Abstract
Background
Pneumonia caused by bacterial pathogens is the leading cause of mortality in children in low‐income countries. Early administration of antibiotics improves outcomes.
Objectives
To identify effective antibiotic drug therapies for community‐acquired pneumonia (CAP) of varying severity in children by comparing various antibiotics.
Search methods
We searched CENTRAL 2012, Issue 10; MEDLINE (1966 to October week 4, 2012); EMBASE (1990 to November 2012); CINAHL (2009 to November 2012); Web of Science (2009 to November 2012) and LILACS (2009 to November 2012).
Selection criteria
Randomised controlled trials (RCTs) in children of either sex, comparing at least two antibiotics for CAP within hospital or ambulatory (outpatient) settings.
Data collection and analysis
Two review authors independently extracted data from the full articles of selected studies.
Main results
We included 29 trials, which enrolled 14,188 children, comparing multiple antibiotics. None compared antibiotics with placebo.
Assessment of quality of study revealed that 5 out of 29 studies were double‐blind and allocation concealment was adequate. Another 12 studies were unblinded but had adequate allocation concealment, classifying them as good quality studies. There was more than one study comparing co‐trimoxazole with amoxycillin, oral amoxycillin with injectable penicillin/ampicillin and chloramphenicol with ampicillin/penicillin and studies were of good quality, suggesting the evidence for these comparisons was of high quality compared to other comparisons.
In ambulatory settings, for treatment of World Health Organization (WHO) defined non‐severe CAP, amoxycillin compared with co‐trimoxazole had similar failure rates (odds ratio (OR) 1.18, 95% confidence interval (CI) 0.91 to 1.51) and cure rates (OR 1.03, 95% CI 0.56 to 1.89). Three studies involved 3952 children.
In children with severe pneumonia without hypoxaemia, oral antibiotics (amoxycillin/co‐trimoxazole) compared with injectable penicillin had similar failure rates (OR 0.84, 95% CI 0.56 to 1.24), hospitalisation rates (OR 1.13, 95% CI 0.38 to 3.34) and relapse rates (OR 1.28, 95% CI 0.34 to 4.82). Six studies involved 4331 children below 18 years of age.
In very severe CAP, death rates were higher in children receiving chloramphenicol compared to those receiving penicillin/ampicillin plus gentamicin (OR 1.25, 95% CI 0.76 to 2.07). One study involved 1116 children.
Authors' conclusions
For treatment of patients with CAP in ambulatory settings, amoxycillin is an alternative to co‐trimoxazole. With limited data on other antibiotics, co‐amoxyclavulanic acid and cefpodoxime may be alternative second‐line drugs. Children with severe pneumonia without hypoxaemia can be treated with oral amoxycillin in an ambulatory setting. For children hospitalised with severe and very severe CAP, penicillin/ampicillin plus gentamycin is superior to chloramphenicol. The other alternative drugs for such patients are co‐amoxyclavulanic acid and cefuroxime. Until more studies are available, these can be used as second‐line therapies.
There is a need for more studies with radiographically confirmed pneumonia in larger patient populations and similar methodologies to compare newer antibiotics. Recommendations in this review are applicable to countries with high case fatalities due to pneumonia in children without underlying morbidities and where point of care tests for identification of aetiological agents for pneumonia are not available.
Plain language summary
Different antibiotics for community‐acquired pneumonia in otherwise healthy children younger than 18 years of age in hospital and outpatient settings
Pneumonia is the leading cause of mortality in children under five years of age. Most cases of community‐acquired pneumonia (CAP) in low‐income countries are caused by bacteria. This systematic review identified 29 randomised controlled trials from many different countries enrolling 14,188 children and comparing antibiotics for treatment of CAP in children. Most were single studies only.
We found that for outpatient treatment of pneumonia, amoxycillin is an alternative treatment to co‐trimoxazole. Oral amoxycillin in children with severe pneumonia without hypoxia (i.e. a decreased level of oxygen), and who are feeding well, may be effective. For very severe pneumonia, a combination of penicillin or ampicillin and gentamycin is more effective than chloramphenicol alone. Reports of adverse events were not available in many studies. Wherever information on adverse events was available, it did not differ between two drugs compared except that gastrointestinal side effects were more commonly reported with erythromycin compared to azithromycin.
Limitations of this review are that only five studies met all the quality assessment criteria and for most comparisons of the efficacy of antibiotics only one or two studies were available.
Background
Pneumonia is the leading single cause of mortality in children aged less than five years, with an estimated incidence of 0.29 and 0.05 episodes per child‐year in low‐income and high‐income countries, respectively. It is estimated that a total of around 156 million new episodes occur each year and most of these occur in India (43 million), China (21 million), Pakistan (10 million) and Bangladesh, Indonesia and Nigeria (six million each) (Rudan 2008). In 2010, out of 7.6 million deaths in children below five years of age, 1.4 million (18.3%) deaths were due to pneumonia (Liu 2012). Reducing mortality due to pneumonia may help in reducing childhood and under five‐year old mortality rates (Liu 2012). The commonest bacterial pathogens isolated in children under five years with pneumonia are Streptococcus pneumoniae (S. pneumoniae) (30% to 50%) and Haemophilus influenzae (H. influenzae) (10% to 30%) (Falade 2011), and 50% of deaths due to pneumonia in this age group are attributed to these two organisms (Shann 1995). To reduce the infant and under five‐year child mortality rate, it is important to reduce mortality due to pneumonia by appropriate intervention in the form of antibiotics. Selection of first‐line antibiotics for empirical treatment of pneumonia is crucial for office practice as well as public health.
Description of the condition
Pneumonia is defined as an infection of the lung parenchyma (alveoli) by microbial agents. It is difficult to identify the causative organism in most cases of pneumonia. The methods used for identification of the aetiologic agents include blood culture, lung puncture, nasopharyngeal aspiration and immune assays of blood and urine tests. Lung puncture is an invasive procedure associated with significant morbidity and hence cannot be performed routinely in most cases. The yield from blood cultures is too low (5% to 15% for bacterial pathogens) to be relied upon (MacCracken 2000). There are few studies that document the aetiology of pneumonia in children below five years of age from low‐income countries. Most studies carried out blood cultures for bacterial aetiology of pneumonia. Some studies carried out nasopharyngeal aspirates and identification of virus and atypical organisms. A review of 14 studies involving 1096 lung aspirates taken from hospitalised children prior to administration of antibiotics reported bacterial pathogens in 62% of cases (Berman 1990). In 27% of patients, the common bacterial pathogens identified were Streptococcus pneumoniae (S. pneumoniae) and Haemophilus influenzae (H. influenzae) (Berman 1990). Studies using nasopharyngeal aspirates for identification of viral agents suggest that about 40% of pneumonia in children below five years of age is caused by viral agents, with the commonest viral pathogen being respiratory syncytial virus (Maitreyi 2000). In infants under three months of age, common pathogens include S. pneumoniae,H. influenzae, gram‐negative bacilli and Staphylococcus (WHOYISG 1999). The causative organisms are different in high‐income countries and include more viral and atypical organisms (Gendrel 1997; Ishiwada 1993; Numazaki 2004; Wubbel 1999). Therefore, treatment regimens may be different in high‐income and low‐income countries. The reference standard for diagnosis of pneumonia is X‐ray film of the chest. However, it does not have the necessary sensitivity and specificity to identify aetiological agents (i e. bacterial or viral). Obtaining an X‐ray film in all suspected pneumonia cases may not be cost‐effective as it does not affect the outcome. Therefore, diagnosis of pneumonia is based on clinical criteria. Treatment of pneumonia includes administration of antibiotics, either in hospital or in an ambulatory setting. Administration of antibiotics for all clinically diagnosed pneumonia may lead to antibiotic prescription even for those cases caused by viral infection. Since clinical or radiological findings cannot differentiate viral or bacterial pneumonia and due to the absence of point of care tests for routine use, empirical treatment with antibiotics in countries with high case fatalities due to pneumonia is recommended by the World health Organization (WHO).
Description of the intervention
Administration of appropriate antibiotics at an early stage of pneumonia improves the outcome of the illness, particularly when the causative agent is bacterial. The WHO has provided guidelines for early diagnosis and assessment of the severity of pneumonia on the basis of clinical features (WHOYISG 1999) and suggests administration of co‐trimoxazole as a first‐line drug. The commonly used antibiotics for community‐acquired pneumonia (CAP) include co‐trimoxazole, amoxycillin, oral cephalosporins and macrolide drugs. Despite evidence of rising bacterial resistance to co‐trimoxazole (IBIS 1999; Timothy 1993), studies conducted in the same time period showed good clinical efficacy of oral co‐trimoxazole for non‐severe pneumonia (Awasthi 2008; Rasmussen 1997; Straus 1998). However, one study reported a doubling of clinical failure rates with co‐trimoxazole treatment when compared to treatment with amoxycillin in severe and radiologically confirmed pneumonia (Straus 1998). A meta‐analysis of all the trials on pneumonia based on the case‐management approach proposed by the WHO (identification of pneumonia on clinical symptoms/signs and administration of empirical antimicrobial agents) has found a reduction in overall mortality as well as pneumonia‐related mortality (Sazawal 2003). Various antibiotics have been used for varying severities of pneumonia. Antibiotics are administered in hospital or in ambulatory settings.
How the intervention might work
Pneumonia is the leading cause of mortality in children below five years of age. It is not easy to identify aetiological agents in children with pneumonia. To meet the public health goal of reducing child mortality due to pneumonia, empirical antibiotic administration is relied upon in most instances. This is necessary in view of the inability of most commonly available laboratory tests to identify causative pathogens.
Why it is important to do this review
Empirical antibiotic administration is the mainstay of treatment of pneumonia in children. Administration of the most appropriate antibiotic as the first‐line treatment may improve the outcome of pneumonia. Many antibiotics are prescribed to treat pneumonia. Therefore, it is important to know which works best for pneumonia in children. The last review of all available randomised controlled trials (RCTs) on antibiotics used for pneumonia in children was published in 2010 (Kabra 2010). Since then, five new trials (Ambroggio 2012; Bari 2011; Nogeova 1997; Ribeiro 2011; Soofi 2012) have been published. Additional information on the epidemiology of pneumonia in children has been published. Therefore, we have updated this review and included new data and also carried out a meta‐analysis on the treatment of severe pneumonia with oral antibiotics.
Objectives
To identify effective antibiotic drug therapies for community‐acquired pneumonia (CAP) of varying severity in children by comparing various antibiotics.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing antibiotics for CAP in children. We considered only those studies using the case definition of pneumonia (as given by the WHO) or radiologically confirmed pneumonia in this review.
Types of participants
We included children under 18 years of age with CAP treated in a hospital or community setting. We excluded studies describing pneumonia post‐hospitalisation in immunocompromised patients (for example, following surgical procedures) or patients with underlying illnesses like congenital heart disease or those in an immune deficient state.
Types of interventions
We compared any intervention with antibiotics (administered by intravenous route, intramuscular route or orally) with another antibiotic for the treatment of CAP.
Types of outcome measures
Primary outcomes
Clinical cure. The definition of clinical cure is symptomatic and involves clinical recovery by the end of treatment.
Treatment failure rates. The definition of treatment failure is the presence of any of the following: development of chest in‐drawing, convulsions, drowsiness or inability to drink at any time, respiratory rate above the age‐specific cut‐off point on completion of treatment, or oxygen saturation of less than 90% (measured by pulse oximetry) after completion of the treatment. Loss to follow‐up or withdrawal from the study at any time after recruitment indicated failure in the analysis.
Secondary outcomes
The clinically relevant outcome measures were as follows.
Relapse rate: defined as children declared 'cured', but developing recurrence of disease at follow‐up in a defined period.
Hospitalisation rate (in outpatient studies only): defined as the need for hospitalisation in children who were getting treatment or in an ambulatory (outpatient) setting.
Length of hospital stay: duration of total hospital stay (from day of admission to discharge) in days.
Need for change in antibiotics: children required change in antibiotics from the primary regimen.
Additional interventions used: any additional intervention in the form of mechanical ventilation, steroids, vaso‐pressure agents, etc.
Mortality rate.
Search methods for identification of studies
We retrieved studies through a search strategy which included cross‐referencing. We checked the cross‐references of all the studies manually.
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 10, part of The Cochrane Library, www.thecochranelibrary.com (accessed 7 November 2012); MEDLINE (September 2009 to October week 4, 2012); EMBASE (September 2009 to November 2012); CINAHL (2009 to November 2012); Web of Science (2009 to November 2012) and LILACS (2009 to November 2012). Details of the previous search are in Appendix 1.
To search CENTRAL and MEDLINE we combined the following search strategy with the validated search strategy for identifying child studies developed by Boluyt (Boluyt 2008). We used the Cochrane Highly Sensitive Search Strategy to identify randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (Appendix 2), CINAHL (Appendix 3), Web of Science (Appendix 4) and LILACS (Appendix 5).
MEDLINE (Ovid)
1 exp Pneumonia/ 2 pneumon*.tw. 3 bronchopneumon*.tw. 4 pleuropneumon*.tw. 5 cap.tw. 6 or/1‐5 7 exp Anti‐Bacterial Agents/ 8 antibiotic*.tw. 9 (amoxycillin* or amoxycillin* or ampicillin* or azithromycin* or augmentin* or benzylpenicillin* or b‐lactam* or beta‐lactam* or clarithromycin* or ceftriaxone* or cefuroxime* or cotrimoxazole* or co‐trimoxazole* or co‐amoxyclavulanic acid or cefotaxime* or ceftriaxone* or ceftrioxone* or cefditoren* or chloramphenicol* or cefpodioxime* or cephradine* or cephalexin* or cefaclor* or cefetamet* or cephalosporin* or erythromycin* or gentamicin* or gentamycin* or levofloxacin* or macrolide* or minocyclin* or moxifloxacin* or penicillin* or quinolone* or roxithromycin* or sulphamethoxazole* or sulfamethoxazole* or tetracyclin* or trimethoprim*).tw,nm. (248104) 10 or/7‐9 11 6 and 10
Searching other resources
We also searched bibliographies of selected articles to identify any additional trials not recovered by the electronic searches.
Data collection and analysis
Selection of studies
Two review authors (SKK, RL) independently selected potentially relevant studies based on their title and abstract. We retrieved the complete texts of these studies electronically or by contacting the trial authors. Two review authors (SKK, RL) independently reviewed the results for inclusion.
Data extraction and management
A person who was not involved in the review gave all relevant studies a serial number to mask the authors' names and institutions, the location of the study, reference lists and any other potential identifiers. Two review authors (SKK, RL) independently reviewed the results for inclusion in the analysis. We resolved differences about study quality through discussion. We recorded data on a pre‐structured data extraction form. We assessed publication bias using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). We included data from cluster‐RCTs after adjustment for the design effect. We calculated the design effect by 1+(M‐1) ICC; where M is the average cluster size and ICC is the intracluster correlation coefficient (Higgins 2011).
Before combining the studies for each of the outcome variables, we carried out an assessment of heterogeneity using Review Manager (RevMan 2012) software. We performed a sensitivity analysis to check the importance of each study in order to see the effect of inclusion and exclusion criteria. We computed both the effect size and summary measures with 95% confidence intervals (CIs) using RevMan 2012. We used a random‐effects model to combine the study results for all the outcome variables.
We collected data on the primary outcome (cure rate/failure rate) and secondary outcomes (relapse rate, rate of hospitalisation and complications, need for change in antibiotics, need for additional interventions and mortality). When available, we also recorded additional data on potential confounders such as prior antibiotic therapy and nutritional status.
We did multiple analyses, firstly on studies comparing the same antibiotics. We also attempted to perform indirect comparisons of various drugs when studies with direct comparisons were not available. For example, we compared antibiotics A and C when a comparison of antibiotics A and B was available and likewise a separate comparison between antibiotics B and C. We only did this type of comparison if the inclusion and exclusion criteria of these studies, the dose and duration of the common intervention (antibiotic B), baseline characteristics and the outcomes assessed were similar (Bucher 1997).
Assessment of risk of bias in included studies
We assessed risk of bias in all included studies using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011).
1. Sequence generation: assessed as yes, no or unclear Yes: when the study described the method used to generate the allocation sequence in sufficient detail. No: sequence not generated. Unclear: when it was not described or incompletely described.
2. Allocation concealment: assessed as yes, no or unclear Yes: when the study described the method used to conceal the allocation sequence in sufficient detail. No: described details where allocation concealment was not done. Unclear: when it was not described or incompletely described.
3. Blinding of participants, personnel and outcome assessors: assessed as yes, no or unclear Yes: when it was a double‐blind study. No: when it was an unblinded study. Unclear: not clearly described.
4. Incomplete outcome data: assessed as yes, unclear Yes: describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. Unclear: either not described or incompletely described.
5. Free of selective outcome reporting: assessed as yes, no or unclear Yes: results of study free of selective reporting. Details of all the participants enrolled in the study are included in the paper. No: details of all the enrolled participants not given in the paper. Unclear: details of all the enrolled participants incompletely described.
6. Other sources of bias Among the other sources of potential bias considered was funding agencies and their role in the study. We recorded funding agencies as governmental agencies, universities and research organisations or pharmaceutical companies. We considered studies supported by pharmaceutical companies to be unclear unless the study defined the role of the pharmaceutical companies. We also considered studies not mentioning the source of funding as unclear under this heading.
Measures of treatment effect
The main outcome variables were failure rates or cure rates. Treatment effect in the form of failure rates was calculated by making 2 x 2 tables and calculating odds ratios (ORs) for each comparison. We expressed the results as ORs with 95% confidence intervals (CIs).
Unit of analysis issues
All except one study were RCTs. One was a cluster‐RCT (Awasthi 2008). We included data from cluster‐RCTs after adjustment for the design effect. We calculated the design effect by 1+(M‐1) ICC; where M is the average cluster size and ICC is the intracluster correlation coefficient (Higgins 2011).
Dealing with missing data
We contacted trial authors for missing data. However, we could not retrieve any missing data from any of the studies. We excluded two new studies in this update (Bari 2011; Soofi 2012).
Assessment of heterogeneity
For each of the outcome variables, we carried out an assessment of heterogeneity with Breslow's test of homogeneity in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
Before combining the study results, we checked for publication bias using a funnel plot. For each of the outcome variables (cure rate, failure rate, relapse rate, rate of hospitalisation, the complications needed for change in antibiotics and mortality rate) we used a 2 x 2 table for each study and performed Breslow's test of homogeneity to determine variation in study results.
Data synthesis
For each comparison, we prepared 2 x 2 tables. We calculated ORs and 95% CIs. We used a random‐effects model for all the comparisons.
Subgroup analysis and investigation of heterogeneity
In this review we included RCTs that compared two antibiotics in children with pneumonia. We performed a subgroup analysis of children with radiologically confirmed pneumonia. For each of the outcome variables, we carried out an assessment of heterogeneity with Breslow's test of homogeneity using RevMan 2012 (see Data collection and analysis).
Sensitivity analysis
Most comparisons were for two to three trials. If there was significant heterogeneity, we conducted a sensitivity analysis. We conducted multiple analyses after excluding one study data at a time.
Results
Description of studies
Results of the search
Two review authors (SKK, RL) screened the article titles. We short‐listed 49 trials as potential RCTs to be included and we attempted to collect the full‐text articles. We obtained the full text for 48 trials. A third person who was not involved in the review masked the papers for identifiers. Two review authors (SKK, RL) independently extracted data by using a pre‐designed data extraction form; the extracted data matched completely.
Included studies
We identified 29 studies for inclusion, with the following comparisons.
Azithromycin with erythromycin: four studies (Harris 1998; Kogan 2003; Roord 1996; Wubbel 1999), involving 457 children aged two months to 16 years.
Clarithromycin with erythromycin: one study (Block 1995), involving 357 children below 15 years of age with clinical or radiographically confirmed pneumonia treated in an ambulatory setting.
Co‐trimoxazole with amoxycillin: three studies (Awasthi 2008; CATCHUP 2002; Straus 1998), involving 2347 children aged two months to 59 months. Total numbers of events and effective sample size in one cluster‐randomised controlled trial (Awasthi 2008) were calculated after adjusting for the design effect.
Co‐trimoxazole with procaine penicillin: two studies (Keeley 1990; Sidal 1994), involving 723 children aged three months to 12 years.
Chloramphenicol with penicillin and gentamycin together: one study (Duke 2002), involving 1116 children aged one month to five years.
Single‐dose benzathine penicillin with procaine penicillin: two studies (Camargos 1997; Sidal 1994), involving 176 children between two and 12 years of age in one study (Sidal 1994) and 105 children aged between three months to 14 years in the other similar study (Camargos 1997).
Amoxycillin with procaine penicillin: one study (Tsarouhas 1998), involving 170 children aged six months to 18 years.
Ampicillin with chloramphenicol plus penicillin: one study (Deivanayagam 1996), involving 115 children aged five months to four years.
Co‐trimoxazole with single‐dose procaine penicillin followed by oral ampicillin: one study (Campbell 1988), involving 134 children aged below five years.
Penicillin with amoxycillin: two studies (Addo‐Yobo 2004; Atkinson 2007), involving 1905 children aged three months to 59 months.
Co‐trimoxazole with chloramphenicol: one study (Mulholland 1995), involving 111 children aged under five years.
Cefpodoxime with co‐amoxyclavulanic acid: one study (Klein 1995), involving 348 children aged three months to 11.5 years.
Azithromycin with amoxycillin: one study (Kogan 2003), involving 47 children aged one month to 14 years.
Amoxycillin with co‐amoxyclavulanic acid: one study (Jibril 1989), involving 100 children aged two months to 12 years.
Chloramphenicol in addition to penicillin with ceftriaxone: one study (Cetinkaya 2004), involving 97 children aged between two to 24 months admitted to hospital with severe pneumonia.
Levofloxacin and comparator (co‐amoxyclavulanic acid or ceftriaxone): one study (Bradley 2007) involving 709 children aged 0.5 to 16 years of age with CAP treated in hospital or in an ambulatory setting.
Parenteral ampicillin followed by oral amoxycillin with home‐based oral amoxycillin: one study (Hazir 2008) involving 2037 children between three months to 59 months of age with WHO‐defined severe pneumonia.
Chloramphenicol with ampicillin and gentamicin: one study (Asghar 2008), involving 958 children between two to 59 months with very severe pneumonia.
Penicillin and gentamicin with co‐amoxyclavulanic acid (Bansal 2006), involving 71 children with severe and very severe pneumonia between two months to 59 months of age.
Co‐amoxyclavulanic acid with cefuroxime or clarithromycin: one study (Aurangzeb 2003), involving 126 children between two to 72 months of age.
Ceftibuten with cefuroxime axetil: one study involving 140 children between one to 12 years of age with CAP that was radiographically confirmed (Nogeova 1997).
Oxacillin/ceftriaxone with co‐amoxyclavulanic acid: one study involving 104 children between age two months to five years with very severe pneumonia (Ribeiro 2011).
Excluded studies
We excluded 20 trials.
Four studies were carried out in adult participants (Bonvehi 2003; Fogarty 2002; Higuera 1996; van Zyl 2002).
Three studies included children with severe infections or sepsis (Haffejee 1984; Mouallem 1976; Vuori‐Holopaine 2000).
One study did not provide separate data for children (Sanchez 1998).
Two cluster‐RCTs (Bari 2011; Soofi 2012) compared oral amoxycillin or standard treatment for severe pneumonia in children below five years of age. Patients on conventional treatment received either intravenous antibiotics in hospital or oral medications at home or no treatment. Results were available as oral treatment with amoxycillin in comparison with standard treatment (referral and antibiotics). Separate data on patients who received intravenous antibiotics were not available and data could not be obtained from the trial authors.
Three studies were not RCTs (Agostoni 1988; Ambroggio 2012; Paupe 1992).
Three studies only compared the duration of antibiotic use (Hasali 2005; Peltola 2001; Ruhrmann 1982); of these, one study (Hasali 2005) also did not report the outcome in the form of cure or failure rates.
One studied only sequential antibiotic use (Al‐Eiden 1999).
One compared azithromycin with symptomatic treatment for recurrent respiratory tract infection only (Esposito 2005).
The full‐text article could not be obtained for one study (Lu 2006).
One study (Lee 2008) was excluded because the outcome was not in the form of cure or failure rates.
Risk of bias in included studies
The overall risk of bias is presented graphically and summarised (Figure 1; Figure 2)
1.
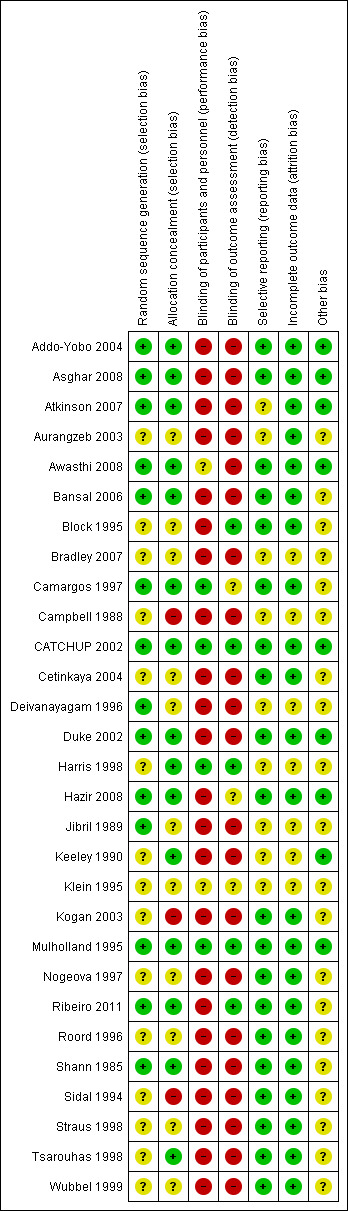
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
2.
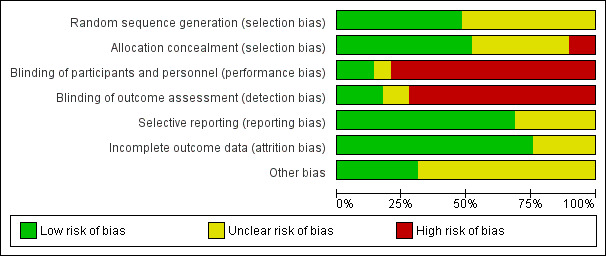
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Details of sequence generation were described in 17 studies (Addo‐Yobo 2004; Asghar 2008; Atkinson 2007; Awasthi 2008; Bansal 2006; Camargos 1997; CATCHUP 2002; Cetinkaya 2004; Deivanayagam 1996;Duke 2002; Hazir 2008; Jibril 1989; Keeley 1990; Mulholland 1995; Ribeiro 2011; Roord 1996; Shann 1985), were not clear in 10 studies (Aurangzeb 2003; Block 1995; Bradley 2007; Campbell 1988; Harris 1998; Klein 1995; Nogeova 1997; Straus 1998; Tsarouhas 1998; Wubbel 1999) and sequence was not generated in two studies (Kogan 2003; Sidal 1994).
Allocation
Allocation concealment was adequate in 17 studies (Addo‐Yobo 2004; Asghar 2008; Atkinson 2007; Awasthi 2008; Bansal 2006; Camargos 1997; CATCHUP 2002; Cetinkaya 2004; Deivanayagam 1996; Duke 2002; Harris 1998; Hazir 2008; Keeley 1990; Mulholland 1995; Ribeiro 2011; Shann 1985; Tsarouhas 1998), it was unclear in nine studies (Aurangzeb 2003; Block 1995; Bradley 2007; Campbell 1988; Jibril 1989; Klein 1995; Nogeova 1997; Straus 1998; Wubbel 1999) and no concealment was done in three studies (Kogan 2003; Roord 1996; Sidal 1994).
Blinding
Only five studies (CATCHUP 2002; Cetinkaya 2004; Harris 1998; Mulholland 1995; Straus 1998) were double‐blinded. The rest of the studies were unblinded.
Incomplete outcome data
Data were fully detailed in 20 studies (Addo‐Yobo 2004; Asghar 2008; Atkinson 2007; Aurangzeb 2003; Awasthi 2008; Bansal 2006; Block 1995; Camargos 1997; CATCHUP 2002; Cetinkaya 2004; Duke 2002; Hazir 2008; Kogan 2003; Mulholland 1995; Nogeova 1997; Ribeiro 2011; Roord 1996; Straus 1998; Tsarouhas 1998; Wubbel 1999) and in the remaining studies details of attrition and exclusions from the analysis were unavailable.
Selective reporting
Selective reporting of data was unclear in 12 studies (Atkinson 2007; Aurangzeb 2003; Bradley 2007; Campbell 1988; Deivanayagam 1996; Harris 1998; Jibril 1989; Keeley 1990; Klein 1995; Shann 1985; Sidal 1994; Wubbel 1999). The rest of the studies were free from selective reporting.
Other potential sources of bias
The source of funding was not mentioned in 15 studies (Aurangzeb 2003; Bansal 2006; Camargos 1997; Campbell 1988; Cetinkaya 2004; Deivanayagam 1996; Jibril 1989; Klein 1995; Kogan 2003; Nogeova 1997; Ribeiro 2011; Shann 1985; Sidal 1994; Straus 1998; Tsarouhas 1998). Five studies were funded by pharmaceutical companies (Block 1995; Bradley 2007; Harris 1998; Roord 1996; Wubbel 1999). Eight studies were supported by the WHO, Medical Research Council or universities (Addo‐Yobo 2004; Asghar 2008; Atkinson 2007; Awasthi 2008; Duke 2002; Hazir 2008; Keeley 1990; Mulholland 1995). One study (CATCHUP 2002) was supported by the WHO in addition to pharmaceutical companies. Information on clearance by Ethics Committees or Institutional Review Boards was available for all except four studies (Aurangzeb 2003; Jibril 1989; Keeley 1990; Sidal 1994).
Effects of interventions
Studies comparing ambulatory setting treatment of non‐severe pneumonia
Azithromycin versus erythromycin (Analysis 1)
Four studies (Harris 1998; Kogan 2003; Roord 1996; Wubbel 1999) compared erythromycin with azithromycin and enrolled 623 children. One study (Harris 1998) was double‐blinded with adequate allocation concealment and three studies (Kogan 2003; Roord 1996; Wubbel 1999) were unblinded and did not have adequate allocation concealment. Information on the presence of wheezing was available in two studies (Harris 1998; Kogan 2003): 104 out of 318 (33%) children experienced wheezing in the azithromycin group, while 62 out of 161 (39%) in the erythromycin group experienced wheezing. The failure rates in the azithromycin and erythromycin groups were six out of 236 (2.5%) and six out of 156 (3.8%), respectively (OR 0.73, 95% CI 0.18 to 2.89) (Analysis 1.5) There were no significant side effects in either group. Three studies reported data on aetiologic organisms separately for each of the two treatment groups (Harris 1998; Kogan 2003; Roord 1996); there were 234 organisms identified in the azithromycin group and 135 in the erythromycin group (Roord 1996). The distribution of different organisms was similar in the two groups. There were 24 organisms identified in the fourth study (Wubbel 1999) in 59 participants tested.
1.5. Analysis.
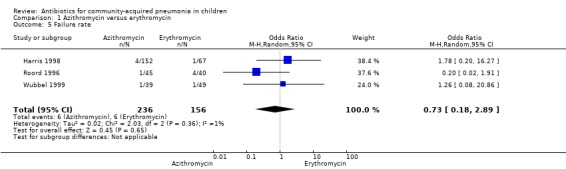
Comparison 1 Azithromycin versus erythromycin, Outcome 5 Failure rate.
