Abstract
Background
Several types of medications have been used for stabilizing heroin users: Methadone, Buprenorphine and levo‐alpha‐acetyl‐methadol (LAAM.) The present review focuses on the prescription of heroin to heroin‐dependent individuals.
Objectives
To compare heroin maintenance to methadone or other substitution treatments for opioid dependence regarding: efficacy and acceptability, retaining patients in treatment, reducing the use of illicit substances, and improving health and social functioning.
Search methods
A review of the Cochrane Central Register of Trials (The Cochrane Library Issue 1, 2005), MEDLINE (1966 to november 2009), EMBASE (1980 to 2005) and CINAHL until 2005 (on OVID) was conducted. Personal communications with researchers in the field of heroin prescription identified ongoing trials.
Selection criteria
Randomised controlled trials of heroin maintenance treatment (alone or combined with methadone) compared with any other pharmacological treatment for heroin‐dependent individuals.
Data collection and analysis
Two reviewers independently assessed trial quality and extracted data.
Main results
Eight studies involving 2007 patients met the inclusion criteria. Five studies compared supervised injected heroin plus flexible dosages of methadone treatment to oral methadone only and showed that heroin helps patients to remain in treatment (valid data from 4 studies, N=1388 Risk Ratio 1.44 (95%CI 1.19‐1.75) heterogeneity P=0.03), and to reduce use of illicit drugs. Maintenance with supervised injected heroin has a not statistically significant protective effect on mortality (4 studies, N=1477 Risk Ratio 0.65 (95% CI 0.25‐1.69) heterogeneity P=0.89), but it exposes at a greater risk of adverse events related to study medication (3 studies N=373 Risk Ratio 13.50 (95% CI 2.55‐71.53) heterogeneity P=0.52). Results on criminal activity and incarceration were not possible to be pooled but where the outcome were measured results of single studies do provide evidence that heroin provision can reduce criminal activity and incarceration/imprisonment. Social functioning improved in all the intervention groups with heroin groups having slightly better results. If all the studies comparing heroin provision in any conditions vs any other treatment are pooled the direction of effect remain in favour of heroin.
Authors' conclusions
The available evidence suggests an added value of heroin prescribed alongside flexible doses of methadone for long‐term, treatment refractory, opioid users, to reach a decrease in the use of illicit substances, involvement in criminal activity and incarceration, a possible reduction in mortaliity; and an increase in retention in treatment. Due to the higher rate of serious adverse events, heroin prescription should remain a treatment for people who are currently or have in the past failed maintenance treatment, and it should be provided in clinical settings where proper follow‐up is ensured.
Plain language summary
Pharmaceutical heroin for heroin maintenance in chronic heroin dependents
Drug dependent heroin users are preoccupied with the desire to obtain and take heroin and so have persistent drug‐seeking behaviours. Those with a long history of treatment attempts and failures may benefit from the provision of heroin and flexible doses of methadone in a maintenance program. When accepted, this treatment may help them to remain in treatment, limit the use of street drugs, reduce illegal activities and possibly reduce mortality. The authors of the review identified eight randomised studies involving 2007 adult patients with a history of previous treatment failures in outpatient settings. The heroin users on the programs were requested to attend the clinic to receive and inject prescribed heroin from two to three times a day. Adverse events were consistently more frequent in the heroin groups. The trialists recommend that the treatment should be properly established so that necessary intensive care can be provided in an emergency. According with the current evidence, heroin prescription should be indicated to people who is currently or have previously failed maintenance treatment, and it should be provided in clinical settings where proper follow‐up is ensured.
Background
Description of the condition
Substance dependence continues to be a major clinical and social problem affecting millions of people worldwide and causing substantial costs to society. Drug dependence has been described by the World Health Organization as "a cluster of physiological, behavioural and cognitive phenomena of variable intensity, in which the use of a psychoactive drug (or drugs) takes on a high priority. The necessary descriptive characteristics are preoccupation with a desire to obtain and take the drug and persistent drug‐seeking behaviour. Determinants and the problematic consequences of drug dependence may be biological, physiological or social, and usually interact." (WHO 1993; WHO 2009).
Heroin is a opioid. Opioids include natural opioids (e.g. morphine), semisynthetics (e.g. heroin), and synthetics with morphine‐like action (e.g. codeine, hydromorphone, methadone, oxycodone, meperidine, fentanyl) (DSM‐IV 1994). Opioids are prescribed as analgesics, anaesthetics, antidiarrhoeal agents, or cough suppressants (Katzung 1999). Heroin is one of the most commonly abused drugs of this class and may be smoked, snorted or injected (EMCDDA 2010 a).
Heroin is abused in many countries. The UNODC estimates the total number of opiates users at the global level between 15.2‐21.1 million people (UNODC 2007). More than half of the world’s opiates using population are thought to live in Asia. The highest levels of use (in terms of the proportion of the population aged 15‐64 years) are found along the main drug trafficking routes out of Afghanistan.
Trends in use appear to indicate a stabilisation of the overall number of heroin users in Europe, but recent data on drug induced deaths are mostly associated with opioid use (EMCDDA 2009). The largest heroin using population in the Americas is found in the USA where approximately 1.2 million heroin users (0.6% of the population aged 15‐64) have been estimated (UNODC 2010) stable since 2002.
The pattern of use described in the USA and in Europe seems to indicate a preference for intravenous use by the elder population of heroin users. Heroin injectors are becoming a largely ageing population with serious health, social and psychiatric problems (EMCDDA 2008; EMCDDA 2010 b). In The Netherlands and in Andalucia, Spain, a prevalence of heroin inhaling users by "chasing the dragon" was reported (van den Brink 1999). This practice foresees that the heroin is heated on tinfoil and the vapours are inhaled (Weil 1998).
Dependent heroin users are characterised by the persistence of use in spite of the difficulties they experience with health, law, social achievements and personal relationships (Ward 1999). Those who seek treatment may have been using heroin for decades (Goldstein 1995; Hser 1993, van den Brink 1999; Ward 1999), experiencing a number of criminal offences, heroin overdoses, and attempts and failures of detoxification.
Description of the intervention
It is well recognised that heroin dependence should be treated as a chronic condition. The course and response to medications and the potential heritability suggest that people who are drug dependent would benefit from patterns of treatment similar to those provided to chronic patients, with continuing care and monitoring over time (McLellan 2000; O'Brien 1997).
Along with prevention, treatment is essential for reducing problems related to heroin dependence. The ultimate goal of interventions is the full reintegration into society of people affected by dependence (Bammer 1999), regardless of the intermediate achievements which may differ substantially (Davoli 2000). The types of available interventions can be divided into the following main categories:
emergency (for overdoses);
detoxification (to reach a drug free condition in a short period);
maintenance (to reduces illicit drug use, criminal offences and to improve health and social behaviour) (Farrell 1998; Ward 1999);
rehabilitation (to achieve the reintegration into the social community).
The present review will consider maintenance treatment, in which the patients enter programs of heroin administration to achieve stabilisation.
How the intervention might work
The first experiences with the prescription of heroin were in the United Kingdom (Metrebian 1998). In 1926, the Rolleston Committee (Strang 1994) supported the role of physicians in the prescription of opiates in the management of chronic opioid dependence (Rolleston 1926). However, in the 1960s, the population of heroin users rapidly increased, and the black market for pharmaceutical heroin grew rapidly and some restrictions were introduced (Hartnoll 1980).
After the clinical trials conducted in Switzerland, the United Kingdom and the Netherlands, a debate arose (Farrell 1994; Venning 1998; Wodak 1998) about the opportunity to introduce the heroin treatment for heroin users.
Some researchers have argued that more resources should be devoted to the consolidation of treatment for which more reliable evidence is already available, such as methadone (Farrell 1998). The general debate has focused on some crucial points: do not offer heroin prescription instead of or at the expense of methadone maintenance, do not prescribe heroin to young users or people with a short history of heroin dependence, do not prescribe heroin as a first choice treatment and minimise the possibility that prescribed heroin will leak into illicit market (Hartnoll 1999).
By the time this review was first published (2003), authorization for heroin trials were obtained in Spain, Germany and Canada while Denmark and Luxembourg were considering the implementation of similar trials. The update of the present review integrate the results with the German trial, the British, the Spanish and the Canadian trials.
The most recent studies were aimed at assessing the provision of supervised self administration of heroin as a treatment for those patients who are severely dependent on heroin and did not show improvement with other treatment options.
Objectives
To compare heroin maintenance to methadone or other substitution treatments for opioid dependence regarding: efficacy and acceptability, retaining patients in treatment, reducing the use of illicit substances, and improving health and social functioning.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were included. As blinding of patients might be difficult to achieve in this field, we also considered open lable controlled clinical trials for inclusion (Bammer 1999; Schellings 1999).
Types of participants
Adults (18 years of age or older) who were diagnosed by any set of criteria as chronically dependent on heroin. We consider "chronic use" to be a pattern of use which was sufficient to convince the responsible clinicians to register the patient in a maintenance program.
Types of interventions
Experimental treatments:
Maintenance treatment with pharmaceutical heroin alone or in combination with methadone irrespective of dosages, preparation, route of administration, setting or duration of treatment.
Control treatments: No intervention Methadone maintenance Waiting list for conventional treatments Any other treatments which are compared against heroin
Types of outcome measures
Primary outcomes
Retention in treatment (number and proportion of patients in treatment at the end of the study for each arm out of the total number of patients allocated to each arm)
Relapse to street heroin use (number and proportion of people who self reported use of heroin during the study for each arm)
Use of other substances (number and proportion of people who self reported use of other substances during the study for each arm)
Death (number and proportion of people died during the study for each arm)
Medical adverse events (number and proportion of people who self‐reported medical adverse events during the study)
Secondary outcomes
Criminal offence (any kind of information about study participants' criminal activities during the study)
Incarceration/imprisonment (any kind of information about study participants' incarceration during the study)
Social functioning (integration at work, family relationship) (any kind of information available about the outcomes in the study)
Search methods for identification of studies
Electronic searches
This is an update of our previous review (Ferri 2005). For the previous review, we searched The Cochrane Central Register of Controlled Trials (CENTRAL) Issue 1, 2005; MEDLINE (1966 to 2005), EMBASE (1980 to 2005), CINAHL (until 2005 on OVID) to identify studies, seeAppendix 1; Appendix 2; Appendix 3; Appendix 4. For this update, we did an additional search of MEDLINE 2005‐2009. In addition, personal communication with researchers in the field of heroin prescription and a review of conference abstracts identified other ongoing trials. There were no language or publication year restrictions.
Searching other resources
In addition, we searched the National Institute for Drug Addiction (USA) web site; the European Monitoring Centre for Drugs and Drug Addiction web site, and the following trials registers: National Research Register, meta‐Register of Controlled Trials, Clinical Trials and Trials Central. We are also in contact with the principal investigators of the ongoing trials (see ongoing trials list and notes) for information about preliminary results.
Data collection and analysis
Selection of studies
One reviewer inspected the search hits by reading the titles and the abstracts. Doubts about inclusion criteria were resolved by discussion. We obtained each potentially relevant article located in the search in full article and two reviewers independently assessed for inclusion
Data extraction and management
Two reviewers independently extracted data.
Assessment of risk of bias in included studies
The risk of bias assessment for RCTs and CCTs in this review was performed using the 5 criteria recommended by the Cochrane Handbbok (Higgins 2008). The recommended approach for assessing risk of bias in studies included in Cochrane Review is a two‐part tool, addressing five specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting). The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement, in terms of "low ", "high" or unclear, relating to the risk of bias for that entry. To make these judgments we will use the criteria indicated by the handbook adapted to the addiction field. SeeAppendix 5 for details.
The domains of sequence generation and allocation concealment (avoidance of selection bias) was addressed in the tool by a single entry for each study.
Blinding of participants, personnel and outcome assessor (avoidance of performance bias and detection bias) was considered separately for objective outcomes (drop out, use of substance of abuse measured by urine‐analysis, subjects relapsed at the end of follow up, subjects engaged in further treatments) and subjective outcomes (duration and severity of signs and symptoms of withdrawal, including patient self‐rating, side effects, social functioning as integration at school or at work, family relationship).
Incomplete outcome data (avoidance of attrition bias) was considered for all outcomes except for the drop out from the treatment, which is very often the primary outcome measure in trials on addiction. It have been assessed separately for results at the end of the study period and for results at follow up.
Two review authors independently assessed the internal validity of the included studies. Any disagreement between the review authors was resolved by discussion, including input from a third independent reviewer if required. Risk of bias assessment was not blinded to trial authors, institutions or journals.
Measures of treatment effect
We calculated the risk ratios (RR) with Review Manager software package(Review Manager (RevMan)) and these are described in Results section. We used 95% confidence intervals (CI). In some cases, where absolute numbers were not provided, we calculated them from the valid percentages published in the studies.
Unit of analysis issues
Three of the included studies were multiple arms studies: 1. CCBH (B) had a third arm in which patients were provided methadone only for the first six months and then they were switched to methadone plus inhaled heroin, and this arm was excluded by the meta‐analysis after an assessment of its contribution to the overall available information. 2. Haasen 2007 is a factorial randomised controlled trial in which the patiens in the heroin + methadone group and in the methadone only group were then provided either with education or with case management. We enclosed in the meta‐analysis the comparison between the two main arms: heroin plus methadone and methadone only . 3. RIOTT 2010 compared heroin+ methadone with oral methadone and injected methadone with oral methadone.We used the first comparison arms (supervised injectable heroin plus methadone vs oral methadone) only.
Assessment of heterogeneity
The presence of heterogeneity between the trials was tested using the I‐squared (I2) statistic. A P‐value of the chi‐square test less than 0.05 indicates a significant heterogeneity.
Assessment of reporting biases
We identified a network of researchers in the field and contacted them for information on published, unpublished and ongoing studies. Considering the few available studies we decided that funnel plot for assessment of publication bias, was not appropriate
Data synthesis
We performed a meta‐analysis of the studies results adopting the Random Effect method based on the inverse‐variance approach, to take the heterogeneity of the enclosed studies into consideration in the analysis.
