Abstract
Our understanding of reflex regulation of veins lags behind that of the arterial system. While the cardiac sympathetic afferent reflex (CSAR) exerts control over sympathetic outflow, its effect on venous tone is not known. We tested the hypothesis that activation of pericardial bradykinin sensitive afferents elicits systemic venoconstriction. Male and female Sprague Dawley rats were chronically instrumented for measurement of arterial pressure and mean circulatory filling pressure, an index of venous tone, and with an indwelling pericardial catheter. Mean arterial pressure, heart rate and mean circulatory filling pressure responses were assessed in conscious rats in response to graded pericardial injections of bradykinin (1.5–20 μg/kg) before and after ganglionic blockade, and to intravenous norepinephrine (0.05–0.8 ug/kg). Bradykinin B2 receptor was assessed by Western blot. Pericardial bradykinin injections caused graded increases in mean arterial pressure, heart rate and mean circulatory filling pressure. These responses were markedly attenuated after autonomic blockade. The increments in mean circulatory filling pressure were attenuated in female rats. There were no differences in the venoconstrictor responses to norepinephrine or ventricular bradykinin receptor expression between male and females. We interpret these findings to indicate that activation of bradykinin sensitive pericardial afferents elicits a sexually dimorphic, autonomically mediated systemic venoconstrictor response. Differences in venous smooth muscle responses to norepinephrine or ventricular bradykinin receptor expression do not account for the sexual dimorphism. We conclude that systemic venoconstriction contributes to the overall hemodynamic response to activation of the cardiac sympathetic afferent reflex and that this effect is sexually dimorphic.
Keywords: cardiac sympathetic afferent reflex, venous tone, blood pressure, sex
1. Introduction
The venous compartment holds approximately 60–75% of the total blood volume and, consequently, plays a major role in the control of venous return, and cardiac output [1–3]. Data from several groups[1, 4–6], including ours[7–9], show that veins participate in blood pressure control. The sympathetic nervous system is a key controller of venous function[1, 10]. Dysregulation of sympathetic control of venous function has been linked to conditions such as heart failure[11, 12], hypovolemia[6] and hypertension [1, 9]. While it is generally accepted that the sympathetic nervous system plays a key role in the control of venous function, the mechanisms that control sympathetic drive to the venous compartment remain less well understood.
The arterial baroreceptor reflex modulates sympathetic drive to the venous compartment. Unloading of the carotid sinus baroreflex decreases venous capacitance[13] whereas increasing carotid sinus pressure increased venous capacitance [14]. Subsequent work used direct denervation to show that venular responses to manipulation of carotid sinus pressures were mediated via the sympathetic nervous system[15]. Greene showed that arterial baroreflex-induced changes in venous return were sufficient to alter cardiac output by up to 40%[2]. More recent work showed that electrical stimulation of the carotid sinus nerve increased venous capacitance while simultaneously decreasing ventricular end diastolic pressure, consistent with an effect on cardiac filling [16]. Arterial baroreflex control of venous function largely involves changes in venous tone, with a lesser effect on venous resistance [10]. In contrast to study of the arterial baroreflex, investigation of the influence of other cardiovascular reflexes modulating venous tone remains sparse.
The heart has an important sensory function via a variety of cardiac receptors that modulate cardiovascular function by neural and humoral reflexes. Ventricular cardiogenic reflexes may be excitatory or depressant on the SNS. Depressant reflexes arise from cardiac receptors with afferents chiefly in the vagus[17, 18]. Activation of this reflex decreased SNS activity and blood pressure[17–19]. This depressant reflex was tied to venous control. Disruption of vagal afferents decreased venous capacitance[20], implying a tonic venodilator effect. Conversely, activation of cardiac vagal afferents decreased venous tone[20–23] and ventricular filling[24]. Thus, cardiac vagal afferents affect venous tone, venous return and cardiac filling. The heart also elicits excitatory responses via cardiac sympathetic afferents resulting in increased SNS activity, tachycardia, hypertension and arterial vasoconstriction[25–27]. This cardiac sympathetic afferent reflex is activated by a number of endogenous stimuli such as hydrogen peroxide or bradykinin released during challenges such as cardiac ischemia [26, 28]. These responses can be also be evoked experimentally by application of bradykinin to sensory afferents in the epicardial layers of the left ventricle [19, 29–31]. While the CSAR has powerful effects on sympathetic outflow[28, 32], its influence on venous tone is not known. This is an interesting question because sympathetic control of the arterial and venous compartments may be distinct. Different sympathetic nerves project to splanchnic arteries and veins [33]. Moreover, sympathetic control of arteries and veins is different in this region [34, 35]. Accordingly, this work was undertaken to test the hypothesis that activation of pericardial bradykinin sensitive afferents elicits systemic venoconstriction. Recent work suggested that estrogen may suppress cardiac afferent modulation of sympathetic drive[36]. Therefore, we compared responses in male and female rats.
2. Materials and Methods
2.1. Surgeries
Sprague Dawley rats were purchased from Harlan Sprague Dawley (Invigo). The rats were maintained in the Animal Resource Center at the University of South Dakota on a 12 hour day/night cycle and allowed free access to rat chow and tap water. The Institutional Animal Care and Use committee of the University of South Dakota reviewed and approved all procedures involving these animals. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Following acclimatization age matched male SD rats (300–400 grams) and female rats (200–300 grams) were used in the following surgical protocols.
