Abstract
Background:
Anti-CD2 treatment provides targeted immunomodulatory properties that have demonstrated clinical usefulness to condition the immune system and to treat transplant rejection. The treatment is species-specific due to structural CD2 antigen differences between non-human primates and humans. Herein, we report the safety profile and efficacy of two modifications of the same anti-CD2 monoclonal antibody in cynomolgus macaques.
Methods:
Twelve subjects received one i.v. anti-CD2 (of rat or rhesus type) dose each, range 1–4 mg/kg, and were followed for 1–7 days. Treatment effects were evaluated with flow cytometry on peripheral blood and histopathological evaluation of secondary lymphoid organs. In vitro inhibitory activity on primary MHC disparate MLRs was determined.
Results:
Upon anti-CD2 treatment, CD4+, CD8+ memory subsets were substantially depleted. Naïve T-cells and Tregs were relatively spared, and exhibited lower CD2 expression than memory T-cells. Early immune reconstitution was noted for naïve cells, while memory counts had not recovered after one week. Both antibodies displayed a concentration-dependent MLR inhibition. Lymph node examination revealed no significant lymphocyte depletion. None of the animals experienced any significant study drug-related adverse events.
Conclusions:
This study outlines the safety and pharmacodynamic profile of primate-specific anti-CD2 treatment, relevant for translation of anti-CD2-based animal models into clinical trials.
Introduction
Treatment with anti-CD2 antibody is an attractive approach to condition the immune system or treat rejection, as this antibody possesses T-cell depleting, co-stimulatory blocking, and donor-specific regulatory T-cell enhancing properties1–7. In humans, the anti-CD2 monoclonal antibody BTI-322 (rat IgG2b) has been shown to reverse first time kidney allograft acute rejections (AR), steroid and antithymocyte globulin (ATG) resistant rejections8, and significantly decrease AR rates compared to standard-of-care when used as induction therapy9. Both BTI-322 and Siplizumab (humanized BTI-322, IgG1k) have favorable safety profiles5,10. The most significant benefit of Siplizumab has been as a key component in a phase I/II tolerance induction clinical trial where the majority of HLA-mismatched kidney allograft recipients could successfully be weaned off all chronic maintenance immunosuppression for at least five years11,12. To bring anti-CD2 treatment to the clinic as a standardized approach, it is important to characterize interventions on this signaling pathway. Cynomolgus macaques are widely used in translational studies due to the biological similarities to humans. To enable experimental use of anti-CD2 treatment in cynomolgus macaque-based animal models, homologous antibodies must be used in lieu of siplizumab and BTI-322, due to the latter’s specificity for a binding site present only on the human and chimpanzee CD2 antigen13. Herein, we report the safety profile and in vitro and in vivo efficacy of two anti-CD2 mAbs, one rodent and one CDR-grafted rhesus version, respectively, in cynomolgus macaques.
Materials and Methods
Test substances
The test substances were manufactured to target the CD2 antigen, and included two modifications of the same anti-CD2 mAb: a rat anti-primate CD2 IgG2b (Immerge BioTherapeutics) (hereafter termed RT-CD2), and the further modified rhesus recombinant anti-primate CD2 IgG1 (hereafter termed RH-CD2). The latter was developed by grafting the CDRs from the RT-CD2 antibody into rhesus frameworks and using rhesus IgG1 and kappa constant regions (NIH Nonhuman Primate Reagent Resource, Boston, MA, USA). RT-CD2 was expressed from stably-transduced Chinese hamster ovary cells. Whereas BTI-322 and its human analogue siplizumab have a narrow species specificity, restricted to only the human and chimpanzee CD2 antigens, the two anti-CD2 antibodies studied herein have a broader primate reactivity. The anti-CD2 mAbs were administered as i.v. infusions over 60 minutes, preceded by prednisolone 0.6 mg/kg and diphenhydramine 1 mg/kg i.v. (Table 1). Antibody dosing was based on the body weight taken on the day of dosing.
Table 1.
Animal characteristics and treatment regimens
| Group | ID | Gender | Weight | Age | Other treatments | Anti-CD2 | Dose | Days followed post anti-CD2 |
|---|---|---|---|---|---|---|---|---|
| A | A1 | M | 7.3 kg | 7 y | Naïve | Rhesus | 1mg/kga | 7 days |
| A2 | M | 10.2 kg | 8 y | Naïve | Rhesus | 2mg/kga | 1 dayd | |
| A3 | M | 7.1 kg | 7 y | Naïve | Rhesus | 2mg/kga | 7 days | |
| A4 | M | 7.4 kg | 9 y | Naïve | Rhesus | 2mg/kga | 7 days | |
| B | B1 | M | 8.5 kg | 7 y | TBIb, Rituximabc | Rat | 1mg/kga | 1 day |
| B2 | M | 8.3 kg | 8 y | TBIb, Rituximabc | Rat | 1mg/kga | 1 day | |
| C | C1 | M | 10.3 kg | 7 y | TBIb, Rituximabc | Rhesus | 1mg/kga | 1 day |
| C2 | M | 10.1 kg | 8 y | TBIb, Rituximabc | Rhesus | 2mg/kga | 1 day | |
| C3 | M | 7.1 kg | 9 y | TBIb, Rituximabc | Rhesus | 2mg/kga | 1 day | |
| C4 | M | 8.1 kg | 6 y | TBIb, Rituximabc | Rhesus | 2mg/kga | 1 day | |
| C5 | M | 9.0 kg | 8.5 y | TBIb, Rituximabc | Rhesus | 2mg/kga | 1 day | |
| C6 | M | 12.5 kg | 7 y | TBIb, Rituximabc | Rhesus | 4mg/kga | 1 day |
Abbreviations: ID (animal identification code); M (male); TBI (total body irradiation).
All animals received premedication with 0.6 mg/kg methylprednisolone and 1 mg/kg diphenhydramine before anti-CD2 dosing on day 0.
