Abstract
A class of aryne precursors, that is, 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates, has been developed through well-established synthetic routes, which allow the formation of arynes under relatively mild conditions. All the aryne precursors were obtained from phenols and 4-chlorobenzenesulfonyl chloride, an inexpensive and easy-to-handle reagent with relatively low toxicity, and subjected to nucleophilic addition reactions, providing addition products in yields of 24 to 92%, and to cycloaddition reactions, affording cycloadducts in yields up to 80%. This work provides interesting insights into the mechanisms of aryne generation. In addition, 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate was successfully employed in the total synthesis of (±)-aporphine.
Introduction
Arynes have been widely employed in preparative organic chemistry, including total syntheses of bioactive natural products1 and preparations of functional materials.2 Accordingly, there are several methods for the formation of benzyne and its derivatives described in the literature.3 Despite the relevance of these methods in the current context of benzyne chemistry, 2-(trimethylsilyl)aryl trifluoromethanesulfonates have emerged as useful reagents for the formation of arynes through a fluoride-induced reaction under mild conditions.4,5 Moreover, some silylaryl triflates are commercially available, and others containing electron-donating and electron-withdrawing groups can be synthesized through well-established routes.4,6
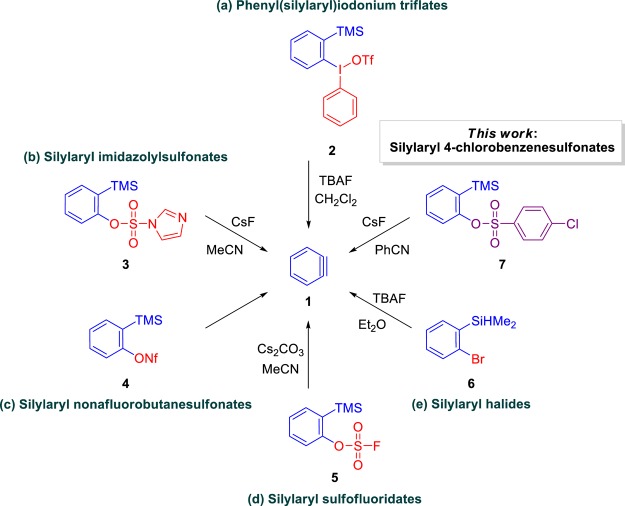
There are advantages to the application of 2-(trimethylsilyl)aryl triflates in organic synthesis; however, trifluoromethanesulfonic anhydride is used in their synthetic routes.4,6 Although trifluoromethanesulfonic anhydride is a commonly used reagent, its use should be discouraged since it is a relatively toxic, expensive, corrosive, and volatile liquid.4,6 From a review of the literature on benzyne chemistry, we found other silylated aryne precursors that were prepared without the use of trifluoromethanesulfonic anhydride: (a) phenyl(silylaryl)iodonium triflates (2),7 (b) silylaryl imidazolylsulfonates (3),8 (c) silylaryl nonafluorobutanesulfonates (4),9 (d) silylaryl sulfofluoridates (5),10 and (e) silylaryl halides (6)11 (Scheme 1). Phenyl(silylaryl)iodonium triflates (2) are well-known compounds that have been used as efficient aryne precursors over the last few decades. The iodonium salts 2 have iodobenzene as the leaving group, but their synthesis involves trifluoromethanesulfonic acid.7 Even though silylaryl imidazolylsulfonates (3) can be considered an attractive alternative for the generation of arynes, they are obtained from 1,1′-sulfonyldiimidazole,8 which is more expensive than trifluoromethanesulfonic anhydride. Silylaryl nonafluorobutanesulfonates (4) are rather interesting because they generate arynes through a domino process from 2-(trimethylsilyl)aryl trimethylsilylethers9b and 2-(trimethylsilyl)phenols;9d however, they employ nonafluorobutanesulfonyl fluoride, a reagent with disadvantages comparable to those of triflic anhydride. In addition, silylaryl sulfofluoridates (5) can generate arynes without the use of cesium fluoride, but they required the use of sulfuryl fluoride,10 which is a neurotoxic gas. The use of silylaryl halides (6) is convenient for the generation of arynes because they can be prepared in one step from commercially available starting materials. However, since these compounds employ aryl bromides as the starting materials and TMPLi as the strong non-nucleophilic base,11 their preparation requires rigorous control to prevent the formation of arynes (Scheme 1).
Scheme 1. Previously Reported Alternatives to Silylaryl Triflates and the Novel Benzyne Precursor Introduced by This Work.
In the search for a substitute for triflic anhydride, we found studies using benzenesulfonates as leaving groups.12,13 Cunico and Dexheimer explored 2-(trimethylsilyl)phenyl 4-methylbenzenesulfonate in the benzyne generation, but they were unsuccessful (the desired products were obtained in very low yields).12 Knochel and co-workers reported the formation of (hetero)arynes using halo(hetero)aryl 4-chlorobenzenesulfonates. Although this approach employed (hetero)aryne precursors obtained from 4-chlorobenzenesulfonyl chloride, which can be considered an inexpensive and easy-to-handle reagent with relatively low toxicity, somewhat strong and nucleophilic bases were required to promote the halo-magnesium exchange reaction followed by the elimination of 4-chlorobenzenesulfonate to afford substituted (hetero)arynes.13 Motivated by previous studies, we selected, synthesized, and evaluated silylphenyl benzenesulfonates and silylphenyl sulfamate, which have considerable potential for the generation of benzyne and its derivatives via fluoride-induced reactions under mild conditions, aiming to obtain a novel class of aryne precursors that employs an inexpensive and easy-to-handle reagent with relatively low toxicity as a substitute for trifluoromethanesulfonic anhydride, with applications in several transformations and in the total syntheses of natural products.
Results and Discussion
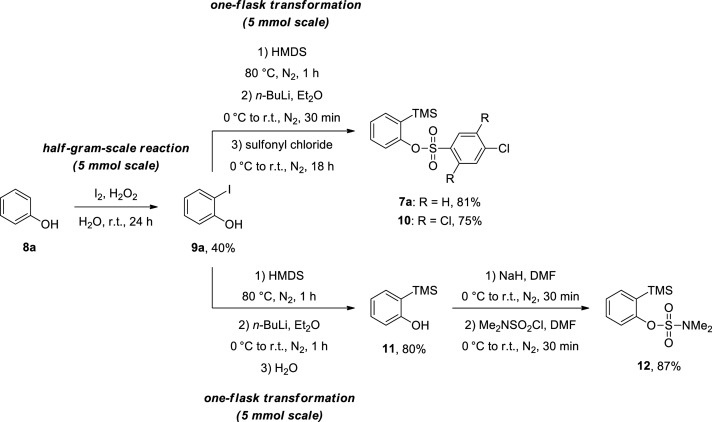
Initially, we synthesized 2-(trimethylsilyl)phenyl benzenesulfonates 7a and 10, starting with the iodination reaction of phenol (8a), which produced 2-iodophenol (9a) in 40% yield.14 Then, we performed a sequence of reactions in the same flask, which required only one purification by column chromatography, with the protection of 2-iodophenol (9a) by hexamethyldisilazane (HMDS),6,15 followed by a retro-Brook rearrangement6,15 and workup with the appropriate sulfonyl chloride to afford 2-(trimethylsilyl)phenyl benzenesulfonates 7a and 10 in yields of 81 and 75%, respectively (Scheme 2).
Scheme 2. Synthesis of 2-(Trimethylsilyl)phenyl Benzenesulfonates 7a and 10 and 2-(Trimethylsilyl)phenyl Sulfamate (12).
We prepared 2-(trimethylsilyl)phenyl dimethylsulfamate (12), inspired by one efficient aryne precursor reported by Daugulis and Mesgar.11 Thus, we carried out the protection of 2-iodophenol (9a) with HMDS,6,15 followed by an iodine-lithium exchange reaction6,15 and workup with water, leading to 2-(trimethylsilyl)phenol (11) in 80% yield. Then, compound 11 was subjected to reaction with sodium hydride, affording a phenolate ion, which was allowed to react with sulfamoyl chloride16 to provide 2-(trimethylsilyl)phenyl dimethylsulfamate (12) in 87% yield (Scheme 2).
To evaluate the efficiency of 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates (7) as aryne precursors, we selected as a model reaction the nucleophilic addition of phenol (8a) to 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a), aiming to obtain diphenyl ether (13a) in a reasonable yield (Table 1).
Table 1. Optimization of Reaction Conditions for the Preparation of Diphenyl Ether (13a)a.
| entry | benzyne precursor (7a, 10, and 12) | F– source (equiv) | additive(s) (equiv) | solvent | temp (°C) | time (h) | isolated yield of 13a (%) |
|---|---|---|---|---|---|---|---|
| 1 | 7a | CsF (3) | MeCN | r.t. | 24 | 0b | |
| 2 | 7a | CsF (3) | MeCN | 50 | 24 | 0b | |
| 3 | 7a | CsF (3) | MeCN | 80 | 24 | 24c | |
| 4 | 7a | CsF (3) | Cs2CO3 (1) | MeCN | 80 | 24 | 32c |
| 5 | 7a | CsF (3) | Cs2CO3 (2) | MeCN | 80 | 24 | 30c |
| 6 | 7a | CsF (3) | Cs2CO3 (1) | PhCN | 80 | 24 | <5b |
| 7 | 7a | CsF (3) | Cs2CO3 (1) | PhCN | 80 | 24 | 48c |
| 18-c-6 ether (1) | |||||||
| 8 | 7a | CsF (3) | Cs2CO3 (1) | PhCN | 100 | 24 | 29c |
| 18-c-6 ether (1) | |||||||
| 9 | 7a | TBAF (1.8) | THF | 0 °C to r.t. | 3 | 0b | |
| 10 | 7a | KF (1.5) | 18-c-6 ether (1.5) | THF | 0 °C to r.t. | 24 | 0b |
| 11 | 10 | CsF (3) | 18-c-6 ether (1) | PhCN | 50 | 24 | 14d,e |
| 12 | 12 | CsF (3) | 18-c-6 ether (1) | PhCN | 80 | 24 | 12f |
Reaction conditions: Phenol (8a) (0.25 mmol), benzyne precursor (7a, 10, and 12) (0.375 mmol), F– source, additive(s), and solvent (4 mL) were maintained under stirring at the temperature and time indicated.
The starting material 7a was partially recovered.
The formation of compound 14 was observed by GC/MS analysis.
The formation of compound 15 was observed by GC/MS analysis.
The formation of compound 16 was observed by GC/MS analysis.
The formation of compound 17 was observed by GC/MS analysis.
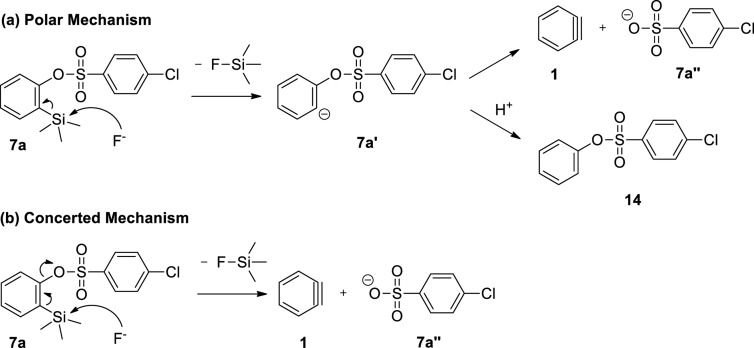
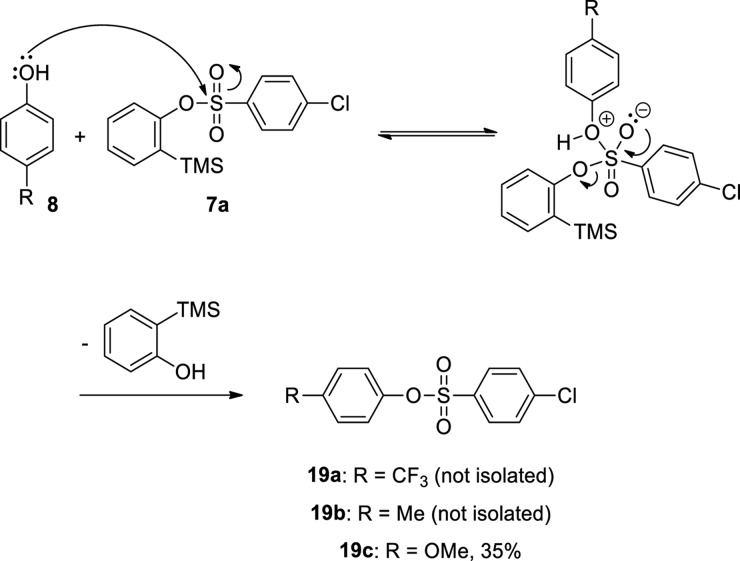
We allowed phenol (8a) to react with 1.5 equiv of 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) employing 3 equiv of cesium fluoride (CsF) in acetonitrile (MeCN) at room temperature and at 50 °C for 24 h, and we did not obtain diphenyl ether (13a) (Table 1, entries 1 and 2). However, when the same reaction was carried out at 80 °C, compound 13a was isolated in 24% yield (entry 3). Note that, as shown in entry 3, compound 14 was obtained as the major product according to GC/MS analysis. We therefore assumed that the reaction to generate benzyne (1) proceeded through a polar mechanism after compound 14 was presumably formed via a carbanion 7a′, which abstracted a relatively acidic hydrogen from the reaction medium (Scheme 3). Nevertheless, we could not rule out the formation of compound 14 through a substitution reaction between phenol (8a) and 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a). When Larock and Liu treated phenol (8a) with 2-(trimethylsilyl)phenyl triflate in the presence of CsF to produce diphenyl ether (13a) in 92% yield, they did not report the formation of phenyl triflate,17 indicating that 2-(trimethylsilyl)aryl triflates possibly generate arynes through a concerted mechanism. In addition, Lan and co-workers published a detailed theoretical work suggesting that the generation of benzyne (1) by the reaction between 2-(trimethylsilyl)phenyl triflate and CsF may proceed through a concerted transition state.18 Thus, in the current work, we considered how the mechanism of benzyne formation can be influenced by the leaving group present in the benzyne precursor.
Scheme 3. Proposed Mechanisms for the Benzyne Generation Using Compound 7a.
In an attempt to prevent the formation of compound 14, we performed a reaction with phenol (8a) and 1.5 equiv of 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) in the presence of 3 equiv of CsF using 1 equiv of cesium carbonate (Cs2CO3) in MeCN at 80 °C for 24 h, which produced diphenyl ether (13a) in 32% yield (entry 4). When 2 equiv of Cs2CO3 was used, compound 13a was obtained in a similar yield of 30% (entry 5).To avoid a possible attack of carbanion 7a′ on the relatively acidic hydrogen of acetonitrile (pKa = 31.3 in DMSO),19 we carried out the reaction between phenol (8a) and 1.5 equiv of 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) with 3 equiv of CsF and 1 equiv of Cs2CO3 using benzonitrile (PhCN) as the solvent, and diphenyl ether (13a) was obtained in less than 5% yield (entry 6). As shown in entry 6, the starting material 7a was not completely consumed. Therefore, we decided to repeat the reaction, adding 1 equiv of 18-crown-6 ether,9d which resulted in the complete consumption of starting material 7a, and diphenyl ether (13a) was isolated in 48% yield (entry 7). When the same transformation was performed at 100 °C, compound 13a was obtained in 29% yield (entry 8). Diphenyl ether (13a) was not produced when TBAF20 (entry 9) and potassium fluoride (KF)21 (entry 10) were employed as the sources of fluoride ions, and the starting material 7a was partially recovered in both experiments (Table 1).
Expecting to generate benzyne (1) through a concerted mechanism to prevent the formation of compound 14, we changed the leaving group present in 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) to 2,4,5-trichlorobenzenesulfonate, a weaker base (and presumably, a better leaving group) than 4-chlorobenzenesulfonate (7a″) (Scheme 3). Thus, we performed several experiments using 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (10) as a benzyne precursor. Nevertheless, the product of interest 13a was obtained in yields of ≤14% (Table S1 in the Supporting Information). The low yields obtained for compound 13a can be partially explained by formation of considerable amounts of compounds 15 and 16. Compound 16 was presumably obtained through an aromatic nucleophilic substitution (SNAr) reaction involving an addition–elimination mechanism between phenol (8a) and compound 15 (Table 1, entry 11).
Still attempting to generate benzyne (1) through a concerted mechanism, we changed the leaving group present in the benzyne precursor 10 to dimethylsulfamate (Scheme 2). In addition, the use of 2-(trimethylsilyl)phenyl dimethylsulfamate (12) in the nucleophilic addition reaction would prevent the formation of the undesired product 16 (Table 1, entry 11). Accordingly, we carried out various experiments using 2-(trimethylsilyl)phenyl dimethylsulfamate (12) as the benzyne precursor. However, the product of interest 13a was isolated in yields of ≤12% (Table S1 in the Supporting Information). The low yields obtained for compound 13a can be partially explained by formation of substantial amounts of compound 17 (entry 12).