Results
Description of studies
Results of the search
The search strategies resulted in 3346 records which were screened by reading the titles and abstracts. Overall twenty two studies were considered eligible (Battersby 1992; CCBH (B) 2002; CCBH (A) 2002; Fischer 1999; Ghodse 1990; Haemmig 2001; Hartnoll 1980; Hendriks 2001; Jasinski 1986; Krausz 1999; McCusker 1996; Mello NK 1980; Metrebian 1998; Mitchell 2002; Moldovanyi 1996; Oppenheimer 1982; Perneger 1998; Rehm 2001; Uchtenhagen 1999; RIOTT 2010; Haasen 2007.).
Included studies
Eight of these met the inclusion criteria ( RIOTT 2010, NAOMI 2009, Haasen 2007; PEPSA 2006CCBH (A) 2002 , CCBH (B) 2002, Perneger 1998; Hartnoll 1980). These 8 studies involved a total of 2007 patients, 1032 of which were randomised in the German study (Haasen 2007). Haasen 2007, NAOMI 2009, RIOTT 2010 and CCBH (A) 2002 and CCBH (B) 2002 were multicenter studies. seeCharacteristics of included studies.
Treatment regimes
Seven studies (RIOTT 2010, NAOMI 2009, Haasen 2007, PEPSA 2006, CCBH (A) 2002, CCBH (B) 2002, Hartnoll 1980) compared heroin (plus flexible dosages of methadone) vs methadone and one (Perneger 1998) compared injectable heroin to a waiting list (control patients were encouraged to select any drug treatment program available in Geneva and were enrolled immediately whenever possible). One of the studies (CCBH (B) 2002) compared inhaled heroin (plus methadone) to methadone, and another study (RIOTT 2010) compared also injectable methadone to oral methadone.
Participants in all the 8 studies were also offered some type of psychosocial support. One study, Hartnoll 1980 provided weekly or two‐weekly voluntary appointments with a psychiatrist, Perneger 1998 the participants were also offered psychological counselling, HIV prevention counselling, social and legal support services and somatic primary care. In Haasen 2007 patients received education (Farnbacher 2002) or case management (Oliva 2001) and in CCBH studies patients in both the comparator groups had the same psychosocial treatment as foreseen: "in a regular methadone program"; in RIOTT the supervised treatment was combined with psychosocial support (RIOTT 2010). See Table 3 for further information on heroin and methadone dosages across groups.
1. Heroin and methadone dosages across groups.
| Study | N Participants | Intervention Heroin mean dosage/day | Intervention Methadone mean dosage/day | Control Methadone mean dosage/day |
| Haasen 2007 | 1015 | 442 mg | 39 mg | 99 mg |
| CCBHA 2002 | 174 | mean heroin dosage 254 mg
per visit (sd=62.5 mg) and 549 mg per day (sd=193 mg). maximum daily dose 1000 mg, and the maximum single dosage 400 mg |
Decided with the help of the treating physician with a minimum daily dose of 30‐50 mg and a maximum of 150 mg. | |
| CCBHB 2002 | 256 | '' | '' | |
| Perneger 1998 | 51 | 509 mg | unspecified | unspecified |
| Hartnoll 1980 | 96 | 30‐120 mg | unspecified | 10‐120 mg |
| RIOTT 2010 | 127 | Injected diamorphine doses in the range of 300 to 600 mg per day, with an upper total daily dose of 900 mg (450 mg per injection) | Injected methadone doses calculated with the formula: injected methadone dose=0·8×oral dose; dose reassessed continually, Maximum dose of injectable methadone: up to 200 mg/day |
Once daily doses of ≥80 mg actively encouraged; optimum doses individually titrated |
| NAOMI 2009 | 226 (+26 INJECTED HYDROMORPHONE) | 392.3 mg | (patients receiving diacetylmorphine plus methadone) mean daily dose of diacetylmorphine was 365.5 mg and the mean daily dose of methadone was 34.0 mg | 96.0 mg. |
| PEPSA 2003 | 62 | DAM dosage was 274.5 mg/day (range: 15–600 mg), | methadone dosage was 42.6 mg/day (range: 18–124 mg). | The daily methadone dosage in the control group was 105 mg/day (range:40–180 mg) |
Setting
In all the enclosed studies treatment was provided in outpatients setting. In seven studies the provision of heroin was supervised and patients were observed before injecting and up to 30 minutes after self injection.
Countries where the studies were conducted
The oldest and the most recent study were conducted in the United Kingdom (respectively in 1972‐75 and in 2004‐8), one study was conducted in Switzerland 1995‐96, two in the Netherlands 1998‐2001 and one in Germany in 2002‐2004. One study was conducted in Canada in 2005‐2008 and one in Spain 2003‐2004.
Duration of the trials
The period of participants inclusion in the trials ranged from 6 to 24 months.
The Swiss study (Perneger 1998) and the recent British study (RIOTT 2010) provided treatment for participants for 6 months.The older British (Hartnoll 1980) , the Dutch (CCBH (A) 2002, CCBH (B) 2002), the Spanish (PEPSA 2006) and the Canadian (NAOMI 2009) studies provided treatment for participants for 12 months, the German study (Haasen 2007) for 12 months in the first phase followed by another 12 months. The first phase of 12 months was a stratified into 4 x 2 randomised control groups comparing heroin (plus availability of methadone at night) treatment to methadone only treatment in similar settings.The subsequent phase 2 consisted of a follow‐up study to monitor long term effects of the treatment and integration into drug addiction services. All patients in the experimental group were provided treatment in phase 2 of the study and only a randomly selected group of the control patients, were offered the vacant heroin treatment places after 12 months of treatment. According to the scope of the present review, only the first 12 month phase results will be considered in the result section.
Participants
To be enrolled in the studies participants needed to be resident in the area of the treatment centres for at least two years (but in some studies duration was not specified), they had to be daily heroin users (for the past 2 to 6 years). Age for enrolment was minimum 18 years (1 study) up to 25 years or older (1 study). In all the studies participants qualified for inclusion if they had a history of previous treatment failures. The Dutch study also included patients who had been prescribed "effective dose methadone" for at least four consecutive weeks in the past five years and had been in regular contact with a methadone maintenance program in the preceding six months.The German study also included patients who have not been in treatment in the previous 6 months. In the RIOTT study the patients, were enrolled in the study if despite receiving conventional oral maintenance treatment (at least 6 months), continued to inject illicit heroin regularly (50% days in preceding 3 months). Exclusion criteria, where specified, were having severe psychiatric disorders, having a pending jail sentence, those who had been abstinent for 2 or more months in the past 12 months and those with a severe physical disorder such as renal or hepatic failure, clinically significant cardiac arrhythmias, chronic obstructive pulmonary problems, or being pregnant or breast‐feeding women.
Comparisons
Six studies compared supervised injected heroin plus flexible doses of methadone, with oral methadone (CCBH (A) 2002; Haasen 2007; NAOMI 2009; PEPSA 2006; Perneger 1998; RIOTT 2010 one arm); one study compared supervised inhalable heroin to oral methadone (CCBH (B) 2002), one study compared supervised injectable heroin to waiting list for methadone and or current treatment (Perneger 1998) and RIOTT 2010 compared also supervised injectable methadone to oral methadone. One study compared heroin maintenance to methadone maintenance (Hartnoll 1980).
Primary Outcomes
CCBHA/B, Haasen 2007, and PEPSA 2006 considered a multi domain primary outcome composed of health, mental and social dimensions, NAOMI 2009 considered retention in treatment at 12 months and reduction in illicit drug use or other illegal activities and RIOTT 2010 considered as a primary outcome, the reduction of regular use of street heroin.
Perneger 1998 considered as primary outcomes self reported drug use, health status and social functioning, and Hartnoll 1980 considered as primary outcomes total opiate consumption (prescribed and illicit), frequency of injection, and involvement with drug sub‐culture.
Table 4 includes a more detailed description of the primary outcomes considered at study level.
2. Primary outcomes of the enclosed studies.
| Study | Definition | Outcome measures |
| Hartnoll 1980 | Total opiate consumption, frequency of injection, involvment with drug subculture. | Interviews (questionnaires not specified), direct observations |
| Perneger 1998 | Self reported drug use, health status , and social functioning | Unpublished questionnaire based on ASI and SF36. |
| CCBHA/B 2002 | Prespecified dichotomous, multidomain outcome index including physical, mental, social dimensions and also completion of treatment and sustained response. | ASI / MAP‐HSS, Case Report Forms (CRF), Composite International Diagnostic Interview (CIDI),SCL‐90, urinalysis |
| PEPSA 2003 | Dichotomous multidimension outcome (MDO) including general health, quality of life, drug‐addiction‐related problems, nonmedical use of heroin, risk behavior for HIV and HCV, and psychological, family, and social status | The ASI, Opiate Treatment Index, Symptom Checklist‐90, and the 12‐item shortform (SF‐12). |
| Haasen 2007 | Two prespecified dichotomous, multidomain primary outcome measures about health and reduction in illicit drug use, were considered. | EuropASI OTI Health Scale (physical health) GSI (mental health) |
| NAOMI 2009 | Retention in addiction treatment at 12 months (defined as receipt of the study medication on at least 10 of the 14 days before the 12‐month assessment, or confirmation of retention in any other treatment program or abstinence from opioids during this interval). Reduction in illicit drug‐use or other illegal activities | Retention in treatment: Data on daily prescription‐drug use and, when possible, with the use of administrative data and pharmacy and physician records Illicit drug use or other illegal activities: Composite scores on the European Addiction Severity Index17 (see the Supplementary Appendix, available with the full text of this article at NEJM.org), |
| RIOTT 2010 | Reduction of regular use of street heroin defined as 50% or more of negative specimens on urinalysis during weeks 14‐26 (responders). Reduction of regular use of street heroin defined as two, one, or zero positive specimens during weeks 14‐26, and a test of zero positive specimens during weeks 23‐26. Self‐reported abstinence from street heroin (zero use) in the past 30 days. |
Urine specimens were obtained at random once a week for 26 weeks; Independent researchers in face‐to‐face interviews with patients at baseline (0 weeks), 13 weeks, and 26 weeks. |
The most recent studies prespecified the achievements (in terms of scoring over some dimensions) that a patient should have reached to be considered "responder" to treatment. The definitions for each study are described in Table 5.
3. Definition of responders across the studies and results of comparisons.
| study name | definition of responder | Measure of effect as reported in the published studies (ARR calculated for NNT) | NNT |
| CCBH (A) 2002 and | Responders: at least 40% improvement in at least one of the 3 domains of inclusion (physical, mental, social) at the end of the treatment compared with baseline; if this improvement was not at the expense of a serious ( ≥ 40%) deterioration in functioning in any of the other outcome domains; and if the improvement was not accompanied by a substantial ( ≥ 20%) increase in use of cocaine or amphetamines. | risk difference difference = 22.8%, 95% CI 11.0%‐ 34.6%; ARR= 0.24 |
NNT=4.2 (95%CI 2.6‐11.1) |
| CCBH (B) 2002 | see above | risk difference 24.3%, 95% CI 9.6% to 39.0%; ARR= 0.23 |
NNT=4.3 (95%CI 2.85‐9.09) |
| Haasen 2007 | Health Responders: at least a 20% improvement and at least 4 points on the OTI Health Scale (physical health) and/or at least a 20% improvement in the GSI (mental health), without a deterioration of more than 20% in the other area of health. Reduction in Illicit drug use Responders: reduction in the use of street heroin with at least 3 of 5 urine samples negative for the drug in the month prior to the 12‐month assessment and no increase in cocaine use (hair analysis). If less than 3 urine samples or no hair was available at 12 months, data from urine or hair testing at 6 months were used (LOCF). |
Health Improvement Adjusted OR=1.54, 95% CI 1.02–2.34, P=0.042. ARR= 0.06 ‘illicit drug use’ Adjusted OR=1.91, 95% CI 1.30–2.79, P=0.001. ARR=0.14 |
NNT=16.7 (95% CI 9.09‐100) NNT=7.2 (95%CI 5‐12.5) |
| NAOMI 2009 | Responders: improvement of at least 20% from the baseline score for illicit‐drug use or legal status (or both). In addition, to rule out deterioration in other variables, a patient with a response could have a decrease of 10% or more on at most one of the remaining composite scores. | Reduction in illicit‐drug use or other illegal activities : 67.0% diacetylmorphine group 47.7% methadone group (rate ratio, 1.40; 95% confidence interval [CI], 1.11 to 1.77; P = 0.004) ARR=0.20 Retention in treatment : 87.8% in the diacetylmorphine group 54.1% in the methadone group (rate ratio, 1.62; 95% CI, 1.35 to 1.95; P<0.001). ARR=0.34 |
NNT=5.3 (95% CI 3.1‐14.3) NNT=3 (95% CI 2.22‐4.34) |
| PEPSA 2006 | Responders: patients showed at least 20% improvement at 9 months, compared with the baseline values, in general health or psychological or family adjustment, without a deterioration superior to 20% in any of these dimensions evaluated with the respective ASI composite scores. | MDO index 70.4% experimental group; 60.9% control group, difference not statistically significant. ARR=0.10 |
NNT=10 (95% CI ‐6.6‐3 *not significant) |
| RIOTT 2010 | Responders: Reduction of regular use of street heroin defined as 50% or more of negative specimens on urinalysis during weeks 14‐26 | ITT weeks 14–26 responders: (72% [n=31]) injectable heroin; oral methadone (27% [n=11], OR 7·42, 95% CI 2·69–20·46, p<0·0001 ARR=0.46 |
NNT=2·17 (95% CI 1·60 to 3·97) |
Excluded studies
Sixteen potentially eligible studies were excluded for the following reasons: design of study not in the inclusion criteria of this review (Battersby 1992Krausz 1999; McCusker 1996; Metrebian 1998;Mitchell 2002; Oppenheimer 1982; Rehm 2001; Uchtenhagen 1999); study outcomes (Ghodse 1990; Haemmig 2001; Hendriks 2001; Jasinski 1986; Moldovanyi 1996; Mello NK 1980); interventions (Mitchell 2002; Mello NK 1980; Strang 2000) or participants (Fischer 1999) differing from inclusion criteria.