TBI 1.5Gy at day −3,
Rituximab 20 mg/kg at day −3.
Severe adverse reaction to ketamine on day 1, unrelated to test substance. Animals B1–B2, C1–C6 could only be followed for one day as they were also included in a separate study as part of a tolerance induction regimen in which they received additional T- and NK-cell acting agents following the anti-CD2 dose.
Animals and experimental design
Three naïve Indonesian-origin cynomolgus macaques (Macaca fascicularis), obtained from AlphaGenesis, Inc (USA), and one Mauritius-origin cynomolgus macaque (Bioculture Ltd, Senneville, Mauritius), were used to evaluate the effect of RH-CD2 monotherapy during seven consecutive study days (group A, Table 1). Eight additional Mauritius cynomolgus monkeys (selected from Bioculture Ltd, Senneville, Mauritius), also part of a longer term liver tolerance study, were evaluated at day 1 post-dosing with either RT-CD2 or RH-CD2 (groups B and C). Groups B and C received low-dose total body irradiation and Rituximab (targeting B-cells only) three days prior to anti-CD2 dosing as part of the liver tolerance inducing regimen (Table 1). During the 24 hours that Groups B and C were monitored for the effects of administered anti-CD2, no other T-cell depleting agents had been infused. All macaques were housed and treated at the Institute of Comparative Medicine (ICM, Columbia University Medical Center, New York, USA). The ICM facility has USDA assurance and is AAALAC-accredited. All experimental procedures were performed in accordance with NIH guidelines for the care and use of primates, and approved by the Columbia University IACUC.
All animals underwent baseline testing before administration of the test substance, consisting of complete blood cell count, serum chemistry, and flow cytometry. Inguinal lymph nodes were excised pre-dose for routine histopathological examination to evaluate the potential treatment effect. Starting on day 0, the animals received i.v. infusions containing the test substance. Flow cytometric analyses were performed on peripheral blood to determine changes in lymphocyte subsets. When applicable, post-dose lymph node biopsies were collected and microscopically assessed by a pathologist for any gross lymphocyte depletion. Safety assessments included careful clinical observation and hematologic and clinical chemistry evaluation before and after dosing.
Flow cytometric immune phenotyping
Fresh peripheral blood samples were stained using a direct cell surface technique to determine the leukocyte subpopulations. The following antibodies were included in serial polychromatic flow cytometric (PFC) measurements: anti-CD2 BV786 (RPA-2–10), MHC-I PE (DX17), MHC-I PeCy7 (G46–2.6), CD3 PerCP Cy5.5 (SP34–2), CD4 APC (L200), CD4 AmCyan (L200), CD8 Pacific Blue (RPA-T8), CD8 APC (RPA-T8), CD56 PE (NCAM16.2), CD95 PE (DX2) (BD Biosciences, San Jose, CA); CD11b AmCyan (M1/70.15.11.5), CD20 APC-Cy7 (LT-20), CD45RA APC-Cy7 (T6D11) (Miltenyi Biotec, Auburn, CA); CCR7 PeCy7 (G043H7), CD28 Pacific Blue (CD28.2) (BioLegend, San Diego, CA). The first step to assess naïve and memory T-cells were based on CCR7 and CD45RA gating; naïve being defined as CCR7+CD45RA+, and all other cells as memory T-cells. The populations were further gated on CD95 and CD28 to define naïve (CD95low/−CD28+), central memory (CD95+CD28+), and effector memory (CD95+CD28−) cells, respectively. Cells were permeabilized using the BioLegend FoxP3 Fixation/Permeabilization Buffer Set according to the manufacturer’s protocol. Prior to permeabilization, cells were stained with CD3 PerCP Cy5.5 (SP34–2) (BD Biosciences, San Jose, CA), CD4 AmCyan (L200) (BD Biosciences, San Jose, CA), CD8 PeCy7 (BW135/80) (Miltenyi), CD127 (HL-7R-M21) (BD Biosciences), CD25 Pacific Blue (BC96) (BioLegend) and CD45RA APC-Cy7 (T6D11) (Miltenyi Biotec, CA). Permeabilized cell staining included FOXP3 (PCH101) (Invitrogen, Carlsbad, CA). Regulatory T cells (Tregs) were defined as CD3+CD4+CD25highFoxP3+. Isotype controls were included throughout, as well as fluorescence minus one (FMO) controls for CD2, and FOXP3. Data was acquired on a BD FACSCanto II flow cytometer (BD Bioscience) and BD Fortessa (BD Bioscience) flow cytometer and analyzed with FlowJo v10.1 Software (TreeStar Inc., Ashland, OR) and FCS Express (De Novo Software, Glendale, CA). Anti-CD2 BV786 (RPA-2–10, BD Biosciences, San Jose, CA) was used for the detection of CD2 expression. Anti-CD2 BV786 and RH-CD2 (secondary detecting antibody, clone HP6017, FITC anti-human IgG Fc, BioLegend) showed partial competitive binding to the CD2 epitope (Figure 1H).
Figure 1. Anti-CD2 treatment pharmacodynamics, and CD2 expression on untreated freshly isolated lymphocytes.
