Abstract
Background
Colorectal cancer (CRC) screening remains underused, especially in safety-net systems. The objective of this study was to determine the effectiveness, costs, and cost-effectiveness of organized outreach using fecal immunochemical tests (FITs) compared with usual care.
Methods
Patients age 50–75 years eligible for CRC screening from eight participating primary care safety-net clinics were randomly assigned to outreach intervention with usual care vs usual care alone. The intervention included a mailed postcard and call, followed by a mailed FIT kit, and a reminder phone call if the FIT kit was not returned. The primary outcome was screening participation at 1 year and a microcosting analysis of the outreach activities with embedded long-term cost-effectiveness of outreach. All statistical tests were two-sided.
Results
A total of 5386 patients were randomly assigned to the intervention group and 5434 to usual care. FIT screening was statistically significantly higher in the intervention group than in the control group (57.9% vs 37.4%, P < .001; difference = 20.5%, 95% confidence interval = 18.6% to 22.4%). In the intervention group, FIT completion rate was higher in patients who had previously completed a FIT vs those who had not (71.9% vs 35.7%, P < .001). There was evidence of effect modification of the intervention by language, and clinic. Outreach cost approximately $23 per patient and $112 per additional patient screened. Projecting long-term outcomes, outreach was estimated to cost $9200 per quality-adjusted life-year gained vs usual care.
Conclusion
Population-based management with organized FIT outreach statistically significantly increased CRC screening and was cost-effective in a safety-net system. The sustainability of the program and any impact of economies of scale remain to be determined.
Colorectal cancer (CRC) is a leading cause of cancer deaths, yet it is often preventable (1,2). Screening remains underused, especially among racial and/or ethnic minorities and low-income populations (3). As a strategy, fecal immunochemical testing (FIT) is accepted by many patients (4) and increasingly used to support population-level screening (5,6). Because FIT testing can be performed at home, CRC screening participation is an ideal preventive health outcome to test the effectiveness and cost of an organized approach to population-level outreach.
We sought to examine the effectiveness and cost of a centrally organized outreach care model, using direct mailing of FIT kits, to improve CRC screening in partnership with multiple primary care clinics serving safety-net patients. Studies to date have examined FIT mailing, but data are scarce regarding implementation effectiveness across varying clinics and populations, the influence of prior screening behavior, and the health economics of such programs. In this study, we quantify the effectiveness of a FIT-based CRC screening outreach intervention within an integrated safety-net system, we determine the time and costs required to deliver the intervention, and we project the program’s clinical impact, cost, and cost-effectiveness over the long term.
Methods
Study Setting
We performed a multisite, pragmatic, randomized, controlled trial in a publicly funded integrated safety-net health system providing services to low-income populations, the San Francisco Health Network (SFHN). The SFHN consists of 12 adult primary care clinics and one specialty medical center, Zuckerberg San Francisco General Hospital. These clinics share an electronic health record system and a centralized clinical laboratory and refer to one gastroenterology unit.
The study team introduced the study protocol to the medical directors, and eight agreed to allow random assignment of empaneled patients to receive outreach intervention vs usual care. Providers and staff were blinded to which patients were assigned to the outreach intervention, although provision of the FIT kit by the outreach team was documented in the electronic medical records. Waiver of informed consent was approved by the University of California San Francisco (institutional review board, 14–14861, NCT02613260), and patients were enrolled between January 2016 and October 2017.
For asymptomatic patients at average risk for CRC, stool-based screening is standard practice within the SFHN. Colonoscopy is recommended for patients at increased risk for CRC and those with abnormal stool-based tests. The FIT brands used in the health system included the OC-Light, which transitioned to OC-Auto Sensor (Polymedco CDP, LLC, Cortlandt Manor, NY) in 2016.
Study Population
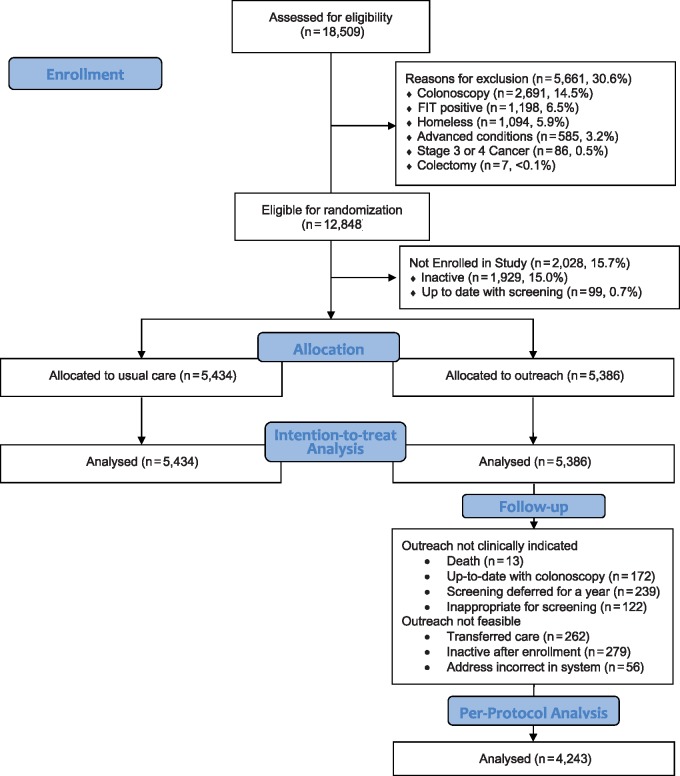
Patients age 50–75 years who were not up to date with CRC screening were eligible. Previously screened patients became eligible 365 days after previous negative FIT, 5 years after previous normal sigmoidoscopy, and 10 years after previous normal colonoscopy. Patients were excluded if they were homeless, had an abnormal FIT but no colonoscopy, colectomy, late-stage cancer, or advanced comorbidities (Figure 1).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. FIT = fecal immunochemical test.
