To the Editor:
Duodenal carcinoids (DC) account for less than 3% of intestinal carcinoids and can be removed endoscopically by simple polypectomy or endoscopic mucosal resection (EMR).1,2 In a study published by our group in the January 2019 issue of Pancreas, we found no differences between these techniques in terms of margin positivity and local tumor recurrence for small lesions between 10-20 mm, at median follow-up of 21 months.3 These findings suggest that simple polypectomy may be equally effective in reducing the risk of local recurrence as compared with EMR for selected lesions.4
We now report a retrospective study comparing operative resection to endoscopic techniques for DCs. Previous studies have only described case reports or case series of endoscopic management of DC.5-7 Detailed comparisons of operative approaches for DCs, or comparisons between operative resection and endoscopic management have not been widely reported.
We identified patients who underwent a dedicated DC resection at our tertiary care referral center between June 2006 and June 2017. After identifying 18 patients who underwent operative resection (OR), we compared their tumor and patient characteristics between those undergoing OR and endoscopic resection (ER) (n = 28). Primary endpoints were margin status, recurrence, and survival. Standard descriptive statistical analyses were performed using Stata/IC 15.1 (College Station, Texas), including Wilcoxon rank sum and Chi squared tests, as indicated.
Eight patients underwent Whipple resection, 8 had targeted resection, and 2 had gastrectomy with Billroth reconstruction. These patients are described in Table 1. Median age at surgery was 54 years, 56% were female, 76% were white, and 81% had no underlying genetic syndrome. 63% underwent surgery for concerning features or possible metastasis; 19% were not amenable to ER; 19% were due to other factors (other concurrent surgery or tumor board discussion). There were no differences in tumor size, margins, penetration, or differentiation among operative subtypes. More Whipple patients had intermediate grade tumors per World Health Organization criteria (P = 0.03). Median post-operative stay was 7 days with no major complications. In total, 89% patients were alive at median follow up 3.8 years after surgery. One patient died at 36 months due to metastatic disease, and 1 was lost to follow up. Of the 4 patients with positive lymph nodes at surgery, all were low grade, with median age was 46 years, with median tumor size was 10.5 mm (vs 5 mm for ER, P = 0.01) and all patients survived.
TABLE 1.
Comparison of Resections
| Surgery (n = 18) | Endoscopy (n = 28) | P | |
|---|---|---|---|
| Age at procedure, median (IQR), y | 54 (44–63) | 57 (52–67) | 0.29 |
| Sex, n (%) | 0.12 | ||
| Female | 10 (56) | 9 (32) | |
| Male | 8 (44) | 19 (68) | |
| Race, n (%) | 0.25 | ||
| White | 13 (76) | 17 (61) | |
| Black | 3 (18) | 9 (32) | |
| Other | 1 (6) | 0 (0) | |
| Unknown | 0 (0) | 2 (7) | |
| Ethnicity, n (%) | 0.25 | ||
| Non-Hispanic | 18 (100) | 27 (96) | 0.42 |
| Hispanic | 0 (0) | 1 (4) | |
| Size of DC, median (IQR), mm | 8 (3–12) | 5 (3–8) | 0.085 |
| Well-differentiated, n (%) | 14 (100) | 27 (96) | 0.47 |
| WHO pathologic grade, n (%) | 0.077 | ||
| Low | 9 (69) | 22 (92) | |
| Intermediate | 4 (31) | 2 (8) | |
| Ki-67 expression, n (%) | 0.028 | ||
| <3 % | 5 (56) | 19 (90) | |
| 3–20% | 4 (44) | 2 (10) | |
| Mitoses, n (%) | 0.082 | ||
| <2/high-powered field | 14 (88) | 23 (100) | |
| 2–10/high-powered field | 2 (12) | 0 (0) | |
| Margins, n (%) | 0.47 | ||
| Positive | 0 (0) | 18 (69) | |
| Clean | 18 (100) | 8 (31) | |
| Surviving* | 16/17 (1 died, 1 lost to follow up) | 21/28 (2 died, 5 lost to follow up) | 0.743 |
Cause of death in surgical patient was metastatic duodenal carcinoid; in endoscopic patients was not related to duodenal carcinoid.