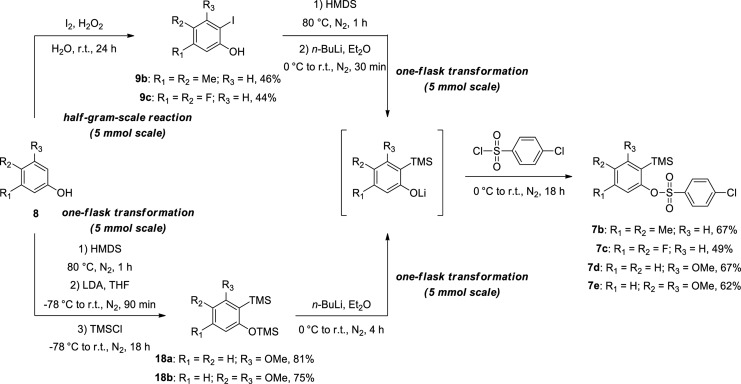
Before the reaction scope was studied, silylaryl 4-chlorobenzenesulfonates 7b and 7c were obtained by a sequence of reactions in the same flask, which required only one purification by column chromatography from iodinated phenols 9b and 9c, and silylaryl benzenesulfonates 7d and 7e were prepared through the production of disilylated intermediates 18a and 18b, as shown in Scheme 4.1g,6,15
Scheme 4. Preparation of 2-(Trimethylsilyl)aryl 4-Chlorobenzenesulfonates 7b–7e.
Employing the optimized conditions for the preparation of diphenyl ether (13a) (Table 1, entry 7), we examined the scope of nucleophilic addition reactions using various nucleophiles 8, 20a, 21, 22, and 23 and 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates (7) (Table 2).
Table 2. Preparation of Diaryl Ethers 13a–13s by Nucleophilic Addition Reactionsa.

Reaction conditions: Nucleophile (8, 20a, and 21–23) (0.25 mmol), 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonate (7a–7e) (0.375 mmol), CsF (0.75 mmol), Cs2CO3 (0.25 mmol), and 18-crown-6 ether (0.25 mmol) in PhCN (4 mL) were maintained under stirring at 80 °C for 24 h.
Isolated yield.
10 mmol scale.
The reactions using 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) and phenols substituted with electron-withdrawing groups led to the formation of nucleophilic addition products 13b and 13c in yields of 51 and 70%, respectively. However, when phenols substituted with electron-donating groups were employed, diphenyl ethers 13d and 13e were achieved in 24 and 30% yields, respectively. In the reactions to obtain compounds 13b, 13d, and 13e, undesired 4-chlorobenzenesulfonates (19) were generated, presumably by the substitution reaction between the phenols 8 and silylphenyl benzenesulfonate 7a, according to GC/MS analyses. The low yields for O-arylation products 13d and 13e can be partially explained by the formation of 4-chlorobenzenesulfonates 19b and 19c in considerable amounts. To provide quantitative information concerning the formation of 4-chlorobenzenesulfonates (19), compound 19c was isolated in 35% yield (Scheme 5). Note that, for the reactions involving the preparation of compounds 13a–13e, we observed the formation of compound 14 by GC/MS analyses (Table 2).
Scheme 5. Proposed Mechanism for the Formation of 4-Chlorobenzenesulfonates 19a–19c.
In the sequence, we performed nucleophilic addition reactions between 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) and halogenated phenols, which resulted in diphenyl ethers 13f and 13g in 80 and 89% yields, respectively. We also carried out the reaction between 2-bromophenol and compound 7a on a gram scale (10 mmol), which provided diphenyl ether 13g in 46% yield. The reactions using 4,5-dimethyl-2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7b) led to compounds 13h–13j in yields of 26 to 76%. As expected, the reaction performed with 4-methoxyphenol showed lower yield compared to the reaction carried out with 4-nitrophenol. We allowed phenol to react with 4,5-difluoro-2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7c), and we did not obtain compound 13k. In this experiment, the attack of phenol on the sulfur atom of the silylaryl benzenesulfonate 7c presumably led to the formation of phenyl 4-chlorobenzenesulfonate (14) in a considerable amount according to GC/MS analysis (Table 2).
We performed reactions with 3-methoxy-2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7d), which resulted in O-arylation products 13l and 13m in 51 and 92% yields, respectively. These reactions were highly regioselective, and mixtures of regioisomers were not detected (GC/MS). When the reaction was carried out using 3,4-dimethoxy-2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7e), we obtained diphenyl ethers 13n and 13o in 55 and 78% yields, respectively. These reactions were also highly regioselective, and mixtures of regioisomers were not detected (GC/MS). In addition to the reactions with phenols, we performed the nucleophilic addition reaction between 2-(trimethylsilyl)phenyl benzenesulfonate (7a) and aniline (20a), which is a stronger nucleophile than phenol, resulting in diphenylamine (13p) in 27% yield. When benzoic acid (21) was used as the nucleophile, we obtained phenyl benzoate (13q) in 28% yield. We subjected benzamide (22) to a nucleophilic addition reaction with 2-(trimethylsilyl)phenyl benzenesulfonate (7a); however, the product of interest 13r was not obtained. When benzenesulfonic acid (23) was used as the nucleophile, compound 13s was obtained in less than 5% yield (Table 2).
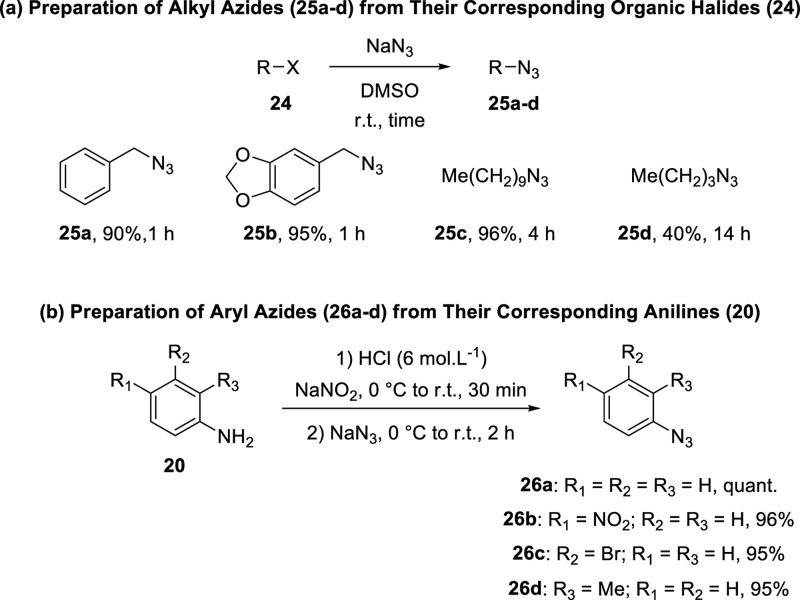
Next, we subjected 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates (7) to [3 + 2] cycloaddition reactions with alkyl azides 25 and aryl azides 26 to obtain cycloadducts 27.8,22 Azides 25 and 26 were prepared according to the procedures described in the literature (Scheme 6).23−25
Scheme 6. Preparation of Alkyl Azides 25a–25d and Aryl Azides 26a–26d.
The [3 + 2] cycloaddition reaction between benzyl azide (25a) and 1.5 equiv of 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) was performed in the presence of 3 equiv of CsF, 1 equiv of 18-crown-6 ether, and 1 equiv of Cs2CO3 in PhCN at 80 °C for 24 h, and benzotriazole 27a was obtained in 60% yield. When the same transformation was carried out without the addition of Cs2CO3, we obtained benzotriazole 27a also in 60% yield. The optimal conditions for the [3 + 2] cycloaddition reaction between azides 25–26 and 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates (7) were evaluated in the preparation of cycloadducts 27 (Table 3).
Table 3. Preparation of Triazoles 27a–27l by the [3 + 2] Cycloaddition Reactiona.

Reaction conditions: Azide (25–26) (0.25 mmol), 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonate (7a–7e) (0.375 mmol), CsF (0.75 mmol), and 18-crown-6 ether (0.25 mmol) in PhCN (4 mL) were maintained under stirring at 80 °C for 24 h.
Isolated yield.
The reactions using alkyl azides 25 and 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) led to the formation of benzotriazoles 27a–27d in yields of 58 to 63%. When the [3 + 2] cycloaddition reaction was performed between benzyl azide (25a) and 4,5-dimethyl-2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7b), we obtained benzotriazole 27e in 52% yield. However, when the reaction was carried out between benzyl azide (25a) and 4,5-difluoro-2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7c), the cycloadduct 27f was isolated in only 25% yield. In the reaction performed between benzyl azide (25a) and 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates 7d and 7e, we obtained benzotriazoles 27g and 27h in yields of 53 and 56%, respectively. These reactions were highly regioselective, and mixtures of regioisomers were not detected (GC/MS). When the reaction between phenyl azide (26a) and 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) was carried out, benzotriazole 27i was obtained in 50% yield. The [3 + 2] cycloaddition reactions between substituted aryl azides 26b–26d and compound 7a gave benzotriazoles 27j–27l in yields of 31 to 80%. The lower yield obtained for compound 27l is ascribed to the use of the sterically hindered ortho-substituted azide 26d (Table 3).
In addition to reactions with azides 25 and 26, we performed [4 + 2] cycloaddition reactions between furans 28 and 2-(trimethylsilyl)aryl benzenesulfonates (7),8,10 and the results achieved are presented in Table 4.
Table 4. Preparation of Adducts 29a–29g by the [4 + 2] Cycloaddition Reactiona.
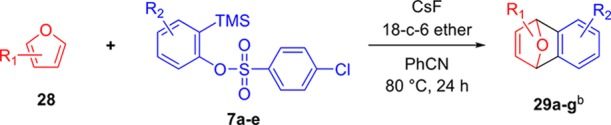
Reaction conditions: Furan (28) (0.25 mmol), 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonate (7a–7e) (0.375 mmol), CsF (0.75 mmol), and 18-crown-6 ether (0.25 mmol) in PhCN (4 mL) were maintained under stirring at 80 °C for 24 h.
Isolated yield.
The reactions using furans 28 and 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) resulted in cycloaddition adducts 29a–29c in yields of 69 to 77%. When the [4 + 2] cycloaddition reaction was performed between furan and 4,5-dimethyl-2-(trimethylsilyl)aryl 4-chlorobenzenesulfonate (7b), the cycloaddition adduct 29d was obtained in 63% yield. However, when the reaction was carried out between furan and 4,5-difluoro-2-(trimethylsilyl)aryl 4-chlorobenzenesulfonate (7c), the cycloadduct 29e was obtained in only 11% yield. When the [4 + 2] cycloaddition reaction was performed between furan and 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates 7d and 7e, the cycloaddition adducts 29f and 29g were obtained in yields of 75 and 69%, respectively (Table 4).
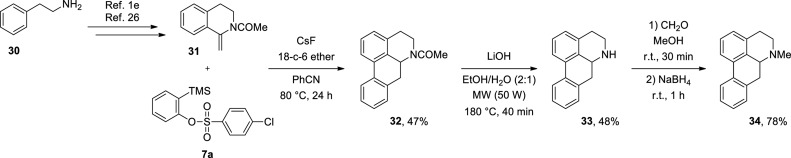
To highlight the applicability of 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates (7), we employed 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) in the total synthesis of the alkaloid (±)-aporphine (34).1e,26 Initially, we prepared 1-methylene-1,2,3,4-tetrahydroisoquinoline 31 from amine 30, as described in the literature.1e,26 Then, we subjected compound 31 to the reaction with compound 7a in the presence of 3 equiv of CsF and 1 equiv of 18-crown-6 ether in PhCN at 80 °C for 24 h, which led to the formation of intermediate 32 in 47% yield. Subsequently, compound 32 was subjected to a basic hydrolysis reaction,1e,26 resulting in (±)-noraporphine (33) in 48% yield. Then, we carried out the N-methylation reaction of intermediate 33 using formaldehyde and sodium borohydride,1e,26 and the alkaloid (±)-aporphine (34) was obtained in 78% yield (Scheme 7).
Scheme 7. Total Synthesis of Alkaloid (±)-Aporphine (34).
Conclusions
In summary, 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates were obtained by well-established synthetic routes and emerged as a novel class of aryne precursors, allowing the formation of benzyne and its derivatives under mild conditions. The novel aryne precursors were obtained from phenols and 4-chlorobenzenesulfonyl chloride, an inexpensive and easy-to-handle reagent with relatively low toxicity that was used as a substitute for the trifluoromethanesulfonic anhydride. All the aryne precursors obtained were subjected to nucleophilic addition reactions, providing addition products in yields of 24 to 92%, and to cycloaddition reactions, affording cycloadducts in yields up to 80%. In addition, 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate was successfully employed in the total synthesis of (±)-aporphine. During the development of this novel class of aryne precursors, we assumed a concerted mechanism for the generation of arynes from 2-(trimethylsilyl)aryl triflates and proposed a polar mechanism for the generation of arynes from 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates. The chemistry reported here complements the classic methods for the generation of arynes and can be applied to the syntheses of molecules with interesting biological properties and to the preparations of useful functional materials.
Experimental Section
General Information
The 1H nuclear magnetic resonance (NMR) spectra were recorded on spectrometers operating at 500 or 300 MHz. 13C NMR spectra were recorded on spectrometers operating at 125 or 75 MHz. The 1H NMR spectra were taken in deuterated solvents, and the chemical shifts were given in parts per million with respect to tetramethylsilane (TMS) used as an internal standard. The 13C NMR spectra were taken in deuterated solvents, and the chemical shifts were given in parts per million with respect to the deuterated solvent used as a reference. The infrared spectra were obtained using attenuated total reflectance (ATR) or KBr pellets in the 4000–400 cm–1 region. The mass spectra were carried out employing a gas chromatograph connected to a mass spectrometer using electron impact ionization at 70 eV. The high-resolution mass spectra were obtained using a time-of-flight mass spectrometer. Melting point values are uncorrected. Microwave-assisted reactions were carried out in sealed vessels (10 mL) using a CEM Discover reactor. Column chromatography separations were carried out using 70–230 mesh silica gel 60. Preparative thin-layer chromatography separations were carried out using silica gel matrix with an inorganic binder and fluorescent indicator. 18-Crown-6 ether was dried and stored over phosphorus pentoxide (P2O5) using a vacuum desiccator. All other commercially obtained reagents were employed without further purification. High-purity cesium fluoride (99.99%) was used in the experiments. THF and diethyl ether were distilled from sodium/benzophenone under a nitrogen atmosphere before use.27n-Butyllithium (n-BuLi) was titrated against sec-butanol using 1,10-phenanthroline as an indicator under a nitrogen atmosphere.28 Lithium diisopropylamide (LDA) was generated following a typical procedure prior to use.29 Acetonitrile and benzonitrile were distilled from calcium hydride under a nitrogen atmosphere before use.27 Solvents were treated when necessary according to the literature.27
Procedure for the Preparation of 2-Iodophenols (9a–9c)14
To a solution containing the appropriate phenol 8 (5 mmol) and molecular iodine (2.5 mmol, 635 mg) in distilled water (50 mL) was added 30% (w/v) aqueous solution of hydrogen peroxide (5 mmol, 0.6 mL). The mixture was maintained under magnetic stirring at room temperature for 24 h. Afterward, a 10% (w/v) aqueous solution of sodium thiosulfate (50 mL) was added to the mixture, which was extracted with ethyl acetate (3 × 100 mL). The organic phase was dried over anhydrous magnesium sulfate (MgSO4). After filtration, the solvent was evaporated under reduced pressure. The residue obtained was purified by column chromatography on silica gel 60 using an appropriate eluent, affording the iodinated phenols of interest 9a–9c.
2-Iodophenol (9a)
Rf = 0.37 (eluent: hexane/methanol (24:1)); yield: 442 mg (40%); off-white solid; mp 40–41 °C (lit.14 mp 40–42 °C); 1H NMR (300 MHz, CDCl3): δ 7.66 (dd, J = 8.0 Hz, 1.3 Hz, 1H), 7.24 (td, J = 7.7 Hz, 1.3 Hz, 1H), 7.00 (dd, J = 8.1 Hz, 1.2 Hz, 1H), 6.68 (td, J = 7.6 Hz, 1.2 Hz, 1H), 5.29 (s, 1H); 13C{1H} NMR (75 MHz, CDCl3): δ 154.7, 138.3, 130.1, 122.4, 115.1, 85.7; IR (KBr, cm–1): 3331, 3057, 2924, 2847, 1574, 1468, 1443, 1194, 1179, 1015, 820, 746; GC/MS (m/z, %): 220 (100.0), 127 (6.3), 93 (37.1), 65 (67.2), 53 (8.4).