Risk of bias in included studies
Overall the risk of bias in the included studies appears sufficiently reduced. The only dimension which appear under the median is the blinding of the subjective outcomes. Below all the dimensions have been addressed for each study Figure 1Figure 2.
1.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
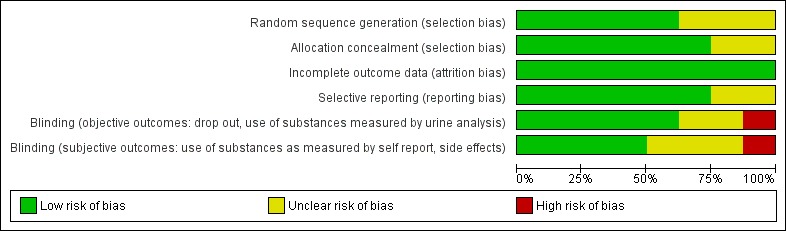
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Adequate sequence generation
The following studies judged to be at low risk of selection bias because described adequate sequence generation procedures: Haasen 2007, NAOMI 2009, RIOTT 2010, Perneger 1998. As for CCBH (A) 2002, CCBH (B) 2002 studies and PEPSA 2006 were judged at unclear risk of selection bias because the information provided was not clear enough.
Allocation concealment
CCBH (A) 2002, CCBH (B) 2002, Haasen 2007, NAOMI 2009, PEPSA 2006 and Perneger 1998 studies , were at low risk of selection bias because had adequate allocation concealment; RIOTT 2010 based on what was reported, was judged at unclear risk of selection bias.
Blinding
Blinding objective outcomes
RIOTT 2010, NAOMI 2009, Hartnoll 1980, CCBH (A) 2002 and CCBH (B) 2002 were judged at low risk of detection bias, PEPSA 2006 and Haasen 2007 were judged at unclear risk and Perneger 1998 at high risk.
Blinding subjective outcomes
RIOTT 2010, NAOMI 2009, CCBH (A) 2002 and CCBH (B) 2002 were judged at low risk of detection bias, Perneger 1998 was judged at high risk and the remaining at unclear risk of bias.
Incomplete outcome data
Alle the studies were judged at low risk of attrition bias .
Selective reporting
Selective reporting has been assessed by checking the outcomes set at protocol stage and comparing them with the published ones, see Table 6. Pre‐published protocols are available for all the studies a part for two older study. Those studies were in fact published even before the first publication of the CONSORT Statement (CONSORT 2010) in which the main criteria for reporting randomised controlled trials, were defined.
4. Assessment of risk of selective publication.
| Study | protocol outcomes | published outcomes | source of protocol information |
| Hartnoll | not available | Health; Use of substances:Total Opiate Consumption (prescribed+illicit); Frequency of Injection during 12 months;Proportion of days spent with other users; Crime activity:Crime as source of outcome during 12 moths;Arrests during 12 months, Employment, Retention in treatment, relapse to street heroin use, death. | info not available |
| Perneger | not available | Consumption of street heroin; frequency of overdoses; risk behaviour for HIV; number of days ill in the past months; use of health services, health status, work status, living arrangements, quality of social relationships, monthly living and drug related expenditures, sources of income, and criminal behaviour, retention in treatment. | info not available |
| CCBH | Physical health, Mental status, Social functioning, Substance use | Dichotomous, multidomain response index, including validated indicators of physical health, mental status, and social functioning. | http://www.ccbh.nl/ |
| PEPSA | General state of health Quality of life Severity of the addiction. Consumption of illegal opiate Consumption of cocaine. Consumption of other psychoactive substances, illegal or legal, not prescribed. Behavior that puts the patient at risk of contracting HIV and hepatitis C Psychological adjustment Symptoms of depression Symptoms of anxiety |
General health status Quality of life Problems related to drug use Use of nonprescribed drugs (in days per month) Heroin HIV risk behavior Related to drug use Related to sexual behavior Psychological adjustment Family and social adjustment Family and social relations, Social functioning Number of days involved in illegal activities (in days per month) | http://www.easp.es/pepsa/inicio/ensayo_english.htm#Protocol http://www.controlled‐trials.com/ISRCTN52023186 |
| NAOMI | A participant was defined as “retained at 12 months” if he or she met any of the following 4 criteria: was compliant with study medication (DAM, HMO and/or MMT) on at least 10 of 14 days prior to the 12‐month date; or was confirmed to be enrolled in detoxification program at the 12‐month date; or was confirmed to be enrolled in a drug‐free program at the 12‐month date; or was confirmed to be abstinent at the 12‐month date. |
The first primary outcome was retention in addiction treatment at 12 months (defined as receipt of the study medication
on at least 10 of the 14 days before the 12‐month assessment, or confirmation of retention in any other treatment program or abstinence from opioids during this interval). The second primary outcome was reduction in illicit‐drug use or other illegal activities. On the basis of composite scores on the European Addiction Severity Index17 patients were considered to have a response at 12 months if they had an improvement of at least 20% from the baseline score for illicit‐drug use or legal status (or both). |
Scientific and political challenges in North America's first randomized controlled trial of heroin‐assisted treatment for severe heroin addiction: rationale and design of the NAOMI study. Oviedo‐Joekes E, Nosyk B, Marsh DC, Guh D, Brissette S, Gartry C, Krausz M, Anis A, Schechter MT. Clin Trials. 2009 Jun;6(3):261‐71. |
| RIOTT | Reduction in illicit heroin, measured by urine drug screens taken on a weekly basis over 6 months. Self‐reported to researcher at baseline, 3 months and 6 months (in or out of treatment): 1. Changes in illicit heroin use: 2. Changes in other illicit opiate drug use (non‐prescribed): 3. Changes in illicit cocaine use: 4. Other illicit drug use and alcohol ‐ benzodiazepines, alcohol, cannabis: 5. Changes in high‐risk injecting practices: 6. Changes in general health status: 7. Changes in psychosocial functioning: 8. Changes in criminality: self‐report using adapted OTI Crime Section of MAP 9. Use of other health and social services: health, social and voluntary sector services used, days off work due to illness, criminal justice sector contacts ‐ adapted REDUCE questionnaire for cost effectiveness analysis 10. Measures of patient expectation of and satisfaction with treatment In addition, we are monitoring injecting practices and complications, any post dosing side effects and serious and non‐serious adverse event data and collecting data on retention. |
Retention; Reduction in street heroin use; Serious adverse events. | Lintzeris et al 2006 (Nicholas Lintzeris, John Strang, Nicola Metrebian, Sarah Byford,Christopher Hallam, Sally Lee, Deborah Zador and RIOTT Group. Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UK. Harm Reduction Journal 2006;3:28.) http://www.controlled‐trials.com/ISRCTN01338071 |
| Haasen | improvement of health, reduction of illicit drug use, decrease of criminal behaviour, increase of accessibility and retainment in treatment, detachment from a social drug context, social stabilisation in the sense of new drug‐free contacts, improved ability to work, financial security, stabilisation of housing situation and enrolment in subsequent treatment. | ‘health’ ‘illicit drug use’ |
http://www.heroinestudie.de/english.html |
Effects of interventions
1.Retention in treatment (number and proportion of patients in treatment at the end of the study for each arm out of the total number of patients allocated to each arm)
Comparison 1, supervised injectable heroin plus flexible dose of methadone vs oral methadone maintenance: Patients in supervised injected heroin plus flexible doses of methadone are retained in treatment more than patients in oral methadone maintenance. The pooled analysis of the 5 relevant studies (RIOTT 2010 1 comparison), NAOMI 2009, Haasen 2007, PEPSA 2006, CCBH (A) 2002), four of which provided valid data for this comparison, enclosing N=1388 patients, shows a measure of effects in favour of heroin RR 1.44 [CI 95% 1.19, 1.75] Heterogeneity: (P = 0.03); I² = 67% (Analysis 1.1 or Figure 3).The results from CCBH studies were not considered valid for this analysis as the authors of the study identified a bias due to the stricter protocol applied to the heroin groups patients.
1.1. Analysis.

Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 1 Retention in treatment.
3.

Supervised injected heroin versus oral methadone: retention in treatment
Comparison 2, provision of heroin (any conditions) vs any other treatment: If the provision of heroin in any condition (supervised or not) is compared with any other treatments (RIOTT 2010, NAOMI 2009, Haasen 2007; PEPSA 2006;CCBH (A) 2002; CCBH (B) 2002; Perneger 1998; Hartnoll 1980; ) and the valid results for the retention in treatment are pooled, the results confirm the favour of heroin provision N= 1535 patients, RR 1.44 [CI 95%1.16, 1.79] and a critical value for heterogeneity: (P=<0.01) I² = 84% (Analysis 2.1 or Figure 4).
2.1. Analysis.
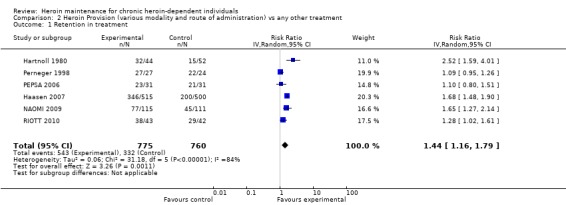
Comparison 2 Heroin Provision (various modality and route of administration) vs any other treatment, Outcome 1 Retention in treatment.
4.

Heroin provision (any route of administration) versus any other treatment: retention in treatment
2.Relapse to street heroin use (number and proportion of people who self reported use of heroin during the study for each arm)
The majority of the studies measured the reduction in illicit drug use, and we therefore renamed this outcome accordingly (see section changes from protocol). The diversity of the criteria adopted at study level to measure this outcome made the meta‐analysis debatable and it was therefore decided to report the results for each individual study.
Each study found a superior reduction in illicit drug use in the heroin arm rather than in the methadone arm. This reduction is measured in different ways and the measures of effect obtained are consistently statistically significant.
Comparison 1, supervised injectable heroin plus flexible dose of methadone vs oral methadone maintenance:
RIOTT defined a reduction of regular use of street heroin as "50% or more of negative specimens on urinalysis during weeks 14–26". The intention to treat analysis found that more patients in the injectable heroin arm were responders than in the oral methadone respectively 31/43 (72%) and 11/42 (27%) OR 7.42, 95% CI 2.69‐20.46.
In NAOMI 2009 the reduction in illicit‐drug use (or other illegal activities) was the second primary outcome and it was measured with the European Addiction Severity Index. Patients were responders at 12 months if they had an improvement of at least 20% from the baseline score for illicit‐drug use or legal status (or both). The reduction in rates of illicit‐drug use or other illegal activity was 67.0% in the diacetylmorphine group and 47.7% in the methadone group (rate ratio, 1.40; 95% CI, 1.11 to 1.77; P = 0.004). Personal communication with the principal investigator of the study brought to our attention that use of street heroin in at least one day in the month preceding the 12 month assessment occurred in 54/115 patients in the dyacetilmorphine arm and 79/111 patients in the methadone arm producing a protective effect for heroin prescription RR 0.66 (95% CI 0.53‐0.83).
In Haasen 2007 reduction in illegal drug use was the second primary outcome and people were considered responders if they showed a reduction in the use of street heroin with at least 3 of 5 urine samples negative for the drug in the month prior to the 12‐month assessment and no increase in cocaine use (hair analysis) with predefined methods to deal with missing data. Were responders 356 patients in the heroin arm (69.1%) and 276 patients ( 55.2%) in the methadone arm with a OR 1.85 (95% CI 1.43‐2.40). The authors adjusted the analysis for target group, study centre and type of psychosocial care and the effect of heroin provision remained significant OR 1.91 ( CI 95%1.30–2.79).
PEPSA 2006 included the use of illicit substances in a primary multi domain outcome (dichotomous multi dimension outcome (MDO)) considering responders the patient who showed at least 20% improvement at 9 months, compared with the baseline values, in general health or psychological or family adjustment, without a deterioration superior to 20% in any of these dimensions. Measured as mean difference (in days per month) at baseline vs end of the study assessment between the two group (with the ASI score McLellan 1992) the use of non prescribed drugs (heroin) gave a mean ratio of 2.36 p.020 in favour of heroin.
In CCBH (A) 2002 were considered responders the patients that, among other results (that will be illustrated later on in the "primary outcomes at study level" section), not showed a substantial ( ≥ 20%) increase in use of cocaine or amphetamines. The dimensions were measured with the Maudsley addiction profile (MAP‐HSS), the symptom checklist (SCL‐90) and self reported data on cocaine consumption were validated against urinalysis . The authors provided unpublished data about heroin consumption (mean days of illicit heroin use during the 30 days before baseline and 12 month assessment) and the mean difference was significantly in favour of injected heroin arm (Mean Difference ‐12.96 (CI 95%‐16.32 to ‐9.6)).
Comparison 2, provision of heroin (any conditions) vs any other treatment
In addition to the previously described results, the following studies pertain to this comparison: CCBH (B) 2002, Perneger 1998, Hartnoll 1980. Only in Hartnoll 1980 no differences among interventions groups in terms of reduction of illicit opioid use are observed as the others found a protective effect of heroin.
In CCBH (B) 2002 were considered responders the patients that not showed a substantial ( ≥ 20%) increase in use of cocaine or amphetamines. The dimensions were measured with the Maudsley addiction profile (MAP‐HSS), the symptom checklist (SCL‐90), self reported data on cocaine consumption were validated against urinalysis. The authors provided unpublished data about heroin consumption (mean days of illicit heroin use during the 30 days before baseline and 12 month assessment) and the mean difference was significantly in favour of inhaled heroin arm (Mean Difference ‐13.9 (95% CI ‐16.62 to ‐11.18)).
