Abstract
When chemical or microbial contaminants are assessed for potential effect or possible regulation in ambient and drinking waters, a critical first step is determining if the contaminants occur and if they are at concentrations that may cause human or ecological health concerns. To this end, source and treated drinking water samples from29 drinking water treatment plants (DWTPs) were analyzed as part of a two-phase study to determine whether chemical and microbial constituents, many of which are considered contaminants of emerging concern, were detectable in the waters. Of the 84 chemicals monitored in the 9 Phase I DWTPs, 27 were detected at least once in the source water, and 21 were detected at least once in treated drinking water. In Phase II, which was a broader and more comprehensive assessment, 247 chemical and microbial analytes were measured in 25 DWTPs, with 148 detected at least once in the source water, and 121 detected at least once in the treated drinking water. The frequency of detection was often related to the analyte’s contaminant class, as pharmaceuticals and anthropogenic waste indicators tended to be infrequently detected and more easily removed during treatment, while per and polyfluoroalkyl substances and inorganic constituents were both more frequently detected and, overall, more resistant to treatment. The data collected as part of this project will be used to help inform evaluation of unregulated contaminants in surface water, groundwater, and drinking water.
Keywords: Pharmaceuticals, Microorganisms, Contaminants of emerging concern, Drinking water, Source water
GRAPHICAL ABSTRACT
1. Introduction
There is increasing public concern over the detection of chemicals in water whose presence results from the diverse array of frequently used consumer, health-, and personal-care products. Chemicals contained in these products—including pharmaceuticals, fragrances, surfactants, and pesticides— may be present in wastewater influent through excretion, bathing, or direct disposal. Many of these chemicals have been documented to survive wastewater treatment and be discharged to surface and groundwaters. Previous reviews (Halling-Sorensen et al., 1998; Daughton and Ternes, 1999; Heberer, 2002; Diaz-Cruz and Barcelo, 2004; Glassmeyer et al., 2008; Kostich et al., 2010; Delgado et al., 2012; Pal et al., 2014; Li et al., 2015; Petrie et al., 2015) have summarized the peer-reviewed literature reporting the occurrences of these chemicals in water resources. Initially termed “emerging contaminants”, there is some misperception that the term suggests that these chemicals have only recently been released into the environment. In fact, these chemicals have been released as long as they have been in use, and some compounds (such as caffeine) have been detected in wastewater (Shuval and Gruener, 1973; Shackelford and Cline, 1986), surface water (Donaldson, 1977; Sheldon and Hites, 1978; Eganhouse et al., 1983; Richardson and Bowron, 1985), and drinking water (Coleman et al., 1980) for several decades. What is emerging is greater awareness by the general public of the presence of these contaminants in the environment and the direct link of environmental presence to household use. The ability of environmental scientists to detect extremely low ambient concentrations of these contaminants, aided by improvements to the analytical instrumentation, further fosters this awareness. Thus, the term “contaminants of emerging concern” (CECs) is a more appropriate choice when describing these contaminants in aggregate.
In the United States, the Safe Drinking Water Act (SDWA), as amended in 1996 (USEPA, 1996) gives the US Environmental Protection Agency (USEPA) the authority to regulate contaminants in finished drinking water, as well as to protect drinking water sources. To regulate a contaminant in drinking water, the SDWA requires that three criteria must be met: 1) the contaminant may have an adverse effect on the health of persons, 2) the contaminant is known to occur or there is a substantial likelihood the contaminant will occur in drinking water with a frequency and at levels of public health concern, and 3) in the sole judgment of the USEPA Administrator, regulation of the contaminant presents a meaningful opportunity for reducing health risks for persons served by public water systems. The SDWA requires the USEPA to evaluate unregulated chemical and microbial contaminants which may necessitate future regulation through the Contaminant Candidate List (CCL) process; the draft fourth CCL (CCL4) was proposed in 2015 (USEPA, 2015). Whether a contaminant is known or anticipated to occur in public water systems is considered as part of the CCL process, along with potential health effects.
Compared to other environmental matrices, there are a paucity of studies that have assessed occurrence of CECs in finished drinking water (Benotti et al., 2009; Stackelberg et al., 2004; Stackelberg et al., 2007; Snyder, 2008; Garcia-Ac et al., 2009; Loos et al., 2007; Togola and Budzinski, 2007), and these studies typically do not examine analytes from multiple contaminant classes. One mechanism to obtain nationally representative drinking water occurrence data is through the Unregulated Contaminant Monitoring Regulation (UCMR), an authority that allows the USEPA to gather occurrence data from all public water systems (PWS) serving >10,000 people, and a representative sample of PWSs serving 10,000 or fewer people, for no more than 30 contaminants in five-year cycles (USEPA, 2012a). Occurrence data of CECs in drinking water in published studies helps determine which analytes would be most appropriate for the UCMR. However, focused, national-scale studies of CEC presence and concentration in source-and treated drinking water samples that use consistent, state-of-the-art sample collection and analysis approaches and assessing the widest array of CECs offer the greatest benefit for identifying the most appropriate contaminants for any detailed UCMR assessments.
This paper is one of a series of papers describing a comprehensive study on the presence, concentrations, and persistence of chemical and microbial CECs in source and treated drinking waters of the United States (Batt et al., 2016; Benson et al., 2016; Conley et al., 2016; Furlong et al., 2016; King et al., 2016; Kostich et al., 2016; Boone et al., unpublished results; Varughese et al., unpublished results). This was a joint effort of the USEPA and the U.S. Geological Survey (USGS), as part of a long-term interagency agreement. A primary goal of the overall study was to provide accurate, objective information for assessing the potential for human exposure to a comprehensive set of CECs via drinking water. A secondary goal was to evaluate removal, if any, of CECs from source waters by currently used drinking water treatment processes under typical plant operating conditions. The interdisciplinary approach of this nationwide study is unique in that it combined both the measurement of CECs along with the evaluation of the potential effects of the contaminants, through both an in vitro estrogenic activity bioassay and screening level human and ecological health impact assessments.
2. Experimental design
This study was conducted in two phases. In Phase I (2007), source and treated drinking water from nine drinking water treatment plants (DWTPs) from eight states across the United States were sampled and analyzed for 84 chemicals using three different analytical methods. The Phase I effort provided an opportunity to test the experimental design, field sampling protocols, and analytical methods as applied to operator-collected samples from DWTPs. In Phase II (2010–2012), the quality assurance/quality control design was refined, the analyte list expanded (247 chemical and microbiological contaminants using 16 different methods, as well as an in vitro estrogenicity bioassay), and the number of DWTPs sampled increased to 25 DWTPs located in 24 states, including five that were also sampled in Phase I. Between the two phases, 29 DWTPs were investigated (five in both Phase I and II, four in Phase I only and 20 in Phase II only). A total of 77 common analytes were measured in both Phase I and II.
2.1. Site selection
An objective of this study was to better determine the upper boundary of CEC concentrations, rather than provide a nationwide average, so DWTP selection was skewed towards sample locations with known wastewater outfalls in the source water. Candidate locations were selected based on water sources with potential for a high wastewater contribution (Swayne et al., 1980), locations with and without existing pharmaceutical concentration data (Associated Press, 2008), nomination by USEPA and USGS regional personnel, and DWTP self-nomination. Sites were chosen to maximize the range in select attributes including geography, diversity in disinfectant type used in the treatment process, and drinking water plant production volume. Participation in the study was voluntary. Table 1 provides a description of each participating DWTP, but the specific identity of each location is not given to provide anonymity of the participating DWTPs.
Table 1.
Background Information on the Phase I (DWTP 1–9) and Phase II (DWTP 1–5; 10–29) locations.
| Location | Pop serveda (1000s) | Production at samplinga (MGD) | Residence time of treatmentb (h) | Sampling intervalb (h) | Primary disinfectantc | GAC depth (feet) | GAC recharge rate (years) | Simplified treatment traine |
|---|---|---|---|---|---|---|---|---|
| DWTP 1 | >500 | >100 | PI4 | PI 4.5 | O3 + NH2Cl | nad | na | O3, coag/floc, NH2Cl, C, floc, C, F |
| PII 10 | PII 8 | |||||||
| DWTP 2 | >500 | >100 | PI 69 | PI 75 | Cl2 | 11.4 | 0.6 | Coag/floc, S, SF, GAC, Cl2 |
| PII 72 | PII 73 | |||||||
| DWTP 3 | 50–500 | 10–100 | 6 | PI 7 | Cl2 + UV | 2.5 | 3 | Coag/floc, C/S, F, GAC, Cl2, UV |
| PII 7 | ||||||||
| DWTP 4 | >500 | 10–100 | PI 38 | PI 45 | Cl2 + NH2Cl | na | na | Pre-Cl2, coag/floc, S, secondary Cl2, SF, NH3 |
| PII 46 | PII 48 | |||||||
| DWTP 5 | <50 | <10 | 0.13 | PI 0 | Cl2 | na | na | Cl2 |
| PII 0 | ||||||||
| DWTP 6 | 50–500 | 10–100 | 24 | 24 | ClO2 + Cl2 | na | na | ClO2, coag, S,pre-Cl2, F, Cl2 |
| DWTP 7 | 50–500 | 10–100 | 8 | 8 | Cl2 | na | na | Coag, pre-Cl2, PAC, floc, S, F, Cl2 |
| DWTP 8 | <50 | <10 | 6 | 6 | ClO2 + Cl2 | 3 | 2 | ClO2, Cl2, coag/floc, S, GAC and SF, Cl2 |
| DWTP 9 | <50 | <10 | 10 | 6 | Cl2 | na | na | S, coag/floc, SF, Cl2 |
| DWTP 10 | 50–500 | >100 | 7 | 9.25 | NH2Cl | na | na | Coag/floc, S, NH2Cl, F |
| DWTP 11 | <50 | <10 | 7 | 2.25 | O3 + Cl2 | 6 | 4 | Coag/floc, S, C, O3, GAC and SF, Cl2 |
| DWTP 12 | <50 | <10 | 30.72 | 23.75 | Cl2 | 1.25 | as needed | Coag/floc, pre-Cl2, C, GAC and SF, post-Cl2 |
| DWTP 13 | >500 | >100 | 1 | 0.75 | Cl2 | na | na | Cl2 |
| DWTP 14 | 50–500 | 10–100 | 10 | 3.25 | ClO2 + Cl2 | 0.75 | 8 | Coag/floc, pre-ClO2, GAC and SF, Cl2 |
| DWTP 15 | <50 | <10 | 1 | 4 | Cl2 | na | na | Coag/floc, S, F, Cl2 |
| DWTP 16 | 50–500 | 10–100 | 6 | 9 | NH2Cl | 2.5 | 3 | Coag/floc, S, GAC and SF, NH2Cl |
| DWTP 17 | <50 | <10 | 2 | 4 | Cl2 | na | na | C, coag/floc, pre-Cl2, F, Cl2 |
| DWTP 18 | <50 | <10 | 7.3 | 7.25 | O3 + NH2Cl | 4 | 2 | O3, floc, S, pre-Cl2, GAC and SF, NH2cl |
| DWTP 19 | 50–500 | 10–100 | 26 | 57.25 | NH2Cl | na | na | Coag/floc, PAC, S, ultrafiltration, NH2Cl |
| DWTP 20 | >500 | 10–100 | 30 | 46.75 | O3 + CI2 | 5 | >4 | Floc, S, O3, GAC and SF, Cl2 |
| DWTP 21 | 50–500 | 10–100 | 90 | 14.5 | Cl2 | na | na | PAC pre-Cl2, coag, S, Cl2 F |
| DWTP 22 | 50–500 | 10–100 | 10 | 1.5 | O3 + Cl2 + UV | 4 | as needed | Pre-O3, coag, S, O3, GAC and SF, UV, Cl2 |
| DWTP 23 | 50–500 | 10–100 | 7 | 6.5 | ClO2 +UV + Cl | na | na | Pre-ClO2, coag/floc, S, dual media F, UV, Cl2 |
| DWTP 24 | 50–500 | 10–100 | 8 | 6.25 | NH2Cl | 1.7 | 3 | PAC, GAC and SF, NH2Cl |
| DWTP 25 | 50–500 | 10–100 | 13.6 | 12 | O3 + NH2CI | 3 | 5–10 | Pre-O3, coag, NH2Cl |
| DWTP 26 | 50–500 | 10–100 | 24–36 | 3.25 | Cl2 | na | na | Pre-Cl2, PAC, coag, S, Cl2, F, Cl2 |
| DWTP 27 | 50–500 | <10 | 4 | 13.75 | NH2Cl + UV | na | na | PAC, coag/floc, S, F, UV, NH2Cl |
| DWTP 28 | >500 | >100 | 1 | 1.5 | O3 + NH2Cl | na | na | NH2cl, O3, F |
| DWTP 29 | <50 | <10 | 8 | 8.75 | Cl2 | na | na | PAC, pre-Cl2, coag/floc, S, Cl2, F |
Population sizes binned to give indication of DWTP size variation while maintaining plant anonymity.
