Abstract
Iatrogenic withdrawal syndrome is an increasingly recognized issue among adult patients in the intensive care unit. The prolonged use of opioids and benzodiazepines during the intensive care unit stay and preexisting disorders associated with their use put patients at risk of developing iatrogenic withdrawal syndrome. Although research to date is scant regarding iatrogenic withdrawal syndrome in adult patients in the intensive care unit, it is important to recognize and adequately manage iatrogenic withdrawal syndrome in order to prevent possible negative outcomes during and after a patient’s intensive care unit stay. This article discusses in depth 8 studies of iatrogenic withdrawal syndrome among adult patients in the intensive care unit. It also addresses important aspects of opioid and benzodiazepine iatrogenic withdrawal syndrome, including prevalence, risk factors, and assessment and considers its prevention and management.
Keywords: benzodiazepines, critical care, opioids, pain management, iatrogenic withdrawal syndrome
Providing appropriate levels of analgesia and sedation is essential to preventing suffering, promoting comfort, and avoiding adverse physiological consequences of pain and agitation among patients in the intensive care unit (ICU). Current ICU practices to provide analgesia and sedation emphasize the minimal use of both opioids (OPs) and benzodiazepines (BNZs) in order to prevent complications from the treatments themselves.1 Some clinical situations, however, may preclude this practice. For example, some patients may require higher and/or prolonged doses of OPs for complicated pain issues and BNZs for deeper sedation levels.
Although BNZs are no longer recommended as sedation agents in the ICU because of their deliriogenic effects,1 they are still used as the main therapy for sedation in low-income1 and some high-income countries.2,3 Patients may develop physical dependence when exposed to large amounts or prolonged use of OPs, BNZs, or both. This puts them at risk for iatrogenic withdrawal syndrome (IWS) when these medications are reduced or discontinued.4,5 Patients who have chronically used these drugs before ICU admission may also be vulnerable to IWS.
In any case, the risk of IWS is poorly recognized. Furthermore, if IWS is present, it may be managed ineffectively in the ICU.6 In other scenarios, per the Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption (PADIS) clinical practice guidelines,1 practice has shifted from sedative-based sedation to analgesic-based sedation, which promotes the use of an OP before sedation. This practice may have increased, or will increase, the selection of OPs over sedatives, which may change the etiology of IWS in years to come.
IWS must be recognized and adequately managed, although research to date is scant regarding IWS in adult patients in the ICU. The purpose of this article is to discuss important aspects of OP and BNZ IWS, including its prevalence and risk factors. The article addresses IWS assessment and considers how to prevent and manage it in light of negative outcomes during and after and ICU stay.
Growing Recognition of OP/BNZ IWS in the ICU
Opioid and BNZ IWS has been widely studied in pediatric patients in the ICU. However, little attention has been given to IWS in adult patients in the ICU. In fact, only 8 reports7–14 have been published since 1998, when the first study addressing IWS in adults was published.15 Six of these reports were published during the years 2017 through 2019.9–14 Current concern about IWS in adult patients in the ICU is evidenced by recent presentations at important critical care congresses: the 2018 Congress of the European Society of Intensive Care Medicine and the 2019 Critical Care Congress of the Society of Critical Care Medicine. This concern is important because IWS can contribute to negative short-term outcomes in patients in the ICU such as prolonged mechanical ventilation (MV) and extended ICU length of stay (LOS).8,10,15 In addition, IWS can contribute to patient suffering, because its signs and symptoms can be extremely unpleasant. To fully understand IWS, clinicians must consider it within the context of other separate but related conditions and their definitions.
Important Definitions
Withdrawal syndrome comprises a constellation of signs and symptoms that can be induced by abruptly interrupting or quickly reducing the dose of a drug, decreasing drug concentrations in the blood, and administering an antagonist of the drug.5 Iatrogenic withdrawal syndrome occurs when providers do not recognize the risk of IWS or do not take measures to prevent it in certain populations. More specifically, IWS can occur when weaning patients from certain drugs they have been using chronically before ICU admission. It can also occur in drug-naive patients who have been taking such drugs while being cared for in the ICU.
Other terms related to IWS have important meanings in relation to treating patients. Tolerance is a decrease in the pharmacological effect of a drug after its continuous use.5 Opioid-induced hyperalgesia (OIH), which can be confused with tolerance, is a state attributed to the continuous use of an OP that can, paradoxically, provoke an exaggerated response to pain.16 Both tolerance and OIH can be misdiagnosed, but they require different interventions and thereby affect patient management. Tolerance is treated by increasing the dose of a particular OP, whereas treating OIH requires discontinuing the OP in order to reverse the hyperalgesia. Patients with OIH can be treated with N-methyl-d-aspartate (NMDA) receptor antagonists such as methadone and ketamine (discussed below in “Other Medications to Prevent or Treat Opioid IWS”). According to the World Health Organization, abstinence syndrome is an older term for withdrawal syndrome, but it is sometimes confused with the term abstinence.17 Tolerance, physical dependence, and psychological dependence are misused or interchanged, but each has its own definition.5 These and other definitions are listed in Table 1.