Study Intervention
Patients were stratified by clinic, sex, race and/or ethnicity, and prior FIT participation and then randomly assigned 1: 1 to outreach intervention or usual care (Figure 1). The study team performed the outreach intervention. Outreach included an informational postcard and up to two phone calls, followed by mailing of a FIT kit packet, and then up to two reminder phone calls if the FIT kit was not returned after 2 weeks. Phone calls were performed during work hours with interpreter services available for all languages. The FIT kit packet included a letter with basic information about CRC, the FIT kit, glove, lab requisition, prepaid return envelope, and low-literacy wordless instructions for completing the test (7). The reasons for not returning the FIT kit were collected during reminder calls. Written materials were provided in English, Spanish, and Chinese.
Usual Care
Usual care was at the discretion of providers in the eight participating clinics. Clinics used medical assistants to offer FIT during patient visits, and panel management software (i2i Population Health, Franklin, TN) was available to identify patients due for screening. Abnormal FIT results are automatically routed to the patient’s primary care provider; follow-up of abnormal FIT results and referral to colonoscopy was at the discretion of primary care providers.
Outcomes
FIT screening status was ascertained by laboratory test completion and results. Health service utilization is populated into the electronic medical records. FIT completion and endoscopy procedures were available in real time and were extracted and linked to the study database to ascertain the clinical outcomes.
Cost Accounting
Prospective cost accounting using a microcosting method was embedded in the intervention arm. Microcosting, which accounts for all the individual components that contribute to an overall cost, distinguished the costs for one-time initial implementation, including initial capital costs and staff training, vs ongoing outreach activities. Staff recorded time spent per specific outreach activity, based on categories previously defined by the Centers for Disease Control and Prevention (8), and normalized by the number of patients receiving outreach during the three outreach cycles (February–March 2016, September–October 2016, and June–July 2017). Average costs per patient were estimated based on individual salaries and benefits of responsible staff and average costs of materials per patient.
Short-Term and Projected Long-Term Cost-Effectiveness
Short-term cost-effectiveness was estimated as the cost per additional patient screened, a measure that incorporates the impact of the intervention on screening participation. To estimate the long-term clinical and economic impact of outreach, we adapted our validated decision analytic model of CRC screening (Supplementary Methods and Supplementary Figure 1, available online) (9,10). In brief, we modeled outreach vs usual care with the screening rates at 1 year and costs observed in the current study (Supplementary Table 1, available online). The simulation’s time horizon was through age 100 years or death, with screening offered from age 50 to 80 years. Based on screening behavior through the second year after randomization in this trial as well as published literature (11–15), we estimated the fractions of consistent, intermittent, and never-screeners over time under outreach vs usual care (10). We accounted for imperfect follow-up colonoscopy rates after abnormal FIT results, which was estimated to be 55.6% based on published data from the SFHN (16). All screening and treatment costs were based on 2018 Medicare reimbursement rates. Primary model outcomes were discounted quality-adjusted life-years (QALYs) and costs per patient. Sensitivity analyses explored the range of intervention effects across individual clinics, a range of outreach program costs, and the potential impact of navigation (17,18) on follow-up colonoscopy completion after abnormal FIT.
Statistical Analysis
Patient demographic characteristics were summarized by treatment group using proportions or means and SD and compared using χ2 or t tests, as appropriate. All tests were two-sided, and a P value of less than .05 was considered statistically significant.
The primary outcome was completion of FIT screening and summarized by the proportions of patients who were up to date 1 year after study enrollment. This intention-to-treat analysis included all patients assigned to outreach, regardless of whether FIT kits were mailed. Evidence for modification of the effect of the outreach intervention by clinic, sex, race and/or ethnicity, insurance coverage, and language was examined using logistic models with interaction terms. Patients who were lost to follow-up, defined as absence of an encounter over 2 years or SFHN no longer designated as their medical home, were assumed not to have completed FIT screening if the patient had not completed screening before being lost to follow-up. For illustrative purposes, a cumulative incidence plot was used to estimate the proportions up to date over time. The between-group difference in proportions was determined and the inverse of the between-group difference was used to estimate the number needed to treat. In addition, we conducted a per-protocol analysis excluding patients who were not sent a FIT kit; reasons included an unreliable address (returned postcard), no longer in the health network, death, or screening deferred (Figure 1).
For patients who had abnormal FIT results and had at least 6 months of follow-up time, we examined the colonoscopy completion rate within the intervention and usual-care groups. Finally, during reminder calls, the reasons for not being up to date were collected and categorized (eg, forgot, already returned the test, needed a new test). We examined the FIT kit return rate after the call, by category.