DWTPs were asked to match the residence time of treatment. Some locations achieved this better than others. PI = Phase I of study; PII = Phase II of study.
O3 = ozone; NH2Cl = chloramine; Cl2 = chlorine; UV = ultraviolet radiation; ClO2 = chlorine dioxide.
na = not applicable.
Major steps in treatment in each plant. Coag = coagulation; floc = flocculation; C = clarification; F = filtration; S = sedimentation; SF = sand filter; NH3 = ammonia; PAC = powdered activated carbon; GAC = granular activated carbon.
2.2. Sample collection
Samples were collected by operating staff at each of the DWTPs with project-provided protocols and sampling materials. Sample collection bottles for each method were pre-spiked (if needed) with an appropriate dechlorination agent. Supplementary information Table 1 details method specific bottles, sample volumes, dechlorination agent, and sample holding times. Although the dechlorination agent was not needed for the source water samples, it was added to all chemical contaminant samples analyzed by a given method, if needed for finished water sample preservation to maintain sample consistency. For the chemical analyses, bioassays, and the majority of the microbial tests, grab samples were collected. Most of the DWTPs were plumbed with sampling taps at different locations in the plant. These taps allow collection either directly, or have piping back to a sink in the facility’s laboratory. The DWTP operators were instructed to collect the source water sample prior to any treatment, including settling basins. The treated water sample was to be collected at a sampling point after final disinfection but prior to the clear well. The DWTP operators were requested to time sampling between the source water and the treated water to match the hydraulic residence time of the plant, so approximately the same parcel of water would be analyzed entering and exiting the plant. In some instances, however, this was not possible (Table 1). Sample collection at most locations was performed by DWTP personnel by simply filling the bottle at the tap to the appropriate volume. DWTP 10 did not have a source water tap, so an empty sampling bottle was dipped into the source water and the sample was decanted into appropriate sample bottles. Since the perfluorinated analytes were known to sorb to container surfaces, and since no dechlorination agent or preservatives were used for that method, the sample bottle was directly dipped into the DWTP 10 source water to collect the sample.
For the protozoa and virus samples in Phase II, field filtration was required. The utilities were supplied with two sets of sterile tubing, filters with appropriate housing cartridges, and flow meters (one for source and one for treated samples). For source water samples, 10 L was filtered on an Envirochek™ (Pall Corporation, Port Washington, NY) for protozoa analysis and up to 200 L was filtered on a NanoCeram® filter (Argonide, Sanford, FL) for viruses. For the treated water samples, since residual chlorine can inactivate viruses attached to the filter, the virus samples were collected at a point just before the introduction of disinfectant. Since 13 of the 25 DWTPs used pre-chlorination, only 12 treated pre-disinfection water samples were collected in Phase II for analyzing viruses. For these treated non-disinfected samples, 2000 L of water was filtered. More details on the virus collection procedure will be provided in a forthcoming manuscript (Varughese et al., unpublished results). Protozoa samples were not collected from the treated water.
In Phase I, all samples were collected in duplicate. One sample was analyzed as the primary sample, and the second analyzed alternately as a replicate sample or as a laboratory fortified matrix sample (matrix spike). In Phase II, all samples for organic chemical analysis were collected in triplicate, with a primary, replicate, and laboratory fortified matrix analyzed at all locations. Only the primary sample was analyzed for inorganic and microbial constituents at all sampling points.
Field blanks were included to monitor for potential contamination during sampling, processing, or transport, because many of the measured analytes occur in products commonly consumed and used by DWTP and other personnel, and gloves and other personal protective equipment may not be sufficient to avert contamination. In Phase I, DWTPs were asked to supply a sample of laboratory grade water, either a decanted bottled sample or produced water, such as Milli-Q (EMD Millipore, Billerica, MA). In Phase II, bottled laboratory grade water (Omni-Solv®, EMD Millipore, Billerica, MA), validated to be free of many organic contaminants, was supplied to all DWTPs for decanting into sample collection bottles on-site.
After collection, all samples and field blanks were immediately packed on ice and shipped overnight to USEPA and USGS laboratories for analysis within sample holding times (Supplementary information Table 1).
2.3. Sample analysis
In Phase I, samples were analyzed using three methods, two for pharmaceuticals (Cahill et al., 2004 adapted as an official USGS method in Furlong et al., 2008; Schultz and Furlong, 2008) and one for a diverse suite of chemicals commonly found in wastewater, such as detergent metabolites, fragrances, and pesticides, described herein as anthropogenic waste indicators (AWIs; Zaugg et al., 2006) These three methods were also utilized in Phase II, along with three additional pharmaceutical methods (a modified version of Ternes et al., 2005; Batt et al., 2008; Furlong et al., 2014), a method for hormones and other endocrine disrupting chemicals (Conley et al., 2016), a per- and polyfluoroalkyl substances (PFAS) method (Boone et al., 2014), a method for fungi (Haugland et al., 2004), two bacteria methods (Covert et al., 1999 and Beumer et al., 2010 for mycobacteria; Donohue et al., 2014 for Legionella), a method for enteric viruses (Varughese et al., unpublished results), and a method for protozoa (USEPA, 2005a). While they are not CECs, three methods for inorganic constituents (USEPA, 2005b; USEPA, 2001; USEPA, 1994) were also used to analyze samples. Three analytes were evaluated in multiple methods in Phase I; a total of 53 compounds (46 organic and 7 inorganic) were measured in multiple methods in Phase II. In addition to the direct concentration measurements, an aliquot of the extracts prepared for the hormone analysis was also evaluated for estrogen receptor-mediated bioactivity using the T47D-KBluc bioassay (Wilson et al., 2004; Conley et al., 2016). Supplementary information Table 1 has a brief summary of each method used for this study. More methodological detail can be found in the above referenced papers, as well as in the accompanying detailed manuscripts on pharmaceuticals (Furlong et al., 2016), hormones (Conley et al., 2016), PFASs (Boone et al., unpublished results), bacteria, fungi and protozoa (King et al., 2016) and viruses (Varughese et al., unpublished results).
2.4. Quality control
Since the concentrations measured in this study were expected to be close to the instrument detection limits, a considerable number of quality assurance/quality control (QA/QC) samples were incorporated into the sampling design. Over 50% of the samples analyzed in Phase I and over 70% of the Phase II samples were for QA/QC purposes. When possible, the lowest concentration minimum reporting level (LCMRL; USEPA, 2010) was determined for each analyte. If the LCMRL could not be calculated, a reporting limit (RL) was used at the quantified detection threshold (USEPA, 2012b). Samples that did not exceed their associated LCMRL or RL but were above the instrument detection limit were considered qualitative detections, and the numerical concentrations were removed from the results. Likewise, samples in Phase II with associated laboratory fortified matrix samples with >150% recoveries were considered as qualitative detections as the matrix exhibited signal enhancement. Sample measurements that did not exceed the concentrations measured in the associated field and/or laboratory blanks by a factor of three were censored from the data set. A detailed discussion of the QA/QC analysis is available in an accompanying manuscript (Batt et al., 2016) as well as in the individual papers on specific aspects of contaminant results (Conley et al., 2016; Furlong et al., 2016; Boone et al., unpublished results; King et al., 2016).
3. Results and discussion
Table 2 lists the analytes qualitatively detected in at least 30% of either the source or treated drinking water samples for both Phase I and II. In this table, and in the remainder of the paper, the analytes are separated into five contaminant classes: 1) pharmaceuticals, 2) perfluoroalkyl and polyfluoroalkyl substances (PFASs), 3) anthropogenic waste indicators (AWIs), 4) inorganic constituents, and 5) microorganisms. Detailed discussions of the individual analytes are presented in the associated papers ( Conley et al., 2016; Furlong et al., 2016; Boone et al., unpublished results; King et al., 2016; Varughese et al., unpublished results). Tables enumerating all analytes detected and not detected are presented in alphabetical order by contaminant class in Supplementary information Tables 2 and 3, respectively. Concentrations of inorganic constituents and AWIs detected at each location are presented in Supplementary information Table 4. Of the 84 analytes in Phase I and 247 analytes in Phase II, 57 and 99 (68% and 40%) were never detected in source water samples and 63 and 126 (75% and 51%) were never detected in treated drinking water samples, respectively.
Table 2.
