Abstract
Varanopids were a group of small to medium-sized synapsids whose fossil record spans the Carboniferous through middle Permian. Although their phylogenetic relationships have received some interest in recent years, little is known about other aspects of their palaeobiology, including their skeletal growth, allometry and habitat preference. Here, we describe varanopid long bone histology based on a sample of well-preserved femora from the lower Permian Richards Spur fissure fill locality, Comanche County, Oklahoma, USA. The sample includes five femora from at least two varanopid taxa—Mycterosaurus and the large varanodontine Varanops brevirostris—and four additional mycterosaurine femora not diagnosed to genus. Prior work on femoral bone compactness provided a baseline to make lifestyle inferences and evaluate whether varanopids were ancestrally terrestrial. Moreover, the large availability of specimens spanning different sizes made possible an assessment of size-related ontogenetic histovariability. All specimens revealed moderately dense cortical bone tissues composed of sparsely vascularized parallel-fibred and lamellar bone with radially arranged rows of longitudinal canals (mostly simple), and many preserved regularly spaced growth marks (annuli and lines of arrested growth) as in modern varanids. We show that bone histology has the potential to explain how ballast was shed and the skeleton lightened for terrestrial mobility in ancestral synapsids and their basal amniote kin, as well as how adjustments in postnatal growth influenced the evolution of larger body sizes in the terrestrial frontier.
This article is part of the theme issue ‘Vertebrate palaeophysiology'.
Keywords: amniote, growth marks, skeletochronology, Permian, bone compactness
1. Introduction
The Synapsida represent one of the two major land-vertebrate clades that diverged from reptile-line amniotes during the late Carboniferous, ca 320 million years ago (Ma) [1–3]. Palaeontologists have long speculated about innovations in the life histories and reproductive physiology of these early amniotes, including how and when their ancestors developed extra-embryonic membranes and detached themselves from dependency on permanent water sources for reproduction, and how this novel ecology might have driven their skeletal biology. Prior work relied on anecdotal observations to address reproduction and ancestral life habitus in stem amniotes or ‘reptiliomorphs' [4–6]. Laurin [7] later evaluated these hypotheses in a phylogenetic context, arguing that the earliest amniotes were larger than previously estimated (snout–vent length up to 24 cm and no less than 12 cm) and that their postnatal skeletal growth was most likely released from the constraint of small egg size. Evolutionary size increases apparently applied to the amniote stem, but not universally to early tetrapods.
While early amniotes and stem amniotes exhibited a variety of body sizes and ecologies by the Carboniferous–Permian transition, synapsid fossils in particular reveal patterns of increasing body length and myriad dietary ecologies [7,8], reflecting either a passive tendency to move away from their presumably small ancestral sizes or relaxation of prior ecophysiological constraints on size [9,10]. Turner & Tracy [11] emphasized the importance of postnatal body size, arguing that in some synapsids large size favoured terrestrial homeothermy (involuntary maintenance of a constant internal body temperature). This transition coincided with the eventual expansion of dryland-adapted faunas in seasonal environments of western Pangea ca 300 Ma, which covered even palaeotropical latitudes [12].
Importantly, whether the amniotic egg is optimized for terrestrial reproduction is tangential to whether the earliest amniotes were primarily terrestrial animals [13]. Ichnological (e.g. trackways) and palaeoenvironmental evidence provide tantalizing albeit circumstantial evidence, but the integration of comparative and functional training datasets in a rigorous phylogenetic framework has begun to offer robust models for ‘retrodicting' the physiology and behaviours of extinct amniotes [14–16]. For example, bone compactness—the distribution of mineralized bone matrix in a cross-section—has been used to retrodict life habitus in some early synapsids, as well as other amniotes. In particular, Kriloff et al. [14] and Canoville & Laurin [15] showed evidence from the tibia and humerus, respectively, that extant amniotes bearing more tubular, less compact limb bone shafts tend toward terrestrial lifestyles, and some stem amniotes and basal synapsids also exhibited this condition. In particular, this challenged earlier notions that the synapsid Ophiacodon was a primarily aquatic ambush predator [17,18], although Canoville & Laurin [15] could not rule out that Ophiacodon was partially amphibious. More recently, Laurin & de Buffrénil [13] analysed femoral cross-sections from additional ophiacodontids, including Clepsydrops, corroborating that their limb bone compactness was best optimized for a terrestrial existence. Contemporary research suggests that the synapsid Dimetrodon, ophiacodontids, and their immediate ancestors included animals that were highly capable terrestrial predators with a capacity for multi-year growth to large body sizes while minimizing overall bone mass [13,19]. Nevertheless, despite Romer's [17] outdated views of ophiacodontid behaviour and phylogeny, the taxa studied to date do not necessarily reflect the ancestral state of Synapsida. Thus, while advances in palaeohistology have begun to address skeletal adaptation in Carboniferous–Permian synapsids with some statistical rigour, description of new material from key clades remains critical for improving the precision and confidence of our retrodictions.
Early investigations of synapsid bone histology were limited to a few well-represented taxa (e.g. Dimetrodon, Edaphosaurus) and isolated bones (e.g. [20,21]), prohibiting an in-depth understanding of histovariation within the group, especially in its basal-most members. The most prolific pioneering work was conducted by Enlow [22,23] and de Ricqlès [24–28], who broadly concluded that pelycosaur-grade synapsids exhibited more ‘reptile-like’ growth and only limited skeletal remodelling compared with the mammal-like Permo-Triassic therapsids. Renewed interest in synapsid palaeohistology in the past decade has revealed previously underappreciated histovariability in some groups, with limited sampling in the basal caseasaurs [29,30], varanopids [31], ophiacodontids [13,32], edaphosaurids [31,33] and sphenacodontids [31,34–36]. For example, the large carnivores Ophiacodon, Sphenacodon and Dimetrodon have shown evidence of rapid bone formation during early growth, in contrast to the caseasaurs and edaphosaurids. However, these are the only basal synapsid taxa to be investigated in a relatively complete ontogenetic series to date.
Of the major basal synapsids, the palaeobiology of Varanopidae remains poorly understood, largely owing to the rarity of their fossil remains. Varanopids represent a long-lived eupelycosaur clade that shared many superficial characteristics with extant monitor lizards, including long, slender limbs and sometimes recurved, ziphodont teeth [37], and survived from the late Carboniferous until the later part of the middle Permian [38]. A few varanopid taxa have been examined histologically, including the varanodontines Varanops [27,29,31] and Watongia [28,39], and a mycterosaurine from the lower Permian of Comanche County, Oklahoma [29,31]. Histologically, varanopid taxa show some degree of variation in tissue textures, vascularity, and growth zone formation. Moreover, the wide-open medulla of varanopid limb bones contrasts with some other early tetrapods, which typically exhibited a more pachyostotic medullary region, highly occluded by the spongiosa. This redistribution of bone mass may suggest evidence of more efficient terrestrial locomotion in varanopids [31].
Here, we present the first detailed descriptions of femoral microanatomy in Varanopidae, updated from the preliminary reports of Huttenlocker & Rega [31] and Shelton [29]. Huttenlocker & Rega [31] described briefly the limb bone histology of two varanopids they referred to Varanops brevirostris and a small mycterosaurine—likely Mycterosaurus—both from near Richards Spur, Oklahoma, USA. Mycterosaurus represents one of the most common vertebrates from this locality, second only to the basal eureptile Captorhinus [40]. For the present study, the sample of femora were combined with additional histological collections produced during Shelton's [29] survey of basal synapsid histology, and were analysed for bone compactness, tissue texture, vascularization, growth marks, and other important histological features. All material comes from the aforementioned lower Permian Richards Spur fissure fill Lagerstätte. The site represents an ‘upland' assemblage dominated by amniotes and presumed terrestrial amphibians, with few anomalous aquatic elements [37,41,42]. Based on their excellent preservation and distinctive histological profile, we show a departure from the stem amniote condition—which was dominated by lamellar cortical bone that is highly compact—and reveal transitional motifs that instead began to resemble adults of more derived eupelycosaurs (e.g. Ophiacodon, Dimetrodon), as well as modern varanid analogues. Palaeohistological data offered here will contribute to a more robust hypothesis of skeletal adaptation in a key fossil clade near the synapsid–reptile dichotomy.
2. Material and methods
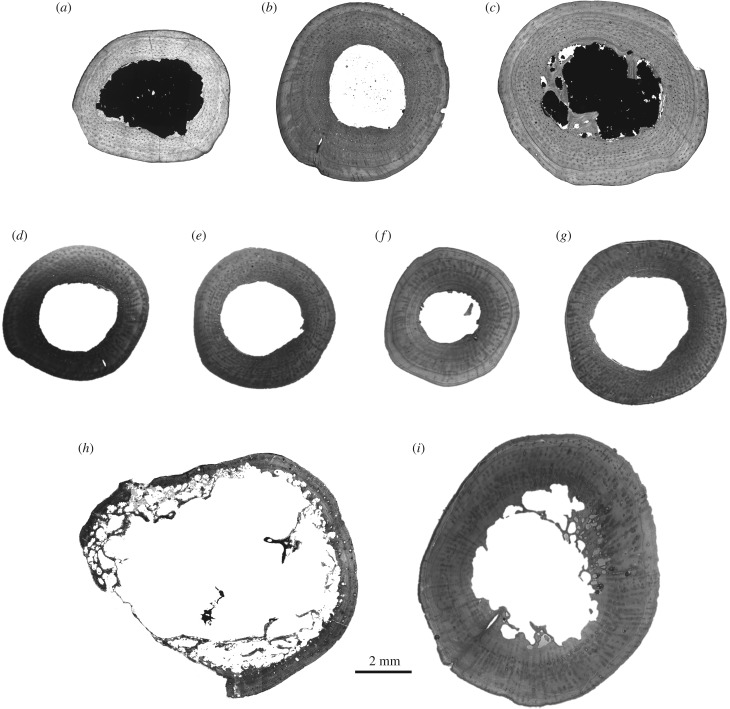
All materials described here come from a single fossil locality in Comanche County, Oklahoma, USA. The Dolese Brother's limestone quarry near Richards Spur preserves a lower Permian fissure fill deposit within an Ordovician limestone, whose fossils have been dated to approximately 289 to 286 Ma (Artinskian global stage) using speleothem U–Pb dating [42,43]. The most abundant synapsid remains from this locality belong to the varanopid Mycterosaurus cf. M. longiceps ([40]; ‘Mycterosaurus sp.' of [44] and [37]). Nine femora belonging to two taxa, Mycterosaurus and Varanops, were sampled for this study. Three femora referred conservatively to the genus Mycterosaurus were included: UWBM 89464, 89466, 98580 (figure 1a–c). Other small varanopid femora sampled here were not as well preserved or lacked key features owing to their small size, and are thus referred only to Mycterosaurinae/Varanopidae, but these most likely also represent small individuals of Mycterosaurus: OMNH 73750a, 73750b, 73750c, 73354 (figure 1d–g). A second varanopid assigned to the varanodontine V. brevirostris is also well known from the locality [44]. Two large femora assigned to V. brevirostris were thin-sectioned here: OMNH 73758, UWBM 89463 (figure 1h,i).
