Abstract
Background
The incidence of delayed graft function in cadaveric grafts has increased over the last few years due in part to the large demand for cadaveric kidneys necessitating the use of kidneys from marginal donors. Calcium channel blockers have the potential to reduce the incidence of post‐transplant acute tubular necrosis (ATN) if given in the peri‐operative period. However, there is controversy surrounding their use in this situation with no consensus as to their efficacy.
Objectives
To evaluate the benefits and harms of using calcium channel blockers in the peri‐transplant period in patients at risk of ATN following cadaveric kidney transplantation.
Search methods
We searched the Cochrane Renal Group's specialised register, the Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library) MEDLINE (from 1966) and EMBASE (from 1980). The Trials Search Coordinator was contacted to develop the search strategy.
Selection criteria
Randomised controlled trials comparing calcium channel blockers given in the peri‐transplant period with controls were included. Quasi‐randomised trials were excluded.
Data collection and analysis
Data was extracted and quality assessed independently by two reviewers, with differences resolved by discussion. Dichotomous outcomes are reported as risk ratio (RR) and measurements on continuous scales are reported as mean differences (MD) with 95% confidence intervals (CI).
Main results
Thirteen trials (724 participants) were suitable for inclusion. Treatment with calcium channel blockers in the peri‐transplant period was associated with a significant decrease in the incidence of post‐transplant ATN (RR 0.62, 95% CI 0.46 to 0.85) and delayed graft function (RR 0.55, 95% CI 0.42 to 0.73). There was no difference between control and treatment groups in graft loss, mortality, requirement for haemodialysis. There was insufficient information to comment on adverse events.
Authors' conclusions
These results suggest that calcium channel blockers given in the peri‐operative period may reduce the incidence of ATN post‐transplantation. The result should be treated with caution due to the heterogeneity of the trials which made comparison of studies and pooling of data difficult.
Keywords: Humans; Calcium Channel Blockers; Calcium Channel Blockers/therapeutic use; Kidney Transplantation; Kidney Transplantation/adverse effects; Kidney Tubular Necrosis, Acute; Kidney Tubular Necrosis, Acute/prevention & control; Randomized Controlled Trials as Topic
Plain language summary
Calcium channel blockers can reduce the death of tubular cells in the kidney after a transplant operation
Acute tubular necrosis (ATN) is the sudden death of tubular cells in the kidney. ATN can happen after a kidney transplant if the kidney does not receive enough oxygen. Calcium channel blockers stop calcium ions flowing into the muscle cells of the heart and blood vessels. These blockers cause the muscles to widen and relax, lowering a person's blood pressure and improving their circulation. The review of 13 studies (724 participants) found that giving calcium channel blockers during a kidney transplant operation reduces the chance of ATN after the operation. The effect of giving the blockers after the operation still needs to be investigated.
Background
The incidence of delayed graft function in cadaveric grafts has increased over the last few years for both primary and re‐grafts. In 1999 the incidence of delayed graft function in Australia was 24% for primary grafts and 42% for re‐grafts (ANZDATA 2000). This high incidence is in part due to the large demand for cadaveric kidneys, which has necessitated the use of kidneys from marginal donors, and also the increase in total ischaemic time (ANZDATA 2000). Grafts with delayed function have a poorer long‐term survival than grafts that function immediately, with the difference between the two appearing after the first year and reaching 10% by nine years post‐transplant (ANZDATA 2000). The United States Renal Data System (USRDS) annual data report for 2006 defines delayed graft function as the need for dialysis during the first week after transplant. The rate of delayed graft function in 2004 was similar to the rate published in 1995 (21.4% and 20.9% respectively) (USRDS 2006).
After ischaemia there is a rise in intracellular calcium which has a number of detrimental effects including; a rise in intra‐mitochondrial calcium concentration which uncouples oxidative phosphorylation and reduces ATP production (Wilson 1984), activation of phospholipases causing an alteration in membrane enzyme and membrane damage (Chien 1980; Matthys 1984) and free radical generation (McCord 1985). Renal vasoconstriction also occurs.
Calcium channel blockers have the potential to protect glomerular filtration rate (GFR) during renal ischaemia by several mechanisms. These include,
Prevention of the rise in intra‐mitochondrial calcium
Prevention of re‐perfusion injury
Increased renal blood flow
Selective dilatation of pre‐glomerular vessels
Restoration of normal autoregulation during increased sympathetic stimulation
Evidence that calcium channel blockers are beneficial if given after the ischaemic event is not forthcoming. Most patients with acute tubular necrosis (ATN) present after the ischaemic event and therefore calcium channel blockers are unlikely to improve GFR in this situation. They do, however, have the potential to reduce the incidence of post‐transplant ATN if given in the peri‐operative period. In reality there is controversy surrounding their use in this particular situation, with authors disagreeing as to their effectiveness in reducing the incidence of initial non‐function of the graft (Donmez 1999; Harper 1996; Ladefoged 1994; Nicholson 1996). In addition, the studies use a variety of calcium channel blockers from different classes, given by different routes over different time periods. The issue is further clouded by the fact that some calcium channel blockers (especially diltiazem) may exert an immunosuppressive effect (Mandreoli 1990; McMillen 1985). Diltiazem interacts with cyclosporin and cyclosporin A pharmacokinetics and lower doses of these immunosuppressive drugs can be given to obtain therapeutic levels (Chrysostomou 1993; Morris 1998). Calcium channel blockers also minimise vasoconstrictive cyclosporin nephrotoxicity (Asberg 1997; Berg 1991).
Our aim was to review the literature and assess the effect calcium channel blockers given in the peri‐transplant period had on the incidence of post‐transplant ATN.
Objectives
Different calcium channel blockers clearly act in a number of ways in the transplant situation and separating out their various effects is difficult. However, we aimed to evaluate the benefits and harms of using calcium channel blockers in the peri‐transplant period in patients at risk of ATN following kidney transplantation. We only looked at the effect on delayed graft function due to amelioration of ATN which all calcium channel blockers have the potential to do. We did not examine in great detail the incidence of acute rejection episodes which is a different issue, and one which is not applicable to all calcium channel blockers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing any calcium channel blocker given in the peri‐transplant period with controls in patients who have had a kidney transplant. Quasi‐RCTs will not be included.
Types of participants
Kidney transplant recipients of any age, sex, or race and with any type of kidney disease causing end‐stage renal failure who receive a kidney transplant. Patients who had previous grafts were included. Patients receiving grafts from live donors were excluded.
Types of interventions
Any calcium channel blocker given by any route pre or immediately post‐transplant to the recipient ± donor or added to the perfusate. Control patients should receive identical treatment but no calcium channel blocker.
Types of outcome measures
The outcome measures include those used by transplant registries to assess the development of ATN and subsequent graft function.
Immediate graft function: defined as a spontaneous fall in serum creatinine of 10% within 24 hours or a spontaneous fall in serum creatinine of 10% within 25‐72 hours post‐transplant.
Poor immediate graft function: no spontaneous fall in creatinine (10%) within 72 hours but no further dialysis required.
No immediate function: no spontaneous fall (> 10%) in serum creatinine. Dialysis required within 72 hours.
Serum creatinine at one week and one month.
GFR at one week and one month.
Adverse effects of therapy.
Incidence of biopsy proven ATN.
Search methods for identification of studies
Relevant trials were initially obtained from the following sources (see Additional Table 1 ‐ Electronic search strategies)
1. Electronic search strategies.
| Database searched | Search terms |
| CENTRAL |
|
| MEDLINE (from 1966) |
|
| EMBASE (from 1980) |
|
Cochrane Renal Group specialised register of RCTs
Cochrane Central Register of Controlled Trials (CENTRAL in The Cochrane Library) for any "New" records not yet incorporated in the specialised register
MEDLINE and Pre MEDLINE (from 1966) were searched using the above terms, combined with the optimally sensitive strategy for the identification of RCTs (Dickersin 1994) (see Cochrane Renal Group Module).
EMBASE (from 1980) was searched using terms similar to those used for MEDLINE and combined with a search strategy for the identification of RCTs (Lefebvre 1996).
Reference lists of nephrology textbooks, review articles and relevant trials.
Conference proceeding's abstracts from nephrology scientific meetings.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous trials.
Studies in languages other than English will be included.
Data collection and analysis
Included and excluded studies
This review was undertaken by two reviewers. Titles and abstracts identified by the search strategy described were screened independently. All potentially relevant reviews were retained and the full text of these studies examined to determine which studies satisfied the inclusion criteria. Data extraction was carried out independently by the same reviewers using standard data extraction forms. Studies reported in languages other than those familiar to the authors were translated and evaluated in the presence of a native speaker of the language. Where more than one publication of one trial existed, only the publication with the most complete data was included. Where important data was not reported, we attempted to contact the original authors to get the necessary information. Discrepancies between the reviewers were resolved by discussion.
Study quality
The quality of studies to be included was assessed independently by two reviewers without blinding to authorship or journal, using the checklist developed for the Cochrane Renal Group. Discrepancies were resolved by discussion. The quality items assessed were allocation concealment, intention‐to‐treat analysis, completeness to follow‐up and blinding of investigators, participants,outcome assessors and data analysis.
Quality checklist
Allocation concealment
Adequate: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study
Unclear: Randomisation stated but no information on method used is available
Inadequate: Method of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group
Blinding
Investigators: Yes/No/Not stated
Participants: Yes/No/Not stated
Outcome assessor/s: Yes/No/Not stated
Data analysis: Yes/No/Not stated
The above are considered not blinded if the treatment group can be identified in >20% of participants because of the side effects of treatment.
Intention‐to‐treat analysis
Yes: Specifically reported by authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment.
Yes: Not stated but confirmed upon study assessment.
No: Not reported and lack of intention‐to‐treat analysis confirmed on study assessment (patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation).
No: Stated but not confirmed upon study assessment.
Completeness of follow‐up
Per cent of participants excluded or lost to follow‐up.
Statistical assessment
Dichotomous outcomes (need for rescue medication, rate of pain recurrence, adverse event rate) results are expressed as risk ratio (RR) with 95% confidence intervals (CI). Data was pooled using the random effects model but the fixed effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers. Where continuous scales of measurement were used to assess the effects of treatment (patient‐rated pain scores, time to pain relief), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used. Heterogeneity was analysed using a Chi squared test on N‐1 degrees of freedom, with a P of 0.05 used for statistical significance and the I² statistic (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity respectively.