Source and treated drinking water qualitative and quantitative frequency of detections, median, and maximum concentrations for analytes detected in at least 30% of collected samples.
| Analytes | CAS registry number | Methoda | Units | RLb | LCMRLb | Source water | Treated drinking water | Analyte class and primary useh | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nc | Quald freq (%) | Quale freq (%) | Med.f conc | Max.g conc | nc | Quald freq (%) | Quale freq (%) | Med.f conc | Max.g conc | |||||||
| Phase I Pharmaceuticals | ||||||||||||||||
| Bupropion | 34,841-39-9 | 4 | ng/L | 0.66 | 9 | 89 | 67 | 1.23 | 3.19 | 9 | 44 | 33 | 1.99 | 3.34 | 1-Antidepressant | |
| Venlafaxine | 93,413-69-5 | 4 | ng/L | 0.58 | 9 | 78 | 78 | 10.8 | 41.9 | 9 | 0 | 0 | nd | nd | 1-Antidepressant | |
| Caffeine | 58-08-2 | 6 | ng/L | 60 | 9 | 67 | 11 | 124 | 124 | 9 | 66 | 11 | 88 | 88 | 1-Psychoactive stimulant | |
| Carbamazepine | 298-46-4 | 6 | ng/L | 40 | 9 | 78 | 11 | 269 | 269 | 9 | 55 | 11 | 586 | 586 | 1-Anticonvulsant and mood stabilizer | |
| Sulfamethoxazole | 723-46-6 | 6 | ng/L | 100 | 9 | 56 | 0 | QL | QL | 9 | 11 | 0 | QL | QL | 1-Sulfonamide antibiotic drug | |
| Citalopram | 59,729-33-8 | 4 | ng/L | 0.9 | 9 | 33 | 11 | 0.90 | 0.90 | 9 | 0 | 0 | nd | nd | 1-Antidepressant | |
| Sertraline | 79,617-96-2 | 4 | ng/L | 0.42 | 9 | 22 | 22 | 0.54 | 0.66 | 9 | 0 | 0 | nd | nd | 1-Antidepressant | |
| Anthropogenic Waste Indicators (AWIs) | ||||||||||||||||
| Tri(2-chloroethyl) phosphate | 115–96-8 | 5 | ng/L | 180 | 9 | 56 | 0 | QL | QL | 9 | 22 | 0 | QL | QL | 5-Fire retardant | |
| Tributyl phosphate | 126-73-8 | 5 | ng/L | 200 | 9 | 33 | 0 | QL | QL | 9 | 11 | 0 | QL | QL | 5-Antifoaming agent and flame retardant | |
| Bromoform | 75-25-2 | 5 | ng/L | 80 | 9 | 22 | 11 | 545 | 545 | 9 | 78 | 78 | 388 | 4060 | 8-Wastewater disinfection byproduct | |
| Phase II Pharmaceuticals | ||||||||||||||||
| Sulfamethoxazole | 723-46-6 | 2 | ng/L | 6.5 | 25 | 60 | 40 | 50.1 | 161.1 | 25 | 4 | 4 | 8.2 | 8.2 | 1-Sulfonamide antibiotic drug | |
| Lithium | 7439-93-2 | 9 | ng/L | 5000 | 25 | 56 | 56 | 10,700 | 46,000 | 25 | 56 | 56 | 10,800 | 42,700 | 1-Treats mania as part of bipolar disorder | |
| Carbamazepine | 298-46-4 | 2 | ng/L | 7.1 | 25 | 56 | 28 | 15.9 | 35.7 | 25 | 8 | 8 | 17.75 | 26.50 | 1-Anticonvulsant and mood stabilizer | |
| Metoprolol | 51,384-51-1 | 2 | ng/L | 4.7 | 25 | 52 | 32 | 11.4 | 37.8 | 25 | 20 | 12 | 8.5 | 18.4 | 1-Antihypertensive | |
| Estrone | 53-16-7 | 3 | ng/L | 0.092 | 25 | 52 | 20 | 0.18 | 0.29 | 25 | 4 | 0 | QL | QL | 3-Hormone | |
| Aciclovir | 59,277-89-3 | 1 | ng/L | 82 | 25 | 44 | 0 | QL | QL | 25 | 12 | 0 | QL | QL | 1-Antiviral | |
| Metformin | 657-24-9 | 1 | ng/L | 23 | 25 | 40 | 0 | QL | QL | 25 | 16 | 0 | QL | QL | 1-Treatment of type 2 diabetes | |
| Methocarbamol | 532-03-6 | 1 | ng/L | 27 | 25 | 36 | 8 | 29.11 | 32.30 | 25 | 16 | 0 | QL | QL | 1-Muscle relaxant | |
| Meprobamate | 57-53-4 | 1 | ng/L | 69 | 25 | 32 | 4 | 14.18 | 14.18 | 25 | 16 | 0 | QL | QL | 1-Anxiolytic | |
| Caffeine | 58-08-2 | 1 | ng/L | 42 | 25 | 32 | 12 | 70.29 | 90.89 | 25 | 8 | 0 | QL | QL | 1-Psychoactive stimulant | |
| Tramadol | 27,203-92-5 | 1 | ng/L | 8.7 | 25 | 32 | 16 | 10.74 | 23.04 | 25 | 0 | 0 | ND | ND | 1-opiate | |
| Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) | ||||||||||||||||
| Perfluorooctanoic acid (PFOA) | 335-67-1 | 8 | ng/L | 0.56 | 25 | 100 | 76 | 6.32 | 112.00 | 25 | 100 | 76 | 4.15 | 104.00 | 12-Perfluorinated | |
| Perfluorobutanesulfonic acid (PFBS) | 375-73-5 | 8 | ng/L | 0.032 | 25 | 100 | 96 | 1.12 | 11.10 | 25 | 100 | 96 | 1.17 | 11.90 | 12-Perfluorinated | |
| Perfluorooctanesulfonic acid (PFOS) | 1763-23-1 | 8 | ng/L | 0.13 | 25 | 96 | 88 | 2.28 | 48.30 | 25 | 92 | 80 | 1.62 | 36.90 | 12-Perfluorinated | |
| Perfluorohexanoic acid (PFHxA) | 307-24-4 | 8 | ng/L | 0.044 | 25 | 96 | 96 | 2.02 | 55.10 | 25 | 100 | 100 | 1.43 | 60.80 | 12-Perfluorinated | |
| Perfluoroheptanoic acid (PFHpA) | 375-85-9 | 8 | ng/L | 0.04 | 25 | 96 | 96 | 1.13 | 184.00 | 25 | 92 | 92 | 0.79 | 177.00 | 12-Perfluorinated | |
| Perfluorononanoic acid (PFNA) | 375-95-1 | 8 | ng/L | 0.094 | 25 | 96 | 96 | 0.86 | 41.40 | 25 | 92 | 88 | 0.74 | 38.60 | 12-Perfluorinated | |
| Perfluorobutanoic acid (PFBA) | 375-22-4 | 8 | ng/L | 0.24 | 25 | 92 | 92 | 3.05 | 96.80 | 25 | 88 | 88 | 3.62 | 104.00 | 12-Perfluorinated | |
| Perfluoropentanoic acid (PFPeA) | 2706-90-3 | 8 | ng/L | 0.051 | 25 | 92 | 92 | 1.95 | 501.00 | 25 | 96 | 96 | 1.78 | 514.00 | 12-Perfluorinated | |
| Perfluorohexanesulfonic acid (PFHxS) | 355-46-4 | 8 | ng/L | 0.034 | 25 | 92 | 92 | 0.86 | 44.80 | 25 | 84 | 84 | 0.86 | 38.40 | 12-Perfluorinated | |
| Perfluorodecanoic acid (PFDA) | 335-76-2 | 8 | ng/L | 0.084 | 25 | 92 | 60 | 0.43 | 31.10 | 25 | 80 | 52 | 0.33 | 24.70 | 12-Perfluorinated | |
| Perfluoroundecanoic acid (PFUnDA) | 2058-94-8 | 8 | ng/L | 0.067 | 25 | 36 | 32 | 0.14 | 2.90 | 25 | 32 | 16 | 0.54 | 1.85 | 12-Perfluorinated | |
| Anthropogenic Waste Indicators (AWIs) | ||||||||||||||||
| Triclocarban (3,4,4′-trichlorocarbanalide) | 101-20-2 | 3 | ng/L | 1.1 | 21 | 57 | 24 | 1.74 | 2.89 | 21 | 19 | 0 | QL | QL | 6-Antimicrobial | |
| Triclosan | 3380–34-5 | 3 | ng/L | 0.68 | 25 | 52 | 12 | 2.71 | 3.50 | 25 | 36 | 0 | QL | QL | 6-Disinfectant, antimicrobial | |
| Benzotriazole methyl-lH | 136-85-6 | 1 | ng/L | 71 | 25 | 48 | 44 | 270 | 1200 | 25 | 36 | 16 | 134 | 247 | 8-Antioxidant and deicing agent | |
| N,N-diethyl-meta-toluamide (DEET) | 134-62-3 | 5 | ng/L | 72 | 25 | 48 | 4 | 98 | 98 | 25 | 24 | 0 | QL | QL | 6-Insect repellant | |
| Atrazine | 1912-24-9 | 1 | ng/L | 22 | 25 | 44 | 24 | 64 | 323 | 25 | 32 | 16 | 154 | 270 | 10-Herbicide | |
| Metolachlor | 51218-45-2 | 5 | ng/L | 49 | 25 | 36 | 12 | 130 | 130 | 25 | 32 | 12 | 95 | 100 | 10-Herbicide | |
| Galaxolide (HHCB) | 1222-05-5 | 5 | ng/L | 2.7 | 25 | 36 | 36 | 28 | 110 | 25 | 24 | 24 | 36.5 | 61 | 7-Fragrance, musk | |
| Tri(2-butoxyethyl) phosphate | 78-51-3 | 5 | ng/L | 410 | 25 | 36 | 4 | 470 | 470 | 25 | 8 | 0 | QL | QL | 8-Plasticizer | |
| Tri(2-chloroethyl) phosphate | 115-96-8 | 5 | ng/L | 91 | 25 | 32 | 4 | 65 | 65 | 25 | 28 | 0 | QL | QL | 5-Fire retardant | |
| Isophorone | 78-59-1 | 5 | ng/L | 28 | 25 | 20 | 0 | QL | QL | 25 | 32 | 4 | 32 | 32 | 8-solvent | |
| Bromoform | 75-25-2 | 5 | ng/L | 100 | 25 | 12 | 12 | 60 | 88 | 25 | 60 | 52 | 180 | 3300 | 8-Wastewater ozonation byproduct | |
| Inorganic Constituents | ||||||||||||||||
| Strontium | 7440-24-6 | 9 | μg/L | 1.00 | 25 | 100 | 100 | 177 | 1014 | 25 | 100 | 100 | 178 | 1000 | 13-Inorganic | |
| Barium | 7440-39-3 | 9 | μg/L | 1.00 | 25 | 100 | 100 | 50.8 | 114 | 25 | 100 | 100 | 29.6 | 110 | 13-Inorganic | |
| Calcium | 7440-70-2 | 9 | mg/L | 0.010 | 25 | 100 | 100 | 38.9 | 129 | 25 | 100 | 100 | 39.3 | 78.4 | 13-Inorganic | |
| Sodium | 7440-23-5 | 9 | mg/L | 0.030 | 25 | 100 | 100 | 24.0 | 128 | 25 | 100 | 100 | 27.8 | 128 | 13-Inorganic | |