Figure 1.
Sample of nine varanopid synapsid femora, sectioned at midshaft, from the Permian Richards Spur locality, Oklahoma, including Mycterosaurus (a–c), small mycterosaurine (d–g) and the varanodontine Varanops brevirostris (h,i). (a) Mycterosaurus, UWBM 89466; (b) Mycterosaurus, UWBM 98580; (c) Mycterosaurus, UWBM 89464; (d) mycterosaurine, OMNH 73750c; (e) mycterosaurine, OMNH 73354; (f) mycterosaurine, OMNH 73750b; (g) mycterosaurine, OMNH 73750a; (h) Varanops, UWBM 89463 (distal shaft cross-section); and (i) Varanops, OMNH 73758. All sections are shown to scale. Measurements are presented in table 1.
Sectioning and imaging protocols followed standard procedures for fossil bone outlined by Wilson et al. [45] and Lamm [46]. After photographing the specimens, each was embedded in a cold-mounting resin that was allowed to set overnight, then sectioned at the diaphysis in 1 mm wafers using a low-speed precision saw with a diamond blade. Transverse sections were cut from the middle diaphysis in most specimens, at about the level of the nutrient foramen, except in the Varanops UWBM 89463, which was sectioned at the distal shaft owing to its completeness. Longitudinal sections were also made for UWBM 98580. Wafers were glued onto frosted petrographic slides with an epoxy resin and then ground further to a thickness of approximately 100 µm using a mechanical grinder/polisher, then polished by hand to remove fine scratches using a polishing cloth. Thin-sections were imaged using normal, elliptical and circularly polarized light settings with a Leica DM 2700 microscope fitted with a motorized stage and digital image capture system.
In order to evaluate femoral compactness profiles for potential terrestrial adaptation, the digital scans of the cross-sections were converted to binary 8-bit images in Adobe Photoshop CC (Adobe Systems) and imported into the software Bone Profiler [47]. Bone Profiler estimates bone compactness (the relative area of a cross-section occupied by mineralized bone tissue) by calculating the proportion of bone area observed in 51 zones averaged across 60 radial sectors, and constructs a ‘compactness profile’ from the centroid to the periphery of the outer bone wall. Several parameters of the compactness profile can be used to describe the distribution of bone mass within a cross-section, some of which have been shown to correlate with lifestyle based on humeral and femoral profiles in a sample of extant amniotes [15,16]. Parameters discussed here include: cortico-diaphyseal index (CDI), the thickness of the cortex divided by the bone radius; P, the position of the inflection point between the less mineralized medulla and the more mineralized cortex; and S, the slope of the medulla–cortex transition zone. The relative medullary diameter, K [48], was also measured, as the ratio between the internal and external diameter of the bone. We predicted that, given the unique upland setting of the Richards Spur assemblage, the sampled varanopid femora should exhibit relatively low compactness and an open or unoccluded medulla reflective of a terrestrial lifestyle. For this purpose, we also compared limb compactness profiles in two sympatric eureptiles, Captorhinus and an indeterminate diapsid. Measurements are presented in table 1.
Table 1.
Varanopid specimens sampled histologically and associated measurements. CDI, cortico-diaphyseal index [47]; Circ, midshaft circumference in mm; K, relative medullary diameter; P, relative position of inflection point; PF-LAM, parallel-fibred to lamellar bone transition (sensu [49]) with simple canals and/or osteons; S, slope of medulla–cortex transition zone; Vascdens, density of vascular canals per mm2.
| identification | no. | element | length (circ) | tissue-type | Vascdens | CDI | K | P | S |
|---|---|---|---|---|---|---|---|---|---|
| Mycterosaurus | UWBM 89466 | femur | 52 (16) | PF-LAM | 56 | 0.468 | 0.51 | 0.53 | 0.02 |
| Mycterosaurus | UWBM 89464 | femur | 65 (23) | PF-LAM | 41 | 0.542 | 0.44 | 0.45 | 0.01 |
| Mycterosaurus | UWBM 98580 | femur | 70 (24) | PF-LAM | 100 | 0.518 | 0.49 | 0.48 | 0.05 |
| Mycterosaurinae | OMNH 73750c | femur | 49 (17) | PF-LAM | 48 | 0.472 | 0.51 | 0.52 | 0.01 |
| Mycterosaurinae | OMNH 73354 | femur | 50 (17) | PF-LAM | 65 | 0.458 | 0.52 | 0.54 | 0.01 |
| Mycterosaurinae | OMNH 73750b | femur | 51 (18) | PF-LAM | 70 | 0.587 | 0.42 | 0.41 | 0.02 |
| Mycterosaurinae | OMNH 73750a | femur | 57 (22) | PF-LAM | 91 | 0.433 | 0.54 | 0.56 | 0.01 |
| Varanops | UWBM 89463 | femur | – (28) | PF-LAM | 24 | 0.172 | 0.72 | 0.82 | 0.04 |
| Varanops | OMNH 73758 | femur | 88 (37) | PF-LAM | 49 | 0.472 | 0.58 | 0.53 | 0.04 |
Histological descriptions were facilitated by the scanned photomicrographs at 50× and 100× total magnification under varied light settings. Elliptical and circular polarization were used to accurately identify fibre orientations and describe tissue texture, which can be somewhat diverse even in basal synapsid taxa [29,31]. The terminology and diagnosis of histotypes used here mainly follows the systems of de Margerie et al. [50] and McFarlin [49,51]. Tissue types and related histological features are abbreviated as follows: LAM, lamellar tissue; PF, parallel-fibred tissue; PF-LAM, parallel-fibred to lamellar bone transition (sensu [49, fig. 5.3]; PFO, parallel-fibred bone with primary osteons; and WB, woven-fibred tissue. Cyclic growth marks were also surveyed and described where noted, including annuli and/or lines of arrested growth (LAGs). Additional histometric measurements, including vascular density (Vascdens), were obtained by analysing the original photomicrographs in ImageJ v.1.47 [52]. Vascular density is defined here as the average number of canals observed per mm2 (see protocol of [53]). In combination with tissue texture, vascular density and osteonal organization have been shown to reflect growth rate in extant amniotes [50,54], and are adopted here as proxies for relative growth in the varanopids. Vascular measurements are also reported in table 1.
In order to build a hypothesis of character histories—including ancestral bone compactness and vascularity—the data were combined with a published phylogenetic dataset that is here expanded from the study of Huttenlocker & Farmer [53]. Because an authoritative source of tetrapod divergence dates is lacking, branch lengths were approximated using median divergence dates from the Time Tree of Life project (http://www.timetree.org). Geological dates for fossil taxa were obtained from FossilWorks (http://www.fossilworks.org). The geological time-scale adopted here is based on the 2014 International Chronostratigraphic Chart circulated by the International Commission on Stratigraphy (updated from [55]). Zero-length branches were converted arbitrarily to a minimum of 1.0 Myr. The reference tree was built in Mesquite 1.0 [56] and phylogenetic character mapping was performed using the PDAP module [57]. Standardized residual contrasts for size (femoral circumference) and vascularity were also regressed in PDAP to test for correlated character evolution that might explain observed variations in cortical vascularity and tissue texture as they relate to body size.
3. Description of histological profiles in sampled femora
(a). Mycterosaurus (figures 1a–c, 2 and 6a)
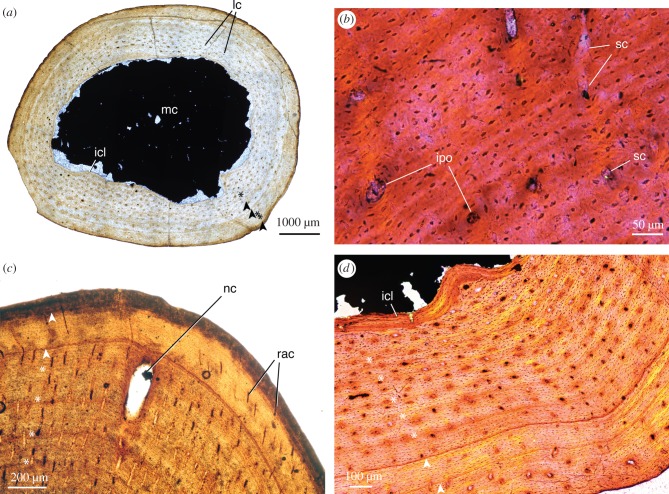
Figure 2.
