Abstract
Intraoperative hypotension (IOH) i.e., low arterial blood pressure (AP) during surgery is common in patients having non-cardiac surgery under general anaesthesia. It has a multifactorial aetiology, and is associated with major postoperative complications including acute kidney injury, myocardial injury and death. Therefore, IOH may be a modifiable risk factor for postoperative complications. However, there is no uniform definition for IOH. IOH not only occurs during surgery but also after the induction of general anaesthesia before surgical incision. However, the optimal therapeutic approach to IOH remains elusive. There is evidence from one small randomised controlled trial that individualising AP targets may reduce the risk of postoperative organ dysfunction compared with standard care. More research is needed to define individual AP harm thresholds, to develop therapeutic strategies to treat and avoid IOH, and to integrate new technologies for continuous AP monitoring.
Key words: Acute kidney injury, blood pressure, haemodynamic monitoring, myocardial injury after non-cardiac surgery, postoperative complications
INTRODUCTION
Each year more than 300 million surgeries are performed worldwide.[1] Rates of major complications and mortality in the first weeks after surgery remain very high.[2,3] Postoperative deaths are a consequence of postoperative complications[3,4] that occur in up to a quarter of patients after in-patient surgery.[5] To avoid postoperative complications, it is crucial to identify and avoid modifiable risk factors for their occurrence. One modifiable risk factor for postoperative complications may be intraoperative hypotension (IOH). In this article, we will discuss the pathophysiology of IOH, its clinical relevance, and current concepts of perioperative arterial blood pressure (AP) management. We searched the electronic databases Pubmed, Web of Science and Cochrane Library using the following search terms (last date of search 15.11.2019): ((hypotension[title and abstract] OR hypotensive [title and abstract]) AND (intraoperative [title and abstract] OR perioperative[title and abstract] OR intraoperatively[title and abstract] OR perioperatively[title and abstract])). In addition, we searched the reference lists of the identified studies and the reference lists of review articles to find additional studies that we had not identified initially. We restricted the search and subsequent bibliographic review to studies (no correspondence or case reports) published in English between 1990 and 2019.
INTRAOPERATIVE HYPOTENSION – DEFINITION
There is no uniform definition for IOH.[6] It even remains unknown whether IOH should be defined based on absolute AP thresholds or on relative thresholds considering a decrease from baseline AP.[7,8] A systematic review identified over 140 different definitions for IOH in 130 studies; definitions were based on systolic AP (SAP) or mean AP (MAP) values, absolute values or relative changes or a combination of them.[6] Frequently used definitions include SAP below 80 mmHg, a decrease in SAP of more than 20% below baseline, and a combination of definitions consisting of an absolute SAP below 100 mmHg and/or 30% decrease below baseline. Depending on which definition for IOH is used, the incidence varies between 5% and 99%.[6]
IOH is not a distinct disease entity that only occurs during surgery. About one third of perioperative hypotensive episodes occur in the period after the induction of general anaesthesia but before surgical incision and can be described as postinduction or pre-incision hypotension.[9,10] Postinduction hypotension should be differentiated against phases of hypotension during surgery, as the causes of hypotension vary in the different phases.[10] While postinduction hypotension is solely caused by anaesthetic management, IOH occurring during surgery can be caused by numerous factors related to general anaesthesia and surgery.
INTRAOPERATIVE HYPOTENSION – PATHOPHYSIOLOGY AND RISK FACTORS
Many pathophysiologic mechanisms can lead to IOH in patients having surgery under general anaesthesia. Thus, the aetiology of IOH is multifactorial. IOH can, among other factors, be caused by vasodilation (anaesthetic drugs, systemic inflammation), intravascular hypovolaemia (bleeding), low cardiac output (bradycardia or low stroke volume), high intra-thoracic pressure (mechanical ventilation), impairment of sympathetic nervous system or compromised baroreflex regulation.
Several risk factors for IOH have been identified, such as older age, high American Society of Anesthesiologists (ASA) class, male sex, lower pre-induction SAP, general anaesthesia with propofol, the combination of general and regional anaesthesia, the duration of surgery, and emergency surgery.[11,12] Additionally, antihypertensive medications such as angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, betablockers, and alpha-2 agonists predispose to the occurrence of hypotensive events during anaesthesia, which is associated with cardiovascular complications and mortality.[13,14,15]
INTRAOPERATIVE HYPOTENSION AND POSTOPERATIVE OUTCOME
IOH is common and associated with major postoperative complications including myocardial injury,[16] acute kidney injury[7,17,18] and death[19,20,21] in patients having non-cardiac surgery under general anaesthesia. The association between IOH and postoperative complications is supported by various database studies.