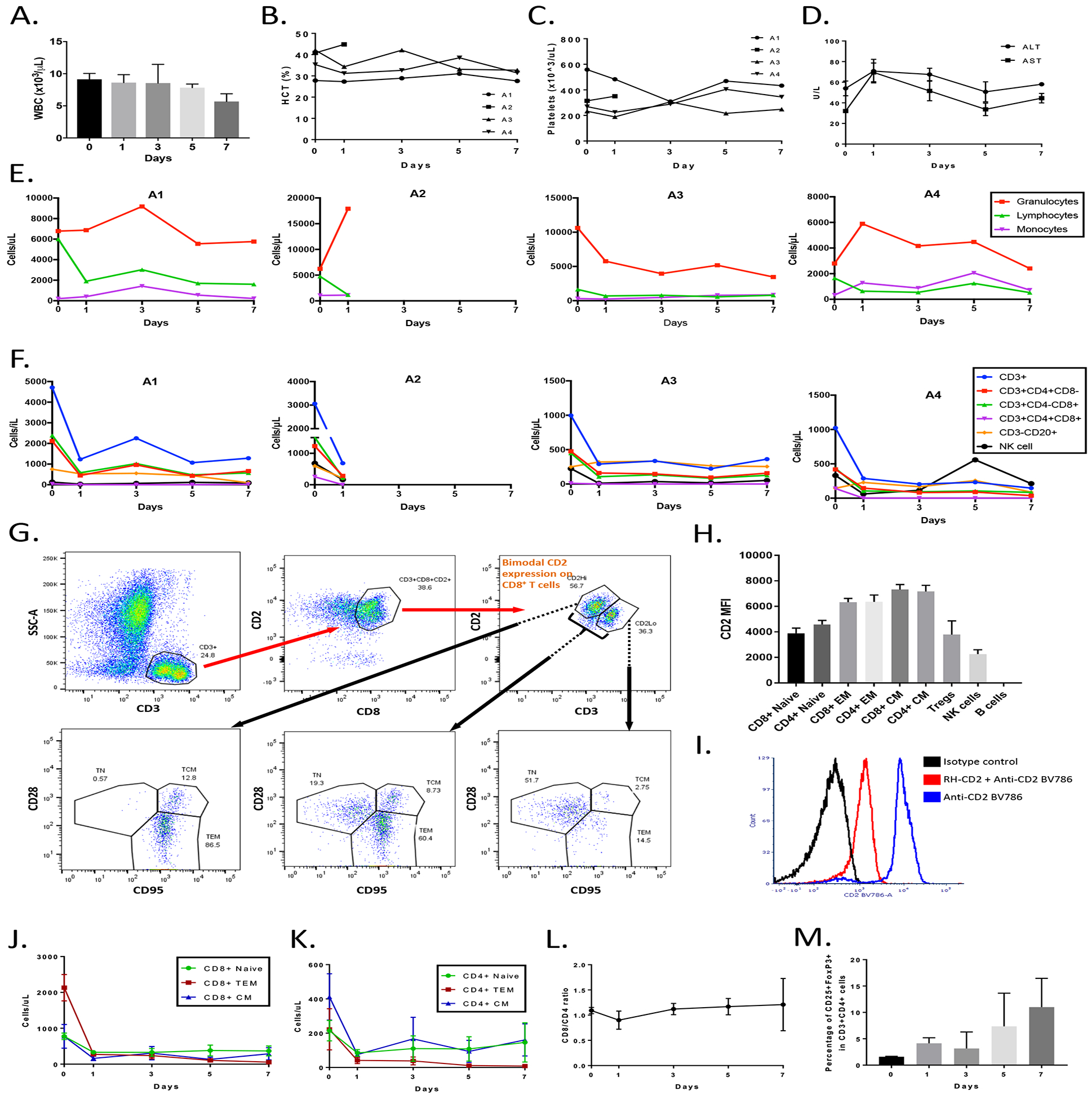
A) WBC counts in all treated animals, groups A-C (n.s.). B, C) Showing treatment impact on hematocrit (Hct%) or platelet levels (n.s.). D) ALT and AST levels pre- and post-anti-CD2 infusion, group A. E, F) Post-treatment levels of leukocyte subsets. G) Showing bimodal CD2 and CD3 T cell expression in naïve cynomolgus monkeys, with memory cells having a higher CD2hi and CD3lo expression. Shown is a representative gate defining the bimodal distribution, and the three subsets of T cells (TN, TEM, TCM) gated for CD2 and CD3. Predominantly, TN are CD3hiCD2lo and memory cells CD3loCD2hi. CCR7/CD45RA gating step not shown. H) Average median fluorescence intensities (MFI) of cell surface CD2 on CD4+-, CD8+-, NK-, B-, and regulatory T cells from naïve animals (n=4). I) Showing partial competitive binding of RH-CD2 and CD2-BV786 antibodies. J, K) RH-CD2 effectively depletes memory cells, while relatively sparing naïve lymphocytes in group A. L) Illustrating the CD8/CD4 ratio before and after RH-CD2 in group A. M) Showing a 695% CD25hiFoxP3+ cell enrichment from day 0 to day 7 after RH-CD2 infusion (p=0.052) in group A. Data is presented as average (±SEM). Abbreviations: WBC, white blood cell count; CD, cluster of differentiation; CM, central memory; EM, effector memory; NK cell, natural killer cell.
Lymph node immunopathology
One portion of each lymph node biopsy specimen was fixed in 10% formalin, and 70% ethanol, paraffin-processed, sectioned, and stained with Gilĺs hematoxylin and eosin (H&E) or CD3 (LN10, Leica). Another portion of the biopsy was placed in RPMI-1640 medium (Gibco, Life Technologies, NY, USA) and lymphocytes were collected by grinding and passing through a 70 μm cell strainer (Corning, NY, USA). Isolated lymphocytes were immunophenotyped by flow cytometry using the same antibody panels as for PBMCs. After euthanasia of each dosed subject, necropsies were performed and major lymphoid tissues were collected for standard histopathological evaluation, performed by a clinical pathologist.
