Abstract
A concise and high-yielding double aza-Michael reaction is presented as an atom-efficient method to access chiral 2-substituted 4-piperidone building blocks from divinyl ketones. The piperidones were further converted into analogues of donepezil, an acetylcholinesterase inhibiting drug used in the treatment of Alzheimer’s disease. The donepezil analogues were obtained as inseparable diastereomeric mixtures with resolved stereochemistry in position 2 of the piperidine ring. Biological evaluation of the acetylcholinesterase inhibition by these analogues provides a new insight into structure–activity relationship studies with regard to donepezil’s piperidine moiety toward stereochemical enhancement.
Introduction
The piperidine ring is an important scaffold in pharmaceutical research and a ubiquitous structural motif which is present in several natural products.1,2 In general, the 1,4-disubstitution pattern on the piperidine ring prevails among drug prototypes due to more accessible synthetic routes and limited stereochemical complications. Nonetheless, it has been shown that additional substitution on different positions of piperidine could show highly beneficial in terms of identifying compounds with increased biological activity.3,4 The acetylcholinesterase (AChE) inhibiting drug donepezil 1 (Figure 1) contains such a 1,4-disubstituted piperidine core.
Figure 1.
Donepezil.
Donepezil is the most commonly prescribed medication for Alzheimer’s diseases (Aricept).5−7 There is a growing interest in the field for the development of analogues to allow for various therapeutic approaches, such as dual- or multifunctional targeting and AChE reactivation.5−10 In this context, medicinal chemistry research efforts have been mainly centered on ligand-based and fragment-based drug design methodologies to generate “fully” organic compounds,5−8 as well as chelators for metals9 and organometallic complexes,10 as donepezil-derived hits for medical applications. The X-ray resolved structure of AChE has also permitted structure-based drug design approaches.11,12 Although these latter studies are reported in a limited number, they have established a structural baseline for efficient AChE inhibitor design.10
In the search for a novel class of acetylcholinesterase inhibitors, studies conducted by the Japanese company Eisai Co., Ltd. demonstrate that the presence of a piperidine ring into the donepezil structure is essential for increased AChE inhibitory effects.13,14 Despite piperidine’s crucial role in the overall activity of donepezil and the various structure–activity relationship studies reported for this lead compound,15−20 to the best of our knowledge, no other modifications on the piperidine ring have been reported in the literature, with the exception of substitutions in positions 1 and 4. Moreover, analysis of the crystal structure of donepezil bound to acetylcholinesterase suggests that additional substituents on the piperidine ring could be accommodated in the binding pocket, thereby leading to an improved pharmacological profile of derived analogue drugs.18,21
In this context, we have used divinyl ketones to set up an efficient aza-Michael synthesis for the preparation of 4-piperidone scaffolds, which are substituted in position 2 of the ring. We have then used the resulting 4-piperidones to develop a new series of donepezil-based derivatives, to biologically evaluate the impact of chiral modification on the piperidine moiety with regard to their acetylcholinesterase inhibition.
Results and Discussion
Chemistry
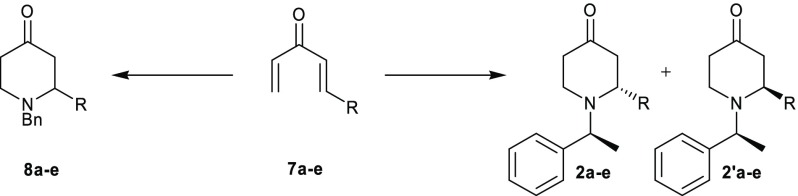
Our approach toward the synthesis of donepezil analogues 4 (Scheme 1) involves the use of chirally resolved 2-substituted 1-S-α-phenylethyl-4-piperidones 2 that are further converted into aldehyde intermediates 3.
Scheme 1. Retrosynthetic Approach for 2-Substituted Donepezil Analogues.
Synthesis of 2-Substituted 1-S-α-Phenylethyl-4-piperidones
The first step toward the synthesis of divinyl ketones 7 was the reaction of suitable vinyl aldehydes 5 with vinylmagnesium bromide under standard Grignard conditions (Scheme 2). Dienols 6a–e were obtained in high yield (Table 1) and sufficiently pure to be used in the next synthetic step without further purification. Also, no degradation was observed for 6a–e, if stored at 4 °C for several weeks (as confirmed by 1H NMR analysis). The desired divinyl ketones 7 (Scheme 2 and Table 1), substituted with aliphatic or aromatic groups, were obtained through the following oxidation reaction under mild conditions of intermediates 6, using manganese(IV) oxide (for 6a,b) or 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (for 6c–e) (Scheme 2). The oxidizing agent was selected according to the reactivity of the substrate and the stability of the product. In this regard, the conversion of methyl- and propyl-substituted 6a,b to ketones 7a,b was straightforwardly achieved using MnO2. In contrast, MnO2-mediated conditions were found not to be suitable to convert the aromatic analogues 6c–e, which were preferentially oxidized using DDQ. Due to the higher stability of ketones 7c–e, column chromatography was successfully performed in this case, allowing the removal of the oxidizing agent and the complete purification of the products, which were obtained in a good to moderate yield (Table 1).
Scheme 2. Preparation of Racemic or Diastereomeric 2-Substituted Piperidones (for R, See Table 1).
Reagents and conditions: (i) CH2=CHMgBr, THF, 0 °C → rt, 1 h; (ii) MnO2, 4 Ȧ MS, CH2Cl2, 50 °C, 3 h (for 7a,b) or, DDQ, 4 Ȧ MS, dioxane, rt, 18 h (for 7c–e); (iii) benzylamine or S-α-phenylethylamine, NaHCO3, CH3CN/H2O (3:1), 16 °C (40 min) → 95 °C, 1.5 h.
Table 1. Grignard Reaction Followed by Oxidation to Yield Mono-Substituted Divinyl Ketones.
| entry | R | aldehyde 5 | alcohol 6 (%)a | ketone 7 (%)b |
|---|---|---|---|---|
| 1 | Me | 5a | 6a (99)c (82)d | 7a (71)c |
| 2 | n-Pr | 5b | 6b (99)c | 7b (62)c |
| 3 | Ph | 5c | 6c (98)c (86)d | 7c (74)d |
| 4 | 4-ClC6H4 | 5d | 6d (100)c | 7d (56)d |
| 5 | 4-OMeC6H4 | 5e | 6e (98)c | 7e (53)d |
Reaction conditions for 6a–e: CH2=CHMgBr, tetrahydrofuran (THF), 0 °C → room temperature (rt), 1 h.
Reaction conditions: MnO2, 4 Ȧ MS, CH2Cl2, 50 °C, 3 h (for 7a,b); DDQ, 4 Ȧ MS, dioxane, rt, 18 h (for 7c–e).
Crude yield.
Isolated yield (by column chromatography).
A double aza-Michael addition of primary amines (i.e., benzylamine or S-α-phenylethylamine) was carried out on ketones 7 as an atom-efficient method to access chiral 4-piperidine-based building blocks 8, 2, and 2′ (Scheme 2). Initial investigations on the appropriate solvent for the double aza-Michael addition were performed with benzylamine and phenyl-substituted ketone 7c. Since neat acetonitrile or dichloromethane proved unsuccessful in these test reactions, we applied a slightly modified procedure in comparison to methods previously reported in the literature.22−24 Specifically, divinyl ketone 7c was slowly added to a mixture of benzylamine in acetonitrile and aqueous sodium bicarbonate at 16 °C over a period of 40 min and then refluxed for 1.5 h (entry 3, Table 2). The 2-phenyl-substituted piperidine 8c was obtained with 79% yield. With these reaction conditions, a range of aliphatically and aromatically substituted piperidones have been prepared (Table 2, 8a–e). As expected, the yields for the methyl- and propyl-substituted piperidones 8a,b (Table 2, entries 1 and 2) were lower due to the less stable nature of the crude starting ketones 7a,b. In contrast, when purified aromatic divinyl ketones 7c–e were used for the cyclization with benzylamine (Table 2, entries 3–5), piperidones 8c–e were obtained in high yield (79–84%). S-α-Phenylethylamine was chosen as a chiral auxiliary to synthesize diastereomeric 2-substituted-4-piperidones.25,26 In line with the analogue series 8a–e, the combined yields for the aliphatically substituted piperidones 2a + 2′a (R = methyl) and 2b + 2′b (R = propyl) were lower (Table 2, entries 1 and 2), while aromatically substituted piperidones 2c + 2′c (entry 3–5) were differently obtained in good, albeit with lower combined yields in comparison to the cyclization using benzylamine. Subsequently, the correct stereochemistry of new compounds 2b–e + 2′b–e was assigned by adopting a similar analysis as conducted by Leshcheva et al. (see Section S2 in the Supporting Information).27
Table 2. Synthesis of 2-Substituted Racemic and Diastereomeric 4-Piperidones.
| entry | R | 8 (%)a | 2 + 2′ (%)b | ratiod of 2:2′ |
|---|---|---|---|---|
| 1 | Me | a (42)c | a (37)c | 1.1:1.0 |
| 2 | n-Pr | b (36)c | b (27)c | 1.4:1.0 |
| 3 | Ph | c (79) | c (63) | 2.6:1.0 |
| 4 | 4-ClC6H4 | d (84) | d (68) | 2.8:1.0 |
| 5 | 4-OMeC6H4 | e (84) | e (57) | 3.7:1.0 |
Reaction conditions: 7a–e, benzylamine, NaHCO3, CH3CN/H2O (3:1), 16 °C (40 min) → 95 °C, 1.5 h.
Reaction conditions: 7a–e, S-α-phenylethylamine, NaHCO3, CH3CN/H2O (3:1), 16 °C (40 min) → 95 °C, 1.5 h.
Crude ketone used as starting material.
The ratio was derived from the isolated product yields (for entries 1, 4, and 5) or determined by analysis of the 1H NMR spectra (for entries 2 and 3).
Synthesis of Donepezil Analogues
By modifying the reported synthesis of donepezil,28 diastereomeric methyl- and phenyl-substituted 4-piperidones building blocks 2a + c and 2′a + c were subjected to a Wittig reaction using [(Ph3)PCH2OCH3]Cl and lithium diisopropylamide (LDA),29 to generate the corresponding methoxymethylene derivatives 9a–d + 9′a–d in good to high yields (Table 3). The chromatographic separation of the two isomers of all substrates allowed the unambiguous determination of their correct stereochemistry due to distinct nuclear Overhauser effect (NOE) correlations found in the isolated products (see Section S1 in the Supporting Information).
Table 3. Combined Yields and Product Ratios for the Synthesized Methoxymethylene Piperidines.
| entry | R | starting material | Wittig products (%)a,b,c |
|---|---|---|---|
| 1 | 2S-Me | 2S-2a | 2S-9 + 9′a (90)–Z:E, 1.2:1.0 |
| 2 | 2R-Me | 2R-2′a | 2R-9 + 9′b (75)–Z:E, 1.2:1.0 |
| 3 | 2R-Ph | 2R-2c | 2R-9 + 9′c (97)–Z:E, 1.0:1.0 |
| 4 | 2S-Ph | 2S-2′c | 2S-9 + 9′d (78)–Z:E, 1.1:1.0 |
Reaction conditions: [(Ph3)PCH2OCH3]Cl, LDA, 4 Ȧ MS, THF, −78 °C → rt, 16 h.
Isolated combined yield.
The ratio was determined by analysis of the 1H NMR spectra (entries 1–3) or derived from the isolated product yields (entry 4).
Sugimoto’s conditions for the originally reported acidic hydrolysis of the methoxymethylene intermediate (in donepezil) lead only to a moderate yield (i.e., 54%),28 due to the need of not trivial column chromatography for the purification of the product. Therefore, we set up a different protocol based on the mild hydrolysis of enol ethers 9 + 9′a–d with a mixture of tetrahydrofuran/1.6 M HCl (1:1)29 (Table 4). The corresponding aldehydes 3 + 3′a–d were obtained as diastereomeric mixtures in excellent yields and high purity. Nonetheless, it was observed (by 1H NMR analysis) that the storage of aldehydes at low temperatures (i.e., −20 °C) is necessary to prevent degradation over time. The diastereomeric ratio was determined by analysis of the 1H NMR spectra (Table 4), and the chirality of the 4-formyl moiety was confidently assigned for all products from the observed nuclear Overhauser effect spectroscopy (NOESY) correlations (see Section S1 in the Supporting Information).
Table 4. Combined Yields and Product Ratios for the Synthesized Aldehydes.
| entry | R | starting material | rearrangement product (%)a,b | ratioc 4R-3/4S-3′ |
|---|---|---|---|---|
| 1 | 2S-Me | 2S-9 + 9′a | 2S-3 + 3′a (97) | 1.0:1.3 |
| Z:E, 1.0:1.7 | ||||
| 2 | 2R-Me | 2R-9 + 9′b | 2R-3 + 3′b (100) | 1.9:1.0 |
| Z:E, 1.2:1.0 | ||||
| 3 | 2R-Ph | 2R-9 + 9′c | 2R-3 + 3′c (100) | 1.0:1.3 |
| Z:E, 1.3:1.0 | ||||
| 4 | 2S-Ph | 2S-9 + 9′d | 2S-3 + 3′d (100) | 2.0:1.0 |
| Z:E, 1.1:1.0 |
Reaction conditions: THF/1.6 M HCl (1:1), 45 °C, 2–3 h.
Diastereomeric combined yield.
The ratio was determined by analysis of the crude 1H NMR spectra.
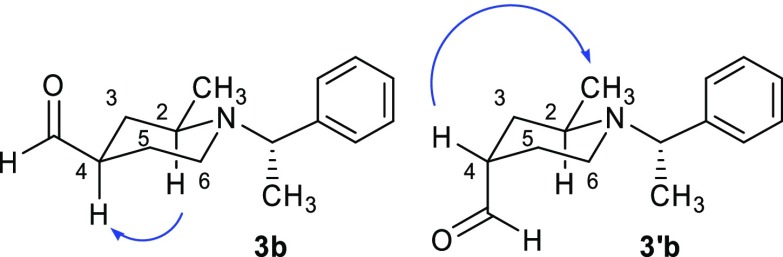
As an example, in Figure 2 are reported the correlations observed between Hax-2 and the proton in position 4 of aldehyde 3b (see Section S1 in the Supporting Information). This indicates an axial position for H-4 and, hence, R-chirality for the stereocenter in position 4. In contrast, no such NOESY correlation between Hax-2 and the proton in position 4 was found in diastereomer 3′b, while the interaction was observed between the protons of the methyl group in position 2 and Heq-4, which confirms S-chirality for this stereocenter in position 4 of the piperidine ring.
Figure 2.
Observed NOESY correlations in diastereomeric aldehydes 3b and 3′b.
With regard to the overall yield and purity, the present conditions for accessing N-benzyl-4-formyl-piperidine are superior to those of the procedures published earlier.28,30,31 The subsequent aldol condensations of 5,6-dimethoxy-1-indanone 10 with aldehyde mixtures 3a–d + 3′a–d were carried out according to a similar protocol developed by Imai et al.,32 by avoiding the low temperatures (−78 °C) and toxic solvents reported by Sugimoto et al.13,28 (Table 5). When a mixture of 2S-methyl-substituted aldehydes 3 + 3′a and 2R-methyl-substituted aldehydes 3 + 3′b was subjected to the aldol condensation reaction (Table 5, entries 1 and 2), not trivially separable methylene products were obtained and their diastereomeric ratios could be determined by analysis of the signals for the olefinic protons in the crude 1H NMR spectra. The corresponding aldol products were obtained as inseparable diastereomeric species for both 2-phenyl-substituted aldehyde mixtures 3 + 3′c and 3 + 3′d (Table 5, entries 3 and 4).
Table 5. Combined Yields and Product Ratios for the Aldol Condensation Products.
R2 = -S-α-phenylethyl. Reaction conditions: 10, NaOMe, MeOH, 80 °C, 1.5–2 h.
Combined isolated yield.
Entry 1 + 2: The ratio was determined by analysis of the crude 1H NMR spectra.
Hydrogenation of alkenes 11a–d + 11′a–d using palladium on activated carbon was investigated according to a protocol by Sugimoto13 and mixtures of the final donepezil analogues 4 + 4′a–h were obtained in a moderate to good yield (Table 6). Standard reversed-phase high-performance liquid chromatography (HPLC) was attempted by using various chromatographic conditions (for details, see representative method development in General procedures, Experimental Section), to isolate the single diasteroisomers from each mixture. All of the screened conditions gave a single peak for 4 + 4′a–h mixtures (see Section S3 in the Supporting Information), suggesting that the separation of chirally resolved single species was not possible in standard reversed-phase HPLC chromatography.
Table 6. Combined Yields and IC50 Values for the Synthesized Donepezil Analogues.
R2 = -S-α-phenylethyl. Reaction conditions: 10% Pd/C, 1 atm, H2, THF, rt, 7–10 h.
Combined isolated yield.
IC50 values are the mean of two separate experiments ± standard deviation. Each experiment was carried out in triplicate.
Not active in the concentration range tested in these experiments.
This IC50 value is the mean of three separate experiments.
As the 2-substituted donepezil analogues also include the chiral N-α-phenylethyl moiety, we envisaged that an unambiguous evaluation of the biological effects (i.e., acetylcholinesterase inhibition) produced by a substituent on the piperidine ring (in comparison to the unsubstituted donepezil) is only feasible with the reference analogues 19 + 19′ (Scheme 3). Indeed, compounds 19 + 19′ bear the chiral N-α-phenylethyl moiety, but no additional substituent is present on the piperidine ring, allowing more accurate structure–activity relationship analyses for this class of compounds. Analogues 19 + 19′ were synthesized in six steps, according to the conditions depicted in Scheme 3. Briefly, intermediates 14 and 15 were synthesized using reported methods.33,34 Piperidone 15 was transformed into methoxymethylene-based compound 16 through an analogous Wittig reaction (with [(Ph3)PCH2OCH3]Cl and LDA) as reported above for 9 + 9′a–d. Intermediate 17 was produced from 16 by mild acidic hydrolysis with THF/1.6 M HCl (1:1) and converted into 18 by aldol condensations of 5,6-dimethoxy-1-indanone 10. The final step is the classical reduction of the alkene group in 18 with H2 and Pd/C (Scheme 3), to afford diastereomeric mixtures of the final analogues 19 + 19′.
Scheme 3. Reaction Conditions.
(i) Methyl iodide, acetone, 25 °C, 2 h;33 (ii) S-α-phenylethylamine, K2CO3, ethanol/water (1:1), 95 °C, 30 min;34 (iii) [(Ph3)PCH2OCH3]Cl, LDA, 4 Ȧ MS, THF, −78 °C → rt, 16 h; (iv) THF/1.6 M HCl (1:1), 45 °C, 2.5 h; (v) 10, NaOMe, MeOH, 80 °C, 75 min; (vi) 10% Pd/C, H2 (1 atm), THF, rt, 8 h.
Biological Activity Studies
It has been reported that the R and S enantiomers of donepezil 1 interconvert in an aqueous solution at 37 °C due to ketoenol tautomerism, with a racemization half-life of 78 h.35 A similar spontaneous racemization of the stereocenter on the indanone moiety is also anticipated for the synthesized donepezil analogues 4 + 4′a–h. Also, it is worth mentioning that donepezil (Aricept) is not chirally resolved and is clinically used as a racemic mixture.18,36 In this view, we opted for a preliminary biological screening of the diastereomeric mixtures 4 + 4′a–h, before engaging in laborious, as well as lengthy, preparative chiral HPLC separations, to obtain initial evidence of the AChE inhibitory activity for this series.
The electric eel AChE (eeAChE) inhibitory activity of the synthesized donepezil analogues was evaluated in comparison to donepezil. A UV–vis spectrophotometry-based assay was conducted to calculate the IC50 values for the diastereomeric mixtures (Table 6), according to Ellman et al.37 and Mohamed et al.38 The half-inhibitory concentrations of all compounds possessing a substituent in position 2 on the piperidine ring (4 + 4′a–h; entries 1–6, Table 6) were evaluated in comparison to the diastereomeric pair of compounds lacking a substituent on the piperidine ring, 19 + 19′ (entry 7, Table 6), and the N-benzyl-substituted reference 1 (i.e., donepezil; entry 8, Table 6).