A retrospective database study including 33,330 non-cardiac surgeries in 27,381 patients demonstrated an independent association between IOH and postoperative acute kidney and myocardial injury.[17] On a population level, the risk for acute kidney and myocardial injury markedly increased below lowest intraoperative MAP values of 55–60 mmHg. Even short durations (i.e., 1–5 minutes) of an intraoperative MAP less than 55 mmHg were associated with acute kidney and myocardial injury, and the odds for these organ injuries additionally increased with longer time periods spent below this MAP threshold.[17]
Another large retrospective database study including 57,315 patients who had non-cardiac surgery also demonstrated that IOH – defined either based on absolute or relative MAP thresholds – was independently associated with both acute kidney and myocardial injury.[7] Both MAP values below 65 mmHg and MAP values 20% below preoperative baseline MAP were progressively associated with postoperative organ injury. At any given MAP threshold, prolonged exposure to IOH was associated with increased odds for postoperative acute kidney and myocardial injury.[7]
Yet another retrospective cohort study investigated the association between intraoperative AP and 30-day mortality in 18,756 patients who underwent non-cardiac surgery using three different approaches to define IOH: population thresholds (individual patient sum of area under threshold two standard deviations from the mean of the population intraoperative AP), absolute thresholds (based on clinical judgement and literature), and percent change from baseline AP.[20] There was an independent association between the occurrence of profound IOH lasting for at least 5 minutes and 30-day mortality.
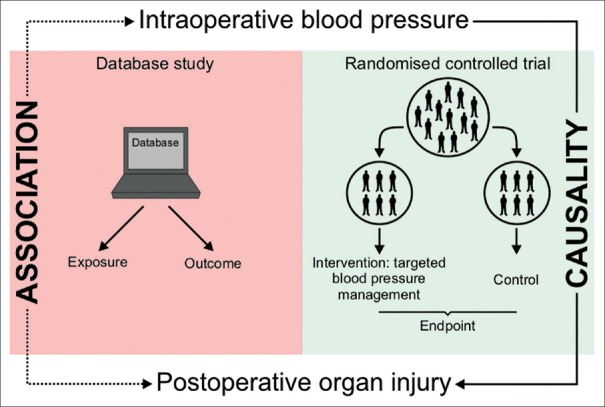
While there are several retrospective database studies that demonstrate an association between IOH and postoperative morbidity and mortality, to date, there is only one randomised controlled trial on perioperative AP management that actually demonstrated a causality between intraoperative AP and postoperative organ function.[22] The multicentre INPRESS trial demonstrated that applying individualised SAP targets significantly reduced the risk of postoperative organ dysfunction compared with standard AP management in high-risk patients having major surgery.[22] It is of crucial importance to understand that there is a fundamental difference between an association and a causal relation between IOH and postoperative organ injury [Figure 1]. While retrospective database studies can only provide insights into the association between an exposure (e.g., IOH) and an outcome (e.g., postoperative organ injury) and can only account for some confounding factors, randomised controlled trials can provide evidence for a causal relation between an exposure (that is modified by an intervention) and an endpoint.
Figure 1.
This figure illustrates that a retrospective database study can only provide insights into the association between an exposure (e.g., intraoperative hypotension) and an outcome. In contrast, a randomised controlled trial can provide evidence for a causal relation between the exposure (that is modified by an intervention) and an endpoint
HOW TO DETECT INTRAOPERATIVE HYPOTENSION?
