Abstract
Medullary bone (MB) is a sex-specific tissue produced by female birds during the laying cycle, and it is hypothesized to have arisen within Avemetatarsalia, possibly outside Avialae. Over the years, researchers have attempted to define a set of criteria from which to evaluate the nature of purported MB-like tissues recovered from fossil specimens. However, we argue that the prevalence, microstructural and chemical variability of MB in Neornithes is, as of yet, incompletely known and thus current diagnoses of MB do not capture the extent of variability that exists in modern birds. Based on recently published data and our own observations of MB distribution and structure using computed tomography and histochemistry, we attempt to advance the discourse on identifying MB in fossil specimens. We propose: (i) new insights into the phylogenetic breadth and structural diversity of MB within extant birds; (ii) a reevaluation and refinement of the most recently published list of criteria suggested for confidently identifying MB in the fossil record; (iii) reconsideration of some prior identifications of MB-like tissues in fossil specimens by taking into account the newly acquired data; and (iv) discussions on the challenges of characterizing MB in Neornithes with the goal of improving its diagnosis in extinct avemetatarsalians.
This article is part of the theme issue ‘Vertebrate palaeophysiology’.
Keywords: medullary bone characterization, computed tomography, bone pathology, endosteal tissue
1. Background
Medullary bone (MB) is a specialized, sex-specific tissue [1,2], unique to female birds (Neornithes) among extant organisms. MB constitutes an ephemeral tissue whose formation and subsequent resorption in the cavities of skeletal elements is induced by fluctuations in steroid hormone levels (i.e. estrogen and androgen) during the egg-laying cycle. These hormones alternatively activate or inhibit the activity of endosteal osteoblasts and osteoclasts [3–8]. This tissue is thus only formed in reproductively mature females shortly before the onset of the laying cycle, during maturation of the ovarian follicles. The tissue is then partly or completely catabolized towards the end of the cycle and the calcium is mobilized for eggshell formation by the shell gland. MB does not contribute directly to the structural stability of bone tissue (unlike the surrounding cortical and trabecular bone; [9]) but is a major component of the calcium metabolism of birds [1,10].
Based almost exclusively on data gathered from poultry research, MB has been characterized by a unique set of microstructural, developmental and molecular criteria. This secondary bone tissue is commonly described as highly vascularized, woven (supporting rapid deposition), oestrogen-dependent and endosteally derived. It is deposited as a trabecular meshwork (MB trabeculae are also called ‘spicules’) within the cavities of skeletal elements [1,2,10]. MB has also been defined by a unique molecular signature and mineral to collagen ratio when compared to surrounding cortical and trabecular bone [9]. Some of its hypothesized molecular markers, such as the sulfated glycosaminoglycan keratan sulfate (KS) and the enzyme tartrate-resistant acid phosphatase (TRAP) have not yet been reported in the calcified matrix of other bone tissue types (although KS is known to be present in other tissues, including some types of cartilage [11–19]).
Several studies have hypothesized that MB originated as an adaptation to offset the demand for reduction of skeletal mass in the lineage leading to birds ([20–22]; see also [23] for discussion on this subject). Indeed, most extant volant birds exhibit overall thinner compact cortices than similarly sized terrestrial and aquatic relatives [24–26]. However, this hypothesis rests on the as yet untested assumption that resorting primarily to the calcium stored in structural bone during the egg-laying cycle would greatly affect the integrity and overall biomechanical properties (for support and locomotion) of the avian skeleton. Another, non-exclusive, hypothesis is that MB, which is only deposited during a limited time period corresponding to the laying cycle, would be a crucial energy saving alternative to storing large (and thus heavy) amounts of calcium in the structural skeleton of flying animals.
Crocodilians, which are the closest relatives to birds among extant amniotes, rely on a different source of calcium for the production of their calcified eggshell. Studies have shown that female alligators do not form MB [21], but rather use the mineral stored in their osteoderms (which are hypothesized to have a physiological, rather than a structural function in crocodilians; see [27–31]) to form the calcified eggshell [32].
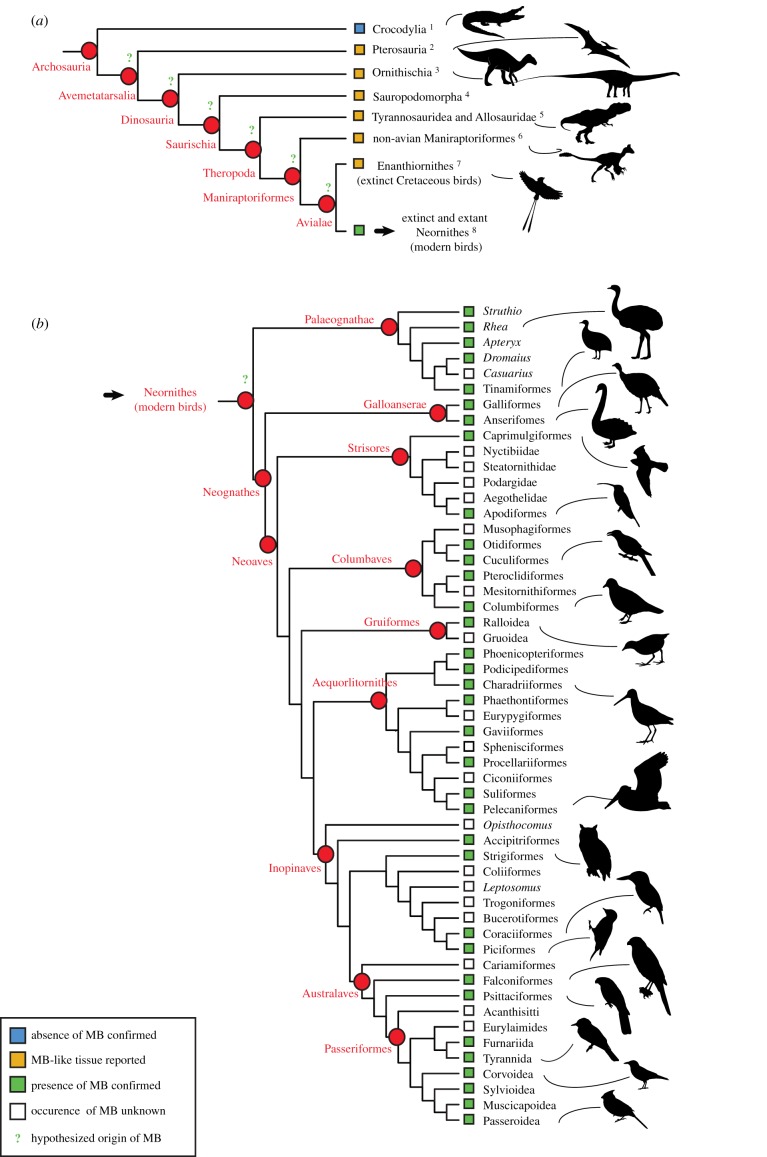
Although the phylogenetic distribution of MB within Neornithes is still incompletely understood, recent studies (e.g. [33,34]) revealed that this tissue is widespread among modern birds and present in the earliest diverging, extant palaeognath clades (figure 1b). If MB evolved after the divergence of crocodilians and birds, it is hypothesized to have arisen somewhere within Avemetatarsalia (bird-line archosaurs, figure 1a), and possibly outside of Avialae (e.g. [41,42]). Indeed, birds and non-avian dinosaurs share a common ancestor (e.g. [51]), and an ever-increasing body of evidence reveals that numerous morphological and physiological adaptations seen exclusively in Neornithes among extant archosaurs (e.g. feathers, powered flight, relatively high growth rates, advanced parental care, monochronic ovulation, relatively thin long bone cortices, pneumatized postcranial skeleton, etc.) were, in fact, progressively acquired from their dinosaurian predecessors (e.g. [51–54]). However, the evolutionary timing of acquisition of MB remains unknown, and the step-wise acquisition of the unique avian reproductive biology, including calcium metabolism, is incompletely understood.
Figure 1.
Phylogenetic distribution of reported MB-like tissues in avemetatarsalians (a); and confirmed occurrences of MB in Neornithes (b; modified from Canoville et al. [34]). These data are based on the following references: 1. Schweitzer et al. [21]; 2. Chinsamy et al. [35]; Prondvai & Stein [36]; 3. Lee & Werning [37,38]; 4. Cerda & Pol [39], Chinsamy et al. [40]; 5. Lee and Werning [37], Schweitzer et al. [41,42]; 6. Skutschas et al. [43]; 7. Chinsamy et al. [44] O'Connor [45], O'Connor et al. [46], Bailleul et al. [47]; 8. Smith & Clarke [48], Angst et al. [49]; Werning [33]; Canoville et al. [34]. The phylogenetic relationships of extant bird groups follow Prum et al. [50]. (Online version in colour.)
Using the microstructural and developmental criteria of MB listed above, Schweitzer et al. [41] were the first to identify MB-like tissues in an extinct taxon; in this case, the hindlimbs of a Tyrannosaurus rex (specimen MOR 1125). This study triggered great interest within the paleontological community, because the unequivocal identification of tissues homologous to avian MB would provide an objective means to recognize sexually mature and gravid females in extinct avemetatarsalians. Furthermore, characterizing these tissues would give access to a wealth of information on different aspects of the reproductive biology (e.g. sexual dimorphism, age at sexual maturity) of these long-extinct animals. Since Schweitzer et al.'s [41] discovery, MB-like tissues have been reported in specimens pertaining to most major groups of Avemetatarsalia, i.e. pterosaurs [35,36], different clades of non-avian dinosaurs [37–40,43], extinct Mesozoic birds outside Neornithes [44–47] and extinct Neornithes [48,49].
However, some of the identifications of MB-like tissues in the fossil record were later challenged, in part because it was noted that these tissues shared many features with avian pathological bone. Indeed, some (avian) bone pathologies meet the set of microstructural (woven, highly vascularized) and developmental criteria (secondary endosteal bone) originally outlined as unique to MB (e.g. [23,40,55]; see also [56]). Furthermore, purported MB tissue was found in reproductively immature individuals of animals forming soft eggshells, such as pterosaurs (e.g. [36]), when MB in living birds is constrained to reproductively mature females. Finally, the anatomical location of some of these proposed MB tissues was seemingly different than any seen for MB in extant birds (e.g. see discussions in [36,40,46]).
