Abstract
Dementia with Lewy bodies and Parkinson’s disease dementia, jointly known as Lewy body dementia, are common neurodegenerative conditions. Patients with Lewy body dementia present with a wide range of cognitive, neuropsychiatric, sleep, motor, and autonomic symptoms. Presentation varies between patients and can vary over time within an individual. Treatments can address one symptom but worsen another, which makes disease management difficult. Symptoms are often managed in isolation and by different specialists, which makes high-quality care difficult to accomplish. Clinical trials and meta-analyses now provide an evidence base for the treatment of cognitive, neuropsychiatric, and motor symptoms in patients with Lewy body dementia. Furthermore, consensus opinion from experts supports the application of treatments for related conditions, such as Parkinson’s disease, for the management of common symptoms (eg, autonomic dysfunction) in patients with Lewy body dementia. However, evidence gaps remain and future clinical trials need to focus on the treatment of symptoms specific to patients with Lewy body dementia.
Introduction
Lewy body dementia comprises both dementia with Lewy bodies and Parkinson’s disease dementia, and is the second most common cause of neurodegenerative dementia.1-3 Dementia with Lewy bodies accounts for 4–8% of patients with dementia in clinic-based studies,1,2 and dementia is a common (up to 80%) outcome for people with Parkinson’s disease.4 Consensus clinical diagnostic criteria have been proposed for both dementia with Lewy bodies3 and Parkinson’s disease dementia,5 but the association between the two disorders remains to be clarified; the two diseases are likely to represent different points along a Lewy body disease continuum with pathological and genetic overlap.6,7 The two diseases are demarcated clinically from one another by the so-called 1-year rule, based on the temporal onset of motor relative to cognitive symptoms (ie, in Parkinson’s disease dementia the motor symptoms precede the onset of dementia by at least one year).3
Dementia with Lewy bodies and Parkinson’s disease dementia are complex and heterogeneous disorders; patients present with a wide range of cognitive, neuropsychiatric, sleep, motor, and autonomic symptoms.3,5 Although clinical guidelines outline some treatment options for patients with dementia with Lewy bodies and those with Parkinson’s disease dementia,8-10 no comprehensive guide to the management of patients with Lewy body dementia exists. Lewy body dementia management has particular challenges: symptoms differ between patients and, even within a patient, can be expressed variably over time; natural fluctuations in symptoms are an inherent part of the disease and frequently treatment of one symptom can worsen another. Furthermore, an individual patient’s symptoms are often managed by different specialists, leading to uncoordinated and suboptimal care.11,12 With the inclusion of dementia with Lewy bodies and Parkinson’s disease dementia in the Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) and WHO’s 11th International Classification of Diseases (ICD-11) and the development of diagnostic toolkits for use in both types of dementia to improve case detection,13 a clear need exists for an inclusive, standardised management approach to improve care and outcomes of patients with Lewy body dementia.
Since 2000, the number of clinical trials in Lewy body dementia has gradually increased. Consequently, it is now possible to conduct robust systematic and meta-analytic reviews to inform management practise. A number of these reviews and meta-analyses have been published since 201514-18 and there is new evidence for the treatment for symptoms, such as parkinsonism19 and daytime somnolence.20 However, some gaps in our knowledge remain. For example, few studies have focused on how to manage autonomic and sleep symptoms in Lewy body dementia. These non-motor symptoms are evident in advanced Parkinson’s disease and therefore drawing upon the wider evidence base, for example in Parkinson’s disease, to inform best practice in Lewy body dementia is appropriate.
In this Review, we present expert opinion developed from a Delphi consensus process (appendix p 4), drawing upon expert clinical experience and data from related disorders, such as Alzheimer’s disease and Parkinson’s disease, to address these gaps in the management of Lewy body dementia. We bring together the evidence base in Lewy body dementia and these expert opinions to form a comprehensive management approach. We cover the management of key domains of cognitive impairment, neuropsychiatric, and motor symptoms before moving on to the treatment of autonomic and sleep symptoms, which has often been neglected in previous reviews. Additionally, we identify key evidence gaps and areas for future consideration, including suggestions of treatment trials for specific symptoms in patients with Lewy body dementia.
Cognitive impairment
Attention, executive, and visuoperceptual functions are disproportionately affected in patients with Lewy body dementia compared with naming and memory abilities,3 with variations in cognitive function (cognitive fluctuation), a key feature and a core diagnostic symptom of dementia with Lewy bodies.3 Systematic reviews and meta-analyses14,15 found that the cholinesterase inhibitors donepezil and rivastigmine were similarly effective at improving cognition for patients with dementia with Lewy bodies and those with Parkinson’s disease dementia. Additionally, both drugs had positive effects on the completion of activities of daily living and reduced caregiver burden. One meta-analysis of two trials15 suggested that rivastigmine was also associated with reduced mortality in patients with Lewy body dementia, although this effect disappeared with a trial sequential analysis (which provides better control of type I and type II errors than the traditional meta-analysis). Both donepezil and rivastigmine are recommended as first-line treatments for dementia with Lewy bodies by the UK National Institute for Health and Care Excellence;10 donepezil is licensed for the treatment of dementia with Lewy bodies in Japan and rivastigmine is licensed for use in Parkinson’s disease dementia in the USA and the UK. Evidence of the efficacy of a third cholinesterase inhibitor, galantamine, in patients with Lewy body dementia is sparse as only open-label trials support its use.14,15
The choice of cholinesterase inhibitors is influenced by the ease of administration, side-effect profile, presence of comorbidities, dose titration regime, cost, care-giver preference, and previous clinical experience.21 Rivastigmine has been associated with more adverse events than donepezil in patients with Parkinson’s disease dementia and those with dementia with Lewy bodies.14,15 However, rivastigmine has a transdermal patch formulation that appears to have fewer gastrointestinal side-effects than oral rivastigmine in patients with Parkinson’s disease dementia.22 An open-label, uncontrolled study of seven patients with Lewy body dementia showed the benefit of high-dose (15 mg donepezil daily) cholinesterase inhibitors,23 but this benefit resulted in increased side-effects.
An absence of improvement should not be a reason for the discontinuation of cholinesterase inhibitors because patients with Lewy body dementia are less likely to deteriorate globally while taking them.14 No randomised controlled trials have assessed cholinesterase inhibitor withdrawal, although an open-label trial of 19 patients with Lewy body dementia found that sudden withdrawal could be associated with deterioration in both cognitive function and neuropsychiatric symptoms.24
Clinical trials of the NDMA receptor antagonist memantine showed that it was well tolerated in patients with Lewy body dementia, but evidence for its efficacy remains mixed.14,15 Two 24-week, double-blind, randomised controlled trials of memantine in Lewy body dementia have been conducted.25,26 The first trial, by Aarsland and colleagues,25 reported significant improvements in their primary outcome (Alzheimer’s disease cooperative study Clinical Global Impression of Change [ADCS-CGIC] scores [a measure based on observation by a clinical assessor of change in the patient’s cognitive, functional, and behavioural performance]) in both patients with Parkinson’s disease dementia and dementia with Lewy bodies (34 patients in active group vs 38 in placebo group), with the effect possibly greater in the Parkinson’s disease dementia group. The second, larger study, by Emre and colleagues,26 showed significant improvements in ADCS-CGIC scores only in the dementia with Lewy body group (34 patients in active group vs 41 in placebo group) and not the Parkinson’s disease dementia group (62 patients in active group vs 58 in placebo group). Further lack of consistency between these trials was also evident for other outcomes: Emre and colleagues26 reported substantial benefits in terms of neuropsychiatric symptoms in dementia with Lewy bodies only with memantine, whereas Aarsland and colleagues25 reported a statistically significant, 1·9 point, improvement in MMSE scores in the active group compared with placebo. Data from 51 patients (21 with dementia with Lewy bodies and 30 with Parkinson’s disease dementia) taken from the study by Aarsland and colleagues25 found that the improvement in ADCS-CGIC score was related to improvements in attention, a cognitive domain which is often profoundly impaired in Lewy body dementia.27 Another post-hoc analysis from this trial of memantine25 reported improvements in patient quality of life as a whole,28 and a 22-week randomised control trial of 25 patients with Parkinson’s disease dementia who received memantine noted a reduction in caregiver burden and improvements in individually set goals.29 A 36-month, open-label, follow-up study of 42 patients from one of the centres in one of the memantine trials25 suggested that a positive response to memantine compared with placebo was associated with improved survival in patients with Lewy body dementia, but the small sample size in this study could have introduced bias and confounders that might not have been adequately controlled for.30
In summary, robust evidence exists for the efficacy of rivastigmine and donepezil in the treatment of cognitive symptoms in patients with Lewy body dementia,14,15 but high-quality randomised controlled studies of galantamine are needed to draw conclusions about this agent. Memantine could have some benefits, but further studies with larger numbers of patients with dementia with Lewy bodies and Parkinson’s disease dementia are needed to determine whether there is an improvement with this drug in either dementia with Lewy bodies or Parkinson’s disease dementia and, if so, which specific symptoms are improved. Whether memantine should be used as a monotherapy or whether it should be combined with cholinesterase inhibitors is also unclear, as only one of the two trials of memantine allowed concomitant cholinesterase inhibitors use.25
Dosing and drug side-effects are important to consider when selecting the cholinesterase inhibitors or memantine for patients with Lewy body dementia (table 1).