An indwelling pericardial catheter was used to deliver bradykinin into the pericardial space to activate bradykinin sensitive afferents[29]. Approximately 1 week before the planned experiments, the rats were anesthetized with isoflurane, intubated and placed on a rodent ventilator (Harvard Apparatus). The left chest was opened at the 4th intercostal space to reveal the pericardial sac after reflection of the thymus gland. A PE10 catheter was introduced into the pericardial space and approximated to the ventral surface of the left ventricle. A small drop of cyanoacrylic glue was used to cement the catheter in place and seal the hole in the pericardial membrane. The chest was closed in layers, the catheter was tunneled subcutaneously and exteriorized at the nape of the neck. The chest was evacuated and the rat was weaned off the ventilator until it resumed spontaneous respiration. The animals were allowed to recover for at least a week from this surgery. Two days before the experimental protocol, the rats were again anesthetized with isoflurane and instrumented with femoral arterial and venous catheters for the measurement of arterial pressure and heart rate. The rats were also instrumented with a balloon tipped catheter introduced into the right atrium via the jugular vein. This catheter was used for the periodic transient interruption of venous return for the estimation of mean circulatory filling pressure as described below. The catheters were tunneled subcutaneously to the nape of the neck and the rats allowed to recover. Mean arterial blood pressure and heart rate were recorded from conscious freely moving rats 48–72 hours later.
2.2. Measurement of Mean Circulatory Filing Pressure
The mean circulatory filling pressure (MCFP) is an integrated estimate of overall venous tone[37]. MCFP was measured as we described previously[38]. Briefly, MCFP can be determined by recording the plateau arterial (PAP) and plateau venous (PVP) pressures during a brief interruption of cardiac output. The right atrial balloon was transiently inflated (4–5 seconds) to interrupt cardiac output during which time arterial pressure fell and venous pressure increased. The PAP and PVP were recorded between the 4th and 5th second after which the balloon was rapidly deflated. Arterial blood pressure and heart were allowed to return to control levels between successive MCFP measurements. MCFP was calculated as MCFP = [PVP + (PAP - PVP) / 60][37].
2.3. Experimental Protocol
Mean arterial pressure (MAP), heart rate (HR) and venous pressure were recorded from conscious unrestrained rats housed in their home cages. Baseline values for these variables were recorded for at least one hour or until the rats were calm and values were stable. Graded doses of bradykinin (1.5–20 μg/kg) were injected (20μl) into the pericardial space. MAP, HR and MCFP responses to pericardial bradykinin were recorded. The rats were allowed to fully recover (~25–30 minutes) prior to the next dose. In some rats, the responses to pericardial bradykinin were obtained after ganglionic blockade (chlorisondamine 10 mg/kg + atropine 0.4 mg/kg). MAP, HR and MCFP responses to intravenous infusion of norepinephrine (0.05–0.8 μg/kg/min) were assessed in a separate cohort of rats.
2.4. Bradykinin Receptor Expression
Hearts were collected from bradykinin naïve male and female Sprague Dawley rats for the assessment of bradykinin receptor expression. A small portion of the left ventricle was then processed for Western blot as previously described, with some minor modifications [39]. The tissue samples were homogenized in homogenization buffer (50mM Tris pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% Deoxycholic acid, 0.1% SDS, 2 mM EDTA, 10 mM NaF, 10 ug/ml leupeptin, 10 ug/ml pepstatin, and 20 ug/ml aprotinin). Equal amounts of protein (45 μg/well) were loaded onto each lane of 12% acrylamide gels, which were then subjected to SDS-PAGE electrophoresis and transferred to PVDF membranes (Bio-Rad). The membranes were probed with bradykinin B2 receptor antibody (BDKRB2; ThermoFisher) (1:500). GAPDH (Abcam 9484; 1:5000) served as a loading control. The protein bands were visualized using fluorescently-labeled secondary antibodies and subsequent image detection (Odyssey Imager, LiCor Biosciences), then quantified by densitometric analysis software on a Licor. Data were expressed as the ratio of BDKRB2 to GAPDH.
Data Analysis
Baseline data and Western blot data were analyzed using an unpaired t-test. Responses to pericardial bradykinin injection or intravenous norepinephrine infusion were analyzed by Two-Factor ANOVA for repeated measures (grouping factors: treatment, sex). Post hoc comparisons were performed with the Sidak-Holm test to correct for multiple comparisons. Values are presented as the mean±SEM.
3. Results
3.1. Baseline Values
Baseline values are shown in table 1. Despite being matched in age, male SD had a significantly greater body weight than female SD (333±8 vs 252±6 grams). For the pericardial injection cohort, (male n=10; female n=11) MAP (male:110±3 vs female: 109±5 mm Hg) and heart rate (male: 371±7 vs female: 376±8 bpm) were not significantly different. On the other hand, MCFP was significantly greater in male (7.5±0.3 mm Hg) compared to female (6.5±0.3 mm Hg) SD rats. In the cohort used for intravenous norepinephrine infusions (male n= 9; female n=9) MAP was modestly but significantly higher in male (117±3 mm Hg) vs females (107±3 mm Hg) as was MCFP male:7.6±0.3 vs female: 5.8±0.3 mm Hg) whereas HR was somewhat higher in females (406±10 bpm) compared to males (373±10 bpm).
Table 1.