2-Iodo-4,5-dimethylphenol (9b)
Rf = 0.42 (eluent: hexane/ethyl acetate (9:1)); yield: 575 mg (46%); yellowish solid; mp 60–61 °C (lit.30 mp 61–63.5 °C); 1H NMR (300 MHz, CDCl3): δ 7.39 (s, 1H), 6.79 (s, 1H), 5.08 (s, 1H), 2.19 (s, 3H), 2.15 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 152.8, 139.0, 138.3, 130.9, 116.1, 81.3, 19.6, 18.4; IR (KBr, cm–1): 3421, 2966, 2938, 1639, 1595, 1394, 1271, 1192, 964; GC/MS (m/z, %): 248 (100.0), 233 (16.4), 121 (40.5), 91 (27.9), 77 (21.8).
4,5-Difluoro-2-iodophenol (9c)
Rf = 0.41 (eluent: hexane/dichloromethane (1:1)); yield: 561 mg (44%); yellowish oil;311H NMR (300 MHz, CDCl3): δ 7.45 (t, J = 8.8 Hz, 1H), 6.85 (dd, J = 11.3 Hz, 7.0 Hz, 1H), 5.28 (s, 1H); 13C{1H} NMR (75 MHz, CDCl3): δ 151.7 (dd, J = 9.8 Hz, 3 Hz, ArF), 151.1 (dd, J = 247.9 Hz, 13.9 Hz, ArF), 144.8 (dd, J = 244.1 Hz, 13.1 Hz, ArF), 125.3 (dd, J = 20.3 Hz, 1.2 Hz, ArF), 103.9 (d, J = 21.0 Hz, ArF), 76.7; IR (ATR, cm–1): 3483, 3119, 3057, 1599, 1499, 1483, 1296, 1188, 1136, 866, 799; GC/MS (m/z, %): 256 (100.0), 227 (2.8), 129 (23.4), 101 (36.7), 75 (12.1), 51 (7.2).
Procedure for the Preparation of Disilylated Compounds (18a and 18b)6l
A solution containing the appropriate phenol 8 (5 mmol) and HMDS (7.5 mmol, 1.21 g, 1.6 mL) was maintained under a nitrogen atmosphere and magnetic stirring at 80 °C for 2 h. Subsequently, the volatile residues were removed under reduced pressure (20 mmHg) for 30 min. In the same flask, THF (7 mL) was added, and the solution was cooled to −78 °C. After that, LDA (5.5 mmol, 9.2 mL of a 0.6 M solution in THF) was added. The resulting mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 90 min. Then, the mixture was cooled to −78 °C, and trimethylsilane chloride (TMSCl) (6.0 mmol, 652 mg, 0.76 mL) was added. The reaction mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 18 h. Afterward, a saturated aqueous solution of ammonium chloride (15 mL) was added to the reaction, which was extracted with ethyl acetate (3 × 50 mL). The organic phase was dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure. The residue obtained was purified by column chromatography on silica gel 60 using hexane/dichloromethane (3:1) as an eluent to afford the disilylated compounds of interest 18a and 18b.
(3-Methoxy-2-(trimethylsilyl)phenoxy)trimethylsilane (18a)
Rf = 0.69 (eluent: hexane/dichloromethane (3:1)); yield: 1.09 g (81%); colorless oil;1g1H NMR (300 MHz, CDCl3): δ 7.15 (t, J = 8.1 Hz, 1H), 6.45 (d, J = 8.2 Hz, 1H), 6.41 (d, J = 8.1 Hz, 1H), 3.73 (s, 3H), 0.30 (s, 9H), 0.28 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 165.7, 161.2, 130.8, 116.7, 110.8, 103.2, 55.1, 1.5, 0.7; IR (ATR, cm–1): 3082, 3051, 2955, 2897, 2832, 1570, 1431, 1234, 1088, 833; GC/MS (m/z, %): 268 (40.9), 253 (100.0), 237 (16.5), 223 (61.1), 207 (52.9).
(3,4-Dimethoxy-2-(trimethylsilyl)phenoxy)trimethylsilane (18b)
Rf = 0.50 (eluent: hexane/dichloromethane (3:1)); yield: 1.12 g (75%); colorless oil;1e1H NMR (300 MHz, CDCl3): δ 6.63 (d, J = 8.7 Hz, 1H), 6.32 (d, J = 8.7 Hz, 1H), 3.65 (s, 3H), 3.63 (s, 3H), 0.17 (s, 9H), 0.15 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 154.6, 154.1, 146.8, 123.2, 114.4, 112.1, 60.8, 56.1, 1.4, 0.6; IR (ATR, cm–1): 2955, 2897, 2832, 1574, 1458, 1429, 1238, 1059, 833, 750, 687; GC/MS (m/z, %): 298 (98.0), 283 (92.0), 268 (23.4), 253 (100.0), 238 (8.7).
Procedure for the Preparation of 2-(Trimethylsilyl)aryl 4-Chlorobenzenesulfonates (7a–7c) and 2-(Trimethylsilyl)phenyl-2,4,5-chlorobenzenesulfonate (10) from the Monoiodinated Phenolic Compounds 9a–9c6,15
A solution containing the appropriate phenols 9a–9c (5 mmol) and HMDS (7.5 mmol, 1.21 g, 1.6 mL) was maintained under a nitrogen atmosphere and magnetic stirring at 80 °C for 1 h. Subsequently, the volatile residues were removed under reduced pressure (20 mmHg) for 30 min. In the same flask, ethyl ether (50 mL) was added, and the solution was cooled to 0 °C and maintained under a nitrogen atmosphere and magnetic stirring. Then, n-BuLi (5.25 mmol, 2.5 mL of a 2.1 M solution in hexane) was added slowly. The resulting mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 30 min. Then, the mixture was cooled to 0 °C, and the appropriate benzenesulfonyl chloride (6 mmol) was added. The reaction mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 18 h. Afterward, a saturated aqueous solution of ammonium chloride (50 mL) was added to the reaction, which was extracted with ethyl acetate (3 × 50 mL). The organic phase was dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure. The residue obtained was purified by column chromatography on silica gel 60 using an appropriate eluent, affording the 2-(trimethylsilyl)aryl benzenesulfonates of interest 7a–7c and 10.
2-(Trimethylsilyl)phenyl 4-Chlorobenzenesulfonate (7a)
Rf = 0.38 (eluent: hexane/methanol (49:1)); yield: 1.38 g (81%); off-white solid; mp 44–45 °C (lit.32 mp 114–115 °C); 1H NMR (300 MHz, CDCl3): δ 7.84–7.80 (m, 2H), 7.48–7.43 (m, 2H), 7.38 (dd, J = 7.1 Hz, 1H), 7.20 (td, J = 7.7 Hz, 2.0 Hz, 1H), 7.13 (td, J = 7.2 Hz, 1.2 Hz, 1H), 6.99 (dd, J = 8.1 Hz, 1.0 Hz, 1H), 0.19 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 155.0, 140.8, 136.0, 135.4, 132.5, 130.6, 129.6, 129.6, 126.3, 119.2, −0.7; IR (KBr, cm–1): 3092, 3065, 2955, 2899, 1591, 1425, 1377, 1196, 1067, 841, 559; GC/MS (m/z, %): 340 (0.1), 325 (100.0), 166 (30.4), 150 (25.8), 135 (30.8); HRMS (ESI): calcd for [C15H18ClO3SSi]+, 341.0429, 343.0400; found, 341.0425, 343,0402.
4,5-Dimethyl-2-(trimethylsilyl)phenyl 4-Chlorobenzenesulfonate (7b)
Rf = 0.43 (eluent: hexane/methanol (49:1)); yield: 1.24 g (67%); off-white solid; mp 87–89 °C; 1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 8.6 Hz, 2H), 7.54 (d, J = 8.6 Hz, 2H), 7.17 (s, 1H), 6.85 (s, 1H), 2.22 (s, 3H), 2.17 (s, 3H), 0.24 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 153.0, 140.7, 139.5, 136.8, 135.6, 134.7, 129.7, 129.5, 129.1, 120.5, 19.9, 19.1, −0.5; IR (KBr, cm–1): 3092, 2957, 2900, 2860, 1585, 1475, 1373, 1192, 1085, 827, 588, 484; GC/MS (m/z, %): 368 (2.3), 353 (100.0), 194 (26.7), 177 (24.7), 163 (21.3); HRMS (ESI): calcd for [C17H22ClO3SSi]+, 369.0742, 371.0713; found, 369.0736, 371.0724.
4,5-Difluoro-2-(trimethylsilyl)phenyl 4-Chlorobenzenesulfonate (7c)
Rf = 0.54 (eluent: hexane/methanol (49:1)); yield: 924 mg (49%); yellowish solid; mp 64–66 °C; 1H NMR (300 MHz, CDCl3): δ 7.91 (d, J = 8.6 Hz, 2H), 7.58 (d, J = 8.6 Hz, 2H), 7.21 (t, J = 9.7 Hz, 1H), 7.02 (dd, J = 10.9 Hz, 6.2 Hz, 1H), 0.25 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 150.3 (dd, J = 252.0 Hz, 14.3 Hz, ArF), 149.5 (dd, J = 7.5 Hz, 3.0 Hz, ArF), 148.5 (dd, J = 248.6 Hz, 11.6 Hz, ArF), 141.4, 134.6, 129.8, 129.8 (d, J = 2.3 Hz, ArF), 129.7, 123.2 (d, J = 15.8 Hz, ArF), 109.8 (d, J = 20.3 Hz, ArF), −0.9; IR (KBr, cm–1): 3092, 2957, 2900, 1606, 1585, 1494, 1381, 1190, 1087, 1018, 840, 611, 486; GC/MS (m/z, %): 361 (100.0), 202 (35.3), 186 (28.7), 171 (32.7), 111 (44.0); HRMS (ESI): calcd for [C15 H16ClF2O3SSi]+, 377.0241, 379.0212; found, 377.0238, 379.0227.
2-(Trimethylsilyl)phenyl 2,4,5-Trichlorobenzenesulfonate (10)
Rf = 0.27 (eluent: hexane); yield: 1.54 g (75%); brownish solid; mp 93–95 °C; 1H NMR (300 MHz, CDCl3): δ 8.17 (s, 1H), 7.76 (s, 1H), 7.53–7.50 (m, 1H), 7.30–7.26 (m, 2H), 6.83–6.80 (m, 1H), 0.34 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 154.9, 139.4, 136.2, 135.3, 133.5, 133.0, 132.2, 132.1, 131.9, 130.7, 126.7, 119.2, −0.6; IR (KBr, cm–1): 3086, 2955, 2899, 1564, 1439, 1383, 1192, 1069, 843, 588; GC/MS (m/z, %): 395 (57.4), 166 (100.0), 150 (46.4), 135 (81.3), 91 (71.9); HRMS (ESI): calcd for [C15H16Cl3O3SSi]+, 408.9650, 410.9620; found, 408.9662, 420,9630.
Procedure for the Preparation of 2-(Trimethylsilyl)aryl 4-Chlorobenzenesulfonates (7d and 7e) from the Disilylated Compounds 18a and 18b(6,15)
In a flask containing the appropriate disilylated compounds 18a and 18b (5 mmol) was added ethyl ether (50 mL). The solution was cooled to 0 °C under a nitrogen atmosphere and magnetic stirring. Then, n-BuLi (5.5 mmol, 2.6 mL of a 2.1 M solution in hexane) was added slowly. The resulting mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 2 h. Then, the mixture was cooled to 0 °C, and 4-chlorobenzenesulfonyl chloride (6 mmol, 1.26 g) was added. The reaction mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 18 h. Afterward, a saturated aqueous solution of ammonium chloride (50 mL) was added to the reaction, which was extracted with ethyl ether (3 × 50 mL). The organic phase was dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure. The obtained residue was purified by column chromatography on silica gel 60 using an appropriate eluent to afford silylphenyl benzenesulfonates of interest 7d and 7e.
3-Methoxy-2-(trimethylsilyl)phenyl 4-Chlorobenzenesulfonate (7d)
Rf = 0.36 (eluent: hexane/methanol (49:1)); yield: 1.24 g (67%); off-white solid; mp 55–57 °C; 1H NMR (300 MHz, CDCl3): δ 7.78 (d, J = 8.6 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 7.20 (t, J = 8.2 Hz, 1H), 6.73 (d, J = 8.2 Hz, 1H), 6.55 (d, J = 8.2 Hz, 1H), 3.78 (s, 3H), 0.26 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 165.4, 154.6, 140.7, 134.8, 131.0, 130.0, 129.4, 121.2, 113.5, 108.6, 55.4, 0.9; IR (KBr, cm–1): 3086, 2957, 2902, 1591, 1429, 1377, 1200, 1067, 852, 621, 543; GC/MS (m/z, %): 355 (100.0), 180 (49.6), 165 (16.5), 135 (21.5), 91 (7.7). HRMS (ESI): calcd for [C16H20ClO4SSi]+, 371.0535, 373.0506; found, 371.0545, 373.0508.
3,4-Dimethoxy-2-(trimethylsilyl)phenyl 4-Chlorobenzenesulfonate (7e)
Rf = 0.41 (eluent: hexane/ethyl acetate (9:1)); yield: 1.24 g (62%); yellowish oil; 1H NMR (300 MHz, CDCl3): δ 7.79–7.76 (m, 2H), 7.52–7.49 (m, 2H), 6.77 (d, J = 8.9 Hz, 1H), 6.64 (d, J = 8.9 Hz, 1H), 3.83 (s, 3H), 3.82 (s, 3H), 0.27 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 154.2, 150.8, 146.9, 140.7, 134.8, 130.0, 129.4, 127.0, 116.5, 113.4, 60.8, 55.8, 1.1; IR (ATR, cm–1): 3094, 3076, 2941, 2900, 1587, 1574, 1454, 1429, 1367, 1200, 1088, 941, 546; GC/MS (m/z, %): 400 (9.4), 385 (59.7), 225 (16.5), 210 (100.0), 195 (51.4); HRMS (ESI): calcd for [C17H22ClO5SSi]+, 401.0640, 403.0611; found, 401.0640, 403.0612.
2-(Trimethylsilyl)phenol (11)6l,11
A solution containing 2-iodophenol (9a) (5 mmol, 1.10 g) and HMDS (7.5 mmol, 1.21 g, 1.6 mL) was maintained under a nitrogen atmosphere and magnetic stirring at 80 °C for 1 h. Subsequently, the volatile residues were removed under reduced pressure (20 mmHg) for 30 min. In the same flask, ethyl ether (50 mL) was added, and the solution was cooled to 0 °C under a nitrogen atmosphere and magnetic stirring. Then, n-BuLi (5.25 mmol, 2.5 mL of a 2.1 M solution in hexane) was added slowly. The resulting mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 2 h. Afterward, distilled water (50 mL) was added to the mixture, which was extracted with ethyl ether (3 × 50 mL). The organic phase was dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure. The residue obtained was purified by column chromatography on silica gel 60 using hexane/ethyl acetate (9:1) as an eluent, affording 2-(trimethylsilyl)phenol (11). Rf = 0.46 (eluent: hexane/ethyl acetate (9:1)); yield: 664 mg (80%); colorless oil;9d1H NMR (300 MHz, CDCl3): δ 7.37 (dd, J = 7.2 Hz, 1.3 Hz, 1H), 7.22 (td, J = 7.7 Hz, 1.4 Hz, 1H), 6.92 (t, J = 7.3 Hz, 1H), 6.64 (d, J = 8.0 Hz, 1H), 4.83 (s, 1H), 0.31 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 160.3, 135.3, 130.7, 125.4, 120.5, 114.4, −1.0; IR (ATR, cm–1): 3470, 3067, 3017, 2955, 2897, 1595, 1572, 1472, 1437, 1323, 1246, 1122, 1072, 852, 754, 621; GC/MS (m/z, %): 166 (27.4), 151 (100.0), 133 (75.0), 123 (72.0), 91 (43.2).
2-(Trimethylsilyl)phenyl Dimethylsulfamate (12)16
To a flask were added sodium hydride (NaH) (5.5 mmol, 132 mg) and DMF (15 mL). The mixture was cooled to 0 °C under a nitrogen atmosphere and magnetic stirring. Then, a solution of 2-(trimethylsilyl)phenol (11) (5 mmol, 832 mg) in DMF (2.5 mL) was added dropwise. The resulting mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 30 min. Then, after cooling to 0 °C, dimethylsulfamoyl chloride (5.5 mmol, 790 mg, 0.6 mL) was added. The mixture was heated to room temperature and maintained under a nitrogen atmosphere and magnetic stirring for 30 min. Afterward, a saturated solution of ammonium chloride (10 mL) and distilled water (75 mL) was added to the reaction, which was extracted with ethyl ether (3 × 100 mL). The organic phase was dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure. The residue obtained was purified by column chromatography on silica gel 60 using hexane/ethyl acetate (9:1) as an eluent to afford 2-(trimethylsilyl)phenyl dimethylsulfamate (12). Rf = 0.40 (eluent: hexane/ethyl acetate (9:1)); yield: 1.19 g (87%); yellowish oil;331H NMR (300 MHz, CDCl3): δ 7.52 (d, J = 8.3 Hz, 1H), 7.47 (dd, J = 7.2 Hz, 1.7 Hz, 1H), 7.38 (td, J = 7.8 Hz, 1.7 Hz, 1H), 7.20 (t, J = 7.3 Hz, 1H), 3.05 (s, 6H), 0.35 (s, 9H); 13C{1H} NMR (75 MHz, CDCl3): δ 156.1, 135.6, 130.8, 130.7, 125.4, 118.7, 38.6, −0.4; IR (ATR, cm–1): 3061, 2955, 2899, 2857, 1591, 1566, 1467, 1435, 1371, 1250, 1126, 1070, 868, 841, 750; GC/MS (m/z, %): 273 (1.2), 258 (100.0), 179 (16.8), 151 (21.1), 135 (23.7).
