Abstract
Background
Although minimally invasive surgery has been accepted for a variety of disorders, laparoscopic resection of colorectal cancer is performed by few. Concern about oncological radicality and long term outcome has limited the adoption of laparoscopic surgery for colorectal cancer.
Objectives
To determine long‐term outcome after laparoscopically‐assisted versus open surgery for non‐metastasised colorectal cancer.
Search methods
The Cochrane library, EMBASE, Pub med and Cancer Lit were searched for published and unpublished randomised controlled trials.
Selection criteria
Randomised clinical trials comparing laparoscopically‐assisted and open surgery for non‐metastasised colorectal cancer were included. Studies that did not report any long‐term outcomes were excluded.
Data collection and analysis
Two reviewers independently assessed the studies and extracted data. RevMan 4.2 was used for statistical analysis.
Main results
Thirty‐three randomised clinical trials (RCT) comparing laparoscopically‐assisted versus open surgery for colorectal cancer were identified. Twelve of these trials, involving 3346 patients, reported long‐term outcome and were included in the current analysis. No significant differences in the occurrence of incisional hernia, reoperations for incisional hernia or reoperations for adhesions were found between laparoscopically assisted and open surgery (2 RCT, 474 pts, 7.9% vs 10.9%;P = 0.32 and 2 RCT, 474 pts, 4.0% vs 2.8%; P = 0.42 and 1 RCT, 391 pts, 1.1% vs 2.5%;P = 0.30, respectively). Rates of recurrence at the site of the primary tumor were similar (colon cancer: 4 RCT, 938 pts, 5.2% vs 5.6%; OR (fixed) 0.84 (95% CI 0.47 to 1.52)(P = 0.57); rectal cancer: 4 RCT, 714 pts, 7.2% vs 7.7%; OR (fixed) 0.81 (95% CI 0.45 to 1.43) (P = 0.46). No differences in the occurrence of port‐site/wound recurrences were observed (P=0.16). Similar cancer‐related mortality was found after laparoscopic surgery compared to open surgery ( colon cancer: 5 RCT, 1575 pts, 14.6% vs 16.4%; OR (fixed) 0.80 (95% CI 0.61 to 1.06) (P=0.15); rectal cancer: 3 RCT, 578 pts, 9.2% vs 10.0%; OR (fixed) 0.66 (95% CI 0.37 to 1.19) (P=0.16). Four studies were included in the meta‐analyses on hazard ratios for tumour recurrence in laparoscopic colorectal cancer surgery. No significant difference in recurrence rate was observed between laparoscopic and open surgery (hazard ratio for tumour recurrence in the laparoscopic group 0.92; 95% CI 0.76‐1.13). No significant difference in tumour recurrence between laparoscopic and open surgery for colon cancer was observed (hazard ratio for tumour recurrence in the laparoscopic group 0.86; 95% CI 0.70‐1.08).
Authors' conclusions
Laparoscopic resection of carcinoma of the colon is associated with a long term outcome no different from that of open colectomy. Further studies are required to determine whether the incidence of incisional hernias and adhesions is affected by method of approach. Laparoscopic surgery for cancer of the upper rectum is feasible, but more randomised trials need to be conducted to assess long term outcome.
Keywords: Humans; Laparoscopy; Laparoscopy/adverse effects; Colonic Neoplasms; Colonic Neoplasms/surgery; Hernia, Ventral; Hernia, Ventral/etiology; Randomized Controlled Trials as Topic; Rectal Neoplasms; Rectal Neoplasms/surgery
Plain language summary
This systematic review focuses on long‐term outcome of laparoscopic versus open surgery for colorectal cancer, including long‐term complications and cancer outcome.
Laparoscopic resection of carcinoma of the colon is associated with a long term outcome no different from that of open colectomy. In the case of rectal cancer, data on long term outcome are scarce and the results of large randomised trails have to be awaited. Laparoscopic approach offers short‐term benefits to patients, such as less pain and quicker recovery. However, concern about port‐site metastases (laparoscopic incision wound) and irradical laparoscopic resections withheld many surgeons from performing laparoscopic surgery for cancer. Minimally invasive surgery for colon and rectal cancer has mainly been performed within the framework of randomized clinical trials.
Background
Colorectal cancer is one of the most common types of cancer in the industrialised nations of Europe, America, Asia and Australia. Radical resection of the tumour‐bearing bowel segment allowing sufficient resection margins and removal of regional lymph nodes is the gold standard in surgery for cancer of the colon and rectum. Five year survival rates after R0‐resection of colorectal cancer vary from almost 100% in patients with tumours staged as UICC I to 50% in patients with lymph node metastases staged as UICC III (Ries 2000). Currently, conventional surgery via laparotomy remains the procedure of choice for elective colorectal resection in both benign and malignant disease. The evolution of minimally invasive surgery allowed laparoscopic colorectal resections, which were first described in 1991(Jacobs 1991; Franklin 1993). During the last decade, laparoscopic colorectal cancer surgery has been mainly performed within randomised trials. Concerns about irradical laparoscopic resections, as was suggested in the early nineties by reports of case series of port‐site metastases (Nduka 1994,Berends 1994), withheld many surgeons from incorporating laparoscopy for colorectal cancer in clinical practice. Short‐term advantages of laparoscopic colorectal surgery compared to conventional surgery include less pain, better pulmonary function, shorter duration of postoperative ileus, less fatigue and a better quality of life (Franklin 1993; Lacy 1995; Milsom 1998; Schwenk 1998). However, the first long‐term results of large, randomised clinical trials comparing laparoscopic and open surgery or colorectal malignancy were published only recently (Lacy 2002; COST 2004; Leung 2004; Braga 2005; Jayne 2007). The aim of this systematic review of randomised controlled trials is to evaluate the long‐term results of laparoscopic and conventional colorectal resection. Cancer outcome, reoperations for adhesions and incisional hernias were studied.
Objectives
Evaluation of long‐term outcome after elective laparoscopic and conventional resection of colorectal cancer.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials that reported long‐term results after laparoscopic and conventional resection of non‐metastasised colorectal carcinoma. Trials that allocated patients according to the availability of staff or instruments or the kind of day (odd or even) were excluded from the analysis. If the method of randomisation was not specified, if a trial was only reported as an abstract or if no measure was given for an outcome variable, the authors were contacted to retrieve more detailed information of the study. When the author could not provide all details of their study methodology, the trial was excluded from further analysis. The decision to exclude a trial was discussed between three observers and disagreements were resolved by discussion. Trials were included irrespectively of the language of publication.
Types of participants
All patients with colorectal cancer undergoing R0‐resection. Patients who underwent transanal excision of rectal cancer were excluded.
Types of interventions
Laparoscopic or laparoscopic‐assisted colorectal resection with intraperitoneal gas insufflation or mechanical abdominal wall lift. Anastomosis were either performed intracorporeally (i. e. 'double‐stapled' colorectal anastomosis) or extracorporeally (i. e. handsewn or stapled).
Types of outcome measures
The following prognostically relevant data were collected in all randomised controlled trials: ‐ neoadjuvant therapy (yes/no), ‐ tumour localisation (colon, rectum), ‐ type of resection (right‐sided, left‐sided, rectal, abdominoperineal excision, extended colectomy, other) ‐ tumour stage (UICC stage I ‐ IV) ‐ number of lymph nodes harvested ‐ resection margins (positive/negative) ‐ adjuvant therapy (yes/no) ‐ total mesorectal excision (for rectal cancer only)
The following outcome measures were collected in all randomised controlled trials: ‐ duration of follow‐up, ‐ incidence of incisional hernia, ‐ incidence of reoperations for incisional hernia or intraperitoneal adhesions, ‐ incidence of local tumour recurrence , ‐ incidence of metachronous metastatic disease and location of metastases, ‐ recurrence free survival and overall survival.
Search methods for identification of studies
See: Collaborative Review Group search strategy. Published and unpublished randomised controlled trials were searched for with no restriction on language in the following electronic databases: Cochrane Library, MEDLINE, EMBASE and CancerLit for the years 1991 to 2005. Search strategies for MEDLINE and EMBASE are shown in Table 1 and Table 2. Searches were carried out using medical subject headings (MeSH) and free text words in combination with the search strategy for randomised controlled trials described by Dickersin et al. (Dickersin 1994). This search was adapted for each database:
1. Search strategy MEDLINE.
| Search |
| #10 #9 not animal #9 #1 and #2 and #3 and #7 and #8 #8 (laparoscop*) #7 Search (colectom*) or (restorative proctocolectomy) or (surgery) or (resection) or (Total mesorectal excision) #6 Search #8 not animal #5 Search #1 and #2 and #3 and #4 #4 Search (laparoscop*) or (colectom*) or (restorative proctocolectomy) or (surgery) or (resection) or (Total mesorectal excision) #3 Search (rect*) or (colorect*) or (colon) or (large intestine) #2 Search neoplasm* or cancer or tumor or tumour or carcinom* or malignan* or laparoscopy or adenomas #1 Search random* or blind* or meta‐analysis or placebo* |
2. Search strategy EMBASE.
| Search |
| #10 #9 not animal #9 #1 and #2 and #3 and #7 and #8 #8 (laparoscop*) #7 (colectom*) or (restorative proctocolectomy) or (surgery) or (resection) or (Total mesorectal exciscion) #6 #5 not animal #5 #1 and #2 and #3 and #4 #4 (laparoscop*) or (colectom*) or (restorative proctocolectomy) or (surgery) or (resection) or (Total mesorectal excision) #3 (rect*) or (colorect*) or (colon) or (large intestine) #2 neoplasm* or cancer or tumor or tumour or carcinom* or malignan* or laparoscopy or adenomas #1 random* or blind* or meta‐analysis or placebo* |
1.Laparoscopy [MeSH] 2.Surgery [MeSH] 3.Colon [MeSH] 4.Colectomy [MeSH] 5.Intestine, Large [MeSH] 6.Restorative Proctocolectomy [MeSH] 7.Colonic Neoplasms [MeSH] 8.Rectal Neoplasms [MeSH]
The following journals were hand‐searched from 1991 to 2004 for randomised controlled trials or clinical controlled trials: British Journal of Surgery, Archives of Surgery, Annals of Surgery, Surgery, World Journal of Surgery, Disease of Colon and Rectum, Surgical Endoscopy, International Journal of Colorectal Disease, Langenbeck's Archives of Surgery, Der Chirurg, Zentralblatt für Chirurgie, Aktuelle Chirurgie/Viszeralchirurgie. Further, abstracts from the following society meetings were handsearched from 1991 to 2004: American College of Surgeons, American Society of Colorectal Surgeons, Royal Society of Surgeons, British Association of Coloproctology, Society of American Gastrointestinal Endoscopic Surgeons , European Association of Endoscopic Surgeons, Asian Society of Endoscopic Surgeons. The reference lists of all relevant articles were searched for further relevant trials. All authors of identified randomised controlled trials were contacted to evaluate whether they had any information on any other recent or ongoing trials. Local opinion leaders in Europe, America and Asia were contacted with the same question.
Data collection and analysis
All studies that met the selection criteria were assessed for methodological quality and details of the randomisation process by two reviewers. In case of differences in opinion, a third reviewer was contacted. All non‐randomised studies were excluded. Each included trial was read independently by two investigators for the criteria: concealed randomisation, time of randomisation (preoperatively, intraoperatively), number of randomised patients, number of patients not randomised and reasons for this, exclusion after randomisation, dealing with drop outs, blinding of patient and observer, and data analysis based on the 'intention‐to‐treat'‐principle. The Halpern and Presley quality score was used to determine quality of the randomised trails (Halpern 1994). Two observers independently extracted the results from each paper; disagreements were resolved by discussion. The software 'REVMAN 4.2 provided by the Cochrane Collaboration was used for statistical analysis. Mean differences with their corresponding 95% confidence intervals were used for the analysis of continuous variables. If no measure of dispersion was given, we would try to obtain these data from the authors. For dichotomous variables odds ratios with their 95% confidence intervals were calculated. Analyses were performed using fixed effects models. Due to the large number of small studies that were included, these analyses were compared with analyses using random effects models. Survival data were extracted by measuring survival curves at predetermined time points, by recording median survival times, and by recording published p‐values for comparisons of survival curves based on Mantel Cox log rank test, Cox model (univariate) or similar statistical tests. Hazard ratios computed by independent study authors (Lacy 2002; COST 2004) were weighed together with approximate hazard ratios computed from reported log‐rank test (Liang 2007; Leung 2004) according to Parmar and colleagues (Parmar 1998).
Results
Description of studies