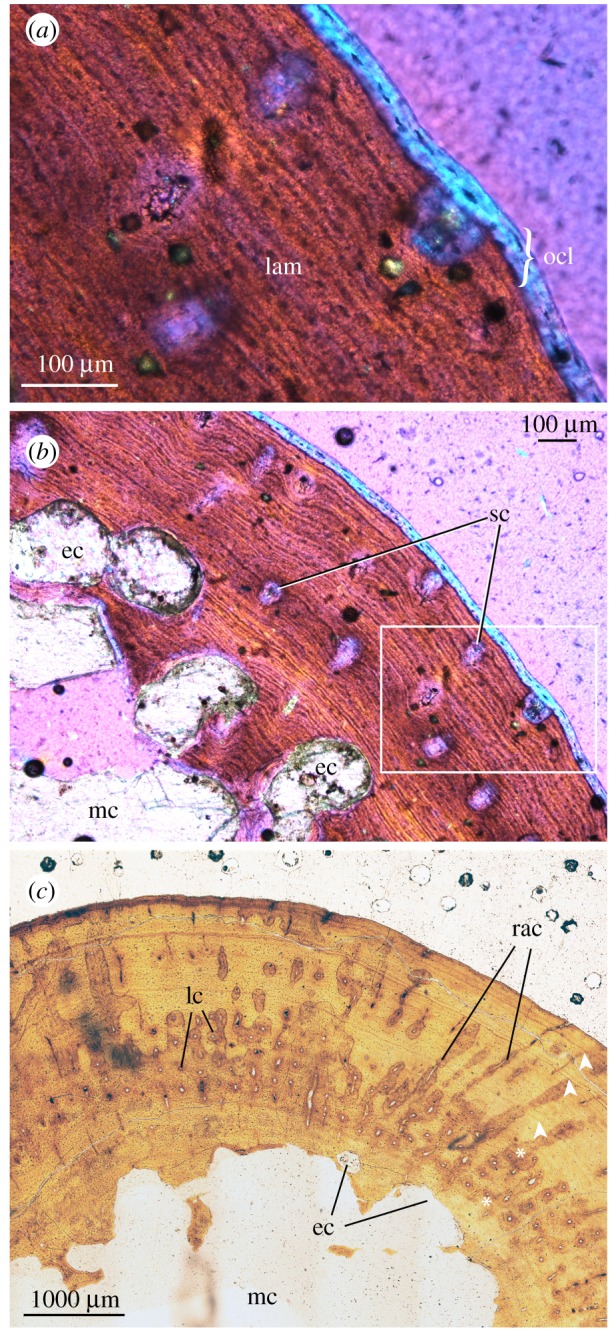
Detailed photomicrographs of mycterosaurine histology. (a) Mycterosaurus, UWBM 89466, femur midshaft at low magnification, normal (non-polarized) light; (b) same as ‘a,' midcortex viewed at high magnification, elliptical polarized light with quarter-wave retardation plate; (c) Mycterosaurus, UWBM 98580, femur midshaft, normal (non-polarized) light; (d) Mycterosaurus, UWBM 89464, femur midshaft viewed at high magnification, elliptical polarized light with quarter-wave retardation plate. Growth marks are present throughout the cortex in all sizes: asterisks indicate annuli, arrowheads indicate lines of arrested growth (LAGs). Abbreviations used in photographs of sections in this article: ec, erosion cavities; icl, inner circumferential lamellae; ipo, incipient primary osteons (sensu [36]); lam, lamellar bone; lc, longitudinal canals; lnv, non-vascular lamellar bone; mc, medullary (marrow) cavity; nc, nutrient canal; ocl, outer circumferential lamellae; pf, parallel-fibred tissue; pf-lam, parallel-fibred–lamellar transition; rac, radial canals; rc, reticular canals; sc, simple canals.
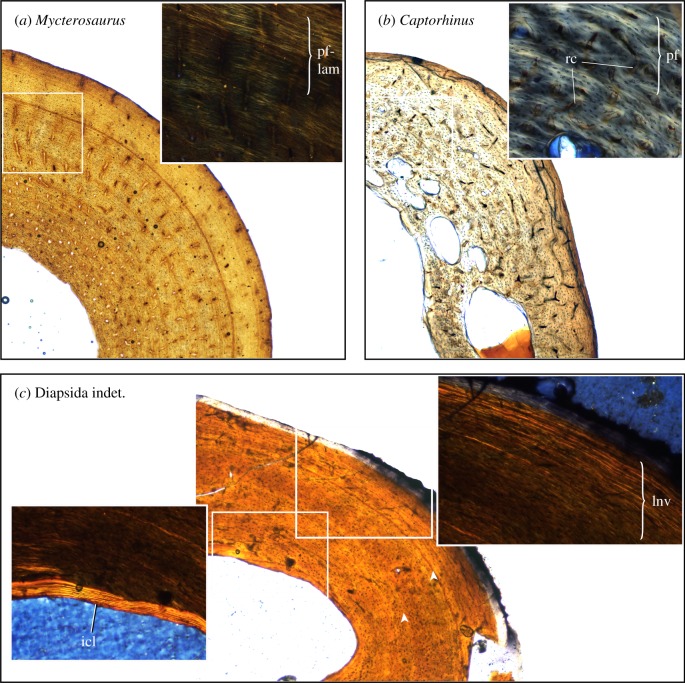
Figure 6.
Comparison of tissue textures between varanopids and two sympatric eureptiles from Richards Spur, viewed in circularly polarized light (insets). (a) Mycterosaurus femur, UWBM 98580, shows cyclic PF-LAM with vascular canals arranged in radial rows; (b) the eureptile Captorhinus femur, UMNH VP 25968, shows disorganized bundles of PF with fewer growth marks and richly vascularized reticular bone; (c) Diapsida indet. (Mycterosaurus longiceps of [29, fig.3.3a]), OMNH 52543, humerus shows nonvascular lamellar bone that is interrupted by two LAGs (arrowheads). Abbreviations as in figure 2. (Online version in colour.)
The three femora sectioned here ranged from approximately 52–70 mm in length (16–24 mm in circumference) (table 1), thus falling in the size range of other Mycterosaurus fossils reported from the locality [40]. The femora are somewhat gracile, slender with a narrow shaft and proximal and distal ends. The intertrochanteric fossa is short with prominent anterior ridge nearly contacting the proximal articular surface, and the internal trochanter and adductor ridge are modestly developed. The smooth, black bone is generally well preserved with little damage or any evidence of recrystallization, presenting ideal optics for demonstrating discrete histological features (e.g. vasculature, growth marks) as well as the organization and texture of the original bone matrix. Minor portions of the bone, mainly internal cavities, are infilled with mud matrix and calcite, and have in places become pyritized (optically extinct areas in figures 1 and 2).
Despite their small, gracile appearance, the cortical walls of the femora are moderately thick and dense at the midshaft, approximately 1.5 to 2 mm or 21% of the cross-sectional diameter. This gives the gestalt of a highly compacted cortex. However, this is offset by an abrupt transition to a wide, open medullary (marrow) cavity with little to no cancellous bone. Coarse trabecular structures are present internally only closer to the proximal and distal diaphysis (near the metaphyses). At the midshaft, the deep portions of the cortex preserve dense primary bone tissue with little endosteal resorption and no evidence of compact-to-cancellous conversion in this area. In places, this smooth boundary is demarcated by endosteally deposited inner circumferential lamellae (icl; figure 2d). Given the large size of the medullary cavity, global compactness of the femoral midshaft was therefore low in all specimens (CDI, 0.468–0.542; K, 0.44–0.51), and showed no clear trend with increasing size.
The size of the medullary cavity limits our interpretation of the earliest growth phase, but subadult growth patterns can be generalized from all three specimens owing to the maintenance of primary bone and limited remodelling throughout the cortex. All femora show excellent preservation of growth marks bearing a naturally dark stain that makes them discernible under normal (non-polarized) light. Multiple zones of densely packed longitudinal vascular canals within a parallel-fibred to lamellar matrix are preserved in the inner cortex. The zones are delimited by faint annuli of denser bone (asterisks, figure 2) that are somewhat regularly spaced, recording a rhythm of lamellated, periosteal bone apposition. Some zones are followed by distinct lines of arrested growth (LAGs: arrowheads, figure 2) instead of annuli. However, there is no clear pattern in the relative distribution of annuli versus LAGs, whose formation may have been somewhat variable across individuals. The bone matrix becomes increasingly dense with fewer vascular spaces in the outermost cortex, particularly in larger individuals (UWBM 98580, 89464; figure 2c,d). The largest femur, UWBM 89464, includes at least one (and possibly a second) stark LAG in the outer one-third of the cortex (figure 2d), followed by poorly vascularized zones composed of mainly lamellar bone. Tissue changes likely represent a gradual decrease in growth rate, but the outer circumferential lamellae are not arranged into a distinct, avascular external fundamental system (EFS, [58]). The lack of a growth asymptote suggests instead that some capacity for skeletal growth was still possible up to the time of death, even in large specimens.
The organization of the vascular and lacunar spaces presents important ontogenetic and phylogenetic considerations. The vascular canals are predominantly simple, with most lacking concentric lamellae along their inner (endosteal) surfaces. Some areas preserve incipient primary osteons, showing the beginnings of concentric lamellation as described in the sphenacodont Dimetrodon [36], particularly within the deeper portions of the cortex representing earlier bone formation. The majority of outer canals, however, are simple canals. The canals are widely spaced (Vascdens, 41.6–100 canals per mm2) and are oriented longitudinally, but are frequently arranged into radial rows. Some may bear slightly radial orientations, particularly within thicker growth zones or in thicker areas of the cross-section (figure 2c). The osteocyte lacunae similarly take on an orderly appearance, being predominantly lenticular in shape, their long-axes aligning with the surrounding lamellae. The sparse vasculature and orderly tissue texture, formed predominantly by alternating patterns of PF-LAM, are consistent with an interpretation of prolonged, cyclic growth.
(b). Mycterosaurinae indet. (figure 1d–g)
The four additional mycterosaurine femora will be summarized together, noting that their histological profiles are nearly identical to that described above in Mycterosaurus. The slender femora, which range in size from 49 to 57 mm in length, have a circular midshaft cross-section with a moderately thick bone wall (CDI, 0.433–0.587; K, 0.42–0.54). Trabecular structures in the medulla are mostly absent at the midshaft level, as shown in Mycterosaurus.
In transverse section, it can be seen that lightly vascularized transitional PF-LAM prevails throughout the cortex. The vascular canals are mainly small, longitudinal canals that may be simple or osteonal. Thin radial canals, arrayed from the inner to outer cortex, also perfuse the cortex, forming a sunburst or ‘bicycle wheel’ pattern as observed in limb bones of other carnivorous eupelycosaurs [29,36]. The canals exhibit a relatively low density (Vascdens, 48–91 canals per mm2). The bone matrix is speckled with densely packed osteocyte lacunae with flattened, lenticular shapes and aligned with the lamellae. The cortical bone contains, on average, a record of three to four growth marks visible in conventional light (not counting earlier growth marks that would have become obliterated by the expansion of the medullary cavity). Sharpey's fibres are not present. As in Mycterosaurus, inner circumferential lamellae are visible in polarized light around the medullary cavity. There is no EFS in the specimens.
(c). Varanops brevirostris (figures 1h–i and 3a–c)
Figure 3.