Clarithromycin versus erythromycin (Analysis 2)
One study (Block 1995) compared erythromycin and clarithromycin; 234 children below 15 years of age with clinical or radiographically confirmed pneumonia were treated in an ambulatory setting. The trial was single‐blinded and allocation concealment was unclear. The following outcomes were similar between the two groups: cure rate (OR 1.61, 95% CI 0.84 to 3.08) (Analysis 2.2), clinical success rate (OR 1.92, 95% CI 0.45 to 8.23) (Analysis 2.3), failure rate (OR 0.52, 95% CI 0.12 to 2.23) (Analysis 2.4) , relapse rate (OR 0.17, 95% CI 0.02 to 1.45) (Analysis 2.5) and adverse events (OR 1.07, 95% CI 0.6 to 1.90) (Analysis 2.9). Resolution of pneumonia (diagnosed radiologically) was more frequent in the clarithromycin group as compared to the erythromycin group (OR 2.51, 95% CI 1.02 to 6.16) (Analysis 2.6). However, there were no differences in the radiologic improvement rates (OR 3.55, 95% CI 0.7 to 18.04) (Analysis 2.7) or radiologic failure rates (OR 0.34, 95% CI 0.06 to 1.80) (,Analysis 2.8) both of which were established with radiological evidence.
2.2. Analysis.
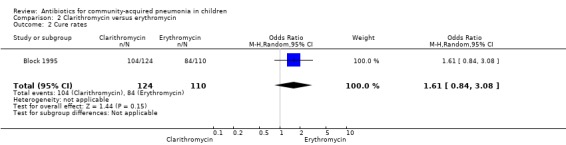
Comparison 2 Clarithromycin versus erythromycin, Outcome 2 Cure rates.
2.3. Analysis.
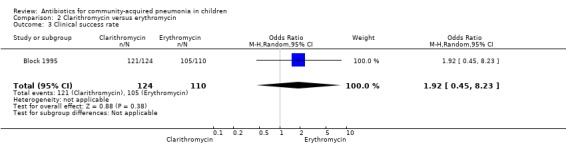
Comparison 2 Clarithromycin versus erythromycin, Outcome 3 Clinical success rate.
2.4. Analysis.
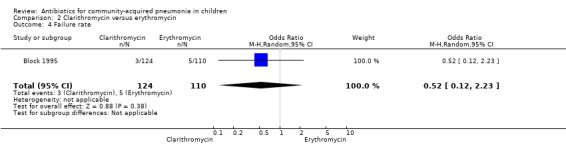
Comparison 2 Clarithromycin versus erythromycin, Outcome 4 Failure rate.
2.5. Analysis.
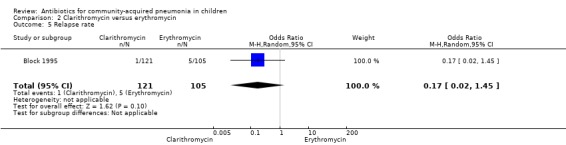
Comparison 2 Clarithromycin versus erythromycin, Outcome 5 Relapse rate.
2.9. Analysis.
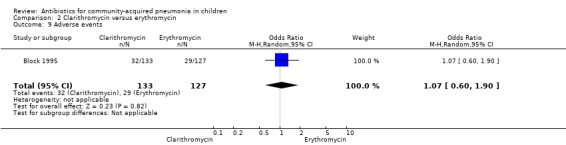
Comparison 2 Clarithromycin versus erythromycin, Outcome 9 Adverse events.
2.6. Analysis.
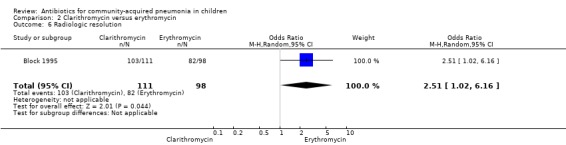
Comparison 2 Clarithromycin versus erythromycin, Outcome 6 Radiologic resolution.
2.7. Analysis.
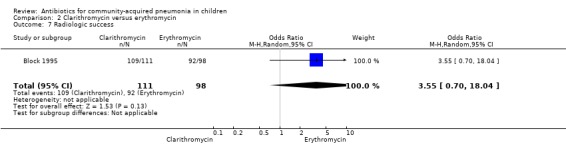
Comparison 2 Clarithromycin versus erythromycin, Outcome 7 Radiologic success.
2.8. Analysis.
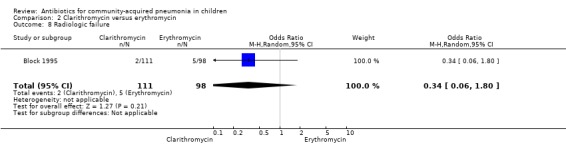
Comparison 2 Clarithromycin versus erythromycin, Outcome 8 Radiologic failure.
Azithromycin versus co‐amoxyclavulanic acid (Analysis 3)
Two studies (Harris 1998; Wubbel 1999) compared these two drugs in 283 children below five years of age. One study (Harris 1998) was double‐blinded and allocation concealment was adequate while the other study (Wubbel 1999) was unblinded with inadequate allocation concealment. The cure rates (available for one study) (OR 1.02, 95% CI 0.54 to 1.95) (Analysis 3.1), failure rates (available for both studies) (OR 1.21, 95% CI 0.42 to 3.53) (Analysis 3.2) and improvement rates (OR 0.85, 95% CI 0.43 to 1.71) (Analysis 3.3) were similar in the two groups. There were fewer side effects reported in the azithromycin group (OR 0.15, 95% CI 0.04 to 0.61) (Analysis 3.4). The organisms isolated were S. pneumoniae in 28 children, H. influenzae in one, Mycoplasma pneumoniae (M. pneumoniae) in 36 and Chlamydia pneumoniae (C. pneumoniae) in 20. The separate data for isolation of organisms in the two groups were available in one study only (Harris 1998). The organisms isolated in this study (Harris 1998) were S. pneumoniae and H. influenzae in one patient each in the azithromycin group. Investigations for mycoplasma were positive in 21 out of the 129 children (16%) tested in the azithromycin group and nine out of the 66 children (14%) tested in the co‐amoxyclavulanic acid group. Investigations for C. pneumoniae were positive in 13 out of the 129 children (10%) tested in the azithromycin group and four out of the 66 children (6%) tested in the co‐amoxyclavulanic acid group.
3.1. Analysis.
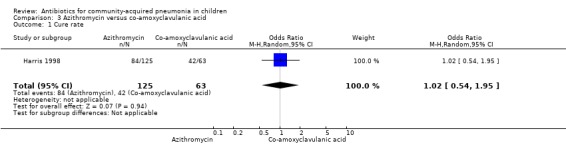
Comparison 3 Azithromycin versus co‐amoxyclavulanic acid, Outcome 1 Cure rate.
3.2. Analysis.
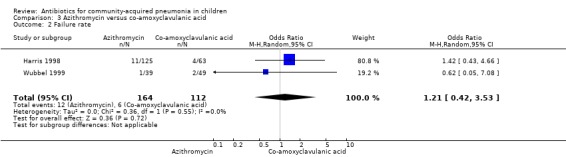
Comparison 3 Azithromycin versus co‐amoxyclavulanic acid, Outcome 2 Failure rate.
3.3. Analysis.
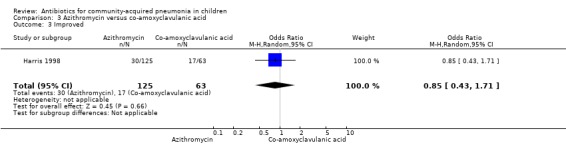
Comparison 3 Azithromycin versus co‐amoxyclavulanic acid, Outcome 3 Improved.
3.4. Analysis.
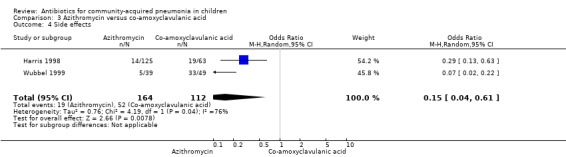
Comparison 3 Azithromycin versus co‐amoxyclavulanic acid, Outcome 4 Side effects.
Azithromycin versus amoxycillin (Analysis 4)
One study involving 47 children aged between one month and 14 years with classical pneumonia compared these two drugs (Kogan 2003). Children treated with azithromycin were older than those treated with amoxycillin (OR 58.1, 95% CI 35.59, 80.61). The study was unblinded and allocation concealment was also inadequate. All children recovered at the end of treatment in both the groups. There were 19 organisms identified in the 47 children tested (10 in the azithromycin group and nine in the amoxycillin group). The identification rates were similar in the two groups. Organisms included M. pneumoniae (in five and three children for the azithromycin and amoxycillin groups, respectively), S. pneumoniae (in four and three, respectively) and others (in one and three, respectively).
Amoxycillin versus procaine penicillin (Analysis 5)
One study involving 170 children aged six months to 18 years was identified (Tsarouhas 1998). The study was unblinded but allocation concealment was adequate. The age distribution in the two groups was comparable. The failure rates were similar in the two groups (OR 0.75, 95% CI 0.17 to 3.25) (Analysis 5.2).
5.2. Analysis.
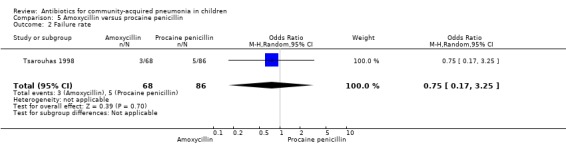
Comparison 5 Amoxycillin versus procaine penicillin, Outcome 2 Failure rate.
Co‐amoxyclavulanic acid versus amoxycillin (Analysis 6)
One study involved 100 children between two and 12 years of age. It was an open‐label study on children suffering from clinically diagnosed bacterial pneumonia (Jibril 1989). The study was unblinded and allocation concealment was also inadequate. Age and sex distribution, presence of wheeze and mean weight in the two groups were comparable. Cure rate was better with co‐amoxyclavulanic acid (OR 10.44, 95% CI 2.85 to 38.21) (Analysis 6.2).
6.2. Analysis.
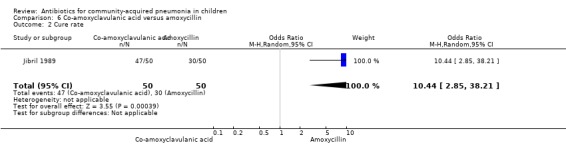
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 2 Cure rate.
Co‐trimoxazole versus amoxycillin (Analysis 7)
Three multicentre studies (Awasthi 2008; CATCHUP 2002; Straus 1998) involving 2346 children (1270 in the co‐trimoxazole group and 1077 in the amoxycillin group) between two months and 59 months of age have compared co‐trimoxazole and amoxycillin. The diagnosis of pneumonia was based on clinical criteria. Two studies (CATCHUP 2002; Straus 1998) were double‐blinded and allocation concealment was adequate. A third study (Awasthi 2008) was open‐label and cluster‐randomisation was done (the randomisation unit was Primary Health Centre) and in this study assessment of the primary outcome of treatment failure was done on day four for the amoxycillin group and day six for the co‐trimoxazole group; total numbers of events and effective sample size in this study (Awasthi 2008) were calculated after adjusting for the design effect. All studies included children with non‐severe pneumonia; one study (Straus 1998) also included 301 children with severe pneumonia. In pooled data the failure rate in non‐severe pneumonia was similar in the two groups (OR 1.18, 95% CI 0.91 to 1.51) (Analysis 7.7). The cure rate could be extracted in two studies (Awasthi 2008; CATCHUP 2002) and it was not different in either treatment group (OR 1.03, 95% CI 0.56 to 1.89) (Analysis 7.14) Loss to follow‐up was comparable in the two groups (OR 0.96, 95% CI 0.59 to 1.57) (Analysis 7.12). There were only two deaths in both the groups. The organisms isolated from blood cultures were H. influenzae in 79 children (52 in the co‐trimoxazole group and 27 in the amoxycillin group) and S. pneumoniae in 49 children (36 in the co‐trimoxazole group and 13 in the amoxycillin group); the distribution was similar in the two groups. In view of the difference in the time of assessment for the primary outcome in one study (Awasthi 2008), we performed analysis for failure rates in non‐severe pneumonia after excluding this study. The results did not alter significantly; failure rates in the two groups were similar (OR 1.19, 95% CI 0.92 to 1.53) (.Analysis 7.16) Failure rate in severe pneumonia available in one study was similar in the two groups (OR 1.71, 95% CI 0.94 to 3.11) (Analysis 7.8)).
7.7. Analysis.
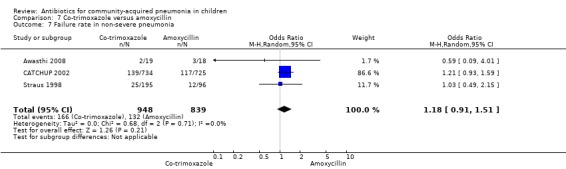
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 7 Failure rate in non‐severe pneumonia.
7.14. Analysis.
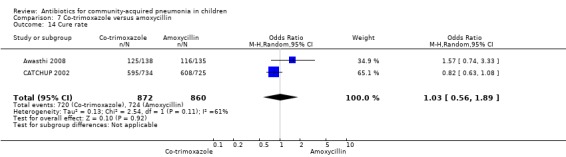
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 14 Cure rate.
7.12. Analysis.
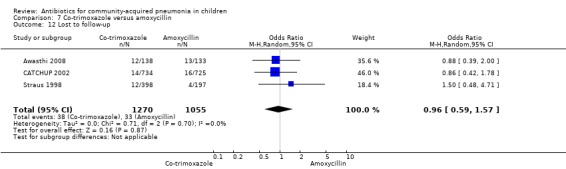
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 12 Lost to follow‐up.
7.16. Analysis.
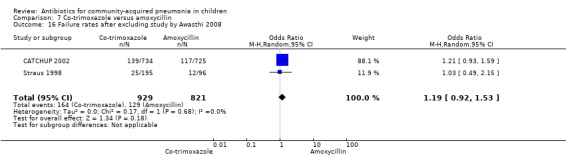
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 16 Failure rates after excluding study by Awasthi 2008.
7.8. Analysis.
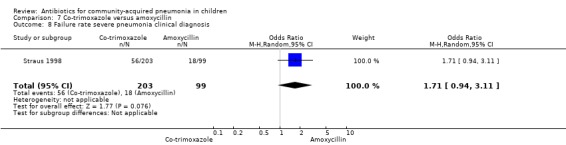
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 8 Failure rate severe pneumonia clinical diagnosis.
Co‐trimoxazole versus procaine penicillin (Analysis 8)
Two studies (Keeley 1990; Sidal 1994) enrolled 723 children between three months and 12 years of age. Both studies were unblinded and allocation concealment was adequate in one study (Keeley 1990). The cure rate was similar in the two groups (OR 1.58, 95% CI 0.26 to 9.69) (.Analysis 8.6) Rate of hospitalisation was available in only one study and was similar in the two groups (OR 2.52, 95% CI 0.88 to 7.25) (.Analysis 8.7) There was only one death.
8.6. Analysis.
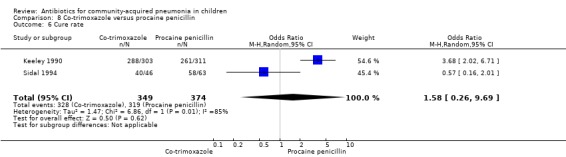
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 6 Cure rate.
8.7. Analysis.
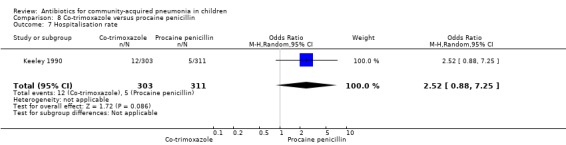
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 7 Hospitalisation rate.
Co‐trimoxazole versus single‐dose procaine penicillin followed by oral ampicillin for five days (Analysis 9)
One study was included that had enrolled 134 children below five years of age with severe pneumonia as defined by WHO criteria (Campbell 1988). The study was unblinded and allocation concealment was not clearly stated. The cure rates (OR 1.15, 95% CI 0.36 to 3.61) (Analysis 9.4), hospitalisation rates (OR 1.57, 95% CI 0.25 to 9.72) (Analysis 9.5) and death rates (OR 0.20, 95% CI 0.01 to 4.25) (Analysis 9.6) were similar for the two groups.
9.4. Analysis.
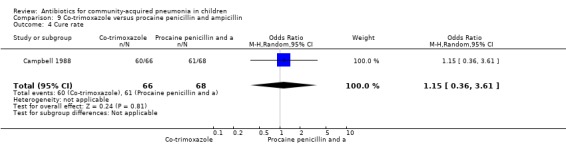
Comparison 9 Co‐trimoxazole versus procaine penicillin and ampicillin, Outcome 4 Cure rate.
9.5. Analysis.
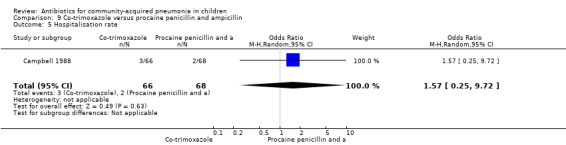
Comparison 9 Co‐trimoxazole versus procaine penicillin and ampicillin, Outcome 5 Hospitalisation rate.
9.6. Analysis.
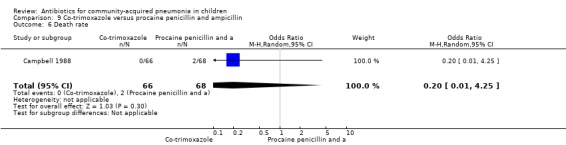
Comparison 9 Co‐trimoxazole versus procaine penicillin and ampicillin, Outcome 6 Death rate.
Cefpodoxime versus co‐amoxyclavulanic acid (Analysis 10)
One multicentre study (Klein 1995) enrolled 348 children between three months and 11.5 years of age. The study was unblinded and allocation concealment was inadequate. The age distribution in the two groups was comparable. The response rate at the end of 10 days of treatment was comparable in the two groups (OR 0.69, 95% CI 0.18 to 2.60) (Analysis 10.1). Organisms were isolated in 59 cases. These organisms were H. influenzae in 28 participants (47.5%), S. pneumoniae in 14 (23%), M. catarrhalis in seven (11.9%) and H. parainfluenzae in four (6.8). There was no significant difference in the bacteriologic efficacy of either group (100% versus 96.4%).
10.1. Analysis.
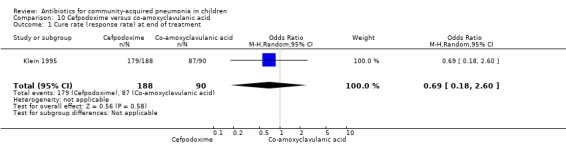
Comparison 10 Cefpodoxime versus co‐amoxyclavulanic acid, Outcome 1 Cure rate (response rate) at end of treatment.
Studies comparing treatment of hospitalised children with severe/very severe pneumonia
Chloramphenicol versus penicillin plus gentamycin (Analysis 11)
One multicentre study including 1116 children aged between one month and five years compared chloramphenicol with penicillin and gentamycin. This was an open‐label RCT in children with severe pneumonia that was carried out in Papua New Guinea (Duke 2002). Allocation concealment was adequate. There was no significant difference between the two groups in positive cultures, children who had received antibiotics earlier and loss to follow‐up. Need for change in antibiotics (OR 0.80, 95% CI 0.54 to 1.18) (Analysis 11.3), death rates (OR 1.25, 95% CI 0.76 to 2.07) (Analysis 11.2) and adverse events (OR 1.26, 95% CI 0.96 to 1.66) (Analysis 11.1) were similar in the two groups. However, re‐admission rates before 30 days favoured the penicillin‐gentamycin combination over chloramphenicol (OR 1.61, 95% CI 1.02 to 2.55) (Analysis 11.4). Bacterial pathogens were identified in 144 children (67 in children receiving chloramphenicol and 77 in the other group). Isolation rates or sensitivity of the organism and failure rates did not differ between the two groups.
11.3. Analysis.
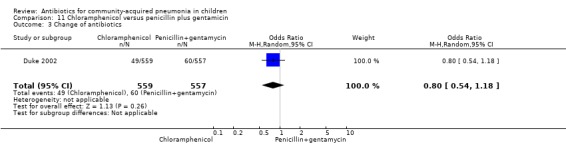
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 3 Change of antibiotics.
11.2. Analysis.
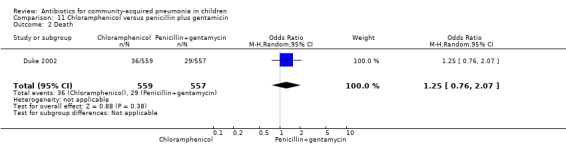
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 2 Death.
11.1. Analysis.
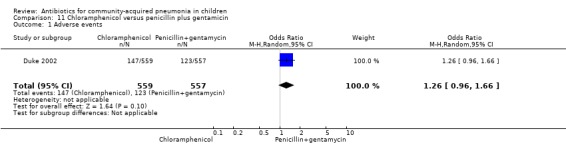
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 1 Adverse events.
11.4. Analysis.
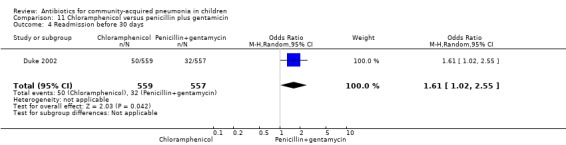
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 4 Readmission before 30 days.
Chloramphenicol with ampicillin and gentamycin (Analysis 12)
One multicentre study was identified; this study enrolled 958 children who were hospitalised with WHO‐defined very severe pneumonia (Asghar 2008). The study was unblinded and allocation concealment was adequate. Mean age, proportion of boys and number of children who had received antibiotics before enrolment were comparable in the two groups. Failure rates on day five (OR 1.51, 95% CI 1.04 to 2.19) (Analysis 12.4), day 10 (OR 1.46, 95% CI 1.04 to 2.06) (Analysis 12.5) and day 21 (OR 1.43, 95% CI 1.03 to 1.98) (Analysis 12.6) were significantly higher in those receiving chloramphenicol as compared to ampicillin and gentamycin. Death rates were higher in those receiving chloramphenicol (OR 1.65, 95% CI 0.99 to 2.77) (Analysis 12.10).
12.4. Analysis.
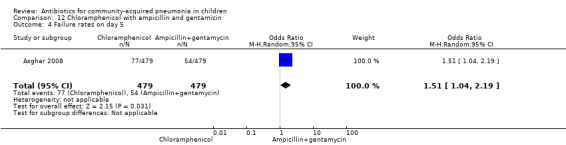
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 4 Failure rates on day 5.
12.5. Analysis.
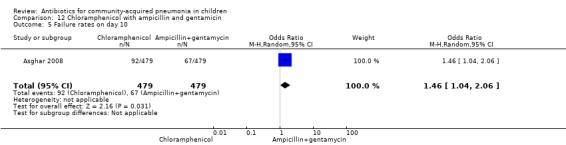
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 5 Failure rates on day 10.
12.6. Analysis.
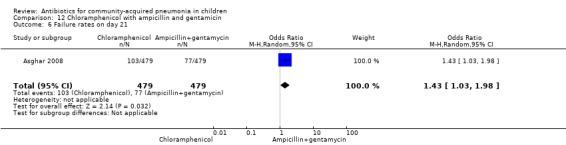
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 6 Failure rates on day 21.
12.10. Analysis.
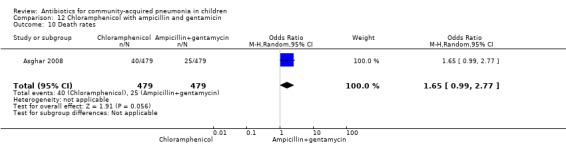
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 10 Death rates.
Chloramphenicol plus penicillin versus ceftriaxone (Analysis 13)
One double‐blind study fulfilled the inclusion criteria; the study enrolled 97 children between 2 and 24 months of age diagnosed with severe CAP with probable bacterial aetiology (Cetinkaya 2004). Allocation concealment was adequate. Ages in the two groups were comparable (details not available). Cure rates in the two groups were similar (OR 1.36, 95% CI 0.47 to 3.93) (Analysis 13.1).
13.1. Analysis.
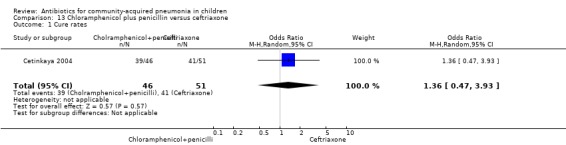
Comparison 13 Chloramphenicol plus penicillin versus ceftriaxone, Outcome 1 Cure rates.
Chloramphenicol alone versus chloramphenicol plus penicillin (Analysis 14)
One study (Shann 1985) from Papua New Guinea involved 748 hospitalised children (age not clear) with severe pneumonia. The study was unblinded but allocation concealment was adequate. Need for change in antibiotics (OR 0.49, 95% CI 0.12 to 1.97) (Analysis 14.1), loss to follow‐up (OR 1.11, 95% CI 0.80 to 1.53) (Analysis 14.3) and deaths rates (OR 0.73, 95% CI 0.48 to 1.09) (Analysis 14.2) were comparable in the two groups.
14.1. Analysis.
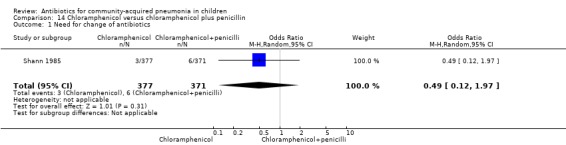
Comparison 14 Chloramphenicol versus chloramphenicol plus penicillin, Outcome 1 Need for change of antibiotics.
14.3. Analysis.
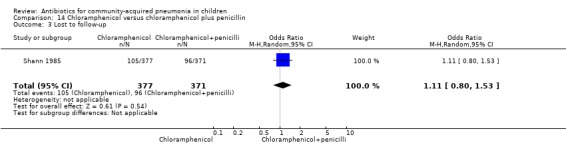
Comparison 14 Chloramphenicol versus chloramphenicol plus penicillin, Outcome 3 Lost to follow‐up.
14.2. Analysis.
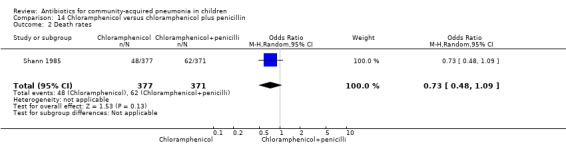
Comparison 14 Chloramphenicol versus chloramphenicol plus penicillin, Outcome 2 Death rates.
Ampicillin alone versus penicillin with chloramphenicol (Analysis 15)
One trial involving 115 children between five months and four years of age was identified (Deivanayagam 1996). The study was unblinded and allocation concealment was adequate. Age and sex distribution and proportion of children with severe malnutrition were comparable in the two groups. The cure rates (OR 0.48, 95% CI 0.15 to 1.51) (Analysis 15.1) and duration of hospitalisation were similar in the two groups (mean difference (MD) 0.1, 95% CI ‐1.13 to 0.93) (Analysis 15.4).
15.1. Analysis.
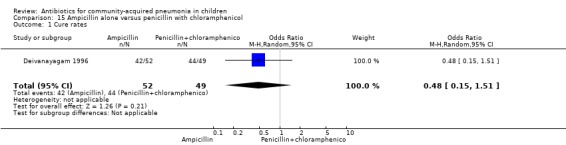
Comparison 15 Ampicillin alone versus penicillin with chloramphenicol, Outcome 1 Cure rates.
15.4. Analysis.
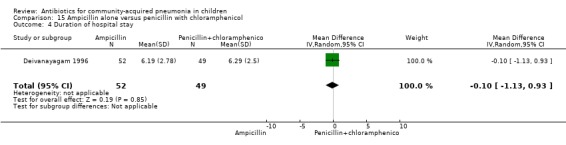
Comparison 15 Ampicillin alone versus penicillin with chloramphenicol, Outcome 4 Duration of hospital stay.
Benzathine penicillin versus procaine penicillin (Analysis 16)
Two studies fulfilled the inclusion criteria; one which included 176 children between two and 12 years of age with chest X‐ray films showing lobar consolidation or infiltration (presumed streptococcal infection) (Camargos 1997) and another study of 105 children between three months and 14 years of age (Sidal 1994). Both studies were unblinded and allocation concealment was adequate in one (Camargos 1997). Cure rates were not significantly different in the two groups (OR 0.53, 95% CI 0.27 to 1.01) (Analysis 16.1). Failure rates were also similar between the groups (OR 3.17, 95% CI 0.9 to 11.11) (Analysis 16.2). Bacterial pathogens were identified in only one study. The isolation rate for S. pneumoniae was six out of 90 blood cultures performed (four participants in the benzathine group and two in the procaine penicillin group). The clinical outcome did not differ in relation to the organism identified.
16.1. Analysis.
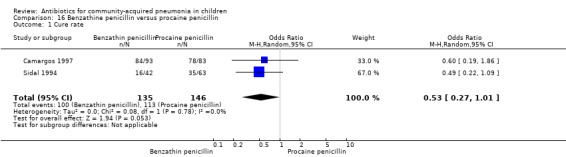
Comparison 16 Benzathine penicillin versus procaine penicillin, Outcome 1 Cure rate.
16.2. Analysis.
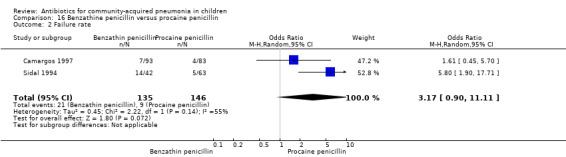
Comparison 16 Benzathine penicillin versus procaine penicillin, Outcome 2 Failure rate.
Amoxycillin versus penicillin (Analysis 17)
Two multicentre non‐blinded studies were identified; these enrolled 1702 children between three months and 59 months of age, suffering from severe pneumonia (diagnosed on the basis of WHO criteria) (Addo‐Yobo 2004) and 203 children with radiographically confirmed pneumonia (Atkinson 2007). The studies were unblinded and allocation concealment was adequate. The second study (Atkinson 2007) measured outcome as time from randomisation until the temperature was < 38 degrees celsius for 24 hours and oxygen requirement had ceased. However, it provided data on need for change of antibiotics due to worsening of respiratory/radiological findings. For the purposes of this analysis we considered them as failure on day five. Age, sex, severe malnutrition, breast feeding and the number of children who had received antibiotics in the last week were similar in both the groups. The failure rates measured at 48 hours (OR 1.03, 95% CI 0.81 to 1.31) (Analysis 17.7), five days (OR 1.15, 95% CI 0.58 to 2.30) (Analysis 17.8) and 14 days (OR 1.04, 95% CI 0.84 to 1.29) (Analysis 17.9) were similar in both groups. There were seven deaths in the group receiving penicillin in one study (Addo‐Yobo 2004) while no deaths were observed in the other study (Atkinson 2007).
17.7. Analysis.
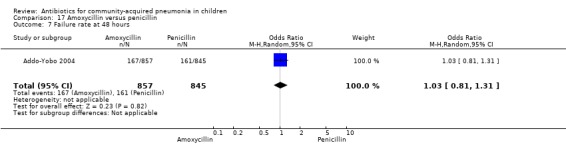
Comparison 17 Amoxycillin versus penicillin, Outcome 7 Failure rate at 48 hours.
