Abstract
Purpose
We explored the prevalence and trends of self-reported complementary and alternative medicine use among patients with prostate cancer using CaPSURE™ (Cancer of the Prostate Strategic Urologic Research Endeavor).
Materials and Methods
A total of 7,989 CaPSURE participants completed questionnaires between 1996 and 2016 on the use of nearly 70 complementary and alternative medicine types. Participants were defined as users if they indicated that they had ever used complementary and alternative medicines. To evaluate trends among 7,696 patients with newly diagnosed prostate cancer we considered complementary and alternative medicine use within 24 months of diagnosis and calculated the percent change in complementary and alternative medicine use between groups defined by the year of diagnosis.
Results
Of patients with prostate cancer 56% reported complementary and alternative medicine use on at least 1 questionnaire. Multivitamin and omega-3 fatty acid use was common at 40% and 24% of patients, respectively. Compared to nonusers greater proportions of complementary and alternative medicine users were college educated, had a higher household income and lived in the West and Midwest. Median prostate specific antigen at diagnosis was 5.8 (IQR 4.4−8.4) and 6.2 ng/ml (IQR 4.7–10.1) among users and nonusers, respectively (p <0.01). Between those diagnosed in 1996 to 2000 and 2011 to 2016, complementary and alternative medicine use increased 128% from 24% to 54%. When comparing participants diagnosed in 2006 to 2010 with those diagnosed in 2011 to 2016, a 108% increase was seen in supplemental vitamin D use and a −48% decrease was seen in supplemental vitamin E use.
Conclusions
Many patients with prostate cancer reported complementary and alternative medicine use. Multivitamins and omega-3 fatty acids were commonly ingested and vitamin D use increased dramatically from 2006 to 2010 compared to 2011 to 2016. These data can guide clinical discussions and decision making such as nutritionist referral and help prioritize future research.
Keywords: prostatic neoplasms, complementary therapies, dietary supplements, vitamins, prevalence
Editor’s Note
This article is the first of 5 published in this issue for which category 1 CME credits can be earned. Instructions for obtaining credits are given with the questions on pages 828 and 829.
In the U.S. PCa makes up 9.5% of all new cancer cases and it is the most common cancer among men. There have been significant improvements in PCa management. According to the National Institutes of Health Surveillance, Epidemiology, and End Results Program data from 2008 to 2014 the 5-year survival rate is 98.6%.1 Conventional PCa treatment includes active surveillance, radiation therapy, RP, hormone therapy and chemotherapy.2
In addition to conventional medicine, patients with PCa may consider CAM. According to the National Center for Complementary and Integrative Health CAM is defined as “a group of diverse medical and health care systems, practices and products that are not generally considered part of conventional medicine.”3 CAM includes various treatment modalities, including natural products (eg supplements and herbs), mind and body practices (eg acupuncture and yoga) and nonwestern medical practices (eg Asian traditional medicine and Ayurveda).3 Patients report that they use CAM to cope with the emotional and physical burdens of the illness and the side effects of treatment. Furthermore, many patients believe that CAM will help them live longer and improve the quality of life.4,5
Still, evidence continues to emerge which challenges prior beliefs of associations of CAM with PCa risk and progression as well as possible interactions with PCa treatment. Studies such as SELECT have shown that something as simple as an over-the-counter nutritional supplement should not be considered benign.6 Furthermore, in vitro metabolism studies have demonstrated that CAMs like St. John’s wort have an inhibitory effect on CYP3A, the enzyme responsible for metabolizing chemotherapeutics like docetaxel.7 Thus, understanding the popularity of specific CAM types and inquiring about patient use are essential to provide high quality care.
Prior studies have shown that 8% to 90% of patients with PCa use CAM.8 This wide range and nonstandardized assessment make it difficult to elucidate how CAM use has changed with time. To our knowledge few groups have reported trends in CAM use among patients with PCa and those which exist are not comprehensive. Using the large, longterm, national CaPSURE™ database of patients with PCa our goal was to report the current prevalence of use of a wide variety of CAM types and observe trends in CAM use among patients newly diagnosed with PCa.
MATERIALS AND METHODS
Cancer of the Prostate Strategic Urologic Research Endeavor
Beginning in 1995, CaPSURE has enrolled approximately 15,310 patients with PCa at a total of 43 institutions across the U.S., including community urology practices, academic medical centers and Veterans Affairs medical centers. The registry holds patient reported and clinical data, including a history of the diagnosis and disease course, biopsy and pathology reports, and treatment modalities.