With more than 10 000 patients, the overall study provided 80% power in two-sided tests with a type-I error rate of 5% to detect a between-group difference of 2.7 percentage points. Within subgroups defined by race, language preferences, and clinic, minimum detectable between-group differences ranged from 5.6 to 13.4 percentage points, depending on the size of the subgroup and the completion rate among controls. We used Stata (version 15.1; StataCorp LP, College Station, TX) and SAS (version 9.4; SAS Institute Inc, Cary, NC) for all statistical analyses, and TreeAge Pro (TreeAge Software, Williamstown, MA) for cost-effectiveness analyses.
Results
Patient Demographics
A total of 18 509 patients age 50–75 years were empaneled in the eight SFHN primary care clinics, and 12 848 (69.4%) were eligible for CRC screening and for random assignment (Figure 1). The leading reasons for exclusion were colonoscopy within the last 10 years (14.5%) and previously abnormal FIT (6.5%) (Figure 1).
Between January 2016 and October 2017, 10 820 patients were enrolled from eight clinic sites: 5434 patients into usual care and 5386 patients into outreach intervention. Demographic characteristics varied by clinic (Table 1). The distribution of age, sex, race, and insurance type were similar among the usual care and intervention groups (data not shown).
Table 1.
Baseline characteristics of patients by clinic
| Characteristics | Clinic 1, % (n = 2565) | Clinic 2, % (n = 1210) | Clinic 3, % (n = 665) | Clinic 4, % (n = 923) | Clinic 5, % (n = 1961) | Clinic 6, % (n = 1694) | Clinic 7, % (n = 934) | Clinic 8, % (n = 868) |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Female | 60.4 | 54.9 | 13.2 | 45.7 | 51.5 | 31.1 | 48.6 | 44.1 |
| Male | 39.6 | 45.1 | 86.8 | 54.3 | 48.5 | 68.9 | 51.4 | 55.9 |
| Age, y | ||||||||
| 50–55 | 26.7 | 23.6 | 45.6 | 28.7 | 21.2 | 26.6 | 24.8 | 28.2 |
| 55–60 | 24.7 | 26.7 | 29.5 | 27.8 | 24.4 | 29.5 | 26.9 | 27.3 |
| 60–65 | 23.1 | 26.5 | 14.7 | 22.9 | 24.7 | 23.9 | 23.9 | 21.9 |
| 65–70 | 16.5 | 15.3 | 7.1 | 13.8 | 18.9 | 14.3 | 16.3 | 16.1 |
| 70–75 | 9.0 | 7.9 | 3.1 | 6.8 | 10.8 | 5.7 | 8.1 | 6.5 |
| Race/Ethnicity | ||||||||
| Hispanic | 33.0 | 8.3 | 18.2 | 25.6 | 27.8 | 14.7 | 40.6 | 11.5 |
| Non-Hispanic black | 9.2 | 24.8 | 21.7 | 24.6 | 13.9 | 34.8 | 5.6 | 61.2 |
| Non-Hispanic white | 12.8 | 26.5 | 38.5 | 19.3 | 17.3 | 37.0 | 29.9 | 7.0 |
| Asian | 28.6 | 28.2 | 4.5 | 16.1 | 27.1 | 7.1 | 7.4 | 6.9 |
| Insurance | ||||||||
| Medicaid | 45.2 | 46.9 | 40.2 | 54.1 | 44.0 | 60.5 | 53.0 | 62.2 |
| Medicare | 17.7 | 12.0 | 40.5 | 11.9 | 21.8 | 20.8 | 12.9 | 14.3 |
| Uninsured | 4.4 | 4.1 | 9.3 | 4.9 | 4.4 | 6.1 | 4.5 | 6.2 |
| County sponsored | 11.4 | 6.0 | 4.7 | 7.7 | 7.9 | 6.1 | 16.7 | 3.3 |
| Healthy Worker* | 17.0 | 27.1 | 1.2 | 15.0 | 18.9 | 3.3 | 8.0 | 9.0 |
| Language | ||||||||
| Chinese | 11.3 | 7.0 | 0.3 | 4.0 | 7.9 | 0.5 | 0.4 | 2.1 |
| Spanish | 26.2 | 3.4 | 6.6 | 15.7 | 17.1 | 6.5 | 32.6 | 7.8 |
| English | 36.5 | 63.4 | 74.7 | 60.8 | 49.9 | 73.1 | 51.1 | 74.1 |
| Other/Unknown | 26.0 | 26.2 | 18.4 | 19.5 | 25.1 | 19.9 | 15.9 | 16.0 |
| Prior FIT | ||||||||
| Yes | 68.1 | 62.8 | 43.5 | 63.2 | 63.1 | 46.6 | 63.7 | 54.4 |
| No | 31.9 | 37.2 | 56.5 | 36.8 | 36.9 | 53.4 | 36.3 | 45.6 |
Insurance type for in-home support service providers and temporary insurance for county employees. FIT = fecal immunochemical testing.
