Introduction:
Human papillomavirus (HPV) mediated Head and Neck Squamous Cell Carcinoma (HNSCC) (HPVmHNSCC) is increasing in prevalence in the United States, surpassing cervical cancer as the most common HPV mediated malignancy1,2. HPVmHNSCC is biologically distinct from non-HPV mediated HNSCC, carrying a significantly improved prognosis3,4. As such, much interest is now focused on de-intensification of therapy via transoral surgical resection and dose-reduced radiation for early stage disease. As prevalence rates continue to rise, and HPV status now routinely reported for oropharyngeal tumors across the world, reports have begun to emerge citing a risk of synchronous or metachronous (multiple) HPVmHNSCCs, with single institution reports suggesting rates as high as 10%5. More comprehensive studies aimed at examining this topic have done so by making inferences about HPV status6-8. Second primary malignancy rates in HNSCC have traditionally been reported to be as high as 36%, and have thus influenced how pracitioners choose to work up and monitor patients with HNSCC13. However, it has generally been assumed that patients with HPVmHNSCC are not at risk for second primary malignancies. The prevalence, patient demographics and implications for treatment decision making of this emerging clinical entity are poorly understood. Here, we performed a multi-tiered analysis of patients with multiple HPVmHNSCC including a systematic review of the literature, a query of the 2017 Surveillance, Epidemiology and End Results (SEER) database, and institutional level reporting at two high volume academic centers, to provide a comprehensive view of this emerging clinical entity
Material and Methods:
Systematic Review
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) system was used for the literature review9. We searched the following databases: PubMed/Medline (1946 to November 26, 2018), Embase.com (1974 to November 26, 2018), and Web of Science Core Collection (1900 to November 26, 2018). This search was run on November 26, 2018. Search terms were included with the combination of keywords and controlled vocabulary terms to represent the search concept of “head and neck cancer”, “HPV,” and “multiple cancers” (see Supplemental Data 1 for the full search strategy). We then conducted a search from the following journals: JAMA Otolaryngology – Head and Neck Surgery, Otolaryngology – Head and Neck Surgery, and Head and Neck. We chose the first three head and neck journals from 2017 Scimago Journal & Country Rank (SJR) in the otorhinolaryngology category (Supplemental Data 2). The search was run using PubMed/Medline and Embase.com on February 1, 2019. It was structured to include terms related to the concepts of “head and neck cancer” and “multiple cancers”. We also performed hand searches for the reference lists of selected full text articles on February 13, 2019. All the searches were created and conducted by a reference librarian (SS). No limitations concerning publication language or publication year were applied to any of the searches. We also included abstracts and posters found in the search that had enough information available to be included. Eligibility criteria: patients with multiple HNSCC tumors with an index tumor of the oropharynx, with positive HPV status by p16 or HPV insitu hybridization (ISH), and second primary tumor of any head and neck site, with either positive or unknown HPV status. Study selection: we first screened the titles and abstracts of retrieved articles in an initial selection, read the full text of the selected articles, and excluded articles that did not meet the review eligibility criteria. This was sequentially conducted by one author (WAS) and subsequently checked by a second (DLF). All processes were managed using the online software Covidence. Data extraction: Data was extracted by hand for each individual article. Prevalence rates for a cohort were included when either: (1) prevalence was reported in the manuscript itself or (2) prevalence could be calculated post hoc using the number of cases of HPVmHNSCC in the overarching cohort from which the multiple HPVmHNSCC cases were identified.
SEER database analysis
Patients diagnosed with HNSCC in 2013-2015 (years in which HPV data is available) were identified from the SEER 18 Regs Custom Data Head and Neck database, published in November 2017. Patients with non-malignant tumors, tumors with stages listed as Tis or T0, and with age less than 18 years were excluded. Patients with an HPV positive oropharynx index tumor by p16 or HPV ISH were then selected, followed by identification of those cases with a second HNSCC with either known or unknown HPV status. Because the implications of p16 positivity is less clear outside the oropharynx, we also analyzed the data using only cases in which both tumors were listed as in the Oropharynx. Tumor site and classification were identified using the 3rd edition International Classification of Diseases for Oncology (ICD-O-3) site and behavior codes. Tumor TNM staging and derived overall staging was determined using the American Joint Committee on Cancer (AJCC) 7th edition definitions as this was the staging system in place for the collected data range.
Institutional level data
University of Pittsburgh: the head and neck cancer database was searched for patients with multiple HNSCC tumors with an index tumor of the oropharynx, with positive HPV status by p16 IHC or HPV ISH, and second primary tumor, with either positive or unknown HPV status. Vanderbilt University Medical Center: the Vanderbilt Cancer Registry was searched from 1/1/2000-12/1/2018 for patients with more than one head and neck cancer and then filtered to patients with at least one oropharyngeal cancer. HPV status was tested by p16 IHC. Missing information was filled in by hand search of the medical record.
HPV Status
HPV status was determined by either p16 immunohistochemistry (IHC), HPV DNA ISH or HPV RNA ISH in all datasets.
Statistical Analysis
Patient and tumor characteristics were compared by using Fisher’s exact tests for non-parametric categorical data, chi-squared tests for parametric categorical data, and unpaired t tests for parametric numerical data, with a p-value <0.05 considered to be significant for each. A sign test was used for determining likelihood of tumor laterality between pairs, again with a p-value <0.05 considered to be significant. Log-rank tests were used to compare survival.