Written consent was obtained from all participants and this study was approved by the institutional review board at University of California-San Francisco as well as at other enrolling sites (IRB No. 70–00881). The specifics of CaPSURE have been published previously.9
Complementary and Alternative Medicine Data
CaPSURE participants completed periodic questionnaires which were sent quarterly to annually depending on the study phase. The questionnaires asked about multiple aspects of quality of life and included a section on CAM use, which was queried as “alternative treatments such as acupuncture, herbal remedies, homeopathic remedies, special diets, any vitamins or other treatments.” Use was evaluated via respondent text starting in 1996 until 1999, at which point a checklist of CAM types was developed. This checklist was updated every few years until 2005 and remained the same through 2016. It inquired about almost 70 CAM types during the 21-year study period.
For analysis the different CAMs were combined into categories of similar products or practices (supplementary table 1, https://www.jurology.com). Overall CAM use was defined as the use of any checklist item or text written by the participant on any survey during the study interval.
Analysis Population
We identified 7,989 participants who were diagnosed between January 1, 1996 and December 31, 2016 who completed at least 1 questionnaire containing CAM questions. Participants were classified as users if they indicated CAM use on any questionnaire during study participation. Nonusers were considered patients who returned a questionnaire and checked no to the high level question or left it blank and did not check yes to any individual checklist item. Of the participants 15% completed 1 questionnaire, 11% completed 2 and 75% completed 3 or more.
To estimate trends in CAM use among patients with newly diagnosed PCa we limited responses to questionnaires submitted within 24 months of diagnosis. That is, participants were classified as users if they indicated CAM use on any questionnaire completed within the first 24 months of diagnosis while nonusers met the mentioned criteria and returned a survey in the first 24 months. We identified 7,696 participants who met these criteria. In each population regardless of the number of questionnaires completed a “yes” response to any individual CAM item was counted only once per participant. If a response to a specific CAM item was left blank, this was interpreted as a “no” response.
Statistical Analysis
To estimate prevalence we generated frequency tables of CAM use by overall and individual CAMs. We examined associations of sociodemographic and clinical characteristics with CAM use and nonuse using the chisquare test, the t-test and the Wilcoxon rank sum test with 2-sided p <0.05 considered statistically significant. Statistical analyses were performed with SAS®, version 9.4. Finally, to estimate trends we grouped patients by diagnosis year in 5 or 6-year intervals and calculated the percent change in use between groups. For overall CAM use we calculated the percent change between patients diagnosed in 1996 to 2000 and 2011 to 2016. For a specific CAM we calculated the percent change between 2006 and 2010, and 2011 and 2016 since all participants in these groups completed the same standardized questionnaire. Among users we calculated the median CAM per participant by averaging the number of CAMs reported on all questionnaires submitted by an individual participant.
RESULTS
Study Population Sociodemographic and Clinical Characteristics
The average ± SD age at diagnosis in the 7,989 participants was 66 ± 9 years. Of the participants 90% were Caucasian, 38% had Gleason 3 + 4 or greater disease on diagnostic biopsy and 91% had clinical stage T1/T2 disease. Median prostate specific antigen at diagnosis was 6.0 ng/ml (IQR 4.5–9.0). The average number of comorbidities at diagnosis was 1.9 ± 1.4. The most common primary treatment modality was RP, which was performed in 51% of cases. Supplementary table 2 (https://www.jurology.com) shows the sociodemographic and clinical characteristics of the study population.
Complementary and Alternative Medicine
Use and Nonuse by Sociodemographic and Clinical Characteristics
The average age of CAM users and nonusers was similar at 65 ± 8 and 66 ± 9 years, respectively (supplementary table 2, https://www.jurology.com). Compared to nonusers, more CAM users were Caucasian (93% vs 87%) and college educated (64% vs 43%), had a higher household income (17% vs 26% reporting less than $30,000 per year), and lived in the West (18% vs 13%) and Midwest (30% vs 26%) regions of the U.S. vs the South and the East (all p <0.01). When comparing clinical characteristics to those of CAM nonusers, median prostate specific antigen at diagnosis was slightly lower in CAM users (5.8, IQR 4.4–8.4 vs 6.2 ng/ml, IQR 4.7–10.1, p <0.01). CAM users had a slightly higher mean number of comorbidities (2.0 ± 1.5 vs 1.8 ± 1.4, p <0.01). More CAM users were treated at an academic hospital (10% vs 5%) and underwent RP (53% vs 48%) (all p <0.01). There was no association between CAM use and insurance type (p = 0.18), biopsy Gleason grade (p = 0.12) or body mass index (p = 0.21).