| Sulfur | 7704-34-9 | 9 | mg/L | 0.003 | 25 | 100 | 100 | 13.3 | 82.7 | 25 | 100 | 100 | 14.5 | 83.9 | 13-Inorganic | |
| Magnesium | 7439-95-4 | 9 | mg/L | 0.005 | 25 | 100 | 100 | 10.6 | 44.6 | 25 | 100 | 100 | 8.81 | 31.7 | 13-Inorganic | |
| Silicon | 7440-21-3 | 9 | mg/L | 0.020 | 25 | 100 | 100 | 2.75 | 22.4 | 25 | 100 | 100 | 2.93 | 22.3 | 13-Inorganic | |
| Potassium | 7440-09-7 | 9 | mg/L | 0.300 | 25 | 100 | 100 | 2.72 | 6.93 | 25 | 100 | 100 | 3.07 | 6.87 | 13-Inorganic | |
| Total dissolved nitrogen | 9 | mg N/L | NA | 23 | 100 | 100 | 1.03 | 5.12 | 23 | 100 | 100 | 0.96 | 4.97 | 13-Inorganic | ||
| Flouride | 16984-48-8 | 9 | mg/L | NA | 23 | 100 | 100 | 0.20 | 0.56 | 24 | 100 | 100 | 0.83 | 1.22 | 13-Inorganic | |
| Nitrate (NO3) | 14797-55-8 | 9 | mg N/L | 0.089 | 24 | 100 | 100 | 0.77 | 5.09 | 25 | 96 | 96 | 0.78 | 4.91 | 13-Inorganic | |
| Aluminum | 7429-90-5 | 9 | μg/L | 4.00 | 25 | 96 | 96 | 91.1 | 949 | 25 | 96 | 96 | 11.1 | 188 | 13-Inorganic | |
| Zinc | 7440-66-6 | 9 | μg/L | 0.50 | 25 | 96 | 96 | 3.30 | 23 | 25 | 68 | 68 | 1.30 | 100 | 13-Inorganic | |
| Sulfate (SO4) | 14808-79-8 | 9 | mg/L | NA | 24 | 96 | 88 | 20.6 | 234 | 24 | 96 | 88 | 43.1 | 241 | 13-Inorganic | |
| Chloride | 16887-00-6 | 9 | mg/L | NA | 24 | 96 | 88 | 15.8 | 52.8 | 24 | 96 | 83 | 26.9 | 60.8 | 13-Inorganic | |
| Iron | 7439-89-6 | 9 | Mg/L | 1.00 | 25 | 92 | 92 | 206 | 1688 | 25 | 80 | 80 | 3.40 | 90.7 | 13-Inorganic | |
| Manganese | 7439-96-5 | 9 | Mg/L | 1.00 | 25 | 92 | 92 | 43 | 1497 | 25 | 64 | 64 | 2.60 | 55.6 | 13-Inorganic | |
| Phosphorus | 7723-14-0 | 9 | mg/L | 0.005 | 25 | 84 | 84 | 0.07 | 0.22 | 25 | 68 | 68 | 0.20 | 0.70 | 13-Inorganic | |
| Copper | 7440-50-8 | 9 | Mg/L | 1.00 | 25 | 84 | 84 | 3.10 | 53.40 | 25 | 64 | 64 | 4.75 | 109 | 13-Inorganic | |
| Phosphate (PO4) | 14265-44-2 | 9 | mg/L | 0.025 | 24 | 83 | 83 | 0.13 | 0.56 | 25 | 68 | 68 | 0.22 | 1.96 | 13-Inorganic | |
| Bromide | 10035-10-6 | 9 | mg/L | 0.005 | 24 | 79 | 79 | 0.06 | 0.26 | 24 | 50 | 50 | 0.04 | 0.24 | 13-Inorganic | |
| Lead | 7439-92-1 | 9 | Mg/L | 0.07 | 22 | 77 | 77 | 0.38 | 2.41 | 24 | 21 | 21 | 0.11 | 0.27 | 13-Inorganic | |
| Uranium | 7440-61-1 | 9 | Mg/L | 0.05 | 22 | 68 | 68 | 0.83 | 8.92 | 24 | 50 | 50 | 0.69 | 3.63 | 13-Inorganic | |
| Ammonia (NH3) | 7664-41-7 | 9 | mg/L | 0.012 | 25 | 64 | 64 | 0.05 | 0.24 | 25 | 48 | 48 | 0.39 | 0.79 | 13-Inorganic | |
| Arsenic | 7440-38-2 | 9 | μg/L | 4.00 | 22 | 64 | 64 | 0.97 | 3.13 | 24 | 54 | 54 | 0.54 | 1.37 | 13-Inorganic | |
| Nitrite (NO2) | 14,797-65-0 | 9 | mg N/L | 0.033 | 24 | 50 | 50 | 0.02 | 0.06 | 25 | 24 | 24 | 0.02 | 0.02 | 13-Inorganic | |
| Nickel | 7440-02-0 | 9 | μg/L | 1.00 | 25 | 44 | 44 | 1.50 | 2.20 | 25 | 20 | 20 | 1.20 | 3.50 | 13-Inorganic | |
| Vanadium | 7440-62-2 | 9 | μg/L | 1.00 | 25 | 44 | 44 | 2.30 | 5.80 | 25 | 16 | 16 | 3.40 | 4.90 | 13-Inorganic | |
| Tin | 7440-31-5 | 9 | μg/L | 1.00 | 25 | 40 | 40 | 3.55 | 17.4 | 25 | 36 | 36 | 6.40 | 15.9 | 13-Inorganic | |
| Chlorate (ClO3) | 14,866-68-3 | 9 | mg/L | 0.010 | 16 | 13 | 13 | 0.05 | 0.07 | 15 | 53 | 53 | 0.08 | 0.32 | 13-Inorganic | |
| Selenium | 7782-49-2 | 9 | μg/L | 1.00 | 22 | 9 | 9 | 1.30 | 1.54 | 24 | 29 | 29 | 1.35 | 1.64 | 13-Inorganic | |
| Microorganisms | ||||||||||||||||
| Aspergillus fumigatus | 10 | cells/L | 25 | 48 | 48 | 10 | 30 | 25 | 0 | 0 | ND | ND | 14-Fungus | |||
| Giardia | 14 | cysts/L | 23 | 48 | 48 | 0.73 | 2.22 | 0 | – | – | – | – | 14-Protozoa | |||
| Adenovirus | 13 | MPN/L | 25 | 36 | 28 | 320 | 5123 | 12i | 17i | 17i | 73i | 105i | 14-Virus | |||
| Norovirus genogroup II | 13 | MPN/L | 25 | 36 | 36 | 471 | 3133 | 12i | 8i | 8i | 98i | 98i | 14-Virus | |||
| Aspergillus terreus | 10 | cells/L | 25 | 28 | 28 | 250 | 4250 | 25 | 0 | 0 | ND | ND | 14-Fungus | |||
| Polyomavirus | 13 | MPN/L | 25 | 28 | 16 | 356 | 848 | 12i | 8i | 0i | Qli | QLi | 14-Virus | |||
Detailed information about each method is presented in Supplementary Information Table 1.
Each analyte has either a reporting limit (RL) or lowest concentration minimum reporting level (LCMRL). See text for discussion on difference.
Number of samples analyzed for a particular analyte. Maximum in Phase Iis9; maximum in Phase II is 25.
Qualitative frequency of detection. Includes measurements below the RL or LCMRL as well as analytes with matrix enhancement in the associated laboratory fortified matrix samples.
Quantitative frequency of detection. Includes only measurements that exceed the RL or LCMRL and did not have matrix enhancement.
Median concentration of quantified detections. QL = all measurements qualitative; ND = non-detect.
Maximum concentration of quantified detections. QL = all measurements qualitative; ND = non-detect.
Analyte classes: 1) pharmaceutical, 2) pharmaceutical metabolite, 3) hormone, 4) detergent metabolite, 5) chlorinated flame retardant, 6) household chemical, 7) fragrance, 8) industrial chemical, 9) polycyclic aromatic hydrocarbon, 10) pesticide,11) plant or fecal sterol, 12) perfluorinated, 13) inorganic analyte, 14) microorganism.
For the virus samples, the treated samples were collected before disinfection, so should be considered only partially treated.
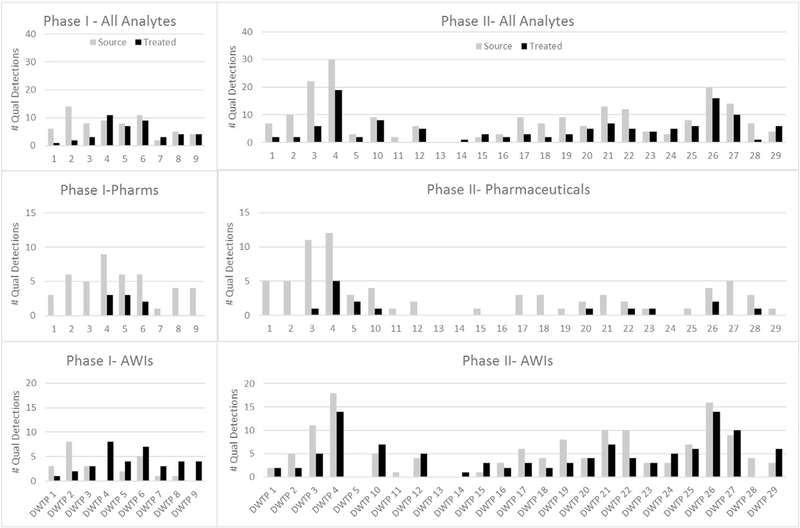
Phases I and II had 77 analytes in common, 24 pharmaceuticals and 53 AWIs. Fig. 1 illustrates the frequency of qualitative detections of these analytes in all of the Phase I and II locations, as a whole as well as separated by chemical class. In general, detections were infrequent, with typically fewer than 5 pharmaceuticals and 10 AWIs detected in any given sample. For the five locations that were sampled in both Phases I and II, the detection trends remained similar, with the exception of the Phase IAWI detections for DWTP 4 (Fig. 1; Supplementary information Table 5). Seven of the AWIs were detected in the field blank from that location, and thus the concentrations in the associated samples were censored. It was field blank detections such as these that triggered the enhanced field blank QC design for Phase II. By supplying a uniform, verified laboratory-grade water in Phase II, better control and assessment of potential contamination from field personnel and/or transport was possible. The similarity of detection at these five locations may be a function of the fact that in both Phases the samples at these five locations were collected between September and March. The concentrations of contaminants in wastewater have been demonstrated to fluctuate diurnally, weekly, and seasonally (Petrie et al., 2015). This variability in wastewater-driven contaminant inputs, as well as temperature-dependent environmental attenuation ability, results in seasonal trends observable in surface waters (Wen et al., 2014; Robles-Molina et al., 2014) and in treated drinking water (Houtman et al., 2014). To fully understand the overall contaminant load at a given location, multiple samples collected on daily, weekly, and monthly time scales are required. A more detailed discussion of the Phase I pharmaceutical detections can be found in Furlong et al. (2016).