17.8. Analysis.
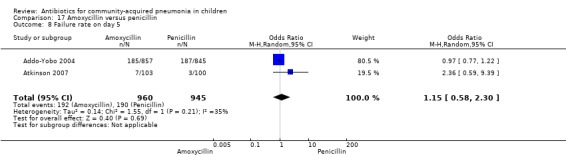
Comparison 17 Amoxycillin versus penicillin, Outcome 8 Failure rate on day 5.
17.9. Analysis.
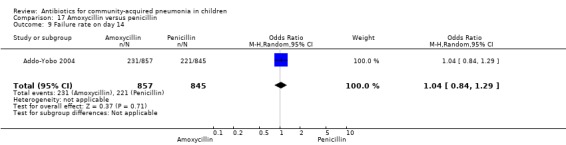
Comparison 17 Amoxycillin versus penicillin, Outcome 9 Failure rate on day 14.
Amoxycillin with intravenous (IV) ampicillin (Analysis 18)
One non‐blinded study involving 237 children between two and 59 months of age with severe pneumonia was identified (Hazir 2008). Allocation concealment was adequate. Number of infants in each group, sex distribution and presence of wheeze were comparable in the two groups. Failure rates (OR 0.86, 95% CI 0.63 to 1.19) (Analysis 18.5), relapse rates (OR 0.78, 95% CI 0.46 to 1.33) (Analysis 18.6) and death rates (OR 0.25, 95% CI 0.03 to 2.21) (Analysis 18.7) were similar in the two groups.
18.5. Analysis.
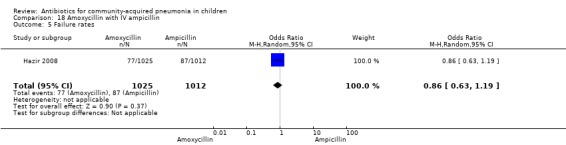
Comparison 18 Amoxycillin with IV ampicillin, Outcome 5 Failure rates.
18.6. Analysis.
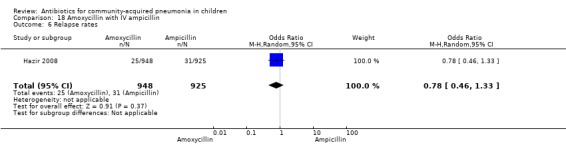
Comparison 18 Amoxycillin with IV ampicillin, Outcome 6 Relapse rates.
18.7. Analysis.
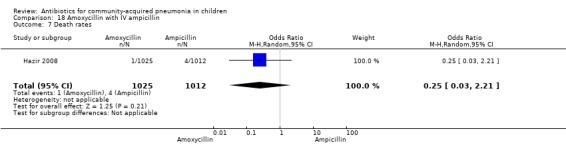
Comparison 18 Amoxycillin with IV ampicillin, Outcome 7 Death rates.
Amoxycillin with cefuroxime (Analysis 19)
One randomised, non‐blinded controlled study was identified; this included 83 children with non‐severe and severe pneumonia (Aurangzeb 2003). Allocation concealment was unclear. Baseline data in the form of mean age and proportion of boys were similar in the two groups. Cure rates (OR 2.05, 95% CI 0.18 to 23.51) (Analysis 19.3) and failure rates (OR 0.49, 95% CI 0.04 to 5.59) (Analysis 19.4) were similar in the two groups.
19.3. Analysis.
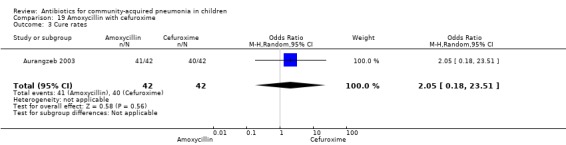
Comparison 19 Amoxycillin with cefuroxime, Outcome 3 Cure rates.
19.4. Analysis.
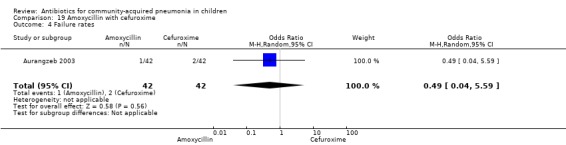
Comparison 19 Amoxycillin with cefuroxime, Outcome 4 Failure rates.
Amoxycillin with clarithromycin (Analysis 20)
One randomised, non‐blinded controlled study compared these two drugs; 85 children with non‐severe and severe pneumonia were enrolled (Aurangzeb 2003). The sequence generation and allocation concealment in the study is not clear. Baseline data in the form of mean age and proportion of boys were similar in the two groups. Cure rates (OR 1.05, 95% CI 0.06 to 17.40) (Analysis 20.3) and failure rates (OR 0.95, 95% CI 0.06 to 15.74) (Analysis 20.4) were similar in the two groups.
20.3. Analysis.
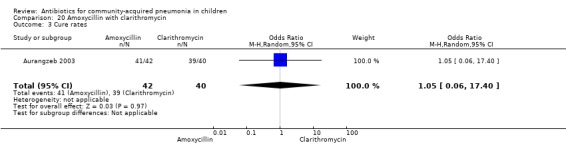
Comparison 20 Amoxycillin with clarithromycin, Outcome 3 Cure rates.
20.4. Analysis.
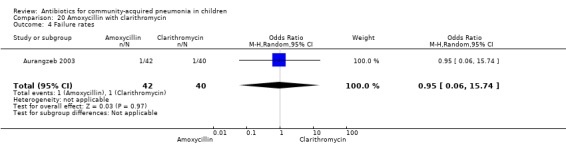
Comparison 20 Amoxycillin with clarithromycin, Outcome 4 Failure rates.
Penicillin and gentamycin with co‐amoxyclavulanic acid (Analysis 21)
One study involving 71 children between two months and 59 months of age with very severe pneumonia fulfilled the inclusion criteria (Bansal 2006). The study was non‐blinded and allocation concealment was adequate. Baseline characteristics, including number of infants and sex distribution, were comparable. Failure rates in the two groups were similar (OR 0.86, 95% CI 0.05 to 14.39) (Analysis 21.3).
21.3. Analysis.
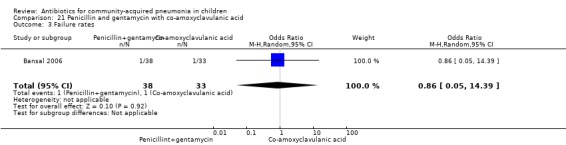
Comparison 21 Penicillin and gentamycin with co‐amoxyclavulanic acid, Outcome 3 Failure rates.
Levofloxacin with comparator group (Analysis 22)
One non‐blinded study, involving 709 children below 16 years of age, compared oral levofloxacin with either ceftriaxone or co‐amoxyclavulanic acid (Bradley 2007). Sequence generation and allocation concealment is not clear from the study. The mean age, sex and number who received antibiotics before enrolment were comparable in the two groups. Cure rates were similar in the two groups (OR 1.05, 95% CI 0.46 to 2.42) (Analysis 22.4).
22.4. Analysis.
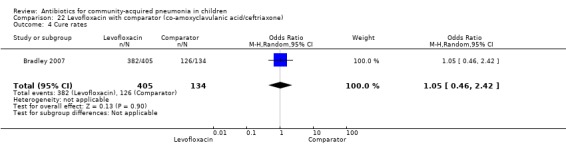
Comparison 22 Levofloxacin with comparator (co‐amoxyclavulanic acid/ceftriaxone), Outcome 4 Cure rates.
Cefuroxime with clarithromycin (Analysis 23)
One randomised, non‐blinded, controlled study involving 85 children with non‐severe and severe pneumonia was identified (Aurangzeb 2003). Allocation concealment was unclear. Baseline data in the form of mean age and proportion of boys were similar in the two groups. Cure rates (OR 0.51, 95% CI 0.04 to 5.89) ( Analysis 23.3) and failure rates (OR 2.05, 95% CI 0.18 to 23.51) (Analysis 23.4) were similar in the two groups.
23.3. Analysis.
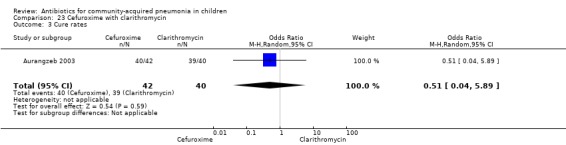
Comparison 23 Cefuroxime with clarithromycin, Outcome 3 Cure rates.
23.4. Analysis.
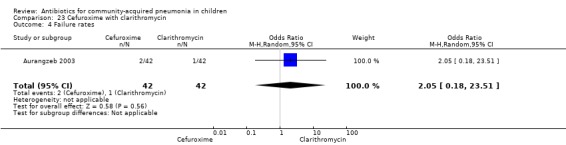
Comparison 23 Cefuroxime with clarithromycin, Outcome 4 Failure rates.
Co‐trimoxazole versus chloramphenicol (Analysis 24)
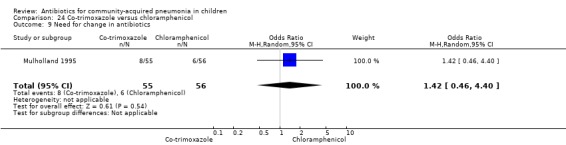
One double‐blind study involving 111 malnourished children under five years of age fulfilled the inclusion criteria for this review (Mulholland 1995). Allocation concealment was adequate. The age and sex distribution, nutritional status, children with wheezing and numbers excluded were similar in the two groups. Cure rates (OR 1.06, 95% CI 0.47 to 2.40) (Analysis 24.5), failure rates (OR 1.03, 95% CI 0.45 to 2.33) (Analysis 24.6), number of participants requiring a change in antibiotics (OR 1.42, 95% CI 0.46 to 4.40) (Analysis 24.9), relapse rates (OR 1.02, 95% CI 0.24 to 4.30) (Analysis 24.8) and death rates (OR 2.21, 95% CI 0.63 to 7.83) (Analysis 24.10) were similar in the two groups.
24.5. Analysis.
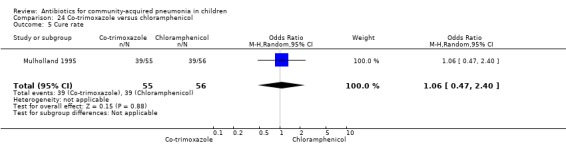
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 5 Cure rate.
24.6. Analysis.
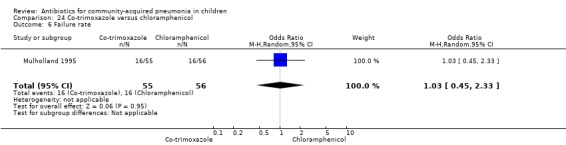
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 6 Failure rate.
24.9. Analysis.
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 9 Need for change in antibiotics.
24.8. Analysis.
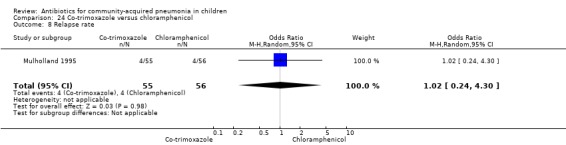
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 8 Relapse rate.
24.10. Analysis.
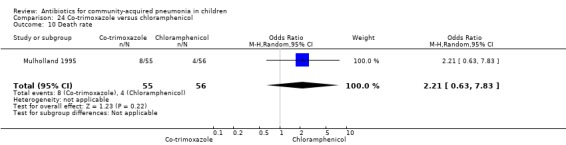
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 10 Death rate.
Ceftibuten with cefuroxime axetil (Analysis 25)
One study involving 140 children between one and 12 years of age with radiographically confirmed CAP compared ceftibuten with cefuroxime axetil (Nogeova 1997). The study was unblinded. Sequence generation and allocation concealment were not clear from the paper. Age and sex distribution were similar in the two groups. Cure rate (OR 0.32 95% CI 0.11 to 0.94) (Analysis 25.4) was significantly higher and failure rate (OR 6.81, 95% CI 1.46 to 31.70) (Analysis 25.5) was significantly lower in children receiving cefuroxime. Organisms were isolated in 83 participants (53 in the ceftibuten group and 30 in the cefuroxime group). Identification of organisms was significantly higher in children who received ceftibuten (OR 3.83, 95% CI 1.87 to 7.83) (Analysis 25.2). Organisms identified in children who received ceftibuten were S. pneumoniae (17), H. influenzae (13), Staphylococcus aureus (S. aureus) (eight), group A beta haemolytic streptococcus (seven), Moraxella catarrhalis (M. catarrhalis) (four), respiratory syncytial virus (onr) and Mycoplasma pneumoniae (M. pneumoniae) (one). The organisms identified in children receiving cefuroxime axetil were: S. pneumoniae (seven), H. influenzae (eight), S. aureus (three), group A beta haemolytic streptococcus (four), Moraxella catarrhalis (seven), respiratory syncytial virus (three) and M. pneumoniae (three).
25.4. Analysis.
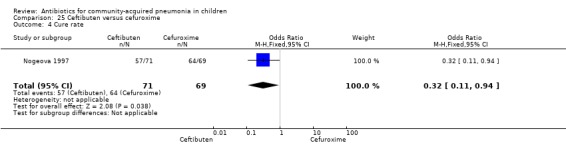
Comparison 25 Ceftibuten versus cefuroxime, Outcome 4 Cure rate.
25.5. Analysis.
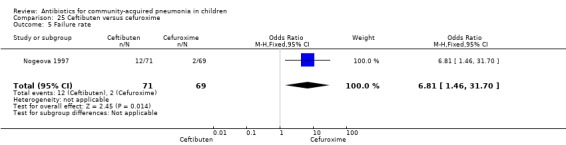
Comparison 25 Ceftibuten versus cefuroxime, Outcome 5 Failure rate.
25.2. Analysis.
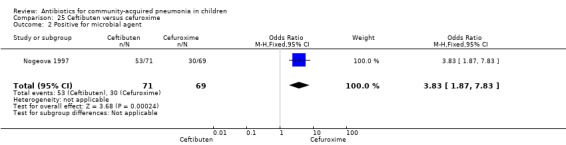
Comparison 25 Ceftibuten versus cefuroxime, Outcome 2 Positive for microbial agent.
Oxacillin/ceftriaxone with co‐amoxyclavulanic acid (Analysis 26)
One study involving 104 children aged between two months to five years with very severe pneumonia was included (Ribeiro 2011). The study was unblinded; random sequence generation, allocation concealment and reporting of data were adequate. Age and sex distribution, days before admission in hospital, receipt of antibiotics before enrolment and failure rates (OR 0.98, 95% CI 0.33 to 2.92) (Analysis 26.5) were similar in the two groups of participants. Mean time for improvement (MD ‐1.00 day, 95% CI ‐1.89 to ‐0.11) (Analysis 26.6) and total hospital stay (MD ‐3.40 days, 95% CI ‐5.46 to ‐1.34) (Analysis 26.7) were significantly better in children receiving co‐amoxyclavulanic acid.
26.5. Analysis.
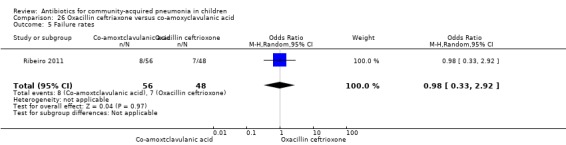
Comparison 26 Oxacillin ceftriaxone versus co‐amoxyclavulanic acid, Outcome 5 Failure rates.
26.6. Analysis.
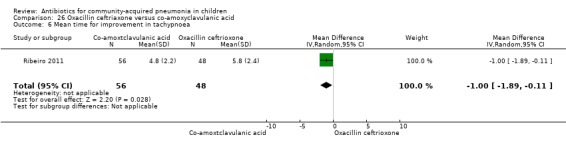
Comparison 26 Oxacillin ceftriaxone versus co‐amoxyclavulanic acid, Outcome 6 Mean time for improvement in tachypnoea.
26.7. Analysis.
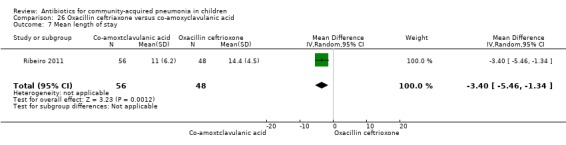
Comparison 26 Oxacillin ceftriaxone versus co‐amoxyclavulanic acid, Outcome 7 Mean length of stay.
Antibiotics in radiographically confirmed pneumonia
Out of 29 studies, 12 (Atkinson 2007; Bansal 2006; Block 1995; Bradley 2007; Camargos 1997; Deivanayagam 1996; Klein 1995; Kogan 2003; Mulholland 1995; Nogeova 1997; Wubbel 1999; Tsarouhas 1998) enrolled children with radiographically confirmed pneumonia. Ten studies (Addo‐Yobo 2004; Asghar 2008; Awasthi 2008; Campbell 1988; CATCHUP 2002; Cetinkaya 2004; Duke 2002; Hazir 2008; Shann 1985; Straus 1998) used clinical criteria to diagnose pneumonia. Three studies (Harris 1998; Ribeiro 2011; Roord 1996) used clinical criteria or radiography for diagnosis of pneumonia. In four studies (Aurangzeb 2003; Jibril 1989; Keeley 1990; Sidal 1994) the role of radiography in the diagnosis of pneumonia was not clear from the description. The following comparisons were carried out in radiographically confirmed pneumonia.
Azithromycin versus erythromycin (Analysis 1)
Out of four studies (Harris 1998; Kogan 2003; Roord 1996; Wubbel 1999), radiographs were performed for diagnosis of pneumonia in only two studies (Kogan 2003; Wubbel 1999). A total of 147 children were enrolled in these two studies. Failure rates (OR 0.62, 95% CI 0.23 to 1.63) (Analysis 1.9) and cure rates (OR 1.72, 95% CI 0.65 to 4.56) (Analysis 1.8) were not different in the two groups.
1.9. Analysis.
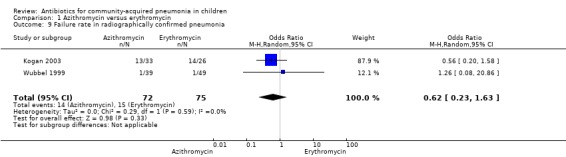
Comparison 1 Azithromycin versus erythromycin, Outcome 9 Failure rate in radiographically confirmed pneumonia.
1.8. Analysis.
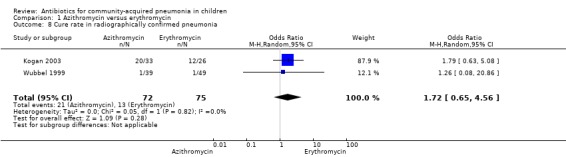
Comparison 1 Azithromycin versus erythromycin, Outcome 8 Cure rate in radiographically confirmed pneumonia.
Clarithromycin versus erythromycin (Analysis 2)
One study (Block 1995) compared erythromycin and clarithromycin; 234 children below 15 years of age with radiographically confirmed pneumonia were treated on in an ambulatory setting. Resolution of pneumonia (diagnosed radiologically) was more frequent in the clarithromycin group compared to the erythromycin group (OR 2.51, 95% CI 1.02 to 6.16) (Analysis 2.6). However, there were no differences in radiologic cure rates (OR 3.55, 95% CI 0.7 to 18.04) (Analysis 2.7) or radiologic failure rates (OR 0.34, 95% CI 0.06 to 1.80) (Analysis 2.8).
Erythromycin versus co‐amoxyclavulanic acid (Analysis 3)
Out of two studies (Harris 1998; Wubbel 1999), one study (Wubbel 1999) involving 88 children enrolled participants with radiographically confirmed pneumonia. Failure rates were similar in the two groups (OR 0.62, 95% CI 0.05 to 7.08) (Analysis 3.7)
3.7. Analysis.
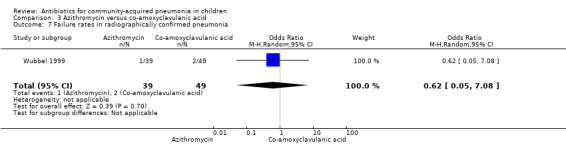
Comparison 3 Azithromycin versus co‐amoxyclavulanic acid, Outcome 7 Failure rates in radiographically confirmed pneumonia.
Azithromycin versus amoxycillin (Analysis 4)
One study involving 47 children aged between one month and 14 years with radiographically confirmed pneumonia compared azithromycin and amoxycillin (Kogan 2003). Children treated with azithromycin were older than those treated with amoxycillin (OR 58.1, 95% CI 35.59 to 80.61) (Analysis 4.1). Cure rates were not significantly different in the two groups (OR 2.85, 95% CI 0.73 to 11.09) (Analysis 4.5).
4.1. Analysis.
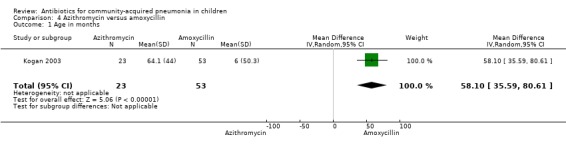
Comparison 4 Azithromycin versus amoxycillin, Outcome 1 Age in months.
4.5. Analysis.
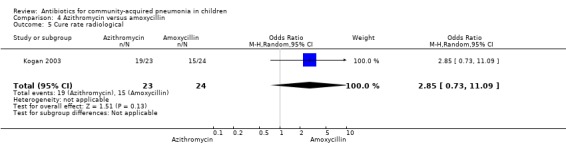
Comparison 4 Azithromycin versus amoxycillin, Outcome 5 Cure rate radiological.
Amoxycillin versus procaine penicillin (Analysis 5)
One study involving 170 children with radiographically confirmed pneumonia, aged six months to 18 years, was identified (Tsarouhas 1998). The failure rates were similar in the two groups (OR 0.75, 95% CI 0.17 to 3.25) (Analysis 5.2).
Cefpodoxime versus co‐amoxyclavulanic acid (Analysis 10)
One multicentre study (Klein 1995) enrolled 348 children with radiographically confirmed pneumonia aged three months to 11.5 years of age. Cure rates in the two groups were similar (OR 0.69, 95% CI 0.18 to 2.60) (Analysis 10.1).
Studies comparing treatment of hospitalised children with severe/very severe pneumonia
Ampicillin alone versus penicillin with chloramphenicol (Analysis 15)
One trial involving 115 children with radiographically confirmed pneumonia, between five months and four years of age, was identified (Deivanayagam 1996). The study was unblinded and allocation concealment was adequate. The cure rates (OR 0.48, 95% CI 0.15 to 1.51) (Analysis 15.1) and duration of hospitalisation were similar in the two groups (MD 0.1, 95% CI ‐1.13 to 0.93) (Analysis 15.4).
Benzathine penicillin versus procaine penicillin (Analysis 16)
Out of two studies, radiographically confirmed pneumonia was only assessed in one study which included 176 children between two and 12 years of age with chest X‐ray films showing lobar consolidation or infiltration (presumed streptococcal infection) (Camargos 1997). Failure rates were similar between the groups (OR 1.61, 95% CI 0.45 to 5.70) (Analysis 16.7).
16.7. Analysis.
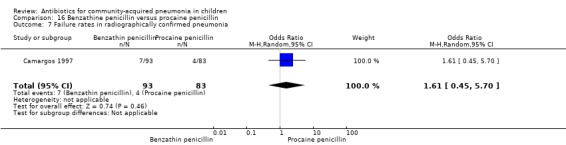
Comparison 16 Benzathine penicillin versus procaine penicillin, Outcome 7 Failure rates in radiographically confirmed pneumonia.
Amoxycillin versus penicillin (Analysis 17)
Out of two studies, children with radiographically confirmed pneumonia were enrolled in one study involving 203 children (Atkinson 2007). The failure rate on day five was similar in the two groups (OR 2.36, 95% CI 0.59 to 9.39) (Analysis 17.15).
17.15. Analysis.
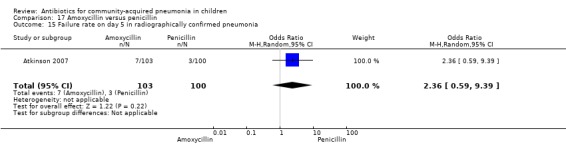
Comparison 17 Amoxycillin versus penicillin, Outcome 15 Failure rate on day 5 in radiographically confirmed pneumonia.
Penicillin and gentamycin with co‐amoxyclavulanic acid (Analysis 21)
One study involving 71 children between two months and 59 months of age with very severe, radiographically confirmed pneumonia fulfilled the inclusion criteria (Bansal 2006). Failure rates in the two groups were similar (OR 0.86, 95% CI 0.05 to 14.39) (Analysis 21.3).
Levofloxacin with comparator group (Analysis 22)
One non‐blinded study, involving 709 children below 16 years of age, compared oral levofloxacin with either ceftriaxone or co‐amoxyclavulanic acid (Bradley 2007). The sequence generation and allocation concealment were not clear from the study. The mean age, sex and number who received antibiotics before enrolment were comparable in the two groups. Cure rates were similar in the two groups (OR 1.05, 95% CI 0.46 to 2.42) (Analysis 22.4).
Co‐trimoxazole versus chloramphenicol (Analysis 24)
One double‐blind study involving 111 malnourished children with radiographically confirmed pneumonia under five years of age fulfilled the inclusion criteria for this review (Mulholland 1995). Cure rates (OR 1.06, 95% CI 0.47 to 2.40) (Analysis 24.5), failure rates (OR 1.03, 95% CI 0.45 to 2.33) (Analysis 24.6), number of participants requiring a change in antibiotics (OR 1.42, 95% CI 0.46 to 4.40) (Analysis 24.9), relapse rates (OR 1.02, 95% CI 0.24 to 4.30) (Analysis 24.8) and death rates (OR 2.21, 95% CI 0.63 to 7.83) (Analysis 24.10) were similar in the two groups.
Ceftibuten with cefuroxime axetil (Analysis 25)
One study (Nogeova 1997) involved 140 children between one and 12 years of age with radiographically confirmed CAP. Cure rate (OR 0.32, 95% CI 0.11 to 0.94) (Analysis 25.4) and failure rate (OR 6.81, 95% CI 1.46 to 31.70) (Analysis 25.5) were significantly better in the children receiving cefuroxime.
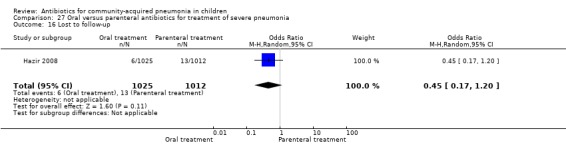
Oral treatment of severe pneumonia with parenteral treatment (Analysis 27)
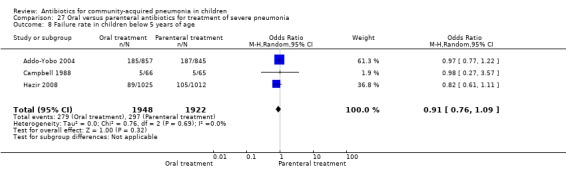
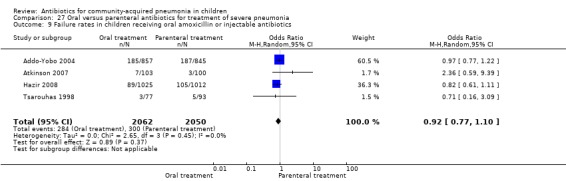
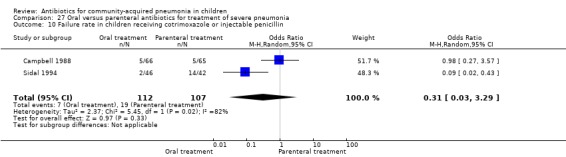
There were six studies (Addo‐Yobo 2004; Atkinson 2007; Campbell 1988; Hazir 2008; Sidal 1994; Tsarouhas 1998) that included children with severe pneumonia and compared oral antimicrobial agents with initial intravenous or intramuscular medications. Four studies compared oral amoxycillin with intravenous penicillin/ampicillin (Addo‐Yobo 2004; Atkinson 2007; Hazir 2008; Tsarouhas 1998). Two studies compared oral cotrimoxazole with intramuscular penicillin (Campbell 1988; Sidal 1994). In four studies (Campbell 1988; Hazir 2008; Sidal 1994; Tsarouhas 1998) children were treated in an ambulatory setting with injections as well as oral medications. A total of 4331 children below 18 years of age were enrolled; 2174 received oral antibiotics (cotrimoxazole or amoxycillin) and 2157 received intravenous or intramuscular antibiotics (penicillin or ampicillin). The baseline characteristics (age and sex distribution) in the two groups and proportion of children who had received antibiotics before enrolment were comparable in the two groups. Failure rates were similar in the two groups (OR 0.84, 95% CI 0.56 to 1.24) (Analysis 27.7). Separate data for children below five years of age were not available. We re‐analysed data after removing studies that also enrolled children above five years of age (Atkinson 2007; Sidal 1994; Tsarouhas 1998). Failure rates were similar in the two groups (OR 0.91, 95% CI 0.76 to 1.09) (Analysis 27.8). Failure rates did not show significant differences when children receiving amoxycillin (OR 0.92, 95% CI 0.77 to 1.10) (Analysis 27.9) or cotrimoxazole (OR 0.31, 95% CI 0.03 to 3.29) (Analysis 27.10) were analysed separately.
27.7. Analysis.
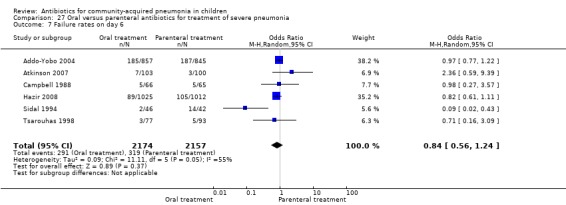
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 7 Failure rates on day 6.
27.8. Analysis.
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 8 Failure rate in children below 5 years of age.
27.9. Analysis.
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 9 Failure rates in children receiving oral amoxicillin or injectable antibiotics.
27.10. Analysis.
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 10 Failure rate in children receiving cotrimoxazole or injectable penicillin.
Analysis of studies that treated both the groups in an ambulatory setting (after removing studies that gave both the treatments in hospital) showed that failure rates in the two groups were not different (OR 0.92, 95% CI 0.77 to 1.10) (Analysis 27.9). Cure rates were available in two studies (Atkinson 2007; Sidal 1994) and were significantly better in children receiving oral antibiotics (OR 5.05, 95% CI 1.19 to 21.33).
Hospitalisation rate in children receiving treatment in an ambulatory setting was available in three studies (Campbell 1988; Sidal 1994; Tsarouhas 1998). The need for hospitalisation was similar in the two groups (OR 1.13, 95% CI 0.38, 3.34) (Analysis 27.13). Relapse rates were available in two studies (Atkinson 2007; Hazir 2008) and there was no significant difference in the two groups (OR 1.28, 95% CI 0.34 to 4.82) (Analysis 27.14). Death rate was available in three studies (Addo‐Yobo 2004; Atkinson 2007; Hazir 2008) and was significantly higher in those who received injectable treatments (OR 0.15, 95% CI 0.03 to 0.87) (Analysis 27.15). There were no deaths in one study (Atkinson 2007) and seven deaths in another study (but only in those receiving intravenous penicillin (Addo‐Yobo 2004)) and five deaths in the third study (one in the oral group and four in the intravenous ampicillin group) (Hazir 2008). Re‐analysis after removing one study with seven deaths in only one group (Addo‐Yobo 2004) suggests no significant difference between the two groups (OR 0.25, 95% CI 0.03, 2.21) (Analysis 27.19). Data on loss to follow‐up were available in one study (Hazir 2008) and were similar in the two groups (OR 0.45, 95% CI 0.17 to 1.20) (Analysis 27.16).
