Abstract
Adipose tissues are complex organs with a wide array of functions, including storage and mobilization of energy in response to local and global needs, uncoupling of metabolism to generate heat, and secretion of adipokines to regulate whole-body homeostasis and immune responses. Emerging research is identifying important regional differences in the developmental, molecular, and functional profiles of adipocytes located in discrete depots throughout the body. Different properties of the depots are medically relevant since metabolic diseases often demonstrate depot-specific effects. This protocol will provide investigators with a detailed anatomic atlas and dissection guide for the reproducible and accurate identification and excision of diverse mouse adipose tissues. Standardized dissection of discrete adipose depots will allow detailed comparisons of their molecular and metabolic characteristics and contributions to local and systemic pathologic states under various nutritional and environmental conditions.
Keywords: Biology, Issue 149, Adipocyte, dissection, subcutaneous adipose tissue, visceral adipose tissue, white adipose tissue (WAT), brown adipose tissue (BAT), bone marrow adipose tissue (BMAT)
Introduction
Adipose tissues play critical roles in whole-body homeostasis, including storage and release of energy in response to local and global needs, thermoregulation, and secretion of adipokines to regulate energy balance, metabolism and immune responses1,2. Adipocytes are distributed throughout the body in discrete depots, and in some cases serve specialized roles within their microenvironments3,4,5. Historically, the study of adipose tissue has centered on white adipose tissue (WAT), and its role in maintaining energy homeostasis. Most adipocytes are distributed throughout the body in subcutaneous and visceral WAT depots. The characteristics of these depots are important for differential susceptibility to metabolic diseases. Subcutaneous adipocytes, located beneath the skin, have been associated with protective metabolic effects5. Visceral adipocytes, which surround the vital organs and are contained within the gonadal, perirenal, retroperitoneal, omental and pericardial depots, are commonly linked to metabolic disorders, including type 2 diabetes and cardiovascular disease2. Brown adipose tissues (BAT) have also been studied extensively. Brown and brown-like adipocytes express uncoupling protein 1 (UCP1) and play important roles in adaptive thermogenesis and glucose homeostasis6,7. Classical brown adipocytes are contained in the interscapular BAT depot8. Clusters of brown adipocytes are also found in other locations, including supraclavicular, infra/subscapular, cervical, paravertebral and periaortic depots8,9.
In addition to their location in major WAT and BAT depots, adipocytes exist in discrete niches throughout the body4, where they may perform specialized functions within their respective microenvironments. For example, bone marrow adipose tissue (BMAT) serves as a lipid reservoir, is a major source of circulating adiponectin, and closely interacts with osteoblasts, osteoclasts, and hematopoietic cells10,11. Dermal adipocytes contribute to widespread processes, including wound healing, immune response, thermoregulation, and hair follicle growth12,13. Further, epicardial adipocytes may produce several adipokines and chemokines that exert local and systemic effects on the development and progression of coronary artery disease14. Expansion of inter/intramuscular WAT has been positively correlated with increased adiposity, systemic insulin resistance, and decreased muscular strength and mobility15. In addition, popliteal adipocytes serve as a lipid reservoir for lymphatic expansion during infection16. While the specific roles of different articular depots are generally unknown, the Hoffa depot (infrapatellar) within the knee is now thought to contribute to pathologies, including anterior knee pain and osteoarthritis17.
Whereas regional differences in adipocyte character and function are under intense study, the field is currently limited by the lack of a standardized protocol for the identification and dissection of diverse mouse depots. Previously published methods have typically described the isolation of one or two specific depots and lack the level of detail required for uniform excision18,19. The protocol described in this manuscript provides a comprehensive guide for the specific anatomic locations and isolation steps of many different mouse adipose depots. Although WAT depots are the primary focus of this manuscript, the excision of interscapular BAT is also described in detail. Adipose tissues excised using this protocol can be used for a wide variety of experimental endpoints, including explant studies, histology, and gene expression analyses.