Piperidine ring unsubstituted diastereomers 19 + 19′ possess an IC50 of 1.83 μM (entry 7, Table 6) and are approximately 27 times less active than the donepezil 1 (entry 8, Table 6), indicating that the additional methyl group has a detrimental effect on the drug’s inhibitory activity. Interestingly, 2S-methyl-4S compounds 4 + 4′a possess a lower half-inhibitory concentration of 1.01 μM (entry 1), demonstrating that the additional methyl group on the piperidine partially compensates for some of the initial drop of activity caused by the N-S-α-phenylethyl moiety in 19 + 19′.
Among the 2-methyl-substituted analogues (entries 1–4, Table 6), those with 2S-chirality (entries 1 and 2, Table 6) are more active than the ones with 2R-chirality (entries 3 and 4, Table 6). The reduced inhibitory potency of these derivatives suggests that donepezil is sensitive to stereoselective substitution in position 2 on the piperidine ring, possibly due to significant changes in the overall conformation within the enzyme’s binding pocket and, therefore, affecting also the binding affinities. Furthermore, both diastereomeric mixtures possessing a phenyl ring in position 2 on the ring (entries 5, 6, Table 6) showed no activity in the tested concentration range (0.001–100 μM). In this regard, previous studies indicate that the bulky phenyl ring could prevent these compounds from entering the narrow, “swinging-gate”-controlled entry site of the binding gorge of the enzyme.21,36
Finally, when comparing the IC50 values of the syn-substituted methyl compounds (entries 1, 4, Table 6) to the anti-substituted methyl analogues (entries 2 and 3, Table 6), the syn-compounds are significantly more active in both cases. 2S,4S-syn-substituted compounds 4 + 4′a are approximately 24 times more active than the 2R,4S-anti-substituted analogues 4 + 4′c (Figure 3), demonstrating that the stereochemistry of the substituent in position 4 in relation to the stereochemistry of the substituent in position 2 (i.e., syn- or anti- conformation) significantly influences the overall inhibitory activity of the diastereomeric mixtures.
Figure 3.
Chemical structures and biological activities (IC50) of syn- and anti-substituted donepezil analogues.
Conclusions
Chirally resolved 2-substituted 4-piperidones were prepared from commercially available starting materials in three steps, thereby providing easy access to building blocks for the assembly of biologically relevant piperidine-based scaffolds. The reaction conditions reported for the synthesis of unstable aldehyde-type intermediates from these piperidine-4-one scaffolds are significantly improved (in terms of both overall conditions and final yield), compared to other procedures found in the literature. Furthermore, the aldehydes have been used to produce novel analogues of donepezil, which are stereochemically enriched through substituents at position 2 of the piperidine ring. AChE inhibition studies strongly indicate that the stereochemistry of substituents on the piperidine ring could play an important role in the binding behavior of such compounds within AChE’s active pocket.
Experiment Section
General
Infrared spectra were obtained on a PerkinElmer 100 Fourier transform infrared spectrometer operating in attenuated total reflection mode. Only significant absorptions (νmax) are reported, and all absorptions are recorded in wavenumbers (cm–1). Melting points were measured with an electrothermal apparatus and are uncorrected. Proton magnetic resonance spectra (1H NMR) were recorded at 400 MHz using a Bruker spectrometer. Chemical shifts (δ) are quoted in parts per million (ppm) and are referenced to the residual protonated solvent peak. The order of citation in parentheses is (i) number of equivalent nuclei (by integration), (ii) multiplicity (s, singlet; d, doublet; t, triplet; q, quartet and m, multiplet), and (iii) coupling constant (J) quoted in Hertz (Hz) to one decimal place. Carbon magnetic resonance spectra (13C NMR) were recorded at 100.6 MHz using a Bruker spectrometer. Chemical shifts (δ) are quoted in parts per million (ppm) and are referenced to the appropriate solvent peak. The assignment is quoted in parentheses. Where necessary, assignments were made with the aid of DEPT, correlation spectroscopy, heteronuclear single quantum coherence, heteronuclear multiple-bond correlation, or NOESY correlation experiments. Low-resolution mass spectra (m/z) were recorded using an LCQ DECA XP instrument by electron spray ionization (ESI). Only molecular ions and major fragments of the molecular ions are reported. Accurate masses were determined using a quadrupole time-of-flight mass spectrometer at King’s College London or a Thermo Fisher LTQ Orbitrap XL instrument at the EPSRC National Mass Spectrometry Facility in Swansea using nanospray ESI (NSI) and atmospheric pressure chemical ionization (APCI). Flash chromatography was carried out using silica gel (Aldrich, 230–400 mesh) as the stationary phase. Thin-layer chromatography was carried out on aluminum plates precoated with silica (Merck silica gel 60 F254 on aluminum), which was visualized by the quenching of ultraviolet fluorescence (λmax = 254 nm) and/or by staining with potassium permanganate solution followed by heat. All reactions were carried out at atmospheric pressure with stirring unless otherwise stated. All reagents were used as received unless otherwise stated. The fractions of light petroleum ether boiling between 40 and 60 °C are referred to as “hexanes”. Optical rotations ([α]DT = α/l.c) were measured by a Bellingham and Stanley ADP 220 polarimeter at 589 nm (sodium-D line). Concentration (c) is in g 100 mL–1. The HPLC analysis was performed on a Hewlett-Packard 1050 system equipped with an autosampler, a reversed-phase HPLC column (Agilent Zorbax 300 Å, C-18, 2.1 mm × 100 mm, particle size 3.5 μm), and a diode-array detector set to monitor 281 nm. The flow rate was 0.2 mL/min, and the column was eluted using three different linear gradients: (i) 0–90% MeCN in 0.1% (v/v) trifluoroacetic acid aqueous solution in 20 min (tR1); (ii) 0–90% MeCN in 0.1% (v/v) trifluoroacetic acid aqueous solution in 30 min (tR2); and (iii) 0–50% MeCN in 0.1% (v/v) trifluoroacetic acid aqueous solution in 50 min (tR3) (see Section S3 in the Supporting Information).
Synthesis
Preparation of Manganese Dioxide
Manganese dioxide (MnO2) was purchased from Alfa Aesar (Cat. No. 014340.22—manganese(IV) oxide, activated, tech. Mn 58% min, 100 g) and further activated by treatment with dilute nitric acid. MnO2 (50 g) was placed on a large Büchner funnel and 10% nitric acid (80 mL) was added slowly. After the addition was completed, the MnO2 cake was washed with water (2–3 L) until the filtrate was neutral. The MnO2 was subsequently dried at 105 °C for 2 days.
E-1,4-Hexadien-3-ol (6a)
Crotonaldehyde (747 mg, 10.70 mmol, 1.3 equiv) in THF (6 mL) was added dropwise to an ice-cooled solution of vinylmagnesium bromide in THF (8.00 mmol, 1.0 M in THF, 1.0 equiv) under an atmosphere of nitrogen. After stirring at room temperature for 1 h, the reaction mixture was poured into a mixture of saturated NH4Cl (10 mL) and ice (10 g) and stirred vigorously for 5 min. The aqueous solution was extracted with ether (3 × 15 mL), and the combined organic phases were dried over MgSO4. Concentration of the solvent under reduced pressure and purification by column chromatography (ethyl acetate/hexane, 1:3) gave alcohol 6a (639 mg, 82%) as a pale yellow oil; Rf 0.17 (5:1, hexane/diethyl ether); νmax (film) 3370, 3296, 2918, 1671, 1634, 1336, 1378, 1074, 990, 967, 924; δH (400 MHz, CDCl3) 5.83 (1H, ddd, J = 17 Hz, 10.4 Hz, 5.9 Hz), 5.66 (1H, dqd, J = 15.3 Hz, 6.5 Hz, 0.8 Hz), 5.46 (1H, ddq, J = 15.3 Hz, 6.7 Hz, 1.5 Hz), 5.18 (1H, dt, J = 17.2 Hz, 1.4 Hz), 5.06 (1H, dt, J = 10.4 Hz 1.2 Hz), 4.51 (1H, t, J = 6.0 Hz), 1.82–1.74 (3H, m); δC (100.6 MHz, CDCl3) 138.78, 131.22, 126.63, 113.68, 72.83, 16.71. In agreement with published data.39
E-Octa-1,4-dien-3-ol (6b)
trans-2-Hexenal (1.37 g, 14.00 mmol, 1.0 equiv) in THF (12 mL) was added dropwise to an ice-cooled solution of vinylmagnesium bromide in THF (16.00 mmol, 0.7 M in THF, 1.1 equiv) under an atmosphere of nitrogen. After allowing the flask to warm to room temperature and stirring for 1 h, the reaction mixture was poured into a mixture of saturated NH4Cl (20 mL) and ice (24 g) and stirred for 10 min. The aqueous solution was extracted with ether (3 × 30 mL), and the combined organic phases were washed with water and brine. The organic phase was dried over MgSO4, filtered, and the solvent was evaporated under reduced pressure. Crude alcohol 6b (1.74 g, 99%) was obtained as a yellow oil; Rf 0.40 (3:1, hexane/ethyl acetate); νmax (film) 3392, 3081, 2960, 2930, 2873, 1692, 1639, 1458, 1380, 1262, 1019, 989, 970, 920 δH (400 MHz, CDCl3) 5.91 (1H, ddd, J = 16.9 Hz, 10.4 Hz, 5.8 Hz), 5.74–5.64 (1H, m), 5.50 (1H, dd, J = 15.4 Hz, 6.7 Hz), 5.26 (1H, d, J = 17.3 Hz), 5.12 (1H, d, J = 10.5 Hz), 4.59 (1H, t, J = 5.5 Hz), 2.03 (2H, app q, J = 7.1 Hz), 1.45–1.36 (2H, m), 0.90 (3H, t, J = 7.4 Hz); δC (100.6 MHz, CDCl3) 140.01, 132.95, 131.21, 114.85, 74.05, 34.44, 22.35, 13.82. In agreement with published data.40
4E-1-Phenyl-penta-1,4-dien-3-ol (6c)
Cinnamaldehyde (925 mg, 10.70 mmol, 1.3 equiv) in THF (6 mL) was added dropwise to an ice-cooled solution of vinylmagnesium bromide in THF (8.00 mmol, 1.0 M in THF, 1.0 equiv) under an atmosphere of nitrogen. After stirring at room temperature for 1 h, the reaction mixture was poured into a mixture of saturated NH4Cl (10 mL) and ice (10 g) and stirred for 5 min. The aqueous solution was extracted with ether (3 × 15 mL), and the combined organic phases were dried over MgSO4. Concentration of the solvent under reduced pressure and purification by column chromatography (ethyl acetate/hexane, 1:8) gave alcohol 6c (1.09 g, 86%) as a pale yellow oil; Rf 0.41 (3:2, hexane/ethyl acetate); νmax (film) 3351, 3027, 1656, 1599, 1494, 1449, 987, 966, 750, 692; δH (400 MHz, CDCl3) 7.37–7.11 (5H, m), 6.55 (1H, d, J = 16.0 Hz), 6.17 (1H, dd, J = 15.9 Hz, 6.4 Hz), 5.91 (1H, ddd, J = 17.2 Hz, 10.4 Hz, 5.9 Hz), 5.28 (1H, dt, J = 17.2 Hz, 1.2 Hz), 5.13 (1H, dt, J = 10.4 Hz, 1.1 Hz), 4.75 (1H, t, J = 6.0 Hz); δC (100.6 MHz, CDCl3) 138.19, 135.51, 129.83, 129.26, 127.56, 126.78, 125.52, 114.45, 72.84. In agreement with published data.41,42
E-1-(4-Chlorophenyl)penta-1,4-dien-3-ol (6d)
E-4-Chlorocinnamaldehyde (1.000 g, 6.00 mmol, 1.0 equiv) in THF (8 mL) was added dropwise to an ice-cooled solution of vinylmagnesium bromide in THF (7.20 mmol, 0.7 M in THF, 1.1 equiv) under an atmosphere of nitrogen. After allowing the flask to warm to room temperature and stirring for 1 h, the reaction mixture was poured into a mixture of saturated NH4Cl (10 mL) and ice (12 g) and stirred for 10 min. The aqueous solution was extracted with ether (3 × 15 mL), and the combined organic phases were washed with water and brine. The organic phase was dried over MgSO4, filtered, and the solvent was evaporated under reduced pressure. Crude alcohol 6d (1.160 g, 100%) was obtained as a yellow oil; Rf 0.14 (1:2.7, diethyl ether:hexane); νmax (film) 3369, 1592, 1491, 1405, 1090, 1013, 967, 926, 807; δH (400 MHz, CDCl3) 7.31–7.26 (m, 4H), 6.56 (dd, 1H, J = 16.0 Hz, 1.1 Hz), 6.20 (dd, 1H, J = 15.9 Hz, 6.3 Hz), 5.96 (ddd, 1H, J = 17.1 Hz, 10.3 Hz, 5.9 Hz), 5.33 (dt, 1H, J = 17.1 Hz, 1.3 Hz), 5.20 (dt, 1H, J = 10.3 Hz, 1.3 Hz), 4.80 (td, 1H, J = 6.2 Hz, 1.3 Hz); δC (100.6 MHz, CDCl3) 139.16, 135.18, 133.50, 131.07, 129.59, 128.86, 127.86, 115.76, 73.79; Mass ion not found (ESI±) Exact mass calcd for C11H11ClO [M-OH]+ requires m/z 177.0461 found 177.0466 (APCI+).
E-1-(4-Methoxyphenyl)penta-1,4-dien-3-ol (6e)
E-4-Methoxycinnamaldehyde (5.000 g, 30.82 mmol, 1.0 equiv) in THF (26 mL) was added dropwise to an ice-cooled solution of vinylmagnesium bromide in THF (35.13 mmol, 0.7 M in THF, 1.1 equiv) under an atmosphere of nitrogen. After allowing the flask to warm to room temperature and stirring for 1 h, the reaction mixture was poured into a mixture of saturated NH4Cl (45 mL) and ice (54 g) and stirred for 5 min. The aqueous solution was extracted with ether (3 × 60 mL), and the combined organic phases were washed with water and brine. The organic phase was dried over MgSO4, filtered, and the solvent was evaporated under reduced pressure. Crude alcohol 6e (5.740 g, 98%) was obtained as a thick, yellow oil; Rf 0.46 (2:3, ethyl acetate/hexane); νmax (film) 3392, 2961, 2837, 1660, 1606, 1512, 1464, 1422, 1300, 1251, 1175, 1109, 1032, 969, 925, 805; δH (400 MHz, CDCl3) 7.33–7.26 (m, 2H), 6.86–6.84 (2H, m), 6.55 (1H, d, J = 16.0 Hz), 6.10 (1H, dd, J = 15.9 Hz, 6.6 Hz), 5.98 (1H, ddd, J = 17.3 Hz, 10.5 Hz, 5.9 Hz), 5.33 (1H, dt, J = 17.3 Hz, 1.5 Hz), 5.18 (1H, dt, J = 10.3 Hz, 1.3 Hz), 3.80 (3H, s); δC (100.6 MHz, CDCl3) 159.47, 139.56, 130.59, 129.42, 128.26, 127.87, 115.32, 114.11, 74.10, 55.40. In agreement with published data.43
E-1,4-Hexadien-3-one (7a)
Crude allylic alcohol 6a (167 mg, 1.70 mmol, 1.0 equiv) was added to 4 Ȧ molecular sieves suspended in CH2Cl2 (13 mL). Activated MnO2 (3.69 g, 42.50 mmol, 25.0 equiv) was added in portions, and the mixture was stirred at 50 °C for 3 h. The mixture was filtered through a pad of Celite, followed by washing with CH2Cl2 (150 mL). Concentration of the solvent under reduced pressure gave crude ketone 7a (115 mg, 71%) as a yellow oil; Rf 0.34 (5:1, hexane/ethyl acetate); νmax (film) 2969, 2931, 1713, 1444, 1377, 1256, 1164, 1129, 1086, 970, 735; δH (400 MHz, CDCl3) 6.96 (1H, dq, J = 15.5 Hz, 6.8 Hz), 6.59 (1H, dd, J = 17.4 Hz, 10.6 Hz), 6.39 (1H, dq, J = 15.6 Hz, 1.6 Hz), 6.28 (1H, dd, J = 17.4 Hz, 1.3 Hz), 5.81 (1H, dd, J = 10.6 Hz, 1.3 Hz), 1.94 (3H, dd, J = 6.8 Hz, 1.6 Hz); δC (100.6 MHz, CDCl3) 189.83, 144.31, 134.98, 129.87, 128.51, 18.64. In agreement with published data.44,45
E-Octa-1,4-dien-3-one (7b)
Crude allylic alcohol 6b (711 mg, 5.64 mmol, 1.0 equiv) was added to 4 Ȧ molecular sieves suspended in CH2Cl2 (30 mL). MnO2 (12.26 g, 141.00 mmol, 25.0 equiv) was added in portions, and the mixture was stirred at 50 °C for 3 h. The mixture was filtered through a pad of Celite, followed by washing with CH2Cl2 (400 mL). Concentration of the solvent under reduced pressure gave crude ketone 7b (433 mg, 62%) as a yellow oil; Rf 0.65 (3:2, hexane/ethyl acetate); νmax (film) 2962, 2932, 2874, 1667, 1633, 1612, 1403, 1219, 1100, 1043; δH (400 MHz, CDCl3) 6.94 (1H, dt, J = 15.6 Hz, 6.9 Hz), 6.61 (1H, dd, J = 17.4 Hz, 10.6 Hz), 6.36 (1H, dt, J = 15.7 Hz, 1.5 Hz), 6.28 (1H, dd, J = 17.4 Hz, 1.3 Hz), 5.81 (1H, dd, J = 10.6 Hz, 1.3 Hz), 2.26–2.20 (2H, m), 1.57–1.47 (2H, m), 0.94 (3H, t, J = 7.4 Hz); δC (100.6 MHz, CDCl3) 189.99, 149.06, 135.04, 128.45 & 128.44, 34.84, 21.50, 13.85; m/z (ESI+) 157 [M + H + CH3OH]+, 125 [M + H]+; Exact mass calcd for C8H12O [M + H]+ requires m/z 125.0961 found 125.0959 (APCI+).
4E-1-Phenyl-penta-1,4-dien-3-one (7c)
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (674 mg, 2.97 mmol, 1.1 equiv) was added to crude allylic alcohol 6c (436 mg, 2.70 mmol, 1.0 equiv) in 1,4-dioxane (8.5 mL). The mixture was stirred at room temperature for 18 h, filtered, and washed with CH2Cl2 (20 mL). Concentration under reduced pressure and purification by column chromatography (ethyl acetate/hexane, 1:8) afforded ketone 7c (317 mg, 74%) as a yellow oil; Rf 0.54 (3:2, hexane/ethyl acetate); νmax (film) 3048, 1655, 1623, 1592, 1497, 1451, 1404, 1348, 1205, 1104, 984, 875, 691; δH (400 MHz, CDCl3) 7.68 (1H, d, J = 16.0 Hz), 7.60–7.58 (2H, m), 7.42–7.40 (3H, m), 7.02 (1H, d, J = 16.0 Hz), 6.72 (1H, dd, J = 17.4 Hz, 10.6 Hz), 6.39 (1H, dd, J = 17.4 Hz 1.2 Hz), 5.90 (1H, dd, J = 10.4 Hz, 1.1 Hz); δC (100.6 MHz, CDCl3) 189.72, 144.14, 135.59, 134.77, 130.76, 129.12, 128.78, 128.54, 124.25. In agreement with published data.46,47
E-1-(4-Chlorophenyl)penta-1,4-dien-3-one (7d)
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (1.45 g, 6.38 mmol, 1.1 equiv) was added to crude allylic alcohol 6d (1.128 g, 5.80 mmol, 1.0 equiv) in dioxane (19 mL). The mixture was stirred at room temperature for 18 h, filtered, and washed with CH2Cl2. Concentration of the solvent under reduced pressure and purification by column chromatography (hexane/diethyl ether, 3:1) gave ketone 7d (627 mg, 56%) as a yellow solid; mp 178–182 °C; Rf 0.20 (1:2.7, diethyl ether:hexane); νmax (solid) 1656, 1603, 1591, 1566, 1491, 1408, 1382, 1313, 1294, 1260, 1240, 1194, 1179, 1090, 1014, 970, 827; δH (400 MHz, CDCl3) 7.61 (1H, d, J = 16.0 Hz), 7.50 (2H, d, J = 8.3 Hz), 7.36 (2H, d, J = 7.4 Hz), 6.97 (1H, d, J = 16.0 Hz), 6.69 (1H, dd, 17.4 Hz, 10.6 Hz), 6.38 (1H, dd, J = 17.4 Hz, 1.1 Hz), 5.89 (1H, dd, J = 10.6 Hz, 1.1 Hz); δC (100.6 MHz, CDCl3) 189.35 (C=O), 142.55, 136.60, 135.56, 133.23, 129.63, 129.36, 128.97, 124.50; Mass ion not found (ESI±) Exact mass calcd for C11H9ClO [M + H]+ requires m/z 193.0413 found 193.0415 (APCI+).