Results:
Systematic Literature Review
After screening, 13 articles were identified that reported on multiple HPVmHNSCCs with at least one HPVmHNSCC located in the oropharynx, from which 48 cases were identified (Figure 1, Table 1). In 33 cases HPV status was positive by p16 staining or HPV ISH in both tumors and in 15 cases the second tumor had unknown HPV status. All reports were either single institution or multi-institution case reports and series. The number of reported cases per article ranged from 1-11 (2-22 cancers). Two out of 13 reports, with confirmed HPV status for both cancers, had population information that allowed calculation of prevalence rates which ranged from 2.34% to 2.50% with a mean of 2.42% and pooled prevalence of 2.37%. By assuming that the second tumor with unknown HPV status is actually HPV mediated, six additional studies had population information that allowed calculation of multiple HPVmHNSCC prevalence rates. Prevalence in this group ranged from 2.11% to 10.0% with a mean of 3.67% and pooled prevalence of 2.64%.
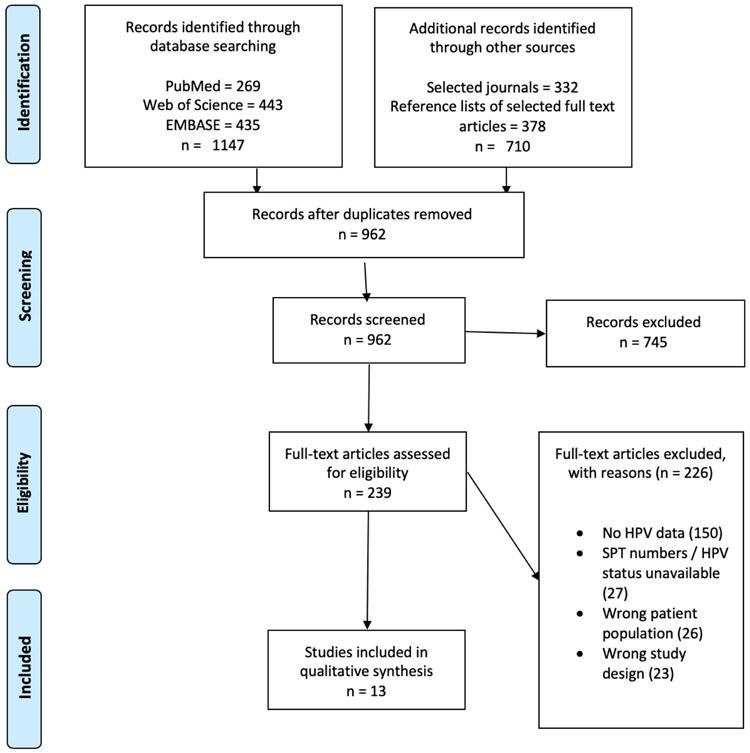
Figure 1. PRISMA diagram for systematic literature review.
After removal of duplicates, 962 reports were initially identified and screened, resulting in 239 full texts available for assessment of eligibility. After full text screening, 13 manuscripts met inclusion criteria.
Table 1. Multiple HPVmHNSCC Identified by Systematic Review.
13 manuscripts were identified for inclusion with 33 pooled cases with confirmed multiple HPVmHNSCC and 48 cases of a first HPVmHNSCC with a second tumor that was either HPV mediated or had unknown HPV status5,14-25. The column titled "Multiple HPVmHNSCCs" refers to the number of cases in which patients had two HPVmHNSCCs, such that the HPV status of both primary tumors was known to be HPV-positive. The column titled "Multiple HPVmHNSCCs w/known or unknown status" refers to the number of cases in which patients had two primary tumors where the index tumor is known to be HPV-positive, but the HPV status of the second primary is either positive or unknown. The column titled "HPVmHNSCC cohort evaluated" refers to the number of cases of index HPVmHNSCC in the overall cohort from which second HPVmHNSCCs were evaluated for. Prevalence of multiple HPVmHNSCC was calculated when: 1. all second primary tumors were confirmed to be HPV-positive and 2. the number of patients in the overall cohort was known.
