Abstract
Background:
Exercises such as walking are prescribed for the patients with breast cancer receiving chemotherapy, but only a few studies include pedometers in conjunction with the routine exercises prescribed. Thus, the aim was to find if the adherence and performance of exercise is improved or has no impact if coupled with the physical activity monitors.
Methods:
A systematic search using the keywords was conducted in PubMed Central, CINAHL, Embase, Cochrane Library, and Scopus. The search revealed 275 articles, of which 3 randomized controlled trials were selected for qualitative analysis based on the inclusion and exclusion criteria for the review.
Results:
The three studies retrieved by the systematic review have used different protocols of pedometer-based walking on patients with breast cancer undergoing chemotherapy and studied the following outcome measures: fatigue, body composition, quality of life, and psychological factors.
Conclusion:
The evidence obtained from the review suggests that pedometer-based walking programs have a positive impact on cancer-related fatigue and overall quality of life of patients with breast cancer receiving chemotherapy.
Keywords: Breast cancer, chemotherapy, pedometer
INTRODUCTION
Impacting around 2.1 million females across the globe every year, breast cancer is considered to be the most frequently occurring cancers and also one of the leading causes of cancer-related deaths among the women according to the 2018 WHO reports.[1]
Remarkable advancement in detection and treatment has led to a consequential rise in percentage of cancer survivors around the world. A 2017 study done in Korea by Shin et al. states that because of the treatment advances, the prognosis after the diagnosis of breast cancer has become better over the last few years and has brought the survival rate of the patients with breast cancer to up to 5 years postdiagnosis.[2]
Although these treatments have increased the survival rates in the breast cancer survivors, at the same time their side effects have a major impact on the functioning of these patients. Anticancer treatments, namely chemotherapy or radiotherapy, result in a group of adverse effects which include fatigue, anxiety, depression, loss of appetite, impaired joint range of motion, exercise intolerance, and physical inactivity.[3]
Of these, one of the major unaddressed and overlooked side effects of all the anticancer treatment is fatigue.[4] Cancer-related fatigue is a distressing, persistent, subjective sense of tiredness or exhaustion related to cancer or its treatment that is not proportional to recent activity and interferes with usual functioning.[5] Among all anticancer treatments, maximum patients (98.30%) undergoing chemotherapy report fatigue as their major concern when compared to other treatments (chemoradiotherapy - 78.57% and radiotherapy - 45%).[4]
It is predicted that the consequences of physical inactivity such as diabetes and hypertension in the population experiencing a chronic disease are subjected to cost billions of dollars each year and are associated with mortality. However, exercises have so far shown positive results with regard to the side effects of physical inactivity, and to promote the same, pedometers have recently gained recognition as an intervention with physical activity.
Thus, this review is conducted to answer specific questions which are:
Does a pedometer when used in conjunction with exercises give any significant changes in cancer-related fatigue and overall quality of life for patients with breast cancer undergoing chemotherapy?
What type of pedometer-based protocols is used in the studies which show a significant difference in overall fitness and health-related outcomes in patients with breast cancer undergoing chemotherapy?
METHODS
Search strategy and selection criteria
Studies were identified using a comprehensive search of PubMed, CINAHL, Embase, Scopus, Cochrane Library, and PEDro. The identification of the articles was done using the search terms pedometers; step count; steps; aerobic exercise; aerobic training; physical activity; physical inactivity; physical therapy; physiotherapy; breast cancer; breast carcinoma; breast tumor; chemotherapy; and Ca breast and breast neoplasm in conjunction with the Boolean operators “AND,” “OR,” and “NOT.”
We included only randomized controlled trials (RCTs) in our review. The search was restricted to studies on humans and articles published in English language. Articles falling in the custom range of 2010–2019 are included in the review because the first study using a pedometer in breast cancer population was done in 2010.[6] Initial searches were carried out in January–February 2019. Other consecutive searches were done in April 2019. Two investigators (AG and SRS) autonomously conducted the searches in the above-mentioned search engines. All the retrieved data were desegregated and individually screened for eligibility criteria by AG and SRS. Any differences in the opinion were resolved by discussions between AG and SRS, and if needed, through mediation by author KVK.
