Abstract
Teleosauridae and Metriorhynchidae were thalattosuchian crocodylomorph clades that secondarily adapted to marine life and coexisted during the Middle to Late Jurassic. While teleosaurid diversity collapsed at the end of the Jurassic, most likely as a result of a global cooling of the oceans and associated marine regressions, metriorhynchid diversity was largely unaffected, although the fossil record of Thalattosuchia is poor in the Cretaceous. In order to investigate the possible differences in thermophysiologies between these two thalattosuchian lineages, we analysed stable oxygen isotope compositions (expressed as δ18O values) of tooth apatite from metriorhynchid and teleosaurid specimens. We then compared them with the δ18O values of coexisting endo-homeothermic ichthyosaurs and plesiosaurs, as well as ecto-poikilothermic chondrichthyans and osteichthyans. The distribution of δ18O values suggests that both teleosaurids and metriorhynchids had body temperatures intermediate between those of typical ecto-poikilothermic vertebrates and warm-blooded ichthyosaurs and plesiosaurs, metriorhynchids being slightly warmer than teleosaurids. We propose that metriorhynchids were able to raise their body temperature above that of the ambient environment by metabolic heat production, as endotherms do, but could not maintain a constant body temperature compared with fully homeothermic ichthyosaurs and plesiosaurs. Teleosaurids, on the other hand, may have raised their body temperature by mouth-gape basking, as modern crocodylians do, and benefited from the thermal inertia of their large body mass to maintain their body temperature above the ambient one. Endothermy in metriorhynchids might have been a by-product of their ecological adaptations to active pelagic hunting, and it probably allowed them to survive the global cooling of the Late Jurassic, thus explaining the selective extinction affecting Thalattosuchia at the Jurassic–Cretaceous boundary.
This article is part of the theme issue ‘Vertebrate palaeophysiology'.
Keywords: Metriorhynchidae, Teleosauridae, Jurassic, thermophysiology, oxygen and carbon isotopes, tooth apatite
1. Introduction
Extant crocodylians are archosaurs that rely on environmental sources of heat in order to raise and maintain their body temperature by behavioural thermoregulation in a restricted range bracketed by ‘critical minimum' and ‘critical maximum' temperatures [1,2]. Within this critical range, crocodylians tend to keep their body temperature within a narrower activity range from about 25°C to 40°C as determined empirically for a few extant species (see Markwick [3] for a review). Consequently, the spatial and temporal distribution of crocodylians is limited by the temperatures of their living environments and by their seasonal fluctuations. Owing to their rather conservative growth morphology and their restricted latitudinal distribution today, and in the geologic record, extant and fossil representatives of the crown group have been used as climate proxies for more than a century [3–6]. Based on observations of extant representatives of crown-group Crocodylia [7], Markwick [3] proposed that the occurrence of fossil representatives implies a living mean annual air temperature ≥ 14.2°C and a coldest month mean temperature ≥ 5.5°C. These minimum limits have been tentatively used to constrain the climatic environment of long-extinct crocodylomorphs [8], as well as the more distantly related clade Choristodera [9].
The crocodylomorph clade Thalattosuchia became secondarily adapted to marine life during the Mesozoic, and is subdivided into the families Teleosauridae [10] and Metriorhynchidae [11]. While teleosaurids retained a morphology reminiscent of typical semi-aquatic longirostrine crocodylomorphs, in having extensive osteoderm coverage and limbs adapted for terrestrial locomotion [12], metriorhynchids had a more hydrodynamic body plan, with a hypocercal tail, and hydrofoil-like forelimbs [13,14]. The bone histology of Callovian teleosaurids and metriorhynchids has been used to hypothesize that thalattosuchians were ectothermic (i.e. they relied on environmental sources of heat in order to raise their body temperature) and poikilothermic (i.e. their body temperature varied along with that of their environment), with teleosaurids being capable of mouth-gape basking on shore, whereas metriorhynchids thermoregulated differently by staying close to the water surface [15]. Hua & de Buffrénil [15] did note that young metriorhynchids may have had a faster growth rate than wild extant crocodylians.
Martin et al. [16] analysed the diversity of marine crocodylomorphs through the Mesozoic and Palaeogene, and observed a significant correlation between sea surface temperatures (SSTs) and generic diversity within four lineages, including the teleosaurids. Teleosaurids, at least European representatives, experienced a diversity crash at the end of the Jurassic during a global cooling of Tethyan waters [17], but according to Fanti et al. [18] they may have continued to survive until at least the Hauterivian along the southern coast of the Tethys Sea. Metriorhynchids, however, appear to have experienced an explosive radiation during the Callovian, surviving through the Late Jurassic until the Aptian [19]. When metriorhynchids became extinct is currently unclear. Young et al. [14] hypothesized a two-step extinction for Metriorhynchidae: first, a diversity crash at the end Jurassic, then a final extinction during the cold icehouse interval of the Valanginian [20]. However, recent re-evaluations of Cretaceous fossils and updated phylogenetic studies have disproved both steps of this hypothesis, with post-Valanginian metriorhynchid specimens known and no fewer than four metriorhynchid lineages that crossed the Jurassic–Cretaceous boundary [19,21,22]. The global thalattosuchian fossil record, however, is still poor; the known diversity of this clade is still heavily biased by the European rock record, as well as global marine record sampling biases for specific time spans (e.g. Aalenian, Oxfordian, Early Cretaceous). Regardless of the geological megabiases, there is still a conspicuous diversity mismatch within Thalattosuchia, which raised the question whether metriorhynchids evolved a distinct thermoregulatory strategy, such as endothermic capabilities, that would explain their diversity and survival under cool SSTs [16].
In order to investigate the thermophysiology of metriorhynchids, we analysed the oxygen isotope composition of the enamel phosphate of their teeth (δ18Op). Indeed, the δ18Op value of vertebrate apatite (the mineral constituting bone, teeth and some fish scales) depends on the animal's body water δ18Obw value, as well as its body temperature [23–25]. For air-breathing vertebrates, the body water has a δ18Obw value controlled by oxygen input coming from drinking water, food and inhaled oxygen (through metabolic water production), as well as oxygen loss as water vapour through transcutaneous evaporation, sweat, exhaled vapour, and liquid water in urine and faeces, some of these losses being associated with oxygen isotope fractionation [23,26]. A fractionation equation that relates the apatite phosphate δ18Op value to that of body water and body temperature (Tb) can be adapted from the phosphate-water temperature scale previously established by Longinelly & Nuti [24] and recently updated by Lécuyer et al. [27]. Such an equation has proven to be valid for a large range of invertebrate and vertebrate bioapatites [24,28–30]:
| 1.1 |
This relationship is commonly used to reconstitute past SSTs based on fish apatite, as most fish have a body temperature similar to that of their surrounding water, and a body water δ18Obw value equal to the ambient one [17,20,31,32]. Air-breathing vertebrates have a body water 18O-enriched relative to environmental water, the magnitude of which depends on the amount of body water loss through exhaled water vapour and transcutaneous evaporation. Direct measurements have shown that terrestrial mammals and birds have body water from about 4 to 7‰ 18O-enriched relative to their drinking water [33–35], whereas the body waters of semi-aquatic to aquatic crocodylians, turtles and birds are about 2 to 3‰ 18O-enriched relative to their drinking water [33,36,37].
In this study, we estimate the body temperatures of metriorhynchid and teleosaurid thalattosuchians recovered from five Jurassic localities in England and France. By comparing Tb of thalattosuchians with those of associated ichthyosaurs or plesiosaurs, as well as with their ambient SST, we show that metriorhynchids may have evolved some degree of endothermic-like thermophysiology, whereas teleosaurids retained a more typical ecto-poikilothermy.