E-1-(4-Methoxyphenyl)penta-1,4-dien-3-one (7e)
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (1.85 g, 8.13 mmol, 1.1 equiv) was added to crude allylic alcohol 6e (1.404 g, 7.39 mmol, 1.0 equiv) and in dioxane (27 mL). The mixture was stirred at room temperature for 18 h, filtered, and washed with CH2Cl2. Concentration of the solvent under reduced pressure and purification by column chromatography (hexane/diethyl ether, 3:1) gave ketone 7e (741 mg, 53%) as a yellow solid; mp 64–67 °C; Rf 0.51 (2:3, ethyl acetate/hexane); νmax (solid) 2936, 2838, 1654, 1601, 1572, 1512, 1463, 1442, 1424, 1404, 1306, 1254, 1222, 1200, 1174, 1103, 1030, 988, 830; δH (400 MHz, CDCl3) 7.65 (1H, d, J = 15.9 Hz), 7.55–7.52 (2H, m), 6.86–6.95 (3H, m), 6.70 (1H, dd, J = 17.4 Hz, 10.6 Hz), 6.36 (1H, dd, J = 17.4 Hz, 1.3 Hz), 5.84 (1H, dd, J = 10.6 Hz, 1.3 Hz), 3.84 (3H, s); δC (100.6 MHz, CDCl3) 189.61 (C=O), 161.83, 143.93, 135.65, 130.28, 128.23, 127.44, 122.12, 114.55, 55.52. In agreement with published data.44
1-Benzyl-2-methylpiperidin-4-one (8a)
Benzylamine (2.90 g, 27.06 mmol, 1.3 equiv) was dissolved in acetonitrile (30 mL) and a solution of aqueous NaHCO3 (6.56 g in 17 mL H2O) was added. The resulting suspension was cooled to 16 °C and crude ketone 7a (2.00 g, 20.83 mmol, 1.0 equiv) in acetonitrile (20 mL) was slowly added over a period of 35 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (60 mL). The solution was stirred for 20 min, the layers were separated, and the aqueous layer was again extracted with ethyl acetate (2 × 60 mL). The organic layers were combined and washed with water (60 mL) and brine (60 mL). The organic layer was dried with anhydrous Na2SO4, filtered, and the solvent was evaporated in vacuo. Purification by column chromatography (ethyl acetate/hexane, 1:2 with 1% triethanolamine (TEA)) afforded product 8a (1.66 g, 39%) as a pale yellow oil; Rf 0.33 (1:1, ethyl acetate/hexane); νmax (film) 2966, 2802, 1720, 1601, 1495, 1453, 1377, 1349, 1332, 1250, 1177, 1137, 1064, 1028, 762, 733; δH (400 MHz, CDCl3) 7.38–7.25 (5H, m), 3.97 (1H, d, J = 13.4 Hz) 3.45 (1H, d, J = 13.5 Hz), 3.02–2.97 (2H, m), 2.56–2.53 (2H, m), 2.40–2.37 (2H, m), 2.31–2.26 (1H, m), 1.18 (3H, d, J = 6.4 Hz); δC (100.6 MHz, CDCl3) 209.89 (C=O), 139.13, 128.80, 128.49, 127.26, 57.18, 56.30, 48.89, 48.75, 41.03, 17.61. In agreement with published data.48
1-Benzyl-2-propylpiperidin-4-one (8b)
Benzylamine (196 mg, 1.83 mmol, 1.3 equiv) was dissolved in acetonitrile (5 mL), and a solution of aqueous NaHCO3 (454 mg in 3 mL H2O) was added. The resulting suspension was cooled to 16 °C, and crude ketone 7b (179 mg, 1.44 mmol, 1.0 equiv) in acetonitrile (5 mL) was slowly added over a period of 40 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (12 mL). The solution was stirred for 20 min, the layers were separated, and the organic layer was washed with water (12 mL) and brine (12 mL). The organic layer was dried over anhydrous Na2SO4 and filtered. Evaporation of the solvent and purification by column chromatography (ethyl acetate/hexane, 1:2.95) afforded product 8b (120 mg, 36%) as a pale yellow oil; Rf 0.15 (5:1, hexane/ethyl acetate); νmax (film) 3028, 2958, 2930, 2872, 2807, 1718, 1495, 1454, 1419, 1350, 1264, 1201, 1174, 1139, 1071, 1027, 942, 733; δH (400 MHz, CDCl3) 7.39–7.25 (5H, m), 3.90 (1H, d, J = 13.5 Hz), 3.66 (1H, d, J = 13.5 Hz), 3.07–2.97 (2H, m), 2.75–2.69 (1H, m), 2.57 (1H, ddd, J = 14.0 Hz, 4.7 Hz, 1.3 Hz), 2.45–2.38 (1H, m), 2.35–2.29 (2H, m), 1.64–1.54 (1H, m), 1.45–1.28 (3H, m), 0.90 (3H, t, J = 7.2 Hz); δC (100.6 MHz, CDCl3) 210.28 (C=O), 139.39, 128.70, 128.53, 127.30, 60.63, 56.11, 48.09, 44.83, 39.62, 33.53, 19.04, 14.27; m/z (ESI+) 264 [M + H + CH3OH]+, 232 [M + H]+; Exact mass calcd for C16H26NO [M + H + CH3OH]+ requires m/z 264.1958 found 264.1960 (NSI+).
1-Benzyl-2-phenylpiperidin-4-one (8c)
Benzylamine (139 mg, 1.30 mmol, 1.3 equiv) was added to a mixture of acetonitrile (3 mL) and aqueous NaHCO3 (315 mg in 2 mL H2O). The resulting suspension was cooled to 15 °C, and ketone 7c (158 mg, 1.00 mmol, 1.0 equiv) in acetonitrile (3 mL) was slowly added over a period of 25 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (8 mL). The solution was stirred for 15 min, the layers were separated, and the aqueous layer was again extracted with ethyl acetate (2 × 10 mL). The organic layers were combined and washed with water (10 mL) and brine (10 mL). The organic phase was dried with anhydrous Na2SO4, filtered, and the solvent was evaporated under reduced pressure. Purification by column chromatography (ethyl acetate/hexane, 1:7) afforded product 8c (210 mg, 79%) as a pale yellow oil; Rf 0.19 (ethyl acetate/hexane, 1:8); νmax (film) 3025, 2797, 1726, 1495, 1454, 1257, 1236, 1160, 1101, 1073, 1023, 907, 823; δH (400 MHz, CDCl3) 7.48–7.24 (10H, m) 3.84 (1H, d, J = 13.4 Hz), 3.59 (1H, dd, J = 11.0 Hz, 3.7 Hz), 3.25–3.20 (1H, m, 1H), 2.94 (1H, d, J = 13.6 Hz), 2.74–2.62 (2H, m), 2.54 (1H, app dq, J = 14.6 Hz), 2.39–2.32 (2H, m); δC (100.6 MHz, CDCl3) 208.68 (C=O), 142.67, 139.10, 129.13, 128.66, 128.45, 127.95, 127.46, 127.20, 68.50, 58.22, 51.33, 50.45, 41.62. In agreement with published data.49
1-Benzyl-2-(4-chlorophenyl)piperidin-4-one (8d)
Benzylamine (74 μL, 0.68 mmol, 1.3 equiv) was dissolved in acetonitrile (1.6 mL) and a solution of aqueous NaHCO3 (164 mg in 1 mL of H2O) was added. The resulting suspension was cooled to 16 °C, and ketone 7d (99 mg, 0.52 mmol, 1.0 equiv) in acetonitrile (1.6 mL) was slowly added over a period of 30 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (10 mL). The solution was stirred for 15 min, the layers were separated, and the aqueous layer was again extracted with ethyl acetate (2 × 10 mL). The organic layers were combined and washed with water (10 mL) and brine (10 mL). The organic phase was dried with anhydrous Na2SO4, filtered, and the solvent was evaporated in vacuo. Purification by column chromatography (ethyl acetate/hexane, 1:5 with 1% TEA) afforded product 8d (131 mg, 84%) as a pale yellow oil; Rf 0.32 (1:4, ethyl acetate/hexane); νmax (film) 2963, 2803, 1721, 1599, 1494, 1453, 1410, 1368, 1326, 1300, 1259, 1240, 1160, 1088, 1015, 836, 736; δH (400 MHz, CDCl3) 7.22–7.42 (9H, m), 3.80 (1H, d, J = 13.5 Hz), 3.58 (1H, dd, J = 10.8 Hz, 3.9 Hz), 3.24–3.19 (1H, m), 2.95 (1H, d, J = 13.5 Hz), 2.69–2.61 (2H, m, 1 × H-2), 2.52 (1H, ddd, J = 14.5 Hz, 3.8 Hz, 2.3 Hz), 2.39–2.32 (2H, m); δC (100.6 MHz, CDCl3) 208.13 (C=O), 141.20, 138.72, 133.59, 129.34, 128.78, 128.60, 128.51, 127.33, 67.68, 58.20, 51.21, 50.20, 41.50; m/z (ESI+) 318 [M + H + H2O]+, 300 [M + H]+, 282, 120, 91; Exact mass calcd for C18H18NO [M + H]+ requires m/z 300.1150 found 300.1153 (NSI+).
1-Benzyl-2-(4-methoxyphenyl)piperidin-4-one (8e)
Benzylamine (409 μL, 3.74 mmol, 1.3 equiv) was dissolved in acetonitrile (9 mL), and a solution of aqueous NaHCO3 (907 mg in 6 mL H2O) was added. The resulting suspension was cooled to 16 °C, and ketone 7e (542 mg, 2.88 mmol, 1.0 equiv) in acetonitrile (9 mL) was slowly added over a period of 35 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (20 mL). The solution was stirred for 15 min, the layers were separated, and the aqueous layer was again extracted with ethyl acetate (2 × 20 mL). The organic layers were combined and washed with water (20 mL) and brine (20 mL). The organic layer was dried with anhydrous Na2SO4, filtered, and the solvent was evaporated in vacuo. Purification by column chromatography (ethyl acetate/hexane, 1:3.2 with 1% TEA) afforded product 8e (712 mg, 84%) as a pale yellow oil; Rf 0.19 (1:5, ethyl acetate/hexane); νmax (film) 3029, 2800, 1720, 1611, 1585, 1512, 1495, 1454, 1367, 1325, 1303, 1248, 1173, 1115, 1032, 837, 816, 770, 738; δH (400 MHz, CDCl3) 7.35–7.39 (2H, m), 7.33–7.21 (5H, m), 7.21–6.93 (2H, m), 3.84 (1H, d, J = 13.6 Hz), 3.81 (3H, s), 3.54 (1H, dd, J = 10.9 Hz, 3.7 Hz), 3.21 (1H, ddd, J = 11.1 Hz, 6.2 Hz, 2.4 Hz), 2.92 (1H, d, J = 13.5 Hz) 2.72–2.61 (2H, m), 2.52 (1H, ddd, J = 14.6 Hz, 3.7 Hz, 2.5 Hz), 2.37–2.30 (2H, m); δC (100.6 MHz, CDCl3) 208.84 (C=O), 159.24, 139.21, 134.68, 128.64, 128.53, 128.41, 127.14, 114.41, 67.76, 58.04, 55.42, 51.25, 50.51, 41.61; m/z (ESI+) 314 [M + H + H2O]+, 296 [M + H]+, 177, 120, 91; Exact mass calcd for C20H26NO2 [M + H + CH3OH]+ requires m/z 328.1907 found 328.1910 (NSI+).
S-2-Methyl-1-(S-1-phenylethyl)piperidin-4-one (2a) and R-2-Methyl-1-(S-1-phenylethyl)piperidin-4-one (2′a)
S-α-Phenylethylamine (4.92 g, 40.63 mmol, 1.3 equiv) was dissolved in acetonitrile (30 mL), and a solution of aqueous NaHCO3 (9.85 g in 20 mL H2O) was added. The resulting suspension was cooled to 16 °C and crude ketone 7a (3.000 g, 31.25 mmol, 1.0 equiv) in acetonitrile (30 mL) was slowly added over a period of 30 min. Upon addition of the ketone, the reaction was stirred at reflux for 1 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (60 mL). The solution was stirred for 15 min, the layers were separated, and the aqueous layer was again extracted with ethyl acetate (2 × 60 mL). The organic layers were combined and washed with water (60 mL) and brine (60 mL). The organic phase was dried with anhydrous Na2SO4, filtered, and the solvent was evaporated under reduced pressure. Purification by column chromatography (ethyl acetate/hexane, 1:6 → 1:3) afforded product 2a (1.280 g, 19%) as a pale yellow oil and product 2′a (1.220 g, 18%) as a pale red solid.
Product 2a, eluting first: [α]D23 21 −47.7 (c 0.30, CHCl3); Rf 0.25 (1:4, ethyl acetate/hexane); νmax (film) 3028, 2969, 2814, 1720, 1601, 1492, 1453, 1380, 1353, 1307, 1262, 1230, 1180, 1140, 766, 70; δH (400 MHz, CDCl3) 7.45–7.43 (2H, m), 7.35–7.31 (2H, m), 7.27–7.23 (1H, m), 4.01 (1H, q, J = 6.7 Hz), 3.41–7.23 (1H, m), 2.75 (1H, ddd, J = 12.5 Hz), 2.70–2.61 (2H, m), 2.37–2.29 (1H, m), 2.24–2.29 (2H, m), 1.33 (3H, d, J = 6.8 Hz), 1.14 (3H, d, J = 6.4 Hz); δC (100.6 MHz, CDCl3) 210.54 (C=O), 145.32, 128.46, 127.34, 126.99, 57.76, 52.54, 48.89, 43.99, 41.46, 16.47, 16.33. In agreement with published data.50
Product 2′a, eluting second: [α]D20 −40.5 (c 0.15, CHCl3); mp 72–75 °C; Rf 0.12 (1:4, ethyl acetate/hexane); νmax (solid) 3027, 2971, 2838, 1719, 1493, 1454, 1418, 1376, 1351, 1285, 1228, 1179, 1122, 1076, 1027, 768, 702; δH (400 MHz, CDCl3) 7.35–7.30 (4H, m), 7.27–7.23 (1H, m), 3.92 (1H, q, J = 6.7 Hz), 3.19–3.12 (1H, m), 3.00–2.90 (2H, m), 2.58–2.49 (2H, m), 2.35–2.28 (1H, m), 2.12–2.07 (1H, m), 1.42 (3H, d, J = 6.7 Hz), 1.03 (3H, d, J = 6.6 Hz); δC (100.6 MHz, CDCl3) 210.54 (C=O), 144.11, 128.55, 127.35, 127.22, 58.84, 52.45, 48.37, 43.19, 41.20, 21.93, 14.78. In agreement with published data.51
S-1-(S-1-Phenylethyl)-2-propylpiperidin-4-one (2b) and R-1-(S-1-Phenylethyl)-2-propylpiperidin-4-one (2′b)
S-α-Phenylethylamine (496 mg, 4.09 mmol, 1.3 equiv) was dissolved in acetonitrile (9 mL), and a solution of aqueous NaHCO3 (1.01 g in 6 mL H2O) was added. The resulting suspension was cooled to 16 °C and crude ketone 7b (400 mg, 3.22 mmol, 1.0 equiv) in acetonitrile (9 mL) was slowly added over a period of 30 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (12 mL). The solution was stirred for 20 min, the layers were separated, and the organic layer was washed with water (12 mL) and brine (12 mL). The organic layer was dried over anhydrous Na2SO4 and filtered. Evaporation of the solvent and purification by column chromatography (ethyl acetate/hexane, 1:5) afforded product 2b (123 mg, 16%) as a pale yellow oil and product 2′b (86 mg, 11%) as pale yellow oil.
Product 2b, eluting first: [α]D22 −36.4 (c 0.52, CHCl3); Rf 0.42 (1:3, ethyl acetate/hexane); νmax (film) 3026, 2959, 2931, 2872, 1716, 1601, 1492, 1454, 1351, 1282, 1219, 1178, 1082, 967, 770; δH (400 MHz, CDCl3) 7.43–7.40 (2H, m), 7.31 (2H, m), 7.26–7.23 (1H, m), 4.00 (1H, q, J = 6.7 Hz), 3.22–3.17 (1H, m), 2.98–2.92 (1H, m), 2.89–2.83 (1H, m), 2.65 (1H, ddd, J = 13.9 Hz, 5.5 Hz, 0.8 Hz), 2.44–2.36 (1H, m), 2.24 (1H, ddd, J = 14.0 Hz), 2.1–2.12 (1H, m), 1.5–1.45 (1H, m), 1.37–1.25 (6H, m) 0.87 (3H, t, J = 7.1); δC (100.6 MHz, CDCl3) 210.89 (C5O), 145.80, 128.55, 127.30, 127.12, 58.19, 57.00, 44.54, 43.72, 40.33, 32.56, 19.30, 19.14, 14.34; m/z (ESI+) 264 [M + H + H2O]+, 246 [M + H]+, 142, 105; Exact mass calcd for C28H26NO [M + H + CH3OH]+ requires m/z 278.2115 found 278.2118 (NSI+).
Product 2′b, eluting second: [α]D21 −25.0 (c 1.08, CHCl3); Rf 0.23 (1:3, ethyl acetate/hexane); νmax (film) 3027, 2960, 2932, 2872, 1713, 1602, 1492, 1454, 1352, 1281, 1219, 1174, 1083, 967, 770; δH (400 MHz, CDCl3) 7.42–7.23 (5H, m), 3.99 (1H, q, J = 6.5 Hz), 3.32–3.27 (1H, m), 2.99–2.88 (2H, m), 2.61–2.49 (2H, m), 2.19–2.11 (2H, m), 1.58–1.47 (1H, m), 1.40 (3H, d, J = 6.5 Hz); 1.37–1.20 (3H, m), 0.88 (3H, t, J = 7.0 Hz); δC (100.6 MHz, CDCl3) 210.79 (C=O), 145.57, 128.65, 127.26, 58.71, 56.18, 44.15, 43.51, 39.48, 32.34, 22.50, 19.50, 14.11; m/z (ESI+) 264 [M + H + H2O]+, 246 [M + H]+, 142, 105; Exact mass calcd for C16H24NO [M + H]+ requires m/z 246.1852 found 246.1855 (NSI+).