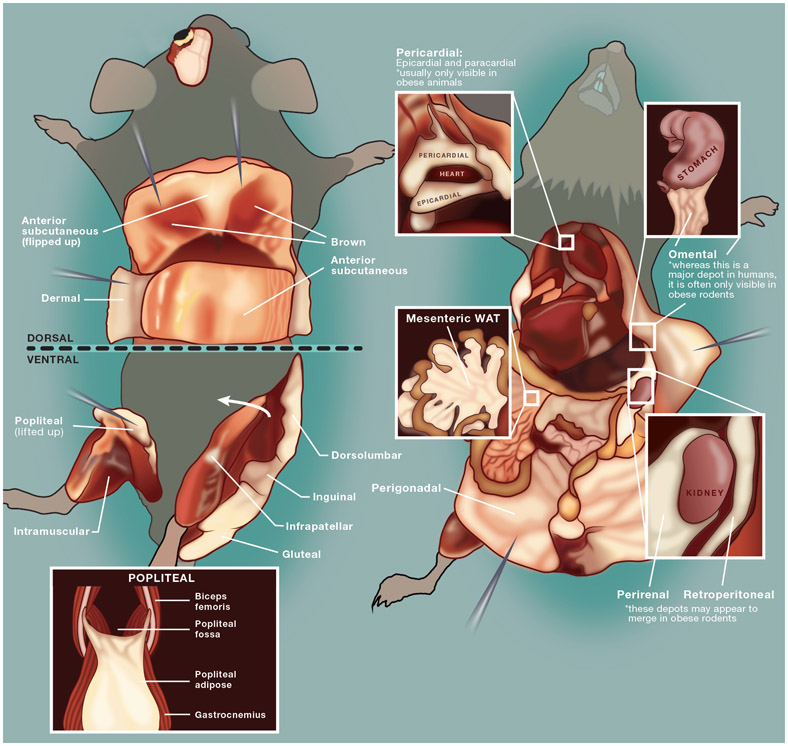
The goal of this manuscript is to provide investigators with a detailed protocol to clearly and precisely identify and isolate both prominent and less-studied mouse adipose depots (Figure 1). This resource will facilitate a more complete investigation of the developmental, molecular, and functional characteristics of adipocytes within diverse niches.
Figure 1: Schematic depiction of mouse adipose depots dissected in this protocol.
This image has been adapted from Bagchi et al., 20184.
Protocol
All animal procedures are performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan.
1. Euthanization
NOTE: For the purpose of this video protocol, 4 to 6 month-old C57BL/6J mice are used.
-
Place the mouse in an isoflurane vaporizer chamber and adjust the isoflurane flow rate to 5% or greater. Continue isoflurane exposure until one minute after breathing stops. Then remove the mouse from the vaporizer chamber and confirm euthanasia using an approved secondary measure.
NOTE: Approved secondary measures will vary by institution and animal protocol and may include cervical dislocation or decapitation.
Place the euthanized mouse on the dissection pan. Sterilize the dorsal and ventral external surfaces of the mouse using 70% ethanol. Ensure that the external surface of the mouse is sufficiently wet to minimize contamination from fur during dissection.
2. Identification and Isolation of Major Subcutaneous Adipose Ddepots (Anterior Subcutaneous, Dorsolumbar, Inguinal, Gluteal) and Interscapular Brown Aadipose Tissue
-
Identifying and isolating anterior subcutaneous WAT
NOTE: Anterior subcutaneous WAT is located between the scapulae, descending from the nape of the neck to the axillae of the mouse7. This depot has alternatively been described as suprascapular8 or interscapular20 WAT and lies directly on top of the interscapular BAT depot.- To isolate the anterior subcutaneous depot, lay the mouse on its stomach in a prone position. Secure the upper and lower limbs to the dissection pan with dissection pins.
- Use forceps to lift the dorsal skin at the nape of the neck. Use iris scissors to make a small (1 mm) cut in the skin.
- Insert one blade of the iris scissors into the initial cut and make a midline vertical incision (2–3 cm) through the skin, beginning at the nape of the neck and descending along the spine to mid-back.
- Make two horizontal incisions (1 cm each) using the iris scissors, extending laterally from midline, at the top and bottom of the initial vertical incision.
- Use forceps to carefully peel back the skin and expose the anterior subcutaneous depot.
-
Use iris scissors to remove the depot following the natural borders of the tissue.NOTE: This method isolates both anterior subcutaneous WAT and interscapular BAT.
- Place the dissected depot on the dissection pan and carefully remove the contaminating BAT using iris scissors.