The need for a set of independent criteria by which to evaluate the hypothesis of MB in extinct avemetatarsalians is apparent, and increasingly important, given the implications of this tissue for understanding the acquisition of certain physiological and reproductive traits in the lineage leading to birds. Over the years, researchers have responded by independently proposing and refining a set of criteria from which to evaluate the nature of purported MB-like tissues (e.g. [40,46,57]), working by necessity from an incompletely known microstructural and chemical framework, because few data were available to inform on the variability and skeletal distribution of this tissue in Neornithes. Without this context, hypotheses of MB may be falsely rejected (false negatives). Indeed, we find that the customary diagnosis of MB does not capture the extent of variability that exists in modern birds (see [34,41] and the present work). This is largely because current diagnoses, drawn from existing literature, are derived from a subsample of avian species relevant to the poultry industry or hormonally treated males (see [23,34] and references therein). This limited taxonomic sample underrepresents Neornithes' phylogenetic diversity and fails to capture variation associated with the range of physiologies and ecologies encountered in wild bird species. Moreover, the majority of studies inferring the presence of MB in extinct taxa have failed to evaluate the criteria used for this diagnosis within a hypothesis-driven framework or a correctly polarized hypothetical framework that would allow for the rejection of an MB hypothesis.
To increase our understanding of MB variability in living birds, recent studies have investigated variations in microstructure, skeletal distribution patterns and prevalence of MB in a broad comparative framework across Neornithes [8,33,34]. However, these new data have not yet been used to advance a hypothetical framework for the confident identification of MB in extinct organisms. Based on the most recently published data and our own observations of MB distribution, structure and composition, using micro- and nano-computed tomography (CT) and histochemistry, we provide: (i) new insights into the phylogenetic breadth and structural diversity of MB within extant birds; (ii) a reevaluation and refinement of the most recently published list of criteria proposed to allow confident identification of MB in the fossil record and their utility within a newly proposed hypothetical framework; (iii) reevaluation of some fossil specimens previously identified to contain MB-like tissues, taking into account the newly acquired data; and (iv) discussions on the challenges of characterizing MB in Neornithes and improving its diagnosis in extinct avemetatarsalians.
2. Phylogenetic distribution of medullary bone
Recent comprehensive studies involving broad sampling across the avian tree confirm that MB is phylogenetically widespread among Neornithes and is most parsimoniously interpreted as a synapomorphy of this clade ([33,34]; figure 1b). However, it remains unclear whether MB is formed and used by females of all major bird clades for the production of mineralized eggshell (figure 1b), and contentious reports of species that lack this reproductive tissue remain (see below).
For example, Maclean [58] studied the bone structure of females of four sandpiper species (Calidris alpine, C. bairdii, C. pusilla and C. melanotos) that died during the egg-laying cycle. This author reported no evidence of calcium storage in the form of MB but instead observed larger quantities of skeletal elements coming from small rodent carcasses in the stomach contents of these individuals than in non-breeding females and males. Maclean thus concluded that females of arctic sandpipers (Calidris spp.) do not resort to MB but rather ingest great quantities of dietary calcium just before egg-laying and directly use this source of mineral to form the eggshell. However, a subsequent study by Piersma et al. [59] showed that considerable amounts of calcium were stored in the skeleton of female Calidris canutus (and not in males) before egg-laying. Unfortunately, the latter study relied on skeletal ash mass and the authors never mentioned whether the calcium was stored in the form of MB or in the form of cortical bone tissue for this species.
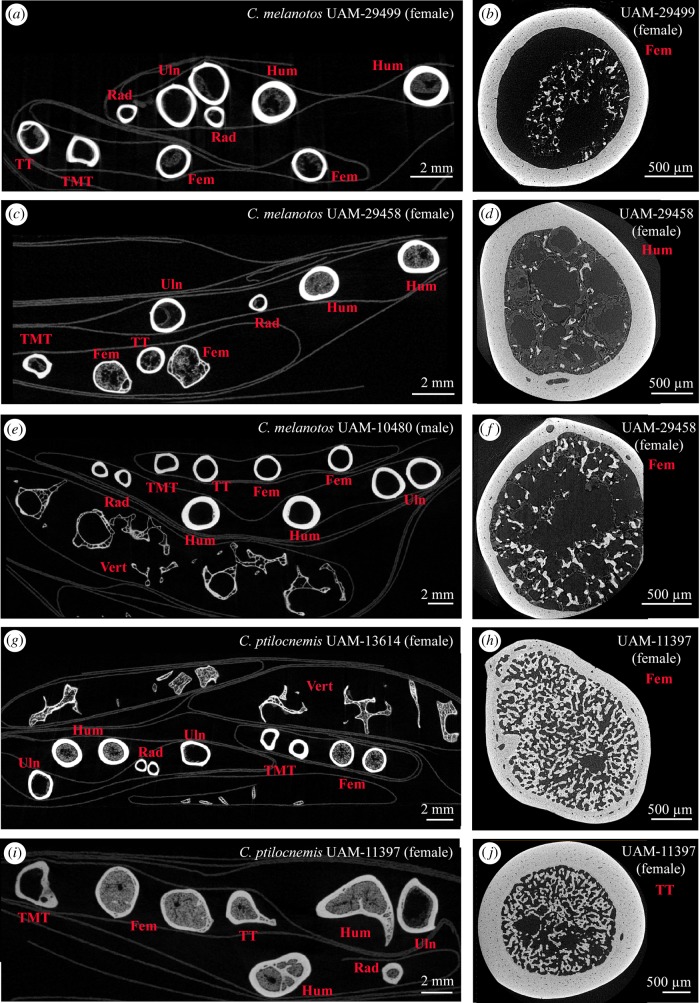
To address the presence of MB in calidrids and reassess the results of Maclean [58] and Piersma et al. [59], we collected female specimens that died during the egg-laying cycle from two Calidris species (the rock sandpiper C. ptilocnemis, and the pectoral sandpiper C. melanotos), as well as conspecific males (as controls; table 1). Our CT data (figure 2) clearly show that MB can form in great quantities in most of the skeletal elements of Calidris females (figure 2g–j) and thus settle the debate on the presence of MB in these sandpipers.
Table 1.
Calidris specimens subjected to CT-scanning examinations to screen for the presence of MB. All specimens are housed in the Bird Collection of the University of Alaska Museum (UAM), Fairbanks, Alaska, USA. n.a, not applicable.
| species/common names | catalogue number | sex | state of female reproductive tract | micro-CT scans - voxel size (in µm) | nano-CT scans - voxel size (in µm) | MB present (YES/NO) |
|---|---|---|---|---|---|---|
| Calidris melanotos pectoral sandpiper | UAM-29499 | female | ovary: 14.5 × 10.3 mm, largest ovum 6.7 mm, oviduct quite enlarged, but no eggs laid yet | 30.02 | 2.17 | YES (small amounts) |
| UAM-29458 | female | ovary: 14 × 7.5 mm, largest ovum 3.2 mm, 4 post-ovulating follicles | 38.01 | 2.21–2.51 | YES (small amounts) | |
| UAM-10480 | male | n.a. | 30.28 | 2.71 | NO | |
|
Calidris ptilocnemis
rock sandpiper |
UAM-13614 | female | ovary: 14.2 × 12.8 mm, largest follicle 6.6 mm | 30.66 | — | YES |
| UAM-11397 | female | ovary: 16.7 × 15.5 mm, partially formed egg in oviduct 28.8 mm | 30.42 | 2.40–3.03 | YES (large amounts) | |
| UAM-9029 | female | ovary: 12X3 mm, ova to 4 mm | 30.02 | — | NO | |
| UAM-11503 | male | n.a. | 29.76 | — | NO | |
| UAM-13787 | male | n.a. | 28.66 | — | NO |
Figure 2.
Micro- (a,c,e,g,i) and nano-CT (b,d,f,h,j) scans of skeletal elements of sandpiper adult specimens. (a–d, f–j) Females that died at different stages of the egg-laying cycle; (e) adult male. (a) Calidris melanotos UAM-29499; (b) femur of UAM-29499 containing small amounts of MB and bone marrow; (c) C. melanotos UAM-29458; (d) humerus of UAM-29458 containing small amounts of MB and bone marrow; (e) C. melanotos UAM-10480; (f) femur of UAM-29458 containing MB and bone marrow; (g) Calidris ptilocnemis UAM-13614; (h) femur of C. ptilocnemis UAM-11397 containing large amounts of MB; (i) C. ptilocnemis UAM-11397; (j) tibiotarsus of C. ptilocnemis UAM-11397 containing large amounts of MB. Abbreviations: Fem, femur; Hum, humerus; MB, medullary bone; Rad, radius; TMT, tarsometatarsus; TT, tibiotarsus; Uln, ulna; Vert, vertebrae. (Online version in colour.)
However, the presence of MB is still undetermined in many bird groups (figure 1b), and the hypothesis that some bird groups may have secondarily lost the need or ability to produce MB during the egg-laying cycle, relying instead on different sources of calcium for shelling, needs to be directly tested. To begin to address this question, Canoville et al. [34] demonstrated that the skeletal distribution of MB depends directly on the combined distributions of pneumaticity and bone marrow. Pneumatized skeletal elements are expected to show only trace amounts or complete absence of MB deposition during the egg-laying cycle. O'Connor [60] classified some bird groups, such as the ground hornbills from the genus Bucorvus, and the Anhimidae, a small group of Anseriformes, as hyperpneumatized. Indeed, these birds show extensive pneumaticity, affecting all postcranial skeletal regions. To our knowledge, MB has not yet been reported in these birds (see electronic supplementary material, S1 of [33]). Whether these species still form MB (at least in some skull elements) or whether extensive pneumatization of their skeletons rendered the deposition and subsequent use of MB impossible has yet to be directly tested.
Finally, whether some secondarily flightless diving bird species with unusually thick bone walls form MB for the production of the eggshell has yet to be directly tested. Spheniscidae (penguins) are particularly relevant to the question of MB in diving birds with thick bone cortices. Our current understanding of MB in Spheniscidae is based on the reported presence of MB in a single specimen of Spheniscus humboldti [61]. However, this report should be considered ambiguous for several reasons. First of all, this identification is based exclusively on gross visual observation and neither this unique observation nor the microstructure of the potential MB are visualized or adequately described. Several studies have shown that, unlike most other birds that possess thin compact cortices and a large open medullary cavity in the limb bones, penguins have thick bone walls and a medullary region filled with a (sometimes dense) network of bony trabeculae [62,63]; it would thus be difficult to discriminate MB from normal trabecular bone tissue when only gross observations are employed. Additionally, the dense bone microstructure of penguins, which is an adaptation to their flightless, diving lifestyle, does not leave large voids for the endosteal deposition of MB that can fill the marrow cavities of most skeletal elements in other bird species (e.g. [8,33,34]). Moreover, the clutch size of all penguins is small (one to two eggs) and most species laying two eggs exhibit relatively long laying intervals [64], thus, their short-lived calcium requirement to form the eggshell may be lower than in most other bird species. Finally, histological studies have shown that some penguin species exhibit cortices that are almost completely formed of dense Haversian bone at maturity, suggesting intense cortical remodelling and mineral homeostasis [62,63]. Based on these observations, it is reasonable to hypothesize that penguin species could resort in part or exclusively to the calcium contained in their thick bone cortices, without having to form large amounts of MB during egg-laying. Further investigations are necessary to test this hypothesis and investigate whether some parameters that could be assessed in extinct avemetatarsalians, such as clutch size or cortical thickness, may be related to the capacity or necessity to form MB.