A total of 33 published randomised controlled trials comparing laparoscopically‐assisted and open surgery for colorectal cancer were identified. These trials were described in 46 different papers. Most of the studies were excluded from the analysis due to the fact that the articles only reported data on short‐term outcome (see Table `Characteristics of excluded studies`). In four cases, articles were excluded because they did not describe any of the long‐term outcomes sought in this analysis or because patients were also included in another article or ongoing trial (Lacy 1998; Weeks 2002; Jayne 2005; Janson 2004; Guillou 2005). Twelve trials, involving 3346 patients, were included in the current analysis. All of the included studies were published as full articles. Baseline characteristics of included studies are described in the Table `Characteristics of included studies`. Most of the studies included patients with colorectal malignancies only. The study by Milsom and colleagues (Milsom 1998) also included patients with benign lesions. Separate data for cancer patients were in this case only available for pathology and survival. Most studies (n=6) only included patients with colon cancer (COST 2004; Curet 2000; Kaiser 2004; Lacy 2002; Winslow 2002; Liang 2007). Two studies focused on patients with rectal cancer (Araujo 2003, Zhou 2004). In four trials, patients with cancer of the rectum and patients with colon cancer were eligible for inclusion (Braga 2005; Milsom 1998; Leung 2004; Jayne 2007). Although all studies reported long‐term outcome, not all of them could be included in the survival analysis. Two studies reported data on local tumour recurrence only (Araujo 2003, Zhou 2004), while two other studies described number of patients with distant metastases and local tumour recurrence (Milsom 1998, Liang 2007). One study focussed on incidences of incisional hernia and reoperations (Winslow 2002). In three of the studies that did report survival, follow‐up was too short to be able to include data in the 5‐year survival analysis (Curet 2000; Kaiser 2004; Jayne 2007). Four studies reported 5‐year survival data (Braga 2005; COST 2004; Lacy 2002; Leung 2004). Data on incisional hernias and adhesions after laparoscopically‐assisted and open surgery for colorectal cancer were scarce. Two trials reported data on incisional hernias (Braga 2005; Winslow 2002) and data on adhesions were described in one case (Braga 2005). Five ongoing trials with primary endpoints on survival were identified (see Table `Characteristics of ongoing trials`). In three of these trials, patient recruitment has been terminated and 3‐year survival data of these studies can be expected in the near future.
Risk of bias in included studies
(See `Characteristics of included studies`)
The Halpern and Preston quality score was used to assess the methodological quality of the studies included (. This score uses the following determinants of trial quality: ‐Allocation concealment ‐Blinding of outcome assessment ‐Availability of an adequate description of outcome measures, included\excluded patients and reasons for exclusion ‐Description of statistical tests, p‐values and sample size calculations ‐Appropriateness of the used statistical tests ‐Availability of a full description of treatment and control groups The maximum obtainable score is 22. Quality varied greatly between different trials, as demonstrated by the difference in scores obtained (range 13‐20).
Randomisation and allocation concealment Seven trials had adequate randomisation and allocation concealment (Braga 2005; COST 2004; Curet 2000; Lacy 2002; Leung 2004; Liang 2007; Jayne 2007). In three cases, sealed numbered envelopes were used in the randomisation process (Braga 2005; Curet 2000; Lacy 2002). In three studies, random sequences were kept concealed, either by an independent operating theatre coordinator (Leung 2004), or by a coordinator based at a central office (COST 2004; Jayne 2007). In one study, block randomisation was used and random numbers were generated by an independent research assistant (Liang 2007). The exact method of randomisation was unclear in four trials and the articles only stated that allocation had been `randomised` (Araujo 2003; Kaiser 2004; Winslow 2002; Zhou 2004). In one trial (Milsom 1998), patients were randomised after diagnostic laparoscopy had been performed. When it was considered too difficult to operate patients laparoscopically, due to for instance adhesions, patients were operated by means of a laparotomy and not included in the study. This method of randomisation could have had a positive effect on outcomes obtained after laparoscopy. In only three cases, data on patients that had not been randomised were available (Lacy 2002; Leung 2004; Milsom 1998). In the other trials, it was unclear whether all patients with colorectal cancer were eligible for inclusion, or whether there were patients that were excluded but not reported on.
Blinding Because of the nature of the trials, it was impossible to perform an analysis in which the patients are blinded for the performed procedure. It was unclear in most of the studies, whether the person assessing the data was unaware of the assigned procedure. Only one article reported blinding of the statistician (Curet 2000).
Description of inclusion/exclusion criteria and reasons for exclusion All studies included in this analysis contain adequate descriptions of inclusion and exclusion criteria. Reasons for excluding patients after randomisation are given in all of the studies. In three studies, no patients were excluded after randomisation. In the other trials, a total number of 93 patients were excluded. The most common reasons were: metastases discovered during surgery (n=62), withdrawal of informed consent (n=11) and lost to follow‐up (n=9). In one study (Milsom 1998), four patients in whom the procedure was converted to open surgery were excluded from the analysis. No data on outcome in these patients is available. In another study (COST 2004), 7 patients were excluded from further analysis because they did not receive the allocated procedure.
Description of statistical tests and p‐values An adequate description of the statistical tests used was available in all cases. In two studies, p‐values were not reported (Curet 2000;Kaiser 2004).
Sample size calculations Sample size calculations were performed in only six trials (COST 2004;Leung 2004; Milsom 1998; Lacy 2002; Liang 2007; Jayne 2007).
Intention‐to‐treat Although intention‐to‐treat was not a determinant in the Halpern and Presley quality score, we did report it in `Characteristics of included studies`, since we consider it to be important for the interpretation of the data. In most trials, data were analysed based on the `intention‐to‐treat`‐principle. In two studies (Kaiser 2004; Curet 2000), converted procedures were analysed separately. In one of these two trials (Kaiser 2004), the conversion rate was 45%, which is high compared to conversion rates in other studies. For the current analysis we included data on converted procedures in the laparoscopic arm of the trial. In the study by Milsom et al. (Milsom 1998) patients (n=4) in whom the procedure was converted were excluded from further analysis. Since no data on this group were available, it was impossible in this case to perform an analysis based on intention‐to‐treat. In one study, converted laparoscopic procedures were included in the open arm of the trial (Winslow 2002).
Effects of interventions
In total, 33 randomised clinical trials (46 citations) were identified. Twelve of these trials, involving 3346 patients, reported long‐term outcome data and were included in the analysis. Six of the included studies (50%) had more than 200 participants (Braga 2005; COST 2004; Lacy 2002; Leung 2004; Liang 2007; Jayne 2007). Four studies (33%) had fewer than 50 participants (Araujo 2003; Curet 2000; Kaiser 2004; Winslow 2002). There was no difference between the laparoscopic or open group regarding the number of patients receiving adjuvant chemotherapy (P=0.35). In one study, all patients that were included had received neoadjuvant therapy (Araujo 2003). For details regarding adjuvant chemotherapy see analysis 01.01. In some of the articles, preoperative localisation of the tumour was reported (Winslow 2002; COST 2004; Kaiser 2004), whereas others reported type of resection (Braga 2005) and again others reported both (Curet 2000; Lacy 2002; Araujo 2003; Zhou 2004; Liang 2007; Jayne 2007). In one study that included both malignant and benign disease, no separate data were given on localisation or type of resection of patients with cancer (Milsom 1998). This study could therefore not be included in the analyses on localisation and type of resection. No significant differences were found in the distribution of the tumours or the types of resections performed. For details see analyses 02.01‐03.02. In 11 out of 12 studies (92 %), data on tumour stage were provided. No significant differences in the distribution of stage 0, I, II, III and IV tumours were present between laparoscopic and open groups (See analyses 05.01‐05.05). Only two studies (Zhou 2004; Jayne 2007) reported whether rectal resections were performed according to TME principles. The other studies that included patients with rectal cancer (Araujo 2003; Braga 2005; Leung 2004) did not state whether TME principles were applied or not.
Number of lymph nodes harvested and number of positive resection margins (See analyses 06.01‐07.01) Nine studies reported data on the number of lymph nodes harvested during the surgical procedure. Three studies only reported median number and ranges and were therefore excluded from the analysis. For details on median number of lymph nodes harvested in excluded studies see Table 06.02. The analysis of the remaining data (6 RCT, 1358 pts) showed that significantly fewer lymph nodes were harvested in patients undergoing laparoscopic surgery: the calculated weighted mean difference (WMD) was ‐1.00 (95% CI ‐1.65 to ‐0.35) (P = 0.003). When using random effects, no significant differences were found (p=0.05). Six trials, including a total number of 2347 patients, reported data on resection margins (Milsom 1998; COST 2004; Kaiser 2004; Zhou 2004; Braga 2005; Jayne 2007). In 5 of these studies, pathological examination of the resected specimens showed negative margins (See analysis 07.01).
Incisional hernia, reoperations for hernia and reoperations for intraperitoneal adhesions (See analyses 09.01‐10.02) In two articles, data on the occurrence of incisional hernia and reoperations for incisional hernia or intraperitoneal adhesions were reported. The analysis on the occurrence of incisional hernia included two studies (Braga 2005; Winslow 2002) with a total number of 474 patients. No significant difference between laparoscopic and open surgery was observed (7.9% vs 10.9%; OR (fixed) 0.72 (95% CI 0.38 to 1.37) (P = 0.32). The same two studies reported reoperations for incisional hernias. No significant difference in the number of reoperations for incisional hernias was found (4.0% vs 2.8%; OR (fixed) 1.52 (95% CI 0.55 to 4.19) (P = 0.42). Only one study including 391 patients reported reoperations for intraperitoneal adhesions (Braga 2005). Again, no significant differences were observed between the laparoscopic and the open arm (1.1% vs 2.5%; OR (fixed) 0.42 (95% CI 0.08 to 2.18) (P = 0.30).
Local recurrences and distant metastases (See analyses 11.01‐12.03) Local recurrences were subdivided into port‐site and wound recurrences, peritoneal recurrences and recurrences at the site of the primary tumour. No significant difference was observed in recurrences at the site of the primary tumour between patient receiving open and laparoscopic surgery (8 RCT, 1987 pts, 5.2% vs 5.3%; OR (fixed) 0.81 (95% CI 0.54 to 1.22) (P = 0.31). Separate analyses for colon and rectal cancer showed no significant differences between laparoscopic and open procedures (for colon cancer: 4 RCT, 938 pts, 5.2% vs 5.6%; OR (fixed) 0.84(95% CI 0.47 to 1.52)(P = 0.57); for rectal cancer: 4 RCT, 714 pts, 7.2% vs 7.7%; OR (fixed) 0.81 (95% CI 0.45 to 1.43) (P = 0.46). No significant differences in the occurrence of port‐site and wound metastases or peritoneal metastases were observed (see comparison 13.07‐13.12). No significant difference in the development of distant metastases was found in colorectal cancer patients, when comparing laparoscopic and open surgery ( 7 RCT, 1853 pts, 13.2% vs 12.6%; OR (fixed) 1.01 (95% CI 0.76 to 1.34) (P = 0.93). When analysing patients with colon and rectal cancer separately, no significant differences were found (for colon cancer: 4 RCT, 938 pts, 11.3% vs 13.6%; OR (fixed) 0.82 (95% CI 0.55 to 1.22) (P = 0.32), for rectal cancer: 3 RCT, 578 pts, 13.5% vs 9.1%; OR (fixed) 1.16 (95% CI 0.66 to 2.05) (P=0.60). Only two studies reported localisation of distant metastases (Kaiser 2004; Liang 2007).
Four studies were included in the meta‐analyses on hazard ratios for tumour recurrence in laparoscopic colorectal cancer surgery. No significant difference in recurrence rate was observed between laparoscopic and open surgery (hazard ratio for tumour recurrence in the laparoscopic group 0.92; 95% CI 0.76‐1.13). No significant difference in tumour recurrence between laparoscopic and open surgery for colon cancer was observed (hazard ratio for tumour recurrence in the laparoscopic group 0.86; 95% CI 0.70‐1.08). Only one study reported hazard ratios for tumour recurrence in rectal cancer patients (Leung 2004). Cancer‐related mortality and overall mortality (See analyses 13.01‐14.03) The majority of the studies (9 out of 12) reported cancer‐related and/or overall mortality at maximum follow‐up. For follow‐up periods of different studies see table 10.01. No significant differences between laparoscopic and open surgery were found in cancer‐related mortality during the follow‐up period of the study (8 RCT, 2490 pts, 13.4% vs 14.4%; OR (fixed) 0.84 (95% CI 0.767 to 1.06) (P = 0.15). In colon cancer patients, cancer‐related mortality was similar after laparoscopic surgery compared to open surgery (5 RCT, 1575 pts, 14.6% vs 16.4%; OR (fixed) 0.80 (95% CI 0.61 to 1.06) (P=0.12). Only 3 studies reported data on cancer‐related mortality after laparoscopic vs open surgery for rectal cancer. No significant differences were found (578 pts, 9.2% vs 10.0%; OR (fixed) 0.66 (95% CI 0.37 to 1.19) (P=0.16). After analysis of data on 2881 patients wit colorectal cancer (9 RCT), overall mortality turned out to be equal in patients who had undergone laparoscopic surgery as compared to patients who underwent open surgery (23.7% vs 25.5%; OR (fixed) 0.84 (95% CI 0.70 to 1.00); P = 0.05). Insufficient data were available to allow for a separate analysis in rectal cancer patients. In 1162 patients with colon cancer (4 RCT), no significant difference in overall mortality after laparoscopic or open surgery was observed (20.4% vs 23.6; OR (fixed) 0.82 (95% CI 0.62 to 1.09) (P = 0.17). Overall mortality for colorectal cancer patients was not significantly different in the laparoscopic and open arm (hazard ratio for overall mortality after laparoscopic surgery 0.89 (95% CI 0.72‐1.08). In colon cancer patients, no significant differences were found (hazard ratio for overall mortality after laparoscopic surgery 0.86 (95% CI 0.86‐1.07).