| Study | Journal | Multiple HPVmHNSCCs |
Multiple primaries w/known or unknown HPV status |
HPVmHNSCC cohort evaluated |
Multiple HPVmHNSCC prevalence (%) |
Multiple primaries prevalence (%) |
|---|---|---|---|---|---|---|
| Baumeister et al. 2014 | J. Cancer Res. Clin. Oncol. | 1 | 1 | 40 | 2.50 | 2.50 |
| Sinha et al. 2018 | The Laryngoscope | 4 | 4 | 171 | 2.34 | 2.34 |
| Evans et al. 2013 | BMC cancer | 2 | 3 | 69 | n/a | 4.35 |
| Huang et al. 2012 | Int. J. Radiat. Oncol. Biol. Phys. | 4 | 6 | 236 | n/a | 2.54 |
| Sims et al. 2017 | Otolaryngol. Head Neck Surg. | 4 | 8 | 379 | n/a | 2.11 |
| Caley et al. 2015 | Head Neck-J. Sci. Spec. Head Neck | 11 | 11 | not reported | n/a | n/a |
| Joseph et al. 2013 | Oral oncology | 4 | 4 | not reported | n/a | n/a |
| Nakahara et al. 2014 | Auris, nasus, larynx | 1 | 1 | not reported | n/a | n/a |
| Rasband-Lindquist et al. 2016 | Ear, Nose, & Throat Journal | 1 | 1 | not reported | n/a | n/a |
| Roeser et al. 2010 | The Laryngoscope | 1 | 1 | not reported | n/a | n/a |
| Dziegielewski et al. 2017 | Head Neck | not reported | 2 | 20 | n/a | 10.00 |
| Maxwell JH et al. 2010 | Clin Cancer Res | not reported | 3 | 102 | n/a | 2.94 |
| Xu et al. 2013 | J. Otolaryngol-Head Neck Surg. | not reported | 3 | 118 | n/a | 2.54 |
| Total | 33 | 48 | 1135 | |||
| Average | 2.42 | 3.67 | ||||
| Pooled Average | 2.37 | 2.64 |
SEER Database
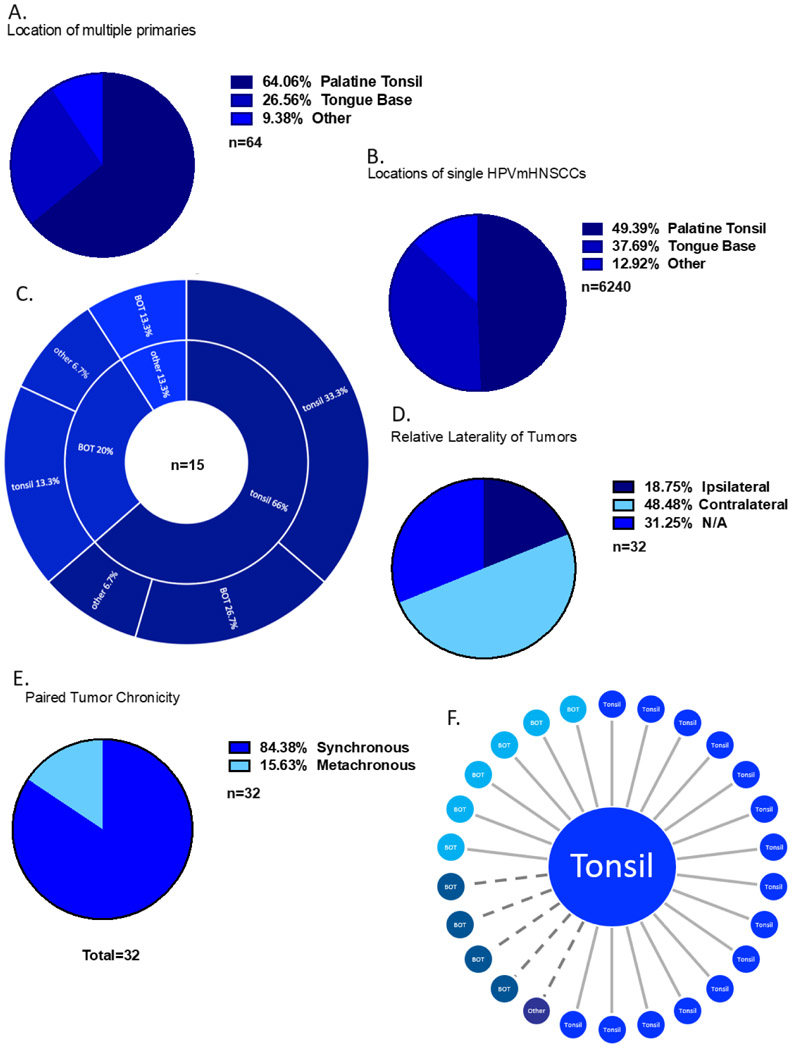
Query of the SEER database from 2013-2015 (the years in which HPV data is available) revealed 6,374 HPVmHNSSCs. Thirty-two (0.51%) of the patients with HPVmHNSCC had two primary HPVmHNSCCs with at least one tumor in the oropharynx (Figures 2, 3, Table 2). We also analyzed the data using more stringent criteria in which both tumors had to be listed as oropharynx, revealing 26 (0.41%) cases (Supplemental Figures 1, 2, Supplemental Table 1). For the remainder of this section, we present the data using the first definition, due to the larger sample size. The second, more stringent cohort is presented in brackets ( [ *** ] ). In instances where statistical significance was different between these cohorts, this is highlighted in the text. Sixty (0.95%) [46 (0.73%)] patients with HPVmHNSCC had a second primary tumor that was either HPV mediated or had unknown HPV status, suggesting the prevalence in this database is between 0.51-0.95% [0.41-0.73%]. Demographic data from multiple HPVmHNSCC cases can be found in Table 2 and Supplemental Table 1. The mean age of first tumor diagnosis was found to be significantly lower in patients with multiple HPVmHNSCCs compared to single HPVmHNSCCs (56.7 vs 60.6 years old, p=0.025) [56.6 vs 60.6 years old, p=0.036]. Patients with multiple HPVmHNSCCs were more likely to present with T1 tumors, no neck adenopathy (N0) and stage 1 disease (p=0.001 for T1 and stage 1, p=0.003 for N0) [p=0.001 for T1 and stage 1, p=0.004 for N0]. They were less likely to have stage IV disease (p=0.001) [p=0.002]. Sex and ethnicity were not statistically different between patients with multiple and single HPVmHNSCCs nor were the proportion of patients who received radiotherapy or chemotherapy. Reported cases of multiple HPVmHNSCCs occurred in the palatine tonsils (64.1%) [76.9%], tongue base (26.6%) [23.1%], and other sites (9.38%) [0%] compared to palatine tonsils (49.4%), tongue base (37.7%), and other sites (12.9%) in the single HPVmHNSCC cohort (Figure 3A-B, Supplemental Figure 2A-B). Cases of multiple HPVmHNSCC were more likely to occur in the tonsil than in the HPVmHNSCC cohort overall (p=0.023) [p=0.001]. If a tumor occurred in the tonsil, the most likely site for the other tumor was the contralateral tonsil (Figure 3C, 3F, Supplemental Figure 2C).