Table 1:
Definitions of Terms Related to Withdrawal Syndrome
| Term | Definition |
|---|---|
| Tolerance | A decrease in response to a drug dose that occurs with continued use; increasing doses are required to achieve the effect originally produced by lower doses5 |
| Physical dependence | A state of adaptation that manifests through a drug class–specific withdrawal syndrome5 |
| Psychological dependence | A subjective sense of need for a specific psychoactive substance, either to obtain its positive effects or to avoid negative effects associated with abstinence5 |
| Abstinence | Refraining from a drug whether as a matter of principle or for other reasons17 |
| Abstinence syndrome | An older term for withdrawal syndrome17 |
| Opioid-induced hyperalgesia | A state of nociceptive sensitization caused by exposure to opioids16 |
Prevalence of IWS Among Adult Patients in the ICU
As noted earlier, research is scant on IWS in adult patients in the ICU. We found in the literature 9 English-language publications related to IWS that were not case reports: 4 full-text articles7–9,15 and 5 abstracts in conference proceedings10–14 (2 from the same study10,11). Most of these studies were observational; only 1 was a randomized clinical trial.8 The majority had small sample sizes (range, 11–54 patients); 1 used a retrospective approach to study a larger sample (126 patients). Table 2 summarizes the characteristics of the 8 studies.
Table 2:
Study Characteristics
| Authors and Study Design | Sample Characteristics | Minimum Duration of Exposure to OP/BNZ | Method of IWS Assessment | Assessment Frequency and Duration | IWS Prevalence and Type |
|---|---|---|---|---|---|
| Cammarano et al (1998)15 Observational, retrospective | n = 28 trauma/surgical patients; MV, drug naive and history of chronic drug use | ≥7 days | Record review; common S&S of WS, diagnosis of analgesic/sedative-related WS, prescription of clonidine to treat OP IWS | Not reported | 32.1% mixed IWS |
| Brown et al (2000)7 Observational, retrospective | n = 11 burn patients; MV, drug naive | ≥ 7 days | Record review; scoring system of common S&S of WS | Not reported | 100% mixed IWS |
| Korak-Leiter et al (2005)8 Interventional RCT (OP/BNZ vs OP/ propofol) | n = 29 general or cardiac surgical patients; MV, drug naive | Not defined | Prospective assessment by 2 independent observers; scoring system of common S&S of WS | Start of MV weaning (beginning of 30% reduction in OP/BNZ), at the end of MV weaning, and 6, 12, and 24 h after stopping sedation | OP IWS 35% sufentanil/midazolam 28% sufentanil/propofol |
| Wang et al (2017)9 Observational, prospective | n = 54 trauma patients; MV, drug naive | > 3 days | Prospective assessment by an ICU physician; S&S of OP IWS from DSM-5 | Once a day upon OP weaning during ICU stay and until 48 h after a DSM-5 positive or extubation, or 14 d after a successful weaning process | 16.7% OP IWS |
| Arroyo-Novoa et al (2018)10,11 Observational, prospective | n = 50 trauma patients; MV or non-MV, drug naive or his- tory of chronic drug use | > 5 days | Prospective assessment by 1 investigator; S&S checklist from DSM-5 or ICD-10, and common S&S from previous studies of WS | Twice a day for 72 h after starting the OP or BNZ weaning; if failed, WS assessment began when weaning was reestablished and continued for up to 72 h | 44% mixed IWS |
| Hyun et al (2018)12 Observational, retrospective | n = 126 medical patients (drug experience and MV status not reported) | ≥ 3 days | Record review; ≥ 2 CNS or autonomic symptoms | Upon OP reduction to half or discontinuation using specific time according to the OP drug (2 h for remifentanil, 10 h for fentanyl, 8 h for morphine) | 16.6% OP IWS |
| Zerrouki et al (2019)14 Observational, prospective | n = 29 MV patients (type of patient and drug experience not reported) | > 3 days | Prospective assessment; standardized form of S&S of WS by investigator (assumed) and with a modified DSM-5 by a physician | Daily after at least 10% OP reduction (duration not reported) | 20.7% OP IWS |
| Taesotikul et al (2019)13Observational, prospective | n = 39 MV patients (type of patient and drug experience not reported) | ≥ 2 days | Prospective assessment by investigator (assumed); S&S of WS in DSM-5 | 1, 3, 6, 24, and 72 h after reduction or discontinuation of fentanyl | 20.5% OP (fentanyl) IWS |
Abbreviations: BNZ, benzodiazepine; CNS, central nervous system; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, 5th edition; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th revision; ICU, intensive care unit; IWS, iatrogenic withdrawal syndrome; MV, mechanically ventilated; OP, opioid; RCT, randomized clinical trial; S&S, signs and symptoms; WS, withdrawal syndrome.
Some methodological issues may be associated with these studies. Observational studies are not associated with high levels of evidence; additionally, small sample sizes, in the majority of studies, may threaten statistical conclusion validity. A retrospective approach to data collection, used in 3 of the studies, can introduce an information bias that is due to incomplete data or misinterpretation of information documented in medical records.
Patients in these studies were exposed to OPs or BNZs for 2 to 7 days before IWS was assessed. The majority of the studies included only patients receiving MV7–9,13–15; 1 included both patients receiving MV and patients who did not require MV.11 Most studies included trauma patients, surgical patients, or both.8,9,11,15 One included patients with burns,7 and another included medical patients.12 Two studies included both drug-naive patients and patients who currently chronically use drugs11,15; 3 included only drug-naive patients7–9; and the rest (n = 3) did not report patients’ drug experience.12–14 The method by which IWS was assessed and the duration of the assessment also varied among studies.