Fig. 1.
Qualitative frequency of detection for analytes monitored in both Phases I and II. Number of analytes in each class - total, 77; pharmaceuticals, 24; anthropogenic waste indicators, 53.
The carbamazepine detections at DWTP 5 triggered another modification to our QA/QC design between Phase I and Phase II. Surprisingly high concentrations of carbamazepine were measured in the treated water sample. Carbamazepine was an analyte in two methods, and this location happened to have the second sample collected as a duplicate rather than a laboratory fortified matrix sample. Therefore, for both the source water and the treated drinking water, we had four independent measurements of the carbamazepine concentration, and all eight measurements pointed to the higher levels in the treated water sample. Since chlorination was the only treatment performed at this location, the time required to collect the samples was enough that slightly different parcels of water were examined before and after treatment. Without the verification of a second method or duplicate sample, the validity of this detection would have been questioned. Because of this, in Phase II, both a duplicate and a laboratory fortified matrix sample were collected for all organic chemicals at all locations. A further discussion of the QA/QC results can be found in the pharmaceutical (Furlong et al., 2016), PFAS (Boone et al., unpublished results), and quality control (Batt et al., 2016) papers.
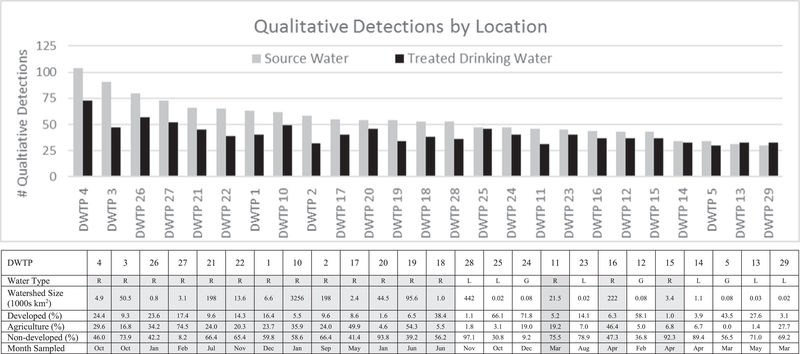
Fig. 2 depicts the number of analytes qualitatively detected in each of the Phase II locations, ordered by the number of detections in the source water. The number of qualitatively detected analytes in the source water ranged from 30 in DWTP 29 to a maximum of 104 in DWTP 4; in the treated drinking water, the number of qualitative detections ranged from 30 in DWTP 5 to 73 in DWTP 4. The number of analytes detected in the source water shows some relation to the type of water body from which the sample was drawn. DWTPs that used rivers or streams as sources tended to have generally higher numbers of analytes than those that used lakes, reservoirs, or groundwater sources (Fig. 2, tabled data); this trend was also observed in previous research (Sun et al., 2015). One explanation for this trend would be that environmental attenuation, including processes such as adsorption and biodegradation, is greater in lakes, reservoirs, and groundwater due to extended residence times. Another possible explanation for the lower number of analytes in lake, reservoir, or groundwater sources is that these sources were, in general, less affected by anthropogenic inputs. The presence of fewer contaminants in reservoirs, lakes, or groundwater is not constant across chemical classes, which is consistent with attenuation processes being chemical specific and with detected analytes originating from various sources. Fig. 3 presents the frequency of detection by the five different contaminant classes. Pharmaceuticals and AWIs generally show the same overall relation between water type and frequency of detection, with the river-based systems showing generally higher frequencies of detection. Additionally, both of these classes of compounds were rather infrequently detected in both source and treated drinking water as compared to the number of analytes in each class.
Fig. 2.
Qualitative detections of all Phase II analytes and watershed characteristics. The qualitative detections ranked according the number of source water analytes detected. The watershed characteristics table lists the type of source water (R, river or stream; L, lake or reservoir; G, groundwater (includes under the influence of surface water)), as well as size and use characteristics of the watershed.
Fig. 3.
Qualitative detections in each Phase II DWTP, separated by chemical/microbial class. Number of analytes in each class: pharmaceuticals, 121; PFASs, 17; AWIs, 55; inorganics, 40; microorganisms, 14.
The PFASs (Boone et al., discussed more fully in a forthcoming publication) and inorganic constituents demonstrated a different relation between frequency of detection and source water type, with the number of analytes measured in each location remaining fairly constant and independent of water type, and a larger percentage of each class detected. This difference, when compared to pharmaceuticals and AWIs, may result from greater detectability due to LCMRLs/RLs for these analytes that are substantially lower than the observed ambient environmental concentrations. The microorganisms presented a more temporal pattern; detections were more related to sampling month, with detections higher in the winter months than the summer months (see Fig. 2, tabled data).
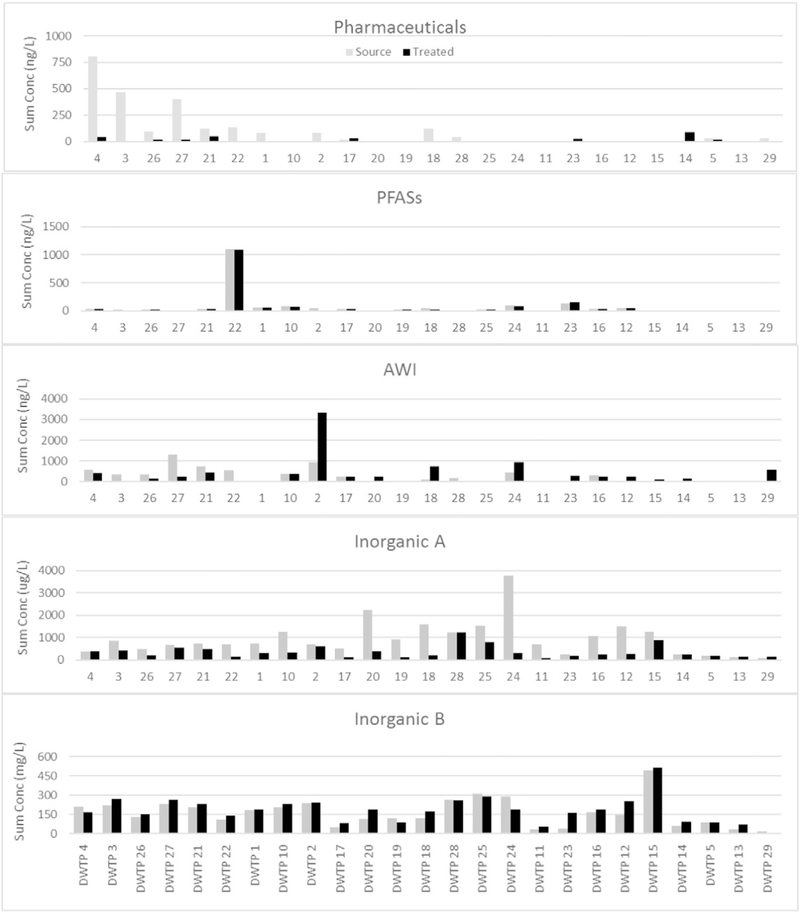
However, frequency of detection does not fully explain analyte distributions. Fig. 4 illustrates the sum of the concentrations of all analytes measured in a given chemical class for each location. Since the inorganic constituents had units of measurement that differed by three orders of magnitude, they were separated into two graphs. The pharmaceuticals still showed the same relation to water source, with samples from river systems having greater summed concentrations. The AWIs were more variable, with a marked total concentration increase in some of the treated waters, due primarily to production of the disinfection byproduct bromoform during treatment. PFASs and inorganics, which showed little variability between locations in terms of frequency of qualitative detection, show greater variability in concentrations between locations.
Fig. 4.
Concentrations summed by chemical class at each Phase IIDWTP. For these figures, lithium is treated as an inorganic analyte instead of a pharmaceutical due to differences in units (μg/L for lithium versus ng/L for the other pharmaceuticals). Inorganics were divided between those with μg/L and mg/L concentrations (see Supplementary information Table 2 for analytes in each class).
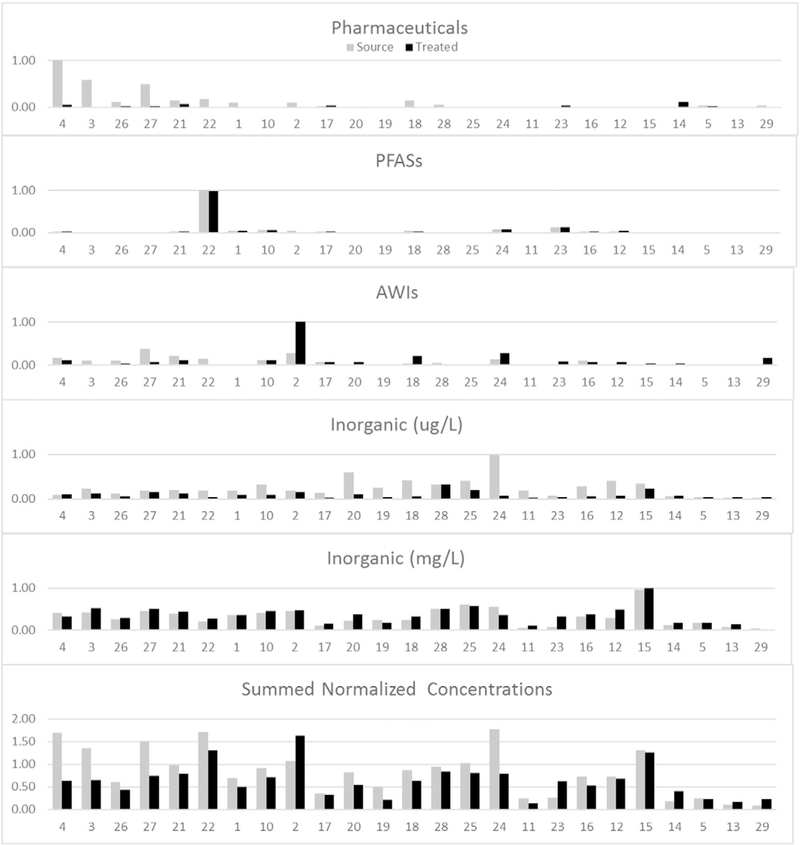
In order to compare total chemical concentrations between locations with analytes that vary by six orders of magnitude (mg/L to ng/L), in Fig. 5 concentrations were normalized for each class by dividing the summed concentrations for each class in all samples by the site with the highest summed concentration for each class (DWTP 4 source for pharmaceuticals, DWTP 22 source for the PFASs, DWTP 2 treated for the AWIs, DWTP 24 source for the inorganics on the μg/L scale and DWTP 15 treated for the inorganics on the mg/L scale). The class-normalized concentrations from all 5 classes were then summed to give a total normalized concentration by DWTP and presented in the bottom panel of Fig. 5. None of the DWTPs had a summed normalized concentration >2, indicating that any one DWTP typically had relative elevated concentrations in only one of the chemical classes and that concentrations were not uniformly elevated among all classes at a particular DWTP.