Disease‐free and overall survival at 5 years (See 15.01‐16.02) For details on survival data reported by individual trials see tables 17.01 and 18.01. For P‐values see tables 17.02 and 18.02. Only 2 studies reported mean follow‐up for each arm. Corresponding authors of studies reporting long‐term data on recurrence (Araujo 2003; Braga 2005; COST 2004; Curet 2000; Kaiser 2004; Lacy 2002; Leung 2004; Milsom 1998; Zhou 2004) were contacted by e‐mail and asked if they could supply us with additional data. Only two authors responded to our mails. In one case, data on mean follow‐up were supplied (COST 2004). In the other case, the author replied that access to data of the trial was no longer available (Curet 2000).
When performing all the analyses using random effects, none of the non‐significant differences became significant. The significant differences in number of lymph‐nodes harvested between laparoscopic and open surgery for colorectal cancer became non‐significant (p=0.05). To show a difference of 15% in 5‐year survival (from 60% to 75%) with 80% probability and with 5% significance, 150 patients are needed in each group. This number is reached for most of the analyses, even without including patients from the COST study.
Discussion
The introduction of laparoscopy caused a wave of changes in abdominal surgery. For several types of surgical procedures, such as cholecystectomy, Nissen fundoplication, appendectomy and gastric bypass, the laparoscopic approach is now preferred. In a recent Cochrane meta‐analysis on short‐term outcome after laparoscopic colorectal surgery, a laparoscopic approach was found to be associated with increased operating time and less intraoperative blood loss compared to open surgery. Furthermore, postoperative pain was less, duration of postoperative ileus shorter, pulmonary function improved, morbidity decreased, and quality of life in the first month was better after laparoscopy compared to open surgery (Schwenk 2005). The authors concluded that if long term outcome of laparoscopic and open procedures showed equivalent results, the laparoscopic approach should be preferred in colorectal cancer surgery. The current Cochrane review focuses on long‐term outcome after laparoscopic compared to open surgery for colorectal cancer. Besides clear long‐term results, such as survival and long‐term complications, factors that could have had an impact on the risk of recurrence, i.e. incidence of positive resection margins and number of harvested lymph nodes, have been analysed. In 5 out of 6 studies that report data on resection margins, none of the margins were found to be positive. Although his is a remarkable finding, it can be explained by the fact that most of the studies (COST 2004; Kaiser 2004; Milsom 1998; Zhou 2004) only reported distal and proximal margins. No data on circumferential margins were available in these cases. The number of lymph nodes harvested during the surgical procedure influences staging of the tumour. The number of retrieved lymph nodes is not only influenced by operative technique or extent of lymphadenectomy, but to an even greater extent by pathological techniques in which specimens were processed. Special pathological techniques such as `fat‐clearing` may increase the number of lymph nodes detected in specimens by tenfold. None of studies reported such sophisticated pathological techniques, and specimens retrieved by either laparoscopic‐assisted or open resection were not processed in different ways. Nevertheless, examining fewer than 13 lymph nodes in a specimen can result in under‐staging. In the current analysis, laparoscopically‐assisted surgery was associated with a significantly lower average number of lymph nodes harvested than open surgery (P = 0.003). Formation of adhesions and occurrence of incisional hernia are two important long‐term complications after abdominal surgery, causing significant morbidity. No fewer than 20% of patients develop an incisional hernia after a midline laparotomy. After abdominal procedures, 70‐90 % of patients develop adhesions (Ellis 1997). Approximately one‐third of all patients undergoing abdominal surgery are later readmitted to hospital for problems related to these adhesions (Ellis 1999; Lower 2000). No fewer than 5% of all patients receiving abdominal surgery are re‐operated for problems possibly related to adhesions (Lower 2000). However, in most randomised trials comparing laparoscopic and open surgery for colorectal or other intraabdominal procedures, very little attention is paid to these complications. In the current analysis, only two of the ten trails reported data on adhesions and incisional hernias. A possible reason for this is the degree of difficulty in studying these kind of complications. For example, in order to study occurrence of incisional hernia a long follow‐up period is necessary. Studying adhesion formation is even more difficult, because objective measurement of adhesion formation is almost impossible. Measuring the number of adhesions requires reoperation. Measuring other objective parameters, such as reoperations due to adhesions, requires very large patient populations. Studies regarding adhesion formation and laparoscopy are therefore mainly studies performed on animals. Standardised tests for scoring adhesions in humans are scarce and if available, difficult to use in practice. However, occurrence of incisional hernia and reoperations for adhesions are being studied in some of the ongoing trials (COLOR; COLOR II). Conversion rates differ greatly between different studies. A possible reason for this is that different definitions of conversion are used in the studies. In only one article (Milsom 1998), authors report which part of the procedure has to be performed laparoscopically in order to consider the procedure `laparoscopic`. Another possible reason for the variability in conversion rates is that the level of experience in laparoscopic colon surgery of surgeons participating in the trials and the number of procedures performed in each of the hospitals per year (hospital case volume) differed. Analysis on hospital case volume and short‐term outcome within the COLOR trial showed that average conversion rates in low case volume hospitals were 24% compared to 9% in hospitals with a high case volume (Kuhry 2005). In this meta‐analysis, data were analysed according to `intention‐to‐treat`, i.e. patients who were randomised to undergo laparoscopic or open surgery, but received the other procedure, where analysed in the group they were randomised for. We did not perform a separate analysis comparing converted procedures, completed laparoscopic procedures and open procedures, since this analysis would be biased since the more difficult cases are converted, leaving the easier cases with better outcomes cancer wise in the laparoscopic arm. However, in one study (Milsom 1998), patients in whom the laparoscopic procedure was converted to open surgery were excluded from the analyses. In another trial (Winslow 2002), converted procedures were analysed in the open group. Although the total number of patients in these trials was small (n=117), this could have caused a bias in favour of laparoscopic surgery. In this review, analyses on cancer‐free survival, overall survival, distant metastases, local recurrence and reoperations for hernias or adhesions were performed using the total number of events at the end of the follow‐up period. Time to event was not taken into account in these analyses, since inadequate data was available in most trials. This could have caused a bias, since patients that are lost to follow‐up are excluded in these analyses, lowering both the power and the validity of the studies. Furthermore, comparing laparoscopic and open surgery at one time point can obscure important differences in outcome between both treatment groups. Also, duration of follow‐up is not taken into account. Only 6 articles reported hazard ratios and/or survival curves. A meta‐analysis on hazard ratios for tumour recurrence and overall mortality was performed, including 4 studies. Since these analyses are more powerful and have less risk of bias, they should be considered as more informative. Analyses on local tumour recurrence, distant metastases, mortality and survival should be performed separately for colon cancer and rectum cancer, since both cancer types are regarded as different types of disease, with different behaviours and outcome. There has been some discussion on how to divide the colorectum anatomically. Division into `colon` and `rectum` is most usual, but some advocate a division between the colon descendens and sigmoid colon, since this is more natural from an epidemiological point of view (Nelson 1998). In this review, we chose to perform separate analyses for colon and rectum cancer. A trend towards a lower overall mortality after laparoscopically‐assisted procedures is found. This could be a sign of bias towards selection of less advanced cases for laparoscopic surgery, as also seen in the distribution of clinical stage. Differences in distribution of clinical stage, however, can also be explained by under staging of tumours that have been removed laparoscopically. Under staging can occur when a lower number of lymph nodes is harvested, or when liver metastases are missed during laparoscopic inspection, that would have be detected during open inspection and palpation. However, in the two large studies that are mainly responsible for the high number of patients with stage I tumours in the laparoscopic group (Lacy 2002; COST 2004), no significant differences in number of lymph nodes harvested and metastases detected during surgery were found. Both studies were of high quality and differences in stage distribution can not be explained based on the study design. In colon cancer patients, several large randomised trials have shown that survival after laparoscopic surgery is at least equal to survival after open surgery (Lacy 2002; COST 2004). Long‐term results of the COLOR trail have not been published yet, but have been presented at the 15 th congress of the European Association for Endoscopic Surgery (EAES) in 2007 (COLOR). No significant differences in disease‐free survival were found. Some have speculated whether the termination of recruitment of patients in the LAPKON II trail before the calculated sample sizes were reached could have been due to significant differences in survival. However, inclusion was terminated due to a decrease in monthly inclusions, which would have resulted in an unacceptable duration of the study and not due to differences in outcome (personal communication). In the near future, we expect to be able to include data on these ongoing trails. Since most of the studies included so far in the analyses are single‐center, inclusion of these large multi‐center trials is very important. The analyses will than reflect a more `real‐life` situation and the risk of bias will be reduced. For rectal cancer, the number of available studies and included patients is too low to draw any reliable conclusions. The results of large randomised studies have to be awaited.
Authors' conclusions
Implications for practice.
Laparoscopic surgery for colon cancer is a safe procedure that is associated with a survival rate equal to survival after open surgery. The procedure can therefore be offered routinely to patients in hospitals where surgeons with sufficient experience in laparoscopic colon surgery are available. In the case of rectal cancer, data on long term outcome are scarce and the results of large randomised trails have to be awaited.
Implications for research.
Trials that compare survival after laparoscopic and open surgery for colorectal cancer should be adequately powered. Articles reporting long‐term outcome should include survival curves and hazard ratios. Analyses should be according to the 'intention to treat' principle, in order to prevent bias caused by conversion of laparoscopic to open procedures. Articles should report the number of patients that were not included in the study, together wit the reason for this. Registration of patients that were not included is important in order to prevent bias that can occur when patients that, for various reasons, are considered difficult to operate laparoscopically, are not asked to participate in the trial. Studies comparing laparoscopic and open surgery for colon cancer in patient groups that were excluded in most of the previously performed trails (i.e. obese patients and patients with tumours in the left flexure and transverse colon) need to be performed. Trials that also include elderly patients with comorbidity are needed, since this study population will resemble the general population more closely. More well‐designed randomised clinical trails on long‐term outcome after laparoscopic versus open surgery for rectal cancer are needed. Studies comparing laparoscopic and open surgery for rectal cancer that include both high rectal resection and low anterior resection are needed. Also, further research is needed to determine whether or not laparoscopic surgery is associated with a reduced risk of development of adhesions and incisional hernias. In the case of adhesions it is difficult to study possible effects, since it is only possible to objectively measure adhesion formation during reoperation. Standardised methods for scoring adhesions during reoperations should be developed.
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2012 | Amended | Additional tables linked to text. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 5 August 2008 | Amended | Converted to new review format. |
| 2 January 2008 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We would like to thank O Haase, JM Muller and J Neudecker for their contributions in writing the protocol for this review.
Data and analyses
Comparison 1. Preoperative adjuvant therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adjuvant chemotherapy | 7 | 2187 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.76, 1.10] |
1.1. Analysis.
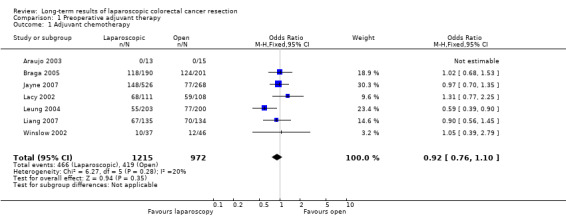
Comparison 1 Preoperative adjuvant therapy, Outcome 1 Adjuvant chemotherapy.
Comparison 2. Type of resection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Right sided | 7 | 1915 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.86, 1.38] |
| 2 Left sided | 7 | 1915 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 3 Rectum resection | 7 | 1915 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.85, 1.39] |
| 4 APR | 7 | 1915 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.46] |
| 5 Subtotal colectomy | 6 | 1121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.39] |
2.1. Analysis.
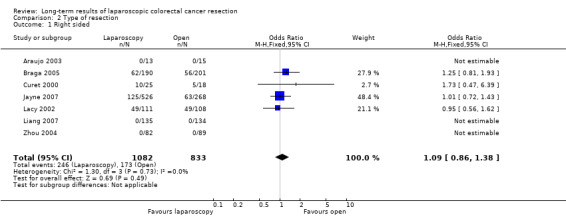
Comparison 2 Type of resection, Outcome 1 Right sided.
2.2. Analysis.