One of the challenges of these IWS studies is that many patients in the ICU concurrently received both OPs and BNZs, and patients were weaned simultaneously from both. This makes it difficult to determine which drug(s) contributed to the IWS. Three of the studies explored mixed IWS (ie, OP and BNZ),7,11,15 and the other 5 explored only OP IWS.8,9,12–14 Patients in half of the studies received a combination of OPs (morphine, fentanyl, or both) and BNZs (lorazepam, midazolam, or both).7,9,11,15 One study compared a specific OP (sufentanil) in combination with either a BNZ (midazolam) or propofol.8 In another study, patients received only fentanyl as the OP.13 Although Wang et al9 measured OP IWS, they noted the coadministration of BNZs as a potential confounder and contributor to OP IWS in their patients. Korak-Leiter et al8 compared 2 analgesic/sedative treatments (sufentanil/midazolam vs sufent-anil/propofol) and suggested that administering an OP and BNZ together could potentiate OP tolerance. This suggestion was based on the need to increase the sufentanil dose in order to achieve sedation at the last stage of treatment for patients receiving sufentanil/midazolam. Korak-Leiter et al8 also noted a long duration of IWS in their patients; they believed this resulted from major suppression and delayed recovery of the synthesis of endogenous OPs, which occurred in the group receiving OP/BNZ but not in the group receiving OP/propofol. In addition, the long duration of IWS could also be due to the increased dose of sufentanil in the group receiving midazolam.
In all, the prevalence of IWS ranged from 32% to 100% in studies of mixed IWS and from 16.6% to 20.7% in studies of OP IWS. The median onset of withdrawal syndrome occurred approximately 2 or 3 days after an OP had been reduced or ceased.9,14 The reported prevalences were based on inclusion criteria, which varied among studies (3%−6% of the total ICU admissions screened).7,9,14,15 Therefore, IWS has been documented in a small percentage of the overall ICU population, limiting the generalizability of the study findings to other patients in the ICU.
Risk Factors for Developing IWS in the ICU
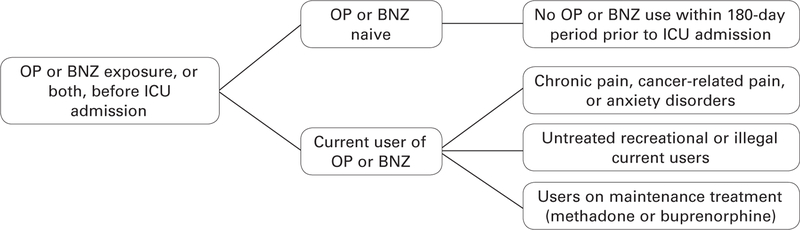
Patients who have never or not recently taken an OP or BNZ and those who currently use OPs/BNZs (whether prescribed or illicit) are admitted to the ICU.6 Patients are considered “naive” when OPs or BNZs have not been used within the previous 180 days.18 Figure 1 categorizes and defines OP/BNZ exposure. It is critical that providers ask patients and their families about prior drug use in order to understand a patient’s potential risk for developing IWS. Important information to ascertain includes duration of OP or BNZ use, types of OPs or BNZs taken, doses, and frequency. This information alerts clinicians to the possibility that a patient could have drug tolerance, have OIH, or be physically or psychologically dependent. Furthermore, such patients could experience withdrawal as a result of insufficient doses of OPs or BNZs, rapid drug discontinuation, or both, which may lead to poor pain management, delirium, anxiety, and agitation. Providers need to consider that not only OPs and BNZs can produce IWS in patients in the ICU. Habitual cigarette smoking, alcohol consumption, or use of cannabis or other drugs can, when discontinued, also induce IWS.4
Figure 1:
Categorization of patients in an intensive care unit based on prior opioid and benzodiazepine experience. BNZ indicates benzodiazepine; ICU, intensive care unit; OP, opioid.
Although findings are not consistent, research has identified other risk factors for developing IWS, such as higher cumulative doses of OPs and BNZs, prolonged exposure to BNZs, high body mass index, young age, history of drug use disorder, a diagnosis of acute respiratory distress syndrome or septic shock, the fentanyl infusion weaning rate, and heavy alcoholism.8,9,11–13,15 Table 3 lists such risk factors. Providers should evaluate patients for relevant associated risk factors, as such assessment could prevent IWS.
Table 3:
Significant Risk Factors for Withdrawal Syndrome
| • Cumulative dose of opioid11,12 |
| • Long duration of benzodiazepine use15 |
| • High mean daily opioid dose15 |
| • High mean daily benzodiazepine dose15 |
| • High mean peak of opioid15 |
| • High weaning rate of opioid infusion7,13 |
| • High weaning rate of benzodiazepine infusion7 |
| • Low mean daily haloperidol dose15 |
| • More frequent use of antipsychotics9 |
| • Long duration of propofol use15 |
| • Use of neuromuscular blocking agents and propofol for > 1 day15 |
| • Increased duration of opioid use15 |
| • Young age15 |
| • High body mass index13 |
| • History of drug use disorder11 |
| • Heavy alcoholism12 |
| • Acute respiratory distress syndrome15 |
| • Septic shock12 |
Assessing OP and BNZ IWS in Patients in the ICU
The signs and symptoms of OP IWS, BNZ IWS, or both in adult patients in the ICU are classified into 3 main categories: (1) central nervous system irritability (eg, anxiety, agitation, restlessness, hallucinations, seizures), (2) gastrointestinal system dysfunction (eg, diarrhea, nausea, vomiting), and (3) sympathetic nervous system activation (eg, tachycardia, tachypnea, fever).4,19–21 These signs and symptoms are categorized in Table 4. They are not specific to withdrawal and can be present in other conditions common among patients in the ICU—another reason why identifying IWS is challenging in these patients.
