Abstract
Background
Corneal densitometry, which is also known as corneal backscattering, is a surrogate measure of corneal clarity. The purpose of the study was to investigate the changes in corneal densitometry (CD) after implanting an implantable collamer lens (ICL-V4c).
Method
Twenty-six high myopic patients (aged 29.3 ± 6.6 years, 6 males and 20 females) who underwent ICL-V4c implantation were enrolled. Intraocular pressure (IOP), corneal topography, corneal densitometry, uncorrected distance visual acuity (UCDVA), manifest refraction, and best corrected distance visual acuity (BCDVA) were evaluated pre-operatively and at 1 day, 1 week, and 1, 3, 6, and 12 months post-operatively. Endothelial cell density (ECD) was measured pre-operatively and at 3, 6, and 12 months post-operatively. The efficacy index (mean post-operative UCDVA / mean pre-operative BCDVA) and the safety index (mean post-operative BCDVA / mean pre-operative BCDVA) were evaluated at 1 month, 3 months, 6 months and 12 months post-operatively.
Results
Over the annular diameters of 0–2 mm, the pre-operative densitometry values of the anterior layer, central layer, posterior layer, and total layer were 20.1 ± 2.8, 11.8 ± 1.1, 10.5 ± 0.9 and 14.1 ± 1.5, respectively. From pre-operatively to post-operative Month 12, the values changed insignificantly (P = 0.177, P = 0.153, P = 0.543 and P = 0.207, respectively). Over the annular diameters of 2–6 mm, the pre-operative mean densitometry values were 17.9 ± 2.2, 10.5 ± 0.9, and 12.6 ± 1.2, respectively. From pre-operatively to post-operative Month 12, the values decreased to 16.5 ± 2.1, 10.0 ± 0.9, and 11.9 ± 1.2, respectively, which were similar to the pre-operative values (all P > 0.05) but significantly lower than the values obtained at post-operative Day 1 (P = 0.013, P = 0.002 and P = 0.010, respectively). The densitometry value of the posterior layer over the annular diameters of 2 to 6 mm remained unchanged (from 9.4 ± 0.7 to 9.1 ± 0.7) over time (P = 0.372). The efficacy and safety indices assessed at 12 months post-operatively were 1.04 ± 0.27 and 1.19 ± 0.23, respectively. The changes in IOP and ECD values were statistically insignificant (P = 0.896 and P = 0.968, respectively).
Conclusion
ICL-V4c implantation may be safe and efficient for high ametropia correction. The corneal densitometry values obtained over the annulus of 0–6 mm increased slightly from before the operation to post-operative Day 1 and then decreased gradually, which indicates that ICL-V4c implantation may not compromise corneal clarity.
Keywords: Implantable collamer lens, Corneal densitometry, Endothelial cell density
Background
Corneal densitometry (also known as corneal backscatter) is an objective method for evaluating corneal clarity and transparency [1–4]. Studies have reported that corneal diseases such as keratitis [5], corneal epithelial oedema [6], Fuchs’ endothelial corneal dystrophy [7] and keratoconus [8] can compromise corneal clarity, thus leading to an increase in corneal densitometry. The normal function of corneal endothelium and the regular arrangement of stromal layers play important roles in maintaining corneal clarity [9]. Otri AM [10] has noted that corneal densitometry can be used as an indicator of corneal heath.
The implantation of a posterior chamber implantable collamer lens (ICL) has been considered an effective procedure for myopia correction [11–13]. Since ICL implantation avoids ablating or removing corneal tissue, the integrity of the corneal structure and the stability of the corneal biomechanics are theoretically maintained; hence, ICL implantation, compared with corneal refractive surgeries, may minimize the risk of ectasia [14–19]. However, complications such as endothelial cell damage and intraocular pressure (IOP) elevation have also been reported [20] intraoperatively or post-operatively.
ICL model V4c (ICL-V4c, STAAR Surgical Company, Monrovia, California, USA) is a modified posterior chamber ICL, which is designed with a central hole instead of iridectomy to improve aqueous humour circulation. Studies have reported that ICL-V4c and conventional ICLs (without a central hole) present similar visual outcomes [21–24]. Nevertheless, surgery-associated endothelial damage, including endothelial cell trauma and endothelial oedema, may not be completely avoided [25]. Moreover, the potential intraoperative or post-operative ocular hypertension (OHT) and IOL rise may also compromise the corneal endothelial barrier and may lead to corneal oedema, affecting corneal transparency [26]. The purpose of this study was to investigate the natural course of corneal densitometry after ICL-V4c implantation.