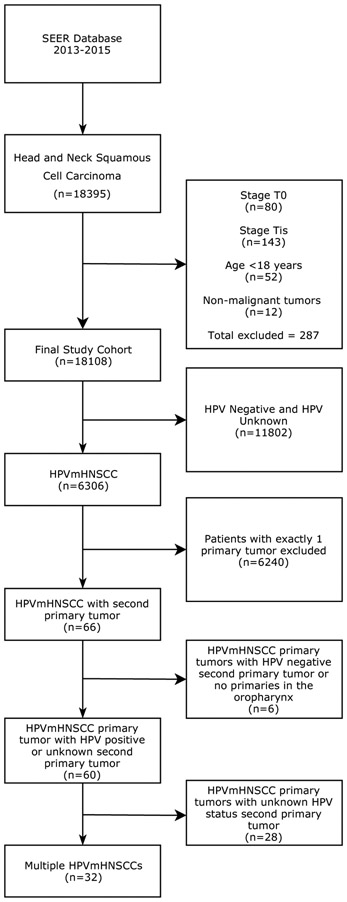
Figure 2. SEER database flow chart.
18,395 cases of HNSCC were identified in SEER version 18. After filtering, 32 patients with confirmed multiple HPVmHNSCCs with at least one located in the oropharynx were identified. An additional 28 patients with HPVmHNSCC had a second tumor that was either HPV mediated or had unknown HPV status.
Figure 3. Multiple HPVmHNSCC with at least one primary in the oropharynx from SEER database.
A) Locations of multiple HPVmHNSCC. “Other” refers to tumors located in the hypopharynx, nasopharynx, or oral cavity and not otherwise included in the first two categories. B) Locations of single HPVmHNSCCs. C) Locations of multiple HPVmHNSCC that were diagnosed at different timepoints. The inner ring corresponds with the first diagnosed primary, and the outer ring corresponds with the second diagnosed primary. D) Tumor laterality. E) Frequency of synchronous tumors (defined as diagnosis within 6 months) compared to metachronous tumors (defined as diagnosis more than 6 months apart). F) Sites of second tumors after a first primary located in the tonsil from SEER. Solid line represents tumors identified synchronously and dotted line represents tumors identified metachronously.
Table 2. Multiple HPVmHNSCC Identified from SEER database.
Patients with multiple HPVmHNSCC with at least one tumor located in the oropharynx were found tc be more likely to present with early T, N and overall stage than single HPVmHNSCCs. When two numbers are included, separated with a "/". The first number represents the number of patients, and the second number represents the number of tumors.
| HNSCC (n=18075/18108) |
HPVmHNSCC (n=6273/6306) |
Single HPVmHNSCC (n=6240) |
HPVmHNSCCs with second primary (HPV status positive or unknown) (n=60/120) |
Multiple HPVmHNSCCs (n=32/64) |
Single vs multiple HPVmHNSCC p-values |
|
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 14575 (80.6) | 5408 (86.2) | 5377 (86.2) | 54 (90) | 29 (90.6) | 0.6123 |
| Female | 3501 (19.4) | 866 (13.8) | 863 (13.8) | 6 (10) | 3 (9.4) | 0.6123 |
| Age | ||||||
| mean | 62.0 | 60.6 | 60.6 | 59.9 | 56.7 | 0.0249 |
| median | 61.0 | 60.0 | 60 | 59.0 | 55.5 | n/a |
| range | 18-103 | 18-95 | 18-95 | 39-82 | 39-81 | n/a |
| Race | ||||||
| White | 14804 (81.9) | 5646 (90) | 5616 (90) | 55 (91.7) | 28 (87.5) | 0.5558 |
| Black | 1771 (9.8) | 338 (5.4) | 335 (5.4) | 4 (6.7) | 3 (9.4) | 0.2467 |
| Japanese | 96 (0.5) | 26 (0.4) | 25 (0.4) | 1 (1.7) | 1 (3.1) | 0.1284 |
| Other | 1299 (7.2) | 231 (3.7) | 231 (3.7) | 0 (0) | 0 (0) | 0.6334 |
| Unknown | 106 (0.6) | 33 (0.5) | 33 (0.5) | 0 (0) | 0 (0) | 1 |