Effect of Organized Outreach to Increase FIT Participation
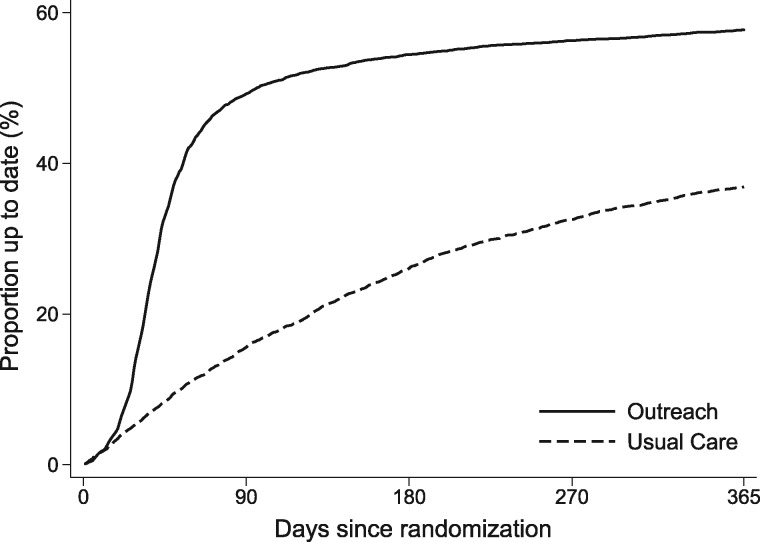
Over 1 year, 11.8% patients were lost to follow-up; this difference was not statistically significantly different between groups (11.5% in outreach vs 12.2% in usual care, P = .25). Of patients in the intervention group, 57.9% (95% confidence interval [CI] = 56.6% to 59.3%) had returned the FIT kit by 1 year vs 37.4% (95% CI = 36.1% to 38.7%) (difference of 20.5%, 95% CI = 18.6% to 22.4%; P < .001; Table 2 and Figure 2). Even at day 28, outreach led to a statistically significant increase in FIT completion (13.3% vs 5.1%; P < .001; data not shown). Most of the screening participation following the outreach intervention occurred within 90 days of the intervention, whereas screening participation under usual care was distributed more evenly over time (Figure 2). The number needed to receive outreach for an additional patient screened was 4.8.
Table 2.
Effect of organized outreach compared with usual care on FIT completion at 1 year
| Characteristics | Usual care arm |
Intervention arm |
1-year difference, % (95% CI) | Interaction OR (95% CI) | ||
|---|---|---|---|---|---|---|
| No. | % Up to date 1 year (95% CI) | No. | % Up to date 1 year (95% CI) | |||
| Total | 5434 | 37.4 (36.1 to 38.7) | 5386 | 57.9 (56.6 to 59.3) | 20.5 (18.6 to 22.4) | — |
| Sex | ||||||
| Female | 2579 | 40.8 (38.9 to 42.7) | 2518 | 61.1 (59.2 to 63.0) | 20.3 (17.5 to 23.0) | 1.00 (Referent) |
| Male | 2855 | 34.5 (32.7 to 36.2) | 2868 | 55.0 (53.2 to 56.9) | 20.6 (17.9 to 23.2) | 1.05 (0.90 to 1.23) |
| Age, y | ||||||
| 50–55 | 1470 | 33.1 (30.6 to 35.5) | 1412 | 54.8 (52.2 to 57.4) | 21.7 (18.0 to 25.4) | 1.00 (Referent) |
| 55–60 | 1446 | 37.5 (35.0 to 40.0) | 1431 | 56.8 (54.2 to 59.4) | 19.3 (15.6 to 23.0) | 0.85 (0.69 to 1.05) |
| 60–65 | 1264 | 39.5 (36.8 to 42.2) | 1261 | 60.6 (57.8 to 63.3) | 21.1 (17.2 to 25.0) | 0.98 (0.79 to 1.23) |
| 65–70 | 834 | 39.9 (36.6 to 43.2) | 853 | 60.9 (57.7 to 64.2) | 21.1 (16.3 to 25.8) | 0.94 (0.73 to 1.21) |
| 70–75 | 420 | 41.2 (36.5 to 45.9) | 429 | 57.8 (53.2 to 62.4) | 16.6 (9.9 to 23.4) | 0.75 (0.55 to 1.03) |
| Race/Ethnicity | ||||||
| Hispanic | 1313 | 42.3 (39.5 to 45.0) | 1264 | 65.2 (62.5 to 67.9) | 22.9 (18.9 to 26.9) | 1.00 (Referent) |
| Non-Hispanic black | 1178 | 32.3 (29.5 to 35.0) | 1173 | 49.7 (46.8 to 52.7) | 17.5 (13.3 to 21.6) | 0.88 (0.70 to 1.11) |
| Non-Hispanic white | 1185 | 28.9 (26.3 to 31.5) | 1205 | 51.6 (48.7 to 54.4) | 22.7 (18.8 to 26.7) | 1.05 (0.83 to 1.33) |
| Asian | 1023 | 48.0 (44.9 to 51.0) | 1012 | 69.3 (66.5 to 72.1) | 21.3 (17.1 to 25.5) | 0.97 (0.76 to 1.24) |
| Insurance | ||||||
| Medicaid | 2706 | 34.1(32.3 to 35.9) | 2711 | 55.1(53.2 to 57.0) | 21.0 (18.3 to 23.7) | 1.00 (Referent) |
| Medicare | 1025 | 38.9 (35.9 to 41.9) | 979 | 59.6 (56.5 to 62.8) | 20.7 (16.3 to 25.2) | 1.00 (0.81 to 1.24) |
| Uninsured | 281 | 23.6 (18.7 to 28.5) | 274 | 48.0 (42.0 to 54.0) | 24.4 (16.5 to 32.3) | 1.40 (0.95 to 2.06) |
| County sponsored | 465 | 40.8 (36.2 to 45.3) | 446 | 64.2 (59.7 to 68.7) | 23.4 (16.8 to 30.0) | 1.03 (0.77 to 1.37) |