R-2-Phenyl-1-(S-1-phenylethyl)piperidin-4-one (2c) and S-2-Phenyl-1-(S-1-Phenylethyl)piperidin-4-one (2′c)
S-α-Phenylethylamine (291 mg, 2.40 mmol, 1.3 equiv) was dissolved in acetonitrile (6 mL), and a solution of aqueous NaHCO3 (599 mg in 4 mL H2O) was added. The resulting suspension was cooled to 16 °C, and ketone 7c (300 mg, 1.90 mmol, 1.0 equiv) in acetonitrile (6 mL) was slowly added over a period of 40 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (12 mL). The solution was stirred for 20 min, the layers were separated, and the organic layer was washed with water (12 mL) and brine (12 mL). The organic phase was dried over anhydrous Na2SO4 and filtered. Evaporation of the solvent and purification by column chromatography (ethyl acetate/hexane, 1:5.5) afforded product 2c (249 mg, 47%) as a pale yellow solid and product 2′c (83 mg, 16%) as a pale yellow oil.
Product 2c, eluting first: [α]D21 −34.2 (c 0.42, CHCl3); mp 87–90 °C; Rf 0.31 (17:3, hexane/ethyl acetate); νmax (solid) 3059, 2972, 2820, 1712, 1600, 1492, 1447, 1421, 1380, 1365, 1331, 1257, 1239, 1168, 1122, 1027, 1011, 805, 785; δH (400 MHz, CDCl3) 7.50–7.47 (4H, m), 7.40–7.22 (6H, m), 3.98–3.90 (2H, m), 2.93–2.88 (1H, m), 2.73 (1H, dd, J = 14.4 Hz, 10.4 Hz), 2.64–2.49 (3H, m), 2.33–2.29 (1H, m), 1.23 (3H, d, J = 6.8 Hz); δ C (100.6 MHz, CDCl3) 209.15 (C=O), 143.93, 142.30, 129.13, 128.24, 127.96, 127.57, 127.49, 126.82, 64.90, 54.97, 50.39, 44.16, 42.05, 9.52; m/z (ESI+) 312 [M + H + CH3OH]+, 280 [M + H]+, 176, 147, 129; Exact mass calcd for C20H42NO2 [M + H + CH3OH]+ requires m/z 312.1958 found 312.1961 (NSI+).
Product 2′c, eluting second: [α]D22 −95.1 (c 0.28, CHCl3); Rf 0.23 (17:3, hexane/ethyl acetate); νmax (film) 3029, 2971, 2817, 1721, 1601, 1493, 1454, 1363, 1309, 1261, 1241, 1108, 1073, 1030, 759, 702; δH (400 MHz, CDCl3) 7.43–7.24 (8H, m), 7.09–7.06 (2H, m), 4.03 (1H, q, J = 7.1 Hz), 3.67 (1H, dd, J = 9.4 Hz, 4.5 Hz), 3.34 (1H, ddd, J = 12.0 Hz, 5.7 Hz, 3.8 Hz), 2.68–2.37 (4H, m), 2.24–2.20 (1H, m), 1.44 (3H, d, J = 7.2 Hz); δC (100.6 MHz, CDCl3) 208.83 (C=O), 143.10, 139.01, 129.12, 128.46, 128.07, 127.67, 127.66, 127.36, 64.63, 56.63, 50.56, 44.52, 41.84, 19.56; m/z (ESI+) 312 [M + H + CH3OH]+, 280 [M + H]+, 176, 147, 129; Exact mass calcd for C19H22NO [M + H]+ requires m/z 280.1696 found 280.1698 (NSI+).
R-2-(4-Chlorophenyl)-1-(S-1-phenylethyl)piperidin-4-one (2d) and S-2-(4-Chlorophenyl)-1-(S-1-phenylethyl)piperidin-4-one (2′d)
S-α-Phenylethylamine (345 μL, 2.68 mmol, 1.3 equiv) was dissolved in acetonitrile (6.5 mL), and a solution of aqueous NaHCO3 (649 mg in 4.3 mL H2O) was added. The resulting suspension was cooled to 16 °C, and ketone 7d (396 mg, 2.06 mmol, 1.0 equiv) in acetonitrile (6.5 mL) was slowly added over a period of 30 min. Upon addition of the ketone, the reaction was stirred at reflux for 1.5 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (15 mL). The solution was stirred for 15 min, the layers were separated, and the aqueous layer was again extracted with ethyl acetate (2 × 15 mL). The organic layers were combined and washed with water (15 mL) and brine (15 mL). The organic layer was dried with anhydrous Na2SO4, filtered, and the solvent was evaporated in vacuo. Purification by column chromatography (ethyl acetate/hexane, 1:5.5 with 1.5% TEA) afforded product 2d (270 mg, 42%) as a pale yellow solid and a mixture of product 2d and product 2′d (170 mg, 26%) as a pale yellow oil. NMR investigation indicated that the mixed fractions of diastereomers were a 0.47:1 ratio of 2d:2′d. Further purification of this diastereomeric mixture afforded pure 2′d.
Product 2d, eluting first: [α]D24 −56.0 (c 0.38, CHCl3); mp 91–94 °C; Rf 0.39 (1:4, ethyl acetate/hexane); νmax (solid) 2989, 2813, 1719, 1600, 1487, 1447, 1412, 1381, 1366, 1299, 1260, 1239, 1166, 1123, 1088, 1014, 835; δH (400 MHz, CDCl3) 7.46–7.23 (9H, m), 3.95–3.89 (2H, m), 2.90 (1H, ddd, J = 11.6 Hz, 5.7 Hz, 3.4 Hz), 2.69–2.47 (4H, m), 2.32 (1H, ddd, J = 14.0 Hz, 5.4 Hz, 2.9 Hz), 1.23 (3H, d, J = 6.8 Hz); δC (100.6 MHz, CDCl3) 208.61 (C=O), 143.62, 140.87, 133.60, 129.35, 128.88, 128.34, 127.41, 126.98, 64.07, 55.16, 49.98, 44.02, 41.86, 9.83; m/z (ESI+) 332 [M + H + H2O]+, 314 [M + H]+, 210, 134, 105; Exact mass calcd for C19H20ClNO [M + H + CH3OH]+ requires m/z 346.1568 found 346.1572 (NSI+).
Product 2′d, eluting second: [α]D23 −116.5 (c 0.13, CHCl3); Rf 0.30 (1:4, ethyl acetate/hexane); νmax (film) 2972, 2815, 1720, 1600, 1487, 1453, 1411, 1370, 1278, 1261, 1240, 1167, 1111, 1088, 1014, 834, 768, 734; δH (400 MHz, CDCl3) 7.44–7.23 (7H, m), 7.06–7.04 (2H, m), 3.97 (1H, q, J = 7.1 Hz), 3.67 (1H, dd, J = 8.5 Hz, 5.4 Hz), 3.33 (1H, ddd, J = 12.0 Hz, 5.7 Hz, 4.0 Hz), 2.51–2.37 (3H, m), 2.25 (1H, td, J = 11.8 Hz, 3.3 Hz), 1.44 (3H, d, J = 7.1 Hz); δ C (100.6 MHz, CDCl3) 208.35 (C=O), 141.65, 138.88, 133.27, 129.31, 128.98, 128.36, 128.16, 127.49, 63.77, 56.87, 50.17, 44.39, 41.70, 19.63; m/z (ESI+) 332 [M + H + H2O]+, 314 [M + H]+, 210, 134, 105; Exact mass calcd for C19H21ClNO [M + H]+ requires m/z 314.1306 found 314.1309 (NSI+).
R-2-(4-Methoxyphenyl)-1-(S-1-phenylethyl)piperidin-4-one (2e) and S-2-(4-Methoxyphenyl)-1-(S-1-phenylethyl)piperidin-4-one (2′e)
S-α-Phenylethylamine (365 μL, 2.83 mmol, 1.3 equiv) was dissolved in acetonitrile (6 mL), and a solution of aqueous NaHCO3 (687 mg in 4 mL of H2O) was added. The resulting suspension was cooled to 16 °C, and ketone 7e (410 mg, 2.18 mmol, 1.0 equiv) in acetonitrile (6 mL) was slowly added over a period of 35 min. Upon addition of the ketone, the reaction was stirred at reflux for 1 h. Evaporation of acetonitrile in vacuo was followed by the addition of ethyl acetate (12 mL). The solution was stirred for 15 min, the layers were separated, and the aqueous layer was again extracted with ethyl acetate (2 × 12 mL). The organic layers were combined and washed with water (12 mL) and brine (12 mL). The organic layer was dried with anhydrous Na2SO4, filtered, and the solvent was evaporated in vacuo. Purification by column chromatography (ethyl acetate/hexane, 1:5.5 with 1.5% TEA) afforded product 2e (284 mg, 42%) as a pale yellow solid and a mixture of product 2e and product 2′e (99 mg, 15%) as a pale yellow oil. NMR investigation indicated that the mixed fractions of diastereomers were a 0.34:1 ratio of 2e:2′e. Further purification of this diastereomeric mixture afforded pure 2′e as a pale yellow oil.
Product 2e, eluting first: [α]D21 −90.9 (c 0.22, CHCl3); mp 76–79 °C; Rf 0.24 (1:6, ethyl acetate/hexane); νmax (solid) 2968, 2835, 1717, 1611, 1584, 1512, 1446, 1380, 1366, 1302, 1250, 1174, 1031, 835, 773, 724, 699; δH (400 MHz, CDCl3) 7.47–7.45 (2H, m), 7.41–7.38 (2H, m), 7.34–7.31 (2H, m), 7.25–7.21 (1H, m), 6.93–6.89 (2H, m), 3.95 (1H, q, J = 6.7 Hz), 3.86 (1H, dd, J = 10.3 Hz, 3.7 Hz), 3.80 (3H, s), 2.92–2.87 (1H, m), 2.70 (1H, dd, J = 14.2 Hz, 10.6 Hz), 2.58–2.47 (3H, m), 2.32–2.27 (1H, m), 1.22 (3H, d, J = 6.8 Hz); δC (100.6 MHz, CDCl3) 209.34 (C=O), 159.25, 144.06, 134.33, 128.61, 128.22, 127.47, 126.77, 114.45, 64.21, 55.41, 54.85, 50.51, 44.13, 42.06, 9.57; m/z (ESI+) 328 [M + H + H2O]+, 310 [M + H]+, 206, 177, 134, 105; Exact mass calcd for C21H28NO2 [M + H + CH3OH]+ requires m/z 342.2064 found 342.2067 (NSI+).
Product 2′e, eluting second: [α]D22 −131.2 (c 0.32, CHCl3); Rf 0.16 (1:6, ethyl acetate/hexane); νmax (film) 3029, 2970, 2835, 1719, 1611, 1585, 1511, 1454, 1365, 1302, 1249, 1173, 1033, 835, 771, 704; δH (400 MHz, CDCl3) 7.35–7.22 (5H, m), 7.09–7.07 (2H, m), 6.98–6.94 (2H, m), 4.04 (1H, q, J = 7.1 Hz), 3.85 (3H, s), 3.63 (1H, dd, J = 9.1 and 4.5 Hz), 3.32 (1H, ddd, J = 12.0 Hz, 5.7 Hz, 3.9 Hz), 2.66–2.36 (4H, m), 2.21 (1H, td, J = 11.5 Hz, 3.3 Hz), 1.43 (3H, d, J = 7.1 Hz); δC (100.6 MHz, CDCl3) 209.01 (C=O), 159.01, 139.25, 135.00, 128.74, 128.42, 128.04, 127.29, 114.40, 63.92, 56.42, 55.42, 50.65, 44.51, 41.85, 19.64; m/z (ESI+) 342 [M + H + CH3OH]+, 310 [M + H]+, 206, 177, 134, 105; Exact mass calcd for C20H24NO2 [M + H]+ requires m/z 310.1802 found 310.1804 (NSI+).
S,Z-4-(Methoxymethylene)-2-methyl-1-(S-1-phenylethyl)piperidine (9a) and S,E-4-(Methoxymethylene)-2-methyl-1-(S-1-phenylethyl)piperidine (9′a)
(Methoxymethyl)triphenylphosphonium chloride (1.419 g, 4.14 mmol, 1.5 equiv) and molecular sieves were placed in an oven-dried flask and added with absolute THF (8 mL) under an atmosphere of nitrogen. The mixture was cooled to −78 °C and a 2 M solution of lithium diisopropylamide (LDA) in THF/heptane/ethylbenzene (2.07 mL, 4.14 mmol, 1.5 equiv) was added slowly. The mixture was stirred at −78 °C for 5 min and then allowed to warm to room temperature while stirring for another 20 min. The reaction mixture was cooled to −20 °C and a solution of 2a (600 mg, 2.76 mmol, 1.0 equiv) in absolute THF (7 mL) was added slowly. The mixture was stirred at −20 °C for 15 min, then allowed to warm to room temperature and stirred for 16 h. 1 M NH4Cl solution (12 mL) and ethyl acetate (25 mL) were added, and the solution was stirred vigorously for 5 min. The phases were separated, and the aqueous phase was again extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over MgSO4, filtered, and the solvent was removed under reduced pressure. Purification by column chromatography (hexane/ethyl acetate, 10:1) afforded 9a and 9′a as a colorless oil (607 mg, 2.48 mmol) in a combined yield of 90%. Several pure fractions of 9a and 9′a could be obtained and were used for spectral analysis. The other fractions contained a mixture of the two diastereoisomers. NMR investigation of the crude residue indicated that the mixture of diastereoisomers was a 1.21:1 ratio of 9a:9′a.
Compound 9a, eluting first: [α]D24 −51.4 (c 0.18, CHCl3); Rf 0.19 (1:10, ethyl acetate/hexane); νmax (film) 2968, 2931, 2833, 1689, 1601, 1492, 1454, 1370, 1315, 1228, 1210, 1126, 1029, 977, 766; δH (400 MHz, CDCl3) 7.44–7.42 (2H, m), 7.32–7.29 (2H, m), 7.22–7.19 (1H, m), 5.80 (1H, s), 3.94 (1H, q, J = 6.7 Hz), 3.53 (3H, s), 3.00–2.93 (1H, m), 2.47 (1H, dd, J = 13.4 Hz, 3.8 Hz), 2.37 (1H, ddd, J = 11.3 Hz, 7.1 Hz, 4.1 Hz), 2.22 (1H, ddd, J = 11.3 Hz, 7.2 Hz, 4.0 Hz), 2.11 (1H, dd, J = 13.4 Hz, 6.8 Hz), 1.93–1.87 (1H, m), 1.85–1.79 (1H, m), 1.26 (3H, d, J = 6.7 Hz), 1.09 (3H, d, J = 6.3 Hz); δC (100.6 MHz, CDCl3) 146.23, 139.97, 128.17, 127.64, 126.47, 114.97, 59.48, 57.68, 51.53, 46.07, 33.36, 30.06, 15.54, 15.09; m/z (ESI+) 246 [M + H]+, 142; Exact mass calcd for C16H24NO [M + H]+ requires m/z 246.1852 found 246.1854 (NSI+).
Compound 9′a, eluting second: [α]D24 −29.2 (c 0.15, CHCl3); Rf 0.13 (1:10, ethyl acetate/hexane); νmax (film) 2968, 2931, 2825, 1692, 1601, 1492, 1453, 1371, 1311, 1265, 1230, 1211, 1186, 1125, 1028, 766; δH (400 MHz, CDCl3) 7.44–7.42 (2H, m), 7.34–7.29 (2H, m), 7.24–7.20 (1H, m), 5.75 (1H, s), 3.91 (1H, q, J = 6.7 Hz), 3.53 (3H, s), 3.00–2.92 (1H, m), 2.36 (1H, ddd, J = 11.5 Hz, 7.3 Hz, 4.3 Hz), 2.25–2.20 (2H, m), 2.17–2.11 (1H, m), 2.08–2.02 (1H, m), 1.85 (1H, dd, J = 13.2 Hz, 6.5 Hz), 1.26 (3H, d, J = 6.7 Hz), 1.06 (3H, d, J = 6.4 Hz); δC (100.6 MHz, CDCl3) 146.24, 139.77, 128.20, 127.61, 126.49, 114.69, 59.51, 57.83, 52.18, 44.65, 37.76, 25.50, 15.42, 15.18; m/z (ESI+) 246 [M + H]+, 142; Exact mass calcd for C16H24NO [M + H]+ requires m/z 246.18524 found 246.18577 (ESI+).
R,E-4-(Methoxymethylene)-2-methyl-1-(S-1-phenylethyl)piperidine (9′b) and R,Z-4-(Methoxymethylene)-2-methyl-1-(S-1-phenylethyl)piperidine (9b)
(Methoxymethyl)triphenylphosphonium chloride (473 mg, 1.38 mmol, 1.5 equiv) and molecular sieves were placed in an oven-dried flask and added with absolute THF (3 mL) under an atmosphere of nitrogen. The mixture was cooled to −78 °C and a 2 M solution of lithium diisopropylamide in THF/heptane/ethylbenzene (690 μL, 1.38 mmol, 1.5 equiv) was added slowly. The mixture was stirred at −78 °C for 5 min and then allowed to warm to room temperature while stirring for another 20 min. The reaction mixture was cooled to −20 °C and a solution of 2′a (200 mg, 0.92 mmol, 1.0 equiv) in absolute THF (2 mL) was added slowly. The mixture was stirred at −20 °C for 15 min, then allowed to warm to room temperature, and stirred for 16 h. 1 M NH4Cl solution (5 mL) and ethyl acetate (10 mL) were added, and the solution was stirred vigorously for 5 min. The phases were separated, and the aqueous phase was again extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over MgSO4, filtered, and the solvent was removed under reduced pressure. Purification by column chromatography (hexane/ethyl acetate, 1.3:1) afforded 9b and 9′b as a colorless oil (170 mg, 0.69 mmol) in a combined yield of 75%. Several pure fractions of 9′b and 9b could be obtained and were used for spectral analysis, and the other fractions contained a mixture of the two diastereoisomers. NMR investigation of the crude residue indicated that the mixture of diastereoisomers was a 1:1.23 ratio of 9′b:9b.
Product 9′b, eluting first: [α]D24 −58.4 (c 0.29, CHCl3); Rf 0.29 (1:1, ethyl acetate/hexane); νmax (film) 2970, 2931, 2828, 1693, 1492, 1453, 1371, 1230, 1211, 1125, 768; δH (400 MHz, CDCl3) 7.32–7.20 (5H, m), 5.67 (1H, s), 3.90 (1H, q, J = 6.8 Hz), 3.51 (3H, s), 2.69 (1H, ddd, J = 11.6 Hz, 7.4 Hz, 4.7 Hz), 2.56 (1H, dq, J = 12.2 Hz, 6.1 Hz), 2.44–2.39 (1H, m), 2.32–2.28 (2H, m), 2.12 (1H, dd, J = 13.3 Hz, 4.1 Hz), 1.73 (1H, dd, J = 13.2 Hz, 6.0 Hz), 1.38 (1H, d, 3H, J = 6.8 Hz), 1.00 (1H, d, 3H, J = 6.3 Hz); δC (100.6 MHz, CDCl3) 143.63, 139.79, 128.15, 127.87, 126.75, 113.89, 59.48, 58.59, 52.43, 43.87, 37.56, 25.63, 21.29, 14.43; m/z (ESI+) 246 [M + H]+, 142; Exact mass calcd for C16H24NO [M + H]+ requires m/z 246.1852 found 246.1854 (NSI+).
Product 9b, eluting second: [α]D24 −126.05 (c 0.12, CHCl3); Rf 0.23 (1:1, ethyl acetate/hexane); νmax (film) 2969, 2929, 2833, 1692, 1492, 1453, 1372, 1229, 1193, 1124, 768; δH (400 MHz, CDCl3) 7.33–7.20 (5H, m), 5.79 (1H, s), 3.95 (1H, q, J = 6.8 Hz), 3.49 (s, 3H), 2.73 (1H, ddd, J = 11.4 Hz, 7.7 Hz, 4.0 Hz), 2.59–2.52 (1H, m), 2.37 (1H, ddd, J = 11.1 Hz, 6.7 Hz, 4.2 Hz), 2.29 (1H, dd, J = 13.5 Hz, 3.5 Hz), 2.13–1.98 (3H, m), 1.38 (3H, d, J = 6.8 Hz), 1.04 (3H, d, J = 6.3 Hz); δC (100.6 MHz, CDCl3) 143.39, 140.04, 128.15, 127.91, 126.78, 114.28, 59.43, 58.31, 51.93, 45.37, 33.18, 30.17, 21.15, 15.03; m/z (ESI+) 246 [M + H]+, 142; Exact mass calcd for C16H24NO [M + H]+ requires m/z 246.18524 found 246.18566 (ESI+).
