Abstract
Background
Overactive bladder syndrome is defined as "urgency with or without urge incontinence, usually with frequency and nocturia". It is a common condition with significant economic and quality of life implications. While the condition's pathophysiology remains to be fully elucidated, pharmacotherapy is the main treatment option. Despite uncertainty as to drug treatment of choice, anticholinergics are increasingly being used in primary and secondary care settings. This review compares anticholinergic drugs with other types or classes of drugs for treating overactive bladder syndromes.
Objectives
To compare anticholinergic drugs with other types or classes of drugs for treating overactive bladder symptoms.
Search methods
We searched the Cochrane Incontinence Group Specialised Trials Register (searched 20 December 2006) and the reference lists of relevant articles. No language or other limits were imposed.
Selection criteria
All randomised and quasi‐randomised controlled trials comparing anticholinergic drugs with other drugs for the treatment of overactive bladder symptoms. At least one arm of the study used an anticholinergic drug and at least one other arm used a non‐anticholinergic drug.
Data collection and analysis
Two reviewers assessed the identified studies for eligibility and methodological quality and independently extracted data from the included studies. Data analysis was performed using RevMan software (version 4.2.8).
Main results
Twelve trials were included in the review. There were seven crossover trials and five parallel group studies. For the comparisons between anticholinergic drugs with tricyclic antidepressants, alpha adrenergic agonists, afferent nerve inhibitors, and calcium channel blocker a single trial was identified for each. Nine trials compared flavoxate with anticholinergics. There was no evidence of a difference in cure rates between anticholinergics and flavoxate. Adverse effects were more frequent in anticholinergic groups versus flavoxate groups (RR 2.28 95% CI 1.45 to 3.56). There was no strong evidence to favour either anticholinergic drugs or the comparators.
Authors' conclusions
Many of the drugs considered in trials in this review are no longer used in clinical practice (and this includes the most commonly tested ‐ flavoxate). There is inadequate evidence as to determine whether any of the available druge are better or worse than anticholinergic medications. Larger randomised controlled trials in clinical settings are required to further establish the role of these medications in the management of overactive bladder syndrome.
Plain language summary
Drugs in overactive bladder syndrome
Overactive bladder syndrome is characterised by a need to rush to urinate ‐ urine is passed frequently and there may be incontinence. The main treatment option is drug therapy. The most commonly used drugs are anticholinergics, but these often have side‐effects, such as dry mouth. This review sought evidence to compare other types of drugs with anticholinergics. Only a few, small‐scale randomised trials were found, many testing drugs that are no longer used clinically. The review found inadequate evidence to assess whether or not available alternative drugs are better or worse than anticholinergics in the management of people with symptoms of overactive bladder syndrome.
Background
Overactive bladder syndrome is defined as "urgency with or without urge incontinence, usually with frequency and nocturia" (Abrams 2002). This is a common condition. In a survey of 16,776 adults done in Europe (France, Germany, Italy, Spain, Sweden and the United Kingdom), 16.6% had overactive bladder, giving the estimated European prevalence of 22 million individuals affected. Urge incontinence was reported by 36% of those with overactive bladder symptoms (Milsom 2001). In a similar study in the United States, the National Overactive Bladder Evaluation (NOBLE) program, the estimated prevalence amongst Americans was 33 million. Of these, an estimated 12 million (37%) were incontinent (Stewart 2001).
The estimated prevalence of overactive bladder amongst people aged 40 years and above is 15.6% and 17.4% in men and women respectively, and the prevalence increases with age in both sexes. The symptoms of urgency and, or, frequency are equally common in men and women but urge incontinence is more common in women (Milsom 2001).
Overactive bladder syndrome has economic and quality of life implications. It has been estimated that the economic cost of overactive bladder was US$12.02 billion in 2000 in the United States (Hu 2003). It is also associated with poorer quality of life indices as shown by the Short Form (SF) questionnaires, King's Health Questionnaire, a higher depression score and a poorer quality of sleep (Kelleher 1997; Stewart 2001; Stewart 2003).
The pathophysiology of the overactive bladder remains to be fully elucidated. However, the involvement of the autonomic nervous system in bladder/detrusor function is recognised (de Groat 1997). The motor supply to the bladder is via the parasympathetic nervous system (via sacral nerves S2,3,4) (Abrams 1988; Ouslander 1982; Ouslander 1986), which effects detrusor muscle contraction. This is mediated by acetylcholine acting on muscarinic receptors at the level of the bladder. The bladder contains both M2 and M3 muscarinic receptor subtypes. Although the M2 subtype is more abundant, it is the M3 subtype which is mainly responsible for bladder contraction (Andersson 2002). The rationale for using anticholinergic drugs in the treatment of overactive bladder syndrome is to block the parasympathetic acetylcholine pathway and thus abolish or reduce the intensity of detrusor muscle contraction. For the purpose of this review, the term 'anticholinergic' will refer to both anticholinergic (inhibiting the action of acetylcholine) and antimuscarinic (muscarinic receptor antagonist) drugs as the common goal is to block acetylcholine transmission at receptor level.
The two main treatment options for overactive bladder are pharmacotherapy and conservative management (eg bladder training or electrical stimulation) or a combination of both. This Cochrane review is confined to drug treatment. One Cochrane review is available for bladder training (Wallace 2004) and two reviews on electrical stimulation are at protocol stage (Berghmans 2004; Herbison 2003) . There is limited evidence to suggest bladder training may be helpful in overactive bladder, however this is a tentative conclusion based on small trials of variable quality (Wallace 2004). The drug treatment with most supporting evidence is the anticholinergic class of drugs. In the recent published Cochrane review comparing anticholinergic medications with placebo, anticholinergics were found to result in statistically significant improvements in symptoms (Nabi 2006).
Anticholinergic drugs have been available in the treatment of overactive bladder for over thirty years and their use is widespread in clinical practice. The number of anticholinergic drugs available on the market is increasing and effectiveness has been assessed in both observational and randomised controlled trials (Thuroff 1991;Van Kerrebroeck 1998). However, in a recent Cochrane review comparing different anticholinergics in overactive bladder only two drugs (oxybutynin and tolterodine) had sufficient supporting evidence from which conclusions could be drawn as to the most effective anticholinergic available (Hay‐Smith 2005). There are also questions regarding the role of anticholinergics in different patient groups (e.g. the elderly, men and women) and the best route of administration. Despite these uncertainties, anticholinergics are increasingly being used in primary and secondary care settings particularly for the treatment of urge incontinence, and this has considerable resource implications (Kobelt 1997). The International Consultation on Incontinence recommends anticholinergics as first line pharmacotherapy in urge incontinence for men and women, the elderly and patients with detrusor hyper‐reflexia. Specialist algorithms using urodynamic studies are suggested before other treatment interventions are considered (ICI 2000).
The main disadvantage in the routine use of anticholinergic medications is the side effect profile of these drugs. While modern day anticholinergic drugs have less side‐effects in comparison with older anticholinergics, dry mouth is still a common adverse effect reported in almost one third of trial participants (Nabi 2006). Moreover, there is much scope for improving treatment in overactive bladder as only 56% of patients on anticholinergics report cure or improvement versus 41% in the placebo group. The anticholinergics versus placebo review also concluded little was known about long term effectiveness of these drugs.
Cure rates are still disappointingly low in this prevalent condition. As almost half of patients do not benefit from anticholinergics, clearly much is still to be done to improve outcomes. New treatments for overactive bladder may improve cure rates. These treatments should be assessed in comparison to existing standards such as anticholinergic drugs. This review seeks to compare the other medications available for overactive bladder with anticholinergic medications.
Four Cochrane reviews will consider anticholinergic drugs for urinary voiding problems. Three published reviews, (Anticholinergics versus placebo for overactive bladder Nabi 2006; Which anticholinergic for overactive bladder? Hay‐Smith 2005; Anticholinergics versus non‐drug therapies for overactive bladder Alhasso 2006); and the current review. This review compares anticholinergic drugs with other types or classes of drugs for treating overactive bladder syndromes. The group of anticholinergic/antimuscarinic medications included emperonium bromide, oxybutynin chloride, propantheline bromide, propiverine, tolterodine and trospium chloride. Terodiline, an anticholinergic previously used in treatment of overactive bladder was excluded as it has been withdrawn from the market due to its association with ventricular tachyarrythmias. Other drugs that have also been used in overactive bladder syndrome can have both peripheral and central action (Andersson 2000). We grouped them as follows:
(1) Tricyclic antidepressants and monoamine reuptake inhibitors: e.g. imipramine, amitryptyline and duloxetine. They have multiple sites of action including: peripheral anticholinergic, norepinephrine reuptake blockage, central effects inhibiting micturition through blockage of norepinephrine and serotonin, and action at Onuf's nucleus via 5‐HT1A receptors. The secondary anticholinergic effects of tricyclic antidepressants are less direct than conventional anticholinergics. The main mechanism of action of these drugs is thought to be mediated via other sites of action. This class of drug was excluded from the anticholinergic groups in the other Cochrane reviews (Hay‐Smith 2005; Nabi 2006) for these reasons. Imipramine is used with success in the treatment of nocturnal enuresis in children (Hunsballe 2001), has been reported to confer benefit in the treatment of urinary incontinence in the elderly (Castleden 1986), but is not in widespread clinical use for overactive bladder symptoms at present.
(2) Afferent nerve inhibitors: e.g. Lidocaine, Dimethyl sulphoxide DMSO, capsaicin and resiniferotoxin, which all act as local anaesthetics and vanilloid receptor agonists, thereby reducing afferent input which might otherwise trigger micturition. None of these drugs are currently in widespread use. Capsaicin has been administered intravesically with success in neurogenic bladder patients but the effect may only last for several months (2nd ICI 2001). Resiniferotoxin has previously produced encouraging results in overactive bladder and detrusor hyperreflexia (Cruz 1997; Lazzeri 1997), however this drug is not in current clinical use (2nd ICI 2001).
(3) Botulinum‐A toxin: This drug selectively blocks the parasympathetic nerve transmission to the detrusor muscle at the local bladder level. The use of intravesical botulinum toxin is increasing especially in resistant cases of neurogenic and idiopathic overactive bladder with encouraging results (Dmochowski 2007).
(4) Alpha‐adrenergic antagonists: e.g. tamsulosin, alfuzosin and doxazosin which act by reducing bladder outlet resistance but may also act centrally to inhibit micturition. This class of drugs has previously been observed to improve bladder overactivity in men with benign prostatic hyperplasia (Eri 1995). Alpha‐blockers when administered long‐term in neurogenic bladders due to suprasacral spinal injury have been observed to significantly improve urodynamic parameters and symptoms (Abrams 2003). The role of this class of drugs in overactive bladder is unclear at present.