Mixed lymphocyte reaction
Mixed lymphocyte reaction (MLR) responses were evaluated on freshly isolated PBMCs from four cynomolgus macaques (including animals A2–A4), creating four MHC-mismatched responder-stimulator pairs, in the presence of increasing doses (0–20 μg/mL) of RT-CD2 and RH-CD2, respectively. Stimulator cells (1×105 cells/well) were irradiated (35 Gy) and plated in triplicate wells together with responder cells (1×105cells/well) in 200 μL MLR media of a 96-well U-bottom plate (Costar, NY). MLR media contained RPMI-1640 (Gibco, Life Technologies, NY, USA), supplemented with 10% human serum (Gemini, Bioproducts, Sacramento, CA, USA), and 4% Nutrient mixture (MEM non-essential amino acids, Life Technologies), L-glutamine (Life Technologies), sodium pyruvate (Corning Cellgro), and penicillin/streptomycin (Life Technologies)). Responders stimulated with anti-CD2CD3CD28 beads (Miltenyi Biotec) (0.5 ×105 beads/well) and responders with MLR media alone served as positive and negative controls, respectively. After a four-day long incubation at 37°C and 5% CO2, plates were pulsed with 1μCi of 3H-thymidine per well (Perkin, Elmer, Waltham, MA), and harvested 24 hours later (Tomtec). Thymidine incorporation was measured on a ß-counter (1450 MicroBeta Scintillation-Luminescence Counter, PerkinElmer, MA, USA). Stimulation indexes (SI) were calculated as: (Mean counts per minute (CPM) of experimental wells) / (Mean CPM of negative control).
Mechanistic FcγR-binding and CDC assays
To determine Fcγ receptor (FcγR) affinity, 6x serial dilutions of RT-CD2 and RH-CD2 were incubated with reporter cells stably transfected with human FcγRI, FcγRIIA-H or FcγRIIIA-V and a luciferase reporter gene (Promega). Luciferase reporter gene expression was triggered if reporter cells bound to the Fc-fragment of a target-bound IgG antibody with their respective FcγR. The reporter cells were from a human Jurkat CD2+ acute T cell leukemia cell line. Reporter cell binding was flow cytometrically confirmed, for RH-CD2 using a BV421 anti-human IgG Fc (clone HP6017, BioLegend), and for RT-CD2 using a BV421 goat anti-rat IgG (clone Poly4054, BioLegend). Since the antibodies have affinity for the reporter cells they can act as a target and reporter in the assay. A human anti-CD2 IgG1 monoclonal antibody was used as a positive control (ITB-Med AB, Sweden), while anti-CD2 Fab fragments (enzymatically produced from the positive control) were used as a negative control. After 23 hours of incubation at 37°C and 5% CO2, Bio-Glo Luciferase assay reagent was added and luminescence measured after 10–15 min of incubation at room temperature and shielded from light using a Synergy HTX Multi-mode plate reader (BioTek). Luminescence values were normalized to the luminescence signal obtained with the highest concentration of the positive control. Each run contained triplicate serial dilutions of each antibody.
For assessment of complement-dependent cytolysis (CDC), Ficoll density-gradient centrifugation isolated PBMCs were incubated with 10x serial dilutions of the respective antibody agent in 10% Ultra low-IgG FBS (Gibco) in PBS at room temperature. Alemtuzumab (Sanofi) served as a positive control while a human anti-CD2 IgG1 mAb (ITB-Med AB, Sweden) known to not activate complement was used as a negative control. Subsequently, an equal volume of reconstituted rabbit complement (InnoTrain) was added followed by incubation at room temperature for 60 minutes. PBMCs were washed in PBS and stained with 7-AAD (Life Technologies), anti-CD56 BV421 (BD, Clone NCAM16.2), anti-CD89 PE (Miltenyi, Clone REA234), anti-CD3 APC and anti-CD19 APC-H7 (BD; Clone SJ25C1). Samples were incubated on ice and shielded from light until sample acquisition using a FACSVerse flow cytometer (BD Biosciences). FlowJo software (FlowJo LLC, Ashland) and GraphPad Prism 8 (GraphPad Software) were used for sample processing and data analysis.
Statistical analysis
To establish statistically significant changes before and after treatment, Student’s t-test or one-way ANOVA were used, and p-values <0.05 were considered significant, where n.s. denoted not significant. Data are expressed as mean ± standard error of the mean (SEM) unless stated otherwise.
Results
Clinical data
The study subjects were treated according to Table 1. None of the animals (groups A-C, Table 1) experienced any adverse events (AEs) related to the study drugs. Animal A2, however, had a severe apneustic event following ketamine injection on day 1, during sedation performed for peripheral blood collection. Prior to RH-CD2 infusion the animal had shown signs of delayed recovery after ketamine injections. The animal was euthanized on day 1. No significant weight loss was noted in animals A1, A3, and A4, which were followed for 7 days.
Chemistry and hematology
Blood chemistry panels and complete blood counts were followed serially. The total WBC did not significantly change post-treatment (n.s., Figure 1A). No clinically significant changes in hemoglobin or hematocrit were observed (Figure 1B). Animal A1 had a mild transient decrease in platelet count only on day 3 post-antibody infusion, which had normalized by day 5 (Figure 1C). Groups B and C were sampled 24h post-infusion, and showed stable WBC, which was in line with group A day 1 measurements. There was a transient low-grade increase in serum transaminases in several animals, which normalized within a few days (Figure 1D). All other chemistry parameters were maintained within normal limits. The RH-CD2 infusion did not alter the number of circulating monocytes (Figure 1E). The granulocyte count was stable in animals A1 and A4, while it was about halved in animal A3, and more than doubled in animal A2 (Figure 1E).
Cell surface CD2 expression in untreated animals and circulating lymphocyte composition before and after anti-CD2 treatment
PFC was used to serially evaluate the effect of anti-CD2 mAb on circulatory lymphocyte subsets post-treatment. All animals developed lymphopenia, characterized by decreased T cell numbers (CD3+, CD3+CD4+CD8−, CD3+CD4–CD8+, CD3+CD4+CD8+), fewer NK cells, with no change in B cell counts (CD3−CD20+) (Figure 1F). Freshly isolated PBMCs from untreated cynomolgus macaques showed bimodal CD2 expression patterns among CD3+CD8+ and CD3+CD4+ cells, with naïve cells exhibiting lower expression than memory cells (Figure 1G). There was no significant CD2 MFI difference between naïve cells, Tregs, and NK cells (n.s.), whereas naïve CD3+CD8+ and CD3+CD4+ cells had lower CD2 expression than their TCM counterparts (p=0.001 and p=0.017, respectively), and TEM and TCM cells within both the CD4 and CD8 compartments had significantly higher expression than Tregs (Figure 1H). Due to partial competitive binding between the treatment (RH-CD2) and detecting (CD2 BV786) antibodies, exact CD2 expression post-infusion could not be determined in group A (Figure 1I).