Baseline Values
| Group | Pericardial Injection Cohort Pre Ganglion Blockade | Pericardial Injection Cohort Post Ganglion Blockade | Norepinephrine Infusion Cohort | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Mean Arterial Pressure (mm Hg) | 110±3 | 109±5 | 69±1* | 68±6* | 117±3 | 107±3 ω |
| Heart Rate (bpm) | 371±7 | 376±8 | 306±8* | 248±19* ω | 373±10 | 406±10 ω |
| Mean Circulatory Filling Pressure (mm Hg) | 7.5±0.3 | 6.5±0.3 ω | 6.5±0.3* | 5.1±0.3* ω | 7.6±0.3 | 5.8±0.3 ω |
p<0.05 preganglionic blockade vs postganglionic blockade
p<0.05 male vs female
3.2. Reponses to Pericardial Injections
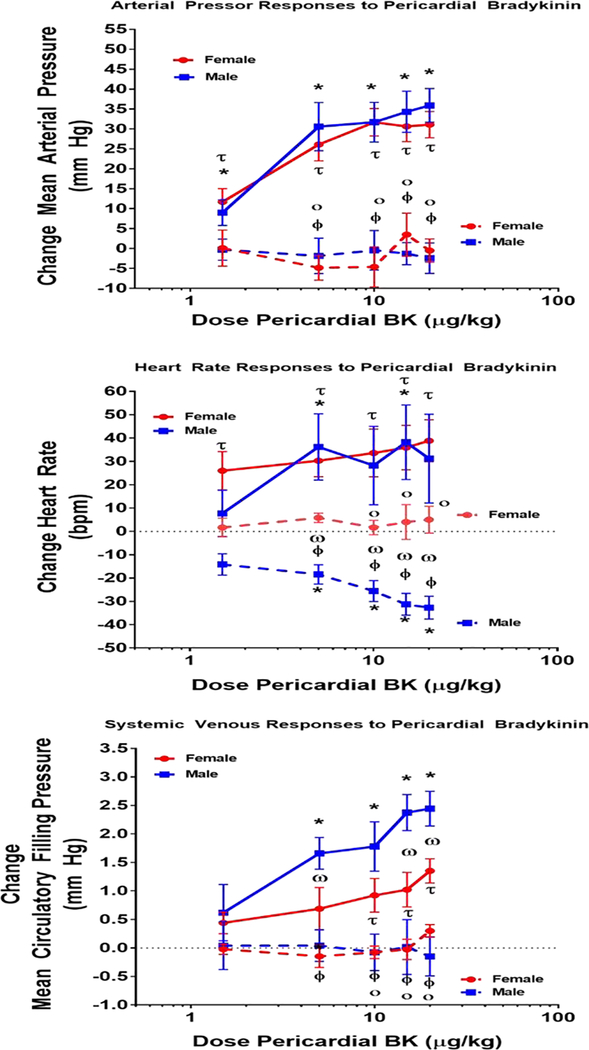
Pericardial injection of saline vehicle did not affect the monitored variables (Males: n=3 MAP 2±2 mm Hg; HR 8±4 bpm; MCFP −0.1±0.1 mm Hg, Females: n=3 MAP 4±2 mm Hg, HR 3±7 bpm, MCFP −0.2±0.1 mm Hg). Pericardial injection of bradykinin was associated with graded increases in MAP, HR and MCFP. These responses reached a plateau in 1– 2 minutes after injection and began to wane about 5 minutes following injection. In male rats, the arterial pressor responses ranged between 9±3 to 36±4 mm Hg. Similar pressor responses were observed in female rats with increases ranging between 12±3 to 31±3 mm Hg. ANOVA and posthoc analysis detected a significant effect of pericardial bradykinin injection on blood pressure in both groups, however there were no differences in responses between male and female rats (Figure 1). The heart rate responses to pericardial bradykinin were quite variable. In males, the tachycardic response to pericardial bradykinin ranged between 8±10 to 38±16 bpm whereas in females these responses spanned from 26±8 to 39±9 bpm (Figure 1). These responses represented significant elevations from baseline heart rate, however these responses were not different between males and females. Pericardial bradykinin injections were also associated with significant increases in MCFP, indicative of systemic venoconstriction. The changes in MCFP in response to pericardial bradykinin injection ranged from 0.6±0.5 to 2.4± 0.3 mm Hg in male rats. In females the responses ranged between 0.4±0.12 to 1.4±0.2 mm Hg and overall these represented significantly smaller responses compared to males.
Figure 1: Responses to Pericardial Bradykinin.
This figure shows the mean arterial pressure (mm Hg; top panel, heart rate (bpm; middle panel) and mean circulatory filling pressure (mm Hg; bottom panel responses to injection of bradykinin into the pericardial space in conscious male and female rats. p< 0.05 * versus pre injection baseline male; Ƭ versus pre injection baseline female; Ф pre vs post ganglion blockade male; ο pre vs post ganglion blockade male; ω male vs female.
3.3. Effect of Ganglionic Blockade
Interruption of autonomic function via ganglionic blockade decreased baseline values for MAP, HR and MCFP. Following ganglion blockade MAP and HR were not different between the groups (MAP: Males; 69±1 mm Hg vs Females; 68±6 mm Hg). Although MCFP decreased significantly in both males and females in response to ganglionic blockade, MCFP remained significantly higher in males (6.5±0.3 mm Hg) compared to females (5.1±0.3 mm Hg). After ganglion blockade, the MAP, HR and MCFP responses to pericardial bradykinin were greatly attenuated. MAP responses to pericardial injection of bradykinin ranged from −0.3±3 mm Hg to −2±4 mm Hg in male rats. In female rats subjected to ganglion blockade, MAP responses to pericardial bradykinin spanned from 0.12±5 mm Hg to 4±5 mm Hg. MAP did not increase significantly above baseline in either group. Similarly, the MCFP responses to pericardial injection of bradykinin were essentially abolished after ganglionic blockade (Males; 0.0±0.4 to −0.2± 0.3 mm Hg vs Females; −0.0±0.1 to 0.4±0.1 mm Hg). Thus ganglionic blockade abolished the pressor responses to pericardial injection of bradykinin. In female rats, ganglionic blockade also abolished the tachycardic response to pericardial bradykinin injections (2±4 bpm to 5±6 bpm). Unexpectedly, after ganglionic blockade pericardial bradykinin injections were associated with significant decreases in heart rate in male rats (−14± 6 to −32±5 bpm). These responses were significantly different from those observed in the female rats.