Table 4:
Signs and Symptoms of Withdrawal Syndrome Assessed in Each Study
| Central Nervous System Irritability | Gastrointestinal System Dysfunction | Sympathetic Nervous System Activation | Other | |
|---|---|---|---|---|
| Opioids | Muscle ache9,13–15,a | Diarrhea8–10,13–15,a | Fever8–10,13,15,a High blood pressure8,10,14,15,a Lacrimation9,10,13,14,a Rhinorrhea9,10,13,14 Mydriasis8–10,13,14 Piloerection9,10,13,14 Tachypnea10,15,a Yawning8–10,13,14 | Drug craving15 Increased sensitivity to pain15 |
| Benzodiazepines | Agitation10,14,a Confusion/delirium7,9,10,15,a Hallucinations/illusions10,14 Muscle twitching7,10,15 Picking motion7 Tremor10 Seizures7,10,15 | |||
| Both opioids and benzodiazepines | Anxiety7,10,14,15 Restlessness/irritability7,8,10,14,15,a Dysphoria9,10,13–15,a Insomnia/sleep disturbance9,10,13–15,a | Nausea8–10,13–15 Vomiting9,10,13–15 | Sweating/diaphoresis7–10,13–15 Tachycardia8,10,15 |
Signs and symptoms reported in 2 or more studies.
In addition, a significant challenge to identifying OP or BNZ IWS in practice or research is the lack of validated IWS assessment tools for use in adult patients. Studies of adult patients have assessed OP and BNZ IWS through the use of graded scoring methods and checklists of common signs and symptoms compiled from previous studies of IWS or the literature; the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5);4 and the International Statistical Classification of Diseases and Related Health Problems, 10th revision. In addition to evaluating signs and symptoms, some studies included 1 or more of the DSM-5 diagnostic criteria for withdrawal syndrome, or modifications of them.9,10,13,14 These criteria include (1) cessation or reduction of an OP or BNZ dose after heavy and prolonged use; (2) development of 3 or more signs or symptoms for OP withdrawal syndrome, 2 or more signs and symptoms of BNZ withdrawal syndrome, or both; and (3) evaluation of the signs or symptoms as not being related to another clinical condition.4 To identify IWS, Cammarano et al15 used signs and symptoms retrieved from the literature, a diagnosis of analgesic- or sedative-related IWS, or a prescription of clonidine to treat OP IWS (as documented in the patient record). The frequency and intervals of assessments also varied among studies (Table 2). Although some studies identified IWS using assessment tools that apply known signs and symptoms of IWS, the lack of validity of those tools threatens the validity of the study findings.
The most frequent signs and symptoms (occurring in patients in ≥ 2 studies) were restlessness, agitation, delirium, dysphoria, diarrhea, fever, high blood pressure, lacrimation, tachypnea, sleep disturbance/insomnia, and muscle ache (Table 4). One study noted that the most common signs were related to autonomic nervous system activation, although it did not report specific signs and symptoms.12
Researchers investigating the pediatric ICU have developed and tested the Withdrawal Assessment Tool-1 (WAT-1) and the Sophia Observation Withdrawal Symptoms–Pediatric Delirium Scale.22 In a secondary analysis23 of the study of Wang and colleagues,9 researchers found twice the number of patients who identified as IWS positive using the WAT-1 when compared with the DSM-5 criteria. Yet they also found that the WAT-1 had low specificity (65.9%) and sensitivity (50%). Thus they concluded that the WAT-1 is not a valid tool with which to measure IWS in adult patients in the ICU.23
Intensity of IWS Signs and Symptoms
Only 2 studies evaluated the intensity of IWS. Brown et al7 found that patients experienced mild to severe signs and symptoms of withdrawal; they determined these levels using a grading system developed specifically for that study. The majority of signs and symptoms, however, were categorized as mild and did not require a change in the weaning process. The intensity of symptoms was related to a rapid drug weaning rate or to abrupt discontinuation of the drug(s). Korak-Leiter et al8 classified signs and symptoms as absent (0), mild (1), moderate (2), or severe (3), and the total score for 9 items ranged from 0 to 27.8 Although they did not operationally define what they reported as “marked withdrawal,” significantly more patients developed “marked withdrawal” in the group receiving sufentanil/midazolam than in the group receiving sufentanil/propofol. In addition, the intensity of IWS for both groups was highest at the sixth hour after ceasing the OP/sedative (with scores of ~ 13). For patients who scored more than 15, clonidine was administered in order to manage the signs and symptoms of withdrawal.
These results must be evaluated with caution, because signs and symptoms are not clearly defined for adult patients in the ICU, nor is a system available to classify the degree of intensity. Once a symptom profile is established, future studies should evaluate the intensity of signs and symptoms of IWS.
Clinical Consequences of IWS
Iatrogenic withdrawal syndrome should be avoided in patients in the ICU for several reasons. First, the multiple signs and symptoms of IWS can cause profound patient discomfort. In addition, patients who develop IWS may require more days on MV and a longer ICU LOS. Two studies showed a statistically significantly longer duration of MV in patients with IWS than in those without IWS.10,15 Three studies found no statistically significant differences between groups9,12,13; 1 of these, however, reported a trend toward a longer duration of MV in the IWS-positive group.9 The difference in duration of MV between the groups that had IWS and those that did not was 4 days and 18 days in two of the studies.9,15 In the study by Korak-Leiter et al,8 patients in the OP/BNZ group required longer MV (4 days more) than patients in the OP/propofol group, although this difference was not statistically significant.
