Abstract
Background
The p.E318K variant of the Melanocyte Inducing Transcription Factor (MITF) has been implicated in genetic predisposition to melanoma as an intermediate penetrance allele. However, the impact of this variant on clinico-phenotypic, as well as on dermoscopic patterns features of affected patients is not entirely defined. The purpose of our study was to assess the association between the p.E318K germline variant and clinic-phenotypical features of MITF+ compared to non-carriers (MITF−), including dermoscopic findings of melanomas and dysplastic nevi.
Methods
we retrospectively analyzed a consecutive series of 1386 patients recruited between 2000 and 2017 who underwent genetic testing for CDKN2A, CDK4, MC1R and MITF germline variants in our laboratory for diagnostic/research purposes. The patients were probands of melanoma-prone families and apparently sporadic single or multiple primary melanoma patients. For all, we collected clinical, pathological information and dermoscopic images of the histopathologically diagnosed melanomas and dysplastic nevi, when available.
Results
After excluding patients positive for CDKN2A/CDK4 pathogenic variants and those affected by non-cutaneous melanomas, our study cohort comprised 984 cutaneous melanoma patients, 22 MITF+ and 962 MITF−. MITF+ were more likely to develop dysplastic nevi and multiple primary melanomas. Nodular melanoma was more common in MITF+ patients (32% compared to 19% in MITF−). MITF+ patients showed more frequently dysplastic nevi and melanomas with uncommon dermoscopic patterns (unspecific), as opposed to MITF− patients, whose most prevalent pattern was the multicomponent.
Conclusions
MITF+ patients tend to develop melanomas and dysplastic nevi with histopathological features, frequency and dermoscopic patterns often different from those prevalent in MITF− patients. Our results emphasize the importance of melanoma prevention programs for MITF+ patients, including dermatologic surveillance with digital follow-up.
Keywords: Melanocyte Inducing Transcription Factor, E318K, Cutaneous melanoma, Renal cell carcinoma, Nevi, Dysplastic nevi, Dermoscopy, Germline variant, Susceptibility, Cancer genetics
Background
Malignant melanoma is a potentially lethal tumor resulting from the malignant transformation of melanocytes. A recent meta-analysis showed that 70.9% of melanomas likely arises de novo from melanocytes located in previously normal skin, mucous membranes or other sites (eye, inner ear, gastrointestinal system) and 29.1% arises from melanocytes in pre-existing lesions (nevi or dysplastic nevi). Ultraviolet light, especially indoor tanning, exposure is a known carcinogen clearly correlated with melanoma.
The worldwide incidence of melanoma is increasing and it is estimated to further increase mainly due to the lengthening of the human lifetime and the aging of population: currently, the lifetime risk of developing melanoma is 1 in 63 in the United States and in other Western countries.
Along with lifestyle, genetic risk factors are significant conditions contributing to melanoma development [1, 2].
A number of novel candidate melanoma predisposition genes, in addition to the well know high penetrance genes such as Cyclin Dependent Kinase Inhibitor 2A (CDKN2A), Cyclin-Dependent Kinase 4 (CDK4) and low-penetrance genes such as Melanocortin 1 Receptor (MC1R) have been uncovered in the last few years. These include genes involved in DNA replication (telomere maintenance) or repair, such as Telomere Reverse Transcriptase (TERT), Protection of Telomeres 1 (POT1), Adrenocortical Dysplasia (ACD), Telomeric Repeat-Binding Factor-2 (TERF2) Interacting Protein (TERF2IP), and Breast Cancer Gene 1 (BRCA1)-Associated Protein 1 (BAP1). Moreover, the p.E318K variant of the Melanocyte Inducing Transcription Factor (MITF) gene has recently been implicated in cancer predisposition [3–5]. The groups of Bertolotto [6] and Yokoyama [7] independently identified the p.E318K variant and categorized MITF as an intermediate penetrance melanoma susceptibility gene. Indeed, patients carrier of this germline variant have a more than fivefold increased risk of developing melanoma (both familial and sporadic), renal cell carcinoma (RCC) or both cancers than non-carriers. The p.E318K variant has also been associated with increased nevus count, non-blue eye color and developing of multiple primary melanoma [7]. MITF is the ‘master regulator’ of differentiation, survival and proliferation of normal melanocytes and is critical in controlling proliferation, migration and invasion of melanoma cells [8]. It has been demonstrated that MITF acts not only as a master transcription factor, involved in cell cycle regulation, but also as a transcriptional repressor [9]. The p.E318K variant alters the SUMOylation of MITF thus impairing MITF inhibitory activity [6, 7]. More specifically, in normal cells under normoxia, the small ubiquitin-like modifier (SUMO) proteins, bind MITF decreasing the transcription of the hypoxia inducible factor 1 A (HIF1A). In addition, HIF1A is hydroxylated for subsequent proteasome mediated degradation of the cells. Under hypoxia, SUMOs are released, allowing the transcription of HIF1A and anaerobic metabolism or glycolysis. The p.E318K variant of MITF in melanoma and renal carcinoma cells severely impaired SUMOylation of MITF, resulting in an increased transcription of HIF1A and other genes compared to wild-type MITF. Even under normoxic conditions, the p.E318K variant allows cancer cells to activate a pseudohypoxic response or aerobic glycolysis and this “Warburg effect” predispose to cancer progression and metastasis [10].
Due to the above-mentioned links with melanoma and kidney cancer susceptibility, current research is focusing on the relationship between p.E318K and the clinico-phenotypic features of individuals carrying this variant [9, 11–16]. However, current literature on dermoscopic features of nevi and melanomas in these patients is still limited, and predisposition to non-melanoma cancers according to MITF germline status needs to be further investigated [11, 14, 16].
The aim of the present work was to retrospectively study genotype–phenotype correlations in melanoma patients carrier of the p. E318K MITF germline variant (MITF+), compared with non-carrier melanoma patients (MITF−). Among the analyzed phenotypic features, we included dermoscopic findings of histopathologically diagnosed dysplastic nevi (DN) and cutaneous melanomas in MITF+ and MITF−.