Prevalence of Use
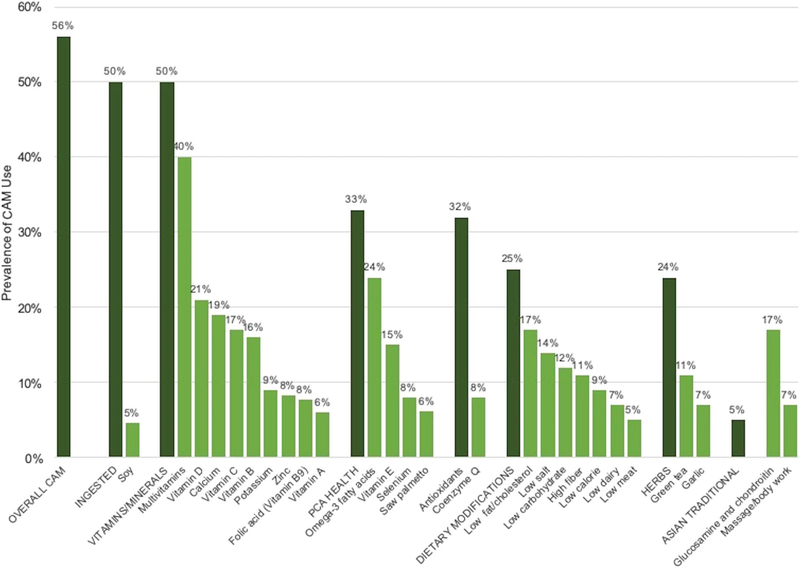
Of the patients with PCa 56% reported using some form of CAM and 50% reported ingesting CAM (not including multivitamins and dietary modifications) during the study period (fig. 1). Supplementary table 1 (https://www.jurology.com) provides a list of individual items in each CAM subcategory. Among CAM users the median number of CAMs per participant averaged across questionnaires was 1.5 (IQR 0.50–3.6). The median number of ingested CAMs per participant was 0.78 (IQR 0.23–2.0). The most prevalent vitamin and mineral supplements were multivitamins, ingested by 40% of participants. The most prevalent CAM for prostate health was omega-3 fatty acids (24%), reported as flaxseed oil, fish oil or other omega-3 fatty acid supplements. The most prevalent dietary modifications were a diet low in fat and/or cholesterol in 17% of participants and a low salt diet in 14%. Other commonly used CAMs were antioxidants in 32% of participants, herbs in 24% and vitamin D in 21%. Figure 1 shows the prevalence of CAM use. Supplementary table 1 (https://www.jurology.com) shows a comprehensive list of CAMs with 2% or greater overall use which were included on the questionnaire or reported as participant written text.
Figure 1.
Prevalence of CAMs with 5% or greater use in 7,989 CaPSURE participants diagnosed between 1996 and 2016. PCA, PCa.