(5) Flavoxate: The main mechanism of action of this drug is not yet fully established. It has been found to have a moderate calcium channel blocking activity, a local anaesthetic activity, as well as an ability to block phosphodiesterase. No anticholinergic activity has been demonstrated. This drug is no longer in widespread clinical use in overactive bladder and at least two randomised controlled trials have demonstrated no benefit versus placebo (Chapple 1990; Dahm 1995). The drug is included in this review for completeness.
(6) Others: This group includes calcium channel blockers like verapamil, potassium channel openers like pinacidil and cromakalim and Gamma‐aminobutyric acid GABA agonists like baclofen. Verapamil has previously been reported to increase bladder capacity in detrusor hyper‐reflexia when instilled intravesically (Mattiasson A), but the drug is not in widespread clinical use at present. Oral nifedipine has been used as prophylaxis for autonomic hyperreflexia during bladder instrumentation with success (Wein 2001). Potassium channel blockers such as cromakalim and pinacidil have been effective in animal models but have not been studied in human overactive bladder. Baclofen has been described in detrusor hyperreflexia but other published evidence is sparse. These drugs are not in current clinical use in overactive bladder (2nd ICI 2001).
Objectives
To determine the effects of anticholinergic drugs compared with other forms of medication in the treatment of overactive bladder syndrome which may or may not include urinary incontinence.
The following hypotheses were addressed. (1) Anticholinergic drugs are better than tricyclic antidepressants and monoamine reuptake inhibitors in the management of overactive bladder syndrome. (2) Anticholinergic drugs are better than afferent nerve inhibitors in the management of overactive bladder syndrome. (3) Anticholinergic drugs are better than Botulinum‐A toxin in the management of overactive bladder syndrome. (4) Anticholinergic drugs are better than alpha adrenergic agonists in the management of overactive bladder syndrome. (5) Anticholinergic drugs are better than flavoxate in the management of overactive bladder syndrome. (6) Anticholinergic drugs are better than "other drugs" as listed in (6) above in the management of overactive bladder syndrome.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials comparing anticholinergic drugs with other drugs for the treatment of overactive bladder symptoms.
Types of participants
All men and women with overactive bladder syndrome.
Types of interventions
At least one arm of the study used an anticholinergic drug and at least one other arm had to use a non‐anticholinergic drug.
Types of outcome measures
The primary measure of outcome was the number of participants whose symptoms were not 'cured' while on treatment. This outcome measure was based on the individual trial authors definition of cure and could be drawn from objective or subjective criteria. Data for the following outcomes were sought:
A. Participant's observations B. Quantification of symptoms C. Clinician's observations D. Quality of life E. Socioeconomic measures F. Adverse events G. Other outcomes
Search methods for identification of studies
This review has drawn on the search strategy developed for the Cochrane Incontinence Review Group (Please see the ‘Specialized Register’ section of the Group’s module in The Cochrane Library). Relevant trials were identified from the Group's Specialised Register of controlled trials which is described, along with the search strategy, under the Incontinence Group's details in The Cochrane Library. The register contains trials identified from MEDLINE, CINAHL, The Cochrane Central Register of Controlled Trials (CENTRAL) and handsearching of journals and conference proceedings. The Incontinence Group Specialised Trials Register was searched using the Group's own keyword system, the search terms used were:
topic.urine.incon* AND ({design.cct*} OR {design.rct*}) AND ({intvent.chem.drug.anticholinergic*} or {relevant.review.anticholinergicVsOtherDrugs}) (All searches were of the keyword field of Reference Manager 9.5 N, ISI ResearchSoft).
Date of the most recent search of the register for this review: 20 December 2006.
The trials in the Incontinence Group Specialised Trials Register are also contained in CENTRAL.
For this review extra specific searches were performed. These are detailed below.
We searched the reference lists of relevant articles for other possible relevant trials.
We did not impose any language or other limits on the searches.
Data collection and analysis
Trials considered for inclusion in the review were assessed independently for their appropriateness by two review authors without prior consideration of their results. Any disagreements that could not be resolved by discussion were considered by a third person.
Assessment of methodological quality (potential for bias) was undertaken by each review author using the Incontinence Group's assessment criteria, which include quality of random allocation and allocation concealment, description of dropouts and withdrawals, analysis by intention to treat, and blinding during treatment and at outcome assessment.
Data was independently abstracted by at least two review authors and cross‐checked. Where data were collected but are not reported, further clarification was sought from the trialists. One author was contacted to clarify information on allocation concealment and randomisation not included in the published paper (Serels 1998).
Included trial data was processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006). Where appropriate, data was combined quantitatively using the Cochrane statistical package. Synthesis used a fixed effects model. We report relative risks (RR) for dichotomous data and weighted mean differences (WMD) for continuous data, accompanied by 95% confidence intervals (CI). Evidence of heterogeneity across studies was determined from visual inspection of the data and from the chi squared test for heterogeneity (at 10%) and the I squared test. If evidence of significant heterogeneity was identified, potential sources of heterogeneity were explored within populations, interventions, outcomes and settings as data permitted. As sufficient information was not reported to allow inclusion in the meta‐analysis, a narrative overview was undertaken of Crossover trials. Sensitivity analyses to assess the impact of study quality was not performed due to limited data available. Planned subgroup analyses to consider differences within types of voiding problem, particularly incontinence compared with other symptoms, was not possible due to the small number of trials, under‐reporting of individual symptoms, and varying definitions of symptoms.
Results
Description of studies
This systematic review included all identified studies making a comparison between anticholinergic and 'other drugs' meeting the review's inclusion criteria. It should be noted that some of the drugs are no longer in everyday clinical use (eg flavoxate) but are included here for completeness. It should also be noted that the anticholinergic drugs used for comparison in some older studies are no longer in regular clinical use but are again included here (eg emepronium bromide).
Twenty‐nine possibly eligible studies were identified. Of these, 17 were excluded from the review (Aagard 1983; Andersen 1988; Athanasopoulos 2002; Barnick 1991; Beisland 1985; Bradley 1970; Clark 1996; Ekstrom 1990; Gruneberger 1984; Homma 1997; Lee 2005; Lukkarinen 1987; Robinson 1983; Sole 1984; Tammela 1999; Zeegers 1987). The reasons for exclusion are given in the table of excluded studies. Common reasons for exclusion were that the drug had been banned (eg terodiline following association with ventricular tachyarrythmias), or that the study used a combination of drugs in one treatment arm; thus the non‐anticholinergic drug was not assessed independently. Of the 12 trials included in the review there were seven crossover trials (Cardozo 1979; Meyhoff 1981; Milani 1993; Riva 1989; Serels 1998; Stanton 1973; Wehnert 1989) and five parallel group studies (Enzelsberger 1991; Frohlich 1998; Gaudenz 1978; Herbst 1970; Takayasu 1990).
Five of the 12 studies included in the review were published after 1990, four were published in the 1980s and three were originally published in the 1970s.
Of the studies used in the review nine trials compared flavoxate with an anticholinergic (three parallel group ‐ Gaudenz 1978; Herbst 1970; Takayasu 1990; and six crossover studies Cardozo 1979; Milani 1993; Meyhoff 1981; Riva 1989; Stanton 1973; Wehnert 1989). Of the remaining trials, one compared anticholinergic medication with the calcium channel blocker verapamil (Frohlich 1998). Another crossover trial used alpha‐blockade with doxazosin as the comparison (Serels 1998). One crossover trial compared intramuscular injection of tricyclic antidepressant imipramine with anticholinergic therapy as well as flavoxate (Cardozo 1979). Enzelberger et al (Enzelsberger 1991) compared intravesical lidocaine gel with oral emepronium.
Some studies did not state the age range for trial participants. The known age range for all studies is 17 to 91 years. Several trials included males although most studies used a female population only. One study did not state the sex of participants (Wehnert 1989). Sample sizes ranged from 15 to 225 participants. Of the anticholinergic versus flavoxate studies (crossover and parallel group), there were 320 women and 146 men, and 224 who took anticholinergics and 222 took flavoxate. For tricyclic antidepressants versus anticholinergics there were 10 women involved in a crossover trial all of whom took both imipramine and anticholinergic (Cardozo 1979). The single parallel group trial comparing verapamil versus anticholinergics included 22 men and 20 women (verapamil n = 21 and oxybutynin n = 21) (Frohlich 1998). Thirty‐one women were included in the crossover trial comparing alpha adrenergic antagonists and anticholinergics (doxazosin n = 25, hyoscyamine n = 31) (Serels 1998). Thirty women were included in the parallel group study comparing intravesical lidocaine and anticholinergics (lidocaine n = 15, emepronium n = 15) (Enzelsberger 1991) .
Of the included studies, three were published in German (Enzelsberger 1991; Frohlich 1998; Wehnert 1989) and one in Japanese (Takayasu 1990). Data was abstracted with the aid of people fluent in these languages.
Inclusion and/or exclusion criteria were generally well defined and in some cases included objective assessment with urodynamic testing. However, some studies included participants with known urinary calculi, post‐prostatectomy patients, and patients with known urinary tract infections. Others actively excluded people with urinary tract infections. Patients with known neurological disease as a basis for their symptoms were again excluded from some trials but included in others. More information about the inclusion/exclusion criteria used by each trial is given in the table of 'Characteristics of Included Studies'.
Crossover trials There are seven crossover trials included in the review. None were suitable for meta‐analysis using RevMan as the required data for crossover trial analysis was not provided (no marginal totals for binary data, no mean values and, or, standard deviations for continuous data), nor did these trials demonstrate the correct statistical analysis had been undertaken (no paired t test).
One of these studies compared an alpha adrenergic antagonist with anticholinergic (Serels 1998). Although not stated in the published paper, the author was contacted to confirm there had been adequate allocation concealment and randomisation. There was no specified run‐in or washout between each of the medications in this study.
Five crossover trials compared flavoxate and an anticholinergics. In two trials (Meyhoff 1981; Milani 1993) patients had a seven day washout between courses of either drug, however, no run‐in was specified in either trial. Three other trials compared flavoxate with anticholinergic medications (Riva 1989; Stanton 1973; Wehnert 1989). One trial prescribed twenty women each medication for four weeks with a two week washout period between each course (Riva 1989). Seven patients dropped out of the study. Wehnert 1989 et al did not state any run in or washout period in their randomised study and measured urodynamic parameters only. Several patients suffered side effects but the authors do not provide figures. One further randomised trial (Stanton 1973) employed a one week washout period between two week courses of each drug. This trial included patients with neurological disease and urinary tract infections. The final crossover trial compared imipramine (tricyclic antidepressant), flavoxate and emepronium (anticholinergic). This trial was conducted under laboratory conditions with one‐off intramuscular doses of each drug with cystometric analysis 30 minutes later. The washout period was variable with a minimum of 30 minutes between drugs (Cardozo 1979).