| T stage | ||||||
| T1 | 3823 (21.1) | 1586 (25.2) | 1551 (24.9) | 49 (40.8) | 34 (53.1) | 0.0001 |
| T2 | 5237 (28.9) | 2286 (36.3) | 2271 (36.4) | 27 (22.5) | 14 (21.9) | 0.018 |
| T3 | 2910 (16.1) | 968 (15.4) | 962 (15.4) | 10 (8.3) | 6 (9.4) | 0.223 |
| T4 | 3058 (16.9) | 794 (12.6) | 789 (12.6) | 13 (10.8) | 5 (7.8) | 0.3417 |
| Unknown | 3080 (17) | 672 (10.7) | 667 (10.7) | 21 (17.5) | 5 (7.8) | 0.6818 |
| N stage | ||||||
| N0 | 3711 (20.5) | 864 (13.8) | 854 (13.7) | 20 (33.3) | 11 (34.4) | 0.0026 |
| N1 | 3233 (17.9) | 1136 (18.1) | 1130 (18.1) | 7 (11.7) | 5 (15.6) | 1 |
| N2 | 8725 (48.3) | 3831 (61.1) | 3816 (61.2) | 26 (43.3) | 14 (43.8) | 0.0673 |
| N3 | 920 (5.1) | 291 (4.6) | 289 (4.6) | 5 (8.3) | 2 (6.3) | 0.6591 |
| Unknown | 1486 (8.2) | 151 (2.4) | 151 (2.4) | 2 (3.3) | 0 (0) | 1 |
| M stage | ||||||
| M0 | 16277 (90.1) | 5991 (95.5) | 5959 (95.5) | 58 (96.7) | 31 (96.9) | 1 |
| M1 | 925 (5.1) | 179 (2.9) | 178 (2.9) | 2 (3.3) | 1 (3.1) | 0.6049 |
| Unknown | 873 (4.8) | 103 (1.6) | 103 (1.7) | 0 (0) | 0 (0) | 1 |
| AJCC Stage | ||||||
| I | 989 (5.5) | 202 (3.2) | 187 (3) | 24 (20) | 14 (21.9) | 0.0001 |
| II | 1330 (7.3) | 355 (5.6) | 348 (5.6) | 12 (10) | 7 (10.9) | 0.0908 |
| III | 2851 (15.7) | 1059 (16.8) | 1049 (16.8) | 12 (10) | 10 (15.6) | 1 |
| IV | 10729 (59.3) | 4357 (69.1) | 4327 (69.3) | 54 (45) | 29 (45.3) | 0.0001 |
| Unknown | 2209 (12.2) | 333 (5.3) | 329 (5.3) | 18 (15) | 4 (6.3) | 0.5803 |
| Chronicity | ||||||
| Synchronous | n/a | n/a | n/a | 46 (76.7) | 27 (84.4) | n/a |
| Metachronous | n/a | n/a | n/a | 14 (23.3) | 5 (15.6) | n/a |
Of the 32 [26] pairs of multiple HPVmHNSCC with confirmed HPV status, six [three] were found on the same side of the oropharynx (ipsilateral), 16 [15] were contralateral, and 10 [8] could not be categorized due to missing data or midline sites (Figure 3D, Supplemental Figure 2D). The second primary tumor was 2.7x more likely to be found on the contralateral side compared to the first lesion (p=0.052) [5x, p=0.008]. 84.4% [88.5%] of the tumor pairs were synchronous (Figure 3E, Supplemental Figure 2E). Synchronous tumors were more likely to have the same grade compared to metachronous tumors (p=0.13) [p=0.24]. Tumor size ranged from 4-84mm [4-84mm], with an average tumor size of 22mm [22mm]. The second diagnosed tumor from a pair was smaller on average than the first diagnosed tumor (1.8cm vs 2.5cm) [1.5cm vs 2.5cm] but this difference did not meet statistical significance (p=0.14) [p=0.0325]. There was no difference in 2-year overall survival between cases of multiple HPVmHNSCC and single HPVmHNSCC (84.4% vs 85.7%, p=0.969) (Supplemental Figure 3).