2. Material and methods
(a). Sample collection
We analysed 88 fossil teeth of Jurassic fish and marine reptiles for their oxygen isotope composition of phosphate (δ18Op), as well as for their oxygen (δ18Oc) and carbon (δ13Cc) isotope composition of apatite carbonate. The first English locality is Smallmouth Sands, Dorset (figure 1), from the lower part of the Kimmeridge Clay Formation, dated as lower Kimmeridgian [39]. The second English locality is the Oxford Clay Formation Peterborough clay pits, a world-famous fossiliferous collection of sites, dated to the middle Callovian, which has yielded a rich marine fauna along with terrestrial elements [40]. The first French locality is Les Vaches Noires of Normandy, dated as upper Callovian, where the Marnes de Dives Formation crops out, consisting of marls where a rich fauna composed of marine and terrestrial elements has been recovered [41]. The second French locality is the excavation of Les Lourdines near the eponymous quarry, dated as middle Callovian, which is composed of white limestone (calcaire des Lourdines), where a marine fauna and some terrestrial plants have been recovered [42]. Finally, the locality of Cintheaux, dated as Bathonian, consists of the Pierre de Caen limestone, which has yielded a marine fauna with some terrestrial elements [43]. Studied tooth specimens include those of metriorhynchid and teleosaurid thalattosuchians, ichthyosaurs and plesiosaurs, as well as of chondrichthyans and osteichthyans (electronic supplementary material, table S1). When possible, tooth enamel was sampled using a spherical diamond-tipped drill bit. For smaller teeth, the bulk enamel and dentine were ground using an agate mortar and pestle.
Figure 1.
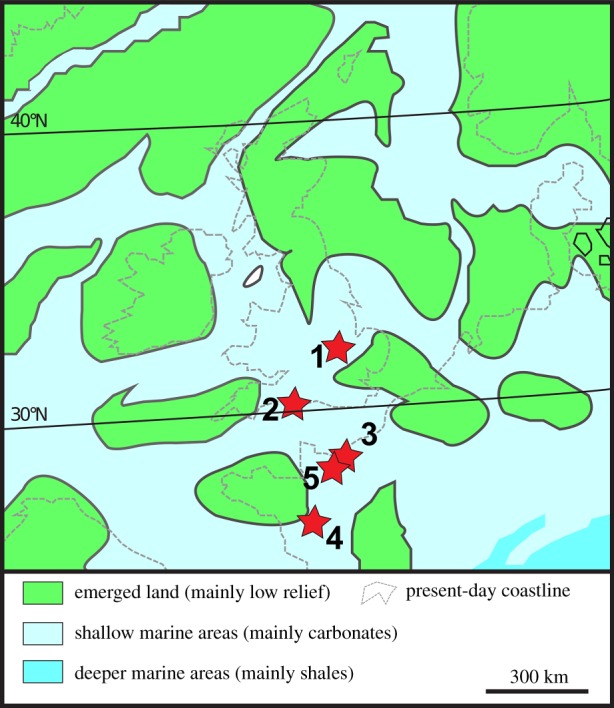
Palaeogeography of northwestern Europe during the Middle–Late Jurassic (modified from Pross et al. [38]). The red stars show the studied localities. 1: Peterborough; 2: Smallmouth Sands; 3: Les Vaches Noires; 4: Les Lourdines excavation; 5: Cintheaux.
(b). Analytical techniques
(i). Oxygen isotope analysis of biogenic apatite phosphate
Apatite powders were treated following the wet chemistry protocol described by Crowson et al. [44] and slightly modified by Lécuyer et al. [45]. This protocol consists in the isolation of phosphate from apatite as silver phosphate (Ag3PO4) crystals using acid dissolution and anion-exchange resin. For each sample, 20–30 mg of enamel powder was dissolved in 2 ml of 2 M HF. The CaF2 residue was separated by centrifugation and the solution was neutralized by adding 2.2 ml of 2 M KOH. Amberlite™ IRN78 anion-exchange resin beads were added to the solution to isolate the ions. After 24 h, the solution was removed, and the resin was rinsed with deionized water and eluted with 27.5 ml of 0.5 M NH4NO3. After 4 h, 0.5 ml of NH4OH and 15 ml of an ammoniacal solution of AgNO3 were added and the solutions were placed in a thermostated bath at 70°C for 7 h, allowing the precipitation of Ag3PO4 crystals. Oxygen isotope compositions were measured using a high-temperature elemental analyser interfaced in continuous flow mode to an isotopic ratio mass spectrometer [46] at the Plateforme d'Ecologie Isotopique du Laboratoire d'Ecologie des Hydrosystèmes Naturels et Anthropisés (LEHNA, UMR5023, Université Claude Bernard Lyon 1). For each sample, five aliquots of 300 μg of Ag3PO4 were mixed with 300 μg of pure graphite powder loaded in silver foil capsules. Pyrolysis was performed at 1450°C using a vario PYRO cube™ elemental analyser (Elementar, Germany) interfaced in continuous flow mode with an Isoprime™ isotopic ratio mass spectrometer (Elementar UK). Measurements have been calibrated against silver phosphate precipitated from the NBS120c (natural Miocene phosphorite from Florida), as well as against the NBS127 (barium sulfate precipitated using seawater from Monterey Bay, California, USA). The value of NBS120c was fixed at 21.7‰ (Vienna standard mean ocean water, V-SMOW) according to Lécuyer et al. [45], and that of NBS127 set at the certified value of 9.3‰ (V-SMOW; see [47,48]) for correction of instrumental mass fractionation during CO isotopic analysis. Silver phosphate precipitated from standard NBS120c along with the silver phosphate samples derived from fossil bioapatites was repeatedly analysed (δ18Op = 21.72 ± 0.22‰, n = 20) to ensure that no fractionation occurred during the wet chemistry. Data are reported as δ18O values with respect to V-SMOW (in ‰ δ units).
(ii). Oxygen and carbon isotope analysis of biogenic apatite carbonate
In order to remove potential organic contaminants as well as secondarily precipitated calcite, about 10 mg of apatite powder was pre-treated following the protocol of Koch et al. [49]. Powders were washed with a 2% NaOCl solution to remove organic matter, then rinsed five times with double deionized water and air-dried at 40°C for 24 h. Acetic acid (0.1 M) was then added and left for 24 h, after which the powder was again rinsed five times with double deionized water and then air-dried at 40°C for 24 h. The powder/solution ratio was kept constant at 0.04 g ml−1 for both treatments. Stable isotope compositions of carbonate oxygen and carbon were carried out at the Plateforme d'Ecologie Isotopique du Laboratoire d'Ecologie des Hydrosystèmes Naturels et Anthropisés (LEHNA, UMR5023). The measurements were performed using an isoFLOW system connected on-line in continuous flow mode to a precisION mass spectrometer (Elementar UK). For each sample, two aliquots of 2 mg of pretreated apatite powder were loaded in LABCO Exetainer® 3.7 ml soda glass vials, round bottomed with Exetainer caps (LABCO UK), and were reacted with anhydrous phosphoric acid. The reaction took place at 90°C in a temperature regulated sample tray. The CO2 gas generated during the acid digestion of the carbonate sample was then transferred to the mass spectrometer via the centrION interface. Calibrated CO2 gas was used as the monitoring gas. Typical reproducibilites are 0.05 and 0.07‰, respectively, for δ13C and δ18O measurements. For tooth apatite, the acid fractionation factor α (CO2–apatite carbonate) of 1.00773 determined for the NBS120c phosphate rock reference material was selected [50]. The calibrated materials used were Carrara marble (δ18OV-PDB = −1.84‰, δ13CV-PDB = +2.03‰ [51]), NBS18 (δ18OV-PDB = −23.2‰, δ13CV-PDB = −5.01‰) and NBS120c (δ18OV-PDB = −1.13‰, δ13CV-PDB = −6.27‰ [50]), Isotopic compositions are quoted in the standard δ notation relative to V-SMOW for oxygen and V-PDB (Vienna Pee Dee belemnite) for carbon.
3. Results
Oxygen isotope compositions of apatite phosphate (δ18Op) and apatite carbonate (δ18Oc), and carbon isotope compositions of apatite carbonate (δ13Cc) are reported in electronic supplementary material, table S1 along with published δ18Op values of teeth and bones of marine vertebrates [52–54]. Analysed teeth have δ18Op values ranging from 18.3 to 21.8‰ V-SMOW, δ18Oc values ranging from 24.1 to 28.7‰ V-SMOW and δ13CC values ranging from −11.2 to 7.7‰ V-PDB. At the three sites of Smallmouth Sands, Peterborough and Les Vaches Noires, ichthyosaurs and plesiosaurs have slightly lower mean δ18OP values than those of co-occurring thalattosuchians (electronic supplementary material, table S2).