Analysis was used to explore possible sources of heterogeneity (e.g. participants, treatments and study quality). Heterogeneity among participants could be related to age, stone size/site, and drug route/dose and where possible these subgroups were explored. Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose and duration of therapy. Where possible, the risk difference (RD) with 95% CI was to be calculated for adverse effects.
The applicability of the results to individual patients will be determined by calculating the reduction in risk of developing ATN post‐transplant in the treatment groups relative to the risk of post‐transplant ATN in the groups not given calcium channel blockers.
Where sufficient RCTs were identified, an attempt was made to examine for publication bias using a funnel plot (Egger 1997).
Results
Description of studies
Thirty two studies were identified from the initial search and 13 met our inclusion criteria. Four were duplicate publications. This left nine studies with 445 participants (Frei 1987; Ladefoged 1994; Lustig 1996; Neumayer 1989; Neumayer 1992a; Neumayer 1992b; Oppenheimer 1992; Tenschert 1991; Wilkie 1994). Wilkie 1994 had three study groups and groups P (placebo) and NS (nifedipine short duration) were suitable for inclusion in the meta‐analysis, and Neumayer 1992a and Neumayer 1992b reported two studies in one paper which have been treated as two separate studies in this review. A second search a year after publication of the review led to the inclusion of a tenth study (Kuypers 2004). The most recent search (January 2007) has led to the inclusion of three further studies (Dawidson 1991; Harper 1992; Morales 1990) giving a total of 724 participants in the review.
A variety of different calcium channel blockers were used;
diltiazem (seven studies: Frei 1987; Ladefoged 1994; Neumayer 1989; Neumayer 1992a; Neumayer 1992b; Oppenheimer 1992; Tenschert 1991),
nifedipine (three studies ‐ all retard preparation: Harper 1992; Morales 1990; Wilkie 1994),
verapamil (Dawidson 1991),
gallopamil (Lustig 1996) and
lacidipine (Kuypers 2004).
In six studies the grafts were perfused with Euro‐Collins solution containing calcium channel blocker ‐ diltiazem (Frei 1987; Ladefoged 1994; Neumayer 1989; Neumayer 1992a; Oppenheimer 1992) and gallopamil (Lustig 1996). Four study populations (Harper 1997Kuypers 2004; Morales 1990; Wilkie 1994) received oral therapy only. One group (Neumayer 1989) only treated the donor kidney by adding diltiazem to the perfusate, recipients did not receive calcium channel blocker. Five treatment groups (recipients) were given a bolus of calcium channel blocker followed by an infusion and then oral calcium channel blocker (Frei 1987; Ladefoged 1994; Neumayer 1992a; Neumayer 1992b; Oppenheimer 1992); two study groups did not receive a bolus of diltiazem prior to the infusion (Lustig 1996; Tenschert 1991). The duration of calcium channel blocker infusion before beginning oral therapy varied between groups. One study group (Dawidson 1991) were given 10 mg verapamil into the newly anastomosed renal artery in 2.5 mg increments to avoid hypotension, followed by oral verapamil.
Immunosuppression post‐transplant was not comparable between groups, although all patients in all studies were on calcineurin inhibitors. Four groups used cyclosporine and prednisolone (Frei 1987; Neumayer 1989; Neumayer 1992a; Neumayer 1992b, ). One group received cyclosporine only (Oppenheimer 1992), five received cyclosporine, prednisolone and azathioprine (Dawidson 1991; Ladefoged 1994; Lustig 1996; Tenschert 1991; Wilkie 1994),one of these also received ATG (Lustig 1996) and another Minnesota antilymphocyte globulin (Dawidson 1991). One study population received cyclosporine, mycophenolate mofetil and prednisone (Kuypers 2004).
SIX studies listed exclusion criteria which included current treatment with a calcium channel blocker, cardiac conduction abnormalities, congestive cardiac failure, liver disease, age less than 18 or greater than 65 years, no consent, PRA greater than 90%, high clinical urgency, intolerant of cyclosporine or azathioprine, intolerant of calcium channel blocker, allografts from donors receiving calcium channel blocker in the seven days prior to harvesting, already on an inducer of cytochrome P450, haplotype matched living related donor, systolic BP less than 90 mm Hg, history of bleeding and pregnant females (Dawidson 1991; Frei 1987; Kuypers 2004; Neumayer 1992a; Neumayer 1992b; Wilkie 1994).
The definitions of immediate, poor and no immediate function in the protocol were based on the definitions used by the Australian and New Zealand Transplant Registry (ANZDATA 2000). In reality the definition of ATN/initial non‐function varied widely between studies. One group did not supply a definition (Tenschert 1991). The other definitions were as follows:
Initial non‐function: need for dialysis during the first post‐operative week (Frei 1987).
Diagnosis of ATN based on the presence of oliguria and/or delayed decrease in serum creatinine, plus morphologic changes of ATN on biopsy (Lustig 1996).
Delayed graft function: continued need for dialysis post‐operatively, and for patients not on dialysis ‐ a lack of decrease in the serum creatinine (Ladefoged 1994).
Primary graft function: graft function without haemodialysis within the first seven days (Neumayer 1989; Neumayer 1992a; Neumayer 1992b).
Definition of ATN: need for dialysis within the first week after transplant (Oppenheimer 1992).
Initial non‐function: requirement for dialysis in the immediate post‐transplant period (Wilkie 1994).
Delayed graft function: need for dialysis post‐transplantation (Kuypers 2004).
Initial non‐function: dialysis dependency by fourth post operative day in the absence of graft rejection (Harper 1992)
The duration of follow up varied widely between studies as follows:
Six months follow‐up (Frei 1987; Oppenheimer 1992; Wilkie 1994).
Three months follow‐up (Ladefoged 1994; Lustig 1996).
GFR reported up to day seven, follow‐up for four weeks (Neumayer 1989).
GFR reported up to day seven, total follow‐up four years (Neumayer 1992a; Neumayer 1992b) .
No duration of follow‐up given (Tenschert 1991).
Two years follow‐up (Kuypers 2004).
Creatinine reported up to day 12, follow‐up from 17.1 ± 5.4 months (controls), 17.9 ± 5.8 (study group) (Dawidson 1991)
Authors were contacted for clarification of characteristics of patients and studies as well as results but no additional information was obtained.
Risk of bias in included studies
Allocation concealment
Eleven studies did not report any concealment approach. Wilkie 1994 used a computerised randomisation system which allocated a trial number and a unique treatment identifier number to each patient. Lustig 1996 used a study coordinator, who had no role in the selection and treatment of patients, to allocate the trial number.
Blinding
Four of the studies (Kuypers 2004; Ladefoged 1994; Lustig 1996; Wilkie 1994) were double‐blind. Two further studies (Frei 1987; Oppenheimer 1992) did not make it clear whether blinding took place or not and the remainder of the studies were not blinded.
Intention‐to‐treat
The use of intention‐to‐treat analysis was present in five studies (Frei 1987; Harper 1997; Morales 1990; Neumayer 1992a; Neumayer 1992b).
Completeness of follow‐up
Loss to follow‐up is uncommon in transplant patients and only one study reports two patients lost to follow‐up (Kuypers 2004).
Effects of interventions
Graft loss
Nine studies reported graft loss (Dawidson 1991; Frei 1987; Ladefoged 1994; Lustig 1996; Morales 1990; Neumayer 1992a; Neumayer 1992b; Oppenheimer 1992; Wilkie 1994). Results for Neumayer 1992a and Neumayer 1992b were given together and have been included as analysis 01.01.01. There was no significant difference between the use of calcium channel blockers and placebo/no treatment in the prevention of graft loss (Analysis 1.1: RR 0.76, 95% CI 0.42 to 1.39, P = 0.38; I² = 18.1%).
1.1. Analysis.
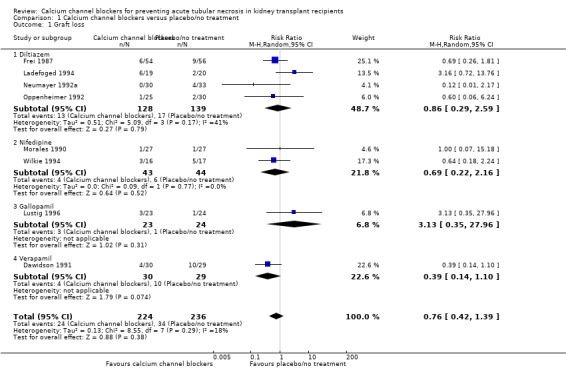
Comparison 1 Calcium channel blockers versus placebo/no treatment, Outcome 1 Graft loss.
Mortality
Seven studies reported mortality (Dawidson 1991; Frei 1987; Ladefoged 1994; Lustig 1996; Morales 1990; Oppenheimer 1992; Wilkie 1994). There was no significant difference between the use of calcium channel blockers and placebo/no treatment on mortality (Analysis 1.2: RR 0.55, 95%CI 0.13 to 2.35, P = 0.42; I² = 0%).
1.2. Analysis.
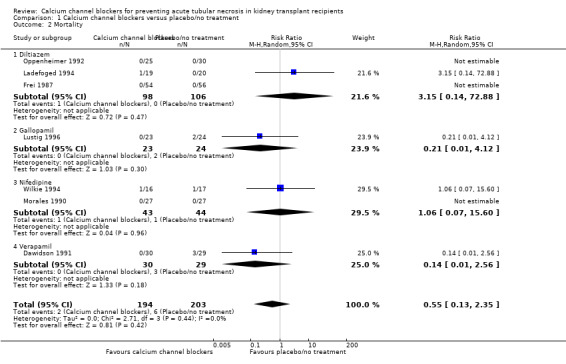
Comparison 1 Calcium channel blockers versus placebo/no treatment, Outcome 2 Mortality.
ATN
Eight studies reported ATN post‐transplant (Frei 1987; Ladefoged 1994; Lustig 1996; Morales 1990; Neumayer 1989; Neumayer 1992a; Neumayer 1992b; Oppenheimer 1992). There was a significant reduction in the number of patients with ATN post‐transplant in patients treated with calcium channel blockers (Analysis 1.3: RR 0.62, 95% CI 0.46 to 0.85, P = 0.003; I² = 18.2%).
