It is a widely believed paradigm that intimal angiogenesis in coronary atherosclerosis contributes to plaque instability, both through hemorrhagic plaque expansion and cholesterol deposition[1]. However, this paper supports an alternative concept in which angiogenesis is actually essential for plaque stabilization and healing[2 3]. The prevailing paradigm has its origins primarily in histopathology work from the 1980s by the Barger et al. group [4]. We argue that misinterpretation of their results has skewed the understanding of vulnerable plaque for over 35 years. This misconception still endures in recent plaque angiogenesis reviews, affecting understanding of acute coronary syndromes (ACS) pathophysiology[1].
Most coronary plaque ruptures/erosions do not lead to ACS. ACS research focuses primarily on vessel breakdown through mechanisms such as inflammation. We have instead proposed that vessel repair mechanisms that oppose instability fail, leading to ACS[3]. ACS is then a ‘double hit’ of rupture/erosion and failed vascular healing. Here we assert that intimal angiogenesis, needed to maintain cellular reparative functions (i.e. healing), if inadequate, leads to coronary plaque instability. Angiogenesis is the supply line to reparative cells in healing regions. In plaques with long necrotic cores, this supply line to compromised regions may not be reliably established.
In the 1984 Barger et. al. paper, researchers injected silicone into human coronary arteries in vitro and filmed the flow into mural microvessels[4]. They described a primarily short centripetal angiogenesis pattern from the vasa vasorum to the intima. Their discussion linked angiogenesis to plaque hemorrhage with rapid plaque expansion as a trigger for ACS. Our data and literature analysis, including re-examination of the Barger et. al. results, does not support primarily centripetal angiogenesis. Instead, we find axial angiogenesis extending many millimeters, yet may still be insufficient to reach compromised intima.
We acknowledge that ‘leaky’ immature microvessels lead to small intimal hemorrhages that contribute RBC cholesterol[2]. However, hemorrhage is not a significant source of coronary plaque rapid expansion[2]. This is unlike human carotids or most animal arteries, often used as models in intimal angiogenesis research, where hemorrhagic plaque expansion is common[1]. We instead propose that coronary angiogenesis is a critical stabilizing factor. Our conclusions are derived from examining human coronary plaque in long axis rather than in conventional cross section, as well as re-evaluating published data including the Barger et. al. work. These conclusions are also supported by data that angiogenesis inhibitors (such as sunitinib and sorafenib) increase, not decrease, the risk of vascular occlusion in humans.
Examining plaques axially rather than in conventional cross section was critical in our questioning current paradigms. The most common morphology found after ACS is thin walled plaques with a large necrotic core by cross section[2]. However, only a minority of these plaques (<20%) progress to ACS. Most heal. This questions whether these plaques should be termed ‘vulnerable plaques’. Since 2014, our data has supported the novel concept that ACS risk is a function of necrotic core axial extent[3 5]. Three recent in vivo optical coherence tomography (OCT) studies, which we reviewed elsewhere, have supported this mechanism[3]. Studying long axial necrotic cores, we observed that intimal microvessels predominately tracked long distances in the luminal intima above the core on a longitudinal rather than centripetal course. A representative long axial plaque is shown in figure 1. The data supports the model that, in the presence of long cores, these immature microvessels track many millimeters in the intima above the core parallel to the lumen. These findings are consistent with our re-examination of the 1980s data in the next paragraph. The cellularity needed for promoting healing requires maintaining this angiogenesis, which is challenging with long cores.
Figure 1. Human Coronary Artery Axial Histopathology Showing a Long Necrotic Core: Microvessels Become Sparse in a Thin Intimal Cap.