Procedure for Nucleophilic Addition Reaction with Arynes17
To a vial (10 mL) were added the appropriate nucleophiles 8, 20a, and 21–23 (0.25 mmol), the appropriate 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates 7a–7e (0.375 mmol), 18-crown-6 ether (0.25 mmol, 66.0 mg), cesium carbonate (0.25 mmol, 81.5 mg), anhydrous benzonitrile (4 mL), and cesium fluoride (0.75 mmol, 114 mg). The vial was capped, and the mixture was maintained under magnetic stirring at 80 °C for 24 h. Afterward, a saturated sodium chloride solution (20 mL) was added to the mixture, which was extracted with ethyl ether (3 × 20 mL). The organic phase was dried over MgSO4. After filtration, the ethyl ether was evaporated under reduced pressure, and the benzonitrile was removed by horizontal distillation under vacuum. The residue obtained was purified by preparative thin-layer chromatography with silica gel using an appropriate eluent, affording the nucleophilic addition products 13a–13s.
Oxidibenzene (13a)
Rf = 0.50 (eluent: hexane); yield: 20.4 mg (48%); yellowish oil;17b1H NMR (300 MHz, CDCl3): δ 7.36–7.29 (m, 4H), 7.12–7.07 (m, 2H), 7.03–6.98 (m, 4H); 13C{1H} NMR (75 MHz, CDCl3): δ 157.2, 129.7, 123.2, 118.9; IR (ATR, cm–1): 3067, 3038, 2924, 2853, 1584, 1487, 1235, 1163, 1072, 866, 748, 690; GC/MS (m/z, %): 170 (100.0), 141 (59.4), 115 (14.2), 77 (31.4), 51 (25.4).
Phenyl 4-Chlorobenzenesulfonate (14)
Rf = 0.25 (eluent: hexane); yellowish solid; mp 86–88 °C (lit.34 mp 82 °C); 1H NMR (300 MHz, CDCl3): δ 7.76 (d, J = 8.6 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 7.33–7.24 (m, 3H), 6.99 (d, J = 6.8 Hz, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 149.4, 141.0, 133.8, 129.9, 129.8, 129.5, 127.4, 122.3; IR (KBr, cm–1): 3096, 3057, 2953, 2853, 1585, 1476, 1381, 1200, 1088, 858, 783, 623; GC/MS (m/z, %): 268 (38.7), 204 (9.3), 175 (100.0), 111 (99.3), 75 (25.7). Undesired product formed in reactions performed to obtain compounds 13a–13e.
1-Phenoxy-4-(trifluoromethyl)benzene (13b)
Rf = 0.62 (eluent: hexane); yield: 30.5 mg (51%); yellowish oil;351H NMR (300 MHz, CDCl3): δ 7.57 (d, J = 8.5 Hz, 2H), 7.42–7.35 (m, 2H), 7.21–7.16 (m, 1H), 7.07–7.03 (m, 4H); 13C{1H} NMR (75 MHz, CDCl3): δ 160.5, 155.8, 130.1, 127.1 (q, J = 3.8 Hz, ArF), 124.9 (q, J = 32.5 Hz, ArF), 124.5, 124.2 (q, J = 269.8 Hz, CF3), 119.9, 117.9; IR (ATR, cm–1): 3055, 2928, 2855, 1618, 1589, 1512, 1491, 1325, 1246, 1167, 1123, 1065, 872, 841, 739; GC/MS (m/z, %): 238 (93.2), 210 (28.0), 169 (22.1), 141 (65.0), 77 (100.0).
1-Nitro-4-phenoxybenzene (13c)
Rf = 0.39 (eluent: hexane); yield: 37.6 mg (70%); yellowish solid; mp 57–58 °C (lit.17b mp 58–59 °C); 1H NMR (300 MHz, CDCl3): δ 8.22–8.17 (m, 2H), 7.43 (t, J = 7.9 Hz, 2H), 7.25 (t, J = 7.4 Hz, 1H), 7.09 (d, J = 7.7 Hz, 2H), 7.03–6.98 (m, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 163.3, 154.7, 142.6, 130.3, 125.9, 125.4, 120.5, 117.0; IR (KBr, cm–1): 3074, 2922, 2837, 1610, 1582, 1483, 1341, 1246, 1163, 1070, 874, 748, 488; GC/MS (m/z, %): 215 (94.9), 185 (51.4), 141 (31.5), 115 (84.0), 77 (100.0).
1-Methyl-4-phenoxybenzene (13d)
Rf = 0.62 (eluent: hexane/ethyl acetate (9:1)); yield: 11.0 mg (24%); yellowish oil;361H NMR (300 MHz, CDCl3): δ 7.30 (t, J = 7.9 Hz, 2H), 7.13 (d, J = 8.3 Hz, 2H), 7.05 (t, J = 7.4 Hz, 1H), 6.97 (dd, J = 8.2 Hz, 0.8 Hz, 2H), 6.91 (d, J = 8.4 Hz, 2H), 2.33 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 157.8, 154.7, 132.9, 130.2, 129.6, 122.8, 119.1, 118.3, 20.7; IR (ATR, cm–1): 3061, 3030, 2922, 2857, 1589, 1489, 1281, 1238, 1165, 1072, 870, 756, 691; GC/MS (m/z, %): 184 (100.0), 169 (6.1), 141 (17.2), 91 (91.8), 77 (22.6).
1-Methoxy-4-phenoxybenzene (13e)
Rf = 0.62 (eluent: hexane/ethyl acetate (9:1)); yield: 14.9 mg (30%); yellowish oil;17b1H NMR (300 MHz, CDCl3): δ 7.28 (t, J = 8.0 Hz, 2H), 7.05–6.85 (m, 7H), 3.78 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 158.5, 155.9, 150.1, 129.6, 122.4, 120.8, 117.5, 114.8, 55.6; IR (ATR, cm–1): 3061, 2934, 2833, 1589, 1504, 1487, 1219, 1163, 1072, 1035, 872, 756, 691; GC/MS (m/z, %): 200 (100.0), 185 (59.1), 141 (1.6), 129 (37.2), 77 (48.1).
4-Methoxyphenyl 4-Chlorobenzenesulfonate (19c)
Rf = 0.33 (eluent: hexane/ethyl acetate (9:1)); brownish solid; mp 88–90 °C (lit.37 mp 90–91 °C); 1H NMR (300 MHz, CDCl3): δ 7.74 (d, J = 8.6 Hz, 2H), 7.49 (d, J = 8.6 Hz, 2H), 6.89 (d, J = 9.1 Hz, 2H), 6.78 (d, J = 9.2 Hz, 2H), 3.77 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 158.4, 142.8, 140.9, 133.7, 129.9, 129.4, 123.2, 114.6, 55.6; IR (KBr, cm–1): 3109, 3069, 2963, 2914, 2839, 1589, 1503, 1442, 1375, 1254, 1196, 1084, 842, 758, 619; GC/MS (m/z, %): 298 (12.8), 219 (0.1), 191 (0.1), 175 (0.8), 123 (100.0). Undesired product formed in the reaction performed to obtain compound 13e.
1-Chloro-4-phenoxybenzene (13f)
Rf = 0.67 (eluent: hexane/ethyl acetate (9:1)); yield: 41.0 mg (80%); yellowish oil;381H NMR (300 MHz, CDCl3): δ 7.36–7.24 (m, 4H), 7.11 (t, J = 7.4 Hz, 1H), 6.99 (dd, J = 8.2 Hz, 1.0 Hz, 2H), 6.96–6.90 (m, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 156.9, 156.0, 129.8, 129.7, 128.2, 123.6, 120.0, 118.9; IR (ATR, cm–1): 3065, 3028, 2955, 2924, 2853, 1584, 1483, 1240, 1163, 1088, 868, 754, 691; GC/MS (m/z, %): 204 (100.0), 169 (18.0), 141 (96.5), 115 (27.7), 77 (49.2).
1-Bromo-2-phenoxybenzene (13g)
Rf = 0.60 (eluent: hexane/ethyl acetate (9:1)); yield: 55.4 mg (89%); brownish solid; mp 42–44 °C (lit.39 mp 43–44 °C); 1H NMR (300 MHz, CDCl3): δ 7.62 (dd, J = 7.9 Hz, 1.5 Hz, 1H), 7.35–7.30 (m, 2H), 7.27–7.21 (m, 1H), 7.10 (t, J = 7.4 Hz, 1H), 7.00–6.93 (m, 4H); 13C{1H } NMR (75 MHz, CDCl3): δ 156.9, 153.7, 133.8, 129.8, 128.6, 125.0, 123.4, 120.6, 118.1, 114.9; IR (KBr, cm–1): 3055, 3012, 2955, 2924, 2853, 1599, 1491, 1464, 1234, 1165, 1045, 866, 748, 658; GC/MS (m/z, %): 250 (31.1), 248 (30.5), 169 (100.0), 141 (60.6), 77 (24.7).
1,2-Dimethyl-4-phenoxybenzene (13h)
Rf = 0.67 (eluent: hexane/ethyl acetate (9:1)); yield: 30.7 mg (62%); yellowish solid; mp 33–35 °C (lit.40 mp 35–37 °C); 1H NMR (300 MHz, CDCl3): δ 7.30 (t, J = 7.9 Hz, 2H), 7.09–7.03 (m, 2H), 6.97 (d, J = 7.8 Hz, 2H), 6.82 (d, J = 2.2 Hz, 1H), 6.75 (dd, J = 8.1 Hz, 2.4 Hz, 1H), 2.23 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3): δ 157.9, 154.8, 138.2, 131.6, 130.6, 129.6, 122.7, 120.5, 118.3, 116.4, 19.9, 19.0; IR (KBr, cm–1): 3061, 3038, 2922, 2857, 1591, 1487, 1251, 1163, 1072, 949, 758, 691; GC/MS (m/z, %): 198 (100.0), 183 (28.1), 155 (25.5), 105 (91.8), 77 (43.1).
1,2-Dimethyl-4-(4-nitrophenoxy)benzene (13i)
Rf = 0.64 (eluent: hexane); yield: 30.7 mg (76%); yellowish solid; mp 86–87 °C (lit.41 mp 87–87.5 °C); 1H NMR (300 MHz, CDCl3): δ 8.20–8.16 (m, 2H), 7.17 (d, J = 8.2 Hz, 1H), 6.99–6.96 (m, 2H), 6.87 (d, J = 2.1 Hz, 1H), 6.82 (dd, J = 8.1 Hz, 2.4 Hz, 1H), 2.28 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3): δ 163.9, 152.4, 142.3, 138.9, 133.8, 131.1, 125.9, 121.7, 117.8, 116.7, 19.9, 19.1; IR (KBr, cm–1): 3107, 3071, 2972, 2922, 2855, 1609, 1591, 1483, 1344, 1254, 1109, 844, 687; GC/MS (m/z, %): 243 (100.0), 228 (17.6), 153 (15.8), 105 (70.1), 77 (27.6).
4-(4-Methoxyphenoxy)-1,2-dimethylbenzene (13j)
Rf = 0.63 (eluent: hexane/ethyl acetate (9:1)); yield: 14.8 mg (26%); yellowish oil;17b1H NMR (300 MHz, CDCl3): δ 7.04 (d, J = 8.1 Hz, 1H), 6.96–6.92 (m, 2H), 6.88–6.84 (m, 2H), 6.76 (d, J = 2.5 Hz, 1H); 6.68 (dd, J = 8.2 Hz, 2.6 Hz, 1H), 3.79 (s, 3H), 2.21 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3): δ 156.3, 155.5, 150.8, 138.0, 130.7, 130.5, 120.3, 119.2, 115.1, 114.7, 55.6, 19.9, 18.9; IR (ATR, cm–1): 3044, 2997, 2924, 2855, 1616, 1581, 1495, 1240, 1211, 1037, 831, 766; GC/MS (m/z, %): 228 (100.0), 213 (31.0), 157 (22.1), 105 (18.2), 77 (18.5).
1-Methoxy-3-phenoxybenzene (13l)
Rf = 0.67 (eluent: hexane/ethyl acetate (9:1)); yield: 25.4 mg (51%); colorless oil;361H NMR (300 MHz, CDCl3): δ 7.33 (t, J = 8.0 Hz, 2H), 7.22 (td, J = 8.9 Hz, 1.3 Hz, 1H), 7.10 (t, J = 7.4 Hz, 1H), 7.02 (dd, J = 8.1 Hz, 1.0 Hz, 2H), 6.65 (dd, J = 9.2 Hz, 1.3 Hz, 1H), 6.59–6.57 (m, 2H), 3.77 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 160.9, 158.5, 157.0, 130.1, 129.7, 123.4, 119.1, 110.9, 108.8, 104.8, 55.3; IR (ATR, cm–1): ν 3067, 3003, 2957, 2928, 2853, 1585, 1487, 1217, 1138, 1041, 951, 760, 689; GC/MS (m/z, %): 200 (100.0), 157 (69.3), 141 (15.7), 129 (30.1), 77 (29.8).
1-Bromo-2-(3-methoxyphenoxy)benzene (13m)
Rf = 0.32 (eluent: hexane/ethyl acetate (9:1)); yield: 64.1 mg (92%); colorless oil;421H NMR (300 MHz, CDCl3): δ 7.62 (d, J = 8.0 Hz, 1H), 7.29–7.19 (m, 2H), 7.04–6.98 (m, 2H), 6.66 (d, J = 8.9 Hz, 1H), 6.54–6.51 (m, 2H), 3.78 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 161.0, 158.1, 153.4, 133.8, 130.2, 128.7, 125.1, 120.9, 115.0, 110.1, 109.0, 104.2, 55.4; IR (ATR, cm–1): 3065, 3001, 2957, 2938, 2833, 1607, 1578, 1487, 1450, 1263, 1140, 1045, 953, 758, 685; GC/MS (m/z, %): 280 (30.7), 278 (30.5), 199 (100.0), 184 (83.1), 77 (17.7).
1,2-Dimethoxy-4-phenoxybenzene (13n)
Rf = 0.32 (eluent: hexane/ethyl acetate (9:1)); yield: 31.5 mg (55%); yellowish solid; mp 48–50 °C (lit.43 mp 50 °C); 1H NMR (300 MHz, CDCl3): δ 7.30 (t, J = 7.9 Hz, 2H), 7.05 (t, J = 7.4 Hz, 1H), 6.96 (d, J = 7.8 Hz, 2H), 6.82 (d, J = 8.7 Hz, 1H), 6.65 (d, J = 2.6 Hz, 1H), 6.56 (dd, J = 8.7 Hz, 2.7 Hz, 1H), 3.87 (s, 3H), 3.83 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 158.2, 150.4, 149.9, 145.4, 129.6, 122.5, 117.6, 111.7, 110.8, 104.5, 56.3, 55.9; IR (KBr, cm–1): 3067, 2999, 2953, 2933, 2833, 1591, 1489, 1219, 1128, 1028, 957, 854, 760, 692; GC/MS (m/z, %): 230 (100.0), 215 (79.4), 187 (24.0), 115 (26.7), 77 (44.2).
4-(2-Bromophenoxy)-1,2-dimethoxybenzene (13o)
Rf = 0.39 (eluent: hexane/ethyl acetate (9:1)); yield: 60.2 mg (78%); yellowish oil; 1H NMR (300 MHz, CDCl3): δ 7.61 (dd, J = 8.0 Hz, 1.5 Hz, 1H), 7.21 (td, J = 7.8 Hz, 1.3 Hz, 1H), 6.95 (td, J = 7.7 Hz, 1.4 Hz, 1H), 6.86 (dd, J = 8.2 Hz, 1.4 Hz, 1H), 6.81 (d, J = 8.7 Hz, 1H), 6.66 (d, J = 2.7 Hz, 1H), 6.50 (dd, J = 8.7 Hz, 2.7 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 154.6, 150.1, 149.9, 145.6, 133.6, 128.5, 124.1, 118.8, 113.6, 111.6, 110.0, 104.0, 56.2, 55.9; IR (ATR, cm–1): 3063, 2999, 2955, 2833, 1600, 1580, 1510, 1470, 1440, 1260, 1229, 1150, 1045, 1028, 959, 854, 754; GC/MS (m/z, %): 310 (98.5), 308 (100.0), 229 (27.7), 186 (50.2), 171 (59.6); HRMS (ESI): calcd for [C14H14BrO3]+, 309.0121, 311.0101; found, 309.0122, 311.0100.