27.13. Analysis.
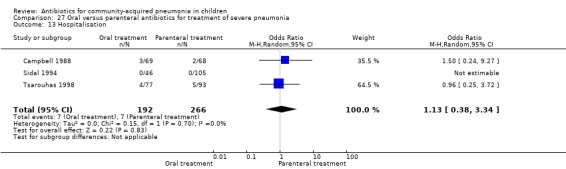
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 13 Hospitalisation.
27.14. Analysis.
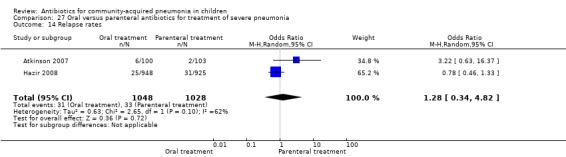
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 14 Relapse rates.
27.15. Analysis.
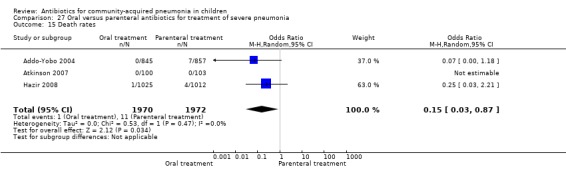
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 15 Death rates.
27.19. Analysis.
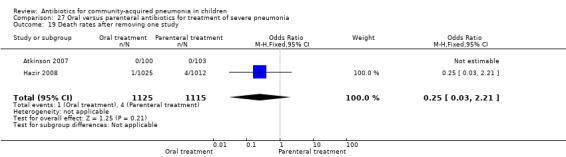
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 19 Death rates after removing one study.
27.16. Analysis.
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 16 Lost to follow‐up.
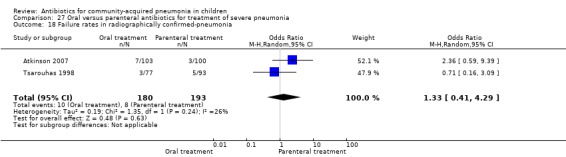
Only two studies (Atkinson 2007; Tsarouhas 1998) enrolled children with radiographically confirmed pneumonia. A total of 373 children were enrolled. The failure rates were similar in the two groups (OR 1.33, 95% CI 0.41 to 4.29) (Analysis 27.18).
27.18. Analysis.
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 18 Failure rates in radiographically confirmed‐pneumonia.
Identification of aetiological agents
Out of 29 studies reviewed, attempts were made to isolate or demonstrate the aetiological organisms in 14 studies. The methods used in these studies for identification of bacteria were a blood culture, sputum examination or urinary antigen detection. For this review, results of a throat swab for bacterial isolation were ignored. Bacterial pathogens could be identified in blood cultures or serology/sputum in 591 (12%) out of 4882 participants tested. Out of the bacterial pathogens identified, 236 (40%) participants had S. pneumoniae, 150 (25%) had H. influenzae, 69 (12%) had S. aureus and 136 (23%) had other pathogens including the gram‐negative bacilli M. catarrhalis and Staphylococcus albus (S. albus) and Group A beta haemolytic streptococcus (Table 1).
1. Bacterial isolation.
| Study/total tested | S. pneumoniae | H. influenzae | Staphylococcus | Others |
| Asghar 2008/958 | 22 | 8 | 47 | 33 |
| Bansal 2006/71 | 3 | 2 | 0 | 0 |
| Block 1995/122 | 2 | 2 | 0 | 0 |
| Bradley 2007/709 | 21 | 7 | 0 | 3 |
| Camargos 1997/90 | 6 | 0 | 0 | 0 |
| Duke 2002/1116 | 4 | 10 | 10 | 36 |
| Harris 1998/351 | 5 | 2 | 0 | 0 |
| Klein 1995/348 | 14 | 28 | 0 | 17 |
| Kogan 2003/47 | 7 | 0 | 0 | 4 |
| Mulholland 1995/111 | 10 | 2 | 0 | 8 |
| Nogeova 1997/140 | 24 | 21 | 11 | 22 |
| Roord 1996/95 | 11 | 19 | 1 | 13 |
| Straus 1998/595 | 79 | 49 | 0 | 0 |
| Wubbel 1999/129 | 35 | 0 | 0 | 0 |
| Total/4882 (12%) | 236 (4.8%) | 150 (3.0%) | 69 (1.4%) | 136 (2.8%) |
| Out of total bacterial isolates (591) | 236/591 (40%) | 150/591 (25%) | 69/591 (12%) | 136/591 (23%) |
Information regarding the sensitivity pattern of bacterial isolates was available in four studies (Asghar 2008; Bansal 2006; Mulholland 1995; Roord 1996). This information was only available for the antibiotics studied and sensitivity was not tested in all the isolates. In the study by Asghar 2008, out of a total of 22 S. pneumoniae isolates, 13/14 were sensitive to chloramphenicol, 12/17 to gentamycin, 15/16 to ampicillin and 12/12 to third‐generation cephalosporins.
Out of a total of eight isolates of H. influenzae, 6/7 were sensitive to chloramphenicol, 12/17 to gentamicin, 15/16 to ampicillin and 6/6 to third‐generation cephalosporins.
Out of a total of 47 isolates of S. aureus, 19/37 were sensitive to chloramphenicol, 29/45 to gentamycin, 15/16 to ampicillin and 6/6 to third‐generation cephalosporins.
In the study by Bansal 2006, all the three isolates of S. pneumoniae were sensitive to penicillin, amoxycillin, erythromycin and gentamycin. However, out of two isolates of H. influenzae, one was sensitive and the other isolate was resistant to penicillin, amoxycillin, erythromycin and gentamycin. The one that was resistant was sensitive to ciprofloxacin, cefotaxime and chloramphenicol. In the study by Mulholland 1995, all 10 isolates of S. pneumoniae were susceptible to co‐trimoxazole and nine of these were also susceptible to chloramphenicol. All three Salmonella spp. isolates were susceptible to co‐trimoxazole and chloramphenicol. A single isolate of H. influenzae was resistant to co‐trimoxazole. In the study by Roord (Roord 1996), all 20 isolates were sensitive to azithromycin while three organisms were resistant to erythromycin.
Respiratory syncytial virus (RSV) was tested in five studies. Nasopharyngeal aspirates were tested for RSV in four studies (Atkinson 2007; Addo‐Yobo 2004; Mulholland 1995; Wubbel 1999) involving 1916 children and RSV was identified by positive serology in one study (Nogeova 1997) involving 140 children. RSV was identified in 407 children (20%).
Identification of atypical organisms was attempted in six studies (Block 1995; Bradley 2007; Harris 1998; Kogan 2003; Nogeova 1997; Wubbel 1999). Out of the 1734 participants tested for M. pneumoniae, 385 (22%) tested positive. In participants aged under five years 141 out of 659 (21%) tested positive for mycoplasma. Tests for Chlamydia spp. were positive in 158 out of 1534 (10%) participants. In children under five years, there were positive test results for Chlamydia spp. in 45 out of 658 (7%) participants.
Indirect comparisons
We attempted to compare various antibiotics (A and C) when comparisons of antibiotics A and B were available and B and C were available. We utilised this process to compare co‐trimoxazole with co‐amoxyclavulanic acid (Analysis 28), amoxycillin with cefpodoxime (Analysis 29) and amoxycillin with chloramphenicol (Analysis 30). Baseline data for age and sex were not comparable in the first two comparisons and therefore no valid comparison could be carried out. In the comparison of amoxycillin with chloramphenicol (CATCHUP 2002; Mulholland 1995; Straus 1998) sex distribution was not comparable although age distribution was. Cure rates were better in the amoxycillin group compared to the chloramphenicol group (OR 4.26, 95% CI 2.57 to 7.08) (Analysis 30.3) and failure rates were lower in the amoxycillin group (OR 0.64, 95% CI 0.41 to 1.00) (Analysis 30.4).
30.3. Analysis.
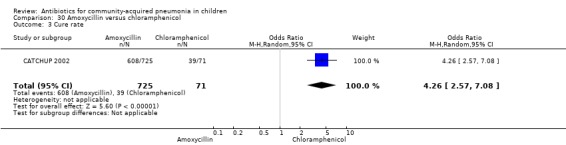
Comparison 30 Amoxycillin versus chloramphenicol, Outcome 3 Cure rate.
30.4. Analysis.
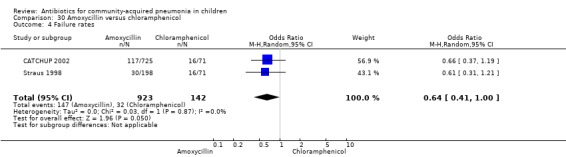
Comparison 30 Amoxycillin versus chloramphenicol, Outcome 4 Failure rates.
Discussion
The aim of this review was to establish the most effective antibiotics for first‐line empirical treatment community‐acquired pneumonia (CAP) of different severity. A limited number of randomised controlled trials (RCTs) fulfilled the inclusion criteria. Most of the antibiotic comparisons were available in single studies only.
Summary of main results
Studies comparing treatment of pneumonia in an ambulatory setting suggest that the failure rate with co‐trimoxazole was comparable to amoxycillin; co‐amoxyclavulanic acid was better than amoxycillin. Resolution of radiographically confirmed pneumonia was better with clarithromycin as compared to erythromycin and side effects were fewer with azithromycin as compared to co‐amoxyclavulanic acid. For children with severe pneumonia, treatment with oral antibiotics was similar to treatment with injectable ampicillin or penicillin. Death rates were higher in children getting chloramphenicol as compared to those getting penicillin/ampicillin plus gentamycin.
For severe/very severe pneumonia, penicillin/ampicillin plus gentamycin was associated with lower re‐admission rates as compared to chloramphenicol.
For very severe pneumonia, failure rates were significantly higher in those receiving chloramphenicol as compared to ampicillin and gentamycin.
The rest of the comparisons for treatment in ambulatory settings involved azithromycin with erythromycin, clarithromycin, clarithromycin with erythromycin, amoxycillin with procaine penicillin, co‐trimoxazole with single‐dose procaine penicillin followed by oral ampicillin and cefpodoxime with co‐amoxyclavulanic acid and there were no statistically significant differences in these comparisons.
Comparisons for severe and very severe pneumonia involved chloramphenicol plus ampicillin with penicillin, amoxycillin with cefuroxime, amoxycillin with clarithromycin, penicillin and gentamycin with co‐amoxyclavulanic acid, levofloxacin with ceftriaxone or co‐amoxyclavulanic acid, cefuroxime with clarithromycin and chloramphenicol with co‐trimoxazole and were comparable. Co‐amoxyclavulanic acid was better than oxacillin/ceftriaxone and cefuroxime was better than ceftibuten.
Overall completeness and applicability of evidence
Treatment of pneumonia depends on the age of the child, the severity of illness, the likely aetiological agents and their resistance pattern. The aetiological agents vary with age and possibly geographic location. Most of the studies included in this review were from underdeveloped countries with age groups below five years, and identification of aetiological agents was limited to a few studies. The burden of pneumonia is significant in infants from developing countries. Attempts to isolate aetiological agents may not be cost‐effective and therefore empirical treatment of pneumonia is justified. The results of this review may therefore be more applicable to the management of pneumonia in developing countries. However, data comparing two different antibiotics may also be useful in guiding antibiotic therapy in industrialised countries.
The World Health Organization (WHO) recommends treatment of non‐severe pneumonia with co‐trimoxazole as a first‐line empirical antimicrobial treatment in countries with an infant mortality rate higher than 40 per 1000 live births (WHO 1991). Concerns about increasing resistance of common pathogens (S. pneumoniae and H. influenzae) to co‐trimoxazole have been raised (Krishnan 2011) and amoxycillin has been suggested as an alternative. This review suggests that amoxycillin and co‐trimoxazole are associated with similar failure rates. Reports of in vitro resistance of common organisms of pneumonia to cotrimoxazole and relatively more sensitivity to amoxycillin have not resulted in more failure rates in the co‐trimoxazole group. All clinical trials included in this review included children with pneumonia diagnosed by the WHO clinical definition of pneumonia. None had chest X‐rays. It can be concluded that there are insufficient data to show superiority of amoxycillin to co‐trimoxazole. It should be noted that amoxycillin is more expensive than co‐trimoxazole for five days of treatment for a child weighing between 5 kg and 10 kg (in India US USD 0.6 versus USD 0.3). Two recent studies (Agarwal 2004; MASCOT Group 2002) reported similar cure rates with amoxycillin given for three or five days. The cost of amoxycillin would be reduced to some extent if the treatment duration of amoxycillin was lowered to three days. Most studies comparing co‐trimoxazole and amoxycillin used clinical case definition of pneumonia (rapid respiration). Respiratory symptoms and rapid respiratory rates in children may be due to bacterial pneumonia, viral infection associated wheeze, asthma etc. In a study from Pakistan chest radiographs were normal in 82% of children diagnosed with non‐severe pneumonia using the WHO case definition (Hazir 2006). The majority of such children, except those with bacterial pneumonia, may not require antimicrobial agents and are likely to recover over three to seven days with supportive care. Giving them co‐trimoxazole or amoxycillin or any other antibiotics may not alter their outcome. Another study from Pakistan observed that children with non‐severe pneumonia treated with amoxycillin or placebo had similar failure rates, suggesting that the specificity of the WHO criteria for diagnosis of true bacterial pneumonia is low. Therefore, it is important to have well‐designed clinical trials in children with true pneumonia (radiologically confirmed/direct or indirect evidence of bacterial pneumonia).
Alternative antibiotics for CAP include macrolides, co‐amoxyclavulanic acid, oral cephalosporins (cefpodoxime, ceftibuten, cefuroxime), procaine penicillin and benzathine penicillin. Comparisons of various macrolides shows similar efficacy, with the exception of more radiological clearance with clarithromycin without any clinical implications. Macrolides may acquire resistance very quickly if used indiscriminately (Inoue 2006). Therefore macrolides should not be used as a first line drug in pneumonia. Amoxycillin was comparable with macrolides (azithromycin and clarithromycin), procaine penicillin and cefuroxime. Amoxycillin may therefore be preferable over these drugs. Co‐amoxyclavulanic acid has been shown to give better results than amoxycillin and oxacillin plus ceftriaxone combination. The results are based on single studies for each drug. In children with severe and very severe pneumonia, co‐amoxyclavulanic acid may be used as an alternative to penicillin. Cefpodoxime was comparable to co‐amoxyclavulanic acid in a single study and may be an alternative where co‐amoxyclavulanic acid cannot be administered. Injectable penicillins (procaine penicillin or benzathine penicillin) are associated with injection‐site problems and therefore have a limited role in non‐severe pneumonia.
The WHO recommends admission to hospital and treatment with penicillin for severe pneumonia and chloramphenicol for very severe pneumonia (WHO 1999). In this review it clearly emerged that children with severe pneumonia without hypoxia, who are feeding well, can be treated with oral amoxycillin. The mortality rates were higher in children receiving injectable antibiotics. Quality assessment of these trials comparing oral with injectable medications reveals adequate allocation concealment but all were unblinded. There is no explanation for the increased death rates in those who received injectable antibiotics, as they were treated with either ampicillin/penicillin or amoxycillin. After excluding one study that reported seven deaths in children receiving injections (Addo‐Yobo 2004), the difference in death rate becomes non‐significant. In view of the similar antimicrobial spectrum of all these drugs (ampicillin/amoxycillin/cotrimoxazole/penicillin) and the possible benefit of better bioavailability with parenteral administration of antibiotics, a better outcome could be expected with use of injectable antibiotics for the treatment of children with severe pneumonia. More recently two cluster‐RCTs (Bari 2011; Soofi 2012) in children with WHO‐defined severe pneumonia without hypoxaemia compared oral amoxycillin with standard care (referral to healthcare services with injectable antibiotics). Results of these studies reveal that the outcome of patients with oral amoxycillin is the same or better than with standard treatment. Many patients on standard treatment did not take injectable medications or follow the instructions. Based on the results of these studies and the observations in the present review, it may be concluded that children with severe pneumonia without hypoxia may be treated with oral amoxycillin. There is a need to re‐define severe pneumonia with or without hypoxia to identify children who may be treated with oral amoxycillin.
In children with severe or very severe pneumonia, it was evident that chloramphenicol was inferior to the combination of penicillin/ampicillin plus gentamycin. Therefore, there is a need to change the WHO guidelines. Alternative antibiotics for hospitalised children with severe and very severe pneumonia include ceftriaxone, levofloxacin, co‐amoxyclavulanic acid and cefuroxime. However, comparisons were based on single studies and these drugs are relatively more expensive. Another study showed that co‐amoxyclavulanic acid is better than an oxacillin‐ceftriaxone combination suggesting that co‐amoxyclavulanic acid may be an alternative to penicillin/ampicillin.
Cure and failure rates of CAP depend not only on the choice of antibiotics but also on the aetiology of the pneumonia, the age of the patient, the sensitivity pattern of the bacterial pathogen, the severity of disease and any antibiotic usage in the recent past. While information on resistance patterns was not included in the studies evaluated in the review, this is likely to be of major importance in the future, in terms of both clinical practice and research.
In the management of CAP, isolation of bacterial pathogens in order to make a decision about the choice of antibiotics is not feasible in most circumstances. Even if bacterial pathogens are isolated, the child will need to be treated with empirical antibiotics until the result of the culture is available. In this review identification of bacterial pathogens was attempted in 14 studies (Asghar 2008; Bansal 2006; Block 1995; Bradley 2007; Camargos 1997; Duke 2002; Harris 1998; Klein 1995; Kogan 2003; Mulholland 1995; Nogeova 1997; Roord 1996; Straus 1998; Wubbel 1999). Bacterial pathogens could be isolated in only 12% of the study participants. S. pneumoniae and H. influenzae constituted 65% of all the bacterial isolates. Therefore, empirical antibiotic therapy for CAP should be effective against these two pathogens.
Respiratory syncytial virus (RSV) could be isolated in 20% of patients, suggesting that a sizeable proportion of patients may have a viral aetiology of CAP. These patients may not need antibiotics. A child with viral pneumonia can be identified from rapid diagnostic tests such as nasopharyngeal aspirates (Maitreyi 2000) and can avoid administration of antibiotics. However, the possibility of mixed infection (bacterial agents with viruses) has been observed in 10% to 40% of cases (Kabra 2003). At present, it is policy to treat all children with pneumonia with antibiotics due to a lack of point of care tests that can reliably rule out bacterial pneumonia.
Another important issue is the aetiological role of atypical organisms (Chlamydia and Mycoplasma spp.) in CAP (Chaudhary 1998; Normann 1998; Pandey 2005). Six studies included in this review identified atypical organisms (Block 1995; Bradley 2007; Harris 1998; Kogan 2003; Nogeova 1997; Wubbel 1999). Out of 1734 children tested forM. pneumoniae, 385 (22%) tested positive. The positivity for Mycoplasma in children under five years age was 21% (141/659). Tests for Chlamydia spp. were positive in 158 out of the 1534 children (10%). In children under five years of age, positive tests for Chlamydia spp. occurred in 45 out of 658 (7%). The most effective antibiotics against atypical organisms are tetracycline and macrolides. In this review, the studies that attempted to identify atypical organisms showed equal cure rates between erythromycin and azithromycin. Two studies (Harris 1998; Wubbel 1999) comparing azithromycin with co‐amoxyclavulanic acid in children under five years of age also showed equal cure and failure rates. In these studies the incidence of atypical organisms in children under five years of age was 15% and 11% for Mycoplasma spp. and Chlamydia, respectively. The cure rates in children receiving co‐amoxyclavulanic acid were comparable to those receiving azithromycin. From this observation it can be inferred that either the diagnostic tests used for atypical organisms in these studies may not indicate invasive infections, or that the study was not adequately powered to detect small differences. A recent retrospective cohort study (Ambroggio 2012) compared the effectiveness of beta‐lactam monotherapy and beta‐lactam and macrolide combination therapy on the outcomes of children hospitalised with CAP. The results of this study suggest that mean hospital stay was 20% less in school‐going children who received macrolide in addition to beta‐lactamase therapy. The study did not observe a difference in re‐admission rates or a difference in length of stay in children below six years of age. More studies are required to recommend the addition of macrolides to beta‐lactamase antibiotics.
Exposure to antibiotics in the recent past may adversely affect the outcome of bacterial pneumonia as the chances of infection with a resistant organism increases (Chenoweth 2000). In this review, information on past antibiotic use was available in eight studies (Addo‐Yobo 2004; Asghar 2008; Atkinson 2007; Bradley 2007; Duke 2002; Hazir 2008; Ribeiro 2011; Straus 1998). The distribution of patients who had received antibiotics in the recent past was similar in the two treatment groups in all the studies. However, subgroup analysis was not available in these studies. In one study (Hazir 2008) antibiotic use in the last week was associated with increased failure rates on univariate analysis. In a study comparing co‐trimoxazole and amoxycillin the number of patients who had received antibiotics in the recent past was higher in the amoxycillin group (34% compared with 25.6% in the co‐trimoxazole group) (Straus 1998). In this study separate data regarding failure rates in those received antibiotics and those did not receive antibiotics are not available. However, failure rates in children with severe pneumonia who received cotrimoxazole was 56/203 (27.5%), is higher than those who received amoxycillin (18/99) (18%). Failure rates in those with non severe pneumonia in the same study were 12.8% and 12.5% in those receiving co‐trimoxazole or amoxycillin respectively (Straus 1998). These results suggest that children suffering from severe pneumonia who have received antibiotics in the recent past may benefit from treatment with amoxycillin. However, these results are based on a single study and care should be taken when drawing any definite conclusions.
Malnutrition may affect the treatment outcome of pneumonia. There was only one study in malnourished children (Mulholland 1995) which compared co‐trimoxazole and chloramphenicol. The study did not show any significant difference in cure rates, failure rates or need for change in antibiotics.
The aetiology of pneumonia depends on the age of the patient. In this review, the majority of enrolled participants were below five years of age and separate data according to age were not available for primary and secondary outcomes in the studies that also enrolled older children. We tried to see the effect on outcomes after removing studies that included children older than five years for severe pneumonia and observed that the age of participants did not change the outcome. Therefore, we feel that the recommendation may be applicable to all age groups. However, more studies are required for children older than five years of age.
There are limitations in reviewing antibiotic usage in CAP. Comparisons are often performed among groups of children for whom identification of aetiological agents is lacking. This means that if the distribution of viral cases is not uniform, the conclusions regarding the efficacy of antibiotics can be debatable. Several individual factors, such as malnutrition, can deeply modify the evolution of CAP and the response to antibiotic therapy. In the present review, only one study addressed this problem; it is highly probable that this issue can influence the correct evaluation of the data. No data regarding antibiotic resistance were reported in the majority of the studies. It is well known that in some cases the level of resistance to commonly used antibiotics can have a great influence on the response to therapy. The role of atypical bacteria in the determination of CAP in children living in low‐income countries is not established, probably because the methods for identifying these pathogens are too complicated or too expensive, or both. These data are needed to more accurately define the best antibiotic therapy. The results may be more applicable for developing countries as most studies were done in these countries.
Quality of the evidence
Five out of 29 studies were double‐blind and allocation concealment was adequate. Another 12 studies were unblinded but had adequate allocation concealment, classifying them as good‐quality studies. Data were fully detailed in 20 studies, selective reporting of data was unclear in 12 studies and 13 studies were funded by WHO or universities. There was more than one study comparing co‐trimoxazole with amoxycillin, oral amoxycillin with injectable penicillin/ampicillin and chloramphenicol with ampicillin/penicillin and studies were of good quality, suggesting the evidence for these comparisons is of high quality compared to other comparisons.
Potential biases in the review process
In this review we included one study (Awasthi 2008) of which one of the authors of the present review (Kabra) was a co‐author.
Agreements and disagreements with other studies or reviews
The important changes in this updated review in comparison to the previous version (Kabra 2010) include the following.
Outcomes of children with radiographically confirmed or clinically diagnosed pneumonia are not different.
WHO‐defined severe pneumonia without hypoxaemia can be managed with oral antibiotics in an ambulatory setting. There is a need to divide WHO‐defined severe pneumonia into those with hypoxia and those without hypoxia to identify children who can be treated with oral antibiotics in an ambulatory setting.
For very severe pneumonia, co‐amoxyclavulanic acid may be an alternative to ceftriaxone or penicillin/ampicillin, gentamycin combination.
A review comparing oral and intravenous antibiotics in pneumonia suggested no difference in cure and failure rates in children getting oral or intravenous antibiotics for the treatment of pneumonia (Rojas‐Reyes 2006). In the present review we also found that oral and intravenous antibiotics (amoxycillin versus penicillin/ampicillin and co‐trimoxazole versus procaine penicillin) for pneumonia are equally effective.
A recent retrospective cohort study (Ambroggio 2012) suggests that the addition of macrolide to beta‐lactam antibiotics in children above six years of age may improve outcomes in the form of reduced hospital stay. In the present review, we did not find any study that compared beta‐lactam antibiotics with and without macrolide for treatment of CAP. Studies comparing macrolides with other antibiotics (amoxycillin, co‐amoxyclavulanic acid) gave similar failure rates suggesting no advantage of macrolides. We conclude that there is a need for RCTs to document the advantages of the addition of macrolide antibiotics to conventional beta‐lactam antibiotics.
Authors' conclusions
Implications for practice.
In children presenting with community‐acquired pneumonia without underlying illness, and where point of care tests for identification of aetiological agents for pneumonia are not available, empirical antibiotics may be used as follows. For the treatment of WHO‐defined non‐severe community‐acquired pneumonia (CAP) in children below five years of age amoxycillin is an alternative to co‐trimoxazole. There are no apparent differences between azithromycin and erythromycin, azithromycin and co‐amoxyclavulanic acid, or cefpodoxime and co‐amoxyclavulanic acid. There are limited data on other antibiotics: co‐amoxyclavulanic acid and cefpodoxime may be alternative second‐line drugs.
Severe pneumonia in children below five years of age, without hypoxia and accepting oral feeds, can be managed with oral amoxycillin on an ambulatory basis.
For children below five years of age, hospitalised with severe and very severe CAP, penicillin/ampicillin plus gentamycin is superior to chloramphenicol. Other alternatives may be co‐amoxyclavulanic acid, ceftriaxone, levofloxacin and cefuroxime. Until more studies are available these can be used as second‐line therapies.
More studies are required to assess the role of the addition of macrolide antibiotics to beta‐lactam antibiotics in children above five years of age.
More randomised controlled trials are required for a review of these antibiotics in order to make more accurate recommendations for their prescription.
There is need for surveillance studies to document antibiotic resistance in different geographic regions for developing empiric antibiotic treatment for pneumonia.
Implications for research.
There is a need to compare various antibiotics for the treatment of pneumonia of varying severity. Studies should include radiographically confirmed pneumonia in place of clinically diagnosed pneumonia. Studies should try to identify the aetiological agents and their susceptibilities to various antibiotics and the risk factors that lead to failure of treatment. The results of such studies will help in the formation of guidelines to identify children at risk of failure who can be managed with second‐line antimicrobials early.
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2012 | New search has been performed | Searches updated. We included two new trials (Nogeova 1997; Ribeiro 2011) and excluded three new trials (Ambroggio 2012; Bari 2011; Soofi 2012). |
| 7 November 2012 | New citation required and conclusions have changed | We have added conclusions about treatment of severe pneumonia with oral antibiotics and a comparison of antibiotics in radiographically confirmed pneumonia. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 4 January 2010 | New citation required and conclusions have changed | Seven new studies included and we have added new information on ambulatory treatment for severe pneumonia and the superiority of ampicillin/penicillin with gentamycin instead of chloramphenicol for the treatment of very severe pneumonia to the conclusions. |
| 18 September 2009 | New search has been performed | Searches conducted. |
| 1 August 2009 | Amended | Converted to new review format. |
| 6 January 2006 | New citation required and major changes | Search conducted. |
Acknowledgements
We acknowledge all the help and infrastructure provided by the All India Institute of Medical Sciences, New Delhi, where all the authors serve as faculty. We acknowledge the help provided by Elizabeth Dooley, Managing Editor and Sarah Thorning, Trials Search Co‐ordinator of the Cochrane Acute Respiratory Infections Group, for doing the EMBASE search and obtaining the full‐text articles of studies. We acknowledge all referees for critically reviewing and suggesting improvements to the quality of review. We also acknowledge the help provided by Mr Bharat Bhusan Pandey and Mr Rajat Prakash in masking the study articles. We are thankful to Dr Shivani Randev for helping in getting full‐text articles for oral treatment of severe pneumonia. We are very thankful to the referees Dr Roger Damoiseaux, Marilyn Bamford, Dr Nicola Principi, Dr Rajni Bhatia and Dr Mark Jones for their input in improving the quality of the previous version of this review.
Appendices
Appendix 1. Previous search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 2), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to September 2009) and EMBASE (1990 to September 2009). There were no language or publication restrictions. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2008).