Post RH-CD2 treatment, we observed a substantial depletion within circulatory CD4+ and CD8+ memory subsets. Naïve cells were relatively spared, while TEM counts were impacted the most (Figure 1 J, K). After one week, at the time of euthanasia, the most pronounced depletion was seen within the CD8+ TEM and CD4+ TEM compartments, with less effect on the naïve cells. There was no major shift in the overall CD3+CD8+ to CD3+CD4+ ratio (Figure 1L). Anti-CD2 treatment relatively spared Tregs, enriching CD25hiFoxP3+ cells among CD3+CD4+ cells. Between day 0 (pre-treatment) and 7 days after RH-CD2 infusion, the average relative Treg frequency had increased 695% (p= 0.052, Figure 1M). Groups B and C were part of a separate tolerance study. Their peripheral lymphocytic depletion could therefore only be tracked 24 hours post anti-CD2 treatment, which supported a similar early (24 hours) depletion profile as in Group A, without any dose-dependent effects observed (data not shown).
Primary mixed lymphocyte reaction (MLR) effects of rat and rhesus anti-CD2 antibodies
To assess the immunosuppressive effects of RH- and RT-CD2 antibodies, varying concentrations of each mAb were added to a primary MLR using MHC disparate PBMCs from untreated macaques. Both antibodies displayed concentration-dependent inhibition of the primary MLR. The addition of beads elicited a strong proliferative response as expected, while culture with either autologous cells or MLR media did not induce proliferation (Figure 2).
Figure 2. Immunosuppressive effects of rat and rhesus anti-CD2 mAb on a primary MLR.
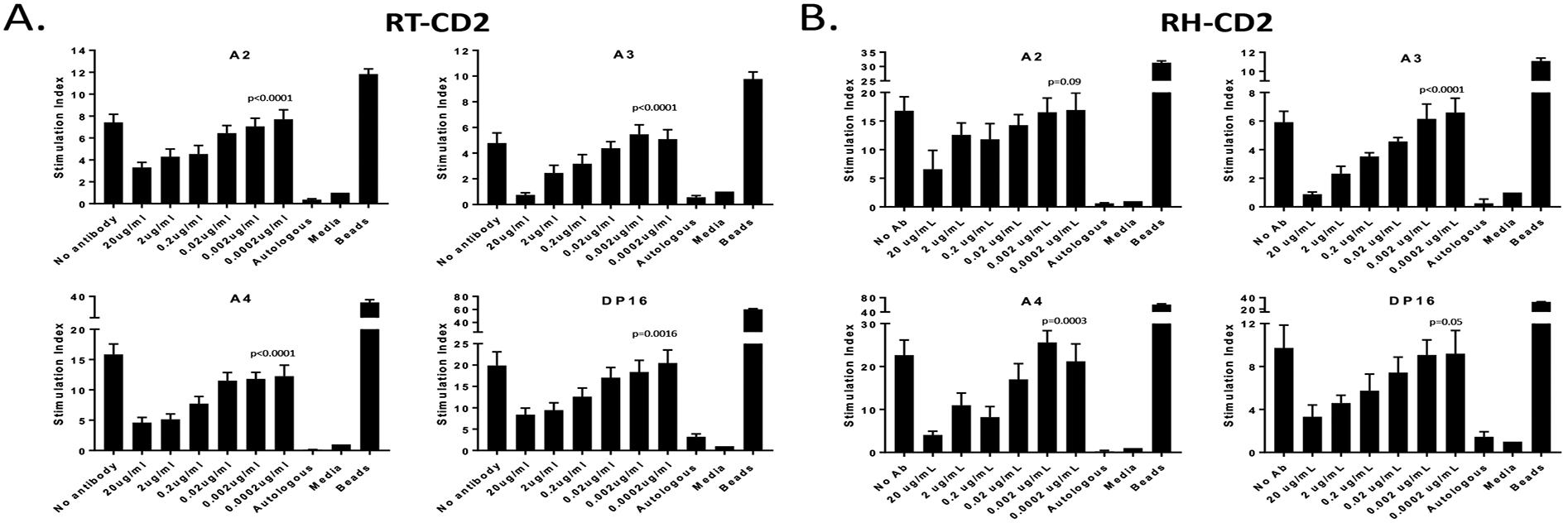
The allogeneic MLRs were inhibited by varying concentrations of RT- and RH-CD2, respectively, at the onset of the host effector cell cultures. After four days, MLRs were pulsed for 24 hours with 1μCi 3H-thymidine per well. The cellular radioactivity was measured in triplicates samples. Statistical analysis were performed of antibody and no antibody treatments using one-way ANOVA. (A) RT-CD2 (B) RH-CD2.
Histopathological and flow cytometric tissue examination
Light microscopic analyses and PFC performed on iliac and mesenteric lymph node samples obtained before and after the treatment with RH-CD2 (group A). No significant cellular reduction was evident, although the presence of a potential minor depletion could not be ruled out. The normal thymus appearance of 6–9 years old cynomolgus monkeys (age span included in this study) is not fully known, but no major cellular depletion could be appreciated at the time of sacrifice (Figure 3). A similar result was noted within the bone marrow, which showed a high cellularity on the last study day.
Figure 3. Histomorphological analysis before (n=2) and after (n=4) anti-CD2 treatment (Group A).