Monitoring AP in patients during anaesthesia is an obligatory part of standard anaesthetic management. Surgery- and patient-related risk factors determine which method should be used to monitor AP. In clinical routine, AP measurements are usually obtained intermittently and non-invasively using oscillometry (upper-arm cuff method), normally at intervals of 2–5 minutes, or continuously and invasively with an arterial catheter.[23] Arterial catheters are used for continuous AP monitoring in patients with high patient-specific or surgery-related risk. An interventional trial with 143 patients revealed that invasive AP monitoring detected, on average, twice as many hypotensive minutes compared to oscillometric measurements.[24] Additionally, patients having invasive AP monitoring received a third more vasopressor boluses than patients with oscillometric AP monitoring.[24]
In the future, technologies for continuous non-invasive AP monitoring – e.g., finger-cuff technologies – may be used in patients undergoing surgery as an alternative to intermittent non-invasive or continuous invasive AP monitoring.[25,26,27] A randomised trial including 316 patients having moderate-to-high risk surgery tested the hypothesis that continuous non-invasive AP monitoring reduces IOH.[28] In all patients, AP was continuously monitored using a finger-cuff technology and standard intermittent oscillometric measurements. In half of the patients, AP values from continuous AP monitoring were available to the clinician. Those patients assigned to continuous AP monitoring had significantly lower time-weighted average MAP below 65 mmHg compared to intermittent AP monitoring. Another randomised trial investigated the impact of continuous non-invasive AP monitoring in 160 patients with a history of chronic hypertension having orthopaedic surgery.[29] AP was monitored in all patients using standard intermittent oscillometric measurements. Patients in the study group were simultaneously monitored using a finger-cuff technology and had significantly fewer hypotensive episodes compared to patients of the control group.
THERAPEUTIC APPROACHES TO INTRAOPERATIVE HYPOTENSION
Given the association between IOH and adverse postoperative outcomes, IOH should be avoided and timely treated. The choice of therapeutic interventions is a subject of an ongoing debate since it remains unclear which treatment strategies substantially affect outcomes.[30] Common therapeutic approaches are the use of vasoactive agents – especially vasopressors – and fluids. However, there is still no uniform consensus on which vasopressors should be used to increase vascular tone and increase AP during surgery. Large randomised trials are needed to answer the questions which treatment strategy should be used to avoid or treat IOH.
Besides pharmacologic treatment options, there might be other strategies to prevent IOH. For example, one randomised controlled trial studied the impact of peristaltic pneumatic compression of the legs on the amount of intraoperative fluid demand and haemodynamic stability in 70 patients having minor surgery.[31] The study revealed that peristaltic pneumatic compression of the legs reduced the rate of hypotensive events as well as the amount of administered intravenous fluids. However, it has to be shown whether this type of intervention actually affects patient-centred outcomes and if it can be transferred to high-risk surgical patients.
WHAT IS THE 'OPTIMAL' BLOOD PRESSURE FOR THE INDIVIDUAL PATIENT?
Knowing that IOH is associated with postoperative complications and death, the remaining key question is which AP is optimal for the individual patient having surgery under general anaesthesia. Based on the results of large retrospective database studies, an absolute MAP threshold of 65 mmHg was recommended as an absolute lower intervention threshold,[30] as lower intraoperative MAP values are associated with harm in the non-cardiac surgical population.[7] However, using this absolute population-based MAP threshold for all patients would ignore the fact that normal AP values vary considerably among individuals[32] and that many patients, especially patients with chronic arterial hypertension may need higher MAP values.[8] Because blood flow autoregulation depends on the individual patient's normal AP, the organ-dependent autoregulation curve is shifted towards higher AP values in patients with arterial hypertension. This leads to a narrowing or complete loss of the plateau range of pressure over which constant blood flow is ensured.[33] The effect of chronic arterial hypertension on the association between IOH and postoperative mortality was nicely shown in a retrospective cohort study including 152,445 non-cardiac surgery patients.[21] The study showed that there is an independent association between the cumulative time accrued below a wide range of MAP thresholds (between 75 and 45 mmHg) and increased all-cause mortality within 30 days after surgery. The increased risk for postoperative mortality was related to a combination of the severity and duration of IOH below a wide range of commonly encountered MAP thresholds over periods of time, that were shorter in patients with a history of hypertension.
Therefore, population harm thresholds for MAP do not necessarily match the individual patient's optimal MAP.[8] In addition, not MAP but organ perfusion pressure is the ultimate target when using strategies for AP optimisation. As the organ perfusion pressure is the organ-specific 'inflow pressure' (which is MAP for most organ systems including the brain and the kidneys) minus the 'outflow pressure', a general recommendation for optimal MAP targets ignoring outflow pressure cannot be given from a physiologic point of view. Rather, MAP thresholds may be adjusted based on the individual patient's outflow pressures, which vary between different organs.[33] For instance, a patient with high intra-cranial pressure might be at relevant risk for cerebral hypoperfusion if a fixed MAP target is applied, since high intra-cranial pressure reduces cerebral perfusion.