3.4. Reponses to Norepinephrine
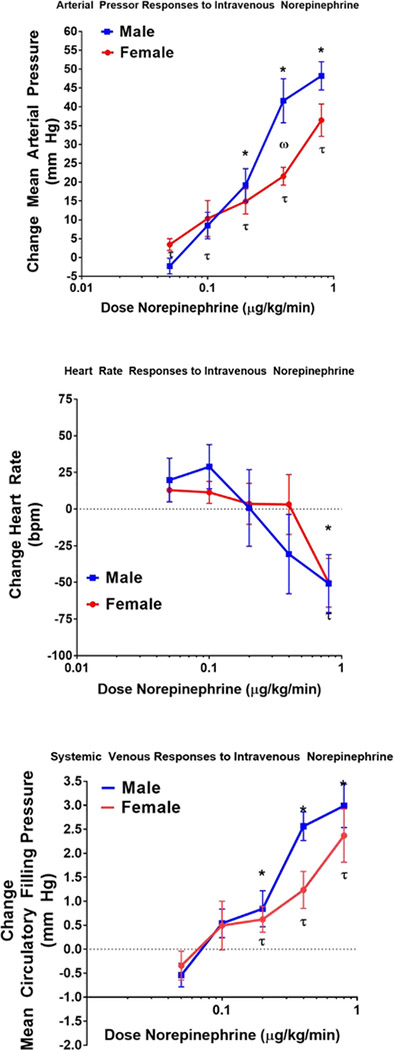
Intravenous administration of norepinephrine (NE) was associated with graded increases in MAP, and MCFP, and decreases in HR. In male rats, the pressor responses ranged between −2±2 to 47±4 mm Hg. Pressor responses observed in female rats were modestly smaller ranging between 3±2 to 36±4 mm Hg. Nevertheless, these pressor responses to NE were only statistically significant between males and females at a single dose. The pattern of heart rate responses to NE was similar in male and female rats consisting of an initial small rise in heart rate following by a bradycardic response at higher doses of NE. Intravenous NE elicited a systemic venoconstrictor response as MCFP increased in both males and female rats. In males the changes in MCFP ranged from −0.7±0.3 to 2.9± 0.5 mm Hg in male rats while in females the responses ranged between −0.3±0.3 to 2.4±0.6 mm Hg. Although there was a significant increase in MCFP in both males and females, these responses were not significantly different.
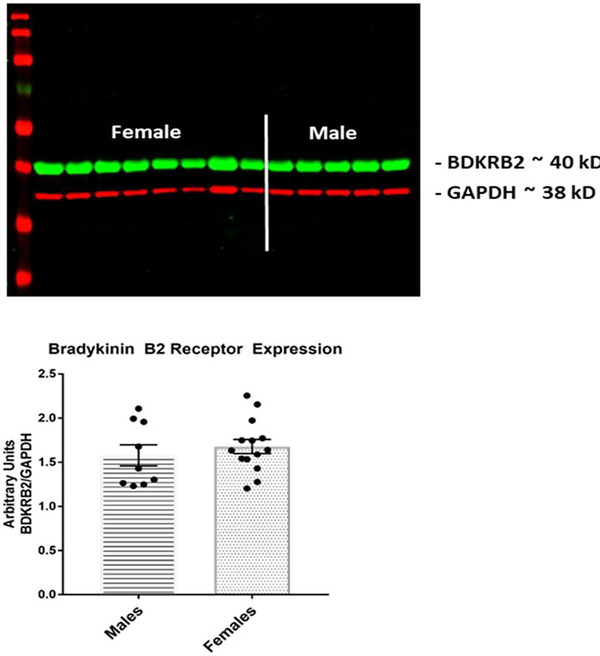
3.5. Bradykinin Receptor Expression
Figure 3 shows a Western blot image and summary data for bradykinin B2 receptor expression in the left ventricle. Bradykinin receptor expression was not significantly different between male and female Sprague Dawley rats.
Figure 3: Ventricular Bradykinin Receptor Expression.
This figure shows BDKRB2 receptor expression in ventricular tissue from male and female rats. The upper panel is an example of a Western blot membrane. The lower panel shows summary data expressed as the ratio of BDKRB2 and the housekeeping protein GAPDH.
4. Discussion
In the present work, we tested the hypothesis that activation of bradykinin sensitive afferents in the heart elicits systemic venoconstriction as assessed by measuring mean circulatory filling pressure. The data demonstrate the following: 1) Pericardial injection of bradykinin elicited an increase in MCFP, indicative of systemic venoconstriction. 2) Interruption of autonomic function abolished these venoconstrictor effects. 3) These responses were attenuated in female versus male Sprague Dawley rats. 4) There were no differences in left ventricular bradykinin B2 receptor expression between male and female Sprague Dawley rats. Together, these findings are consistent with the view that, in addition to their other actions on the cardiovascular system, the cardiac sympathetic afferents contribute to the control of peripheral venous tone.