Methods
Participants
Twenty-six high myopic patients (6 males and 20 females) who had not yet received the ICL-V4c operation were selected for this study. Each patient had the operation on only 1 eye. The mean age of the patients was 29.3 ± 6.6 years (range 18 to 44 years). The mean value of manifest refraction spherical equivalent (MRSE) was − 12.48 ± 3.79 dioptres (D) (range − 26.63 to − 6.63 D).
The inclusion criteria were the following: age ≥ 18 years, MRSE of higher than − 6.00 D, astigmatism of up to − 5.00 D, best corrected distance visual acuity (BCDVA) of 20/40 or better, anterior chamber depth of ≥2.8 mm, and endothelial cell density of ≥2000 cell/mm2.
The exclusion criteria were the following: a history of suspected keratectasia, cornea or lens opacity, retinal detachment, glaucoma, macular degeneration, or neuro-ophthalmic disease, a history of ocular surgery, inflammation or trauma, systemic disease, and pregnancy.
Ethics approval and consent to participate were obtained. This study adhered to the Declaration of Helsinki and was approved by the Ethical Committee Review Board of Fudan University Eye and ENT Hospital. Written informed consent was obtained from each patient after the procedure was thoroughly explained.
Ophthalmic examinations
Each patient underwent routine pre-operative evaluations. Intraocular pressure, corneal topography and corneal densitometry were assessed using a non-contact tonometer and an anterior segment analyser (Pentacam HR, Typy70900, Oculus Optikgeräte GmbH, Germany), respectively. The evaluations were administered before the operation and at 1 day, 1 week, 1 month, 3 months, 6 months and 12 months after the operation.
Corneal densitometry values measured from the Scheimpflug images are expressed in grey scale units (range 0 to 100) and are presented in the Average Cornea Densitometry Table (version 1.20r29). The mean densitometry values on the diameters from 0 to 2 mm and 2–6 mm of the corneas’ anterior layer (the first 120 μm of the complete corneal thickness), posterior layer (the last 60 μm of the complete corneal thickness), central layer (the volume between the anterior layer and the posterior layer), and the total layer (the volume between the epithelium and endothelium of a cornea) were analysed since densitometry measurements were much more accurate and repeatable in the central areas than in the peripheral areas [27, 28].
The efficacy index (mean post-operative UCDVA / mean pre-operative BCDVA) and the safety index (mean post-operative BCDVA / mean pre-operative BCDVA) were also evaluated at 12 months post-operatively. UCDVA, manifest refraction, BCDVA, and IOP (non-contact tonometer) were all assessed before the operation and at 1 month, 3 months, 6 months and 12 months after the operation. Corneal endothelial cell density (ECD) was measured with a specular microscope (SP-2000P; Topcon Corporation, Japan) before the operation and at 6 months and 12 months after the operation.
Surgical techniques
All ICL-V4c procedures were performed by the same surgeon (XYW) under cycloplegia (2.5% phenylephrine and 1% tropicamide, Alcon, China) and topical anaesthesia (0.4% oxybuprocaine hydrochloride, Santen, Japan). A lid speculum was used to fully expose the ocular surface after the sterilization of the ocular surface and periocular skin (povidone-iodine 5%). Then, a 3 mm incision was created at the temporal or superior corneoscleral limbus. After a viscoelastic surgical agent (1.7% sodium hyaluronate; Bausch & Lomb, China) was injected into the anterior chamber to maintain the anterior chamber depth, an ICL-V4c was inserted into the anterior chamber through the incision with an injector cartridge. An additional viscoelastic agent was then placed on the top of the ICL (between the corneal endothelium and the upper surface of the ICL-V4c), and an ICL positioning instrument was used to sweep the 4 footplates of the ICL beneath the iris. Subsequently, both the anterior chamber and the posterior chamber were irrigated thoroughly using a balanced salt solution. Finally, the overfilled perfusate was released from the incision, and the IOP was estimated with “finger touch” before the end of the procedure.
Post-operative topical therapies
Each patient was required to use antibiotic eyedrops (0.5% left ofloxacin, Santen, Japan), steroidal eye drops (prednisolone acetate ophthalmic suspension 1%, Allergan Pharmaceuticals, Ireland), and miotic eye drops (pilocarpine nitrate 0.5%, Bausch & Lomb, USA) 4 times every 10 min immediately after the procedure. Antibacterial and steroidal medications (0.1% tobramycin dexamethasone, Alcon, China) were prescribed to be taken 4 times daily for 3 days, followed by fluorometholone eye drops that were tapered gradually over 2 weeks. Then, antibiotic eyedrops (0.5% left ofloxacin, Santen, Japan) were prescribed to be taken 4 times daily for 1 week, non-steroidal anti-inflammatory eyedrops (NSAID, pranoprofen, Senju, Japan) were prescribed to be taken 4 times daily for 2 weeks and artificial tears were prescribed to be taken 4 times daily for 1 month [29].