R,E-4-(Methoxymethylene)-2-phenyl-1-(S-1-phenylethyl)piperidine (9′c) and R,Z-4-(Methoxymethylene)-2-phenyl-1-(S-1-phenylethyl)piperidine (9c)
(Methoxymethyl)triphenylphosphonium chloride (1.028 g, 3.00 mmol, 1.5 equiv) and molecular sieves were placed in an oven-dried flask and added with absolute THF (6 mL) under an atmosphere of nitrogen. The mixture was cooled to −70 °C, and a 2 M solution of lithium diisopropylamide in THF/heptane/ethylbenzene (1.500 mL, 3.00 mmol, 1.5 equiv) was added slowly. The mixture was stirred at −70 °C for 5 min and then allowed to warm to room temperature while stirring for another 20 min. The reaction mixture was cooled to −20 °C, and a solution of 2c (558 mg, 2.00 mmol, 1.0 equiv) in absolute THF (4 mL) was added slowly. The mixture was stirred at −20 °C for 15 min, then allowed to warm to room temperature and stirred for 16 h. 1 M NH4Cl solution (8 mL) and ethyl acetate (15 mL) were added, and the solution was stirred vigorously for 5 min. The phases were separated, and the aqueous phase was again extracted with ethyl acetate (2 × 15 mL). The combined organic layers were washed with water and brine, dried over MgSO4, filtered, and the solvent was removed under reduced pressure. Purification by column chromatography (ethyl acetate/hexane, 1:36 → 1:28) afforded 9′c (128 mg, 21%) as a colorless oil, a mixture of 9′c and 9c (392 mg, 64%) as a colorless oil, and 9c (72 mg, 12%) as a colorless oil. NMR investigation of the crude residue indicated that the mixture of diastereoisomers was a 1.03:1 ratio of 9′c:9c.
Product 9′c, eluting first: [α]D23 −69.8 (c 0.94, CHCl3); Rf 0.31 (1:20, ethyl acetate/hexane); νmax (film) 3060, 3027, 2967, 2931, 2901, 2814, 1692, 1493, 1452, 1372, 1228, 1211, 1126, 1029, 761, 732; δH (400 MHz, CDCl3) 7.50–7.46 (4H, m), 7.36–7.29 (4H, m), 7.27–7.18 (2H, m), 5.80 (1H, s), 3.85 (1H, q, J = 6.8 Hz), 3.55 (3H, s), 3.47 (1H, dd, J = 10.7 Hz, 3.2 Hz), 2.66–2.60 (2H, m), 2.34–2.28 (1H, m), 2.19 (1H, td, J = 11.6 Hz, J = 3.0 Hz), 2.14–2.10 (1H, m), 1.86 (1H, td, J = 13.1 Hz, 4.9 Hz), 1.16 (3H, d, J = 6.8 Hz); δC (100.6 MHz, CDCl3) 144.71, 144.41, 139.55, 128.73, 127.96, 127.69, 127.60, 127.28, 126.29, 115.95, 67.01, 59.58, 54.96, 45.16, 40.69, 25.83, 8.50; m/z (ESI+) 308 [M + H]+, 204, 172; Exact mass calcd for C21H26NO [M + H]+ requires m/z 308.2009 found 308.2009 (NSI+).
Product 9c, eluting second: [α]D24 −149.6 (c 0.12, CHCl3); Rf 0.28 (1:20, ethyl acetate/hexane); νmax (film) 2931, 2833, 1692, 1600, 1492, 1451, 1378, 1245, 1225, 1204, 1127, 1029, 975, 774, 758; δH (400 MHz, CDCl3) 7.52–7.46 (4H, m), 7.36–7.28 (4H, m), 7.27–7.18 (2H, m), 5.81 (1H, t, J = 1.6 Hz), 3.87 (1H, q, J = 6.8 Hz), 3.54 (3H, s), 3.49 (1H, dd, J = 10.9 Hz, 3.3 Hz), 2.86 (1H, ddd, J = 13.6 Hz, 2.8 Hz, 2.0 Hz), 2.65 (1H, ddd, J = 10.5 Hz, 3.9 Hz, 2.9 Hz), 2.19 (1H, “dt”, J = 11.8 Hz, 2.6 Hz), 2.12–2.04 (2H, m), 1.93–1.88 (1H, m), 1.17 (3H, d, J = 6.8 Hz); δC (100.6 MHz, CDCl3) 144.81, 144.51, 128.65, 127.94, 127.75, 127.62, 127.18, 126.27, 115.72, 65.41, 59.52, 54.98, 46.37, 35.81, 30.11, 8.63; m/z (ESI+) 308 [M + H]+, 204, 172; Exact mass calcd for C21H26NO [M + H]+ requires m/z 308.2009 found 308.2010 (NSI+).
S,E-4-(Methoxymethylene)-2-phenyl-1-(S-1-phenylethyl)piperidine (9′d) and S,Z-4 (Methoxymethylene)-2-phenyl-1-(S-1-phenylethyl)piperidine (9d)
(Methoxymethyl)triphenylphosphonium chloride (922 mg, 2.69 mmol, 1.5 equiv) and molecular sieves were placed in an oven-dried flask and added with absolute THF (6 mL) under an atmosphere of nitrogen. The mixture was cooled to −78 °C, and a 2 M solution of lithium diisopropylamide in THF/heptane/ethylbenzene (1.345 mL, 2.69 mmol, 1.5 equiv) was added slowly. The mixture was stirred at −78 °C for 5 min and then allowed to warm to room temperature while stirring for another 20 min. The reaction mixture was cooled to −20 °C and a solution of 2′c (500 mg, 1.79 mmol, 1.0 equiv) in absolute THF (4 mL) was added slowly. The mixture was stirred at −20 °C for 15 min, then allowed to warm to room temperature and stirred for 16 h. 1 M NH4Cl solution (8 mL) and ethyl acetate (15 mL) were added, and the solution was stirred vigorously for 5 min. The phases were separated, and the aqueous phase was again extracted with ethyl acetate (2 × 15 mL). The combined organic layers were washed with water and brine, dried over MgSO4, filtered, and the solvent was removed under reduced pressure. Purification by column chromatography (ethyl acetate/hexane, 1:28 →1:10) afforded product 9′d (20 mg, 4%) as a colorless oil, a mixture of 9′d and 9d (304 mg, 55%, ratio of 9′d:9d was 1.62:1) as a colorless oil, and 9d (104 mg, 19%) as a colorless oil. NMR investigation of the crude residue indicated that the mixture of diastereoisomers was a 1.06:1 ratio of 9d:9′d.
Product 9′d, eluting first: [α]D23 −116.4 (c 0.19, CHCl3); Rf 0.42 (1:8, ethyl acetate/hexane); νmax (film) 2823, 1692, 1492, 1453, 1227, 1122, 944, 822, 759, 701; δH (400 MHz, CDCl3) 7.45–7.19 (8H, m), 7.02–7.00 (2H, m), 5.66 (1H, s), 3.96 (1H, q, J = 7.1 Hz), 3.47 (3H, s), 3.17 (1H, ddd, J = 10.9 Hz, 4.4 Hz, 2.9 Hz), 3.11 (1H, dd, J = 10.5 Hz, 3.5 Hz), 2.72–2.67 (1H, m), 2.16–2.09 (1H, m), 2.04–1.96 (2H, m), 1.75 (1H, td, J = 12.2 Hz, 2.9 Hz), 1.39 (3H, s, J = 7.2 Hz); δC (100.6 MHz, CDCl3) 145.33, 139.30, 139.04, 128.93, 128.75, 127.86, 127.64, 127.03, 126.85, 115.42, 67.20, 59.46, 56.76, 45.87, 41.47, 25.93, 18.98; m/z (ESI+) 308 [M + H]+, 204, 172; Exact mass calcd for C21H26NO [M + H]+ requires m/z 308.20089 found 308.20020 (ESI+).
Product 9d, eluting second: [α]D24 −148.2 (c 0.16, CHCl3); Rf 0.36 (1:8, ethyl acetate/hexane); νmax (film) 2833, 1692, 1492, 1453, 1225, 1196, 1124, 912, 838, 757, 700; δH (400 MHz, CDCl3) 7.48–7.19 (8H, m), 7.02 (2H, d, J = 7.2 Hz), 5.69 (1H, s), 3.99 (1H, q, J = 7.1 Hz), 3.45 (3H, s), 3.20–3.13 (2H, m), 2.75–2.70 (1H, m), 2.20 (1H, ddd, J = 12.8 Hz, 4.1 Hz, 2.3 Hz), 2.03–1.98 (1H, m), 1.95–1.89 (1H, m), 1.74 (1H, td, J = 11.6 Hz, 2.9 Hz), 1.39 (3H, d, J = 7.1 Hz); δC (100.6 MHz, CDCl3) 145.41, 139.24, 139.13, 128.90, 128.69, 127.90, 127.63, 126.93, 126.85, 115.19, 65.63, 59.40, 56.67, 47.08, 36.59, 30.15, 18.98; m/z (ESI+) 308 [M + H]+, 204, 172; Exact mass calcd for C21H26NO [M + H]+ requires m/z 308.20089 found 308.20013 (ESI+).
2S,4R-2-Methyl-1-(S-1-phenylethyl)piperidine-4-carbaldehyde (3a) and 2S,4S-2-Methyl-1-(S-1-phenylethyl)piperidine-4-carbaldehyde (3′a)
1.6 N HCl (2.4 mL) was added to a solution of a diastereomeric mixture of 9a and 9′a (ratio of 9a:9′a was 1:1.70) (274 mg, 1.12 mmol) in THF (2.4 mL) and stirred at 45 °C for 2 h. THF was evaporated under reduced pressure, and the residue was diluted with water (10 mL). The pH was adjusted to 10 by the addition of an aqueous Na2CO3 solution. The aqueous phase was extracted with CH2Cl2 (3 × 15 mL), and the combined organic layers were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure, and crude aldehydes 3a and 3′a were obtained as a colorless oil (253 mg, 97%). The products could be used for the next step without the need of further purification. NMR investigation indicates that the mixture of diastereoisomers is a 1:1.29 ratio of 3a:3′a. NOe analysis suggests that compound 3′a is the major product and compound 3a is the minor compound (labeled as M).
Products 3a and 3′a as a mixture. Product 3a is labeled as M. Rf 0.08 (1:3, ethyl acetate/hexane); νmax (film) 2968, 2935, 2803, 1724, 1601, 1493, 1446, 1376, 1331, 1286, 1267, 1194, 1145, 1073, 912, 782, 765; δH (400 MHz, CDCl3) 9.65 (1H, d, J = 1.2 Hz, HM), 9.59 (1H, d, J = 1.6 Hz), 7.46–7.44 (2H, m), 7.37–7.28 (6H, m, 4 × Ar-HM), 7.24–7.20 (2H, m, 1 × Ar-HM), 4.35 (1H, q, J = 6.8 Hz), 3.71 (1H, q, J = 6.6 Hz, HM), 3.37–3.29 (1H, m, HM), 2.64 (1H, dqd, J = 12.4 Hz, 6.2 Hz, 2.6 Hz), 2.56–2.45 (2H, m, 1 × HM), 2.40–2.22 (3H, m, 2 × HM, 1 × H-5), 2.12 (1H, “td”, J = 11.7 Hz, 2.5 Hz), 1.97–1.87 (2H, m, 1 × HM), 1.76–1.54 (4H, m, 3 × HM), 1.45–1.31 (2H, m), 1.27–1.24 (9H, m, 3 × HM), 1.09 (3H, d, J = 6.6 Hz, 3 × HM); δC (100.6 MHz, CDCl3) 205.15 (CM), 204.18, 146.41 (ArM), 144.49, 128.36, 128.04, 127.86, 127.39, 126.72 (ArM), 126.46, 59.43 (CM), 54.35, 52.85, 49.64, 48.25 (CM), 44.45 (CM), 44.00, 42.48 (CM), 35.05, 32.18 (CM), 26.15, 25.51 (CM), 20.70, 18.43 (CM), 12.38 (CM), 8.55; m/z (ESI+) 264 [M + H + CH3OH]+, 232 [M + H]+, 128, 105; Exact mass calcd for C15H22NO [M + H]+ requires m/z 232.1696 found 232.17028 (ESI+).
2R,4R-2-Methyl-1-(S-1-phenylethyl)piperidine-4-carbaldehyde (3b) and 2R,4S-2-Methyl-1-(S-1-phenylethyl)piperidine-4-carbaldehyde (3′b)
1.6 N HCl (850 μL) was added to a solution of a diastereomeric mixture of 9b and 9′b (ratio of 9b:9′b was 1.16:1) (94 mg, 0.38 mmol) in THF (850 μL) and stirred at 45 °C for 2 h. THF was evaporated under reduced pressure, and the residue was diluted with water (4 mL). The pH was adjusted to 10 by the addition of an aqueous Na2CO3 solution. The aqueous phase was extracted with CH2Cl2 (3 × 6 mL), and the combined organic layers were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure, and crude aldehydes 3b and 3′b were obtained as a colorless oil (87 mg, 100%). The products could be used for the next step without the need of further purification. NMR investigation indicates that the mixture of diastereoisomers is a 1:1.85 ratio of 3′b:3b. NOe analysis suggests that compound 3b is the major product and compound 3′b is the minor compound (labeled as M).
Products 3b and 3′b as a mixture. Product 3′b is labeled as M. Rf 0.10 (2:1, ethyl acetate/hexane); νmax (film) 3027, 2971, 2932, 2805, 2741, 1724, 1493, 1452, 1376, 1278, 1070, 762, 703; δH (400 MHz, CDCl3) 9.60 (1H, d, J = 1.36 Hz, HM), 9.55 (1H, d, J = 1.42 Hz), 7.35–7.20 (10H, m, 5 × Ar-HM), 4.33 (1H, q, J = 7.1 Hz), 3.72 (1H, q, J = 6.5 Hz, HM), 3.15 (1H, dt, J = 11.5 Hz, 3.7 Hz), 2.94–2.87 (1H, m, HM), 2.80 (1H, dt, J = 11.5 Hz, 4.3 Hz, 1 × HM), 2.57 (1H, dt, J = 11.7 Hz, 3.0 Hz, 1 × HM), 2.50–2.42 (1H, m, HM), 2.21 (1H, dqd, J = 12.0 Hz, 6.0 Hz, 2.9 Hz), 2.08 (1H, ttd, J = 12.2 Hz, 3.9 Hz, 1.5 Hz), 1.91–1.70 (5H, m, 3 × HM), 1.62–1.43 (5H, m, 1 × HM), 1.41–1.24 (7H, m, 3 × HM), 0.97 (3H, d, J = 6.6 Hz, HM); δC (100.6 MHz, CDCl3) 204.97 (CM), 203.86, 144.90 (ArM), 139.48, 128.46, 128.40, 127.94, 127.42, 127.04, 126.89, 59.67 (CM), 55.86, 52.93, 48.93 (CM), 44.95, 43.89 (CM), 41.03 (CM), 34.93, 32.14 (CM), 26.20, 25.76 (CM), 22.03 (CM), 21.13, 19.09, 11.30 (CM); m/z (ESI+) 264 [M + H + CH3OH]+, 232 [M + H]+, 128, 105; Exact mass calcd for C15H22NO [M + H]+ requires m/z 232.1696 found 232.1696 (NSI+).
2R,4R-2-Phenyl-1-(S-1-phenylethyl)piperidine-4-carbaldehyde (3c) and 2R,4S-2-Phenyl-1-(S-1-phenylethyl)piperidine-4-carbaldehyde (3′c)
1.6 N HCl (2.8 mL) was added to a solution of a diastereomeric mixture of 9c and 9′c (ratio of 9c:9′c was 1.32:1) (365 mg, 1.19 mmol) in THF (2.8 mL) and stirred at 45 °C for 3 h. THF was evaporated under reduced pressure, and the residue was diluted with water (10 mL). The pH was adjusted to 10 by the addition of an aqueous Na2CO3 solution. The aqueous phase was extracted with CH2Cl2 (3 × 15 mL), and the combined organic layers were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure, and crude aldehydes 3c and 3′c were obtained as a colorless oil (349 mg, 100%). The products could be used for the next step without the need of further purification. NMR investigation indicates that the mixture of diastereoisomers is a 1:1.33 ratio of 3c:3′c. NOe analysis indicates that compound 3′c is the major product and compound 3c is the minor compound (labeled as M).
Products 3c and 3′c as a mixture. Product 3c is labeled as M. Rf 0.13 (1:20, ethyl acetate/hexane); νmax (film) 2968, 2938, 2808, 2712, 1723, 1601, 1492, 1446, 1384, 1365, 1266, 1204, 1129, 1071, 1025, 953, 910, 760; δH (400 MHz, CDCl3) 9.82 (1H, s, HM), 9.61 (1H, d, J = 1.5 Hz), 7.51–7.18 (20H, m, 10 × Ar-HM), 3.89 (1H, q, J = 6.8 Hz), 3.83 (1H, q, J = 6.9 Hz, HM), 3.63–3.58 (2H, m, 1 × HM), 2.72 (1H, dt, J = 11.6 Hz, 3.4 Hz), 2.63–2.60 (1H, m, HM), 2.50 (1H, dt, J = 12.0 Hz, 3.6 Hz, 1 × HM), 2.43–2.23 (4H, m, 2 × HM), 2.15–2.09 (1H, m, 1 × HM), 2.08–1.95 (2H, m, 1 × HM), 1.89–1.79 (2H, m, 1 × HM), 1.76–1.67 (1H, m), 1.58–1.48 (1H, m), 1.20 (3H, d, J = 6.8 Hz), 1.13 (3H, d, J = 6.8 Hz, HM); δC (100.6 MHz, CDCl3) 205.17 (CM), 203.58, 144.44 (CM), 144.19, 144.10 (CM), 143.81, 128.89, 128.81, 128.02, 128.00, 127.64, 127.59, 127.55, 127.39, 126.44, 126.40, 64.48, 61.89 (CM), 55.19 (CM), 54.82, 49.86, 46.16 (CM), 44.01, 41.86 (CM), 36.13, 34.73 (CM), 26.05, 24.97(CM), 8.88 (CM), 8.20; m/z (ESI+) 326 [M + H + CH3OH]+, 294 [M + H]+, 190; Exact mass calcd for C20H24NO [M + H]+ requires m/z 294.1852 found 294.1850 (NSI+).
2S,4R-2-phenyl-1-(S-1-Phenylethyl)piperidine-4-carbaldehyde (3d) and 2S,4S-2-Phenyl-1-(S-1-phenylethyl)piperidine-4-carbaldehyde (3′d)
1.6 N HCl (3 mL) was added to a solution of a diastereomeric mixture of 9d and 9′d (ratio of 9d:9′d was 1.10:1) (395 mg, 1.29 mmol) in THF (3 mL) and stirred at 45 °C for 2 h. THF was evaporated under reduced pressure, and the residue was diluted with water (10 mL). The pH was adjusted to 10 by the addition of an aqueous Na2CO3 solution. The aqueous phase was extracted with CH2Cl2 (3 × 20 mL), and the combined organic layers were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure, and crude aldehydes 3d and 3′d were obtained as a colorless oil (379 mg, 100%). The products could be used for the next step without the need of further purification. NMR investigation indicates that the mixture of diastereoisomers is a 2.04:1 ratio of 3d:3′d. NOe analysis indicates that compound 3d is the major product and compound 3′d is the minor compound (labeled as M).