3. Criteria previously used to diagnose medullary bone
Since the first report of MB-like tissues in a T. rex specimen by Schweitzer et al. [41], criteria based upon known features of MB in extant birds have been explicitly or implicitly used to recognize MB-like tissues in fossil specimens. These were defined and progressively refined based on our growing understanding of MB in extant birds. However, our understanding of the formation, distribution and chemistry of this unique bone type is incomplete. The extent of MB microstructural and chemical variability is still poorly constrained, because it has only been observed and described for a handful of wild bird species to date.
The first three criteria commonly used to describe MB by the paleontological community were related to the developmental origin (endosteum), microstructure (trabecular architecture, woven and highly vascularized) and location (primarily found in the marrow cavity of limb bones) of this tissue [35,37,41].
However, in a subsequent study, Chinsamy & Tumarkin-Deratzian [55] urged caution in using these criteria alone. In a paper describing bone pathologies in the ulna of a turkey vulture (Cathartes aura) and in a long bone of a non-avian dinosaur, they noted that both specimens presented a reactive periosteal bone associated with a pathological endosteal tissue. The authors showed that on the basis of origin, microstructure and location, these pathological bone tissues could not be clearly discriminated from MB and recommended caution in recognizing all unusual endosteal tissue as homologous to MB in the fossil record. Moreover, they proposed that the MB-like tissue recovered in the tibia of Allosaurus specimen UUVP 5300 and initially hypothesized to belong to a reproductively mature female by Lee & Werning [37] might actually have a pathological origin because it was coupled with a reactive periosteal tissue. Subsequently, studies included a fourth criterion for the identification of MB in extinct avemetatarsalians: skeletal elements exhibiting MB-like tissues should be devoid of any external bone pathologies to be considered homologous to avian MB [38,44].
In 2014, Prondvai & Stein reconsidered the original function of purported MB in extinct archosaurs after observing such tissues in the mandibular symphyses of several, supposedly young pterosaur specimens. Considering the early ontogenetic stage of these animals, the fact that pterosaurs laid thin-shelled eggs, and the unusual anatomical location of the observed MB-like tissues, Prondvai & Stein [36] argued that these tissues did not serve a reproductive function. Following works reflected on these four criteria and called for further independent evidence to unambiguously identify MB in the fossil record [23,40,57]. As Prondvai & Stein [36] had described before them, Chinsamy et al. [40] noted the wide skeletal distribution of MB-like tissues in extinct avemetatarsalians. They suggested purported MB was not restricted to long limb bones, but also commonly occurred in the axial and dermal skeleton, and proposed that this location raised additional doubt regarding the homology of these tissues to avian MB (e.g. [40]). Canoville et al. [34] responded by recording the pattern and extent of MB skeletal distribution in the skeletons of 38 bird species representative of the taxonomic, body size and ecological diversity of Neornithes. Their main findings revealed that MB is a systemic bone tissue that can be deposited within virtually all skeletal elements, including cranial bones, and that its distribution pattern is directly linked to the coupled distributions of pneumaticity and bone marrow. These authors thus showed that the ‘unusual’ location is an invalid criterion against the potential reproductive function of MB-like tissues.
Werning et al. [57] proposed four additional criteria to include for diagnosing MB: (i) hormonal stimulus (MB is an oestrogen-dependent tissue); (ii) duration (MB is an ephemeral tissue formed and subsequently resorbed during the egg-laying cycle); (iii) timing in context of life history (MB is only naturally formed in reproductively mature female birds); and (iv) chemical composition (different from surrounding cortical and trabecular bone tissues). Although Werning et al. [57] acknowledged that the first of these criteria (i.e. hormonal stimulus) could not yet be evaluated in fossil specimens, it seems evident that the second proposed criterion (i.e. duration) is also currently impossible to assess in extinct taxa.
Schweitzer et al. [42] were the first to test the fourth of Werning et al.'s [57] additional criteria (i.e. unique chemical composition). Based on a decade of evidence suggesting that some organic molecules are preserved post-fossilization (numerous studies have demonstrated the presence of endogenous organic molecules in fossilized bone, e.g. [65–69]) and that MB has a unique chemical signature as compared to structural bone tissue (e.g. [9]), it was expected that some identifying chemical signals might remain in these fossil tissues. Using techniques traditionally employed to highlight the presence of KS in the matrix of MB (i.e. chemical staining using alcian blue or high iron diamine (HID), and immunochemistry using monoclonal X-KS antibody (5D4); e.g. [12,18]), Schweitzer et al. [42] reassessed the nature of the MB-like tissue found in the T. rex specimen MOR 1125. Results of these chemical methods were positive, when compared to similar tests on MB in extant birds. Moreover, the absence of reactivity in a pathological avian control led them to conclude that the tissue observed was indeed MB and that this method could reliably distinguish MB from pathological bone.
The most recent study discussing a revisited ensemble of MB characteristics was carried out by O'Connor et al. [46]. These authors combined features discussed in the paleontological literature over the past 15 years, together with observations from newly published data, to establish an updated checklist of 11 criteria that should be taken into account to clearly diagnose MB. O'Connor et al. [46] attribute the first three criteria (#1–3) listed in their table 1 to the features traditionally recognized in the literature as characteristic of MB. They propose eight additional criteria (#4–11) that are either based on their own observations or on published data and suggest that to be robustly diagnosed as MB, the tissue under investigation must fulfill at least the first 9 (#1–9) out of 11 of their criteria.
This list is meant to summarize the most inclusive knowledge of avian MB to date, and the validity of each criterion, when applied to fossil specimens, will be considered as a baseline for our following discussion. Here we evaluate and refine the features listed by O'Connor et al. ([46]: table 1) as diagnostic criteria for identifying MB in fossil specimens in light of the most recently published data on MB, and new observations from our ongoing work.
(a). Criterion #1: ‘occur in the medullary cavity and other cancellous spaces throughout the appendicular and axial skeletons'
This is a valid criterion for the identification of MB; MB indeed forms in the medullary cavities and cancellous spaces of skeletal elements, and can be present in any skeletal region (including cranial elements; [34]). However, studies have demonstrated that the amount of MB present throughout the skeleton fluctuates during the egg-laying cycle and differs intra-, but more specifically interspecifically (e.g. [8,33,34]). Indeed, recent research demonstrates that the pattern of MB distribution in the avian skeleton is based on several physiological variables, and is thus rather complex. Therefore, we can refine this criterion to be more precise.
Studies have shown that MB only forms in bone cavities containing haematopoietic tissues [7,34]. Thus, MB is predicted to be absent or only present in trace amounts in the pneumatic cavities of skeletal elements of extant birds and extinct avemetatarsalians, in general [34].
To begin to establish a refined, location-based framework for the identification of MB in the skeleton, Canoville et al. [34] investigated the skeletal distribution of this tissue across Neornithes. Results showed that, in modern birds, MB deposition greatly varies between species, but follows predictable patterns, and is closely correlated to the skeletal distributions of pneumaticity and bone marrow. Thus, the extent of MB deposition across the skeleton is high when the extent of skeletal pneumaticity is low and the reciprocal relationship is also true. Our results for the genus Calidris are in agreement with this conclusion. These small- to medium-sized scolopacids show an extensive skeletal distribution of MB (e.g. figure 2g–j) but present limited skeletal pneumaticity, restricted to a few vertebrae (see [60]: table 2).
Considering the hypothesized homology of lung tissues between birds and some extinct avemetatarsalians, Canoville and colleagues proposed a series of location-based predictions that can be used to critically evaluate MB-like tissues in fossil specimens. For example, they showed that if found in the autopod, MB should also be present in the adjacent zeugopod. Similarly, if the axis presents MB, the associated atlas should show MB as well (see [34] for a detailed list of location-based predictions). This ‘skeletal distribution pattern’ criterion is unique to MB and not valid for other bone tissue types, especially pathological ones that usually concern restricted skeletal portions (that have been affected by infection, fracture damage, etc.) and often only one of the paired elements (A Canoville 2019, personal observation; see also figure 4a–d).
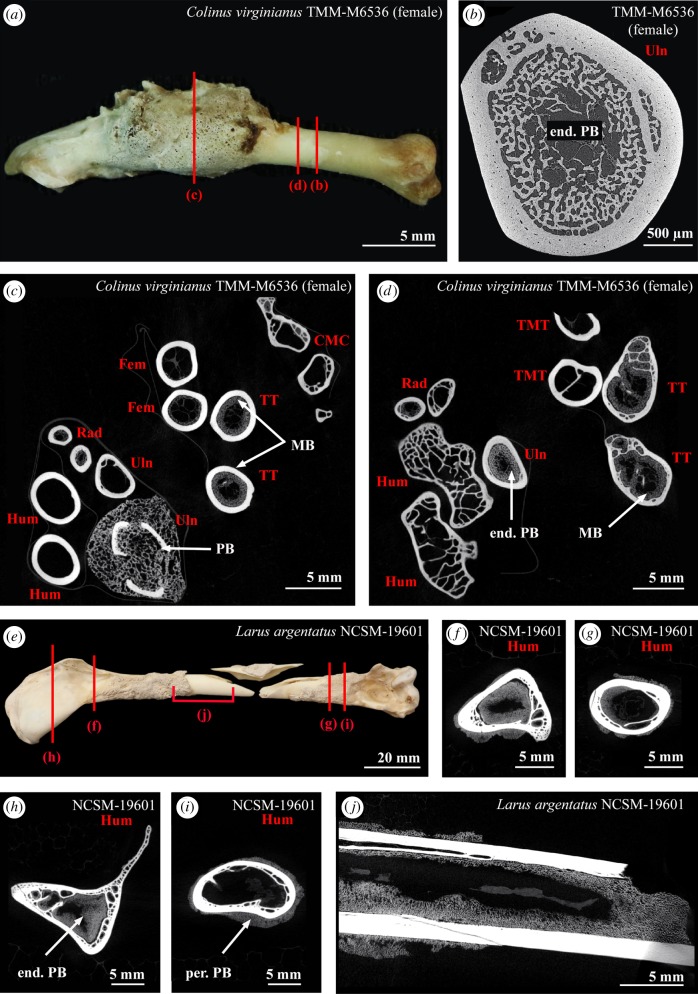
Figure 4.
(a) Pathological ulna of Colinus virginianus specimen TMM-M6536. (b) Virtual cross-section of the ulna in (a). An endosteal PB tissue fills up most of the medullary cavity and resorption spaces. (c,d) Virtual cross-sections of the different limb bones of Colinus virginianus specimen TMM-M6536. The femora, tibiotarsi and radii contain MB. One ulna contains large amounts of an endosteal PB tissue. The microstructure and density of this PB is different from the MB observed in this specimen. (e) Pathological humerus of Larus argentatus specimen NCSM-19601; (f–i) virtual cross-sections of the humerus in (e). (j) Virtual longitudinal section of the humerus in (e). Abbreviations: end. PB, endosteal pathological bone tissue; Fem, femur; Hum, humerus; MB, medullary bone; per. PB, periosteal pathological bone tissue; Rad, radius; TMT, tarsometatarsus; TT, tibiotarsus; Uln, ulna.