Detailed photomicrographs of Varanops histology. (a) Varanops, UWBM 89463, femur distal shaft at high magnification, elliptical polarized light with quarter-wave retardation plate; (b) same as (a) (inset), viewed at low magnification, elliptical polarized light with quarter-wave retardation plate; (c) Varanops, OMNH 73354, femur midshaft, normal (non-polarized) light. Abbreviations as in figure 2. (Online version in colour.)
The two sampled Varanops femora show broad similarities to Mycterosaurus, suggesting important family-level features of varanopid histology, but there are notable differences between the genera. The robust elements included a complete left femur sectioned at the midshaft (OMNH 73758), and a second partial left femur sectioned at the distal shaft (UWBM 89463). As expected, the well-preserved bone walls appear generally thinner closer to the metaphysis than at the midshaft, but both sections are proportionately thinner-walled than in the mycterosaurines. For example, the midshaft bone wall is of similar thickness to that in the mycterosaurines, being approximately 1.5 to 2 mm thick, despite the larger femoral diameter of Varanops. The relatively compact cortex transitions to coarse cancellous bone with prominent secondary trabecular structures (remodelled primary bone) and a relatively cavernous marrow space. Unlike the mycterosaurine samples, an inner circumferential layer was not established, and the endosteal lining of the medullary cavity was instead festooned by large erosion cavities carving into the cortical wall (figure 3b,c). Overall bone compactness was lower in Varanops than the majority of mycterosaurine femora described above, although values overlapped with some (CDI, 0.172–0.472; K, 0.58–0.72).
Growth rhythms are best recorded in the midshaft section of OMNH 73354, where a thicker wall of primary bone is preserved without as much internal erosion (figure 1i). There were at least five and as many as six growth marks (including both annuli and LAGs) visible in the specimen under normal light, although the innermost was largely obliterated by the expanding medullary cavity. The inner growth marks appear as annuli, whereas the outer three zones were punctuated by distinct LAGs that became more closely spaced peripherally (figure 3c). In UWBM 89463, there is an intriguing shell of avascular outer circumferential lamellae, about two or three lamellae thick (ocl, figure 3a), with fibre orientations apparently perpendicular to that of the underlying bone matrix. This switch in matrix organization is detectable on the basis of the elliptical polarization and reorientation of the long-axis of the osteocyte lacunae, which are considerably longer on the transverse plane in this zone.
The vascular motifs and tissue textures exhibited by Varanops were similar to those found in the mycterosaurine samples. The majority of the cortex was formed by alternating PF-LAM, with lamellar bone becoming more predominant toward the periphery. Primary osteons and simple canals were sparsely distributed throughout the highly lamellated bone matrix, their orientation being mainly longitudinal, but with a few radial canals in some locations forming a distinctive starburst or ‘bicycle wheel' motif (figure 3c). Vascular density was low overall (Vascdens, 24–49 canals per mm2). In UWBM 89463 (figure 3a,b), the cortex was dominated by lamellar bone with sparse simple canals having longitudinal orientations. The osteocyte lacunae are densely packed, lenticular in shape, and arranged into rows that parallel the sheet-like lamellae. Sharpey's fibres are present in localized areas, best visualized under polarized light.
4. Discussion of results
(a). Bone compactness and lifestyle inference in Richards Spur varanopids
In general, femur compactness profiles of Richards Spur varanopids reveal a relatively tubular cross-section, having a dense, moderately thickened cortex but with limited internal cancellous structure (figure 4). Cancellous trabeculae are more obvious in the Varanops sections than in mycterosaurines. The resulting ‘hollow cylinder' structure is noteworthy given that many other basal amniotes, such as the eureptile Captorhinus (figure 4c), and stem amniotes preserve a more oval or teardrop cross-section, partly due to the hypertrophied adductor ridge that spans much of the femur length in the latter. The more cylindrical build in varanopids permits greater resistance to torsion and multidirectional bending [59,60], and possibly indicates a wider range of hindlimb motion in varanopids than in other early amniotes such as captorhinid eureptiles.
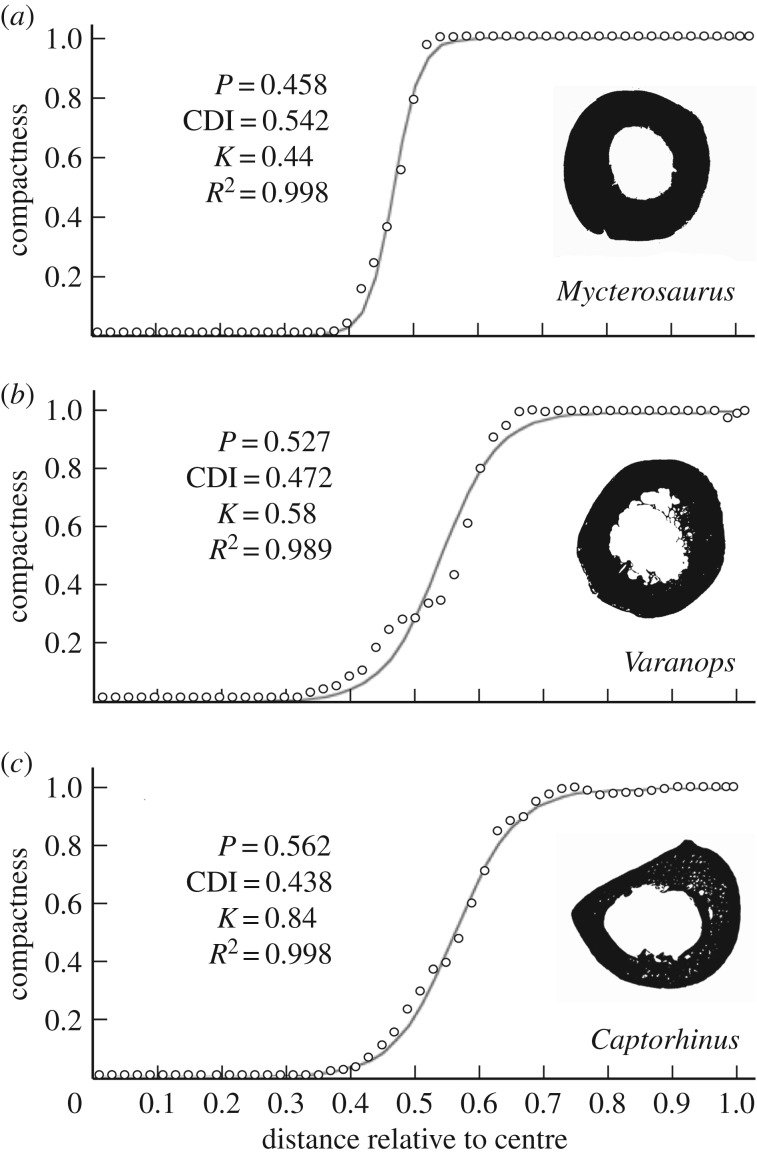
Figure 4.
Femur midshaft compactness profiles of representative varanopid and Captorhinus specimens. (a) Mycterosaurus, UWBM 98580; (b) Varanops, OMNH 73758; (c) the eureptile Captorhinus, UMNH VP 25967. Values represent: P, position of the inflection point; CDI, cortico-diaphyseal index; and K, relative medullary diameter. Measurements for individual varanopids analysed in Bone Profiler [47] are listed in table 1.
In terms of the circular distribution of mineralized bone matrix, the bone compactness parameters are consistent with a terrestrial life habitus in varanopids (as suggested for other eupelycosaurs; [13]). The perimedullary margin of mycterosaurine femora is smoothly finished by endosteally deposited inner circumferential lamellae, whereas that of Varanops is highly irregular. The relative medullary diameter is also more restricted in mycterosaurines (mean K, 0.49) than in Varanops, where the transition from the thin cortical wall to the medulla was more gradual (mean K, 0.65) (figure 4a,b). Nevertheless, only moderate levels of femoral compactness are observed in mycterosaurines (mean CDI, 0.496; mean P, 0.49) and especially in Varanops (mean CDI, 0.322; mean P, 0.67). Despite the difference in eccentricity mentioned above, these values are broadly similar to Captorhinus, also from the Richards Spur assemblage (figure 4c). These contrast, however, with published values for more aquatic taxa, where bone mass is distributed closer to the centroid (see [16]).
Germain & Laurin [18] originally argued for an aquatic lifestyle in the synapsid Ophiacodon owing to the compactness of the radius, but inferred this condition with low confidence given their meagre sample size. Improved predictive models based on extant amniote tibiae [14] and humeri [15] led the authors to revise this inference, showing instead that a terrestrial or only partially amphibious mode of life was more likely in Dimetrodon and Ophiacodon. Building on these studies, Laurin & de Buffrénil [13] included additional ophiacodontids, including femora of Clepsydrops and Ophiacodon uniformis, as well as the radius of Ophiacodon retroversus originally analysed by Germain & Laurin [18]. The authors concluded that ophiacodontids were primitively terrestrial, and the initial discrimination of O. retroversus as aquatic might have been based on variation in the proximo-distal location of the radial thin-section. Perhaps another reason for the incongruous results is the universally thick, compact radial cortex in synapsid quadrupeds compared with more proximal and hindlimb elements (e.g. [61]), making discrimination of a terrestrial or semiterrestrial animal less certain.
Overall, sampled synapsid limb bones suggest an ancestral condition that may have overlapped with values observed in semi- and fully terrestrial taxa, but their lifestyle discrimination based on femoral compactness tends toward terrestriality [13], broadly congruous with their functional anatomy [19] and palaeoenvironmental settings [12,37,41,42]. Any aquatic adaptations in the group were likely secondary. Significantly, Varanops is also notable for its larger size compared with mycterosaurines. A rough survey of other large-bodied basal synapsids shows that, as in Varanops, they too sometimes preserve cancellous structure within the medulla despite discriminating as terrestrial animals (e.g. see [36, figs 6,7; 32, figs. 1,6–14]). Thus, ‘spongiosity' may also be size-related in basal synapsids, even though terrestrial mammal cursors show no such constraint by generally maintaining an open marrow cavity. Future investigation may identify a connection between bone size or robusticity and ‘spongiosity' in basal synapsids, which could shed further light on locomotor changes during the basal synapsid-to-mammal transition. This constraint was presumably lifted during synapsid evolution in larger- (heavier-) limbed animals that were more erect and experienced more habitual bending but less frequent torsional loads as inferred in some therapsids [60].