Convincing data shows that the venous compartment plays an important role in circulatory regulation by controlling venous return, cardiac filling and cardiac output [1, 3, 16]. A major mechanism controlling venous tone and thus venous return is sympathetic drive to the veins system [1]. The CSAR is recognized as a powerful sympathoexcitatory reflex originating from the heart [26, 28, 40]. The CSAR is invoked by activation of chemoreceptors in the ventricular wall that respond to endogenous factors such as adenosine, bradykinin, and reactive oxygen species (e.g. hydrogen peroxide [28, 40]. These substances are released in response to stimuli such as myocardial ischemia [26, 28, 40], presumably as a mechanism to increase coronary blood flow via increases in pressure gradient or cardiac output. Accordingly, we hypothesized that activation of the CSAR by stimulating bradykinin sensitive afferents on the heart would increase venous tone. We chose to assess systemic venous tone by measuring the mean circulatory filling pressure (MCFP). We showed previously that MCFP is increased in settings of increased sympathetic drive [41–43], consistent with the view that the MCFP is a reliable estimate of systemic venous tone [37].
It is relatively well established activation of the CSAR by application of bradykinin to the epicardial surface of the heart elicits a strong sympathetically mediated pressor response [19, 28, 32, 44, 45]. Many of the studies investigating the effects of the CSAR on cardiovascular function have used open chest vagotomized preparations to allow application of bradykinin directly to the epicardial surface. However, given the sensitivity of the venous circulation to changes in chest pressure, we chose to perform our studies in baroreflex intact conscious rats to avoid potential confounds in the interpretation of the venous pressure responses. Our approach most closely resembles that described by McDermott et al. [29] which reported that intrapericardial injection of bradykinin in awake rats caused dose dependent increases in blood pressure with a maximum effect of 25–35 mm Hg. We observed a graded pressor response ranging between 10–35 mm Hg, which compares favorably with previously reported responses in conscious rats. Our observations also align with findings in a broader context of anesthetized vagotomized animals and using different methods for activation of the cardiac sympathetic afferents [19, 44, 46, 47]. Thus, despite methodological differences, we believe our findings are generally consistent with the established literature for bradykinin activation of the CSAR.
Coupled with the pressor and tachycardic responses, we also observed dose dependent increases in MCFP in response to pericardial injection of bradykinin. These responses ranged between 0.6 to 2.2 mm Hg. Although these are seemingly small changes in pressure, it is important to recognize that the venous compartment operates at substantially lower pressures/resistances than the arterial compartment. Thus, even small changes in venous pressure can have substantial impact on venous function. The changes in MCFP that we observed represent changes ranging from 6–28% of baseline MCFP. Moreover, Greene has reported that in anesthetized dogs, the maximum baroreflex change in MCFP was approximately 3.4 mm Hg and that this MCFP response altered cardiac output by 40% [2]. Accordingly, the CSAR-induced increases in MCFP observed in our study could be predicted to increase CO by as much as 26%. In fact, epicardial application of bradykinin increased left ventricular end diastolic volume and cardiac output in rats with heart failure [44]. Thus activation of bradykinin sensitive afferents in the pericardial space induces a venoconstrictor response of sufficient magnitude to influence systemic hemodynamics.
Another important consideration is that the present study was conducted in conscious rats with intact arterial and vagal baroreflexes. Given the relatively large pressor responses to activation of the CSAR, it is possible that pressor and venoconstrictor responses to pericardial bradykinin injection were damped to a certain degree by reflex compensatory mechanisms. Consistent with this possibility, denervation of arterial baroreceptors or cervical vagotomy potentiated the pressor response to activation of the CSAR by epicardial application of capsaicin [48] suggesting that these reflex mechanisms inhibit the CSAR. On the other hand, it was also shown that activation of the CSAR inhibited the arterial baroreflex [49]. Thus, the extent to which any reflex compensatory mechanisms impacted our observations is unclear. Nevertheless, we believe that collectively these data suggest that the CSAR is capable of inducing marked increases in venous tone and is an important reflex system regulating venous function.
It is generally accepted that sex affects cardiovascular function, yet the interaction of sex with circulatory function remains complex and under active investigation. In the present work we assessed the impact of sex on the CSAR by comparing responses in male and female rats. While we did not observe a difference in blood pressure between male and female rats, MCFP was significantly lower in females compared to males. This finding is supported by our earlier work [38, 50] and that of others showing venous tone as assessed by MCFP was lower in female rats or by treatment with estrogen[51]. We observed that the pressor and tachycardic responses to pericardial injection of bradykinin with an intact autonomic nervous system were not different in male and female rats. This is consistent with previous work showing that the increases in blood pressure and heart rate to epicardial application of capsaicin were not sexually dimorphic [36]. However, following ganglionic blockade, surprisingly, we observed that pericardial bradykinin injections were associated with a reduction in heart rate in male rats, a response that was not observed in female rats. A direct negative chronotropic effect of bradykinin was reported in dogs[52], however in rats a positive chronotropic effect dependent on sympathetic ganglionic stimulation was reported [53]. It is possible that in the presence of a ganglion blocker, a direct negative chronotropic effect was revealed. To our knowledge there are no reports addressing sex differences in the direct chronotropic effects of bradykinin. Given that we did not observe a similar response in female rats, the response is not related to injection volume or vehicle, but may be sexually dimorphic. However, at present this observation is challenging to explain. We also observed that the venoconstrictor responses to injection of bradykinin into the pericardial sac were attenuated in female compared to male rats. To our knowledge, this is the first report of sex differences in CSAR reflex control of venous tone. The physiological implications of the reduced CSAR-mediated venoconstriction remain to be explored. However, it is possible that females exhibit a lesser venoconstrictor response because other variables controlling cardiac filling (e.g. resistance to venous return, cardiac pressures, ventricular compliance) promote venous return at lower MCFP. Direct measurement of cardiac volumes and pressures will be needed to address this question.