For each locality, SST was calculated from the δ18Op values of fish using equation (1.1) (electronic supplementary material, table S2), considering that Tb ≈ Tsw, and that δ18Obw ≈ δ18Osw [28]. Because average seawater δ18Osw value may have varied between −1 and 0‰ V-SMOW depending on the amount of seawater stored as polar ice [55], an average value of −0.5‰ was arbitrarily selected for temperature calculation, keeping in mind that the associated error in temperature calculation is about 2.3°C (based on the slope of 4.5 of equation (1.1)).
The body temperatures of studied marine reptiles have also been estimated using equation (1.1) and assuming a seawater–body water 18O-enrichment of 2‰, a general enrichment previously observed among semi-aquatic and aquatic air-breathing vertebrates, including extant crocodylomorphs [33,36,37]. While ichthyosaurs and plesiosaurs have a body temperature within the 32–40°C range compatible with their known endo-homeothermy [53], teleosaurids have lower Tb ranging from 27 to 31°C, and metriorhynchids show intermediate body temperatures ranging from 29 to 37°C (electronic supplementary material, table S2).
4. Discussion
(a). Original preservation of the stable isotope compositions
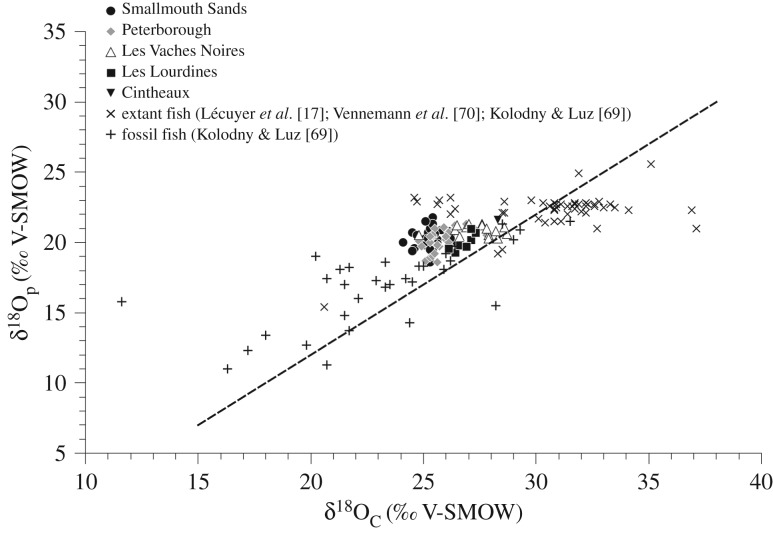
Before discussing the thermophysiological significance of the oxygen and carbon isotope compositions of vertebrate apatites, pristine preservation of the isotopic record needs to be assessed. Indeed, biotic and abiotic processes leading to the decomposition, burial and fossilization of living organisms may alter the original isotopic composition of bioapatite through processes of secondary precipitation, ion adsorption or dissolution–recrystallization of bioapatite [56–61]. Although no method can definitely demonstrate whether the original isotope compositions have been kept, several ways to assess the preservation state of the isotopic record have been proposed [57,58,61–65]. In modern skeletal tissues of vertebrates, carbonate and phosphate precipitate close to equilibrium with body water, so the δ18Op and δ18Oc values are positively correlated. Because isotopic exchange rates between carbonate-water and phosphate-water are significantly different, re-equilibration of both compounds during diagenesis is not expected and altered enamel should show isotopic shifts from the empirical δ18Op–δ18Oc line. Therefore, it is expected that the distribution of pristine δ18O values of fish and reptile tooth enamel should follow a line with a slope close to unity, mimicking those established between the δ18Oc and δ18Op values of modern mammals [61,63,66–68]. Despite the narrow range in the distribution of oxygen isotope compositions (figure 2), both δ18Oc and δ18Op values fall within the range observed in extant and fossil marine vertebrates [17,69,70]. This correlation shows that the oxygen isotope compositions of structural carbonate in both tooth and bone apatites have preserved to a certain degree their original record.
Figure 2.
Oxygen isotope compositions of tooth phosphate reported against their corresponding oxygen isotope composition of structural carbonate, as well as published values from modern and fossil fish for comparison [17,69,70]. The dashed line with a slope a = 1 illustrates the correlation between oxygen isotope composition of phosphate and carbonate.
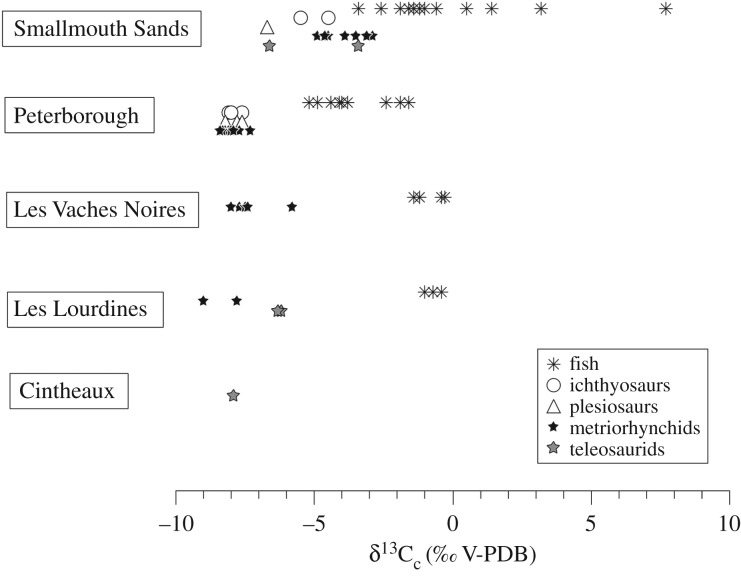
A clue to the primary preservation of stable carbon isotope composition of apatite carbonate is the systematic and significant difference in δ13Cc values between fish and marine reptiles (figure 3). Air-breathing reptiles have δ13Cc values mainly reflecting those of their diets, with an isotope fractionation that depends on their digestive physiology [71], as expected. In aquatic environments, the relationship between fish carbonate and diet δ13C values is complicated as a substantial amount of the carbon may be derived from dissolved inorganic carbon in their ambient water [72,73] with a higher δ13C value [74]. Finally, the weight percentage of carbonate in analysed fossil apatites (electronic supplementary material, table S1) lies within the expected biological range of modern vertebrate apatites of 2–13% [70,75,76]. From these lines of evidence, we can assume that oxygen isotope compositions of apatite phosphate and carbonate have kept at least a significant part of their original information, and might be interpreted in terms of seawater temperature and thermophysiology.
Figure 3.
Carbon isotope compositions of apatite carbonates (δ13Cc) from fish and marine reptile samples. For each locality, the sizable difference between fish and coexisting marine reptiles' δ13Cc values is considered evidence for primary preservation of the stable isotope compositions of studied specimens.