(b). Tissue texture, vascularity and evolution of growth patterns
In addition to lifestyle inferences, histological data from varanopids shed light on growth patterns in the basalmost synapsids and among early amniotes in general. Although recent years have seen a resurgence of studies on long bone growth in basal synapsids [13,29–32,36], prior sampling in varanopids was limited to two zeugopodial elements of Varanops (tentatively interpreted as a radius and fibula) [27]; an unsourced element belonging to the large varanodontine Watongia [28,39], and a handful of mycterosaurine and Varanops elements from Richards Spur which were described only briefly [31]. Our inspection reveals modest vascularization, formed mostly by simple canals and some incipient primary osteons, in an alternating PF-LAM matrix spanning multiple growth zones. Growth marks are abundant and suggest multi-year growth to moderate-to-large sizes (snout–vent length >12 cm) as in other large, predatory eupelycosaurs (e.g. Dimetrodon; [36]). Although their tissues are not as strongly lamellated or avascular as in most modern reptiles of similar size (e.g. iguanians; [62]), the samples bear striking resemblance to varanids (figure 5). Despite a range of growth strategies among varanid species [63], many larger varanids exhibit moderate cortical vascularity—mainly simple canals in longitudinal and radial orientations, with osteonal canals located in deeper regions—within an alternating PF-LAM matrix that becomes increasingly lamellar toward the periphery of the bone [54].
Figure 5.
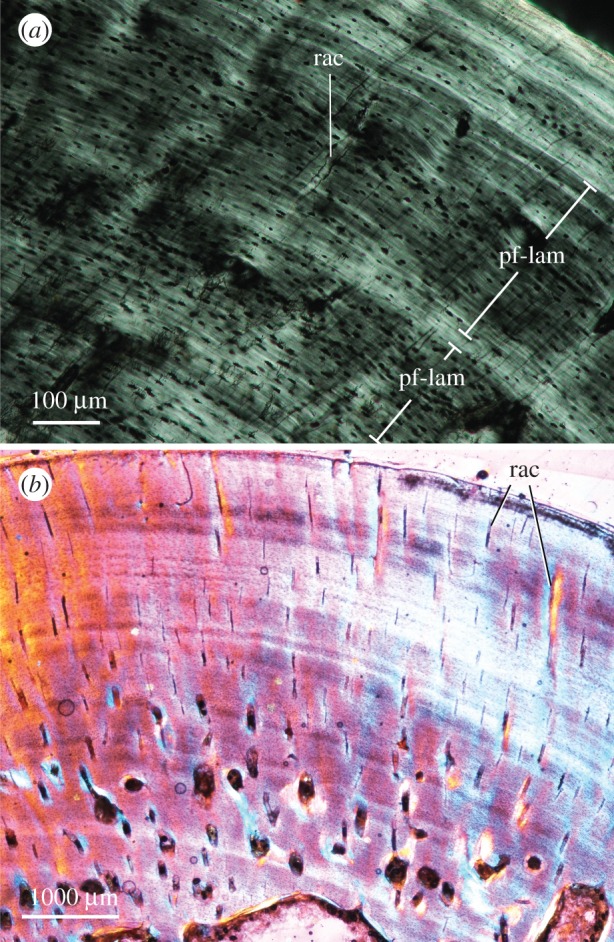
Exemplar photomicrographs of extant varanid analogues. (a), Varanus niloticus, MNHN FAOML-1996-69, femur midshaft, linear polarized light; (b), Varanus komodoensis, UWBM 8082, femur midshaft, elliptical polarized light with quarter-wave retardation plate. Abbreviations as in figure 2. (Online version in colour.)
From a palaeoecological perspective, the diverse assemblage of Richards Spur also offers a window into the growth rhythms of Permian amniotes living at approximately palaeoequatorial latitudes, and, by extension, potential environmental impacts on their life histories. Histological data have been applied widely to other palaeoecological systems, including the Permian-aged Briar Creek assemblage [64] and the Beaufort Group assemblages of the Karoo Basin [65], as well as dinosaur assemblages of Inner Mongolia [66] and the Hell Creek Formation in Montana [67], to name a few. Peabody [68] offered the first survey of cyclic growth mark formation in Richards Spur vertebrates, recognizing at least two or three zones in different skeletal elements attributed to Captorhinus. He concluded that cyclic growth mark formation likely indicated seasonal climatic variations in western Pangea, despite the palaeotropical position of the locality during Permian times. New data from the varanopids sampled here are congruent with this hypothesis. Nevertheless, noteworthy histovariation is apparent among amniotes from this assemblage, supporting that some histological features are influenced by additional historical (phylogenetic) and niche-specific factors (diet, foraging behaviour) [69]. For example, Captorhinus has been suggested to record fewer skeletal growth marks than contemporary synapsids and diadectomorphs [70], an observation that is confirmed by our sectioning of additional comparative specimens from the Richards Spur assemblage (figure 6b). Other small eureptiles (figure 6c) may show strong incremental lamellar tissues with poor to no vascularization, as in iguanians [62]. The apparent histological diversity at the base of Amniota reinforces arguments that some histological traits carry an important phylogenetic signal (e.g. [15,69]), and suggests that phylogenetic comparative approaches are most appropriate to establish ancestral life-history patterns in Amniota.
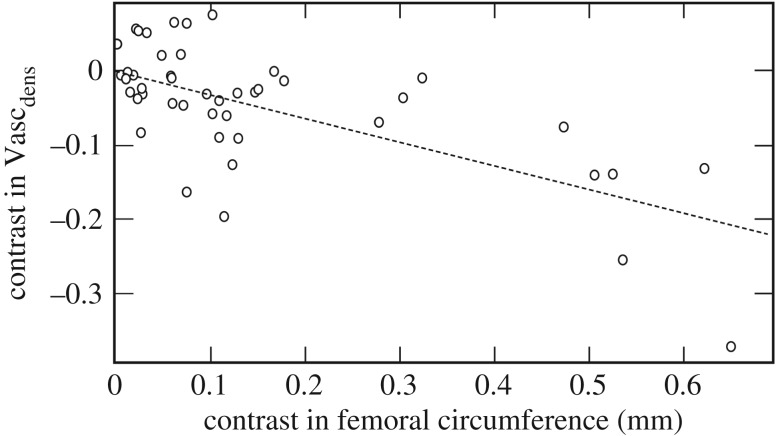
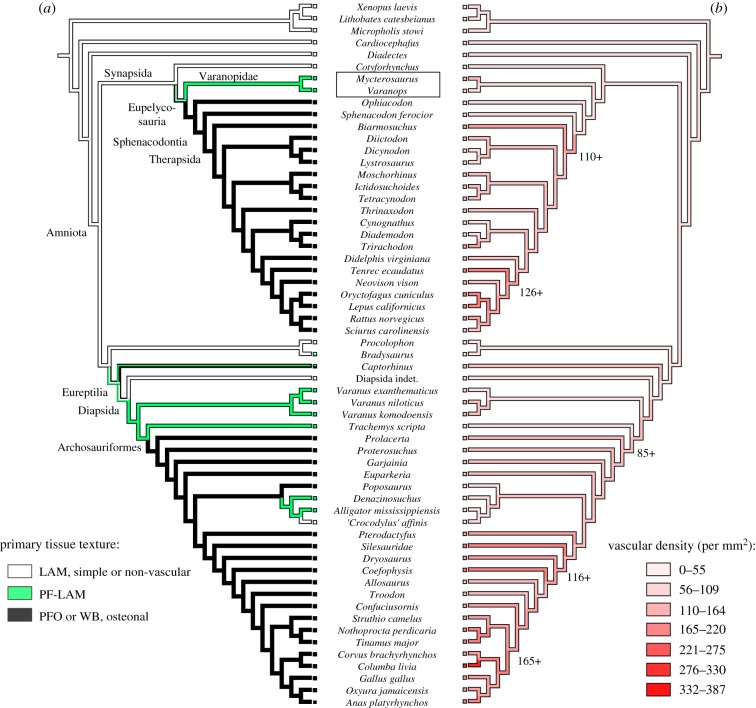
The incorporation of primary vascular canals within the mineral matrix is somewhat variable in early amniotes and stem amniotes, but most crown amniotes that have been analysed in a phylogenetic context preserve moderate vasculature that permits inference of relative bone growth rate and vascular supply [71,72] (figures 7 and 8). In general, rapidly deposited bone incorporates a rich vascular supply, which becomes progressively less vascularized as growth slows nearing maturity [50,54]. Vascular density is modest in the varanopid femora sampled here, with slightly greater vascularization in the mycterosaurines than in the larger-bodied Varanops (Mycterosaurus: Vascdens 74 canals per mm2; Varanops: Vascdens 36 canals per mm2). Notably, larger tetrapods in our phylogenetic sample typically showed less vascular density than in their small-bodied relatives (Pearson's r, −0.764; p < 0.001) (figure 7), as highlighted by the apparent disparity between mycterosaurine and Varanops femora. This might suggest that selection for larger adult body size in some basal amniotes was driven initially by a capacity for prolonged, cyclic (and not necessarily accelerated) skeletal growth. However, some eupelycosaurs like Ophiacodon may have bucked this trend by further accelerating their subadult growth rates [32]. Optimization of histological traits on a phylogeny of early tetrapods suggests that the terrestrial (or semi-terrestrial) ancestors of amniotes were small to moderate-sized animals with modest skeletal growth rates and primary cortical tissues dominated by lamellar bone. Unlike eupelycosaurs, however, this tissue-type incorporated little to no primary vasculature and was highly lamellated, as seen in microsaurs, diadectomorphs, caseids, parareptiles and early diapsids, resembling the familiar cortical bone tissues of extant small to mid-sized lizards (e.g. iguanians). Among early amniotes, non-lamellar tissues (as in woven-fibred bone or incipient fibrolamellar bone; e.g. [32]) were encountered frequently only in the carnivorous lineages leading to therapsids and archosauromorphs (figure 8a), showing associated stepwise increases in their cortical vascularization (figure 8b). Systemic non-lamellar tissues were apparently rare to absent in the herbivorous caseids and edaphosaurids. [29], although woven-fibred tissues were localized in the tubercles of Edaphosaurus [33].
Figure 7.
Standardized residual contrasts for femur circumference and vascular canal density (Vascdens) based on a phylogeny of tetrapods sampled histologically in a prior study (dataset updated from [53]; taxon sampling and tree shown in figure 8). Evolutionary increases in adult bone size were strongly correlated with fewer vascular canals per unit area (N, 45 contrasts; r, −0.764; p < 0.001) and thus a higher outer cortical bone density, despite having large canal diameters and high vascular area during the early growth phase. Data were log-transformed prior to analysis.