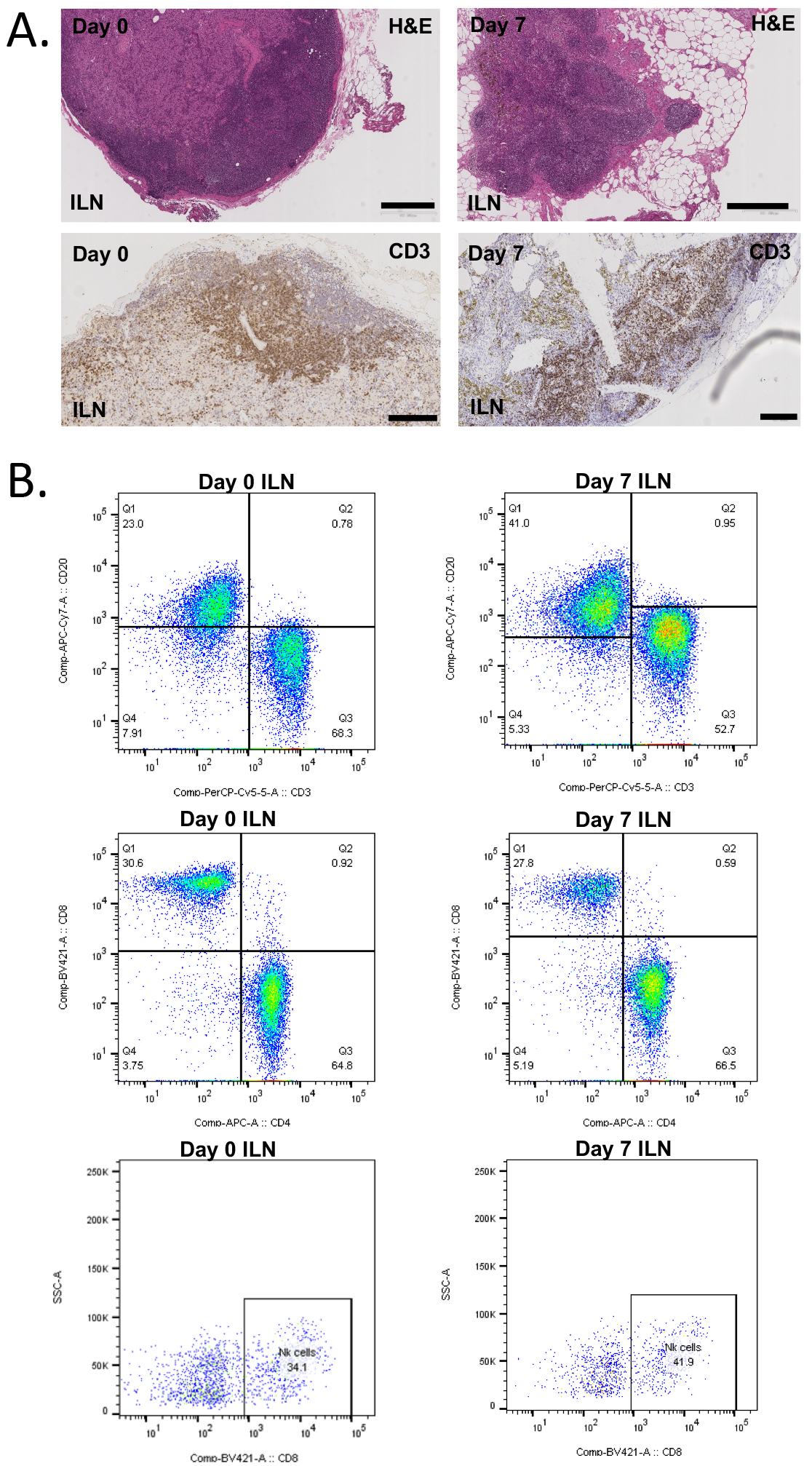
A) Light microscopic analysis showing representative iliac lymph node H&E and CD3 staining, suggesting no significant lymphocyte reduction seven days post-treatment. Bar width, 500 um. B) Polychromatic flow cytometric analysis of lymph nodes showing no major treatment impact on CD3+, CD4+, CD8+, and NK cells subpopulations. Abbreviations: BM, bone marrow; ILN, iliac lymph node.
Mechanistic FcγR-binding and CDC assays
RH-CD2 induced a clear dose-dependent signaling through human FcγRI, FcγRIIA and FcγRIIIA, respectively, although at a lower level than the human anti-CD2 IgG1 reference antibody that was used as a positive control. In contrast, RT-CD2 induced minimal FcγR-mediated signaling (Figure 5 A-C). Both RT-CD2 and RH-CD2 induced significant dose-dependent CDC of lymphocytes (p<0.05) (Figure 5D).
Discussion
Blocking the interaction between the CD2 molecule on T cells and LFA-3 (CD58) on antigen-presenting cells has dual effects, leading to inhibition of T cell proliferation and co-stimulation blockade1,2,14,15. Depending on the structure of anti-CD2 mAbs, they can mediate T- and NK-cell depletion5. The humanized anti-CD2 monoclonal antibody Siplizumab induces lytic antibody-dependent cell-mediated cytotoxicity (ADCC)3,16 via Fc receptor binding. More recently, using quantitative functional analyses and high-throughput T-cell receptor sequencing, we have shown that pre-existing donor-specific Tregs are expanded early in human recipients of combined kidney transplant and bone marrow cell infusion, who received a nonmyeloablative preparative regimen in which Siplizumab was a key component6. In vivo, the enrichment of CD3+CD4+CD25highCD127lowFoxp3+ Tregs in blood constituted almost 75% of the CD3+CD4+ PBMCs at the peak4. These results were further supported by in vitro cellular assays, where the ability of Siplizumab monotherapy to enrich donor-specific Tregs while effectively depleting memory T cells was demonstrated7. This differential response is likely an effect of the lower CD2 expression on human Tregs and naïve cells and higher CD2 cell surface expression on memory lymphocyte subsets7. The current work built on these previous findings about the anti-CD2 effects in humans to determine if similar effects can be generated through intravenous administration of homologous anti-CD2 mAbs in non-human primates.
None of the cynomolgus monkeys dosed with either the RH- or RT-CD2 showed any study drug-related AEs. However, all animals received pre-medication with methylprednisolone and diphenhydramine, potentially masking any first dose effects. The only detectable change in serum chemistries post-dosing was a transient low-grade increase in serum transaminases, which normalized spontaneously without clinically impacting the animals (Figure 1D).