Methods
Patients characteristics
Between 2000 and 2017 we recruited a consecutive series of 1386 patients. This cohort included probands of melanoma-prone families and apparently sporadic patients diagnosed with multiple primary melanomas who underwent genetic testing for diagnostic or research purposes, as well as apparently sporadic patients with melanoma, tested for research purposes only. All patients, except one case already characterized for germline status, were subjected to genetic testing for CDKN2A, CDK4, MC1R and MITF germline variants in our laboratory. For all patients, we collected and stored clinical and pathological information. In addition, when available, we collected dermoscopic images of the histopathologically diagnosed DN and cutaneous melanomas.
Indeed, the histopathological diagnosis of DN is based on the presence of both of the two major criteria (proliferation of atypical melanocytes extending beyond the dermal component; atypical melanocytes arranged in a lentiginous/epithelioid-cell pattern) and at least two minor criteria (lamellar/eosinophilic fibrosis; neovascularization; inflammatory response; fusion of rete ridges) [17].
For 667 of the patients included in this study, molecular and, partly, clinical information have been previously described [13].
Collection of clinical, pathological and dermoscopic data
Clinical information were collected through a questionnaire, administered by a trained interviewer, and included phenotype and personal/family history of melanomas and other tumors, as previously described [18, 19]. Either clinical records or local cancer registry data were used to collect pathological information, including tumor histological type and staging according to the American Joint Committee on Cancer (AJCC)’s TNM staging system [20, 21]. For both MITF+ and MITF− patients, the following phenotypical and clinico-pathological features were studied: phototype, freckles, hair and eye color, total number of nevi with diameter > 2 mm, number of histopathologically diagnosed DN and number of histopathologically diagnosed cutaneous melanomas. For first diagnosed melanomas, we also gathered information on age at diagnosis, anatomical site, histotype, Breslow thickness (mm), sentinel lymph node and stage.
Dermoscopic images of lesions clinically suggestive of melanomas were collected through the FotoFinder dermoscope Medicam 1000 (FotoFinder Systems GmbH, Bavaria, Germany) during dermatologic visits performed for screening (first visit) or for follow-up at the Dermatologic Clinic of the San Martino Hospital (Genoa, Italy) and at the Dermatologic outpatient clinic, Division of Oncology, Centro di Riferimento Oncologico, Aviano National Cancer Institute (Aviano, Italy).
The analysis of global dermoscopic pattern was retrospectively performed on all available dermoscopic images of non-acral lesions according to the dermoscopic classification of acquired melanocytic nevi, as follows: reticular, globular, homogenous, multicomponent, reticular-globular, reticular-homogenous, globular-homogenous and unspecific pattern. This latter was defined as a pattern lacking specific features related to a melanocytic or non melanocytic lesions. Conversely, the following dermoscopic patterns were considered to assess acral melanocytic lesions: parallel furrow, parallel ridge, lattice-like, fibrillary [22, 23]. All the dermoscopic images were evaluated by a panel of three independent observers; the dermoscopic features were scored based on the agreement of two observer (G.C. and F.D.) and in case of disagreement between the two observers, a third observer (M.A.P.) was consulted. The evaluation of the dermoscopic criteria was made when 3/3 or 2/3 observers agreed.
Molecular analysis
All patients provided a blood sample from which we extracted genomic DNA. Purified DNA samples were then amplified by conventional polymerase chain reaction (PCR) and analyzed by Sanger sequencing to assess the germline status of CDKN2A, CDK4, MC1R and MITF. Samples processing and analysis were performed as previously described [24, 25].
Patients selection
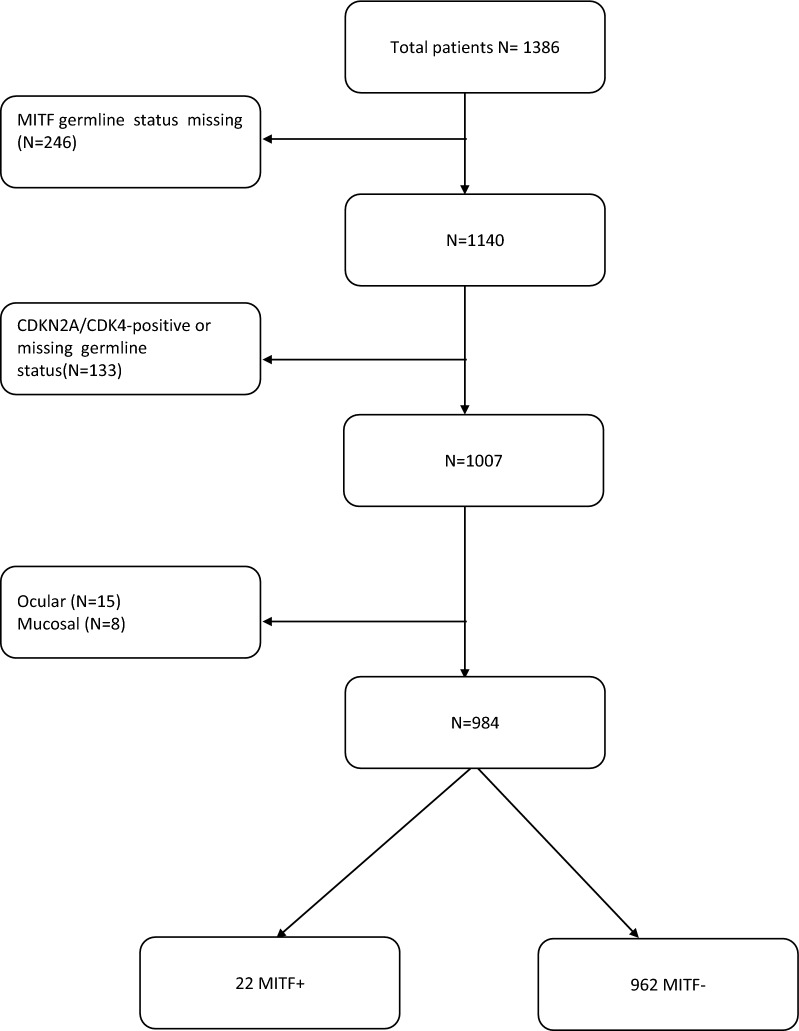
From the melanoma cohort, we excluded the patients lacking information on germline status and tumor stage, as well as those with non-cutaneous melanoma (ocular and mucosal melanomas). Moreover, to avoid confounding effects by CDKN2A and CDK4, patients with concurrent CDKN2A and CDK4 pathogenic variants were also excluded from this study. Subsequently, we gathered MITF+ and MITF− patients into two separate study groups. All patients signed a written informed consent according to local ethics committee approved protocol prior to enrolment in the study.