-
Identifying and isolating classical BAT
NOTE: Classical BAT is located beneath the anterior subcutaneous WAT depot.- To isolate BAT, use iris scissors to cut horizontally along the bottom edge of the anterior subcutaneous tissue, following the natural border of the depot.
- Then, use iris scissors to make two vertical incisions along the lateral edges of the depot, following the natural borders of the tissue.
- Use forceps to carefully flip the depot up and reveal the butterfly-shaped interscapular BAT embedded within the WAT. Carefully dissect the BAT out from the surrounding WAT.
- Identifying and isolating posterior subcutaneous WAT,
- Lay the mouse on its back in a supine position.
- After securing the upper and lower limbs with dissection pins, use forceps to lift up the skin at the base of the sternum and make a small (1 mm) cut in the skin.
- Insert one blade of the iris scissors into the initial cut and make a midline incision (4—5 cm) through the skin, beginning at the base of the sternum and descending to the base of the tail. Exercise caution when making this incision because the ventral skin is very thin and is closely associated with the underlying peritoneal cavity wall.
- Make two horizontal incisions (1 cm each) using iris scissors, extending laterally from midline, at the top of the initial vertical incision.
-
Use forceps to carefully peel back the skin from the peritoneal cavity and the leg to find posterior subcutaneous WAT, which should remain associated with the skin. Secure the outstretched skin with dissection pins to ease complete excision of the WAT.NOTE: Although the posterior subcutaneous WAT appears to be continuous, it is actually comprised of three discrete depots: dorsolumbar, inguinal, and gluteal1. The dorsolumbar depot extends from the lumbar spine to the base of the hindlimb. The triangular inguinal depot extends from the base of the hindlimb ventrally across the groin and contains a prominent lymph node. The gluteal depot extends inferiorly from the base of the groin and wraps around the leg to the base of the tail.
3. Identification and Isolation of Visceral Adipose Depots (Gonadal, Perirenal, Retroperitoneal, Omental, Pericardial)
-
Identifying and isolating visceral WAT depots
NOTE: WAT depots, which are largely contained within the thoracic and peritoneal cavities, keep the mouse in a supine position. Secure the upper and lower limbs to the dissection pan with dissection pins.- Use forceps to lift up the thin peritoneal cavity wall at the base of the sternum and make a small cut (1 mm) using iris scissors.
- Insert one blade of the iris scissors into the cut and make a descending vertical incision (4–5 cm) from the top of the peritoneal cavity (base of the sternum) to the rectum.
- Make two horizontal incisions (1 cm each) with iris scissors, extending laterally from midline, at the top and bottom of the vertical incision.
- Use forceps to peel back the peritoneum and expose the abdominal cavity contents. Pin the outstretched peritoneum to the dissection pan.
-
Identifying and isolating gonadal WAT
NOTE: Gonadal WAT surrounds the uterus and ovaries in females (ovarian or parametrial) and the epididymis and testes in males (epididymal). Ovarian WAT surrounds the ovaries, uterus, and bladder. In obese animals, gonadal and perirenal WAT can appear to be continuous — in this case, separate the depots at the uterine horn and ovaries. Epididymal WAT is found bound to the epididymis, testes, and the prominent epididymal blood vessel.- Locate the gonads (testes or ovaries) and use forceps to lift up the associated gonadal WAT.
- Use iris scissors to carefully excise the WAT from the gonads.
-
Identifying and isolating perirenal WAT
NOTE: Perirenal WAT surrounds the kidneys bilaterally. In obese animals, this depot can appear to extend inferiorly to the top of the uterine horn and ovaries. Although the perirenal WAT has traditionally been classified as a visceral depot21, several groups have identified it as a brown depot based on lineage tracing and radiolabeled glucose uptake studies8,9,20. Histologically it is comprised of a mixture of white and brown adipocytes.- To excise the perirenal depot, locate the kidney and use forceps to lift it up and pull it midline to see a clear division between the perirenal and retroperitoneal depots.
- Excise the WAT directly associated with the kidneys. Ensure that the adrenal glands, located above the kidneys, are removed from the WAT.
-
Identifying and isolating retroperitoneal WAT
NOTE: Retroperitoneal WAT is located in a paravertebral position, along the border between the posterior abdominal wall and the spinal cord.-
To excise this depot, use forceps to lift the kidney up and towards midline to clearly see the border between the perirenal and retroperitoneal depots.NOTE: In obese animals, identifying this border can be challenging.