Table 1:
Cholinesterase inhibitors and memantine for the treatment of cognitive and neuropsychiatric symptoms in patients with Lewy body dementia
| Dosing | Adverse effects | Comment | |
|---|---|---|---|
| Donepezil | 5 mg once daily for 4-6 weeks, increased to 10 mg daily if tolerated | Overall cholinesterase inhibitors are well tolerated; adverse effects include: gastrointestinal symptoms, postural hypotension, urinary frequency, drooling, watering eyes, runny nose, and worsening of extrapyramidal motor symptoms, particularly fine tremor; these effects only occurs in a few patients, particularly those with more advanced disease; given the high incidence of autonomic dysfunction (eg, orthostatic hypotension, syncope or presyncope, or cardiac dysrhythmia or conduction disturbances), examination for these must be done before treatment; if the patient has a history of these signs and symptoms, cardiac issues, or autonomic dysfunction, an electrocardiogram might be appropriate and specialist cardiology advice might need to be sought, particularly if a pacemaker is required | Double-blind randomised control trial evidence in both patients with dementia with Lewy bodies and those with Parkinson’s disease dementia support the use of donepezil; meta-analyses suggest a similar effectiveness to rivastigmine14,15 |
| Rivastigmine (oral) | 1·5 mg twice daily for 4 weeks, increased to 3 mg twice daily. Dose can be increased up to 6 mg twice daily if tolerated | Similar to donepezil | Double-blind randomised control trial evidence in both patients with dementia with Lewy bodies and those with Parkinson’s disease dementia support the use of rivastigmine;14,15 rivastigmine might be associated with more adverse effects than donepezil14,15 |
| Rivastigmine patch | 4·6 mg/24 h for 4 weeks, increased to 13·3 mg/24 h if tolerated |
Similar to donepezil | Might have advantages in patients with swallowing difficulties; those who have gastrointestinal side-effects in response to oral agents; those that have compliance issues; or if the patient has a history of a variable response to oral dosing |
| Galantamine | 8 mg/day increased to the initial maintenance dose of 16 mg/day after a minimum of 4-6 weeks; if tolerated after 4 weeks at 16 mg/day, a further dose increase to 24 mg/day of galantamine can be attempted |
Similar to donepezil | Open-label trial data only, but galantamine might have positive effects on cognition and neuropsychiatric symptoms14 |
| Memantine | Dosing of memantine should be increased gradually (typically by 5 mg per week) to 20 mg once daily (some patients might prefer divided dosing) over 4 weeks according to tolerance | Side-effects of memantine include gastrointestinal symptoms, confusion, somnolence, hypertension, and dizziness; be cautious when prescribing memantine to individuals with a history of seizures or poor renal function; memantine might enhance the effects of dopaminergics, such as selegiline, and can be toxic when given with, for example, amantadine | In clinical trials memantine was superior to placebo on Global Impression of Change scores, but not on cognitive function or other outcomes, with inconsistent findings reported between patients with dementia with Lewy bodies and those with Parkinson’s disease dementia14,17 |
Neuropsychiatric symptoms
Patients with Lewy body dementia present with a variety of neuropsychiatric symptoms, including visual hallucinations and hallucinations in other sensory modalities, systematised delusions, apathy, aggression, anxiety, and depression.31 Symptoms might not always need treatment (eg, hallucinations can be regarded neutrally or as being comforting or pleasurable, with little or no effect on psychosocial function).3,32 Including collateral information from the care-giver or an informant in the clinical assessment of patients is essential to diagnosis and management because patients frequently lack insight or awareness of the extent of their neuropsychiatric symptoms. Rating scales can provide a useful framework for assessing the severity and frequency of symptoms and monitoring treatment response (eg, for visual hallucinations).33 Scales can either be specific to a symptom or a composite; composite scales, such as the Neuropsychiatric Inventory,34 aggregate several symptoms and are commonly used as measures in clinical trials in patients with Lewy body dementia.25,26,35,36
As for other types of dementias, non-pharmacological interventions (eg, musical therapy and environmental modifications) are usually advocated as a first-line treatment for neuropsychiatric symptoms;10 however, the evidence base for this in patients with Lewy body dementia is weak, based only on case report and case series data.17,18 In this context, the application of approaches shown to be effective in patients with Alzheimer’s disease might also be helpful in patients with Lewy body dementia, but no specific consensus exists as to if, or how, they could be adapted.18
If symptoms are severe or distressing, or if non-pharmacological interventions do not succeed, then pharmacotherapy might be indicated.10 Studies of donepezil and rivastigmine have found improvements in composite scores of neuropsychiatric symptoms in patients with Lewy body dementia;15 however, evidence on whether a treatment leads to the improvement of a specific symptom is more challenging to obtain, because these are not commonly reported in studies. Scores that aggregate apathy, delusions, depression, and hallucinations have indicated a benefit to patients with dementia with Lewy bodies from donepezil, but not rivastigmine.35,36 One systematic review14 suggested that donepezil, but not rivastigmine, might reduce delusions, hallucinations, and cognitive fluctuations in patients with dementia with Lewy bodies. In patients with Parkinson’s disease dementia, donepezil does not appear to be beneficial for hallucinations, hostility, suspiciousness, or unusual thought content.37 Despite the absence of consistent effects on specific neuropsychiatric symptoms, expert opinion from our Delphi consensus group and national guideline bodies10 have endorsed the use of rivastigmine and donepezil for neuropsychiatric symptoms in patients with Lewy body dementia. Open-label trial data of galantamine provides preliminary evidence of improved cognitive fluctuation, sleep, and psychiatric symptoms in patients with dementia with Lewy bodies,38 and an improvement in hallucinations, anxiety, apathy, and sleep symptoms in patients with Parkinson’s disease dementia;39 therefore, galantamine could be an alternative if other cholinesterase inhibitors are not tolerated. As noted previously, the reported efficacy of memantine on neuropsychiatric symptoms in patients with Lewy body dementia is mixed.14,15
If, despite cholinesterase inhibitor or memantine treatment, psychotic symptoms remain, an antipsychotic agent could be considered, although its use needs to be balanced against a scarcity of documented efficacy of these agents when assessed systematically14 and the high-risk of severe sensitivity reactions that can occur in up to 50% of patients with Lewy body dementia, which can be lifethreatening, as well as an enhanced mortality risk in the longer term.40-42 If antipsychotics are prescribed in patients with Lewy body dementia, there needs to be a high degree of caution in their use.43 No evidence supports the use of any antipsychotic drug in patients with Lewy body dementia. Quetiapine appears to have the fewest side-effects, but evidence for its efficacy in patients with Parkinson’s disease9 and those with Lewy body dementia is insufficient.14 Clozapine, effective in patients with Parkinson’s disease psychosis,44 might also help in patients with Lewy body dementia, but no trials have been done with this drug in patients exclusively with Lewy body dementia. Pimavanserin is a new antipsychotic drug with specific inverse agonism for the 5-HT2A receptor. It is available in the USA but not in Europe, and has shown antipsychotic effects in patients with Parkinson’s disease psychosis;45 its safety and efficacy have not been formally evaluated in patients with Lewy body dementia, although a phase 3 clinical trial of pimavanserin () in patients with dementia related psychosis is ongoing that will include patients with Lewy body dementia.
Depression occurs in about a third of patients with Lewy body dementia46,47 and is often accompanied by anxiety. Pharmacological treatments for depression and anxiety in patients with Lewy body dementia have not been adequately evaluated. A small randomised controlled trial comparing citalopram, a selective serotonin reuptake inhibitor, with risperidone in 14 patients with dementia with Lewy bodies did not show efficacy and found high overall burden of side-effects.48 More studies with antidepressants have been conducted in Parkinson’s disease.9 A randomised control trial of paroxetine (n=42), venlafaxine (n=34), or placebo (n=39) for the treatment of depression in patients with Parkinson’s disease, reviewed by Sepp and colleagues,9 found a reduction in depressive symptoms for both drugs. However, other studies are less conclusive and provide mixed results for selective serotonin-reuptake inhibitors and tricyclics.9 Therefore, it is difficult to conclude which drugs are best to use in the treatment of depression in patients with dementia with Lewy bodies or Parkinson’s disease dementia and there are concerns that antidepressants might affect sleep and worsen REM sleep behaviour disorder symptoms.49
Electroceutical approaches are increasingly being investigated in patients with Lewy body dementia, but many of these techniques are primarily research based rather than being used clinically. Statistically significant reduction in depression scores after repetitive transcranial magnetic stimulation was shown in one case series of six patients with dementia with Lewy bodies.50 By contrast, two randomised sham-controlled trials of transcranial direct current stimulation in patients with Lewy body dementia have not shown statistically significant improvements in cognition (42 patients)51 or hallucinations (36 patients).52 A study of six patients with Parkinson’s disease dementia53 reported that deep brain stimulation of the nucleus basalis of Meynert improved neuropsychiatric inventory scores compared with sham stimulation, but findings remain tentative and numbers small on which to base any firm conclusions.