MEDLINE (OVID)
1 exp PNEUMONIA/ 2 pneumonia 3 or/1‐2 4 exp Anti‐Bacterial Agents/ 5 antibiotic$ 6 or/4‐5 7 exp CHILD/ 8 exp INFANT/ 9 (children or infant$ or pediatric or paediatric) 10 or/7‐9 11 3 and 6 and 10
EMBASE (WebSPIRS)
#1 explode 'pneumonia‐' / all subheadings in DEM,DER,DRM,DRR #2 (pneumonia in ti) or (pneumonia in ab) #3 #1 or #2 #4 'antibiotic‐agent' / all subheadings in DEM,DER,DRM,DRR #5 (antibiotic* in ti) or (antibiotic* in ab) #6 #4 or #5 #7 'child‐' / all subheadings in DEM,DER,DRM,DRR #8 (child in ti) or (child in ab) #9 (children in ti) or (children in ab) #10 'infant‐' / all subheadings in DEM,DER,DRM,DRR #11 (infant* in ti) or (infant* in ab) #12 #7 or #8 or #9 or #10 or #11 #13 #3 and #6 and #12 #14 explode 'randomized‐controlled‐trial' / all subheadings #15 explode 'controlled‐study' / all subheadings #16 explode 'single‐blind‐procedure' / all subheadings #17 explode 'double‐blind‐procedure' / all subheadings #18 explode 'crossover‐procedure' / all subheadings #19 explode 'phase‐3‐clinical‐trial' / all subheadings #20 (randomi?ed controlled trial in ti) or (randomi?ed controlled trial in ab) #21 ((random* or placebo* or double‐blind*)in ti) or ((random* or placebo* or double‐blind*)in ab) #22 (controlled clinical trial* in ti) or (controlled clinical trial* in ab) #23 #14 or #15 or #16 or #17 or 318 or #19 or #290 or #21 or #22 #24 (nonhuman in der) not ((human in der) and (nonhuman in der)) #25 #23 not #24 #26 #13 and #25
Appendix 2. EMBASE search strategy
#9 #7 AND #8 #8 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR (random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti #7 #1 AND #6 #6 #2 OR #3 OR #4 OR #5 #5 kindergar*:ab,ti OR highschool*:ab,ti OR (school* NEAR/1 (nursery OR primary OR secondary OR elementary OR high)):ab,ti #4 (school NEAR/2 (age* OR child*)):ab,ti #3 infant*:ab,ti OR infanc*:ab,ti OR baby*:ab,ti OR babies:ab,ti OR newborn*:ab,ti OR child*:ab,ti OR schoolchild*:ab,ti OR preschool*:ab,ti OR kid:ab,ti OR kids:ab,ti OR toddler*:ab,ti OR adolescen*:ab,ti OR teen*:ab,ti OR boy*:ab,ti OR girl*:ab,ti OR minor*:ab,ti OR puberty:ab,ti OR pediatric*:ab,ti OR paediatric*:ab,ti #2 'infant'/exp OR 'child'/exp OR 'adolescent'/exp OR 'pediatrics'/exp OR 'juvenile'/exp OR 'puberty'/exp #1 #1.9 #1.4 AND #1.8 #1.8 #1.5 OR #1.6 OR #1.7 #1.7 amoxycillin:ab,ti OR amoxicillin:ab,ti OR ampicillin:ab,ti OR azithromycin:ab,ti OR augmentin:ab,ti OR benzylpenicillin:ab,ti OR 'b‐lactam':ab,ti OR 'beta‐lactam':ab,ti OR 'beta‐lactams':ab,ti OR clarithromycin:ab,ti OR cefuroxime:ab,ti OR cotrimoxazole:ab,ti OR 'co‐trimoxazole':ab,ti OR cefotaxime:ab,ti OR ceftriaxone:ab,ti OR ceftrioxone:ab,ti OR cefditoren:ab,ti OR chloramphenicol:ab,ti OR cefpodixime:ab,ti OR cephradine:ab,ti OR cephalexin:ab,ti OR cefetanet:ab,ti OR cefaclor:ab,ti OR cephalosporin*:ab,ti OR erythromycin:ab,ti OR gentamicin:ab,ti OR genamycin:ab,ti OR levofloxacin:ab,ti OR minocyclin:ab,ti OR moxifloxacin:ab,ti OR penicllin*:ab,ti OR quinolone*:ab,ti OR roxithromycin:ab,ti OR sulphamethoxazole:ab,ti OR sulfamethoxazole:ab,ti OR trimethoprim:ab,ti #1.6 antibiotic*:ab,ti #1.5 'antibiotic agent'/exp #1.4 #1.1 OR #1.2 OR #1.3 #1.3 'community‐acquired‐pneumonia':ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti OR cap:ab,ti #1.2 pneumon*:ab,ti #1.1 'pneumonia'/exp
Appendix 3. CINAHL (Ebsco) search strategy
S37 S35 and S36 S36 S11 and S20 S35 S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 S34 TI school* OR AB school* S33 TI (nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*) OR AB (nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*) S32 (MH "Schools") OR (MH "Schools, Elementary") OR (MH "Schools, Middle") OR (MH "Schools, Nursery") OR (MH "Schools, Secondary") OR (MH "Schools, Special") 8229 Edit S32 S31 TI (pediatric* or paediatric*) OR AB (pediatric* or paediatric*) S30 (MH "Pediatrics+") S29 TI (minor* or pubert* or pubescen*) OR AB (minor* or pubert* or pubescen*) S28 (MH "Puberty") S27 (adoles* or teen* or boy* or girl*) OR (adoles* or teen* or boy* or girl*) S26 (MH "Adolescence+") S25 TI (child* or schoolchild* or school age* or preschool* or kid or kids or toddler*) OR AB (child* or schoolchild* or school age* or preschool* or kid or kids or toddler*) S24 (MH "Child+") S23 TI (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur*) OR AB (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur*) 66234 S22 (MH "Infant+") S21 (S11 and S20) S20 S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 S19 (MH "Placebos") S18 (MH "Quantitative Studies") S17 TI placebo* OR AB placebo* S16 TI random* OR AB random* S15 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or trebl* mask* or tripl* mask*) OR AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or trebl* mask* or tripl* mask*) S14 TI clinic* trial* OR AB clinic* trial* S13 PT clinical trial S12 (MH "Clinical Trials+") S11 S9 and S10 S10 S5 or S6 or S7 or S8 S9 S1 or S2 or S3 or S4 S8 AB amoxycillin* or amoxicillin* or ampicillin* or azithromycin* or augmentin* or benzylpenicillin* or b‐lactam* or beta‐lactam* or clarithromycin* or ceftriaxone* or cefuroxime* or cotrimoxazole* or co‐trimoxazole* or co‐amoxyclavulanic acid or cefotaxime* or ceftriaxone* or ceftrioxone* or cefditoren* or chloramphenicol* or cefpodioxime* or cephradine* or cephalexin* or cefaclor* or cefetamet* or cephalosporin* or erythromycin* or gentamicin* or gentamycin* or levofloxacin* or macrolide* or minocyclin* or moxifloxacin* or penicillin* or quinolone* or roxithromycin* or sulphamethoxazole* or sulfamethoxazole* or tetracycline* or trimethoprim* S7 TI amoxycillin* or amoxicillin* or ampicillin* or azithromycin* or augmentin* or benzylpenicillin* or b‐lactam* or beta‐lactam* or clarithromycin* or ceftriaxone* or cefuroxime* or cotrimoxazole* or co‐trimoxazole* or co‐amoxyclavulanic acid or cefotaxime* or ceftriaxone* or ceftrioxone* or cefditoren* or chloramphenicol* or cefpodioxime* or cephradine* or cephalexin* or cefaclor* or cefetamet* or cephalosporin* or erythromycin* or gentamicin* or gentamycin* or levofloxacin* or macrolide* or minocyclin* or moxifloxacin* or penicillin* or quinolone* or roxithromycin* or sulphamethoxazole* or sulfamethoxazole* or tetracycline* or trimethoprim* S6 TI (antibiotic* or antibacter* or anti‐bacter*) OR AB (antibiotic* or antibacter* or anti‐bacter*) S5 (MH "Antibiotics+") S4 TI cap OR AB cap S3 TI (bronchopneumon* or pleuropneumon*) OR AB (bronchopneumon* or pleuropneumon*) S2 TI pneumon* OR AB pneumon* S1 (MH "Pneumonia+")
Appendix 4. Web of Science (Thomson ISI) search strategy
| # 6 | 113 | #4 AND #3 Refined by: Publication Years=( 2011 OR 2012 ) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 5 | 796 | #4 AND #3 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 4 | 1,302,084 | Topic=(random* or placebo* or allocat* or crossover* or "cross over" or ((singl* or doubl*) NEAR/1 blind*)) OR Title=(trial) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 3 | 5,848 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 2 | 1,414,126 | Topic=(infant* or infancy or newborn* or baby or babies or neonat* or preterm* or prematur* or child* or schoolchild* or "school age*" or preschool* or kid or kids or toddler* or adoles* or teen* or boy* or girl* or pediatric* or paediatric*) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 1 | 29,498 | Topic=(pneumon* or bronchopneumon* or pleuropneumon* or cap) AND Topic=(antibiotic* or amoxycillin* or amoxicillin* or ampicillin* or azithromycin* or augmentin* or benzylpenicillin* or b‐lactam* or beta‐lactam* or clarithromycin* or ceftriaxone* or cefuroxime* or cotrimoxazole* or co‐trimoxazole* or co‐amoxyclavulanic acid or cefotaxime* or ceftriaxone* or ceftrioxone* or cefditoren* or chloramphenicol* or cefpodioxime* or cephradine* or cephalexin* or cefaclor* or cefetamet* or cephalosporin* or erythromycin* or gentamicin* or gentamycin* or levofloxacin* or macrolide* or minocyclin* or moxifloxacin* or penicillin* or quinolone* or roxithromycin* or sulphamethoxazole* or sulfamethoxazole* or tetracyclin* or trimethoprim*) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
Appendix 5. LILACS (Brieme) search strategy
> Search > (MH:pneumonia OR Neumonía OR MH:C08.381.677$ OR MH:C08.730.610$ OR Pulmonía OR "Inflamación Experimental del Pulmón" OR "Inflamación del Pulmón" OR "Neumonía Lobar" OR Neumonitis OR "Inflamación Pulmonar" OR "Inflamação Experimental dos Pulmões" OR "Inflamação do Pulmão" OR "Pneumonia Lobar" OR Pneumonite OR "Inflamação Pulmonar" OR Pulmonia OR bronchopneumon$ OR Bronconeumonía OR Broncopneumonia OR Pleuropneumonia OR Pleuroneumonía OR Pleuropneumonia) AND (MH:"Anti‐Bacterial Agents" OR antibiotic$ OR Antibacterianos OR Antibióticos OR MH:D27.505.954.122.085$ OR MH:amoxicillin OR amoxicillin$ OR Amoxicilina OR MH:ampicillin OR Ampicilina OR ampicillin$ OR MH:Azithromycin OR Azitromicina OR azithromycin$ OR augmentin$ OR benzylpenillin OR MH:"penicillin g" OR "penicilina g" OR MH:"beta‐lactams" OR "beta‐lactams" OR "beta‐lactamas" OR MH:clarithromycin OR claritromicina OR clarithromycin OR MH:ceftriaxone OR ceftriaxone OR ceftriaxona OR MH:cefroxime OR cefroxime$ OR cefuroxima OR cotrimoxazol$ OR "Trimethoprim‐Sulfamethoxazole Combination" OR "Combinación Trimetoprim‐Sulfametoxazol" OR "Combinação Trimetoprima‐sulfametoxazol" OR "co‐amoxyclavulanic acid" OR cefotaxime OR MH:cefotaxime OR cefotaxima OR MH:ceftriaxone OR ceftriaxone OR ceftriaxona OR ceftrioxone OR cefditoren$ OR chloramphenicol OR cloranfenicol OR MH:chloramphenicol OR cefpodixime OR MH:cephradine OR cephradin$ OR cefradina OR MH:cephalexin OR cefalexina OR cephalexin$ OR cefaclor OR MH:cefaclor OR cefetamet OR cephalosporin$ OR MH:cephalosporins OR cefalosporinas OR MH:erythromycin OR erythromycin OR eritomicina OR MH:gentamicins OR gentamicin$ OR gentamycin$ OR Gentamicinas OR levofloxacin OR MH:ofloxacin OR ofloxacin$ OR MH:macrolides OR macrolide$ OR Macrólidos OR Macrolídeos OR minocyclin$ OR MH:minocycline OR Minociclina OR moxifloxacin OR penicillin$ OR MH:penicillins OR penicilinas OR MH:quinolones OR quinolon$ OR roxithromycin OR MH:roxithromycin OR roxitromicina OR MH:sulfamethoxazole OR sulfamethoxazole$ OR sulphamethoxazole OR Sulfametoxazol OR MH:tetracyclines OR tetracycline$ OR Tetraciclinas OR MH:trimethoprim OR trimetoprim OR trimetoprima) > clinical_trials
Data and analyses
Comparison 1. Azithromycin versus erythromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean age (months) | 3 | 369 | Mean Difference (IV, Random, 95% CI) | ‐4.48 [‐18.54, 9.57] |
| 2 Male sex | 3 | 564 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.18] |
| 3 Wheezing present | 2 | 479 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.31, 4.87] |
| 4 Cure rate | 3 | 363 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.50, 2.94] |
| 5 Failure rate | 3 | 392 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.18, 2.89] |
| 6 Side effects | 2 | 153 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.18, 4.73] |
| 7 Organisms identified by serology or nasopharyngeal cultures | 3 | 368 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.30, 1.87] |
| 8 Cure rate in radiographically confirmed pneumonia | 2 | 147 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.65, 4.56] |
| 9 Failure rate in radiographically confirmed pneumonia | 2 | 147 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.63] |
1.1. Analysis.
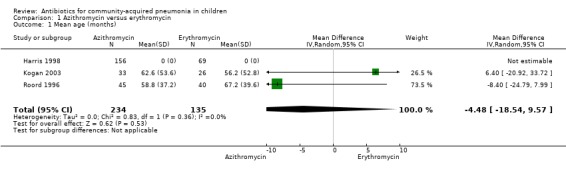
Comparison 1 Azithromycin versus erythromycin, Outcome 1 Mean age (months).
1.2. Analysis.
Comparison 1 Azithromycin versus erythromycin, Outcome 2 Male sex.
1.3. Analysis.
Comparison 1 Azithromycin versus erythromycin, Outcome 3 Wheezing present.
1.4. Analysis.
Comparison 1 Azithromycin versus erythromycin, Outcome 4 Cure rate.
1.6. Analysis.
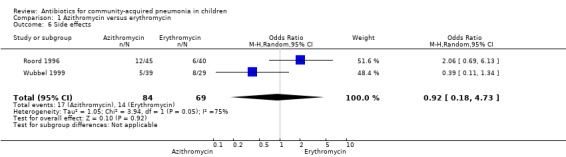
Comparison 1 Azithromycin versus erythromycin, Outcome 6 Side effects.
1.7. Analysis.
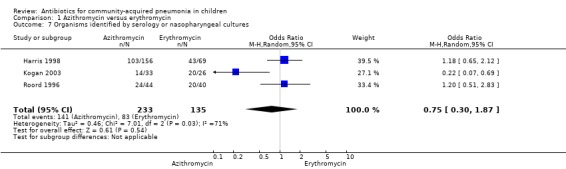
Comparison 1 Azithromycin versus erythromycin, Outcome 7 Organisms identified by serology or nasopharyngeal cultures.
Comparison 2. Clarithromycin versus erythromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age below 5 years | 1 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.56, 1.55] |
| 2 Cure rates | 1 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [0.84, 3.08] |
| 3 Clinical success rate | 1 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [0.45, 8.23] |
| 4 Failure rate | 1 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.12, 2.23] |
| 5 Relapse rate | 1 | 226 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.45] |
| 6 Radiologic resolution | 1 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [1.02, 6.16] |
| 7 Radiologic success | 1 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 3.55 [0.70, 18.04] |
| 8 Radiologic failure | 1 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.06, 1.80] |
| 9 Adverse events | 1 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.90] |
| 10 Bacteriologic response | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.67] |
2.1. Analysis.
Comparison 2 Clarithromycin versus erythromycin, Outcome 1 Age below 5 years.
2.10. Analysis.
Comparison 2 Clarithromycin versus erythromycin, Outcome 10 Bacteriologic response.
Comparison 3. Azithromycin versus co‐amoxyclavulanic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rate | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.95] |
| 2 Failure rate | 2 | 276 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.42, 3.53] |
| 3 Improved | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.71] |
| 4 Side effects | 2 | 276 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.61] |
| 5 Organisms isolated | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.24, 6.74] |
| 6 Mycoplasma serology positive | 1 | 192 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.64, 2.22] |
| 7 Failure rates in radiographically confirmed pneumonia | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.05, 7.08] |
3.5. Analysis.
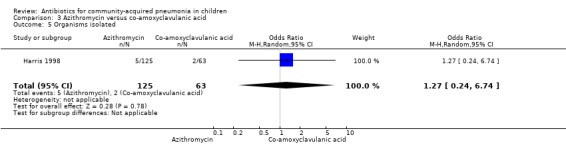
Comparison 3 Azithromycin versus co‐amoxyclavulanic acid, Outcome 5 Organisms isolated.
3.6. Analysis.
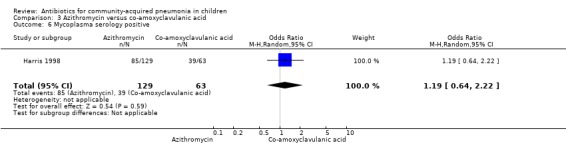
Comparison 3 Azithromycin versus co‐amoxyclavulanic acid, Outcome 6 Mycoplasma serology positive.
Comparison 4. Azithromycin versus amoxycillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age in months | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 58.10 [35.59, 80.61] |
| 2 Duration of illness | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.50, 1.30] |
| 3 Wheezing present | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 2.02 [0.59, 6.96] |
| 4 Cure rate clinical | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cure rate radiological | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 2.85 [0.73, 11.09] |
| 6 Fever day 7 | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.41, 4.61] |
4.2. Analysis.
Comparison 4 Azithromycin versus amoxycillin, Outcome 2 Duration of illness.
4.3. Analysis.
Comparison 4 Azithromycin versus amoxycillin, Outcome 3 Wheezing present.
4.4. Analysis.
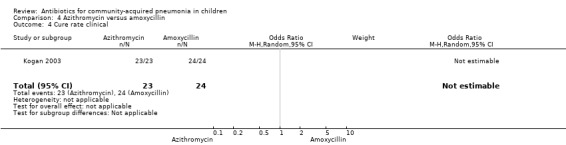
Comparison 4 Azithromycin versus amoxycillin, Outcome 4 Cure rate clinical.
4.6. Analysis.
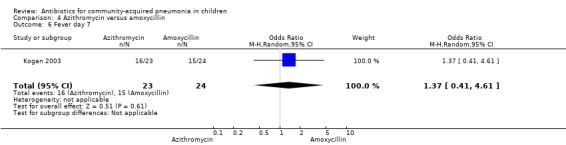
Comparison 4 Azithromycin versus amoxycillin, Outcome 6 Fever day 7.
Comparison 5. Amoxycillin versus procaine penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median age | 1 | 170 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.52, 1.12] |
| 2 Failure rate | 1 | 154 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.17, 3.25] |
5.1. Analysis.
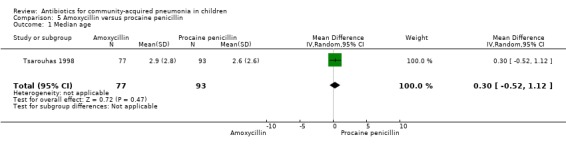
Comparison 5 Amoxycillin versus procaine penicillin, Outcome 1 Median age.
Comparison 6. Co‐amoxyclavulanic acid versus amoxycillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor or no response | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.01, 0.67] |
| 2 Cure rate | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 10.44 [2.85, 38.21] |
| 3 Complications | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 5.21 [0.24, 111.24] |
| 4 Age (months) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐8.09, 17.69] |
| 5 Weight | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐1.06, 3.26] |
| 6 Male sex | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.57, 3.03] |
| 7 Wheeze present | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.18, 1.92] |
| 8 Side effects | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 5.21 [0.24, 111.24] |
6.1. Analysis.
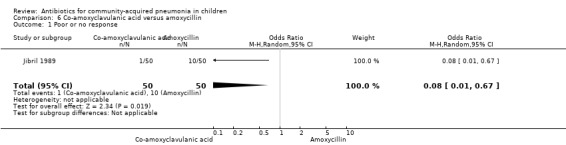
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 1 Poor or no response.
6.3. Analysis.
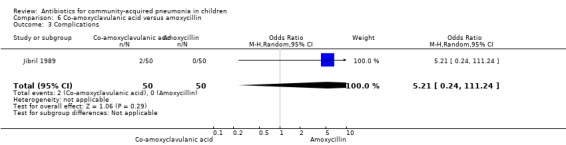
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 3 Complications.
6.4. Analysis.
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 4 Age (months).
6.5. Analysis.
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 5 Weight.
6.6. Analysis.
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 6 Male sex.
6.7. Analysis.
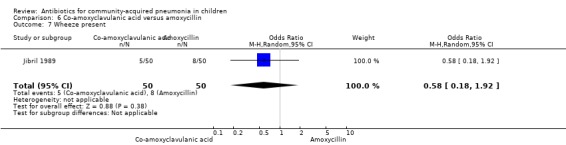
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 7 Wheeze present.
6.8. Analysis.
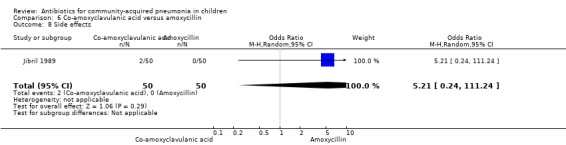
Comparison 6 Co‐amoxyclavulanic acid versus amoxycillin, Outcome 8 Side effects.
Comparison 7. Co‐trimoxazole versus amoxycillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age less than 1 year | 3 | 2347 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.29] |
| 2 Male sex | 3 | 2318 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.59, 0.83] |
| 3 Mean Z score for weight | 2 | 2066 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.27, 0.15] |
| 4 Non‐severe pneumonia | 1 | 595 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.69, 1.37] |
| 5 Received antibiotics in previous week | 1 | 595 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.97] |
| 6 Severe pneumonia | 1 | 595 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.73, 1.45] |
| 7 Failure rate in non‐severe pneumonia | 3 | 1787 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.91, 1.51] |
| 8 Failure rate severe pneumonia clinical diagnosis | 1 | 302 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [0.94, 3.11] |
| 9 Failure rate radiological positive pneumonia | 1 | 153 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [0.96, 4.78] |
| 10 Failure rate radiological negative pneumonia | 1 | 424 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.96, 3.09] |
| 11 Death rate | 2 | 2050 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.22, 20.06] |
| 12 Lost to follow‐up | 3 | 2325 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.59, 1.57] |
| 13 Wheeze positive | 1 | 1471 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.49, 1.19] |
| 14 Cure rate | 2 | 1732 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.56, 1.89] |
| 15 Change of antibiotics | 1 | 1459 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.95, 1.69] |
| 16 Failure rates after excluding study by Awasthi 2008 | 2 | 1750 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.92, 1.53] |
7.1. Analysis.
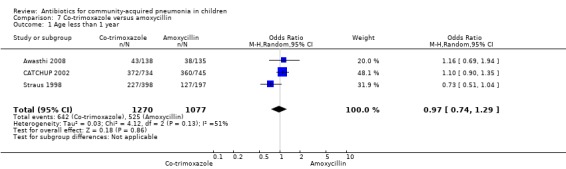
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 1 Age less than 1 year.
7.2. Analysis.
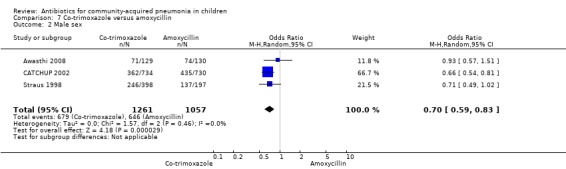
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 2 Male sex.
7.3. Analysis.
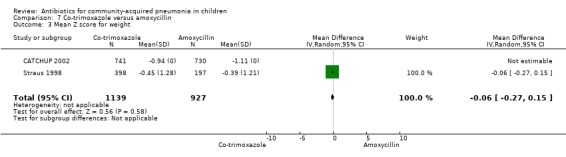
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 3 Mean Z score for weight.
7.4. Analysis.
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 4 Non‐severe pneumonia.
7.5. Analysis.
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 5 Received antibiotics in previous week.
7.6. Analysis.
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 6 Severe pneumonia.
7.9. Analysis.
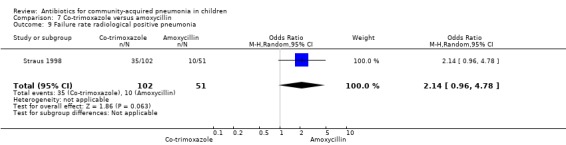
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 9 Failure rate radiological positive pneumonia.
7.10. Analysis.
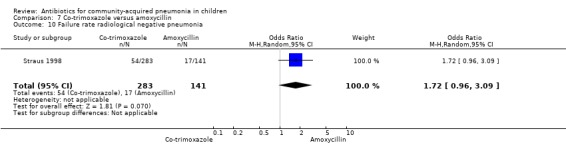
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 10 Failure rate radiological negative pneumonia.
7.11. Analysis.
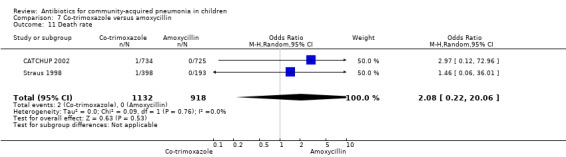
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 11 Death rate.
7.13. Analysis.
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 13 Wheeze positive.
7.15. Analysis.
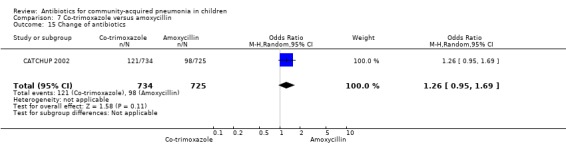
Comparison 7 Co‐trimoxazole versus amoxycillin, Outcome 15 Change of antibiotics.
Comparison 8. Co‐trimoxazole versus procaine penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age less than 1 year | 2 | 723 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.96, 1.75] |
| 2 Age 1 to 5 years | 1 | 614 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.16] |
| 3 Age 5 to 12 years | 2 | 723 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.45, 1.39] |
| 4 Duration of illness in days | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.49, 0.20] |
| 5 Male sex | 1 | 614 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.67, 1.27] |
| 6 Cure rate | 2 | 723 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [0.26, 9.69] |
| 7 Hospitalisation rate | 1 | 614 | Odds Ratio (M‐H, Random, 95% CI) | 2.52 [0.88, 7.25] |
| 8 Well at end of follow‐up | 1 | 614 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.51, 1.57] |
| 9 Death | 1 | 614 | Odds Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 76.13] |
| 10 Treatment failure | 1 | 614 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.41, 7.27] |
8.1. Analysis.
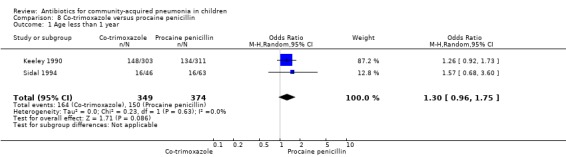
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 1 Age less than 1 year.
8.2. Analysis.
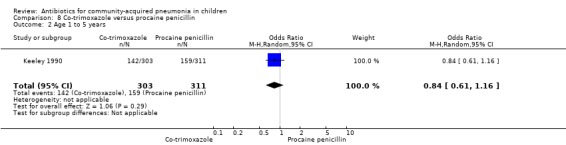
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 2 Age 1 to 5 years.
8.3. Analysis.
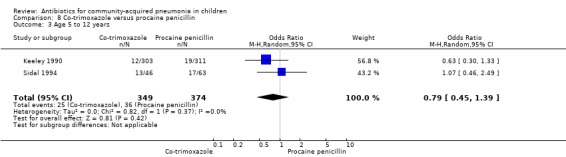
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 3 Age 5 to 12 years.
8.4. Analysis.
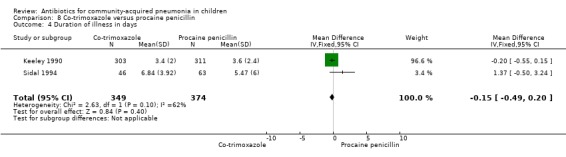
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 4 Duration of illness in days.
8.5. Analysis.
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 5 Male sex.
8.8. Analysis.
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 8 Well at end of follow‐up.
8.9. Analysis.
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 9 Death.
8.10. Analysis.
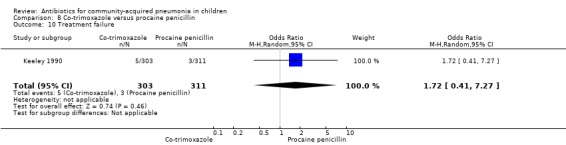
Comparison 8 Co‐trimoxazole versus procaine penicillin, Outcome 10 Treatment failure.
Comparison 9. Co‐trimoxazole versus procaine penicillin and ampicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean age in months | 1 | 134 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Age less than 1 year | 1 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.39, 1.64] |
| 3 Male sex | 1 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.65, 2.58] |
| 4 Cure rate | 1 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.36, 3.61] |
| 5 Hospitalisation rate | 1 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.25, 9.72] |
| 6 Death rate | 1 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.25] |
9.1. Analysis.
Comparison 9 Co‐trimoxazole versus procaine penicillin and ampicillin, Outcome 1 Mean age in months.
9.2. Analysis.
Comparison 9 Co‐trimoxazole versus procaine penicillin and ampicillin, Outcome 2 Age less than 1 year.
9.3. Analysis.
Comparison 9 Co‐trimoxazole versus procaine penicillin and ampicillin, Outcome 3 Male sex.
Comparison 10. Cefpodoxime versus co‐amoxyclavulanic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rate (response rate) at end of treatment | 1 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.18, 2.60] |
| 2 Mean age (months) | 1 | 348 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse effects | 1 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.16, 1.35] |
| 4 Age in years | 1 | 348 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Follow‐up | 1 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.31] |
10.2. Analysis.
Comparison 10 Cefpodoxime versus co‐amoxyclavulanic acid, Outcome 2 Mean age (months).
10.3. Analysis.
Comparison 10 Cefpodoxime versus co‐amoxyclavulanic acid, Outcome 3 Adverse effects.
10.4. Analysis.
Comparison 10 Cefpodoxime versus co‐amoxyclavulanic acid, Outcome 4 Age in years.
10.5. Analysis.
Comparison 10 Cefpodoxime versus co‐amoxyclavulanic acid, Outcome 5 Follow‐up.
Comparison 11. Chloramphenicol versus penicillin plus gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.96, 1.66] |
| 2 Death | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.76, 2.07] |
| 3 Change of antibiotics | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.18] |
| 4 Readmission before 30 days | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.02, 2.55] |
| 5 Absconded | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.83, 2.09] |
| 6 Age (months) | 1 | 1116 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Culture positive | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.21] |
| 8 Male sex | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.12] |
| 9 Received antibiotics in previous 1 week | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.75, 1.22] |
| 10 Lost to follow‐up | 1 | 1116 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.83, 2.09] |
11.5. Analysis.
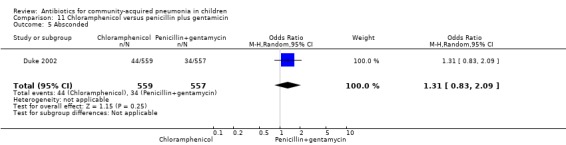
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 5 Absconded.
11.6. Analysis.
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 6 Age (months).
11.7. Analysis.
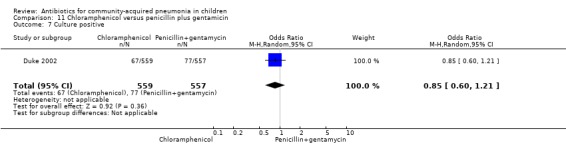
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 7 Culture positive.
11.8. Analysis.
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 8 Male sex.
11.9. Analysis.
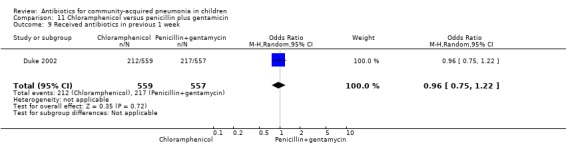
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 9 Received antibiotics in previous 1 week.
11.10. Analysis.
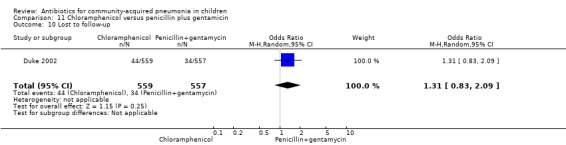
Comparison 11 Chloramphenicol versus penicillin plus gentamicin, Outcome 10 Lost to follow‐up.