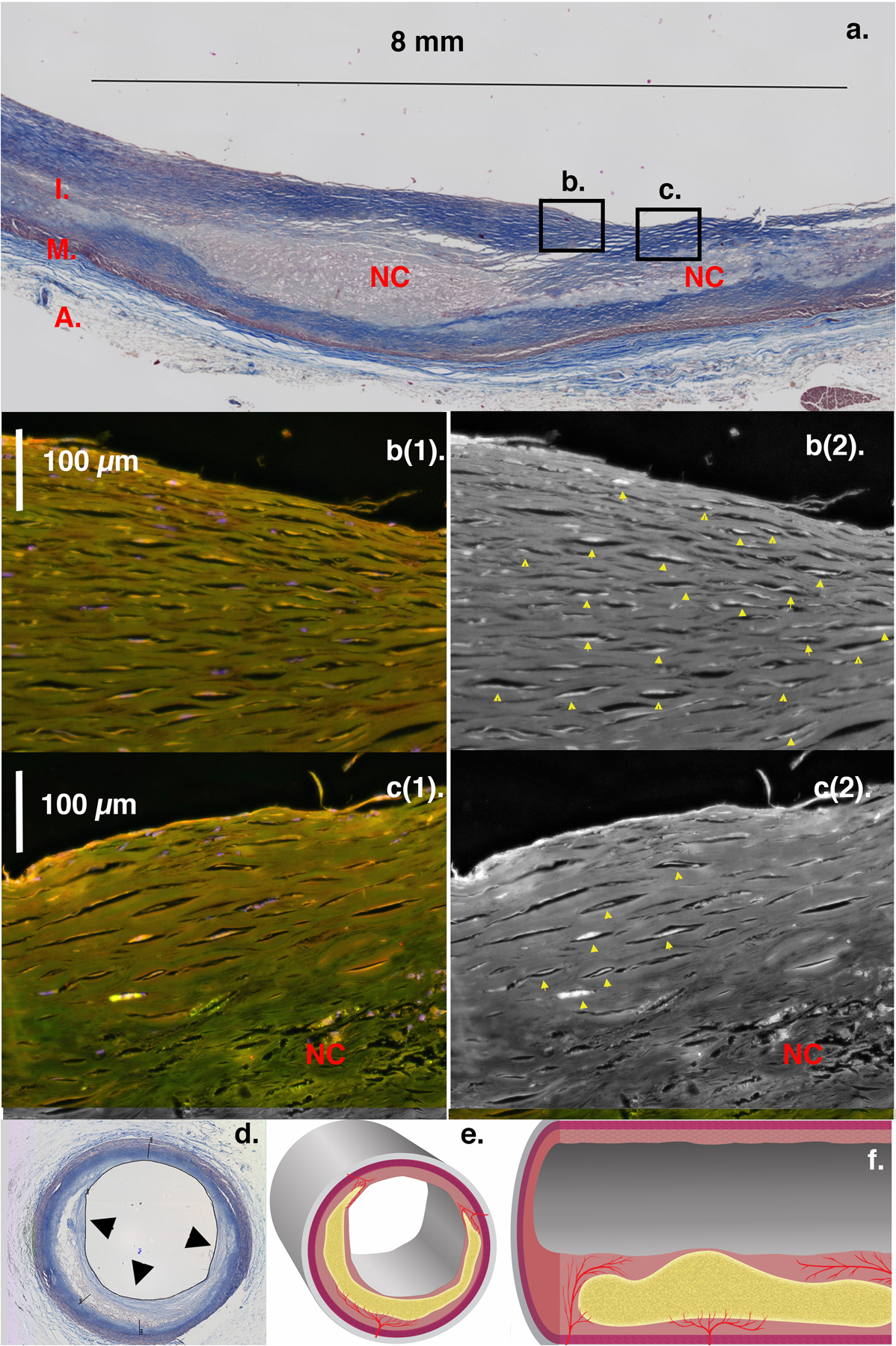
Image a: Coronary artery axial section (Masson trichrome stain) with intima (I), media (M), and adventitia (A). The long necrotic core (NC) extends beyond the image and approaches the right luminal surface (thin intimal cap). Microvessels course longitudinally over the core with decreasing density as the intima thins, seen in magnifications of boxes b and c. Images b(1), c(1): Endothelium fluorescently stained for von Willebrand Factor (red/orange). Images b(2), c(2): Contrast enhanced endothelium appears white within vessel lumens (yellow arrows) by converting the red channel to gray scale. Intima thickness diminishes from b(1,2) across c(1,2). Microvessel density is highest in the thicker cap of b(1,2) and tapers across c(1,2) till absent (no yellow arrows) where the intima is thinnest (< 200 μm). We assert angiogenesis occurs axially over long NCs and is essential for intimal cap healing. Inadequate microvessel extension over long axial NCs, as in this figure, confers vulnerability. Thin-capped fibroatheroma (TCFA) NCs are defined only in cross-section (Image d). This may explain why less than 20% lead to ACS, as most may have limited axial extent and therefore maintain adequate angiogenesis to heal. Concept illustrated in drawings of NC in cross-section (Image e) and long axis (Image f). (Artist Oran Suta)
The angiogenesis patterns we observed are in conflict with currently held paradigms and initially seemed to be in conflict with Barger et al., leading us to re-examine their data [4]. In their second paper, the authors quantified regional microvessel densities from the coronary cinematography described in their first paper. They published a table of microvessel densities for the adventitia, inner media, and intima from cross sections of diseased human coronaries. The average microvessel densities (not paired data) were 9.8 ±1.3 and 10.3 ± 4.6 in the adventitia and intima respectively. However, the inner media vessel density was only 2.2 ±0.7 and was not explained in the paper. This is inconsistent with predominately centripetal penetration. Microvessel densities should correlate across artery wall layers if penetration is centripetal with microvessels growing directly from adventitia through media to intima. We found no significant paired correlation between the microvessel densities of the intima and inner media (paired t-test p < 0.0009). Nor was there a significant correlation between the adventitia and intima microvessel densities for each vessel (Pearson paired correlation r = 0.25, p = 0.24). In arteries with significant plaque, all data are consistent with intimal microvessel propagation in a predominately