Comparison 2 Type of resection, Outcome 2 Left sided.
2.3. Analysis.

Comparison 2 Type of resection, Outcome 3 Rectum resection.
2.4. Analysis.

Comparison 2 Type of resection, Outcome 4 APR.
2.5. Analysis.

Comparison 2 Type of resection, Outcome 5 Subtotal colectomy.
Comparison 3. Location of the tumor.
3.1. Analysis.

Comparison 3 Location of the tumor, Outcome 1 Colon.
3.2. Analysis.

Comparison 3 Location of the tumor, Outcome 2 Rectum.
Comparison 5. Tumor stage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stage 0 | 11 | 3309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.33, 1.02] |
| 2 Stage I | 11 | 3309 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.00, 1.43] |
| 3 Stage II | 11 | 3309 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.17] |
| 4 Stage III | 11 | 3309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.08] |
| 5 Stage IV | 11 | 3309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.22] |
| 6 Unknown | 11 | 3309 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.67] |
5.1. Analysis.

Comparison 5 Tumor stage, Outcome 1 Stage 0.
5.2. Analysis.

Comparison 5 Tumor stage, Outcome 2 Stage I.
5.3. Analysis.

Comparison 5 Tumor stage, Outcome 3 Stage II.
5.4. Analysis.

Comparison 5 Tumor stage, Outcome 4 Stage III.
5.5. Analysis.

Comparison 5 Tumor stage, Outcome 5 Stage IV.
5.6. Analysis.

Comparison 5 Tumor stage, Outcome 6 Unknown.
Comparison 6. Number of lymph nodes harvested.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lymph nodes harvested | 6 | 1358 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐1.65, ‐0.35] |
| 2 Median number of lymph nodes harvested | Other data | No numeric data |
6.1. Analysis.

Comparison 6 Number of lymph nodes harvested, Outcome 1 Number of lymph nodes harvested.
6.2. Analysis.
Comparison 6 Number of lymph nodes harvested, Outcome 2 Median number of lymph nodes harvested.
| Median number of lymph nodes harvested | |||||
|---|---|---|---|---|---|
| Study | Laparoscopy | Open | |||
| COST 2004 | 12 | 12 | |||
| Curet 2000 | 11 (1‐29) | 10 (1‐21) | |||
| Milsom 1998 | 19 (5‐59) | 25 (4‐74) | |||
Comparison 7. Resection margins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of positive resection margins | 6 | 2347 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.71, 2.10] |
7.1. Analysis.

Comparison 7 Resection margins, Outcome 1 Number of positive resection margins.
Comparison 8. Duration of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Follow‐up | Other data | No numeric data |
8.1. Analysis.
Comparison 8 Duration of follow‐up, Outcome 1 Follow‐up.
| Follow‐up | |
|---|---|
| Study | |
| Araujo 2003 | Mean 47.2 months |
| Braga 2005 | Median 36 months (range: 15‐60 months) |
| COST 2004 | Median 4.4 years| |
| Curet 2000 | Mean 4.9 years |
| Jayne 2007 | Median 36.8 months (range 20.0‐61.5 months) |
| Kaiser 2004 | Median 35 months (range: 3‐69 months) |
| Lacy 2002 | Median 43 months (range: 27‐85 months) |
| Leung 2004 | Mean 52.7 months in lap. and 49.2 in open arm |
| Liang 2007 | Median 40 months (range: 18‐72 months) |
| Milsom 1998 | Median 1.5 years (range: 1.5‐46 months) |
| Winslow 2002 | Mean 30.1 months |
| Zhou 2004 | Range 1‐16 months |
Comparison 9. Incisional hernia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incisional hernia | 2 | 474 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.38, 1.37] |
9.1. Analysis.

Comparison 9 Incisional hernia, Outcome 1 Incisional hernia.
Comparison 10. Reoperations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incisional hernia | 2 | 474 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.55, 4.19] |
| 2 Intraperitoneal adhesions | 1 | 391 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.08, 2.18] |
10.1. Analysis.

Comparison 10 Reoperations, Outcome 1 Incisional hernia.
10.2. Analysis.
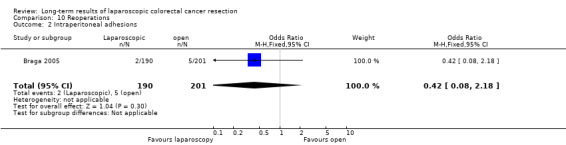
Comparison 10 Reoperations, Outcome 2 Intraperitoneal adhesions.
Comparison 11. Local tumour recurrence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4 Recurrence in operating area, colorectal | 8 | 1987 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.22] |
| 5 Recurrence in the operating area, colon | 4 | 938 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.52] |
| 6 Recurrence in the operating area, rectum | 4 | 714 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.43] |
| 7 Peritoneal, colorectal | 7 | 1193 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.19, 1.86] |
| 8 Peritoneal, colon | 5 | 782 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.19, 1.86] |
| 9 Peritoneal, rectum | 4 | 335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Port‐site or wound recurrences, colorectal | 10 | 3187 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.77, 5.02] |
| 11 Port‐site or wound recurrences, colon | 3 | 525 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 7.00] |
| 12 Port‐site or wound recurrences, rectum | 3 | 452 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.94] |
11.4. Analysis.

Comparison 11 Local tumour recurrence, Outcome 4 Recurrence in operating area, colorectal.
11.5. Analysis.

Comparison 11 Local tumour recurrence, Outcome 5 Recurrence in the operating area, colon.
11.6. Analysis.

Comparison 11 Local tumour recurrence, Outcome 6 Recurrence in the operating area, rectum.
11.7. Analysis.

Comparison 11 Local tumour recurrence, Outcome 7 Peritoneal, colorectal.
11.8. Analysis.

Comparison 11 Local tumour recurrence, Outcome 8 Peritoneal, colon.
11.9. Analysis.

Comparison 11 Local tumour recurrence, Outcome 9 Peritoneal, rectum.
11.10. Analysis.

Comparison 11 Local tumour recurrence, Outcome 10 Port‐site or wound recurrences, colorectal.
11.11. Analysis.

Comparison 11 Local tumour recurrence, Outcome 11 Port‐site or wound recurrences, colon.
11.12. Analysis.

Comparison 11 Local tumour recurrence, Outcome 12 Port‐site or wound recurrences, rectum.
Comparison 12. Distant metastases.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Distant metastases, colorectal | 7 | 1853 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 2 Distant metastases, colon | 4 | 938 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.22] |
| 3 Distant metastases, rectum | 3 | 578 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.66, 2.05] |
| 4 Lung | 2 | 317 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.29, 4.94] |
| 5 Liver | 2 | 317 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.33, 2.69] |
12.1. Analysis.

Comparison 12 Distant metastases, Outcome 1 Distant metastases, colorectal.
12.2. Analysis.

Comparison 12 Distant metastases, Outcome 2 Distant metastases, colon.
12.3. Analysis.

Comparison 12 Distant metastases, Outcome 3 Distant metastases, rectum.
12.4. Analysis.

Comparison 12 Distant metastases, Outcome 4 Lung.
12.5. Analysis.