Data analysis and statistical evaluation
All the values are expressed as the mean ± standard deviation. Statistical analyses were performed using SPSS 19 (SPSS Inc., IBM). A normality check was conducted using the Kolmogorov–Smirnov test. A mixed linear model with Bonferroni-adjusted post hoc comparisons was used to evaluate the changes in MRSE, CD, ECD and IOP values over time. The cut-off P value was 0.05 for indicating statistical significance.
Results
All procedures were completed without complications. At 2 h after ICL-V4c implantation, each post-operative eye had mild epithelial oedema and endothelial oedema, which were identified with slit lamp biomicroscopy. However, the cornea recovered the day after the operation. No severe intraoperative or post-operative complications occurred.
The natural course of corneal densitometry after ICL implantation
The mean densitometry values obtained before and after ICL implantation are listed in Table 1. Over the annular diameters of 0–2 mm, no significant changes were detected in the densitometry values of the anterior layer (AL 0–2 mm), the central layer (CL 0–2 mm), the posterior layer (PL 0–2 mm) or the total layer (TL 0–2 mm) (F = 1.510, P = 0.177; F = 1.589, P = 0.153; F = 0.836, P = 0.543 and F = 1.426, P = 0.207, respectively) within the first year after the operation (Fig. 1).
Table 1.
The natural course of corneal densitometry (n = 26)
| Variables | Pre-op | 1 Day | 1 Week | 1 Month | 3 Months | 6 Months | 1 Year | F valuea | P value |
|---|---|---|---|---|---|---|---|---|---|
| Post-op | Post-op | Post-op | Post-op | Post-op | Post-op | ||||
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | |||
| AL0-2 mm | 20.1 ± 2.8 | 20.1 ± 2.6 | 19.5 ± 2.3 | 19.5 ± 2.7 | 19.5 ± 2.2 | 18.8 ± 1.7 | 18.5 ± 2.7 | 1.510 | 0.177 |
| CL0-2 mm | 11.8 ± 1.1 | 11.7 ± 1.1 | 11.5 ± 1.1 | 11.5 ± 1.1 | 11.4 ± 0.8 | 11.2 ± 0.7 | 11.1 ± 1.1 | 1.589 | 0.153 |
| PL0-2 mm | 10.5 ± 0.9 | 10.3 ± 1.0 | 10.4 ± 0.8 | 10.4 ± 0.9 | 10.5 ± 0.7 | 10.3 ± 0.8 | 10.0 ± 0.9 | 0.836 | 0.543 |
| TL0-2 mm | 14.1 ± 1.5 | 14.1 ± 1.5 | 13.8 ± 1.3 | 13.8 ± 1.5 | 13.8 ± 1.1 | 13.5 ± 1.0 | 13.2 ± 1.5 | 1.426 | 0.207 |
| AL2-6 mm | 17.9 ± 2.2 | 18.5 ± 2.2 | 17.5 ± 2.0 | 17.3 ± 2.3 | 17.2 ± 1.8 | 16.7 ± 1.4b | 16.5 ± 2.1b | 2.798 | 0.013* |
| CL2-6 mm | 10.5 ± 0.9 | 11.0 ± 1.1 | 10.3 ± 0.9 | 10.3 ± 1.0 | 10.2 ± 0.8b | 10.0 ± 0.7b | 10.0 ± 0.9b | 3.750 | 0.002** |
| PL2-6 mm | 9.4 ± 0.7 | 9.5 ± 0.9 | 9.4 ± 0.7 | 9.4 ± 0.8 | 9.4 ± 0.7 | 9.3 ± 0.7 | 9.1 ± 0.7 | 1.087 | 0.372 |
| TL2-6 mm | 12.6 ± 1.2 | 13.0 ± 1.3 | 12.4 ± 1.2 | 12.3 ± 1.4 | 12.3 ± 1.0 | 12.0 ± 0.9 | 11.9 ± 1.2b | 2.719 | 0.015* |
Pre-op Pre-operation, Post-op Post-operation, AL Anterior Layer, CL Central Layer, PL Posterior Layer, TL Total Layer
* P<0.05; ** P<0.01
a Mixed linear model
b significant difference was detected when compared with the value obtained at the 1 day mark
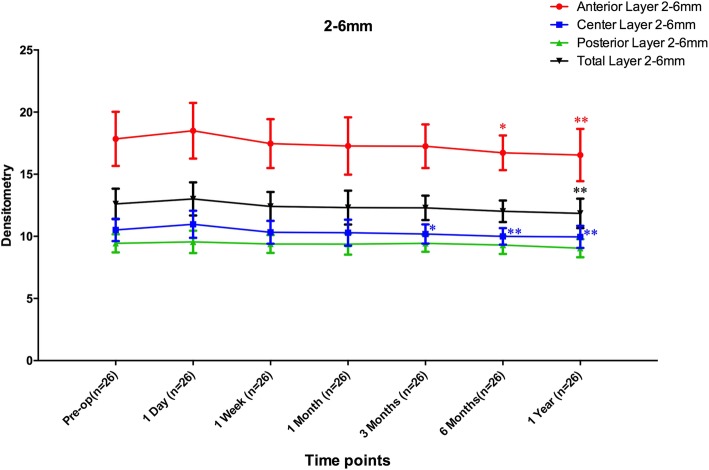
Fig. 1.