(b). Body temperature reconstruction
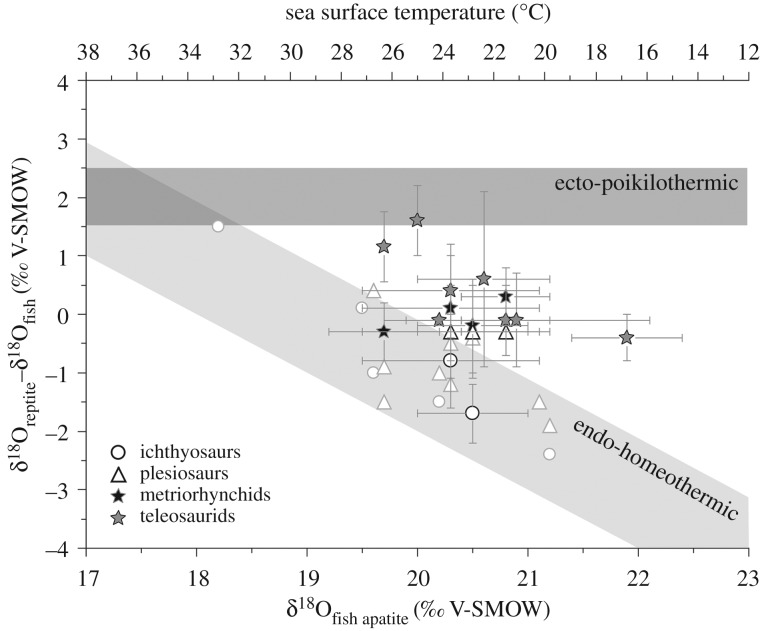
In a previous study of marine reptile thermophysiology, Bernard et al. [53] interpreted the oxygen isotope differences between marine reptiles and coexisting fish from a large range of water temperatures (estimated from 14 to 34°C) in terms of Tb differences. They concluded that the three lineages Ichthyosauria, Plesiosauria and Mosasauridae were most likely endothermic and homeothermic marine reptiles, although for mosasaurids this was later debated [77,78]. The method used in Bernard et al. [53] cannot clearly identify the thermophysiology of thalattosuchians (figure 4). Indeed, the available sample-set of thalattosuchian specimens is restricted to five localities with a narrow range of low palaeolatitudes from about 29° to 36° N (electronic supplementary material, table S2; palaeolatitudes calculated using the online application of van Hinsbergen et al. [79]), as well as a narrow range of estimated palaeotemperatures from 22 ± 2 to 27 ± 2°C. Consequently, the sampled sites do not allow the two strategies of thermophysiology to be clearly distinguished. Most metriorhynchids and teleosaurids show intermediate values between the expected range for endotherms and ectotherms, with the exception of the metriorhynchids from the middle Callovian of Les Lourdines, which fall within the expected range of endotherms, and one teleosaurid from the Bathonian of Cintheaux, which has a typical ectotherm signature (figure 4).
Figure 4.
Model variation of the differences in the δ18Op values of tooth phosphate between marine reptiles and fish against the variation of the δ18Op values of fish teeth, assuming (1) an ectothermic and poikilothermic reptile (body water δ18Obw values 2‰ enriched relative to seawater value, and body temperature (T) equal to seawater temperature); (2) an endothermic reptile with body temperature ranging from 35 to 39°C and body water 2‰ enriched relative to a seawater value ranging from −1 to 0‰ (modified from [53]). For comparison, metriorhynchid and teleosaurid values are reported, along with newly measured (black band) and published (grey band) ichthyosaur and plesiosaur values.
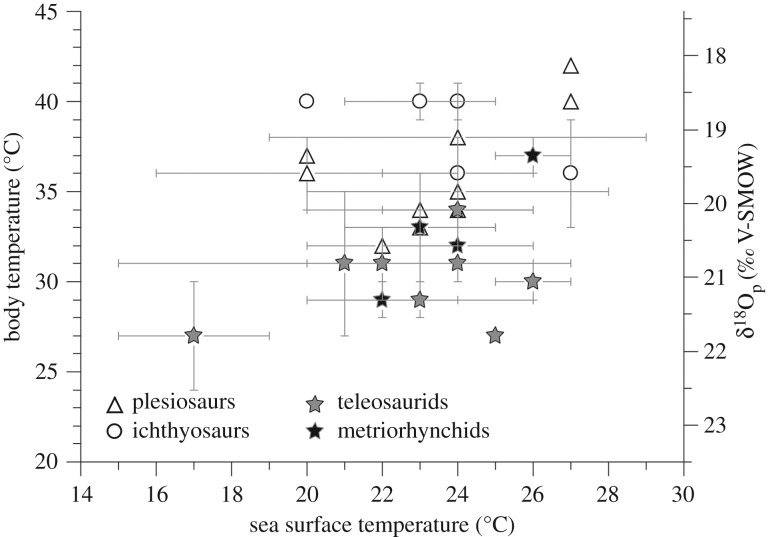
Using equation (1.1) and assuming that thalattosuchians have a δ18Obw value about 2‰ more positive than their ambient seawater, both metriorhynchids and teleosaurids have a body temperature above their environmental one, metriorhynchids being slightly warmer than teleosaurids on average (electronic supplementary material, table S2; figure 5). However, thalattosuchian Tb values are systematically below the calculated ones of co-occurring ichthyosaurs and plesiosaurs, for which the body temperature was calculated from the same equation (1.1) and using a body water δ18Obw value similar to that of thalattosuchians.
Figure 5.
Estimated body temperature of marine reptiles (left axis) and corresponding δ18Op values (right axis) are plotted against their environmental sea surface temperature estimated from fish δ18Op values.
An endothermic-like thermophysiology interpreted from metriorhynchid δ18Op values seems likely according to their known morphology and ecology as active pelagic predators [15,80–84]. Active predation would require elevated metabolic rates compatible with an endothermic thermophysiology. This seems plausible considering the large suite of evidence for an endothermic ancestral condition for Archosauria and a reversal within the crocodylomorph lineage to an ectothermic state coming from different fields of biology, including developmental biology [85], physiology [86], anatomy [87], palaeohistology [88] and phylogenetic signal extraction [89]. Endothermy within metriorhynchids might have been inherited from their archosaur ancestors, and ‘reactivated' along with the acquisition of morphological adaptations to active pelagic predation. However, body temperature regulation seems to have been limited as observed Tb values vary with varying seawater temperature (figure 5). Limited thermoregulatory capacities would be compatible with the restricted range of palaeolatitudinal occurrences compared with fully endo-homeothermic ichthyosaurs and plesiosaurs having been found from equatorial to polar seas [90].
Interpretation of the oxygen isotope composition of teleosaurid apatite in terms of body temperature may be strongly biased by their possible semi-aquatic and eurhyaline ecology, some species having been found in estuarine or freshwater environments [91–94]. As most teleosaurids retained a typical semi-aquatic crocodylomorph morphology (external mandibular fenestrae, extensive osteoderm cover and limbs adapted to terrestrial locomotion), it is hypothesized that they were ectothermic and poikilothermic ambush predators spending most of their time motionless, and mouth-gape basking like modern crocodylians. However, during the late Kimmeridgian–early Tithonian there is evidence for a subclade of teleosaurids that became more pelagic [95]. The oxygen isotope composition of teleosaurid apatite indicates that they kept a body temperature lower than those of ichthyosaurs and plesiosaurs, but close to those of metriorhynchids. However, more estuarine living environments can be characterized by more negative δ18O values as a result of the mixing between seawater and river waters having negative δ18O values such as in the case of San Francisco Bay [96]. In this example, waters from the Sacramento River having δ18O values ranging from about −12 to −10‰ mix with seawater of 0 and result in estuarine waters that can have δ18O values of −3 to −10‰. Keeping this in mind, then the calculated body temperature of teleosaurids living in estuarine environments would be lower, and their apatite δ18O values would fit within the expected range of ectotherms. Moreover, the possible semi-aquatic lifestyle of teleosaurids could at least partly account for their elevated δ18Op values as a result of body water loss through transcutaneous evaporation. In the studied localities where teleosaurid specimens were found, fossil remains of continental vertebrates and plants have been found, indicating a proximity to landmasses and a possible estuarine origin of teleosaurids. Gigantothermy and behavioural thermoregulation may also account for the calculated body temperatures of teleosaurids close to those of metriorhynchids. Today, large marine crocodylians, such as approximately 1000 kg adult individuals of the saltwater crocodile (Crocodylus porosus), are able to raise their body temperature well above ambient temperatures through mouth-gape basking behaviours and can retain this elevation by the thermal inertia of their large body size [97,98]. Large teleosaurids may have used a similar behavioural thermoregulation as extant marine crocodiles (C. porosus) and would have raised their body temperatures close to that of Jurassic metriorhynchids and maintained it within a narrow range. This would explain the apparent tendency of teleosaurid-fish isotope difference to parallel those of endo-homeothermic ichthyosaurs and plesiosaurs (figure 4). Most metriorhynchid specimens were of smaller body-size than teleosaurids [99,100], and metriorhynchids would have been unable to mouth-gape bask onshore [14].
Based on the available isotopic dataset, it seems likely that teleosaurids retained a typical ecto-poikilothermic thermophysiology in agreement with their morphology and ecology, whereas metriorhynchids may have been endothermic, being able to raise their body temperature above the ambient one, and close to that of other warm-blooded marine reptiles. However, metriorhynchids could not have achieved efficient thermoregulation, as suggested from their varying body temperature along with varying SST.