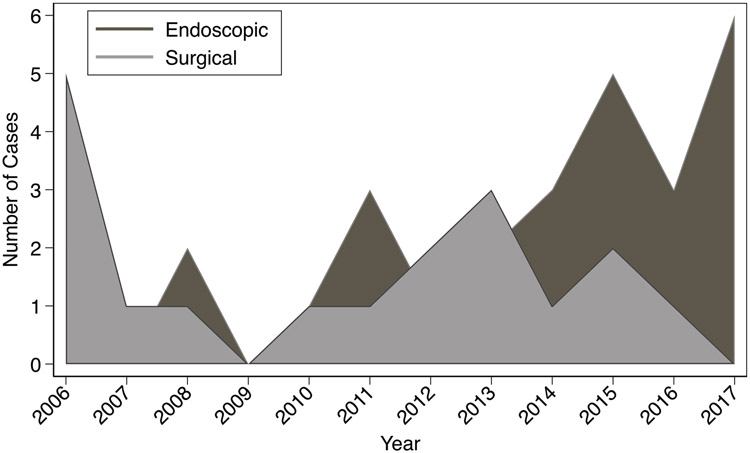
Comparing OR and ER, there were no differences in tumor differentiation, grade, size, patient demographics, or survival (Table 1). Endoscopic resection left more positive margins (69 vs 0% in surgery, P < 0.01). Local recurrence was confirmed in 3/28 (11%) of ER after 1, 3, and 7 months vs distant recurrence in 3/19 (16%) of OR, at 17, 18, and 37 months (P = 0.61). The cause of death in the surgical patient was metastatic duodenal carcinoid; in endoscopic patients, death was not related to duodenal carcinoid. There was an increased use of ER vs OR over time (Fig. 1, P < 0.01).
FIGURE 1.
Graphical depiction of trends of resection of duodenal carcinoids from 2006-2017, comparing endoscopic and surgical resection.
Operative resection of DC is rare, even in our referral center. In this retrospective analysis, operative modalities have excellent resection margins and survival, and outcomes appear similar to ER. Patients with larger tumors may be most appropriate for surgery. There has been a trend towards ER over time, reflecting the expanding role of endoscopy. Further studies are warranted to identify carcinoid tumors that may be appropriate for endoscopic resection. However, our findings would support a consideration of initial endoscopic resection of DC <10-15 mm, with OR reserved for those lesions with higher grade, high mitotic rate and positive resection margins in the ER specimen.
Acknowledgments
Grant support:
Shria Kumar, MD is supported by an NIH training grant (5 T32 DK 7740-22)
Nadim Mahmud, MD is supported by an NIH training grant (2 T32 DK007740 21A1)
Footnotes
Disclosures:
Shria Kumar, MD: Travel (Boston Scientific Corporation)
Nadim Mahmud, MD: none
Robert E. Roses, MD: none
Bryson W. Katona, MD PhD: Consulting (Exact Sciences), Travel (Janssen)
Gregory G. Ginsberg, MD: Consulting (Boston Scientific Corporation), Consulting (Olympus Corporation)
David C. Metz, MBBCh: Consulting (Takeda, Lexicon, AAA. Novartis), Grant Support (Lexicon, Wren Laboratories, Ipsen, AAA)
REFERENCES
- 1.Riddell RH, Petras RE, Williams GT, et al. Tumors of the intestines AFIP Atlas of Tumor Pathology. Series 3, Fascicle 32. Washington, DC: Armed Forces Institute of Pathology, 2003. [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. [DOI] [PubMed] [Google Scholar]
- 3.Mahmud N, Tomizawa Y, Stashek K, et al. Endoscopic Resection of Duodenal Carcinoid Tumors: A Single-Center Comparison Between Simple Polypectomy and Endoscopic Mucosal Resection. Pancreas. 2019;48:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JS, Kim BW. Endoscopic resection or surgical management for nonampullary duodenal neoplasms? Transl Gastroenterol Hepatol. 2018;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim GH, Kim JI, Jeon SW, et al. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29:318–324. [DOI] [PubMed] [Google Scholar]
- 6.Abraham A, Singh J, Siddiqui G, et al. Endoscopic management of a primary duodenal carcinoid tumor. Case Rep Gastroenterol. 2012;6:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navaneethan U, Lourdusamy D, Mehta D, et al. Endoscopic resection of large sporadic non-ampullary duodenal polyps: efficacy and long-term recurrence. Surg Endosc. 2014;28:2616–2622. [DOI] [PubMed] [Google Scholar]