Institutional Data
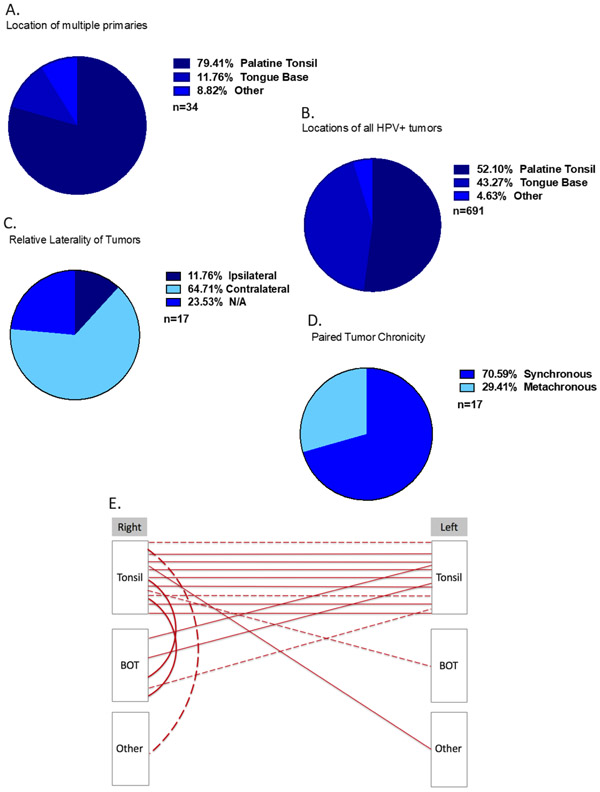
Twelve patients at the University of Pittsburgh were identified with multiple HPVmHNSCCs, constituting 1.69% of the 708 patients with HPVmHNSCC from 2000-2018. Similar to the SEER database, patients with multiple HPVmHNSCC were more likely to present with a T1 tumor (p=0.007) (Table 3). Between 2007-2018 five patients at Vanderbilt University were identified with multiple HPVmHNSCC, representing 0.55% of patients with OPSCC (916 total) in this timeframe (Table 3). The HPV status of all cases was not available at Vanderbilt for the complete time frame and thus prevalence could not be calculated. When examining the pooled Pittsburgh and Vanderbilt data, similar to single HPVmHNSCC, patients with multiple HPVmHNSCCs were most commonly white (100%), male (77%), and had a mean age of presentation of 54 years old. Cases of multiple HPVmHNSCCs occurred in the palatine tonsils (79.4%), tongue base (11.8%), and other sites (8.8%), compared to palatine tonsils (52.1%), tongue base (43.3%), and other sites (4.6%) in the single HPVmHNSCC cohort (Figure 4A-B). Second primary HPVmHNSCCs were more likely to occur in the contralateral tonsil (p=0.002) (Figure 4E).
Table 3. Institutional HPVmHNSCC Patient characteristics.
17 patients were identified at the University of Pittsburgh and Vanderbilt University with confirmed multiple HPVmHNSCCs. When comparing single vs multiple HPVmHNSCC at the University of Pittsburgh, similar to the SEER data, patients with multiple HPVmHNSCCs were more likely to present with early T stage.
| Pittsburgh Single HPVmHNSCC (n=691) |
Pittsburgh Multiple HPVmHNSCC (n=12) |
Vanderbilt Multiple HPVmHNSCC (n=5) |
Pooled Pittsburgh and Vanderbilt Multiple HPVmHNSCC (n=17) |
Pitt Single vs Multiple HPVmHNSCC p-values |
|
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Sex | |||||
| Male | 600 (86.8) | 9 (75) | 4 (80) | 13 (76.5) | 0.2084 |
| Female | 91 (13.2) | 3 (25) | 1 (20) | 4 (23.5) | 0.2084 |
| Age | |||||
| Mean | 57.4 | 53.8 | 52.6 | 53.8 | 0.1866 |
| Median | 57 | 54.5 | 52 | 52 | n/a |
| Range | 21-90 | 36-69 | 44-61 | 36-69 | n/a |
| Race | |||||
| White | 656 (94.9) | 12 (100) | 5 (100) | 17 (100) | 1 |
| Black | 26 (3.8) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Japanese | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Other | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Unknown | 7 (1) | 0 (0) | 0 (0) | 0 (0) | 1 |
| T Stage | |||||
| T1 | 232 (33.6) | 15 (62.5) | 5 (50) | 20 (58.8) | 0.0072 |
| T2 | 275 (39.8) | 5 (20.8) | 3 (30) | 8 (23.5) | 0.0869 |
| T3 | 65 (9.4) | 1 (4.2) | 1 (10) | 2 (5.9) | 0.7165 |
| T4 | 63 (9.1) | 1 (4.2) | 1 (10) | 2 (5.9) | 0.7145 |
| Unknown | 56 (8.1) | 2 (8.3) | 0 (0) | 2 (5.9) | 1 |
| N stage | |||||
| N0 | 81 (11.7) | 3 (25) | 1 (20) | 4 (23.5) | 0.1637 |
| N1 | 136 (19.7) | 4 (33.3) | 2 (40) | 6 (35.3) | 0.2696 |
| N2 | 439 (63.5) | 5 (41.7) | 2 (40) | 7 (41.2) | 0.1376 |
| N3 | 28 (4.1) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Unknown | 7 (1) | 0 (0) | 0 (0) | 0 (0) | 1 |
| M stage | |||||
| M0 | 658 (95.2) | 12 (100) | 5 (100) | 17 (100) | 1 |
| M1 | 13 (1.9) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Unknown | 20 (2.9) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Chemotherapy | |||||
| Yes | 446 (64.5) | 8 (66.7) | 5 (100) | 13 (76.5) | 1 |
| No/unknown | 245 (35.5) | 4 (33.3) | 0 (0) | 4 (23.5) | 1 |
| Radiotherapy | |||||
| Yes | 585 (84.7) | 10 (83.3) | 4 (80) | 14 (82.4) | 1 |
| No/unknown | 106 (15.3) | 2 (16.7) | 1 (20) | 3 (17.6) | 1 |
| Chronicity | |||||
| Synchronous | n/a | 9 (75) | 3 (60) | 12 (70.6) | n/a |
| Metachronous | n/a | 3 (25) | 2 (40) | 5 (29.4) | n/a |
| Smoking history | |||||
| Yes | 476 (68.9) | 8 (66.7) | 1 (20) | 9 (52.9) | 1 |
| No/unknown | 215 (31.1) | 4 (33.3) | 4 (80) | 8 (47.1) | 1 |
Figure 4. Multiple HPVmHNSCCs from Institutional Data.