5. Concluding remarks
The possible difference in thermophysiologies between metriorhynchids and teleosaurids inferred from their stable oxygen isotope composition of apatite can at least partly explain the peculiar thalattosuchian biodiversity pattern of the Jurassic and Early Cretaceous [16,22]. Teleosaurid diversity crashed at the end of the Jurassic, a time of global marine temperature decline, probably as a result of the temperature change or the global regression affecting their ecological niches [90]. Endothermy may have helped metriorhynchids to cope with global cooling, and owing to their pelagic ecology, they might not have been as affected by the marine regressions as teleosaurids. This may explain their success across the Jurassic–Cretaceous boundary [19,22,101]. At this point, we cannot speculate when metriorhynchids became extinct, or why. All we can say is that Jurassic metriorhynchids had an imperfect endothermic and poikilothermic thermophysiology. We hope future studies will investigate whether the Late Jurassic pelagic subclade of teleosaurids began to develop endothermic capabilities, and test whether Cretaceous metriorhynchids, the most marine-adapted of all thalattosuchians [14,102], evolved towards enhanced thermoregulatory abilities.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank the Hunterian Museum of Glasgow, Mr and Mrs Pennetier for providing material from England and France, Gilles Cuny for his constructive comments and the two anonymous reviewers for their constructive comments.
Data accessibility
Stable oxygen and carbon isotope compositions are provided in Excel tables as electronic supplementary material.
Authors' contributions
All authors contributed to the study and the writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
N.S. and R.A. were financially supported by the ANR OXYMORE. M.T.Y. is financially supported by Leverhulme Trust Research Project grant no. RPG-2017-167.
References
- 1.Cowles RB, Bogert CM. 1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus. Nat. Hist. 83, 261–296. [Google Scholar]
- 2.Pough FH, Gans C. 1982. The vocabulary of reptilian thermoregulation. In Physiology C. Physiological Ecology. Biology of the Reptilia, vol. 12 (eds Gans C, Pough FH), pp. 17–23. London: Academic Press. [Google Scholar]
- 3.Markwick PJ. 1998. Fossil crocodilians as indicators of Late Cretaceous and Cenozoic climates: implications for using palaeontological data in reconstructing palaeoclimate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 137, 205–271. ( 10.1016/S0031-0182(97)00108-9) [DOI] [Google Scholar]
- 4.Berg DE. 1965. Krokodile als Klimazeugen [Crocodile as climate indicator]. Geol. Rundsch. 54, 328–333. [In German, with an English abstract.] ( 10.1007/BF01821186) [DOI] [Google Scholar]
- 5.Crichton A. 1825. On the climate of the antediluvian world and its independence of solar influence: and on the formation of granite. Ann. Phil. 9, 207–217. [Google Scholar]
- 6.Owen R. 1850. On the fossil Crocodilia of England. Edinb. New Philos. J. 49, 248–250. [Google Scholar]
- 7.Owen R. 1842. Report on British Fossil Reptiles. Part II. Rep. Br. Assoc. Adv. Sci. Plymouth Meet. 1841, 60–240. [Google Scholar]
- 8.Amiot R, et al. 2011. Oxygen isotopes of East Asian dinosaurs reveal exceptionally cold Early Cretaceous Climates. Proc. Natl Acad. Sci. USA 108, 5179–5183. ( 10.1073/pnas.1011369108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarduno JA, Brinkman DB, Renne PR, Cottrell RD, Scher H, Castillo P. 1998. Evidence for extreme climatic warmth from Late Cretaceous Arctic vertebrates. Science 282, 2241–2244. ( 10.1126/science.282.5397.2241) [DOI] [PubMed] [Google Scholar]
- 10.Saint-Hilaire G. 1831. Recherches sur de grands sauriens trouvés à l’état fossile aux confins maritimes de la Basse-Normandie, attribués d'abord au crocodile, puis déterminés sous les noms de Teleosaurus et Steneosaurus. [Research on large fossil saurians found in marine deposits of Lower Normandy, previously attributed to crocodilians and then determined under the names of Teleosaurus and Steneosaurus]. Mém. Acad. Sci. 12, 1–138. [In French.] [Google Scholar]
- 11.Fitzinger L. 1843. Systema Reptilium, Fasciculus Primus, Amblyglossae. Vienna: Braumüller et Seidel. [Google Scholar]
- 12.Eudes-Deslongchamps E. 1869. Notes Paléontologiques. Caen, France: Le Blanc Hardel & Savy. [Google Scholar]
- 13.Fraas E. 1902. Die Meer-Krocodilier (Thalattosuchia) des oberen Jura unter specieller Berücksichtigung von Dacosaurus und Geosaurus. [The sea crocodiles (Thalattosuchia) of the upper Jurassic, with special consideration of Dacosaurus and Geosaurus]. Palaeontographica 49, 1–72. [In German.] [Google Scholar]
- 14.Young MT, Brusatte SL, Ruta M, de Andrade MB. 2010. The evolution of Metriorhynchoidea (Mesoeucrocodylia, Thalattosuchia): an integrated approach using geometric morphometrics, analysis of disparity, and biomechanics. Zool. J. Linn. Soc. 158, 801–859. ( 10.1111/j.1096-3642.2009.00571.x) [DOI] [Google Scholar]
- 15.Hua S, De Buffrénil V. 1996. Bone histology as a clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia). J. Vertebr. Paleontol. 16, 703–717. ( 10.1080/02724634.1996.10011359) [DOI] [Google Scholar]
- 16.Martin JE, Amiot R, Lécuyer C, Benton MJ. 2014. Sea surface temperature contributes to marine crocodilian evolution. Nat. Commun. 5, 4658 ( 10.1038/ncomms5658) [DOI] [PubMed] [Google Scholar]
- 17.Lécuyer C, Picard S, Garcia J-P, Sheppard SM, Grandjean P, Dromart G. 2003. Thermal evolution of Tethyan surface waters during the Middle-Late Jurassic: evidence from δ18O values of marine fish teeth. Paleoceanogr. Paleoclimatol. 18, 1076–1091. ( 10.1029/2002PA000863) [DOI] [Google Scholar]
- 18.Fanti F, Miyashita T, Cantelli L, Mnasri F, Dridi J, Contessi M, Cau A. 2016. The largest thalattosuchian (Crocodylomorpha) supports teleosaurid survival across the Jurassic-Cretaceous boundary. Cret. Res. 61, 263–274. ( 10.1016/j.cretres.2015.11.011) [DOI] [Google Scholar]
- 19.Chiarenza AA, Foffa D, Young MT, Insacco G, Cau A, Carnevale G, Catanzariti R. 2015. The youngest record of metriorhynchid crocodylomorphs, with implications for the extinction of Thalattosuchia. Cret. Res. 56, 608–616. ( 10.1016/j.cretres.2015.07.001) [DOI] [Google Scholar]
- 20.Pucéat E, Lécuyer C, Sheppard SM, Dromart G, Reboulet S, Grandjean P. 2003. Thermal evolution of Cretaceous Tethyan marine waters inferred from oxygen isotope composition of fish tooth enamels. Paleoceanogr. Paleoclimatol. 18, 1029 ( 10.1029/2002PA000823) [DOI] [Google Scholar]