1.3. Analysis.
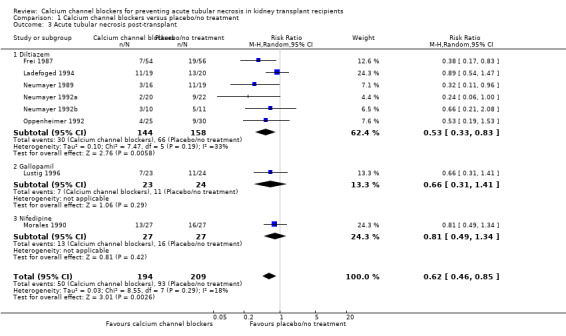
Comparison 1 Calcium channel blockers versus placebo/no treatment, Outcome 3 Acute tubular necrosis post‐transplant.
Only Lustig 1996 routinely biopsied all grafts to confirm the clinical diagnosis of ATN. Ladefoged 1994 routinely biopsied 34/39 patients comprising the study population. Frei 1987 and Oppenheimer 1992 make no mention of biopsies and the remaining five studies performed biopsies to diagnose episodes of acute rejection.
Renal function
GFR and creatinine clearance
Four studies reported GFR or creatinine clearance at days one and seven and at one, three and six months (Kuypers 2004; Ladefoged 1994; Neumayer 1989; Wilkie 1994). These measures have been included on the same plot, with the summary point turned off. There were no significant differences in GFR/creatinine clearance at any of the measured time points (Analysis 1.4). Data from one study (Dawidson 1991) has not been included in this outcome as it is not clear from the text if only data from patients with GFR > 10 mL/min has been reported. Clarification has been sought.
1.4. Analysis.
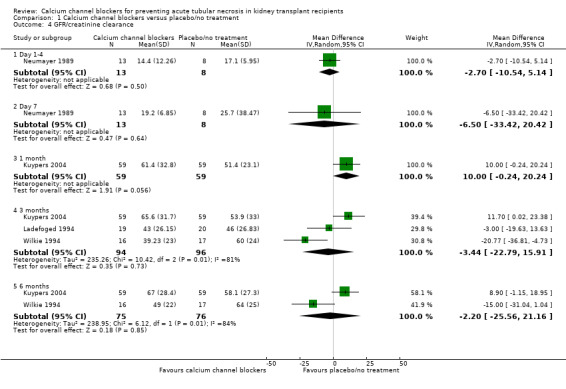
Comparison 1 Calcium channel blockers versus placebo/no treatment, Outcome 4 GFR/creatinine clearance.
Serum creatinine
Nine studies reported serum creatinine levels at various time points (Dawidson 1991; Frei 1987; Harper 1997; Kuypers 2004; Ladefoged 1994; Lustig 1996; Morales 1990; Oppenheimer 1992; Tenschert 1991). There was a significant reduction in serum creatinine at day two for the calcium channel blocker group (Analysis 1.5.1: MD ‐248.00 μmol/L, 95%CI ‐441.17 to ‐54.83, P = 0.01). Similar significant reductions were seen at day 7 (analysis 01.05.02: MD ‐87.61 μmol/L, 95% CI ‐147.95 to ‐27.27, P = 0.004; I² = 0%) and day 14 (Analysis 1.5.3: MD ‐129.00 μmol/L, 95% CI ‐149.74 to ‐108.26, P < 0.00001). However for one, three and six months post‐transplant there were no significant differences between the calcium channel blocker and the placebo/no treatment groups.
1.5. Analysis.
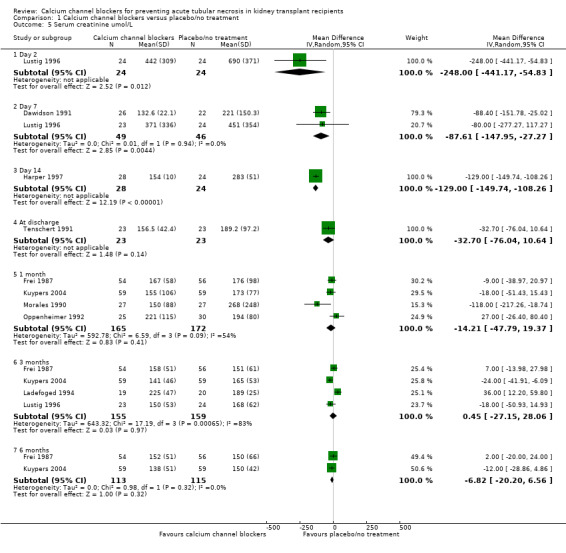
Comparison 1 Calcium channel blockers versus placebo/no treatment, Outcome 5 Serum creatinine umol/L.
Delayed graft function
Nine studies reported delayed graft function (Dawidson 1991Frei 1987; Harper 1997; Kuypers 2004; Morales 1990; Neumayer 1989; Neumayer 1992a; Neumayer 1992b; Wilkie 1994). There was a significant reduction in the number of patients with delayed graft function in the calcium channel blocker group (Analysis 1.6: RR 0.55, 95% CI 0.42 to 0.73; P = 0.00003; I² = 2.2%).
1.6. Analysis.
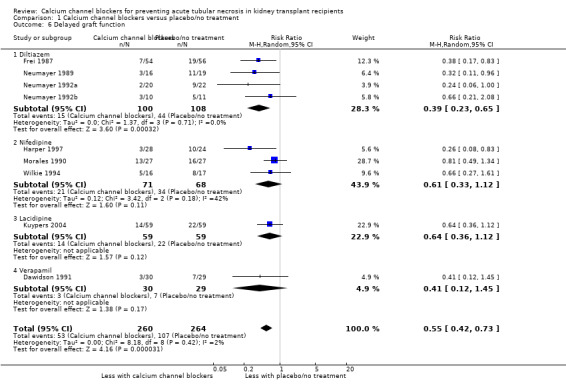
Comparison 1 Calcium channel blockers versus placebo/no treatment, Outcome 6 Delayed graft function.
Requirement for haemodialysis post‐operatively
Five studies reported the number of haemodialysis sessions/patient required post‐operatively (Morales 1990; Neumayer 1989; Neumayer 1992a; Neumayer 1992b; Tenschert 1991). All three studies by Neumayer give the results as the mean number of dialysis sessions/patient for the whole group (i.e. those with immediate graft function are included) rather than only those with delayed graft function who required dialysis. Neumayer 1992a, two patients with delayed graft function in the diltiazem group required a total of 12 dialysis sessions between them, and in the control group with delayed graft function nine patients required a total of 78 haemodialysis sessions. The number of dialysis/patient in the remaining two studies (Morales 1990; Tenschert 1991) tended to be higher in the control groups but this did not reach statistical significance.
Adverse events
Four studies reported adverse events. Ladefoged 1994 reported one death due to cytomegalovirus (CMV) infection and one episode of life threatening sepsis in the diltiazem group. Wilkie 1994 reported two cases of headache and oedema associated with nifedipine therapy, eight cases of CMV infection, and two cases of pneumocystis pneumonia (group/s not stated). One patient in the nifedipine group also developed Epstein Barr virus‐induced cerebral lymphoma and one patient in the control group had a cerebrovascular event. It is not clear whether the side effects reported in the nifedipine group occurred in different patients. Kuypers 2004 reports an extensive list of side effects although there was no significant difference in the incidence of adverse or serious adverse events between the calcium channel blocker and placebo groups. The most commonly reported side effects in this study were hypertension, constipation, oedema, diarrhoea, palpitations and tachycardia. Dawidson 1991 reported three deaths in the control group due to intestinal perforation, pneumonia and liver failure.
Discussion
Very few studies have been designed specifically to look at the effect that calcium channel blockers have on the development of ATN post‐transplantation. This meta‐analysis of thirteen studies showed that the use of calcium channel blockers in the peri‐operative period reduced the incidence of ATN post‐transplantation. However, this result should be treated with caution due to the small number and heterogeneity of trials. Immunosuppression post‐transplant was not comparable between groups, although all patients were on a calcineurin inhibitor. In addition, the type and administration of the calcium channel blockers varied between studies. In seven of the studies the graft was not perfused with perfusate containing calcium channel blocker and one study (Neumayer 1989) assessed the effect of calcium channel blocker by only treating the donor kidney. The use of different types of calcium channel blockers may have affected the outcome.
Lustig 1996 did not show a significant difference between calcium channel blocker and placebo groups but, when analysed separately, the clinical course of recipients who received kidneys from donors aged greater than 50 years showed a significantly higher rate of ATN in the placebo group compared to matched calcium channel blocker recipients (91% versus 36%, P < 0.02). Donor age and cold ischaemic times were apparently comparable (no data provided) but the age of recipients in the treatment group was significantly lower. By three months post‐transplant there was no significant difference in serum creatinine. This finding needs further investigation as the use of kidneys from older donors is becoming much more common place due to the shortage of kidneys for transplantation. If this result can be reduplicated calcium channel blockers may have a role in reducing the risk of ATN in high‐risk patients receiving kidneys from older donors.
Only one study routinely performed biopsies to confirm the clinical diagnosis of ATN, and the definition of ATN differed widely between studies, which contributes to the difficulty in comparing studies and pooling data. The small number of studies available for review is disappointing and makes it difficult to come to any definite conclusion about the effects of calcium channel blockers on ATN in the post‐transplant period. Overall study quality was poor with only one study having adequate allocation concealment. Inadequate allocation concealment may over exaggerate the efficacy of the experimental treatment and meta‐analysis of trials with inadequate allocation concealment can overestimate the benefits of treatment.
Exclusion of unpublished trials will result in publication bias. As unpublished trials are more likely to show no effect of treatment, publication bias will also over estimate the benefits of treatment. Funnel plot asymmetry will indicate whether or not bias is present but due to the small number of studies was not possible.
It is difficult to assess what the long‐term gain of a decrease in the incidence of ATN, as patients were followed up at different time periods and for different lengths of time. It would appear from the limited data available that calcium channel blockers given in the peri‐operative period have no effect on renal function at three and six months post‐transplant.
Authors' conclusions
Implications for practice.