Statistical analysis
To assess the difference of a numerical variable between the two study groups (age at diagnosis, Breslow thickness, number of melanomas diagnosed, total number of nevi, number of dysplastic nevi) we used the Mann–Whitney U test.
To assess the association between MITF+ germline status and a categorical variable (hair-eye color, familial status, sentinel node status, familiarity for pancreatic and kidney cancer, site and histotype of first melanoma, dermoscopic pattern of DN and melanomas grouped together, MC1R germline status, histopathologically diagnosed melanomas and DN analyzed as a dichotomous variable), we used the Fisher’s exact test.
Kruskal–Wallis test was used to analyze the association between MITF germline status and an ordinal variable (phototype, freckles, tumor stage and number of nevi grouped in three categories).
Results
After excluding 246 patients with missing information on MITF mutational status, 133 patients either positive for CDKN2A/CDK4 pathogenic variants or with missing information on CDKN2A/CDK4 germline status, and 23 patients affected by ocular or mucosal melanomas, our study cohort comprised 984 cutaneous melanoma patients, 22 MITF+ and 962 MITF− (Fig. 1). Of the 22 MITF+ patients, 5 had a positive family history of melanoma, whereas the remaining 17 were apparently sporadic cases. Overall, 6 patients, all apparently sporadic cases, developed multiple melanomas. Even though the overall prevalence of the MITF p.E318K variant was 2.2% (22 of 984), MITF p.E318K was more common among multiple primary melanoma (MPM) patients (5% compared to 2% in single melanoma patients). All MPM MITF+ patients were sporadic, whereas among single primary melanoma (SPM) patients MITF p.E318K rate was similar in familial and sporadic subgroups.
Fig. 1.
Patients selection workflow
The distribution of MC1R variants did not significantly differ between the two study groups (p = 0.45, Table 1). In the MITF+ group, two patients had amelanotic/hypomelanotic melanomas; both patients carried one red-hair-color (RHC) MC1R variant (R169W, R142H).
Table 1.
Clinical, pathological and molecular characteristics of the study groups
| N | MITF+ N (%) | MITF− N (%) | ORa | Lower CI | Upper CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Phototype | 927 | I | 0 (0) | 52 (6) | 0.27 | |||
| II | 15 (75) | 478 (53) | ||||||
| III | 5 (25) | 353 (39) | ||||||
| IV | 0 (0) | 24 (3) | ||||||
| Freckles | 349 | None | 8 (4) | 77 (23) | 0.37 | |||
| Rare | 4 (2) | 126 (38) | ||||||
| Few | 7 (35) | 89 (27) | ||||||
| Many | 1 (5) | 37 (11) | ||||||
| Hair color | 932 | Albino | 0 (0) | 1 (0) | 0.06 | |||
| Red | 1 (5) | 49 (5) | ||||||
| Blond | 9 (43) | 201 (22) | ||||||
| Blond_red | 0 (0) | 9 (1) | ||||||
| Brown | 7 (33) | 565 (62) | ||||||
| Black | 4 (19) | 86 (9) | ||||||
| Eye color | 931 | Light blue | 7 (37) | 214 (23) | 0.50 | |||
| Blue | 1 (5) | 38 (4) | ||||||
| Green | 2 (11) | 98 (11) | ||||||
| Grey | 0 (0) | 21 (2) | ||||||
| Light brown | 1 (5) | 206 (23) | ||||||
| Dark brown | 8 (42) | 324 (36) | ||||||
| Black | 0 (0) | 5 (1) | ||||||
| Hazel | 0 (0) | 6 (1) | ||||||
| Number of nevi | 492 | < 10 | 3 (17) | 200 (42) | 0.04 | |||
| 10–50 | 10 (55) | 185 (39) | ||||||
| > 50 | 5 (28) | 89 (19) | ||||||
| Histologically diagnosed Dysplastic nevi | 866 | Median (IQR) | 0 (0–1) | 0 (0–0) | < 0.001 | |||
| 0 | 10 (50) | 769 (91) | 9.93 | 3.59 | 27.53 | < 0.001 | ||
| 1+ | 10 (50) | 77 (9) | ||||||
| Familial | 984 | Spo | 17 (77) | 833 (87) | 1.9 | 0.54 | 5.48 | 0.21 |
| Fam | 5 (23) | 129 (13) | ||||||
| Age at first melanoma | 858 | Median (IQR) | 44 (32.25–58.75) | 49 (38.99–61.71) | 0.20 | |||
| N. of melanomas removed | 983 | Median (IQR) | 1 (1–1.75) | 1 (1–1) | 0.02 | |||
| 1 | 16 (73) | 855 (89) | 3.02 | 0.95 | 8.35 | 0.03 | ||
| 2+ | 6 (27) | 106 (11) | ||||||
| Breslow mm | 930 | Median (IQR) | 1 (0.6–2.025) | 1 (0.35–1.765) | 0.22 | |||
| Sentinel node | 337 | Neg | 6 (27) | 266 (28) | 1.41 | 0.14 | 8.1 | 0.65 |
| Pos | 2 (9) | 63 (7) | ||||||
| Stage | 771 | IS | 2 (17) | 122 (16) | 0.65 | |||
| I | 8 (67) | 473 (62) | ||||||
| II | 2 (17) | 75 (10) | ||||||
| III | 0 (0) | 56 (7) | ||||||
| IV | 0 (0) | 33 (4) | ||||||
| Pancreatic cancer in family | 972 | No | 19 (86) | 901 (94) | 2.90 | 0.53 | 10.35 | 0.11 |
| Yes | 3 (14) | 49 (5) | ||||||
| Kidney cancer in family | 971 | No | 18 (82) | 910 (95) | 5.17 | 1.21 | 16.74 | 0.01 |
| Yes | 4 (18) | 39 (5) | ||||||
| Site of first melanoma | 943 | Head and neck | 1 (5) | 74 (8) | 0.27 | |||
| Trunk | 8 (40) | 464 (50) | ||||||
| Arms | 6 (30) | 125 (14) | ||||||
| Legs | 5 (25) | 260 (28) | ||||||