- 21.Ősi A, Young MT, Galácz A, Rabi M. 2018. A new large-bodied thalattosuchian crocodyliform from the Lower Jurassic (Toarcian) of Hungary, with further evidence of the mosaic acquisition of marine adaptations in Metriorhynchoidea. PeerJ 6, e4668 ( 10.7717/peerj.4668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young MT, de Andrade MB, Cornée J-J, Steel L, Foffa D. 2014. Re-description of a putative Early Cretaceous ‘teleosaurid’ from France, with implications for the survival of metriorhynchids and teleosaurids across the Jurassic-Cretaceous Boundary. Ann. Paléontol. 100, 165–174. ( 10.1016/j.annpal.2014.01.002) [DOI] [Google Scholar]
- 23.Kohn MJ. 1996. Predicting animal δ18O: accounting for diet and physiological adaptation. Geochim. Cosmochim. Acta 60, 4811–4829. ( 10.1016/S0016-7037(96)00240-2) [DOI] [Google Scholar]
- 24.Longinelli A, Nuti S. 1973. Revised phosphate-water isotopic temperature scale. Earth Planet. Sci. Lett. 19, 373–376. ( 10.1016/0012-821X(73)90088-5) [DOI] [Google Scholar]
- 25.Luz B, Kolodny Y, Horowitz M. 1984. Fractionation of oxygen isotopes between mammalian bone-phosphate and environmental drinking water. Geochim. Cosmochim. Acta 48, 1689–1693. ( 10.1016/0016-7037(84)90338-7) [DOI] [Google Scholar]
- 26.Langlois C, Simon L, Lécuyer C. 2003. Box-modeling of bone and tooth phosphate oxygen isotope compositions as a function of environmental and physiological parameters. Isotopes Environ. Health Stud. 39, 259–272. ( 10.1080/10256010310001621146) [DOI] [PubMed] [Google Scholar]
- 27.Lécuyer C, Amiot R, Touzeau A, Trotter J. 2013. Calibration of the phosphate δ18O thermometer with carbonate–water oxygen isotope fractionation equations. Chem. Geol. 347, 217–226. ( 10.1016/j.chemgeo.2013.03.008) [DOI] [Google Scholar]
- 28.Kolodny Y, Luz B, Navon O. 1983. Oxygen isotope variations in phosphate of biogenic apatites, I. Fish bone apatite—rechecking the rules of the game. Earth Planet. Sci. Lett. 64, 398–404. ( 10.1016/0012-821X(83)90100-0) [DOI] [Google Scholar]
- 29.Lécuyer C, Grandjean P, Emig C. 1996. Determination of oxygen isotope fractionation between water and phosphate from living lingulids: potential application to palaeoenvironmental studies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 126, 101–108. ( 10.1016/S0031-0182(96)00073-9) [DOI] [Google Scholar]
- 30.Longinelli A, Nuti S. 1973. Oxygen isotope measurements of phosphate from fish teeth and bones. Earth Planet. Sci. Lett. 20, 337–340. ( 10.1016/0012-821X(73)90007-1) [DOI] [Google Scholar]
- 31.Dera G, Pucéat E, Pellenard P, Neige P, Delsate D, Joachimski MM, Reisberg L, Martinez M. 2009. Water mass exchange and variations in seawater temperature in the NW Tethys during the Early Jurassic: evidence from neodymium and oxygen isotopes of fish teeth and belemnites. Earth Planet. Sci. Lett. 286, 198–207. ( 10.1016/j.epsl.2009.06.027) [DOI] [Google Scholar]
- 32.Picard S, Garcia J-P, Lécuyer C, Sheppard SMF, Cappetta H, Emig C. 1998. δ18O values of coexisting brachiopods and fish: temperature differences and estimates of paleo–water depths. Geology 26, 975–978. () [DOI] [Google Scholar]
- 33.Lazzerini N, et al. 2016. Oxygen isotope fractionation between bird eggshell calcite and body water: application to fossil eggs from Lanzarote (Canary Islands). Sci. Nat. 103, 81 ( 10.1007/s00114-016-1404-x) [DOI] [PubMed] [Google Scholar]
- 34.Longinelli A. 1984. Oxygen isotopes in mammal bone phosphate: a new tool for paleohydrological and paleoclimatological research? Geochim. Cosmochim. Acta 48, 385–390. ( 10.1016/0016-7037(84)90259-X) [DOI] [Google Scholar]
- 35.Wolf N, Newsome SD, Fogel ML, Del Rio CM. 2013. The relationship between drinking water and the hydrogen and oxygen stable isotope values of tissues in Japanese quail (Cortunix japonica). Auk 130, 323–330. ( 10.1525/auk.2013.12075) [DOI] [Google Scholar]
- 36.Amiot R, Lécuyer C, Escarguel G, Billon-Bruyat J-P, Buffetaut E, Langlois C, Martin S, Martineau F, Mazin J-M. 2007. Oxygen isotope fractionation between crocodilian phosphate and water. Palaeogeogr. Palaeoclimatol. Palaeoecol. 243, 412–420. ( 10.1016/j.palaeo.2006.08.013) [DOI] [Google Scholar]
- 37.Barrick RE, Fischer AG, Showers WJ. 1999. Oxygen isotopes from turtle bone: applications for terrestrial paleoclimates? Palaios 14, 186–191. ( 10.2307/3515374) [DOI] [Google Scholar]
- 38.Pross J, Link E, Ruf M, Aigner T. 2006. Delineating sequence stratigraphic patterns in deeper ramp carbonates: quantitative palynofacies data from the Upper Jurassic (Kimmeridgian) of southwest Germany. J. Sediment. Res. 76, 524–538. ( 10.2110/jsr.2006.031) [DOI] [Google Scholar]
- 39.Young MT, Steel L, Middleton H. 2014. Evidence of the metriorhynchid crocodylomorph genus Geosaurus in the Lower Kimmeridge Clay Formation (Late Jurassic) of England. Hist. Biol. 26, 551–555. ( 10.1080/08912963.2013.801468) [DOI] [Google Scholar]
- 40.Martill DM, Hudson JD (eds) 1991. Fossils of the Oxford clay, palaeontological association field guide to fossils. London, UK: The Palaeontological Association. [Google Scholar]
- 41.Lebrun P, Courville P. 2013. Le Jurassique des falaises des Vaches-noires [The Jurassic of the Vaches-noires cliffs]. Fossiles Hors Ser. 4, 16–33. [In French.] [Google Scholar]
- 42.Barale G, Cariou E, Radureau G. 1974. Etude biostratigraphique et paléobotanique des gisements de calcaire blanc callovien au Nord de Poitiers [Biostratigraphic and palaeobotanical study of Callovian white limestone deposits north of Poitiers]. Geobios 7, 43–69. [In French with an English abstract.] ( 10.1016/S0016-6995(74)80018-5) [DOI] [Google Scholar]
- 43.Rioult M. 1963. Le Calcaire de Caen, dépôt de rivage du Bathonien normand [The Caen limestone, a shore deposit of the Normandy Bathonian]. Bull. Soc. Linn. Normandie 3, 119–141. [In French.] [Google Scholar]
- 44.Crowson RA, Showers WJ, Wright EK, Hoering TC. 1991. Preparation of phosphate samples for oxygen isotope analysis. Anal. Chem. 63, 2397–2400. ( 10.1021/ac00020a038) [DOI] [Google Scholar]
- 45.Lécuyer C, Grandjean P, O'Neil JR, Cappetta H, Martineau F. 1993. Thermal excursions in the ocean at the Cretaceous–Tertiary boundary (northern Morocco): δ18O record of phosphatic fish debris. Palaeogeogr. Palaeoclimatol. Palaeoecol. 105, 235–243. ( 10.1016/0031-0182(93)90085-W) [DOI] [Google Scholar]
- 46.Fourel F, Martineau F, Lécuyer C, Kupka H-J, Lange L, Ojeimi C, Seed M. 2011. 18O/16O ratio measurements of inorganic and organic materials by elemental analysis–pyrolysis–isotope ratio mass spectrometry continuous-flow techniques. Rapid Commun. Mass Spectrom. 25, 2691–2696. ( 10.1002/rcm.5056) [DOI] [PubMed] [Google Scholar]
- 47.Halas S, Szaran J. 2001. Improved thermal decomposition of sulfates to SO2 and mass spectrometric determination of δ34S of IAEA SO-5, IAEA SO-6 and NBS-127 sulfate standards. Rapid Commun. Mass Spectrom. 15, 1618–1620. ( 10.1002/rcm.416) [DOI] [Google Scholar]
- 48.Hut G. 1987. Consultants' group meeting on stable isotope reference samples for geochemical and hydrological investigations. IAEA, Vienna 16 - 18 September 1985, Report to the Director General. See http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/18/075/18075746.pdf.