Quality assessment and methodological rating
All the retrieved trials that were eligible for the systematic review were assessed for quality of study following which a methodological rating was given to each by authors AG and KVK using the Physiotherapy Evidence Database scale for RCTs.[7] The scale has 11 components with a simple answering method through “yes” or “no.” The maximum score rewarded is 10 as the first component is not scored.[8] The scores were allotted by author AG and scrutinized by author KVK. Any differences in the opinion were resolved by discussions between AG and KVK, and if needed, through mediation by author SRS.
A data extraction sheet was made and revised to cover all the data regarding the objectives of the study, site and stage of cancer, type of exercise intervention, primary and secondary outcome measures, study design, sample size, participant selection, medical and surgical intervention, details of exercise intervention, and adverse events as reported by the study. Data were organized to highlight study characteristics, methodological rating score, site and stage of cancer, type of exercise interventions, and outcomes reported from each of the selected study.
RESULTS
All the retrieved data, which summed up to 275 articles, were fed in Paul Foeckler, Victor Henning, Jan Reichelt. Elsevier copyright. London, UK. Mendeley Desktop v1.19.2. Duplicates were checked and merged. The articles were then screened through the titles, and around 54 articles were found eligible. Furthermore, through abstract reading, 210 articles were filtered out, following which full-text reading was done and 45 were excluded. Of those 165 articles, 3 articles were found eligible after screening for the inclusion and exclusion criteria formulated in the outline of the search review. The PRISMA flow diagram in Figure 1 summarizes the identification, screening, eligibility, and inclusion of the clinical trials.
Figure 1.
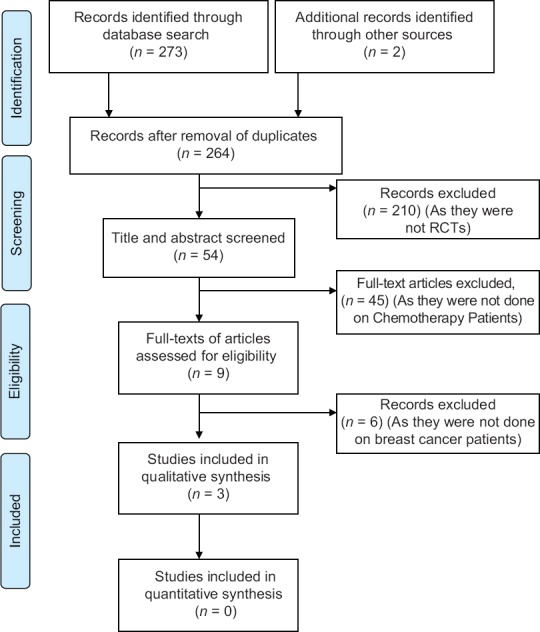
PRISMA flow diagram
All the retrieved trials that were eligible for the systematic review underwent a quality rating following which a methodological rating was given to each using the PEDro scale for RCTs. The scores ranged from 7 to 10 of 11, and therefore, the trials were categorized as high quality (6–10). All the studies were reasonably reliable to assess the effect of intervention on their principle outcome measures. The total and element scores of PEDro scale are summarized in Table 1.
Table 1.