Perneger 1998 reported the "Use of street heroin in past month" (measured with an unpublished questionnaire, based on addiction severity index) and showed a reduction in the daily use in the heroin arm passing from N= 27 (all the enrolled patients) at baseline to 1 patient at follow up in the heroin arm, and from 19 at baseline to 10 at follow up in the other treatments arm, being the difference statistically significant P= 0.002.
In Hartnoll 1980 the outcome is named "daily average of illicit opioid use during twelve months" and was checked by regular urine testing and interviews by an independent researcher. The results are grouped by amount of substance used in grams (0‐4 mg, 5‐39 mg, 40+) showing not significant differences among the groups. People in the two higher categories were 27/42 in the heroin group and 27/46 in the methadone group RR 1.10 95% IC (0.79 to 1.53), if all the categories were compared a limited significant result in favour of heroin is found RR 0.88 (95% IC 0.78‐0.99).
3. Use of other substances (number and proportion of people who self reported use of other substances during the study for each arm)
Most of the studies measured and reported the use of illicit substances without distinguishing between heroin and other substances. If further data will be made available in the future, these will be included in an update version of the present review.
Perneger 1998 reports significant results in the reduction of use of hashish/cannabis and non prescribed benzodiazepines, Hartnoll 1980 narratively comments about not having found differences between the groups (p.880).
4. Death (number and proportion of people died during the study for each arm)
Comparison 1, supervised injectable heroin plus flexible dose of methadone vs oral methadone maintenance.
In four out of the five studies comparing supervised injected heroin to oral methadone some fatalities occurred (4 studies N=1477). There were overall 6 deaths in the heroin groups and 10 in the methadone ones giving a not statistically significant protective measure of effect in favour of heroin 0.65 [CI 95% 0.25, 1.69] Heterogeneity (P = 0.89); I² = 0% (Analysis 1.2 or Figure 5).
1.2. Analysis.
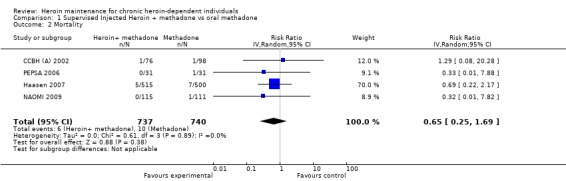
Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 2 Mortality.
5.
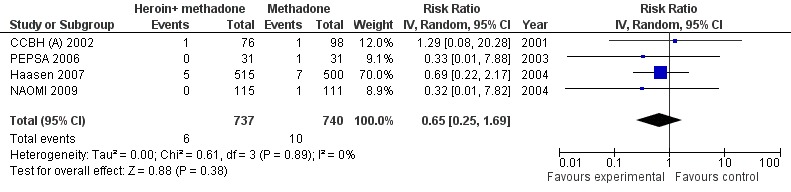
Supervised Injected Heroin + methadone vs oral methadone, outcome: Mortality.
Comparison 2, provision of heroin (any conditions) vs any other treatment . The comparison among the studies providing heroin in any condition and route of administration against any other treatment and including death events (5 studies N=1573) gave a not statistically significant protective effect in favour of heroin 0.78 [95% CI 0.32, 1,89] Hetherogeneity (P = 0.81); I² = 0% (Analysis 2.2 or Figure 6).
2.2. Analysis.
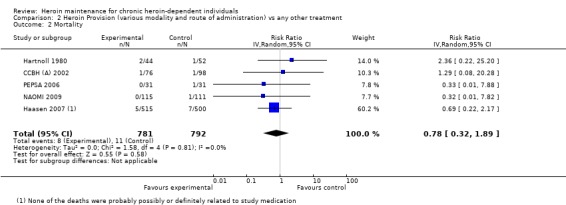
Comparison 2 Heroin Provision (various modality and route of administration) vs any other treatment, Outcome 2 Mortality.
6.
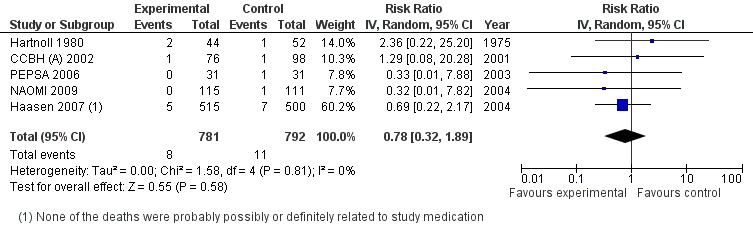
Heroin Provision (various modality and route of administration) vs methadone different modalities, outcome: Mortality.
5. Medical adverse events (number and proportion of people who self‐reported medical adverse events during the study)
Comparison 1, supervised injectable heroin plus flexible dose of methadone vs oral methadone maintenance. Five studies reported the number of adverse events related to study prescriptions (RIOTT 2010, NAOMI 2009; PEPSA 2006; Haasen 2007;CCBH (A) 2002), nevertheless the results by CCBHA and Haasen 2007 cannot be pooled with the others. CCBHA only reported the events that were related to prescribed heroin so that these cannot be used for comparison with the methadone arm; and Haasen 2007 did not report the outcomes at individual patient level. The cumulative results from the three included studies gave a significantly higher risk in the heroin arms RR 13.50 [ CI 95% 2.55, 71.53] (Analysis 1.3).
1.3. Analysis.
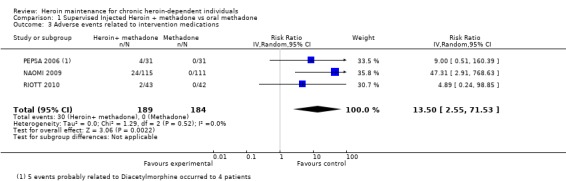
Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 3 Adverse events related to intervention medications.
In Haasen 2007 the average number of adverse events per patients was 8.99 in the heroin group (290 days of treatment) and 8.11 in the methadone group (195 days of treatment) (data provided by authors). The adjusted analysis of the adverse events that were possibly, probably or definitely related to the study medication gave a 2.5 higher risk in the heroin groups.
If all the events (related or not related to study medication) are considered the risk remains higher in the heroin groups but the measure of effect is lower RR 1.61 (CI 95% 1.11 to 2.33).
Comparison 2, provision of heroin (any conditions) vs any other treatment.
In Hartnoll 1980 during the year 21% of the heroin group patients and 11% of the Oral Methadone Patients were admitted to hospital and in Perneger 1998 4 patients in the experimental group and 6 in the control group had at least 1 overdose in the last 6 months but none were related to study medications.
Secondary outcomes
Criminal offence
Comparison 1 supervised injectable heroin plus flexible dose of methadone vs oral methadone maintenance.
In Haasen 2007 the risk ratio of criminal activity shows a protective effect of heroin provision RR= 0.68 [CI 95% 0.57, 0.81], NAOMI 2009 measures the outcome as reduction in illicit drug use and illegal activity and when the reduction in illegal activity alone is showed, the results do not appear significantly different between the study arms. Both the above mentioned studies measured the outcome through the scoring in the EuropASI questionnaire (Kokkevi 1995).
In PEPSA 2006 the outcome is measured in number of days involved in illegal activities (in days per month) at baseline and at 9 months assessment and a more marked reduction is observed in the heroin arm (mean ratio baseline/9month 18.34 p.001) than in the methadone arm mean ratio baseline/9month 1.94 p.0.15).
RIOTT 2010 did not report the outcome.
CCBH (A) 2002 (injected heroin), unpublished data were provided by the authors about mean days of illegal activities during 30 days before baseline and 12month assessment and mean difference was significantly in favour of injected heroin arm Mean Difference ‐5.81 (95% CI ‐8.68 to ‐2.94).
Comparison 2, provision of heroin (any conditions) vs any other treatment .
In Hartnoll 1980, those offered methadone had a trend for being more criminally active at 12 months but if taken into consideration that this trend was already present at intake, the significance of the trend is reduced, in Perneger 1998 the results are significantly in favour of heroin provision in RR 0.32 (95% CI 0.14 to 0.78) for any charge in the last 6 months, and in CCBH (B) 2002 (inhaled heroin) unpublished data were provided by the authors about mean days of illegal activities during 30 days before baseline and 12month assessment and mean difference was significantly in favour of inhaled heroin arm Mean Difference ‐4.27 (95% CI ‐6.62‐1.92).
Incarceration/imprisonment
Comparison 1 supervised injectable heroin plus flexible dose of methadone vs oral methadone maintenance.
The only study pertaining to this comparison and providing the information is Haasen 2007. During the first 12 months of study period, convictions occurred among 49.7% of patients in the heroin groups compared to 65.9% among patients in the methadone groups, and imprisonments occurred among 13.8% of heroin patients compared to 23.6% among methadone patients showing a protective factor of the heroin provision.
In CCBH (A) 2002 (injected heroin) no disaggregated information was available. NAOMI 2009, PEPSA 2006, RIOTT 2010 did not report data on incarceration and imprisonment.
Comparison 2, provision of heroin (any conditions) vs any other treatment .
The cumulative analysis of the studies including this outcome (Haasen 2007; Hartnoll 1980, N=1103) suggest a protective effect of the provision of heroin regarding arrests and imprisonments (RR=0.64, 95%CI=0.51, 0.79, heterogeneity P=0.31).
None of the other studies pertaining to this comparison reported the outcome.
Social functioning (integration at work, family relationship)
Comparison 1 supervised injectable heroin plus flexible dose of methadone vs oral methadone maintenance.
Integration at work
No cumulative analysis was possible for this outcome and results will be described for each individual study. Overall both the groups improved on this dimension in all the studies, heroin gave slightly better results in one study.
In Haasen 2007, employment status improved generally among study participants, from 4.4% at baseline to 10.6% at month 12, with heroin groups participants doing slightly better than methadone participants.
PEPSA 2006 reported this outcome as a mean score change in the Opiate Treatment Index with a statistically significant improvement in both the groups.
NAOMI 2009 reported the outcome as a mean score change in the European Addiction Severity Scale Index with employment satisfaction and social relation giving significant results of improvement in the heroin groups.
In CCBH (A) 2002 (injected heroin) no disaggregated information available and RIOTT did not report the outcome.
Family relationships
No significant differences were observed in the studies.
Haasen 2007 reported a slight improvement in having a stable relationship at month 12 from around 30% at baseline. Having stable housing (living in their own apartment, partner's apartment, parents/relatives and flat sharing) changed from around 69% in both groups at baseline to 72.2% in the heroin groups and 67.6% in the methadone groups at month 12.
RIOTT 2010, CCBH (A) 2002, did not analyse this outcome. In NAOMI 2009 the comparison between the scoring of the European Addiction Severity Index at baseline and at 12 months was not statistically significant (p=0.21) in PEPSA 2006 the same comparisons is borderline significant (p=0.07).
Comparison 2, provision of heroin (any conditions) vs any other treatment.
No substantial differences were observed among interventions groups.
Hartnoll 1980, reported no substantial differences in the two groups in employment. At 12 months 18/42 people were employed in the heroin group and 23/46 in the methadone group (RR=0.86, 95%CI=0.54, 1.35). Comparing with work status at baseline, fewer participants in both groups were working full time at the end of the study.
Perneger 1998 reported that 6/27 in the heroin group and 3/21 in the control group were employed at follow‐up (RR=1.56, 95%CI=0.44, 5.50), which was not different from baseline either.
CCBH (B) 2002 (inhaled heroin), no disaggregated information available.
Family relationship
CCBH (B) 2002 and Hartnoll 1980 did not analyse this outcome.
Perneger 1998 found that 12/27 people in the heroin group and 7/21 people in the control group had a stable partner (RR=1.33, 95%CI=0.64, 2.79). There was no substantial difference when compared with the baseline information.
The primary outcomes assessed by the primary studies enclosed in this review.
The most recent studies comparing supervised injected heroin with oral methadone, set prespecified composite outcomes to identify the patients that succeeded in the treatment. Detailed definitions of the achievements that make a patient responding to treatment are described in the Table 5.
All the studies found positive results upon the prespecified composite outcomes and the number needed to treat ranged from 2.17 (95%CI 1.60‐3.97)(RIOTT 2010) to 16.7 for health improvement outcome and 7.2 for illicit drug use in the German trial.
Discussion
Heroin provision has been studied during the last thirty years in several European countries and in Canada. Even though all the studies have acceptable methods, the most recently published studies have more rigorous methodologies, especially considering the availability of study protocols, which allow the assessment of possible selection and reporting bias.
Each of the studies provided some unique piece of information. The German study obtained a considerable number of patients included, and the study from the UK compared heroin provision with a optimised dose of oral methadone clarifying the doubts about methadone dosages provided in the comparison arms in some of the previous studies. In fact, the >=80‐180 mg dosages reported in RIOTT 2010 and the PEPSA study (Table 3) include those recommended in the recent evidence‐based guidelines (WHO 2009).
The available results showed statistically significant positive effects of supervised injectable heroin plus flexible dosages of methadone in retention in treatment and reduction of illicit drug use, criminal offence and incarceration. A non statistically significant protective effect on mortality was also observed.
This intervention is intended to offer an alternative to those patients that have failed methadone maintenance treatments. Patients are required to attend the clinic to receive and inject the prescribed heroin between 2 to 3 times per day and this might hinder social reintegration and family life, which were supposed to be improved by participating in the treatment.
All the authors of the studies highlight the risks of adverse events that warrant the provision of heroin only to patients that clearly had failed methadone treatment and only in centres that are equipped to respond to emergencies.
Drug addiction has been widely accepted as a chronic medical illness, such as hypertension and diabetes, for which adherence and ultimately outcome are poorest by definition in particular among patients with low socioeconomic status, lack of family and social support. Poverty, lack of family support, and psychiatric comorbidity were described as major predictors of noncompliance and relapse across all the chronic illnesses mentioned.
It remains therefore necessary to identify the definition of treatment failure, which could form the basis for indication to heroin prescription.