Diphenylamine (13p)
Rf = 0.52 (eluent: hexane/ethyl acetate (15:1)); yield: 11.4 mg (27%); greenish solid; mp 49–51 °C (lit.17a mp 40–50 °C); 1H NMR (300 MHz, CDCl3): δ 7.26 (t, J = 7.8 Hz, 4H), 7.07 (d, J = 7.7 Hz, 4H), 6.92 (t, J = 7.3 Hz, 2H), 5.69 (s, 1H); 13C{1H} NMR (75 MHz, CDCl3): δ 143.1, 129.3, 121.0, 117.8; IR (KBr, cm–1): 3383, 3034, 2922, 1595, 1493, 1319, 1172, 1084, 876, 745, 689, 505; GC/MS (m/z, %): 169 (100.0), 139 (3.3), 115 (3.4), 83 (17.6), 51 (7.6).
Phenyl Benzoate (13q)
Rf = 0.50 (eluent: hexane/ethyl acetate (9:1)); yield: 13.8 mg (28%); off-white solid; mp 68–69 °C (lit.17c mp 69–70 °C); 1H NMR (300 MHz, CDCl3): δ 8.21 (d, J = 7.4 Hz, 2H), 7.62 (t, J = 7.4 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.42 (t, J = 7.8 Hz, 2H), 7.29–7.20 (m, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 165.1, 151.0, 133.5, 130.1, 129.6, 129.5, 128.5, 125.8, 121.7; IR (KBr, cm–1): 3088, 3057, 3017, 2922, 2855, 1728, 1589, 1485, 1261, 1198, 1063, 752, 692, 503; GC/MS (m/z, %): 198 (7.1), 168 (0.1), 105 (100.0), 77 (42.3), 51 (11.8).
Procedure for the Preparation of Alkyl Azides (25a–25c)23
To a flask were added sodium azide (3.3 mmol, 215 mg) and dimethylsulfoxide (DMSO) (6.6 mL). The mixture was maintained under anhydrous conditions and magnetic stirring at room temperature for 24 h. Then, the appropriate alkyl bromide 24 (3 mmol) was added slowly. The resulting mixture was maintained under anhydrous conditions and stirring at room temperature for the indicated time. Then, ice-cold distilled water (15 mL) was added to the mixture, which was extracted with ethyl ether (3 × 9 mL). The organic phase was washed with distilled water (2 × 15 mL) and brine (15 mL) and dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure to afford the azides 25a–25c, which were employed in the next step without further purification.
(Azidomethyl)benzene (25a)
Reaction time: 1 h; Rf = 0.61 (eluent: hexane/ethyl acetate (9:1)); yield: 359 mg (90%); colorless liquid;231H NMR (300 MHz, CDCl3): δ 7.39–7.27 (m, 5H), 4.29 (s, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 135.3, 128.7, 128.2, 128.1, 54.7; IR (ATR, cm–1): 3088, 3032, 2930, 2876, 2097, 1497, 1454, 1256, 876, 698; GC/MS (m/z, %): 133 (23.1), 104 (87.6), 91 (100.0), 77 (77.8), 51 (40.5).
5-(Azidomethyl)benzo[d][1,3]dioxole (25b)
Reaction time: 1 h; Rf = 0.46 (eluent: hexane/ethyl acetate (9:1)); yield: 504 mg (95%); yellowish liquid;441H NMR (300 MHz, CDCl3): δ 6.80–6.74 (m, 3H), 5.95 (s, 2H), 4.21 (s, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 148.0, 147.6, 129.0, 121.9, 108.7, 108.3, 101.2, 54.6; IR (ATR, cm–1): 3073, 2992, 2968, 2897, 2097, 1608, 1504, 1489, 1445, 1250, 1099, 1040, 930, 810, 689, 505; GC/MS (m/z, %): 177 (28.7), 148 (88.7), 135 (100.0), 121 (29.6), 77 (22.3).
1-Azidodecane (25c)
Reaction time: 4 h; Rf = 0.71 (eluent: hexane/ethyl acetate (9:1)); yield: 527 mg (96%); colorless liquid;451H NMR (300 MHz, CDCl3): δ 3.25 (t, J = 6.9 Hz, 2H), 1.59 (q, J = 7.0 Hz, 2H), 1.38–1.27 (m, 14H), 0.88 (t, J = 6.6 Hz, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 51.4, 31.8, 29.5, 29.5, 29.3, 29.1, 28.8, 26.7, 22.6, 14.0; IR (ATR, cm–1): 2953, 2926, 2855, 2095, 1464, 1348, 1261, 895, 721, 654; GC/MS (m/z, %): 155 (0.9), 140 (0.2), 70 (49.0), 56 (52.4), 43 (100.0).
1-Azidobutane (25d)24
To a flask were added 1-chlorobutane (20 mmol, 1.85 g, 2.1 mL), sodium azide (40 mmol, 2.6 g), and DMSO (40 mL). The resulting mixture was maintained under anhydrous conditions and magnetic stirring at room temperature for 14 h. Then, ice-cold distilled water (40 mL) was added to the mixture, which was extracted with ethyl ether (3 × 50 mL). The organic phase was washed with a saturated sodium bicarbonate solution (5 × 30 mL) and dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure, affording the azide 25d, which was employed in the next step without further purification. Rf = 0.31 (eluent: hexane/ethyl acetate (9:1)); yield: 792 mg (40%); yellowish liquid;241H NMR (300 MHz, CDCl3): δ 3.26 (t, J = 6.9 Hz, 2H), 1.59 (q, J = 7.1 Hz, 2H), 1.41 (s, J = 7.4 Hz, 2H), 0.94 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 51.1, 30.8, 19.8, 13.4; IR (ATR, cm–1): ν 2955, 2922, 2853, 2091, 1595, 1450, 1431, 1261, 802; GC/MS (m/z, %): 99 (0.3), 70 (10.1), 56 (18.7), 43 (83.6), 43 (100.0).
Procedure for the Preparation of Aryl Azides (26a–26d)25
To a flask were added the appropriate aniline 20 (3 mmol) and hydrochloric acid (3 mL of a 6 M aqueous solution), and the mixture was cooled to 0 °C. Then, a solution of sodium nitrite (9 mmol, 310 mg) in distilled water (7.5 mL) was added slowly. The reaction mixture was maintained under magnetic stirring at room temperature for 30 min. Then, a solution of sodium azide (12 mmol, 780 mg) in distilled water (15 mL) was added dropwise. The resulting mixture was maintained under stirring at room temperature for 2 h. Afterward, the mixture was extracted with dichloromethane (3 × 30 mL), and the organic phase was washed with brine (90 mL) and dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure to afford the azides 26a–26d, which were employed in the next step without further purification.
Azidobenzene (26a)
Rf = 0.67 (eluent: hexane/ethyl acetate (3:1)); yield: 357 mg (quantitative); brownish liquid;251H NMR (300 MHz, CDCl3): δ 7.31 (t, J = 7.8 Hz, 2H), 7.11 (t, J = 7.4 Hz, 1H), 6.99 (d, J = 8.0 Hz, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 139.9, 129.7, 124.8, 118.9; IR (ATR, cm–1): 2957, 2922, 2851, 2112, 1618, 1501, 1261, 1103, 1020, 810, 751, 629.
1-Azido-4-nitrobenzene (26b)
Rf = 0.50 (eluent: hexane/ethyl acetate (9:1)); yield: 472 mg (96%); orange solid; mp 70–71 °C (lit.46 mp 69–70 °C); 1H NMR (300 MHz, CDCl3): δ 8.27–8.22 (m, 2H), 7.17–7.12 (m, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 146.9, 144.6, 125.6, 119.4; IR (KBr, cm–1): 3103, 3067, 2994, 2922, 2127, 1605, 1516, 1491, 1350, 1288, 1107, 847, 746, 681.
1-Azido-3-bromobenzene (26c)
Rf = 0.58 (eluent: hexane/ethyl acetate (9:1)); yield: 564 mg (95%); brownish liquid;471H NMR (300 MHz, CDCl3): δ 7.26–7.14 (m, 3H), 6.93 (ddd, J = 7.8 Hz, 1.9 Hz, 1.1 Hz, 1H); 13C{1H} NMR (75 MHz, CDCl3): δ 141.5, 130.8, 127.9, 123.2, 122.1, 117.6; IR (ATR, cm–1): ν 3061, 2925, 2853, 2102, 1587, 1470, 1283, 1067, 854, 771, 673.
1-Azido-2-methylbenzene (26d)
Rf = 0.81 (eluent: hexane/ethyl acetate (9:1)); yield: 379 mg (95%); brownish liquid;481H NMR (300 MHz, CDCl3): δ 7.18 (t, J = 7.6 Hz, 1H), 7.12 (d, J = 7.3 Hz, 1H), 7.05 (d, J = 7.7 Hz, 1H), 7.00 (t, J = 7.4 Hz, 1H), 2.18 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 138.3, 131.0, 129.5, 127.0, 124.5, 117.8, 17.1; IR (ATR, cm–1): 3061, 2926, 2855, 2018, 1612, 1503, 1462, 1275, 1069, 746, 581.
Procedure for the [3 + 2] Cycloaddition Reaction with Arynes8,22
To a vial (10 mL) were added the appropriate azides 25 and 26 (0.25 mmol), the appropriate 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates 7a–7e (0.375 mmol), 18-crown-6-ether (0.25 mmol, 66.0 mg), anhydrous benzonitrile (4.0 mL), and cesium fluoride (0.75 mmol, 114 mg). The vial was capped, and the mixture was maintained under magnetic stirring at 80 °C for 24 h. Afterward, a saturated sodium chloride solution (20 mL) was added to the mixture, which was extracted with ethyl ether (3 × 20 mL). The organic phase was dried over MgSO4. After filtration, the ethyl ether was evaporated under reduced pressure, and the benzonitrile was removed by horizontal distillation under vacuum. The residue obtained was purified by preparative thin-layer chromatography with silica gel using an appropriate eluent, affording the benzotriazoles 27a–27l.
1-Benzyl-1H-benzo[d][1,2,3]triazole (27a)
Rf = 0.16 (eluent: hexane/ethyl acetate (9:1)); yield: 31.3 mg (60%); brownish solid; mp 114–116 °C (lit.49 mp 113–115 °C); 1H NMR (300 MHz, CDCl3): δ 8.07 (d, J = 7.9 Hz, 1H), 7.43–7.26 (m, 8H), 5.84 (s, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 146.3, 134.7, 132.8, 129.0, 128.4, 127.5, 127.4, 123.9, 120.0, 109.7, 52.2; IR (KBr, cm–1): 3086, 3028, 2976, 2945, 2868, 1601, 1587, 1456, 1224, 1070, 947, 746, 623; GC/MS (m/z, %): 209 (51.8), 180 (100.0), 152 (10.2), 91 (70.2), 65 (17.4).
1-(Benzo[d][1,3]dioxol-5-yl-methyl)-1H-benzo[d][1,2,3]triazole (27b)
Rf = 0.43 (eluent: hexane/ethyl acetate (9:1)); yield: 36.6 mg (58%); off-white solid; mp 134–136 °C (lit.50 mp 128–130 °C); 1H NMR (300 MHz, CDCl3): δ 8.08 (d, J = 8.1 Hz, 1H), 7.45–7.32 (m, 3H), 6.84–6.74 (m, 3H), 5.93 (s, 2H), 5.74 (s, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 148.3, 147.8, 146.3, 132.7, 128.4, 127.4, 124.0, 121.3, 120.1, 109.7, 108.5, 108.1, 101.3, 52.2; IR (KBr, cm–1): 3061, 2978, 2914, 1614, 1489, 1449, 1250, 1088, 758, 740; GC/MS (m/z, %): 253 (100.0), 224 (47.0), 167 (45.8), 135 (85.4), 77 (60.8).
1-Decyl-1H-benzo[d][1,2,3]triazole (27c)
Rf = 0.71 (eluent: dichloromethane); yield: 38.8 mg (60%); yellowish oil;81H NMR (300 MHz, CDCl3): δ 8.06 (d, J = 8.3 Hz, 1H), 7.54–7.45 (m, 2H), 7.36 (t, J = 7.4 Hz, 1H), 4.63 (t, J = 7.2 Hz, 2H), 2.01 (t, J = 6.9 Hz, 2H), 1.34–1.24 (m, 14H), 0.87 (t, J = 6.5 Hz, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 146.0, 132.9, 127.1, 123.7, 120.0, 109.3, 48.2, 31.8, 29.7, 29.4, 29.3, 29.2, 29.0, 26.7, 22.6, 14.1; IR (ATR, cm–1): 3065, 2924, 2853, 1616, 1495, 1454, 1159, 745; GC/MS (m/z, %): 259 (38.0), 146 (50.7), 132 (83.0), 106 (100.0), 91 (68.3).
1-Butyl-1H-benzo[d][1,2,3]triazole (27d)
Rf = 0.29 (eluent: hexane/ethyl acetate (9:1)); yield: 27.5 mg (63%); yellowish oil;511H NMR (300 MHz, CDCl3): δ 8.06 (d, J = 8.3 Hz, 1H), 7.55–7.45 (m, 2H), 7.39–7.36 (m, 1H), 4.65 (t, J = 7.1 Hz, 2H), 2.00 (q, J = 7.4 Hz, 2H), 1.38 (s, J = 7.5 Hz, 2H), 0.96 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 146.0, 132.9, 127.1, 123.7, 120.0, 109.3, 47.9, 31.7, 19.9, 13.5; IR (ATR, cm–1): 3065, 2959, 2874, 1614, 1495, 1454, 1225, 1051, 746; GC/MS (m/z, %): 175 (14.2), 133 (20.4), 118 (11.5), 91 (100.0), 77 (41.3).
1-Benzyl-5,6-dimethyl-1H-benzo[d][1,2,3]triazole (27e)
Rf = 0.18 (eluent: hexane/ethyl acetate (9:1)); yield: 30.8 mg (52%); brownish solid; mp 161–163 °C (lit.22 mp 158–161 °C); 1H NMR (300 MHz, CDCl3): δ 7.79 (s, 1H), 7.33–7.29 (m, 3H), 7.26–7.23 (m, 2H), 7.10 (s, 1H), 5.78 (s, 2H), 2.37 (s, 3H), 2.34 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 145.5, 137.7, 135.1, 133.7, 131.9, 128.9, 128.3, 127.4, 119.1, 109.0, 52.0, 20.9, 20.4; IR (KBr, cm–1): 3086, 3028, 2976, 2949, 2924, 2853, 1628, 1449, 1217, 1101, 837, 704; GC/MS (m/z, %): 237 (18.6), 208 (23.7), 194 (16.0), 91 (100.0), 65 (16.5).
1-Benzyl-5,6-difluoro-1H-benzo[d][1,2,3]triazole (27f)
Rf = 0.15 (eluent: hexane/ethyl acetate (9:1)); yield: 15.3 mg (25%); off-white solid; mp 127–128 °C (lit.22 mp 119–122 °C); 1H NMR (300 MHz, CDCl3): δ 7.80 (dd, J = 9.2 Hz, 7.1 Hz, 1H), 7.40–7.34 (m, 3H), 7.28–7.25 (m, 2H), 7.09 (dd, J = 8.6 Hz, 6.6 Hz, 1H), 5.80 (s, 2H); 13C NMR (75 MHz, CDCl3): δ 151.4 (dd, J = 251.6 Hz, 16.9 Hz, ArF), 149.0 (dd, J = 246.8 Hz, 16.5 Hz, ArF), 141.4 (d, J = 9.0 Hz, ArF), 133.9, 129.2, 128.8, 128.5 (d, J = 11.3 Hz, ArF), 127.6, 106.4 (dd, J = 19.9 Hz, 1.9 Hz, ArF), 97.1 (d, J = 22.5 Hz, ArF), 52.6; IR (KBr, cm–1): 3086, 3055, 2972, 2947, 2866, 1599, 1499, 1479, 1233, 1139, 837, 702, 633; GC/MS (m/z, %): 245 (54.8), 216 (100.0), 188 (11.4), 91 (69.8), 77 (10.9).
1-Benzyl-4-methoxy-1H-benzo[d][1,2,3]triazole (27g)
Rf = 0.11 (eluent: hexane/ethyl acetate (9:1)); yield: 31.7 mg (53%); brownish solid; mp 90–92 °C (lit.22 mp 92–94 °C); 1H NMR (300 MHz, CDCl3): δ 7.32–7.24 (m, 6H), 6.90 (d, J = 8.3 Hz, 1H), 6.66 (d, J = 7.7 Hz, 1H), 5.81 (s, 2H), 4.10 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 151.8, 138.3, 134.8, 128.9, 128.6, 128.4, 127.5, 103.3, 101.8, 56.2, 52.2; IR (KBr, cm–1): 3086, 2961, 2916, 2851, 1638, 1514, 1454, 1396, 1248, 1069, 725, 637; GC/MS (m/z, %): 239 (48.1), 210 (32.4), 180 (20.9), 167 (12.7), 91 (100.0). The position of the methoxy group was attributed by the NOESY 2D NMR experiment. The methylene hydrogens show two cross-peaks with two aromatic hydrogens, which can only be explained when the methoxy group is placed in position 4.