PEDro scores of the included trials
| Authors | Components of PEDro scale | Maximum total score (10) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specified eligibility criteria | Random allocation | Allocation was concealed | Groups similar at baseline | Blinding of subjects | Blinding of therapists | Blinding of assessors | At least 1 key outcome from 85% of subjects | From dropouts, at least 1 key outcome was measured by ITT | Results for at least 1 between-group comparison are reported | Both point measures and variability for at least 1 key outcome | ||
| Djuric et al. (2011) | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 8/10 |
| Pelekasis et al. (2016) | Yes | Yes | No | Yes | Yes | No | No | Yes | No | Yes | Yes | 6/10 |
| Gokal et al. (2018) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9/10 |
ITT: Intention-to-treat
All the trials had a homogeneous group of patients, that is, patients with breast cancer undergoing chemotherapy. Three distinct brackets of exercise interventions were noted which are home-based walking program, dietetic intervention, and psychological therapy. All the study-related details regarding the cancer site, study design, sample size, cancer management, type of exercise intervention, and outcome measures assessed are summarized in Table 2, whereas the detailed explanation of exercise protocols and their respective results are mentioned in Table 3.
Table 2.
Details of all the trials included in the review
| References | Cancer site/stage | Study design | Sample size | Cancer management | Exercise intervention | Outcome assessment |
|---|---|---|---|---|---|---|
| Djuric et al.[9] | Breast; I-IIIA | RCT | 40 | Chemotherapy | Dietary management, tele-based motivational interviewing, pedometer-based walking | BMI, body fat percentage, waist circumference, weight, dietary fat intake, fatigue (FACT-G, FACT-B), total physical activity |
| Pelekasis et al.[10] | Breast cancer; Stage I-IV | RCT | 61 | Chemotherapy | Pedometer-based walking (8300-10,000 steps), DB, PMR, dietary consulting, guided imagery, CBT | BMI, QoL (SRH, HLC), night sleep duration, sleep onset latency, DASS-21 score, and spiritual health (SWBS) |
| Gokal et al.[11] | Breast cancer | RCT | 50 | Adjuvant and neoadjuvant chemotherapy | Home-based walking program (150 min/week) | Executive function (Stroop task), working memory (WAIS-III), attention (SART), and cognitive functioning (CFQ) |
RCT: Randomized controlled trial, BMI: Body mass index, FACT-G: Functional Assessment of Cancer Treatment-General, FACT-B: Functional Assessment of Cancer Treatment-Breast, DB: Diaphragmatic breathing, PMR: Progressive muscle relaxation, CBT: Cognitive behavioral therapy, SRH: Self-reported health, HLC: Health locus of control, DASS-21: Depression Anxiety Stress Scale-21, SWBS: Spiritual Well-Being Scale, WAIS-III: Wechsler Adult Intelligence Scale-III, SART: Sustained Attention to Response Task, CFQ: Cognitive Failures Questionnaire, QoL: Quality of life
Table 3.
Details of exercise protocols and their respective results
| Author (year) | Exercise intervention | Results | ||
|---|---|---|---|---|
| Outcome measure | Written material group (after 12 months) | Telephone counseling group (after 12 months) | ||
| Djuric et al. (2011)[9] | Intervention group | Body fat (%) | 40.8±2.0 | 37.3±1.3 |
| Received written educational materials | Body lean (%) | 57.1±1.8 | 60.1±1.3 | |
| Fruit and vegetable goals=8/day=1700-1900 kcal/day | Waist circumference | 93.4±3.7 | 91.8±3.4 | |
| 30 min/day of moderate to vigorous activity with pedometers | Weight | 72.0±4.2 | 70.9±3.7 | |
| Permitted for dietary counseling | BMI | 26.9±1.3 | 26.1±1.3 | |
| Control group | Dietary fat intake | 32.6±1.8 | 26.9±2.2 | |
| “My Pyramid” written plan from the USDA | FACT-B | 116±4 | 116±5 | |
| Daily exercise (30 min/day) | FACT-G | 89±3 | 88±4 | |
| Dietary Guidelines for Americans 2005 | Total physical activity | 265±39 | 364±55 | |
| No dietary or exercise counseling provided | Adverse events: None | |||
| Only telephone counseling | ||||
| Author (year) | Exercise intervention | Results | ||