Arroyo-Novoa et al10 found that patients with IWS spent statistically significantly more time in both the ICU and the hospital than did those without IWS. Two other studies found prolonged ICU LOSs among patients in the IWS-positive group versus those who did not have IWS; the LOS differences between groups were 4 days9 and 18 days.15 Two studies reported only that differences in ICU LOS were not significant between groups.12,13 Hyun et al12 evaluated mortality but did not find any differences between groups. Finally, given that most patients in the ICU receive OPs to manage pain, it is not clear how IWS affects patients’ pain. Future research is warranted to clearly identify the clinical consequences of IWS.
Strategies to Prevent and Treat IWS
To prevent IWS, it is important to identify the patient’s previous experience with OPs and BNZs (Figure 1). This will help providers develop an individualized plan for managing pain and sedation while accounting for the patient’s specific clinical condition. For example, patients who currently take drugs (prescribed or illicit) may require a higher dose of OPs or BNZs than drug-naive patients in order to account for tolerance, or a maintenance or substitution therapy such as methadone may be needed.
Strategies to Prevent Opioid IWS
The recent PADIS guidelines note a specific approach to managing pain and agitation that is relevant to preventing OP IWS.1 The guidelines encourage the use of multimodal analgesia and nonpharmacologic interventions (eg, massage, music therapy, or relaxation techniques) for pain that may prevent OP IWS by reducing the use of OPs. Multimodal analgesia occurs when 2 or more medications with different mechanisms are administered in order to improve pain relief by producing an additive or synergistic effect, or both. The guidelines mention acetaminophen, ketamine, and neuropathic pain medications as candidates for multimodal analgesia.1
Acetaminophen.
Acetaminophen has been tested primarily in postsurgical patients after major abdominal or cardiac surgeries, or after craniotomy. The results were inconsistent regarding OP consumption and corresponding reductions in pain score.24 Nevertheless, the PADIS guidelines conditionally recommend acetaminophen, warning about the hypotensive effect that may preclude the use of intravenous acetaminophen in some patients.1
Ketamine.
Ketamine is an NMDA receptor antagonist that, in low doses, has an analgesic and antihyperalgesic effect. Researchers tested its use in surgical and trauma patients in the ICU and found a significant reduction in postoperative OP consumption.25–28 Three of these studies are recent retrospective investigations of the use of ketamine: 2 evaluated postoperative OP consumption without comparison groups, which reduced their quality,26,27 and 1 was a randomized controlled trial,28 which per the PADIS guidelines was rated as having low-quality evidence because of a high risk of bias. Nevertheless, the guidelines conditionally recommend the use of low-dose ketamine as an adjunct to OP therapy. Ketamine should be avoided in patients with heart failure and a risk of myocardial ischemia.29 Providers must recognize that it is potentially addictive.30
Neuropathic Pain Medications.
The PADIS guidelines strongly recommend, on the basis of moderate-quality evidence, neuropathic pain medications as an adjunct to OPs when managing neuropathic pain in patients in the ICU. The guidelines conditionally recommended their use in cardiac patients in the ICU because of a low quality of evidence.1 These guideline recommendations were based on studies of patients with Guillain-Barré syndrome and patients undergoing cardiac surgery, in whom both pain scores and OP consumption significantly decreased.1 In a recent study of trauma patients with rib fractures, however, Moskowitz et al31 found no differences in pain scores or OP consumption when gabapentin was used. The study had a small sample size, which may threaten the statistical validity of the conclusions, and it had attrition, which may threaten its internal validity. Nevertheless, the authors recommended discontinuing gabapentin in trauma patients with rib fractures, pending further studies.
Other Medications to Prevent or Treat Opioid IWS
Specific drugs have been tested for their effectiveness in preventing or treating OP IWS. For example, methadone has been recommended in order to decrease the time required to wean from OP infusions patients treated with high doses of short-acting OPs and especially patients with a history of chronic OP use.32 Methadone is a long-acting OP μ-receptor agonist and an NMDA receptor antagonist. The latter characteristic may attenuate the effects of tolerance and OP IWS.33
One study evaluated patients before and after a sedation protocol was implemented in a medical ICU that used methadone to manage patients’ pain; it found that patients in the group that used methadone were weaned from fentanyl infusions earlier than those who did not use methadone.34 Iatrogenic withdrawal syndrome was not assessed, however, and clinical outcomes such as duration of MV and ICU LOS were not different before and after the protocol was implemented. Although methadone is associated with a prolonged QTc interval, Al-Qadheeb et al34 found no differences in the proportion of patients who experienced a prolonged QTc interval.34 A randomized controlled trial tested the ability of methadone to reduce the duration of weaning from MV in patients taking OPs over the long term. However, IWS occurrence, as measured by the need for supplemental doses of fentanyl to treat signs of OP withdrawal, was similar in both groups.35
Ketamine, in addition to decreasing OP consumption (as described earlier), may help patients in the ICU who have a history of OP dependence. This effect is due to ketamine blocking the NMDA receptor, thereby inhibiting or reversing OP tolerance and OIH.36 In a case series, 4 patients in the ICU who had a history of OP abuse received a ketamine infusion after failing OP or BNZ standard therapy.37 Analgesia and sedation improved in 3 of these patients, and they required smaller amounts of analgesics and sedatives and reported minimal adverse effects associated with ketamine use.