Densitometry over the annular diameters 0–2 mm of the cornea at different follow-up times after ICL V4c implantation
Over the annular diameters of 2–6 mm, the mean densitometry values of the anterior layer (AL 2–6 mm), the central layer (CL 2–6 mm), and the total layer (TL 2–6 mm) changed significantly (F = 2.798, P = 0.013; F = 3.750, P = 0.002 and F = 2.719, P = 0.015, respectively). Bonferroni-adjusted post hoc comparisons revealed that all the post-operative densitometry values obtained from the four layers were similar to the values measured pre-operatively (all P values > 0.05). However, the densitometry values of the AL 2–6 mm obtained at 6 months and 12 months after the operation were significantly lower than the value obtained at 1 day after the operation (P = 0.039 and P = 0.013). The values of CL 2–6 mm assessed at 3 months, 6 months and 12 months after the operation significantly decreased compared with the value obtained at post-operative Day 1 (P = 0.046; P = 0.004 and P = 0.002, respectively). The densitometry values of TL 2–6 mm obtained at post-operative Month 12 were significantly lower than those obtained at 1 day after the operation (P = 0.010) (Fig. 2).
Fig. 2.
Densitometry over the annular diameters 2–6 mm of the cornea at different follow-up times after ICL V4c implantation. (* refers to the P value < 0.05; ** refers to the P value < 0.01)
Visual outcomes
As demonstrated in Fig. 3a to d, 19 eyes (73.1%) had a UCDVA of 20/20 or better, 22 eyes (84.6%) had a UCDVA of 20/25 or better, 25 eyes (96.2%) had a UCDVA of 20/40 or better, and all 26 eyes (100%) had a UCDVA of 20/70 or better (Fig. 3a). No statistically significant difference was detected between the BCDVA before the operation and UCDVA (F = 1.032, P = 0.394) after the operation. The efficacy indices, assessed at 1 month, 3 months, 6 months and 12 months, were 1.13 ± 0.32 (n = 24), 1.13 ± 0.24 (n = 26), 1.09 ± 0.25 (n = 26) and 1.04 ± 0.27 (n = 26), respectively. At the 12-month mark, no patient showed a decline in BCDVA; 10 eyes (38.5%) had unchanged BCDVA, another 10 eyes gained one line (38.5%), and the remaining 6 eyes (23.1%) gained 2 or more lines (Fig. 3b). The safety indices assessed at the 1-month, 3-month, 6-month and 12-month marks were 1.27 ± 0.26 (n = 24), 1.29 ± 0.23 (n = 26), 1.19 ± 0.26 (n = 26), and 1.19 ± 0.23 (n = 26), respectively. A scatterplot and the best linear fit line of the attempted versus the achieved spherical equivalent refraction (SER) correction are shown in Fig. 3c. The values of manifest spherical equivalent refraction (MSER) measured before the operation and at 1 month, 3 months, 6 months, and 12 months after were − 12.48 ± 3.79 D, − 0.24 ± 1.04 D, − 0.44 ± 1.23 D, − 0.37 ± 0.97 D and − 0.36 ± 0.98 D, respectively. As shown in Fig. 3d, the SER before the operation was significantly lower than those after the operation (all P values < 0.001). However, there were no significant differences among the SER values after the operation (all P values > 0.05).
Fig. 3.
Refractive outcomes (a: Efficacy b: Safety c: Predictability d: Stability) at different follow-up times after the implantation of an implantable collamer lens with a central hole (ICL V4c). UCDVA = uncorrected distance visual acuity; BCDVA = best corrected distance visual acuity; D = dioptres; SD = standard deviation
IOP and ECD outcomes
The mean IOP values measured before the procedure and at the 1-day, 1-week, 1-month, 3-month, 6-month and 12-month marks were 15.43 ± 3.00 mmHg, 14.58 ± 3.76 mmHg, 15.27 ± 3.77 mmHg, 14.90 ± 3.46 mmHg, 15.48 ± 3.84 mmHg, 14.55 ± 3.78 and 15.52 ± 2.87 mmHg, respectively. The mean ECD values before the procedure and at 3 months, 6 months and 12 months after were 3273.9 ± 342.9/mm2, 3263.9 ± 437.6/mm2, 3311.7 ± 459.2/mm2 and 3261.4 ± 355.1/mm2, respectively. The mean IOP (F = 0.372, P = 0.896) and ECD (F = 0.085, P = 0.968) values remained unchanged over time.