| Histotype of first melanoma | 722 | Acral | 2 (9) | 11 (2) | 0.04 | |||
| Lentigo maligna | 0 (0) | 36 (5) | ||||||
| Nodular | 7 (32) | 132 (19) | ||||||
| SSM | 13 (59) | 454 (64) | ||||||
| Other | 0 (0) | 67 (10) | ||||||
| MC1R | 576 | –/– | 6 (30) | 165 (30) | 0.45 | |||
| r/– | 4 (20) | 158 (28) | ||||||
| r/r | 2 (10) | 24 (4) | ||||||
| R/– | 6 (30) | 111 (21) | ||||||
| R/r | 1 (5) | 74 (13) | ||||||
| R/R | 1 (5) | 24 (4) |
Significant p-values are italicized
N = number of patients, % = percentage of patients, OR = odds ratio, lower CI = lower confidence interval limit, upper CI = upper confidence interval limit, IQR = inter-quartile range, Spo = sporadic, Fam = familial, Neg = negative, Pos = positive, SSM = superficial spreading melanoma, IS = in situ melanoma, R = MC1R red hair color variant, r = MC1R non-red hair color variant
aOdds that the outcome occurs in the MITF+ group compared to the odds of the outcome occurring in the MITF− group
Clinico-pathological features
The two study groups displayed significant differences with regards to: total number of nevi, number of histopathologically diagnosed DN and melanomas, histotype of first melanoma and family history of kidney cancer (Table 1). More specifically, MITF+ patients had, in median, a higher total number of nevi compared to MITF− patients: 28% of MITF+ patients had more than 50 nevi, compared to 19% of MITF− patients (p = 0.04).
The number of melanomas removed was higher in MITF+ compared to MITF− negative patients: 27% of the MITF+ patients have removed more than 2 melanomas versus 11% of the MITF− patients (p = 0.03).
Patients with at least one histologically diagnosed DN were more frequent in the MITF+ group, (50% vs. 10% in the MITF− group, p < 0.001) with a higher median DN removal compared to MITF− (median = 0.5, IQR = 0–1 and median = 0, IQR = 0–0, respectively; p < 0.001).
Concerning the histotype of first melanoma, MITF+ patients showed a higher rate of nodular melanomas than MITF− patients (32% and 19%, respectively, p = 0.04).
Patients with MPM were more frequently MITF+ (27% compared to 11% of MITF− patients, p = 0.03). A positive family history for kidney cancer was more frequent among MITF+ patients (18% versus 5% of MITF− patients; p = 0.01).
We also compared phenotypical features between the MITF+ and MITF− patients, and no significant differences were found as regards phototype, hair and eyes color, freckles, age at first melanoma diagnosis, anatomical site, Breslow thickness, sentinel lymph node, stage of first melanoma and family history of melanoma or pancreatic cancer (Table 1).
Dermoscopic features
The dermoscopic patterns of 23 lesions (including DN and melanomas) belonging to four MITF+ patients were compared with those of 47 lesions (DN and melanomas) belonging to 37 MITF− patients (Table 2).
Table 2.
Dermoscopic patterns of MITF + and MITF− dysplastic nevi and cutaneous melanomas
| Dermoscopic pattern | MITF+ | MITF− | p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Structureless | 6 | 26 | 4 | 8 | < 0.001 |
| Reticular | 1 | 4 | 1 | 2 | |
| Globular | 0 | 0 | 3 | 6 | |
| Homogeneous | 2 | 9 | 0 | 0 | |
| Globular-homogenous | 5 | 22 | 2 | 5 | |
| Reticular-homogenous | 6 | 26 | 3 | 6 | |
| Reticular-globular | 0 | 0 | 12 | 26 | |
| Multicomponent | 2 | 9 | 22 | 47 | |
| Parallel ridges (or other patterns typical of acral melanoma) | 1 | 4 | 0 | 0 | |
| Total | 23 | 100 | 47 | 100 | |
Significant p-values are italicized
N = number of dysplastic nevi/melanomas; % = percentage of dysplastic nevi/melanomas
When dermoscopically evaluating only melanomas, nine lesions belonging to three MITF+ were compared with those of 23 lesions belonging to 22 MITF− (Table 3).
Table 3.
Dermoscopic patterns of MITF+ and MITF− cutaneous melanomas
| Dermoscopic pattern | MITF+ | MITF− | ||
|---|---|---|---|---|
| N | % | N | % | |
| Structureless | 4 | 45 | 3 | 13 |
| Reticular | 1 | 11 | 0 | 0 |
| Globular | 0 | 0 | 2 | 9 |
| Homogeneous | 0 | 0 | 0 | 0 |
| Globular-homogenous | 1 | 11 | 1 | 4 |
| Reticular-homogenous | 0 | 0 | 0 | 0 |
| Reticular-globular | 0 | 0 | 2 | 9 |
| Multicomponent | 2 | 22 | 15 | 65 |
| Parallel ridges (or other patterns typical of acral melanoma) | 1 | 11 | 0 | 0 |
| Total | 9 | 100 | 23 | 100 |
N = number of melanomas; % = percentage of melanomas
Of the 23 dermoscopic images from the four MITF+ patients, seven melanomas (four with structureless, two with multicomponent and one with globular-homogenous pattern) and ten DN (two with homogenous, four with reticular-homogenous and four with globular-homogenous pattern) belonged to one single patients. This patient actually developed 10 melanomas, only 7 of which had dermoscopic images.