Even electroconvulsive therapy, an established clinical treatment has surprisingly little evidence in Lewy body dementia although a comprehensive review18 in this area noted a reduction in depressive symptoms with electroconvulsive therapy across four uncontrolled studies, in a total of 22 patients.
In summary, a number of case studies have examined the non-pharmacological management of neuropsychiatric symptoms in patients with Lewy body dementia; future studies are needed to address this gap. Cholinesterase inhibitors might help, but further studies focusing on which particular symptom domains are most likely to improve are needed. The effect of memantine on neuropsychiatric symptoms needs to be confirmed in large randomised controlled studies. Studies of pimavanserin in patients with Lewy body dementia for psychosis and trials of antidepressants for depression have not yet been done and should be a future priority.
Motor symptoms
Up to 85% of patients with dementia with Lewy bodies experience motor difficulties,3 although resting tremor is less prevalent than in those with Parkinson’s disease.54 By contrast, parkinsonism in patients with Parkinson’s disease dementia can be moderate-to-severe and patients have often been exposed to long-term and high-dose antiparkinsonian medications with commensurate side-effects, including motor fluctuations and psychosis.55,56 Thus, the management of motor symptoms can differ markedly between patients with dementia with Lewy bodies and those with Parkinson’s disease dementia (table 2).
Table 2:
Pharmacological interventions for motor symptoms in patients with Parkinson’s disease dementia and those with dementia with Lewy bodies
| Dosing | Adverse effects | Comment | |
|---|---|---|---|
| Parkinson’s disease dementia | |||
| Simplification of antiparkinsonian treatment regime | Withdraw one at a time in the order: (1) anticholinergic drugs, (2) amantadine, (3) selegiline, (4) dopamine agonists, and (5) catechol-O-methyltransferase inhibitors | Reduction in antiparkinsonian medications can lead to the worsening of motor symptoms | Despite the poor correlation between dopaminergic drug exposure and psychosis,55 a stepwise withdrawal approach might be useful, especially if psychosis is present57 |
| Dementia with Lewy bodies | |||
| Levodopa monotherapy | Either co-careldopa or co-beneldopa can be used; start with a low dose and increase slowly; commonly, dementia with Lewy bodies initiation doses (50 mg levodopa equivalent dose, for example co-careldopa 12·5 mg/50 mg, taken one to three times daily) are lower than in patients with Parkinson’s disease | Psychosis, postural hypotension, sedation, nausea, and vomiting | Up to a third of patients might experience improvement; however, a third of these patients might also experience psychotic symptoms (eg, hallucinations or delusions)58,59 |
| Zonisamide | 25–50 mg once a day as an adjunct to levodopa | Side-effects include weight loss and decreased appetite | Evidence for use in patients with dementia with Lewy bodies comes from one phase 2 randomised control trial19 |
To date, no double-blind randomised controlled trials have investigated levodopa therapy in patients with dementia with Lewy bodies, or whether changing to a levodopa monotherapy regimen in patients with Parkinson’s disease dementia is beneficial. However, open-label studies suggest that both acute and chronic levodopa monotherapy can improve motor function and reduce tremor in patients with dementia with Lewy bodies and patients with Parkinson’s disease dementia.58,59 Motor function appears to improve more in patients with Parkinson’s disease dementia (65–70%) than in those with dementia with Lewy bodies (32–50%).14 Approximately one in three patients with dementia with Lewy bodies treated with levodopa will experience psychotic symptoms.59 A meta-analysis of four double-blind randomised controlled trials, which recruited a total of 1068 patients with Parkinson’s disease across these four studies,60 and a phase 2 trial of 158 patients with dementia with Lewy bodies have reported a statistically significant improvement in motor function compared with patients receiving placebo with zonisamide, an antiepileptic agent, when used as adjunctive treatment to levodopa.19
In terms of non-pharmacological approaches, deep brain stimulation is an effective treatment for motor symptoms in patients with Parkinson’s disease that are medication refractory, display significant on–off fluctuations, tremor, or dyskinesias.61 However, pre-existing cognitive impairment is a contraindication for deep brain stimulation because stimulation impairs cognitive function and exacerbates any pre-existing cognitive impairment.62
Falls
Falls are common in patients with Lewy body dementia and are associated with substantial morbidity and mortality.63,64 Contributors to fall risk in patients with Lewy body dementia are commonly multifactorial, including parkinsonism, dysautonomia, and frailty.64 The use of physiotherapy has a robust evidence base in patients with Parkinson’s disease and can help to improve balance, power, flexibility, and enhance mobility—all factors that can decrease the risk of falls and improve functional independence.64,65 Hypothetically, cognitive impairment could influence engagement with therapy in Lewy body dementia. Unfortunately, no evidence exists for the use of physical therapy in Lewy body dementia and such studies are needed.
Autonomic dysfunction
There are a wide range of autonomic signs and symptoms in patients with Lewy body dementia and these are associated with more rapid disease progression and shorter survival.66 Despite the prominence and effect of these symptoms, no evidence base is yet established for their treatment; as a result, opinion on best management is largely drawn upon from the more established evidence base in patients with Parkinson’s disease.8,67,68
Orthostatic hypotension
Non-pharmacological approaches are not evidence based but our Delphi panel recommended advising the patient to stand slowly, raising the head of the bed for those with morning orthostatic hypotension, increasing fluid intake and for some, the use of compression hosiery, and increased salt intake, when appropriate. Pharmacologically, fludrocortisone, a drug with significant mineralocorticoid effects, and midodrine, whose active metabolite, desglymidodrine, is a α1-receptor agonist with vasopressive effects, have both been suggested for the treatment of orthostatic hypotension in patients with Parkinson’s disease on the basis of a small number of trials (table 3).8,9,69 No trials with these drugs have been specifically conducted in Lewy body dementia; however, consensus from our Delphi panel supported the use of these agents in Lewy body dementia. Both agents require specific monitoring of supine blood pressure. Electrolytes should also be monitored in patients taking fludrocortisone, as should hepatic and renal function in patients taking midodrine. Droxidopa (a prodrug that converts to norepinephrine) is licensed for the treatment of orthostatic hypotension in patients with Parkinson’s disease in some countries, including the USA and Japan. Although no data for droxidopa exist in patients with Lewy body dementia, given its low side-effect profile,71 the use of droxidopa in these patients could be a viable treatment option if licensed or available (table 3). The importance of treating orthostatic hypotension in patients with Lewy body dementia is highlighted by the link between orthostatic hypotension and attention-executive impairments in patients with Parkinson’s disease, which raises the possibility that treatment of the low blood pressure might have benefits beyond the hypotension itself, but randomised controlled trials are needed to assess this effect.74
Table 3:
Potential pharmacological interventions for orthostatic hypotension in patients with Lewy body dementia
| Dosing | Adverse effects | Comment | |
|---|---|---|---|
| Midodrine | A 2·5–10 mg dose taken up to three times daily; avoid evening doses of midodrine; last dose should be taken at least 4 h before bed; monitor hepatic and renal function | Risk of supine hypertension | Several trials of patients with orthostatic hypotension (which have included patients with Parkinson’s disease) with some suggestion of efficacy9 |
| Fludrocortisone | 50–300 μg/day; titrate slowly and monitor electrolytes | Electrolyte disturbances, hypertension (especially supine), and oedema | A crossover clinical trial in 17 patients with Parkinson’s disease showed statistically significant subjective benefits with fludrocortisone compared to a range of non-pharmacological interventions70 |
| Droxidopa | 100–600 mg three times daily | Risk of supine hypertension, worsening heart disease or heart failure, and arrhythmias | A phase 3 trial of 162 patients with Parkinson’s disease with orthostatic hypotension reported subjective improvements in symptoms and a mean standing systolic blood pressure increase of 11·2 mm Hg vs 3–9 mm Hg compared with placebo;71 however, an interim analysis of a double-blind randomised controlled trial in patients with Parkinson’s disease did not show subjective benefits of droxidopa compared with placebo with regard to orthostatic hypotension symptoms,72 although a revised primary outcome in the full trial, which specifically focused on feelings of dizziness, light headedness, and feeling faint, suggested short-term benefits73 |
Gastrointestinal dysfunction
The full extent of the alimentary tract can be affected in patients with Lewy body dementia with symptoms ranging from sialorrhoea to dysphagia, gastroparesis, and constipation.75-77 When reviewed,77 the prevalence of excessive drooling in patients with Parkinson’s disease has been reported to range from 10% to 81% of patients in case controlled studies. In part, the wide variation might reflect differences in how drooling was assessed, cohort ascertainment, and the lack of established diagnostic criteria.78 Excessive drooling can have substantial negative effects on the quality of life and social and emotional function of the patient. Drooling has been linked to inefficient swallowing, which leads to high prevalence of aspiration (>80%) in patients with Lewy body dementia.78 A randomised controlled trial of 132 patients with Parkinson’s disease dementia with dysphagia investigated the effects of the interventions that prevent aspiration. Fewer patients using honey-thickened fluids aspirated compared with those receiving nectar-thickened liquids or completing chin-down posturing, as evidenced by videofluoroscopy.79 Another observational study reported objective improvements in swallowing function after the consumption of carbonated liquids in 48 patients with Lewy body dementia referred for videofluoroscopy.78 Whether such interventions have clinically meaningful effects (eg, prevention of aspiration pneumonia) remains to be resolved. A randomised, double-blind, placebo-controlled, crossover trial of glycopyrrolate (1 mg two or three times a day) in 23 patients with Parkinson’s disease reported that nine (39·1%) patients had a clinically meaningful improvement in sialorrhoea over a 4-week period;80 however, the efficacy of this agent in patients with Lewy body dementia is not known. Botulium toxin injection into the salivary glands appears effective and safe in patients with Parkinson’s disease,77 and consensus opinion suggests that it would be similarly effective in patients with Lewy body dementia, although repeated injections are often needed.