Parallel group trials Three parallel group randomised trials compared flavoxate with anticholinergic medication. One study with 225 participants randomised patients into two treatment groups of flavoxate and an anticholinergic (Takayasu 1990). The study duration was two weeks. In the second of these trials, patients were randomised to take one of four medications for twelve weeks (emepronium bromide, propantheline, flavoxate or placebo) (Gaudenz 1978). In another trial which included patients post prostatectomy, those with urinary calculi, cystitis and prostatitis, patients were placed randomly into two treatment groups with 11 out of 21 patients in one group receiving flavoxate alone, the rest receiving flavoxate in addition to antibiotic or other medication (Herbst 1970). In the other treatment group 12 out of 22 patients received propantheline alone whilst the remainder had a second drug in addition to the anticholinergic. This trial had no objective inclusion criteria (i.e. urodynamic assessment) and the length of the trial was seven days.
One randomised placebo‐controlled parallel group trial compared intravesical verapamil with the intravesical anticholinergics, oxybutynin and trospium chloride (Frohlich 1998). This study was performed single blinded (to the patient) under laboratory conditions with one‐off doses of each drug. This study also included post prostatectomy patients and those with neurological disease and urinary tract infections. Another compared intravesical lidocaine with oral emepronium (Enzelsberger 1991). Patients were treated with each medication for 30 days. Participants with illness involving the central nervous system were excluded.
Risk of bias in included studies
The methods for the individual trials is summarised in the table of "Characteristics of Included Studies".
Allocation concealment The study methods were not described in detail in any of the thirteen trials included in the review. One trial used coded packages of drugs (Herbst 1970) to ensure adequate allocation concealment. We have assumed that if an author states that a trial was randomised, double blind and placebo controlled then allocation concealment was classed as A (adequate allocation concealment). Those trials that do not state whether there was any allocation concealment or those which were stated as single blind (Frohlich 1998) were classed as B (unclear) or C (inadequate). Based on this classification, seven trials were classed A and five trials classed B. One of the trials was unclear regarding randomisation and allocation concealment and in this case the author was contacted and necessary information obtained via e‐mail (Serels 1998).
Power calculation and intention to treat None of the studies stated whether there had been power calculation or intention to treat analysis. In terms of intention to treat analysis, if a study had no dropouts, then the results may have been analysed with intention to treat. However it should be acknowledged that in the older studies, details of drop‐outs may simply have been omitted from published data. Four studies had no dropouts (Cardozo 1979; Riva 1989; Serels 1998 and Stanton 1973). For the remaining trials intention to treat analysis was unclear (Enzelsberger 1991; Herbst 1970; Riva 1989) or did not appear to have been undertaken (Frohlich 1998; Gaudenz 1978; Milani 1993; Takayasu 1990; Wehnert 1989).
Adverse effects Side effects and dropouts were generally well reported. Two trials did not give clear information on the number of adverse effects or dropouts in the trial (Enzelsberger 1991; Wehnert 1989). The remaining 10 trials included figures for adverse effects. This included two studies (Cardozo 1979; Frohlich 1998) that were performed as one‐off dosing under laboratory conditions with no demonstrable adverse effects in one study (Frohlich 1998). However it is apparent that any potential delayed adverse effects of either drug would not be observed in these studies. Eight of the trials used objective assessment with urodynamics to grade response to treatment. Nine trials used a form of symptomatic assessment with only one study (Serels 1998) using a recognised scoring system (AUA symptom score).
Statistical significance Eleven studies quoted p values or stated statistical significance was or was not present. One study did not mention whether results were significant or not (Herbst 1970).
Statistical analysis None of the seven crossover trials undertook an appropriate statistical analysis using a paired t test or presented the data required to allow statistical analysis within the review (i.e. marginal totals for binary data or standard deviations for continuous data etc) and were thus excluded from meta‐analysis (Cardozo 1979; Meyhoff 1981; Milani 1993; Serels 1998; Stanton 1973; Riva 1989Wehnert 1989). One trial (Cardozo 1979) did not provide standard deviations when reporting continuous data.
Pre‐specified outcomes and definition of cure In terms of the pre‐specified outcome measures assessed by the review, three trials provided subjective data (number cured, improved etc) on symptomatic assessment derived from patient's or physician's observations (Enzelsberger 1991; Gaudenz 1978; Takayasu 1990) and four trials provided objective data that was possible to use in the meta‐analysis (Enzelsberger 1991; Frohlich 1998; Gaudenz 1978; Herbst 1970). Only two of the studies (Enzelsberger 1991; Takayasu 1990) provided a definition of cure. None of the above trials provided any outcome measures that assessed socioeconomic viability, general health status or psychological status.
Effects of interventions
Hypothesis 1: Anticholinergic drugs are better than tricyclic antidepressants and monoamine reuptake inhibitors One randomised crossover trial was identified (Cardozo 1979) comparing one‐off intramuscular injection of imipramine (tricyclic antidepressant) with intramuscular emepronium. Fifteen female patients were studied with three different drugs (imipramine, emepronium bromide and flavoxate). A single dose of one of the drugs was given intravenously and depending on whether or not cystometric changes occurred a second drug was administered after 30 minutes or they were asked to return at a later date. Ten patients received the three drugs.
Only objective outcomes were assessed with urodynamic assessment at 10 and 30 minutes post‐drug administration. Emepronium led to a clinically significant improvement in urodynamic parameters whereas imipramine did not. Four of the 10 patients in the anticholinergic group experienced adverse effects. No adverse effects were reported in the imipramine group.
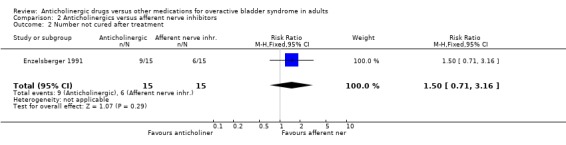
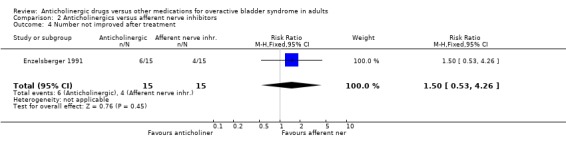
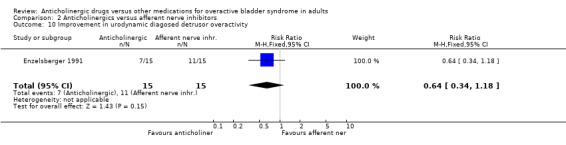
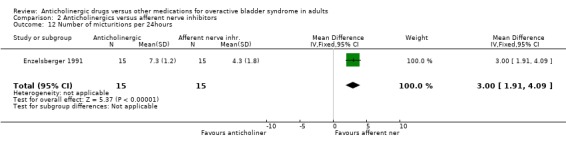
Hypothesis 2: Anticholinergic drugs are better than afferent nerve inhibitors One parallel group trial was identified (Enzelsberger 1991) which compared oral emepronium bromide (200 mg three times daily) with intravesical lidocaine gel (administered in 6 ml ampoules via a bladder catheter for four consecutive days followed by three days off) for a period of four weeks. Two groups of 15 female patients were assessed. The intravesical lidocaine gel group had fewer patients not improved (4 out of 15 versus 6 out of 15; comparison 02.04; RR 1.50 95% CI 0.53, 4.26) or cured (9 out of 15 versus 6 out of 15; comparison 02.02; RR 1.50 95% CI 0.71, 3.16) in comparison to oral emepronium though these differences were not significant. The mean number of micturitions was statistically significantly higher in the anticholinergic group (MD 3.00 micturitions in 24 hours; 95% CI 1.91 to 4.09 comparison 02.12). The number with improvement in urodynamic diagnosed detrusor overactivity favoured, though not statistically, the intravesical lidocaine gel group (7 out of 15 versus 11 out of 15; comparison 02.10; RR 0.64 95% CI 0.34, 1.18). No dropouts or adverse effects were reported. Firm conclusions are tenuous as the trial's power is low, study length is short and the routes of drug administration are different (intravesical versus oral).
Hypothesis 3: Anticholinergic drugs are better than Botulinum‐A toxin etc. No eligible trials were identified.
Hypothesis 4: Anticholinergic drugs are better than alpha adrenergic antagonists One crossover trial was identified (Serels 1998). Thirty‐one of the 34 women in the trial took hyoscyamine 0.375 mg twice daily and 25 women took 2 mg doxazosin four times daily for a minimum of one month on each treatment. Thirteen women took a combination of both drugs. Some patients who had a good response to treatment did not wish to cross over to the other drug. These patients were included in the authors analysis. This is the reason for different sized treatment groups in this crossover study. Mean improvements in AUA symptom score was similar in both groups: 34% for hyoscyamine versus 30% for doxazosin. A higher incidence of adverse effects was seen in the anticholinergic group. No statistically significant differences in urodynamic parameters were reported. The study suggests those who failed to respond to one medication responded to the other (50% of women not responding to hyoscyamine responded to the introduction of doxazosin whilst 38% of women not responding to doxazosin improved with hyoscyamine).
Hypothesis 5: Anticholinergic drugs are better than flavoxate Nine eligible trials were identified, six crossover trials (Cardozo 1979; Meyhoff 1981; Milani 1993; Riva 1989; Stanton 1973; Wehnert 1989) and three parallel group trials (Gaudenz 1978; Herbst 1970; Takayasu 1990). Two trials compared flavoxate with oxybutynin (Milani 1993; Riva 1989), three with propantheline (Gaudenz 1978; Herbst 1970; Takayasu 1990), three with emepronium (Cardozo 1979; Gaudenz 1978; Stanton 1973) and one with propiverine (Wehnert 1989). For meta analysis, the comparison between flavoxate and propantheline was abstracted from the Gaudenz trial. Whilst nine trials assessed oral medications only, one trial compared the parenteral administration of flavoxate with emepronium (Cardozo 1979).