Trends in Use among Patients with Newly Diagnosed Prostate Cancer
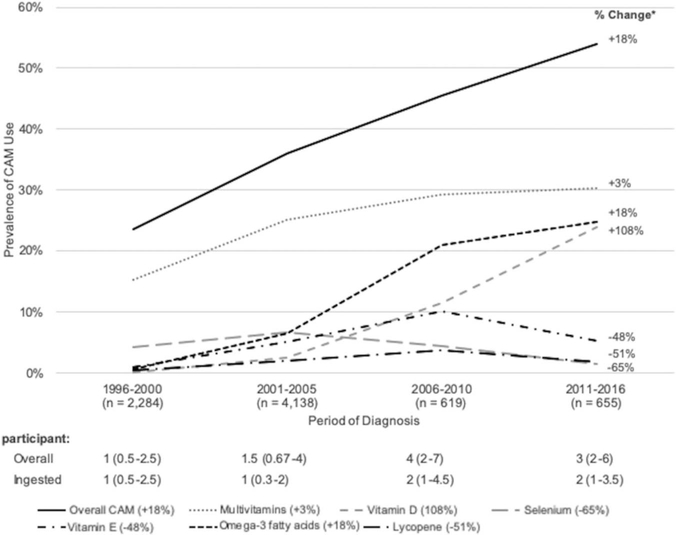
Of participants diagnosed in 1996 to 2000 and 2011 to 2016 overall CAM use increased 128% from 24% to 54%. Between 2006 to 2010 and 2011 to 2016 a meaningful increase was seen in supplemental vitamin D and a meaningful decrease was seen in supplemental vitamin E (108% and −48%, respectively). There was also a large increase in acupuncture (259%) and prostate health combination products (67%) as well as a large decrease in selenium (−65%) and lycopene (−51%). However, absolute use of these items was low at less than 5% of each group. Figure 2 shows trends in the use of select CAMs and the median number of CAMs per participant by group. Supplementary table 1 (https://www.jurology.com) provides a more comprehensive list of trends.
Figure 2.
Solid black curve indicates overall CAMs (18%). Gray dotted curve indicates multivitamins (3%). Gray curve of short dashes indicates vitamin D (108%). Gray curve of long dashes indicates selenium (−65%). Black curve of dashes and dots indicates vitamin E (−48%). Black dashed curve indicates omega-3 fatty acids (18%). Black curve of long dashes and dots indicates lycopene (−51%).
DISCUSSION
We report novel data on self-reported CAM use in a large, national cohort of patients with PCa, reflecting trends between 1996 and 2016 in the U.S. Bishop et al reviewed CAM use among 11,736 patients with PCa in a total of 39 studies published between 1999 and 2009.8 However, the heterogeneity of reporting between studies precluded a meta-analysis. The reported median of 30% overall CAM use differs from these reported findings of 56% ever use from 1996 to 2016, although other, more recent studies have shown findings similar to these data.10–12 The observation that CAM users were more likely to be Caucasian and college educated, and have a higher income is consistent with studies done in other Western countries.13,14 Still, a nonnegligible amount of patients across all education levels and income categories reported CAM use.
These data demonstrate that overall CAM use is common and increasing. Many professional organizations recognize the usefulness of CAM for cancer survivorship.15–17 For example, the ACS® (American Cancer Society) recommends a plant based diet low in saturated fat as a part of PCa survivorship guidelines.17 Also, the American Society of Clinical Oncology recently endorsed the clinical practice guidelines of the Society for Integrative Oncology on CAM use in patients with breast cancer.15
Clinicians should have an open conversation with their patients about commonly used CAMs. For example, given the common use of ingested CAMs, it is important that patients understand the lack of regulation of nutritional supplements by the U.S. Food and Drug Administration, which raises concerns about quality and accuracy of labeling.18 These data also indicate that multivitamin use is high and remained high across the study period. Thus, it is important to warn that although daily multivitamin use may reduce the overall cancer risk, there appears to be no effect on the prostate cancer risk while excessive intake of multivitamins alone or combined with other single supplements has been associated with a higher risk of advanced and lethal PCa.19,20 It is also important to ensure that ingested CAM supplementation among users is not used as a proxy for healthful nutrition and exercise.
Emerging data on the risks of certain CAMs may also be guiding use. In the cohort there was a reduction in the use of selenium and vitamin E between 2006 to 2010 and 2011 to 2016 (−65% and −48%, respectively). In 2009 SELECT was terminated early due to futility.21 In 2011 a followup publication described a statistically significant increase in PCa risk with vitamin E use (HR 1.17, p = 0.008) and a nonstatistically significant increase with selenium use (HR 1.09, p = 0.18).6 These observational data cannot be linked directly to the publication of SELECT data. However, this trend may suggest that information from this trial reached the general public. Unfortunately, certain prostate health combination products continue to contain selenium and vitamin E. Although the use of these products was low overall in this population (3%), use may be increasing (67%).