(b). Criterion #2: ‘be of endosteal origin’
MB is indeed deposited and mineralized centripetally into the (medullary) cavity by endosteal osteoblasts [10,70,71]. However, this criterion is not specific to MB. Indeed, the innermost part of bone cortices is often formed of an endosteal lamellar bone in adult tetrapods. Furthermore, several studies have shown that some pathological bone tissues have an endosteal origin (e.g. [55]). We agree with O'Connor et al. [46], that although the absence of this criterion can be used to reject a hypothesis of MB in fossil vertebrates, its presence alone cannot eliminate alternative hypotheses for endosteal tissues.
(c). Criterion #3: ‘primarily have a woven arrangement of the collagen fibres indicative of rapid formation (may also be partially parallel-fibred or lamellar in some instances)’
MB has been traditionally described as a primarily woven bone tissue with a cancellous/trabecular arrangement [12,41,72]. This histological description relies on direct observations, using electron microscopy, of the random orientation of the collagen fibrils and hydroxyapatite crystals of MB matrix in a few bird species (e.g. [41,72]). This tissue organization is directly associated with the highly labile and ephemeral nature of MB, which is rapidly deposited at the beginning of the egg-laying cycle and subsequently resorbed when the egg reaches the shell gland in the oviduct. A few studies used the term ‘lamellae’ to describe the overall microstructure of MB [3,73]. However, it is unclear whether these authors refer here to the organization of the collagen fibres in the matrix, or to the individual units of bone tissue successively deposited through time and forming the heterogeneous cores of MB trabeculae [73, pp. 450–451]. To our knowledge, a lamellar component for MB has been reported for only one ostrich specimen thus far [74] and additional work is necessary to determine whether or not this is a common occurrence in Neornithes or at least large ratites.
A parallel-fibred to lamellar component (with anisotropy) has been more frequently reported in MB-like tissues recovered from extinct avemetatarsalians (e.g. [37,46,47]). However, criteria pertaining to the histology of the MB matrix and used to evaluate the nature of purported MB-like tissues in extinct species should not be defined by features based primarily on observations of fossil taxa. This reasoning is circular and does not lead to objective inferences. Instead, a large comparative investigation across Neornithes should be conducted to investigate the extent of MB microstructural variability.
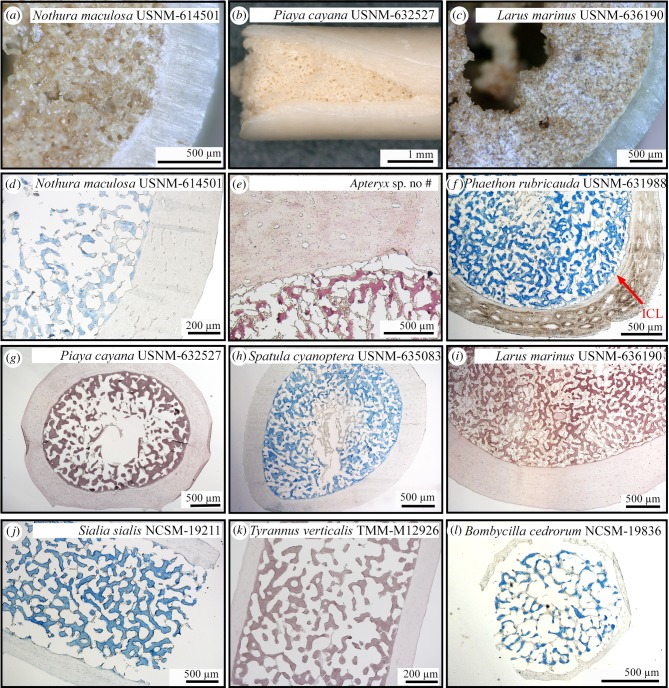
With respect to the currently available data, MB-like tissues should thus be primarily woven (among other criteria) to be considered as potentially homologous to avian MB. This requirement is not met, for example, in the case of the newly described Avimaia schweitzerae specimen IVPP V25371 [47]. Indeed, its femur presents a very thin layer of endosteal bone tissue separated from the cortex by a thin inner circumferential layer. This tissue presents only a few elongated osteocyte lacunae and has a completely lamellar organization. Nonetheless, Bailleul et al. [47] hypothesized it to be MB because this Early Cretaceous enantiornithine also preserves eggshell material in its body cavity, consistent with it being gravid and female. Another recently described pengornithid enantiornithine (specimen IVPP V15576A) also presents an unusual endosteal tissue in the cavities of several hindlimb bones, and is interpreted as homologous to MB by O'Connor et al. [46]. In the femur and tibiotarsus, this tissue consists of both parallel-fibred and woven bone. To explain the partial anisotropy of these endosteal tissues, O'Connor et al. [46] and Bailleul et al. [47] hypothesized that MB may have formed more slowly in Enanthiornithines than in most similar-sized Neornithes, considering the overall slower growth rates observed in these Cretaceous birds relative to modern birds [75–77]. Indeed, whereas most Neornithes reach skeletal maturity in less than a year and exhibit high growth rates (translated into cortices formed of well-vascularized fibrolamellar bone tissue), some enanthiornithes took several years to reach adult size and thus grew at relatively slower rates (cortices often medium to poorly vascularized and formed of a woven to parallel-fibred bone tissue interrupted by lines of arrested growth; [75–77]). One way to test their hypothesis would be to investigate the structure of MB in a modern bird species that grows slowly over several years. The kiwi (Apteryx spp.) is an exception among extant Neornithes, because it exhibits a relatively slow and discontinuous growth, unlike most other extant bird species [78]. Preliminary observations of MB in a female Apteryx sp. (figure 3e; A Canoville 2019, personal observation), however, reveal that its microstructure does not differ from similar-sized palaeognaths (such as Nothura maculosa USNM-614501, figure 3d) and other neognaths (figure 3f–l). This suggests that the proposed microstructural differences observed between the hypothesized MB of Avimaia and those of extant avialans cannot reasonably be attributed to a slower growth rate and this criterion cannot be used to support or refute the presence of MB in this specimen.
Figure 3.
Hand samples (a–c) and stained paraffin sections (with alcian blue: d, f, h, j, l, or HID: e, g, i, k) of the femoral shafts of diverse female birds that died during or just after the egg-laying cycle and present MB. (a) Nothura maculosa USNM-614501; (b) Piaya cayana USNM-632527; (c) Larus marinus USNM-636190; (d) cross-section of Nothura maculosa USNM-614501; (e) cross-section of Apteryx sp.; (f) cross-section of Phaethon rubricauda USNM-631988; (g) cross-section of Piaya cayana USNM-632527; (h) cross-section of Spatula cyanoptera USNM-635083; (i): cross-section of Larus marinus USNM-636190; (j) longitudinal section of Sialia sialis NCSM-19211; (k) longitudinal section of Tyrannus verticalis TMM-M12926; (l) cross-section of Bombycilla cedrorum NCSM-19836. ICL, inner circumferential layer. (Online version in colour.)
(d). Criterion #4: ‘occur with periosteal surface free from pathological indicators'
This criterion sets up a false dichotomy. Pathological bone tissues and MB are not mutually exclusive; therefore, an individual can exhibit both pathological bone and MB. Bird specimens containing large amounts of MB throughout the skeleton and yet still exhibiting bone pathologies have been observed (see below). For example, one female of Colinus virginianus (TMM-M6536) was found with MB in about 74% of its skeletal elements [34]. However, one of its ulnae exhibits a pathology, clearly affecting its external morphology, but only on a restricted portion of the shaft (figure 4a). Micro- and nano-CT data revealed that whereas the pathology is only externally visible on a restricted portion of the shaft, the medullary cavity of this element is filled with an endosteal pathological bone tissue throughout the length of the ulna (figure 4a–d). This endosteal bone tissue could have been mistaken for potential MB when considered on its own (because of its endosteal origin and its woven and trabecular architecture; see for example figure 4b, as compared to figure 2h,j). However, this diagnosis is not supported for two reasons: first, CT data clearly show that the MB seen in the associated skeletal elements (including the contralateral ulna) does not have the same structure and mineralization rate as the endosteal pathological bone tissue in this ulna (figure 4c,d); and second, the contralateral ulna contains only small amounts of MB, where the pathological ulna is completely filled with the endosteal pathological bone tissue (figure 4c). By contrast, Canoville et al. [34] have shown that contralateral elements usually exhibit the same distribution and amount of MB. Rejecting a hypothesis of MB based on the presence of pathological periosteal tissue can increase the rate of type II (false negative) errors. Thus, in the fossil record, the nature of MB-like tissues in a specimen should not be questioned only on the basis of additional evidence of pathology.
Moreover, although not directly related to this criterion, some endosteal bone pathologies, at least when considered in cross-section, are observed to have no associated periosteal reactive bone tissue on parts of the shaft. We observed this in several modern bird specimens, such as in the ulna of Colinus virginianus TMM-M6536 mentioned above, but also one specimen of Larus argentatus humerus (NCSM 19601, figure 4e–j). In the case of the latter specimen, the rest of the skeleton did not contain any sign of MB. However, whereas some parts of the shaft or the epiphysis did show large amounts of endosteal pathological bone tissue, the periosteal surface did not exhibit any sign of pathology. This is important to recognize because not only can the presence of pathological bone tissues on the periosteal surface not be reliably used to reject a hypothesis of MB, the absence of pathological bone tissue on the periosteal surface cannot be used to reject a hypothesis that the tissue observed is pathological. The localization of periosteal reactive pathological bone is expected to be highly problematic when dealing with fragmentary, incomplete fossils or single cross-sectional samples. In most cases of reported MB-like tissues in extinct avemetatarsalians, the majority of histological descriptions are based on cross-sections of limb bones or other skeletal elements, and usually do not entail examination of more than one element from a single individual; thus the critical element of systemic distribution of MB cannot be evaluated in most fossil specimens.
(e). Criterion #5: ‘line a majority of the medullary cavity (including trabecular surfaces)’
Although this is true in most specimens observed (e.g. [8,34]), the resorption, deposition and mineralization processes of MB occur at different locations within the medullary cavity. MB trabecular scaffold follows the three-dimensional architecture of the bone marrow stroma [33], thus MB can be dispersed throughout the cavity. It may or may not form a ‘ring’ lining a majority of the medullary cavity, depending on the stage in the egg-laying cycle, as illustrated here in some Calidris specimens (figure 2b,d,f). This criterion is therefore not a reliable requirement for an MB identification.