Figure 8.
Evolution of bone tissue texture and cortical vasculature in synapsids and other early amniotes (see table 8). (a) Phylogenetic distribution and hypothesized character changes in tissue texture based on three categorical states: lamellar primary bone, parallel-fibred to lamellar transition, and non-lamellar, osteonal primary tissues, including PFO and woven-fibred bone. (b) Ancestral state reconstructions of vascular density (Vascdens) in early amniotes. Phylogenetic character mapping was performed using the PDAP module ([57] in Mesquite 1.0 [56]). LAM, lamellar primary bone; PF-LAM, parallel-fibred to lamellar transition; PFO, parallel-fibred bone with primary osteons; WB, woven-fibred bone. (Online version in colour.)
5. Conclusion
Histological data are consistent with recently accepted notions of synapsid palaeobiology, including their ancestral life histories, habitat preferences, and locomotor behaviour. Varanopid synapsids and their close relatives were likely capable terrestrial locomotors. Their cortical bone tissues provide evidence of continuous growth and internal bone resorption with low compactness, suggesting versatile limb mobility and strength, while also demonstrating multi-year growth to moderate-to-large body sizes. Their bone physiology mimicked that of extant varanid lizards, a group that includes active foragers capable of moderately sustained exercise and high aerobic scope [53], making them an uncanny modern analogue. More generally, optimization of histological traits on our reference phylogeny indicates that other basal amniotes and stem amniotes were typified by poorly vascularized bone dominated by lamellar-zonal tissues. Notable exceptions included the basal eureptile Captorhinus, which formed more vascular, disorganized bony tissues than similar-sized sympatric amniotes, likely reflective of its more rapid growth and/or shorter longevity. Varanopids also contrast in recording a pattern of vascular PF-LAM that broadly resembles adults of other carnivorous eupelycosaurs (e.g. Ophiacodon, Sphenacodon). These intriguing outliers highlight an underappreciated histological diversity during the early radiation of Amniota, even among species from the same assemblage.
Acknowledgements
We thank W. May for donating specimens for thin-sectioning. We also thank the repositories that accepted the resulting histology collections: C. Sidor and the University of Washington Burke Museum (UWBM), Seattle; R. Cifelli and the Sam Noble Oklahoma Museum of Natural History (OMNH), Norman; and R. Irmis and C. Levitt-Bussian and the Natural History Museum of Utah (UMNH), Salt Lake City. We thank J. Gardner and E.-T. Lamm for access to additional fossil samples in the collection of Museum of the Rockies (MOR), Bozeman, and S. Echols, DVM, DABVP for providing bird tissue samples analysed for figure 8. For their support and insightful discussions, we also thank our collaborators J. Botha, C. Farmer, M. Sander and K. Stein. V. de Buffrénil is thanked for access to comparative material, including the extant varanid analogue V. niloticus shown in figure 5 (FAOML, UN Food and Agriculture Organization).
Appendix A
Table 2.
List of taxa and specimens analysed for phylogenetic comparative analysis in figure 8. This sample is expanded from Huttenlocker & Farmer [53].
| taxon | specimen/voucher no. | element | ref. |
|---|---|---|---|
| Temnospondyli | |||
| Micropholis stowi | NMQR 3621; UCMP 78395b | humerus | [53] |
| Stem amniotes | |||
| Cardiocephalus | OMNH 56886 | femur | [73,74] |
| Diadectes | FMNH UR2514 | femur | new |
| Reptilia (Sauropsida) | |||
| Allosaurus | UUVP 2656; UUVP 3694 | femur | [75] |
| Bradysaurus | SAM-PK-9137, 9165, 5127, 12057 | femur | [76] |
| 12129, 9355 | |||
| Captorhinus sp. | UMNH VP 25964; UMNH VP 25966; | femur | [53], new |
| UMNH VP 259 67; UMNH VP 25968 | |||
| Coelophysis | UCMP 129618 | femur | [77] |
| Confuciusornis | MOR 1063 | femur | [77] |
| ‘Crocodylus' affinis | UWBM 89252; UWBM 88113 | femur | [53] |
| Denazinosuchus | UMNH VP 16733 | femur | new |
| Diapsida indet. | OMNH 52543 | humerus | new |
| Dryosaurus | CM 1949 | femur | [78] |
| Euparkeria capensis | SAM-PK-K10548; SAM-PK-K10010 | femur | [79] |
| Garjainia madiba | BP/1/6232; BP/1/7217; BP/1/7219 | femur | [80] |
| Poposaurus gracilis | YPM 57100 | femur | [53] |
| Procolophon | NMQR 3944b; 3555b | femur | [81] |
| Prolacerta broomi | NMQR 3763 | humerus | [53,79] |
| Proterosuchus fergusi | SAM-PK-K11208; SAM-PK-K140 | femur | [79] |
| Pterodactylus | CM 11430 | femur | [77] |
| Silesauridae | NHCC LB54; NHCC LB79 | femur | [5,82] |
| Troodon | MOR 748 | femur | [77] |
| Synapsida | |||
| Biarmosuchus tener | PIN/401.1.1.T | humerus | [27] |
| Bienotherium yunnanense | YPM 13-I | femur | [24] |
| Cynognathus crateronotus | NMQR 3019; SAM-PK-6235 | femur | [83] |
| Diademodon tetragonus | UCMZ T493; UCMZ T495; UCMZ T503 | femur | [83] |
| Dicynodon | SAM-PK-5576 | femur | [84] |
| Diictodon feliceps | SAM-PK-K5035; SAM-PK-K5282; | femur | [84] |
| SAM-PK-10174; SAM-PK-11099 | |||
| Ictidosuchoides longiceps | SAM-PK-K8659; SAM-PK-K10423 | femur | [85] |
| Lystrosaurus | NMQR 835a | femur | [84] |
| Moschorhinus kitchingi | NMQR 3939; NMQR 3351; NMQR 3684 | femur | [61,85] |
| Mycterosaurus | UWBM 89464; UWBM 89466; | femur | [53] |
| UWBM 98580 | |||
| Sphenacodon ferocior | UCMP 34209; UCMP 83628 | femur | [31] |
| Tetracynodon darti | UCMP 78395; UCMP 78396 | femur | [85] |
| Thrinaxodon liorhinus | SAM-PK-1395; SAM-PK-K8004 | femur | [86] |
| Trirachodon berryi | SAM-PK-5881 | femur | [87] |
| Varanops brevirostris | OMNH 73758; UWBM 89463 | femur | new |
Institutional abbreviations: BP, Evolutionary Studies Institute (former Bernard Price Institute collection), Johannesburg; CM, Carnegie Museum of Natural History, Pittsburgh; MOR, Museum of the Rockies, Bozeman; NHCC, National Heritage Conservation Commission, Lusaka; NMQR, National Museum, Bloemfontein; OMNH, Sam Noble Oklahoma Museum of Natural History, Norman; PIN, Paleontological Institute, Moscow; SAM, Iziko South African Museum, Cape Town; UCMP, University of California Museum of Paleontology, Berkeley; UCMZ, University Museum of Zoology, Cambridge, UK; UMNH/UUVP, Natural History Museum of Utah, Salt Lake City; UWBM, University of Washington Burke Museum of Natural History and Culture, Seattle; YPM, Yale Peabody Museum, New Haven.
Data accessibility
Data are available from the corresponding author (A.K.H.) by request.
Authors' contributions
A.K.H. and C.D.S. analysed the data, prepared figures and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
A.K.H. was supported by the National Science Foundation DEB-1209018 and DBI-1309040; C.D.S. was supported by DFG grant no. SA 469/34-1 and the University of Bonn.
References
- 1.Gauthier J, Kluge AG, Rowe T. 1988. Amniote phylogeny and the importance of fossils. Cladistics 4, 105–209. ( 10.1111/j.1096-0031.1988.tb00514.x) [DOI] [PubMed] [Google Scholar]
- 2.Gauthier JA. 1994. The diversification of the amniotes. Short Courses Paleontol. 7, 129–159. ( 10.1017/S247526300000129X) [DOI] [Google Scholar]
- 3.Angielczyk KD. 2009. Dimetrodon is not a dinosaur: using tree thinking to understand the ancient relatives of mammals and their evolution. Evol. Educ. Outreach 2, 257 ( 10.1007/s12052-009-0117-4) [DOI] [Google Scholar]
- 4.Romer AS. 1957. Origin of the amniote egg. Scient. Monthly 85, 57–63. [Google Scholar]
- 5.Carroll RL. 1970. Quantitative aspects of the amphibian-reptilian transition. Forma Functio 3, 165–178. [Google Scholar]
- 6.Carroll RL. 1991. The origin of reptiles. In Origins of the higher groups of tetrapods: controversy and consensus (eds H-P Schultze, L Trueb), pp. 331–353. Ithaca, NY: Comstock Publishing Associates. [Google Scholar]
- 7.Laurin M. 2004. The evolution of body size, Cope's rule and the origin of amniotes. Syst. Biol. 53, 594–622. ( 10.1080/10635150490445706) [DOI] [PubMed] [Google Scholar]
- 8.Reisz RR, Fröbisch J. 2014. The oldest caseid synapsid from the Late Pennsylvanian of Kansas, and the evolution of herbivory in terrestrial vertebrates. PLoS ONE 9, e94518 ( 10.1371/journal.pone.0094518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley SM. 1973. An explanation for Cope's rule. Evolution 27, 1–26. ( 10.1111/j.1558-5646.1973.tb05912.x) [DOI] [PubMed] [Google Scholar]
- 10.McShea DW. 1994. Mechanisms of large-scale evolutionary trends. Evolution 48, 1747–1763. ( 10.1111/j.1558-5646.1994.tb02211.x) [DOI] [PubMed] [Google Scholar]
- 11.Turner JS, Tracy CR. 1986. Body size, homeothermy and the control of heat exchange in mammal-like reptiles. In The ecology and biology of mammal-like reptiles (eds N Hotton, PD MacLean, J Roth, EC Roth), pp. 185–194. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 12.Pardo JD, Small BJ, Milner AR, Huttenlocker AK. 2019. Carboniferous–Permian climate change constrained early land vertebrate radiations. Nat. Ecol. Evol. 3, 200 ( 10.1038/s41559-018-0776-z) [DOI] [PubMed] [Google Scholar]
- 13.Laurin M, de Buffrénil V. 2016. Microstructural features of the femur in early ophiacodontids: a reappraisal of ancestral habitat use and lifestyle of amniotes. C. R. Palevol 15, 115–127. ( 10.1016/j.crpv.2015.01.001) [DOI] [Google Scholar]
- 14.Kriloff A, Germain D, Canoville A, Vincent P, Sache M, Laurin M. 2008. Evolution of bone microanatomy of the tetrapod tibia and its use in palaeobiological inference. J. Evol. Biol. 21, 807–826. ( 10.1111/j.1420-9101.2008.01512.x) [DOI] [PubMed] [Google Scholar]
- 15.Canoville A, Laurin M. 2010. Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on palaeobiological inferences. Biol. J. Linn. Soc. 100, 384–406. ( 10.1111/j.1095-8312.2010.01431.x) [DOI] [Google Scholar]
- 16.Quemeneur S, De Buffrenil V, Laurin M. 2013. Microanatomy of the amniote femur and inference of lifestyle in limbed vertebrates. Biol. J. Linn. Soc. 109, 644–655. ( 10.1111/bij.12066) [DOI] [Google Scholar]
- 17.Romer AS. 1961. A large ophiacodont pelycosaur from the Pennsylvanian of the Pittsburgh region. Bull. Mus. Comp. Zool. 144, 1–7. [Google Scholar]
- 18.Germain D, Laurin M. 2005. Microanatomy of the radius and lifestyle in amniotes (Vertebrata, Tetrapoda) . Zool. Scr. 34, 335–350. ( 10.1111/j.1463-6409.2005.00198.x) [DOI] [Google Scholar]
- 19.Felice RN, Angielczyk KD. 2014. Was Ophiacodon (Synapsida, Eupelycosauria) a swimmer? A test using vertebral dimensions. In Early evolutionary history of the Synapsida (eds C Kammerer, K Angielczyk, J Fröbisch), pp. 25–51. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 20.Moodie RL. 1923. Paleopathology. An introduction to the study of ancient evidences of disease. Urbana, IL: University of Illinois Press.