- 49.Koch PL, Tuross N, Fogel ML. 1997. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J. Archaeol. Sci. 24, 417–429. ( 10.1006/jasc.1996.0126) [DOI] [Google Scholar]
- 50.Passey BH, Cerling TE, Levin NE. 2007. Temperature dependence of oxygen isotope acid fractionation for modern and fossil tooth enamels. Rapid Commun. Mass Spectrom. 21, 2853–2859. ( 10.1002/rcm.3149) [DOI] [PubMed] [Google Scholar]
- 51.Fourel F, Martineau F, Tóth EE, Görög A, Escarguel G, Lécuyer C. 2016. Carbon and oxygen isotope variability among Foraminifera and ostracod carbonated shells. Ann. Univ. Mariae Curie-Sklodowska AAA Physica 70, 133–156. [Google Scholar]
- 52.Anderson TF, Popp BN, Williams AC, Ho L-Z, Hudson JD. 1994. The stable isotopic records of fossils from the Peterborough Member, Oxford Clay Formation (Jurassic), UK: palaeoenvironmental implications. J. Geol. Soc. 151, 125–138. ( 10.1144/gsjgs.151.1.0125) [DOI] [Google Scholar]
- 53.Bernard A, et al. 2010. Regulation of body temperature by some Mesozoic marine reptiles. Science 328, 1379–1382. ( 10.1126/science.1187443) [DOI] [PubMed] [Google Scholar]
- 54.Billon-Bruyat J-P, Lécuyer C, Martineau F, Mazin J-M. 2005. Oxygen isotope compositions of Late Jurassic vertebrate remains from lithographic limestones of western Europe: implications for the ecology of fish, turtles, and crocodilians. Palaeogeogr. Palaeoclimatol. Palaeoecol. 216, 359–375. ( 10.1016/j.palaeo.2004.11.011) [DOI] [Google Scholar]
- 55.Shackleton NJ, Kennett JP. 1975. Paleotemperature history of the Cenozoic and the initiation of Antarctic glaciation: oxygen and carbon isotope analyses in DSDP sites 277, 279 and 281. DSDP Initial Rep. Deep Sea Drill. Proj. 29, 743–756. ( 10.2973/dsdp.proc.29.117.1975) [DOI] [Google Scholar]
- 56.Blake RE, O'Neil JR, Garcia GA. 1997. Oxygen isotope systematics of biologically mediated reactions of phosphate: I. Microbial degradation of organophosphorus compounds. Geochim. Cosmochim. Acta 61, 4411–4422. ( 10.1016/S0016-7037(97)00272-X) [DOI] [Google Scholar]
- 57.Kolodny Y, Luz B, Sander M, Clemens WA. 1996. Dinosaur bones: fossils or pseudomorphs? The pitfalls of physiology reconstruction from apatitic fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 126, 161–171. ( 10.1016/S0031-0182(96)00112-5) [DOI] [Google Scholar]
- 58.Lécuyer C, Bogey C, Garcia J-P, Grandjean P, Barrat JA, Floquet M, Bardet N, Pereda-Superbiola X. 2003. Stable isotope composition and rare earth element content of vertebrate remains from the Late Cretaceous of northern Spain (Laño): did the environmental record survive? Palaeogeogr. Palaeoclimatol. Palaeoecol. 193, 457–471. ( 10.1016/S0031-0182(03)00261-X) [DOI] [Google Scholar]
- 59.Trueman C, Chenery C, Eberth DA, Spiro B. 2003. Diagenetic effects on the oxygen isotope composition of bones of dinosaurs and other vertebrates recovered from terrestrial and marine sediments. J. Geol. Soc. 160, 895–901. ( 10.1144/0016-764903-019) [DOI] [Google Scholar]
- 60.Zazzo A, Lécuyer C, Mariotti A. 2004. Experimentally-controlled carbon and oxygen isotope exchange between bioapatites and water under inorganic and microbially-mediated conditions. Geochim. Cosmochim. Acta 68, 1–12. ( 10.1016/S0016-7037(03)00278-3) [DOI] [Google Scholar]
- 61.Zazzo A, Lécuyer C, Sheppard SMF, Grandjean P, Mariotti A. 2004. Diagenesis and the reconstruction of paleoenvironments: a method to restore original δ18O values of carbonate and phosphate from fossil tooth enamel. Geochim. Cosmochim. Acta 68, 2245–2258. ( 10.1016/j.gca.2003.11.009) [DOI] [Google Scholar]
- 62.Fricke HC, Clyde WC, O'Neil JR, Gingerich PD. 1998. Evidence for rapid climate change in North America during the latest Paleocene thermal maximum: oxygen isotope compositions of biogenic phosphate from the Bighorn Basin (Wyoming). Earth Planet. Sci. Lett. 160, 193–208. ( 10.1016/S0012-821X(98)00088-0) [DOI] [Google Scholar]
- 63.Iacumin P, Bocherens H, Mariotti A, Longinelli A. 1996. Oxygen isotope analyses of co-existing carbonate and phosphate in biogenic apatite: a way to monitor diagenetic alteration of bone phosphate? Earth Planet. Sci. Lett. 142, 1–6. ( 10.1016/0012-821X(96)00093-3) [DOI] [Google Scholar]
- 64.Pucéat E, Reynard B, Lécuyer C. 2004. Can crystallinity be used to determine the degree of chemical alteration of biogenic apatites? Chem. Geol. 205, 83–97. ( 10.1016/j.chemgeo.2003.12.014) [DOI] [Google Scholar]
- 65.Tütken T, Vennemann TW, Pfretzschner HU. 2008. Early diagenesis of bone and tooth apatite in fluvial and marine settings: constraints from combined oxygen isotope, nitrogen and REE analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 266, 254–268. ( 10.1016/j.palaeo.2008.03.037) [DOI] [Google Scholar]
- 66.Bryant DJ, Koch PL, Froelich PN, Showers WJ, Genna BJ. 1996. Oxygen isotope partitioning between phosphate and carbonate in mammalian apatite. Geochim. Cosmochim. Acta 60, 5145–5148. ( 10.1016/S0016-7037(96)00308-0) [DOI] [Google Scholar]
- 67.Chenery CA, Pashley V, Lamb AL, Sloane HJ, Evans JA. 2012. The oxygen isotope relationship between the phosphate and structural carbonate fractions of human bioapatite. Rapid Commun. Mass Spectrom. 26, 309–319. ( 10.1002/rcm.5331) [DOI] [PubMed] [Google Scholar]
- 68.Lécuyer C, et al. 2010. Oxygen isotope fractionation between apatite-bound carbonate and water determined from controlled experiments with synthetic apatites precipitated at 10–37°C. Geochim. Cosmochim. Acta 74, 2072–2081. ( 10.1016/j.gca.2009.12.024) [DOI] [Google Scholar]
- 69.Kolodny Y, Luz B. 1991. Oxygen isotopes in phosphate of fossil fish—Devonian to Recent. In Stable isotope geochemistry: a tribute to Samuel Epstein. Geochem. Soc. Spec. Publ. (eds Taylor HP Jr, O'Neil JR, Kaplan IR), pp. 105–119.