Gastric emptying is slow in patients with Parkinson’s disease, but this process might be even slower in patients with dementia with Lewy bodies,81 and slow emptying correlates with severity of motor impairment.82 Impairments in gastric motility can lead to fullness, reflux and excess eructation, and, importantly, affect drug absorption. Management includes the avoidance of high fat foods, drinking during meals, walking after meals, and an awareness that dopaminergic medications can exacerbate gastroparesis.68,75 Domperidone, a peripheral dopamine blocker, might have efficacy in the treatment of gastroparesis in patients with Parkinson’s disease,68,83 but it is associated with cardiotoxicity and the risk of QT prolongation.
Constipation is one of the most common symptoms in patients with Lewy body dementia,84 with prolonged colon transit time and pelvic floor dyssynergia as plausible causes.75,76 Constipation can also be exacerbated by opiates and anticholinergics,84,85 poor fluid intake, reduced fibre intake, and sedentary behaviour. Polyethylene glycol (also known as macrogrol) and psyllium increase bowel frequency in patients with Parkinson’s disease,86,87 and experts advocate dietary modification, increased fluid intake, and suppositories as treatments for constipation in patients with Lewy body dementia. Stronger laxatives, suppositories, or enemas might be needed in severely affected patients.68 Lubiprostone, a bicyclic fatty acid that activates type-2 chloride channels in the gut and enhances intestinal secretions, has been shown to have short-term benefits in patients with Parkinson’s disease.84,88
Although randomised controlled trial evidence supports the use of thickened liquids to reduce aspiration in patients with Lewy body dementia, further studies of interventions to help other symptoms, such as constipation, are needed. Until then, the evidence base for Parkinson’s disease can be used to inform management of gastrointestinal symptoms in patients with Lewy body dementia.
Urinary symptoms
Urinary symptoms in patients with Lewy body dementia are very common and include urgency, frequency, and incontinence.89 Despite being common, no trials for patients with Lewy body dementia have been conducted; thus, treatment recommendations are based on data from Parkinson’s disease studies. A double-blind randomised placebo-controlled trial of solifenacin over 12 weeks in 23 patients with Parkinson’s disease and urinary frequency, incontinence, or nocturia reported a statistically significant reduction in urinary incontience over a 24 h period.90 However, antimuscarinics (such as solifenacin) have a high risk of adverse effects, including cognitive side-effects,91 which might be a contraindication for their use in patients with Lewy body dementia. An alternative drug without cognitive side-effects is the β3-adrenoceptor agonist mirabegron (25–50 mg once per day); a retrospective cohort study, which considered 50 patients with Parkinson’s disease between 2012 and 2017, suggested that this agent was well tolerated in patients with Parkinson’s disease and offered benefit.92 A wide range of medications are available to treat urinary symptoms, such as over active bladder, frequency, and nocturia. Trials specific to Lewy body dementia patients are needed given the major effect of these symptoms have on patient quality of life.
Excess sweating
Hyperhidrosis is reported by two-thirds of patients with Parkinson’s disease;93 it is associated with disease severity and might be linked with fluctuations in motor symptom severity,94,95 but how common hyperhidrosis is in patients with Lewy body dementia is not known. It has substantial social and emotional effects and might occur with other autonomic disturbances.93 No treatment trials have been done, but the consensus from our Delphi panel group is that patients might benefit from the use of loose fitting clothing, cotton bedding for night sweats, antiperspirants, and avoidance of triggers, including alcohol, spicy foods, and rooms with a high ambient temperature, to control symptoms. For those with dyskinesias and hyperhidrosis, reducing dopaminergic medication should be considered.93,95
Sleep disturbances
Sleep disturbances in patients with Lewy body dementia can be severe and include insomnia, sleep fragmentation, rapid eye movement (REM) sleep behaviour disorder, motor-related sleep disturbances, restless legs syndrome, periodic limb movements, obstructive sleep apnoea, and excessive daytime sleepiness.3 Most of the evidence base for the management of these symptoms comes from studies done in patients with Parkinson’s disease and those with idiopathic REM sleep behaviour disorder rather than from studies of patients with Lewy body dementia. Management begins with education on good sleep hygiene and avoidance of any drugs that can affect sleep or alertness.8
For insomnia, a meta-analysis of melatonin from nine randomised controlled trials in patients with different neurodegenerative diseases, including patients with Parkinson’s disease, found improvements in subjective sleep quality and the drug appears to be well tolerated.96,97 Non-benzodiazepines (Z-drugs), such as eszopiclone, zopiclone, and zolpidem, have not been trialled in patients with Lewy body dementia, but the consensus opinion from experts is that they could be considered for short-term treatment of insomnia if sleep apnoea is not evident, with the caveat that they might have negative effects on cognition, daytime sleepiness, and increase the risk of fractures and falls.98 If sleep disturbances occur secondary to nocturnal parkinsonism, long-acting levodopa preparations might be useful.99 Randomised controlled studies have shown that dopaminergic medications, such as ropinirole, pramipexole, and rotigotine, have efficacy in treating idiopathic restless legs syndrome,100 but have not been trialled in patients with Lewy body dementia. A network meta-analysis of 35 studies, which collectively included 7333 participants, found that gabapentin and pregabalin were statistically superior to placebo and as effective as dopaminergic drugs, such as rotigotine, for the treatment of restless legs syndrome.100 However, all of the drugs need to be used with caution in patients with Lewy body dementia given their cognitive side-effects.
Obstructive sleep apnoea might occur in up to a third of patients with Lewy body dementia101 and is often unrecognised. Patients might experience excessive daytime somnolence, worsening cognitive function, unrefreshing sleep, and early morning headaches.102 Pauses in breathing during sleep and regular snoring raise the suspicion of this particular sleep symptom. A number of associated risk factors exist, including being overweight, male sex, smoker, use of sedatives, alcohol use, reflux, and anatomical considerations (eg, collar size >43 cm [>17”]),103 which should be assessed for in every patient.