Products 3d and 3′d as a mixture. Product 3′d is labeled as M. Rf 0.16 (1:8, ethyl acetate/hexane); νmax (film) 2809, 1720, 1492, 1453, 1374, 959, 911, 759, 734, 701; δH (400 MHz, CDCl3) 9.62 (1H, s, HM), 9.53 (1H, s), 7.46–7.18 (16H, m, 8 × ArM), 7.03 (2H, d, J = 7.8 Hz), 6.98 (2H, d, J = 7.7 Hz, 2 × ArM), 3.99 (1H, q, J = 7.0 Hz), 3.93 (1H, q, J = 7.0 Hz, HM), 3.31–3.24 (2H, m, 1 × HM), 3.20 (1H, dd, J = 11.1 Hz, 2.3 Hz), 3.01 (1H, dt, J = 11.8 Hz, 3.5 Hz, 1 × HM), 2.50–2.46 (1H, m, HM), 2.22–2.10 (3H, m, 1 × HM), 1.97–1.80 (6H, m, 3 × HM), 1.66–1.44 (2H, m), 1.38 (3H, d, J = 7.2 Hz), 1.35 (3H, d, J = 7.1 Hz, 3 × HM); δC (100.6 MHz, CDCl3) 204.81 (CM), 203.38, 144.97, 144.85, 139.13 (ArM), 138.33, 128.95, 128.93, 128.83, 128.63, 127.83, 127.79, 127.73, 127.35, 127.14, 127.08, 127.04, 64.73, 62.12 (CM), 56.87 (CM), 56.56, 49.35, 45.65 (CM), 44.68, 42.78 (CM), 37.14, 35.36 (CM), 26.14, 25.15 (CM), 19.14 (CM), 18.66; m/z (ESI+) 326 [M + H + CH3OH]+, 294 [M + H]+, 190; Exact mass calcd for C20H24NO [M + H]+ requires m/z 294.18524 found 294.18498 (ESI+).
E-5,6-Dimethoxy-2-((2S,4S-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methylene)-2,3-dihydro-1H-inden-1-one (11a) and E-5,6-Dimethoxy-2-((2S,4R-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)methylene)-2,3-dihydro-1H-inden-1-one (11′a)
5,6-Dimethoxy-1-indanone (349 mg, 1.82 mmol, 1.0 equiv) was added to a solution of a mixture of 3a and 3′a (420 mg, 1.82 mmol, 1.0 equiv, ratio of 3a:3′a was 1:1.42) in methanol (10 mL), and the system was put under an atmosphere of nitrogen. The mixture was heated to 80 °C and a sodium methoxide solution (28% in methanol, 382 μL, 2.18 mmol, 1.2 equiv) was added. Stirring under reflux was continued for 90 min, then the reaction was allowed to cool to room temperature. The solvent was evaporated under reduced pressure, and the residue was dissolved in CH2Cl2 (25 mL). Water (15 mL) was added, and the phases were separated. The aqueous phase was extracted again with CH2Cl2 (2 × 20 mL), and the organic phases were combined and washed with brine and dried over MgSO4. The solvent was evaporated under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 3:2 → 100% ethyl acetate) afforded product 11a (368 mg, 50%) as white crystals, a mixture of 11a and 11′a (129 mg, 18%) as white crystals, and 11′a (144 mg, 19%) as pale yellow crystals. NMR analysis of the crude residue indicated that the mixture of diastereomers was a 1.63:1 ratio of 11a:11′a. NOe analysis of the purified products indicated the correct stereochemistry.
Product 11a, eluting first: [α]D21 11.2 (c 0.22, CHCl3); mp 84–87 °C; Rf 0.30 (3:1, ethyl acetate/hexane); νmax (solid) 2911, 1694, 1652, 1606, 1591, 1502, 1427, 1367, 1307, 1251, 1214, 1130, 1071, 997, 802, 761, 720, 697; δH (400 MHz, CDCl3) 7.49–7.47 (2H, m), 7.35–7.20 (4H, m), 6.90 (1H, s, H-5), 6.60 (1H, dt, J = 9.6 Hz, 1.8 Hz), 4.38 (1H, q, J = 6.8 Hz), 3.97 (3H, s), 3.92 (3H, s), 3.58 (2H, s), 2.69–2.61 (1H, m), 2.49 (1H, dt, J = 11.4 Hz, 3.3 Hz), 2.39–2.35 (1H, m), 2.17 (1H, dt, J = 11.7 Hz, 2.2 Hz), 1.73–1.68 (1H, m), 1.56–1.50 (1H, m), 1.45–1.32 (2H, m), 1.29–1.21 (6H, m); δC (100.6 MHz, CDCl3) 192.82, 155.35, 149.58, 144.65, 144.55, 140.33, 135.36, 132.03., 128.01, 127.96, 126.41, 107.35, 105.12, 56.37, 56.27, 54.29, 53.23, 44.39, 41.11, 38.68, 31.87, 29.62, 20.91, 8.15; m/z (ESI+) 406 [M + H]+, 302; Exact mass calcd for C26H32NO3 [M + H]+ requires m/z 406.23767 found 406.23728 (ESI+); HPLC tR1 = 14.1 min, tR2 = 16.9 min, tR3 = 31.2 min.
Product 11′a, eluting second: [α]D20 −95.0 (c 0.50, CHCl3); mp 66–69 °C; Rf 0.16 (3:1, ethyl acetate/hexane); νmax (solid) 2927, 1693, 1648, 1604, 1587, 1499, 1454, 1369, 1302, 1253, 1216, 1124, 1084, 996, 913, 801, 763, 727, 700; δH (400 MHz, CDCl3) 7.19–7.38 (6H, m), 6.90 (1H, s), 6.68 (1H, dt, J = 9.4 Hz, 1.7 Hz), 3.96 (3H, s), 3.92 (3H, s), 3.64–3.59 (3H, m), 3.51–3.44 (1H, m), 2.69–2.59 (1H, m), 2.46 (1H, dt, J = 12.2 Hz, 4.0 Hz), 2.33 (1H, dt, J = 11.7 Hz, 3.0 Hz), 1.87–1.80 (1H, m), 1.63–1.58 (1H, m), 1.55–1.50 (1H, m), 1.48–1.38 (1H, m), 1.30 (3H, d, J = 6.6 Hz), 1.11 (3H, d, J = 6.6 Hz); δC (100.6 MHz, CDCl3) 192.80, 155.38, 149.60, 144.65, 140.56, 135.61, 132.04, 128.43, 127.39, 126.76, 107.35, 105.15, 60.57, 56.38, 56.29, 47.94, 42.96, 37.59, 32.35, 31.28, 29.62, 20.28, 10.99; m/z (ESI+) 406 [M + H]+, 302; Exact mass calcd for C26H32NO3 [M + H]+ requires m/z 406.23767 found 406.23770 (ESI+).
E-5,6-Dimethoxy-2-((2R,4S-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)methylene)-2,3-dihydro-1H-inden-1-one (11b) and E-5,6-Dimethoxy-2-((2R,4R-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)methylene)-2,3-dihydro-1H-inden-1-one (11′b)
5,6-Dimethoxy-1-indanone (340 mg, 1.77 mmol, 1.0 equiv) was added to a solution of a mixture of 3b and 3′b (410 mg, 1.77 mmol, 1.0 equiv, ratio of 3b:3′b was 2:1) in methanol (10 mL), and the system was put under an atmosphere of nitrogen. The mixture was heated to 80 °C, and a sodium methoxide solution (28% in methanol, 372 μL, 2.12 mmol, 1.2 equiv) was added. Stirring under reflux was continued for 90 min; then, the reaction was allowed to cool to room temperature. The solvent was evaporated under reduced pressure, and the residue was dissolved in CH2Cl2 (25 mL). Water (15 mL) was added and the phases were separated. The aqueous phase was extracted again with CH2Cl2 (2 × 20 mL), and the organic phases were combined and washed with brine and dried over MgSO4. The solvent was evaporated under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 6:1 → 100% ethyl acetate) afforded product 11b (64 mg, 9%) as pale yellow crystals, a mixture of 11b and 11′b (121 mg, 17%, 11b:11′b 1.36:1) as white crystals, and a mixture of 11b and 11′b (274 mg, 38%, 11b:11′b, 1:5.39) as white crystals. NMR analysis of the crude residue indicated that the mixture of diastereomers was a 1:1.68 ratio of 11b:11′b. NOe analysis of the purified products indicated the correct stereochemistry.
Product 11b, eluting first: [α]D22 −177.6 (c 0.11, CHCl3); mp 66–68 °C; Rf 0.28 (19:1.5, ethyl acetate:methanol); νmax (solid) 1693, 1648, 1605, 1587, 1500, 1454, 1303, 1253, 1217, 1127, 1080, 996, 801, 763; δH (400 MHz, CDCl3) 7.37–7.21 (6H, m), 6.90 (1H, s), 6.63 (1H, dt, J = 9.5 Hz, 2.0 Hz), 3.97 (3H, s), 3.92 (3H, s), 3.67–3.58 (3H, m), 2.99–2.93 (2H, m), 2.66–2.52 (2H, m), 1.73–1.64 (3H, m), 1.51–1.37 (1H, m), 1.32 (3H, d, J = 5.8 Hz), 0.98 (3H, d, J = 5.9 Hz); δC (100.6 MHz, CDCl3) 192.76 (C=O), 155.36, 149.58, 145.66, 144.63, 140.69, 135.55, 132.02, 128.45, 127.40, 126.84, 107.34, 105.13, 60.36, 56.37, 56.28, 48.86, 41.36, 37.74, 31.99, 31.73, 29.61, 22.57, 10.16; m/z (ESI+) 406 [M + H]+, 302; Exact mass calcd for C26H32NO3 [M + H]+ requires m/z 406.2377 found 406.2376 (NSI+).
Product 11′b, eluting second: [α]D22 −62.5 (c 0.19, CHCl3); mp 76–78 °C; Rf 0.23 (19:1.5, ethyl acetate:methanol); νmax (film) 1693, 1648, 1605, 1588, 1499, 1454, 1302, 1254, 1216, 1129, 1080, 996, 748, 702, 665; δH (400 MHz, CDCl3) 7.37–7.23 (6H, m), 6.84 (1H, s), 6.56 (1H, dt, J = 9.7 Hz, 2.0 Hz), 4.39 (1H, q, J = 6.7 Hz), 3.95 (3H, s), 3.91 (3H, s), 3.47 (2H, d, J = 1.9 Hz), 3.15 (1H, app d, J = 11.3 Hz), 2.23–2.14 (2H, m), 1.86 (1H, app t, J = 11.8 Hz), 1.71–1.37 (7H, m), 1.30 (3H, d, J = 5.8 Hz); δC (100.6 MHz, CDCl3) 192.71 (C=O), 155.34, 149.55, 144.61, 144.59, 139.89, 135.53, 131.95, 128.74, 127.86, 127.04, 107.30, 105.08, 56.37 & 56.26, 55.72, 53.31, 45.29, 40.91, 38.01, 31.85, 29.59, 21.36, 19.00; m/z (ESI+) 406 [M + H]+, 302; Exact mass calcd for C26H32NO3 [M + H]+ requires m/z 406.23767 found 406.23771 (ESI+).
E-5,6-Dimethoxy-2-((2R,4S-2-phenyl-1-(S-1-phenylethyl)piperidin-4-yl)-methylene)-2,3-dihydro-1H-inden-1-one (11c) and E-5,6-Dimethoxy-2-((2R,4R-2-phenyl-1-(S-1-phenylethyl)piperidin-4-yl)methylene)-2,3-dihydro-1H-inden-1-one (11′c)
5,6-Dimethoxy-1-indanone (206 mg, 1.07 mmol, 1.0 equiv) was added to a solution of a mixture of 3c and 3′c (314 mg, 1.07 mmol, 1.0 equiv, ratio of 3c:3′c was 1:1.33) in methanol (5 mL), and the system was put under an atmosphere of nitrogen. The mixture was heated to 80 °C, and a sodium methoxide solution (28% in methanol, 225 μL, 1.28 mmol, 1.2 equiv) was added. Stirring under reflux was continued for 2 h, then the reaction was allowed to cool to room temperature. The solvent was evaporated under reduced pressure, and the residue was dissolved in CH2Cl2 (25 mL). Water (15 mL) was added, and the phases were separated. The aqueous phase was extracted again with CH2Cl2 (2 × 20 mL); the organic phases were combined and washed with brine and dried over MgSO4. The solvent was evaporated under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 1:3 with 1% TEA) afforded products 11c and 11′c (317 mg, 64%) as white crystals as an inseparable mixture.
Products 11c and 11′c as a mixture: Rf 0.18 (1:1, ethyl acetate/hexane); νmax (solid) 2930, 1694, 1650, 1604, 1586, 1499, 1453, 1302, 1254, 1214, 1127, 1076, 1029, 998, 801, 759, 699; δH (400 MHz, CDCl3) 7.49–7.44 (4H, m), 7.36–7.19 (7H, m), 6.90 (1H, s), 6.62 (1H, dt, J = 9.4 Hz, 1.8 Hz), 3.97 (3H, s), 3.95–3.86 (4H, m), 3.66–3.56 (3H, m), 2.67 (1H, dt, J = 11.5 Hz, 3.3), 2.54–2.44 (1H, m), 2.36 (1H, td, J = 11.7 Hz, 2.4 Hz), 1.89–1.83 (1H, m), 1.78–1.72 (1H, m), 1.69–1.65 (1H, m), 1.61–1.51 (1H, m), 1.26–1.21 (3H, m); δC (100.6 MHz, CDCl3) 192.68, 155.38, 149.59, 144.60, 144.33, 144.13, 139.65, 135.60, 131.97, 128.81, 127.99, 127.64, 127.61, 127.39, 126.37, 107.33, 105.12, 64.86, 56.37, 56.25, 54.85, 44.33, 41.89, 38.79, 31.79, 29.64, 8.10; m/z (ESI+) 468 [M + H]+, 381, 363, 293; Exact mass calcd for C31H34NO3 [M + H]+ requires m/z 468.2533 found 468.2527 (NSI+); HPLC tR1 = 15.8 min, tR2 19.5 min, tR3 38.2 min.
E-5,6-Dimethoxy-2-((2S,4S-2-phenyl-1-(S-1-phenylethyl)piperidin-4-yl)-methylene)-2,3-dihydro-1H-inden-1-one (11d) and E-5,6-Dimethoxy-2-((2S,4R-2-phenyl-1-(S-1-phenylethyl)piperidin-4-yl)methylene)-2,3-dihydro-1H-inden-1-one (11′d)
5,6-Dimethoxy-1-indanone (236 mg, 1.23 mmol, 1.0 equiv) was added to a solution of a mixture of 3d and 3′d (360 mg, 1.23 mmol, 1.0 equiv, ratio of 3d:3′d was 2.04:1) in methanol (5 mL), and the system was put under an atmosphere of nitrogen. The mixture was heated to 80 °C, and a sodium methoxide solution (28% in methanol, 258 μL, 1.47 mmol, 1.2 equiv) was added. Stirring under reflux was continued for 2 h, then the reaction was allowed to cool to room temperature. The solvent was evaporated under reduced pressure, and the residue was dissolved in CH2Cl2 (25 mL). Water (15 mL) was added, and the phases were separated. The aqueous phase was extracted again with CH2Cl2 (2 × 20 mL); the organic phases were combined and washed with brine and dried over MgSO4. The solvent was evaporated under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 1:3 → 1:1) afforded inseparable products 11d and 11′d (335 mg, 59%) as white crystals.
Products 11d and 11′d as a mixture: Rf 0.30 (1:2, ethyl acetate/hexane); νmax (solid) 2928, 1695, 1649, 1605, 1587, 1499, 1453, 1303, 1253, 1215, 1129, 1976, 802, 760, 702; δH (400 MHz, CDCl3) 7.46–7.26 (9H, m), 7.08–7.06 (2H, m), 6.85 (1H, s), 6.56 (1H, dt, J = 9.5 Hz, 1.8 Hz), 4.02 (1H, q, J = 7.2 Hz), 3.96 (3H, s), 3.91 (3H, s), 3.50–3.49 (2H, d, J = 1.5 Hz), 3.27–3.22 (2H, m), 2.32–2.22 (1H, m), 1.90 (1H, dt, J = 11.8 Hz, 2.3 Hz), 1.80–1.63 (3H, m), 1.62–1.52 (1H, m), 1.41 (3H, d, J = 7.2 Hz); δC (100.6 MHz, CDCl3) 192.56, 155.44, 149.66, 145.10, 144.55, 139.51, 138.54, 135.65, 131.99, 129.08, 128.87, 127.72, 127.65, 127.15, 126.98, 107.38, 105.20, 65.14, 56.62, 56.35, 56.25, 45.05, 42.90, 38.28, 31.89, 29.62, 18.64; m/z (ESI+) 468 [M + H]+, 381, 363, 293; Exact mass calcd for C31H34NO3 [M + H]+ requires m/z 468.25332 found 468.25197 (ESI+); HPLC tR1 = 16.1 min, tR2 19.8 min, tR3 39.7 min.
1,1-Dimethyl-4-oxopiperidin-1-ium Iodide (14)
Methyl iodide (4.126 mL, 66.28 mmol, 2.5 equiv) was added to a stirring solution of 1-methyl-4-piperidone (3.261 mL, 26.51 mmol, 1.0 equiv) in acetone (140 mL) at 25 °C. After stirring for 2 h, the precipitate was isolated by filtration and subsequent washing with acetone (40 mL). Product 14 (6.713 g, 99%) was obtained as a pale yellow solid; mp 185–189 °C (decomposition); νmax (solid) 1726, 1476, 1386, 1333, 1295, 1169, 1025, 909, 773, 757; δH (400 MHz, DMSO-d6) 3.76 (4H, t, J = 6.6 Hz), 3.29 (6H, s) 2.71 (4H, t, J = 6.6 Hz); δC (100.6 MHz, CDCl3) 201.66 (C=O), 60.02, 50.90, 35.13. In agreement with published data.33
S-1-(1-Phenylethyl)piperidin-4-one (15)
S-α-Phenylethylamine (2.128 mL, 16.50 mmol, 1.0 equiv) was added to a solution of ethanol (25 mL), potassium carbonate (4.790 g, 34.66 mmol, 2.65 equiv), and water (12 mL), and the resulting mixture was heated to 95 °C. 14 (4.210 g, 16.50 mmol, 1.0 equiv) in water (14 mL) was added dropwise to the mixture. The mixture was heated at reflux for another 30 min and then cooled to room temperature. The ethanol was removed under reduced pressure, and the aqueous residue was extracted with ether (3 × 100 mL). The combined organic layers were dried over MgSO4, the solvent was evaporated under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 1:1) afforded product 15 (2.327 g, 69%) as a yellow oil; Rf 0.29 (1:1, ethyl acetate/hexane); [α]D23 −29.3 (c 0.72, CHCl3); νmax (film) 3028, 2971, 2908, 2806, 2756, 1718, 1493, 1454, 1412, 1386, 1342, 1316, 1284, 1220, 1131, 1080, 1011, 767, 703; δH (400 MHz, CDCl3) 7.24–7.37 (5H, m), 3.62 (1H, q, J = 6.7 Hz), 2.69–2.80 (4H, m), 2.42 (4H, t, J = 6.2 Hz), 1.42 (3H, d, J = 6.7 Hz); δC (100.6 MHz, CDCl3) 209.84 (C=O), 143.58, 128.47, 127.48, 127.27, 63.53, 50.14, 41.68, 19.50. In agreement with published data.34
S-4-(Methoxymethylene)-1-(1-phenylethyl)piperidine (16)
(Methoxymethyl)triphenylphosphonium chloride (2.785 g, 8.12 mmol, 1.5 equiv) and molecular sieves were placed in an oven-dried flask and added with absolute THF (17 mL) under an atmosphere of nitrogen. The mixture was cooled to −78 °C and a 2 M solution of lithium diisopropylamide (LDA) in THF/heptane/ethylbenzene (4.06 mL, 8.12 mmol, 1.5 equiv) was added slowly. The mixture was stirred at −78 °C for 5 min and then allowed to warm to rt while stirring for another 20 min. The reaction mixture was cooled to −20 °C, and a solution of 15 (1.100 g, 5.42 mmol, 1.0 equiv) in absolute THF (12 mL) was added slowly. The mixture was stirred at −20 °C for 15 min, then allowed to warm to room temperature and stirred for 16 h. 1 M NH4Cl solution (25 mL) and ethyl acetate (50 mL) were added, and the solution was stirred vigorously for 5 min. The phases were separated, and the aqueous phase was again extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over MgSO4, filtered, and the solvent was removed under reduced pressure. Purification by column chromatography (hexane/ethyl acetate, 3:2) afforded 16 as a colorless oil (1.165 g, 93%); [α]D23 −42.6 (c 1.21, CHCl3); Rf 0.25 (1.5:1, ethyl acetate/hexane); νmax (film) 1691, 1452, 1223, 1190, 1121, 1075, 837, 758, 735, 700; δH (400 MHz, CDCl3) 7.37–7.23 (5H, m), 5.77 (1H, s), 3.54 (3H, s), 3.49 (1H, q, J = 6.9 Hz), 2.48–2.36 (4H, m), 2.31 (2H, t, J = 5.8 Hz), 2.06 (2H, t, J = 5.6 Hz), 1.41 (3H, d, J = 6.8 Hz); δC (100.6 MHz, CDCl3) 143.74, 139.42, 128.18, 127.85, 126.87, 115.30, 64.60, 59.43, 52.38, 51.14, 30.04, 25.53, 19.28; m/z (ESI+) 232 [M + H]+, 128; Exact mass calcd for C15H22NO [M + H]+ requires m/z 232.16959 found 232.16937 (ESI+).