(f). Criterion #6: ‘Be clearly demarcated from the cortical tissue without a graded transition’
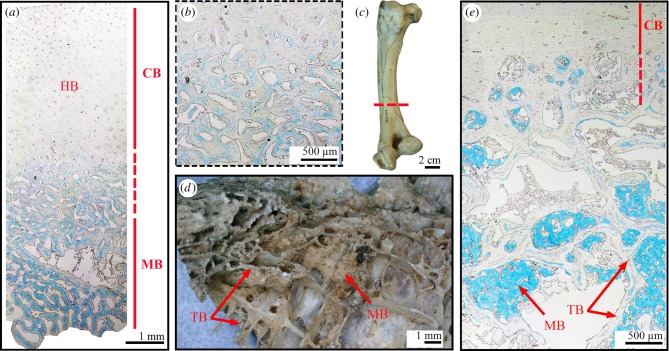
Although this is the case at the mid-diaphyseal level in most Neornithes specimens studied to date (e.g. [34]; figures 2 and 3), we document contradictory examples herein, and thus we find this trait should not be considered as a necessary criterion for diagnosing MB. The most common pattern of MB deposition is well illustrated by the long bone mid-diaphysal cross-sections of different bird species in figures 2 and 3. MB tissue usually shows a trabecular architecture (the density of the trabecular network, as well as the size of the trabeculae vary as illustrated in figure 3) and is clearly demarcated from the surrounding compact cortical bone tissue, even on hand samples (figure 3a–c). Moreover, along the shaft, cortical bone and MB are commonly separated by a layer of endosteal lamellar bone (also called internal circumferential layer (ICL) in birds; e.g. [76,79]) that forms after the centrifugal expansion of the medullary cavity has ceased (e.g. ICL clearly visible on figure 3f). This ICL is thus separated from the overlying periosteal bone tissue by a scalloped resorption line. However, this interface between cortical bone and MB is not always so conspicuous. Indeed, at least two large palaeognath specimens observed here illustrate that the transition between the compact cortex and MB is not necessarily well demarcated (figure 5). The femur of a female ostrich (already described in [41,42]) that died during the egg-laying cycle (table 2) was sectioned along the shaft and shows a gradual transition between the compact cortex and the centripetally deposited MB (figure 5a,b). The compact cortex has been heavily remodelled and is formed of a dense Haversian bone tissue. Therefore, the interface between the endosteal and periosteal bone territories is no longer visible (figure 5a,b). Moreover, the femur of a female Rhea pennata pennata USNM-631769 that has been sectioned at the distal diaphyseal level (figure 5c), exhibits similar structure. In this specimen, the deep cortex presents large erosion cavities and grades into a loose trabecular network in the medullary region. MB fills some of the resorption cavities and is deposited between trabeculae (figure 5d,e). Thus, there is no clear separation between the cortical and trabecular domains on the one hand, and MB on the other hand—the distribution areas of these tissues are intertwined (figure 5d,e). Moreover, in this specimen, MB does not clearly exhibit a trabecular organization. The MB spicules seem to be randomly aggregated along the trabecular surfaces (figure 5d,e). Future research is needed to assess whether these differences in MB microstructure and spatial deposition observed between these palaeognaths and the other sampled neornithes are linked to specific factors such as phylogeny, body size, flightlessness or even age of the individual.
Figure 5.
Stained paraffin sections (with alcian blue: a,b,e) and hand samples (c,d) of the femora of two palaeognath females presenting MB. (a) Cross-section of a specimen of Struthio camelus. Note that the transition between the compact cortex (made of Haversian bone) and the MB is gradual. (b) Higher magnification of the transition zone in (a). (c) Sampled femur of Rhea pennata pennata USNM-631769. The sampling location is highlighted by the red dashed line. (d) Bone fragment sampled from (c). MB spicules are aggregated along the trabecular surfaces. (e) Cross-section of Rhea pennata pennata USNM-631769. Note that the transition between the compact cortex and the MB is gradual. MB is deposited between trabeculae. CB, cortical bone; HB, Haversian bone; MB, medullary bone; TB, trabecular bone. (Online version in colour.)
Table 2.
Female bird specimens subjected to histochemical staining. n.a., not applicable.
| clade | species/common names | catalogue number | skeletal element sampled | sex | state of reproductive tract |
|---|---|---|---|---|---|
| Struthioniformes |
Struthio camelus
common ostrich |
n.a. | femur (shaft) | female | unshelled eggs in reproductive tract |
| Rheiformes |
Rhea pennata pennata
lesser rhea |
USNM-631769 | femur (distal shaft) | female | n.a. |
| Tinamiformes |
Nothura maculosa
spotted nothura |
USNM-614501 | femur (midshaft) | female | laying condition; unshelled eggs, oviduct enlarged |
| Apterygiformes |
Apteryx sp. kiwi |
n.a. | femur (proximal shaft) | female | cloacal inflammation from recent egg laying |
| Anseriformes |
Spatula cyanoptera
cinnamon teal |
USNM-635083 | femur (midshaft) | female | ovary: laying, unshelled egg in oviduct |
| Columbaves |
Piaya cayana
squirrel cuckoo |
USNM-632527 | femur (midshaft) | female | ovary 13 × 10 mm, 1 collapsed follicle; ova: 3 × 3 mm (×4) |
| Aequorlitornithes |
Phaethon rubricauda
red-tailed tropicbird |
USNM-631988 | femur (midshaft) | female | ovary enlarged, 1 collapsed follicle |
| Aequorlitornithes |
Larus marinus
great black-backed gull |
USNM-636190 | femur (midshaft) | female | ovary: enlarged, collapsed follicles |
| Passeriformes |
Sialia sialis
Eastern bluebird |
NCSM-19211 | femur (midshaft) | female | unshelled egg in oviduct; found dead in a nest on four eggs |
| Passeriformes |
Tyrannus verticalis
Western kingbird |
TMM-M12926 | femur (midshaft) | female | n.a. |
| Passeriformes |
Bombycilla cedrorum
cedar waxwing |
NCSM-19836 | femur (midshaft) | female | fully formed egg in oviduct, other follicule 10 × 10 mm; three burst follicules |
We argue that, once again, this character is not specific to MB and the absence of this feature is not a sufficient cause to reject a hypothesis of MB in fossils. Indeed, some endosteal pathological bone tissues can be clearly demarcated from the surrounding cortical bone. This is illustrated in figure 4b,d,f,g,h,j.
(g). Criterion #7: ‘occur in multiple elements including the tibiotarsus'
Canoville et al. [34] investigated the skeletal distribution of MB in 40 female birds that died during the egg-laying cycle. Results showed that when present (38 of the sampled specimens), MB was universally found in at least the proximal half of the tibiotarsi examined, regardless of its distribution in other elements. Our new dataset on the genus Calidris support this statement. However, a few bird species might be exceptions. Indeed, in his comparative study of the skeletal distribution of pneumaticity in birds, O'Connor [60] observed that the genus Pelecanus is unique among Neornithes. Most of the postcranial skeleton of these animals is pneumatized (including the tibiotarsus), with the exception of the femur, which is filled with red bone marrow. In this genus, we can thus predict MB deposition to be restricted to the femur, although this has not been directly tested. MB has been reported in the femur of a female specimen of Pelecanus erythrorhynchos by Foote [80] and Simkiss [81], but never in the tibiotarsus. Future studies investigating the distribution of MB in the complete skeleton of Pelecanus females will answer this question.
(h). Criterion #8: ‘coincide with reduced growth rates indicative of sexual maturity’
O'Connor et al. [46] formulated this criterion based on the fact that, in most amniotes, a relative decrease in growth rates during ontogeny usually accompanies the onset of sexual maturity, reflecting energy allocation shifts from growth to reproduction. However, this criterion might be inadequate when broadly applied and might be difficult to observe in fossil specimens.
First, this criterion, as listed, is problematic because the vast majority of Neornithes commonly reach skeletal maturity long before sexual maturity [82]. A shift in growth rate during ontogeny thus coincides with the attainment of skeletal maturity, and does not necessarily correlate to reproductive maturity that might be attained several years after growth had ceased and will not necessarily be recorded in the bone microstructure of the organism.
This criterion is invalid for some extinct avemetatarsalians outside Neornithes where the reverse is documented; these organisms have been hypothesized to reach sexual maturity several years before reaching somatic maturity (e.g. [44,83–85]). Although sexual maturity in these animals (such as Tyrannosaurus rex, see [86]) is hypothesized to occur when the growth rate has initially slowed, these organisms could still grow at relatively high rates (and deposit highly vascularized fibrolamellar bone) for several years after this initial attenuation.
Furthermore, this criterion is not validated by newly described enanthiornithine specimens hypothesized to contain endosteal tissues homologous to avian MB. Indeed, Avimaia schweitzerae (IVPP V25371) does not exhibit any sign of a marked decrease in growth rates in its sampled femur [47]. Rather, the cortical bone presents an overall uniform histology, with a poorly vascularized, parallel-fibred bone matrix interrupted by a single line of arrested growth. This bone histology pattern is common among Enanthiornithines and used to support the hypothesis that this group grew slower than most modern-day birds and sometimes took several years to reach skeletal maturity [75–77]. According to the authors, there is no outer circumferential layer (OCL) in the outermost cortex. The only sign of sexual maturity in this specimen is the presence of egg material in its body cavity; thus this criterion, proposed by O'Connor et al. [46], is not met in this specimen.
Similarly, specimen IVVP V15576, described by O'Connor et al. [46] as containing MB, also does not clearly exhibit slowed growth rates. The cortical bone of its femur and tibiotarsus is rather homogeneous and consists of parallel-fibred bone, with simple, longitudinal canals located close to the periosteal surface. Neither an OCL nor an ICL can be confidently identified in these skeletal elements, and only one line of arrested growth is visible in the mid-cortex of the femur.
(i). Criterion #9: ‘often have vascular canals with a doublet or triplet pattern within osteon-like structures (vascular sinuses)’
We argue that this is not, to date, a valid criterion for rejecting a hypothesis of MB. This statement is based on limited observations. Such structures have been described in the fossil record (e.g. [46]) and only two specimens of extant ratites [41]. Whether this is a common feature unique to avian MB (and not present in pathological bone tissues, for example), has not yet been rigorously tested for the entire avian tree; it should not be considered a necessary criterion for the identification of MB in extinct organisms.
(j). Criterion #10: ‘have a histochemistry comparable to that of extant avian medullary bone (e.g. shows higher amounts of glycosaminoglycans than that of cortical bone) (unique histochemical signature, yet to be determined)’
As mentioned previously, various studies have shown that MB has a different chemical composition than the surrounding structural bone tissues (i.e. [9,11–18]). To date, only a single study has investigated this question in fossils. Schweitzer et al. [42] developed techniques to document the presence of keratan sulfate (KS) in the matrix of MB and used these data to support the identification of a tissue consistent with MB in a fossil specimen (T. rex MOR 1125). The results they obtained were similar to tests on MB in extant birds, leading them to conclude that the tissue was indeed MB. As a control, the authors also tested the hypothesis that MB is chemically different from avian pathological bone, and included in their analyses one specimen with avian osteopetrosis (a viral-induced skeletal lesion common in domesticated birds; [87]). They found no evidence of reactivity with stains or antibodies, suggesting the lack of KS in this pathology and rejecting a hypothesis of pathological origin for the endosteal tissue.