When we analyzed the global patterns of DN and melanomas, grouped together as a single variable, the unspecific, globular-homogenous and reticular-homogenous patterns were more frequent in MITF+ compared to MITF− patients; conversely, the multicomponent pattern was more common in MITF− than in MITF+ patients, as shown in Table 2 (p < 0.001). We could not perform the same analysis on a melanoma-only subset because of the small resulting sample size. However, as regards melanomas, the frequency of global dermoscopic patterns among the two study groups is reported in Table 3. The unspecific pattern was found more frequently in melanomas of MITF+ (45% of the lesions) than in those of MITF− patients (13% of the lesions), while the multicomponent pattern was seen more frequently among melanomas of MITF− (65% of the lesions) than those of MITF+ patients (22%) (Fig. 2). Taking into account that a considerable number of melanocitic lesions belonged to the same patient, we performed again the analysis excluding this outlier patient, to reduce the risk that such a relevant number of non-independent samples could bias our results. Even without the outlier patient, melanocytic lesions in MITF+ and MITF− patients showed a different distribution of dermoscopic patterns (p = 0.001). Namely, the unspecific was the most frequent dermoscopic pattern found in DN/melanomas of MITF+ patients (40%, as opposed to 9% in MITF− lesions). Conversely, the multicomponent and the reticular-globular patterns (47% and 28% respectively in MITF− lesions) were absent in MITF+ lesions.
Fig. 2.
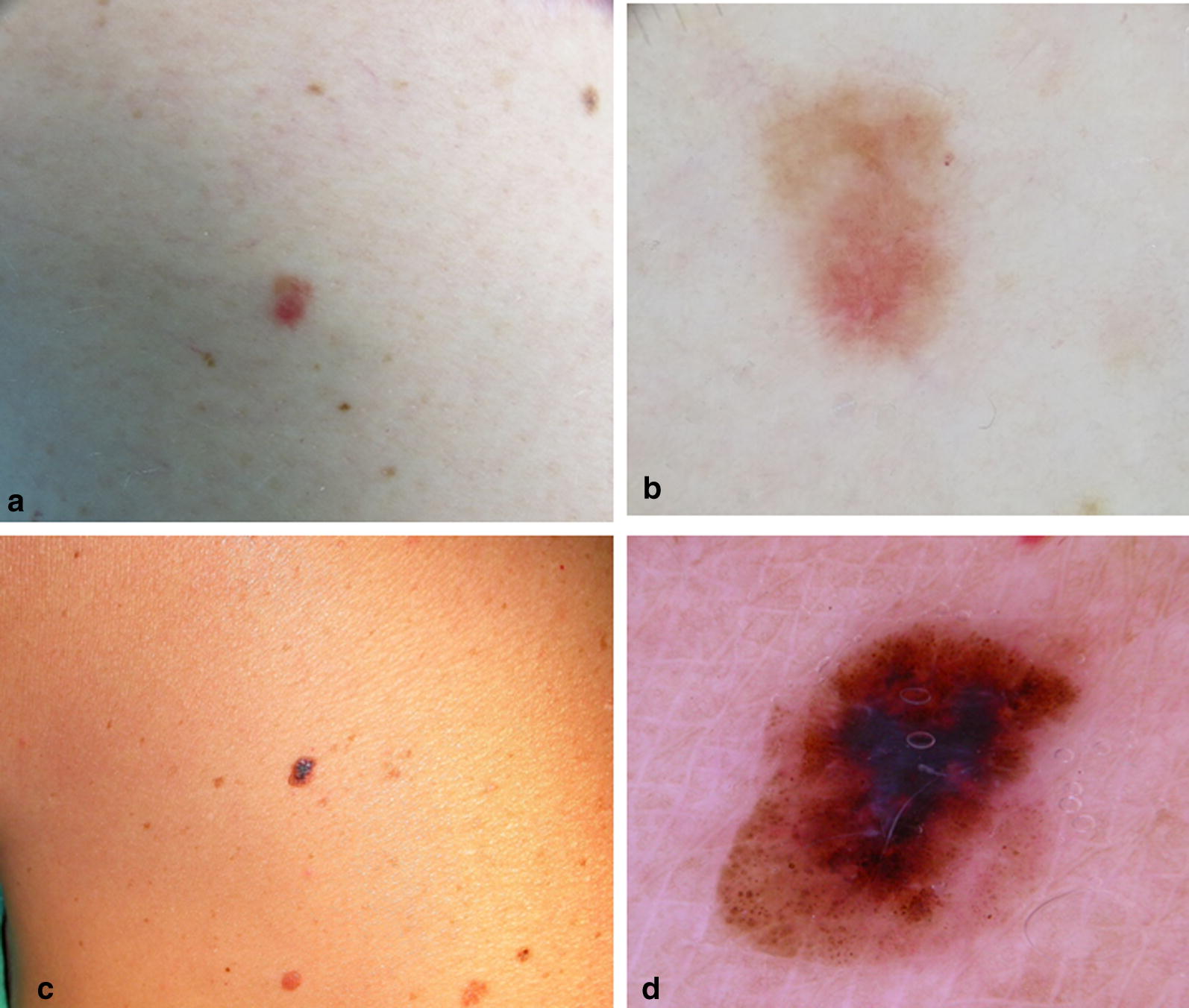
a Clinical and b dermoscopic images of a superficial spreading melanoma of the right thigh with an unspecific dermoscopic pattern in a MITF+ patient; this patient also carries one RHC variant (R142H) of MC1R that could be responsible for the hypomelanotic aspect of this lesion; c clinical and d dermoscopic images of a superficial spreading melanoma of the left shoulder with a multicomponent pattern in a MITF− patient
Discussion
In our study cohort, the prevalence of the p.E318K germline variant in CDKN2A/CDK4-negative patients was 2.2%, slightly higher than we previously reported in a smaller series of melanoma patients (1.8%) [13], but in line with Spanish (1.9%) [14], French (2.8%) [6], Australian (3.4–3.6%) [16] and American (2.8%) [12] studies.