Calcium channel blockers given in the peri‐operative period appear to reduce the incidence of ATN post‐transplantation. The result should be treated with caution due to the small number of trials available and the heterogeneity of the trials, in particular the use of different calcium channel blockers given by different routes, the different definitions of ATN/initial non‐function, marked differences in the immunosuppressive regimens and different lengths of follow‐up.
Implications for research.
Studies designed specifically to determine the relationship between the post‐operative administration of calcium channel blockers and the incidence of ATN post‐transplantation are still desirable. They should also assess the long‐term benefits of any reduction in ATN. Subsequent trials should include protocol biopsies.
What's new
| Date | Event | Description |
|---|---|---|
| 22 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 15 February 2007 | New search has been performed | Three new studies included |
Acknowledgements
We would like to thank Mr Peter Cook and Drs Bryan Becker, Jeremy Chapman, Vincent Lee, Alison MacLeod, Teut Risler and Robert Walker for their editorial advice during the preparation of this review.
Data and analyses
Comparison 1. Calcium channel blockers versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft loss | 8 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.42, 1.39] |
| 1.1 Diltiazem | 4 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.29, 2.59] |
| 1.2 Nifedipine | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.16] |
| 1.3 Gallopamil | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [0.35, 27.96] |
| 1.4 Verapamil | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.10] |
| 2 Mortality | 7 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.13, 2.35] |
| 2.1 Diltiazem | 3 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.14, 72.88] |
| 2.2 Gallopamil | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.12] |
| 2.3 Nifedipine | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.07, 15.60] |
| 2.4 Verapamil | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.56] |
| 3 Acute tubular necrosis post‐transplant | 8 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.85] |
| 3.1 Diltiazem | 6 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.83] |
| 3.2 Gallopamil | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.31, 1.41] |
| 3.3 Nifedipine | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.49, 1.34] |
| 4 GFR/creatinine clearance | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Day 1‐4 | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐10.54, 5.14] |
| 4.2 Day 7 | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐6.50 [‐33.42, 20.42] |
| 4.3 1 month | 1 | 118 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐0.24, 20.24] |
| 4.4 3 months | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐3.44 [‐22.79, 15.91] |
| 4.5 6 months | 2 | 151 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐25.56, 21.16] |
| 5 Serum creatinine umol/L | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Day 2 | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐248.0 [‐441.17, ‐54.83] |
| 5.2 Day 7 | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐87.61 [‐147.95, ‐27.27] |
| 5.3 Day 14 | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐129.0 [‐149.74, ‐108.26] |
| 5.4 At discharge | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐32.70 [‐76.04, 10.64] |
| 5.5 1 month | 4 | 337 | Mean Difference (IV, Random, 95% CI) | ‐14.21 [‐47.79, 19.37] |
| 5.6 3 months | 4 | 314 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐27.15, 28.06] |
| 5.7 6 months | 2 | 228 | Mean Difference (IV, Random, 95% CI) | ‐6.82 [‐20.20, 6.56] |
| 6 Delayed graft function | 9 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| 6.1 Diltiazem | 4 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.23, 0.65] |
| 6.2 Nifedipine | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.33, 1.12] |
| 6.3 Lacidipine | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.12] |
| 6.4 Verapamil | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dawidson 1991.
| Methods | Randomised: yes Blinded: unknown Intention to treat: no | |
| Participants | INCLUSION CRITERIA
No history of calcium channel blocker use. TREATMENT GROUP (verapamil) Number: 30 Age: 36.4 ± 12.4 years. Cold ischaemic time: 19.3 ± 8.6 hours HLA A, B, DR mismatches: 1.0 ± 0.9 DR mismatches 1.3 ± 0.7 PRA: 15.3% ± 29.6 CONTROL GROUP Number: 29 Age: 41.1 ± 12.2 years Cold ischaemic time: 20.1 ± 8.1 hours. HLA A, B, DR mismatches: 1.0 ± 1.0 DR mismatches 1.5 ± 0.6 PRA: 20.6 ± 28.2 EXCLUSION CRITERIA Pre‐op CCB use, cardiac arrythmia, no consent, failure to randomise. |
|
| Interventions | TREATMENT GROUP
Graft perfused with Euro‐Collins (13) or University of Wisconsin (17).
10 mg verapamil given intra‐arterially into newly anastomosed renal artery in 2.5 mg increments.
Post operatively 120 mg slow release verapamil given twice daily for 14 days. CONTROL GROUP Graft perfused with Euro‐Collins (14) or university of Wisconsin (15). IMMUNOSUPPRESSION Identical in both groups. Methylprednisolone 375 mg iv on the day of surgery (day 0) tapered to prednisolone 20 mg/d by day 10. Azathioprine 100 mg (day 0) and decreased to 25 mg daily for 5 days. Minnesota antilymphocyte globulin 15 mg/kg/d iv on post‐op days 1 to 5. If not dialysis dependent CSA A 7 mg/kg started on day 6 and increased to 12 mg/kg on day 7. Dose adjusted according to renal function and trough level. |
|
| Outcomes | 1. Number with immediate graft function 2. Number with delayed graft function 3. Serum creatinine days 1 to 12 4. GFR days 1‐3, 7‐9. | |
| Notes | Definition of delayed graft function: GFR < 10 mL/min at day 7. Title of table including daily creatinine for days 1‐12 indicated results are for 30 patients receiving verapamil and 29 controls. Text comments on results for day 7 and says that patients with DGF have been removed i.e. number for verapamil 26 and control 22. Only results for day 7 used as not clear how many patients have DGF on other days. Only GFR for days 7‐9 included as number of patient included in days 1‐3 not clear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Frei 1987.
| Methods | Randomised: yes Blinded: unknown Intention‐to‐treat: yes | |
| Participants | TREATMENT GROUP (diltiazem)
Number: 54
Age: 41.2 ± 10 years
Donor age: 33.5 ± 14 years
Cold ischaemia time (hours): 26.43 ± 3.15
Warm ischaemia time (min): 7 ± 8
HLA mismatch (AB): 2.3 ± 1.1
HLA mismatch (DR): 0.4 ± 0.4
Diuresis ‐ donor (mL/h) 383 ± 243
Donor creatinine (umol/L): 102.1 ± 48 CONTROL GROUP Number: 56 Age: 40.7 ± 12 years Donor age: 35.3 ± 12 years Cold ischaemia time (hours): 25.49 ± 3.62 Warm ischaemia time (min): 37 ± 7 HLA mismatch (AB): 2.3 ± 1 HLA mismatch (DR): 0.6 ± 0.5 Diuresis ‐ donor (mL/h): 391 ± 307 Donor creatinine (umol/L): 91.2 ± 38 EXCLUSION CRITERIA Cardiac conduction disturbances, CCF, liver disease, age < 18 or > 65, no consent |
|
| Interventions | TREATMENT GROUP (diltiazem)
Graft perfused with Euro‐Collins.
Diltiazem 100 mg/L added to perfusate.
IV diltiazem 0.28 mg/kg 2 hours pre‐op followed by an infusion of diltiazem 0.12 mg/kg/h for up to 72 hours. Then 90 mg orally bd until day 30. IMMUNOSUPPRESSION Same in both groups ‐ prednisolone 1 mg/kg tapered to 7.5 mg daily by 3 months, CSA A 10 mg/kg/d 6 hours post‐op orally in 2 divided doses. Dose adjusted ‐ trough (RIA) 400‐600 ng/mL. |
|
| Outcomes | 1. Number with immediate graft function 2. Number with delayed graft function 3. GFR at 1 month | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Harper 1997.
| Methods | Randomised: yes Blinded: unknown Intention to treat: yes | |
| Participants | TREATMENT GROUP (nifedipine retard)
Number: 28
PRA status: 14.5%. CONTROL GROUP Number: 24 PRA status: 2.3%. |
|
| Interventions | TREATMENT GROUP (nifedipine retard)
Oral nifedipine retard 10 mg three times daily for one week then 20 mg twice daily.
Increased to 40 mg twice daily if necessary for BP. First dose given pre‐operatively. IMMUNOSUPPRESSION Same in both groups: CSA 17 mg/kg/d reduced by 2 mg/kg/wk to maintenance of 7mg/kg/d at 6 weeks. Identical prednisolone regimens in both groups (dose not given) |
|
| Outcomes | 1. Number with immediate graft function 2. Number with delayed graft function 3. Creatinine at day 14 | |
| Notes | Three study groups. Groups A and B suitable for inclusion. Definition of DGF: dialysis dependence by 4th post operative day in the absence of graft rejection. No significant difference between groups in donor or recipient age, HLA mismatches, total and cold ischaemic time, anastomosis time or graft perfusion fluid. No details given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kuypers 2004.
| Methods | Randomised: yes Blinded: no Intention to treat: no | |
| Participants | INCLUSION CRITERIA
Age: 18‐65 years
Primary cadaveric kidney transplantation
Donor age 10‐65 years
Written informed consent TREATMENT GROUP (lacidipine) Number: 66 Age: 46.5 ± 12.6 years Donor age: 37.9 ± 16 years Cold ischaemia time (hours): 17.42 ± 3.73 HLA mismatch: 2.48 ± 1.3 CONTROL GROUP Number: 65 Age: 48.3 ± 12.6 years Donor age: 43.5 ± 14.8 years Cold ischaemia time (hours): 17.7 ± 5.4 HLA mismatch: 2.43 ± 1.2 EXCLUSION CRITERIA Pregnancy. Women of childbearing age had to be taking adequate contraception for the duration of the study. |
|
| Interventions | TREATMENT GROUP
Lacidipine 2 mg daily immediately after transplant. Dose increased at 1 and 3 weeks if diastolic BP remained elevated.
Thereafter other antihypertensives (excluding CCB) were added in.
