Nooshkam M.; Madadlou A. Maillard conjugation of lactulose with potentially bioactive peptides. Food Chem. 2016, 192, 831–836. 10.1016/j.foodchem.2015.07.094. [DOI] [PubMed] [Google Scholar]
- Aider M.; Gimenez-Vidal M. Lactulose synthesis by electro-isomerization of lactose: Effect of lactose concentration and electric current density. Innovative Food Sci. Emerging Technol. 2012, 16, 163–170. 10.1016/j.ifset.2012.05.007. [DOI] [Google Scholar]
- Zokaee F.; Kaghazchi T.; Zare A.; Soleimani M. Isomerization of lactose to lactulose—study and comparison of three catalytic systems. Process Biochem. 2002, 37, 629–635. 10.1016/S0032-9592(01)00251-5. [DOI] [Google Scholar]
- Aissa A. A.; Aïder M. Ion exchange membranes controlled electro-catalytic synthesis of lactulose from lactose under refrigerated conditions. Innovative Food Sci. Emerging Technol. 2013, 20, 299–309. 10.1016/j.ifset.2013.09.013. [DOI] [Google Scholar]
- Cardelle-Cobas A.; Olano A.; Irazoqui G.; Giacomini C.; Batista-Viera F.; Corzo N.; Corzo-Martínez M. synthesis of Oligosaccharides Derived from lactulose (Oslu) Using soluble and immobilized Aspergillus oryzae β-galactosidase. Front. Bioeng. Biotechnol. 2016, 4, 21 10.3389/fbioe.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aider M.; Gnatko E.; Benali M.; Plutakhin G.; Kastyuchik A. Electro-activated aqueous solutions: theory and application in the food industry and biotechnology. Innovative Food Sci. Emerging Technol. 2012, 15, 38–49. 10.1016/j.ifset.2012.02.002. [DOI] [Google Scholar]
- Hung C.-Y.; Wang C.-C.; Chen C.-Y.; Li S.-D. Influences of a bipolar membrane and an ultrasonic field on alkaline water electrolysis. J. Membr. Sci. 2012, 389, 197–204. 10.1016/j.memsci.2011.10.050. [DOI] [Google Scholar]
- Djouab A.; Aïder M. Whey permeate integral valorisation via in situ conversion of lactose into lactulose in an electro-activation reactor modulated by anion and cation exchange membranes. Int. Dairy J. 2019, 89, 6–20. 10.1016/j.idairyj.2018.07.019. [DOI] [Google Scholar]
- Paseephol T.; Small D. M.; Sherkat F. Lactulose production from milk concentration permeate using calcium carbonate-based catalysts. Food Chem. 2008, 111, 283–290. 10.1016/j.foodchem.2008.03.051. [DOI] [PubMed] [Google Scholar]
- Sprinchan E.; Bologa M.; Stepurina T.; Bologa A. M.; Polikarpov A. Peculiarities of the electric activation of whey. Surf. Eng. Appl. Electrochem. 2011, 47, 66–69. 10.3103/S1068375511010182. [DOI] [Google Scholar]
- Bologa M.; Sprinchan E.; Bologa A. M. Recovery of lactulose products and protein-mineral concentrate. Surf. Eng. Appl. Electrochem. 2008, 44, 410–414. 10.3103/S1068375508050128. [DOI] [Google Scholar]
- Aissa A. A.; Aïder M. Lactose isomerization into lactulose in an electro-activation reactor and high-performance liquid chromatography (HPLC) monitoring of the process. J. Food Eng. 2013, 119, 115–124. 10.1016/j.jfoodeng.2013.05.011. [DOI] [Google Scholar]
- Kareb O.; Champagne C. P.; Aïder M. Contribution to the production of lactulose-rich whey by in situ electro-isomerization of lactose and effect on whey proteins after electro-activation as confirmed by matrix-assisted laser desorption/ionization time-of-flight-mass spectrometry and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Dairy Sci. 2016, 99, 2552–2570. 10.3168/jds.2015-10037. [DOI] [PubMed] [Google Scholar]
- Aissa A. A.; Aïder M. Electro-catalytic isomerization of lactose into lactulose: The impact of the electric current, temperature and reactor configuration. Int. Dairy J. 2014, 34, 213–219. 10.1016/j.idairyj.2013.08.010. [DOI] [Google Scholar]
- Song Y. S.; Lee H. U.; Park C.; Kim S. W. Batch and continuous synthesis of lactulose from whey lactose by immobilized β-galactosidase. Food Chem. 2013, 136, 689–694. 10.1016/j.foodchem.2012.08.074. [DOI] [PubMed] [Google Scholar]
- Sakkas L.; Moutafi A.; Moschopoulou E.; Moatsou G. Assessment of heat treatment of various types of milk. Food Chem. 2014, 159, 293–301. 10.1016/j.foodchem.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Hashemi S. A.; Ashtiani F. Z. The isomerization kinetics of lactose to lactulose in the presence of sodium hydroxide at constant and variable pH. Food Bioprod. Proces. 2010, 88, 181–187. 10.1016/j.fbp.2009.11.001. [DOI] [Google Scholar]
- Lu Y.; Yi S.; Luo G. Modeling of the mass transfer and conduction behavior in electro-electrodialysis with oil/water emulsion as the catholyte. J. Membr. Sci. 2008, 322, 265–274. 10.1016/j.memsci.2008.05.056. [DOI] [Google Scholar]
- Fiegenbaum F.; Martini E. M.; de Souza M. O.; Becker M. R.; de Souza R. F. Hydrogen production by water electrolysis using tetra-alkyl-ammonium-sulfonic acid ionic liquid electrolytes. J. Power Sources 2013, 243, 822–825. 10.1016/j.jpowsour.2013.06.077. [DOI] [Google Scholar]
- Cifuentes-Araya N.; Pourcelly G.; Bazinet L. Impact of pulsed electric field on electrodialysis process performance and membrane fouling during consecutive demineralization of a model salt solution containing a high magnesium/calcium ratio. J. Colloid Interface Sci. 2011, 361, 79–89. 10.1016/j.jcis.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Dykstra C. E. External electric field effects on the water trimer. Chem. Phys. Lett. 1999, 299, 132–136. 10.1016/S0009-2614(98)01270-6. [DOI] [Google Scholar]
- Nabok M.; Plutahin G.. Study and development of new technology for baking wheat bread by using electro-activated water as replacement to ordinary water, In Scientific Communications of the Kuban State Agrarian University; Kuban State Agrarian University, 2005. [Google Scholar]
- Antonchenko V. Y.; Davydov A.; Il’in V.. Fundamentals of Water Physics; Naukova Dumka: Kiev, 1991. [Google Scholar]
- Shironosov V.; Shironosov E. In Non-Contact Electrochemical Water Activation Experiments, Collection of Abstracts of the 2nd International Symposium on “Electrochemical Activation in Medicine, Farming and Industry”, Moscow, VNIIIMT AO NPO Screen; 1999, pp 66–68..