| Healthy Worker* | 728 | 52.2 (48.5 to 55.8) | 762 | 70.1 (66.8 to 73.4) | 17.9 (12.9 to 22.9) | 0.95 (0.74 to 1.21) |
| Language | ||||||
| Chinese | 232 | 54.0 (47.6 to 60.4) | 367 | 80.0 (75.9 to 84.1) | 26.0 (18.3 to 33.6) | 1.49 (1.02 to 2.17) |
| Spanish | 674 | 41.9 (38.2 to 45.6) | 1046 | 68.2 (65.4 to 71.1) | 26.4 (21.7 to 31.0) | 1.30 (1.04 to 1.64) |
| English | 2636 | 31.5 (29.7 to 33.3) | 3461 | 51.3 (49.6 to 52.9) | 19.8 (17.3 to 22.2) | 1.00 (Referent) |
| Other/Unknown† | 1892 | 43.7 (41.5 to 45.9) | 512 | 58.8 (54.5 to 63.0) | 15.0 (10.2 to 19.9) | 0.81 (0.65 to 1.01) |
| Prior FIT | ||||||
| Yes | 3231 | 49.0 (47.2 to 50.8) | 3240 | 71.0 (69.4 to 72.5) | 22.0 (19.5 to 24.4) | 1.06 (0.89 to 1.25) |
| No | 2203 | 20.9(19.2 to 22.6) | 2146 | 37.4 (35.3 to 39.5) | 16.5 (13.8 to 19.3) | 1.00 (Referent) |
| Clinic | ||||||
| 1 | 1286 | 44.2 (41.5 to 47.0) | 1279 | 67.4 (64.8 to 70.0) | 23.2 (19.3 to 27.0) | 1.00 (Referent) |
| 2 | 597 | 39.3 (35.3 to 43.2) | 613 | 53.6 (49.7 to 57.6) | 14.4 (8.7 to 20.0) | 0.73 (0.55 to 0.97) |
| 3 | 338 | 31.2 (26.2 to 36.3) | 327 | 58.4 (52.9 to 64.0) | 27.3 (19.4 to 35.1) | 1.30 (0.91 to 1.85) |
| 4 | 450 | 36.1 (31.7 to 40.6) | 473 | 66.1 (61.9 to 70.4) | 30.0 (23.8 to 36.3) | 1.31 (0.96 to 1.80) |
| 5 | 999 | 41.4 (38.3 to 44.5) | 962 | 57.0 (53.8 to 60.2) | 15.7 (9.8 to 19.0) | 0.73 (0.57 to 0.93) |
| 6 | 856 | 26.2 (23.3 to 29.2) | 838 | 50.1(46.6 to 53.5) | 23.8 (19.1 to 28.6) | 1.14 (0.88 to 1.48) |
| 7 | 473 | 32.4 (28.0 to 36.7) | 461 | 49.9 (45.2 to 54.6) | 17.5 (10.8 to 24.2) | 0.82 (0.60 to 1.12) |
| 8 | 435 | 38.7 (34.0 to 43.4) | 433 | 52.4 (47.6 to 57.1) | 13.6 (6.8 to 20.5) | 0.67 (0.49 to 0.92) |
Insurance for those who work as in-home support service providers and temporary insurance for county employees. CI = confidence interval; FIT = fecal immunochemical test; OR = odds ratio.
The percentage with other or unknown language decreased in the intervention arm as outreach workers verified language preference.
Figure 2.
Cumulative proportion of patients up to date with colorectal cancer screening in the outreach intervention and usual care groups. At 1 year, 57.9% of patients in the intervention group had returned the FIT kit vs 37.4% in the usual care group. By day 28, outreach statistically significantly increased FIT completion (13.3% vs 5.1%). FIT = fecal immunochemical test.
In a per-protocol analysis excluding 1143 patients who were not mailed FIT kits (Figure 1), FIT completion increased by 10.7 percentage points to 68.6% (95% CI = 67.2% to 69.9%). The between-group difference in participation increased to 31.3%, with a number needed to mail a FIT kit for an additional patient screened of 3.2.
Effect of Organized Outreach by Clinic
The effect of the outreach intervention consistently increased screening participation; however, the magnitude of the effect differed by clinic with evidence of effect modification (odds ratio [OR] range = 1.75–3.54; percentage point increase across clinics, 13.6% to 30.0%) (Table 2). FIT completion by clinic ranged from 49.9% to 67.4% in the intervention arm vs 26.2% to 44.2% in usual care (Table 2).