Preservation fluid: University of Wisconsin (67.2%), HTK (5.2%), Euro‐Collins (20.7), other (6.9%). CONTROL GROUP Preservation fluid: University of Wisconsin (58.6%), HTK (12%), Euro‐Collins (19%), Other (8.6%). IMMUNOSUPPRESSION Both groups received CSA (dose adjusted to maintain trough 100‐250 ng/mL), MMF 1 g twice daily, prednisone 0.5 mg/kg/d for one month, tapering to a minimum maintenance dose of 5 mg/d. |
|
| Outcomes | 1. Delayed graft function 2. Rejection episodes 3. Serum creatinine 1, 3, 6, 12, 18 and 24 months 4. Creatinine clearance and GFR 1, 3, 6, 12, 18 and 24 months 5. Adverse events and hospitalisation | |
| Notes | Definition of DGF: need for dialysis post transplantation. 131 enrolled, 41 patients withdrawn (adverse events 21, patient's refusal 7, investigator's decision 7, lost from follow up 2). All 131 patients evaluated in the safety analysis, 118 included in the ITT analysis. 13 excluded from ITT because of missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ladefoged 1994.
| Methods | Randomised: yes Double blind: yes Intention‐to‐treat: no | |
| Participants | TREATMENT GROUP (diltiazem)
Number: 19
Age: 42 years (range 21‐64)
Donor age: 45 years (range 12‐64)
Cold ischaemia time (hours): 22.6 (15‐35)
Warm ischaemia time (min): 9.0 (0‐22)
HLA mismatch (AB): 1.9 (0‐3)
HLA mismatch (DR): 0.4 (0‐2)
Diuresis ‐ donor (mL/h): not reported
Donor creatinine: not reported CONTROL GROUP Number: 20 Age: 45 years (range 20‐64) Donor age: 41 years (range 16‐61) Cold ischaemia time (hours): 23.7 (12‐39) Warm ischaemia time (min): 9.6 (0‐33) HLA mismatch (AB): 1.6 (0‐3) HLA mismatch (DR): 0.6 (0‐2) Diuresis ‐ donor (mL/h): not reported Donor creatinine: not reported |
|
| Interventions | Grafts perfused with Euro‐Collins perfusate. TREATMENT GROUP Grafts also perfused with diltiazem 20 mg/L. Recipients given diltiazem bolus 0.3 mg/kg pre‐op, then infusion 3 mg/kg/24 h then 60‐120 mg tid orally. CONTROL GROUP Placebo 0.3 mg/kg bolus, then infusion 3 mg/kg/24 h then 60‐120 mg tid orally. IMMUNOSUPPRESSION Same in both groups ‐ CSA, prednisolone and azathioprine. |
|
| Outcomes | 1. DGF 2. Rejection 3. re‐rejection 4. Graft survival 5. Creatinine clearance 6. Serum creatinine | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lustig 1996.
| Methods | Randomised: yes Double blind: yes Intention‐to‐treat: no | |
| Participants | TREATMENT GROUP (gallopamil)
Number: 23
Age: 37 ± 12 years
Donor age (years): 51 ± 15
Cold ischaemia time (hours): 19 ± 5
Warm ischaemia time (min): not reported
HLA mismatch: not reported
Diuresis, donor (mL/h): not reported
Donor creatinine mg/dL (umol/L): 1.2 ± 0.3 (106 ± 26.5) CONTROL GROUP Number: 24 Age: 35 ± 17 years Donor age (years): 42 ± 21 Cold ischaemia time (hours): 19 ± 6 Warm ischaemia time (min): not reported HLA mismatch: not reported Diuresis, donor (mL/h): not reported Donor creatinine mg/dL (umol/L): 1.2 ± 0.3 (106 ± 26.5) |
|
| Interventions | Grafts perfused with Euro‐Collins solution. TREATMENT GROUP Gallopamil 12 mg/L added to perfusate. Recipient given gallopamil 0.00015 mg/min/kg infusion for 12 hours then 75 mg bd for 3 months. CONTROL GROUP Placebo 0.00015 mg/min/kg infusion for 12 hours followed by placebo 75 mg bd for 3 months. IMMUNOSUPPRESSION Same in both groups: ATG 100 mg/d for 10 days, CSA from day 5, azathioprine 2mg/kg/d from day 5, prednisolone from day 1. |
|
| Outcomes | 1. Oliguric ATN. 2. Serum creatinine days 2, 7, 120. 3. Graft survival. 4. Patient survival. | |
| Notes | DEFINITION OF ATN Oliguria and /or delayed decrease in serum creatinine, together with morphologic changes typical of ATN on aspiration or core biopsy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Morales 1990.
| Methods | Randomised: yes Double blind: no Intention‐to‐treat: yes | |
| Participants | TREATMENT GROUP (nifedipine retard)
Number: 27
Recipient age: 46.8 ± 9.5
Cold ischaemic time (hours): 23.8 ± 1.9
HLA DR : Rx 1.7 ± 0.8 CONTROL GROUP Number: 27 Recipient age: 38.9 ± 13.5 Cold ischaemic time (hours): 22.6 ± 3.8 HLA DR: 1.8 ± 0.4 |
|
| Interventions | TREATMENT GROUP
Nifedipine retard 20 mg pre‐op followed by 40 mg /day for the first 15 post‐op days. CONTROL GROUP Placebo IMMUNOSUPPRESSION The same in both groups. Steroids 0.5 mg/kg/d. CSA A 10 mg/kg before surgery and at t = 24 hours, dose reduced gradually according to trough levels or side effects. |
|
| Outcomes | 1. Immediate graft function 2. Acute tubular necrosis ‐ not biopsy proven 3. Rejection episodes 4. Length of hospital stay 5. Number of haemodialysis/patient 6. Plasma creatinine | |
| Notes | Definition of DGF | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Neumayer 1989.
| Methods | Randomised: yes Blinded: no Intention‐to‐treat: yes | |
| Participants | TREATMENT GROUP (Diltiazem)
Number: 16
Age: 46 ± 12 years
Donor age: 47 ± 16 years
Ischaemia time
Cold ischaemia time (hours): 21 ± 4
Warm ischaemia time (min): 34 ± 4
Mismatches: 2.8 ± 0.8
Diuresis ‐ donor (mL/h): not reported
Donor creatinine: 102 ± 68 umol/L CONTROL GROUP Number: 19 Age: 48 ± 13 years Donor age: 42 ± 17 years Ischaemia time Cold ischaemia time (hours): 21 ± 4 Warm ischaemia time (min): 37 ± 9 Mismatches: 2 ± 0.9 Diuresis ‐ donor (mL/h): not reported Donor creatinine: 141 ± 144 umol/L |
|
| Interventions | Grafts perfused with Euro‐Collins solution. TREATMENT GROUP Diltiazem 20 mg/L added to perfusate of study group. Recipients not given calcium antagonist. IMMUNOSUPPRESSION CSA, steroids |
|
| Outcomes | 1. Primary graft function 2. DGF 3. Haemodialysis 4. Renal perfusion 5. Rejection episodes | |
| Notes | Results have been converted from mean ± SEM to mean ± SD. Four groups: 1. control, 2. diltiazem, 3. iloprost and 4. diltiazem plus iloprost. Data from control and diltiazem groups extracted. No exclusion criteria given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Neumayer 1992a.
| Methods | Randomised: yes Blinded: no Intention‐to‐treat: yes | |
| Participants | TREATMENT GROUP (diltiazem)
Number: 20
Age: 42 ± 4 years
Donor age: 44 ± 4 years
Cold ischaemia time (hours): 19 ± 0
Warm ischaemia time (min): 31 ± 0
HLA mismatch: 2.7 ± 0.4
Diuresis ‐ donor (mL/h): 269 ± 54
Donor creatinine: 92 ± 4 umol/L CONTROL GROUP Number: 22 Age: 40 ± 5 years Donor age: 38 ± 5 years Cold ischaemia time (hours): 21 ± 0 Warm ischaemia time (min): 38 ± 0 HLA mismatch: 2.9 ± 0.5 Diuresis ‐ donor (mL/h): 292 ± 52 Donor creatinine: 105 ±9 umol/L EXCLUSION CRITERIA No consent, systolic BP < 91 mm Hg, CCF, heart block without pacemaker, history of bleeding |
|
| Interventions | Grafts perfused with Euro‐Collins solution. TREATMENT GROUP Perfusion fluid also contained diltiazem 20 mg/L. Recipient: bolus diltiazem 0.28 mg/kg then infusion 0.002 mg/min/kg for 2 days, then 60 mg bd for 396 ± 79 days. IMMUNOSUPPRESSION For both groups: CSA, prednisolone. |
|
| Outcomes | 1. Primary graft function. 2. Delayed graft function 3. HD post transplant 4. Rejection episodes | |
| Notes | Three randomised studies in one paper. Study one suitable for inclusion. Results have been converted from mean ± SEM to mean ± SD. Primary graft function defined as no HD required within the first 7 days post transplant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Neumayer 1992b.
| Methods | Randomised: yes Blinded: no Intention‐to‐treat: yes | |
| Participants | TREATMENT GROUP (diltiazem)
Number: 10
Age: 43 ± 6 years
Donor age: 34 ± 6 years
Cold ischaemia time (hours): 25 ± 3
Warm ischaemia time (min): 37 ± 6
HLA mismatch: 1.7 ± 0.9
Diuresis ‐ donor (mL/h): 324 ± 76
Donor creatinine: 102 ±13 umol/L CONTROL GROUP Number: 11 Age: 48 ± 3 years Donor age: 35 ± 3 years Cold ischaemia time (hours): 25 ± 0 Warm ischaemia time (min): 40 ± 7 HLA mismatch: 1.7 ± 1.3 Diuresis ‐ donor (mL/h): 385 ± 90 Donor creatinine: 104 ± 10 umol/L EXCLUSION CRITERIA No consent, systolic BP < 91 mm Hg, CCF, heart block without pacemaker, history of bleeding |
|
| Interventions | Grafts perfused with Euro‐Collins solution. Kidney not pre treated with calcium antagonist. Treatment group: Recipient ‐ bolus diltiazem 0.28 mg/kg then infusion 0.002 mg/kg/m for 2 days, then 60 mg bd for 396 +/‐ 79 days. Immunosuppression: For both groups ‐ cyclosporin, prednisolone. | |
| Outcomes | 1. Primary graft function. 2. Delayed graft function 3. HD post transplant 4. Rejection episodes | |
| Notes | Three randomised studies in one paper. Study two suitable for inclusion. Results have been converted from mean ± SEM to mean ± SD. Primary graft function defined as no HD required within the first 7 days post transplant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Oppenheimer 1992.