- Then, use iris scissors to carefully dissect the retroperitoneal WAT from the posterior peritoneal wall.
-
-
Identifying and isolating omental WAT
NOTE: Omental WAT is located along the greater curvature of the stomach. Although omental adipose is an important depot in humans, it is generally present only in morbidly obese mice.- To identify visceral omental WAT, use forceps to lift the stomach up. Use iris scissors to remove the associated adipose tissue along the inferior border of the stomach. Do not confuse omental WAT with the pancreas.
-
Identifying and isolating mesenteric WAT
NOTE: Mesenteric WAT is a web-like structure surrounding and associated with the small and large intestines.- To excise this depot, remove the intestines from the rest of the digestive tract by cutting at the base of the stomach and the rectum. Use forceps to lift the intestines out of the visceral cavity and unravel them.
- Use iris scissors to carefully dissect the mesenteric WAT away from the intestines, beginning at the duodenum and continuing to the end of the colon. Carefully remove lymph nodes that are closely associated with the mesenteric depot.
-
Identifying and isolating pericardial WAT
NOTE: Pericardial WAT is located outside the visceral pericardium and on the external surface of the parietal pericardium, often along the inferior aspect of the heart14.- To gain access to the thoracic cavity, keep the mouse in a supine position. Secure the upper and lower limbs to the dissection pan with dissection pins.
- Use forceps to lift the xyphoid process (cartilage at the base of the sternum) and make a small (1 mm) cut in the thoracic cavity wall.
- Insert one blade of the iris scissors into the cut and make two horizontal incisions (1 cm each) extending laterally from the base of the sternum. This will expose the diaphragm and inferior border of the thoracic cavity.
- Use dissecting scissors to make two ascending vertical incisions (3–4 cm) through the ribcage, extending superiorly from the lateral borders of the thoracic cavity to the clavicle.
- Use forceps to lift up the ventral half of the ribcage. Use dissecting scissors to make a final cut (2 cm) along the inferior length of the clavicle to remove the ribcage and expose the thoracic cavity contents.
- If present, carefully dissect the pericardial adipose from the outer surface of the pericardium.
4. Identification and Isolation of Other Adipose Depots
-
Identifying and isolating epicardial WAT
NOTE: Epicardial WAT is contained within the visceral pericardium and is directly associated with the surface of the myocardium.- If present, epicardial adipocytes can be observed histologically after isolation and fixation of the perfused heart in 10% neutral buffered formalin for 24 h.
-
Identifying and isolating popliteal WAT
NOTE: Popliteal WAT is located in the popliteal fossa in the posterior knee and contains a large lymph node. This depot is not typically visible in young animals.- To isolate popliteal WAT, use iris scissors to carefully remove the skin from the base of the hind limb to the foot.
- Place the patella against the dissection pan and secure the outstretched leg with a pin in the foot. Ensure that the popliteal fossa at the back of the knee is facing upward.
- Use iris scissors to make a cut at the inferior border of the medial and lateral heads of the gastrocnemius muscle. Use forceps to lift up the muscle and reveal the triangular popliteal depot.
- Use iris scissors to excise the depot along the natural tissue borders.
-
Identifying and isolating dermal WAT
NOTE: Dermal WAT is a thin layer of adipocytes located between the reticular dermis and the panniculus carnosus muscle layer.- To identify dermal adipocytes by histological methods, place the mouse on its stomach in a prone position. After securing the upper and lower limbs with dissection pins, spray the external surface of the mouse with 70% ethanol to wet the skin.
- Use a scalpel to remove the wetted fur from a square portion of skin on the back of the mouse.
- Use iris scissors to carefully excise the shaved portion of skin, which will include the reticular dermis, dermal WAT, panniculus carnosus, and some subcutaneous WAT.
- Use a scalpel to cut the excised skin into thin vertical strips.
- Position the thin vertical strips with the sticky subcutaneous WAT layer facing down.
- Starting at one end, roll the strip onto itself to form a spiral. The sticky subcutaneous WAT layer on the outside of the spiral will allow the roll to maintain its shape during fixation.
- Place the spiral in a well of a 24 well plate containing 10% neutral buffered formalin for 24 h prior to histological processing22.