Besides, as in all the countries (at different level) the resources for treatment are limited, the open question is wether it is advisable to allocate patients to the provision of more expensive medications instead of trying to address more effectively the identified health and social predictors of non‐compliance and relapse. Nevertheless some specific publications are available to expand the assessment of cost utility of heroin provision (Dijkgraaf 2005; Gutzwiller 2000) and other publications are expected as complementary analysis of the existing studies. A meta‐analysis of the relevant results will contribute to a clearer picture of the cost‐utility of such an intervention.
Summary of main results
Supervised injected heroin plus flexible dosages of methadone treatment compared to oral methadone only helps patients to remain in treatment (4 studies, N=1388 Risk Ratio 1.44 (95%CI 1.19‐1.75)), and helps patients to reduce use of illicit drugs. It also have a not statistically significant protective effects on mortality (4 studies, N=1477 Risk Ratio 0.65 (95% CI 0.25‐1.69)), but it exposes at a greater risk of adverse events related to study medication (3 studies N=373 RR=14.42 95% CI 2.74‐75.95). Heroin provision can also have a protective effect for criminal activity and incarceration/imprisonement. Social functioning improved in all the interventions group with heroin groups having slightly better results, although it is difficult to assess this type of outcome in the relatively short timeframe of an experimental study.
Heroin provision in any conditions vs any other treatment. When considering altoghether the studies comparing heroin provision in any route of administration to other treatments they reach the same effects of those on supervised injected heroin vs oral methadone, but the measures of effects are slightly lower. Retention in treatment (6 studies, 4 valid for this outcome, N=1535 Risk Ratio 1.44 (95% CI 1.12‐1.84)); mortality (5 studies, N=1561 RR 0.77 95% CI 0.32‐1.87)).
Overall completeness and applicability of evidence
The studies included in the present review answer the main questions posed by this review. They were carried out in different countries and in some cases in multi‐site centres. The characteristics of patients that would most benefit from this intervention (more than from the methadone maintenance) might be better clarified.
Quality of the evidence
Overall the risk of bias in the included studies appears sufficiently reduced.The number of participants enrolled in the 8 considered studies range from 50 to 1000. The results are consistent across studies. The most recent studies, thanks to the publication of the protocols, provide full information about the process of enrolling patients and randomly assigning them to the intervention arms. For the study conducted in the Netherlands the existence of stricter disciplinary discharge rules in the heroin group was argued as a reason for biasing the results about retention in treatment. ("Altogether 11% of patients were removed from the program as a result of repeated violations of the house rules, or were not able to visit the program anymore due to incarceration. In the control condition, on the other hand, patients had no alternative, because methadone maintenance treatment was already their last treatment option" CCBH (A) 2002).
Potential biases in the review process
The present review has been updated three times over nine years. It is likely that all the relevant available studies have been enclosed. Most of the studies adopted composite outcomes and measured them with standardised questionnaires, reporting the scoring as continuous variables. Individual patient data might be obtained from the most recent studies and a deeper analysis could be performed.
Agreements and disagreements with other studies or reviews
As far as we know there are not at the moment other systematic meta‐analyses on this topic. A review on substitution treatment for reducing criminal activities among drug users (Egli 2009) found positive effect of heroin provision. A project is being undertaken by the European Monitoring Centre for Drugs and Drug Addiction in collaboration with the National Addiction Centre. The project entitled "Heroin (diacetylmorphine) assisted treatment: evidence and current practices in Europe and beyond" will integrate the results of this review with information from observational data. A recent publication about a sub‐analysis of the results from the German study assessed this intervention for patients that were seriously addicted to heroin but had not undergone previous methadone treatment (Haasen 2010).
Authors' conclusions
Implications for practice.
Heroin provision provides added value to methadone treatment. Considering the higher rate of serious adverse events, the eventual risk‐benefit balance of heroin prescription should be carefully evaluated before its implementation in clinical practice. Heroin prescription should be considered for people who have failed maintenance treatment and it should be provided in clinical settings where proper follow‐up is ensured. The capacity of addiction services and the economical sustainability should be carefully assessed before undertaking such an intervention.
Implications for research.
As heroin provision is now currently provided in several countries, studies based on data collected at treatment centres level could provide valuable information on the effectiveness of this approach in real‐world conditions.
Feedback
Post Publication comments from Heroin triallists
Summary
After the publication of the last update of the present systematic review (August 2010) the contact author received individual messages with comments and criticisms from the authors of the most recent trials: M. Schetcher, W. van Den Brink, C.Haasen and J.Strang.
The comments can be synthetised as follows:
‐about the retention in treatment results some possible explanation of the behaviour of people assigned to the heroin or the methadone arms, were proposed.
‐ it was discussed whether it was the case for providing the meta‐analysis of the relapse to street heroin use as the outcomes were measured in different ways across the studies and sometimes without distinguishing between heroin and other illicit drugs;
‐ the same discussion applied, as a consequence, to the meta‐analysis of the results of other illicit drugs use.
‐ it was commented that the most recent studies compared the provision of supervised injected heroin versus oral methadone and this comparison should have been made clearer by restricting the analysis to the studies focusing on it.
‐ it was argued that only the adverse events considered to be related to the study medications should have been included in the meta‐analisis. It was also pointed out that it was more likely to register adverse events in the heroin arms patients who were observed 2‐3 times per day than in the methadone patients who were seen only 1 time per day.
‐ it was underlined that the studies were powered to measure their prespecified outcomes and this was not reflected in the systematic review.
‐it was discussed that in the RIOTT study the comparison with the injectable methadone arm should not have been included in the meta‐analysis as this was an experimental arm not to be compared with the injected heroin one.
‐it was criticised that mentioning the higher cost of heroin as medication for stabilisation in the discussion was inappropriate as some studies about cost‐utility of heroin provision provided positive results.
Reply
Each single comment was answered individually to the sender and they are available from the first author of the present review. The comments have been considered as a base for the present update which includes the following:
The results of the review are presented in two main comparisons: one about supervised injected heroin plus flexible doses of methadone versus oral methadone, and the second comparison about provision of heroin in any route of administration compared with any other treatments.
The retention in treatment results were maintained as before but the exclusion of the two studies in which the data were not comparable was made clearer. An extended individual answer based on a debate about the possible motivation of patients assigned to different interventions (http://www.bmj.com/content/327/7410/310.abridged/reply#bmj_el_35634) concluded that a systematic review (and surely a meta‐analysis) might not be the right place to deepen the analysis of motivations to behave.
The outcome "relapse to street heroin use" was renamed "reduction in illicit drug use" and the meta‐analysis was substituted by a description of the results at study level. The meta‐analysis of the use of other substances was withdrawn.
The meta‐analysis of the adverse events was restricted to the only cases associated with the study medications. For the higher probability of registering adverse events in the experimental arms, it was answered that this is due to ascertainment bias and this can only be dealt at study level.
The injected methadone comparison arm (RIOTT study) was excluded by the analysis.
An additional paragraph and one additional table describe the primary outcomes for which the studies were powered and their results.
The references to the cost‐utility studies on heroin provision were included in the discussion.
The conclusions were slightly modified to reflect the different comparisons provided.
Contributors
Marica Ferri, Marina Davoli, Silvia Minozzi.
What's new
| Date | Event | Description |
|---|---|---|
| 18 April 2012 | Amended | minor correction in number of participants included in two included studies (Naomi and Riott) |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 9 November 2011 | New citation required but conclusions have not changed | it is to amend a previous error, the review needs a new citation |
| 14 September 2011 | Amended | minor correction in the text in results section |
| 21 December 2010 | Feedback has been incorporated | unpublished new data, new analysis, new tables |
| 7 July 2010 | New search has been performed | updated |
| 14 February 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Professor Wim van Den Brink, Professor Mike Clarke and Ms Elizabeth Pienaar for refereeing the protocol; and Professor Michael Farrell for acting as Contact Editor. Professor Christian Haasen, Professor John Strang, Professor Martin Schechter, Dr Eugenia Oviedo‐Joekes and Professor Wim van den Brink provided further data and information on ongoing publications for their respective studies. Special thanks to Dr Silvia Minozzi for providing quality advice on the manuscript, and to Dr V. Anna Gyarmathy, EMCDDA, for editing the text.
Appendices
Appendix 1. CENTRAL search strategy
1 SUBSTANCE‐RELATED‐DISORDERS:ME 2 SUBSTANCE‐ABUSE‐INTRAVENOUS:ME 3 SUBSTANCE‐WITHDRAWAL‐SYNDROME:ME 4 WITHDRAW* 5 ABSTINEN* 6 ABSTAIN* 7 ABUSE OR ABUSES OR ABUSING 8 EXCESSIVE* NEAR USE* 9 USE* NEAR DISORDER* 10 ADDICT* 11 OVERDOSE 12 OVER‐DOSE 13 INTOXICAT* 14 SUBSTANCE‐RELATED DISORDER*:ME AND ((CRIMINAL NEXT OFFENCE) OR CRIME)) 15 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 16 HEROIN 17 METHADONE 18 OPIOID* 19 OPIAT* 20 SUBSTANCE‐ABUSE‐TREATMENT‐CENTERS*: ME 21 16 OR 17 OR 18 OR 19 OR 20 22 15 OR 21
Appendix 2. MEDLINE search strategy
1 exp substance related disorders/ 2 withdraw$ or abstinen$ or abstain$ or abuse or abuses or abusing or addict$ or overdos$ or over‐dos$ or intoxicat$ 3 (drug and narcotic control) 4 exp street drugs/ 5 substance abuse 6 drug withdrawal symptoms 7 1 or 2 or 3 or 4 or 5 or 6 8 exp heroin/ 9 exp methadone/ 10 methadone 11 *Narcotics 12 Opioids 13 Opioid 14 opiat$ 15 exp substance abuse centers 16 substance ADJ abuse ADJ treatment cent$ 17 drug ADJ rehabilitation ADJ cent$ 18 heroin ADJ prescription 19 heroin ADJ maintenance ADJ therapy 20 heroin ADJ maintenance ADJ programme 21 OR 8/20
Appendix 3. EMBASE search strategy
1 exp Heroin Dependence/ 2 heroin ADJ depen$ 3 Heroin ADJ abus$ 4 Heroin ADJ Us$ 5 Heroin ADJ inject$. 6 heroin ADJ smok$. 7 heroin ADJ snort$. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 heroin ADJ crav$ 10 heroin ADJ withdrawal 11 8 or 9 or 10 12 heroin 13 Diamorphine/ 14 Heroin Dependence/dt [Drug Therapy] 15 heroin ADJ prescription 16 heroin ADJ provision 17 heroin ADJ maintenance ADJ treatment 18 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
Appendix 4. CINAHL search strategy
1 exp HEROIN/ 2 heroin 3 heroin ADJ dependence 4 substance ADJ related ADJ disorders 5 Substance Abusers/ or Intravenous Drug Users/ 6 1 or 2 or 3 or 4 or 5 7 (diamorphine and therapy) 8 6 or 7
Appendix 5. Risk of bias table methods
|
Item |
Judgment |
Description |
||||||
| 1 | Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization | |||||
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |||||||
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |||||||
| 2 | Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. | |||||
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |||||||
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |||||||
| 3 | blinding of outcome assessor (detection bias) Objective outcomes |
Low risk |
Blinding of participants, providers and outcome assessor and unlikely that the blinding could have been broken; Either participants or providers were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. No blinding, but the objective outcome measurement are not likely to be influenced by lack of blinding |
|||||
| 4 | Blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk |
Blinding of participants, providers and outcome assessor and unlikely that the blinding could have been broken; Either participants or providers were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. |
|||||
| High risk | No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; Either participants or outcome assessor were not blinded, and the non‐blinding of others likely to introduce bias |
|||||||
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |||||||
| 5 | Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
Low risk |
No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
|||||
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; |
|||||||
| Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group); | |||||||
| 6.Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
||||||
| High risk | Not all of the study’s pre‐specified primary outcomes have been reported; One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|||||||
| Unclear risk | Insufficient information to permit judgement of ‘Yes’ or ‘No’ |
Data and analyses
Comparison 1. Supervised Injected Heroin + methadone vs oral methadone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 4 | 1388 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.19, 1.75] |
| 2 Mortality | 4 | 1477 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.25, 1.69] |
| 3 Adverse events related to intervention medications | 3 | 373 | Risk Ratio (IV, Random, 95% CI) | 13.50 [2.55, 71.53] |
Comparison 2. Heroin Provision (various modality and route of administration) vs any other treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 6 | 1535 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.16, 1.79] |
| 2 Mortality | 5 | 1573 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.32, 1.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CCBH (A) 2002.
| Methods | Allocation: randomised controlled trial;
Randomization performed centrally;
Blindness of the patients and or care providers in respect to treatment: not performed, all the patients were orally informed about the treatment they had been allocated Duration of treatment within the study: 12 months |
|
| Participants | Diagnosis: heroin addicts (intravenous use) registered in the local methadone maintenance programs, who had failed several methadone programs
N= 174 Age=38.5 years (5.7 SD) Sex= male 82.2% History=a history of heroin dependency (DSM‐IV) of at least five years; a minimum dose level of 50 mg (inhaling) or 60 mg (injecting) of methadone per day for an uninterrupted period of at least four week in the previous five years; in the previous year registered in a methadone program, and during the previous six months in regular contact with the methadone program; chronic heroin addiction and unsuccessfully treated in methadone maintenance treatment; daily or nearly daily use of illicit heroin; poor physical, and/or mental, and/or social functioning; Criminal activity=at study entrance at least six days in the previous month of drug‐related illegal activities Mental State= at study entrance a SCL‐90 total score of at least 41 (males) or 60 (females) |
|
| Interventions | Group A (no. = 98) 12 months methadone; Group B (no. = 76) 12 months methadone+heroin injectable Psychosocial interventions: Psychosocial treatment was offered throughout |
|
| Outcomes | Dichotomous, multidomain response index, including validated indicators of physical health, mental status, and social functioning. Information on Substance use; Retention in treatment, Death, Adverse events | |
| Notes | Country: The Netherlands 1998‐2001 website: www.ccbh.nl/ENG/publications.htm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There are no information about procedure of sequence generationand in agreement with the CDAG rule, the judgment has to be "unclear". |
| Allocation concealment (selection bias) | Low risk | "the randomization was organized centrally by an independent monitoring organization, and conducted separately for the trials on injectable heroin and inhalable heroin." Authors were contacted for further details and correspondence is available by the reviewer's author. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "In the present study, it could not be ruled out that the probability of response would systematically and considerably differ between patients with and without endpoint‐assessments. However, since the primary analysis of effectiveness concerned the total treatment‐offer, regardless of possible deviations from the protocol, statistical methods to correct for bias in the findings caused by missing endpoint‐assessments, like multiple imputation or propensity score estimation, were only of limited applicability. It was therefore considered crucial to minimize the occurrence of missing endpoint‐assessments as much as possible, by conducting intensive field work and by providing additional financial compensation for participating in the endpoint‐assessments. Nevertheless it could not be excluded that some missing endpoint‐assessments would occur in the study population. In case of such missing endpoint‐assessments, and because of lack of satisfactory alternatives, the "last observation carried forward" (LOCF) method was used in the primary analysis." |
| Selective reporting (reporting bias) | Low risk | prespecified outcomes available from the website of the study, details available on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | In order to reduce the risk of information bias, outcome assessments were conducted by independent assessors, who used standardized instruments and evaluation procedures. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | The validity of the self‐report data was checked through the application of urinalysis, with regard to the concurrent use of illicit drugs, and collection of registered data from the police and justice system, with regard to committed offenses and periods of detention. These latter types of data are insensitive to information bias. |
CCBH (B) 2002.
| Methods | Allocation: randomised controlled trial;
Randomization performed centrally;
Blindness of the patients and or the care providers in respect to treatment: not performed, all the patients were orally informed about the treatment they had been allocated Duration of treatment within the study: 12 months |
|
| Participants | Diagnosis: heroin addicts (inhaling use) registered in the local methadone maintenance programs, who had failed several methadone programs.