Comparison 12. Chloramphenicol with ampicillin and gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean age | 1 | 958 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.12, 0.92] |
| 2 Male sex | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.66, 1.11] |
| 3 Number received antibiotics in past 7 days | 1 | 950 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.14] |
| 4 Failure rates on day 5 | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [1.04, 2.19] |
| 5 Failure rates on day 10 | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.04, 2.06] |
| 6 Failure rates on day 21 | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [1.03, 1.98] |
| 7 Need for change in antibiotics (day 5) | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [1.10, 2.98] |
| 8 Need for change in antibiotics (day 10) | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [1.10, 2.66] |
| 9 Need for change in antibiotics (day 21) | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [1.09, 2.49] |
| 10 Death rates | 1 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.99, 2.77] |
12.1. Analysis.
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 1 Mean age.
12.2. Analysis.
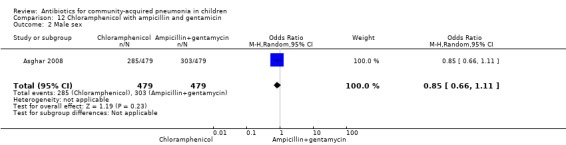
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 2 Male sex.
12.3. Analysis.
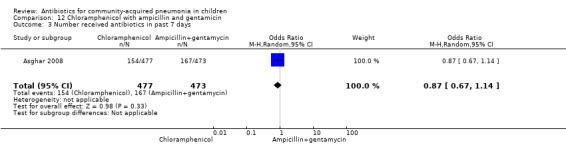
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 3 Number received antibiotics in past 7 days.
12.7. Analysis.
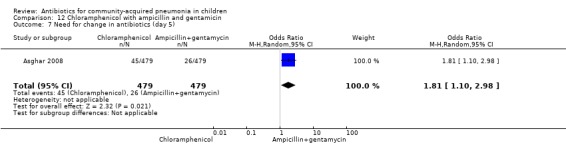
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 7 Need for change in antibiotics (day 5).
12.8. Analysis.
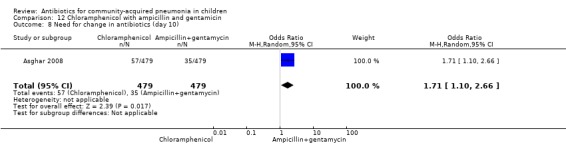
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 8 Need for change in antibiotics (day 10).
12.9. Analysis.
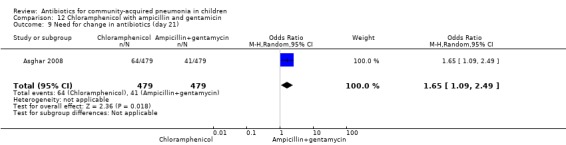
Comparison 12 Chloramphenicol with ampicillin and gentamicin, Outcome 9 Need for change in antibiotics (day 21).
Comparison 13. Chloramphenicol plus penicillin versus ceftriaxone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.47, 3.93] |
Comparison 14. Chloramphenicol versus chloramphenicol plus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for change of antibiotics | 1 | 748 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.97] |
| 2 Death rates | 1 | 748 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.48, 1.09] |
| 3 Lost to follow‐up | 1 | 748 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.80, 1.53] |
Comparison 15. Ampicillin alone versus penicillin with chloramphenicol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.51] |
| 2 Age (months) | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐5.54, 2.16] |
| 3 Male sex | 1 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.41, 1.93] |
| 4 Duration of hospital stay | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.13, 0.93] |
| 5 Grade 2 to 4 malnutrition | 1 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.41, 1.93] |
15.2. Analysis.
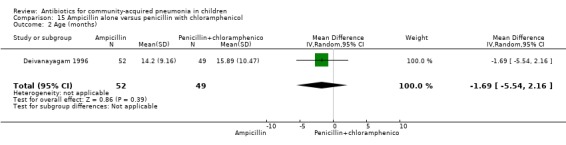
Comparison 15 Ampicillin alone versus penicillin with chloramphenicol, Outcome 2 Age (months).
15.3. Analysis.
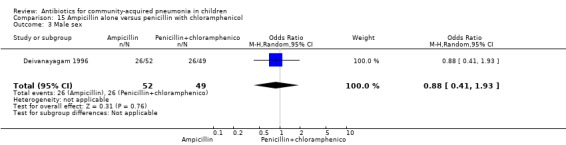
Comparison 15 Ampicillin alone versus penicillin with chloramphenicol, Outcome 3 Male sex.
15.5. Analysis.
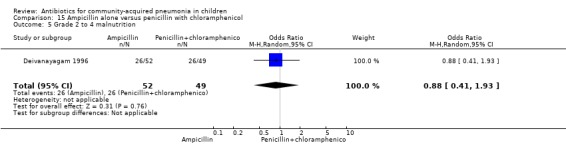
Comparison 15 Ampicillin alone versus penicillin with chloramphenicol, Outcome 5 Grade 2 to 4 malnutrition.
Comparison 16. Benzathine penicillin versus procaine penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rate | 2 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.27, 1.01] |
| 2 Failure rate | 2 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 3.17 [0.90, 11.11] |
| 3 Male sex | 2 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.67, 1.76] |
| 4 Age between 2 to 6 years | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.47, 2.48] |
| 5 Age between 7 to 12 years | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.88] |
| 6 Lost to follow‐up | 1 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [0.16, 20.25] |
| 7 Failure rates in radiographically confirmed pneumonia | 1 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [0.45, 5.70] |
16.3. Analysis.
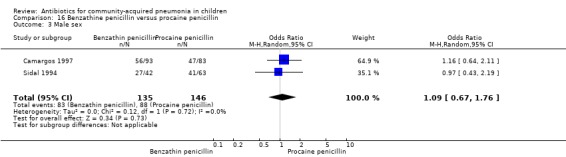
Comparison 16 Benzathine penicillin versus procaine penicillin, Outcome 3 Male sex.
16.4. Analysis.
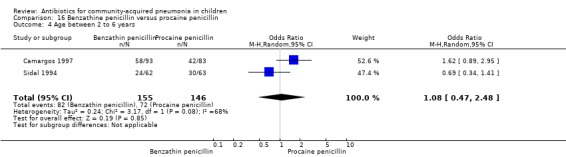
Comparison 16 Benzathine penicillin versus procaine penicillin, Outcome 4 Age between 2 to 6 years.
16.5. Analysis.
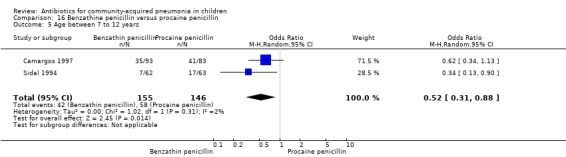
Comparison 16 Benzathine penicillin versus procaine penicillin, Outcome 5 Age between 7 to 12 years.
16.6. Analysis.
Comparison 16 Benzathine penicillin versus procaine penicillin, Outcome 6 Lost to follow‐up.
Comparison 17. Amoxycillin versus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nasopharyngeal aspirates for S. pneumoniae | 1 | 1486 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.72, 1.13] |
| 2 Age less than 1 year | 1 | 1702 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.87, 1.29] |
| 3 Male sex | 2 | 1905 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.25] |
| 4 Weight below 2 Z score (indicating severe malnutrition) | 1 | 1686 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.19] |
| 5 Breast fed | 1 | 1702 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.37] |
| 6 Received antibiotics in last 7 days | 2 | 1905 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.69, 1.38] |
| 7 Failure rate at 48 hours | 1 | 1702 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.81, 1.31] |
| 8 Failure rate on day 5 | 2 | 1905 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.58, 2.30] |
| 9 Failure rate on day 14 | 1 | 1702 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.84, 1.29] |
| 10 Death rates | 2 | 1905 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.18] |
| 11 Nasopharyngeal H. influenzae | 1 | 1482 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.78, 1.29] |
| 12 Respiratory syncytial virus (RSV) in nasopharyngeal swabs | 2 | 1731 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.83, 1.31] |
| 13 Mean age | 1 | 203 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Blood culture positive for S. pneumoniae | 1 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.03, 3.29] |
| 15 Failure rate on day 5 in radiographically confirmed pneumonia | 1 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 2.36 [0.59, 9.39] |
17.1. Analysis.
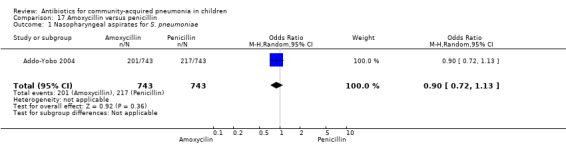
Comparison 17 Amoxycillin versus penicillin, Outcome 1 Nasopharyngeal aspirates for S. pneumoniae.
17.2. Analysis.
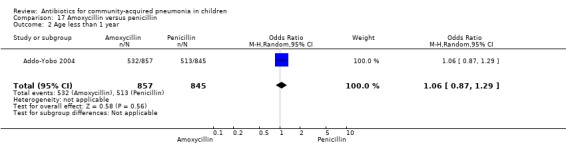
Comparison 17 Amoxycillin versus penicillin, Outcome 2 Age less than 1 year.
17.3. Analysis.
Comparison 17 Amoxycillin versus penicillin, Outcome 3 Male sex.
17.4. Analysis.
Comparison 17 Amoxycillin versus penicillin, Outcome 4 Weight below 2 Z score (indicating severe malnutrition).
17.5. Analysis.
Comparison 17 Amoxycillin versus penicillin, Outcome 5 Breast fed.
17.6. Analysis.
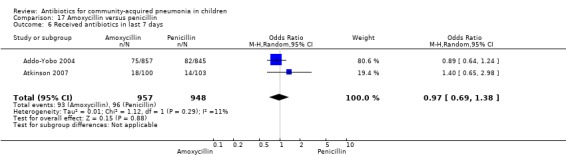
Comparison 17 Amoxycillin versus penicillin, Outcome 6 Received antibiotics in last 7 days.
17.10. Analysis.
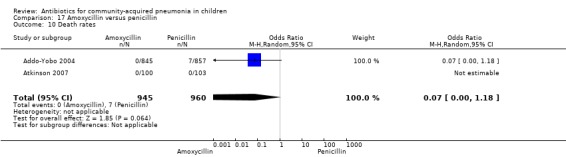
Comparison 17 Amoxycillin versus penicillin, Outcome 10 Death rates.
17.11. Analysis.
Comparison 17 Amoxycillin versus penicillin, Outcome 11 Nasopharyngeal H. influenzae.
17.12. Analysis.
Comparison 17 Amoxycillin versus penicillin, Outcome 12 Respiratory syncytial virus (RSV) in nasopharyngeal swabs.
17.13. Analysis.
Comparison 17 Amoxycillin versus penicillin, Outcome 13 Mean age.
17.14. Analysis.
Comparison 17 Amoxycillin versus penicillin, Outcome 14 Blood culture positive for S. pneumoniae.
Comparison 18. Amoxycillin with IV ampicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age below one year | 1 | 2037 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.13] |
| 2 Male sex | 1 | 2037 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.91, 1.30] |
| 3 Wheezing in infants | 1 | 1311 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.37] |
| 4 Wheezing in age group one to five years | 1 | 726 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.04] |
| 5 Failure rates | 1 | 2037 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.19] |
| 6 Relapse rates | 1 | 1873 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.46, 1.33] |
| 7 Death rates | 1 | 2037 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.21] |
| 8 Lost to follow‐up | 1 | 2037 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.20] |
| 9 Protocol violation | 1 | 2037 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.43, 1.96] |
18.1. Analysis.
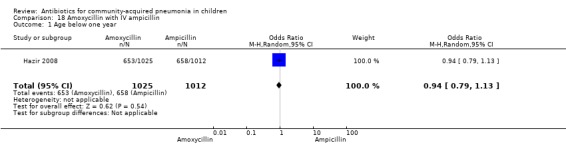
Comparison 18 Amoxycillin with IV ampicillin, Outcome 1 Age below one year.
18.2. Analysis.
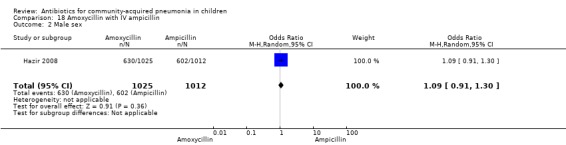
Comparison 18 Amoxycillin with IV ampicillin, Outcome 2 Male sex.
18.3. Analysis.
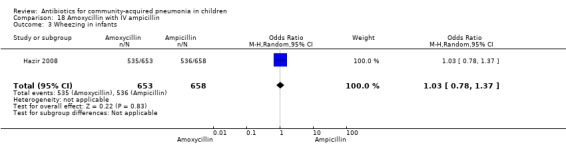
Comparison 18 Amoxycillin with IV ampicillin, Outcome 3 Wheezing in infants.
18.4. Analysis.
Comparison 18 Amoxycillin with IV ampicillin, Outcome 4 Wheezing in age group one to five years.
18.8. Analysis.
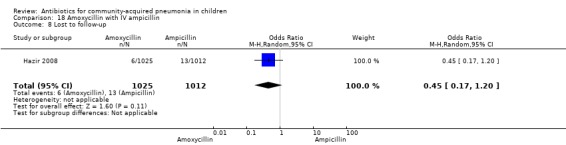
Comparison 18 Amoxycillin with IV ampicillin, Outcome 8 Lost to follow‐up.
18.9. Analysis.
Comparison 18 Amoxycillin with IV ampicillin, Outcome 9 Protocol violation.
Comparison 19. Amoxycillin with cefuroxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean age in months | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 4.47 [‐1.45, 10.39] |
| 2 Male sex | 1 | 85 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.90] |
| 3 Cure rates | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.18, 23.51] |
| 4 Failure rates | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.04, 5.59] |
19.1. Analysis.
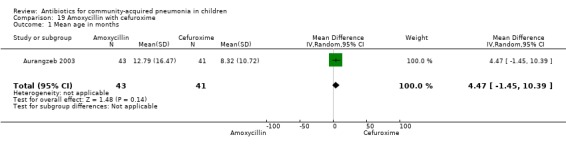
Comparison 19 Amoxycillin with cefuroxime, Outcome 1 Mean age in months.
19.2. Analysis.
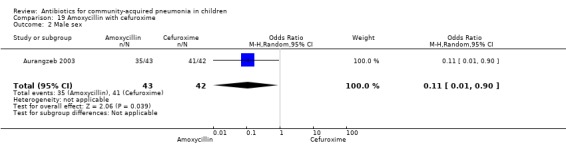
Comparison 19 Amoxycillin with cefuroxime, Outcome 2 Male sex.
Comparison 20. Amoxycillin with clarithromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean age | 1 | 85 | Mean Difference (IV, Random, 95% CI) | ‐3.16 [‐10.30, 3.98] |
| 2 Male sex | 1 | 85 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.55, 4.35] |
| 3 Cure rates | 1 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.06, 17.40] |
| 4 Failure rates | 1 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.06, 15.74] |
20.1. Analysis.
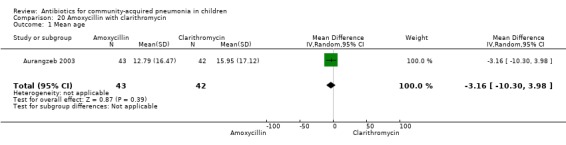
Comparison 20 Amoxycillin with clarithromycin, Outcome 1 Mean age.
20.2. Analysis.
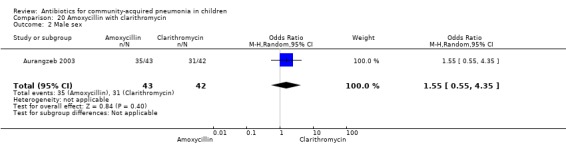
Comparison 20 Amoxycillin with clarithromycin, Outcome 2 Male sex.
Comparison 21. Penicillin and gentamycin with co‐amoxyclavulanic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of children less than 1 year age | 1 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.20, 1.43] |
| 2 Male sex | 1 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.42, 4.32] |
| 3 Failure rates | 1 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.05, 14.39] |
21.1. Analysis.
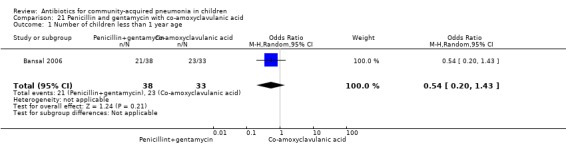
Comparison 21 Penicillin and gentamycin with co‐amoxyclavulanic acid, Outcome 1 Number of children less than 1 year age.
21.2. Analysis.
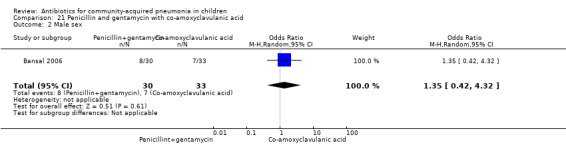
Comparison 21 Penicillin and gentamycin with co‐amoxyclavulanic acid, Outcome 2 Male sex.
Comparison 22. Levofloxacin with comparator (co‐amoxyclavulanic acid/ceftriaxone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean age | 1 | 709 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.64, 0.74] |
| 2 Male sex | 1 | 709 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.69, 1.36] |
| 3 Numbers received antibiotics in past 1 week | 1 | 709 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.35] |
| 4 Cure rates | 1 | 539 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.46, 2.42] |
22.1. Analysis.
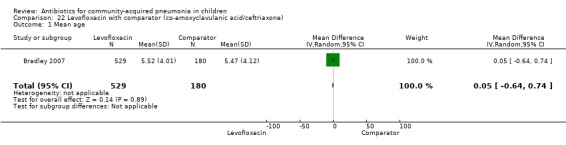
Comparison 22 Levofloxacin with comparator (co‐amoxyclavulanic acid/ceftriaxone), Outcome 1 Mean age.
22.2. Analysis.
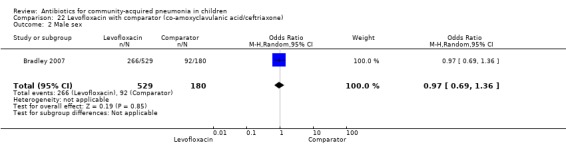
Comparison 22 Levofloxacin with comparator (co‐amoxyclavulanic acid/ceftriaxone), Outcome 2 Male sex.
22.3. Analysis.
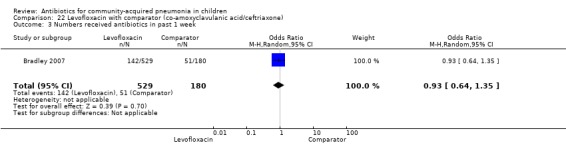
Comparison 22 Levofloxacin with comparator (co‐amoxyclavulanic acid/ceftriaxone), Outcome 3 Numbers received antibiotics in past 1 week.
Comparison 23. Cefuroxime with clarithromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean age | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐7.03 [‐13.16, ‐0.90] |
| 2 Male sex | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 14.55 [1.78, 118.76] |
| 3 Cure rates | 1 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.04, 5.89] |
| 4 Failure rates | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.18, 23.51] |
23.1. Analysis.
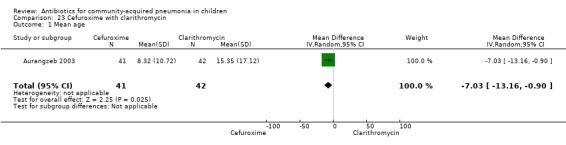
Comparison 23 Cefuroxime with clarithromycin, Outcome 1 Mean age.
23.2. Analysis.
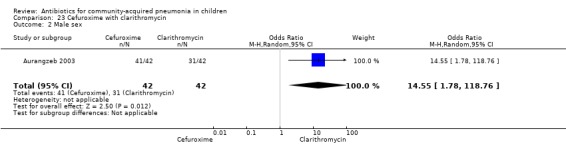
Comparison 23 Cefuroxime with clarithromycin, Outcome 2 Male sex.
Comparison 24. Co‐trimoxazole versus chloramphenicol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age in months | 1 | 111 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐0.64, 4.44] |
| 2 Male sex | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.42, 1.89] |
| 3 Weight for age | 1 | 111 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.11, 3.11] |
| 4 Wheezing positive | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 4.15] |
| 5 Cure rate | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.47, 2.40] |
| 6 Failure rate | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.45, 2.33] |
| 7 Excluded | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.12] |
| 8 Relapse rate | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.24, 4.30] |
| 9 Need for change in antibiotics | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.46, 4.40] |
| 10 Death rate | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 2.21 [0.63, 7.83] |
| 11 Organisms isolated on blood culture or lung puncture | 1 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.47, 3.30] |
24.1. Analysis.
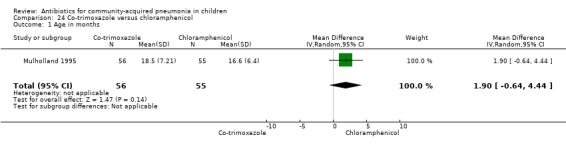
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 1 Age in months.
24.2. Analysis.
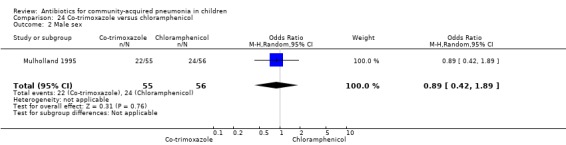
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 2 Male sex.
24.3. Analysis.
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 3 Weight for age.
24.4. Analysis.
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 4 Wheezing positive.
24.7. Analysis.
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 7 Excluded.
24.11. Analysis.
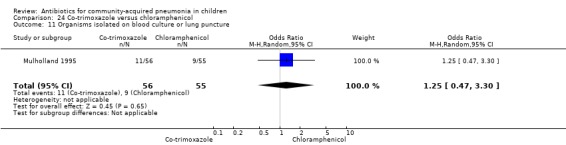
Comparison 24 Co‐trimoxazole versus chloramphenicol, Outcome 11 Organisms isolated on blood culture or lung puncture.
Comparison 25. Ceftibuten versus cefuroxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Male sex | 1 | 140 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.41, 1.54] |
| 2 Positive for microbial agent | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.83 [1.87, 7.83] |
| 3 Adverse reaction | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.35, 11.29] |
| 4 Cure rate | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.94] |
| 5 Failure rate | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.81 [1.46, 31.70] |
25.1. Analysis.
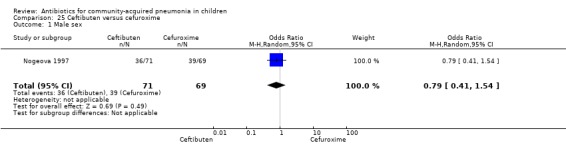
Comparison 25 Ceftibuten versus cefuroxime, Outcome 1 Male sex.
25.3. Analysis.
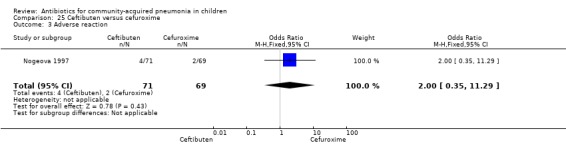
Comparison 25 Ceftibuten versus cefuroxime, Outcome 3 Adverse reaction.
Comparison 26. Oxacillin ceftriaxone versus co‐amoxyclavulanic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median age (months) with IQR | Other data | No numeric data | ||
| 2 Male sex | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.32, 1.54] |
| 3 Mean number of days before admission | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.28, 0.48] |
| 4 Received antibiotics before enrolment | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.50, 2.76] |
| 5 Failure rates | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.33, 2.92] |
| 6 Mean time for improvement in tachypnoea | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.89, ‐0.11] |
| 7 Mean length of stay | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐5.46, ‐1.34] |
26.1. Analysis.
Comparison 26 Oxacillin ceftriaxone versus co‐amoxyclavulanic acid, Outcome 1 Median age (months) with IQR.
| Median age (months) with IQR | ||
|---|---|---|
| Study | Amoxicillin clavulanic acid group | Oxacillin+ ceftriaxone group |
| Ribeiro 2011 | 11.5 (3 to 60) | 10.5 (2 to 60) |
26.2. Analysis.
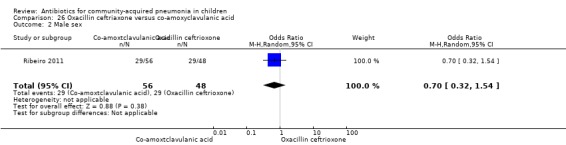
Comparison 26 Oxacillin ceftriaxone versus co‐amoxyclavulanic acid, Outcome 2 Male sex.
26.3. Analysis.
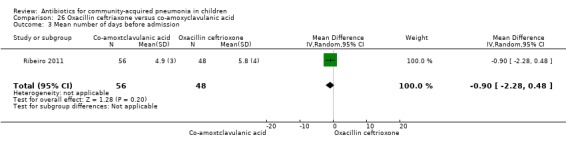
Comparison 26 Oxacillin ceftriaxone versus co‐amoxyclavulanic acid, Outcome 3 Mean number of days before admission.
26.4. Analysis.
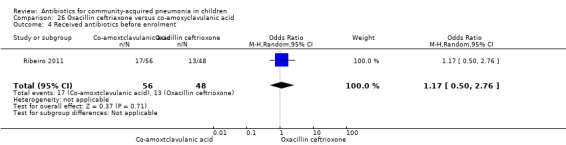
Comparison 26 Oxacillin ceftriaxone versus co‐amoxyclavulanic acid, Outcome 4 Received antibiotics before enrolment.
Comparison 27. Oral versus parenteral antibiotics for treatment of severe pneumonia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Male sex | 5 | 4164 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.21] |
| 2 Age below 12 months | 4 | 3961 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.30] |
| 3 Received antibiotics in the past week | 3 | 3942 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.86, 1.52] |
| 4 Children with wheezing | 2 | 3739 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.70, 1.68] |
| 5 RSV positivity | 2 | 1634 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.82, 1.31] |
| 6 Failure rates on day 3 | 3 | 3942 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.15] |
| 7 Failure rates on day 6 | 6 | 4331 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.56, 1.24] |
| 8 Failure rate in children below 5 years of age | 3 | 3870 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.76, 1.09] |
| 9 Failure rates in children receiving oral amoxicillin or injectable antibiotics | 4 | 4112 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.10] |
| 10 Failure rate in children receiving cotrimoxazole or injectable penicillin | 2 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.29] |
| 11 Failure rate in children treated with oral or parenteral antibiotics on ambulatory basis | 4 | 2426 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.24, 1.32] |
| 12 Failure rate after removing one study | 2 | 2240 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.44, 2.83] |
| 13 Hospitalisation | 3 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.38, 3.34] |
| 14 Relapse rates | 2 | 2076 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.34, 4.82] |
| 15 Death rates | 3 | 3942 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.03, 0.87] |
| 16 Lost to follow‐up | 1 | 2037 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.20] |
| 17 Cure rate | 2 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 5.05 [1.19, 21.33] |
| 18 Failure rates in radiographically confirmed‐pneumonia | 2 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.41, 4.29] |
| 19 Death rates after removing one study | 2 | 2240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.21] |
27.1. Analysis.
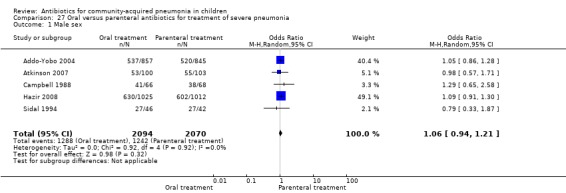
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 1 Male sex.
27.2. Analysis.
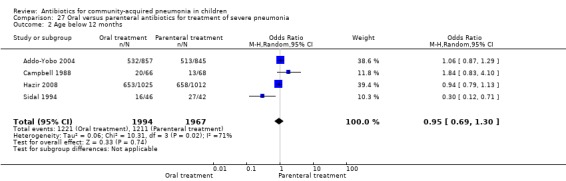
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 2 Age below 12 months.
27.3. Analysis.
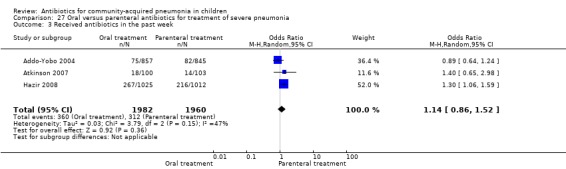
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 3 Received antibiotics in the past week.
27.4. Analysis.
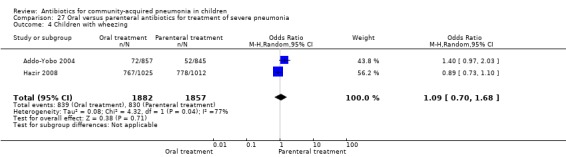
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 4 Children with wheezing.
27.5. Analysis.
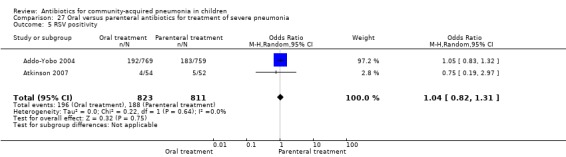
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 5 RSV positivity.
27.6. Analysis.
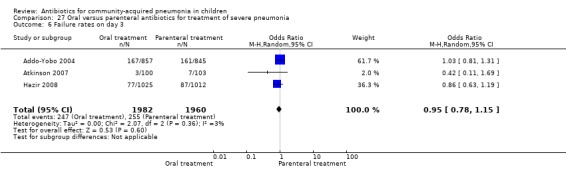
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 6 Failure rates on day 3.
27.11. Analysis.
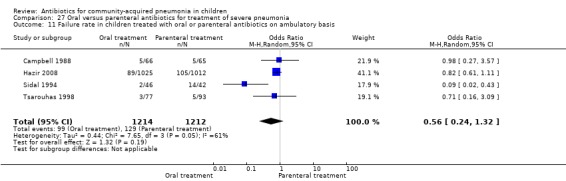
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 11 Failure rate in children treated with oral or parenteral antibiotics on ambulatory basis.
27.12. Analysis.
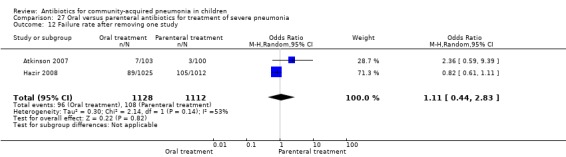
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 12 Failure rate after removing one study.
27.17. Analysis.
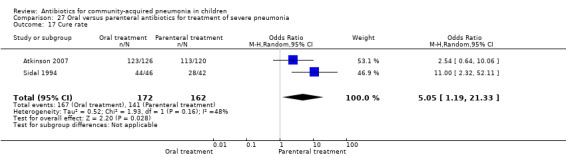
Comparison 27 Oral versus parenteral antibiotics for treatment of severe pneumonia, Outcome 17 Cure rate.
Comparison 28. Co‐trimoxazole versus co‐amoxyclavulanic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children below 1 year of age | 2 | 1232 | Odds Ratio (M‐H, Random, 95% CI) | 117.90 [16.39, 848.37] |
| 2 Male sex | 2 | 1232 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.88] |
| 3 Failure rate | 2 | 1232 | Odds Ratio (M‐H, Random, 95% CI) | 12.98 [3.18, 53.06] |
28.1. Analysis.
Comparison 28 Co‐trimoxazole versus co‐amoxyclavulanic acid, Outcome 1 Children below 1 year of age.
28.2. Analysis.
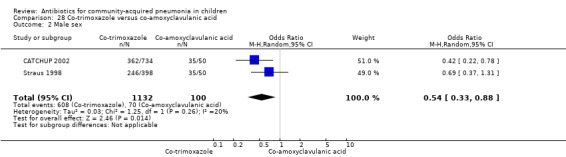
Comparison 28 Co‐trimoxazole versus co‐amoxyclavulanic acid, Outcome 2 Male sex.