S-1-(1-Phenylethyl)piperidine-4-carbaldehyde (17)
1.6 N HCl (9 mL) was added to a solution of 16 (1.000 g, 4.33 mmol) in THF (9 mL) and stirred at 45 °C for 2.5 h. THF was evaporated under reduced pressure, and the residue was diluted with water (20 mL). The pH was adjusted to 10 by the addition of an aqueous Na2CO3 solution. The aqueous phase was extracted with CH2Cl2 (3 × 40 mL), and the combined organic layers were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure, and the crude aldehyde 17 was obtained as a colorless oil (863 mg, 92%). The product could be used for the next step without the need of further purification; [α]D24 −71.8 (c 0.20, CHCl3); Rf 0.23 (1.5:1, ethyl acetate/hexane); νmax (film) 1723, 1492, 1450, 1373, 1147, 1132, 941, 769, 758, 701; δH (400 MHz, CDCl3) 9.62 (1H, s), 7.35–7.22 (5H), 3.45 (1H, q, J = 6.7 Hz), 2.96–2.93 (1H, m), 2.79–2.73 (1H, m), 2.22–2.15 (1H, m), 2.13–2.03 (2H, m), 1.91–1.83 (2H, m), 1.75–1.58 (2H, m), 1.38 (3H, d, J = 6.8 Hz); δC (100.6 MHz, CDCl3) 204.25, 143.56, 128.35, 127.78, 127.10, 64.84, 49.75, 49.56, 48.24, 25.75, 19.31; m/z (ESI+) 236 [M + H + H2O]+, 218 [M + H]+, 132, 114, 105; Exact mass calcd for C14H20NO [M + H]+ requires m/z 218.15394 found 218.15448 (ESI+).
E-5,6-Dimethoxy-2-((1-(S-1-phenylethyl)piperidin-4-yl)methylene)-2,3-dihydro-1H-inden-1-one (18)
5,6-Dimethoxy-1-indanone (679 mg, 3.53 mmol, 1.0 equiv) was added to a solution of 17 (767 mg, 3.53 mmol, 1.0 equiv) in methanol (18 mL), and the system was put under an atmosphere of nitrogen. The mixture was heated to 80 °C, and a sodium methoxide solution (28% in methanol, 740 μL, 4.24 mmol, 1.2 equiv) was added. Stirring under reflux was continued for 75 min, then the reaction was allowed to cool to room temperature. The solvent was evaporated under reduced pressure, and purification by column chromatography (100% ethyl acetate → ethyl acetate:methanol, 19:1.5) afforded product 18 (926 mg, 67%) as a white foamy solid; [α]D23 −53.5 (c 0.35, CHCl3); mp 76–79 °C; Rf 0.26 (19:1.5 ethyl acetate:methanol); νmax (solid) 1693, 1648, 1605, 1587, 1499, 1453, 1424, 1303, 1253, 1216, 1126, 1075, 984, 802, 762, 731, 701; δH (400 MHz, CDCl3) 7.34–7.22 (6H, m), 6.88 (1H, s), 6.64 (1H, dt, J = 9.5 Hz, 1.6 Hz), 3.96 (3H, s), 3.91 (3H, s), 3.55 (2H, “d”, J = 1.4 Hz), 3.46 (1H, q, J = 6.6 Hz), 3.10–3.06 (1H, m), 2.89–2.84 (1H, m), 2.32–2.22 (1H, m), 2.08–2.02 (1H, m), 1.98–1.93 (1H, m), 1.74–1.50 (4H, m), 1.40 (3H, J = 6.8 Hz); δC (100.6 MHz, CDCl3) 192.70, 155.40, 149.60, 144.62, 143.36, 139.98, 135.70, 131.97, 128.28, 127.90, 127.05, 107.34, 105.13, 65.09, 56.35, 56.25, 50.41, 50.06, 37.53, 31.49, 29.60, 19.49; m/z (ESI+) 392 [M + H]+, 288; Exact mass calcd for C25H30NO3 [M + H]+ requires m/z 392.2220 found 392.2216 (NSI+); HPLC tR1 = 14.1 min, tR2 = 16.9 min, tR3 = 31.2 min.
R-5,6-Dimethoxy-2-((1-(S-1-phenylethyl)piperidin-4-yl)methyl)-2,3-dihydro-1H-inden-1-one (19) and S-5,6-Dimethoxy-2-((1-(S-1-phenylethyl)piperidin-4-yl)methyl)-2,3-dihydro-1H-inden-1-one (19′)
A solution of 18 (300 mg, 0.77 mmol) in THF (29 mL) was hydrogenated over palladium 10% on activated charcoal (48 mg) for 8 h at ambient temperature and pressure. The reaction mixture was filtered through Celite and washed with CH2Cl2 (100 mL). The solvent was removed under reduced pressure, and purification by column chromatography (100% ethyl acetate → ethyl acetate:methanol, 10:0.5) afforded product 19 + 19′ (176 mg, 58%) as a colorless sticky solid. Rf 0.23 (19:2.5 ethyl acetate:methanol); νmax (film) 2920, 1693, 1606, 1591, 1499, 1453,1312, 1262, 1121, 1036, 912, 763, 728, 701, 647; δH (400 MHz, CDCl3) 7.34–7.22 (5H), 7.15 (1H, s), 6.83 (1H, s), 3.95 (3H, s), 3.89 (1H, s), 3.53–3.47 (1H, m), 3.20 (1H, dd, J = 17.6 Hz, 8.1 Hz), 3.11–3.07 (1H, m), 2.90–2.84 (1H, m), 2.71–2.64 (2H, m), 2.03–1.95 (1H, m), 1.93–1.84 (2H, m), 1.77–1.60 (2H, m), 1.46–1.23 (7H, m); δC (100.6 MHz, CDCl3) 207.91, 155.57, 149.55, 148.87, 143.07, 129.44, 128.27, 128.04, 127.11, 107.47, 104.50, 65.02, 56.34, 56.22, 51.15 & 51.13, 50.50, 45.54, 45.53, 38.77, 34.57, 33.49, 33.47, 33.01, 31.95, 31.89, 19.46, 19.43; m/z (ESI+) 394 [M + H]+, 290; Exact mass calcd for C25H32NO3 [M + H]+ requires m/z 394.23767 found 394.23764 (ESI+); HPLC tR1 = 14.1 min, tR2 = 16.9 min, tR3 = 31.3 min.
R-5,6-Dimethoxy-2-((2S,4S-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4a) and y-5,6-Dimethoxy-2-((2S,4S-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′a)
A solution of 11a (150 mg, 0.37 mmol) in THF (15 mL) was hydrogenated over palladium 10% on activated charcoal (25 mg) for 8 h at ambient temperature and pressure. The reaction mixture was filtered through Celite and washed with CH2Cl2 (40 mL). The solvent was removed under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 2:1 → 100% ethyl acetate) afforded an inseparable mixture of product 4a and product 4′a (103 mg, 68%) as a pale yellow solid. Rf 0.32 (8:1, ethyl acetate:methanol); νmax (solid) 2919, 1693, 1590, 1499, 1454, 1420, 1307, 1262, 1221, 1119, 1034, 764, 721, 698; δH (400 MHz, CDCl3) 7.48–7.46 (4H, m), 7.33–7.30 (4H, m), 7.23–7.19 (2H, m), 7.16 (2H, s), 6.85–6.84 (2H, m), 4.35 (2H, q, J = 6.1 Hz), 3.96 (6H, s), 3.90 (6H, s), 3.23 (2H, dd, J = 17.5 Hz, 8.1 Hz), 2.71–2.65 (4H, m), 2.61–2.53 (2H, m), 2.47–2.42 (2H, m), 2.12–2.06 (2H, m), 1.90–1.82 (2H, m), 1.78–1.68 (2H, m), 1.59–1.48 (4H, m), 1.31–1.02 (18H, m); δC (100.6 MHz, CDCl3) 208.13, 208.12, 155.51, 149.49, 148.97, 148.96, 144.89, 129.47, 129.44, 127.95, 127.92, 126.27, 56.34, 56.22, 54.26, 53.54, 45.68 & 45.52, 44.88, 43.48, 42.35, 39.20, 39.12, 35.66, 35.57, 33.72, 32.45, 33.54, 21.16, 21.14, 8.15, 8.12; m/z (ESI+) 408 [M + H]+, 304; Exact mass calcd for C26H34NO3 [M + H]+ requires m/z 408.25332 found 408.25278 (ESI+); HPLC tR1 = 14.2 min, tR2 = 17.0 min, tR3 = 31.3 min.
R-5,6-Dimethoxy-2-((2S,4R-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4b) and S-5,6-Dimethoxy-2-((2S,4R-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′b)
A solution of 11′a (72 mg, 0.18 mmol) in THF (7 mL) was hydrogenated over palladium 10% on activated charcoal (10 mg) for 8 h at ambient temperature and pressure. The reaction mixture was filtered through Celite and washed with CH2Cl2 (30 mL). The solvent was removed under reduced pressure, and purification by column chromatography (100% ethyl acetate → ethyl acetate:methanol, 10:1) afforded an inseparable mixture of product 4b and product 4′b (26 mg, 35%) as a pale yellow sticky solid. Rf 0.20 (9:1, ethyl acetate:methanol); νmax (film) 2921, 1693, 1606, 1591, 1500, 1454, 1310, 1263, 1122, 1039, 751, 701, 649; δH (400 MHz, CDCl3) 7.41–7.35 (4H, m), 7.32–7.28 (4H, m), 7.23–7.20 (2H, m), 7.16 (2H, s), 6.85 (2H, “ds”), 3.95 (6H, s), 3.90 (6H, s), 3.63–3.45 (4H, m), 3.26–3.19 (2H, m), 2.73–2.65 (4H, m), 2.52–2.42 (2H, m), 2.36–2.28 (2H, m), 1.92–1.78 (4H, m), 1.65–1.48 (6H, m), 1.33–1.07 (16H, m); δC (100.6 MHz, CDCl3) 208.04, 155.54, 155.53, 149.50, 148.93, 147.03, 129.43, 129.39, 128.50, 127.48, 126.93, 107.44, 104.43, 104.42, 61.15, 56.34, 56.21, 48.68, 45.64, 45.39, 43.87, 38.95, 38.89, 37.77, 33.54, 33.48, 31.42, 28.97, 28.67, 20.94, 10.64; m/z (ESI+) 408 [M + H]+, 304; Exact mass calcd for C26H34NO3 [M + H]+ requires m/z 408.25332 found 408.25349 (ESI+); HPLC tR1 = 14.4 min, tR2 = 17.4 min, tR3 = 32.2 min.
R-5,6-Dimethoxy-2-((2R,4S-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4c) and S-5,6-Dimethoxy-2-((2R,4S-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′c)
A solution of 11b (72 mg, 0.18 mmol) in THF (8 mL) was hydrogenated over palladium 10% on activated charcoal (14 mg) for 9 h at ambient temperature and pressure. The reaction mixture was filtered through Celite and washed with CH2Cl2 (30 mL). The solvent was removed under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 8:1 → 100% ethyl acetate → ethyl acetate:methanol, 10:1) afforded an inseparable mixture of product 4c and product 4′c (31 mg, 42%) as a pale yellow sticky solid. Rf 0.09 (100% ethyl acetate); νmax (film) 2921, 1692, 1606, 1591, 1499, 1454,1311, 1263, 1121, 1037, 913, 767, 728, 702, 646; δH (400 MHz, CDCl3) 7.43–7.41 (2H, m), 7.32 (2H, t, J = 7.4 Hz), 7.26–7.22 (1H, m), 7.15 (1H, s), 6.85–6.84 (1H, ‘m’), 3.95 (3H, s), 3.89 (3H, s), 3.72–3.67 (1H, m), 3.23 (1H, dd, J = 17.4 Hz, 8.0 Hz), 3.11–3.01 (2H, m), 2.71–2.54 (3H, m), 1.88–1.76 (3H, m), 1.57–1.23 (7H, m), 0.98–1.00 (3H, m); δC (100.6 MHz, CDCl3) 207.93, 207.91, 155.58, 149.54, 148.91, 142.64, 129.40, 129.37, 128.61, 127.60, 127.22, 107.46, 104.43, 61.12, 56.33, 56.20, 50.08, 45.46, 45.34, 42.40, 38.91, 38.84, 37.73, 33.63, 33.54, 31.57, 28.25, 28.14, 22.02, 10.49; m/z (ESI+) 408 [M + H]+, 304; Exact mass calcd for C26H34NO3 [M + H]+ requires m/z 408.25332 found 408.25396 (ESI+); HPLC tR1 = 14.5 min, tR2 = 17.6 min, tR3 = 32.7 min.
R-5,6-Dimethoxy-2-((2R,4R-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4d) and S-5,6-Dimethoxy-2-((2R,4R-2-methyl-1-(S-1-phenylethyl)piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′d)
A solution 11′b (85 mg, 0.21 mmol) in THF (10 mL) was hydrogenated over palladium 10% on activated charcoal (16 mg) for 7 h at ambient temperature and pressure. The reaction mixture was filtered through Celite and washed with CH2Cl2 (40 mL). The solvent was removed under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 3:1 → 100% ethyl acetate) afforded an inseparable mixture of product 4d and product 4′d (57 mg, 67%) as a pale yellow foamy solid. Rf 0.25 (9:1, ethyl acetate:methanol); νmax (film) 2918, 1693, 1605, 1590, 1499, 1453,1307, 1262, 1119, 1035, 762, 733, 702, 651; δH (400 MHz, CDCl3) 7.36–7.23 (10H, m), 7.14 (2H, s), 6.81 (2H, s), 4.39 (2H, q, J = 7.1 Hz), 3.94 (6H, s), 3.89 (6H, s), 3.19–3.12 (4H, m), 2.68–2.58 (4H, m), 2.17–2.14 (2H, m), 1.87–1.58 (8H, m), 1.50 (6H, d, J = 7.0 Hz), 1.37–1.10 (14 H, m); δC (100.6 MHz, CDCl3) 207.83, 207.79, 155.40, 149.37, 148.74, 138.96, 129.29, 129.27, 128.68, 127.71, 126.94, 107.32, 104.33, 56.21, 56.09, 55.72, 55.69, 53.71, 45.71, 45.35, 45.26, 42.98, 41.88, 38.85, 38.77, 34.68, 33.33, 32.09, 21.34, 21.29, 18.88, 18.87; m/z (ESI+) 408 [M + H]+, 304; Exact mass calcd for C26H34NO3 [M + H]+ requires m/z 408.25332 found 408.25274 (ESI+); HPLC tR1 = 14.5 min, tR2 = 17.6 min, tR3 = 32.9 min.
R-5,6-Dimethoxy-2-((2R,4S-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4e), R-5,6-Dimethoxy-2-((2R,4R-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4f), S-5,6-Dimethoxy-2-((2R,4S-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′e) and S-5,6-Dimethoxy-2-((2R,4R-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′f)
A solution of a mixture of 11c and 11′c (139 mg, 0.30 mmol) in THF (13 mL) was hydrogenated over palladium 10% on activated charcoal (14 mg) for 10 h at ambient temperature and pressure. The reaction mixture was filtered through Celite and washed with CH2Cl2 (40 mL). The solvent was removed under reduced pressure, and purification by column chromatography (ethyl acetate/hexane, 1:3.5) afforded an inseparable mixture of products 4 + 4′e and 4 + 4′f (108 mg, 77%) as a foamy white solid; Rf 0.47 (1:1, ethyl acetate/hexane); νmax (solid) 2910, 1693, 1606, 1591, 1499, 1453,1309, 1262, 1120, 1032, 909, 762, 728, 690; δH (400 MHz, CDCl3) 7.50–7.44 (4H, m), 7.36–7.16 (7H, m), 6.85–6.84 (1H, m), 3.95–3.94 (3H, m), 3.89 (3H, s), 3.92–3.84 (1H, m), 3.61–3.55 (1H, m), 3.26–3.19 (1H, m), 2.73–2.59 (3H, m), 2.32–2.26 (1H, m), 1.94–1.86 (2H, m), 1.74–1.60 (2H, m), 1.53–1.41 (1H, m), 1.38–1.20 (5H, m); δC (100.6 MHz, CDCl3) 207.89, 207.85, 155.51, 155.50, 149.48, 148.86, 148.83, 144.95, 144.90, 144.64, 129.3, 128.69, 127.89, 127.63, 127.55, 127.17, 127.13, 126.20, 107.43, 107.41, 104.43, 65.18, 56.29, 56.16, 54.79, 45.45, 45.38, 44.69, 44.67, 44.42, 43.31, 39.12, 39.03, 35.85, 35.63, 33.69, 33.43, 33.38, 32.32, 8.12; m/z (ESI+) 470 [M + H]+, 366; Exact mass calcd for C31H36NO3 [M + H]+ requires m/z 470.26897 found 470.26805 (ESI+); HPLC tR1 = 15.7 min, tR2 = 19.4 min, tR3 = 37.8 min.
R-5,6-Dimethoxy-2-((2S,4S-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4g), R-5,6-Dimethoxy-2-((2S,4R-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4h), S-5,6-Dimethoxy-2-((2S,4S-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′g) and S-5,6-Dimethoxy-2-((2S,4R-2-phenyl-1-(S-1-phenylethyl)-piperidin-4-yl)-methyl)-2,3-dihydro-1H-inden-1-one (4′h)
A solution of a mixture of 11d and 11′d (200 mg, 0.43 mmol) in THF (16 mL) was hydrogenated over palladium 10% on activated charcoal (26 mg) for 8 h at ambient temperature and pressure. The reaction mixture was filtered through Celite and washed with CH2Cl2 (100 mL).The solvent was removed under reduced pressure, and purification by column chromatography (chloroform:methanol, 4:0.1) afforded an inseparable mixture of products 4 + 4′g and 4 + 4′h (171 mg, 84%) as a colorless solid. Rf 0.31 (1:2, ethyl acetate/hexane); νmax (film) 2923, 1693, 1591, 1500, 1453, 1311, 1263, 1216, 1119, 1033, 844, 759, 735, 701; δH (400 MHz, CDCl3) 7.45–7.37 (4H, m), 7.32–7.22 (4H, m), 7.13 (1H, s), 7.05–7.03 (2H, m), 6.81–6.79 (1H, m), 3.97 (1H, q, J = 7.2 Hz), 3.94–3.93 (3H, s), 3.88 (3H, s), 3.21–3.10 (3H, m), 2.67–2.55 (2H, m), 1.86–1.66 (4H, m), 1.45–1.26 (6H, m), 1.21–1.12 (1H, m); δC (100.6 MHz, CDCl3) 207.82, 207.73, 155.58, 155.55, 149.55, 148.83, 148.78, 145.86, 145.84, 138.66, 129.43, 129.06, 128.78, 127.75, 127.57, 126.95, 126.91, 126.88, 107.49, 107.47, 104.52, 65.56, 56.62, 56.60, 56.31, 56.20, 45.51, 45.48, 45.43, 45.34, 44.34, 38.97, 38.89, 35.32, 35.05, 33.57, 33.30, 32.32, 18.73, 18.70; m/z (ESI+) 470 [M + H]+, 366; Exact mass calcd for C31H36NO3 [M + H]+ requires m/z 470.26897 found 470.26726 (ESI+); HPLC tR1 = 16.0 min, tR2 = 20.0 min, tR3 = 40.0 min.