Additionally, the type of surgery together with various critical surgery-related events (e.g., changes in position, clamping of arteries, bleeding) plays an important role for defining the 'optimal' MAP for the individual patient. For instance, a patient having trauma surgery in beach chair position or a patient having carotid endarterectomy needs a higher MAP to ensure adequate cerebral perfusion pressure.
One randomised controlled trial tested the hypothesis that individualising AP targets is superior to standard AP management in terms of postoperative outcomes. The INPRESS trial compared individualised and standard AP management in 292 high-risk patients having major surgery.[22] In this multicentre trial, patients of the standard treatment group received ephedrine boluses for any decrease in SAP below 80 mmHg or lower than 40% from the patient's reference value (patient's resting SAP). Patients in the individualised treatment group received a continuous infusion of norepinephrine during surgery and for four postoperative hours to achieve a SAP within 10% of the reference value. Using individualised SAP targets significantly reduced the risk of postoperative organ dysfunction in patients in the intervention group compared with patients in the standard management group. However, the INPRESS trial has several major limitations that limit the internal validity of the trial, including the use of two different drugs for AP management in the treatment groups or the assessment of the baseline reference value based on single non-standardised AP measurements.[34,35]
HOW TO ASSESS THE INDIVIDUAL NORMAL BLOOD PRESSURE?
Given that individualising AP target values during surgery under general anaesthesia might be an innovative and promising approach[36] a key question is how to assess the individual patient's normal AP.
In clinical practice, AP measurements taken just before induction of general anaesthesia are often used as a surrogate for the patient's normal AP. However, a prospective observational study comparing ambulatory and perioperative AP in 370 patients showed that pre-induction MAP values do not reflect mean daytime MAP values.[32]
Another observational study including 101 patients having elective surgery showed that high pre-induction AP (SAP ≥160 mmHg and/or diastolic AP (DAP) ≥100 mmHg), as well as AP measurements from the day of surgery, are likely to overestimate ambulatory baseline AP.[37] Pre-induction MAP values thus should not serve as a surrogate for the individual normal daytime MAP.[32,37] A recent consensus statement of the Perioperative Quality Initiative states that 'ambulatory AP measurement is the optimal method to establish baseline values'.[30]
FUTURE RESEARCH AND PERSPECTIVES
Further research is needed to define individual AP harm thresholds, to develop therapeutic strategies to avoid or treat IOH, and to integrate new technologies and improve their measurement performance to detect and predict IOH. So far, there is a lack of large randomised trials that investigate the causality between IOH and postoperative complications and the impact of IOH treatment strategies on postoperative outcomes. Nevertheless, there are major advances in the development of continuous non-invasive AP monitoring devices and algorithms using machine learning and artificial intelligence for the prediction of hypotension. The latter use machine-learning models that analyse features of the AP waveform to predict hypotension in real-time several minutes before a hypotensive event becomes clinically apparent.[38] Studies that investigate whether these algorithms substantively reduce hypotension during non-cardiac surgery are currently in progress.[39,40] Besides machine learning and artificial intelligence, the development of new monitoring technologies that enable the clinician to define individual AP thresholds based on the measurement of cerebral blood flow autoregulation might play an important role in the near future.[41] The impact of new AP monitoring technologies and innovative individualised therapeutic strategies on IOH-related postoperative complications and death needs to be studied in randomised trials.
SUMMARY
IOH is common in patients having non-cardiac surgery under general anaesthesia and is associated with acute kidney injury, myocardial injury and death. Many observational database studies show the association between IOH and postoperative complications. Therefore, IOH may be a modifiable risk factor for postoperative complications. However, there is no uniform definition for IOH. IOH not only occurs during surgery but also after the induction of general anaesthesia before surgical incision. However, the optimal therapeutic approach to IOH remains elusive. There is evidence from one small randomised controlled trial that individualising AP targets may reduce the risk of postoperative organ dysfunction compared with standard care. More research is needed to define individual AP harm thresholds, to develop therapeutic strategies to treat and avoid IOH, and to integrate new technologies for continuous AP monitoring.
Financial support and sponsorship
Support was provided only from institutional and/or departmental funds.
Conflicts of interest
KK has no conflicts of interest to declare.
PH has no conflicts of interest to declare.
LB has no conflicts of interest to declare.