Comparison 12 Distant metastases, Outcome 5 Liver.
Comparison 13. Mortality, cancer related.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cancer‐related mortality at maximum follow‐up, colorectal | 8 | 2490 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.67, 1.06] |
| 2 Cancer‐related mortality at maximum follow‐up, colon | 5 | 1575 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.06] |
| 3 Mortality, cancer‐related, rectum | 3 | 578 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.37, 1.19] |
13.1. Analysis.

Comparison 13 Mortality, cancer related, Outcome 1 Cancer‐related mortality at maximum follow‐up, colorectal.
13.2. Analysis.

Comparison 13 Mortality, cancer related, Outcome 2 Cancer‐related mortality at maximum follow‐up, colon.
13.3. Analysis.

Comparison 13 Mortality, cancer related, Outcome 3 Mortality, cancer‐related, rectum.
Comparison 14. Mortality, overall.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality at maximum follow‐up, colorectal | 9 | 2881 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.00] |
| 2 Overall mortality at maximum follow‐up, colon | 4 | 1162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.62, 1.09] |
| 3 Mortality, overall, rectum | 2 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Meta‐analysis of hazard ratios for death in the laparoscopic group | Other data | No numeric data |
14.1. Analysis.

Comparison 14 Mortality, overall, Outcome 1 Overall mortality at maximum follow‐up, colorectal.
14.2. Analysis.

Comparison 14 Mortality, overall, Outcome 2 Overall mortality at maximum follow‐up, colon.
14.3. Analysis.