A) Locations of multiple HPVmHNSCC. “Other” refers to tumors located in the oropharynx, nasopharynx, or palate and not otherwise included in the first two categories. B) Locations of single HPVmHNSCC. C) Tumor laterality. D) Frequency of synchronous tumors (defined as diagnosis within 6 months) compared to metachronous tumors (defined as diagnosis more than 6 months apart). E) Sites of paired multiple HPVmHNSCC from institutional data. Solid line represents tumors identified synchronously and dotted line represents tumors identified metachronously.
Of the 17 pairs of multiple HPVmHNSCC primaries, 4 were found to be ipsilateral and 12 were contralateral (Figure 4C) (p=0.038). 70.6% of the tumor pairs were synchronous (Figure 4D). Tumor size ranged from 2-47mm, with an average tumor size of 20.9mm. The second diagnosed tumor from a pair was smaller on average than the first diagnosed tumor (2.2cm vs 3.7cm) but this difference was not significant (p=0.17).
Survival was also compared between the two groups (Supplemental Figure 4). 2-year and 5-year survival for patients with multiple HPVmHNSCCs were both found to be 90.9%. 2-year and 5-year survival for patients with single HPVmHNSCCs was found to be 88.7% and 78.2% respectively. The difference in 5-year overall survival (90.9% vs 78.2%) was not significant (p=0.454). Of the patients with multiple HPVmHNSCCs, two patients had local recurrence and two patients had distant metastases.
Discussion:
HPVmHNSCC is etiologically, epidemiologically, prognostically, and biologically distinct from non-HPVmHNSCC. Unlike non-HPVmHNSCC, HPVmHNSCC arises from a complex interplay of viral- and host-induced genomic alterations, is increasing in prevalence world-wide, portends improved survival and treatment response, and has distinct growth and metastasis patterns3,10,11. Due in part to the recent emergence of HPVmHNSCC, there is a paucity of basic information on how HPVmHNSCCs originate, grow and behave.
Traditionally, the risk of second primary malignancy in HNSCC is reported to be ~14%, although some metanalyses demonstrate 20-year cumulative prevalence rates as high as 36%12,13. Contrary to traditional HNSCC, patients with HPVmHNSCC are assumed to not be at risk for a second primary malignancy. This concept has begun to factor into emerging practice patterns; for example, some head and neck surgeons now choose not to perform bronchoscopy and esophagoscopy in HPVmHNSCC prior to treatment due to the lack of “field cancerization”. However, single institution reports have emerged of patients with multiple HPVmHNSCCs, challenging this assumption. Here, using a multi-tiered analysis, we examined the prevalence and demographics of multiple HPVmHNSCCs.
Combining a systematic literature review, query of the SEER database and institutional level reporting, we found the prevalence of multiple confirmed HPVmHNSCCs ranged from 0.51%-2.5% of HPVmHNSCC cases. If we allowed the second tumor to be either HPV mediated or have unknown HPV status, the prevalence ranged from 0.95%-10%. Of note, the 10% reported by Dziegielewski et al is more than double the next highest prevelence of 4.35% and thus, is an outlier. The pooled prevalence, using all datasets was 0.70% for cases with confirmed HPV-positive second primaries and 1.27% for either positive or unconfirmed second cases. These prevalence rates should be interpreted with caution. Prevalence rates from the systematic literature review are plagued by small sample sizes, non-standardized work up and detection techniques, and variable HPV status reporting. Database review (SEER and Institutional level reporting) similarly likely underestimate the actual prevalence of multiple HPVmHNSCC for the same reasons. Further, established treatments may unknowingly treat small synchronous primary tumors that go forever undetected. For example, most patients with HPVmHNSCC receive either primary, or adjuvant radiation to the oropharynx, which may eradicate small second primary tumors without ever being identified. In patients treated with surgery, “uninvolved” oropharyngeal subsites are frequently resected. For example, the contralateral tonsil may be removed in the setting of an ipsilateral tonsil cancer, or a multi-subsite oropharyngectomy may be performed as part of an unknown primary work up. In this situation pathologic workup may stop, or become less rigorous, once the first tumor is identified, resulting in unknowing surgical resection of a small second tumor. This concept fits with our data, showing that the second identified tumor, on average, is smaller in size than the first. Prospective, well-controlled, and pathologically rigorous studies are be needed to more accurately address true prevalence rates.
If second HPVmHNSCCs are so easily missed, or treated as part of the standard management of the first tumor, is their presence clinically revelant? Treatment of HPVmHNSCC is shifting to “de-intensification” regimens including unilateral transoral surgery with no adjuvant treatment and unilateral radiation fields, for early stage disease. These protocols are based on the premise that most HPVmHNSCC patients respond better to treatment and live longer after treatment due to their younger age. Assuming de-intensifcation trends continue, we would predict an increase in the identification of multiple HPVmHNSCCs, as more cases go “incidentally un-treated”. This increase would be independent of the increase in multiple HPVmHNSCCs which is occurring due to the continued rising prevalence rates of HPVmHNSCCs overall.