N=256 Age=39.6 (5.7 SD) Sex= 79.7% male; History=a history of heroin dependency (DSM‐IV) of at least five years; a minimum dose level of 50 mg (inhaling) or 60 mg (injecting) of methadone per day for an uninterrupted period of at least four week in the previous five years; in the previous year registered in a methadone program, and during the previous six months in regular contact with the methadone program; chronic heroin addiction and unsuccessfully treated in methadone maintenance treatment; daily or nearly daily use of illicit heroin; poor physical, and/or mental, and/or social functioning; Criminal activity=at study entrance at least six days in the previous month of drug‐related illegal activities Mental State= at study entrance a SCL‐90 total score of at least 41 (males) or 60 (females) |
|
| Interventions | Group A (no. = 139) 12 months methadone; Group B (no. = 117) 12 months heroin (inhalable) + methadone; Group C (n=119) 6months methadone+ 6 months Psychosocial interventions: Psychosocial treatment was offered throughout |
|
| Outcomes | Dichotomous, multidomain response index, including validated indicators of physical health, mental status, and social functioning. Information on Substance use; Retention in treatment, Death, Adverse events | |
| Notes | Country: The Netherlands 1998‐2001 Website: www.ccbh.nl/ENG/publications.htm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description of sequence generation could be traced in the articles and the report of the study and in agreement with the CDAG rule, the judgment has to be "unclear". |
| Allocation concealment (selection bias) | Low risk | "the randomization was organized centrally by an independent monitoring organization, and conducted separately for the trials on injectable heroin and inhalable heroin." Authors were contacted for further details and correspondence is available by the reviewer's author. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "In the present study, it could not be ruled out that the probability of response would systematically and considerably differ between patients with and without endpoint‐assessments. However, since the primary analysis of effectiveness concerned the total treatment‐offer, regardless of possible deviations from the protocol, statistical methods to correct for bias in the findings caused by missing endpoint‐assessments, like multiple imputation or propensity score estimation, were only of limited applicability. It was therefore considered crucial to minimize the occurrence of missing endpoint‐assessments as much as possible, by conducting intensive field work and by providing additional financial compensation for participating in the endpoint‐assessments. Nevertheless it could not be excluded that some missing endpoint‐assessments would occur in the study population. In case of such missing endpoint‐assessments, and because of lack of satisfactory alternatives, the "last observation carried forward" (LOCF) method was used in the primary analysis." |
| Selective reporting (reporting bias) | Low risk | Yes prespecified outcomes available on the website of the study, details on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | In order to reduce the risk of information bias, outcome assessments were conducted by independent assessors, who used standardized instruments and evaluation procedures. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | The validity of the self‐report data was checked through the application of urinalysis, with regard to the concurrent use of illicit drugs, and collection of registered data from the police and justice system, with regard to committed offenses and periods of detention. These latter types of data are insensitive to information bias. |
Haasen 2007.
| Methods | Allocation: Randomized controlled trial, multicenter Randomization: 4x2 stratified randomization Blindness of patients and or care providers in respect to treatment: not specified Duration of threatment within the study: 12 months |
|
| Participants | Diagnosis: ICD10 for opiate dependence for at least 5 years (World Health Organization, 1993). (a) people with heroin dependence who were insufficiently responding to treatment owing to continuous intravenous heroin use (n=492); and (b) people with heroin dependence who were not in treatment in the previous 6 months (n=540). N=1032 Age=35.9 ( SD 6.8) Sex= Male 81.8 History=Intravenous drug use in the past month days 26.5 (SD 7.4) Criminal activity=Illegal activities past month, days,mean 22.6 (SD9.8) Mental State=At least one lifetime psychiatric diagnosis 59.3%; Previous suicide attempts,39.7% |
|
| Interventions | Arms 1,2 of each stratum: Heroin+education or Heroin + Case management Maximum single dose of 400mg and a maximum daily dose of 1000mg (none to take home) individually adjusted dose of injectable heroin self‐administered under direct supervision of medical staff Maximally three times a day, 7 days a week out‐patient setting; Up to 60mg of methadone could also be given for take‐home nighttime use to suppress withdrawal Arms 3,4 of each stratum: Methadone + education or Methadone + case‐management minimum daily dose of 60mg oral individually adjusted according to clinical judgement |
|
| Outcomes | Two prespecified dichotomous, multidomain primary outcome measures: Primary outcome measure on health. Second primary outcome measure, people were considered responders if they showed a reduction in the use of street heroin and no increase in cocaine use (hair analysis). |
|
| Notes | Country: Germany, 2002‐2004 Website: http://www.heroinstudie.de/english.html |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated list of numbers |
| Allocation concealment (selection bias) | Low risk | "Randomisation took place separately for each target group (methadone treatment failure and not in treatment), and treatment allocation was performed using sealed and consecutively numbered envelopes at each study site". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the intent‐to‐treat analysis, all those randomised were assessed regardless of treatment retention. Data from the baseline and 12‐month assessments were used for analysis of the primary outcome measures; the last‐observation‐carried‐forward (LOCF) procedure from data at 6 months was used if data at 12 months were missing. If no data were available for 6 and 12 months, the outcome was coded according to a worst‐case analysis (i.e. as a responder in the methadone group and a non‐responder in the heroin group). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes available from the website of the study, details on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Unclear risk | No mention of blinding of assessors.The assessment by independent research assistants included administration of the European version of the Addiction Severity Index (EuropASI; Kokkevi & Hartgers, 1995), and gathering data on criminal behaviour and on subjective aspects of treatment. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Unclear risk | see above |
Hartnoll 1980.
| Methods | Allocation: computer generated list of random number; Randomization: unspecified Blindness of patients and or care providers in respect of treatment: patients were aware of the treatment provided but they were not aware of being part of a trial; blindness over interventions by patients and treatment providers and outcomes measurers not mentioned; Duration of treatment within the study: 12 months |
|
| Participants | Diagnosis: regular opiate use including daily heroin injection in the last three months sufficient to convince clinical staff to be entered in a substitution program N= 96 Age= 18‐35 years; mean 23.9 Sex= 75% male; 25% female; History= mean duration of opiate use : 5.9 years; age at first use: 18.0yrs Criminal activity: 87% had criminal convictions; the mean number of convictions 4.3; the 52% had been convicted at least once in the 12 months before intake Mental State: at intake 60% were recorded as mildly; 27% anxious; 35% mildly depressed and 3% euphoric | |
| Interventions | Heroin Maintenance (N= 44); dosages 30‐120 mg/day;
Oral Methadone (N=52) dosages 10‐120 mg/day Psychosocial interventions: Interviews by a clinic psychiatrist at 3,9 and 12 months; |
|
| Outcomes | Health; Use of substances:Total Opiate Consumption (prescribed+illicit); Frequency of Injection during 12 months; Proportion of days spent with other users; Crime activity: Crime as source of outcome during 12 moths; Arrests during 12 months Employment. Retention in treatment, relapse to street heroin use, death |
|
| Notes | Country: UK 1972‐75 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated list of random numbers was consulted (without patients being aware of this) and the patients prescribed either HM or refused it and offered Oral Methadone instead." |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information where obtained by almost all of the patients some of them where interviewed in the United States and for others family members were interviewed an parallel sources of informations were compared. |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the study not available, due to the older date of the study. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | Data collected by independent research but no blinding of assessors mentioned nevertheless any limitations to validity of objective results are described and taken into consideration in the study results. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Unclear risk | Data collected by independent research but no blinding of assessors mentioned nevertheless any limitations to validity of subjective results are described and taken into consideration in the study results. |
NAOMI 2009.
| Methods | Allocation: "A computer‐generated randomisation list of permuted blocks of two, four, and six was used. Patients were assigned to receive diacetylmorphine, methadone, or hydromorphone in a 45:45:10 ratio. Randomization was stratified according to centre and according to the number of previous methadone treatments (two or fewer vs. three or more)." Blindness of patients and or care providers in respect to treatment:"The investigators and participants were aware of whether the assigned study drug was oral methadone or one of the injectable drugs, but diacetylmorphine and hydromorphone were administered in a double‐blind fashion". "Evaluations were performed at a separate research office that operated independently from the treatment clinic in each city" there is no mention whether the assessors were blinded to treatment allocation. Duration of treatment within the study: 12 months | |
| Participants | Opiate‐dependent using injected heroin on regular basis,
not responding in the past or currently in MMT. Diagnosis: opioid dependence (meeting three or more of seven criteria listed in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition,14 including tolerance or withdrawal) N= 251 Age=25 years or older Sex= Male154 (61.4) History= duration of injecting drug use yr 16.5±9.8 |
|
| Interventions | Injected heroin + oral methadone (N = 115) oral methadone (N = 111) injected Dilaudid + oral methadone (N = 25) Psychosocial interventions: All patients were offered a comprehensive range of psychosocial and primary care services in keeping with Health Canada best practices. |
|
| Outcomes | retention in addiction treatment reduction in illicit‐drug use or other illegal activity |
|
| Notes | Country: Canada 2005‐2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors describe "a computer‐generated randomisation list of permuted blocks of two, four, and six". "Randomization was stratified according to center and according to the number of previous methadone treatments (two or fewer vs. three or more)." |
| Allocation concealment (selection bias) | Low risk | central allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Almost no missing outcome data: "We obtained 12‐month retention data on 245 of 251participants (97.6%) and response data on 240 of 251 participants (95.6%). The baseline characteristics of the groups were similar" |
| Selective reporting (reporting bias) | Low risk | Details from the published protocol on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | No blinding but the objective outcome measurement were not likely to be influenced by lack of blinding: "Retention was assessed with the use of detailed data on daily prescription‐drug use and, when possible, with the use of administrative data and pharmacy and physician records." |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | Self‐reported nonuse of illicit heroin was confirmed by means of urine testing at 100% of 46 visits in the group of patients randomly assigned to receive hydromorphone (the double‐blind portion of the study). (information obtained by the authors on request) |
PEPSA 2006.
| Methods | Allocation: Permuted blocks of two, four, and six were used (not generated by a list, but ‘manually’ (see risk of bias documents). Patients were assigned to receive diacetylmorphine or methadone in a 50:50 ratio.