Discussion
ICL procedures correct refractive errors without removing corneal tissue, thus avoiding the risk of secondary corneal ectasia. Currently, ICL procedures are becoming increasingly popular, especially for high ametropia correction [13, 14].
The results of the current study demonstrated that the densitometry values of AL 0–2 mm, CL 0–2 mm, PL 0–2 mm and TL 0–2 mm remained unchanged during the 12-month follow-up period, which was consistent with the ECD results. In addition, the values of AL 2–6 mm, CL 2–6 mm, PL 2–6 mm and TL 2–6 mm measured at 1 day, 1 month, 3 months, 6 months and 12 months after the procedure were also unchanged compared with those measured before the procedure, indicating that the effects of ICL-V4c implantation on central corneal densitometry may be insignificant and that the corneal histological structure is intact. The mean values of AL 2–6 mm, CL 2–6 mm and TL 2–6 mm increased slightly at the 1-day mark but then decreased gradually until post-operative Month 12. Significant differences in the values of AL 2–6 mm, CL 2–6 mm and TL 2–6 mm were detected between post-operative Day 1 and post-operative Month 12. We hypothesize that the surgical operation may slightly compromise the transparency of the peripheral cornea. Dong et al. [30] investigated the differences in corneal densitometry between high myopic patients and normal individuals and found that densitometry values of the high myopic patients were significantly lower than those of the normal individuals. The authors hypothesize that the natural loss of endothelial cell density in a high myopic population might lead to a decrease in corneal densitometry. However, in the present study, although we detected a significant decrease in corneal densitometry between post-operative Day 1 and post-operative Month 12, we did not detect any remarkable changes in endothelial cell density. We suggest that changes in corneal densitometry may be caused by various factors, including the density and function of endothelial cells, the balance between the stromal swelling pressure and the endothelial pump rate [31, 32], the corneal wound healing reaction and even the quality of tear film [33]. Additional studies are needed to determine the primary cause of a decrease in corneal densitometry.
Ní Dhubhghaill S et al. [27] investigated normative corneal densitometry data in a normal population. The authors found that the densitometry value is the lowest in the central zone and the highest in the anterior layer, which is consistent with our results. However, Ní Dhubhghaill S’s data showed that the accurate corneal densitometry values of each zone and each layer were much higher than our corresponding values. We suggest that racial difference may also be a major factor that affects corneal densitometry values, as Caucasians’ corneas are thicker than Asians’ corneas [34].
Pentacam HR was able to assess the corneal densitometry values over the annular zones of 0–12 mm; however, as most Asians’ palpebral fissures are narrow, their eyelids and eyelashes cover the peripheral areas of annuli 6–10 mm and 10–12 mm; thus, we have to analyse the zones over the annuli of 0–2 mm and 2–6 mm instead of the entire cornea. This method of measurement is a limitation of the study.
In this study, we found that at post-operative Month 12, both the MSER and visual acuity (VA) remained stable. The safety and efficacy indices were 1.19 ± 0.23 and 1.04 ± 0.27, there were no declines in BCDVA, and all the patients were satisfied with their vision, implying that ICL-V4c implantation is safe and efficient for high ametropia correction. Pérez-Vives C [35] and Ganesh S [36] investigated the differences in visual and refractive results between the ICL procedure and corneal refractive surgery. The outcomes demonstrated that the quality of vision and patient satisfaction is better with ICL than with femtosecond-LASIK. Tian Y [37] and Hyun J [38] compared the differences in visual and refractive results between ICL-V4 and ICL-V4c. Both authors found that the central hole did not significantly affect the visual and refractive outcomes. However, Eppig T et al. analysed the optical effect of the central hole on the ICL. The authors found that all the eye models showed ghost images and noted that the central hole may cause stray light rays and ghost images; however, the on-axis visual quality was mostly unaffected [39]. Additional studies are required to determine the effects of the central hole on visual quality and patient satisfaction.
Ocular hypertension (OHT) is considered one of the most commonly observed symptoms after ICL implantation. The possible causes include steroid responses, retained viscoelastic agents, acute pupillary blocks, and pigment dispersion [26]. In the current study, the IOP values changed slightly over time. The possible reasons may be as follows.
The short-term usage of steroidal eye drops and the application of an NSAID may maximally avoid steroid-related ocular hypertension, which was reported as the most common cause of OHT after ICL implantation.