Effect of Organized Outreach by Patient Subgroup
There was an increase in FIT participation among patients assigned to outreach intervention vs usual care across all patient subgroups (Table 2). The subgroups with highest screening participation at 1 year were ethnic minorities (eg, Asian 69.3% and Hispanic 65.2%), patients with Healthy Worker insurance (70.1%), and patients who used non-English languages (Chinese 80.0% and Spanish 68.2%), with language showing evidence of effect modification. Patients who had previously completed a FIT were more likely to complete a FIT than those with no record of prior FIT completion (70.2% vs 34.8%; P < .001). OC-Light transitioned to OC-Sensor during the study period. There was no statistically significant difference in completion rates by the two brands of FIT tests after adjustment for clinical covariates.
Colonoscopy Follow-up After Abnormal FIT
Colonoscopy completion was ascertained for patients with abnormal FIT results. Among patients who had at least 6 months of follow-up time after an abnormal FIT, a similar proportion of patients in both groups completed a colonoscopy: 51.0% (106 of 208) of patients in intervention vs 51.4% (57 of 111) in usual care (P = .94) (Supplementary Table 2, available online).
Reasons for Not Being Up to Date
At the time of the reminder call, the most common reasons stated for not being up to date were forgot/not a priority/busy (26.4%), patients reporting that they already returned or completed the test but had not mailed it yet (24.0%), did not receive/lost/damaged test (15.3%), and did not understand how to complete the test (10.6%) (Supplementary Table 3, available online). After the reminder call, the percentage of patients becoming up to date according to stated reasons were as follows: forgot/not a priority/busy (62.7%), already returned or completed the test (90.7%), did not receive test/lost test/test damaged (61.8%), and did not understand how to complete the test (77.4%).
Cost Accounting
Initial start-up implementation costs, including equipment and training, totaled $15 997 (Supplementary Table 4, available online). The average time spent in outreach per patient (range = 21.5–24.2 minutes) and the average total outreach cost per patient (range = $22.2–$24.1) did not change substantially across the three sampling cycles (Table 3). Labor costs exceeded material costs (Supplementary Figure 2, available online), and 86%–91% of labor time was devoted to direct patient activities as opposed to program-related activities, including 25%–36% of time spent on phone calls (Table 3; Supplementary Figure 3, available online).
Table 3.
Prospective cost accounting of outreach activities
| Cost categories | Cycle 1, Feb–Mar 2016 (n = 1833 receiving some outreach activity) |
Cycle 2, Sep–Oct 2016 (n = 1270 receiving some outreach activity) |
Cycle 3, Jun–Jul 2017 (n = 1007 receiving some outreach activity) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Proportion of patients who received this activity, % | Time per patient, minutes, weighted mean* | Cost per patient, weighted mean* | Proportion of patients who received this activity, % | Time per patient, minutes, weighted mean* | Cost per patient, weighted mean* | Proportion of patients who received this activity, % | Time per patient, minutes, weighted mean* | Cost per patient, weighted mean* | |
| Labor | |||||||||
| Direct patient-related activities | |||||||||
| Phone calls | |||||||||
| Pre-FIT call | 66.1 | 3.78 | $2.21 | 61.7 | 3.48 | $2.09 | 34.6 | 1.96 | $1.17 |
| Updating addresses | 11.0 | 0.88 | $0.52 | 5.2 | 0.37 | $0.22 | 6.0 | 0.51 | $0.31 |
| Responding to patients (voicemail/calls) | 2.4 | 0.25 | $0.15 | 3.1 | 0.35 | $0.20 | 6.1 | 0.66 | $0.40 |
| Reminder call | 62.3 | 3.43 | $1.98 | 85.7 | 3.32 | $2.00 | 63.6 | 2.89 | $1.75 |
| Postcards (address, stamp, mail) | 100 | 0.70 | $0.41 | 100 | 0.48 | $0.29 | 100 | 0.83 | $0.51 |
| FIT kits (letter, requisition, assemble, stamp, mail) | 76.8 | 8.00 | $4.82 | 58.5 | 3.26 | $1.96 | 86.4 | 7.23 | $4.39 |
| Data entry | 100 | 4.18 | $2.35 | 100 | 7.98 | $4.83 | 100 | 6.73 | $4.19 |
| Subtotal | NA | 21.22 | $12.44 | NA | 19.23 | $11.60 | NA | 20.81 | $12.73 |
| Program activities | |||||||||
| Program management | NA | 1.69 | $1.43 | NA | 1.59 | $1.76 | NA | 2.64 | $2.29 |
| Office management | NA | 0.47 | $0.34 | NA | 0.63 | $0.40 | NA | 0.73 | $0.49 |
| Subtotal | NA | 2.15 | $1.77 | NA | 2.22 | $2.16 | NA | 3.37 | $2.78 |
| Materials | |||||||||
| Direct patient-related materials | |||||||||
| Postcards, mailing and related | NA | NA | $0.54 | NA | NA | $0.53 | NA | NA | $0.52 |
| FIT kits, mailing and related | NA | NA | $7.53 | NA | NA | $7.48 | NA | NA | $7.47 |
| Program materials/expenses | NA | NA | $0.32 | NA | NA | $0.47 | NA | NA | $0.58 |
| Total | |||||||||
| Totals per patient | NA | 23.4 | $22.6 | NA | 21.5 | $22.2 | NA | 24.2 | $24.1 |
Reflects all patients, including those who received the intervention components as well as those who did not. FIT = fecal immunochemical test; NA = not applicable.