These data also suggest types of CAM requiring further investigation. For example, nearly a quarter of participants used omega-3 fatty acids while vitamin D use increased dramatically from 12% to 24% between 2006 to 2010 and 2011 to 2016. A secondary analysis of the SELECT cohort showed that some fatty acids (eg eicosapentaenoic acid and docosahexaenoic acid) were associated with high grade disease.22 However, a subsequent randomized controlled trial comparing patients on a low fat diet with fish oil supplementation to those on a Western diet reported that men in the intervention arm had a reduced proliferation index.23 Still, the Vitamin D and Omega-3 Trial revealed no significant reduction in death from PCa in those treated with omega-3 fatty acids (HR 1.15, 95% CI 0.94–1.39) or with vitamin D (HR 0.88, 95% CI 0.72–1.07) for a median of 5.3 years compared to placebo.24,25
The strengths of this study include the cohort size and the diversity of CAM types. In addition, these CAM data were obtained from patients across the U.S. at various practice types with detailed information on participant sociodemographic and clinical characteristics. Limitations include the fact that this inquiry did not assess reasons for use (ie cancer specific, secondary to a provider/peer recommendation, etc). Some trends in CAM use might reflect general population trends. Rooney et al reported a similar spike in vitamin D use among the general U.S. population between 1999 and 2014 (0.3% to 18.2%, p <0.01).26 Duration and frequency of use, association between using specific CAM types and other complementary or conventional therapies, CAM use in patient social networks, an individual change in CAM use during the treatment period and the cost to patients were also not assessed. Furthermore, underrepresentation of African American and Latino patients (551 or 7% and 90 or 1%, respectively) may limit study generalizability.
The definition of conventional vs complementary medicine can be ambiguous. For example, some may argue that a low saturated fat diet is not complementary, given its inclusion in the ACS guidelines. Still, dietary modifications fall outside the National Cancer Institute definition of conventional medicine (ie drugs, radiation and surgery).17,27 Lastly, self-reported data carry the risk of measurement error. However, because the questionnaires included a detailed checklist of CAM items and an open-ended question for participants to write a response, this risk may have been minimal. Furthermore, questionnaires were mailed to participants to be completed at home and/or online, which may have minimized misreporting secondary to social desirability.
CONCLUSIONS
Overall CAM use is common among patients with PCa and it is increasing among newly diagnosed patients. From 1996 to 2016 some of the most prevalently used CAMs were multivitamins and omega-3 fatty acids. Between 2006 and 2010, and 2011 and 2016 the use of vitamin D increased dramatically while the use of vitamin E decreased. This information reinforces the importance of discussing CAM use with patients, including referral to specialists (eg nutritionists) when indicated, and may help prioritize future research.
Supplementary Material
Acknowledgments
Supported by National Center for Complementary and Integrative Health T32AT003997 (KBZ), the University of California-San Francisco Department of Urology, Department of Defense W81XWH-13-2-0074 (PRC), and the Steven and Christine Burd Safeway Distinguished Professorship (JMC).
Abbreviations and Acronyms
- CAM
complementary and alternative medicine
- CaPSURE™
Cancer of the Prostate Strategic Urologic Research Endeavor
- PCa
prostate cancer
- RP
radical prostatectomy
- SELECT
Selenium and Vitamin E Cancer Prevention Trial
- U.S.
United States
Footnotes
No direct or indirect commercial, personal, academic, political, religious or ethical incentive is associated with publishing this article.
Contributor Information
Kyle B. Zuniga, Osher Center for Integrative Medicine, University of California-San Francisco, San Francisco, California; College of Physicians and Surgeons, Columbia University Medical Center, New York, New York.
Shoujun Zhao, Department of Urology, University of California-San Francisco, San Francisco, California.
Stacey A. Kenfield, Department of Urology, University of California-San Francisco, San Francisco, California
Benjamin Cedars, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, Pennsylvania.
Janet E. Cowan, Department of Urology, University of California-San Francisco, San Francisco, California
Erin L. Van Blarigan, Department of Urology, University of California-San Francisco, San Francisco, California Department of Epidemiology and Biostatistics, University of California-San Francisco, San Francisco, California.
Jeanette M. Broering, Department of Urology, University of California-San Francisco, San Francisco, California
Peter R. Carroll, Department of Urology, University of California-San Francisco, San Francisco, California
June M. Chan, Department of Urology, University of California-San Francisco, San Francisco, California; Department of Epidemiology and Biostatistics, University of California-San Francisco, San Francisco, California.
REFERENCES
- 1.Noone AM, Howlander N, Krapcho M et al. : SEER Cancer Statistics Review, 1975–2015, based on November 2017 SEER data submission. Available at https://seer.cancer.gov/archive/csr/1975_2015/ Accessed July 24, 2018.