| Methods | Randomised: yes Blinded: unknown Intention‐to‐treat: unknown | |
| Participants | No inclusion or exclusion criteria specified. TREATMENT GROUP (diltiazem) Number: 25 Age: 46.91 years Donor age: 34.86 years Cold ischaemia time (hours): 14.8 Warm ischaemia time (min): not reported HLA mismatch: not reported Diuresis ‐ donor (mL/h): not reported Donor creatinine: not reported CONTROL GROUP Number: 30 Age: 43.9 years Donor age: 29.66 years Cold ischaemia time (hours): 15.9 Warm ischaemia time (min): not reported HLA mismatch: not reported Diuresis ‐ donor (mL/h): not reported Donor creatinine: not reported |
|
| Interventions | Graft perfused with Euro‐Collins ± diltiazem 20 mg/L. TREATMENT GROUP Did not receive dopamine Recipients: Given Diltiazem bolus 0.28 mg/kg followed by an infusion 0.12 mg/kg/h for 72 hours, then 60 mg bd from day 4‐6 weeks. CONTROL GROUP Had dopamine infusion. IMMUNOSUPPRESSION CSA only (both groups). |
|
| Outcomes | 1. Incidence and duration of ATN 2. Rejection episodes 3. Renal function 4. CSA level | |
| Notes | 25 patients randomised to diltiazem group in text but table only has 23 patients in diltiazem group, with no mention in the text as to what happened to the other 2 patients ?typographical error Definition of ATN: Dialysis needed within first week post transplant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tenschert 1991.
| Methods | Randomised: yes Blinded: no Intention‐to‐treat: yes | |
| Participants | TREATMENT GROUP (diltiazem)
Number: 23
Age: 42.65 ± 14.22 years
Donor age: not reported
Cold ischaemia time (hours): 27.75 ± 8.33
Warm ischaemia time (min): not reported
HLA mismatch: not reported
Diuresis ‐ donor (mL/h): not reported
Donor creatinine: not reported CONTROL GROUP Number: 23 Age: 43.37 ± 15.12 years Donor age: not reported Cold ischaemia time (hours): 27.89 ± 5.51 Warm ischaemia time (min): not reported HLA mismatch: not reported Diuresis ‐ donor (mL/h): not reported Donor creatinine: not reported |
|
| Interventions | Grafts not perfused. TREATMENT GROUP Recipients: diltiazem 1.7 mg/kg/24 h immediately post‐op for 72 hours, then 30 mg tid or qid. IMMUNOSUPPRESSION For both groups ‐ prednisolone, azathioprine, CSA. |
|
| Outcomes | 1. Rejection episodes 2. Dialysis therapy 3. Length of hospital stay | |
| Notes | HD stopped when creatinine < 442 umol/L during the dialysis free interval | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wilkie 1994.
| Methods | Randomised: yes Double blind: yes Intention‐to‐treat: no | |
| Participants | TREATMENT GROUP (nifedipine)
Number: 16
Age: 40 ± 14 years
Donor age: not reported
Cold ischaemia time (hours): not reported
Warm ischaemia time (min): not reported
HLA mismatch: not reported
Diuresis ‐ donor (mL/h): not reported
Donor creatinine: not reported CONTROL GROUP Number: 17 Age: 44 ± 13 years Donor Age: not reported Cold ischaemia time (hours): not reported Warm ischaemia time (min): not reported HLA mismatch: not reported Diuresis ‐ donor (mL/h): not reported Donor creatinine: not reported |
|
| Interventions | TREATMENT GROUP
Nifedipine LA 20 mg bd for 48 hours followed by placebo for a total of three months CONTROL GROUP Placebo for 3 months IMMUNOSUPPRESSION Azathioprine, prednisolone and CSA |
|
| Outcomes | 1. Early graft function 2. Renal function at 3 and 6 months post transplantation | |
| Notes | Paper includes three study groups P (placebo) and NS (Nifedipine) are suitable for inclusion. Definition of DGF ‐ requirement for dialysis in the immediate post transplant period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
BP ‐ blood pressure; CCB ‐ calcium channel blocker; CCF ‐ chronic cardiac failure; CSA ‐ cyclosporin; DGF ‐ delayed graft function; MMF ‐ mycophenolate mofetil
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aros 2005 | Study investigated the correlation between concentration of CSA 2 hours after dosing with absorption area under the curve over the first 4 hours. |
| Barenbrock 1995 | Looking at effect of nitrendipine on graft function ‐ randomisation into study 6‐12 weeks post‐transplant. |
| Berg 1991 | Not given in peri‐operative period. Not RCT, no data re type of donors. |
| Calo 2002 | Study looking at the anti‐oxidant effect of carvedilol in post‐transplant patients. Uses nifedipine as control. |
| Chanard 2003 | Study is comparing the effect of amlodipine versus tertatolol on CSA‐induced hyperuricaemia in post‐renal transplant recipients with hypertension. |
| Cuharadoglu 1993 | Study presented in abstract form and looks at effect of verapamil on graft survival and CSA levels. |
| Dawidson 1989 | Calcium antagonist not started until day three post‐operatively ‐ study is really looking at the effect of calcium antagonist on rejection. |
| Dawidson 1992 | Summary of two prospective and one retrospective studies. Study 1 published and possible for inclusion but excluded because verapamil not started until day 3 post operatively. Study 2 has already been included in review. Study 3 retrospective. |
| Donmez 1999 | Includes living‐related transplants. |
| Duggan 1985 | Only gave verapamil to the donor. |
| el‐Agroudy 2003 | This study evaluates the effect of losartan on TGF‐Beta 1 plasma levels and proteinuria in hypertensive transplant recipients. Two comparison groups ‐ one receiving captopril, the other amlodipine. |
| Ferguson 1990 | Retrospective study. |
| Gossmann 2002 | Study looks at the effect of gallopamil on renal plasma flow and GFR in patients transplanted at least 6 months before. |
| Harper 1992 | Paper is assessing the effect of nifedipine on high dose CSA. |
| Harper 1996 | Includes living‐related donors. |
| Kelly 1990 | Didn't look at the data we are interested in. |
| Kumana 2003 | Study assesses whether diltiazem co‐treatment achieves worthwhile dose reduction of Neoral. |
| Lehtonen 2000 | Unable to confirm donor population. |
| Madsen 1998 | Didn't look at the outcome data we are interested in. Includes living donors. |
| McLaughlin 2005 | Assesses effect of theophylline and loop diuretic in acute tacrolimus nephrotoxicity. |
| McNally 1990 | Looks at the effect of nifedipine on renal haemodynamics 6 months post‐transplant. |
| Midtvedt 1999 | Study is looking at the effect of nifedipine on acute rejection in hypertensive post‐transplant patients. |
| Midtvedt 2001 | Study compared the effect of lisinopril with controlled release nifedipine in treatment of post‐transplant hypertension focusing on changes in LVH. |
| Parrott 1990 | Didn't use calcium antagonist. |
| Pedersen 1995 | Abstract. Study examines the effect of felodipine on CSA nephrotoxicity. |
| Pedersen 1996 | Didn't look at the outcome data we are interested in. |
| Pirsch 1993 | Didn't look at the outcome data we are interested in. Not all patients started verapamil immediately post‐op (7.9 ± 0.9 days post‐transplant). |
| Po 1994 | Study examines the effect of calcium channel blockers on proteinuria in renal transplant patients. |
| Propper 1989 | Didn't look at the outcome data we are interested in. |
| Puig 1991 | Retrospective controls. |
| Santos 2002 | Study looks at the effect of diltiazem on dose of CSA. |
| Scheuermann 1995 | Looks at the effect of gallopamil on renal function in post‐transplant patients on gallopamil. Patients at least 6 months post‐transplant. |
| Schott 1994 | Didn't look at the data we are interested in. |
| Sennesael 1996 | Study looks at effects of amlodipine and perindopril on blood pressure, glomerular haemodynamics and tubule function in hypertensive cyclosporin treated renal transplant patients. |
| Sobh 1989 | Includes living‐related donors. |
| Sonzogni 1995 | Study looking at effect of lacidipine on renal haemodynamics and cyclosporin pharmacokinetics ‐ stable post transplant patients. |
| van Riemsdijk 2000 | Includes living‐related transplants ‐ details not split. |
| Venkat 1995 | Study is looking at the effect amlodipine has on pharmacokinetics of CSA in stable post‐transplant patients. |
| Wahlberg 1992 | Didn't look at the outcome data we are interested in. |
Contributions of authors
Ilona Shilliday: Design, trial selection, quality assessment, data extraction, data analysis, interpretation of results, reporting (first reviewer) Mohammed Sherif: trial selection, quality assessment, data extraction, data analysis, interpretation of results, reporting (second reviewer)
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Dawidson 1991 {published data only}
- Dawidson I, Rooth P, Lu C, Sagalowsky A, Diller K, Palmer B, et al. Verapamil improves the outcome after cadaver renal transplantation. Journal of the American Society of Nephrology 1991;2(5):983‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frei 1987 {published data only}
- Frei U, Margreiter R, Harms A, Bosmuller C, Neumann KH, Viebahn R, et al. Preoperative graft reperfusion with a calcium antagonist improves initial function: preliminary results of a prospective randomized trial in 110 kidney recipients. Transplantation Proceedings 1987;19(5):3539‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Harper 1997 {published data only}
- Harper SJ, Moorhouse J, Veitch PS, Bell PR, Horsburgh T, Walls J, et al. Improved immediate graft function with nifedipine in cyclosporine‐treated renal allograft recipients‐‐a randomized prospective study. Transplantation 1992;54(4):742‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Harper SJ, Moorhouse J, Walls J, Veitch S, Bell PR, Donnelly J, et al. Protective effects of nifedipine in immediate and 6‐month graft function in cyclosporin A treated renal allograft recipients [abstract]. Nephrology Dialysis Transplantation 1991;6(11):904. [CENTRAL: CN‐00260679] [Google Scholar]
Kuypers 2004 {published data only}
- Kuypers DR, Neumayer HH, Fritsche L, Budde K, Rodicio JL, Vanrenterghem Y. Calcium channel blockade and preservation of renal graft function in cyclosporine‐treated recipients: a prospective randomized placebo‐controlled 2‐year study. Transplantation 2004;78(8):1204‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kuypers DR, Neumayer HH, Fritsche L, Budde K, Vanrenterghem Y. Calcium channel blockade and prevention of renal graft function deterioration in cyclosporine treated recipients: a multi‐centre prospective, randomised , placebo controlled, 2 year study [abstract]. American Journal of Transplantation 2004;4(Suppl 8):432. [CENTRAL: CN‐00509299] [DOI] [PubMed] [Google Scholar]
Ladefoged 1994 {published data only}