-
Identifying and isolating intermuscular WAT
NOTE: Intermuscular WAT is broadly defined as adipocytes located beneath the deep fascia of muscles. This term includes adipocytes interspersed between muscle fibers of skeletal muscle, also known as intramuscular WAT, and adipocytes located within muscle bundles themselves. Wildtype mice generally do not have large numbers of intermuscular adipocytes. However, it is possible under certain conditions to identify intermuscular WAT by histological methods. Under some conditions, intermuscular adipocytes can also be found in smooth muscles, such as the diaphragm.- To isolate the tibialis anterior (TA) muscle, for example, place the mouse on its back in a supine position and secure the lower limbs to the dissection pan using dissection pins. Wet the skin with 70% ethanol to minimize contamination with fur.
-
Use iris scissors to carefully remove the skin from the leg and expose the quadriceps muscle group, located above the knee on the femur shaft, and the TA located below the knee on the ventral surface of the tibia.NOTE: The TA is thick near the proximal end of the tibia and more tendinous near the distal end.
- Use iris scissors to excise a piece of the muscle and fix in 10% neutral buffered formalin for 24 h prior to histological processing22.
-
Identifying and isolating intra-articular WAT depots
NOTE: Intra-articular WAT depots are located within synovial joints.- To identify infrapatellar WAT, for example, by histological methods, harvest leg bones as detailed in the BMAT section.
- After the removing the femur-tibial complex, use iris scissors and gauze pads to remove as much muscle and connective tissue as possible. Do not break the tibiofemoral joint.
- Fix the femur-tibial complex in 10% neutral buffered formalin for 24 h prior to decalcification and histological processing (described below, step 4.6.8).
-
Identifying and isolating bone marrow adipose tissue
NOTE: Bone marrow adipose tissue (BMAT) is contained within bones, interspersed with hematopoietic cells. Anatomically, BMAT can be classified as constitutive (distal tibia and caudal vertebrae) or regulated (mid-to-proximal tibia, femur, and lumbar vertebrae)10,11. Clean isolation of bone marrow adipocytes for RNA and protein analyses is challenging in mice. However, mouse bones can readily be harvested, fixed, decalcified and paraffin-embedded for histological analyses.- To harvest leg bones, for example, first remove the legs from the mouse. Use dissection scissors to cut the acetabulofemoral joint, keeping the femoral head intact.
- Use iris scissors to carefully remove the skin from the leg, revealing the leg muscles.
- Carefully dissect away the main muscles from the femur and tibia using iris scissors and gauze pads.
- When the femur is exposed, follow the edge of the bone to the articulation of the pelvis and femur and carefully release the femoral head from the acetabulofemoral joint. Use gauze to clean any remaining tissue from the femur.
- Follow the border of the tibia to the ankle joint. Carefully release the medial malleolus, located at the tip of the tibia, from the ankle joint.
- Once the femur-tibial complex has been isolated, remove as much muscle and connective tissue as possible using iris scissors and/or gauze.
- Separate the tibia from the femur by inserting one blade of the iris scissors into the tibiofemoral joint and gently cut through the medial and lateral collateral ligaments and the anterior and posterior cruciate ligaments. Do not remove the capsule of the knee joint to ensure that the tibia remains intact. Use gauze to clean any remaining tissue from the tibia.
- Fix bones in 10% neutral buffered formalin for 24 h at room temperature and then wash and store according to future needs.
- To analyze bone parameters using microcomputed tomography (μCT), store bones in Sorensen’s phosphate buffer, pH 7.423.
- For BMAT quantification and histological analyses, decalcify bones in 14% EDTA, pH 7.4, for 10-14 days.