1-Benzyl-4,5-dimethoxy-1H-benzo[d][1,2,3]triazole (27h)
Rf = 0.21 (eluent: dichloromethane/hexane (2:1)); yield: 37.7 mg (56%); brownish oil; 1H NMR (300 MHz, CDCl3): δ 7.34–7.25 (m, 5H), 7.16 (d, J = 8.8 Hz, 1H), 6.86 (d, J = 8.8 Hz, 1H), 5.78 (s, 2H), 4.58 (s, 3H), 3.90 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 144.7, 140.1, 139.8, 134.7, 130.7, 129.0, 128.4, 127.5, 117.9, 101.1, 61.4, 58.6, 52.1; IR (ATR, cm–1): 3088, 2992, 2936, 2835, 1591, 1512, 1452, 1244, 1098, 714; GC/MS (m/z, %): 269 (41.0), 240 (7.1), 226 (12.8), 91 (100.0), 65 (26.8); HRMS (ESI) calcd for [C15H16N3O2]+, 270.1237, 271.1271; found, 270,1247, 271.1258.
1-Phenyl-1H-benzo[d][1,2,3]triazole (27i)
Rf = 0.19 (eluent: hexane/ethyl acetate (9:1)); yield: 24.5 mg (50%); brownish solid; mp 85–87 °C (lit.22 mp 86–87 °C); 1H NMR (300 MHz, CDCl3): δ 8.15 (d, J = 8.3 Hz, 1H), 7.79–7.73 (m, 3H), 7.63–7.40 (m, 5H); 13C{1H} NMR (75 MHz, CDCl3): δ 146.4, 136.9, 132.2, 129.8, 128.6, 128.2, 124.3, 122.8, 120.2, 110.3; IR (KBr, cm–1): 3096, 3055, 2918, 2851, 1616, 1501, 1458, 1275, 1059, 748, 707; GC/MS (m/z, %): 195 (27.9), 167 (100.0), 139 (19.6), 77 (51.5), 51 (31.8).
1-(4-Nitrophenyl-1H-benzo[d][1,2,3]triazole (27j)
Rf = 0.14 (eluent: hexane/ethyl acetate (9:1)); yield: 42.6 mg (71%); brownish solid; mp 242–243 °C (lit.52 mp 243–244 °C); 1H NMR (300 MHz, DMSO-d6): δ 8.53 (d, J = 8.9 Hz, 2H), 8.27 (d, J = 9.0 Hz, 3H), 8.12 (d, J = 8.2 Hz, 1H), 7.77 (t, J = 7.7 Hz, 1H), 7.60 (t, J = 7.7 Hz, 1H); 13C{1H} NMR (75 MHz, DMSO-d6): δ 147.0, 146.6, 141.7, 131.9, 130.0, 126.1, 125.8, 123.4, 120.6, 111.8; IR (KBr, cm–1): 3100, 2957, 2920, 2851, 1593, 1518, 1346, 1109, 851, 748; GC/MS (m/z, %): 240 (22.2), 212 (10.0), 166 (100.0), 140 (42.4), 77 (5.9).
1-(3-Bromophenyl)-1H-benzo[d][1,2,3]triazole (27k)
Rf = 0.32 (eluent: hexane/ethyl acetate (9:1)); yield: 54.6 mg (80%); yellowish solid;53 mp 106–108 °C; 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.3 Hz, 1H), 8.00 (s, 1H), 7.77 (d, J = 8.2 Hz, 2H), 7.66–7.57 (m, 2H), 7.53–7.44 (m, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 146.6, 138.1, 132.0, 131.6, 131.1, 128.7, 125.8, 124.7, 123.4, 121.2, 120.5, 110.1; IR (KBr, cm–1): 3072, 2957, 2922, 2851, 1589, 1479, 1283, 1063, 791, 683; GC/MS (m/z, %): 275 (10.6), 273 (10.9), 166 (100.0), 139 (18.9), 77 (2.6).
1-o-Tolyl-1H-benzo[d][1,2,3]triazole (27l)
Rf = 0.36 (eluent: hexane/ethyl acetate (9:1)); yield: 16.2 mg (31%); yellowish solid; mp 65–66 °C (lit.10 mp 60–63 °C); 1H NMR (300 MHz, CDCl3): δ 8.16 (d, J = 8.2 Hz, 1H), 7.54–7.40 (m, 6H), 7.35 (d, J = 8.2 Hz, 1H), 2.14 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 145.6, 135.3, 135.2, 133.9, 131.7, 130.0, 128.0, 127.0, 126.9, 124.1, 120.1, 110.1, 17.8; IR (KBr, cm–1): 3059, 2957, 2924, 2853, 1614, 1503, 1273, 1182, 1069, 785, 746; GC/MS (m/z, %): 209 (14.5), 180 (100.0), 152 (8.7), 91 (14.1), 65 (23.3).
Procedure for the [4 + 2] Cycloaddition Reaction with Arynes8,10
To a vial (10 mL) were added the appropriate 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates 7a–7e (0.375 mmol), 18-crown-6-ether (0.25 mmol, 66.0 mg), anhydrous benzonitrile (4 mL), cesium fluoride (0.75 mmol, 114 mg), and the appropriate furan 28 (0.25 mmol). The vial was capped, and the mixture was maintained under magnetic stirring at 80 °C for 24 h. Afterward, a saturated sodium chloride solution (20 mL) was added to the mixture, which was extracted with ethyl ether (3 × 20 mL). The organic phase was dried over MgSO4. After filtration, the ethyl ether was evaporated under reduced pressure, and the benzonitrile was removed by horizontal distillation under vacuum. The residue obtained was purified by preparative thin-layer chromatography with silica gel using an appropriate eluent to afford the cycloadducts 29a–29g.
1,4-Dihydro-1,4-epoxynaphthalene (29a)
Rf = 0.39 (eluent: hexane/ethyl acetate (9:1)); yield: 24.8 mg (69%); yellowish solid; mp 52–54 °C (lit.54 mp 54.5–55.5 °C); 1H NMR (300 MHz, CDCl3): δ 7.25–7.24 (m, 2H), 7.03 (s, 2H), 6.97 (dd, J = 5.1 Hz, 3.0 Hz, 2H), 5.71 (s, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 149.0, 143.0, 125.0, 120.3, 82.3; IR (KBr, cm–1): 2955, 2924, 2853, 1638, 1616, 1454, 1381, 1279, 1175, 847, 758, 623; GC/MS (m/z, %): 144 (11.2), 128 (50.5), 115 (100.0), 102 (7.4), 89 (25.0).
1,4-Dihydro-1,4-dimethyl-1,4-epoxynaphthalene (29b)
Rf = 0.46 (eluent: hexane/ethyl acetate (9:1)); yield: 33.1 mg (77%); colorless oil;551H NMR (300 MHz, CDCl3): δ 7.13 (dd, J = 5.1 Hz, 3.0 Hz, 2H), 6.97 (dd, J = 5.1 Hz, 3.0 Hz, 2H), 6.77 (s, 2H), 1.89 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3): δ 152.7, 146.8, 124.7, 118.3, 88.6, 15.2; IR (ATR, cm–1): 3071, 2974, 2930, 2866, 1638, 1618, 1452, 1383, 1306, 1142, 860, 760, 692, 648; GC/MS (m/z, %): 172 (9.0), 146 (28.8), 129 (47.5), 77 (15.7), 43 (100.0).
1-(1,4-Epoxynaphthalen-1(4H)-yl)ethanone (29c)
Rf = 0.44 (eluent: hexane/ethyl acetate (9:1)); yield: 32.5 mg (70%); yellowish oil;561H NMR (300 MHz, CDCl3): δ 7.26 (td, J = 6.4 Hz, 1.4 Hz, 2H), 7.07–6.96 (m, 4H), 5.80 ( s, 1H), 2.40 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 205.2, 148.0, 147.5, 143.4, 142.2, 125.6, 125.2, 120.6, 119.6, 95.7, 82.3, 26.8; IR (ATR, cm–1): 3071, 2974, 3012, 2924, 2853, 1713, 1587, 1456, 1362, 1306, 1150, 874, 758, 677, 554; GC/MS (m/z, %): 186 (5.8), 160 (7.5), 115 (61.8), 89 (17.6), 43 (100.0).
1,4-Dihydro-6,7-dimethyl-1,4-epoxynaphthalene (29d)
Rf = 0.48 (eluent: hexane/ethyl acetate (9:1)); yield: 27.0 mg (63%); yellowish oil;571H NMR (300 MHz, CDCl3): δ 7.05 (s, 2H), 7.00 (s, 2H), 5.66 (s, 2H), 2.19 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3): δ 146.6, 143.1, 132.5, 122.1, 82.2, 19.8; IR (ATR, cm–1): 3071, 2974, 2930, 2866, 1638, 1618, 1452, 1383, 1305, 1142, 860, 760, 692, 648; GC/MS (m/z, %): 172 (21.6), 156 (15.6), 144 (45.5), 129 (100.0), 51 (13.8).
6,7-Difluoro-1,4-dihydro-1,4-epoxynaphthalene (29e)
Rf = 0.28 (eluent: hexane/ethyl acetate (9:1)); yield: 5.0 mg (11%);colorless oil;571H NMR (300 MHz, CDCl3): δ 7.09–7.02 (m, 4H), 5.68 (s, 2H); 13C{1H} NMR (75 MHz, CDCl3): δ 147.2 (dd, J = 246.4 Hz, 14.6 Hz, ArF), 145.1 (t, J = 4.9 Hz, ArF), 143.0, 111.0–110.4 (m, ArF), 82.0; IR (ATR, cm–1): 3094, 3019, 2926, 2855, 1626, 1618, 1366, 1254, 1040, 852, 789, 696; GC/MS (m/z, %): 180 (15.6), 151 (100.0), 125 (16.5), 101 (8.4), 75 (11.7).
1,4-Dihydro-5-methoxy-1,4-epoxynaphthalene (29f)
Rf = 0.36 (eluent: hexane/ethyl acetate (9:1)); yield: 32.6 mg (75%); brownish oil;581H NMR (300 MHz, CDCl3): δ 7.07 (dd, J = 5.5 Hz, 1.6 Hz, 1H), 7.02 (dd, J = 5.5 Hz, 1.7 Hz, 1H), 6.96–6.91 (m, 2H), 6.59 (dd, J = 7.7 Hz, 1.0 Hz, 1H), 5.95 (s, 1H), 5.70 (s, 1H), 3.82 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 152.9, 151.5, 143.0, 142.9, 135.0, 126.9, 113.7, 110.3, 82.5, 80.0, 55.7; IR (ATR, cm–1): 3067, 2959, 2936, 2836, 1616, 1597, 1479, 1263, 1092, 999, 856, 721, 623; GC/MS (m/z, %): 174 (7.2), 146 (65.7), 115 (100.0), 103 (64.5), 77 (38.3).
1,4-Dihydro-5,6-dimethoxy-1,4-epoxynaphthalene (29g)
Rf = 0.18 (eluent: dichloromethane/hexane (1:1)); yield: 35.0 mg (69%); brownish solid; mp 88–90 °C (lit.59 mp 92–93 °C); 1H NMR (300 MHz, CDCl3): δ 7.00 (s, 2H), 6.86 (d, J = 7.5 Hz, 1H), 6.43 (d, J = 7.6 Hz, 1H), 6.05 (s, 1H), 5.64 (s, 1H), 3.94 (s, 3H), 3.81 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 149.7, 143.8, 143.0, 141.8, 141.4, 136.5, 114.4, 107.2, 81.9, 81.2, 60.4, 56.2; IR (KBr, cm–1): 3015, 2999, 2932, 2843, 1638, 1616, 1489, 1350, 1244, 1059, 868, 718, 588; GC/MS (m/z, %): 204 (60.9), 176 (80.0), 161 (100.0), 115 (80.5), 89 (57.8).
1-(6a,7-Diidro-4H-dibenzo[de,g]quinolina-6(5H)-il)etanona (32)26
To a vial were added the enamide 31 (0.25 mmol, 46.8 mg), 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate (7a) (0.375 mmol, 128 mg), 18-crown-6 ether (0.25 mmol, 66.0 mg), anhydrous benzonitrile (4.0 mL), and cesium fluoride (0.75 mmol, 114 mg). The vial was capped, and the mixture was maintained under magnetic stirring at 80 °C for 24 h. Afterward, a saturated sodium chloride solution (20 mL) was added to the mixture, which was extracted with ethyl ether (3 × 20 mL). The organic phase was dried over MgSO4. After filtration, the ethyl ether was evaporated under reduced pressure, and the benzonitrile was removed by horizontal distillation under vacuum. The residue was purified by preparative thin-layer chromatography with silica gel using ethyl acetate as an eluent to give the product of interest 32. Rf = 0.48 (eluent: ethyl acetate); yield: 30.8 mg (47%); brownish solid; mp 210–212 °C (lit.26 mp 210–212 °C); 1H NMR (500 MHz, CDCl3, mixture of rotamers): δ 7.81 (d, J = 7.7 Hz, 0.5H), 7.77 (d, J = 7.7 Hz, 1H), 7.62 (t, J = 4.2 Hz, 1.5H), 7.33–7.24 (m, 6H), 7.14 (d, J = 7.5 Hz, 0.5H), 7.10 (d, J = 7.5 Hz, 1H), 5.28 (dd, J = 14.1 Hz, 4.4 Hz, 1H), 4.99 (dd, J = 13.1 Hz, 4.8 Hz, 0.5H), 4.77 (dd, J = 14.4 Hz, 3.7 Hz, 0.5H), 4.03 (d, J = 12.8 Hz, 1H), 3.33 (t, J = 12.3 Hz, 1H), 3.19 (dd, J = 14.0 Hz, 4.4 Hz, 1H), 3.13 (d, J = 14.5 Hz, 0.5H), 2.92–2.74 (m, 5H), 2.25 (s, 3H), 2.21 (s, 1.5H); 13C{1H} NMR (125 MHz, CDCl3, mixture of rotamers): δ 169.8, 169.2, 135.4, 135.2, 134.8, 134.6, 134.2, 134.0, 133.8, 133.6, 132.6, 131.8, 129.0, 128.5, 128.1, 128.0, 128.0, 127.8, 127.4, 127.3, 126.8, 123.9, 123.6, 122.5, 122.5, 53.6, 50.4, 42.1, 36.6, 35.5, 32.9, 30.7, 29.8, 29.6, 22.6, 21.5; IR (KBr, cm–1): 3011, 2924, 2897, 1634, 1612, 1435, 1273, 1261, 1173, 1036, 768, 648; GC/MS (m/z, %): 263 (61.5), 248 (0.7), 235 (1.3), 204 (26.3), 192 (100.0).
(±)-Noraporphine (33)26
To a vessel at room temperature were added compound 32 (0.19 mmol, 51.2 mg), ethanol/water (2:1) (3.0 mL), and lithium hydroxide (1.9 mmol, 46.6 mg). The mixture was heated under microwave with a closed system at 180 °C (50 W) for 40 min. Afterward, the solvent was evaporated under reduced pressure. Then, water (10 mL) was added, and the mixture was extracted with dichloromethane (3 × 15 mL). The organic phase was dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure. The residue was purified by preparative thin-layer chromatography with silica gel using methanol as an eluent to give the desired product 33. Rf = 0.28 (eluent: methanol); yield: 20.7 mg (48%); yellowish oil;261H NMR (300 MHz, CDCl3): δ 7.66 (d, J = 7.7 Hz, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.25–7.22 (m, 2H), 7.18–7.16 (m, 2H), 7.01 (d, J = 7.5 Hz, 1H), 3.98 (dd, J = 13.8 Hz, 4.9 Hz, 1H), 3.35 (d, J = 7.3 Hz, 1H), 3.00 (d, J = 9.2 Hz, 1H), 2.90–2.68 (m, 4H), 2.57 (s, 1H); 13C{1H} NMR (75 MHz, CDCl3): δ 134.9, 134.3, 134.2, 133.5, 133.2, 128.4, 128.3, 127.6, 127.4, 127.1, 123.9, 121.8, 53.3, 43.0, 36.3, 28.8; IR (ATR, cm–1): 3393, 3331, 3065, 3022, 2922, 2851, 1570, 1462, 1194, 1120, 1069, 756, 470; GC/MS (m/z, %): 220 (100.0), 204 (13.6), 192 (20.7), 178 (7.7), 165 (10.7).