- Ladefoged SD, Pederson E, Hammer M, Rasmussen KC, Hansen FM, Andersen CB. Influence of diltiazem on renal function and rejection in renal allograft recipients receiving triple‐drug immunosuppression: a randomized, double‐blind, placebo‐controlled study. Nephrology Dialysis Transplantation 1994;9(5):543‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lustig 1996 {published data only}
- Lustig S, Shmueli D, Boner G, Bar‐Nathan N, Nakache R, Yussim A, et al. Gallopamil reduces the post‐transplantation acute tubular necrosis in kidneys from aged donors. Israel Journal of Medical Sciences 1996;32(12):1249‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Morales 1990 {published data only}
- Morales JM, Andres A, Alvarez C, Prieto C, Ortuno B, Ortuno T, et al. Calcium channel blockers and early cyclosporine nephrotoxicity after renal transplantation: a prospective randomized study. Transplantation Proceedings 1990;22(4):1733‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Neumayer 1989 {published data only}
- Neumayer HH, Schreiber M, Wagner K. Prevention of delayed graft function by diltiazem and iloprost. Transplantation Proceedings 1989;21(1):1221‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Neumayer HH, Wagner K. Prevention of delayed graft function in cadaver kidney transplants by diltiazem: outcome of two prospective, randomized clinical trials. Journal of Cardiovascular Pharmacology 1987;10 Suppl 10:S170‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Wagner K, Albrecht S, Neumayer HH. Prevention of posttransplant acute tubular necrosis by the calcium antagonist diltiazem: a prospective randomized study. American Journal of Nephrology 1987;7(4):287‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wagner K, Neumayer HH. Influence of the calcium antagonist diltiazem on delayed graft function in cadaveric kidney transplantation: results of a 6‐month follow‐up. Transplantation Proceedings 1987;19(1 Pt 2):1353‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Neumayer 1992a {published data only}
- Neumayer HH, Kunzendorf U, Schreiber M. Protective effects of calcium antagonists in human renal transplantation. Kidney International ‐ Supplement 1992;36:87‐93. [MEDLINE: ] [PubMed] [Google Scholar]
- Neumayer HH, Kunzendorf U, Schreiber M. Protective effects of diltiazem and the prostazycline analogue iloprost in human renal transplantation. Renal Failure 1992;14(3):289‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neumayer 1992b {published data only}
- Neumayer HH, Kunzendorf U, Schreiber M. Protective effects of calcium antagonists in human renal transplantation. Kidney International ‐ Supplement 1992;36:87‐93. [MEDLINE: ] [PubMed] [Google Scholar]
- Neumayer HH, Kunzendorf U, Schreiber M. Protective effects of diltiazem and the prostazycline analogue iloprost in human renal transplantation. Renal Failure 1992;14(3):289‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oppenheimer 1992 {published data only}
- Alcaraz A, Oppenheimer F, Talbot‐Wright R, Fernandez‐Cruz L, Manalich M, Garcia‐Pages E, et al. Effect of diltiazem in the prevention of acute tubular necrosis, acute rejection, and cyclosporine levels. Transplantation Proceedings 1991;23(5):2383‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Oppenheimer F, Alcaraz A, Manalich M, Ricart MJ, Vilardell J, Campistol JM, et al. Influence of the calcium blocker diltiazem on the prevention of acute renal failure after renal transplantation. Transplantation Proceedings 1992;24(1):50‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Tenschert 1991 {published data only}
- Tenschert W, Harfmann P, Meyer‐Moldenhauer WH, Arndt R, Klosterhalfen H. Kidney protective effect of diltiazem after renal transplantation with long cold ischaemia time and triple‐drug immunosuppression. Transplantation Proceedings 1991;23(1 Pt 2):1334‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Wilkie 1994 {published data only}
- Wilkie ME, Beer JC, Evans S, Lord RH, Raferty M, Marsh FP. Prospective trial of oral nifedipine on early renal allograft function [abstract]. Nephrology Dialysis Transplantaion. 1993; Vol. 8:1053‐4. [CENTRAL: CN‐00260901] [PubMed]
- Wilkie ME, Beer JC, Evans SJ, Raftery MJ, Lord RH, Moore R, et al. A double‐blind, randomized, placebo‐controlled study of nifedipine on early renal allograft function. Nephrology Dialysis Transplantation 1994;9(7):800‐4. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Aros 2005 {published data only}
- Aros CA, Schneider HO, Flores CA, Ardiles LG, Alruiz PA, Jerez V, et al. Correlation between C2 and AUC(0‐4) in renal transplant patients treated with diltiazem. Transplantation Proceedings 2005;37(3):1580‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barenbrock 1995 {published data only}
- Barenbrock M, Breuer J, Schroder K, Wagner K, Neumayer HH, Rahn KH. A study of the nehroprotective effect of nitrendipine after renal transplantation [abstract]. XIIIth International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:387. [CENTRAL: CN‐00509083]
Berg 1991 {published data only}
- Berg KJ, Holdaas H, Endresen L, Fauchald P, Hartmaan A, Pran T, et al. Effects of isradipine on renal function in cyclosporin‐treated renal transplanted patients. Nephrology Dialysis Transplantation 1991;6(10):725‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Calo 2002 {published data only}
- Calo L, Giacon B, Davis PA, Pagnin E, Piccin A, Riegler P, et al. Oxidative stress and TGFbeta in kidney‐transplanted patients with cyclosporin‐induced hypertension. Effect of carvedilol and nifedipine. Clinical Nephrology 2002;58(2):103‐10. [MEDLINE: ; CN‐00409977] [DOI] [PubMed] [Google Scholar]
Chanard 2003 {published data only}
- Chanard J, Toupance O, Lavaud S, Hurault de Ligny B, Bernaud C, Moulin B. Amlodipine reduces cyclosporin‐induced hyperuricaemia in hypertensive renal transplant recipients. Nephrology Dialysis Transplantation 2003;18(10):2147‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cuharadoglu 1993 {published data only}
- Cuharadoglu S, Demirbas S, Haberal M. Results of verapamil treatment in 40 kidney transplant recipients [abstract]. Nephrology Dialysis Transplantation 1993;8:1038. [CENTRAL: CN‐00260880] [Google Scholar]
Dawidson 1989 {published data only}
- Dawidson I, Rooth P, Fry WR, Sandor Z, Wilms C, Coorpender L, et al. Prevention of acute cyclosporine‐induced renal blood flow inhibition and improved immunosuppression with verapamil. Transplantation 1989;48(4):575‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Dawidson 1992 {published data only}
- Dawidson I, Lu C, Palmer B, Peters P, Rooth P, Risser R, et al. Verapamil (VP) improves the outcome after renal transplantation. Transplant International 1992;5 Suppl 1:S60‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donmez 1999 {published data only}
- Donmez A, Karaaslan D, Sekerci S, Akpek E, Karakayali H, Arslan G. The effects of diltiazem and dopamine on early graft function in renal transplant recipients. Transplantation Proceedings 1999;31(8):3305‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duggan 1985 {published data only}
- Duggan KA, MacDonald GJ, Charlesworth JA, Pussell BA. Verapamil prevents post‐transplant oliguric renal failure. Clinical Nephrology 1985;24(6):289‐91. [MEDLINE: ] [PubMed] [Google Scholar]
el‐Agroudy 2003 {published data only}
- el‐Agroudy AE, Hassan NA, Foda MA, Ismail AM, el‐Sawy EA, Mousa O, et al. Effect of angiotensin II receptor blocker on plasma levels of TGF‐beta 1 and interstitial fibrosis in hypertensive kidney transplant patients. American Journal of Nephrology 2003;23(5):300‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferguson 1990 {published data only}
- Ferguson CJ, Hillis AN, Williams JD, Griffin PJ, Salaman JR. Calcium‐channel blockers and other factors influencing delayed function in renal allografts. Nephrology Dialysis Transplantation 1990;5(9):816‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gossmann 2002 {published data only}
- Gossmann J, Mondorf U, Dietz A, Kramer W, Kachel HG, Geiger H, et al. A randomized prospective double‐blind placebo‐controlled study of gallopamil, calcium antagonist of the verapamil type, in stable cyclosporine‐treated renal transplant recipients. Transplantation Proceedings 2002;34(5):1767‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harper 1992 {published data only}
- Harper SJ, Moorhouse J, Veitch PS, Horsburgh T, Walls J, Bell PR, et al. Nifedipine improves immediate, and 6‐ and 12‐month graft function in cyclosporin A (CyA) treated renal allograft recipients. Transplant International 1992;5 Suppl 1:S69‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harper 1996 {published data only}
- Harper SJ, Harris K, Jurewicz A, Walls J, Veitch P, Feehally J. Beneficial effects of oral nifedipine on cyclosporin (cya) treated renal allograft recipients ‐ a randomised prospective study [abstract]. XIIIth International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:381. [CENTRAL: CN‐00509226]
- Harper SJ, Moorhouse J, Abrams K, Jurewicz A, Nicholson M, Horsburgh T, et al. The beneficial effects of oral nifedipine on cyclosporin‐treated renal transplant recipients ‐ a randomised prospective study. Transplant International 1996;9(2):115‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kelly 1990 {published data only}
- Kelly JJ, Walker RG, D'Apice AJ, Kincaid‐Smith P. A prospective study of the effect of diltiazem in renal allograft recipients receiving cyclosporine A: preliminary results. Transplantation Proceedings 1990;22(5):2127‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Kumana 2003 {published data only}
- Kumana CR, Tong MK, Li CS, Lauder IJ, Lee JS, Kou M, et al. Diltiazem co‐treatment in renal transplant patients receiving microemulsion cyclosporin. British Journal of Clinical Pharmacology 2003;56(6):670‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehtonen 2000 {published data only}
- Lehtonen S, Isoniemi H, Salmela K. A randomised placebo controlled study on initial isradipine therapy in renal transplantation; long‐term results [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A276. [CENTRAL: CN‐00461150] [Google Scholar]
Madsen 1998 {published data only}
- Madsen JK, Sorensen SS, Hansen HE, Pederson EB. The effect of felodipine on renal function and blood pressure in cyclosporin‐treated renal transplant recipients during the first three months after transplantation. Nephrology Dialysis Transplantation 1998;13(9):2327‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McLaughlin 2005 {published data only}