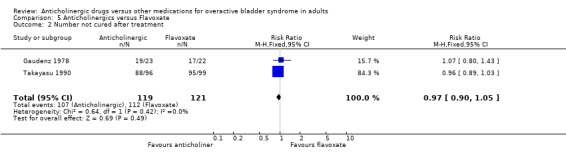
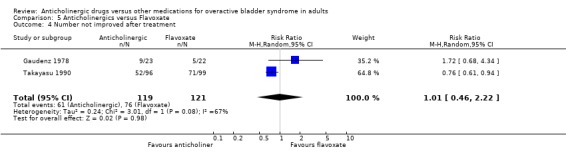
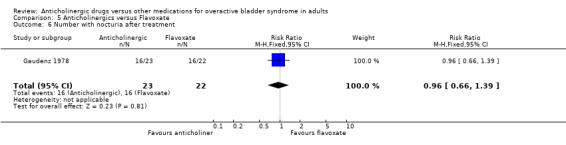
No evidence of a difference was found in the subjective cure rates after treatment in two trials included in the meta‐analysis (Gaudenz 1978; Takayasu 1990) (RR 0.97; 95% CI 0.90 to 1.05 comparison 05.02); or in the subjective improvement rate (RR 1.01; 95% CI 0.46 to 2.22 comparison 05.04). There was evidence of heterogeniety between trials for the latter comparison. The participants in these two studies differed: one study (Gaudenz 1978) had exclusively female participants in a European population and the other study (Takayasu 1990) included both male and female participants drawn from a Japanese population. Based upon one small trial (Gaudenz 1978) there was no evidence of a difference in the number with nocturia after treatment (RR 0.96; 95% CI 0.66 to 1.39 comparison 05.06). Two trials reported results of symptomatic assessment favouring the use of flavoxate (Herbst 1970; Stanton 1973). It is worth noting that these trials favouring flavoxate were published in the early 1970s. Four trials (Gaudenz 1978; Meyhoff 1981; Riva 1989; Wehnert 1989) reported that there were no statistically significant differences between flavoxate and anticholinergic drugs. Gaudenz 1978 found more patients preferred flavoxate although objective assessment with urodynamics was equivocal. Milani 1993 et al found flavoxate was the preferred drug. Two crossover trials (Cardozo 1979; Milani 1993) reported favourable results for anticholinergics.
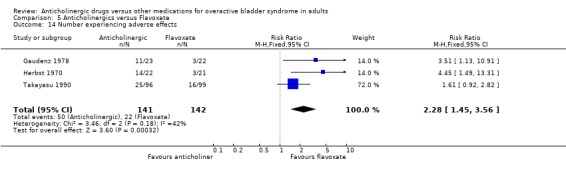
Based on three studies (Gaudenz 1978; Herbst 1970; Takayasu 1990) included in the meta‐analysis adverse effects were generally worse in the anticholinergic groups (RR 2.28 95% CI 1.45 to 3.56 comparison 5.14). Reported adverse events included dryness of mouth, dizziness, nausea, blurred vision, swelling of lips, diarrhoea and constipation. Four crossover trials (Cardozo 1979; Milani 1993; Riva 1989; Stanton 1973) stated in the reports of the trials that there were significant differences between flavoxate and the anticholinergic drug with more adverse effects reported in the anticholinergic group. A further crossover trial (Meyhoff 1981) stated that there was an increase in adverse effects in the anticholinergic group; the differences between flavoxate and anticholinergics, no comment was made on statistical significance.
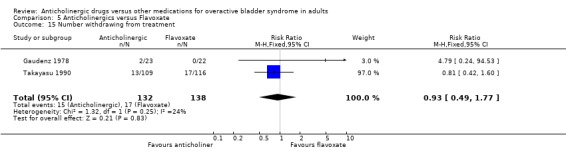
The combined results of two trials showed no evidence of a difference in the number of patients withdrawing between anticholinergics and flavoxate (RR 0.93 95% CI 0.49 to 1.77 comparison 5.15) (Gaudenz 1978; Takayasu 1990). No dropouts were reported in four trials (Herbst 1970; Meyhoff 1981; Riva 1989; Stanton 1973). Wehnert 1989 et al did not provide figures for adverse effects or dropouts.
Hypothesis 6: Anticholinergic drugs are better than "other drugs" as listed in (6) above One clinical trial was identified which compared the intra‐vesically administered calcium channel blocker verapamil with an anticholinergic (Frohlich 1998). One‐off doses of the anticholinergics trospium chloride and oxybutynin as well as the calcium channel blocker verapamil and a placebo were administered in a parallel group study. There were 84 participants with equal sex distribution. The study included neurological disease, post trans‐urethral resection of prostate (TURP) patients and those with recurrent urinary tract infections. Only urodynamic parameters were assessed 30 minutes post‐drug administration. The anticholinergic medications led to a significant improvement in urodynamic parameters. Verapamil had no significant effect versus placebo. No adverse effects were reported.
Discussion
There were 12 eligible trials used in this review. Of the 12 studies, five provided data that could be used for potential meta‐analysis (Enzelsberger 1991; Frohlich 1998; Gaudenz 1978; Herbst 1970; Takayasu 1990). Hypothesis 5 was the only comparison for which there is more than one published trial to allow meta‐analysis. None of the seven crossover trials in the review provided information suitable for meta‐analysis for reasons already given. Five different anticholinergics were used in the anticholinergic arm of the twelve trials: emepronium in four studies, oxybutynin in four studies, propantheline in three studies, hyoscyamine in one study and propiverine in one study. Pre‐planned subgroup analysis was not possible due to under‐reporting of outcomes and low number of studies.
Based on a single crossover study performed under laboratory conditions in 10 patients there is no evidence to suggest tricyclic antidepressants are better or worse than anticholinergics in overactive bladder when administered parenterally (Cardozo 1979). This study employed one‐off dosing of drugs and assessed for urodynamic effects at 10 and 30 minutes post dose. The authors do not make any adjustments for the variation in washout period and no consideration is given to potential combined or synergistic effects in the patients receiving the second drug after only 30 minutes. The data were not presented in a way that allowed clarification of this. Little is known about the drugs pharmacokinetics of imipramine in overactive bladder when administered parenterally. Certainly, an immediate response was not seen; however, the authors do not consider whether a delayed effect exists. The study was of low statistical power and larger, randomised, controlled trials performed in a clinical setting would be required to further establish the role of tricyclic antidepressants in overactive bladder.
The single parallel group study with 30 female participants, involving the afferent nerve inhibitor lidocaine, reported significantly better urodynamic results in the lidocaine group (Enzelsberger 1991). This study drew comparisons between lidocaine given via bladder catheter and oral emepronium. A high placebo effect from drugs administered in overactive bladder is well documented (Nabi 2006). Potential differences in placebo effects of the two routes of administration were not considered. Intravesical drug administration could be considered a more invasive or aggressive treatment by the patient versus oral drugs. This is a potential source of bias and could provide an explanation for the difference in results. In addition, afferent nerve inhibitors have previously been observed to diminish in effectiveness after several months treatment (eg capsaicin) (2nd ICI 2001). This trial was 30 days duration and long‐term effects of lidocaine are not known. The trial was of low statistical power with 15 patients in each treatment group. Conclusions are tenuous but the results do suggest this is an area requiring further research to fully establish the role of afferent nerve inhibitors in overactive bladder.
In a single crossover study comparing the use of the anticholinergic hyoscyamine and the alpha receptor antagonist doxazosin, both drugs led to a significant improvement in symptom scores (Serels 1998). There was no significant difference between the anticholinergic group and the alpha blocker groups. The trial was a crossover study and washout periods study duration variable. One of the drugs was taken for a minimum of one month over a two year period. Some patients did not crossover to the other drug through individual choice and the author includes these patients in the results. The potential bias from variable treatment times has not been addressed by the authors. It was also noted that 50% patients not responding to the anticholinergic drug responded to doxazosin. Moreover, side effects were reported more often in the anticholinergic group. Whist the study methods are open to criticism this study suggests there may be a role for doxazosin in overactive bladder, possibly in patients not responding to anticholinergic drugs. The role of alpha receptor antagonists in this condition remains to be fully elucidated.
Flavoxate is no longer in use in overactive bladder and two large randomised trials have shown flavoxate to have no beneficial effect versus placebo in treatment of incontinence (Chapple 1990; Dahm 1995). The review of nine studies comparing flavoxate and anticholinergics found results favoured the use of anticholinergics in preference to flavoxate for overactive bladder. However there were only four trials with significantly better clinical results for anticholinergics. Side effect profiles were worse in the anticholinergic groups. This may reflect use of some of the older anticholinergic drugs such as emepronium and propantheline which have well established side effect profiles. Modern day anticholinergic drugs for overactive bladder have fewer adverse effects and should these studies be repeated with modern drugs the number of side effects in the anticholinergic group would undoubtedly be lower. There is no evidence to suggest flavoxate should be used in treatment of overactive bladder.
Anticholinergic medication (oxybutynin) was significantly better than verapamil when administered intravesically in one‐off doses in a single trial (Frohlich 1998). There were no demonstrable improvements in urodynamic parameters with verapamil. This is a single parallel group study with patient groups numbering 21 individuals. The speed of onset and time to effectiveness of intravesical verapamil is not known. Single dose studies provide limited data and larger randomised controlled trials conducted in a clinical setting would be required to further elicit any place for verapamil in the management of overactive bladder. From published evidence there is no evidence currently to suggest a role in the management of overactive bladder.
Limitations Although drugs other than anticholinergics have been used in the treatment of overactive bladder for some years, it is clear there are few randomised controlled trials that make direct comparisons with anticholinergic medications. As anticholinergic drugs are the most commonly prescribed treatment in overactive bladder, it is perhaps surprising that new alternative treatments have not had more comparative studies with this current 'standard' treatment. The above trials span a period of almost 30 years, with the earliest included study originally published in 1970. There are clear differences between study design and statistical analysis undertaken in studies in the earlier days of evidence based medicine in comparison to modern methods. This is apparent in that most of the early crossover trials failed to undertake paired analysis as modern crossover trials would do.
This review has included comparisons involving flavoxate, a drug no longer in clinical use. Whilst the inclusion of flavoxate may not prove clinically relevant to modern day practice, the drug is included here for academic and historical interest as well as completeness. The review sought to compare anticholinergics with other medications and in achieving this goal older anticholinergic drugs such as emepronium and propantheline are used in several studies. These drugs are unlikely to return to clinical use as modern day anticholinergics have much improved side‐effect profiles.
Disappointingly, no studies were identified comparing botulinum toxin with anticholinergics. This drug is administered intravesically and unlike other drugs discussed here this is in current clinical use in overactive bladder. The lack of direct comparative evidence underlines the requirements for existing treatments for overactive bladder to be tested against another in randomised clinical trials. A wider systematic review of botulinum toxin has recently been published as a Cochrane Review (Duthie 2007).
It is also worth noting the difference in terminology used in the management of overactive bladder. The term overactive bladder is a relatively new term which has been defined by the International Continence Society (Abrams 2002). In this respect some of the older trials use terms such as "bladder spasm" which may seem self explanatory, but was not given a definition in the published paper (Herbst 1970). It is not clear whether the participants in these older trials would be diagnosed with overactive bladder using modern criteria. The modern definition of overactive bladder is based solely on patients symptoms with or without urge incontinence. Urodynamic studies are not required to make the diagnosis. Of the trials included in this review, five trials employed inclusion criteria requiring urodynamic evidence of detrusor instability (Cardozo 1979; Frohlich 1998; Gaudenz 1978; Meyhoff 1981; Wehnert 1989) and three trials excluded patients without incontinence (Cardozo 1979; Enzelsberger 1991; Wehnert 1989). These patients may have overactive bladder syndrome, however such symptoms and signs might reflect a more severe form of the condition. Any future studies should aim to stratify the patients appropriately to determine effectiveness of treatments on these subgroups. Along with modern day definitions, standardised symptom scoring has been developed. Only one of the trials (Serels 1998) used a recognised symptoms scoring chart (AUA Score).