- 2.Thompson I, Thrasher JB, Aus G et al. : Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007; 177: 2106. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Complementary and Alternative Medicine: The Use of Complementary and Alternative Medicine in the United States. Available at https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm Accessed July 24, 2018.
- 4.Bar-Sela G, Danos S, Visel B et al. : Understanding the attitudes of patients with cancer toward complementary and alternative therapies. J Palliat Med 2016; 19: 496. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson S, Gomella LG, Smith JA et al. : Attitudes and use of complementary medicine in men with prostate cancer. J Urol 2002; 168: 2505. [DOI] [PubMed] [Google Scholar]
- 6.Klein EA, Thompson IM Jr, Tangen CM et al. : Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306: 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goey AKL, Mooiman KD, Beijnen JH et al. : Relevance of in vitro and clinical data for predicting CYP3A4-mediated herb-drug interactions in cancer patients. Cancer Treat Rev 2013; 39: 773. [DOI] [PubMed] [Google Scholar]
- 8.Bishop FL, Rea A, Lewith H et al. : Complementary medicine use by men with prostate cancer: a systematic review of prevalence studies. Prostate Cancer Prostatic Dis 2011; 14: 1. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Broering JM, Litwin MS et al. : The contemporary management of prostate cancer in the United States: lessons from the Cancer of the Prostate Strategic Urologic Research Endeavor (CapSURE), a national disease registry. J Urol 2004; 171: 1393. [DOI] [PubMed] [Google Scholar]
- 10.Egger S, Hughes S, Smith DP et al. : Factors associated with the use of complementary and alternative medicines for prostate cancer by long-term survivors. PLoS One 2018; 13: e0193686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahall M: Prevalence, patterns, and perceived value of complementary and alternative medicine among cancer patients: a cross-sectional, descriptive study. BMC Complement Altern Med 2017; 17: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott CL, Blough DK, Fedorenko CR et al. : Complementary and alternative medicine use among newly diagnosed prostate cancer patients. Support Care Cancer 2012; 20: 65. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey SD, Zeliadt SB, Blough DK et al. : Complementary and alternative medicine use, patient-reported outcomes, and treatment satisfaction among men with localized prostate cancer. Urology 2012; 79: 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boon H, Westlake K, Stewart M et al. : Use of complementary/alternative medicine by men diagnosed with prostate cancer: prevalence and characteristics. Urology 2003; 62: 849. [DOI] [PubMed] [Google Scholar]
- 15.Lyman GH, Greenlee H, Bohlke K et al. : Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol 2018; 36: 2647. [DOI] [PubMed] [Google Scholar]
- 16.Van Vu D, Molassiotis A, Ching SS et al. : Effects of Qigong on symptom management in cancer patients: a systematic review. Complement Therapies Clin Pract 2017; 29: 111. [DOI] [PubMed] [Google Scholar]
- 17.Skolarus TA, Wolf AM, Erb NL et al. : American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin 2014; 64: 225. [DOI] [PubMed] [Google Scholar]
- 18.Marcus DM: Dietary supplements: what’s in a name? What’s in the bottle? Drug Test Anal 2016; 8: 410. [DOI] [PubMed] [Google Scholar]
- 19.Hollenbeck A, Subar A, Schatzkin A et al. : Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. JNCI 2007; 99: 754. [DOI] [PubMed] [Google Scholar]
- 20.Gaziano JM, Sesso HD, Christen WG et al. : Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2012; 308: 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippman SM, Klein EA, Goodman PJ et al. : Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brasky TM, Till C, White E et al. : Serum phospholipid fatty acids and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Am J Epidemiol 2011; 173: 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aronson WJ, Kobayashi N, Barnard RJ et al. : Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2011; 4: 2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manson JE, Cook NR, Lee IM et al. : Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019; 380: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manson JE, Cook NR, Lee IM et al. : Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019; 380: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rooney MR, Harnack L, Michos ED et al. : Trends in use of high-dose vitamin D supplements exceeding 1000 or 4000 international units daily, 1999–2014. JAMA 2017; 317: 2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCI Dictionary of Cancer Terms: conventional medicine. Available at https://www.cancer.gov/publications/dictionaries/cancer-terms/def/conventional-medicine. Accessed March 24, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.