Sex differences in cardiovascular responses may be mediated at the level of the vascular smooth muscle [54], via the peripheral autonomic nervous system [55]or via effects in the brain[56]. Since estrogen receptors are found in vascular smooth muscle [57] and reportedly decrease venous responsiveness to alpha adrenergic stimulation[54], we assessed whether post synaptic responses to norepinephrine were different in male and female rats. We observed no marked differences between male and female rats in the norepinephrine dose response curves for the changes in arterial pressure or mean circulatory filling pressure. The pressor responses to norepinephrine were modestly lower at higher doses, although only one dose showed a statistically significant effect. The MCFP responses to norepinephrine showed a similar pattern, although no significant differences were detected. We interpret these data to indicate that the differential MCFP responses to pericardial injection of bradykinin are not due to changes in vascular smooth muscle responsiveness to norepinephrine. Another possibility is that bradykinin receptor expression was lower in the female hearts. We assessed BDKRB2 protein expression in ventricular tissue and found no differences between male and female rats, which is consistent with previous work show that neither estradiol nor ovariectomy affected bradykinin mRNA expression in the heart [58]. Alternatively, the male female differences may be mediated modulation of sympathetic drive to the veins since sex differences in renal sympathetic nerve activity responses to CSAR activation were reported [36].
There are some limitations to this work. An important consideration is the experimental design. We chose to use cumulative dosing and a paired design wherein bradykinin injections were given sequential before and after ganglionic blockade. A limitation to this approach is that bradykinin responses are known to desensitize with repeated activation (tachyphylaxis) [29]. It is possible that the magnitude the responses at the higher doses may have been attenuated somewhat due to desensitization. Similarly, it could be argued that attenuation of the response following ganglion blockade was due to desensitization. While we cannot exclude these possibilities, our experimental design incorporated a rest period between doses of 25–30 minutes. McDermott showed that this time interval was sufficient to avoid development of tachyphylaxis. We have also done a limited number of experiments with repeated dose response curves showing that this interval largely avoids desensitization of the response (Data supplement). A second limitation is that in addition or in conjunction with activation of the sympathetic nervous system, cardiac afferents also may convey pain signals [18, 40]. We did not observe any overt signs of pain such as vocalization or biting. However we cannot rule out the possibility that activation of pain pathways contributed to the sympathetic activation and the venoconstrictor and pressor responses.
Collectively, we believe that these data show that pericardial injection of bradykinin causes systemic venoconstriction sufficient to participate in the overall cardiovascular response to activation of the CSAR. Convincing data show that activity of the CSAR is enhanced in the settings of heart failure [44, 59] and hypertension [28, 60]. Venous tone is increased in heart failure [51] and in hypertension [1, 9, 61]. It is possible that augmented activity of the CSAR contributes to the elevation in systemic venous tone observed in these pathophysiological conditions. Studies employing cardiac afferent ablation in hypertensive animals coupled with measurement of venous tone will be needed to answer this question. Recent work is consistent with this possibility since ablation of cardiac sensory afferents attenuated hypertension development in the SHR[60], a model in which elevation in venous tone and cardiac output are associated with hypertension development.
Supplementary Material
Figure 2:
Responses to Intravenous Norepinephrine
Acknowledgements
This work was supported by funding from the National Institutes of Health (R01HL136741) and the Sanford School of Medicine University of South Dakota. The authors are grateful for the technical assistance of Teresa Grant, Rebecca Redetzke and Bart Kjellsen.
Footnotes
Competing Interests: The authors have no conflicts of interests or competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Literature Cited
- 1.Fink GD, Arthur C Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long-term regulation of arterial pressure. Hypertension, 2009. 53(2): p. 307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene AS and Shoukas AA, Changes in canine cardiac function and venous return curves by the carotid baroreflex. Am. J. Physiol, 1986. 251: p. H288–H296,. [DOI] [PubMed] [Google Scholar]
- 3.Tyberg JV, How changes in venous capacitance modulate cardiac output. Pflugers Arch, 2002. 445(1): p. 10–7. [DOI] [PubMed] [Google Scholar]
- 4.Guyton AC, Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev, 1955. 35: p. 123–128. [DOI] [PubMed] [Google Scholar]
- 5.Tyberg JV, et al. , Ventricular interaction and venous capacitance modulate left ventricular preload. Can J Cardiol, 1996. 12(10): p. 1058–64. [PubMed] [Google Scholar]