- 21.Gross W. 1934. [The types of bone microstructure in the fossil stegocephalians and reptiles]. Z. Anat. Entwickl. 103, 731–764. [In German.] ( 10.1007/BF02118752) [DOI] [Google Scholar]
- 22.Enlow DH, Brown SO. 1957. A comparative histological study of fossil and recent bone tissues. Part II. Texas J. Sci. 9, 136–214. [Google Scholar]
- 23.Enlow DH. 1969. The bone of reptiles. In Biology of the Reptilia volume 1: morphology A (eds Gans C, Bellairs A, Parsons T), pp. 45–80. London, UK: Academic Press. [Google Scholar]
- 24.de Ricqlès A. 1969. [Palaeohistological study of the long bones of some tetrapods, II: some observations on the long bone structure of some theriodonts]. Ann. Paléontol. Vertébrés 55, 1–52. [In French.] [Google Scholar]
- 25.de Ricqlès A. 1972. [Palaeohistological study of the long bones of some tetrapods, III: titanosuchians, dinocephalians and dicynodonts]. Ann. Paléontol. Vertébrés 58, 17–60. [In French.] [Google Scholar]
- 26.de Ricqlès A. 1974. Evolution of endothermy: histological evidence. Evol. Theory 1, 51–80. [Google Scholar]
- 27.de Ricqlès A. 1974. Paleohistological research on the long bones of tetrapods IV: eotheriodonts and pelycosaurs. In Ann. Paléontol. 60, 3–39. [Google Scholar]
- 28.de Ricqlès A. 1976. On bone histology of fossil and living reptiles, with comments on its functional and evolutionary significance. Morphol. Biol. Reptiles 3, 123–149. [Google Scholar]
- 29.Shelton CD. 2015. Origins of Endothermy in the mammalian lineage. PhD dissertation, Universitäts und Landesbibliothek Bonn. [Google Scholar]
- 30.Lambertz M, Shelton CD, Spindler F, Perry SF. 2016. A caseian point for the evolution of a diaphragm homologue among the earliest synapsids. Ann. N. Y. Acad. Sci. 1385, 3–20. ( 10.1111/nyas.13264) [DOI] [PubMed] [Google Scholar]
- 31.Huttenlocker AK, Rega E. 2012. The paleobiology and bone microstructure of pelycosaurian-grade synapsids. In The forerunners of mammals: radiation, histology and biology (ed. Chinsamy-Turan A.), pp. 90–119. Bloomington, IN: Indiana University Press. [Google Scholar]
- 32.Shelton CD, Sander PM. 2017. Long bone histology of Ophiacodon reveals the geologically earliest occurrence of fibrolamellar bone in the mammalian stem lineage. C. R. Palevol. 16, 397–424. ( 10.1016/j.crpv.2017.02.002) [DOI] [Google Scholar]
- 33.Huttenlocker AK, Mazierski D, Reisz R. 2011. Comparative osteohistology of hyperelongate neural spines in the Edaphosauridae (Amniota: Synapsida). Palaeontology 54, 573–590. ( 10.1111/j.1475-4983.2011.01047.x) [DOI] [Google Scholar]
- 34.Huttenlocker AK, Rega E, Sumida S. 2010. Comparative anatomy and osteohistology of hyperelongate neural spines in the sphenacodontids Sphenacodon and Dimetrodon (Amniota: Synapsida). J. Morphol. 271, 1407–1421. ( 10.1002/jmor.10876) [DOI] [PubMed] [Google Scholar]
- 35.Rega EA, Noriega K, Sumida SS, Huttenlocker A, Lee A, Kennedy B. 2012. Healed fractures in the neural spines of an associated skeleton of Dimetrodon: implications for dorsal sail morphology and function. Fieldiana Life Earth Sci. 2012, 104–111. ( 10.3158/2158-5520-5.1.104) [DOI] [Google Scholar]
- 36.Shelton CD, Sander PM, Stein K, Winkelhorst H. 2013. Long bone histology indicates sympatric species of Dimetrodon (Lower Permian, Sphenacodontidae). Earth Environ. Sci. Trans. R. Soc. Edinb. 103, 217–236. ( 10.1017/S175569101300025X) [DOI] [Google Scholar]
- 37.Evans DC, Maddin HC, Reisz RR. 2009. A re-evaluation of sphenacodontid synapsid material from the Lower Permian fissure fills near Richards Spur, Oklahoma. Palaeontology 52, 219–227. ( 10.1111/j.1475-4983.2008.00837.x) [DOI] [Google Scholar]
- 38.Modesto SP, Smith RM, Campione NE, Reisz RR. 2011. The last “pelycosaur”: a varanopid synapsid from the Pristerognathus Assemblage Zone, Middle Permian of South Africa. Naturwissenschaften 98, 1027–1034. ( 10.1007/s00114-011-0856-2) [DOI] [PubMed] [Google Scholar]
- 39.Bennett AA, Ruben JA. 1986. The metabolic and thermoregulatory status of therapsids. In The ecology and biology of mammal-like reptiles (eds Hotton N, MacLean PD, Rothand JJ, Roth EC), pp. 207–218. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 40.Reisz RR, Wilson H, Scott D. 1997. Varanopseid synapsid skeletal elements from Richards Spur, a Lower Permian fissure fill near Fort Sill, Oklahoma. Oklahoma Geol. Notes 57, 160–170. ( 10.1111/j.1475-4983.2008.00837.x) [DOI] [Google Scholar]
- 41.Olson EC. 1991. An eryopid (Amphibia: Labyrinthodontia) from the Fort Sill fissures, Lower Permian, Oklahoma. J. Vertebr. Paleontol. 11, 130–132. ( 10.1080/02724634.1991.10011379) [DOI] [Google Scholar]
- 42.MacDougall MJ, Tabor NJ, Woodhead J, Daoust AR, Reisz RR. 2017. The unique preservational environment of the Early Permian (Cisuralian) fossiliferous cave deposits of the Richards Spur locality, Oklahoma. Palaeogeogr. Palaeoclimatol. Palaeoecol. 475, 1–11. ( 10.1016/j.palaeo.2017.02.019) [DOI] [Google Scholar]
- 43.Woodhead J, Reisz R, Fox D, Drysdale R, Hellstrom J, Maas R, Cheng H, Edwards RL. 2010. Speleothem climate records from deep time? Exploring the potential with an example from the Permian. Geology 38, 455–458. ( 10.1130/G30354.1) [DOI] [Google Scholar]
- 44.Maddin HC, Evans DC, Reisz RR. 2006. An Early Permian varanodontine varanopid (Synapsida: Eupelycosauria) from the Richards Spur locality, Oklahoma. J. Vertebr. Paleontol. 26, 957–966. ( 10.1671/0272-4634(2006)26[957:aepvvs]2.0.co;2) [DOI] [Google Scholar]
- 45.Wilson JW, Leiggi P, May P. 1994. Histological techniques. Vertebr. Paleontol. Tech. 1, 205–234. [Google Scholar]
- 46.Lamm E-T. 2013. Preparation and sectioning of specimens. In Bone histology of fossil tetrapods: advancing methods, analysis, and interpretation (eds K Padian and E-T Lamm), pp. 55–160. Berkeley, CA: University of California Press. [Google Scholar]
- 47.Girondot M, Laurin M. 2003. Bone profiler: a tool to quantify, model, and statistically compare bone-section compactness profiles. J. Vertebr. Paleontol. 23, 458–461. ( 10.1671/0272-4634(2003)023[0458:BPATTQ]2.0.CO;2) [DOI] [Google Scholar]
- 48.Currey JD, Alexander RM. 1985. The thickness of the walls of tubular bones. J. Zool. 206, 453–468. ( 10.1111/j.1469-7998.1985.tb03551.x) [DOI] [Google Scholar]
- 49.McFarlin SC. 2006. Ontogenetic variation in long bone microstructure in catarrhines and its significance for life history research. PhD dissertation, City University of New York. [Google Scholar]
- 50.de Margerie E, Cubo J, Castanet J. 2002. Bone typology and growth rate: testing and quantifying ‘Amprino's rule'in the mallard (Anas platyrhynchos). C. R. Biol. 325, 221–230. ( 10.1016/S1631-0691(02)01429-4) [DOI] [PubMed] [Google Scholar]
- 51.McFarlin SC, Terranova CJ, Zihlman AL, Bromage TG. 2016. Primary bone microanatomy records developmental aspects of life history in catarrhine primates. J. Hum. Evol. 92, 60–79. ( 10.1016/j.jhevol.2015.12.004) [DOI] [PubMed] [Google Scholar]
- 52.Rasband WS. 1997–2015 ImageJ. Bethesda, MD: U.S. National Institutes of Health; See http://imagej.nih.gov/ij/. [Google Scholar]
- 53.Huttenlocker AK, Farmer CG. 2017. Bone microvasculature tracks red blood cell size diminution in Triassic mammal and dinosaur forerunners. Curr. Biol. 27, 48–54. ( 10.1016/j.cub.2016.10.012) [DOI] [PubMed] [Google Scholar]
- 54.de Buffrénil V, Houssaye A, Böhme W. 2008. Bone vascular supply in monitor lizards (Squamata: Varanidae): influence of size, growth, and phylogeny. J. Morphol. 269, 533–543. ( 10.1002/jmor.10604) [DOI] [PubMed] [Google Scholar]
- 55.Gradstein FM, Ogg JG, Schmitz M, Ogg G. 2012. The geologic time scale 2012. Oxford, UK: Elsevier. [Google Scholar]
- 56.Maddison WP, Maddison DR.. 2014. Mesquite: a modular system for evolutionary analysis. V. 1.0. See http://www.mesquiteproject.org.