REM sleep behaviour disorder is a parasomnia manifested by recurrent dream enactment behaviour, which includes movements mimicking dream content, and is associated with an absence of normal REM sleep atonia. Between half and three-quarters of patients with Lewy body dementia have REM sleep behaviour disorder,104,105 and it is a core symptom for the diagnosis of dementia with Lewy bodies.3 It can precede the onset of Parkinson’s disease and Lewy body dementia by many years to decades or can emerge during the dementia phase.106,107 However, obstructive sleep apnoea, narcolepsy, and nocturnal arousal events coupled with confusion might mimic REM sleep behaviour disorder.101,107 As a result, the origin of sleep disturbance in patients with Lewy body dementia might require polysomnography.3 A number of non-pharmacological strategies have been used anecdotally in patients with Lewy body dementia with REM sleep behaviour disorder, including lowering bed height or placing a mattress on the floor; removal of potentially dangerous objects in the bedroom, such as sharp or glass objects; or, if necessary, asking bed partners to sleep separately from the patient. Some medications can worsen REM sleep behaviour disorder (eg, antidepressants),108 and retrospective case series in patients with idiopathic and secondary REM sleep behaviour disorder supported the use of clonazepam, although caution is needed when prescribing it to patients with Lewy body dementia who are more prone to gait disturbance, sleep apnoea, cognitive impairment, and are at high risk of falls.109 Pramipexole has been assessed in observational studies as a potentially effective treatment for REM sleep behaviour disorder in patients with Parkinson’s disease,99 but it is associated with an increased risk of psychosis.110 Melatonin (3–12 mg before bedtime) is well tolerated and has a clinical trial evidence base in the treatment of idiopathic REM sleep behaviour disorder.109,111 Another treatment option is memantine, which decreased physical activity during sleep over 24 weeks in a randomised controlled study of 20 patients with Lewy body dementia, while the 22 patients in the placebo group worsened over the same period.112
Excessive daytime sleepiness is common in patients with Lewy body dementia,113 and it can make daily function challenging for patients and carers. Management is difficult and primarily draws upon ensuring good sleep hygiene and assessing for other potential causes or factors that might exacerbate the sleepiness.69 A single-arm, open label, pilot study investigating the treatment of 20 patients with dementia with Lewy bodies and hypersomnia with armodafinil reported improvements in sleepiness, neuropsychiatric symptoms, and carer quality of life.20 An open label trial of methylphenidate for gait dysfunction in 17 patients with Parkinson’s disease114,115 reported improvements in excessive sleepiness as a secondary outcome; however, sleepiness has not been assessed as a primary efficacy outcome in patients with Parkinson’s disease or Lewy body dementia in any clinical trials. Other treatments, such as atomoxetine, sodium oxybate, istradefylline, and caffeine have been investigated for sleepiness in patients with Parkinson’s disease, but evidence to support their efficacy in this patient population or those with Lewy body dementia remains insufficient.116 In patients with Lewy body dementia, memantine did not improve day time sleepiness in a small controlled trial of 42 patients.112
Extrapolating from the evidence base in patients with Parkinson’s disease and related disorders, management of sleep problems in patients with Lewy body dementia includes attention to sleep hygiene and the avoidance of exacerbating factors. REM sleep behaviour disorder treatment can include clonazepam, melatonin, or potentially memantine. Management of obstructive sleep apnoea is best undertaken by specialist sleep services. Further studies of management strategies for specific sleep disturbances in patients with dementia with Lewy bodies, including REM sleep behaviour disorder and excessive daytime sleepiness, are needed.
Conclusions and future directions
In this Review, we have summarised the new evidence base for pharmacological and non-pharmacological management of Lewy body dementia. Treatment of any single symptom should not be done in isolation, as benefit in one domain might be gained at the cost of deterioration in another. A multispecialist or interdisciplinary approach is likely to produce the greatest therapeutic gains, although delivery of that might present practical and logistical challenges for health-care services.
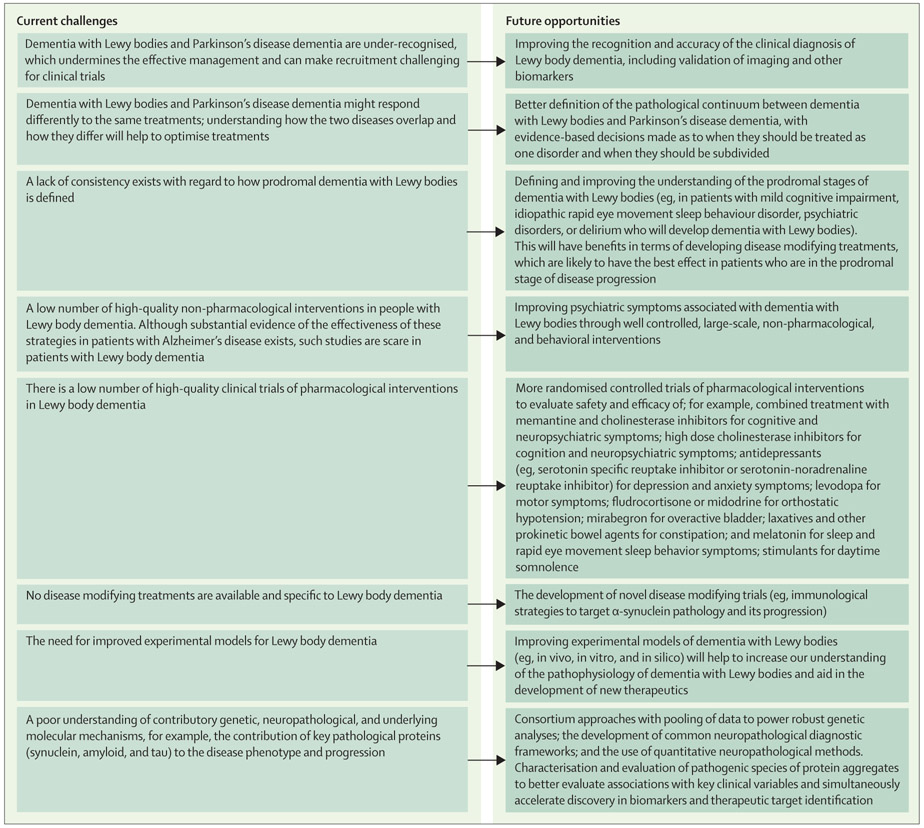
Many aspects of Lewy body dementia care need further research (figure)117 and a major unmet challenge is the paucity of evidence from high-quality, large-scale clinical trials in patients with Lewy body dementia. Given the heterogeneous nature of Lewy body dementia and the different constellations of symptoms with which patients can present, trial design and definition of outcome measures remain problematic and need to be prioritised and agreed upon with the regulatory bodies before large trials are embarked upon. We also need to improve our understanding of the underlying molecular mechanisms behind the disease and to identify novel targets for therapeutic intervention. Inclusion of Lewy body dementia in formal diagnostic classifications, such as DSM-5 and ICD-11, is a substantial step forward, which is expected to drive interest in developing therapeutics for these conditions (table 4). International strategic efforts, such as the European Dementia with Lewy Bodies Consortium and the US-based Lewy Body Dementia Association Research Centres of Excellence network, are also providing important research infrastructure to support such work, and resources need to be directed into developing and strengthening these collaborative efforts. The scale of costs and unmet needs in patients with Lewy body dementia is high and the management is complex. However the potential benefits of properly managing Lewy body dementia and its wide array of symptoms are substantial.