BS has received honoraria for consulting, honoraria for giving lectures, and refunds of travel expenses from Edwards Lifesciences Inc. (Irvine, CA, USA). BS has received honoraria for consulting, institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from Pulsion Medical Systems SE (Feldkirchen, Germany). BS has received institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from CNSystems Medizintechnik GmbH (Graz, Austria). BS has received institutional restricted research grants from Retia Medical LLC. (Valhalla, NY, USA). BS has received honoraria for giving lectures from Philips Medizin Systeme Böblingen GmbH (Böblingen, Germany). BS has received honoraria for consulting, institutional restricted research grants, and refunds of travel expenses from Tensys Medical Inc. (San Diego, CA, USA).
REFERENCES
- 1.Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(Suppl 2):S11. doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 2.Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. European Surgical Outcomes Study (EuSOS) group for the Trials groups of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–65. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators. Spence J, LeManach Y, Chan MT, Wang CY, Sigamani A, Xavier D, et al. Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019;191:E830–7. doi: 10.1503/cmaj.190221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373:2258–69. doi: 10.1056/NEJMra1502824. [DOI] [PubMed] [Google Scholar]
- 5.International Surgical Outcomes Study Group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016;117:601–9. doi: 10.1093/bja/aew316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107:213–20. doi: 10.1097/01.anes.0000270724.40897.8e. [DOI] [PubMed] [Google Scholar]
- 7.Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: A retrospective cohort analysis. Anesthesiology. 2017;126:47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 8.Saugel B, Reuter DA, Reese PC. Intraoperative mean arterial pressure targets: Can databases give us a universally valid “magic number” or does physiology still apply for the individual patient? Anesthesiology. 2017;127:725–6. doi: 10.1097/ALN.0000000000001810. [DOI] [PubMed] [Google Scholar]
- 9.Maheshwari K, Turan A, Mao G, Yang D, Niazi AK, Agarwal D, et al. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223–8. doi: 10.1111/anae.14416. [DOI] [PubMed] [Google Scholar]
- 10.Sudfeld S, Brechnitz S, Wagner JY, Reese PC, Pinnschmidt HO, Reuter DA, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119:57–64. doi: 10.1093/bja/aex127. [DOI] [PubMed] [Google Scholar]
- 11.Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. doi: 10.1213/01.ANE.0000175214.38450.91. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Taffe P, Sicard N, Pittet V, Pichard S, Burnand B. The occurrence of intra-operative hypotension varies between hospitals: observational analysis of more than 147,000 anaesthesia. Acta Anaesthesiol Scand. 2009;53:995–1005. doi: 10.1111/j.1399-6576.2009.02032.x. [DOI] [PubMed] [Google Scholar]
- 13.Devereaux PJ, Sessler DI, Leslie K, Kurz A, Mrkobrada M, Alonso-Coello P, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–13. doi: 10.1056/NEJMoa1401106. [DOI] [PubMed] [Google Scholar]
- 14.POISE Study Group. Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 15.Roshanov PS, Rochwerg B, Patel A, Salehian O, Duceppe E, Belley-Côté EP, et al. withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An analysis of the vascular events in noncardiac surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126:16–27. doi: 10.1097/ALN.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 16.Sessler DI, Khanna AK. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018;44:811–22. doi: 10.1007/s00134-018-5224-7. [DOI] [PubMed] [Google Scholar]
- 17.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 18.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–23. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 19.Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123:79–91. doi: 10.1097/ALN.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 20.Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping ST, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–19. doi: 10.1097/ALN.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 21.Stapelfeldt WH, Yuan H, Dryden JK, Strehl KE, Cywinski JB, Ehrenfeld JM, et al. The SLUScore: A novel method for detecting hazardous hypotension in adult patients undergoing noncardiac surgical procedures. Anesth Analg. 2017;124:1135–52. doi: 10.1213/ANE.0000000000001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Futier E, Lefrant JY, Guinot PG, Godet T, Lorne E, Cuvillon P, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: A randomized clinical trial. JAMA. 2017;318:1346–57. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saugel B, Dueck R, Wagner JY. Measurement of blood pressure. Best Pract Res Clin Anaesthesiol. 2014;28:309–22. doi: 10.1016/j.bpa.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Naylor AJ, Sessler DI, Maheshwari K, Khanna AK, Yang D, Mascha EJ, et al. Arterial catheters for early detection and treatment of hypotension during major noncardiac surgery: A randomized trial. Anesth Analg. 2019 doi: 10.1213/ANE.0000000000004370. doi: 10.1213/ANE.0000000000004370. [DOI] [PubMed] [Google Scholar]