However, it is reasonable to hypothesize that different avian bone ‘pathologies’ that are triggered by diverse factors (pathogens, traumas, etc.), grow at different rates, and exhibit a variety of microstructures, could have different chemical compositions. It is thus absolutely necessary to test the techniques used to chemically identify KS on a larger and more diverse sample of avian pathological bone tissues. Preliminary analyses carried out in our laboratory revealed that alcian blue, HID and the anti-KS antibody 5D4 reacted positively with some avian pathological bones, but not the surrounding cortical tissue [74]. These results suggest that (i) some avian pathological bone may contain KS; therefore, KS may not be used as a unique molecular marker of MB; and (ii) these chemical analyses may not be reliable to unambiguously discriminate MB from pathological tissues in fossil specimens. In addition to ruling out obvious pathologies in specimens for which MB is claimed, more diagnostic assays are thus required. As of yet, a unique identifier of MB that does not appear in any pathologies has yet to be found.
(k). Criterion #11: ‘have a mineral to collagen ratio significantly greater than that of cortical bone’
Although this statement is true at certain stages of the egg-laying cycle, this is not due to a higher mineral concentration, but rather to a lower collagen content in MB than in cortical bone [9,10], because non-collagenous proteins and proteoglycans constitute a larger percentage of the organic phase in MB than in cortical bone. However, several studies have shown that the degree of mineralization of cortical bone is usually higher than, or similar to, MB [9,10,19,88]. This is consistent with CT observations showing that MB is always as or less dense to the X-rays than the surrounding cortical bone tissue (see, for example, virtual sections in [8,34]; see also figures 2 and 5 in present study).
We argue that the mineral to collagen ratio of MB greatly varies during the egg-laying cycle and criterion #11 might thus be difficult to evaluate in fossil specimens. Indeed, several studies reported that the MB matrix formation (and initial mineralization) and its subsequent increasing calcification occur at different periods of the cycle and that the degree of mineralization (mineral concentration) of MB greatly varies throughout the cycle [19,89].
4. Concluding remarks and perspectives
For all studies attempting to determine if an unknown tissue-type observed in a fossil specimen can be classified as MB, it is critical to establish a rigorous hypothesis-driven framework that explicitly weighs MB against all alternative hypotheses and takes into account all data—(e.g. gross morphology, microstructure, chemistry). The key to such determination is to establish criteria specific enough to exclusively characterize MB, alternative tissue types (such as various pathologies, cortical or trabecular bone), or some unique combination thereof, and purposely refuting alternative hypotheses. Specifically, it is not sufficient to determine that MB is present because several criteria regarding its microstructure and chemical composition are met. The present work demonstrates that, unfortunately, many criteria that are representative of MB, can also be found in other tissue types. Thus, alternative hypotheses regarding the origin of endosteal tissues (e.g. pathology) must also be rejected to confidently identify the presence of MB. Similarly problematic, some studies refute the identification of MB when features that characterize some, but not all, examples of MB are observed. However, we find that many of the criteria previously proposed as necessary for the identification of MB are not present in MB of some extant avialans and thus the absence of some features may not be a suitable reason for rejecting a hypothesis of MB.
In short, the morphological and chemical diversity of MB, its taxonomically variable skeletal distribution, ephemeral nature and poor characterization among extant birds create complications for identifying consistent and unique characteristics of MB, underscoring the need for additional research in both neontological and paleontological communities. For example, more neontological work is needed to better document the microstructural, chemical and depositional variations of MB phylogenetically, and individually, both during the egg-laying cycle and throughout adulthood of a female individual. The same attention is needed to develop criteria that can distinguish MB from pathological bone and can be used to reject these hypotheses, as opposed to traits that variably characterize both tissues. We suggest the complexity of identifying MB in the fossil record outlined herein creates a wealth of opportunities for future research. Such studies would add greatly to the establishment of a refined set of criteria to reassess hypotheses on the nature of MB-like tissues in extinct avemetatarsalians and on the origin and evolution of this unique tissue.
5. Material and methods
(a). Biological sample
Here, we only present a subset of the data collected for a large comparative study aiming at better documenting the phylogenetic distribution and microstructural variability of MB in Neornithes.
To further document the phylogenetic distribution of MB within Neornithes and reassess the results of Maclean [58] and Piersma et al. [59], we collected female sandpiper specimens that died of natural causes during the egg-laying cycle from two Calidris species (the rock sandpiper C. ptilocnemis, and the pectoral sandpiper C. melanotos), as well as conspecific males (as controls; table 1).
Two bone pathological specimens were also analysed in order to compare the skeletal distribution pattern and overall microstructure of such tissues with MB. These specimens constitute the humerus of a non-breeding female of the European herring gull Larus argentatus NCSM-19601 and the ulna of a Northern bobwhite female Colinus virginianus TMM-M6536 that died during the egg-laying cycle.
Finally, to illustrate some of the MB microstructural diversity encountered in Neornithes, we present the hindlimb bone cross-sections of a few female specimens that died during the egg-laying cycle (table 2). These specimens represent various bird groups with different body sizes and ecologies.
(b). Methods
The postcranial skeletons of each Calidris specimen listed in table 1 were submitted to CT-scanning observations to record the presence/absence of MB. Indeed, several studies have shown that CT-scanning allows discrimination of MB from other tissue types (trabecular, cortical, pathological bone tissues, bone marrow contents, etc.) based on differential relative density to the X-rays, distribution pattern, and overall microstructure (e.g. [34]).
All Calidris specimens, as well as the two pathological specimen, were scanned with a high-resolution micro-CT scanner (Nikon XTH 225 ST) at the Shared Materials Instrumentation Facility of the Duke University, Durham, NC, USA. The resolution of our micro-CT scans ranged from about 28.7 to 38 µm for the Calidris specimens and from 31.4 to 49.9 µm for the pathological specimens. In some cases, the CT-scan resolution was not high enough to clearly discriminate between potential trace amounts of MB and bone marrow content in our Calidris sample. We thus subjected some skeletal elements to nano-CT scan examination using the Zeiss Xradia 510 Versa microscope housed at the Analytical Instrumentation Facility of the North Carolina State University, Raleigh, NC, USA. The resolution of our nano-CT scans ranged from about 2.17 to 3.03 µm and revealed new microstructural details. Data were imported into Avizo Lite (v. 9.0.0) for visualization. All CT scans supporting the results of this article are available in the MorphoSource repository, under project # P873 at https://www.morphosource.org [90].
Finally, the bone histology of all specimens listed in table 2 was analysed using decalcified paraffin sections and chemical staining (with alcian blue or HID, two stains commonly used to discriminate MB from the surrounding cortical and trabecular bone tissues; e.g. [18]), using the protocols detailed in Schweitzer et al. [42] and Canoville et al. [34].
Acknowledgements
The authors are grateful to the different (assistant) curators and colleagues for facilitating access to bird specimens: Brian O'Shea and John Gerwin (North Carolina Museum of Natural Sciences, Raleigh, NC); Timothy B. Rowe, Julia Clarke and J. Chris Sagebiel (Jackson School Museum of Earth History, Austin, TX); Kevin Winker and Jack Withrow (University of Alaska Museum, Fairbanks, AK); Helen James and Christopher Milensky (National Museum of Natural History, Smithsonian Institution, Washington, DC); Holly Woodward (Oklahoma State University Center for Health Sciences, Tulsa, USA). Our thanks also go to Wenxia Zheng (Biological Science Department, North Carolina State University, Raleigh, NC) for sharing her practical knowledge on paraffin thin sectioning and chemical staining techniques. Justin Gladman (Shared Materials Instrumentation Facility, Duke University, Durham, NC) and Roberto Garcia, Hanhan Zhou, Ching-Chang Chung and Toby Tung (Analytical Instrumentation Facility, North Carolina State University, Raleigh, NC) are acknowledged for their assistance with micro- and nano-CT scanning, respectively. We would also like to thank the guest editors Adam Huttenlocker and Jorge Cubo for inviting us to contribute to the ‘Vertebrate Palaeophysiology’ issue of Philosophical Transactions B. Finally, we are grateful to two anonymous reviewers for their constructive comments and suggestions that improved our manuscript.
Data accessibility
Most of the new data are provided in the tables and figures of the article. All CT scans supporting the results of this article are available in the MorphoSource repository, under project # P873 at https://www.morphosource.org [90].
Authors' contributions
A.C., M.H.S. and L.E.Z. designed the study. A.C. collected and analysed the data. A.C., L.E.Z. and M.H.S. wrote the manuscript and approved its final version.
Competing interests
The authors declare that they have no competing interests.
Funding
This research was funded by a National Science Foundation award # 1552328 and Brimley Postdoctoral Fellowship.