Figure:
Current challenges and future opportunities for improving care and outcomes of patients with Lewy body dementia
Table 4:
Examples of clinical trials assessing treatment interventions in patients with Lewy body dementia
| NCT number | Study design | Proposed mechanism of action | Main outcomes | Comment | |
|---|---|---|---|---|---|
| Intepirdine (RVT-101) | , , and | Double-blind, randomised, placebo-controlled study of RVT-101 in patients with dementia with Lewy bodies () and a double-blind randomised placebo-controlled study of gait impairment in patients with either Alzheimer’s disease, dementia with Lewy bodies, or Parkinson’s disease (); additionally, there was a planned 6 month extension study with RVT-101 in patients with dementia with Lewy body () | Serotonin 6 receptor antagonist that causes the release of acetylcholine and other neurotransmitters | Primary outcome for first study () was Unified Parkinson’s Disease Rating Scale-Part III and, for the second study (), quantitative gait function; secondary outcomes included cognitive and Clinician’s Interview-Based Impression of Change score Plus Caregiver Input () and safety outcomes () | , , and were terminated in 2018. The results indicated a lack of efficacy on all outcomes for . No formal results have been posted for and |
| Nelotanserin | , , and | Double-blind, randomised, placebo-controlled cross-over study in patients with Lewy body dementia with visual hallucinations () and a double-blind, randomised, placebo-controlled parallel-arm study in patients with dementia with Lewy bodies or Parkinson’s disease dementia who have REM sleep behaviour disorder (); additionally, an open-label study was planned in patients with Lewy body dementia with frequent visual hallucinations or REM Sleep Behavior Disorder () | Serotonin receptor subtype 5-HT2A inverse agonist | Visual hallucinations and safety data as well as motor function in first trial (), and REM sleep behaviour disorder symptoms in the second () | No formal results from these trials have been posted or published but further development of this drug has been discontinued |
| SYN120 | Double-blind randomised placebo-controlled study in patients with Parkinson’s disease dementia | Dual HT2A with a dual 5-HT6 or 5-HT5 antagonist | Primary outcomes were attention measures | Results posted (May 2019) to clinicaltrials.gov indicated a lack of statistical difference on primary outcomes between active and placebo arms | |
| LY3154207 | Double-blind randomised placebo-controlled study in patients with Parkinson’s disease dementia | Enhancer of dopamine receptor D1 | Attention measures (primary) and cognitive, neuropsychiatric outcomes, sleep, motor, and functional measures (secondary) | Recruiting | |
| E2027 | Double-blind randomised placebo-controlled study of patients with dementia with Lewy Bodies | A selective phosphodiesterase 9 inhibitor that might maintain cyclic GMP concentration in the brain | Cognitive measures and Global Impression of Change scores with wide range of secondary outcomes including, for example, neuropsychiatric, cognitive fluctuations, global impression of change, and safety data | Recruiting | |
| Ambroxol | Double-blind randomised placebo-controlled parallel study in patients with Parkinson’s disease dementia | Raises the concentrations of the enzyme β-glucocerebrosidase, which might lead to reduced concentrations of α-synuclein | Cognitive measures and Global Impression of Change scores with wide range of secondary outcomes including, for example, motor performance measures, cerebrospinal fluid concentrations of α-synuclein, tau, phospho-tau and β amyloid 42 and structural magnetic resonance biomarkers (ventricular volumes and hippocampal atrophy) | Recruiting | |
| Deep brain stimulation | and | Pilot studies assessing stimulation of the nucleus basalis of Meynert in patients with dementia with Lewy bodies | Enhance cholinergic output of the nucleus basalis of Meynert | Various cognitive and neuropsychiatric outcomes | Completed: awaiting results |
| HTL0018318 | Double-blind randomised placebo-controlled trial of patients with dementia with Lewy bodies | Muscarinic M1 agonist | Primary outcome was to assess safety and secondary outcomes included measures of cognition and psychosis | Trial suspended pending investigation of an unexpected animal toxicology finding (development of tumours) | |
| Ramelteon | and | Double blind, randomised placebo-controlled pilot studies for REM sleep behaviour disorder which included patients with dementia with Lewy body | Selective agonist of melatonin receptors (MT1 and MT2) | Primary outcomes included sleep efficiency and other sleep parameters with a wide range of secondary outcomes (motor, cognitive, and functional etc) | Trials terminated due to low participant enrolment and recruitment |
| Pimavanserin | Double-blind randomised placebo-controlled trial for the treatment of hallucinations and delusions associated with dementia related psychosis; it will include patients with dementia with Lewy bodies or Parkinson’s disease dementia | Selective 5-HT2A inverse agonist | Time to relapse in double blind period or discontinuation for any reason | Recruiting | |
| Bosutinib | Double blind randomised placebo-controlled study in dementia with Lewy bodies | Tyrosine kinase inhibitor targeting c-Abelson and Src tyrosine kinases. In models of neurodegeneration, it reduces alpha-synuclein, tau, and amyloid β | Primary outcome is safety with secondary outcomes including cerebrospinal fluid markers of, for example, bosutinib levels, cell death, tau, phosphorylated tau, amyloid β, and inflammation | Recruiting |
REM=rapid eye movement.
Supplementary Material
Search strategy and selection criteria.
We identified clinical trials and intervention studies for patients with Lewy body dementia through bibliographic databases, trials registers, and grey literature. Search terms for identification of these studies included (Lewy OR Park*or Parkinson) and dementia from Jan 1, 1990 to Feb 13, 2019. We prioritised articles published in the past five years. Older articles for citation were chosen for their historical value, importance, ease of access, and timeliness. At least two reviewers J-PT, IGM, JTO’B independently assessed search results for inclusion by title and abstract with papers reviewed in full if patients had a diagnosis of dementia with Lewy bodies, Parkinson’s disease dementia, or Lewy body dementia (or were the caregivers of patients with these diagnoses) and were relevant. We also examined reference lists of relevant studies and previous systematic reviews. In addition, we also sought input from members of the Delphi expert consensus panel for any missing literature and relevant trials in patients with Alzheimer’s disease and those with Parkinson’s disease and papers pertinent to Lewy body dementia symptom cause and epidemiology.
Acknowledgments
The two systematic reviews informing this Review14,18 and the Delphi process were funded as part of a UK NIHR programme grant for applied research entitled Improving the diagnosis and management of Lewy body dementia (DIAMOND-Lewy; grant reference number DTC-RP-PG-0311-12001). This evidence presented in this Review informed our development of formal Lewy body dementia management guidelines and toolkits as part of the Diamond Lewy research programme. These clinical guidelines were developed for clinical practice in the UK and based on UK drug availability, but adaptable for other countries. We also acknowledge infrastructure support provided by Newcastle Biomedical Research Centre hosted by Newcastle upon Tyne Hospitals National Health Service (NHS) Foundation Trust and Newcastle University, the Cambridge Biomedical Research Centre hosted by the Cambridge University Hospitals NHS Foundation Trust and the Cambridgeshire and Peterborough NHS Foundation Trust, and the Cambridge Centre for Parkinson-Plus within the University of Cambridge. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Declaration of interests
J-PT, IGM, DJB, CB, LMA, AJT, and JTO’B report grants from the UK National Institute of Health Research (NIHR) during the conduct of this study. J-PT reports non-financial support from Axovant and personal fees from GE Healthcare, outside the submitted work. IGM reports personal fees from Axovant, Eisai, GE Healthcare, Sumitomo Dainippon Pharma, and Heptares, outside the submitted work. BFB reports personal fees for scientific advisory board membership from the Tau Consortium, grants from GE Healthcare, National Institutes of Health, Mangurian Foundation, and Axovant, outside the submitted work. DW reports personal fees from Acadia, outside the submitted work. JTO’B reports personal fees from TauRx, Axon, GE Healthcare, and Eisai, outside the submitted work.
Contributor Information
John-Paul Taylor, Institute of Neuroscience, Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK.
Ian G McKeith, Institute of Neuroscience, Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK.
David J Burn, Institute of Neuroscience, Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK.
Brad F Boeve, Department of Neurology, Mayo Clinic, Rochester, MN, USA.
Daniel Weintraub, Department of Psychiatry and Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA; Parkinson’s Disease and Mental Illness Research, Education and Clinical Centers, Philadelphia Veterans Affairs Medical Center, Philadelphia, PA, USA.
Claire Bamford, Institute of Health and Society, Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK.
Louise M Allan, University of Exeter Medical School, University of Exeter, Exeter, UK.
Alan J Thomas, Institute of Neuroscience, Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK.
John T O’Brien, Department of Psychiatry, School of Clinical Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
References
- 1.Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 2014; 44: 673–83. [DOI] [PubMed] [Google Scholar]
- 2.Kane JPM, Surendranathan A, Bentley A, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther 2018; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the dementia with Lewy bodies Consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008; 23: 837–44. [DOI] [PubMed] [Google Scholar]
- 5.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Dis 2007; 22: 1689–707. [DOI] [PubMed] [Google Scholar]
- 6.Jellinger KA. Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm (Vienna) 2018; 125: 615–50. [DOI] [PubMed] [Google Scholar]
- 7.Orme T, Guerreiro R, Bras J. The genetics of dementia with Lewy bodies: current understanding and future directions. Curr Neurol Neurosci Rep 2018; 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NICE. Parkinson’s disease in adults. 2017. https://www.nice.org.uk/guidance/ng71 (accessed March 21, 2019).