Fig. 5.
Normalized chemical concentrations. Concentrations in each chemical class were normalized to the location with the greatest concentration. The summed normalized concentration figure is the sum of the normalized concentrations of five chemical class subunits (pharmaceuticals, PFASs, AWIs, inorganics with μg/L units and inorganics with mg/L units).
The number of qualitatively detected analytes and their concentration typically vary between the source and treated drinking water samples from each location (Figs. 3 and 4). It is also apparent that these changes are analyte-class specific. These trends in qualitative and quantitative detections are summarized in Table 3. Since many of the detections of pharmaceuticals and AWIs were less than the LCRML or RL, typical statistical analyses requiring uniformly numerical concentrations were not appropriate. To examine these concentration trends, the percent change between the source and the treated sample was calculated for each analyte by dividing the difference between the source and treated samples by the concentration in the source water. Non-detects and blank corrected detections were assumed to have a concentration of zero. Changes between qualitatively detected analytes and non-detects were assumed to be either a −100% or a 100% change, depending on if the qualitative detection was in the source or treated water, respectively. Changes between quantitatively detected analytes and qualitatively detected analytes were assigned a −50% or a 50% change, also depending on if the qualitative detection was in the source or treated water, respectively. No calculation was made if both the source and the treated sample had a qualitative detection, or if both were non-detects. The calculated percent change trends are in general agreement with the relations graphically depicted in Figs. 1 and 2. Grand median (median of median) percent changes of −100% and −67% were observed for pharmaceuticals and AWIs, respectively, indicating that the treated water concentrations were lower than the source water concentrations. Conversely, the grand median percent changes for PFASs and inorganics were −1% and −3%, respectively. The calculation for the microorganisms were difficult, since the protozoa were not collected in any of the source water samples, and the viruses could not be collected in nearly half of the DWTPs due to the use of pre-chlorination, and the virus samples that were collected were before final disinfection. But, for those DWTPs where source and treated water pairs were collected, a grand median of −100% was observed, indicating generally lower microorganism densities in the treated water as compared to the source. The locations in Table 3 were ranked by increasing percent change between the source and treated samples. The locations with the greatest percent change tended to be the river systems, presumably because lake/reservoir and groundwater systems provide greater environmental attenuation, thus making the efficacy of engineered treatment difficult to evaluate based solely on a comparison of source and treated water samples.
Table 3.
Analysis of qualitative and quantitative detections by location in Phase II. Values in bold indicate statistically significant change between detections in source and treated samples.
| Analysis of qualitative detections | ||||||||||||
| Location | All | Pharmaceuticals | PFASs | AWIs | Inorganic | Microorganisms | ||||||
| Number of pairs | Med % change | Number of pairs | Med % change | Number of pairs | Med % change | Number of pairs | Med % change | Number of pairs | Med % change | Number of pairs | Med % change | |
| DWTP 2 | 61 | −100 | 15 | −100 | 12 | −98 | 8 | −100 | 24 | −3 | 2 | −100 |
| DWTP 3 | 89 | −100 | 31 | −100 | 12 | −25 | 10 | −100 | 30 | −9 | 6 | −100 |
| DWTP 22 | 65 | −80 | 14 | −100 | 14 | −7 | 10 | −100 | 27 | −3 | 0 | |
| DWTP 19 | 54 | −57 | 6 | −100 | 9 | −1 | 11 | −100 | 28 | −57 | 0 | |
| DWTP 18 | 55 | −56 | 11 | −100 | 12 | −49 | 5 | −100 | 26 | −7 | 1 | −100 |
| DWTP 21 | 64 | −54 | 13 | −100 | 10 | 1 | 10 | −100 | 30 | −9 | 1 | −100 |
| DWTP 11 | 45 | −50 | 4 | −100 | 8 | −29 | 2 | −100 | 25 | −11 | 6 | −100 |
| DWTP 4 | 86 | −48 | 33 | −100 | 11 | 0 | 13 | −50 | 25 | 9 | 4 | 0 |
| DWTP 27 | 70 | −45 | 21 | −100 | 10 | −39 | 8 | −46 | 27 | −6 | 4 | −96 |
| DWTP 1 | 67 | −34 | 18 | −100 | 14 | −9 | 1 | 50 | 30 | −23 | 4 | −97 |
| DWTP 10 | 63 | −11 | 8 | −100 | 13 | −2 | 10 | −16 | 25 | −1 | 7 | −100 |
| DWTP 24 | 43 | −10 | 1 | −2 | 9 | −4 | 6 | 25 | 27 | −43 | 0 | |
| DWTP 20 | 54 | −6 | 9 | −100 | 7 | 15 | 3 | −67 | 28 | −4 | 7 | 100 |
| DWTP 26 | 68 | −6 | 12 | −100 | 10 | 7 | 14 | −75 | 26 | −2 | 6 | −100 |
| DWTP 17 | 50 | −6 | 8 | −100 | 11 | 4 | 4 | −100 | 24 | −3 | 3 | −100 |
| DWTP 25 | 48 | −5 | 3 | −100 | 10 | −4 | 7 | −100 | 23 | −5 | 5 | 100 |
| DWTP 15 | 45 | −5 | 3 | −100 | 8 | −3 | 5 | 100 | 28 | −4 | 1 | −100 |
| DWTP 28 | 50 | −3 | 8 | −100 | 10 | 2 | 4 | −100 | 25 | −1 | 3 | −100 |
| DWTP 16 | 45 | −1 | 4 | 0 | 9 | 9 | 3 | −27 | 27 | −4 | 2 | 0 |
| DWTP 29 | 35 | −1 | 4 | −100 | 7 | −1 | 5 | 100 | 16 | −3 | 3 | 100 |
| DWTP 12 | 44 | −1 | 3 | −100 | 10 | 4 | 5 | 100 | 25 | −1 | 1 | −100 |
| DWTP 5 | 34 | 0 | 7 | −100 | 1 | 25 | 0 | 25 | 0 | 1 | 100 | |
| DWTP 14 | 39 | 2 | 4 | −100 | 8 | 1 | 1 | 100 | 26 | 10 | 0 | |
| DWTP 13 | 36 | 9 | 4 | −100 | 9 | 9 | 0 | 23 | 9 | 0 | ||
| DWTP 23 | 47 | 18 | 2 | 75 | 12 | 7 | 6 | −25 | 23 | 77 | 4 | −100 |
| Grand Median | −6 | −100 | −1 | −67 | −3 | −100 | ||||||
| Analysis of quantitative detections | ||||||||||||
| Location | All | Pharmaceuticals | PFASs | AWIs | Inorganic | Microorganisms | ||||||
| Number of pairs | Wilcoxon P | Number of pairs | Wilcoxon P | Number of pairs | Wilcoxon P | Number of pairs | Wilcoxon P | Number of pairs | Wilcoxon P | Number of pairs | Wilcoxon | |
| DWTP 2 | 47 | 0.000 | 4 | 0.063 | 11 | 0.000 | 6 | 0.281 | 24 | 0.126 | 2 | 0.250 |
| DWTP 18 | 49 | 0.000 | 9 | 0.002 | 11 | 0.000 | 2 | 0.750 | 26 | 0.079 | 1 | |
| DWTP 3 | 65 | 0.000 | 17 | 0.000 | 10 | 0.019 | 2 | 0.250 | 30 | 0.301 | 6 | 0.016 |
| DWTP 22 | 53 | 0.000 | 11 | 0.000 | 12 | 0.367 | 3 | 0.125 | 27 | 0.098 | 0 | |
| DWTP 24 | 39 | 0.001 | 1 | 9 | 0.180 | 2 | 0.750 | 27 | 0.001 | 0 | ||
| DWTP 28 | 44 | 0.002 | 4 | 0.063 | 10 | 0.884 | 2 | 0.250 | 25 | 0.051 | 3 | 0.125 |
| DWTP 27 | 53 | 0.003 | 10 | 0.001 | 9 | 0.064 | 3 | 0.625 | 27 | 0.314 | 4 | 0.313 |
| DWTP 21 | 52 | 0.012 | 6 | 0.016 | 10 | 0.646 | 5 | 0.219 | 30 | 0.319 | 1 | |
| DWTP 1 | 52 | 0.016 | 6 | 0.156 | 12 | 0.291 | 0 | 30 | 0.076 | 4 | 0.188 | |
| DWTP 17 | 41 | 0.024 | 3 | 0.625 | 10 | 0.470 | 1 | 24 | 0.137 | 3 | 0.125 | |
| DWTP 11 | 36 | 0.032 | 0 | 6 | 0.656 | 0 | 25 | 0.221 | 5 | 0.031 | ||
| DWTP 4 | 59 | 0.052 | 17 | 0.000 | 11 | 0.232 | 2 | 0.750 | 25 | 0.862 | 4 | 0.438 |
| DWTP 10 | 49 | 0.056 | 2 | 0.250 | 10 | 0.080 | 5 | 0.313 | 25 | 0.468 | 7 | 0.344 |
| DWTP 19 | 38 | 0.062 | 1 | 9 | 0.500 | 0 | 28 | 0.029 | 0 | |||
| DWTP 25 | 41 | 0.095 | 2 | 0.500 | 10 | 0.044 | 1 | 23 | 0.035 | 5 | 0.594 | |
| DWTP 26 | 51 | 0.152 | 6 | 0.078 | 10 | 1.000 | 3 | 0.875 | 26 | 0.460 | 6 | 0.016 |
| DWTP 20 | 43 | 0.203 | 1 | 6 | 0.953 | 2 | 0.750 | 28 | 0.094 | 6 | 0.656 | |
| DWTP 16 | 42 | 0.206 | 1 | 9 | 0.994 | 3 | 0.125 | 27 | 0.226 | 2 | 0.500 | |
| DWTP 15 | 38 | 0.267 | 1 | 7 | 0.469 | 1 | 28 | 0.321 | 1 | |||
| DWTP 12 | 38 | 0.307 | 0 | 10 | 0.941 | 2 | 0.750 | 25 | 0.173 | 1 | ||
| DWTP 29 | 28 | 0.375 | 1 | 7 | 0.289 | 1 | 16 | 0.281 | 3 | 0.625 | ||
| DWTP 5 | 30 | 0.598 | 3 | 0.625 | 1 | 0 | 25 | 0.466 | 1 | |||
| DWTP 23 | 39 | 0.761 | 0 | 11 | 0.761 | 1 | 23 | 0.951 | 4 | 0.063 | ||
| DWTP 14 | 36 | 0.946 | 1 | 8 | 0.727 | 1 | 26 | 0.838 | 0 | |||
| DWTP 13 | 34 | 0.981 | 2 | 0.500 | 9 | 0.914 | 0 | 23 | 0.980 | 0 | ||
| Median P | 0.056 | 0.063 | 0.470 | 0.469 | 0.226 | 0.250 | ||||||
For quantitative detections, a statistical analysis was possible. The bottom of Table 3 presents the results of the Wilcoxon paired sample test (statistiXL, Nedlands, Western Australia) between the source and treated drinking water samples, for all quantitative detections at a given DWTP, as well as for each analyte class. A one-tailed test was used, with the concentration in the source assumed to be greater than the treated drinking water samples. For these calculations, non-detects, blank corrected samples, and values lower than the LCMRL or RL were assumed to be equal to zero. The limitations of left censoring data have been recognized (Helsel, 2010); however, since the Wilcoxon test is nonparametric, the impact to the conclusions is minimal. Either the source water or the treated drinking water for a contaminant at a given location had to have a quantitated detection for the pair to be included in the analysis. Numbers in bold indicate statistically significant differences between the source and treated samples at the 0.05 significance level. The locations are again ranked in order of decreasing difference between the source and treated water samples. Eleven of the DWTPs showed statistically significant overall differences between the source and treated drinking water. Nine of the 11 DWTPs with statistically significant differences were from river systems. The two non-river locations, DWTPs 24 (groundwater) and 28 (lake/reservoir), were the locations that had greater numbers of qualitative detections of analytes than some of the river systems, as depicted in the qualitative detection ranking in Fig. 2. Part of the high number of locations showing statistically significant differences may be attributed to the high degree of freedom due to the number of pairs across all analyte classes. When one examines the differences between source and treated water concentrations within an analyte class, the number of statistically significant differences decreases substantially. No location showed statistically significant differences for the AWIs and only three locations showed significant differences in the number of microorganisms. Three DWTPs show significant differences between the source and treated drinking water samples for the PFASs. The inorganic constituents had the largest number of pairs at each location, but only three DWTPs exhibited statistically significant differences between the source and treated water samples. For the pharmaceuticals, six locations have statistically significant decreases between the source and treated samples. Out of the five analyte classes investigated in this paper, pharmaceuticals have the most paired source and treated water data available in the literature. These studies (Benotti et al., 2009; Simazaki et al., 2015; Cai et al., 2015) show similar reductions during drinking water treatment. Detailed analyte-specific discussions can be found in the pharmaceutical (Furlong et al., 2016), PFAS (Boone et al., unpublished results), and microorganism (King et al., 2016) papers.