Short-Term and Projected Long-Term Cost-Effectiveness
The approximate outreach cost of $23 per patient, coupled with the absolute increase in FIT completion rate of 20.5% at 1 year, translated to a cost of $112 per additional patient screened. Projecting the clinical trial results over the long term, and incorporating the observed implementation and ongoing costs of outreach, the estimated QALYs and cost per patient were 19.6259 QALYs and $2960 with outreach, and 19.6103 QALYs and $2816 with usual care, yielding a cost of $9200 per QALY gained with outreach (Supplementary Table 5, available online).
In clinic-level sensitivity analyses across the range of gains in FIT participation rates (Table 2), the cost of outreach was $900–$23 400 per QALY gained (Supplementary Table 5, available online). If outreach cost decreased to less than $14 per patient, outreach achieved better outcomes at lower costs than usual care. At outreach costs of $50 per patient, outreach cost $37 400 per QALY gained. If screening outreach was coupled with navigation to increase completion of colonoscopy after abnormal FIT from 55% to a hypothetical 75% with navigation, as has been reported for navigation with screening colonoscopy, then outreach plus navigation cost $3000 per QALY gained.
Discussion
In this trial of an organized outreach intervention, implemented in partnership with eight primary care clinics, direct mailing of FIT kits to patients not up to date with CRC screening statistically significantly increased CRC screening participation at each site. An estimated three to five additional individuals needed to receive outreach to increase screening by one. Because this study was conducted in multiple clinics with diverse populations, the effectiveness reflects a range of real-world estimates of the initiative depending on the clinical setting. The prospective cost accounting embedded in the trial provides real-world estimates of the staff time, resources, and costs required to deliver outreach. Our long-term cost-effectiveness analysis, informed by the clinical results of the study and the cost accounting, suggests that organized outreach is highly cost-effective across multiple clinical scenarios.
The cost to perform outreach for one individual ($23) and the cost per additional patient screened ($112) both appear acceptable at face value based on the established clinical benefits of CRC screening. Our cost of outreach are in line or lower than prior estimates, which could be attributed to a central structure with increased scale (19,20). In addition, our estimate of the long-term cost-effectiveness of outreach ($9200 per QALY gained vs usual care in the base case) is novel and within acceptable ranges for services adopted by health systems (21). Moreover, we used conservative assumptions for colonoscopy completion at 55.6% after abnormal FIT that are based on an earlier study in this safety-net health system (16), and that are consistent with the rates observed in other safety-net settings (22,23). When outreach effectiveness was varied across the range observed across all clinics, organized outreach to improve CRC screening remained highly cost-effective. To further drive down cost, economies of scale and automation might be achieved through use of communication and mailing vendors, which could be cost saving at outreach costs of less than $14 per patient in our models.
Organized outreach has been shown to be effective in several private and public health settings (24–30). By randomly assigning patients in eight clinical sites, this study may be the most comprehensive to date to characterize the range of effectiveness achieved across clinics and demography and prior testing behavior while accounting for implementation and operation costs and long-term cost-effectiveness. This level of detail in diverse safety-net clinics extends the knowledge by demonstrating that a centrally administered FIT outreach program is highly cost-effective. We expect the comprehensive nature of this study to inform health-care systems as they address population-wide CRC screening.
We attempted to understand barriers to screening faced by patients in our health system. Our results suggest that reminder phone calls addressing patient concerns may improve completion, as reported previously by others (30,31). However, there remains a proportion of patients who do not complete screening despite outreach and follow-up efforts. The intervention consistently raised the level of screening, but it did not close disparity gaps.
Our study has limitations. First, the clinics that did not participate in this study tended to have CRC screening rates of 70% or higher. The impact of organized outreach would have been more modest among these clinics, but it could have offset clinic staff time currently dedicated to panel management and screening. As such, it is possible that the results do not generalize to other health-care settings and populations. Second, there were patients excluded from this study based on homelessness and advanced comorbidities, who are less likely to be screened and could have lowered the screening rates. Third, stool-based screening programs address screening, but colonoscopy completion for patients with abnormal screening results is necessary for screening to reduce CRC incidence and mortality; our intervention did not address colonoscopy follow-up, which was similar between both groups. Nonetheless, our long-term simulation suggests that outreach remains highly cost-effective even with suboptimal follow-up colonoscopy rates. Lastly, we have not examined the individual contribution of each component of the intervention, although prior studies have attempted to disentangle these components (31,32).
In conclusion, organized outreach using direct mailing of FIT kits can improve CRC screening, and the favorable health economic results support the widespread adoption of this service, assuming that the results generalize to other locations. Reimbursement models that facilitate population-level preventive health management strategies could further improve CRC screening.
Funding
This work was supported by the Centers for Disease Control and Prevention (CDC) U48DP004998 (SIP 14–012), the UCSF Academic Research Systems, and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR991872, and the SF Cancer Initiative.