Representative Results
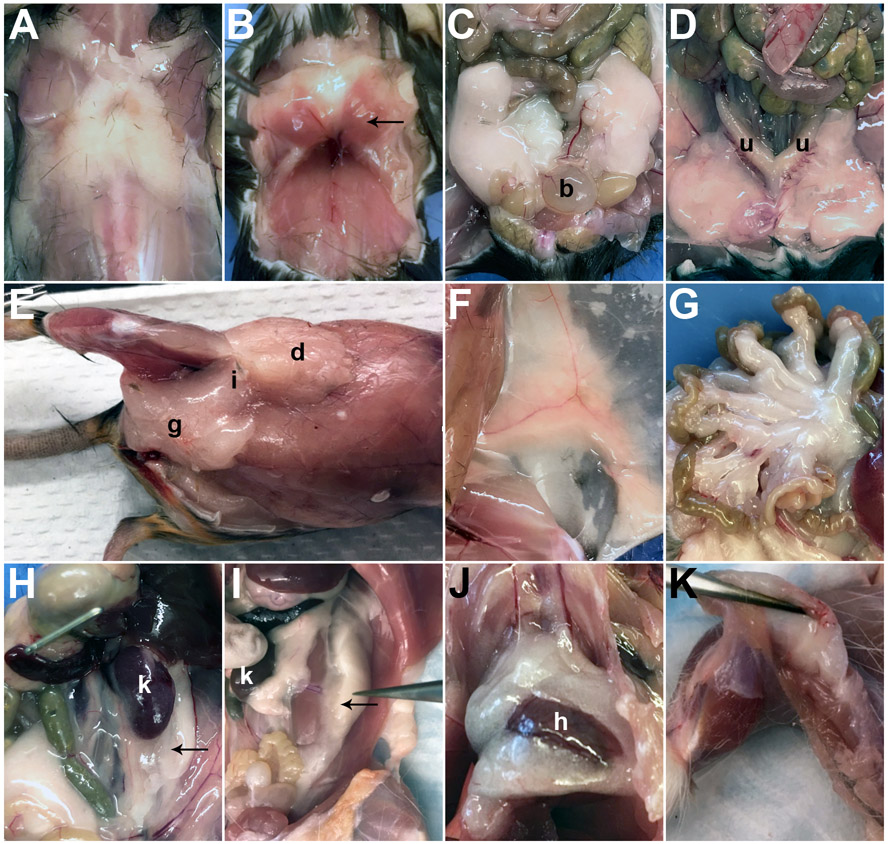
Successful identification and isolation of various mouse adipose depots can be achieved using the protocol described above. The gross anatomical locations of subcutaneous (A, E-F), brown (B), visceral (C, D, G-J), and popliteal (K) depots are shown in Figure 2.
Figure 2: Gross anatomical locations of mouse adipose depots.
Gross anatomy of (A) anterior subcutaneous, (B) brown, (C) epididymal (b, bladder), (D) ovarian (u, uterine horns), (E) posterior subcutaneous (dorsolumbar (d), inguinal (i), gluteal (g)), (F) inguinal, (G) mesenteric, (H) perirenal (k, kidney), (I) retroperitoneal (k, kidney), (J) pericardial (h, heart), and (K) popliteal adipose depots in C57BL/6J adult mice. Arrows point to specific depots if multiple depots are depicted. Relevant organs are designated by appropriate letters.
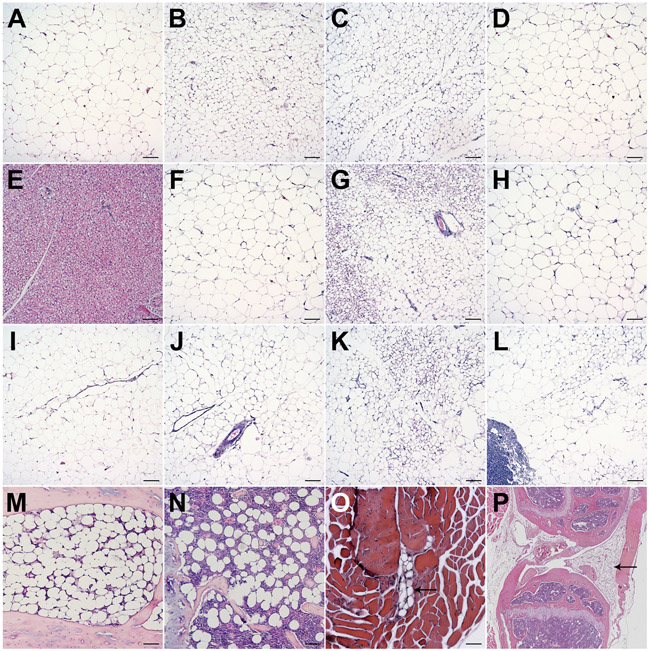
Histological characteristics of subcutaneous (A-D), brown (E), visceral (F-K), popliteal (L), constitutive (M) and regulated marrow adipose (N), intramuscular (O), and infrapatellar (P) adipose depots were evaluated by Hematoxylin and Eosin (H&E) staining22 and are shown in Figure 3.