(±)-Aporphine (34)26
To a vial were added compound 33 (0.094 mmol, 20.7 mg), methanol (3 mL), and 37% (w/v) aqueous solution of formaldehyde (12 mmol, 1.0 mL). The vial was capped, and the mixture was maintained under magnetic stirring at room temperature for 30 min. Thereafter, sodium borohydride (106 mg, 2.8 mmol) was added to the reaction mixture, which was maintained under stirring at room temperature for 1 h. Afterward, a saturated aqueous solution of sodium chloride (20 mL) was added to the mixture, which was extracted with ethyl acetate (3 × 30 mL). The organic phase was dried over MgSO4. After filtration, the solvent was evaporated under reduced pressure. The residue was purified by preparative thin-layer chromatography with silica gel using methanol as an eluent to give (±)-aporphine (34). Rf = 0.62 (eluent: methanol); yield: 17.1 mg (78%); yellowish oil;261H NMR (300 MHz, CDCl3): δ 7.65 (d, J = 7.7 Hz, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.28–7.17 (m, 4H), 7.02 (d, J = 7.5 Hz, 1H), 3.29–3.05 (m, 4H), 2.74–2.65 ( m, 2H), 2.59–2.50 (m, 4H), 2.54 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3): δ 135.0, 134.2, 133.5, 133.2, 133.1, 128.4, 128.0, 127.6, 127.4, 127.1, 123.8, 122.0, 61.9, 53.2, 33.8, 29.7, 28.6; IR (ATR, cm–1): 3055, 2954, 2921, 2851, 1590, 1465, 1284, 1128, 757, 746, 602; GC/MS (m/z, %): 234 (100.0), 219 (19.4), 204 (8.8), 192 (35.7), 178 (5.2).
Acknowledgments
We are grateful to São Paulo Research Foundation (FAPESP) (grant number 2017/21990-0) and to National Council for Scientific and Technological Development (CNPq) for the financial support. A.C.A.M thanks the Coordination for the Improvement of Higher Education Personnel (CAPES) and the FAPESP (grant number 2016/10894-7) for the fellowships.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03989.
Table S1, optimization of reaction conditions for the preparation of diphenyl ether (13a); copies of 1H and 13C NMR spectra of all compounds 9a–9c, 7a–7e, 10–12, 13a–13j, 13l–13q, 14, 18a, 18b, 19c, 25a–25d, 26a–26d, 27a–27l, 29a–29g, and 32–34) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews, see:; a Takikawa H.; Nishii A.; Sakai T.; Suzuki K. Aryne-Based Strategy in the Total Synthesis of Naturally Occuring Polycyclic Compounds. Chem. Soc. Rev. 2018, 47, 8030–8056. 10.1039/C8CS00350E. [DOI] [PubMed] [Google Scholar]; b Tadross P. M.; Stoltz B. M. A Comprehensive History of Arynes in Natural Product Total Synthesis. Chem. Rev. 2012, 112, 3550–3577. 10.1021/cr200478h. [DOI] [PubMed] [Google Scholar]; c Gampe C. M.; Carreira E. M. Arynes and Cyclohexyne in Natural Product Synthesis. Angew. Chem., Int. Ed. 2012, 51, 3766–3778. 10.1002/anie.201107485. [DOI] [PubMed] [Google Scholar]; For selected examples, see:; d Corsello M. A.; Kim J.; Garg N. K. Total Synthesis of (−)-Tubingensin B Enabled by the Strategic Use of an Aryne Cyclization. Nat. Chem. 2017, 9, 944–949. 10.1038/nchem.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Muraca A. C. A.; Perecim G. P.; Rodrigues A.; Raminelli C. Convergent Total Synthesis of (±)-Apomorphine via Benzyne Chemistry: Insights into the Mechanisms Involved in the Key Step. Synthesis 2017, 49, 3546–3557. 10.1055/s-0036-1588855. [DOI] [Google Scholar]; f Gouthami P.; Chegondi R.; Chandrasekhar S. Formal Total Synthesis of (±)-Cephalotaxine and Congeners via Aryne Insertion Reaction. Org. Lett. 2016, 18, 2044–2046. 10.1021/acs.orglett.6b00659. [DOI] [PubMed] [Google Scholar]; g Rossini A. F. C.; Muraca A. C. A.; Casagrande G. A.; Raminelli C. Total Syntheses of Aporphine Alkaloids via Benzyne Chemistry: An Approach to the Formation of Aporphine Cores. J. Org. Chem. 2015, 80, 10033–10040. 10.1021/acs.joc.5b01634. [DOI] [PubMed] [Google Scholar]; h Allan K. M.; Stoltz B. M. A Concise Total Synthesis of (−)-Quinocarcin via Aryne Annulation. J. Am. Chem. Soc. 2008, 130, 17270–17271. 10.1021/ja808112y. [DOI] [PubMed] [Google Scholar]
- For reviews, see:; a Pozo I.; Guitián E.; Pérez D.; Peña D. Synthesis of Nanographenes, Starphenes, and Sterically Congested Polyarenes by Aryne Cyclotrimerization. Acc. Chem. Res. 2019, 52, 2472–2481. 10.1021/acs.accounts.9b00269. [DOI] [PubMed] [Google Scholar]; b Roy T.; Biju A. T. Recent Advances in Molecular Rearrangements Involving Aryne Intermediates. Chem. Commun. 2018, 54, 2580–2594. 10.1039/C7CC09122B. [DOI] [PubMed] [Google Scholar]; c Shi J.; Li Y.; Li Y. Aryne Multifunctionalization with Benzdiyne and Benztriyne Equivalents. Chem. Soc. Rev. 2017, 46, 1707–1719. 10.1039/C6CS00694A. [DOI] [PubMed] [Google Scholar]; d García-López J.-A.; Greaney M. F. Synthesis of Biaryls Using Aryne Intermediates. Chem. Soc. Rev. 2016, 45, 6766–6798. 10.1039/C6CS00220J. [DOI] [PubMed] [Google Scholar]; e Wu D.; Ge H.; Liu S. H.; Yin J. Arynes in the Synthesis of Polycyclic Aromatic Hydrocarbons. RSC Adv. 2013, 3, 22727–22738. 10.1039/c3ra43804j. [DOI] [Google Scholar]; f Pérez D.; Peña D.; Guitián E. Aryne Cycloaddition Reactions in the Synthesis of Large Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2013, 5981–6013. 10.1002/ejoc.201300470. [DOI] [Google Scholar]; For selected examples, see:; g Mizukoshi Y.; Mikami K.; Uchiyama M. Aryne Polymerization Enabling Straightforward Synthesis of Elusive Poly(ortho-arylene)s. J. Am. Chem. Soc. 2015, 137, 74–77. 10.1021/ja5112207. [DOI] [PubMed] [Google Scholar]; h Li J.; Zhao Y.; Lu J.; Li G.; Zhang J.; Zhao Y.; Sun X.; Zhang Q. Double [4 + 2] Cycloaddition Reaction To Approach a Large Acene with Even-Number Linearly Fused Benzene Rings: 6,9,16,19-Tetraphenyl-1.20,4.5,10.11,14.15-Tetrabenzooctatwista-cene. J. Org. Chem. 2015, 80, 109–113. 10.1021/jo5021163. [DOI] [PubMed] [Google Scholar]; i Shen Y.-M.; Grampp G.; Leesakul N.; Hu H.-W.; Xu J.-H. Synthesis and Emitting Properties of the Blue-Light Fluorophores Indolizino[3,4,5-ab]isoindole Derivatives. Eur. J. Org. Chem. 2007, 3718–3726. 10.1002/ejoc.200700250. [DOI] [Google Scholar]; j Chen Y.-L.; Wong M.-S.; Wong W.-Y.; Lee A. W. M. Synthesis and Characterization of Oxadisilole Fused Benzo[b]triphenylene. Tetrahedron Lett. 2007, 48, 2421–2425. 10.1016/j.tetlet.2007.01.174. [DOI] [Google Scholar]
- For reviews, see:; a Yoshimura A.; Saito A.; Zhdankin V. V. Iodonium Salts as Benzyne Precursors. Chem. – Eur. J. 2018, 24, 15156–15166. 10.1002/chem.201802111. [DOI] [PubMed] [Google Scholar]; b Yoshida S.; Hosoya T. The Renaissance and Bright Future of Synthetic Aryne Chemistry. Chem. Lett. 2015, 44, 1450–1460. 10.1246/cl.150839. [DOI] [Google Scholar]; c Bhunia A.; Yetra S. R.; Biju A. T. Recent Advances in Transition-Metal-Free Carbon–Carbon and Carbon–Heteroatom Bond-Forming Reactions Using Arynes. Chem. Soc. Rev. 2012, 41, 3140–3152. 10.1039/c2cs15310f. [DOI] [PubMed] [Google Scholar]; d Kitamura T. Synthetic Methods for the Generation and Preparative Application of Benzyne. Aust. J. Chem. 2010, 63, 987–1001. 10.1071/CH10072. [DOI] [Google Scholar]; e Dyke A. M.; Hester A. J.; Lloyd-Jones G. C. Organometallic Generation and Capture of ortho-Arynes. Synthesis 2006, 4093–4112. 10.1055/s-2006-950370. [DOI] [Google Scholar]; f Pellissier H.; Santelli M. The Use of Arynes in Organic Synthesis. Tetrahedron 2003, 59, 701–730. 10.1016/S0040-4020(02)01563-6. [DOI] [Google Scholar]; For selected examples, see:; g Matsumoto T.; Hosoya T.; Katsuki M.; Suzuki K. New Efficient Protocol for Aryne Generation. Selective Synthesis of Differentially Protected 1,4,5-Naphthalenetriols. Tetrahedron Lett. 1991, 32, 6735–6736. 10.1016/S0040-4039(00)93589-5. [DOI] [Google Scholar]; h Campbell C. D.; Rees C. W. Reactive Intermediates. Part II. Some Addition Reactions of Benzyne. J. Chem. Soc. C. 1969, 748–751. 10.1039/j39690000748. [DOI] [Google Scholar]; i Stiles M.; Miller R. G.; Burckhardt U. Reactions of Benzyne Intermediates in Non-basic Media. J. Am. Chem. Soc. 1963, 85, 1792–1797. 10.1021/ja00895a022. [DOI] [Google Scholar]; j Le Goff E. Aprotic Generation of Benzyne from Diphenyliodonium-2-Carboxylate. J. Am. Chem. Soc. 1962, 84, 3786. 10.1021/ja00878a050. [DOI] [Google Scholar]; k Wittig G.; Knauss E. Dehydrobenzol und Cyclopentadien. Chem. Ber. 1958, 91, 895–907. 10.1002/cber.19580910502. [DOI] [Google Scholar]
- Himeshima Y.; Sonoda T.; Kobayashi H. Fluoride-induced 1,2-Elimination of o-Trimethylsilylphenyl Triflate to Benzyne under Mild Conditions. Chem. Lett. 1983, 12, 1211–1214. 10.1246/cl.1983.1211. [DOI] [Google Scholar]
- For reviews, see:; a Dubrovskiy A. V.; Markina N. A.; Larock R. C. Use of Benzynes for the Synthesis of Heterocycles. Org. Biomol. Chem. 2013, 11, 191–218. 10.1039/C2OB26673C. [DOI] [PubMed] [Google Scholar]; b Yoshida H.; Takaki K. Aryne Insertion Reactions into Carbon–Carbon σ-Bonds. Synlett 2012, 23, 1725–1732. 10.1055/s-0031-1290401. [DOI] [Google Scholar]; c Yoshida H.; Ohshita J.; Kunai A. Aryne, ortho-Quinone Methide, and ortho-Quinodimethane: Synthesis of Multisubstituted Arenes Using the Aromatic Reactive Intermediates. Bull. Chem. Soc. Jpn. 2010, 83, 199–219. 10.1246/bcsj.20090245. [DOI] [Google Scholar]
- a Sumida Y.; Sumida T.; Hashizume D.; Hosoya T. Preparation of Aryne-Nickel Complexes from ortho-Borylaryl Triflates. Org. Lett. 2016, 18, 5600–5603. 10.1021/acs.orglett.6b02831. [DOI] [PubMed] [Google Scholar]; b García-López J.-A.; Greaney M. F. Use of 2-Bromophenylboronic Esters as Benzyne Precursors in the Pd-Catalyzed Synthesis of Triphenylenes. Org. Lett. 2014, 16, 2338–2341. 10.1021/ol5006246. [DOI] [PubMed] [Google Scholar]; c Sumida Y.; Kato T.; Hosoya T. Generation of Arynes via Ate Complexes of Arylboronic Esters with an ortho-Leaving Group. Org. Lett. 2013, 15, 2806–2809. 10.1021/ol401140d. [DOI] [PubMed] [Google Scholar]; d Hamura T.; Chuda Y.; Nakatsuji Y.; Suzuki K. Catalytic Generation of Arynes and Trapping by Nucleophilic Addition and Iodination. Angew. Chem. Int. Ed. 2012, 51, 3368–3372. 10.1002/anie.201108415. [DOI] [PubMed] [Google Scholar]; e Atkinson D. J.; Sperry J.; Brimble M. A. Improved Synthesis of the Benzyne Precursor 2-(Trimethylsilyl)phenyl Trifluoromethanesulfonate. Synthesis 2010, 911–913. 10.1055/s-0029-1218631. [DOI] [Google Scholar]; f Bronner S. M.; Garg N. K. Efficient Synthesis of 2-(Trimethylsilyl)phenyl Trifluoromethanesulfonate: A Versatile Precursor to o-Benzyne. J. Org. Chem. 2009, 74, 8842–8843. 10.1021/jo9020166. [DOI] [PubMed] [Google Scholar]; g Arisawa T.; Hamura T.; Uekusa H.; Matsumoto T.; Suzuki K. Linearly Fused Dicyclobutabenzenes via Dual, Regioselective Cycloadditions of 1,4-Benzdiyne Equivalent and Ketene Silyl Acetals. Synlett 2008, 2008, 1179–1184. 10.1055/s-2008-1072729. [DOI] [Google Scholar]; h Wu Q.-C.; Li B.-S.; Shi C.-Q.; Chen Y.-X. Synthesis of Aryne Precursor o-Trimethylsilylphenyl Triflate. Hecheng Huaxue 2007, 15, 111–113. [Google Scholar]; i Peña D.; Iglesias B.; Quintana I.; Pérez D.; Guitián E.; Castedo L. Synthesis and Reactivity of New Strained Cyclic Allene and Alkyne Precursors. Pure Appl. Chem. 2006, 78, 451–455. 10.1351/pac200678020451. [DOI] [Google Scholar]; j Hamura T.; Arisawa T.; Matsumoto T.; Suzuki K. Two-Directional Annelation: Dual Benzyne Cycloaddtions Starting from Bis(sulfonyloxy)diiodobenzene. Angew. Chem., Int. Ed. 2006, 45, 6842–6844. 10.1002/anie.200602539. [DOI] [PubMed] [Google Scholar]; k Peña D.; Cobas A.; Pérez D.; Guitián E. An Efficient Procedure for the Synthesis of ortho-Trialkylsilylaryl Triflates: Easy Access to Precursors of Functionalized Arynes. Synthesis 2002, 1454–1458. 10.1055/s-2002-33110. [DOI] [Google Scholar]; l Peña D.; Pérez D.; Guitián E.; Castedo L. Palladium-Catalyzed Cocyclization of Arynes with Alkynes: Selective Synthesis of Phenanthrenes and Naphthalenes. J. Am. Chem. Soc. 1999, 121, 5827–5828. 10.1021/ja9907111. [DOI] [Google Scholar]
- a Kitamura T.; Yamane M.; Inoue K.; Todaka M.; Fukatsu N.; Meng Z.; Fujiwara Y. A New and Efficient Hypervalent Iodine-Benzyne Precursor, (Phenyl)[o-(trimethylsilyl)phenyl]iodonium Triflate: Generation, Trapping Reaction, and Nature of Benzyne. J. Am. Chem. Soc. 1999, 121, 11674–11679. 10.1021/ja992324x. [DOI] [Google Scholar]; b Kitamura T.; Yamane M. (Phenyl)[o-(trimethylsilyl)phenyl]iodonium triflate. A New and Efficient Precursor of Benzyne. J. Chem. Soc., Chem. Commun. 1995, 983–984. 10.1039/c39950000983. [DOI] [Google Scholar]
- Kovács S.; Csincsi A. I.; Nagy T. Z.; Boros S.; Timári G.; Novák Z. Design and Application of New Imidazolylsulfonate-Based Benzyne Precursor: An Efficient Triflate Alternative. Org. Lett. 2012, 14, 2022–2025. 10.1021/ol300529j. [DOI] [PubMed] [Google Scholar]