Notes
The funds from CDC were granted through a cooperative agreement in which we worked closely with a CDC program officer to design and implement the study. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or US National Institutes of Health.
The authors have no conflicts of interest to disclose.
Specific author contributions: Study concept and design: MS, UL, EV, JAS. Acquisition of data: MS, CDR, VL, DG, BG. Administrative, technical, or material support: MS, CDR, EC, UL. Statistical analysis: MS, BG, EV, AM, UL. Drafting of the manuscript: MS, CDR, RBI, BG, EV, AM, UL, JAS. Critical revision of the manuscript for important intellectual content: MS, CDR, AM, EV, UL, JAS. Approval of the final manuscript: all authors. Study supervision: MS, EV, UL.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–2575. [DOI] [PubMed] [Google Scholar]
- 3. Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106(4):dju032.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening. Arch Intern Med. 2012;172(7):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu HM, Chen SL, Yen AM, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121(18):3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin TR, Corley DA, Jensen CD, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large, community-based population. Gastroenterology. 2018;155(5):1383–1391.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang A, Rachocki C, Shapiro JA, et al. Low-literacy level instructions and reminder calls improve patient handling of fecal immunochemical test samples [published online ahead of print Nov. 29, 2018]. Clin Gastroenterol Hepatol. 2018. doi:10.1016/j.cgh.2018.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subramanian S, Tangka FK, Hoover S, et al. Clinical and programmatic costs of implementing colorectal cancer screening: evaluation of five programs. Eval Program Plann. 2011;34(2):147–153. [DOI] [PubMed] [Google Scholar]
- 9. Ladabaum U, Song K.. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129(4):1151–1162. [DOI] [PubMed] [Google Scholar]
- 10. Ladabaum U, Mannalithara A.. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology. 2016;151(3):427–439.e6. [DOI] [PubMed] [Google Scholar]
- 11. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening. Ann Intern Med. 2016;164(7):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duncan A, Turnbull D, Wilson C, et al. Behavioural and demographic predictors of adherence to three consecutive faecal occult blood test screening opportunities: a population study. BMC Public Health. 2014;14:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lo SH, Halloran S, Snowball J, et al. Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut. 2015;64(2):282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapidzic A, Grobbee EJ, Hol L, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol. 2014;109(8):1257–1264. [DOI] [PubMed] [Google Scholar]
- 15. Wong MC, Ching JY, Lam TY, et al. Prospective cohort study of compliance with faecal immunochemical tests for colorectal cancer screening in Hong Kong. Prev Med. 2013;57(3):227–231. [DOI] [PubMed] [Google Scholar]
- 16. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net system. Am J Gastroenterol. 2017;112(2):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ladabaum U, Mannalithara A, Jandorf L, et al. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority individuals. Cancer. 2015;121(7):1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jandorf L, Stossel LM, Cooperman JL, et al. Cost analysis of a patient navigation system to increase screening colonoscopy adherence among urban minorities. Cancer. 2013;119(3):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lara CL, Means KL, Morwood KD, et al. Colorectal cancer screening interventions in 2 health care systems serving disadvantaged populations: screening uptake and cost-effectiveness. Cancer. 2018;124(21):4130–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liss DT, French DD, Buchanan DR, et al. Outreach for annual colorectal cancer screening: a budget impact analysis for community health centers. Am J Prev Med. 2016;50(2):e54–e61. [DOI] [PubMed] [Google Scholar]
- 21. Nord E, Johansen R.. Concerns for severity in priority setting in health care: a review of trade-off data in preference studies and implications for societal willingness to pay for a QALY. Health Policy. 2014;116(2–3):281–288. [DOI] [PubMed] [Google Scholar]
- 22. Martin J, Halm EA, Tiro JA, et al. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med. 2017;130(1):93.e1–93.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thamarasseril S, Bhuket T, Chan C, et al. The need for an integrated patient navigation pathway to improve access to colonoscopy after positive fecal immunochemical testing: a safety-net hospital experience. J Community Health. 2017;42(3):551–557. [DOI] [PubMed] [Google Scholar]
- 24. Issaka RB, Avila P, Whitaker E, et al. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: a systematic review. Prev Med. 2019;118:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–1241. [DOI] [PubMed] [Google Scholar]
- 27. Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety-net health system. Cancer. 2016;122(3):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldman SN, Liss DT, Brown T, et al. Comparative effectiveness of multifaceted outreach to initiate colorectal cancer screening in community health centers: a randomized controlled trial. J Gen Intern Med. 2015;30(8):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coronado GD, Petrik AF, Vollmer WM, et al. Effectiveness of a mailed colorectal cancer screening outreach program in community health clinics: the STOP CRC cluster randomized clinical trial. JAMA Intern Med. 2018;178(9):1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levy BT, Daly JM, Xu Y, et al. Mailed fecal immunochemical tests plus educational materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. J Am Board Fam Med. 2012;25(1):73–82. [DOI] [PubMed] [Google Scholar]
- 31. Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–2091. [DOI] [PubMed] [Google Scholar]
- 32. Senore C, Ederle A, DePretis G, et al. Invitation strategies for colorectal cancer screening programmes: the impact of an advance notification letter. Prev Med. 2015;73:106–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.