Considering that the p.E318K variant is not common in melanoma patients, attempts to determine its effects on MITF+ patients’ phenotypical features and cancer predisposition are generally limited by sample size. To our best knowledge, the present study describes the largest cohort of MITF+ patients reported to date from a dermoscopic point of view (DN and melanomas), in addition to a genetic, clinical, and pathological perspective.
Concerning the histotype of the first diagnosed melanoma, we validated the association between the p.E318K variant and the nodular subtype previously reported by our group [13]. Indeed, seven out of 22 p.E318K patients (32%) developed a first melanoma with nodular histotype, a significantly higher percentage than the one observed in MITF− patients (16%). Our results differ from those of previous studies by other groups, which did not find significant associations of p.E318K with pathological features, possibly due to underpowered study samples [14, 16].
However, Potrony and colleagues reported that during 10 years of dermatological surveillance of patients at high risk of melanoma, the only two fast-growing melanomas (growth rate greater than 0.4 mm per month) were diagnosed in MITF+ patients. Of these two lesions, one was a nodular melanoma and the other one was a superficial spreading melanoma (SSM) [14]. However, in our MITF+ study group, all nodular melanomas were first diagnosed melanomas, identified during dermatological screening with digital follow-up or clinical examination. Conversely, all subsequent melanomas diagnosed in our MITF+ cohort during dermatological follow-up were SSM, and Breslow thickness of melanomas in patients with MPM was always lower than that of the preceding ones, except for one patient, possibly reflecting the intensive dermatological follow-up after the first melanoma diagnosis. However, further investigations with larger series of patients are needed to confirm the association between the p.E318K variant and nodular-type melanoma, and to study the prognostic role of this variant.
Concerning the role of the p.E318K variant in the predisposition to tumors other than melanoma, we confirm the association with renal cell carcinoma (RCC) previously described [6, 7, 13–15]. The association with pancreatic cancer we previously observed in a smaller series of patients [13] was not confirmed here, and therefore remains to be further explored. Although none of our p.E318K patients developed RCC, 18% of them reported a positive family history, as opposed to 4% of MITF− patients. Apart from melanoma, the most frequent tumor in MITF+ patients was basal cell carcinoma (14% of the patients), in line with previous data reported by Potrony et al. [14].
The finding that MITF+ p.E318K was associated with a higher number of histopathologically confirmed DN in our cohort was never reported to date, differently from CDKN2A variants, whose possible role in influencing the development of dysplastic melanocytic lesions has already been described [26, 27].
Our study confirms that MITF+ patients have an increased risk of developing multiple melanomas and a higher total nevi count compared to MITF− patients, as previously reported [6, 7, 13, 14]. Indeed, 28% of MITF+ patients in our cohort had more than 50 nevi with > 2 mm diameter, ascompared to 19% of MITF− patients. Similarly, previous studies reported high nevi counts in MITF+ patients, corroborating the hypothesis that MITF is involved in nevogenesis [11, 14, 16].
Of course, as MITF p.E318K is considered an intermediate penetrance allele, the possibility that other additional gene’s effects may have affected our results cannot be completely ruled out. However, patients with CDKN2A pathogenic variants were excluded from this study, in order to avoid a confounding effect by this gene. Moreover, MC1R variants, which influence phototype and are associated with melanoma risk [19, 28], had a similar distribution in the two study groups, therefore not affecting our analyses. MC1R RHC variants have also been associated with the likelihood of developing amelanotic/hypomelanotic melanomas [29]. In our cohort, both MITF+ patients with amelanotic/hypomelanotic melanomas carried one RHC variants. However, due to the retrospective nature of this study, standardized information on pigmentation was not available, and therefore we could not assess the impact of RHC variants on melanoma pigmentation according to MITF germline status.
Although dermoscopic patterns of melanocytic nevi in MITF+ and MITF− patients have already been reported [11, 14, 16], our study is the first to assess the dermoscopic characterization of DN and melanomas in MITF+ patients compared to MITF− patients. Previous studies [11, 14, 16] found that the predominant dermoscopic pattern of nevi was the reticular one, both in MITF+ and in MITF− patients. Moreover, Sturm et al. reported that the frequency of globular nevi was greater in MITF+ patients, albeit not significant [16]. In DN and melanomas of our series of MITF+ patients, we found 3 prevalent dermoscopic patterns: unspecific, globular-homogeneous and reticular-homogeneous. The unspecific pattern was defined as devoid of structures or with too few structures to identify a pattern, except for the presence of blood vessels. This latter pattern is most frequently found in amelanotic/hypomelanotic melanocytic lesions including amelanotic/hypomelanotic nodular melanomas where it can be associated with polymorphous atypical vessels [30].
While the reticular pattern is suggestive of photoinduced nevogenesis, the globular-homogeneous one, with globules at the periphery of the lesion, expression of lesion growth, suggests that p.E318K variant may also act to force the continuous growth of the nevi/melanomas [31].
Considering only melanomas, the prevalent pattern among the MITF+ patients was the unspecific one, a finding that has never been associated with the MITF+ variant to date.
Conversely, the multicomponent pattern was prevalent among the MITF− patients, as already reported in the literature [32, 33].
Noteworthy, as a rule, all lesions with unspecific patterns should be biopsied, also in the context of lesions clinically appearing benign, to avoid missing melanomas [23].
Therefore, the detection of this pattern in MITF+ patients should alert dermatologist raising the level of suspicion of malignancy.