- 70.Vennemann TW, Hegner E, Cliff G, Benz GW. 2001. Isotopic composition of recent shark teeth as a proxy for environmental conditions. Geochim. Cosmochim. Acta 65, 1583–1599. ( 10.1016/S0016-7037(00)00629-3) [DOI] [Google Scholar]
- 71.Passey BH, Robinson TF, Ayliffe LK, Cerling TE, Sponheimer M, Dearing MD, Roeder BL, Ehleringer JR. 2005. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals J. Archaeol. Sci. 32, 1459–1470. ( 10.1016/j.jas.2005.03.015) [DOI] [Google Scholar]
- 72.McConnaughey TA, Burdett J, Whelan JF, Paull CK. 1997. Carbon isotopes in biological carbonates: respiration and photosynthesis. Geochim. Cosmochim. Acta 61, 611–622. ( 10.1016/S0016-7037(96)00361-4) [DOI] [Google Scholar]
- 73.Thorrold SR, Campana SE, Jones CM, Swart PK. 1997. Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish. Geochim. Cosmochim. Acta 61, 2909–2919. ( 10.1016/S0016-7037(97)00141-5) [DOI] [Google Scholar]
- 74.Santos GM, Ferguson J, Acaylar K, Johnson KR, Griffin S, Druffel E. 2011. Δ14C and δ13C of seawater DIC as tracers of coastal upwelling: a 5-year time series from Southern California. Radiocarbon 53, 669–677. ( 10.1017/S0033822200039126) [DOI] [Google Scholar]
- 75.Brudevold F, Soremark R. 1967. Chemistry of the mineral phase of enamel. In Structural and chemical organization of teeth, vol. 2 (ed. Mills A.), pp. 247–277. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 76.Rink WJ, Schwarcz HP. 1995. Tests for diagenesis in tooth enamel: ESR dating signals and carbonate contents. J. Archaeol. Sci. 22, 251–255. ( 10.1006/jasc.1995.0026) [DOI] [Google Scholar]
- 77.Harrell TL Jr, Pérez-Huerta A, Suarez CA. 2016. Endothermic mosasaurs? Possible thermoregulation of Late Cretaceous mosasaurs (Reptilia, Squamata) indicated by stable oxygen isotopes in fossil bioapatite in comparison with coeval marine fish and pelagic seabirds. Palaeontology 59, 351–363. 10.1111/pala.12240 [DOI] [Google Scholar]
- 78.Motani R. 2010. Warm-blooded ‘sea dragons’? Science 328, 1361–1362. ( 10.1126/science.1191409) [DOI] [PubMed] [Google Scholar]
- 79.van Hinsbergen DJ, de Groot LV, van Schaik SJ, Spakman W, Bijl PK, Sluijs A, Langereis CG, Brinkhuis H. 2015. A paleolatitude calculator for paleoclimate studies. PLoS ONE 10, e0126946 ( 10.1371/journal.pone.0126946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Andrade MB, Young MT, Desojo JB, Brusatte SL. 2010. The evolution of extreme hypercarnivory in Metriorhynchidae (Mesoeucrocodylia: Thalattosuchia) based on evidence from microscopic denticle morphology. J. Vertebr. Paleontol. 30, 1451–1465. ( 10.1080/02724634.2010.501442) [DOI] [Google Scholar]
- 81.Fernández M, Gasparini Z. 2000. Salt glands in a Tithonian metriorhynchid crocodyliform and their physiological significance. Lethaia 33, 269–276. ( 10.1080/002411600750053835) [DOI] [Google Scholar]
- 82.Massare JA. 1987. Tooth morphology and prey preference of Mesozoic marine reptiles. J. Vertebr. Paleontol. 7, 121–137. ( 10.1080/02724634.1987.10011647) [DOI] [Google Scholar]
- 83.Young MT, de Andrade MB, Brusatte SL, Sakamoto M, Liston J. 2013. The oldest known metriorhynchid super-predator: a new genus and species from the Middle Jurassic of England, with implications for serration and mandibular evolution in predacious clades. J. Syst. Palaeontol. 11, 475–513. ( 10.1080/14772019.2012.704948) [DOI] [Google Scholar]
- 84.Young MT, Brusatte SL, Beatty BL, De Andrade MB, Desojo JB. 2012. Tooth-on-tooth interlocking occlusion suggests macrophagy in the Mesozoic marine crocodylomorph Dakosaurus. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 295, 1147–1158. ( 10.1002/ar.22491) [DOI] [PubMed] [Google Scholar]
- 85.Seymour RS, Bennett-Stamper CL, Johnston SD, Carrier DR, Grigg GC. 2004. Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. Physiol. Biochem. Zool. 77, 1051–1067. ( 10.1086/422766) [DOI] [PubMed] [Google Scholar]
- 86.Farmer CG, Sanders K. 2010. Unidirectional airflow in the lungs of alligators. Science 327, 338–340. ( 10.1126/science.1180219) [DOI] [PubMed] [Google Scholar]
- 87.Summers AP. 2005. Evolution: warm-hearted crocs. Nature 434, 833 ( 10.1038/434833a) [DOI] [PubMed] [Google Scholar]
- 88.de Ricqlès A, Padian K, Knoll F, Horner JR. 2008. On the origin of high growth rates in archosaurs and their ancient relatives: complementary histological studies on Triassic archosauriforms and the problem of a ‘phylogenetic signal’ in bone histology. In Ann. Paléontol. 94, 57–76. [Google Scholar]
- 89.Legendre LJ, Guénard G, Botha-Brink J, Cubo J. 2016. Palaeohistological evidence for ancestral high metabolic rate in archosaurs. Syst. Biol. 65, 989–996. ( 10.1093/sysbio/syw033) [DOI] [PubMed] [Google Scholar]
- 90.Bardet N, Falconnet J, Fischer V, Houssaye A, Jouve S, Suberbiola XP, Perez-García A, Rage J-C, Vincent P. 2014. Mesozoic marine reptile palaeobiogeography in response to drifting plates. Gondwana Res. 26, 869–887. ( 10.1016/j.gr.2014.05.005) [DOI] [Google Scholar]
- 91.Buffetaut E. 1982. Radiation évolutive, paléoécologie et biogéographie des crocodiliens mésosuchiens [Evolutionary radiation, palaeoecology and biogeography of mesosuchian crocodilians] Mém. Soc. Géol. France 142, 1–88. [Google Scholar]
- 92.Martin JE, et al. 2019. A new freshwater teleosaurid from the Jurassic of northeastern Thailand. J. Vertebr. Paleontol. 38, e1549059 ( 10.1080/02724634.2018.1549059) [DOI] [Google Scholar]
- 93.Martin JE, et al. 2016. Strontium isotopes and the long-term residency of thalattosuchians in the freshwater environment. Paleobiology 42, 143–156. ( 10.1017/pab.2015.42) [DOI] [Google Scholar]
- 94.Wilberg EW, Turner AH, Brochu CA. 2019. Evolutionary structure and timing of major habitat shifts in Crocodylomorpha. Scient. Rep. 9, 514 ( 10.1038/s41598-018-36795-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foffa D, Johnson MM, Young MT, Steel L, Brusatte SL. 2019. Revision of the Late Jurassic deep-water teleosauroid crocodylomorph Teleosaurus megarhinus Hulke, 1871 and evidence of pelagic adaptations in Teleosauroidea. PeerJ 7, e6646 ( 10.7717/peerj.6646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ingram BL, Conrad ME, Ingle JC. 1996. Stable isotope and salinity systematics in estuarine waters and carbonates: San Francisco Bay. Geochim. Cosmochim. Acta 60, 455–467. ( 10.1016/0016-7037(95)00398-3) [DOI] [Google Scholar]
- 97.Grigg GC, Seebacherd F, Beard LA, Morris D. 1998. Thermal relations of large crocodiles, Crocodylus porosus, free-ranging in a naturalistic situation. Proc. R. Soc. Lond. B 265, 1793–1799. ( 10.1098/rspb.1998.0504) [DOI] [Google Scholar]
- 98.Seebacher F, Grigg GC, Beard LA. 1999. Crocodiles as dinosaurs: behavioural thermoregulation in very large ectotherms leads to high and stable body temperatures. J. Exp. Biol. 202, 77–86. [DOI] [PubMed] [Google Scholar]
- 99.Young MT, Rabi M, Bell MA, Foffa D, Steel L, Sachs S, Peyer K. 2016. Big-headed marine crocodyliforms and why we must be cautious when using extant species as body length proxies for long-extinct relatives. Palaeontol. Electron. 19, 1–14. ( 10.26879/648) [DOI] [Google Scholar]
- 100.Young MT, Bell MA, De Andrade MB, Brusatte SL. 2011. Body size estimation and evolution in metriorhynchid crocodylomorphs: implications for species diversification and niche partitioning. Zool. J. Linn. Soc. 163, 1199–1216. ( 10.1111/j.1096-3642.2011.00734.x) [DOI] [Google Scholar]
- 101.Tennant JP, Mannion PD, Upchurch P, Sutton MD, Price GD. 2017. Biotic and environmental dynamics through the Late Jurassic–Early Cretaceous transition: evidence for protracted faunal and ecological turnover. Biol. Rev. 92, 776–814. ( 10.1111/brv.12255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hua S, Vignaud P, Atrops F, Clément A. 2000. Enaliosuchus macrospondylus Koken, 1883 (Crocodylia, Metriorhynchidae) du Valanginien de Barret-le-Bas (Hautes Alpes, France): un cas unique de remontée des narines externes parmi les crocodiliens [Enaliosuchus macrospondylus Koken, 1883 (Crocodylia, Metriorhynchidae) of the Valanginian from Barret-le-Bas (Hautes Alpes, France): a unique case of external nostril shift among crocodilians]. Géobios 33, 467–474. [In French.] ( 10.1016/S0016-6995(00)80080-7) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Stable oxygen and carbon isotope compositions are provided in Excel tables as electronic supplementary material.