Comparison 14 Mortality, overall, Outcome 3 Mortality, overall, rectum.
14.4. Analysis.
Comparison 14 Mortality, overall, Outcome 4 Meta‐analysis of hazard ratios for death in the laparoscopic group.
| Meta‐analysis of hazard ratios for death in the laparoscopic group | ||||
|---|---|---|---|---|
| Study | Hazard ratio (HR) | Ln(HRi) | Var(Ln(HRi)) | |
| COST 2004 | 0.91 | ‐0.094 | 0.022 | |
| Lacy 2002 | 0.77 | ‐0.261 | 0.036 | |
| Leung 2004 | 1.01 | 0.0085 | 0.051 | |
Comparison 15. Disease‐free survival at 5 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease‐free survival at 5 years | Other data | No numeric data | ||
| 2 p‐value | Other data | No numeric data | ||
| 3 Meta‐analysis on hazard ratios for tumour recurrence in the laparoscopic group | Other data | No numeric data |
15.1. Analysis.
Comparison 15 Disease‐free survival at 5 years, Outcome 1 Disease‐free survival at 5 years.
| Disease‐free survival at 5 years | ||
|---|---|---|
| Study | Laparoscopic | Open |
| Braga 2005 | 66% | 52% |
| Lacy 2002 | 86% | 68% |
| Leung 2004 | 75.3% | 78.3% |
15.2. Analysis.
Comparison 15 Disease‐free survival at 5 years, Outcome 2 p‐value.
| p‐value | |
|---|---|
| Study | |
| Braga 2005 | 0.78 |
| Lacy 2002 | 0.029 |
| Leung 2004 | 0.45 |
15.3. Analysis.
Comparison 15 Disease‐free survival at 5 years, Outcome 3 Meta‐analysis on hazard ratios for tumour recurrence in the laparoscopic group.
| Meta‐analysis on hazard ratios for tumour recurrence in the laparoscopic group | ||||
|---|---|---|---|---|
| Study | Hazard ratio (HR) | Ln(HR) | Var(Ln(HRi)) | |
| COST 2004 | 0.86 | ‐0.151 | 0.025 | |
| Lacy 2002 | 0.72 | ‐0.329 | 0.039 | |
| Leung 2004 | 1.24 | 0.219 | 0.06 | |
| Liang 2007 | 1.29 | 0.2528 | 0.077 | |
Comparison 16. Overall survival at 5 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival at 5 years (%) | Other data | No numeric data | ||
| 2 p‐value | Other data | No numeric data |
16.1. Analysis.
Comparison 16 Overall survival at 5 years, Outcome 1 Overall survival at 5 years (%).
| Overall survival at 5 years (%) | ||
|---|---|---|
| Study | Laparoscopic | Open |
| Braga 2005 | 72% | 64% |
| COST 2004 | 78% | 76% |
| Lacy 2002 | 80% (probability of overall survival) | 64% (probability of overall survival) |
| Leung 2004 | 76.1% (probability of overall survival) | 72.9% (probability of overall survival) |
16.2. Analysis.
Comparison 16 Overall survival at 5 years, Outcome 2 p‐value.
| p‐value | |
|---|---|
| Study | |
| COST 2004 | 0.51 |
| Lacy 2002 | 0.052 |
| Leung 2004 | 0.61 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Araujo 2003.
| Methods | RCT Single centre No sample size calculation Not clear when patients were randomised Number of patients not randomised: unclear No conversions Follow‐up 47.2 months (mean) | |
| Participants | n=28 Inclusion criteria: distal rectal adenocarcinoma., incomplete response after chemoradiation, preop. staging favourable to radical resection by APR Two exclusions after randomisation due to peroperative metastases. | |
| Interventions | Laparoscopic vs open Tumour location: distal rectum Adj. therapy: chemoradiation Type of resection: APR | |
| Outcomes | Adjuvant therapy, type of resection, tumor localisation, tumor stage, number of lymph nodes harvested, duration of follow‐up, local tumour recurrence | |
| Notes | Halpern and Preston quality score: 13 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Braga 2005.
| Methods | RCT Single centre No sample size calculation Preoperative randomisation Number of patients not randomised: unclear Conversion rate 4.2% Intention to treat Follow‐up 36 months (median) | |
| Participants | n=391 Inclusion criteria: age >18, histologically confirmed colorectal cancer, suitable for elective surgery Exclusion criteria: cancer infiltrating adjacent organs assessed by CT or MRI, cardiovascular dysfunction (arterial pO2 < 70 mmHg), hepatic dysfunction (Child‐Pugh class C), ongoing infection, plasma neutrophil level < x 109/l. | |
| Interventions | Laparoscopic vs open Tumour location: colon or rectum Adj. therapy: postoperative chemotherapy was given to over 60% of patients. Type of resection: right or left hemicolectomy, sigmoidectomy, rectal resection | |
| Outcomes | Adjuvant therapy, type of resection, tumor stage, number of lymph nodes harvested, TME, duration of follow‐up, incisional hernia, reoperation for incisional hernia or adhesions, recurrence free survival and overall survival | |
| Notes | Halpern and Preston quality score: 16 out of 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
COST 2004.
| Methods | RCT Multicenter Sample size calculation Preoperative randomisation Number of patients not randomised: unclear Conversion rate 21% Intention to treat Follow‐up: 4.4 years (median) | |
| Participants | n=863 Inclusion criteria: adenocarcinoma of the colon, age of at least 18 years, absence of prohibitive abdominal adhesions Exclusion criteria: advanced local or metastatic disease, rectal or transverse colon cancer, acute bowel obstruction or perforation from cancer, severe medical illness, inflammatory bowel disease, pregnancy, concurrent or previous malignant tumour Seven patients were excluded after randomisation due to the fact that they did not receive the allocated treatment. | |
| Interventions | Laparoscopically assisted vs open Tumour location: left, right or sigmoid colon Adj. therapy: postoperative chemotherapy was allowed at the physician`s discretion Type of resection: right or left hemicolectomy, sigmoidectomy | |
| Outcomes | Tumor localisation, tumor stage, number of lymph nodes harvested, duration of follow‐up, recurrence free survival and overall survival | |
| Notes | Halpern and Preston quality score: 20 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Curet 2000.
| Methods | RCT Single centre No sample size calculation Preoperative randomisation Number of patients not randomised: unclear Conversion rate 28% Converted procedures were analysed separately Follow‐up 4.9 years (mean) | |
| Participants | n=43 Inclusion criteria: colon cancer Exclusion criteria: patients undergoing colostomy placement or removal alone, age less than 18 years, pregnancy, complete or near colon obstruction resulting in significant proximal distention, malignant fistulization or fixation to adjacent tissues. | |
| Interventions | Laparoscopically assisted vs open Tumour location: left, right, transverse or sigmoid colon Adj. therapy: unclear Type of resection: right or left hemicolectomy, sigmoidectomy, low anterior resection | |
| Outcomes | Type of resection, tumor localisation, tumor stage, number of lymph nodes harvested, duration of follow‐up, recurrence free survival and overall survival | |
| Notes | Halpern and Preston quality score: 14 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Jayne 2007.
| Methods | RCT Multicenter Sample size calculation Conversion rate 29% Intention to treat Preoperative randomisation Follow‐up 36.8 months (median) | |
| Participants | n=794 Inclusion criteria: adenocarcinoma of the colon or rectum Exclusion criteria: Transverse colon cancer, contraindications to pneumoperitoneum (chronic cardiac or pulmonary disease), acute intestinal obstruction, malignant disease in the past 5 years, synchronous adenocarcinomas, pregnancy, associated gastrointestinal diseases needing surgical intervention. | |
| Interventions | Laparoscopically‐assisted vs open Tumour location: colon or rectum Type of resection: right or left Adj. therapy: radiotherapy and chemotherapy were allowed at the physician`s discretion Type of resection: left or right hemicolectomy, sigmoidectomy, anterior resection, APR | |
| Outcomes | Adjuvant therapy, type of resection, localisation of the tumor, tumor stage, TME, number of positive resection margins, duration of follow‐up, local tumor recurrence, distant metastases, cancer‐related mortality. | |
| Notes | Halpern and Preston quality score: 20 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kaiser 2004.
| Methods | RCT Single center No sample size calculation Preoperative randomisation Number of patients not randomised: unclear Conversion rate 45% Converted procedures were analysed separately Follow‐up 35 months (median) | |
| Participants | n=48 Inclusion criteria: Primary left, right or sigmoid colon adenocarcinoma, elective surgery with curative intent, age over 18 years, Ability to participate in follow‐up evaluation, ASA class I‐III Exclusion criteria: Emergency surgery, tumour stage IV, rectal or transverse colon cancer, known prohibitive adhesions from previous abdominal surgery, associated gastrointestinal disease (Crohn`s disease, chronic ulcerative colitis, FAP), pregnancy One patient was excluded after randomisation because he was lost to follow‐up. | |
| Interventions | Laparoscopically‐assisted vs open Tumour location: Left, right or sigmoid colon Adj. therapy: not clear Type of resection: right or left hemicolectomy, sigmoidectomy | |
| Outcomes | Tumor localisation, tumor stage, number of lymph nodes harvested, number of positive resection margins, duration of follow‐up, recurrence free survival and overall survival | |
| Notes | Halpern and Preston quality score: 15 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lacy 2002.
| Methods | RCT Single center Sample size calculation Preoperative randomisation Number of patients not randomised: 223, reasons given Conversion rate 11% Intention to treat Follow‐up 43 months (median) | |
| Participants | n=219 Inclusion criteria: adenocarcinoma of the colon, 15 cm above the anal verge Exclusion criteria: cancer located at the transverse colon, distant metastases, adjacent organ invasion, intestinal obstruction, past colonic surgery, no consent Thirteen patients excluded after randomisation due to metastases during surgery (11) or lost to follow‐up (2). | |
| Interventions | Laparoscopically assisted vs open Tumour location: left, right or sigmoid colon Adj. therapy: unless contraindicated, patients with stage II and III disease received postoperative chemotherapy Type of resection: left hemicolectomy, right hemicolectomy, sigmoidectomy, high anterior resection subtotal colectomy, Hartmann procedure | |
| Outcomes | Adjuvant therapy, type of resection, tumor localisation, tumor stage, number of lymph nodes harvested, duration of follow‐up, local tumour recurrence, distant metastasis, type of metastasis, recurrence free survival and overall survival | |
| Notes | Halpern and Preston quality score: 20 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Leung 2004.
| Methods | RCT Single surgeon, 2 centres Sample size calculation Preoperative randomisation Number of patients not randomised: 422, reason given Conversion rate 23% Intention to treat Follow‐up 5 years | |
| Participants | n=403 Inclusion criteria: patients with rectosigmoid carcinoma Exclusion criteria: distal tumour needing anastomosis within 5 cm within the dentate line, tumour larger than 6 cm, tumour infiltration to adjacent organs on sonography or CT, previous abdominal operations near the region of the colorectal operation, no informed consent, intestinal obstruction or perforation 36 patients excluded after randomisation due to stage IV disease discovered during surgery. | |
| Interventions | Laparoscopic assisted vs open Tumour location: sigmoid, rectum Adj. therapy: not clear Type of resection: sigmoidectomy, TME | |
| Outcomes | Adjuvant therapy, tumor stage, number of lymph nodes harvested, duration of follow‐up, local tumor recurrence, distant metastasis, type of metastasis, recurrence free survival and overall survival | |
| Notes | Halpern and Preston quality score: 20 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Liang 2007.
| Methods | RCT Single surgeon Sample size calculation Follow up ( Intention to treat Number of patients not randomised unclear Preoperative randomisation Conversion rate 3% Intention to treat Follow‐up 40 months (median) | |
| Participants | n=269 Inclusion criteria: left‐sided primary colon cancer that requires the mobilisation of the splenic flexure, TNM stage II or III, curative and elective surgery, ASA I‐III, age>18 yrs Exclusion criteria: Cecal. ascending, proximal transverse, middle and distal sigmoid and rectal cancer; emergency or palliative surgery, evidence of disseminated disease or adjacent organ invasion, tumor> 8 cm in diameter, BMI >40 kg/m2, previous major upper abdominal surgery | |
| Interventions | Laparoscopic versus open Tumour location: distal transverse colon, splenic flexure, descending colon, S‐D junction, proximal sigmoid colon Adj. therapy: postoperative chemotherapy in stage III patients Type of resection: (extended) left‐sided hemicolectomy | |
| Outcomes | Adjuvant therapy, type of resection, localisation of the tumor, tumor stage, number lymph nodes harvested, duration of follow‐up, local recurrence, distant metastases. | |
| Notes | Halpern and Preston quality score: 18 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Milsom 1998.
| Methods | RCT Single centre Sample size calculation Number of patients not randomised: 27, reason unclear Peroperative randomisation Conversion rate 10,5% Converted procedures were excluded from the analysis Follow‐up 1,6 years (median) | |
| Participants | n=80 Inclusion criteria: curative elective surgery, primary right, left or sigmoid colon cancers or polyps, upper or lower rectal cancers or polyps, ASA I‐III, age over 18 years Exclusion criteria: emergency surgery, evidence of metastasised disease or adjacent organ invasion, primary tumour size over 8 cm in diameter, transverse or descending colon cancers or polyps, mid rectal cancers or polyps, BMI over 32 kg\m2 Eleven patients were excluded after randomisation due to redrawn of informed consent (7) or conversion(4). | |
| Interventions | Laparoscopic vs open Tumour location: right colon, sigmoid colon, rectum Adj. therapy: not clear Type of resection: right hemicolectomy, proctosigmoidectomy, APR | |
| Outcomes | Tumor stage, number of lymph nodes harvested, TME, duration of follow‐up, local tumor recurrence, distant metastasis | |
| Notes | Halpern and Preston quality score: 17 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Winslow 2002.
| Methods | RCT Single centre No sample size calculation Preoperative randomisation Number of patients not randomised: unclear Conversion rate 15% Conversions were analysed in the open arm of the trial Follow‐up 30.1 months (mean) | |