- 57.Midford PE, Garland T, Maddison WP.2011. PDAP: PDTREE module for Mesquite (version 1.16). See http://mesquiteproject.org/pdap_mesquite/index.html .
- 58.Cormack DH. 1987. Ham's histology, 9th edn, p. 732 New York, NY: Lippincott. [Google Scholar]
- 59.Blob RW. 2000. Interspecific scaling of the hindlimb skeleton in lizards, crocodilians, felids and canids: does limb bone shape correlate with limb posture? J. Zool. 250, 507–531. ( 10.1111/j.1469-7998.2000.tb00793.x) [DOI] [Google Scholar]
- 60.Blob RW. 2001. Evolution of hindlimb posture in nonmammalian therapsids: biomechanical tests of paleontological hypotheses. Paleobiology 27, 14–38. () [DOI] [Google Scholar]
- 61.Huttenlocker AK, Botha-Brink J. 2013. Body size and growth patterns in the therocephalian Moschorhinus kitchingi (Therapsida: Eutheriodontia) before and after the end-Permian extinction in South Africa. Paleobiology 39, 253–277. ( 10.1666/12020) [DOI] [Google Scholar]
- 62.Hugi J, Sánchez-Villagra MR. 2012. Life history and skeletal adaptations in the Galapagos marine iguana (Amblyrhynchus cristatus) as reconstructed with bone histological data—a comparative study of iguanines. J. Herpetol. 46, 312–324. ( 10.1670/11-071) [DOI] [Google Scholar]
- 63.Buffrénil V, Ineich I, Böhme W. 2005. Comparative data on epiphyseal development in the family Varanidae. J. Herpetol. 39, 328–336. ( 10.1670/0022-1511(2005)039[0328:CDOEDI]2.0.CO;2) [DOI] [Google Scholar]
- 64.Konietzko-Meier D, Shelton CD, Sander PM. 2016. The discrepancy between morphological and microanatomical patterns of anamniotic stegocephalian postcrania from the Early Permian Briar Creek Bonebed (Texas). C. R. Palevol 15, 103–114. ( 10.1016/j.crpv.2015.06.005) [DOI] [Google Scholar]
- 65.Botha-Brink J, Codron D, Huttenlocker AK, Angielczyk KD, Ruta M. 2016. Breeding young as a survival strategy during Earth's greatest mass extinction. Scient. Rep. 6, 24053 ( 10.1038/srep24053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varricchio DJ, Sereno PC, Xijin Z, Lin T, Wilson JA, Lyon GH. 2008. Mud-trapped herd captures evidence of distinctive dinosaur sociality. Acta Palaeontol. Pol. 53, 567–579. ( 10.4202/app.2008.0402) [DOI] [Google Scholar]
- 67.Horner JR, Goodwin MB, Myhrvold N. 2011. Dinosaur census reveals abundant Tyrannosaurus and rare ontogenetic stages in the Upper Cretaceous Hell Creek Formation (Maastrichtian), Montana, USA. PLoS ONE 6, e16574 ( 10.1371/journal.pone.0016574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peabody FE. 1961. Annual growth zones in living and fossil vertebrates. J. Morphol. 108, 11–62. ( 10.1002/jmor.1051080103) [DOI] [Google Scholar]
- 69.Cubo J, Ponton F, Laurin M, De Margerie ED, Castanet J. 2005. Phylogenetic signal in bone microstructure of sauropsids. Syst. Biol. 54, 562–574. ( 10.1080/10635150591003461) [DOI] [PubMed] [Google Scholar]
- 70.Warren JW. 1963. Growth zones in the skeleton of recent and fossil vertebrates. PhD dissertation, University of California Los Angeles. [Google Scholar]
- 71.Cubo J, Le Roy N, Martinez-Maza C, Montes L. 2012. Paleohistological estimation of bone growth rate in extinct archosaurs. Paleobiology 38, 335–349. ( 10.1666/08093.1) [DOI] [Google Scholar]
- 72.Legendre LJ, Segalen L, Cubo J. 2013. Evidence for high bone growth rate in Euparkeria obtained using a new paleohistological inference model for the humerus. J. Vertebr. Paleontol. 33, 1343–1350. ( 10.1080/02724634.2013.780060) [DOI] [Google Scholar]
- 73.Ricqlès A. 1981. [Palaeohistological study of the long bones of some tetrapods, VI: stegocephalians]. Ann. Paléontol. 67, 141–160. [In French.] [Google Scholar]
- 74.Organ CL, Canoville A, Reisz RR, Laurin M. 2011. Paleogenomic data suggest mammal-like genome size in the ancestral amniote and derived large genome size in amphibians. J. Evol. Biol. 24, 372–380. ( 10.1111/j.1420-9101.2010.02176.x) [DOI] [PubMed] [Google Scholar]
- 75.Bybee PJ, Lee AH, Lamm ET. 2006. Sizing the Jurassic theropod dinosaur Allosaurus: assessing growth strategy and evolution of ontogenetic scaling of limbs. J. Morphol. 267, 347–359. ( 10.1002/jmor.10406) [DOI] [PubMed] [Google Scholar]
- 76.Canoville A, Chinsamy A. 2017. Bone microstructure of pareiasaurs (Parareptilia) from the Karoo Basin, South Africa: implications for growth strategies and lifestyle habits. Anat. Rec. 300, 1039–1066. ( 10.1002/ar.23534) [DOI] [PubMed] [Google Scholar]
- 77.Padian K, Horner JR, De Ricqlès A. 2004. Growth in small dinosaurs and pterosaurs: the evolution of archosaurian growth strategies. J. Vertebr. Paleontol. 24, 555–571. ( 10.1671/0272-4634(2004)024[0555:GISDAP]2.0.CO;2) [DOI] [Google Scholar]
- 78.Horner JR, de Ricqlès A, Padian K, Scheetz RD. 2009. Comparative long bone histology and growth of the ‘hypsilophodontid’ dinosaurs Orodromeus makelai, Dryosaurus altus, and Tenontosaurus tillettii (Ornithischia: Euornithopoda). J. Vertebr. Paleontol. 29, 734–747. ( 10.1671/039.029.0312) [DOI] [Google Scholar]
- 79.Botha-Brink J, Smith RMH. 2011. Osteohistology of the Triassic archosauromorphs Prolacerta, Proterosuchus, Euparkeria, and Erythrosuchus from the Karoo Basin of South Africa. J. Vertebr. Paleontol. 31, 1238–1254. ( 10.1080/02724634.2011.621797) [DOI] [Google Scholar]
- 80.Gower DJ, Hancox PJ, Botha-Brink J, Sennikov AG, Butler RJ. 2014. A new species of Garjainia Ochev, 1958 (Diapsida: Archosauriformes: Erythrosuchidae) from the Early Triassic of South Africa. PLoS ONE 9, e111154 ( 10.1371/journal.pone.0111154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Botha-Brink J, Smith RMH. 2012. Palaeobiology of Triassic procolophonids, inferred from bone microstructure. C. R. Palevol. 11, 419–433. ( 10.1016/j.crpv.2012.03.002) [DOI] [Google Scholar]
- 82.Peecook BR, Steyer JS, Tabor NJ, Smith RM. 2017. Updated geology and vertebrate paleontology of the Triassic Ntawere Formation of northeastern Zambia, with special emphasis on the archosauromorphs. J. Vertebr. Paleontol. 37(Suppl. 1), 8–38. ( 10.1080/02724634.2017.1410484) [DOI] [Google Scholar]
- 83.Botha J, Chinsamy A. 2001. Growth patterns deduced from the bone histology of the cynodonts Diademodon and Cynognathus. J. Vertebr. Paleontol. 20, 705–711. ( 10.1671/0272-4634(2000)020[0705:GPDFTB]2.0.CO;2) [DOI] [Google Scholar]
- 84.Botha-Brink J, Angielczyk KD. 2010. Do extraordinarily high growth rates in Permo-Triassic dicynodonts (Therapsida, Anomodontia) explain their success before and after the end-Permian extinction? Zool. J. Linn. Soc. 160, 341–365. ( 10.1111/j.1096-3642.2009.00601.x) [DOI] [Google Scholar]
- 85.Huttenlocker AK, Botha-Brink J. 2014. Bone microstructure and the evolution of growth patterns in Permo-Triassic therocephalians (Amniota, Therapsida) of South Africa. PeerJ 2, e325 ( 10.7717/peerj.325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Botha J, Chinsamy A. 2005. Growth patterns of Thrinaxodon liorhinus, a non-mammalian cynodont from the Lower Triassic of South Africa. Palaeontology 48, 385–394. ( 10.1111/j.1475-4983.2005.00447.x) [DOI] [Google Scholar]
- 87.Botha J, Chinsamy A. 2004. Growth and life habits of the Triassic cynodont Trirachodon, inferred from bone histology. Acta Palaeontol. Pol. 49, 619–627. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author (A.K.H.) by request.