References
- 1.Simkiss K. 1961. Calcium metabolism and avian reproduction. Biol. Rev. 36, 321–359. ( 10.1111/j.1469-185X.1961.tb01292.x) [DOI] [Google Scholar]
- 2.Dacke CG, Arkle S, Cook DJ, Wormstone IM, Jones S, Zaidi M, Bascal ZA. 1993. Medullary bone and avian calcium regulation. J. Exp. Biol. 184, 63–88. [Google Scholar]
- 3.Kyes P, Potter TS. 1934. Physiological marrow ossification in female pigeons. Anat. Rec. 60, 377–379. ( 10.1002/ar.1090600402) [DOI] [Google Scholar]
- 4.Zondek B. 1936. Impairment of anterior pituitary functions by follicular hormone. Lancet 228, 842–847. ( 10.1016/S0140-6736(00)48290-5) [DOI] [Google Scholar]
- 5.Pfeiffer CsA, Gardner WU. 1938. Skeletal changes and blood serum calcium level in pigeons receiving estrogens. Endocrinology 23, 485–491. ( 10.1210/endo-23-4-485) [DOI] [Google Scholar]
- 6.Gardner WU, Pfeiffer CA. 1943. Influence of estrogens and androgens on the skeletal system. Physiol. Rev. 23, 139–165. ( 10.1152/physrev.1943.23.2.139) [DOI] [Google Scholar]
- 7.Landauer W, Zondek B. 1944. Observations on the structure of bone in estrogen-treated cocks and drakes. Am. J. Pathol. 20, 179. [PMC free article] [PubMed] [Google Scholar]
- 8.Squire ME, Veglia MK, Drucker KA, Brazeal KR, Hahn TP, Watts HE. 2017. Estrogen levels influence medullary bone quantity and density in female house finches and pine siskins. Gen. Comp. Endocrinol. 246, 249–257. ( 10.1016/j.ygcen.2016.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knott L, Bailey AJ. 1999. Collagen biochemistry of avian bone: comparison of bone type and skeletal site. Brit. Poultry Sci. 40, 371–379. ( 10.1080/00071669987476) [DOI] [PubMed] [Google Scholar]
- 10.Dacke CG, Sugiyama T, Gay CV. 2015. The role of hormones in the regulation of bone turnover and eggshell calcification. In Sturkie's avian physiology (ed. Scanes CG.), pp. 549–575. New York, NY: Academic Press; ( 10.1016/B978-0-12-407160-5.00025-7) [DOI] [Google Scholar]
- 11.Candlish JK, Holt FJ. 1971. The proteoglycans of fowl cortical and medullary bone. Comp. Biochem. Physiol. B Biochem. 40, 283–290. ( 10.1016/0305-0491(71)90084-8) [DOI] [PubMed] [Google Scholar]
- 12.Bonucci E, Gherardi G. 1975. Histochemical and electron microscope investigations on medullary bone. Cell Tissue Res. 163, 81–97. ( 10.1007/BF00218592) [DOI] [PubMed] [Google Scholar]
- 13.Fisher LW, Schraer H. 1980. The glycosaminoglycans of estrogen-induced medullary bone in Japanese quail. Arch. Biochem. Biophys. 205, 396–403. ( 10.1016/0003-9861(80)90122-8) [DOI] [PubMed] [Google Scholar]
- 14.Schraer H, Hunter SJ. 1985. The development of medullary bone: a model for osteogenesis. Comp. Biochem. Physiol. A Physiol. 82, 13–17. ( 10.1016/0300-9629(85)90697-8) [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Nagai H. 1992. A histochemical study of acid phosphatases in medullary bone matrix and osteoclasts in laying Japanese quail. J. Bone Miner. Res. 7, 1267–1273. ( 10.1002/jbmr.5650071121) [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Nagai H. 1994. Tartrate-resistant acid phosphatase accumulated in the matrix of developing medullary bone induced by estrogen treatment of male Japanese quail. J. Bone Miner. Res. 9, 1153–1157. ( 10.1002/jbmr.5650090804) [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Nakamura H, Tsuji T, Hirata A. 2001. Ultracytochemical study of medullary bone calcification in estrogen injected male Japanese quail. Anat. Rec. 264, 25–31. ( 10.1002/ar.1101) [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Nagaoka N, Hirata A, Nakamura H, Inoue M, Kawai M, Ikegame M. 2005. Ultrastructural and immunohistochemical studies of medullary bone calcification, with special reference to sulphated glycosaminoglycans. J. Electron Microsc. 54, 29–34. ( 10.1093/jmicro/dfh097) [DOI] [PubMed] [Google Scholar]
- 19.Kerschnitzki M, Zander T, Zaslansky P, Fratzl P, Shahar R, Wagermaier W. 2014. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone 69, 109–117. ( 10.1016/j.bone.2014.08.019) [DOI] [PubMed] [Google Scholar]
- 20.Miller SC, Bowman BM. 1981. Medullary bone osteogenesis following estrogen administration to mature male Japanese quail. Dev. Biol. 87, 52–63. ( 10.1016/0012-1606(81)90060-9) [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer MH, Elsey RM, Dacke CG, Horner JR, Lamm ET. 2007. Do egg-laying crocodilian (Alligator mississippiensis) archosaurs form medullary bone? Bone 40, 1152–1158. ( 10.1016/j.bone.2006.10.029) [DOI] [PubMed] [Google Scholar]
- 22.Reynolds SJ, Perrins CM. 2010. Dietary calcium availability and reproduction in birds. Curr. Ornithol. 17, 31–74. ( 10.1007/978-1-4419-6421-2_2) [DOI] [Google Scholar]
- 23.Prondvai E. 2017. Medullary bone in fossils: function, evolution and significance in growth curve reconstructions of extinct vertebrates. J. Evol. Biol. 30, 440–460. ( 10.1111/jeb.13019) [DOI] [PubMed] [Google Scholar]
- 24.Currey JD, Alexander RM. 1985. The thickness of the walls of tubular bones. J. Zool. 206, 453–468. ( 10.1111/j.1469-7998.1985.tb03551.x) [DOI] [Google Scholar]
- 25.Habib MB, Ruff CB. 2008. The effects of locomotion on the structural characteristics of avian limb bones. Zool. J. Linnean Soc. 153, 601–624. ( 10.1111/j.1096-3642.2008.00402.x) [DOI] [Google Scholar]
- 26.Canoville A, de Buffrénil V, Laurin M.. 2016. Microanatomical diversity of amniote ribs: an exploratory quantitative study. Biol. J. Linnean Soc. 118, 706–733. ( 10.1111/bij.12779) [DOI] [Google Scholar]
- 27.Jackson DC, Andrade DV, Abe AS. 2003. Lactate sequestration by osteoderms of the broad-nose caiman, Caiman latirostris, following capture and forced submergence. J. Exp. Biol. 206, 3601–3606. ( 10.1242/jeb.00611) [DOI] [PubMed] [Google Scholar]
- 28.Clarac F, De Buffrénil V, Cubo J, Quilhac A.. 2018. Vascularization in ornamented osteoderms: physiological implications in ectothermy and amphibious lifestyle in the crocodylomorphs? Anat. Rec. 301, 175–183. ( 10.1002/ar.23695) [DOI] [PubMed] [Google Scholar]
- 29.Clarac F, Goussard F, de Buffrénil V, Sansalone V.. 2019. The function(s) of bone ornamentation in the crocodylomorph osteoderms: a biomechanical model based on a finite element analysis. Paleobiology 45, 182–200. ( 10.1017/pab.2018.48) [DOI] [Google Scholar]
- 30.Clarac F, Scheyer TM, Desojo JB, Cerda IA, Sanchez S. 2020. The evolution of dermal shield vascularisation in Testudinata and Pseudosuchia: phylogenetic constraints versus ecophysiological adaptations. Phil. Trans. R. Soc. B 375, 20190132 ( 10.1098/rstb.2019.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarac F, Quilhac A. 2019. The crocodylian skull and osteoderms: a functional exaptation to ectothermy? Zoology 132, 31–40. ( 10.1016/j.zool.2018.12.001) [DOI] [PubMed] [Google Scholar]
- 32.Dacke CG, Elsey RM, Trosclair PL, Sugiyama T, Nevarez JG, Schweitzer MH. 2015. Alligator osteoderms as a source of labile calcium for eggshell formation. J. Zool. 297, 255–264. ( 10.1111/jzo.12272) [DOI] [Google Scholar]
- 33.Werning S. 2018. Medullary bone is phylogenetically widespread and its skeletal distribution varies by taxon. J. Ornithol. 159, 527–543. ( 10.1007/s10336-017-1514-z) [DOI] [Google Scholar]
- 34.Canoville A, Schweitzer MH, Zanno LE. 2019. Systemic distribution of medullary bone in the avian skeleton: ground truthing criteria for the identification of reproductive tissues in extinct Avemetatarsalia. BMC Evol. Biol. 19, 71 ( 10.1186/s12862-019-1402-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinsamy A, Codorniú L, Chiappe L. 2009. Palaeobiological implications of the bone histology of Pterodaustro guinazui. Anat. Rec. 292, 1462–1477. ( 10.1002/ar.20990) [DOI] [PubMed] [Google Scholar]
- 36.Prondvai E, Stein KH. 2014. Medullary bone-like tissue in the mandibular symphyses of a pterosaur suggests non-reproductive significance. Sci. Rep. 4, 6253 ( 10.1038/srep06253) [DOI] [Google Scholar]
- 37.Lee AH, Werning S. 2008. Sexual maturity in growing dinosaurs does not fit reptilian growth models. Proc. Natl Acad. Sci. USA 105, 582–587. ( 10.1073/pnas.0708903105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hübner TR. 2012. Bone histology in Dysalotosaurus lettowvorbecki (Ornithischia: Iguanodontia)–variation, growth, and implications. PLoS ONE 7: e29958 ( 10.1371/journal.pone.0029958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerda IA, Pol D. 2013. Evidence for gender-specific reproductive tissue in a basal sauropodomorph dinosaur from the Late Triassic of Argentina. Ameghiniana 50, 11–12R. [Google Scholar]
- 40.Chinsamy A, Cerda I, Powell J. 2016. Vascularised endosteal bone tissue in armoured sauropod dinosaurs. Sci. Rep. 6, 24858 ( 10.1038/srep24858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweitzer MH, Wittmeyer JL, Horner JR. 2005. Gender-specific reproductive tissue in ratites and Tyrannosaurus rex. Science 308, 1456–1460. ( 10.1126/science.1112158) [DOI] [PubMed] [Google Scholar]
- 42.Schweitzer MH, Zheng W, Zanno L, Werning S, Sugiyama T. 2016. Chemistry supports the identification of gender-specific reproductive tissue in Tyrannosaurus rex. Sci. Rep. 6, 23099 ( 10.1038/srep23099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skutschas PP, Boitsova EA, Averianov AO, Sues H-D. 2017. Ontogenetic changes in long-bone histology of an ornithomimid theropod dinosaur from the Upper Cretaceous Bissekty Formation of Uzbekistan. Hist. Biol. 29, 715–729. ( 10.1080/08912963.2016.1233180) [DOI] [Google Scholar]
- 44.Chinsamy A, Chiappe LM, Marugán-Lobón J, Chunling G, Fengjiao Z. 2013. Gender identification of the Mesozoic bird Confuciusornis sanctus. Nat. Commun. 4, 1381 ( 10.1038/ncomms2377) [DOI] [PubMed] [Google Scholar]
- 45.O'Connor JK. 2017. Definitive occurrence of medullary bone in an enantiornithine (Aves: Ornithothoraces). In 4th Int. Symp. Paleohistology (eds Pellegrini RA, Parris DC), p. 78 Trenton, NJ: New Jersey State Museum. [Google Scholar]