Since among the 22 MITF+ patients one patient developed 10 melanomas (of which 7 dermoscopic images were available), and the different distribution of clinico-pathological-dermoscopic features between the two groups could have been influenced by this single patient, we repeated the analysis excluding this patient. Even though the observed patterns were actually influenced by this patient, the unspecific pattern remained prevalent in MITF+ patients and the association remained significant. Dermoscopically, the most common patterns of DN and melanomas (multicomponent, reticular-globular) were almost absent in MITF+ patients, while the multicomponent was the most frequent pattern among MITF− patients.
The major limitation in this study is the small number of images included for assessment of dermoscopic pattern in relation to MITF variant which may influence the reliability of these results (only 23 dermoscopic images belonging to four MITF+ patients were available).
Conclusions
Besides confirming previous results on the association of the p.E318K variant with high number of nevi (> 50 units) and higher risk of melanoma and kidney cancers compared to MITF− patients, our study adds the finding that MITF+ patients have a higher risk of developing DN than MITF− patients. This result underlines the necessity for MITF+ patients to follow melanoma prevention programs, including dermatologic surveillance with digital follow-up.
In MITF+ patients, any melanocytic lesion with a dermoscopic pattern that digresses from the most commonly dermoscopic patterns reported among the MITF− patients, such as multicomponent and reticular-globular patterns, should be examined with caution to avoid missing melanomas that are devoid of structures.
Further studies through an international collaborative effort are crucial to increase the sample size and validate these findings.
Acknowledgements
BD, WB, LP, VA, PQ, FS, ET, MAP and PG are on behalf of Italian Melanoma Intergroup (IMI).
Abbreviations
- ACD
Adrenocortical dysplasia
- AJCC
American Joint Committee on Cancer
- BAP1
Breast cancer gene 1 (BRCA1)-associated protein 1
- CDKN2A
Cyclin Dependent Kinase Inhibitor 2A
- CDK4
Cyclin-dependent kinase 4
- DN
Dysplastic nevi
- MC1R
Melanocortin 1 Receptor
- MITF
Microphthalmia-associated transcription factor
- PCR
Polymerase chain reaction
- POT1
Protection of telomeres 1
- RCC
Renal cell carcinoma
- TERF2
Telomeric repeat-binding factor-2
- TERF2IP
Telomeric repeat interacting protein
- TERT
Telomere reverse transcriptase
Authors’ contributions
GC: acquisition and analysis of data, drafting of submitted version. GC, BD: substantial contribution to the design of the work; BD, LP, VA, GP and MAP: analysis and interpretation of data, drafting of submitted version. WB: acquisition and interpretation of data, drafting of the work. PQ, FS, ET, CM, FD, AP, GG: acquisition of data, drafting of submitted version. GP: acquisition of data. PG: substantial contribution to the conception of the work, analysis and interpretation of data, drafting of the submitted version. All authors read and approved the final manuscript.
Funding
Ricerca finalizzata—Italian Ministry of Health (RF-2016-02362288) and Ricerca Corrente to IRCCS Ospedale Policlinico San Martino, Genoa.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon request.
Ethics approval and consent to participate
The present study, conducted in agreement with the principles of the Declaration of Helsinki, was approved by our internal committee and written informed consent form for testing, approved by the hosting Institution, was obtained from all patients.
Consent for publication
Not applicable since the clinic and dermoscopic images of the nevi and melanomas do not allow to trace the identity of the patients.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giulia Ciccarese and Bruna Dalmasso should be considered joint first author
Contributor Information
William Bruno, Email: william.bruno@unige.it.
Italian Melanoma Intergroup (I.M.I.):
Bruna Dalmasso, William Bruno, Lorenza Pastorino, Virginia Andreotti, Paola Queirolo, Francesco Spagnolo, Enrica Tanda, Maria Antonietta Pizzichetta, and Paola Ghiorzo
References
- 1.Pampena R, Kyrgidis A, Lallas A, et al. A meta-analysis of nevus-associated melanoma: prevalence and practical implications. J Am Acad Dermatol. 2017;77:938–945. doi: 10.1016/j.jaad.2017.06.149. [DOI] [PubMed] [Google Scholar]
- 2.Carr S, Smith C, Wernberg J. Epidemiology and risk factors of melanoma. Surg Clin N Am. 2020;100:1–12. doi: 10.1016/j.suc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Bressac-de Paillerets B, Vabres P, Thomas L. Genetic testing for melanoma—where are we with moderate-penetrance genes? JAMA Dermatol. 2016;152:375. doi: 10.1001/jamadermatol.2015.4359. [DOI] [PubMed] [Google Scholar]
- 4.Potrony M, Badenas C, Aguilera P, et al. Update in genetic susceptibility in melanoma. Ann Transl Med. 2015;3:210. doi: 10.3978/j.issn.2305-5839.2015.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Read J, Wadt KA, Hayward NK. Melanoma genetics. J Med Genet. 2016;53:1–14. doi: 10.1136/jmedgenet-2015-103150. [DOI] [PubMed] [Google Scholar]
- 6.Bertolotto C, Lesueur F, Giuliano S, et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;480:94. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama S, Woods SL, Boyle GM, et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koludrovic D, Davidson I. MITF, the Janus transcription factor of melanoma. Future Oncol. 2013;9:235. doi: 10.2217/fon.12.177. [DOI] [PubMed] [Google Scholar]