| Participants | n=37 Inclusion criteria: adenocarcinoma of the right, left, or sigmoid colon, 18 years of age or older, able to participate in close follow‐up evaluations postoperatively Exclusion criteria: prohibitive scars from previous abdominal surgery, advanced local disease, stage IV colon cancer, rectal cancer, acutely perforated or obstructing cancers, cancers of the transverse colon, ASA IV or V, associated gastrointestinal diseases requiring extensive operative intervention, concurrent or previous malignant tumour within 5 years (excl. non melanoma skin cancer), pregnancy Six patients were lost to follow‐up. | |
| Interventions | Laparoscopic vs open Tumour location: right, left, or sigmoid colon Adj. therapy: postoperative chemotherapy according to tumour stage and other patient‐related factors, no postoperative radiation Type of resection: Right or left hemicolectomy, sigmoidectomy | |
| Outcomes | Adjuvant therapy, tumor localisation, duration of follow‐up, incidence of incisional hernia, reoperations for incisional hernia | |
| Notes | Halpern and Preston quality score: 15 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zhou 2004.
| Methods | RCT Single centre No sample size calculation Not clear when patients were randomised Number of patients not randomised: unclear No conversions Follow‐up ranged from 1 to 16 months | |
| Participants | n=171 Inclusion criteria: rectal adenocarcinoma, lowest margin of the tumour under the peritoneal deflection and 1.5 cm above the dentate line Exclusion criteria: emergency surgery required, Dukes D with local infiltration of adjacent organs, no informed consent. | |
| Interventions | Laparoscopic vs open Tumour location: rectal cancer, lowest margin 1.5‐8 cm above the dentate line Adj. therapy: not clear Type of resection: TME with anal sphincter preservation | |
| Outcomes | Tumor localisation, tumor stage, number of positive resection margins, TME, duration of follow‐up, local tumor recurrence | |
| Notes | Halpern and Preston quality score: 15 out of 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Basse 2003 | RCT on gastrointestinal recovery after laparoscopic versus open colonic resection (n=32). Includes also patients with benign disease. No follow‐up. |
| Basse 2005 | RCT on functional recovery after laparoscopic versus open colonic resection (n=60). Includes also patients with benign disease. No follow‐up. |
| Bohm 1999 | RCT on liver and renal function after laparoscopic compared to open colorectal resection for cancer. No follow‐up. |
| Braga 2002 a | RCT on short‐term outcome after laparoscopic vs open colorectal resection for cancer. Patients are also included in Brage 2005. |
| Braga 2002 b | RCT on immune responses and metabolism in laparoscopic versus open colorectal surgery (n=79). No follow‐up. |
| Danelli 2002 | RCT on temperature control and recovery of bowel function after laparoscopic and conventional colorectal surgery (n=44). No follow‐up. Includes also benign disease, no separate data for cancer resections. |
| Delgado 2001 | RCT on acute phase responses before and after laparoscopic versus open colectomy for cancer (n=97). No follow‐up. |
| Guillou 2005 | did not describe any of the long‐term outcomes sought in this analysis or because patients were also included in another article or ongoing trial |
| Hewitt 1998 | RCT on immune responses in laparoscopic‐assisted versus open surgery for colorectal cancer (n=25). No follow‐up. |
| Hildebrandt 2003 | RCT on stress responses in laparoscopic and open colonic resections (n=42). Includes also patients with benign disease. No follow‐up. |
| Hu 2003 | RCT on immune responses in laparoscopic versus open total mesorectal excision for rectal cancer (n=45). Patients were enrolled as part of the trail Zhou 2004. No follow‐up. |
| Janson 2004 | RCT on the costs of laparoscopic versus open surgery for colonic cancer (n=210). Only data on costs are given. Patients were enrolled as part of the COLOR trial. |
| Jayne 2005 | RCT on bladder and sexual function following laparoscopic‐assisted versus open colorectal resection for cancer. Patients were enrolled as part of the CLASICC trial. |
| Kang 2004 | RCT on perioperative and short‐term results in laparoscopic versus open colectomy (n=60). Includes also patients with benign disease. No follow‐up. |
| Kim 1998 | RCT on spillage of tumour cells in the peritoneal cavity during laparoscopic versus conventional surgery for colorectal cancer (n=38). No follow‐up. |
| Lacy 1995 | RCT on laparoscopic versus open colectomy for colon cancer. Preliminary short‐term results of the trial Lacy 2002. Data and patients are also included in Lacy 2002. No follow‐up. |
| Lacy 1998 | RCT on port‐site metastases and recurrences after laparoscopic‐assisted versus open colectomy for cancer (n=91). Data and patients are also included in Lacy 2002. |
| Leung 2000 | RCT on systemic cytokine responses after laparoscopic‐assisted versus open resection of rectosigmoid carcinoma (n=34). No follow‐up. |
| Leung 2003 | RCT on immune responses after laparoscopically‐assisted versus open resection of rectosigmoid carcinoma (n=40). No follow‐up. |
| Neudecker 2002 | RCT on peritoneal fibrinolytic capacity after laparoscopic and conventional colorectal resection for cancer (n=30) No follow‐up. Patients were enrolled as part of the LAPKON II trial. |
| Neudecker 2003 | RCT on intravasal fibrinolytic activity after laparoscopic and conventional colorectal resection for cancer (n=30). No follow‐up. Patients were enrolled as part of the LAPKON II trial. |
| Neudecker 2005 | RCT on fibrinolytic capacity after laparoscopic and conventional colorectal surgery for cancer (n=30). No follow‐up. Patients were enrolled as part of the LAPKON II trial. |
| Ordemann 2001 | RCT on inflammatory responses after laparoscopic versus conventional colorectal resections for cancer (n=40). No follow‐up. |
| Ortiz 1996 | RCT on early oral feeding after laparoscopic versus open colonic resections (n=40). Includes also patients with benign disease. No follow‐up. |
| Schwenk 1998 a | RCT on postoperative ileus after laparoscopic compared to open colorectal resection for cancer (n=60). No follow‐up. Data and patients are included in Schwenk 2002. |
| Schwenk 1998 b | RCT on pain and fatique after laparoscopic compare to open colorectal resection for cancer (n=60). No follow‐up. Data and patients are also included in Schwenk 2002. |
| Schwenk 1998 c | RCT on quality of life after laparoscopic compared to open colorectal resection for cancer (n=60). No follow‐up. Data and patients are also included in Schwenk 2002. |
| Schwenk 1999 | RCT on pulmonary function after laparoscopic compared to open colorectal resection for cancer (n=60). No follow‐up. Data and patients are also included in Schwenk 2002. |
| Schwenk 2002 | RCT on short‐term outcome after laparoscopic compared to open colorectal resection for cancer (n=102). No follow‐up. |
| Stage 1997 | RCT on short‐term outcome, immunologic parameters and pathological evaluation of the resected specimen after laparoscopic compared to open surgery for cancer (n=34). No follow‐up. |
| Tang 2001 | RCT on systemic immunity in patients undergoing laparoscopically‐assisted versus open colectomy for colorectal cancer (n=236). No follow‐up. |
| Weeks 2002 | RCT on quality‐of‐life after laparoscopic‐assisted colectomy versus open colectomy for colon cancer (n=428). Only data on quality‐of‐life, pain and hospital stay are given. Patients were enrolled as part of the COST trial. |
| Wu 2003 | RCT on peritoneal and systemic immune responses after laparoscopic compared to open surgery for colon cancer (n=26). No follow‐up. Patients were enrolled as part of the COLOR trial. |
| Wu 2004 | RCT on local and systemic angiogenic responses after laparoscopic compared to open surgery for colon cancer (n=26). No follow‐up Patients were enrolled as part of the COLOR trial. |
Characteristics of ongoing studies [ordered by study ID]
COLOR.
| Trial name or title | COLOR: COlon cancer Laparscopic or Open resecxtion? |
| Methods | |
| Participants | Patients with non‐metastasised colon cancer |
| Interventions | Laparoscopic versus open rescection of colon cancer |
| Outcomes | Primary endpoint: disease‐free survival at 3 years |
| Starting date | 1997 |
| Contact information | Prof. dr H.J. Bonjer, Department of General Surgery, Health Sciences Centre, Halifax, Canada |
| Notes | Data analyzed |
COLOR II.
| Trial name or title | COLOR II: Laparoscopic versus open surgery for rectal cancer |
| Methods | |
| Participants | Patients with non‐metastasised rectal cancer |
| Interventions | Laparoscopic versus open resection of rectal cancer |
| Outcomes | Primary endpoint: locoregional recurrence rate 3 years after surgery Secondary endpoints: disease‐free and overall survival rate 3 and 5 years after surgery, rate of distant metastases, macroscopic evaluation of the resected specimen, 8‐week mortality and morbidity, quality of life, costs |
| Starting date | 2003 |
| Contact information | Prof. dr H.J. Bonjer Department of General Surgery, Health Sciences Centre, Halifax, Canada |
| Notes | Patient recruitment ongoing |
Hasegawa.
| Trial name or title | RCT of laparoscopic versus open colectomy for advanced colorectal cancer |
| Methods | |
| Participants | Patients with T2 or T3 colorectal cancer |
| Interventions | Laparoscopic versus open resection of colorectal cancer |
| Outcomes | Primary endpoint: overall and disease‐free survival Secondary endpoints: short‐term clinical outcomes |
| Starting date | 1998 |
| Contact information | H. Hasegawa Department of Surgery, Keio University School of Medicine, Tokyo, Japan |
| Notes | Patient recruitment terminated October 2000 |
JCOG 0404.
| Trial name or title | Randomised controlled trial to evaluate laparoscopic surgery for colorectal cancer: Japan Clinical Oncology Group JCOG 0404 |
| Methods | |
| Participants | Patients with T3 and T4 tumours of the colon or rectum Sample size: 818 |
| Interventions | Laparoscopic versus open resection of colorectal cancer |
| Outcomes | Primary endpoint: overall survival Secondary endpoints: disease‐free survival, short‐term clinical outcome, adverse events, conversion rate, proportion of completion of laparoscopic surgery |
| Starting date | 2004 |
| Contact information | S. Kitano Department of gastroenterogical Surgery, Oita University Faculty of Medicine |
| Notes | Patient recruitment ongoing |
LAPKON II.
| Trial name or title | Prospective‐randomised multicenter trial on the long‐term results of laparoscopic or open resection of colorectal cancer (LAPKON II) |
| Methods | |
| Participants | Patients with non‐metastasised cancer of the colon or rectum Sample size: 900. Recruitment stopped after 477 inclusions |
| Interventions | Laparoscopic versus open resection of colorectal cancer |
| Outcomes | Primary endpoint: local recurrence after 3 years, survival after 5 years Secondary endpoints: morbidity,mortality, incidence of incisional hernia |
| Starting date | 1998 |
| Contact information | Prof. dr W. Schwenk Department of General‐, Visceral‐, Vascular‐, and Thoracic Surgery Charite campus Mitte, Berlin, Germany |
| Notes | Patient recruitment terminated 01.10.2004 |
Contributions of authors
Esther Kuhry, MD, Ph.D 1st reviewer to search literature, assess quality of trials and collect data, manage the data, coordinate the review, help write the review.
Prof. dr H. Jaap Bonjer, MD, Ph.D Interpretation of data, help write the review, providing general advice on the review.
Robin Gaupset, MD 2nd reviewer to search literature, assess quality of trials and collect data.
Ulla Romild, statistician Analysis of the data, interpretation of data.
Prof. dr. med. Wolfgang Schwenk, MD, Ph.D Interpretation of data, writing the protocol, help write the review
Declarations of interest
Two of the authors (H.J. Bonjer and E. Kuhry) have been involved in COLOR and COLOR II trials in which laparoscopic and open surgery for respectively colon and rectum cancer are compared. One of authors (W. Schwenk) was involved in the LAPKON II trial, in which laparoscopic versus open surgery for colon cancer are compared. Long‐term outcome data of these trials have not been published yet and these studies have therefore not been included in the current analyses.
Edited (no change to conclusions)
References
References to studies included in this review
Araujo 2003 {published data only}
- Araujo SE, Silva eSousa AH Jr, Campos FG, Habr‐Gama A, Dumarco RB, Caravatto PP, Nahas SC, Silva J, Kiss DR, Gama‐Rodriques JJ. Conventional approach x laparoscopic abdominoperineal resection for rectal cancer treatment after neoadjuvant chemoradiation: results of a prospective randomized trial. Rev Hosp Clin Fac Med Sao Paulo 2003;58(3):133‐40. [DOI] [PubMed] [Google Scholar]
Braga 2005 {published data only}
- Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Carlo V. Laparoscopic versus open colectomy in cancer patients: long‐term complications, quality of life, and survival. Dis Colon Rectum 2005;48:2217‐2223. [DOI] [PubMed] [Google Scholar]
COST 2004 {published data only}
- Clinical Outcomes of Surgical Therapy Group. A comparison of laparoscopically assisted and open colectomy for cancer. N Eng J Med 2004;350(20):2050‐9. [DOI] [PubMed] [Google Scholar]
Curet 2000 {published data only}
- Curet MJ, Putrakul K, Pitcher DE, Josloff RK, Zucker KA. Laparoscopically assisted colon resection for colon carcinoma: perioperative results and long‐term outcome. Surg Endosc 2000;14(11):1062‐6. [DOI] [PubMed] [Google Scholar]
Jayne 2007 {published data only}
- Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith A, Heath RM, Brown J. Randomized trial of laparoscopic‐assisted resection of colorectal carcinoma: 3‐year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25(21):3061‐8. [DOI] [PubMed] [Google Scholar]
Kaiser 2004 {published data only}
- Kaiser AM, Kang JC, Chan LS, Vukasin P, Beart RW Jr. Laparoscopic‐assisted vs. open colectomy for colon cancer: a prospective randomized trial. J Laparoendosc Adv Surg Tech A 2004;14(6):329‐34. [DOI] [PubMed] [Google Scholar]
Lacy 2002 {published data only}
- Lacy AM, Garcia Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J. Laparoscopic‐assisted colectomy versus open colectomy for treatment of non‐metastatic colon‐cancer: a randomised clinical trial. Lancet 2002;359(9325):2224‐9. [DOI] [PubMed] [Google Scholar]
Leung 2004 {published data only}
- Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomized trial. Lancet 2004;363(9416):1187‐92. [DOI] [PubMed] [Google Scholar]
Liang 2007 {published data only}