Figure 3: Histological evaluation of discrete mouse adipose depots.
Histology of (A) anterior subcutaneous, (B) dorsolumbar, (C) inguinal, (D) gluteal, (E) brown, (F) gonadal, (G) perirenal, (H) retroperitoneal, (I) omental, (J) mesenteric, (K) pericardial, (L) popliteal, (M) constitutive and (N) regulated bone marrow adipose, (O) intermuscular, and (P) infrapatellar depots in C57BL/6J adult mice. Tissues were isolated according to the given protocol, fixed overnight in 10% neutral buffered formalin, processed, and embedded in paraffin. 5 μm sections were stained with H&E. Most images were taken at 100x magnification; Scale bar = 100 μm.
Discussion
As the importance of the diverse molecular and functional characteristics of discrete adipocyte clusters is increasingly recognized, it is crucial that investigators within the field uniformly identify and excise adipose depots for further analyses. To date, few protocols exist for standardized localization and isolation of the wide range of mouse adipose depots. Previously published methods are focused primarily on one or two depots and lack the details necessary for uniform identification and excision by different investigators18,19. This manuscript is a novel and important contribution to the field of adipose biology since it provides an in-depth guide to the anatomic location and precise dissection of commonly studied and lesser known depots throughout the mouse. Gross anatomical location and histological analyses are provided to demonstrate the diversity of adipocyte niches.
Successful isolation of discrete adipose depots using this protocol is dependent on several critical steps. Maintenance of a clean dissection environment is crucial; 70% ethanol can be used as necessary to remove contaminating hairs or blood from the dissection tray and tools. When isolating posterior subcutaneous WAT, it is imperative that the initial incision in the skin does not penetrate the closely associated peritoneal cavity so that the two layers can be efficiently separated to expose the WAT. Similarly, when cutting through the peritoneal wall to expose the abdominal cavity contents, incisions must not perforate the underlying intestines to prevent contamination with digestive elements. Interscapular BAT must be carefully excised from the surrounding tissue to prevent WAT contamination, which will skew future analyses. Consistent identification of the posterior subcutaneous dorsolumbar, inguinal, and gluteal depots depends on the utilization of precise anatomic landmarks described in the protocol. The distinction between these depots is particularly important; although the depots can appear to be continuous, centrally located inguinal adipocytes acquire brown-like characteristics more readily than their surrounding counterparts.
The adipose tissues isolated using this protocol can be analyzed using a variety of techniques. In addition to histological evaluation (e.g. H&E staining, immunohistochemistry), adipose tissues can be used for a wide array of molecular analyses, from regulation of transcription through to posttranslational protein modifications. Adipocytes and stromal vascular cells within individual depots can be isolated by collagenase digestion and differential centrifugation. These fractions can then be used for molecular, metabolic, and cell culture studies. Depot explants can also be used for ex vivo metabolic and enzymatic assays.
Several challenges and limitations arise during the isolation and excision of mouse adipose tissues. First, some depots that are physiologically important in humans are not commonly present in lean, adult mice. For example, omental WAT, which is the major visceral depot in humans, can only be seen in genetically or diet-induced obese mice. The expansion of some depots can, however, be induced by nutritional challenges or drug treatments. For example, high fat diet feeding can cause the expansion of omental and pericardial WAT. Intermuscular WAT can be induced by skeletal muscle injury25,26 or high fat diet27. Regulated BMAT expands in response to various challenges, including high fat diet, calorie restriction and thiazolidinedione treatment11,28. Second, due to the small size of mice, obtaining enough sample for molecular or metabolic analyses can be difficult. For example, isolation of enough pure bone marrow adipocytes for subsequent analyses is challenging, even after pooling bones from multiple mice. Third, accurately defining borders of certain depots can be difficult. For example, posterior subcutaneous WAT appears to be one continuous depot but is actually comprised of discrete dorsolumbar, inguinal, and gluteal depots. Additionally, the perirenal can appear to be fused to both the gonadal and retroperitoneal depots in very obese animals. The lack of distinct borders between adjacent depots can make clean isolation of these tissues challenging. However, this dissection protocol provides investigators with a detailed atlas and step-by-step guidance for accurate and reproducible dissection of a wide range of mouse adipose depots. Field-wide standardization of the identification and isolation of discrete mouse adipose depots described above will undoubtedly help further elucidate differences in the development, gene expression, and local and systemic functions of previously under-studied adipocyte niches.