28.3. Analysis.
Comparison 28 Co‐trimoxazole versus co‐amoxyclavulanic acid, Outcome 3 Failure rate.
Comparison 29. Amoxycillin versus cefpodoxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age in months | 1 | 284 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Male sex | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [0.07, 44.09] |
| 3 Response/cure rate | 1 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.08, 0.53] |
29.1. Analysis.
Comparison 29 Amoxycillin versus cefpodoxime, Outcome 1 Age in months.
29.2. Analysis.
Comparison 29 Amoxycillin versus cefpodoxime, Outcome 2 Male sex.
29.3. Analysis.
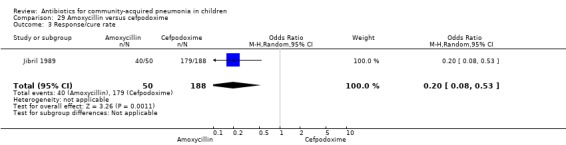
Comparison 29 Amoxycillin versus cefpodoxime, Outcome 3 Response/cure rate.
Comparison 30. Amoxycillin versus chloramphenicol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age (mean/median) | 2 | 1032 | Mean Difference (IV, Random, 95% CI) | ‐6.60 [‐10.52, ‐2.68] |
| 2 Male sex | 2 | 1032 | Odds Ratio (M‐H, Random, 95% CI) | 2.34 [1.55, 3.53] |
| 3 Cure rate | 1 | 796 | Odds Ratio (M‐H, Random, 95% CI) | 4.26 [2.57, 7.08] |
| 4 Failure rates | 2 | 1065 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.41, 1.00] |
30.1. Analysis.
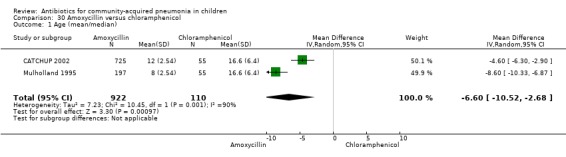
Comparison 30 Amoxycillin versus chloramphenicol, Outcome 1 Age (mean/median).
30.2. Analysis.
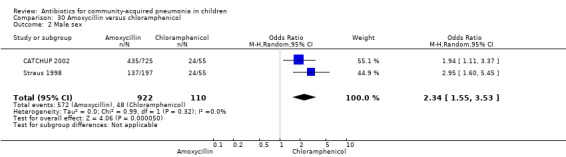
Comparison 30 Amoxycillin versus chloramphenicol, Outcome 2 Male sex.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Addo‐Yobo 2004.
| Methods | RCT comparing amoxycillin and penicillin | |
| Participants | Children 3 to 59 months with severe pneumonia | |
| Interventions | Daily IM penicillin 200,000 IU/kg or PO amoxycillin 45 mg/kg/day | |
| Outcomes | Failure rate at 48 hours, 5 days and 14 days and death rate | |
| Notes | Exclusion criteria: asthma, audible wheeze, non‐severe pneumonia, very severe disease, clinical HIV, persistent vomiting, penicillin allergy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by World Health Organization (WHO) |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were sealed in opaque envelopes in accordance with allocation sequence, stratified by site and prepared in advance by the WHO |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by World Heath Organization and Applied Research Child Health Project, Boston University |
Asghar 2008.
| Methods | Randomised, non‐blinded, multi‐site efficacy study | |
| Participants | Children between 2 to 59 months of age with very severe pneumonia | |
| Interventions | Ampicillin plus gentamicin (ampicillin 200 mg/kg/d in 4 doses every 6 hours, and gentamicin 7.5 mg/kg/d as in a single daily dose) or chloramphenicol (75 mg/kg/d given in 3 doses) every 8 hours for minimum of 5 days. After that first group received oral amoxycillin (45 mg/kg/day in 3 divided doses) and the other group received oral chloramphenicol 75 mg/kg/day to complete 10 days | |
| Outcomes |
Primary outcome
Secondary outcomes Treatment failure as defined above at 48 to 60 hours Treatment failure as defined above plus relapse (hypoxaemic pneumonia at 10 to 12 days and 21 to 30 days, with oxygen saturations ≤ 90%, or ≤ 88% in the 2 high altitude sites in Mexico and Yemen) Death by 30 days after enrolment Bacterial pathogens isolated from blood or other sterile sites Antimicrobial susceptibility of the isolated pathogens |
|
| Notes | Exclusion criteria: wheezing, with a history of 3 or more attacks, or known asthma, known heart disease, duration of present illness more than 10 days, history of serious adverse reaction to any of the study drugs, previous enrolment in the study Admission to hospital for more than 24 hours within past 7 days Documented evidence of injectable antibiotic treatment for more than 24 hours before enrolment, stridor, known renal failure or not passed urine during past 6 hours, evidence of cerebral malaria Evidence of bacterial meningitis, clinical jaundice, residence of patient in an area where follow‐up was not possible, empyema or presence of pneumatoceles on chest radiograph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by World Health Organization by using variable size blocks |
| Allocation concealment (selection bias) | Low risk | Separate randomisation lists were prepared for each site examination, and individual patient assignments were placed in opaque, sealed envelopes. Before opening each envelope the doctor in charge signed and dated the opening flap of the envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label randomised controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label randomised controlled trial |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by World Health Organization and Center of International Health and Development, Boston University and Johns Hopkins Bloomberg School of Public Health, Baltimore |
Atkinson 2007.
| Methods | Multicentre randomised controlled trial | |
| Participants | Children admitted with pneumonia in 8 hospitals. At least 3 inclusion criteria for diagnosis of pneumonia. Respiratory symptoms or signs, fever > 37.5 °C, radiographically confirmed pneumonia (defined as confluent area of consolidation agreed subsequently by 2 independent radiologists) | |
| Interventions | Oral amoxycillin (doses for 6 months to 12 years of age 8 mg/kg/dose 3 times a day above 12 years of age 500 mg 3 times a day) or IV benzyl penicillin (doses 25 mg/kg/dose 4 times a day) | |
| Outcomes | The primary outcome measure was time from randomisation until the temperature was 38 °C for 24 continuous hours and oxygen requirement had ceased (the latter only applicable to those children who required oxygen during the admission) Secondary outcomes included time in hospital, complications (empyema, re‐admission, further courses of antibiotics), duration of oxygen requirement and time to resolution of illness |
|
| Notes | Exclusion criteria were wheeze, oxygen saturations, 85% in air, shock requiring 20 ml/kg fluid resuscitation, immunodeficiency, pleural effusion at presentation requiring drainage, chronic lung condition (excluding asthma), penicillin allergy and age 6 months Treatment with oral antibiotics in the 5 days prior to admission, including amoxycillin, was not an exclusion criterion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation list generated |
| Allocation concealment (selection bias) | Low risk | A block randomisation sequence stratified by centre was produced using a random number generator. The sequence was accessed via the Internet, therefore allowing concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Unclear risk | While the authors mention the primary outcome as "the time from randomisation until the temperature was less than 38 degree celsius for 24 continuous hours and oxygen requirement had ceased", they calculated the sample size based on the proportion meeting the primary outcome measure at any time. The authors have not reported on these proportions in the results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Other bias | Low risk | Funded by the British Lung Foundation |
Aurangzeb 2003.
| Methods | Randomised, non‐blinded controlled clinical trial | |
| Participants | Children between 3 to 72 months of age, admitted in the hospital with community‐acquired pneumonia | |
| Interventions | The patients were randomly allotted to 1 of the 3 groups Group 1 was given amoxycillin 75 mg/kg/d IV in 3 divided doses, Group 2 was given cefuroxime 75 mg/kg/d IV in 3 divided doses and Group 3 was given clarithromycin 15 mg/kg/d IV divided into 2 divided doses | |
| Outcomes | 1. Improvement defined as slower respiratory rate (either back to normal for the age of the child), or more than 5 as compared to the previous day evaluation without retractions. The same defined as still breathing fast as before as or higher than that with no chest in drawing or danger signs 2. Worse was defined as development of severe pneumonia or very severe disease 3. Cure was defined as return of respiratory rate to age specific normal range |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Unclear risk | There are discrepancies in the number of patients in different study arms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Other bias | Unclear risk | Source of funding not mentioned |
Awasthi 2008.
| Methods | Cluster‐randomised, open‐label trial | |
| Participants | Children of either sex, between 2 months to 59 months with WHO‐defined non‐severe pneumonia | |
| Interventions | Eligible children were randomised to receive oral dispersible scored amoxycillin (125 mg per tablet) given thrice a day (tds) for 3 days or co‐trimoxazole (20 mg trimethoprim per tablet) given twice a day (bd) for 5 days. Doses of amoxycillin were between 31 to 51 mg/kg/day and trimethoprim 7 to 11 mg/kg/day | |
| Outcomes | Primary outcome measure was clinical failure defined as presence of at least 1 of the following: (i) development of signs of WHO‐defined severe pneumonia or very severe disease (ii) respiratory rate above age specific cut‐off, (iii) documented axillary temperature > 38.3 °C on the day of outcome assessment, that is day 4 for amoxycillin and day 6 for co‐trimoxazole arm, (iv) death within the follow‐up period of 14 days, (v) lost to follow‐up on day 4 or day 6 in the amoxycillin and co‐trimoxazole arms, respectively, or (vi) withdrawal at any time (requirement of intention‐to‐treat (ITT) analysis) The secondary outcome measure was clinical relapse on day 13 to 15, defined as development of signs of WHO‐defined pneumonia among the clinically cured in either arm |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Low risk | This was an open‐label study and the unit of randomisation was primary health centre |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label randomised controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label randomised controlled trial |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by Indian Council of Medical Research |
Bansal 2006.
| Methods | Open‐label randomised controlled trial | |
| Participants | Children aged 2 to 59 months with WHO‐defined severe or very severe pneumonia with hypoxaemia (Sp02 < 90%) were included in the study | |
| Interventions | Patients in Group A received crystalline penicillin (benzyl penicillin) ‐ 50,000 IV/kg IV, q6h and gentamycin 2.5 mg/kg, IV, q8h for at least 3 days. After that, oral amoxycillin 15 mg/kg 8‐hourly was substituted for crystalline penicillin. Group B patients were given amoxycillin‐clavulanate 30 mg/kg IV q12h for at least 3 days and were changed to oral amoxycillin‐clavulanic acid when able to feed | |
| Outcomes | Treatment failure was defined as any change, modification or discontinuation of allocated antibiotic therapy because of deterioration in patient's condition, development of serious intercurrent illness or complications such as refractory septic shock, acute renal failure, meningitis etc., persistence of danger signs such as inability to drink after 48 hours of treatment or relapse of the hypoxaemic pneumonia during the following 2 weeks | |
| Notes | Patients with fever > 10 days, bacterial meningitis, prior antibiotic therapy > 24 hours, stridor, heart disease and allergy to any of the study drugs were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Randomisation list was prepared before starting the study and random treatment assignment was placed in serially labelled sealed envelopes. The assignment was opened when the patient had met all the inclusion and exclusion criteria and written consent was available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label randomised controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label randomised controlled trial |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Block 1995.
| Methods | RCT comparing clarithromycin with erythromycin in children with pneumonia | |
| Participants | Children between 3 to 16 years of age with radiographically confirmed pneumonia | |
| Interventions | PO clarithromycin (15 mg/kg/day) for 10 days or erythromycin 40 mg/kg/day for 10 days | |
| Outcomes | Cure rates, resolution of signs and symptoms, improvement, improved but non‐resolution of signs and symptoms, failure or worsening | |
| Notes | Exclusion: hypersensitivity to macrolides, severe renal or hepatic diseases, active tuberculosis, severe infections requiring intravenous antibiotics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned clearly. Open‐label study. Study drugs were dispensed and compliance was monitored by third party |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator‐blinded study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Funded by Abbott Laboratories and role of funding agency not clear |
Bradley 2007.
| Methods | This was a randomised (3:1, levofloxacin:comparator), open‐label, active‐comparator, non‐inferiority, multicentre study | |
| Participants | Children between 0.5 to 16 years old with a diagnosis of community‐acquired pneumonia (CAP). A diagnosis of CAP was defined as radiographic evidence of pulmonary infiltrate consistent with acute infection requiring antibiotic therapy, and the presence of 2 or more of the indications of pneumonia: fever (rectal or oral temperature 38 °C for children 2 years, or 38.3 °C for children 0.5 to 2 years), shortness of breath, cough, chest pain, abnormal white blood cell count (15,000/L or 5000/L), or physical signs of pneumonia on examination (e.g. rales on auscultation, dullness to percussion, egophony) | |
| Interventions | Levofloxacin or a comparator antibiotic for 10 days. Levofloxacin and comparators were given either orally or by intravenous (IV) administration. The patients were randomised in a 3:1 levofloxacin:comparator ratio within 14 strata in the study (1 for the 2 age groups within each country). For Group I (6 months to 5 years), levofloxacin was administered (a) 10 mg/kg/dose as oral suspension bd (up to 500 mg/d) or (b) 10 mg/kg/dose IV q12 hours (up to 500 mg/d). The comparator administration was (a) amoxycillin and clavulanic acid (7:1) oral suspension bd, with dose determined by calculating amoxycillin 22.5 mg/kg/dose (up to 875 mg/d), or (b) ceftriaxone 25 mg/kg/dose IV q12 hours (up to 4 G/d) For Group II (5 to 16 years), levofloxacin was administered (a) as 10 mg/kg/dose as oral suspension qd (up to 500 mg/d), (b) as 1 250 mg tablet qd (for children weighing 22.5 to 27.5 kg) or 2 250 mg tablets qd (for children weighing 45.5 kg), or (c) 10 mg/kg/dose IV q24 hours (up to 500 mg/d). The comparator administration was (a) clarithromycin 7.5 mg/kg/dose as oral suspension (or as a 250 mg tablet) bd (up to 250 mg bd), clarithromycin 250 mg oral tablet bd, or (b) ceftriaxone 25 mg/kg/dose IV q12 hours (up to 4 G/d), with either erythromycin lactobionate 10 mg/kg/dose IV q6 hours (up to 4 G/24 hours) or clarithromycin 7.5 mg/kg/dose as oral suspension (or as a 250 mg tablet) bd (up to 250 mg bd) | |
| Outcomes | Clinical response was categorised as cured, improved, clinical failure, relapse at test of cure visit (TOCV) 10 to 17 days after the last dose of study drug: (1) cured: resolution of signs and symptoms associated with active infection along with an improvement or lack of progression of abnormal findings of chest roentgenogram; (2) improved: continued incomplete resolution of signs and symptoms with no deterioration or relapse after post‐therapy visit (PTV) and no requirement for additional antimicrobial therapy; (3) clinical relapse: resolution or improvement of signs and symptoms at PTV evaluation with reappearance or deterioration of signs and symptoms of infection at test of cure visit (TOCV); (4) failure: patient was considered a clinical failure at PTV, response was carried forward to TOCV; and (5) unable to evaluate: unable to determine response because patient was not evaluated after PTV | |
| Notes | Exclusion criteria: received systemic antibiotics for more than 24 hours immediately before enrolment, required a systemic antibiotic other than the study drugs, or had a suspected infection with micro‐organisms known to be resistant to the study drugs. Other exclusion criteria included hospitalisation or residence in a long‐term care facility for 14 or more days before the onset of symptoms; infection acquired in a hospital (48 hours after hospital admission and 7 days after hospital discharge); signs and symptoms of a bacterial infection of the central nervous system; history or presence of arthropathy or periarticular disease or any other musculoskeletal signs or symptoms that in the opinion of the investigator may have confounded a future safety evaluation of musculoskeletal complaints qd: once a day bd: twice a day |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned clearly in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label randomised controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label randomised controlled trial |
| Selective reporting (reporting bias) | Unclear risk | Information not clear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not provided clearly |
| Other bias | Unclear risk | Funded by Johnson & Johnson Pharmaceutical Research and Development; details of role of funding agency not mentioned |
Camargos 1997.
| Methods | RCT comparing benzathine penicillin and procaine penicillin | |
| Participants | Children 2 years to 12 years with non‐severe pneumonia | |
| Interventions | Single dose of benzathine penicillin (600,000 U for patients below 20 kg weight and 1,200,000 U for those above 20 kg), procaine penicillin 300,000 IU/kg/day IM for 7 days | |
| Outcomes | Cure rate, failure rate, lost to follow‐up | |
| Notes | Exclusion criteria: severe disease, atelectasis, post‐measles pneumonia, sickle cell cardiomyopathy, immunodeficiency, allergic to penicillin, hospitalisation in previous 2 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients assigned randomly |
| Allocation concealment (selection bias) | Low risk | Randomisation done by trained staff member blinded to control or treatment using 4 identifying letters randomly selected for benzathine (W and Z) and procaine (X Y) enclosed in sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation done by trained staff member blinded to control or treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not provided |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Campbell 1988.
| Methods | RCT comparing co‐trimoxazole for 5 days and procaine penicillin single dose with ampicillin for 5 days | |
| Participants | Children 1 month to 4 years of age with non‐severe pneumonia | |
| Interventions | Daily co‐trimoxazole PO for 5 days or single‐dose procaine penicillin with daily PO ampicillin | |
| Outcomes | Cure rate, hospitalisation rate and death rate | |
| Notes | Exclusion criteria: very severe disease, refusal of consent, unable to take tablets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation and randomisation not clear |
| Allocation concealment (selection bias) | High risk | Eligible children were allocated sequentially to 2 treatment groups by study physician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Unclear risk | Data not recorded clearly |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data not recorded clearly |
| Other bias | Unclear risk | Source of funding not mentioned |
CATCHUP 2002.
| Methods | RCT comparing amoxycillin and co‐trimoxazole in non‐severe pneumonia | |
| Participants | Children 2 to 59 months with non‐severe pneumonia | |
| Interventions | PO amoxycillin 25 mg/kg/day for 5 days or co‐trimoxazole 20/4 mg/kg/day for 5 days | |
| Outcomes | Cure rate, failure rate, change of antibiotics | |
| Notes | Blinded, exclusion criteria: severe pneumonia, very severe disease, chronic illness, past history of 2 or more episodes of wheeze, acute bronchial asthma, antibiotics in past 48 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generated using a computer program |
| Allocation concealment (selection bias) | Low risk | The drug assignment was concealed from patients parents and study personnel |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind randomised controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind randomised controlled trial |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by World Health Organization |
Cetinkaya 2004.
| Methods | RCT comparing chloramphenicol in combination with penicillin with ceftriaxone | |
| Participants | Children aged 6 months to 16 years with clinical or graphically confirmed pneumonia | |
| Interventions | IV chloramphenicol 15 mg/kg every 6 hours plus penicillin 25,000 IU/kg every 4 hours for 10 days and ceftriaxone 50 mg/kg every 12 hours | |
| Outcomes | Clinical recovery | |
| Notes | Blinded, children clinically diagnosed with bacterial pneumonia were enrolled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of random list/numbers not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Deivanayagam 1996.
| Methods | RCT comparing ampicillin in combination with penicillin with chloramphenicol for pneumonia diagnosed by clinical/radiological evidence | |
| Participants | Children 5 months to 4 years with pneumonia admitted to hospital | |
| Interventions | IM/IV ampicillin (100 mg/kg/day) for 48 hours than PO, IV penicillin (100,000 IU/kg/day) plus chloramphenicol (100 mg/kg/day) | |
| Outcomes | Cure rate, failure rate | |
| Notes | Not blinded. Exclusion criteria: acute bronchiolitis, allergy to penicillin, antibiotics in past 2 days, other drugs by treating physician receiving anti‐tuberculosis drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random listen |
| Allocation concealment (selection bias) | Unclear risk | Information not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Unclear risk | Data not completely described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No intention‐to‐treat analysis and details of excluded patients not clear |
| Other bias | Unclear risk | Source of funding not mentioned |
Duke 2002.
| Methods | RCT comparing chloramphenicol with combination of penicillin and gentamicin in children with severe pneumonia | |
| Participants | Children aged 1 to 59 months age, with severe pneumonia | |
| Interventions | IM chloramphenicol (25 mg/kg 6‐hourly for at least 5 days) versus penicillin (50 mg/kg 6‐hourly) and gentamycin (7.5 mg/kg/d single dose) for at least 5 days | |
| Outcomes | Adverse outcome (death, change in antibiotics, absconded, readmission within 30 days), rate of hospitalisation, duration of hospital stay | |
| Notes | Not blinded Exclusion criteria: wheezing, bronchiolitis, meningitis, tuberculosis, CHD, renal failure, jaundice, received study antibiotics for more than 48 hours in last 1 week | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by the World Health Organization and the Papua New Guinea Health Department |
Harris 1998.
| Methods | RCT comparing azithromycin, co‐amoxyclavulanic acid and erythromycin in pneumonia | |
| Participants | Children aged 6 months to 16 years with clinical or radiological evidence of pneumonia | |
| Interventions | PO azithromycin (10 mg/kg/day 1 followed by 5 mg/kg/day for 4 days) or amoxycillin clavulanic acid (40 mg/kg/day) for 10 days or erythromycin (40 mg/kg/day) for 10 days | |
| Outcomes | Cure rate (day 15 to 19), improvement rate, failure rate | |
| Notes | Exclusion criteria: known hypersensitivity, intolerance to drugs, pregnancy, lactation, need for parental antibiotics, severe pneumonia, antibiotics in past 72 hours, chronic steroid therapy, on carbamazepine, ergotamine, terfenadine, loratadine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not mentioned |
| Allocation concealment (selection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis not performed and no details of excluded patients |
| Other bias | Unclear risk | Funded by Pfizer Inc., New York |
Hazir 2008.
| Methods | Randomised, open‐label equivalency trial | |
| Participants | Children aged 3 to 59 months with WHO‐defined severe pneumonia | |
| Interventions | Oral amoxycillin syrup (80 to 90 mg/kg per day in 2 doses) and sent home (ambulatory group), or to receive intravenous ampicillin (100 mg/kg per day in 4 doses) for 48 hours as an inpatient (hospitalised group) | |
| Outcomes | Primary outcome (treatment failure up to or on day 6). Any of the following: clinical deterioration; inability to take oral medication due to persistent vomiting; development of a comorbid condition requiring an antibiotic; persistence of fever > 38 ºC with lower chest in‐drawing (LCI) from day 3 to day 6; either fever or lower chest in‐drawing alone at day 6; hospitalisation related to pneumonia; serious adverse event; left against medical advice or lost to follow‐up; voluntary withdrawal of consent; death Secondary outcome (treatment failure between day 6 and day 14; relapse). Any of the following: clinical deterioration; development of a comorbid condition requiring an antibiotic; development of lower chest in‐drawing or fast breathing non‐responsive to 3 trials in children with wheeze | |
| Notes | Exclusion criteria: known asthma, those with a history of 3 or more episodes of wheezing in 1 year, or those in whom lower chest in‐drawing resolved after 3 doses of a bronchodilator over 30 minutes were excluded. Children showing signs of WHO‐defined very severe pneumonia (panel 1) were also excluded; such individuals were admitted to hospital for treatment with intravenous antibiotics. Children who were known to have anaphylactic reactions to penicillin or amoxycillin, those with persistent vomiting, those who had been hospitalised within the previous 2 weeks, and those with other infectious diseases that needed antibiotic treatment, were also excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated by uneven block size |
| Allocation concealment (selection bias) | Low risk | The randomisation scheme was generated by a computer program in uneven blocks of 4, 6 and 8 by an individual not involved in study. Randomisation codes were sealed in opaque envelopes in accordance with the allocation sequence and stratified by site. After being deemed eligible for enrolment, participants were assigned the next envelope in the sequence to determine treatment assignment. The randomisation code was held at the co‐ordinating centre and was broken at the time of data analysis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by the World Health Organization and Family Applied Research Project, Boston University |
Jibril 1989.
| Methods | RCT comparing amoxycillin and co‐amoxyclavulanic acid with amoxycillin alone in bacterial pneumonia (non‐severe) | |
| Participants | Children aged 2 years to 12 years age, with non‐severe pneumonia | |
| Interventions | Amoxycillin and co‐amoxyclavulanic acid (250 mg + 62.5 mg or 500 + 125 mg tds) with amoxycillin (250 mg or 500 mg tds) for 10 days | |
| Outcomes | Poor or no response; cure rate | |
| Notes | Exclusion criteria: renal/hepatic impairment; hypersensitivity to penicillin/cephalosporin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Serial number selected on random basis |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded study |
| Selective reporting (reporting bias) | Unclear risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Keeley 1990.
| Methods | RCT comparing co‐trimoxazole and procaine penicillin | |
| Participants | Children aged 3 months to 12 years with non‐severe pneumonia | |
| Interventions | Co‐trimoxazole per oral for 5 days. Procaine penicillin IM daily for 5 days | |
| Outcomes | Cure rate, treatment failure, hospitalisation, well at final follow‐up and death rate | |
| Notes | Exclusion criteria: children with chest in‐drawing, unable to feed and requiring immediate referral | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided in the paper |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Selective reporting (reporting bias) | Unclear risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by University of Zimbabwe, Harare |
Klein 1995.
| Methods | RCT comparing cefpodoxime and co‐amoxyclavulanic acid in LRTI | |
| Participants | Children aged 3 months to 11.5 years | |
| Interventions | Cefpodoxime 5 to 12 mg/kg/day PO for 10 days or co‐amoxyclavulanic acid 6 to 13 mg/kg/day for 10 days | |
| Outcomes | Response rate | |
| Notes | Exclusion criteria: nosocomial infection, antibiotics in past 48 hours, allergy to beta‐lactam antibiotics, suspected/confirmed TB, congenital anomalies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided |
| Allocation concealment (selection bias) | Unclear risk | No mention about details of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information not provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not provided |
| Selective reporting (reporting bias) | Unclear risk | No intention‐to‐treat analysis. No details of children excluded from the analysis |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No intention‐to‐treat analysis. No details of children excluded from the analysis |
| Other bias | Unclear risk | Source of funding not mentioned |
Kogan 2003.
| Methods | RCT comparing azithromycin and amoxycillin | |
| Participants | Children aged 1 month to 14 years with non‐severe pneumonia | |
| Interventions | Azithromycin (10 mg/kg/day) PO for 3 days or amoxycillin PO 75 mg/kg/day for 7 days | |
| Outcomes | Clinical and radiological cure rates, fever on day 3 and day 7, chest X‐ray on day 14 | |
| Notes | Exclusion criteria: chronic pathology, preterm, received antibiotics in past 5 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information on sequence generation not mentioned |
| Allocation concealment (selection bias) | High risk | Allocated by investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Mulholland 1995.
| Methods | RCT comparing chloramphenicol and co‐trimoxazole in malnourished children with clinical or radiological pneumonia | |
| Participants | Children below 5 years of age with malnutrition and clinical or radiological evidence of pneumonia | |
| Interventions | Enrolled subjects received either chloramphenicol with TMP/SMX placebo or TMP/SMX with chloramphenicol placebo | |
| Outcomes | Cure rate, relapse rate, failure rate and exclusion, death rate | |
| Notes | Blinded
Exclusion criteria: already receiving antibiotics, clinical or radiological signs of TB, severe pneumonia TMP/SMX: trimethoprim/ sulphamethoxazole (co‐trimoxazole) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list generated in advance |
| Allocation concealment (selection bias) | Low risk | Double‐blind study. Randomisation codes were kept with senior nurse and pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Low risk | Funded by the World Health Organization |
Nogeova 1997.
| Methods | Randomised, controlled, multicentre trial on children between 1 to 12 years of age with radiologically documented pneumonia to compare efficacy of ceftibuten with cefuroxime axetil. Sputum and blood were tested for aetiological agents | |
| Participants | Children 1 to 12 years of age with radiographically confirmed pneumonia | |
| Interventions | Ceftibuten or cefuroxime axetil in 2 divided doses | |
| Outcomes | Cure or failure rates | |
| Notes | Duration of treatment not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation including sequence generation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. Details not included in the text |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. Details not included in the text |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | No mention about ethical clearance and source of funding |
Ribeiro 2011.
| Methods | Randomised controlled trial comparing oxacillin + ceftriaxone with co‐amoxyclavulanic acid for treatment of very severe pneumonia that was radiologically diagnosed | |
| Participants | Children between 2 months to 5 years of age, hospitalised with very severe pneumonia | |
| Interventions | Patients received either intravenous (IV) oxacillin 200 mg/kg/day every 6 hours for 10 days and ceftriaxone 100 mg/kg/day every 12 hours for 10 days or co‐amoxyclavulanic acid 100 mg/kg/day every 8 hours (amoxycillin base). Children receiving oxacillin + ceftriaxone continued to get IV antibiotics for 10 days while those receiving co‐amoxyclavulanic acid were switched over to oral medications after improvement at 48 hours | |
| Outcomes | Cure rate, failure rate, time for response in tachypnoea, total hospital stay | |
| Notes | Unblinded study. Source of funding not mentioned. 5 bacterial isolates from blood culture. In oxacillin + ceftriaxone group: Enterobacter and S. aureus (1 each); in co‐amoxyclavulanic acid group: coagulase‐negative staphylococci (2 patients), Pseudomonas aeruginosa (1 patient) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Kept in opaque envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians were blinded |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Roord 1996.
| Methods | RCT comparing azithromycin and erythromycin in non‐severe pneumonia (acute LRTI) | |
| Participants | Children aged 2 months to 16 years with non‐severe pneumonia (acute LRTI) | |
| Interventions | Azithromycin 10 mg/kg/day for 3 days or erythromycin 40 mg/kg/day for 10 days | |
| Outcomes | Cure rate, failure rate at day 10 to 14, improvement at day 10 and between days 25 to 30 | |
| Notes | Exclusion criteria: not able to take oral medications, known hypersensitivity to azithromycin or erythromycin, cystic fibrosis, immunodeficiency, need for oxygen, nosocomial pneumonia, leucocyte count less than 300,000 per litre, bacteraemia, receiving alternative treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided |
| Allocation concealment (selection bias) | Unclear risk | Open‐label randomised controlled trial. Block randomisation. No mention about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Funded by Pfizer ‐ BV |
Shann 1985.
| Methods | RCT comparing chloramphenicol and chloramphenicol in combination with penicillin in severe pneumonia | |
| Participants | Children | |
| Interventions | IM chloramphenicol daily until switched over to oral, or IM chloramphenicol with benzyl penicillin until switched over to oral | |
| Outcomes | Discharge from hospital and good improvement of symptoms | |
| Notes | Not blinded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table was prepared |
| Allocation concealment (selection bias) | Low risk | Sealed numbered envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Sidal 1994.
| Methods | RCT comparing co‐trimoxazole and penicillin in non‐severe pneumonia (including moderate pneumonia) | |
| Participants | Children aged 3 months to 14 years with non‐severe pneumonia (including moderate pneumonia) | |
| Interventions | PO co‐trimoxazole (40 mg/kg/day) for 10 days or IM procaine penicillin (50,000 IU/kg/day) for 10 days | |
| Outcomes | Cure rate at day 5 and day 10, evident improvement at day 5 and day 10, failure rate | |
| Notes | Exclusion criteria: severe chest in‐drawing, inability to eat or drink, moderate to severe malnutrition, antibiotics in last 2 weeks, wheezing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided |
| Allocation concealment (selection bias) | High risk | No details of randomisation or allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Straus 1998.