- 25.Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42:1350–9. doi: 10.1007/s00134-016-4375-7. [DOI] [PubMed] [Google Scholar]
- 26.Thiele RH. Cardiac bulldozers, backhoes, and blood pressure. Anesth Analg. 2015;121:1417–9. doi: 10.1213/ANE.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 27.Saugel B, Cecconi M, Wagner JY, Reuter DA. Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth. 2015;114:562–75. doi: 10.1093/bja/aeu447. [DOI] [PubMed] [Google Scholar]
- 28.Maheshwari K, Khanna S, Bajracharya GR, Makarova N, Riter Q, Raza S, et al. A randomized trial of continuous noninvasive blood pressure monitoring during noncardiac surgery. Anesth Analg. 2018;127:424–31. doi: 10.1213/ANE.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meidert AS, Nold JS, Hornung R, Paulus AC, Zwissler B, Czerner S. The impact of continuous non-invasive arterial blood pressure monitoring on blood pressure stability during general anaesthesia in orthopaedic patients: A randomised trial. Eur J Anaesthesiol. 2017;34:716–22. doi: 10.1097/EJA.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 30.Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–74. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Kiefer N, Theis J, Putensen-Himmer G, Hoeft A, Zenker S. Peristaltic pneumatic compression of the legs reduces fluid demand and improves hemodynamic stability during surgery: A randomized, prospective study. Anesthesiology. 2011;114:536–44. doi: 10.1097/ALN.0b013e31820c3973. [DOI] [PubMed] [Google Scholar]
- 32.Saugel B, Reese PC, Sessler DI, Burfeindt C, Nicklas JY, Pinnschmidt HO, et al. Automated ambulatory blood pressure measurements and intraoperative hypotension in patients having noncardiac surgery with general anesthesia: A prospective observational study. Anesthesiology. 2019;131:74–83. doi: 10.1097/ALN.0000000000002703. [DOI] [PubMed] [Google Scholar]
- 33.Kato R, Pinsky MR. Personalizing blood pressure management in septic shock. Ann Intensive Care. 2015;5:41. doi: 10.1186/s13613-015-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daoud M. Organ dysfunction after surgery in patients treated with individualized or standard blood pressure management. JAMA. 2018;319:720–1. doi: 10.1001/jama.2017.20931. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell K, Adams D, McHugh SM. Organ dysfunction after surgery in patients treated with individualized or standard blood pressure management. JAMA. 2018;319:719–20. doi: 10.1001/jama.2017.20948. [DOI] [PubMed] [Google Scholar]
- 36.Saugel B, Vincent JL, Wagner JY. Personalized hemodynamic management. Curr Opin Crit Care. 2017;23:334–41. doi: 10.1097/MCC.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 37.Drummond JC, Blake JL, Patel PM, Clopton P, Schulteis G. An observational study of the influence of “white-coat hypertension” on day-of-surgery blood pressure determinations. J Neurosurg Anesthesiol. 2013;25:154–61. doi: 10.1097/ANA.0b013e31827a0151. [DOI] [PubMed] [Google Scholar]
- 38.Saugel B, Kouz K, Hoppe P, Maheshwari K, Scheeren TW. Predicting hypotension in perioperative and intensive care medicine. Best Pract Res Clin Anaesthesiol. 2019;33:189–97. doi: 10.1016/j.bpa.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Wijnberge M, Schenk J, Terwindt LE, Mulder MP, Hollmann MW, Vlaar AP, et al. The use of a machine-learning algorithm that predicts hypotension during surgery in combination with personalized treatment guidance: study protocol for a randomized clinical trial. Trials. 2019;20:582. doi: 10.1186/s13063-019-3637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maheshwari K, Shimada T, Fang J, Ince I, Mascha EJ, Turan A, et al. Hypotension Prediction Index software for management of hypotension during moderate- to high-risk noncardiac surgery: protocol for a randomized trial. Trials. 2019;20:255. doi: 10.1186/s13063-019-3329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brady KM, Hudson A, Hood R, DeCaria B, Lewis C, Hogue CW. Personalizing the definition of hypotension to protect the brain. Anesthesiology. 2020;132:170–9. doi: 10.1097/ALN.0000000000003005. [DOI] [PubMed] [Google Scholar]