Acknowledgments
O.A.M. is supported by NIH grants DK062876 and DK092759; D.P.B. is supported by the University of Michigan Medical Scientist Training Program (T32GM007863), University of Michigan Training Program in Organogenesis (T32HD007605), University of Michigan Rackham Merit Fellowship, and Tylenol Future Care Fellowship.
Footnotes
Disclosures
The authors have nothing to disclose.
Video Link
The video component of this article can be found at https://www.jove.com/video/59499/
References
- 1.Cinti S The adipose organ at a glance. Disease Models & Mechanisms. 5 (5), 588–594 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM What we talk about when we talk about fat. Cell. 156 (1-2), 20–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Gurmaches J, Guertin DA Adipocyte lineages: tracing back the origins of fat. Biochimica et Biophysica Acta. 1842 (3), 340–351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagchi DP, Forss I, Mandrup S, MacDougald OA Snapshot: Niche Determines Adipocyte Character I. Cell Metabolism. 27 (1), 264–264 e261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchkonia T et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metabolism. 17 (5), 644–656 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajimura S, Spiegelman BM, Seale P Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metabolism. 22 (4), 546–559 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frontini A, Cinti S Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metabolism. 11 (4), 253–256 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Zhang F et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metabolism. 27, 252–262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Gurmaches J, Guertin DA Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nature Communications. 5, 4099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Hardij J, Bagchi DP, Scheller EL, MacDougald OA Development, regulation, metabolism and function of bone marrow adipose tissues. Bone. 110, 134–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA Marrow Adipose Tissue: Trimming the Fat. Trends in Endocrinology & Metabolism. 27 (6), 392–403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander CM et al. Dermal white adipose tissue: a new component of the thermogenic response. The Journal of Lipid Research. 56 (11), 2061–2069 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruglikov IL, Scherer PE Dermal Adipocytes: From Irrelevance to Metabolic Targets? Trends in Endocrinology & Metabolism. 27 (1), 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobellis G Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nature Reviews Endocrinology. 11 (6), 363–371 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Addison O, Marcus RL, LaStayo PC, Ryan AS Intermuscular Fat: A Review of the Consequences and Causes. International Journal of Endocrinology. 2014, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pond CM Adipose tissue and the immune system. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 73 (1), 17–30 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Kloppenburg AI-FM An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Research & Therapy. 15 (225), 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann A, Thompson A, Robbins N, Blomkalns AL Localization, Identification, and Excision of Murine Adipose Depots. Journal of Visualized Experiments. (94), e52174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casteilla L, Cousin B, Calise D Choosing an adipose tissue depot for sampling: factors in selection and depot specificity. Methods in Molecular Biology. 155, 1–22 (2008). [DOI] [PubMed] [Google Scholar]
- 20.de Jong JM, Larsson O, Cannon B, Nedergaard J A stringent validation of mouse adipose tissue identity markers. American Journal of Physiology-Endocrinology and Metabolism. 308 (12), E1085–1105 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Cinti S The adipose organ. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 73 (1), 9–15 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Parlee SD, Lentz SI, Mori H, MacDougald OA Quantifying size and number of adipocytes in adipose tissue. Methods in Enzymology. 537, 93–122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheller EL et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nature Communications. 6, 7808 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheller EL et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods in Enzymology. 537, 123–139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukjanenko L, Brachat S, Pierrel E, Lach-Trifilieff E, Feige JN Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS One. 8 (8), e71084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagano AF et al. Muscle Regeneration with Intermuscular Adipose Tissue (IMAT) Accumulation Is Modulated by Mechanical Constraints. PLoS One. 10 (12), e0144230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan IM et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. International Journal of Obesity. 39 (11), 1607–1618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulston RJ et al. Increased Circulating Adiponectin in Response to Thiazolidinediones: Investigating the Role of Bone Marrow Adipose Tissue. Frontiers in Endocrinology. 7, 128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]