- 6.Tiniakov R, Pahan K, and Scrogin KE, Sympathetic innervation of the splanchnic region mediates the beneficial hemodynamic effects of 8-OH-DPAT in hemorrhagic shock. Am J Physiol Regul Integr Comp Physiol, 2012. 303(5): p. R527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin DS and McNeill JR, Arginine vasopressin increases apparent whole body capacity in anesthetized cats. Am J Physiol, 1990. 258(3 Pt 2): p. H722–8. [DOI] [PubMed] [Google Scholar]
- 8.Martin DS and McNeill JR, Whole body vascular capacitance response to vasopressin is mediated by autonomic function. Am. J. Physiol, 1991. 261: p. H493–H499. [DOI] [PubMed] [Google Scholar]
- 9.Martin DS, Rodrigo MC, and Appelt CW, Venous tone in the developmental stages of spontaneous hypertension. Hypertension, 1998. 31: p. 139–144. [DOI] [PubMed] [Google Scholar]
- 10.Pang C, Autonomic control of the venous system in health and disease. Effect of drugs. Pharmacology and Therapeutics, 2001. 90: p. 179–230. [DOI] [PubMed] [Google Scholar]
- 11.Burchell AE, et al. , Chemohypersensitivity and autonomic modulation of venous capacitance in the pathophysiology of acute decompensated heart failure. Curr Heart Fail Rep, 2013. 10(2): p. 139–46. [DOI] [PubMed] [Google Scholar]
- 12.Fallick C, Sobotka PA, and Dunlap ME, Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail, 2011. 4(5): p. 669–75. [DOI] [PubMed] [Google Scholar]
- 13.Shoukas AA and Sagawa K, Control of total systemic vascular capacity by the carotid sinus baroreceptor reflex. Circ. Res, 1973. 33: p. 22–33. [DOI] [PubMed] [Google Scholar]
- 14.Hoka S, et al. , Dynamic changes in venous outflow by baroreflex and left ventricular distension. Am. J. Physiol, 1988. 254: p. R212–R221. [DOI] [PubMed] [Google Scholar]
- 15.Haase EB and Shoukas AA, Carotid sinus baroreceptor reflex control of venular pressure-diameter relations in rat intestine. Am J Physiol, 1991. 260(3 Pt 2): p. H752–8. [DOI] [PubMed] [Google Scholar]
- 16.Burgoyne S, et al. , Systemic vascular effects of acute electrical baroreflex stimulation. Am J Physiol Heart Circ Physiol, 2014. 307(2): p. H236–41. [DOI] [PubMed] [Google Scholar]
- 17.Hainsworth R, Reflexes from the heart. Physiological Reviews, 1991. 71: p. 617–658. [DOI] [PubMed] [Google Scholar]
- 18.Longhurst JC, Cardiac receptors: Their function in health and disease. Progress in Cardiovascular Diseases, 1984. 27: p. 201–222. [DOI] [PubMed] [Google Scholar]
- 19.Veelken R, et al. , Epicardial bradykinin B2 receptors elicit a sympathoexcitatory reflex in rats. Hypertension, 1996. 28: p. 615–621. [DOI] [PubMed] [Google Scholar]
- 20.Carneiro JJ and Donald DE, Change in liver blood flow and blood content in dogs during direct and reflex activation of hepatic sympathetic nerve activity. Circ. Res, 1976. 40: p. 150–158. [DOI] [PubMed] [Google Scholar]
- 21.Ross J, Frahm CJ, and Braunwald E, The influence of intracardiac baroreceptors on venous return, systemic vascular volume and peripheral resistance. J. Clin. Invest, 1961. 40: p. 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tutt SM, McGregor KH, and Hainsworth R, Reflex vascular responses to changes in left ventricular pressure in anesthetized dogs. Quarterly Journal of Experimental Physiology, 1988. 73: p. 425–437. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, et al. , Changes in regional vascular capacitance during prostacyclin induced cardiac vagal reflex in pigs. Am. J. Physiol, 1994. 267: p. H535–H539. [DOI] [PubMed] [Google Scholar]
- 24.Nganele DM and Hintze TH, Cardiac chemical reflex control of preload in conscious dogs. Am. J. Physiol, 1990. 258: p. H1055–H1063. [DOI] [PubMed] [Google Scholar]
- 25.Hainsworth R, Cardiovascular reflexes from ventricular and coronary receptors. Adv Exp Med Biol, 1995. 381: p. 157–74. [DOI] [PubMed] [Google Scholar]
- 26.Longhurst JC, Tjen ALSC, and Fu LW, Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann N Y Acad Sci, 2001. 940: p. 74–95. [DOI] [PubMed] [Google Scholar]
- 27.Malliani A, et al. , Cardiovascular reflexes mediated by sympathetic afferent fibers. J Auton Nerv Syst, 1983. 7(3–4): p. 295–301. [DOI] [PubMed] [Google Scholar]
- 28.Chen WW, et al. , Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf), 2015. 213(4): p. 778–94. [DOI] [PubMed] [Google Scholar]
- 29.McDermott DA, et al. , Use of an indwelling catheter for examining cardiovascular responses to pericardial administration of bradykinin in rat. Cardiovasc Res, 1995. 30(1): p. 39–46. [PubMed] [Google Scholar]
- 30.Pagnani M, et al. , Analysis of the pressor sympathetic reflex produced by intracoronary injections of bradykinin in conscious dogs. Circ. Res, 1985. 56: p. 175–183. [DOI] [PubMed] [Google Scholar]
- 31.Staszewska-Woolley J, Woolley G, and Regoli D, Specific receptors for bradykinin-induced cardiac sympathetic chemoreflex in the dog. European J. Pharmacol, 1988. 156: p. 309–314. [DOI] [PubMed] [Google Scholar]
- 32.Xu B, Zheng H, and Patel KP, Relative contributions of the thalamus and the paraventricular nucleus of the hypothalamus to the cardiac sympathetic afferent reflex. Am J Physiol Regul Integr Comp Physiol, 2013. 305(1): p. R50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Browning KN, et al. , Two populations of sympathetic neurons project selectively to mesenteric artery or vein. Am J Physiol, 1999. 276(4): p. H1263–72. [DOI] [PubMed] [Google Scholar]