| Methods | RCT comparing co‐trimoxazole and amoxycillin in non‐severe pneumonia | |
| Participants | Children aged 2 months to 59 months with non‐severe pneumonia | |
| Interventions | PO co‐trimoxazole 20 mg/kg/day for 5 days or amoxycillin 45 mg/kg/day for 5 days | |
| Outcomes | Failure rate, determined by clinical and radiological evidence | |
| Notes | Blinded. Exclusion criteria: very severe pneumonia, antibiotics in past 48 hours, hospitalisation in past 7 days, hypoxaemia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Drug allotment was concealed from participants. Details not clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. Details not included |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. Details not included |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Tsarouhas 1998.
| Methods | RCT comparing procaine penicillin and amoxycillin for radiographically confirmed pneumonia | |
| Participants | Children aged 6 months to 18 years with pneumonia | |
| Interventions | PO amoxycillin (50 mg/kg/day) or procaine penicillin IM (50,000 IU/kg/day) | |
| Outcomes | Hospitalisation rate, failure rate, temperature more than 38.5 °C, ill appearance, increased respiratory rate | |
| Notes | Unblinded Exclusion criteria: chronic illness, asthma, sickle cell disease, cystic fibrosis, allergy to amoxycillin, or penicillin, antibiotics in past 1 week, wheezing, concurrent febrile illness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not mentioned |
| Allocation concealment (selection bias) | Low risk | Sealed envelope opened by Emergency Department nurse |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Source of funding not mentioned |
Wubbel 1999.
| Methods | RCT comparing azithromycin and erythromycin in children over 5 years of age with pneumonia; and comparing azithromycin with co‐amoxyclavulanic acid in children under 5 years of age | |
| Participants | Children aged between 6 months a 16 years with pneumonia | |
| Interventions | PO azithromycin (10 mg/kg on day 1 followed by 5 mg/kg/day for next 4 days) or co‐amoxyclavulanic acid 40 mg/kg/day for 10 days in children under 5 years of age; and erythromycin 40 mg/kg/day for 10 days in children over 5 years | |
| Outcomes | Clinically diagnosed cure rates, failure rates and improvement | |
| Notes | Non‐blinded. Exclusion criteria: hypersensitivity to study drugs, nosocomial pneumonia, hospitalisation, antibiotics in last 7 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded study |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Other bias | Unclear risk | Funded by Pfizer Inc. |
bd: twice a day CHD: congenital heart disease CPZ: carbamazepine IM: intramuscular IV: intravenous LRTI: lower respiratory tract infection PO: orally PTV: post‐therapy visit q6h: every 6 hours q8h: every 8 hours q12h: every 12 hours RCT: randomised controlled trial Sp02: oxygen saturation TB: tuberculosis tds: three times a day TOCV: test of cure visit WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agostoni 1988 | Compares minocycline and amoxycillin in 23 children between 3 to 11.5 years with pneumonia. Not a RCT |
| Al‐Eiden 1999 | Describes results of sequential antibiotic therapy (SAT) in 89 patients with severe lower respiratory tract infection. The sequential antibiotic use was the reason for exclusion |
| Ambroggio 2012 | A retrospective cohort study, analysed 20,743 patients hospitalised with community‐acquired pneumonia. Of these, 24% received beta‐lactam and macrolide combination therapy on admission. Compared outcome in form of hospital stay and relapse rates between children who received beta‐lactam monotherapy or children who received beta‐lactam plus macrolide combination therapy Excluded because it was not randomised controlled trial |
| Bari 2011 | A cluster‐randomised controlled trial on children below 5 years of age with severe pneumonia compared oral amoxicillin with standard treatment (referral and parenteral/oral antibiotics). The study compared 2 modalities of treatment (oral amoxicillin with standard treatment); did not compare 2 antibiotics |
| Bonvehi 2003 | Compared clarithromycin and co‐amoxyclavulanic acid in adult patients with CAP due to penicillin‐resistant and/or macrolide‐resistant S. pneumoniae. The study was excluded because of its adult study population |
| Esposito 2005 | Compared azithromycin in addition to symptomatic treatment with symptomatic treatment alone in children with recurrent respiratory tract infections. The study did not compare 2 or more antibiotics for pneumonia |
| Fogarty 2002 | Compared cefditoren with co‐amoxyclavulanic acid in the management of community‐acquired pneumonia in adult patients. The study had an adult population |
| Haffejee 1984 | A single‐blind therapeutic trial using cefotaxime or a benzyl‐penicillin‐gentamycin combination in 68 hospitalised paediatric patients with 72 episodes of severe infection (septicaemia, pneumonia, neonatal meningitis and others). No separate data were available for pneumonia |
| Hasali 2005 | A randomised comparative study of clarithromycin and erythromycin in the treatment of community‐acquired pneumonia in children. Outcome in form of cure or failure available only for children with mycoplasma or chlamydia pneumonia |
| Higuera 1996 | Compared oral cefuroxime axetil and oral co‐amoxyclavulanic acid in the treatment of community‐acquired pneumonia in adult patients. The study was in adult patients |
| Lee 2008 | A randomised controlled trial comparing ampicillin versus ampicillin + gentamycin in children with community‐acquired pneumonia. Outcome variables were total hospital stay and time taken for improvement in clinical symptoms. No clear data on cure or failure rates |
| Lu 2006 | Full paper could not be obtained |
| Mouallem 1976 | Compared cephradine and cephalexin for the treatment of bacterial infections in 162 children between 4 months and 11 years of age. There were no separate data for pneumonia |
| Paupe 1992 | Compares cefetamet (2 doses) with cefaclor. The doses of antibiotics were inconsistent |
| Peltola 2001 | Describes results of treatment with a short (4‐day) duration of antibiotics |
| Ruhrmann 1982 | Randomised controlled study. Compared erythromycin with amoxycillin in the treatment of 120 children with community‐acquired pneumonia. Measured outcomes were duration of clinical symptoms, aetiology of pneumonia and side effects of antibiotics. The study does not provide cure rates, failure rates, death rates or relapse rates |
| Sanchez 1998 | Randomised controlled trial involving 409 patients admitted to internal medicine department. Compared ceftriaxone, cefuroxime and amoxycillin‐clavulanic acid. Study does not provide separate data for children |
| Soofi 2012 | A cluster‐randomised controlled trial on children below 5 years of age with severe pneumonia compared oral amoxycillin with standard treatment (referral and parenteral/oral antibiotics). The study compared 2 modalities of treatment (oral amoxicillin with standard treatment); did not compare 2 antibiotics |
| van Zyl 2002 | Randomised controlled trial compared cefditoren with cefpodoxime in community‐acquired pneumonia in adult patients. The study had an adult study population |
| Vuori‐Holopaine 2000 | Compared procaine penicillin and cefuroxime in children between 3 months and 15 years of age with suspected sepsis. There were no separate data for pneumonia available |
CAP: community‐acquired pneumonia RCT: randomised controlled trial
Differences between protocol and review
In the protocol we decided to include studies with an outcome in the form of cure rates. However, there were a few studies that did not report cure rates. We therefore decided to include studies that gave either cure rates or treatment failure rates as one of the outcomes.
Contributions of authors
Dr Sushil K Kabra (SK) and Dr Rakesh Lodha (RL) jointly prepared and edited the review. Dr RM Pandey (RP) contributed to the sections on data extraction, data analysis, quality assessment and statistical methods, in addition to editing the review.
Sources of support
Internal sources
All India Institute of Medical Sciences, New Delhi, India.
External sources
No sources of support supplied
Declarations of interest
One of the authors (Kabra) was co‐author of one study (Awasthi 2008) included in the review. Other two authors do not have any competing conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Addo‐Yobo 2004 {published data only}
- Addo‐Yobo E, Chisaka N, Hassan M, Hibberd P, Lozano JM, Jeena P, et al. Oral amoxycillin versus injectable penicillin for severe pneumonia in children aged 3‐59 months: a randomized multicenter equivalency study. Lancet 2004;364:1141‐8. [DOI] [PubMed] [Google Scholar]
Asghar 2008 {published data only}
- Asghar R, Banajeh S, Egas J, Hibberd P, Iqbal I, Katep‐Bwalya M, et al. Chloramphenicol versus ampicillin plus gentamicin for community acquired very severe pneumonia among children aged 2‐59 months in low resource settings: multicentre randomised controlled trial (SPEAR study). BMJ 2008;336:80‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkinson 2007 {published data only}
- Atkinson M, Lakhanpaul M, Smyth A, Vyas H, Weston V, Sithole J, et al. Comparison of oral amoxicillin and intravenous benzylpenicillin for community acquired pneumonia in children (PIVOT trial): a multicentre pragmatic randomised controlled equivalence trial. Thorax 2007;62:1102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aurangzeb 2003 {published data only}
- Aurangzeb B, Hameed A. Comparative efficacy of amoxycillin, cefuroxime and clarithromycin in the treatment of community acquired pneumonia in children. Journal of College of Physicians and Surgeons ‐ Pakistan 2003;13:704‐7. [PubMed] [Google Scholar]
Awasthi 2008 {published data only}
- Awasthi S, Agarwal G, Singh JV, Kabra SK, Pillai RM, Singhi S, et al. Effectiveness of 3‐day amoxycillin vs. 5‐day co‐trimoxazole in the treatment of non‐severe pneumonia in children aged 2–59 months of age: a multi‐centric open labelled trial. Journal Tropical Pediatrics 2008;54:382‐9. [DOI] [PubMed] [Google Scholar]
Bansal 2006 {published data only}
- Bansal A, Singhi SC, Jayashree M. Penicillin and gentamicin therapy vs amoxicillin/clavulanate in severe hypoxemic pneumonia. Indian Journal of Pediatrics 2006;73:305‐9. [DOI] [PubMed] [Google Scholar]
Block 1995 {published data only}
- Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community‐acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatric Infectious Disease Journal 1995;14:471‐7. [DOI] [PubMed] [Google Scholar]
Bradley 2007 {published data only}
- Bradley JS, Arguedas A, Blumer JL, Sa´ez‐Llorens X, Melkote R, Noel GJ. Comparative study of levofloxacin in the treatment of children with community‐acquired pneumonia. Pediatric Infectious Disease Journal 2007;26:868–78. [DOI] [PubMed] [Google Scholar]
Camargos 1997 {published data only}
- Camargos PAM, Guimarlies MDC, Ferreira CS. Benzathine penicillin for unilateral lobar or segmental infiltrates presumptively caused by Streptococcus pneumoniae in children 2‐12 years old. Journal of Tropical Pediatrics 1997;43:353‐60. [DOI] [PubMed] [Google Scholar]
Campbell 1988 {published data only}
- Campbell H, Byass P, Forgie IM, O'Neill KP, Lloyd Evans N, Greenwood BM. Trial of cotrimoxazole versus procaine penicillin with ampicillin in the treatment of community acquired pneumonia in young Gambian children. Lancet 1988;2:1182‐4. [DOI] [PubMed] [Google Scholar]
CATCHUP 2002 {published data only}
- CATCHUP Study Group. Clinical efficacy of cotrimoxazole versus amoxicillin twice daily for treatment of pneumonia: a randomized controlled clinical trial in Pakistan. Archives of Diseases in Childhood 2002;86:113‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cetinkaya 2004 {published data only}
- Cetinkaya F, Gogremis A, Kutluk G. Comparison of two antibiotic regimens in the empirical treatment of severe childhood pneumonia. Indian Journal of Pediatrics 2004;71:969‐72. [DOI] [PubMed] [Google Scholar]
Deivanayagam 1996 {published data only}
- Deivanayagam N, Nedunchelian K, Ashok TP, Mala N, Sheela D, Rathnam SR. Effectiveness of ampicillin and combination of penicillin and chloramphenicol in the treatment of pneumonia: randomized controlled trial. Indian Pediatrics 1996;33:813‐6. [PubMed] [Google Scholar]
Duke 2002 {published data only}
- Duke T, Poka H, Dale F, Michael A, Mgone J, Wal T. Chloramphenicol versus benzylpenicillin and gentamycin for the treatment of severe pneumonia in children in Papua New Guinea. Lancet 2002;359:474‐80. [DOI] [PubMed] [Google Scholar]
Harris 1998 {published data only}
- Harris JS, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community acquired pneumonia in children. Pediatric Infectious Disease Journal 1998;17:865‐71. [DOI] [PubMed] [Google Scholar]
Hazir 2008 {published data only}
- Hazir T, Fox LM, Nisar YB, Fox MP, Ashraf YP, MacLeod WB, et al. Ambulatory short‐course high‐dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. Lancet 2008;371:49–56. [DOI] [PubMed] [Google Scholar]
Jibril 1989 {published data only}
- Jibril HB, Ifere OAS, Odumah DU. An open label comparative evaluation of amoxycillin and amoxycillin plus clavulanic acid (Augmentin) in the treatment of bacterial pneumonia in children. Current Medical Research and Opinion 1989;11(9):585‐92. [DOI] [PubMed] [Google Scholar]
Keeley 1990 {published data only}
- Keeley DJ, Nkrumah FK, Kapuyanyika C. Randomized trial of sulphamethoxazole + trimethoprim versus procaine penicillin for out patient treatment of childhood pneumonia in Zimbabwe. WHO Bulletin 1990;68:185‐92. [PMC free article] [PubMed] [Google Scholar]
Klein 1995 {published data only}
- Klein M, The International Study Group. Multicenter trial of cefpodoxime proxetil vs. amoxycillin‐clavulanate in acute lower respiratory tract infections in childhood. Pediatric Infectious Disease Journal 1995;14(Suppl):19‐22. [DOI] [PubMed] [Google Scholar]
Kogan 2003 {published data only}
- Kogan R, Martinez MA, Rubilar L, Paya E, Quevedo I, Puppo H, et al. Comparative randomized trial of azithromycin versus erythromycin and amoxycillin for treatment of community acquired pneumonia in children. Pediatric Pulmonology 2003;35:91‐8. [DOI] [PubMed] [Google Scholar]
Mulholland 1995 {published data only}
- Mulholland EK, Falade AG, Corrah PT, Omoshigho C, Giadom PNRB, Adegbola RA, et al. A randomized trial of chloramphenicol vs trimethoprim sulphamethoxazole for the treatment of malnourished children with community acquired pneumonia. Pediatric Infectious Diseases Journal 1995;14:959‐65. [DOI] [PubMed] [Google Scholar]
Nogeova 1997 {published data only}
- Nogeova A, Galova K, Krizan L, Sufliarska S, Cizmarova E, Raskova J, et al. Ceftibuten vs. cefuroxime‐axetil in initial therapy for community‐acquired bronchopneumonia: randomized multicentric study in 140 children. Infectious Diseases in Clinical Practice 1997;6:133‐4. [Google Scholar]
Ribeiro 2011 {published data only}
- Ribeiro CF, Ferrari GF, Fioretto JR. Antibiotic treatment schemes for very severe community‐acquired pneumonia in children: a randomized clinical study. Pan American Journal of Public Health 2011;6:444‐50. [PubMed] [Google Scholar]
Roord 1996 {published data only}
- Roord JJ, Wolf BH, Gossens MM, Kimpen JL. Prospective open randomized study comparing efficacies and safety of 3 day course of azithromycin and 10 day course of erythromycin in children with community acquired acute lower respiratory tract infections. Antimicrobial Agents and Chemotherapy 1996;40:2765‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shann 1985 {published data only}
- Shann F, Barker J, Poore P. Chloramphenicol alone versus chloramphenicol plus penicillin for severe pneumonia in children. Lancet 1985;2(8457):684‐6. [DOI] [PubMed] [Google Scholar]
Sidal 1994 {published data only}
- Sidal M, Oguz F, Unuvar A, Sarbat G, Neyzi O. Trial of cotrimoxazole versus procaine penicillin G and benzathine penicillin + procaine penicillin G in the treatment of childhood pneumonia. Journal of Tropical Pediatrics 1994;40:301‐4. [DOI] [PubMed] [Google Scholar]
Straus 1998 {published data only}
- Straus WL, Qazi SA, Kundi Z, Nomani NK, Schwartz B, The Pakistan Cotrimoxazole Study Group. Antimicrobial resistance and clinical effectiveness of cotrimoxazole versus amoxycillin for pneumonia among children in Pakistan: randomised controlled trial. Lancet 1998;352:270‐4. [DOI] [PubMed] [Google Scholar]
Tsarouhas 1998 {published data only}
- Tsarouhas N, Shaw KN, Hodinka RL, Bell LM. Effectiveness of intramuscular penicillin versus oral amoxicillin in the early treatment of outpatient pediatric pneumonia. Pediatric Emergency Care 1998;14:338‐41. [PubMed] [Google Scholar]
Wubbel 1999 {published data only}
- Wubbel L, Muniz L, Ahmed A, Trujillo M, Carubelli C, McCoig C, et al. Etiology and treatment of community‐acquired pneumonia in ambulatory children. Pediatric Infectious Disease Journal 1999;18:98‐104. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agostoni 1988 {published data only}
- Agostoni C, Giovannini M, Fraschini F, Scaglione F, Galluzzo C, Riva E, et al. Comparison of minocyclin versus amoxycillin in lower respiratory tract infections in children: clinical response and effect on natural killer activity. Journal of International Medical Research 1988;16:305‐11. [DOI] [PubMed] [Google Scholar]
Al‐Eiden 1999 {published data only}
- Al‐Eidan FA, McElnay JC, Scott MG, Kearney MP, Troughton KE, Jenkins J. Sequential antimicrobial therapy; treatment of severe lower respiratory tract infections in children. Journal of Antimicrobial Chemotherapy 1999;44:709‐15. [DOI] [PubMed] [Google Scholar]
Ambroggio 2012 {published data only}
- Ambroggio L, Taylor JA, Tabb LP, Newschaffer CJ, Evans AA, Shah SS. Comparative effectiveness of empiric β‐lactam monotherapy and β‐lactam‐macrolide combination therapy in children hospitalized with community‐acquired pneumonia. Journal of Pediatrics 2012;161:1097‐103. [DOI] [PubMed] [Google Scholar]
Bari 2011 {published data only}
- Bari A, Sadruddin S, Khan A, Khan IU, Khan A, Lehri IA, et al. Community case management of severe pneumonia with oral amoxicillin in children aged 2‐59 months in Haripur district, Pakistan: a cluster randomised trial. Lancet 2011;378:1796‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonvehi 2003 {published data only}
- Bonvehi P, Weber K, Busman T, Shortridge D, Notario G. Comparison of clarithromycin and amoxicillin/clavulanic acid for community‐acquired pneumonia in an era of drug‐resistant Streptococcus pneumoniae. Clinical Drug Investigation 2003;23:491‐501. [DOI] [PubMed] [Google Scholar]
Esposito 2005 {published data only}
- Esposito S, Bosis S, Faelli N, Begliatti E, Droghetti R, Tremolati E, et al. Role of atypical bacteria and azithromycin therapy for children with recurrent respiratory tract infections. Pediatric Infectious Disease Journal 2005;24:438‐44. [DOI] [PubMed] [Google Scholar]
Fogarty 2002 {published data only}
- Fogarty CM, Cyganowski M, Palo WA, Hom RC, Craig WA. A comparison of cefditoren pivoxil and amoxicillin/clavulanate in the treatment of community acquired pneumonia: a multicenter prospective randomized investigator‐blinded parallel group study. Clinical Therapeutics 2002;24:1854‐70. [DOI] [PubMed] [Google Scholar]
Haffejee 1984 {published data only}
- Haffejee IE. A therapeutic trial of cefotaxime versus penicillin‐gentamycin for severe infections in children. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):147‐52. [DOI] [PubMed] [Google Scholar]
Hasali 2005 {published data only}
- Hasali MA, Ibrahim MI, Sulaiman SA, Ahmad Z, Hasali JB. A clinical and economic study of community‐acquired pneumonia between single versus combination therapy. Pharmacy World and Science 2005;27:249‐53. [DOI] [PubMed] [Google Scholar]
Higuera 1996 {published data only}
- Higuera F, Hidalgo H, Feris J, Giguere G, Collins JJ. Comparison of oral cefuroxime axetil and oral amoxycillin/clavulanate in the treatment of community‐acquired pneumonia. Journal of Antimicrobial Chemotherapy 1996;37:555‐64. [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee PI, Wu MH, Huang LM, Chen JM, Lee CY. An open, randomized, comparative study of clarithromycin and erythromycin in the treatment of children with community‐acquired pneumonia. Journal of Microbiology, Immunology and Infection 2008;41(1):54‐61. [PubMed] [Google Scholar]
Lu 2006 {published data only}
- Lu Q, Chen HZ, Zhang LE, Qing M, Yang YJ. A prospective multi‐center randomized parallel study on efficacy and safety of cefaclor vs. amoxicillin‐clavulanate in children with acute bacterial infection of lower respiratory tract. Chinese Journal of Infection and Chemotherapy 2006;6:77‐81. [Google Scholar]
Mouallem 1976 {published data only}
- Mouallem R. Comparative efficacy and safety of cephradine and cephalexin in children. Journal of International Medical Research 1976;4:265‐71. [DOI] [PubMed] [Google Scholar]
Paupe 1992 {published data only}
- Paupe J, Sarbeji M, Scheinmann P, Delacourt C, Sorin M. Clinical trial of pediatric lower respiratory tract infection: results and comments with cefetamet pivoxil. Chemotherapy 1992;38:29‐32. [DOI] [PubMed] [Google Scholar]
Peltola 2001 {published data only}
- Peltola H, Vuori‐Holopainen E, Kallio MJ, SE‐TU Study Group. Successful shortening from seven to four days of parenteral beta‐lactam treatment for common childhood infections: a prospective and randomized study. International Journal of Infectious Diseases 2001;5:3‐8. [DOI] [PubMed] [Google Scholar]
Ruhrmann 1982 {published data only}
- Ruhrmann H, Blenk H. Erythromycin versus amoxycillin for the treatment of pneumonia in children. Infection 1982;10(Suppl 2):86‐91. [DOI] [PubMed] [Google Scholar]
Sanchez 1998 {published data only}
- Sanchez ME, Gomez J, Gomez Vargas J, Banos V, Ruiz Gomez J, Munoz L, et al. Prospective and comparative study between cefuroxime, ceftriaxone and amoxicillin‐clavulanic acid in the treatment of community‐acquired pneumonia [Estudio prospectivo y comparativo entre cefuroxima, ceftriaxona y amoxicilina ‐clavulanico en el tratamiento de la neumonia adqirida en en la comunidad]. Revista Espanola de Quimioterapia 1998;11(2):132‐8. [PubMed] [Google Scholar]
Soofi 2012 {published data only}
- Soofi S, Ahmed S, Fox MP, MacLeod WB, Thea DM, Qazi SA, et al. Effectiveness of community case management of severe pneumonia with oral amoxicillin in children aged 2‐59 months in Matiari district, rural Pakistan: a cluster‐randomised controlled trial. Lancet 2012;379:729‐37. [DOI] [PubMed] [Google Scholar]
van Zyl 2002 {published data only}
- Zyl L, Roux JG, LaFata JA, Volk RS, Palo WA, Flamm R, et al. Cefditoren pivoxil versus cefpodoxime proxetil for community‐acquired pneumonia: results of a multicenter, prospective, randomized, double‐blind study. Clinical Therapeutics 2002;24:1840‐53. [DOI] [PubMed] [Google Scholar]
Vuori‐Holopaine 2000 {published data only}
- Vuori‐Holopainen E, Peltola H, Kallio MJ, SE‐TU Study Group. Narrow versus broad spectrum parenteral antimicrobials against common infections of childhood: a prospective randomized comparison between penicillin and cefuroxime. European Journal of Pediatrics 2000;159:878‐84. [DOI] [PubMed] [Google Scholar]
Additional references
Agarwal 2004
- Agarwal G, Awasthi S, Kabra SK, Kaul A, Singhi S, Walter SD, et al. Three day versus five day treatment with amoxicillin for non‐severe pneumonia in young children: a multicentre randomised controlled trial. BMJ 2004;328:791‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berman 1990
- Berman S. Epidemiology of acute respiratory tract infection in children of developing countries. Reviews of Infectious Diseases 1990;13(Suppl 60):454‐67. [DOI] [PubMed] [Google Scholar]
Boluyt 2008
- Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatrics & Adolescent Medicine 2008;162(2):111‐6. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50:683‐91. [DOI] [PubMed] [Google Scholar]
Chaudhary 1998
- Chaudhary R, Nazima N, Dhawan B, Kabra SK. Prevalence of mycoplasma pneumonia and chlamydia pneumoniae in children with community acquired pneumonia. Indian Journal of Pediatrics 1998;65:717‐21. [DOI] [PubMed] [Google Scholar]
Chenoweth 2000
- Chenoweth CE, Saint S, Martinez F, Lynch JP 3rd, Fendrick AM. Antimicrobial resistance in Streptococcus pneumoniae: implications for patients with community‐acquired pneumonia. Mayo Clinic Proceedings 2000;75:1161‐8. [DOI] [PubMed] [Google Scholar]
Falade 2011
- Falade AG, Ayede AI. Epidemiology, aetiology and management of childhood acute community‐acquired pneumonia in developing countries‐‐a review. African Journal of Medicine and Medical Sciences 2011;40(4):293‐308. [PubMed] [Google Scholar]
Gendrel 1997
- Gendrel D, Raymond J, Moulin F, Iniguez JL, Ravilly S, Habib F, et al. Etiology and response to antibiotic therapy of community‐acquired pneumonia in French children. European Journal of Clinical Microbiology and Infectious Diseases 1997;16:388‐91. [DOI] [PubMed] [Google Scholar]
Hazir 2006
- Hazir T, Nisar YB, Qazi SA, Khan SF, Raza M, Zameer S, et al. Chest radiography in children aged 2‐59 months diagnosed with non‐severe pneumonia as defined by World Health Organization: descriptive multicentre study in Pakistan. BMJ 2006;33:629‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
IBIS 1999
- Invasive Bacterial infection Surveillance (IBIS) Group and INCLEN. Prospective multicentre hospital surveillance of Streptococcus pneumoniae disease in India. Invasive Bacterial Infection Surveillance (IBIS) Group, International Clinical Epidemiology Network (INCLEN). Lancet 1999;353:1216‐21. [PubMed] [Google Scholar]
Inoue 2006
- Inoue M, Kaneko K, Akizawa K, Fujita S, Kaku M, Igari J, et. Antimicrobial susceptibility of respiratory tract pathogens in Japan during PROTEKT years 1‐3 (1999‐2002). Journal of Infection and Chemotherapy 2006;12:9‐21. [DOI] [PubMed] [Google Scholar]
Ishiwada 1993
- Ishiwada N, Kurosaki T, Toba T, Niimi H. Etiology of pediatric in patients with pneumonia. Kansenshogaku Zasshi 1993;67:642‐7. [DOI] [PubMed] [Google Scholar]
Kabra 2003
- Kabra SK, Lodha R, Broor S, Chaudhary R, Ghosh M, Maitreyi RS. Etiology of acute lower respiratory tract infection. Indian Journal of Pediatrics 2003;70:33‐6. [DOI] [PubMed] [Google Scholar]
Krishnan 2011
- Krishnan P, Rajendran P, Sambandan AP, Anitha C, Chavda RK, Khobragade KJ. Evaluation of coamoxiclav and other antibiotics against S pneumoniae and H influenzae from paediatric cases of acute respiratory infections. Journal of the Indian Medical Association 2011;109:241‐2. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Chichester: Wiley‐Blackwell, 2011. [Google Scholar]
Liu 2012
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379(9832):2151‐61. [DOI] [PubMed] [Google Scholar]
MacCracken 2000
- MacCracken Jr GH. Diagnosis and management of pneumonia in children. Pediatric Infectious Disease Journal 2000;19:924‐8. [DOI] [PubMed] [Google Scholar]
Maitreyi 2000
- Maitreyi RS, Broor S, Kabra SK, Ghosh M, Seth P, Dar L, et al. Rapid detection of respiratory viruses by centrifugation enhanced cultures from children with acute lower respiratory tract infections. Journal of Clinical Virology 2000;16:41‐7. [DOI] [PubMed] [Google Scholar]
MASCOT Group 2002
- MASCOT Group. Clinical efficacy of three day versus five days of oral amoxicillin for the treatment of childhood pneumonia: a multicenter randomised blind trial. Lancet 2002;360:835‐41. [DOI] [PubMed] [Google Scholar]
Normann 1998
- Normann E, Gnarpe J, Gnarpe H, Wettergren B. Chlamydia pneumoniae in children with acute respiratory tract infection. Acta Paediatrica Scandinavica 1998;87:23‐7. [DOI] [PubMed] [Google Scholar]
Numazaki 2004
- Numazaki K, Chiba S, Umetsu M, Tanaka T, Yoshimura H, Kuniya Y, et al. Etiological agents of lower respiratory tract infections in Japanese children. In Vivo 2004;18:67‐71. [PubMed] [Google Scholar]
Pandey 2005
- Pandey A, Chaudhry R, Kapoor L, Kabra SK. Acute lower respiratory tract infection due to Chlamydia species in children under five years of age. Indian Journal of Chest Diseases & Allied Sciences 2005;47:97‐101. [PubMed] [Google Scholar]
Rasmussen 1997
- Rasmussen Z, Bari Z, Qazi SA. Standard versus double strength co‐trimoxazole for the treatment of childhood pneumonia: a double blind, randomized multicentric trial in Pakistan. International Journal of Tuberculosis and Lung Diseases 1997;1:119. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rojas‐Reyes 2006
- Rojas‐Reyes MX, Granados Rugeles C. Oral antibiotics versus parenteral antibiotics for severe pneumonia in children. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004979.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudan 2008
- Rudan I, Boschi‐Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization 2008;86:408‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sazawal 2003
- Sazawal S, Black RE. Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta‐analysis of community‐based trials. Lancet 2003;3:547‐56. [DOI] [PubMed] [Google Scholar]
Shann 1995
- Shann F. The management of pneumonia in children in developing countries. Clinical Infectious Diseases 1995;21(Suppl):218‐25. [DOI] [PubMed] [Google Scholar]
Timothy 1993
- Timothy D, Mastro MD, Nomani N, Ishaq Z, Ghafoor A, Shaukat NF, et al. Use of nasopharyngeal isolates of Streptococcus pneumoniae and Haemophilus influenzae from children in Pakistan for surveillance for antimicrobial resistance. Pediatric Infectious Diseases Journal 1993;12:824‐30. [DOI] [PubMed] [Google Scholar]
WHO 1991
- World Health Organization. Technical basis of WHO recommendation on the management of pneumonia in children at first level health facility. WHO/ARI/91.20, Geneva, World Health Organization 1991.
WHO 1999
- World Health Organization. Making a difference. World Health Report 1999, Geneva 1999.
WHOYISG 1999
- The WHO Young Infant Study Group. Bacterial etiology of serious infections in young infants in developing countries: results of multicentre study. Pediatric Infectious Diseases Journal 1999;18(Suppl):17‐22. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kabra 2006
- Kabra SK, Lodha R, Pandey RM. Antibiotics for community acquired pneumonia in children. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004874.pub2] [DOI] [PubMed] [Google Scholar]
Kabra 2010
- Kabra SK, Lodha R, Pandey RM. Antibiotics for community‐acquired pneumonia in children. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004874.pub3] [DOI] [PubMed] [Google Scholar]