- Hricova D.; Stephan R.; Zweifel C. Electrolyzed water and its application in the food industry. J. Food Prot. 2008, 71, 1934–1947. 10.4315/0362-028X-71.9.1934. [DOI] [PubMed] [Google Scholar]
- Podkolzin A.; Dontsov V.; Chernilevskii V.; Megreladze A.; Mrakaeva O. S.; Zhukova E. Effects of electroactivated solutions on antioxidant enzymes. Bul. Exp. Biol. Med. 2001, 131, 53–55. 10.1023/A:1017534713845. [DOI] [PubMed] [Google Scholar]
- Kareb O.; Gomaa A. I.; Champagne C. P.; Jean J.; Aïder M. Impact of electro-activation on antioxidant properties of defatted whey. Int. Dairy J. 2017, 65, 28–37. 10.1016/j.idairyj.2016.09.009. [DOI] [Google Scholar]
- The United States Pharmacopeia. National Formulary. 2008, 14.
- Martinez-Castro I.; Olano A.; Corzo N. Modifications and interactions of lactose with mineral components of milk during heating processes. Food Chem. 1986, 21, 211–221. 10.1016/0308-8146(86)90019-1. [DOI] [Google Scholar]
- Corzo-Martínez M.; Copoví P.; Olano A.; Moreno F. J.; Montilla A. Synthesis of prebiotic carbohydrates derived from cheese whey permeate by a combined process of isomerisation and transgalactosylation. J. Sci. Food Agric. 2013, 93, 1591–1597. 10.1002/jsfa.5929. [DOI] [PubMed] [Google Scholar]
- Hicks K. B.; Raupp D. L.; Smith P. W. Preparation and purification of lactulose from sweet cheese whey ultrafiltrate. J. Agric. Food Chem. 1984, 32, 288–292. 10.1021/jf00122a028. [DOI] [Google Scholar]
- Olano A.; Calvo M. Kinetics of lactulose, galactose and epilactose formation during heat-treatment of milk. Food Chem. 1989, 34, 239–248. 10.1016/0308-8146(89)90101-5. [DOI] [Google Scholar]
- Rendleman J. A. Jr; Hodge J. E. Complexes of carbohydrates with aluminate ion. Aldose-ketose interconversion on anion-exchange resin (aluminate and hydroxide forms). Carbohydr. Res. 1979, 75, 83–99. 10.1016/S0008-6215(00)84629-7. [DOI] [Google Scholar]
- Seo Y. H.; Park G. W.; Han J.-I. Efficient lactulose production from cheese whey using sodium carbonate. Food Chem. 2015, 173, 1167–1171. 10.1016/j.foodchem.2014.10.109. [DOI] [PubMed] [Google Scholar]
- Schuster-Wolff-Bühring R.; Fischer L.; Hinrichs J. Production and physiological action of the disaccharide lactulose. Int. Dairy J. 2010, 20, 731–741. 10.1016/j.idairyj.2010.05.004. [DOI] [Google Scholar]
- Corbett W.; Kenner J. 462. The degradation of carbohydrates by alkali. Part II. Lactose. J. Chem. Soc. 1953, 2245–2247. 10.1039/jr9530002245. [DOI] [Google Scholar]
- Dendene K.; Guihard L.; Nicolas S.; Bariou B. Kinetics of lactose isomerisation to lactulose in an alkaline medium. J. Chem. Technol. 1994, 61, 37–42. 10.1002/jctb.280610106. [DOI] [Google Scholar]
- Suzuki T.; Itakura J.; Watanabe M.; Ohta M.; Sato Y.; Yamaya Y. Inactivation of Staphylococcal enterotoxin-A with an electrolyzed anodic solution. J. Agric. Food Chem. 2002, 50, 230–234. 10.1021/jf010828k. [DOI] [PubMed] [Google Scholar]
- Petrushanko I. I.; Lobyshev V. I. Physico-chemical properties of aqueous solutions, prepared in a membrane electrolyzer. Biofizika 2004, 49, 22–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.