Acetylcholinesterase Inhibition Assay
The inhibitory activity of compounds 4 + 4′a, 4 + 4′b, 4 + 4′c, 4 + 4′d, 4 + 4′e–f, 4 + 4′g–h, and 19 + 19′ toward AChE (enzyme commission number 3.1.1.7, type VI-S from Electrophorus electricus (electric eel)) was measured using a protocol based on Ellman’s reagent.37 The 96-well plate format of this assay, as described by Mohamed,38 was further adjusted to result in the following protocol. The control compound donepezil (Bertin Pharma, Cayman, France), and the test compounds were dissolved in methanol to obtain stock solutions of 10 mM. Twelve further dilutions were made of each compound using methanol, resulting in final assay concentrations of 0.001–100 μM. To obtain a 1.5 mM solution, 29.73 mg of 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB, Ellman’s reagent, Alfa Aesar) was dissolved in 50 mL of 50 mM tris(hydroxymethyl)aminomethane (TRIS)-HCl buffer (pH = 8.0) containing 0.1 M NaCl and 0.02 M MgCl2 · 6 H2O. A 259 U/mL stock solution of AChE in buffer (50 mM Tris–HCl, pH = 8.0, 0.1% w/v bovine serum albumin (BSA)) was prepared, and aliquots of 21 μL were frozen in Eppendorf tubes and stored at −20 °C. Before running the assay, one of these aliquots was diluted with buffer (50 mM Tris–HCl, pH = 8.0, 0.1% w/v BSA) to obtain 25 mL of a solution with an enzyme concentration of 0.22 U/mL. A 15 mM solution of acetylthiocholine iodide (ATCI, Sigma-Aldrich) was prepared by dissolving 108.45 mg in ultrapure water (25 mL). All of these solutions were prepared freshly directly before the assay was run. In 96-well plates, 160 μL of the 1.5 mM DTNB solution was first added, followed by 10 μL of different concentrations of test compounds. Then, a 0.22 U/mL enzyme solution was added (50 μL) and incubated at room temperature for 7 min. The background absorbance was measured at a wavelength of 412 nm on a POLARstar OPTIMA (BMG Labtech) microplate reader. The addition of 30 μL of the 15 mM ATCI solution initiated the enzymatic reaction, and the time elapsed between the beginning of the pipetting and the first measurement (t = 0) was noted and kept consistently at 2 min. Further kinetic measurements were made at different time intervals (t = 1, 2, 3, 4, 5 min) after a short episode of shaking (10 s). Each experiment was carried out in triplicate. Various control incubations containing 10 μL of methanol were run alongside the test compounds on each plate. The IC50 values were calculated using GraphPad Prism software (version 6.01) by applying a nonlinear regression analysis (sigmoidal dose–response fit with a variable slope).
Acknowledgments
AP is thankful to Dr Vincenzo Abbate (King’s College London) for his advice on the HPLC experiments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03808.
HRMS spectra and full 1H and 13C NMR spectra for all of the new derivatives reported in the manuscript, stereochemical assignment for 2-substituted piperidones, and HPLC chromatograms for the analogues tested as AChE inhibitors (PDF)
Author Present Address
† Albrechtskreithgasse 27/4/7, Vienna 1160, Austria (A.P.).
The authors declare no competing financial interest.
Supplementary Material
References
- Szoke E.; Lemberkovics E.; Kursinszki L.. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes, 1st ed.; Springer: Berlin, 2013; Chapter 11, p 303. [Google Scholar]
- Cordell G. A.; Quinn-Beattie M. L.; Farnsworth N. R. The potential of alkaloids in drug discovery. Phytother. Res. 2001, 15, 183–205. 10.1002/ptr.890. [DOI] [PubMed] [Google Scholar]
- Van Bever W. F. M.; Niemegeers C. J. E.; Janssen P. A. J. Synthetic analgesics. Synthesis and pharmacology of the diastereoisomers of N-(3-methyl-1-(2-phenylethyl)-4-piperidyl)-N-phenylpropanamide and N-(3-methyl-1-(1-methyl-2-phenylethyl)-4-piperidyl)-N-phenylpropanamide. J. Med. Chem. 1974, 17, 1047–1051. 10.1021/jm00256a003. [DOI] [PubMed] [Google Scholar]
- Wang Z. X.; Zhu Y. C.; Chen X. J.; Ji R. Y. Stereoisomers of 3-methylfentanyl: synthesis, absolute configuration and analgesic activity. Acta Pharm. Sin. 1993, 28, 905–910. [PubMed] [Google Scholar]
- Green K. D.; Fosso M. Y.; Garneau-Tsodikova S. Multifunctional donepezil analogues as cholinesterase and BACE1 inhibitors. Molecules 2018, 23, 3252 10.3390/molecules23123252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou J.; Dias J.; Mercey G.; Verdelet T.; Rousseau C.; Gastellier A.-J.; Arboleas M.; Touvrey-Loiodice M.; Baati R.; Jean L.; Nachon F.; Renard P.-Y. Synthesis and in vitro evaluation of donepezil based reactivators and analogues for nerve agent inhibited human acetylcholinesterase. RSC Adv. 2016, 6, 17929–17940. 10.1039/C5RA25477A. [DOI] [Google Scholar]
- Costanzo P.; Cariati L.; Desiderio D.; Sgammato R.; Lamberti A.; Arcone R.; Salerno R.; Nardi M.; Masullo M.; Oliverio M. Design, synthesis, and evaluation of donepezil-like compounds as AChE and BACE-1 inhibitors. ACS Med. Chem. Lett. 2016, 7, 470–475. 10.1021/acsmedchemlett.5b00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabr M. T.; Abdel-Raziq M. S. Design and synthesis of donepezil analogues as dual AChE and BACE-1 inhibitors. Bioorg. Chem. 2018, 80, 245–252. 10.1016/j.bioorg.2018.06.031. [DOI] [PubMed] [Google Scholar]
- Piemontese L.; Tomás D.; Hiremathad A.; Capriati V.; Candeias E.; Cardoso S. M.; Chaves S.; Santos M. A. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J. Enzyme Inhib. Med. Chem. 2018, 33, 1212–1224. 10.1080/14756366.2018.1491564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junaid M.; Islam N.; Hossain M. K.; Ullah M. O.; Halim M. A. Metal based donepezil analogues designed to inhibit human acetylcholinesterase for Alzheimer’s disease. PLoS One 2019, 14, e0211935 10.1371/journal.pone.0211935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y.; Grassi J.; Bougis P. E.; Marchot P. Conformational flexibility of the acetylcholinesterase tetramer suggested by x-ray crystallography. J. Biol. Chem. 1999, 274, 30370–30376. 10.1074/jbc.274.43.30370. [DOI] [PubMed] [Google Scholar]
- Cheung J.; Rudolph M. J.; Burshteyn F.; Cassidy M. S.; Gary E. N.; Love J.; Franklin M. C.; Height J. J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- Sugimoto H.; Iimura Y.; Yamanishi Y.; Yamatsu K. Synthesis and Structure-Activity Relationships of Acetylcholinesterase Inhibitors: 1-Benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-yl)methyl]piperidine Hydrochloride and Related Compounds. J. Med. Chem. 1995, 38, 4821–4829. 10.1021/jm00024a009. [DOI] [PubMed] [Google Scholar]
- Sugimoto H.; Tsuchiya Y.; Sugumi H.; Higurashi K.; Karibe N.; Iimura Y.; Sasaki A.; Kawakami Y.; Nakamura T. Novel piperidine derivatives. Synthesis and anti-acetylcholinesterase activity of 1-benzyl-4-[2-(N-benzoylamino)ethyl]piperidine derivatives. J. Med. Chem. 1990, 33, 1880–1887. 10.1021/jm00169a008. [DOI] [PubMed] [Google Scholar]
- Sugimoto H. The new approach in development of anti-Alzheimer’s disease drugs via the cholinergic hypothesis. Chem.-Biol. Interact. 2008, 175, 204–208. 10.1016/j.cbi.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Kawakami Y.; Inoue A.; Kawai T.; Wakita M.; Sugimoto H.; Hopfinger A. J. The rationale for E2020 as a potent acetylcholinesterase inhibitor. Bioorg. Med. Chem. 1996, 4, 1429–1446. 10.1016/0968-0896(96)00137-X. [DOI] [PubMed] [Google Scholar]
- Sugimoto H.; Tsuchiya Y.; Sugumi H.; Higurashi K.; Karibe N.; Iimura Y.; Sasaki A.; Yamanishi Y.; Yamatsu K.; et al. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-benzyl-4-(2-phthalimidoethyl)piperidine, and related derivatives. J. Med. Chem. 1992, 35, 4542–4548. 10.1021/jm00102a005. [DOI] [PubMed] [Google Scholar]
- Sugimoto H.; Yamanishi y.; Iimura Y.; Kawakami Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 2000, 7, 303–339. 10.2174/0929867003375191. [DOI] [PubMed] [Google Scholar]
- Korabecny J.; Spilovska K.; Mezeiova E.; Benek O.; Juza R.; Kaping D.; Soukup O. A Systematic Review on Donepezil-Based Derivatives as Potential Cholinesterase Inhibitors for Alzheimer’s Disease. Curr. Med. Chem. 2018, 26, 5625–5648. 10.2174/0929867325666180517094023. [DOI] [PubMed] [Google Scholar]
- van Greunen D. G.; Cordier W.; Nell M.; van der Westhuyzen C.; Steenkamp V.; Panayides J. L.; Riley D. L. Targeting Alzheimer’s Disease by Investigating Previously Unexplored Chemical Space Surrounding the Cholinesterase Inhibitor Donepezil. Eur. J. Med. Chem. 2017, 127, 671–690. 10.1016/j.ejmech.2016.10.036. [DOI] [PubMed] [Google Scholar]
- Cheung J.; Rudolph M. J.; Burshteyn F.; Cassidy M. S.; Gary E. N.; Love J.; Franklin M. C.; Height J. J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- Guzzo P. R.; Henderson A. J.; Isherwood M.; Lee C. Y.; Ghosh A.; Zhao H.. Benzofuro[3,2-C] pyridines and related analogs as serotonin sub-type 6 (5-HT6) modulators for the treatment of obesity, metabolic syndrome, cognition and schizophrenia. U.S. Patent US2012/184531 A12012.
- Blanco-Pilado M.-J.; Benesh D. R.; Filla S. A.; Hudziak K. J.; Mathes B. M.. (Piperidinyloxy)phenyl, (Piperidinyloxy)pyridinyl, (Piperidinylsulfanyl)phenyl and (Piperidinylsulfanyl)pyridinyl Compounds as 5-HT Agonists. WO Patent WO2004/094380 A12004.
- Tang X.-J.; Yan Z.-L.; Chen W.-L.; Gao Y.-R.; Mao S.; Zhang Y.-L.; Wang Y.-Q. Aza-Michael reaction promoted by aqueous sodium carbonate solution. Tetrahedron Lett. 2013, 54, 2669–2673. 10.1016/j.tetlet.2013.03.043. [DOI] [Google Scholar]
- Pawłowska J.; Krawczyk K. K.; Wojtasiewicz K.; Maurin J. K.; Czarnocki Z. (S)-(−)-α-Methylbenzylamine as chiral auxiliary in the synthesis of (+)-lortalamine. Monatsh. Chem. 2008, 140, 83–86. 10.1007/s00706-008-0017-2. [DOI] [Google Scholar]
- Ziółkowski M.; Czarnocki Z.; Leniewski A.; Maurin J. K. (S)-(−)-α-Methylbenzylamine as an efficient chiral auxiliary in enantiodivergent synthesis of both enantiomers of N-acetylcalycotomine. Tetrahedron: Asymmetry 1999, 10, 3371–3380. 10.1016/S0957-4166(99)00370-5. [DOI] [Google Scholar]
- Leshcheva I. F.; Sergeev N. M.; Grishina G. V.; Potapov V. M. The 13C NMR spectra, isomerism, and conformational analysis of substituted piperidin-4-ones. Chem. Heterocycl. Compd. 1986, 22, 1214–1225. 10.1007/BF00471805. [DOI] [Google Scholar]
- Sugimoto H.; Tsuchiya Y.; Higurashi K.; Karibe N.; Iimura Y.; Sasaki A.; Yamanishi Y.. Cyclic Amine Compounds with Activity against Acetylcholinesterase. U.S. Patent US48958411990.
- Janssens F. E.; Schoentjes B.; Coupa S.; Poncelet A. P.; Simonnet Y. R. F.. Substituted Diaza-spiro-[5,5]-undecane Derivatives and Their Use as Neurokinin Antagonists. WO Patent WO2005/097795 A12005.
- Niphade N.; Mali A.; Jagtap K.; Ojha R. C.; Vankawala P. J.; Mathad V. T. An improved and efficient process for the production of donepezil hydrochloride: substitution of sodium hydroxide for n-butyl lithium via phase transfer catalysis. Org. Process Res. Dev. 2008, 12, 731–735. 10.1021/op800066m. [DOI] [Google Scholar]
- Sheng R.; Hu Y. Novel and Efficient Method to Synthesize N-Benzyl-4-Formyl-Piperidine. Synth. Commun. 2004, 34, 3529–3533. 10.1081/SCC-200030987. [DOI] [Google Scholar]
- Imai A.; Shimotani A.; Tsurugi T.; Narabe Y.. Process for Producing 1-Benzyl-4-[(5,6-dimethoxy-1-indanone)-2-ylidene]methylpiperidine. EP Patent EP1911745 A12008.
- Amato J. S.; Chung J. Y. L.; Cvetovich R. J.; Reamer R. A.; Zhao D.; Zhou G.; Gong X. Acrylate as an Efficient Dimethylamine Trap for the Practical Synthesis of 1-tert-Butyl-4-piperidone via Transamination. Org. Process Res. Dev. 2004, 8, 939–941. 10.1021/op049860g. [DOI] [Google Scholar]
- Kuehne M. E.; Matson P. A.; Bornmann W. G. Enantioselective syntheses of vinblastine, leurosidine, vincovaline and 20′-epi-vincovaline. J. Org. Chem. 1991, 56, 513–528. 10.1021/jo00002a008. [DOI] [Google Scholar]
- Matsui K.; Oda Y.; Ohe H.; Tanaka S.; Asakawa N. Direct determination of E2020 enantiomers in plasma by liquid chromatography-mass spectrometry and column-switching techniques. J. Chromatogr. A 1995, 694, 209–218. 10.1016/0021-9673(94)01005-Y. [DOI] [PubMed] [Google Scholar]
- Kryger G.; Silman I.; Sussman J. L. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. 10.1016/S0969-2126(99)80040-9. [DOI] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K.; Andres V.; Featherstone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Mohamed T.; Yeung J. C. K.; Vasefi M. S.; Beazely M. A.; Rao P. P. N. Development and evaluation of multifunctional agents for potential treatment of Alzheimer’s disease: application to a pyrimidine-2,4-diamine template. Bioorg. Med. Chem. Lett. 2012, 22, 4707–4712. 10.1016/j.bmcl.2012.05.077. [DOI] [PubMed] [Google Scholar]
- Birtwistle D. H.; Brown J. M.; Foxton M. W. Diastereoselectivity in the intramolecular diels-alder reaction of dienylpropynoates. Tetrahedron 1988, 44, 7309–7318. 10.1016/S0040-4020(01)86103-2. [DOI] [Google Scholar]
- Radha Krishna P.; Ramana D. V. Titanium(IV)-promoted regioselective nucleophilic ring-opening reaction of chiral epoxyallyl alcohols with acids as a tool for ready access to chiral 1,2,3-triol monoesters: application to stereoselective total synthesis of macrolides. J. Org. Chem. 2012, 77, 674–679. 10.1021/jo202199g. [DOI] [PubMed] [Google Scholar]
- Bouziane A.; Carboni B.; Bruneau C.; Carreaux F.; Renaud J.-L. Pentamethylcyclopentadienyl ruthenium: an efficient catalyst for the redox isomerization of functionalized allylic alcohols into carbonyl compounds. Tetrahedron 2008, 64, 11745–11750. 10.1016/j.tet.2008.09.095. [DOI] [Google Scholar]
- Bertus P.; Drouin L.; Laroche C.; Szymoniak J. Pentadienyl transfer reagents based on zirconium: preparation and reactions with carbonyl compounds. Tetrahedron 2004, 60, 1375–1383. 10.1016/j.tet.2003.08.065. [DOI] [Google Scholar]
- Takacs J. M.; Jiang X.; Leonov A. P. Palladium-catalyzed regiocontrolled and stereoselective alkylations of bis(trifluoroethyl) malonates with dienyl alcohols. Tetrahedron Lett. 2003, 44, 7075–7079. 10.1016/S1268-7731(03)00074-2. [DOI] [Google Scholar]
- Han S. B.; Krische M. J. Reductive aldol coupling of divinyl ketones via rhodium-catalyzed hydrogenation: syn-diastereoselective construction of beta-hydroxyenones. Org. Lett. 2006, 8, 5657–5660. 10.1021/ol0624023. [DOI] [PubMed] [Google Scholar]
- Kjeldsen G.; Knudsen J. S.; Ravn-Petersen L. S.; Torssell K. B. Synthesis of 1,4-dien-3-ones and 2-cyclopentenones. Tetrahedron 1983, 39, 2237–2239. 10.1016/S0040-4020(01)91945-3. [DOI] [Google Scholar]
- Brozek L. A.; Sieber J. D.; Morken J. P. Catalytic enantioselective conjugate allylation of unsaturated methylidene ketones. Org. Lett. 2011, 13, 995–997. 10.1021/ol102982b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenajdenko V. G.; Lebedev M. V.; Balenkova E. S. Reactions of acylsulfonium salts with unsaturated hydrocarbons. Tetrahedron 2001, 57, 9017–9024. 10.1016/S0040-4020(01)00897-3. [DOI] [Google Scholar]
- Barco A.; Benetti S.; Risi C. D.; Marchetti P.; Pollini G. P.; Zanirato V. 4-[(4′-Methylphenyl)sulfonyl]-1-(triphenylphosphoranylidene)-2-butanone as a New Four-Carbon Synthon for Substituted Divinyl Ketones. Eur. J. Org. Chem. 2001, 2001, 975–986. . [DOI] [Google Scholar]
- Cui L.; Peng Y.; Zhang L. A two-step, formal [4 + 2] approach toward piperidin-4-ones via Au catalysis. J. Am. Chem. Soc. 2009, 131, 8394–8395. 10.1021/ja903531g. [DOI] [PubMed] [Google Scholar]
- Miltz W.; Oberhauser B.; Vaupel A.; Velcicky J.; Weigand K.; Leletti R.; Liu Y.; Du Z.. Pyrazolo Pyrimidine Derivatives. U.S. Patent US2012/252778 A12012.
- Grunewald G. L.; Seim M. R.; Bhat S. R.; Wilson M. E.; Criscione K. R. Synthesis of 4,5,6,7-tetrahydrothieno[3,2-c]pyridines and comparison with their isosteric 1,2,3,4-tetrahydroisoquinolines as inhibitors of phenylethanolamine N-methyltransferase. Bioorg. Med. Chem. 2008, 16, 542–559. 10.1016/j.bmc.2007.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.