The individual study methods differ significantly between trials included in the review. Two of the trials were performed under laboratory conditions using urodynamic assessment following one‐off dosing of drugs (Cardozo 1979; Frohlich 1998). In each case the route of administration was the same: intravesical (Frohlich 1998) or parenteral (Cardozo 1979). One trial made a comparison between two drugs with differing modes of administration: intravesical lidocaine and the oral anticholinergic (Enzelsberger 1991). Such different comparisons increased possibility of bias and no study listed here appears to have considered this.
Considerable placebo effects exist in prescribing for overactive bladder and none of the studies made allowances for this potential effect. Trials were generally of short duration and potential placebo effects may wear off with time. No data were available for long term effects of any of the drugs in this review.
There are several differences between the inclusion criteria for the trials that should be mentioned. One trial did not state specific criteria (Serels 1998). Neurological causes for overactive bladder were excluded from four of the trials (Enzelsberger 1991; Gaudenz 1978; Meyhoff 1981; Milani 1993) and included in two of the trials (Cardozo 1979; Wehnert 1989). The remaining trials did not mention neurological causes in their inclusion/exclusion criteria. Two trials (Frohlich 1998 and Herbst 1970) included patients with known prostatitis. One trial included patients with recurrent urinary tract infections (Frohlich 1998). Other trials included patients with diagnoses such as cystitis cystica (Stanton 1973), post‐TURP (Herbst 1970; Stanton 1973), urethral calculi (Herbst 1970).
Adequate and methodologically robust, randomised, controlled trials are lacking in the assessment of anticholinergics versus other medications in overactive bladder. Any future studies should be powered appropriately so as to draw sound conclusions. The adverse effects reported in the anticholinergic groups in the included studies should be noted with caution. Many trials are 20 to 30 years ago and anticholinergic medication has much improved side‐effect profiles in modern day practice. Future trials aimed at assessing anticholinergic drugs versus other medications for overactive bladder should employ modern anticholinergics with the lower side effect profiles. A number of the existing studies included in the review are crossover studies in which adequate statistical analysis has not been undertaken. Future studies using crossover methodology should make consideration to the statistical analysis which if performed correctly will also enable their data to be entered into meta‐analysis in systematic reviews.
In designing further trials, inclusion and exclusion criteria must be clear. Stratification of patient subgroups may identify pathologies or conditions in which different drug regimens may be more or less appropriate. From the studies included in this review, the inclusion criteria vary significantly with some studies actively excluding patients that others include. In addition, the application of universally recognised symptom scores and other subjective measurements as well as objective criteria such as urodynamic assessment will allow closer and more meaningful comparison of outcomes in future studies. When planning future studies, it may be more appropriate to ensure the route of administration is the same or to control for this difference. A patient's subjective views of use of intravesical medication may be very different from the non‐invasive and simple oral administration of a drug.
Drugs that may merit further study based on the published evidence included in this review include afferent nerve inhibitors and alpha antagonists. New medications such as botulinum toxin have no published comparisons with anticholinergics. New drugs available should be tested against existing standards and at present anticholinergics are the most wide‐spread drugs prescribed. In addition as yet no research has been carried out to assess socioeconomic or quality of life factors in comparing anticholinergics versus other medications in overactive bladder. There is clearly more work to be done in assessing efficacy, effectiveness, cost‐effectiveness and safety of drugs in overactive bladder.
Authors' conclusions
Implications for practice.
In this review we attempt to make several different comparisons in an area where there are few published trials available. The trials included here are of only moderate quality and all have small numbers of participants. In small trials of this nature there is a danger of both bias and imprecision and firm conclusions often cannot be drawn. In addition, a number of the studies are crossover trials which creates difficulties in meta‐analysis. None of the crossover trials had undertaken appropriate statistical tests as to allow the data to be used in this review's analysis. Thus, there is essentially no evidence in this review to suggest that any of the other drugs available in the management of overactive bladder are better or worse than anticholinergic medications.
One low power trial reported a non‐significant improvement in cure rates with intravesical lidocaine versus oral emepronium, but it is evident further large randomised controlled trials would be required to establish this drug's use. A single trial also suggests a potential role for alpha receptor antagonists in overactive bladder; however, the trial design and methods make firm conclusions tenuous. Further research is needed. Anticholinergic drugs had a worse side effect profile in most studies and this resulted in more patients discontinuing the anticholinergic drug than the other drug assessed. However, modern‐day anticholinergic drugs used in overactive bladder do have a better side effect profile and so it is likely that fewer adverse effects would be seen if further trials were undertaken.
Implications for research.
Larger randomised controlled trials versus anticholinergics and conducted in clinical settings are required to further establish the role of these other medications in the management of overactive bladder. There are very few trials at present and all are small. Some drugs with a growing evidence base and increasing use, such as botulinum toxin, did not have any published randomised controlled trials comparisons with anticholinergics. The inclusion criteria for the trials in this review were variable and studies focussed upon patient subgroups are needed. For example, patients with neurological causes for symptoms and those post prostatectomy should be studied. Better reporting of the definition of cure and a standardised approach to subjective assessment is needed with improved definitions and standard symptom scores. At present no comparison of socio‐economic or quality of life status has been made between anticholinergics and other medications.
What's new
| Date | Event | Description |
|---|---|---|
| 16 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 19 June 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank A Alhasso and L Stewart who were authors of the protocol. The authors also wish to thank Hans Glazener, Terumasa Matsuoka, Christiane Pflanz‐Sinclair and Neil Scott for translating non‐English papers. We thank Dr S. Serels for providing non‐published data for a study used in the review.
Data and analyses
Comparison 1. Anticholinergics versus tricyclics or monoamine inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number not cured during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number not cured after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number not improved during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number not improved after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with nocturia during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with nocturia after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of pad changes over 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number not cured on Urodynamics | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number of incontinent episodes over 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number with no Improvement in urodynamic diagnosed detrusor overactivity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Mean volume or weight of urine loss on pad test | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Number of micturitions per 24hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Frequency of sensation of urgency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Number experiencing adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Number withdrawing from treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Number changing dose of treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Anticholinergics versus afferent nerve inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number not cured during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number not cured after treatment | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.71, 3.16] |
| 3 Number not improved during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number not improved after treatment | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.53, 4.26] |
| 5 Number with nocturia during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with nocturia after treament | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of pad changes over 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number not cured on urodynamics | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number of incontinent episodes over 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Improvement in urodynamic diagosed detrusor overactivity | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.34, 1.18] |
| 11 Mean volume or weight of urine loss on pad test | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Number of micturitions per 24hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [1.91, 4.09] |
| 13 Frequency of sensation of urgency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Number experiencing adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Number withdrawing from treament | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Number changing dose of treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.2. Analysis.
Comparison 2 Anticholinergics versus afferent nerve inhibitors, Outcome 2 Number not cured after treatment.
2.4. Analysis.
Comparison 2 Anticholinergics versus afferent nerve inhibitors, Outcome 4 Number not improved after treatment.
2.10. Analysis.
Comparison 2 Anticholinergics versus afferent nerve inhibitors, Outcome 10 Improvement in urodynamic diagosed detrusor overactivity.
2.12. Analysis.
Comparison 2 Anticholinergics versus afferent nerve inhibitors, Outcome 12 Number of micturitions per 24hours.
Comparison 3. Anticholinergics versus Botulinum Toxin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number not cured during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number not cured after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number not improved during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number not improved after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with nocturia during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with nocturia after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of pad changes per 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number not cured on urodynamics | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number of incontinent episodes per 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Improvement in urodynamic diagnosed overactivity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Mean volume or weight urine loss on pad test | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Number of micturitions per 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Frequency of sensation of urgency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Number experiencing adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Number withdrawing from treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Number changing dose of treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Anticholinergics versus alpha adrenergic adonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number not cured during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number not cured after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number not improved during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number not improved after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with nocturia during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with nocturia after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of pad changes per 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number not cured on urodynamics | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number of incontinent episodes per 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Improvement in urodynamic diagnosed overactivity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Mean volume or weight urine loss on pad test | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Number of micturations per 24hrs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Frequency of sensation of urgency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Number experiencing adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Number withdrawing from treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Number changing dose or treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Anticholinergics versus Flavoxate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number not cured during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number not cured after treatment | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.05] |
| 3 Number not improved during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number not improved after treatment | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.46, 2.22] |
| 5 Number with nocturia during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with nocturia after treatment | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.39] |
| 7 Number of pad changes over 24 hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number not cured on urodynamics | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number of incontinent episodes per 24 hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number with no improvement in urodynamic diagnosed detrusor overactivity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Mean volume or weight urine loss on pad test | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Number of micturitions per 24 hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Frequency of sensation of urgency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Number experiencing adverse effects | 3 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.45, 3.56] |
| 15 Number withdrawing from treatment | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.49, 1.77] |
| 16 Number changing dose of treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.2. Analysis.
Comparison 5 Anticholinergics versus Flavoxate, Outcome 2 Number not cured after treatment.
5.4. Analysis.
Comparison 5 Anticholinergics versus Flavoxate, Outcome 4 Number not improved after treatment.
5.6. Analysis.
Comparison 5 Anticholinergics versus Flavoxate, Outcome 6 Number with nocturia after treatment.
5.14. Analysis.
Comparison 5 Anticholinergics versus Flavoxate, Outcome 14 Number experiencing adverse effects.
5.15. Analysis.
Comparison 5 Anticholinergics versus Flavoxate, Outcome 15 Number withdrawing from treatment.
Comparison 6. Anticholinergics versus other medications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number not cured during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number not cured after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number not improved during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number not improved after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with nocturia during treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with nocturia after treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of pad changes over 24 hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number not cured on urodynamics | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number of incontinent episodes per 24 hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number with no improvement in urodynamic diagnosed detrusor overactivity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Mean volume or weight urine loss on pad test | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Number of micturitions per 24 hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Frequency of sensation of urgency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Number experiencing adverse effects | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Number withdrawing from treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Number changing dose of treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.14. Analysis.