- 9.Soura E, Eliades PJ, Shannon K, Stratigos AJ, Tsao H. Hereditary melanoma: update on syndromes and management: emerging melanoma cancer complexes and genetic counseling. J Am Acad Dermatol. 2016;74:411. doi: 10.1016/j.jaad.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohh M. Tumor strengths and frailties: cancer SUMmOns Otto’s metabolism. Nat Med. 2012;18(1):30–31. doi: 10.1038/nm.2631. [DOI] [PubMed] [Google Scholar]
- 11.Bassoli S, Pellegrini C, Longo C, et al. Clinical, dermoscopic, and confocal features of nevi and melanomas in a multiple primary melanoma patient with the MITF p.E318K homozygous mutation. Melanoma Res. 2018;28:166. doi: 10.1097/CMR.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 12.Berwick M, MacArthur J, Orlow I, et al. MITF E318K’s effect on melanoma risk independent of, but modified by, other risk factors. Pigment Cell Melanoma Res. 2014;27:485. doi: 10.1111/pcmr.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiorzo P, Pastorino L, Queirolo P, et al. Prevalence of the E318K MITF germline mutation in Italian melanoma patients: associations with histological subtypes and family cancer history. Pigment Cell Melanoma Res. 2013;26:259. doi: 10.1111/pcmr.12047. [DOI] [PubMed] [Google Scholar]
- 14.Potrony M, Puig-Butille JA, Aguilera P, et al. Prevalence of MITF p.E318K in patients with melanoma independent of the presence of CDKN2A causative mutations. JAMA Dermatol. 2016;152:405. doi: 10.1001/jamadermatol.2015.4356. [DOI] [PubMed] [Google Scholar]
- 15.Stoehr CG, Walter B, Denzinger S, et al. The microphthalmia-associated transcription factor p.E318K mutation does not play a major role in sporadic renal cell tumors from caucasian patients. Pathobiology. 2016;83:165. doi: 10.1159/000443311. [DOI] [PubMed] [Google Scholar]
- 16.Sturm RA, Fox C, McClenahan P, et al. Phenotypic characterization of nevus and tumor patterns in MITF E318K mutation carrier melanoma patients. J Invest Dermatol. 2014;134:141. doi: 10.1038/jid.2013.272. [DOI] [PubMed] [Google Scholar]
- 17.Rosendahl CO, Grant-Kels JM, Que SK. Dysplastic nevus: fact and fiction. J Am Acad Dermatol. 2015;73:507. doi: 10.1016/j.jaad.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Bruno W, Pastorino L, Ghiorzo P, et al. Multiple primary melanomas (MPMs) and criteria for genetic assessment: multiMEL, a multicenter study of the Italian Melanoma Intergroup. J Am Acad Dermatol. 2016;74:325. doi: 10.1016/j.jaad.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 19.Ghiorzo P, Bonelli L, Pastorino L, et al. MC1R variation and melanoma risk in relation to host/clinical and environmental factors in CDKN2A positive and negative melanoma patients. Exp Dermatol. 2012;21:718. doi: 10.1111/j.1600-0625.2012.01549.x. [DOI] [PubMed] [Google Scholar]
- 20.Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol. 2018;25:2105. doi: 10.1245/s10434-018-6513-7. [DOI] [PubMed] [Google Scholar]
- 22.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the internet. J Am Acad Dermatol. 2003;48:679. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 23.Argenziano G, Zalaudek I, Ferrara G, et al. Dermoscopy features of melanoma incognito: indications for biopsy. J Am Acad Dermatol. 2007;56:508. doi: 10.1016/j.jaad.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Ghiorzo P, Gargiulo S, Pastorino L, et al. Impact of E27X, a novel CDKN2A germ line mutation, on p16 and p14ARF expression in Italian melanoma families displaying pancreatic cancer and neuroblastoma. Hum Mol Genet. 2006;15:2682. doi: 10.1093/hmg/ddl199. [DOI] [PubMed] [Google Scholar]
- 25.Ghiorzo P, Fornarini G, Sciallero S, et al. CDKN2A is the main susceptibility gene in Italian pancreatic cancer families. J Med Genet. 2012;49:164. doi: 10.1136/jmedgenet-2011-100281. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein AM, Chaudru V, Ghiorzo P, et al. Cutaneous phenotype and MC1R variants as modifying factors for the development of melanoma in CDKN2A G101W mutation carriers from 4 countries. Int J Cancer. 2007;121:825. doi: 10.1002/ijc.22712. [DOI] [PubMed] [Google Scholar]
- 27.Liang X, Pfeiffer RM, Li WQ, et al. Association of genetic variants in CDK6 and XRCC1 with the risk of dysplastic nevi in melanoma-prone families. J Invest Dermatol. 2014;134:481. doi: 10.1038/jid.2013.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tagliabue E, Gandini S, Bellocco R, et al. MC1R variants as melanoma risk factors independent of at-risk phenotypic characteristics: a pooled analysis from the M-SKIP project. Cancer Manag Res. 2018;10:1143. doi: 10.2147/CMAR.S155283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curchin C, Wurm E, Jagirdar K, Sturm R, Soyer P. Dermoscopy, reflectance confocal microscopy and histopathology of an amelanotic melanoma from an individual heterozygous for MC1R and tyrosinase variant alleles. Australas J Dermatol. 2012;53:291. doi: 10.1111/j.1440-0960.2012.00882.x. [DOI] [PubMed] [Google Scholar]
- 30.Pizzichetta MA, Kittler H, Stanganelli I, et al. Dermoscopic diagnosis of amelanotic/hypomelanotic melanoma. Br J Dermatol. 2017;177:538. doi: 10.1111/bjd.15093. [DOI] [PubMed] [Google Scholar]
- 31.Zalaudek I, Catricalà C, Moscarella E, Argenziano G. What dermoscopy tells us about nevogenesis. J Dermatol. 2011;38:16. doi: 10.1111/j.1346-8138.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 32.Ciudad-Blanco C, Avilés-Izquierdo JA, Lázaro-Ochaita P, Suárez-Fernández R. Dermoscopic findings for the early detection of melanoma: an analysis of 200 cases. Actas Dermosifiliogr. 2014;105:683. doi: 10.1016/j.ad.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Pizzichetta MA, Canzonieri V, Soyer PH, Rubegni P, Talamini R, Massone C. Negative pigment network and shiny white streaks: a dermoscopic-pathological correlation study. Am J Dermatopathol. 2014;36:433. doi: 10.1097/DAD.0000000000000019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon request.