The effectiveness of α2-adrenergic agonists has also been tested in managing OP IWS. Clonidine and dexmedetomidine are both α2-agonists and have sedative, analgesic, and sympatholytic properties.38 A protocol for intravenous clonidine was tested in mechanically ventilated patients who had failed at least 1 remifentanil/propofol weaning attempt and developed IWS after sedation was interrupted.39 Among 30 patients, 25 responded to clonidine treatment during MV, during the drug weaning process, or both. Use of clonidine was associated with decreases in the hemodynamic, metabolic, and respiratory demands that occur with IWS.39
One study reported the use of dexmedetomidine to manage IWS in 2 patients with substance use disorders who were admitted to the ICU for acute intoxication and other related complications.40 Dexmedetomidine infusion, with the addition of lorazepam during the night, helped to control the signs and symptoms of IWS.40 In the same study, clonidine was administered orally in order to taper dexmedetomidine infusion.40
Strategies to Prevent or Treat Benzodiazepine IWS
The PADIS guidelines do not recommend use of BNZs for sedation.1 Nevertheless, the guidelines establish, as an ungraded recommendation, interrupting sedative use daily or having nurses apply protocolized sedation in order to maintain a light sedation level.1 The guidelines also recommend avoiding deep sedation unless clinically necessary. The guidelines suggest using non-BNZ agents such as propofol or dexmedetomidine instead of BNZs.1 All of these approaches may help to prevent IWS related to BNZ use. Another recommended strategy for preventing and treating BNZ IWS is to use long-acting agents (eg, diazepam, chlordiazepoxide, or clonazepam) as maintenance therapy or as a substitute for treating signs and symptoms of withdrawal.41
Other Considerations for Avoiding IWS
Although it has not been well studied, another strategy to decrease the occurrence of IWS is reducing the OP/BNZ infusion rate. For patients being weaned from OPs, one recommendation is to reduce the daily infusion dose by 10%.42 Psychiatrists or addiction medicine specialists may be helpful in developing individual treatment plans for preventing and treating OP or BNZ IWS, especially in patients with drug-use disorders.
Nursing Implications for Preventing, Identifying, and Treating IWS in Adult ICU Patients
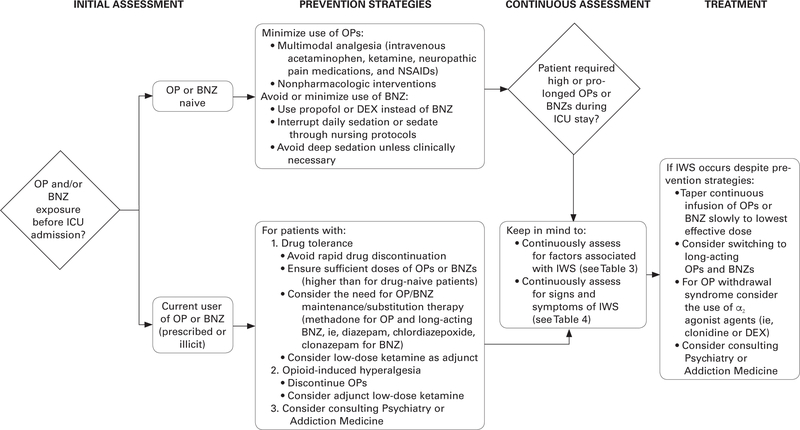
Although the evidence is limited for identifying and treating IWS in adult patients in the ICU, nurses can create an “index of suspicion” to identify IWS in patients who have risk factors (eg, high drug doses, long duration of administration, history of substance use disorder) or who demonstrate some of the signs and symptoms noted earlier. After identifying patients who are at risk, clinicians can observe signs and symptoms that might be indicators of IWS. Providers can develop an individualized plan with the goal of preventing IWS or minimizing and treating it when present. Figure 2 provides an algorithm for preventing, assessing, and treating IWS in adult patients in the ICU. The validity of this algorithm needs to be tested, but in the meantime, its use may decrease patient discomfort and the negative consequences associated with IWS.
Figure 2:
Clinical approach to preventing, identifying, and treating opioid or benzodiazepine iatrogenic withdrawal syndrome in adult patients in the intensive care unit. BNZ indicates benzodiazepine; DEX, dexmedetomidine; ICU, intensive care unit; IWS, iatrogenic withdrawal syndrome; NSAIDs, nonsteroidal anti-inflammatory drugs; OP, opioid.
Future Research
Future research in the adult ICU population should focus on identifying the main signs and symptoms of, and risk factors for, IWS, and it should validate an IWS assessment tool. Future studies should include larger sample sizes and patients with varying diagnoses. Such studies need to investigate the prevention and management of IWS and the short- and long-term clinical consequences of IWS.
Conclusion
Opioid and BNZ IWS may occur in only a select group of patients in the ICU who are exposed to high doses of OPs or BNZs, those who have used these drugs before, and those with a long duration of OP or BNZ infusion, among others. However, the syndrome may be under-recognized because the signs and symptoms related to IWS lack specificity.