- Liang JT, Huang KC, Lai HS, Lee PH, Jeng YM. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left‐sided colon cancers: a randomized controlled trial. Ann Surg 2007;14(1):109‐17. [DOI] [PubMed] [Google Scholar]
Milsom 1998 {published data only}
- Milsom JW, Bohm B, Hammerhofer KA, Fazio Z, Steiger E, Elson P. A prospective randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg 1998;187(1):46‐54. [DOI] [PubMed] [Google Scholar]
Winslow 2002 {published data only}
- Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparocopic vs open colectomy. Surg Endosc 2002;16(10):1420‐5. [DOI] [PubMed] [Google Scholar]
Zhou 2004 {published data only}
- Zhou ZG, Hu M, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 2004;18:1211‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Basse 2003 {published data only}
- Basse L, Madsen JL, Billesbolle P, Bardram L, Kehlet H. Gastrointestinal transit after laparoscopic versus open colonic resection. Surg Endosc 2003;17(12):1919‐22. [DOI] [PubMed] [Google Scholar]
Basse 2005 {published data only}
- Basse L, Jakobsen DH, Bardram L, Billesbolle P, Lund C, Mogensen T, Rosenberg J, Kehlet H. Functional recovery after open versus laparoscopic colonic resction: a rndomized, blinded study. Ann Surg 2005;241(3):416‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohm 1999 {published data only}
- Bohm B, Junghans T, Neudecker J, Schwenk W. Liverfunction and renal function following laparoscopic and conventional resection of colorectal tumors [Leber‐und nierenfunktion nach laparoskopischer und konventioneller resektion kolorektaler tumoren‐ergebnisse aus einer prospektiv‐randomiserten studie]. Viszeralchirurgie 1999;34:20‐4. [Google Scholar]
Braga 2002 a {published data only}
- Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Carlo V. Laparoscopic versus open colorectal surgery. A randomized trial on short‐term outcome. Ann Surg 2002;236(6):759‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braga 2002 b {published data only}
- Braga M, Vignali A, Zuliani W, Radaelli G, Gianotti L, Martani C, Tousoun G, Carlo V. Metabolic and functional results after laparoscopic colorectal surgery: a randomized, controlled trial. Dis Colon Rectum 2002;45(8):1070‐7. [DOI] [PubMed] [Google Scholar]
Danelli 2002 {published data only}
- Danelli G, Berti M, Perotti V, Albertin A, Baccri P, deni F, Fanelli G, Casati A. Temperature control and recovery of bowel function after laparoscopic or laparotomic colorectal surgery in patients receving combined epidural\general anesthesia and postoperative anesthesia. Anesth Analg 2002;95(2):467‐71. [DOI] [PubMed] [Google Scholar]
Delgado 2001 {published data only}
- Delgado S, Lacy AM, Filela X, Castells A, Garcia‐Valdecasas JC, Pique JM, Mombln D, Visa J. Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum 2001;44(5):638‐46. [DOI] [PubMed] [Google Scholar]
Guillou 2005 {published data only}
- Guillou PJ, Quirke P, Thorpe H, Walker J. Short‐term endpoints of conventional versus laparoscopic‐assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentere, randomised controlled trial.. The Lancet 2005;365(9472):1718‐27. [DOI] [PubMed] [Google Scholar]
Hewitt 1998 {published data only}
- Hewitt PM, Ip SM, Kwok SPY, Somers SS, Li K, Leung KL, Lau WY, Li AKC. Laparoscopic‐assisted vs. open surgery for colorectal cancer: comparative study of immune effects. Dis Colon Rectum 1998;41(7):901‐9. [DOI] [PubMed] [Google Scholar]
Hildebrandt 2003 {published data only}
- Hildebrandt U, Kessler K, Plusczyk T, Pisorius G, Vollmar B, Menger MD. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc 2003;17(2):242‐6. [DOI] [PubMed] [Google Scholar]
Hu 2003 {published data only}
- Hu JK, Zhou ZG, Chen ZX, Wang LL, Yu YY, Liu J, Zhang B, Li L, Shu Y, Chen JP. Comparative evaluation og immune respons after laparoscopical and open total mesorectal excisions with anal sphincter preservation in patients with rectal cancer. World J Gastroenterol 2003;9(12):2690‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janson 2004 {published data only}
- Janson M, Bjørholt I, Carlsson P, Haglind E, Hendriksson M, Lindholm E, Anderberg B. Randomized clinical trial of the costs of open and laparoscopic surgery for colonic cancer. Br J Surg 2004;91:409‐17. [DOI] [PubMed] [Google Scholar]
Jayne 2005 {published data only}
- Jayne DG, Brwon JM, Thorpe H, Walker J, Quirke P, Guillou PJ. Bladder and sexual function following resection for rectal cancer in a randomized clinical trial of laparoscopic versus open technique. Br J Surg 2005;92(9):1124‐32. [DOI] [PubMed] [Google Scholar]
Kang 2004 {published data only}
- Kang JC, Chung MH, Chao PC, Yeh CC, Hsiao CW, Lee TY, Jao SW. Hand‐assisted laparoscopic colectomy versus open colectomy: a prospective randomized study. Surg Endosc 2004;18(4):577‐81. [DOI] [PubMed] [Google Scholar]
Kim 1998 {published data only}
- Kim SH, Milsom JW, Gramlich TL, Toddy SM, Shore GI, Okuda J, Fazio VW. Does laparoscopic vs. conventional surgery increase exfoliated cancer cells in the peritoneal cavity during resection of colorectal cancer?. Dis Colon Rectum 1998;41(8):971‐8. [DOI] [PubMed] [Google Scholar]
Lacy 1995 {published data only}
- Lacy AM, Garcia‐Valdecasas JC, Pique JM, Delgado S, Campo E, Bordas JM, Taura P, Grande L, Fuster J, Pacheco JL, et al. Short‐term outcome analysis of a randomized study comparing laparoscopic vs open colectomy for colon cancer. Surg Endosc 1995;9(10):1101‐5. [DOI] [PubMed] [Google Scholar]
Lacy 1998 {published data only}
- Lacy AM, Delgado S, Garcia‐Valdecasas, Castells A, Pique JM, Grande L, Fuster J, Targarona EM, Pera M, Visa J. Port site metastases and recurrence after laparoscopic colectomy. A randomized trial. Surg Endosc 1998;12:1039‐42. [DOI] [PubMed] [Google Scholar]
Leung 2000 {published data only}
- Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, Lau WY. Systemic cytokine response after laparoscopic‐assisted resection of rectosigmoid carcinoma: A prospective randomized trial. Ann Surg 2000;231(4):506‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2003 {published data only}
- Leung KL, Tsang KS, Ng MH, Leung KJ, Lai PB, Lee JF, Lau WY. Lymphocyte subsets and natural killer cells cytotoxity after laparoscopically assisted resection of rectosigmoid carcinoma. Surg Endosc 2003;17(8):1305‐10. [DOI] [PubMed] [Google Scholar]
Neudecker 2002 {published data only}
- Neudecker J, Junghans T, Ziemer S, Raue W, Schwenk W. Effect of laparoscopic and conventional colorectal resection on peritoneal fibrinolytic cpacity: a prospective randomized clinical trial. Int J Colorectal Dis 2002;17(6):426‐9. [DOI] [PubMed] [Google Scholar]
Neudecker 2003 {published data only}
- Neudecker J, Junghans T, Ziemer S, Raue W, Schwenk W. Prospective randomized tril to determine the influence of laparoscopic and conventional colorectal resection on intravasal fibrinolytic capacity. Surg Endosc 2003;17(1):73‐7. [DOI] [PubMed] [Google Scholar]
Neudecker 2005 {published data only}
- Neudecker J, Junghans T, Raue W, Ziemer S, Schwenk W. Fibrinolytic capacity in peritoneal fluid after laparoscopic and conventional resection: data from a randomized controlled trial. Langenbecks Arch Surg 2005;390(6):523‐7. [DOI] [PubMed] [Google Scholar]
Ordemann 2001 {published data only}
- Ordemann J, Jacobi CA, Schwenk W, Stosslein R, Muller JM. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc 2001;15(6):600‐8. [DOI] [PubMed] [Google Scholar]
Ortiz 1996 {published data only}
- Ortiz H, Armendariz P, Yarnoz C. Early postoperative feeding after elective colorectal surgery is not a benefit unique to laparoscopy‐asisted procedures. Int J Colorectal Dis 1996;11(5):246‐9. [DOI] [PubMed] [Google Scholar]
Schwenk 1998 a {published data only}
- Schwenk W, Bohm B, Haase O, Junghans T, Muller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch Surg 1998;383(1):49‐55. [DOI] [PubMed] [Google Scholar]
Schwenk 1998 b {published data only}
- Schwenk W, Bohm B, Muller JM. Postoperative pain and fatique after laparoscopic or conventional colorectal resections. A prospective randomized trial. Surg Endosc 1998;12(9):1131‐6. [DOI] [PubMed] [Google Scholar]
Schwenk 1998 c {published data only}
- Schwenk W, Bohm B, Haase O, Junghans T, Muller JM. Laparoscopic or conventional colorectal resection ‐ is there an influence of the postoperative technique on postoperative quality of life? [Laparoskopische oder konventionelle kolorektale Resektionen ‐ beenflusst die Operasionstechnik die postoperative Lebensqualitat?]. Zentralbl Chir 1998;123:483‐90. [PubMed] [Google Scholar]
Schwenk 1999 {published data only}
- Schwenk W, Bohm B, Witt C, Junghans T, Grundel K, Muller JM. Pulmonary function following laparoscopic or conventional colorectal resection: a randomized controlled evaluation. Arch Surg 1999;134(1):6‐12. [DOI] [PubMed] [Google Scholar]
Schwenk 2002 {published data only}
- Schwenk W, Neudecker J, Bohm B, Muller J. Postoperative short‐term course following laparoscopic or conventional resection of colorectal tumours [Kurzfristiger postoperativer verlauf nach laparoskopischen oder konventionellen resektionen kolorektaler tumoren]. Minimal Invasive Chirurgie 2002;11(2):112‐8. [Google Scholar]
Stage 1997 {published data only}
- Stage JG, Schulze S, Moller P, Overgaard H, Andersen M, Rebsdorf‐Pedersen VB, Nielsen HJ. Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma. Br J Surg 1997;84(3):391‐6. [PubMed] [Google Scholar]
Tang 2001 {published data only}
- Tang CL, Eu KW, Tai BC, Soh JG, MacHin D, Seow‐Choen F. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg 2001;88(6):801‐7. [DOI] [PubMed] [Google Scholar]
Weeks 2002 {published data only}
- Weeks JC, Nelson H, gelber S, Sargent D, Schroeder G, Clinical Outcomes of Surgical Therapy (COST) Study Group. Short‐term quality‐of‐life outcomes following laparoscpic‐asisted colectomy vs open colectomy for colon cancer: a randomdized trial. JAMA 2002;287(3):321‐8. [DOI] [PubMed] [Google Scholar]
Wu 2003 {published data only}
- Wu FPK, Sietses C, Blomberg BME, Leeuwen PAM, Meijer S, Cuesta MA. Systemic and peritoneal inflammatory response after laparoscopic or conventional colon resection in cancer patients. Dis Colon Rectum 2003;46(2):147‐55. [DOI] [PubMed] [Google Scholar]
Wu 2004 {published data only}
- Wu FP, Hoekman K, Sietses C, Blomberg BM, Meijer S, Bonjer HJ, Cuesta MA. Systemic and peritoneal angiogenic reponse after laparoscopic or conventional colonresction in cancer patients: a prospective, randomized trial. Dis Colon Rectum 2004;47(10):1670‐4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
COLOR {unpublished data only}
- Hazebroek EJ, COLOR Study Group. COLOR. A randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc 2002;16:949‐53. [DOI] [PubMed] [Google Scholar]
- M. Buunen, COLOR Study Group. Long‐term outcome of the COLOR trial. Abstracts of the 15th congress of the European Association for Endoscopic Surgery (EAES). 2007.
- Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy AM, COlon cancer Laparoscopic or Open Resection. Laparoscopic surgery versus open surgery for colon cancer: short‐term outcomes of a randomised trial. Lancet Oncol 2005;6(7):477‐84. [DOI] [PubMed] [Google Scholar]
COLOR II {unpublished data only}
- COLOR II: Laparoscopic versus open surgery for rectal cancer. Ongoing study 2003.
Hasegawa {published data only}
- Hasegawa H, Kabeshima Y, Watanabe S, Yamamoto S, Kitajima M. Randomized controlled trial of laparoscopic versus open colectomy for advanced colorectal cancer. Surg Endosc 2003;17:636‐40. [DOI] [PubMed] [Google Scholar]
JCOG 0404 {published data only}
- Kitano S, Inomata M, Sato A, Yoshimura K, Moriya Y, Japan Clinical Oncology Group. Randomized controlled trial to evaluate laparoscopic surgery for colorectal cancer: Japan Clinical Oncology Group Study JCOG 0404. Jpn J Clin Oncol 2005;35(8):475‐7. [DOI] [PubMed] [Google Scholar]
LAPKON II {unpublished data only}
- Prospective‐randomised multicenter trial on the long‐term results of laparoscopic or open resection of colorectal cancer (LAPKON II). Ongoing study 1998.
Additional references
Berends 1994
- Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet 1994;344(8914):58. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 1997
- Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg 1997;Suppl(557):5‐9. [PubMed] [Google Scholar]
Ellis 1999
- Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, McGuire A, Lower AM, Hawthorn RJ, O`Brien F, Bucchan S, Crowe AM. Adhesion‐related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet 1999;3533(9163):1476‐1480. [DOI] [PubMed] [Google Scholar]
Franklin 1993
- Franklin ME, Ramos R, Rosenthal D, Schuessler W. Laparoscopic colonic procedures. World J Surg 1993;17:51‐56. [DOI] [PubMed] [Google Scholar]
Halpern 1994
- Halpern S, Preston R. Postdural puncture headache and spinal needle design. Anesthesiology 1994;81:1376‐83. [DOI] [PubMed] [Google Scholar]
Jacobs 1991
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144‐150. [PubMed] [Google Scholar]
Kuhry 2005
- Kuhry E, Bonjer HJ, Haglind E, Hop WC, Veldkamp R, Cuesta MA, Påhlman L, Morino M, Lacy A, Delgado S, COLOR Study Group. Impact of hospital case volume on short‐term outcome after laparoscopic operation for colonic cancer. Surg Endosc 2005;19(5):687‐92. [DOI] [PubMed] [Google Scholar]
Lower 2000
- Lower AM, Hawthorn RJ, Ellis H, O`Brien F, Buchan S, Crowe AM. The impact of adhesions on hospital readmissions after abdominal and pelvic surgery: a retrospective study. BJOG 2000;107(7):855‐862. [DOI] [PubMed] [Google Scholar]
Nduka 1994
- Nduka CC, Monson JR, Menzies‐Gow N, Darzi A. Abdominal wall metastases following laparoscopy. Br J Surg 1994;81(5):648‐52. [DOI] [PubMed] [Google Scholar]
Nelson 1998
- Nelson RL. Division of the colorectum into anatomic subsites: why and where?. J of Surg Oncol 1998;69:1‐3. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Ries 2000
- Ries LAG, Wingo RAW, Miller S, Howe HL, Weir HK, Rosenberg HM, Vernone SW, Cronin K, Edwards BK. The annual report to the nation on the status of cancer, 1973 ‐ 1997, with a special section on colorectal cancer. Cancer 2000;88(10):2398‐2424. [DOI] [PubMed] [Google Scholar]
Schwenk 1998
- Schwenk W, Böhm B, Müller JM. Postoperative pain and fatigue after laparoscopic or conventional colorectal resections. A prospective randomized trial. Surg Endosc 1998;12:1131‐1136. [DOI] [PubMed] [Google Scholar]
Schwenk 2005
- Schwenk W, Haase O, Neudecker J, Muller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003145.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