Randomization was not stratified (information provided by the authors on request). Blindnessof patients and or care providers in respect to treatment: given the administration routes and different treatment schedules in each group, it was impossible to blind health care personnel to the treatment condition. Nevertheless, the professional who made the assessments and the statistical analysis was blind to the treatment condition. Duration: 12 months |
|
| Participants | Diagnosis: opiate dependency for more than 2 years in line with International Statistical Classification of Diseases, 10th Revision criteria; ongoing intravenous opioid habit; have been in MT in the past at least twice according to official certificates issued by authorized centers N= 62 Age= mean 37.2 (SD 5.5) Sex= male 90.3% History= opiate dependency for more than 2 years , resident in Granada over the preceding year. Criminal activity:number of days in the prior month M=9,8 (SD=12,2) (information provided by the authors on request) Mental State:mental health problems, and social maladjustment (according to scores of the assessment of severity by the interviewerQ in the social/family situation and legal Addiction Severity Index [ASI] subscales) |
|
| Interventions | Experimental group: injected DAM, twice a day, plus oral methadone, once a day. The average DAM dosage was 274.5 mg/day (range: 15–600 mg), and average methadone 42.6 mg/day (range: 18–124 mg). Control group: daily methadone (once a day) 105 mg/day (range:40–180 mg). Comprehensive clinical, psychological, social, and legal support was given to both groups. Psychosocial interventions: see above |
|
| Outcomes | General state of health, Quality of life, Severity of the addiction. Consumption of illegal opiate. Consumption of cocaine. Consumption of other psychoactive substances, illegal or legal, not prescribed. Behavior that puts the patient at risk of contracting HIV and hepatitis C Psychological adjustment Symptoms of depression Symptoms of anxiety Family situation Social support Rate of retention. Level of utilization of the psychosocial services of the trial |
|
| Notes | Country: Spain 2001‐2004 Website: http://www.easp.es/pepsa/inicio/ensayo_english.htm#Design |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information |
| Allocation concealment (selection bias) | Low risk | Treatment name ‘Metadona’ or ‘Heroína’ were introduced in identical opaque envelopes at a 50/50 ratio (i.e. six patients, 3 envelopes will say methadone, 3 will say heroin). There were shuffled in the presence of the participants and each participant will pick an envelope. Our guys had ‘trust’ issues. We early realized that a physician calling the center and tell the participant the treatment he or she randomly got, would generate problems. We knew this from our contact with them, some of them where convinced we were going to ‘cheat’ in the randomisation. Therefore, we had to show them that they had a fifty fifty chance to enter the heroin or methadone arm. (information provided by the authors) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat and per protocol analysis were performed (completers in the experimental group: 23/27( 85.1%), and in the control group: 21/23 (91.3%). |
| Selective reporting (reporting bias) | Low risk | Protocol available and study registered, details on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Unclear risk | "Independent interviewers of the research and clinical teams were responsible for applying the assessment instruments". |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Unclear risk | Our outcomes were based on the ASI, administered by independent interviewers. This instrument is based on self‐report. We tested the use of street heroin with the acetylcodeine test. It did not work; 95% of the test came back ‘negative’ regardless of the allocation group and self‐reported use of street heroin. |
Perneger 1998.
| Methods | Allocation: randomised controlled trial; Randomization Blindness of patients in respect to treatment: non described. Duration of treatment within the study: 6 months |
|
| Participants | Diagnosis: Heroin addicts; N=51 age >20; use >2yrs (12 years on average); more previous attempt of drug treatments (average 8 range 2‐21) and had experienced four drug overdoses (range 0‐30); high prevalence of mental disorders and health status scores 1‐2 SD below population norms. | |
| Interventions | Heroin injected by the patients themselves + oral methadone if the patient travels or want to reduce the attendance of the clinic; mean daily dosages of intravenous heroin was 509 mg/day in one to three injections; in addition to heroin all the patients occasionally received oral opiates and 16 patients received clorazepate substitution therapy (median dose 60 mg/day)
psychological support (N=27) Any other conventional drug treatment (N=24) control: waiting list (control patients were encouraged to select any drug treatment program available in Geneva and were enrolled immediately whenever possible). Psychosocial treatment: all patients received psychological counselling, HIV prevention counselling, social and legal support services, and somatic primary care. |
|
| Outcomes | Consumption of street heroin; frequency of overdoses; risk behaviour for HIV; number of days ill in the past months; use of health services, health status, work status, living arrangements, quality of social relationships, monthly living and drug related expenditures, sources of income, and criminal behaviour, retention in treatment. | |
| Notes | Country: Switzerland 1995‐1996 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated list of numbers placed in sealed envelopes". |
| Allocation concealment (selection bias) | Low risk | Patients are allocated by the psychiatrist during the first visit through the sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All experimental group patients and 22 in the control group were reassessed 196 days on average after enrolment (range 168248); one person from the control group filled only the SF36 questionnaire. The two remaining patients in the control group were alive at follow up but refused to cooperate. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified probably due to the date of the study. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | High risk | no objective measures adopted: all outcome measures were self reported, which raises the issue of information bias. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | High risk | no actions to reduce the risks reported |
RIOTT 2010.
| Methods | Allocation: Randomisation was undertaken independently by the Clinical Trials Unit (IoP). Blindness: Researchers unblinded to treatment allocation, informed both clinicians and patients together of treatment allocation prior to treatment commencing. Duration of treatment within the study: 6 months |
|
| Participants | Diagnosis:regular heroin injecting no active significant medical condition N=127 Age= Aged between 18 and 65 years at recruitment to study History= >3 years injecting, in treatment >6 months Mental state= no active significant psychiatric conditions |
|
| Interventions | experimental group:supervised injectable heroin (SIH) attendance at clinic twice daily for prescribed split daily dose of injectable heroin; self‐administered under supervision; supplementary oral methadone available (as take‐home dose at clinician's discretion). experimental group :supervised injectable methadone (SIM) attendance at clinic once daily for prescribed single daily dose of injectable methadone; self‐administered under supervision; supplementary oral methadone available (as take‐home dose at clinician's discretion); control group:optimised oral methadone (OOM) enhanced oral methadone treatment, daily doses of >80mg actively encouraged, consumed under supervision on >5 days per week for 3 months; thereafter frequency of supervision reduced if clinically appropriate; |
|
| Outcomes |
1.a. objective: (operationally defined as urinalysis negative for street‐heroin for 50% or more of weekly random urines between weeks 14‐26); 1.b. subjective: self‐reported street‐heroin use (over 30 days prior to interview) was elicited through face‐to‐face research interviews with patients and 1.undertaken by independent researchers at baseline, three‐months and six months. |
|
| Notes | Country: UK 2005‐2008 Website:http://www.iop.kcl.ac.uk/projects/?id=10114 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible consenting patients were randomised by minimisation in the ratio (1:1:1) to one of three treatment options (OOM, SIM, SIH), with stratification for a) regular cocaine/crack use (>50% days in previous 4 weeks), b) previous optimised oral methadone treatment (doses of >80mg/day; supervised >5 days/week) and c) clinic site (London, Darlington, Brighton). Randomisation was undertaken independently by the Clinical Trials Unit (IoP). |
| Allocation concealment (selection bias) | Unclear risk | Researchers unblinded to treatment allocation, informed both clinicians and patients together of treatment allocation prior to treatment commencing |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For the primary outcome measure, missing data were handled using multiple imputation in cases where missing urines occurred due to hospitalisation, imprisonment, pre‐agreed absence (holiday), safety reasons or clinical omission/error. Missing urines from a patient who attempted abstinence were similarly managed. Urine samples not provided due to non‐compliance (refusal to provide or unplanned non‐attendance) were presumed positive. Data were analysed on an Intention‐To‐Treat basis for the primary analysis (all patients randomised included in analyses). Per‐Protocol analyses (only patients who received trial interventions according to protocol) were also conducted: any substantial differences in findings are reported alongside the primary ITT analyses. All the data for each of the groups to which participants were randomised were presented so that the risk of bias of multiple‐intervention study are minimised. |
| Selective reporting (reporting bias) | Low risk | Protocol published and study registered see table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | Urinalysis (primary outcome measure) was preformed by laboratory personnel blinded to treatment allocation and the statistician was blinded to injectable group. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | Interviews with patients and undertaken by independent researchers at baseline, three‐months and six months. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Battersby 1992 | Study design: retrospective study (audit of 40 patients treated in substitution therapy centres) excluded as the design not in the scope of the review. |
| Fischer 1999 | Study design: Open randomised clinical trial Allocation: randomised Participants: pregnant women Excluded as the type of participants were not in the scope of the review. |
| Ghodse 1990 | Study design: Controlled Clinical Trial Allocation: double blind, randomisation not mentioned Participants: opiate dependents aged 19‐42 yr Interventions: heroin or methadone oral Outcomes: appropriate dosage of heroin to obtain stabilization Excluded as the outcomes were not in the scope of the review. |
| Haemmig 2001 | Study design: Randomised Controlled Trial Allocation: Randomisation by Central Pharmacy Participants: opiate users mean age 29.9 Intervention: heroin or morphine Outcomes: reaction to substances: euphoria, itching, pain nausea, side effects Excluded as the outcomes were not in the scope of the review. |
| Hendriks 2001 | Study design: Controlled Clinical Trial Outcome: bioavailability of heroin comparison between "chasing the dragon" of inhaled heroin Excluded as the outcomes were not in the scope of the review. |
| Jasinski 1986 | Study design: Controlled Clinical Trial Participants: non‐dependent adult prisoners with history of long term opiate abuse Intervention: Methadone, Morphine and Heroin Outcomes: effects of the substance Excluded as the outcomes were not in the scope of the review. |
| Krausz 1999 | Study design: review Excluded for not being a study but a review of studies |
| McCusker 1996 | Study design: cross sectional study Excluded as the design not in the inclusion criteria |
| Mello NK 1980 | Study design: double blinded, randomised study Participants: 12 patients 25.8 yrs, abused heroin for 7.8 yrs Intervention: All the participants were detoxified with methadone, then remained drug free for 7 days after which they were given naltrexone. People were then offered to work to earn money or point for heroin self‐administration. Outcomes: The potential of Naltrexone to help people remain abstinent Excluded because the intervention and the outcomes considered were not in the scope of the review. |
| Metrebian 1998 | Study design: prospective observational study Participants: patients admitted to the clinic and observed for a period of 18 months. Intervention: Patients self selected whether they received methadone or heroin Excluded as the design was not in the scope of the review. |
| Mitchell 2002 | Study design: open‐label crossover design Participants: 18 methadone maintenance patients, 36 yrs, a median of 1 previous methadone maintenance treatment episodes and a median duration of treatment of 28 months. Intervention: Patients were transferred from methadone to Slow Release Oral Morphine for six weeks before resuming methadone maintenance. Excluded as the design and the intervention not in the scope of the review. |
| Moldovanyi 1996 | Study design: Controlled Clinical Trial
Participants: 16 opiate dependence
Intervention: morphine intravenous different dosages
Outcomes: side effects Excluded as the design and the intervention not in the scope of the review. |
| Oppenheimer 1982 | Study design: follow‐up study Excluded as the design is not in the scope of the review. |
| Rehm 2001 | Study design: Cohort study Participants: 1969 opioid dependent drug users Intervention: heroin assisted treatment Outcomes: retention in treatment, social integration, referral to abstinence oriented treatment. Excluded for the design not in the scope of the review |
| Strang 2000 | Study design: randomised controlled trial Participants: 40 opiate dependent injectors Intervention: injectable vs oral methadone Excluded as the intervention is not in the scope of the review. |
| Uchtenhagen 1999 | Study design: cohort study Excluded as the study design is not in the scope of the review. |
Characteristics of ongoing studies [ordered by study ID]
Universite' de Liege 2010.
| Trial name or title | Projet pilote de traitement assisté par diacétylmorphine: comparaison entre un traitement par diacétylmorphine et un traitement par méthadone. |
| Methods | Muticenter study, allocation performed by a neutral person with the help of informatic procedure (unclear whether randomisation will occur or not) |
| Participants | 2 groups 100 patients each· Beglian citizens or legal residence in Belgium ; · being living in the area, · >= 20 years; · history of daily heroin consumption >=5 years; · inject or inhal; · multiple treatment failures OR · not having access to the treatment because of psychological or social problems; · use effective contraception; |
| Interventions | Intervention: diacetylmorphine (inhaled, injected or in tablets) supervised by a nurse+ psychosocial interventions; Control: Oral methadone + psychosocial interventions. |
| Outcomes | retention in treatment, use of substances and social integration related outcomes |
| Starting date | 2007 expected results: 2010 |
| Contact information | |
| Notes |
Differences between protocol and review
The outcome "relapse to street heroin" was changed into "reduction of illicit drug use" for consistency with the outcome measures available at study level.
Contributions of authors
Marica Ferri wrote the protocol, selected the search strategy results, assessed the trials for inclusion/exclusion, evaluated the methodological quality of each enclosed study, extracted data from the studies analysed them, and wrote the review. Marina Davoli overviewed the protocol, assessed the trials for inclusion/exclusion, evaluated the methodological quality of each enclosed study, extracted data from the studies and wrote the review. Carlo A. Perucci commented on the first draft of the review and contributed to the final version of the review.
Sources of support
Internal sources
Department of Epidemiology, ASL RM E, Italy.
Agency for Public Health, Italy.
European Monitoring Centre for Drugs and Drug Abuse EMCDDA, Not specified.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
CCBH (A) 2002 {published data only}
- Brink W, Hendriks VM, Blanken P, Koeter MWJ, Zwieten BJ, Ree JM. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ 2003;327:310‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink W, Hendriks VM, Blanken P, Ree JM. Dutch research on the effectiveness of medical prescription of heroin; background, research design and preliminary results. Ned Tijdschr Geneeskd 2000;144(3):108‐12. [PubMed] [Google Scholar]
- Brink W, Hendriks Vincent M, Ree Jan M. Medical co‐prescription of heroin to chronic, treatment‐resistant methadone patients in the Netherlands. Journal of Drug Issues 1999;29(3):587‐606. [Google Scholar]
- Brink Wim, Hendriks VM, Blanken P, Huijsman IA, Ree JM. Medical Co‐prescription of Heroin. Two trials. Central Committee on the treatment of heroin addicts ‐ The Netherlands 2002.