The current literature on IWS in the adult ICU population is scarce, and findings should be interpreted with caution. The available studies provide only low-quality evidence because of various limitations (eg, small sample sizes and a lack of validated tools for assessing IWS). Furthermore, the heterogeneity of characteristics of IWS studies makes it difficult to establish clear recommendations for preventing, assessing, and managing IWS. However, attention to risk factors identified in the literature and cautious application of suggested strategies in practice may help to alleviate patient discomfort, avoid the negative consequences of IWS, and improve patient outcomes.
Acknowledgments
FINANCIAL DISCLOSURE
This work was supported by the Hispanic Clinical and Translational Research Education and Career Development Program (grant R25MD007607).
Contributor Information
Carmen Mabel Arroyo-Novoa, Graduate Department, University of Puerto Rico School of Nursing, Medical Sciences Campus, PO Box 365067, San Juan, PR 00936-5067.
Milagros I. Figueroa-Ramos, Graduate Department, University of Puerto Rico School of Nursing, San Juan, Puerto Rico..
Kathleen A. Puntillo, Physiological Nursing Department, University of California, San Francisco, School of Nursing, San Francisco, California.
REFERENCES
- 1.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46(9):e825–e873. [DOI] [PubMed] [Google Scholar]
- 2.García-Sánchez M, Caballero-López J, Ceniceros-Rozalén I, et al. ; Miembros del GTSAD. Management of analgesia, sedation and delirium in Spanish Care Units: A national two-part survey [in Spanish]. Med Intensiva. 2019;43(4):225–233. [DOI] [PubMed] [Google Scholar]
- 3.Urkmez S, Erdogan E, Utku T, Dikmen Y. Sedation practices and preferences of Turkish intensive care physicians: a national survey. Turk J Anaesthesiol Reanim. 2019;47(3):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM-5. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.American Society of Addiction Medicine. The ASAM national practice guideline for the use of medications in the treatment of addiction involving opioid use. http://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf?sfvrsn=24#search=%22guideline%22.pdf?sfvrsn=24. Adopted June 1, 2015. Accessed September 27, 2019. [DOI] [PMC free article] [PubMed]
- 6.Puntillo KA, Naidu R. Chronic pain disorders after critical illness and ICU-acquired opioid dependence. Curr Opin Crit Care. 2016;22(5):506–512. [DOI] [PubMed] [Google Scholar]
- 7.Brown C, Albrecht R, Pettit H, McFadden T, Schermer C. Opioid and benzodiazepine withdrawal syndrome in adult burn patients. Am Surg. 2000;66(4):367–371. [PubMed] [Google Scholar]
- 8.Korak-Leiter M, Likar R, Oher M, et al. Withdrawal following sufentanil/propofol and sufentanil/midazolam. Sedation in surgical ICU patients: correlation with central nervous parameters and endogenous opioids. Intensive Care Med. 2005;31(3):380–387. [DOI] [PubMed] [Google Scholar]
- 9.Wang PP, Huang E, Feng X, et al. Opioid-associated iatrogenic withdrawal in critically ill adult patients: a multicenter prospective observational study. Ann Intensive Care. 2017;7(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo-Novoa CM, Figueroa-Ramos M, Puntillo K. Identifying opioid and benzodiazepine withdrawal in trauma intensive care unit (TICU) patients [abstract 1614]. Crit Care Med. 2018;46(suppl 1):791.29443814 [Google Scholar]
- 11.Arroyo-Novoa CM, Figueroa-Ramos MI, Puntillo KA. Factors associated with probable withdrawal syndrome in trauma intensive care unit patients [abstract 1184]. Intensive Care Med Exp. 2018;6(suppl 2):1184. [Google Scholar]
- 12.Hyun D- G, Lim C- M, Huh JW, Hong S- B, Koh Y. Iatrogenic opioid withdrawal syndrome in critically ill patients: a retrospective cohort study [abstract 1186]. Intensive Care Med Exp. 2018;6(suppl 2):1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taesotikul S, Tangsujaritvijit S, Trisataya A, Suthisisang C, Dilokpattanamongkol P. Withdrawal reactions after discontinuation or rate reduction of fentanyl infusion in ventilated critically ill adults. Crit Care. 2019;23 (suppl 2):404.31829216 [Google Scholar]
- 14.Zerrouki K, Li Q, Delucilla L, et al. Symptomatology of opioid-associated withdrawal syndrome in critically ill adults: a descriptive study. Crit Care. 2019;23(suppl 2):403.31829221 [Google Scholar]
- 15.Cammarano WB, Pittet J- FF, Weitz S, Schlobohm RM, Marks JD. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit Care Med. 1998; 26(4):676–684. [DOI] [PubMed] [Google Scholar]
- 16.Carullo V, Fitz-James I, Delphin E. Opioid-induced hyperalgesia: a diagnostic dilemma. J Pain Palliat Care Pharmacother. 2015;29(4):378–384. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Lexicon of alcohol and drug terms published by the World Health Organization. https://www.who.int/substance_abuse/terminology/who_lexicon/en. Published 1992. Accessed May 20, 2019.