- a Devaraj K.; Ingner F. J. L.; Sollert C.; Gates P. J.; Orthaber A.; Pilarski L. T. Arynes and Their Precursors from Arylboronic Acids via Catalytic C–H Silylation. J. Org. Chem. 2019, 84, 5863–5871. 10.1021/acs.joc.9b00221. [DOI] [PubMed] [Google Scholar]; b Ikawa T.; Masuda S.; Nakajima H.; Akai S. 2-(Trimethylsilyl)phenyl Trimethylsilyl Ethers as Stable and Readily Accessible Benzyne Precursors. J. Org. Chem. 2017, 82, 4242–4253. 10.1021/acs.joc.7b00238. [DOI] [PubMed] [Google Scholar]; c Michel B.; Greaney M. F. Continuous-Flow Synthesis of Trimethylsilylphenyl Perfluorosulfonate Benzyne Precursors. Org. Lett. 2014, 16, 2684–2687. 10.1021/ol500959e. [DOI] [PubMed] [Google Scholar]; d Ikawa T.; Nishiyama T.; Nosaki T.; Takagi A.; Akai S. A Domino Process for Benzyne Preparation: Dual Activation of o-(Trimethylsilyl)phenols by Nonafluorobutanesulfonyl Fluoride. Org. Lett. 2011, 13, 1730–1733. 10.1021/ol200252c. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Yu H.; Xu Z.; Lin L.; Jiang X.; Wang R. Development and Application of O-(Trimethylsilyl)aryl Fluorosulfates for the Synthesis of Arynes. J. Org. Chem. 2015, 80, 6890–6896. 10.1021/acs.joc.5b00923. [DOI] [PubMed] [Google Scholar]
- Mesgar M.; Daugulis O. Silylaryl Halides Can Replace Triflates as Aryne Precursors. Org. Lett. 2016, 18, 3910–3913. 10.1021/acs.orglett.6b01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunico R. F.; Dexheimer E. M. Generation of 1,2-Dehydrobenzene from the Dehalosilylation of (o-Halophenyl)trimethylsilanes. J. Organomet. Chem. 1973, 59, 153–160. 10.1016/S0022-328X(00)95030-7. [DOI] [Google Scholar]
- a Lin W.; Chen L.; Knochel P. Preparation of Functionalized 3,4-Pyridynes via 2-Magnesiated Diaryl Sulfonates. Tetrahedron 2007, 63, 2787–2797. 10.1016/j.tet.2007.01.027. [DOI] [Google Scholar]; b Lin W.; Ilgen F.; Knochel P. Preparation of Highly Functionalized Arylmagnesium Reagents by the Addition of Magnesium Phenylselenide to Arynes. Tetrahedron Lett. 2006, 47, 1941–1944. 10.1016/j.tetlet.2006.01.071. [DOI] [Google Scholar]; c Lin W.; Sapountzis I.; Knochel P. Preparation of Functionalized Aryl Magnesium Reagents by the Addition of Magnesium Aryl Thiolates and Amides to Arynes. Angew. Chem., Int. Ed. 2005, 44, 4258–4261. 10.1002/anie.200500443. [DOI] [PubMed] [Google Scholar]; d Sapountzis I.; Lin W.; Fischer M.; Knochel P. Preparation of Polyfunctional Arynes via 2-Magnesiated Diaryl Sulfonates. Angew. Chem., Int. Ed. 2004, 43, 4364–4366. 10.1002/anie.200460417. [DOI] [PubMed] [Google Scholar]
- Gallo R. D. C.; Gebara K. S.; Muzzi R. M.; Raminelli C. Efficient and Selective Iodination of Phenols Promoted by Iodine and Hydrogen Peroxide in Water. J. Braz. Chem. Soc. 2010, 21, 770–774. 10.1590/S0103-50532010000400026. [DOI] [Google Scholar]
- Moreira B. V.; Muraca A. C. A.; Raminelli C. Synthesis of Silylbiaryl Triflates by Chemoselective Suzuki Reaction. Synthesis 2017, 49, 1093–1102. 10.1055/s-0036-1588332. [DOI] [Google Scholar]
- Macklin T. K.; Snieckus V. Directed Ortho Metalation Methodology. The N,N-Dialkyl Aryl O-Sulfamate as a New Directed Metalation Group and Cross-Coupling Partner for Grignard Reagents. Org. Lett. 2005, 7, 2519–2522. 10.1021/ol050393c. [DOI] [PubMed] [Google Scholar]
- a Liu Z.; Larock R. C. Facile N-Arylation of Amines and Sulfonamides and O-Arylation of Phenols and Arenecarboxylic Acids. J. Org. Chem. 2006, 71, 3198–3209. 10.1021/jo0602221. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu Z.; Larock R. C. Facile O-Arylation of Phenols and Carboxylic Acids. Org. Lett. 2004, 6, 99–102. 10.1021/ol0361406. [DOI] [PubMed] [Google Scholar]; c Liu Z.; Larock R. C. Facile N-Arylation of Amines and Sulfonamides. Org. Lett. 2003, 5, 4673–4675. 10.1021/ol0358612. [DOI] [PubMed] [Google Scholar]
- Liu S.; Li Y.; Lan Y. Mechanistic Study of the Fluoride-Induced Activation of a Kobayashi Precursor: Pseudo-SN2 Pathway via a Pentacoordinated Silicon Ate Complex. Eur. J. Org. Chem. 2017, 6349–6353. 10.1002/ejoc.201701249. [DOI] [Google Scholar]
- Suh S.-E.; Chenoweth D. M. Aryne Compatible Solvents are not Always Innocent. Org. Lett. 2016, 18, 4080–4083. 10.1021/acs.orglett.6b01977. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Larock R. C. Intermolecular C–N Addition of Amides and S–N Addition of Sulfinamides to Arynes. J. Am. Chem. Soc. 2005, 127, 13112–13113. 10.1021/ja054079p. [DOI] [PubMed] [Google Scholar]
- Gebara K. S.; Casagrande G. A.; Raminelli C. An Efficient Fluoride-mediated O-Arylation of Sterically Hindered Halophenols with Silylaryl Triflates under Mild Reaction Conditions. Tetrahedron Lett. 2011, 52, 2849–2852. 10.1016/j.tetlet.2011.03.124. [DOI] [Google Scholar]
- Shi F.; Waldo J. P.; Chen Y.; Larock R. C. Benzyne Click Chemistry: Synthesis of Benzotriazoles from Benzynes and Azides. Org. Lett. 2008, 10, 2409–2412. 10.1021/ol800675u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez S. G.; Alvarez M. T. A Practical Procedure for the Synthesis of Alkyl Azides at Ambient Temperature in Dimethyl Sulfoxide in High Purity and Yield. Synthesis 1997, 413–414. 10.1055/s-1997-1206. [DOI] [Google Scholar]
- Kamalraj V. R.; Senthil S.; Kannan P. One-pot Synthesis and the Fluorescent Behavior of 4-Acetyl-5-methyl-1,2,3-triazole Regioisomers. J. Mol. Struct. 2008, 892, 210–215. 10.1016/j.molstruc.2008.05.028. [DOI] [Google Scholar]
- Dai Z.-C.; Chen Y.-F.; Zhang M.; Li S.-K.; Yang T.-T.; Shen L.; Wang J.-X.; Qian S.-S.; Zhu H.-L.; Ye Y.-H. Synthesis and Antifungal Activity of 1,2,3-Triazole Phenylhydrazone Derivatives. Org. Biomol. Chem. 2015, 13, 477–486. 10.1039/C4OB01758G. [DOI] [PubMed] [Google Scholar]
- Perecim G. P.; Rodrigues A.; Raminelli C. A Convenient Formation of Aporphine Core via Benzyne Chemistry: Conformational Analysis and Synthesis of (R)-Aporphine. Tetrahedron Lett. 2015, 56, 6848–6851. 10.1016/j.tetlet.2015.10.083. [DOI] [Google Scholar]
- Armarego W. L. F; Perrin D. D.. Purification of Laboratory Chemicals; 4th Ed.; Butterworth-Heinemann: Oxford, 1996. [Google Scholar]
- Watson S. C.; Eastham J. F. Colored Indicators for Simple Direct Titration of Magnesium and Lithium Reagents. J. Organomet. Chem. 1967, 9, 165–168. 10.1016/S0022-328X(00)92418-5. [DOI] [Google Scholar]
- Leonard J.; Lygo B.; Procter G.. Advanced Practical Organic Chemistry; 2nd Ed.; CRC Press: Boca Raton, 1998, 102. [Google Scholar]
- Kajigaeshi S.; Kakinami T.; Yamasaki H.; Fujisaki S.; Kondo M.; Okamoto T. Iodination of Phenols by Use of Benzyltrimethylammonium Dichloroiodate(1−). Chem. Lett. 1987, 16, 2109–2112. 10.1246/cl.1987.2109. [DOI] [Google Scholar]
- Francke R.; Schnakenburg G.; Waldvogel S. R. Efficient and Reliable Iodination and O-Methylation of Fluorinated Phenols. Eur. J. Org. Chem. 2010, 2357–2362. 10.1002/ejoc.201000161. [DOI] [Google Scholar]
- Rasheed O. K.; Hardcastle I. R.; Raftery J.; Quayle P. Aryne Generation vs Truce-Smiles and Fries Rearrangements During the Kobayashi Fragmentation Reaction: A New Bi-Aryl Synthesis. Org. Biomol. Chem. 2015, 13, 8048–8052. 10.1039/C5OB01239B. [DOI] [PubMed] [Google Scholar]
- Quasdorf K. W.; Riener M.; Petrova K. V.; Garg N. K. Suzuki–Miyaura Coupling of Aryl Carbamates, Carbonates, and Sulfamates. J. Am. Chem. Soc. 2009, 131, 17748–17749. 10.1021/ja906477r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. H.; Lee B. C.; Lee H. W.; Lee I. Competitive Reaction Pathways in the Nucleophilic Substitution Reactions of Aryl Benzenesulfonates with Benzylamines in Acetonitrile. J. Org. Chem. 2002, 67, 1277–1281. 10.1021/jo0161835. [DOI] [PubMed] [Google Scholar]
- Senecal T. D.; Parsons A. T.; Buchwald S. L. Room Temperature Aryl Trifluoromethylation via Copper-Mediated Oxidative Cross-Coupling. J. Org. Chem. 2011, 76, 1174–1176. 10.1021/jo1023377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A. B.; Jaseer E. A.; Sekar G. General, Mild, and Intermolecular Ullmann-Type Synthesis of Diaryl and Alkyl Aryl Ethers Catalyzed by Diol–Copper(I) Complex. J. Org. Chem. 2009, 74, 3675–3679. 10.1021/jo900438e. [DOI] [PubMed] [Google Scholar]
- Slagh H.; Britton E. New Compounds. Aryl Esters of Substituted Benzenesulfonic Acids. J. Am. Chem. Soc. 1950, 72, 2808. 10.1021/ja01162a605. [DOI] [Google Scholar]
- Zhang J.; Zhang Z.; Wang Y.; Zheng X.; Wang Z. Nano-CuO-Catalyzed Ullmann Coupling of Phenols with Aryl Halides under Ligand-Free Conditions. Eur. J. Org. Chem. 2008, 5112–5116. 10.1002/ejoc.200800637. [DOI] [Google Scholar]
- Ames D. E.; Opalko A. Synthesis of Dibenzofurans by Palladium-Catalysed Intramolecular Dehydrobromination of 2-Bromophenyl Phenyl Ethers. Synthesis 1983, 234–235. 10.1055/s-1983-30296. [DOI] [Google Scholar]
- Nollau E. H.; Daniels L. C. A Study of the Reaction of Alkali Salts of Sulfonic Acids with Alkali Phenolates by Dry Distillation. J. Am. Chem. Soc. 1914, 36, 1885–1891. 10.1021/ja02186a010. [DOI] [Google Scholar]
- Salgado-Zamora H. A Novel Diaryl Ether Ullmann-Type Synthesis using Thallium Derivatives as Both Aryl Components. Rev. Soc. Quim. Mex. 2003, 47, 258–259. [Google Scholar]
- Lockner J. W.; Dixon D. D.; Risgaard R.; Baran P. S. Practical Radical Cyclizations with Arylboronic Acids and Trifluoroborates. Org. Lett. 2011, 13, 5628–5631. 10.1021/ol2023505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavill G. W. K.; Robertson A.; Whalley W. B. 355. The Chemistry of Fungi. Part VIII. The Oxidation of Methylene Groups in Compounds Analogous to O-Dimethylcitromycin. J. Chem. Soc. 1949, 1567–1570. 10.1039/jr9490001567. [DOI] [Google Scholar]
- Ritschel J.; Sasse F.; Maier M. E. Synthesis of a Benzolactone Collection using Click Chemistry. Eur. J. Org. Chem. 2007, 78–87. 10.1002/ejoc.200600681. [DOI] [Google Scholar]
- Nguyen T.-T.-T.; Simon F.-X.; Schmutz M.; Mésini P. J. Direct Functionalization of Self-Assembled Nanotubes Overcomes Unfavorable Self-Assembling Processes. Chem. Commun. 2009, 3457–3459. 10.1039/b903797g. [DOI] [PubMed] [Google Scholar]
- Gassman P. G.; Schaffhausen J. G. Synthesis, Photolysis, and Pyrolysis of 10-Substituted exo-3,4,5-Triazatricyclo[5.2.1.02,6]dec-3-enes. Preparation of 8-Substituted exo- and endo-3-Aryl-3-azatricyclo[3.2.1.02,4]octanes. J. Org. Chem 1978, 43, 3214–3223. 10.1021/jo00410a024. [DOI] [Google Scholar]
- Knepper K.; Vanderheiden S.; Bräse S. Nitrogen Functionalities in Palladium-Catalyzed Reactions on Solid Supports: A Case Study. Eur. J. Org. Chem. 2006, 1886–1898. 10.1002/ejoc.200500779. [DOI] [Google Scholar]
- Zhu W.; Ma D. Synthesis of Aryl Azides and Vinyl Azides via Proline-Promoted CuI-Catalyzed Coupling Reactions. Chem. Commun 2004, 888–889. 10.1039/b400878b. [DOI] [PubMed] [Google Scholar]
- Ankati H.; Biehl E. Microwave-Assisted Benzyne-Click Chemistry: Preparation of 1H-benzo[d][1,2,3]triazoles. Tetrahedron Lett. 2009, 50, 4677–4682. 10.1016/j.tetlet.2009.06.004. [DOI] [Google Scholar]
- Katritzky A. R.; Abdel-Fattah A. A. A.; Tymoshenko D. O.; Belyakov S. A. Nucleophilic Substitution of Benzotriazolylalkyl Chlorides with Grignard Reagents: A Direct Route to Benzotriazoloalkyl(hetero)aromatic Compounds. Synthesis 1999, 1437–1440. 10.1055/s-1999-3662. [DOI] [Google Scholar]
- Lee H.-G.; Won J.-E.; Kim M.-J.; Park S.-E.; Jung K.-J.; Kim B. R.; Lee S.-G.; Yoon Y.-J. TBAF-Assisted Copper-Catalyzed N-Arylation and Benzylation of Benzazoles with Aryl and Benzyl Halides under the Ligand/Base/ Solvent-Free Conditions. J. Org. Chem. 2009, 74, 5675–5678. 10.1021/jo900752z. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Moses J. E. Benzyne Click Chemistry with in Situ Generated Aromatic Azides. Org. Lett. 2009, 11, 1587–1590. 10.1021/ol9002338. [DOI] [PubMed] [Google Scholar]
- Kim H.; Kim Y.; Kim H.; Son H.; Bae H.. Organic Compound and Organic Electroluminescent Element Comprising Same. Patent WO 2018093107, May 24, 2018.
- Gilman H.; Gorsich R. D. Some Reactions of o-Halophenyllithium Compounds. J. Am. Chem. Soc. 1957, 79, 2625–2629. 10.1021/ja01567a072. [DOI] [Google Scholar]
- Zhou H.; Li J.; Yang H.; Xia C.; Jiang G. Triarylphosphines as Aryl Donors for Pd(II)-Catalyzed Aromatic Coupling of Oxabenzonorbornadienes. Org. Lett. 2015, 17, 4628–4631. 10.1021/acs.orglett.5b02366. [DOI] [PubMed] [Google Scholar]
- Villeneuve K.; Tam W. Ruthenium(II)-Catalyzed Cyclization of Oxabenzonorbornenes withPropargylic Alcohols: Formation of Isochromenes. Eur. J. Org. Chem. 2006, 5449–5453. 10.1002/ejoc.200600836. [DOI] [Google Scholar]
- Nishimura T.; Kawamoto T.; Sasaki K.; Tsurumaki E.; Hayashi T. Rhodium-Catalyzed Asymmetric Cyclodimerization of Oxa- and Azabicyclic Alkenes. J. Am. Chem. Soc. 2007, 129, 1492–1493. 10.1021/ja068488c. [DOI] [PubMed] [Google Scholar]
- Webster R.; Boyer A.; Fleming M. J.; Lautens M. Practical Asymmetric Synthesis of Bioactive Aminotetralins from a Racemic Precursor Using a Regiodivergent Resolution. Org. Lett. 2010, 12, 5418–5421. 10.1021/ol1022239. [DOI] [PubMed] [Google Scholar]
- Slamet R.; Wege D. Preparation of Isobenzofurandiones by Flash Vacuum Pyrolysis Involving Retro-Diels-Alder Expulsion of Ethylene and Concomitant C-O Cleavage of Methoxy or Ethylenedioxy Groups. Tetrahedron 2007, 63, 12621–12628. 10.1016/j.tet.2007.10.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.