CCBH (B) 2002 {published data only}
- Brink W, Hendriks VM, Blanken P, Koeter WJ, Zwieten BJ, Ree JM. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ 2003;327:310‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink W, Hendriks Vincent M, Blanken P, Huijsman IA, Ree Jan M. Medical co‐prescription of Heroin. Two trials. The Hague: Central Committee on the treatment of heroin addicts, 2002. [Google Scholar]
Haasen 2007 {published and unpublished data}
- Haasen C, Verthein U, Degwitz P, Berger J, Krausz M, Naber D. Heroin‐assisted treatment for opioid dependence. Randomised controlled trial. British Journal of Psychiatry 2007;191:55‐62. [DOI] [PubMed] [Google Scholar]
- Löbmann R, Verthein U. Explaining the effectiveness of heroin‐assisted treatment on crime reductions. Law Hum Behav 2009;33(1):83‐95. [DOI] [PubMed] [Google Scholar]
Hartnoll 1980 {published data only}
- Hartnoll RL. Evaluation of heroin maintenance in controlled trial. Archives of General Psychiatry 1980;37:877‐84. [DOI] [PubMed] [Google Scholar]
NAOMI 2009 {published data only}
- Oviedo‐Joekes E, Brissette S, Marsh DC, Lauzon P, Guh D, Anis A, Schechter MT. Diacetylmorphine versus methadone for the treatment of opioid addiction. New England Journal of Medicine 2009;361(8):777‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
PEPSA 2006 {published data only}
- March JC, Oviedo‐Joekes E, Perea‐Milla E, Carrasco F, PEPSA team. Controlled trial of prescribed heroin in the treatment of opioid addiction.. Journal of Substance Abuse Treatment 2006;31(2):203‐11. [DOI] [PubMed] [Google Scholar]
- Perea‐Milla E, Ayçaguer LC, Cerdà JC, Saiz FG, Rivas‐Ruiz F, Danet A, Vallecillo MR, Oviedo‐Joekes E. Efficacy of prescribed injectable diacetylmorphine in the Andalusian trial: Bayesian analysis of responders and non‐responders according to a multi domain outcome index. Trials 2009;10:70. [PMID: 19682360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perneger 1998 {published data only}
- Perneger TV, Giner F, Rio M, Mino A. Randomised trial of heroin maintenance programme for addicts who fail in conventional drug treatments. BMJ 1998;317(7150):13‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RIOTT 2010 {published and unpublished data}
- Nicholas Lintzeris, John Strang, Nicola Metrebian, Sarah Byford, Christopher Hallam, Sally Lee, Deborah Zador and RIOTT Group. Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UK. Harm Reduction Journal 2006;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Metrebian N, Lintzeris N, Potts L, Carnwath T, Mayet S, et al. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial.. Lancet 2010;375(9729):1885‐95. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Battersby 1992 {published data only}
- Battersby M, Farrell M, Gossop M, Robson P, Strang J. Horse trading: Prescribing Injectable Opiates to Opiate Addicts ‐ A descriptive study. Drug and Alcohol Dependence 1992;7:35‐42. [DOI] [PubMed] [Google Scholar]
Fischer 1999 {published data only}
- Fischer G, Reinhold J, Eder H, Gombas W, Etzersdorfer P, Schmidl‐Mohl K, et al. Comparison of methadone and slow release morphine maintenance in pregnant addicts. Addiction 1999;94(2):231‐9. [DOI] [PubMed] [Google Scholar]
Ghodse 1990 {published data only}
- Ghodse AH, Creighton FJ, Bhat AV. Comparison of oral preparations of heroin and methadone to stabilise opiate misusers as inpatients. BMJ 1990;300:719‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haemmig 2001 {published data only}
- Haemmig RB, Tschacher W. Effects of High‐Dose Heroin vs Morphine in Intravenous Drug Users: a Randomised Double‐Blind Crossover Study. Journal of Psychoactive Drugs 2001;33(2):105‐10. [DOI] [PubMed] [Google Scholar]
Hendriks 2001 {published data only}
- Hendriks VM, Brink W, Blanken P, Bosman IJ, Ree JM. Heroin self‐administration by means of 'chasing the dragon?: pharmacodynamics and bioavailability of inhaled heroin. European Neuropsychopharmacology 2001;11:241‐52. [DOI] [PubMed] [Google Scholar]
Jasinski 1986 {published data only}
- Jasinnski DR, Preston KL. Comparison of intravenously administered methadone, morphine and heroin. Drug and Alcohol Dependence 1986;17:301‐10. [DOI] [PubMed] [Google Scholar]
Krausz 1999 {published data only}
- Krausz M, Uchtenhagen A, Brink W. Medically indicated heroin prescription in the treatment of drug addicts [Medizinish indizierte Heroinverschreibung in der Behandlung Drogenabhangiger]. Sucht 1999;45(3):171‐86. [Google Scholar]
McCusker 1996 {published data only}
- McCusker C, Davies M. Prescribing drug of choice to illicit heroin users: the experience of a U.K. community drug team. Journal of Substance Abuse Treatment 1996;13(6):521‐31. [DOI] [PubMed] [Google Scholar]
Mello NK 1980 {published data only}
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self‐administration and the effects of Naltrexone. The journal of pharmacology and experimental therapeutics 1981;216(1):45‐54. [PubMed] [Google Scholar]
Metrebian 1998 {published data only}
- Metrebian N, Shanahn W, Wells B, Stimson G. Feasibility of prescribing injectable heroin and methadone to opiate‐dependent drug users: associated health gains and harm reductions. Medical Journal of Australia 1998;168:596‐600. [DOI] [PubMed] [Google Scholar]
Mitchell 2002 {published data only}
- Mitchell TB, White JM, Somogyl AA, Bochner Felix. Slow‐release oral morphine versus methadone: a crossover comparison of patient outcomes and acceptability as maintenance pharmacotherapies for opiod dependence. Addiction 2004;99(8):940. [DOI] [PubMed] [Google Scholar]
Moldovanyi 1996 {published data only}
- Moldovany A, Ladewig D, Affentranger P, Natsch C, Stohler R. Morphine maintenance treatment of opioid‐dependent out‐patients. European Addiction Research 1996;2:208‐12. [Google Scholar]
Oppenheimer 1982 {published data only}
- Oppenheimer E, Stimson GV. Seven year follow‐up of heroin addicts: life history summarised. Drug and Alcohol Dependence 1982;9:153‐9. [DOI] [PubMed] [Google Scholar]
Rehm 2001 {published data only}
- Rehm J, Gschwend P, Steffen T, Gutzwiller F, Dobler‐Mikola A, Uchtenhagen A. Feasibility, safety, and efficacy of injectable heroin prescription for refractory opioid addicts : a follow‐up study. The Lancet 2001;358:1417‐20. [DOI] [PubMed] [Google Scholar]
Strang 2000 {published data only}
- Strang J, Marsden J, Cummins M, Farrell M, Finch E, Gossop M, et al. Randomized trial of supervised injectable versus oral methadone maintenance: report of feasibility and 6‐month outcome. Addiction 2000;95(11):1631‐45. [DOI] [PubMed] [Google Scholar]
Uchtenhagen 1999 {published data only}
- Uchtnehagen A, Dobler‐Mikola A, Steffen T, Gutzwiller F, Blattler R, Pfeifer S. Prescription of Narcotics for Heroin Addicts. Vol. 1, Basel: Karger, 1999. [Google Scholar]
References to ongoing studies
Universite' de Liege 2010 {published data only}
- Projet pilote de traitement assisté par diacétylmorphine: comparaison entre un traitement par diacétylmorphine et un traitement par méthadone.. Ongoing study 2007 expected results: 2010.
Additional references
Bammer 1999
- Bammer G, Dobler‐Mikola A, Philip M, Strang J, Uchtenhagen A. The heroin prescribing debate: integrating science and politics. Science 1999;284(5418):1277‐8. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- CONSORT group. How CONSORT began. CONSORT‐Statement.org last updated 2 April 2008:URL: http://www.consort‐statement.org/about‐consort/history/.
Davoli 2000
- Davoli M. Health Regional Plan 2000‐2. Piano Sanitario Regionale triennio 2000‐2. Assessorato alla salvaguardia e Cura della Salute Regione Lazio, 2000. [Google Scholar]
Dijkgraaf 2005
- Dijkgraaf MG, Zanden BP, Borgie CA, Blanken P, Ree JM, Brink W. Cost utility analysis of co‐prescribed heroin compared with methadone maintenance treatment in heroin addicts in two randomised trials. BMJ 2005;330(4):1297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
DSM‐IV 1994
- American Psychiatric Association (Pub.). Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Egli 2009
- Egli N, Pina M, Skovbo Christensen P, Aebi MF, Killias M. Effects of drug substitution programs on offending among drug‐addicts. Campbell Systematic Reviews 2009, Issue 3. [Google Scholar]
EMCDDA 2008
- Scalia Tomba GP, Rossi C, Taylor C, Klempova D, Wiessing L. Guidelines for Estimating the Incidence ofProblem Drug Use. Lisbon: EMCDDA, 2008. [ISBN 92‐9168‐097‐4] [Google Scholar]
EMCDDA 2009
- EMCDDA 2009. Annual report on the state of the drugs problem in Europe. Lisbon: EMCDDA, 2009. [Google Scholar]
EMCDDA 2010 a
- EMCDDA. Trends in injecting drug use in Europe. Selected Issue. Lisbon: EMCDDA, 2010. [Google Scholar]
EMCDDA 2010 b
- EMCDDA. Treatment and care for older drug users. Selected Issue. November. Lisbon: EMCDDA, 2010. [Google Scholar]
Farnbacher 2002
- Farnbacher G, Basdekis‐Josza R, Krausz, M. Psychoeducation as a method in addiction services. In Practice, Legislation and Policy on Drugs [Psychoedukation als Methode in derDrogenhilfe]. In: L.Boellinger, H. Stover editor(s). Drogenpraxis Drogenrecht Drogenpolitik. Fachhochschulverlag, 2002:386‐402. [Google Scholar]
Farrell 1994
- Farrell M, Ward J, Mattick RP, Hall W, Stimson GV, Jarlais D, et al. Fortnightly Review: Methadone maintenance treatment in opiate dependence: a review. BMJ 1994;309:997‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 1998
- Farrell M, Hall W. The Swiss heroin trials: testing alternative approaches. BMJ 1998;316:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 1995
- Goldstein A, Herrera J. Heroin addicts and methadone treatment in Albuquerque: a 22 year follow‐up. Drug and Alcohol Dependence 1995;40:139‐50. [DOI] [PubMed] [Google Scholar]
Gutzwiller 2000
- Gutzwiller F, Steffen T eds. Cost‐benefit analysis of heroin maintenance treatment. Medicalprescription of narcotics.. Vol. 2, Basel: Karger, 2000. [Google Scholar]
Haasen 2010
- C. Haasen U. Verthein F.J. Eiroa‐Orosa I. Schäfer J. Reimer. Is Heroin‐Assisted Treatment Effective for Patientswith No Previous Maintenance Treatment? Resultsfrom a German Randomised Controlled Trial. European Addiction Research 2010;16:124–30. [DOI] [PubMed] [Google Scholar]
Hartnoll 1999
- Hartnoll Richard. Heroin Prescription and public health research. Epidemiological problems. Abstract book. International Symposium: heroin assisted treatment for dependent drug users. University of Bern, Switzerland 10‐12 March 1999. 1999.
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2009]. The Cochrane Collaboration Available from www.cochrane‐handbook.org. September 2008.
Hser 1993
- Hser Y, Anglin MD, Powers K. A 24‐follow‐up of California narcotic addicts. Archives of General Psychiatry 1993;50:577‐84. [DOI] [PubMed] [Google Scholar]
Katzung 1999
- Katzung BC. Basic and Clinical Pharmacology. London: Prentice Hall International, 1999. [Google Scholar]
Kokkevi 1995
- Kokkevi, A, Hartgers. C. EuropASI: European adaptation of a multidimensional assessment instrument for drug and alcohol dependence. European Addiction Research 1995;1:208‐210. [info available at: http://www.emcdda.europa.eu/html.cfm/index3647EN.html] [Google Scholar]
McLellan 1992
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 1992;9(3):199‐213. [information at: http://www.emcdda.europa.eu/html.cfm/index3538EN.html] [DOI] [PubMed] [Google Scholar]
McLellan 2000
- McLellan T, Lewis DC, O'Brien CP, Kleber HD. Drug Dependence, a Chronic Medical Illness. Implications for Treatment, Insurance, and Outcomes Evaluation. Journal of American Medical Association 2000;284(13):1689‐95. [DOI] [PubMed] [Google Scholar]
O'Brien 1997
- O'Brien CP. A range of research‐based pharmacotherapies for addiction. Science 1997;278(5335):66‐70. [DOI] [PubMed] [Google Scholar]
Oliva 2001
- Oliva H, Gorgen W, Schlanstedt G, et al. Case Management in der Suchtkranken‐ und Drogenhilfe. Nomos, 2001. [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre. Review Manager. Version 5.0. Copenhagen: The Cochrane Collaboration, 2008.
Rolleston 1926
- Departmental Committee on Morphine and Heroin Addiction. Rolleston Report of the Departmental Committee on Morphine and Heroin Addiction. London: Ministry of Health, 1926. [Google Scholar]
Schellings 1999
- Schellings R, Kessels AG, ter Riet G, Sturmans F. The Zelen design may be the best choice for a heroin‐provision experiment. Journal of Clinical Epidemiology 1999;6(52):503‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strang 1994
- Strang J, Ruben S, Farrell M, Gossop M. Prescribing Heroin and other Injectable Drugs. In: John Strang, Michael Gossop editor(s). Heroin Addiction and Drug Policy The British System. Oxford University Press, 1994:192‐206. [Google Scholar]
UNODC 2007
- United Nations Office on Drugs and Crime. World Drug Report. New York USA: United Nation, 2007. [Google Scholar]
UNODC 2010
- UNODC. 2010 Report: Promoting health,security and justice. New York USA: United Nations, 2010. [Google Scholar]
van den Brink 1999
- Brink W, Hendriks VM, Ree JM. Medical co‐prescription of heroin to chronic treatment‐resistant methadone patients in The Netherlands. Journal of Drug Issues 1999;29(3):587‐608. [Google Scholar]
Venning 1998
- Venning GR. Trial is needed comparing decriminalisation of heroin with existing policy of prohibition. BMJ 1998;317:1011. [PMC free article] [PubMed] [Google Scholar]
Ward 1999
- Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. The Lancet 1999;353(9148):221‐6. [DOI] [PubMed] [Google Scholar]
Weil 1998
- Weil A, Rosen W. From chocolate to morphine. 3rd Edition. Boston New York: Houghton Mifflin Company, 1998. [Google Scholar]
WHO 1993
- WHO Expert Committee on Drug Dependence. World Drug Report 28th. Vol. 836, Geneva: World Health Organization, 1997. [ISBN 92 4 120 836] [Google Scholar]
WHO 2009
- WHO. Guidelines for the PsychosociallyAssisted Pharmacological Treatmentof Opioid Dependence. Geneva: World Health Organization, 2009. [Google Scholar]
Wodak 1998
- Wodak Alex. Further studies of heroin treatment are needed. BMJ 1998;317:1011. [PubMed] [Google Scholar]
References to other published versions of this review
Ferri 2005
- Ferri M, Davoli M, Perucci CA. Heroin maintenance for chronic heroin dependents. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003410.pub2] [DOI] [PubMed] [Google Scholar]