Both anti-CD2 mAbs (RT- and RH-derived) effectively and significantly depleted circulating CD8+ and CD4+ effector memory T cells within 24 hours post-dosing, while relatively sparing naïve cells and Tregs (Figure 1J, 1K, 1M). The depletion profile for memory T cells lasted during at least the studied seven days, and is likely reflected by the high CD2 expression on cynomolgus monkey PBMCs (Figure 1H), which has similarities to the findings on human PBMCs7. The post-dosing CD2 expression analysis was incomplete due to partial competing binding between the treatment (RH-CD2) and detection (CD2 BV786) antibodies (Figure 1I). The low-dose TBI given in Groups B and C is not expected to significantly influence lymphocyte number at the time of anti-CD2 administration. Therefore, the early (24 hours) anti-CD2 depletion profile in Group B-C further supports the effects seen in Group A. However, no dose-response conclusions can be drawn.
Results obtained from MLR assays that compared the effect of titrated doses of RT- and RH-CD2 on the proliferation of stimulated PBMCs confirmed that the in vitro addition of the anti-CD2 mAbs resulted in a concentration-dependent inhibition (Figure 2A–B), suggesting the same effect could occur in vivo. This in vitro inhibition was not as strong as for Siplizumab treatment of human PBMCs in equal dose ranges7.
Histopathological data that compared lymph node samples obtained before and after RH-CD2 treatment (group A) showed no significant changes in cell number, cell type or distribution in lymph nodes (Figure 3). Similarly, thymus and bone marrow samples obtained at the time of sacrifice suggested no significant changes in the cell populations compared to the standard cell distribution in cynomolgus macaques in these tissues17.
The mode of depletion for RT-CD2 is mainly mediated via CDC while RH-CD2 mediated both CDC and FcgR signaling (indicating ADCC/ADCP). However, consideration must be taken towards possible differences in the affinity of the Fc-region of the respective antibodies towards human Fc-receptors used in the assays (Figure 4).
Figure 4. FcγR Reporter Bioassay and flow-cytometry based assessment of CDC.
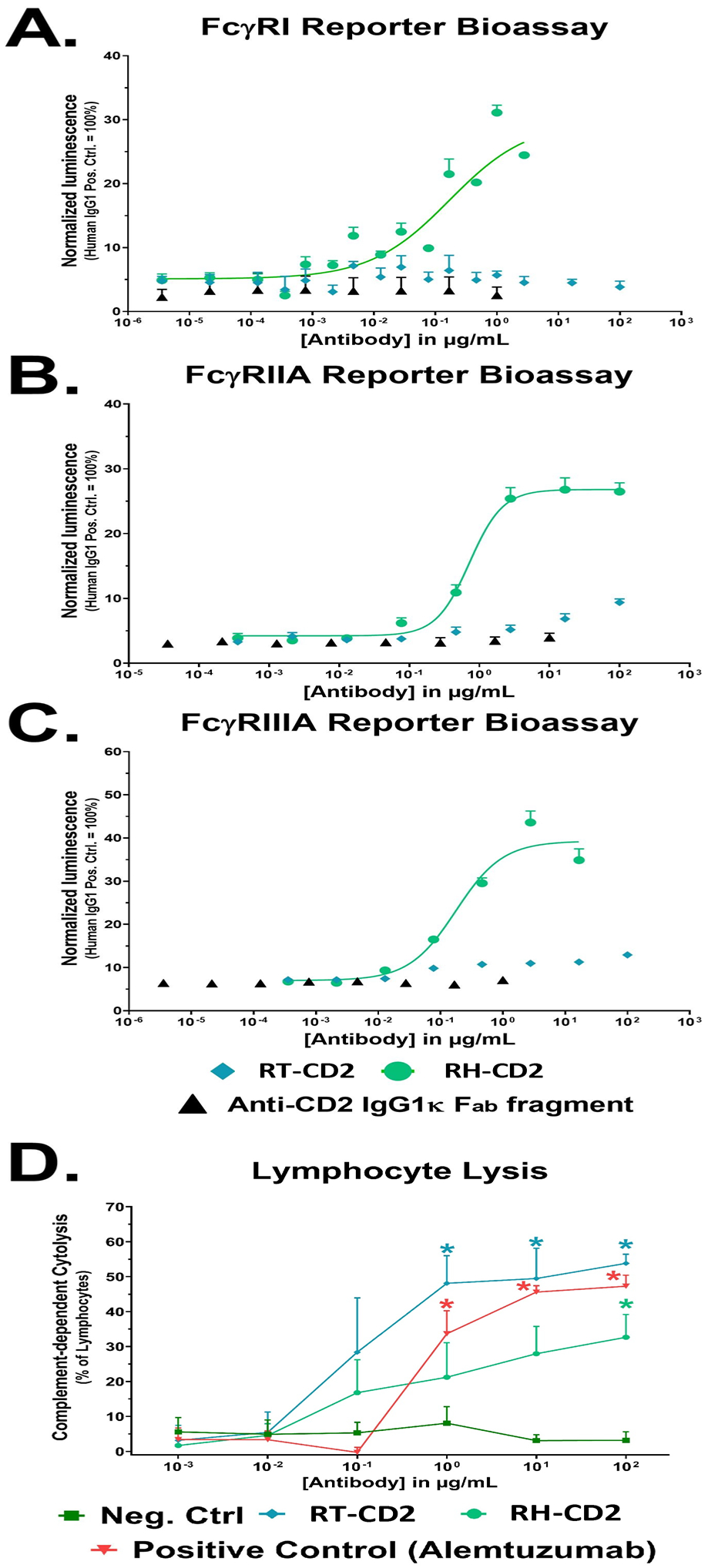
Effector cells stably transfected with A) FcγRI (n=3), B) FcγRIIA (n=3) or C) FcγRIIIA (n=2) and a luciferase reporter gene were incubated with serial dilutions of antibody. Upon binding of a target-bound IgG antibody (the reporter cells expressed CD2) luciferase expression was induced. Luminescence values were normalized to the highest concentration of the positive control. RH-CD2 displayed clear dose-dependent FcγR-mediated signaling but RT-CD2 did not. D) Both antibodies induced dose-dependent CDC of lymphocytes relative to untreated controls (n=4).