As the central hole joins the anterior chamber and the posterior chamber, laser peripheral iridotomy is no longer routinely recommended before ICL-V4c implantation. Hence, the risks of pigment dispersion and acute pupillary block may be remarkably reduced. Thorough irrigation of the anterior and posterior chambers may be another key step that prevents retained viscoelastic agent-related OHT.
During the ICL-V4c implantation procedure, the intraocular space for surgical operation is limited, and endothelial cell damage (including endothelial cell loss or endothelial oedema) is a potential complication, especially when an ICL is being injected into the anterior chamber or positioned into the posterior chamber [20, 40, 41]. In the current study, we monitored the ECD values for 12 months and found that the mean ECD values measured pre-operatively and at 3 months, 6 months and 12 months post-operatively were indistinctive, implying that ECD may have been unaffected after ICL-V4c implantation. Therefore, ICL-V4c implantation may be safe for endothelial cells.
However, because the specular microscope evaluates ECD by analysing the central corneal image, which was taken through a central fixation area of the cornea [42, 43], peripheral endothelial damage may not be detected. Corneal densitometry assessments by using a Pentacam HR are able to detect various abnormalities in a wider zone than is a specular microscope. Corneal densitometry analysis may be employed to assess endothelial function in peripheral zones [2, 4, 9, 10].
Conclusions
In summary, ICL-V4c implantation may be safe and efficient for high ametropia correction. The corneal densitometry values obtained over the annulus of 0–6 mm increase slightly at post-operative Day 1 and then decrease gradually, indicating that ICL-V4c implantation may not compromise corneal clarity.
Acknowledgements
Not Applicable.
Abbreviations
- AL
Anterior layer
- BCDVA
Best corrected distance visual acuity
- CD
Corneal densitometry
- CL
Central layer
- D
Dioptres
- ECD
Endothelial cell density
- ICL
Implantable collamer lens
- IOP
Intraocular pressure
- MRSE
Manifest refraction spherical equivalent
- NSAID
Nonsteroidal anti-inflammatory drug
- OHT
Ocular hypertension
- PL
Posterior layer
- SER
Spherical equivalent refraction
- TL
Total layer
- UCDVA
Uncorrected distance visual acuity
- VA
Visual acuity
Authors’ contributions
Literature screening and selection was performed by XC and YS. XYW and XTZ participated in the design of the study. XC and YS drafted the manuscript. XC and YS carried out the statistical analysis. XC, YS and HPX prepared and reviewed the manuscript. XYW and XTZ have approved the final version to be published. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 81770955), the National Natural Science Foundation of China (Grant No. 81570879), the Project of Shanghai Science and Technology (Grant No .17140902900), the Project of Shanghai Science and Technology (Grant No .17411950200), the Project of Shanghai Science and Technology (Grant No. 19140900700), and the Shanghai Shenkang Hospital Development Center (Grant No. SHDC12016207).
The funding organization had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Data and materials are available upon request from the corresponding author at doctxiaoyingwang@163.com.
Ethics approval and consent to participate
This study adhered to the Declaration of Helsinki and was approved by the Ethical Committee Review Board of Fudan University Eye and ENT Hospital. Written informed consent was obtained from each patient after the procedure was thoroughly explained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xun Chen and Yang Shen contributed equally to this work.
Contributor Information
Xun Chen, Email: chenxun19910923@163.com.
Yang Shen, Email: doctshenyang@163.com.
Haipeng Xu, Email: xu_haipeng@hotmail.com.
Xiaoying Wang, Email: doctxiaoyingwang@163.com.
Xingtao Zhou, Email: doctxiaoyingwang@163.com.