Comparison 6 Anticholinergics versus other medications, Outcome 14 Number experiencing adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cardozo 1979.
| Methods | Randomised crossover trial. Allocation concealment not stated. No run‐in, drugs given as one‐off dose, washout period variable (minimum 30 mins). Intention to treat analysis. Single centre British study. | |
| Participants | 15 female patients (age 21‐ 28). Inclusion criteria: Frequency, urgency or urge incontinance and diagnosis of detrusor instability on videocystourethr‐ography. Exclusion criteria: Not stated. | |
| Interventions | Single dose 50mg Intramuscular imipramine. Single dose 50mg Intramuscular emepronium. Single dose 200mg flavoxate hydrochloide intravenously | |
| Outcomes | Urodynamic outcomes (measuring 1st sensation filling pressures and bladder capacity) performed at 10 and 30 mins after administration of drug. | |
| Notes | Full text English Included patients with previous CVA or MS. Crossover trial ‐ not siutable for metaanalysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Enzelsberger 1991.
| Methods | Randomised parallel group trial. Allocation concealment not stated. Single centre in Germany. No run in/ 30 days treatment. Baseline comparison. | |
| Participants | 30 females Mean age 51.9 Diagnosis by bladder pressure studies and cystoscopy Inclusion criteria: Female with urge incontinance. Exclusion criteria: CNS Illness. | |
| Interventions | Group 1: (n=15) Lidocain gel (2 x 6ml ampoules) via bladder catheter ‐ 4 days then 3 days without for 3 weeks. Group 2 (n=15) Oral emepronium bromide 200mg 3x/day for 3 weeks. | |
| Outcomes | Number not cured after treatment, number not improved after treatment, number of micturitions over 24hrs, urodynamic parameters, depression quotient, nocturia. | |
| Notes | German translated. Dropouts not stated. No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Frohlich 1998.
| Methods | Randomised, controlled, parallel group study. Allocation concealment ‐ 'single blind'. 4 treatment arms. One off ‐ intravesical instillation with UDS before and 30 mins after. No run in stated. No intention to treat analysis. German single centre study. | |
| Participants | 84 participants (43 males and 41 females). Inclusion criteria: abnormal frequent urination with diagnosis by urodynamics. Exclusion criteria: patients on calcium channel blockers, those with a denervated bladder, severe cardiac, renal, hepatic disease, contraindication to anticholinergic, pregnancy. | |
| Interventions | Group 1 (n=21) Single dose intravesical oxybutynin 30mg. Group 2 (n=21) Single dose intravesical trospium chloride 40mg. Group 3 (n=21) Single dose intravesical verapamil 80mg Group 4 (n=21) Placebo | |
| Outcomes | Urodynamic parameters. | |
| Notes | German translated. No reported adverse effects. Included patients with prostatitis, post‐ TURP, neurological disease and recurrent UTI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gaudenz 1978.
| Methods | Randomised, controlled parallel group study. Allocation concealment not stated. No intention to treat analysis. Single centre in Switzerland. | |
| Participants | 85 female patients. Age range 17‐79yrs. Inclusion criteria: motor urge incontinance with uninhibited detrusor contractions exceeding 20cmH20 Exclusion criteria: neurogenic bladder, UTI | |
| Interventions | Group 1: (n=25) Emepronium 200mg 3x/day. Group 2: (n=22) Flavoxate 200mg 3x/day. Group 3: (n=23) Propantheline 30mg 3x/day. Group 4: (n=15) Placebo | |
| Outcomes | Side effects, patient views, symptomatic assessment, urodynamic parameters. | |
| Notes | Full text English. Included patients with previous operative treatment for incontinence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Herbst 1970.
| Methods | Randomised, parallel group trial. Allocation concealment. Single centre USA. No baseline comparison. Unclear if Intention to treat analysis. | |
| Participants | 43 patients (20 males 23 females) 75% of patients over 50. Inclusion criteria 'Bladder spasm'. Exclusion criteria: patients were not on other drugs capable of giving symptom relief. | |
| Interventions | Group 1: (n=21) 11 had flavoxate alone 200mg 4x/day, 10 had flavoxate in addition to antibiotics, diuretics, meperidine and a glucocorticoid. Group 2: (n=22) 12 patients had propantheline 30mg 4x/day alone, 10 patients had propantheline in addition to meperidine, codeine and antibiotics. | |
| Outcomes | Symptom assessment (poor/ fair/ good/ excellent), side effects. | |
| Notes | Full text English. Patient group also included prostatitis, urethral calculi, post prostatectomy. Small numbers, short time, no delineation between different subsections within groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Meyhoff 1981.
| Methods | Randomised, crossover trial. Allocation concealment. Single centre. No run in, 14 days on each drug with one week break between. Intention to treat analysis. Danish single centre study. | |
| Participants | 20 females Age range 22‐79yrs. Inclusion criteria: Urodynamic parameters, negative culture, residual volume <50ml, max urinary flow rate >15ml/s, no calculi. Exclusion: Neurological disease, detrusor sphincter dysynergia, glaucoma, severe heart failure, patients on medications affecting the autonomic nervous system or smooth muscle. | |
| Interventions | Group 1: Emepronium 200mg 4x/day. Group 2: Flavoxate 200mg 4x/day. Group 3: Placebo 4x/day. | |
| Outcomes | Assessed 2 days following completion of treatment. Frequency charts, patient preference, side effects. | |
| Notes | Full text English. Crossover trial ‐ not suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Milani 1993.
| Methods | Randomised crossover trial. Allocation concealment. 2 centres in Italy. No run‐in. 4 weeks of each drug with 7 day washout between. No intention to treat analysis. | |
| Participants | 50 Females Age range 19‐78 Inclusion criteria: Motor or sensory urgency according to International Continence Society criteria Exclusion criteria: Severely ill or overt neurological disease, UTIs, obstruction, pregnancy, taking concomittant medications. | |
| Interventions | Group 1: Flavoxate 400mg 3x/day. Group 2: Oxybutynin 5mg 3x/day. | |
| Outcomes | Assessed at 4 weeks. Urodynamic parameters, patient symptom evaluation, patients drug preference, side effects. | |
| Notes | Full text English. Crossover trial ‐ not suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Riva 1989.
| Methods | Randomised, crossover study. Allocation concealment. 4 weeks of each drug with 2 week washout between. Single centre Italian study. Intention to treat analysis unclear. | |
| Participants | 20 females. 7 withdrew (4 due to protocol violation, 3 due to oxybutynin side effects). Inclusion/exclusion criteria not stated. | |
| Interventions | Group 1: Flavoxate 1200mg per day. Group 2: Oxybutynin 15mg once daily. | |
| Outcomes | Side effect profiles, urodynamics and symptomatic evaluation. | |
| Notes | Abstract from Proceedings of The International Continence Society 1989 Not suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Serels 1998.
| Methods | Randomised crossover trial with allocation concealment. 3 patient groups on each medication for at least 1 month over a 2 year period. No stated washout period. Single centre USA. Baseline comparison. Intention to treat analysis. | |
| Participants | 34 female patients. Age Range ‐ 29‐91yrs Inclusion criteria: not stated Exclusion criteria: urinalysis evidence of UTI | |
| Interventions | Group 1 n=31 Hyoscyamine 0.375mg 2x/day Group 2 n=25 Doxazosin 2mg 4x/day. Group 3 n=13 Combination of 1 and 2 | |
| Outcomes | AUA symptom score assessment, side effects, urodynamic parameters. | |
| Notes | Full text English. S Serels contacted directly for information not clearly given in text. Crossover trial ‐ Not suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Stanton 1973.
| Methods | Randomised crossover trial with allocation concealment. No run in, 2 weeks of treatment with 1 week washout in between. Single centre British study. | |
| Participants | 38 patients ( 6 male, 32 female) Inclusion criteria: patients with frequency, urgency and incontinance. Exclusion criteria: not stated. | |
| Interventions | Group 1: Emepronium 200mg 3x/day Group 2: Flavoxate 200mg 3x/day. Number of paricipants in each group not stated. | |
| Outcomes | Urinalysis, urodynamic parameters, side effects, symptom assessment. | |
| Notes | Full text English. Included 3 patients with UTI, 3 post prostatectomy, 2 diabetics, 18 had sphincter dysfunction, 1 had cystitis cystica. Not suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Takayasu 1990.
| Methods | Randomised controlled parallel group study. Study duration 2 weeks. Multicentre Japanese study No intention to treat analysis. | |
| Participants | 225 patients (117 male, 78 female) Inclusion criteria: age >20, pollakisuria, ability to record frequency/volume chart. Exclusion criteria: Bladder outflow obstruction, UTI, Bladder necrosis, glaucoma. | |
| Interventions | Group 1: (n=99) Flavoxate 600mg daily Group 2: (n=96) Propantheline 20mg daily | |
| Outcomes | Subjective symptom assessment, patients impressions, efficacy and uselfulness | |
| Notes | Translated Japanese trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wehnert 1989.
| Methods | Randomised cross‐over trial. Allocation concealment unclear. 4 weeks on each drug, washout not stated. German study. No intention to treat analysis. | |
| Participants | 46 participants (sex not stated). Mean age 47.3yrs. Inclusion criteria: neuropathic bladder and urgency or urge incontinance (assessed with symptom scoring and urodynamics) Exclusion criteria: UTI, other urological or gynaecological illness. | |
| Interventions | Group 1: Flavoxate 300mg daily. Group 2: Propiverine 45mg daily Group 3: Placebo daily | |
| Outcomes | Urodynamics before, during and after treatment, subjective symptom scoring. | |
| Notes | German translated trial. Crossover trial ‐ not suitable for meta‐analysis. No dropouts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Key to Abbreviations: CVA ‐ Cerebro‐vascular accident; MS ‐ Multiple Sclerosis; TURP ‐ Trans‐urethral resection of prostate; UDS ‐ Urodynamic Studies; UTI ‐ Urinary Tract Infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aagard 1983 | Randomised controlled trial. Combination study: flavoxate and emepronium (anticholinergic) versus empronium alone. Flavoxate not assessed independently in single treatment arm of study. |
| Andersen 1988 | Randomised controlled trial. Comparison: emepronium (anticholinergic) versus terodiline (anticholinergic and calcium channel blocking effects) (now banned due to assosciation with ventricular tacharythmias). |
| Athanasopoulos 2002 | Randomised controlled trial. Combination study: alpha blocker (tamsulosin) plus anticholinergic (tolterodine) versus alpha blocker alone. Tamsulosin not assessed independently in single treatment arm of study. |
| Barnick 1991 | Double blind cross‐over study. Comparison: Oxybutinin (anticholinergic) versus terodiline (calcium channel blocker and anticholinergic). Terodiline banned due to association with ventricular tacharythmias. |
| Beisland 1985 | Crossover study. Comparison: meladrazine (poysynaptic inhibitor in CNS) with terodiline (now banned due to assosciation with ventricular tacharythmias). |
| Bradley 1970 | Study design unclear. May not be randomised or even quasi‐randomised. Contacted authors but no reply. |
| Clark 1996 | Crossover study. Combination study: imipramine (tricyclic antidepressant) and propantheline (anticholinergic) versus penthionate (anticholinergic) alone. Imipramine not assessed independently in single treatment arm of the study. |
| Ekstrom 1990 | Abstract only available. Not a randomised controlled trial. Study using intravesical instillations of verapamil (calcium‐channel blocker), atropine (anticholinergic), phentolamine (alpha receptor antagonist) and terodiline (anticholinergic/calcium channel blocker) in parallel groups. No comparisons between groups stated. Outcome assessed is whether intravesical instillation has any efficacy. |
| El Bahnasawy 2002 | Abstract only available ‐ randomised crossover study Oxybutinin versus verapamil. Study on patients with orthotopic ileal reservoirs not human bladder tissue. |
| Gruneberger 1984 | Randomised controlled trial. Clenbuterol (beta‐2 receptor antagonist) versus flavoxate. No anticholinergic treatment arm in this study. Flavoxate thought at time to exert effects through predominantly anticholinergic pathways. This has since been disproven. Thus not suitable for current review. |
| Homma 1997 | Abstract only available ‐ randomised controlled trial. Temiverine (predominantly anticholinergic with calcium channel antagonistic qualities) versus propiverine Comparison of 2 anticholinergics. Temiverine is a new drug not yet liscensed or in clinical use. |
| Lee 2005 | Randomised controlled trial. Combination study: Doxazosin (alpha blocker) plus propiverine (anticholinergic) versus doxazosin alone. Doxazosin not assessed inedpendently in single treatment arm of the study. |
| Lukkarinen 1987 | Randomised crossover trial. Emepronium (anticholinergic) versus terodiline (anticholinergic and calcium channel blocker) (banned due to association with ventricular tacharythmias). |
| Robinson 1983 | Randomised double blind crossover study. Comparitive study: Flavoxate plus emepronium versus placebo. Flavoxate not assessed independently in single treatment arm. |
| Sole 1984 | Randomised crossover study. Emepronium versus terodiline (banned due to assosciation with ventricular tacharythmias). |
| Tammela 1999 | Abstract only available ‐ randomised controlled trial. Temiverine (predominantly anticholinergic with calcium channel antagonistic qualities) versus propiverine (anticholinergic). Comparison of 2 anticholinergic drugs. Temiverine is a new drug not yet licensed or in clinical use. |
| Zeegers 1987 | Randomised crossover study. Crossover was only partial as participants did not receive all treatments. Flavoxate (anticholinergic) versus Emepronium (anticholinergic) versus Oxybutinin (anticholinergic) chloride versus Placebo. |
Characteristics of ongoing studies [ordered by study ID]
Shaw.