Overall, source and treated water samples from DWTPs of diverse volume and water sources that employ typical treatment processes contain a range of CECs and other associated contaminants. These overview results indicate that while the majority of CECs are either not observed in source or treated water samples, or are below detection after treatment, many CECs are incompletely removed during treatment and thus are present in water distributed for potable use. The concentrations of most CECs are low, typically in the part-per-trillion range; even so, their persistent presence suggests that there is exposure via water consumption. Taken together these results identify the range of CECs and other contaminants that may be found in source and treated waters where discharged wastewater effluent is potentially a substantial component in source water. It should be noted that the measurements in this study may not represent global maximum concentrations, and greater exposures are possible, if not probable, in developing countries (Rehman et al., 2015). It is also critical to note that most of the results from this study were collected at a single point in time and thus comprise a snapshot in time; future studies would benefit from more detailed and focused time series sample collection designs that better capture temporal variation. Nevertheless, the use of a stringent QA/QC design and consistent field protocols and laboratory methods has resulted in a unique, consistent dataset of chemical and microbiological contaminants reflective of water supply conditions in typical DWTPs during the time of the sampling campaign (2007–2012). As a result, this dataset provides a benchmark and framework for future monitoring of CECs.
Four associated papers further explore the implications, if any, of the detections of these analytes to aquatic life and human health. The first two papers conduct risk quotient assessments on the source water for aquatic life (Kostich et al., 2016) and the margin-of-exposure assessments for the detected unregulated chemicals in treated drinking water for human health (Benson et al., 2016); the concentrations of the 17 chemicals in this study which are regulated in the United States (Code of Federal Regulations, 2015; USEPA, 2016) were compared to the regulatory thresholds in Supplementary information Table 6. The third paper compares the measured endocrine disrupting chemicals to bioactivity results from an estrogenicity bioassay (Conley et al., 2016). The fourth paper examines the microorganism detection (King et al., 2016). This health-based context is vital in determining the impact of these contaminants in the environment and to human health.
Supplementary Material
HIGHLIGHTS.
Nationwide study of 29 paired source water and treated drinking water samples
Chemicals: pharmaceuticals, PFASs, anthropogenic waste indicators, and inorganics
Microorganisms: bacteria, fungi, protozoa and viruses
148 contaminants detected in source water; 121 detected in treated drinking water.
Provides a baseline for future drinking water monitoring for these constituents
Acknowledgments
Disclaimers and acknowledgements
The research described in this article has been funded in part by the U.S. Environmental Protection Agency through Interagency Agreement DW14922330 to the U.S. Geological Survey, and through programmatic support of the USGS Toxic Substances Hydrology Program and the USEPA’s Office of Research and Development, Office of Water, Office of Chemical Safety and Pollution Prevention, and Region 8. Information Collection Rule approval for the Phase II Questionnaire was granted under EPA ICR No. 2346.01, OMB Control No. 2080–0078. This document has been reviewed in accordance with USEPA and USGS policy and approved for publication. Approval does not signify that the contents reflect the views of the USEPA and mention of trade names or commercial products does not constitute endorsement or recommendation for use by USEPA. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the USEPA, the USGS, or the U.S. Government. The authors would like to thank all participating drinking water treatment plants for their involvement in the project and for their assistance in collecting the samples. The authors would also like to thank the following personnel for sample and data analysis assistance: Steve Werner, Steve Zaugg, Mary Noriega, Richard Miltner, Bing Guan, Craig Vigo, Tripp Boone, Christian Byrne, Joseph Ferrario, Nicola Evans, Justin Conley, Laura Rosenblum, Michael Ware, Megan Vogel, Robert Flick, William Sander, Nichole Brinkman, Emily Anneken, and Scott Keely.
Abbreviations:
- AWI
anthropogenic waste indicator
- CEC
contaminant of emerging concern
- CCL
Contaminant Candidate List
- DWTP
drinking water treatment plant
- LCMRL
lowest concentration minimum reporting level
- PFAS
per- and polyfluoroalkyl substances
- PWS
public water system
- QA/QC
quality assurance/quality control
- RL
reporting level
- SDWA
Safe Drinking Water Act
- UCMR
Unregulated Contaminant Monitoring Rule
- USEPA
United States Environmental Protection Agency
- USGS
United States Geological Survey
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scitotenv.2016.12.004.
This paper belongs to the special section on Emerging contaminants in drinking water and it has been inadvertently missed.
References
- Associated Press, 2008. An AP Investigation: Pharmaceuticals Found in Drinking Water. http://hosted.ap.org/specials/interactives/pharmawater_site/
- Batt AL, Furlong ET, Mash HE, Glassmeyer ST, Kolpin DW, 2017. The importance of quality control in validating concentration of contaminants of emerging concern in source and treated drinking water samples. Sci. Total Environ. 579,1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt AL, Kostich MS, Lazorchak JM, 2008. Analysis of ecologically relevant pharmaceuticals in wastewater and surface water using selective solid-phase extraction and UPLC-MS/MS. Anal. Chem. 80 (13), 5021–5030. [DOI] [PubMed] [Google Scholar]
- Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA, 2009. Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ. Sci. Technol. 43 (3), 597–603. [DOI] [PubMed] [Google Scholar]
- Benson R, Conerly O, Sander W, Batt AL, Boone JS, Furlong ET, Glassmeyer ST, Kolpin DW, Mash HE, Schenck KM, Simmons JE, 2017. Human health screening and public health significance of contaminants of emerging concern detected in public water supplies. Sci. Total Environ. 579,1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer A, King D, Donohue M, Mistry J, Covert T, Pfaller S, 2010. Detection of Mycobacterium avium subsp. paratuberculosis in drinking water and biofilms by quantitative PCR Appl. Environ. Microbiol. 76 (21 ), 7367–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone JS, Guan B, Vigo C, Boone T, Byrne C, Ferrario J, 2014. A method for the analysis of perfluorinated compounds in environmental and drinking waters and the determination of their lowest concentration minimal reporting levels. J. Chromatogr. A 1345, 68–77. [DOI] [PubMed] [Google Scholar]
- Boone JS, Guan B, Vigo C, Boone T, Byrne C, Ferrario J, Glassmeyer ST, Furlong ET, Kolpin DW Per- and Poly-fluorinated Alkyl Substances in Source and Treated Drinking Waters of the United States. (Unpublished Results). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill JD, Furlong ET, Burkhardt MR, Kolpin D, Anderson LG, 2004. Determination of pharmaceutical compounds in surface- and ground-water samples by solidphase extraction and high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A1041 (1–2), 171–180. [DOI] [PubMed] [Google Scholar]
- Cai MQ, Wang R, Feng L, Zhang LQ, 2015. Determination of selected pharmaceuticals in tap water and drinking water treatment plant by high-performance liquid chromatography-triple quadrupole mass spectrometer in Beijing, China. Environ. Sci. Pollut. Res. 22 (3), 1854–1867. [DOI] [PubMed] [Google Scholar]
- Code of Federal Regulations, 2015. National Primary Drinking Water Regulations 40 C.F.R.§ 141. (Available online). http://www.ecfr.gov/cgi-bin/text-idx?tpl=/ecfrbrowse/Title40/40cfr141_main_02.tpl. [Google Scholar]
- Coleman WE, Melton RG, Kopfler FC, Barone KA, Aurand TA, Jellison MG, 1980. Identification of organic-compounds in a mutagenic extract of surface drinking-water by a computerized gas chromatography-mass spectrometry system (GC-MS-COM). Environ. Sci. Technol. 14 (5), 576–588. [Google Scholar]
- Conley JM, Evans N, Mash H, Rosenblum L, Schenck K, Glassmeyer S, Furlong ET, Kolpin DW, Wilson VS, 2017. Comparison of in vitro estrogenic activity and estrogen concentrations in source and treated waters from 25 drinking water treatment plants. Sci. Total Environ. 579, 1610–1617. [DOI] [PubMed] [Google Scholar]
- Covert TC, Rodgers MR, Reyes AL, Stelma GN Jr., 1999. Occurrence of nontuberculous mycobacteria in environmental samples. Appl. Environ. Microbiol. 65, 2492–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton CG, Ternes TA, 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 107, 907–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado LF, Charles P, Glucina K, Morlay C, 2012. QSAR-like models: a potential tool for the selection of PhACs and EDCs for monitoring purposes in drinking water treatment systems-a review. Water Res. 46 (19), 6196–6209. [DOI] [PubMed] [Google Scholar]
- Diaz-Cruz S, Barcelo D, 2004. Occurrence and analysis of selected pharmaceuticals and metabolites as contaminants present in waste waters, sludge and sediments. Emerging Organic Pollutants in Waste Waters and Sludge. 1, pp. 227–260 (5). [Google Scholar]
- Donaldson WT, 1977. Trace organics in water. Environ. Sci. Technol. 11 (4), 348–351. [Google Scholar]