longitudinal course. Our efforts to obtain the original silicone injection films were unsuccessful. On close examination of their still images (see reference 4, figure 1), silicone is rarely seen penetrating directly from the adventitia into the luminal intima [4]. These uncommon centripetal penetrations connect to microvessels coursing above the core, parallel to and near the lumen. These were not the conclusions drawn from their paper. Reexamination of their data is instead consistent with the longitudinal intimal angiogenesis shown in our analysis of plaques axially.
Intact healing mechanisms, dependent on the microvasculature, are critical for preventing intimal thinning, plaque rupture/erosion, and ACS[3]. This includes maintaining the presence and function of non-contractile, synthetic (-ACTA2) smooth muscle cells[2 3]. We have shown that longitudinal angiogenesis occurs over long necrotic cores and fails to reach some areas in the intimal cap (figure 1). We propose that these areas are at risk for thinning, rupture/erosion, and ACS due to impaired healing mechanisms. In this model, ACS requires a ‘double hit’ of both rupture/erosion and impaired vascular healing from inadequate angiogenesis. Clinically, this model supports moving away from angiogenesis inhibition to a strategy of augmenting healing.
Acknowledgements:
We thank Peter Caradonna for his technical support in histopathology generation.
Sources of Funding: Histopathology was funded for by NIH R01 HL55686 (MEB), NIH R01 EB02638/HL63953 (MEB), and a UNE Office of Scholarship and Research Grant (MEB/FW)
Footnotes
Disclosure Statement: None
References:
- 1.Camare C, Pucelle M, Negre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol 2017; 12:18–34. doi: 10.1016/j.redox.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer MC, Rittersma SZ, de Winter RJ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol 2010; 55:122–32. doi: 10.1016/j.jacc.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 3.Brezinski ME. Comparing the Risk Factors of Plaque Rupture and Failed Plaque Healing in Acute Coronary Syndromes. JAMA Cardiology 2019;4(4):329–31. doi: 10.1001/jamacardio.2019.0312 [DOI] [PubMed] [Google Scholar]
- 4.Kamat BR, Galli SJ, Barger AC, Lainey LL, Silverman KJ. Neovascularization and coronary atherosclerotic plaque: cinematographic localization and quantitative histologic analysis. Hum Pathol 1987;18(10):1036–42. [DOI] [PubMed] [Google Scholar]
- 5.Brezinski ME, Harjai KJ. Longitudinal necrotic shafts near TCFAs--a potential novel mechanism for plaque rupture to trigger ACS? Int J Cardiol 2014;177(3):738–41. doi: 10.1016/j.ijcard.2014.09.144 [DOI] [PMC free article] [PubMed] [Google Scholar]