| Outcome measure | Intervention group | Control group | ||
| Pelekasis et al. (2016)[10] | Intervention group | BMI | 0.21±0.38 | 0.11±0.23 |
| 8-week stress management and health promotion program | SRH | 0.56±1.61 | 0.25±1.55 | |
| 1st week - physical activity-walking at least | Night sleep duration | 0.89±0.89 | 0.02±0.68 | |
| 8300-10,000 steps/day | Sleep onset latency | 12.12±16.6 | 2.35±9.74 | |
| 2nd week - DB (practicing 2 times/day) | DASS-21 score | 12.16±10.15 | 1.93±8.7 | |
| 3rd week - PMR (practicing twice a day) | HLC (internal) | 2.48±4.40 | 0.39±4.00 | |
| 4th week - CBT | SWBS | 6.88±14.8 | 3.43±6.67 | |
| 5th week - dietary consulting | Adverse events: NR | |||
| 6th week - guided imagery | ||||
| 7th week - no intervention | ||||
| 8th week - no intervention | ||||
| Control group | ||||
| Approximately 15-min placebo-effect meeting was carried out | ||||
| Author (year) | Exercise intervention | Results | ||
| Outcome measure | Intervention group (after 12 weeks) | Control group (after 12 weeks) | ||
| Gokal et al. (2018)[11] | Intervention group | Stroop task | 118.93±125.98 | 177.08±172.71 |
| 12 weeks of home-based walking | WAIS-III | |||
| Begin with completing 10-min walking | Digits backward | 5.21±1.71 | 4.88±1.45 | |
| Gradually increase to 30-min walking | Digits forward | 7.84±1.21 | 6.81±1.09 | |
| Frequency - five times/week | SART | |||
| Recommended guidelines: 150 min of moderate to vigorous physical activity in a week | Errors of omission | 6.56±3.35 | 9.08±4.41 | |
| Control group | Correct | 407.59±35.02 | 386.22±97.92 | |
| Usual medical care alone | Incorrect | 357.69±80.38 | 353.47±68.22 | |
| CFQ | 32.48±7.05 | 39.20±10.12 | ||
| Adverse events: NR | ||||
USDA: United States Department of Agriculture, BMI: Body mass index, FACT-G: Functional Assessment of Cancer Treatment-General, FACT-B: Functional Assessment of Cancer Treatment-Breast, DB: Diaphragmatic breathing, PMR: Progressive muscle relaxation, CBT: Cognitive behavioral therapy, SRH: Self-reported health, DASS-21: Depression Anxiety Stress Scale-21, HLC: Health locus of control, SWBS: Spiritual Well-Being Scale, WAIS-III: Wechsler Adult Intelligence Scale-III, SART: Sustained Attention to Response Task, CFQ: Cognitive Failures Questionnaire, NR: Nothing reported, →Its plus or minus standard deviation
DISCUSSION
This review yields some major research findings so as to how pedometer-based exercise intervention plays an important role in improving the quality of life in patients with breast cancer who are undergoing chemotherapy. A wide range of studies were identified for the review, but only a few studies on pedometer-based exercises have been done on breast cancer patients undergoing chemotherapy. Pedometer is an inexpensive, portable, and a user-friendly device; therefore, it is very easy to administer in the clinical setup. The use of a pedometer is now gradually taking up the pace. Nevertheless, the studies done till now, using a pedometer, show potential benefits on various health-related parameters during breast cancer survivorship.
The studies retrieved by this review help us to understand that pedometer-based exercises are feasible during the course of chemotherapy in patients with breast cancer. To summarize, the studies included in this review studied the outcome measures such as fatigue, quality of life, body composition, and psychosocial factors in various combinations.
All the three trials selected for the review adopted different protocols using pedometers. Of these, two studies[9,11] used the ACSM guidelines with regard to the duration of exercise, step counts per day, and the frequency of the exercise.[12] The other trials[10] used their own pedometer-based protocols in conjunction with other treatment therapies such as cognitive behavioral therapy and diaphragmatic breathing. The duration of pedometer-based protocols ranged from 8 weeks to 14 weeks. The reason for this variation was cited as the time period required in adapting to the physical activity by patients. Furthermore, the step counts prescribed did not exceed 10,000 which usually is considered normal for a healthy individual.