References
- 1.Cankaya AB, et al. Assessment of corneal backward light scattering in the healthy cornea and factors affecting corneal transparency. Jpn J Ophthalmol. 2018;62(3):335–341. doi: 10.1007/s10384-018-0584-7. [DOI] [PubMed] [Google Scholar]
- 2.Chan T, et al. Corneal backward scattering and higher-order aberrations in children with vernal keratoconjunctivitis and normal topography. Acta Ophthalmol. 2018;96(3):e327–e333. doi: 10.1111/aos.13566. [DOI] [PubMed] [Google Scholar]
- 3.Alnawaiseh M, et al. Changes in corneal transparency after cross-linking for progressive Keratoconus: long-term follow-up. J Refract Surg. 2015;31(9):614–618. doi: 10.3928/1081597X-20150820-07. [DOI] [PubMed] [Google Scholar]
- 4.Alnawaiseh M, et al. Corneal densitometry, central corneal thickness, and corneal central-to-peripheral thickness ratio in patients with Fuchs endothelial dystrophy. Cornea. 2016;35(3):358–362. doi: 10.1097/ICO.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 5.Orucoglu F, et al. Corneal densitometry evaluation in archipelago keratitis. Int Ophthalmol. 2014;34(1):99–102. doi: 10.1007/s10792-013-9736-4. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa S, Kato N, Takeuchi M. Quantitative evaluation of corneal epithelial edema after cataract surgery using corneal densitometry: a prospective study. BMC Ophthalmol. 2018;18(1):334. doi: 10.1186/s12886-018-0998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen E, et al. Fuchs' endothelial corneal dystrophy: a controlled prospective study on visual recovery after endothelial keratoplasty. Acta Ophthalmol. 2016;94(8):780–787. doi: 10.1111/aos.13126. [DOI] [PubMed] [Google Scholar]
- 8.Lopes B, Ramos I, Ambrosio RJ. Corneal densitometry in keratoconus. Cornea. 2014;33(12):1282–1286. doi: 10.1097/ICO.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 9.Tekin K, et al. Corneal densitometry in healthy corneas and its correlation with endothelial Morphometry. Cornea. 2017;36(11):1336–1342. doi: 10.1097/ICO.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 10.Otri AM, et al. Corneal densitometry as an indicator of corneal health. Ophthalmology. 2012;119(3):501–508. doi: 10.1016/j.ophtha.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi A, Shimizu K, Kamiya K. Eight-year follow-up of posterior chamber Phakic intraocular Lens implantation for moderate to high myopia. Am J Ophthalmol. 2014;157(3):532–539. doi: 10.1016/j.ajo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Moya T, et al. Implantable Collamer Lens for myopia: assessment 12 years after implantation. J Refract Surg. 2015;31(8):548–U54. doi: 10.3928/1081597X-20150727-05. [DOI] [PubMed] [Google Scholar]
- 13.Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059-77. 10.2147/OPTH.S111620. [DOI] [PMC free article] [PubMed]
- 14.Parkhurst GD. A prospective comparison of phakic collamer lenses and wavefront-optimized laser-assisted in situ keratomileusis for correction of myopia. Clin Ophthalmol. 2016;10:1209–1215. doi: 10.2147/OPTH.S106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders D, Vukich JA. Comparison of implantable collamer lens (ICL) and laser-assisted in situ keratomileusis (LASIK) for low myopia. Cornea. 2006;25(10):1139–1146. doi: 10.1097/ICO.0b013e31802cbf3c. [DOI] [PubMed] [Google Scholar]
- 16.Sanders DR. Matched population comparison of the Visian implantable Collamer Lens and standard LASIK for myopia of −3.00 to −7.88 diopters. J Refract Surg. 2007;23(6):537–553. doi: 10.3928/1081-597X-20070601-02. [DOI] [PubMed] [Google Scholar]
- 17.Sanders DR, Vukich JA. Comparison of implantable contact lens and laser assisted in situ keratomileusis for moderate to high myopia. Cornea. 2003;22(4):324–331. doi: 10.1097/00003226-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi A, et al. Visual Performance after Implantable Collamer Lens Implantation and Wavefront-Guided Laser In Situ Keratomileusis for High Myopia. Am J Ophthalmol. 2009;148(1):164–170. doi: 10.1016/j.ajo.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya K, et al. Visual Performance After Posterior Chamber Phakic Intraocular Lens Implantation and Wavefront-Guided Laser In Situ Keratomileusis for Low to Moderate Myopia. Am J Ophthalmol. 2012;153(6):1178–1186. doi: 10.1016/j.ajo.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes P, et al. Implantable collamer posterior chamber intraocular lenses: a review of potential complications. J Refract Surg. 2011;27(10):765–776. doi: 10.3928/1081597X-20110617-01. [DOI] [PubMed] [Google Scholar]