- McLaughlin GE, Abitol CL. Reversal of oliguric tacrolimus nephrotoxicity in children. Nephrology Dialysis Transplantation 2005;20(7):4171‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McNally 1990 {published data only}
- McNally PG, Walls J, Feehally J. The effect of nifedipine on renal function in normotensive cyclosporin‐A‐treated renal allograft recipients. Nephrology Dialysis Transplantation 1990;5(11):962‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Midtvedt 1999 {published data only}
- Midtvedt K, Hatmann A, Fauchald P, Sund S, Foss A, Nordal KP, et al. Nifedipine slow release reduces the incidence of acute rejections in hypertensive renal transplant recipients [abstract]. Nephrology Dialysis Transplanatation 1999;14(9):A308. [CENTRAL: CN‐00485098] [Google Scholar]
Midtvedt 2001 {published data only}
- Midtveddt K, Hartmaan A, Foss A, Fauchald P, Nordal KP, Rootwelt K, et al. Sustained improvement of renal graft function for two years in hypertensive renal transplant recipients treated with nifedipine as compared to lisinopril. Transplantation 2001;72(11):1787‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Midtvedt K, Ihlen H, Hartmann A, Bryde P, Bjerkely BL, Foss A, et al. Reduction of left ventricular mass by lisinopril and nifedipine in hypertensive renal transplant recipients: a prospective randomized double‐blind study. Transplantation 2001;72(1):107‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Midtvedt kK, Hartmann A, Holdaas A, Fauchald P. Efficacy of nifedipine or lisinopril in the treatment of hypertension after renal transplantation: a double blind randomised comparative trial. Clinical Transplantation 2001;15(6):426‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parrott 1990 {published data only}
- Parrott NR, Forsythe JL, Mathews JN, Lennard TWJ, Rigg KM, Proud G, et al. Late perfusion. A simple remedy for renal allograft primary nonfunction. Transplantation 1990;49(5):913‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Pedersen 1995 {published data only}
- Pedersen EB, Madsen JK, Kornerup HJ, Sorensen SS, Zachariae H. Ciclosporine nephrotoxicity can be counteracted by a calcium antagonist (felodipine) in acute and short‐term studies [abstract]. Journal of the American Society of Nephrology 1995;6(3):1102. [CENTRAL: CN‐00485392] [Google Scholar]
Pedersen 1996 {published data only}
- Pedersen EB, Madsen JK, Sorensen SS, Zachariae H. Improvement in renal function by felodipine during cyclosporine treatment in acute and short‐term studies. Kidney International ‐ Supplement 1996;55:94‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Pirsch 1993 {published data only}
- Pirsch JD, D'Alessandro AM, Roecker EB, Knechtle SJ, Reed A, Sollinger HW, et al. A controlled, double‐blind, randomized trial of verapamil and cyclosporine in cadaver renal transplant patients. American Journal of Kidney Diseases 1993;21(2):189‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Po 1994 {published data only}
- Po C, Alvez L, Caldwell I, Raja R. Long term effects of calcium channel blockers on proteinuria in renal transplant patients on cyclosporine [abstract]. Journal of the American Society of Nephrology 1994;5(3):1030. [Google Scholar]
Propper 1989 {published data only}
- Propper DJ, Whiting PH, Power DA, Edward N, Catto GR. The effect of nifedipine on graft function in renal allograft recipients treated with cyclosporin A. Clinical Nephrology 1989;32(2):62‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Puig 1991 {published data only}
- Puig JM, Lloveras J, Oliveras A, Costa A, Aubia J, Masramon J. Usefulness of diltiazem in reducing the incidence of acute tubular necrosis in Euro‐Collins‐preserved cadaveric renal grafts. Transplantation Proceedings 1991;23(5):2368‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Santos 2002 {published data only}
- Santos AF, Keitel E, Bittar A, Neto JP, Alves MD, Schaefer PG, et al. Long term results of diltiazem use associated to cyclosporin in renal transplantation. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (USA). 2002. [CENTRAL: CN‐00416584]
Scheuermann 1995 {published data only}
- Scheuermann EH, Gossmann J, Peschke B, Kachel HG, Schoeppe W. A randomized, double blind, placebo controlled study of gallo‐pamil in cyclosporine treated renal transplant recipients [abstract]. Journal of the American Society of Nephrology 1995;6(3):1114. [CN‐00485739] [Google Scholar]
Schott 1994 {published data only}
- Schott G, Prom T. Intraoperative cortical PO2 measurement in kidney transplantation. the effect of the calcium antagonist diltiazem. Urologe 1994;33(5):415‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Sennesael 1996 {published data only}
- Sennesael JJ, Lamote JG, Violet I, Tasse S, Verbeelen DL. Divergent effects of calcium channel and angiotensin converting enzyme blockade on glomerulotubular function in cyclosporine‐treated renal allograft recipients. American Journal of Kidney Diseases 1996;27(5):701‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sobh 1989 {published data only}
- Sobh MA, Shehab el‐Din AB, Moustafa FE, el‐Far MA, Hussein ME, Gad HM, et al. A prospective randomized study of the protective effect of verapamil on ischemic renal injury in renal allotransplants. Transplantation Proceedings 1989;21(1):1230‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Sonzogni 1995 {published data only}
- Sonzogni G, Perico N, Gaspari F, Amuchastegui CS, Ruggenenti P. Effect of calcium‐channel blockade on renal hemodynamics and cyclosporine (csa) pharmacokinetcis in randomized double‐blind dose‐response study in stable renal allograft recipients [abstract]. Journal of the American Society of Nephrology 1995;6(3):1116. [CENTRAL: CN‐00485960] [Google Scholar]
van Riemsdijk 2000 {published data only}
- Riemsdijk IC, Mulder PG, Fijter JW, Bruijn JA, Hooff JP, Hoitsma AJ, et al. Addition of isradipine (Lomir) results in a better renal function after kidney transplantation: a double‐blind, randomized, placebo‐controlled, multi‐center study. Transplantation 2000;70(1):122‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Venkat 1995 {published data only}
- Venkat Raman G, Griffin P, Moore R, Goodship T. Effects of amlodipine on renal function and cyclosporine pharmacokinetics in normotensive renal transplant recipients. XIIIth International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:381.
Wahlberg 1992 {published data only}
- Wahlberg J, Hanas E, Bergstrom C, Fellstrom B, Skarp‐Orbeg I, Frodin L. Diltiazem treatment with reduced dose of cyclosporine in renal transplant recipients. Transplantation Proceedings 1992;24(1):311‐2. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Frei 1990 {published data only}
- Frei U, Harms A, Bakovic‐Alt R, Pichlmayr R, Koch K. Calcium channel blockers for kidney protection. Journal of Cardiovascular Pharmacology 1990;16 Suppl 6:S11‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
ANZDATA 2000
- Australian and New Zealand Society of Nephrology. Australian and New Zealand Dialysis and Transplant Registry. http://www.anzdata.org.au/anzdata/AnzdataReport/download.htm (accessed May 2000).
Asberg 1997
- Asberg A, Christensen H, Hartmann A, Berg KJ. Diltiazem modulates cyclosporin A induced renal hemodynamic effects but not its effect on plasma endothelin‐1. Clinical Transplantation 1998;12(5):363‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Chien 1980
- Chien KR, Sherman SC, Mittnacht S Jr, Farber JL. Microsomal membrane structure and function subsequent to calcium activation of an endogenous phospholipase. Archives of Biochemistry & Biophysics 1980;205(2):614‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chrysostomou 1993
- Chrysostomou A, Walker RG, Russ GR, d'Apice AJ, Knicaid‐Smith P, Mathew TH. Diltiazem in renal allograft recipients receiving cyclosporine. Transplantation 1993;55(2):300‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium; 1996 Oct 20‐24; Adelaide (Australia). 1996.
Mandreoli 1990
- Mandreoli M, Esposti ED, Cocchi R, Fabbri A, Guerrini L, Fusaroli M. Do calcium channel blockers have any influence on the immunological status of renal graft recipients on ciclosporin therapy?. American Journal of Nephrology 1990;10(1):58‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matthys 1984
- Matthys E, Patel Y, Kreisberg J, Stewart JH, Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney International 1984;26(2):153‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCord 1985
- McCord JM. Oxygen‐derived free radicals in postischemic tissue injury. New England Journal of Medicine 1985;312(3):159‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McMillen 1985
- McMillen MA, Lewis T, Jaffe BM, Wait RB. Verapamil inhibition of lymphocyte proliferation and function in vitro. Journal of Surgical Research 1985;39(1):76‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morris 1998
- Morris RG, Jones TE. Diltiazem disposition and metabolism in recipients of renal transplants. Therapeutic Drug Monitoring 1998;20(4):365‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicholson 1996
- Nicholson ML, Veitch PS, Bell PR. A prospective randomised study of the effect of nicardipine on ischaemic renal injury in renal allografts. Transplant International 1996;9(1):51‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2006
- United States Renal Data System. www.usrds.org/atlas.htm (accessed April 2007).
Wilson 1984
- Wilson DR, Arnold PE, Burke TJ, Schrier RW. Mitochondrial calcium accumulation and respiration in ischemic acute renal failure in the rat. Kidney International 1984;25(3):519‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shilliday 2003
- Shilliday I, Woo YM. Calcium antagonists for preventing acute tubular necrosis in renal transplant recipients. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003421] [DOI] [Google Scholar]
Shilliday 2004
- Shilliday IR, Sherif M. Calcium channel blockers for preventing acute tubular necrosis in kidney transplant recipients. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003421.pub2] [DOI] [PubMed] [Google Scholar]
Shilliday 2005
- Shilliday IR, Sherif M. Calcium channel blockers for preventing acute tubular necrosis in kidney transplant recipients. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003421.pub3] [DOI] [PubMed] [Google Scholar]