Taken together, there is a resemblance of CD2 expression patterns on naive cynomolgus monkeys, rhesus macaques and human PBMCs. The safety and pharmacodynamic profiles are similar with both antibodies, and with no obvious dose-response effects.
Acknowledgements
The study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through Grant Number UL1TR001873. These studies used the resources of the Herbert Irving Comprehensive Cancer Center Flow Cytometry Shared Resources funded in part through Center Grant P30CA013696 and the Diabetes and Endocrinology Research Center Flow Core Facility funded in part through Center Grant 5P30DK063608. Primate recombinant antibody was provided by the NIH Nonhuman Primate Reagent Resource funded through U24AI126683 and R24OD010976. The authors acknowledge Mr. Michael and Mrs. Susan Kerr, Jodie Glickman and the Glickman family for generous support of this work.
Abbreviations
- 7-AAD
7-aminoactinomycin D
- AR
acute rejection
- AEs
adverse events
- APC
antigen presenting cell
- ATG
antithymocyte globulin
- CBC
complete blood cell count
- CD
cluster of differentiation
- CDC
complement-dependent cytolysis
- CM
central memory
- CPM
counts per minute
- EM
effector memory
- FcγR
Fc γ receptor
- i.v
intravenous
- KTx
kidney transplantation
- mAb
monoclonal antibody
- MLR
mixed lymphocyte reaction
- NK cells
natural killer cells
- PBMC
peripheral blood mononuclear cell
- WBC
white blood cell
Footnotes
Disclosure: EB, DHS and DB are shareholders in ITB-MED AB. MS is a scientific advisor to ITB-MED AB. FS, CB are employees of ITB-MED AB. All other authors have no conflict of interest to disclose.
References
- 1.Moingeon P, Chang HC, Wallner BP, Stebbins C, Frey AZ, Reinherz EL. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature. 1989;339(6222): 312–314. [DOI] [PubMed] [Google Scholar]
- 2.Huet S, Wakasugi H, Sterkers G, et al. T cell activation via CD2 [T, gp50]: the role of accessory cells in activating resting T cells via CD2. J Immunol. 1986;137(5): 1420–1428. [PubMed] [Google Scholar]
- 3.Branco L, Barren P, Mao SY, et al. Selective deletion of antigen-specific, activated T cells by a humanized MAB to CD2 (MEDI-507) is mediated by NK cells. Transplantation. 1999;68(10): 1588–1596. [DOI] [PubMed] [Google Scholar]
- 4.Sprangers B, DeWolf S, Savage TM, et al. Origin of Enriched Regulatory T Cells in Patients Receiving Combined Kidney-Bone Marrow Transplantation to Induce Transplantation Tolerance. Am J Transplant. 2017;17(8): 2020–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langley RG, Papp K, Bissonnette R, et al. Safety profile of intravenous and subcutaneous siplizumab, an anti-CD2 monoclonal antibody, for the treatment of plaque psoriasis: results of two randomized, double-blind, placebo-controlled studies. Int J Dermatol. 2010;49(7): 818–828. [DOI] [PubMed] [Google Scholar]
- 6.Savage TM, Shonts BA, Obradovic A, et al. Early expansion of donor-specific Tregs in tolerant kidney transplant recipients. JCI Insight. 2018;3(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podesta MA, Binder C, Sellberg F, et al. Siplizumab selectively depletes effector memory T-cells and promotes a relative expansion of alloreactive regulatory T-cells in vitro. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mourad M, Besse T, Malaise J, et al. BTI-322 for acute rejection after renal transplantation. Transplant Proc. 1997;29(5): 2353. [DOI] [PubMed] [Google Scholar]
- 9.Besse T, Malaise J, Mourad M, et al. Prevention of rejection with BTI-322 after renal transplantation (results at 9 months). Transplant Proc. 1997;29(5): 2425–2426. [DOI] [PubMed] [Google Scholar]
- 10.Pruett TL, McGory RW, Wright FH, Pescovitz MD, Yang H, McClain JB. Safety profile, pharmacokinetics, and pharmacodynamics of siplizumab, a humanized anti-CD2 monoclonal antibody, in renal allograft recipients. Transplant Proc. 2009;41(9): 3655–3661. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4): 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7): 1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damschroder MM, Kozhich AA, Woods RM, et al. Analysis of human and primate CD2 molecules by protein sequence and epitope mapping with anti-human CD2 antibodies. Mol Immunol. 2004;41(10): 985–1000. [DOI] [PubMed] [Google Scholar]
- 14.Bockenstedt LK, Goldsmith MA, Dustin M, Olive D, Springer TA, Weiss A. The CD2 ligand LFA-3 activates T cells but depends on the expression and function of the antigen receptor. J Immunol. 1988;141(6): 1904–1911. [PubMed] [Google Scholar]
- 15.Latinne D, De La Parra B, Nizet Y, et al. An anti-CD2 mAb induces immunosuppression and hyporesponsiveness of CD2+ human T cells in vitro. Int Immunol. 1996;8(7): 1113–1119. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Kolber-Simonds D, Hope JA, et al. The anti-CD2 monoclonal antibody BTI-322 generates unresponsiveness by activation-associated T cell depletion. Clin Exp Immunol. 2004;138(3): 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zitsman JS, Alonso-Guallart P, Ovanez C, et al. Distinctive Leukocyte Subpopulations According to Organ Type in Cynomolgus Macaques. Comp Med. 2016;66(4): 308–323. [PMC free article] [PubMed] [Google Scholar]