- Donohue MJ, O’Connell K, Vesper SJ, Mistry JH, King D, Kostich M, Pfaller S, 2014. Widespread molecular detection of Legionella pneumophila serogroup 1 in cold water taps across the United States. Environ. Sci. Technol. 48 (6), 3145–3152. [DOI] [PubMed] [Google Scholar]
- Eganhouse RP, Blumfield DL, Kaplan IR, 1983. Long-chain alkulbenzenes as molecular tracers of domestic wastes in the marine-environment. Environ. Sci. Technol. 17 (9), 523–530. [DOI] [PubMed] [Google Scholar]
- Furlong ET, Batt AL, Glassmeyer ST, Noriega MC, Kolpin DW, Mash H, Schenck KM, 2017. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the Unites States: pharmaceuticals. Sci. Total Environ. 579, 1629–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong ET, Noriega MC, Kanagy CJ, Kanagy LK, Coffey LJ, Burkhardt MR, 2014. Determination of human-use pharmaceuticals in filtered water by direct aqueous injection—high-performance liquid chromatography/tandem mass spectrometry. U.S. Geological Survey Techniques and Methods. book 5, chap. B10 (49 pp.). 10.3133/tm5B10. [DOI] [Google Scholar]
- Furlong ET, Werner SL, Anderson BD, Cahill JD, 2008. Determination of human-health pharmaceuticals in filtered Water by chemically modified styrene-divinylbenzene resin-based solid-phase extraction and high-performance liquid chromatography/mass spectrometry. U.S. Geological Survey Techniques and Methods (book 5, sec. B, chap. B5, 56 p). [Google Scholar]
- Garcia-Ac A, Segura PA, Viglino L, Furtos A, Gagnon C, Prevost M, Sauve S, 2009. On-line solid-phase extraction of large-volume injections coupled to liquid chromatography-tandem mass spectrometry for the quantitation and confirmation of 14 selected trace organic contaminants in drinking and surface water. J. Chromatogr. A 1216 (48), 8518–8527. [DOI] [PubMed] [Google Scholar]
- Glassmeyer ST, Kolpin DW, Furlong ET, Focazio MJ, 2008. Environmental presence and persistence of pharmaceuticals: an overview Fate of the Pharmaceuticals in the Environment and in Water Treatment Systems. Ed Diana Aga CRC/Taylor and Francis Press, Boca Raton, FL, pp. 3–51. [Google Scholar]
- Halling-Sorensen B, Nielsen SN, Lanzky PF, Ingerslev F, Lutzhoft HCH, Jorgensen SE, 1998. Occurrence, fate and effects of pharmaceutical substances in the environment - a review. Chemosphere 36 (2), 357–394. [DOI] [PubMed] [Google Scholar]
- Haugland RA, Varma M, Wymer LJ, Vesper SJ, 2004. Quantitative PCR analysis of selected Aspergillus, Penicillium and Paecilomyces species. Syst. Appl. Microbiol. 27 (2), 198–210. [DOI] [PubMed] [Google Scholar]
- Heberer T, 2002. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol. Lett. 131 (1–2), 5–17. [DOI] [PubMed] [Google Scholar]
- Helsel D, 2010. Summing nondetects: incorporating low-level contaminants in risk assessment. Integr. Environ. Assess. Manag. 6 (3), 361–366. [DOI] [PubMed] [Google Scholar]
- Houtman CJ, Kroesbergen J, Lekkerkerker-Teunissen K, van der Hoek JP, 2014. Human health risk assessment of the mixture of pharmaceuticals in Dutch drinking water and its sources based on frequent monitoring data. Sci. Total Environ. 496 (1), 54–62. [DOI] [PubMed] [Google Scholar]
- King DN, Donohue MJ, Vesper SJ, Villegas EN, Ware MW, Vogel ME, Furlong ET, Kolpin DW, Glassmeyer ST, Pfaller SL, 2016. Microbial pathogens in source and treated waters from drinking water treatment plants in the United States and implications for human health. Sci. Total Environ. 562, 987–995. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Batt AL, Glassmeyer ST, Lazorchak JM, 2010. Predicting variability of aquatic concentrations of human pharmaceuticals. Sci. Total Environ. 408 (20), 4504–4510. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Flick RW, Batt AL, Mash HE, Boone JS, Furlong ET, Kolpin DW, Glassmeyer ST, 2017. Aquatic concentrations of chemical analytes compared to ecotoxicity estimates. Sci. Total Environ. 579, 1649–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dong Z, Weng Q, Chang CC, Liu B, 2015. Emerging pollutants-part I: occurrence, fate and transport. Water Environ. Res. 87 (10), 1849–1872. [DOI] [PubMed] [Google Scholar]
- Loos R, Wollgast J, Huber T, Hanke G, 2007. Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around Lake Maggiore in Northern Italy. Anal. Bioanal. Chem. 387 (4), 1469–1478. [DOI] [PubMed] [Google Scholar]
- Pal A, He Y, Jekel M, Reinhard M, Gin KYH, 2014. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 71, 46–62. [DOI] [PubMed] [Google Scholar]
- Petrie B, Barden R, Kasprzyk-Hordern B, 2015. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 72 (1), 3–27. [DOI] [PubMed] [Google Scholar]
- Rehman MSU, Rashid N, Ashfaq M, Saif A, Ahmad N, Han JI, 2015. Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere 138 (1), 1045–1055. [DOI] [PubMed] [Google Scholar]
- Richardson ML, Bowron JM, 1985. The fate of pharmaceutical chemicals in the aquatic environment. J. Pharm. Pharmacol. 37 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- Robles-Molina J, Gilbert-López B, García-Reyes JF, Molina-Díaz A, 2014. Monitoring of selected priority and emerging contaminants in the Guadalquivir River and other related surface waters in the province of Jaén, South East Spain. Sci. Total Environ. 479, 247–257. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Furlong ET, 2008. Trace analysis of antidepressant pharmaceuticals and their select degradates in aquatic matrixes by LC/ESI/MS/MS. Anal. Chem. 80 (5), 1756–1762. [DOI] [PubMed] [Google Scholar]
- Shackelford WM, Cline DM, 1986. Organic-compounds in water. Environ. Sci. Technol. 20 (7), 652–657. [DOI] [PubMed] [Google Scholar]
- Sheldon LS, Hites RA, 1978. Organic-compounds in Delawater River. Environ. Sci. Technol. 12(10), 1188–1194. [Google Scholar]
- Shuval HI, Gruener N, 1973. Health considerations in renovating waste-water for domestic use. Environ. Sci. Technol. 7 (7), 600–604. [Google Scholar]
- Simazaki D, Kubota R, Suzuki T, Akiba M, Nishimura T, Kunikane S, 2015. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 76,187–200. [DOI] [PubMed] [Google Scholar]
- Snyder SA, 2008. Occurrence, treatment, and toxicological relevance of EDCs and pharmaceuticals in water. Ozone Sci. Eng. 30 (1), 65–69. [Google Scholar]
- Stackelberg PE, Furlong ET, Meyer MT, Zaugg SD, Henderson AK, Reissman DB, 2004. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water treatment plant. Sci. Total Environ. 329 (1–3), 99–113. [DOI] [PubMed] [Google Scholar]
- Stackelberg PE, Gibs J, Furlong ET, Meyer MT, Zaugg SD, Lippincott RL, 2007. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 377 (2–3), 255–272. [DOI] [PubMed] [Google Scholar]
- Sun J, Luo Q, Wang D, Wang Z, 2015. Occurrences of pharmaceuticals in drinking water sources of major river watersheds, China. Ecotoxicol. Environ. Saf. 2015 (117), 132–140. [DOI] [PubMed] [Google Scholar]
- Swayne MD, Boone GH, Bauer D, Lee JS, 1980. Wastewater in Receiving Waters at Water Supply Abstraction Points EPA 600/2–80-044. [Google Scholar]
- Ternes TA, Bonerz M, Herrmann N, Löffler D, Keller E, Lacida BB, Alder AC, 2005. Determination of pharmaceuticals, iodinated contrast media and musk fragrances in sludge by LC/tandem MS and GC/MS. J. Chromatogr. A 1067 (1–2), 213–223. [DOI] [PubMed] [Google Scholar]
- Togola A, Budzinski H, 2007. Multi-residue analysis of pharmaceutical compounds in aqueous samples. J. Chromatogr. A1177 (1), 150–158. [DOI] [PubMed] [Google Scholar]
- USEPA, 1994. EPA Method 200.8 Determination of Trace Elements in Waters and Wastes by ICP-MS. [Google Scholar]
- USEPA, 1996. Pub.L. 104–182,110 Stat. 1613. “Safe Drinking Water Act Amendments of 1996.” 1996–08-06.
- USEPA, 2001. EPA Method 200.7 Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. [Google Scholar]
- USEPA, 2005a. EPA Method 1623 Cryptosporidium and Giardia in Water by Filtration/IMS/FA. [Google Scholar]
- USEPA, 2005b. EPA Method 332.0 Determination of Perchlorate in Drinking Water by Ion Chromatography with Suppressed Conductivity and Electrospray Ionization Mass Spectrometry. [Google Scholar]
- USEPA, 2010. Technical Basis for the Lowest Concentration Minimum Reporting Level (LCMRL) Calculator. In. http://water.epa.gov/scitech/drinl<ingwater/labcert/analyticalmethods_ogwdw.cfm (24 pp)
- USEPA, 2012a. Revisions to the Unregulated Contaminant Monitoring Regulation (UCMR 3) for Public Water Systems 77 FR 26071(May 02, 2012; revised July 25, 2012). [Google Scholar]
- USEPA, 2012b. Definition and procedure for the determination of the method detection limit. U.S. Environmental Protection Agency: 40 CFR 104.1. Part 136, App B, pp. 343–346. [Google Scholar]
- USEPA, 2015. Drinking Water Contaminant Candidate List 4-Draft 80 FR 6076 (February 4, 2015). [Google Scholar]
- USEPA, 2016. Table of Regulated Drinking Water Contaminants. https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-water-contaminants.
- Varughese EA, Brinkman NE, Anneken EM, Glassmeyer ST, Furlong ET, Kolpin DW, Keely SP Use of Bayesian Modeling to Assess Occurrence of Viral Pathogens in Multiple US Drinking Water Systems. (Unpublished results). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZH, Chen L, Meng XZ, Duan YP, Zhang ZS, Zeng EY, 2014. Occurrence and human health risk of wastewater-derived pharmaceuticals in a drinking water source for Shanghai, East China. Sci. Total Environ. 490, 987–993. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LE, 2004. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol. Sci. 81 (1), 69–77. [DOI] [PubMed] [Google Scholar]
- Zaugg SD, Smith SG, Schroeder MP, Barber LB, Burkhardt MR, 2006. Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory–determination of wastewater compounds by polystyrene-divinylbenzene solid-phase extraction and capillary-column gas chromatography/mass spectrometry. U.S. Geological Survey Water-Resources Investigations Report 01–4186 (37 pp). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.