- 9.Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence-based medicine review. Mov Disord 2019; 34: 180–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NICE. Dementia: assessment, management and support for people living with dementia and their carers. 2018. https://www.nice.org.uk/guidance/ng97 (accessed March 21, 2019). [PubMed]
- 11.Killen A, Flynn D, De Brun A, et al. Support and information needs following a diagnosis of dementia with Lewy bodies. Int Psychogeriatr 2016; 28: 495–501. [DOI] [PubMed] [Google Scholar]
- 12.Zweig YR, Galvin JE. Lewy body dementia: the impact on patients and caregivers. Alzheimers Res Ther 2014; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas AJ, Taylor JP, McKeith I, et al. Revision of assessment toolkits for improving the diagnosis of Lewy body dementia: the DIAMOND lewy study. Int J Geriatr Psychiatry 2018; 33: 1293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry 2015; 172: 731–42. [DOI] [PubMed] [Google Scholar]
- 15.Wang HF, Yu JT, Tang SW, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 2015; 86: 135–43. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien JT, Holmes C, Jones M, et al. Clinical practice with antidementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol 2017; 31: 147–68. [DOI] [PubMed] [Google Scholar]
- 17.Morrin H, Fang T, Servant D, Aarsland D, Rajkumar AP. Systematic review of the efficacy of non-pharmacological interventions in people with Lewy body dementia. Int Psychogeriatr 2018; 30: 395–407 [DOI] [PubMed] [Google Scholar]
- 18.Connors MH, Quinto L, McKeith I, et al. Non-pharmacological interventions for Lewy body dementia: a systematic review. Psychol Med 2018; 48: 1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata M, Odawara T, Hasegawa K, et al. Adjunct zonisamide to levodopa for dementia with Lewy bodies parkinsonism: a randomized double-blind phase 2 study. Neurology 2018; 90: e664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapid MI, Kuntz KM, Mason SS, et al. Efficacy, safety, and tolerability of armodafinil therapy for hypersomnia associated with dementia with Lewy bodies: a pilot study. Dement Geriatr Cogn Disord 2017; 43: 269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings JL, Isaacson RS, Schmitt FA, Velting DM. A practical algorithm for managing Alzheimer’s disease: what, when, and why? Ann Clin Trans Neurol 2015; 2: 307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emre M, Poewe W, De Deyn PP, et al. Long-term safety of rivastigmine in Parkinson disease dementia: an open-label, randomized study. Clin Neuropharmacol 2014; 37: 9–16. [DOI] [PubMed] [Google Scholar]
- 23.Pakrasi S, Thomas A, Mosimann UP, et al. Cholinesterase inhibitors in advanced dementia with Lewy bodies: increase or stop? Int J Geriatr Psychiatry 2006; 21: 719–21. [DOI] [PubMed] [Google Scholar]
- 24.Minett TS, Thomas A, Wilkinson LM, et al. What happens when donepezil is suddenly withdrawn? An open label trial in dementia with Lewy bodies and Parkinson’s disease with dementia. Int J Geriatr Psychiatry 2003; 18: 988–93. [DOI] [PubMed] [Google Scholar]
- 25.Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2009; 8: 613–18. [DOI] [PubMed] [Google Scholar]
- 26.Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9: 969–77. [DOI] [PubMed] [Google Scholar]
- 27.Wesnes KA, Aarsland D, Ballard C, Londos E. Memantine improves attention and episodic memory in Parkinson’s disease dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry 2015; 30: 46–54. [DOI] [PubMed] [Google Scholar]
- 28.Larsson V, Engedal K, Aarsland D, Wattmo C, Minthon L, Londos E. Quality of life and the effect of memantine in dementia with Lewy bodies and Parkinson’s disease dementia. Dement Geriatr Cogn Disord 2011; 32: 227–34. [DOI] [PubMed] [Google Scholar]
- 29.Leroi I, Atkinson R, Overshott R. Memantine improves goal attainment and reduces caregiver burden in Parkinson’s disease with dementia. Int J Geriatr Psychiatry 2014; 29: 899–905. [DOI] [PubMed] [Google Scholar]
- 30.Stubendorff K, Larsson V, Ballard C, Minthon L, Aarsland D, Londos E. Treatment effect of memantine on survival in dementia with Lewy bodies and Parkinson’s disease with dementia: a prospective study. BMJ Open 2014; 4: e005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30: 603–11. [DOI] [PubMed] [Google Scholar]
- 32.Collerton D, Taylor JP. Advances in the treatment of visual hallucinations in neurodegenerative diseases. Future Neurol 2013; 8: 433–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosimann UP, Collerton D, Robert D, et al. A semi-structured interview to assess visual hallucinations in older people. Int J Geriatric Psychiatry 2008; 23: 712–18. [DOI] [PubMed] [Google Scholar]
- 34.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–14. [DOI] [PubMed] [Google Scholar]
- 35.Mori E, Ikeda M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol 2012; 72: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356: 2031–36. [DOI] [PubMed] [Google Scholar]
- 37.Ravina B, Putt M, Siderowf A, et al. Donepezil for dementia in Parkinson’s disease: a randomised, double blind, placebo controlled crossover study. J Neurol Neurosurg Psychiatry 2005; 76: 934–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord 2007; 23: 401–05. [DOI] [PubMed] [Google Scholar]
- 39.Litvinenko IV, Odinak MM, Mogil’naia VI, Emelin A. Efficacy and safety of galantamine (reminyl) in the treatment of dementia in patients with Parkinson’s disease (open-label controlled trial). Zh Nevrol Psikhiatr Im S S Korsakova 2007; 107: 25–33 (in Russian). [PubMed] [Google Scholar]
- 40.McKeith I, Fairbairn A, Perry R, Thompson P, Perry E. Neuroleptic sensitivity in patients with senile dementia of lewy body type. BMJ 1992; 305: 673–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aarsland D, Perry R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson’s disease and parkinsonian dementias. J Clin Psychiatry 2005; 66: 633–37 [PubMed] [Google Scholar]
- 42.Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol 2016; 73: 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry 2016; 173: 543–46. [DOI] [PubMed] [Google Scholar]
- 44.Friedman JH. Pharmacological interventions for psychosis in Parkinson’s disease patients. Expert Opin Pharmacother 2018; 19: 499–505. [DOI] [PubMed] [Google Scholar]
- 45.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014; 383: 533–40. [DOI] [PubMed] [Google Scholar]
- 46.Kuring JK, Mathias JL, Ward L. Prevalence of depression, anxiety and PTSD in people with dementia: a systematic review and meta-analysis. Neuropsychol Rev 2018; 28: 393–416. [DOI] [PubMed] [Google Scholar]
- 47.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord 2008; 23: 183–89. [DOI] [PubMed] [Google Scholar]
- 48.Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord 2010; 24: 360–64. [DOI] [PubMed] [Google Scholar]
- 49.St Louis EK, Boeve B. REM sleep behavior disorder: diagnosis, clinical implications, and future directions. Mayo Clin Proc 2017. 92: 1723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi S, Mizukami K, Yasuno F, Asada T. Depression associate with dementia with Lewy bodies (dementia with Lewy bodies) and the effect of somatotherapy. Psychogeriatrics 2009; 9: 56–61. [DOI] [PubMed] [Google Scholar]
- 51.Elder GJ, Ashcroft J, da Silva Morgan K, et al. Transcranial direct current stimulation in Parkinson’s disease dementia: a randomised double-blind crossover trial. Brain Stim 2017; 10: 1150–51. [DOI] [PubMed] [Google Scholar]
- 52.Elder GJ, Colloby SJ, Firbank MJ, McKeith IG, Taylor JP. Consecutive sessions of transcranial direct current stimulation do not remediate visual hallucinations in Lewy body dementia: a randomised controlled trial. Alzheimers Res Ther 2019; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gratwicke J, Zrinzo L, Kahan J, et al. Bilateral deep brain stimulation of the nucleus basalis of meynert for Parkinson disease dementia: a randomized clinical trial. JAMA Neurol 2018; 75: 169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onofrj M, Varanese S, Bonanni L, et al. Cohort study of prevalence and phenomenology of tremor in dementia with Lewy bodies. J Neurol 2013; 260: 1731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Factor SA, McDonald WM, Goldstein FC. The role of neurotransmitters in the development of Parkinson’s disease-related psychosis. Eur J Neurol 2017; 24: 1244–54. [DOI] [PubMed] [Google Scholar]
- 56.Ray Chaudhuri K, Poewe W, Brooks D. Motor and nonmotor complications of levodopa: phenomenology, risk factors, and imaging features. Mov Disord 2018; 33: 909–19. [DOI] [PubMed] [Google Scholar]
- 57.Hindle JV. The practical management of cognitive impairment and psychosis in the older Parkinson’s disease patient. J Neural Transm (Vienna) 2013; 120: 649–53. [DOI] [PubMed] [Google Scholar]
- 58.Molloy S, McKeith IG, O’Brien JT, Burn DJ. The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2005; 76: 1200–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman JG, Goetz CG, Brandabur M, Sanfilippo M, Stebbins GT. Effects of dopaminergic medications on psychosis and motor function in dementia with Lewy bodies. Mov Disord 2008; 23: 2248–50. [DOI] [PubMed] [Google Scholar]
- 60.Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis 2017; 56: 1229–39. [DOI] [PubMed] [Google Scholar]
- 61.Bratsos S, Karponis D, Saleh SN. Efficacy and safety of deep brain stimulation in the treatment of Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. Cureus 2018; 10: e3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehanna R, Bajwa JA, Fernandez H, Wagle Shukla AA. Cognitive impact of deep brain stimulation on Parkinson’s disease patients. Parkinsons Dis 2017; published online Nov 22. DOI:10.1155/2017/3085140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiorth YH, Alves G, Larsen JP, Schulz J, Tysnes OB, Pedersen KF. Long-term risk of falls in an incident Parkinson’s disease cohort: the Norwegian ParkWest study. J Neurol 2017; 264: 364–72. [DOI] [PubMed] [Google Scholar]