- 21.Alfonso JF, et al. Clinical outcomes after implantation of a posterior chamber collagen copolymer phakic intraocular lens with a central hole for myopic correction. J Cataract Refract Surg. 2013;39(6):915–921. doi: 10.1016/j.jcrs.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Huseynova T, et al. Comparative study of 2 types of implantable Collamer lenses, 1 with and 1 without a central artificial hole. Am J Ophthalmol. 2014;157(6):1136–1143. doi: 10.1016/j.ajo.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, et al. Long-term comparison of posterior chamber Phakic intraocular Lens with and without a central hole (hole ICL and conventional ICL) implantation for moderate to high myopia and myopic astigmatism. Medicine. 2016;95(14):e3270. doi: 10.1097/MD.0000000000003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu K, et al. Intraindividual comparison of visual performance after posterior chamber phakic intraocular lens with and without a central hole implantation for moderate to high myopia. Am J Ophthalmol. 2012;154(3):486–494. doi: 10.1016/j.ajo.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Price MO, Price FJ. Evaluation of the toric implantable collamer lens for simultaneous treatment of myopia and astigmatism. Expert Rev Med Devices. 2015;12(1):25–39. doi: 10.1586/17434440.2015.984685. [DOI] [PubMed] [Google Scholar]
- 26.Senthil S, et al. Etiology and Management of Raised Intraocular Pressure following posterior chamber Phakic intraocular Lens implantation in myopic eyes. PLoS One. 2016;11(11):e0165469. doi: 10.1371/journal.pone.0165469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni DS, et al. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014;55(1):162–168. doi: 10.1167/iovs.13-13236. [DOI] [PubMed] [Google Scholar]
- 28.Elflein HM, et al. Measuring corneal clouding in patients suffering from mucopolysaccharidosis with the Pentacam densitometry programme. Br J Ophthalmol. 2013;97(7):829–833. doi: 10.1136/bjophthalmol-2012-302913. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, et al. Comparison of early changes in and factors affecting vault following posterior chamber phakic implantable Collamer Lens implantation without and with a central hole (ICL V4 and ICL V4c) BMC Ophthalmol. 2016;16(1):161. doi: 10.1186/s12886-016-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong J, et al. Corneal densitometry in high myopia. BMC Ophthalmol. 2018;18(1):182. doi: 10.1186/s12886-018-0851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamorita T, Uozato H, Shimizu K. Fluid dynamics simulation of aqueous humour in a posterior-chamber phakic intraocular lens with a central perforation. Graefes Arch Clin Exp Ophthalmol. 2012;250(6):935–939. doi: 10.1007/s00417-011-1850-2. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, et al. Experimental research on intraocular aqueous flow by PIV method. Biomed Eng Online. 2013;12(108):1-8. 10.1186/1475-925X-12-108. [DOI] [PMC free article] [PubMed]
- 33.Koh S, et al. Ocular forward light scattering and corneal backward light scattering in patients with dry eye. Invest Ophthalmol Vis Sci. 2014;55(10):6601–6606. doi: 10.1167/iovs.14-15125. [DOI] [PubMed] [Google Scholar]
- 34.Semes L, et al. The relationship among race, iris color, central corneal thickness, and intraocular pressure. Optom Vis Sci. 2006;83(7):512–515. doi: 10.1097/01.opx.0000225117.55813.e9. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Vives C, et al. Implantable collamer lens and femtosecond laser for myopia: comparison using an adaptive optics visual simulator. Arq Bras Oftalmol. 2014;77(2):103–109. doi: 10.5935/0004-2749.20140026. [DOI] [PubMed] [Google Scholar]
- 36.Ganesh S, Brar S, Pawar A. Matched population comparison of visual outcomes and patient satisfaction between 3 modalities for the correction of low to moderate myopic astigmatism. Clin Ophthalmol. 2017;11:1253–1263. doi: 10.2147/OPTH.S127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Y, et al. Comparison of implantable Collamer Lens Visian ICL V4 and ICL V4c for high myopia: a cohort study. Medicine (Baltimore) 2017;96(25):e7294. doi: 10.1097/MD.0000000000007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyun J, et al. A comparison of visual outcome and rotational stability of two types of toric implantable collamer lenses (TICL) : V4 versus V4c. PLoS One. 2017;12(8):e0183335. doi: 10.1371/journal.pone.0183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eppig T, et al. Ghost-image analysis in phakic intraocular lenses with central hole as a potential cause of dysphotopsia. J Cataract Refract Surg. 2015;41(11):2552–2559. doi: 10.1016/j.jcrs.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 40.Goukon H, et al. Comparison of corneal endothelial cell density and morphology after posterior chamber phakic intraocular lens implantation with and without a central hole. Br J Ophthalmol. 2017;101(11):1461–1465. doi: 10.1136/bjophthalmol-2016-309363. [DOI] [PubMed] [Google Scholar]
- 41.Edelhauser HF, et al. Corneal endothelial assessment after ICL implantation. J Cataract Refract Surg. 2004;30(3):576–583. doi: 10.1016/j.jcrs.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 42.Huang J, et al. Comparison of manual & automated analysis methods for corneal endothelial cell density measurements by specular microscopy. J Optom. 2018;11(3):182-191. 10.1016/j.optom.2017.06.001. [DOI] [PMC free article] [PubMed]
- 43.Huang J, et al. Comparison of noncontact specular and confocal microscopy for evaluation of corneal endothelium. Eye Contact Lens. 2018;44 Suppl 1:S144-S150. 10.1097/ICL.0000000000000362. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available upon request from the corresponding author at doctxiaoyingwang@163.com.