- 46.O'Connor J, Erickson GM, Norell M, Bailleul AM, Hu H, Zhou Z. 2018. Medullary bone in an Early Cretaceous enantiornithine bird and discussion regarding its identification in fossils. Nat. Commun. 9, 5169 ( 10.1038/s41467-018-07621-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailleul AM, O'Connor J, Zhang S, Li Z, Wang Q, Lamanna MC, Zhu X, Zhou Z. 2019. An Early Cretaceous enantiornithine (Aves) preserving an unlaid egg and probable medullary bone. Nat. Commun. 10, 1275 ( 10.1038/s41467-019-09259-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith NA, Clarke JA. 2014. Osteological histology of the Pan-Alcidae (Aves, Charadriiformes): correlates of wing-propelled diving and flightlessness. Anat. Rec. 297, 188–199. ( 10.1002/ar.22841) [DOI] [PubMed] [Google Scholar]
- 49.Angst D, Chinsamy A, Steel L, Hume JP. 2017. Bone histology sheds new light on the ecology of the dodo (Raphus cucullatus, Aves, Columbiformes). Sci. Rep. 7, 7993 ( 10.1038/s41598-017-08536-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573. ( 10.1038/nature15697) [DOI] [PubMed] [Google Scholar]
- 51.Brusatte SL, O'Connor JK, Jarvis ED. 2015. The origin and diversification of birds. Curr. Biol. 25, R888-R898. ( 10.1016/j.cub.2015.08.003) [DOI] [PubMed] [Google Scholar]
- 52.Varricchio DJ, Jackson FD. 2003. Origins of avian reproduction: answers and questions from dinosaurs. Palaeovertebrata 32, 149–169. [Google Scholar]
- 53.Brusatte SL, Lloyd GT, Wang SC, Norell MA. 2014. Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Curr. Biol. 24, 2386–2392. ( 10.1016/j.cub.2014.08.034) [DOI] [PubMed] [Google Scholar]
- 54.Pan Y, et al. 2019. The molecular evolution of feathers with direct evidence from fossils. Proc. Natl Acad. Sci. USA 116, 3018–3023. ( 10.1073/pnas.1815703116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chinsamy A, Tumarkin-Deratzian A. 2009. Pathologic bone tissues in a turkey vulture and a nonavian dinosaur: implications for interpreting endosteal bone and radial fibrolamellar bone in fossil dinosaurs. Anat. Rec. 292, 1478–1484. ( 10.1002/ar.20991) [DOI] [PubMed] [Google Scholar]
- 56.Kato KM, Rega E, Sidor C, Huttenlocker A. 2020. Investigation of a bone lesion in a gorgonopsian (Synapsida) from the Permian of Zambia and periosteal reactions in fossil non-mammalian tetrapods. Phil. Trans. R. Soc. B 375, 20190144 ( 10.1098/rstb.2019.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Werning S, Schweitzer M, Padian K. 2016. Microstructure isn't enough: additional diagnostic criteria to test among hypotheses of bone tissue identity. Anat. Rec. 299, 76. [Google Scholar]
- 58.MacLean SF. 1974. Lemming bones as a source of calcium for arctic sandpipers (Calidris spp .). Ibis 116, 552–557. ( 10.1111/j.1474-919X.1974.tb07653.x) [DOI] [Google Scholar]
- 59.Piersma T, Gudmundsson GA, Davidson NC, Morrison RG. 1996. Do arctic-breeding Red Knots (Calidris canutus) accumulate skeletal calcium before egg laying? Can. J. Zool. 74, 2257–2261. ( 10.1139/z96-257) [DOI] [Google Scholar]
- 60.O'Connor PM. 2009. Evolution of archosaurian body plans: skeletal adaptations of an air-sac based breathing apparatus in birds and other archosaurs. J. Exp. Zool. A: Ecol. Genet. Physiol. 311, 629–646. ( 10.1002/jez.548) [DOI] [PubMed] [Google Scholar]
- 61.Matthiesen DG. 1988. Preceramic animal use on the central coast. In Economic prehistory of the Central Andes (B.A.R. International Series 427) (eds Wing ES, Wheeler JC), pp. 18–30. Oxford, UK: BAR Publishing. [Google Scholar]
- 62.Meister W. 1962. Histological structure of the long bones of penguins. Anat. Rec. 143, 377–387. ( 10.1002/ar.1091430408) [DOI] [PubMed] [Google Scholar]
- 63.Ksepka DT, Werning S, Sclafani M, Boles ZM. 2015. Bone histology in extant and fossil penguins (Aves: Sphenisciformes). J. Anat. 227, 611–630. ( 10.1111/joa.12367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams AJ. 1981. Why do penguins have long laying intervals? Ibis 123, 202–204. ( 10.1111/j.1474-919X.1981.tb00925.x) [DOI] [Google Scholar]
- 65.Cadena EA, Schweitzer MH. 2014. A Pelomedusoid turtle from the Paleocene–Eocene of Colombia exhibiting preservation of blood vessels and osteocytes. J. Herpetol. 48, 461–465. ( 10.1670/13-046) [DOI] [Google Scholar]
- 66.Schweitzer MH, Zheng W, Cleland TP, Bern M. 2013. Molecular analyses of dinosaur osteocytes support the presence of endogenous molecules. Bone 52, 414–423. ( 10.1016/j.bone.2012.10.010) [DOI] [PubMed] [Google Scholar]
- 67.Lindgren J, et al. 2011. Microspectroscopic evidence of Cretaceous bone proteins. PLoS ONE 6, e19445 ( 10.1371/journal.pone.0019445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindgren J, et al. 2014. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 506, 484–488. ( 10.1038/nature12899) [DOI] [PubMed] [Google Scholar]
- 69.Lindgren J, et al. 2018. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 564, 359–365. ( 10.1038/s41586-018-0775-x) [DOI] [PubMed] [Google Scholar]
- 70.Bloom MA, McLean FC, Bloom W. 1942. Calcification and ossification. The formation of medullary bone in male and castrate pigeons under the influence of sex hormones. Anat. Rec. 83, 99–120. ( 10.1002/ar.1090830108) [DOI] [Google Scholar]
- 71.Kusuhara S, Schraer H. 1982. Cytology and autoradiography of estrogen-induced differentiation of avian endosteal cells. Calcif. Tissue Int. 34, 352–358. ( 10.1007/BF02411267) [DOI] [PubMed] [Google Scholar]
- 72.Ascenzi A, Francois C, Bocciarelli DS. 1963. On the bone induced by estrogens in birds. J. Ultrastruct. Res. 8, 491–505. ( 10.1016/S0022-5320(63)80051-9) [DOI] [PubMed] [Google Scholar]
- 73.Bloom W, Bloom MA, McLean FC. 1941. Calcification and ossification. Medullary bone changes in the reproductive cycle of female pigeons. Anat. Rec. 81, 443–475. ( 10.1002/ar.1090810404) [DOI] [Google Scholar]
- 74.Canoville A, Zanno LE, Zheng W-X, Schweitzer MH. 2018. New data on the skeletal distribution, microstructure, and chemistry of medullary bone in Neornithes – paleobiological implications. In 5th International Paleontological Congress – Abstract Book, p. 912.
- 75.Chinsamy A, Chiappe LM, Dodson P. 1995. Mesozoic avian bone microstructure: physiological implications. Paleobiology 21, 561–574. ( 10.1017/S0094837300013543) [DOI] [Google Scholar]
- 76.de Ricqlès A, Padian K, Horner JR. 2001. The bone histology of basal birds in phylogenetic and ontogenetic perspectives. In New Perspectives on the Origin and Early Evolution of Birds: Proc. Int. Symp. in Honor of John H. Ostrom (eds Gauthier J, Gall LF), pp. 411–426. Newhaven, CT: Peabody Museum of Natural History. [Google Scholar]
- 77.Erickson GM, Rauhut OW, Zhou Z, Turner AH, Inouye BD, Hu D, Norell MA. 2009. Was dinosaurian physiology inherited by birds? Reconciling slow growth in Archaeopteryx. PLoS ONE 4, e7390 ( 10.1371/journal.pone.0007390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bourdon E, Castanet J, De Ricqlès A, Scofield P, Tennyson A, Lamrous H, Cubo J.. 2009. Bone growth marks reveal protracted growth in New Zealand kiwi (Aves, Apterygidae). Biol. Lett. 5, 639–642. ( 10.1098/rsbl.2009.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchell J, Legendre LJ, Lefèvre C, Cubo J. 2017. Bone histological correlates of soaring and high-frequency flapping flight in the furculae of birds. Zoology 122, 90–99. ( 10.1016/j.zool.2017.03.004) [DOI] [PubMed] [Google Scholar]
- 80.Foote JS. 1916. A contribution to the comparative histology of the femur (Vol. 35, No. 3). Washington, DC: Smithsonian; ( 10.5962/bhl.title.83960) [DOI] [Google Scholar]
- 81.Simkiss K. 1967. Calcium in reproductive physiology. London, UK: Chapman and Hall Ltd. [Google Scholar]
- 82.Ricklefs RE. 1968. Patterns of growth in birds. Ibis 110, 419–451. ( 10.1111/j.1474-919X.1968.tb00058.x) [DOI] [Google Scholar]
- 83.Erickson GM. 2005. Assessing dinosaur growth patterns: a microscopic revolution . Trends Ecol. Evol. 20, 677–684. ( 10.1016/j.tree.2005.08.012) [DOI] [PubMed] [Google Scholar]
- 84.Erickson GM, Curry Rogers K, Varricchio DJ, Norell MA, Xu X. 2007. Growth patterns in brooding dinosaurs reveals the timing of sexual maturity in non-avian dinosaurs and genesis of the avian condition. Biol. Lett. 3, 558–561. ( 10.1098/rsbl.2007.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woodward HN, Freedman Fowler EA, Farlow JO, Horner JR. 2015. Maiasaura, a model organism for extinct vertebrate population biology: a large sample statistical assessment of growth dynamics and survivorship. Paleobiology 41, 503–527. ( 10.1017/pab.2015.19) [DOI] [Google Scholar]
- 86.Erickson GM, Makovicky PJ, Currie PJ, Norell MA, Yerby SA, Brochu CA. 2004. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature 430, 772–775. ( 10.1038/nature02699) [DOI] [PubMed] [Google Scholar]
- 87.Banes AJ, Smith RE. 1977. Biological characterization of avian osteopetrosis. Infect. Immun. 16, 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shipov A, Sharir A, Zelzer E, Milgram J, Monsonego-Ornan E, Shahar R. 2010. The influence of severe prolonged exercise restriction on the mechanical and structural properties of bone in an avian model. Vet. J. 183, 153–160. ( 10.1016/j.tvjl.2008.11.015) [DOI] [PubMed] [Google Scholar]
- 89.Van de Velde JP, Vermeiden JPW, Bloot AM.. 1985. Medullary bone matrix formation, mineralization, and remodeling related to the daily egg-laying cycle of Japanese quail: a histological and radiological study. Bone 6, 321–327. ( 10.1016/8756-3282(85)90322-9) [DOI] [PubMed] [Google Scholar]
- 90.Canoville A, Schweitzer MH, Zanno LE.. 2019. Data from: Identifying medullary bone in extinct avemetatarsalians: challenges, implications, and perspectives. MorphoSource repository, project # P873. See https://www.morphosource.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Canoville A, Schweitzer MH, Zanno LE.. 2019. Data from: Identifying medullary bone in extinct avemetatarsalians: challenges, implications, and perspectives. MorphoSource repository, project # P873. See https://www.morphosource.org.
Data Availability Statement
Most of the new data are provided in the tables and figures of the article. All CT scans supporting the results of this article are available in the MorphoSource repository, under project # P873 at https://www.morphosource.org [90].