- 64.Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson’s disease: a complex and evolving picture. Mov Disord 2017; 32: 1524–36. [DOI] [PubMed] [Google Scholar]
- 65.Shen X, Wong-Yu IS, Mak MK. Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair 2016; 30: 512–27 [DOI] [PubMed] [Google Scholar]
- 66.De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT. Association of autonomic dysfunction with disease progression and survival in parkinson disease. JAMA Neurol 2017; 74: 970–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfeiffer RF. Management of autonomic dysfunction in Parkinson’s disease. Semin Neurol 2017; 37: 176–85. [DOI] [PubMed] [Google Scholar]
- 68.Palma JA, Kaufmann H. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord 2018; 33: 372–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol 2013; 20: 5–15. [DOI] [PubMed] [Google Scholar]
- 70.Schoffer KL, Henderson RD, O’Maley K, O’Sullivan JD. Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson’s disease. Mov Disord 2007; 22: 1543–9. [DOI] [PubMed] [Google Scholar]
- 71.Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension. A randomized, placebo-controlled, phase 3 trial. Neurology 2014; 83: 328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hauser RA, Hewitt LA, Isaacson S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J Parkinsons Dis 2014; 4: 57–65. [DOI] [PubMed] [Google Scholar]
- 73.Hauser RA, Isaacson S, Lisk JP, Hewitt LA, Rowse G. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (nOH306B). Mov Disord 2015; 30: 646–54 [DOI] [PubMed] [Google Scholar]
- 74.Centi J, Freeman R, Gibbons CH, Neargarder S, Canova AO, Cronin-Golomb A. Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology 2017; 88: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukherjee A, Biswas A, Das SK. Gut dysfunction in Parkinson’s disease. World J Gastroenterol 2016; 22: 5742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol 2017; 134: 811–26. [DOI] [PubMed] [Google Scholar]
- 77.Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 2015; 14: 625–39. [DOI] [PubMed] [Google Scholar]
- 78.Larsson V, Torisson G, Bulow M, Londos E. Effects of carbonated liquid on swallowing dysfunction in dementia with Lewy bodies and Parkinson’s disease dementia. Clinical Interv Aging 2017; 12: 1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Logemann JA, Gensler G, Robbins J, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J Speech Lang Hear Res 2008; 51: 173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arbouw MEL, Movig KLL, Koopmann M, et al. Glycopyrrolate for sialorrhea in Parkinson disease. A randomized, double-blind, crossover trial. Neurology 2010; 74: 1203–07 [DOI] [PubMed] [Google Scholar]
- 81.Doi H, Sakakibara R, Masuda M, et al. Gastrointestinal function in dementia with Lewy bodies: a comparison with Parkinson disease. Clin Auton Res 2019; published online Feb 11. DOI: 10.1007/s10286-019-00597-w. [DOI] [PubMed] [Google Scholar]
- 82.Goetze O, Nikodem AB, Wiezcorek J, et al. Predictors of gastric emptying in Parkinson’s disease. Neurogastroenterol Motil 2006; 18: 369–75. [DOI] [PubMed] [Google Scholar]
- 83.Soykan I, Sarosiek I, Shifflett J, Wooten GF, McCallum RW. Effect of chronic oral domperidone therapy on gastrointestinal symptoms and gastric emptying in patients with Parkinson’s disease. Mov Dis 1997; 12: 952–57. [DOI] [PubMed] [Google Scholar]
- 84.Pedrosa Carrasco AJ, Timmermann L, Pedrosa DJ. Management of constipation in patients with Parkinson’s disease. NPJ Parkinsons Dis 2018; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pagano G, Tan EE, Haider JM, Bautista A, Tagliati M. Constipation is reduced by beta-blockers and increased by dopaminergic medications in Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 120–25. [DOI] [PubMed] [Google Scholar]
- 86.Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled study. Mov Disord 2007; 22: 1239–44. [DOI] [PubMed] [Google Scholar]
- 87.Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EMM. Constipation in Parkinson’s disease: objective assessment and response to psyllium. Mov Disord 1997; 12: 946–51. [DOI] [PubMed] [Google Scholar]
- 88.Ondo WG, Kenney C, Sullivan K, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology 2012; 78: 1650–54. [DOI] [PubMed] [Google Scholar]
- 89.Tateno F, Sakakibara R, Ogata T, et al. Lower urinary tract function in dementia with Lewy bodies (dementia with Lewy bodies). Mov Disord 2015; 30: 411–15. [DOI] [PubMed] [Google Scholar]
- 90.Zesiewicz TA, Evatt M, Vaughan CP, et al. Randomized, controlled pilot trial of solifenacin succinate for overactive bladder in Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 514–20. [DOI] [PubMed] [Google Scholar]
- 91.Vouri SM, Kebodeaux CD, Stranges PM, Teshome BF. Adverse events and treatment discontinuations of antimuscarinics for the treatment of overactive bladder in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2017; 69: 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peyronnet B, Vurture G, Palma JA, et al. Mirabegron in patients with Parkinson disease and overactive bladder symptoms: a retrospective cohort. Parkinsonism Relat Disord 2018; 57: 22–26. [DOI] [PubMed] [Google Scholar]
- 93.Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A, Quinn N. Sweating dysfunction in Parkinson’s disease. Mov Disord 2003; 18:1459–63. [DOI] [PubMed] [Google Scholar]
- 94.Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology 2002; 59: 408–13. [DOI] [PubMed] [Google Scholar]
- 95.Schaeffer E, Berg D. Dopaminergic therapies for non-motor symptoms in Parkinson’s disease. CNS Drugs 2017; 31: 551–70. [DOI] [PubMed] [Google Scholar]
- 96.Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhaes MC, de Lourdes Seabra M, de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J Neurol 2007; 254: 459–64. [DOI] [PubMed] [Google Scholar]
- 97.Zhang W, Chen XY, Su SW, et al. Exogenous melatonin for sleep disorders in neurodegenerative diseases: a meta-analysis of randomized clinical trials. Neurol Sci 2016; 37: 57–65. [DOI] [PubMed] [Google Scholar]
- 98.Asaly A, Kolenberg Geron L, Treves N, Matok I, Perlman A. Z-drugs and risk for falls and fractures in older adults—a systematic review and meta-analysis. Age Ageing 2018; 47: 201–08. [DOI] [PubMed] [Google Scholar]
- 99.Schaeffer E, Berg D. Dopaminergic therapies for non-motor symptoms in Parkinson’s disease. CNS Drugs 2017; 31: 551–70. [DOI] [PubMed] [Google Scholar]
- 100.Iftikhar IH, Alghothani L, Trotti LM. Gabapentin enacarbil, pregabalin and rotigotine are equally effective in restless legs syndrome: a comparative meta-analysis. Eur J Neurol 2017; 24: 1446–56. [DOI] [PubMed] [Google Scholar]
- 101.Terzaghi M, Arnaldi D, Rizzetti MC, et al. Analysis of video-polysomnographic sleep findings in dementia with Lewy bodies. Mov Disord 2013; 28: 1416–23. [DOI] [PubMed] [Google Scholar]
- 102.Mery VP, Gros P, Lafontaine A-L, et al. Reduced cognitive function in patients with Parkinson disease and obstructive sleep apnea. Neurology 2017; 88: 1120–28. [DOI] [PubMed] [Google Scholar]
- 103.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383: 736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 2011; 77: 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X, Sun X, Wang J, Tang L, Xie A. Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: a meta and meta-regression analysis. Neurol Sci 2017; 38: 163–70. [DOI] [PubMed] [Google Scholar]
- 106.Iranzo A, Fernändez-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014; 9: e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.St Louis EK, Boeve AR, Boeve Bradley F. REM sleep behavior disorder in parkinson’s disease and other synucleinopathies. Mov Disord 2017; 32: 645–58. [DOI] [PubMed] [Google Scholar]
- 108.Gagnon J-F, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology 2006; 67: 742–47. [DOI] [PubMed] [Google Scholar]
- 109.Jung Y, St Louis EK. Treatment of REM sleep behavior disorder. Curr Treat Options Neurol 2016; 18: 50. [DOI] [PubMed] [Google Scholar]
- 110.Ecker D, Unrath A, Kassubek J, Sabolek M. Dopamine agonists and their risk to induce psychotic episodes in Parkinson’s disease: a case-control study. BMC Neurol 2009; 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McGrane IR, Leung JG, St Louis EK, Boeve BF. Melatonin therapy for REM sleep behavior disorder: a critical review of evidence. Sleep Med 2015; 16: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson’s disease dementia. Int J Geriatr Psychiatry 2010; 25: 1030–38. [DOI] [PubMed] [Google Scholar]
- 113.Ferman TJ, Smith GE, Dickson DW, et al. Abnormal daytime sleepiness in dementia with Lewy bodies compared to Alzheimer’s disease using the multiple sleep latency test. Alzheimers Res Ther 2014; 6: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 2007; 78: 470–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moreau C, Delval A, Defebvre L, et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol 2012; 11: 589–96. [DOI] [PubMed] [Google Scholar]
- 116.Shen Y, Huang J-Y, Li J, Liu C-F. Excessive daytime sleepiness in Parkinson’s disease: clinical implications and management. Chin Medl J (Engl) 2018; 131: 974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Outeiro TF, Koss DJ, Erskine D, et al. Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 2019; 14: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.