| Trial name or title | Leistershire MRC Incontinence Study |
| Methods | |
| Participants | Unknown |
| Interventions | Oxybutynin versus imipramine |
| Outcomes | Unknown |
| Starting date | 1999 |
| Contact information | |
| Notes |
Contributions of authors
Campbell Roxburgh ‐ designed the data extraction sheet, assessed the eligible studies for inclusion, extracted the data and wrote the first draft of the review. Jonathan Cook ‐ assessed the eligible studies for inclusion, extracted the data and commented on the draft of the review. Norman Dublin ‐ wrote the original protocol and commented on the draft version of the review.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Cardozo 1979 {published data only}
- Cardozo LD, Stanton SL. An objective comparison of the effects of parenterally administered drugs in patients suffering from detrusor instability. Journal of Urology 1979;122:58‐9. [Ref ID 2627] [DOI] [PubMed] [Google Scholar]
Enzelsberger 1991 {published data only}
- Enzelsberger H, Schatten C, Kurz C. Comparison of emepronium bromide with intravesical administration of lidocaine gel in women with urge incontinance [Vergleeich von Emeproniumbromid mit intravesikal appliziertem Lidocain‐gel bei Frauen mit Urge‐Inkontinenz]. Geburtschilfe und Frauenheilkunde 1991;51(1):54‐7. [Ref ID 272] [DOI] [PubMed] [Google Scholar]
Frohlich 1998 {published data only}
- Frohlich G, Burmeister S, Weidermann A, Bulitta M. Intravesical instillation of trospium chloride, oxybutinin and verapamil for relaxation of the bladder detrusor muscle. A placebo contolled randomized clinical test. [Intravesikal instillation von trospiumchlorid, oxybutinin und verapamil zur relaxation des harnblasen‐detrusors. Ein placebo‐kontrollierte, randomisierte klinische prufung]. Arzneimittelforschung 1998;48(5):486‐91. [Ref ID 7874] [PubMed] [Google Scholar]
Gaudenz 1978 {published data only}
- Gaudenz R, Weil A. Motor urge incontinence: diagnosis and treatment. Urologia Internationalis 1980;35(1):1‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herbst 1970 {published data only}
- Herbst WP. Double blind comparison of flavoxate and propantheline as urologic antispasmodics. American Journal of Clinical Research 1970;1:65‐7. [Ref ID: 5154] [Google Scholar]
Meyhoff 1981 {published data only}
- Meyhoff HH, Gerstenberg TC, Nordling J. Placebo ‐ the drug of choice in female motor urge incontinence?. British Journal of Urology 1983;55(1):34‐7. [Ref ID:685] [DOI] [PubMed] [Google Scholar]
Milani 1993 {published data only}
- Milani R, Scalambrino S, Milia R, Sambruni I, Riva D, Pulici L, et al. Double blind crossover comparison of flavoxate and oxybutynin in women affected by urinary urge syndrome. International Urogynaecology Journal 1993;4:3‐8. [Ref ID: 6169] [Google Scholar]
Riva 1989 {published data only}
- Riva D, Vigano R, Azzolini E, Colli E, Sartanj A, Scatigna M. A randomised, double‐blind, crossover trial on the effecacy and tolerance of falvoxate and oxybutynin in the treatment of urge incontinence. Proceedings of the International Continence Society (ICS), 13th Annual Meeting; 1989 Sept 7‐9; Ljubljiana, Slovenia. 1989:218. [Ref ID: 12036]
Serels 1998 {published data only}
- Serels S, Stein M. Prospective study comparing hyoscyamine, doxazosin and combination therapy for the treatment of urgency and frequency in women. Neurourology & Urodynamics 1998;17(1):31‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stanton 1973 {published data only}
- Stanton SL. A comparison of emepronium bromide and flavoxate hydrochloride in the treatment of urinary incontinence. Journal of Urology 1973;110(5):529‐32. [Ref ID: 2649] [DOI] [PubMed] [Google Scholar]
Takayasu 1990 {published data only}
- Takayasu H, Ueno A, Tsuchida S, Koiso K, Kurita T, Kawabe K, et al. Clinical evaluation of propiverine hydrochloride (P‐4) for the treatment of urinary frequency ‐ A double‐blind controlled study using flavoxate hydrochloide. Nishinihon Journal of Urology 1990;52(2):248‐58. [Ref ID: 12897] [Google Scholar]
Wehnert 1989 {published data only}
- Wehnert J, Sage S. Comparative studies of the effect of mictonorm (propiverin hydrochloride) and Spasuret (flavoxate hydrochloride) on the bladder detrusor muscle (GERMAN) [Vergleichende Untersuchungen zur Wirkung von Mictonorm (Propiverin hydrochlorid) und Spasuret (flavoxat hydrochlorid) auf den Detrusor vesicae]. Zeitschrift Fur Urologie und Nephrologie 1989;82(5):259‐63. [Ref ID:408] [PubMed] [Google Scholar]
References to studies excluded from this review
Aagard 1983 {published data only}
- Aagaard J, Reuther K, Stimpel H. A comparision between the combination emepronium bromide/flavoxate and emepronium bromide in the treatment of detrusor instability. Urologia Internationalis 1983;38(3):191‐2. [DOI] [PubMed] [Google Scholar]
Andersen 1988 {published data only}
- Andersen JR, Lose G, Norgaard M, Stimpel H, Andersen JT. Terodiline, Emepronium bromide or placebo for treatment of female detrusor overactivity? A randomized, double ‐blind cross‐over study. British Journal of Urology 1988;61(4):310‐3. [DOI] [PubMed] [Google Scholar]
Athanasopoulos 2002 {published data only}
- Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis I, Perimenis P, Barbalias G. Combination treatment with an a‐blocker plus an anticholinergic improves quality of life in patients with bladder outflet obstruction. A prospective, randomized, controlled study (Abstract). Neurourology and Urodynamics 2002;21(4):308‐9. [Google Scholar]
Barnick 1991 {published data only}
- Barnick CGW, Cutner A, Cardozo LD. Detrusor instability: a double blind, dose titrated, cross‐over study of Oxybutinin vesus Terodiline. Proceedings of the International Continence Society (ICS), 21st Annual Meeting; 1991 Oct 10‐12; Hannover, Federal Republic of Germany. 1991:181‐2.
Beisland 1985 {published data only}
- Beisland HO, Fossberg E. The effects of terodiline and melardazine on sever motor urge incontinence in geriatric patients. Journal of American Geriatric Society 1985;33(1):29‐32. [DOI] [PubMed] [Google Scholar]
Bradley 1970 {published data only}
- Bradley DV, Cazort RJ. Relief of bladder spasm by Flavoxate. A comparative study. Journal of Clinical Pharmacology 1970;10(1):65‐8. [PubMed] [Google Scholar]
Clark 1996 {published data only}
- Clarke B. Anticholinergic medication for the unstable bladder: Prospective trials of imipramine/propantheline versus penthienate and oxybutinin versus penthienate. International Urogynecology Journal of Pelivic Floor Dysfunction 1996;7:191‐5. [DOI] [PubMed] [Google Scholar]
Ekstrom 1990 {published data only}
- Ekstrom B, Andersson KE, Mattiasson A. Intravesical administration of drugs in patients with detrusor hyperactivity. Neurourology and Urodynamics 1990;9(4):340. [Google Scholar]
El Bahnasawy 2002 {published data only}
- Bahnasawy MS, Shaaban HS, Gomha MA, Nabeeh A. Management of noctural incontinence in orthotopic ileal reservoirs using oxybutinin versus verapamil: A proscpective randomised crossover study. Journal of Urology 2002;167:86. [Google Scholar]
Gruneberger 1984 {published data only}
- Gruneberger A. Treatment of motor urge incontinence with clenbuterol and flavoxate hydrochloride. British Journal of Obstetrics and Gynaecology 1984;91(3):275‐8. [PubMed] [Google Scholar]
Homma 1997 {published data only}
- Homma Y, Aso Y, Kawabe K, Kunamoto Y, Tsuchida M, et al. Randomized double blind study to compare clinical efficacy of temeverine and propiverine for unstable bladder and detrusor hyperreflexia. Neurourology and Urodynamics 1997;16(5):345‐6. [Google Scholar]
Lee 2005 {published data only}
- Lee KS, Choo MS, Kim DY, Kim JC, Kim HJ, Min KS, et al. Combination treatment with propiveriene hydrochloride plus doxazosin controlled release gastrointestinal therapeutic system formulation for overactive bladder and coexisting benign prostatic obstruction: a prospective randomised controlled multicentre study. Journal of Urology 1005;174:1334‐8. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim DY, Kim JC, Kim HJ, Min KS, Park WH, et al. Combination treatment with propiverine hydrochloride and doxazosin GITS in men with overactive bladder coexisting benign prostatic obstruction: a prospective, randomized, controlled, multicentre study (ABSTRACT). Proceedings from the International Continence Society (34th Annual Meeting) and the International Urogynaecological Association; 2004 Aug 23‐27; Paris. 2004; Vol. 207.