- 18.Jeffery MM, Hooten WM, Hess EP, et al. Opioid prescribing for opioid-naive patients in emergency departments and other settings: characteristics of prescriptions and association with long-term use. Ann Emerg Med. 2018;71(3):326–336.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devlin JW, Mallow-Corbett S, Riker RR. Adverse drug events associated with the use of analgesics, sedatives, and antipsychotics in the intensive care unit. Crit Care Med. 2010;38(6)(suppl):S231–S243. [DOI] [PubMed] [Google Scholar]
- 20.Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation. Crit Care Med. 2008;36(8):2427–2432. [DOI] [PubMed] [Google Scholar]
- 21.Birchley G Opioid and benzodiazepine withdrawal syndromes in the paediatric intensive care unit: a review of recent literature. Nurs Crit Care. 2009;14(1):26–37. [DOI] [PubMed] [Google Scholar]
- 22.Chiu AW, Contreras S, Mehta S, et al. Iatrogenic opioid withdrawal in critically ill patients: a review of assessment tools and management. Ann Pharmacother. 2017; 51(12):1099–1111. [DOI] [PubMed] [Google Scholar]
- 23.Capilnean A, Martone A, Rosu VA, et al. Validation of the Withdrawal Assessment Tool–1 in adult intensive care patients. Am J Crit Care. 2019;28(5):361–369. [DOI] [PubMed] [Google Scholar]
- 24.Wampole CR, Smith KE. Beyond opioids for pain management in adult critically ill patients. J Pharm Pract. 2019;32(3):256–270. [DOI] [PubMed] [Google Scholar]
- 25.Walters MK, Farhat J, Bischoff J, Foss M, Evans C. Ketamine as an analgesic adjuvant in adult trauma intensive care unit patients with rib fracture. Ann Pharmacother. 2018;52(9):849–854. [DOI] [PubMed] [Google Scholar]
- 26.Buchheit JL, Yeh DD, Eikermann M, Lin H. Impact of low-dose ketamine on the usage of continuous opioid infusion for the treatment of pain in adult mechanically ventilated patients in surgical intensive care units. J Intensive Care Med. 2019;34(8):646–651. [DOI] [PubMed] [Google Scholar]
- 27.Pruskowski KA, Harbourt K, Pajoumand M, Chui SHJ, Reynolds HN. Impact of ketamine use on adjunctive analgesic and sedative medications in critically ill trauma patients. Pharmacotherapy. 2017;37(12):1537–1544. [DOI] [PubMed] [Google Scholar]
- 28.Guillou N, Tanguy M, Seguin P, Branger B, Campion J- P, Mallédant Y. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003;97(3):843–847. [DOI] [PubMed] [Google Scholar]
- 29.Erstad BL, Patanwala AE. Ketamine for analgosedation in critically ill patients. J Crit Care. 2016;35:145–149. [DOI] [PubMed] [Google Scholar]
- 30.Karlow N, Schlaepfer CH, Stoll CRT, et al. A systematic review and meta-analysis of ketamine as an alternative to opioids for acute pain in the emergency department. Acad Emerg Med. 2018;25(10):1086–1097. [DOI] [PubMed] [Google Scholar]
- 31.Moskowitz EE, Garabedian L, Harden K, et al. A doubleblind, randomized controlled trial of gabapentin vs. placebo for acute pain management in critically ill patients with rib fractures. Injury. 2018;49(9):1693–1698. [DOI] [PubMed] [Google Scholar]
- 32.Elefritz JL, Murphy CV, Papadimos TJ, Lyaker MR. Methadone analgesia in the critically ill. J Crit Care. 2016;34:84–88. [DOI] [PubMed] [Google Scholar]
- 33.Chhabra S, Bull J. Methadone. Am J Hosp Palliat Med. 2008;25(2):146–150. [DOI] [PubMed] [Google Scholar]
- 34.Al-Qadheeb NS, Roberts RJ, Griffin R, Garpestad E, Ruthazer R, Devlin JW. Impact of enteral methadone on the ability to wean off continuously infused opioids in critically ill, mechanically ventilated adults: a case-control study. Ann Pharmacother. 2012;46(9):1160–1166. [DOI] [PubMed] [Google Scholar]
- 35.Wanzuita R, Poli-de-Figueiredo LF, Pfuetzenreiter F, Cavalcanti AB, Westphal GA. Replacement of fentanyl infusion by enteral methadone decreases the weaning time from mechanical ventilation: a randomized controlled trial. Crit Care. 2012;16(2):R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorlin AW, Rosenfeld DM, Ramakrishna H. Intravenous sub-anesthetic ketamine for perioperative analgesia. J Anaesthesiol Clin Pharmacol. 2016;32(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treu CN, Groth CM, Patel JH. The use of continuous ketamine for analgesia and sedation in critically ill patients with opioid abuse: a case series. J Crit Care Med. 2017; 3(4):148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28(1):3–6. [DOI] [PubMed] [Google Scholar]
- 39.Liatsi D, Tsapas B, Pampori S, Tsagourias M, Pneumatikos I, Matamis D. Respiratory, metabolic and hemodynamic effects of clonidine in ventilated patients presenting with withdrawal syndrome. Intensive Care Med. 2009;35(2):275–281. [DOI] [PubMed] [Google Scholar]
- 40.Upadhyay SP, Mallick PN, Elmatite WM, Singh RK. Prolonged dexmedetomidine infusion to facilitate drug detoxification and withdrawal in patients with multiple drugs addiction. Crit Care Shock. 2011;14(3): 84–88. [Google Scholar]
- 41.Jenkins DH. Substance abuse and withdrawal in the intensive care unit. Contemporary issues. Surg Clin North Am. 2000;80(3):1033–1053. [DOI] [PubMed] [Google Scholar]
- 42.American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 6th ed. Glenview, IL: American Pain Society; 2008. [Google Scholar]