- 34.Hottenstein OD, K.D.L., Comparison of the frequency dependence of venous and arterial responses to sympathetic nerve stimulation in guinea-pigs. J. Physiol, 1987. 384: p. 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J Galligan JJ. Fink GD., Swain GM Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins a probed by in vitro continuous amperometry and video imaging. J. Physiol, 2007. 584: p. 819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinkham MI and Barrett CJ, Estradiol alters the chemosensitive cardiac afferent reflex in female rats by augmenting sympathoinhibition and attenuating sympathoexcitation. Clin Exp Pharmacol Physiol, 2015. 42(6): p. 622–31. [DOI] [PubMed] [Google Scholar]
- 37.Rothe CF, Mean circulatory filling pressure:its meaning and measurement. J. Applied Physiol, 1993. 74: p. 499–509. [DOI] [PubMed] [Google Scholar]
- 38.Martin DS, et al. , Effect of ovariectomy on blood pressure and venous tone in female spontaneously hypertensive rats. Am J Hypertens, 2008. 21(9): p. 983–8. [DOI] [PubMed] [Google Scholar]
- 39.Song J and Martin DS, Rho kinase contributes to androgen amplification of renal vasoconstrictor responses in the spontaneously hypertensive rat. J Cardiovasc Pharmacol, 2006. 48(3): p. 103–9. [DOI] [PubMed] [Google Scholar]
- 40.Fu LWLJC, Regulation of Cardiac Afferent Excitability in Ischemia. Handbook of experimental pharmacology, 2009. 194: p. 185–225. [DOI] [PubMed] [Google Scholar]
- 41.Martin D, et al. , Acute stress increases venomotor tone in conscious rats. Am. J. Physiol, 1996. 271: p. H1375–H1383. [DOI] [PubMed] [Google Scholar]
- 42.Martin DS, et al. , Adrenergic nerves mediate the venoconstrictor response to PVN stimulation. Brain Res, 2006. 1076(1): p. 93–100. [DOI] [PubMed] [Google Scholar]
- 43.Martin DS, et al. , Disinhibition of the hypothalamic paraventricular nucleus increases mean circulatory filling pressure in conscious rats. Brain Res, 1997. 756: p. 106–113. [DOI] [PubMed] [Google Scholar]
- 44.Wang HJ, Rozanski GJ, and Zucker IH, Cardiac sympathetic afferent reflex control of cardiac function in normal and chronic heart failure states. Journal of Physiology-London, 2017. 595(8): p. 2519–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, et al. , Involvement of enhanced cardiac sympathetic afferent reflex in sympathetic activation in early stage of diabetes. J Appl Physiol (1985), 2012. 113(1): p. 47–55. [DOI] [PubMed] [Google Scholar]
- 46.Gao L, et al. , Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension, 2005. 45(6): p. 1173–81. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, et al. , Intrapericardial capsaicin and bradykinin induce different cardiac-somatic and cardiovascular reflexes in rats. Auton Neurosci, 2016. 198: p. 28–32. [DOI] [PubMed] [Google Scholar]
- 48.Xu B, et al. , Responses of neurons in paraventricular nucleus to activation of cardiac afferents and acute myocardial ischaemia in rats. Exp Physiol, 2011. 96(3): p. 295–304. [DOI] [PubMed] [Google Scholar]
- 49.Gao L, et al. , Cardiac sympathetic afferent stimulation impairs baroreflex control of renal sympathetic nerve activity in rats. Am J Physiol Heart Circ Physiol, 2004. 286(5): p. H1706–11. [DOI] [PubMed] [Google Scholar]
- 50.Martin D, et al. , Castration reduces blood pressure and autonomic venous tone in spontaneously hypertensive rats. J. Hypertens, 2005. 23: p. 2229–36. [DOI] [PubMed] [Google Scholar]
- 51.Nekooeian AA and Pang CCY, Effects of estrogen on venous function in rats with chronic heart failure. Am. J. Physiol, 2000. 278: p. H1941–H1947. [DOI] [PubMed] [Google Scholar]
- 52.R.C.G.D.C.R.R.D.N. R, In vivo B2 receptor mediated negative chronotropic effect of bradykinin in canine sinus node‥ Am. J. Physiol, 1993. 265: p. H876. [DOI] [PubMed] [Google Scholar]
- 53.L.J.F.Z.J.P.M.v.Z. PA, Positive chronotropic activity of bradykinin in the pithed normotensive rat‥ Fundamental and Clinical Pharmacology 1998. 12 [DOI] [PubMed] [Google Scholar]
- 54.Raffetto JD, et al. , Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins. J Vasc Surg, 2010. 51(4): p. 972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bengtsson B, Estrogen induced inhibition of 3H noradrenaline release in the uterus and portal vein of the rat. Acta Physiol. Scand, 1978. 104: p. 287–298. [DOI] [PubMed] [Google Scholar]
- 56.Hay M, Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin Sci (Lond), 2016. 130(1): p. 9–18. [DOI] [PubMed] [Google Scholar]
- 57.Bracamonte MP, Jayachandran M, and Miller VM, Acute effects of 17 B estradiol on femoral veins from adult gonadally intact and ovariectomized female pigs. Am. J. Physiol, 2002. 283: p. H2389–H2396. [DOI] [PubMed] [Google Scholar]
- 58.Madeddu P, et al. , Regulation of bradykinin B2-receptor expression by oestrogen. Br J Pharmacol, 1997. 121(8): p. 1763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HJ, et al. , Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension, 2014. 64(4): p. 745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shanks J, et al. , TRPV1 (Transient Receptor Potential Vanilloid 1) Cardiac Spinal Afferents Contribute to Hypertension in Spontaneous Hypertensive Rat. Hypertension, 2019. 74(4): p. 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fink GD, Johnson RJ, and Galligan JJ, Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate hypertension. Hypertension, 2000. 35: p. 464–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.