Lukkarinen 1987 {published data only}
- Lukkarinen O, Grohn P, Wilen‐Rosenquvist G, Juusela H, Sotarauta M, Lehtonen T. A controlled, double blind, cross over study of terodiline in motor urge incontinence. Annales Chirurgiae et Gynaecologiae 1987;76(2):121‐32. [PubMed] [Google Scholar]
Robinson 1983 {published data only}
- Robinson JM, Brocklehurst JC. Emepronium bromide and flavoxate hydrochloride in the treatment of urinary incontinence associated with detrusor instability in elderly women. British Journal of Urology 1983;55(4):371‐6. [DOI] [PubMed] [Google Scholar]
Sole 1984 {published data only}
- Sole GM, Arkell DG. A symptomatic and cystomtric comparison of terodiline with emepronium in the treatment of women with frequency, urgency and incontinence. Scandanavian Journal of Urology and Nephrology Supplementum 1984;87:55‐7. [PubMed] [Google Scholar]
Tammela 1999 {published data only}
- Tammela TLJ, Mattiasson A, Chapple CR, Moller B, Bakke A, Happonen P. Short term efficacy and safety of temeverine in the treatment of bladder overactivity. A randomized clinical trial in man and women. Nerourology and Urodynamics 2000;18(4):334‐5. [Google Scholar]
Zeegers 1987 {published data only}
- Zeegers A, Keisswetter H, Kramer A, Jonas U. Conservative therapy of urgency and urge incontinence: a double blind clinical trial of flavoxate hydrochloride, oxybutinin chloride, emepronium bromide and placebo [Medicamenteuze behandeling van overmatige mictiedrang: Dubbel‐blind onderzoek van Cetiprin, Dridase, Urispas en placebo]. World Journal of Urology 1987;5(1):57‐61. [Ref ID: 5158] [Google Scholar]
References to ongoing studies
Shaw {unpublished data only}
- Leistershire MRC Incontinence Study. Ongoing study 1999.
Additional references
2nd ICI 2001
- Abrams P, Cardozo L, Khoury S, Wein A. Incontinence: 2nd International Consultation for Incontinence. Plymouth: Health Publication Ltd, 2001:481. [Google Scholar]
Abrams 1988
- Abrams PH, Blaivas JG, Stanton SL, Andersen JT. Standardisation of terminology of lower urinary tract function. Neurourology & Urodynamics 1988;7(5):403‐27. [PubMed] [Google Scholar]
Abrams 2002
- Abrams P, Cardoza L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub‐committee of the International Continence Society. Neurology & Urodynamics 2002;21(2):167‐78. [DOI] [PubMed] [Google Scholar]
Abrams 2003
- Abrams P, Amarenco G, Bakke A, Bukzynski A, Castro‐Diaz D, et al. Tamsulosin: Efficacy and safety in patients with neurogenic lower urinary tract dysfunction due to suprasacral spinal cord injury. Journal of Urology 2003;170:1242‐51. [DOI] [PubMed] [Google Scholar]
Alhasso 2006
- Alhasso AA, McKinley J, Patrick K, Stewart L. Anticholinergic drugs versus non‐drug therapies for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. [Art. No.: CD003193. DOI: 10.1002/14651858.CD003193.pub3] [DOI] [PubMed] [Google Scholar]
Andersson 2000
- Andersson KE. Treatment of overactive bladder: other drug mechanisms. Urology 2000;55 (Suppl 5A):51‐7. [DOI] [PubMed] [Google Scholar]
Andersson 2002
- Andersson, KE. Potential benefits of Muscarinic M3 receptor selectivity. European Urology Supplements 2002;1(4):23‐8. [Google Scholar]
Berghmans 2004
- Berghmans B, Bo K, Hendriks E, Kempen M, die Bie R. Electrical stimulation with non‐implanted electrodes for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI] [PMC free article] [PubMed]
Castleden 1986
- Castleden CM, Duffin HM, Gulati RS. Double‐blind study of imipramine and placebo for incontinence due to bladder instability. Age Ageing 1986;15:299. [DOI] [PubMed] [Google Scholar]
Chapple 1990
- Chapple CR, Parkhouse H, Gardener C, et al. Double‐blind, placebo‐controlled, crossover study of flavoxate in the treatment of idiopathic detrusor instability. British Journal of Urology 1990;66:491. [DOI] [PubMed] [Google Scholar]
Cruz 1997
- Cruz F, Guimaraes M, Silva C, et al. Suppression of bladder hyperreflexia by intravesical instillation of resiniferatoxin. Lancet 1997;350:640. [DOI] [PubMed] [Google Scholar]
Dahm 1995
- Dahm TL, Ostri P, Kristensen JK, et al. Flavoxate treatment of micturiton disorders accompanying benign prostatic hypertrophy: a double‐blind, placebo‐controlled, multicentre investigation. Urology International 1995;55:205. [DOI] [PubMed] [Google Scholar]
de Groat 1997
- Groat WC. A neurologic basis for the overactive bladder. Urology 1997;50 (6A Suppl):36‐52. [DOI] [PubMed] [Google Scholar]
Dmochowski 2007
- Dmochowski R, Sand PK. Botulinum toxin A in the overactive bladder: current status and future directions. BJU International 2007;99(2):247‐62. [DOI] [PubMed] [Google Scholar]
Duthie 2007
- Duthie J, Wilson DI, Herbison GP, Wilson D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database of Systematic Reviews 2007, Issue 3. [Art. No.: CD005493. DOI: 10.1002/14651858.CD005493] [DOI] [PubMed] [Google Scholar]
Eri 1995
- Eri LM, Tveter KJ. Alpha blockade in the treatment of syptomatic benign prostatic hyperplasia. Journal of Urology 1995;154:923. [PubMed] [Google Scholar]
Hay‐Smith 2005
- Hay‐Smith J, Herbison P, Ellis G, Morris A. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [Art. No.: CD005429. DOI: 10.1002/14651858.CD005429] [DOI] [PubMed] [Google Scholar]
Herbison 2003
- Herbison P, Arnold E. Neuromodulation with implanted electrodes for urinary storage and voiding dysfunction in adults. Cochrane Database of Systematic Reviews 2003, Issue 2. [Art. No.: CD004202. DOI: 10.1002/14651858.CD004202] [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S. Editors. Cochrane Handbook of Systematic Reviews of Interventions (Updated Sept 2006). Vol. 4.2.6, Oxford: The Cochrane Collaboration, 2006. [Google Scholar]
Hu 2003
- Hu TW, Wagner TH, Bentkover JD, Leblanc K, Piancentini A, Stewart WF, et al. Estimated economic costs of overactive bladder in the United States. Urology 2003;61:1123‐8. [DOI] [PubMed] [Google Scholar]
Hunsballe 2001
- Hunsballe JM, Djurhuus JC. Clinical options for imipramine in management of urinary incontinence. Urological Research 2001;29:118. [DOI] [PubMed] [Google Scholar]
ICI 2000
- Anonymous. Assessment and treatment of urinary incontinence. Scientific Commitee of the First International Consultation on Incontinence. Lancet 2000;355(9221):2153‐8. [PubMed] [Google Scholar]
Kelleher 1997
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to access the quality of life of urinary incontinent women. British Journal of Obstetrics & Gynaecology 1997;104:1374‐9. [DOI] [PubMed] [Google Scholar]
Kobelt 1997
- Kobelt G. Economic considerations and outcome measurement in urge incontinence. Urology 1997;50(6A suppl):100‐7. [DOI] [PubMed] [Google Scholar]
Lazzeri 1997
- Lazzeri M, Beneforti P, Turini D. Urodynamic effects of intravesical resiniferatoxin in humans: preliminary results in stable and unstable detrusor. Journal of Urology 1997;158:2093. [DOI] [PubMed] [Google Scholar]
Mattiasson A
- Mattiasson A, Ekstrom B, Andersson K‐E. Effects of intravesical instillation of verapamil in patients with detrusor hyperactivity. Journal of Urology 1989;141:174. [DOI] [PubMed] [Google Scholar]
Milsom 2001
- Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population‐based prevalence study. BJU International 2001;87(9):760‐6. [DOI] [PubMed] [Google Scholar]
Nabi 2006
- Nabi G, Cody JD, Ellis G, Herbison P, Hay‐Smith J. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. [Art. No.: CD003781. DOI: 10.1002/14651858.CD003781.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouslander 1982
- Ouslander JG, Kane RL, Abrass IB. Urinary incontinence in elderly nursing home patients. Journal of the American Medical Association 1982;248(10):1194‐8. [PubMed] [Google Scholar]
Ouslander 1986
- Ouslander JG, Hepps K, Raz S, Su HL. Genitourinary dysfunction in a geriatric outpatient population. Journal of the American Geriatrics Society 1986;34(7):507‐14. [DOI] [PubMed] [Google Scholar]
Stewart 2001
- Stewart WF, Herzog AR, Wein AJ, Abrams P, Payne C, Corey R, et al. Prevalence and impact of overactive bladder in the United States: Results from the NOBLE program. Neurology & Urodynamics 2001;20:403‐22. [Google Scholar]
Stewart 2003
- Stewart WF, Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World Journal of Urology 2003;20(6):327‐36. [DOI] [PubMed] [Google Scholar]
Thuroff 1991
- Thuroff JW, Bunke B, Ebner A, Faber P, Geeter P, Hannappel J, et al. Randomized, double‐blind, multicenter trial on treatment of frequency, urgency and incontinence related to detrusor hyperactivity: oxybutynin versus propantheline versus placebo. Journal of Urology 1991;145:813‐6. [DOI] [PubMed] [Google Scholar]
Van Kerrebroeck 1998
- Kerrebroeck PE, Amarenco G, Thuroff JW, Madersbacher HG, Lock MT, Messelink EJ, et al. Dose‐ranging study of tolterodine in patients with detrusor hyperreflexia. Neurourlogy & Urodynamics 1998;17(5):499‐512. [DOI] [PubMed] [Google Scholar]
Wallace 2004
- Wallace S, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD001308. DOI: 10.1002/14651858.CD001308.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wein 2001
- Wein AJ. Pharmacological agents for the treatment of urinary incontinence due to overactive bladder. Expert Opinion on Investigational Drugs 2001;10:65. [DOI] [PubMed] [Google Scholar]