Various combinations of outcome measures were observed in the three trials. The study conducted by Gokal et al. in 2018 has focused more on psychological outcomes. They concluded in their studies that simple physical activity interventions such as walking have a great impact on cognitive functioning, memory, attention, anxiety, fatigue, and depression which in turn has a positive impact on the quality of life. Whereas, the other two studies done by Djuric et al., 2011, and Pelekasis et al., 2016, have studied the effect of physical activity on body mass index (BMI) and body composition and shown that pedometer-based exercise intervention throughout the course of chemotherapy decreases the skeletal muscle loss and reduces the body fat percentage.
Except for the study by Gokal et al., all the other studies had incorporated dietetic intervention or consultation along with the pedometer-based exercise program. Even these studies show positive results on psychological health, BMI, body composition, and other health-related domains of quality of life.
The current review suggests that pedometers have started gaining more importance due to its feasibility when compared to its counterparts such as accelerometers which are much more expensive. Researchers, therefore, have started using it as a physical activity monitor to quantify a walking program. We further suggest that future studies should use the above-mentioned outcomes and design a pedometer-based training for patients with breast cancer to strengthen the current body of evidence in this field of exercise oncology.
CONCLUSION
This systematic review concludes that pedometer-based exercise interventions are feasible and beneficial in patients with breast cancer receiving chemotherapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1. [Last accessed on 2019 Jul 01]. Available from: https://www.who.int/cancer/prevention/diagnosisscreening/breast-cancer/en/
- 2.Shin WK, Song S, Jung SY, Lee E, Kim Z, Moon HG, et al. The association between physical activity and health-related quality of life among breast cancer survivors. Health Qual Life Outcomes. 2017;15:132. doi: 10.1186/s12955-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyob T, Ng T, Chan R, Chan A. Impact of chemotherapy on cancer-related fatigue and cytokines in 1312 patients: A systematic review of quantitative studies. Curr Opin Support Palliat Care. 2016;10:165–79. doi: 10.1097/SPC.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 4.Karthikeyan G, Jumnani D, Prabhu R, Manoor UK, Supe SS. Prevalence of fatigue among cancer patients receiving various anticancer therapies and its impact on quality of life: A cross-sectional study. Indian J Palliat Care. 2012;18:165–75. doi: 10.4103/0973-1075.105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardwell WA, Ancoli-Israel S. Breast cancer and fatigue. Sleep Med Clin. 2008;3:61–71. doi: 10.1016/j.jsmc.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swenson KK, Nissen MJ, Henly SJ. Physical activity in women receiving chemotherapy for breast cancer: Adherence to a walking intervention. Oncol Nurs Forum. 2010;37:321–30. doi: 10.1188/10.ONF.321-330. [DOI] [PubMed] [Google Scholar]
- 7. [Last accessed on 2019 Jul 01]. Available from: https://www.pedro.org.au/wp-content/uploads/PEDro_scale.pdf .
- 8. [Last accessed on 2019 Jul 01]. Available from: https://www.strokengine.ca/en/glossary/pedro-score/
- 9.Djuric Z, Ellsworth JS, Weldon AL, Ren J, Richardson CR, Resnicow K, et al. Adiet and exercise intervention during chemotherapy for breast cancer. Open Obes J. 2011;3:87–97. doi: 10.2174/1876823701103010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelekasis P, Zisi G, Koumarianou A, Marioli A, Chrousos G, Syrigos K, et al. Forming a stress management and health promotion program for women undergoing chemotherapy for breast cancer: A pilot randomized controlled trial. Integr Cancer Ther. 2016;15:165–74. doi: 10.1177/1534735415598225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gokal K, Munir F, Ahmed S, Kancherla K, Wallis D. Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy. Results from a small randomised controlled trial? PLoS One. 2018;13:e0206874. doi: 10.1371/journal.pone.0206874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]