The tonsil is the most likely location to develop an HPVmHNSCC (Figure 3B, Supplemental Figure 2B). Similarly in this cohort, the most common site to develop a second HPVmHNSCC was the contralateral tonsil (Figure 3A, Supplemental Figure 2A). Interestingly, the chance of a tumor from a patient with multiple primary tumors occurring in the tonsil, as opposed to the tongue base, was elevated even above the risk of tonsil being the primary tumor location in patients with just one HPVmHNSCC (p=0.023 for SEER data and p=0.0018 for institutional data). One hypothesis for the higher rate of second primary tonsil cancers compared to the prevalence of HPV mediated tonsil cancers overall, is that in patients who undergo definitive radiation for a tonsil cancer, radiation fields are more likely to cover and treat the tongue base at suffient dosing to eradicate a small incidental second tongue base second malignancy, while undertreating a contralateral tonsil malignancy. A second hypothesis is that this ratio reflects a surgical discovery bias. For example, some surgeons perform elective contralateral tonsillectomy in the setting of definitive transoral surgical treatment for a tonsil HPVmHNSCC. This would again shift the rate of tonsil to tongue base cancers as the tonsil tissue would be pathologically interograted while the tongue base would not. Thus, whether the contralateral tonsil is indeed the most common location to develop a second cancer, or simply is a reflection of treatment and discovery effects, remains to be seen.
How patients develop two HPVmHNSCCs remains an unanswered question. Various theories have been suggested to explain this phenomenon including: 1. a single viral infection resulting in infection of cells at more than one anatomic location via intra-host spread (lympahtic, hematogenous, or salivary) 2. infection of different tissue locations with different viruses, 3. infection of different tissue locations with the same virus at the same time, or on subsequent exposure, and 4. intralymphatic intraoropharyngeal metastases of tumor cells14. Joseph et al reported viral concordance between four pairs of HPV tonsil cancers using viral E6 sequencing, suggesting theories 1, 3, or 4. However, while viral sublineage can be inferred through E6 sequencing, sequencing of just the E6 exon does not prove the virus at both sites is indeed indentical, and in fact, the majority of HPV genome SNPs occurs outside of the E6 gene. Interestingly, in our dataset, tumors that presented synchronously were more likely to be the same grade than tumors that were metachronous. One could then hypothesize that the same virus may be causative in synchronous cases but perhaps different viruses play a role in metachronous cases. More in-depth studies of the viral genome and tumor genome are needed to answer this question.
Why certain patients develop multiple HPVmHNSCCs also remains unknown. Both patient-specific (genetic and immunologic) and virus-specific (such as particularly aggressive sublineages) could play a role. Here, patients with multiple HPVmHNSCCs were more likely to be younger than patients with a single HPVmHNSCC (SEER database: 56.7 vs 60.6 (p=0.025), institutional data: 53.8 vs 57.4 (p=0.187)), have a small (T1) tumor (SEER database: 53% vs 25% (p=0.0001), institutional data: 59% vs 34% (p=0.007)) and have no neck adenopathy (N0) (SEER database: 34% vs 14% (p=0.003), institutional data: 24% vs 12% (p=0.164)). This suggests that patients with multiple HPVmHNSCCs may have a biologically distinct process from patients with one tumor.
In light of emerging treatment schemes focused on de-intensification and unilateral treatments, prospective translational studies are needed to better understand the true prevalence of multiple HPVmHNSCC and the genomic and immunologic underpinnings of this phenomenon. In the meantime, thorough interrogation of all oropharyngeal subsites should be performed as part of the initial workup for HPVmHNSCC. Consideration should be given to contralateral tonsillectomy at the time of surgical resection for an HPV mediated tonsil cancer, as it is the most common location for synchronously identified tumors.
Supplementary Material
References
- 1.SEER: Cervical Cancer - Cancer Stat Facts.
- 2.Mourad M, Jetmore T, Jategaonkar AA, et al. : Epidemiological Trends of Head and Neck Cancer in the United States: A SEER Population Study. J Oral Maxillofac Surg 75:2562–2572, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. : Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillison ML, Akagi K, Xiao W, et al. : Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res 29:1–17, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dziegielewski PT, Boyce BJ, Old M, et al. : Transoral robotic surgery for tonsillar cancer: Addressing the contralateral tonsil. Head Neck 39:2224–2231, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Diaz DARI, Weed DT, Elsayyad N, Samuels M, Abramowitz MC: Head and neck second primary cancer rates in the human papillomavirus era: A population-based analysis. Head & Neck 38:E873–83, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Jain KSSA, Baxi SS, Morris LG: Synchronous cancers in patients with head and neck cancer. Cancer 119:1832–1837, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Morris LGSA, Patel SG, Hayes RB, Ganly I: Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. . Clin. Oncol. 29:739–46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A. Liberati DGA, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med., 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faden DL, Thomas S, Cantalupo PG, et al. : Multi-modality analysis supports APOBEC as a major source of mutations in head and neck squamous cell carcinoma. Oral Oncol 74:8–14, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. : Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haughey BH, Gates GA, Arfken CL, et al. : Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol 101:105–12, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Chuang SC, Scelo G, Tonita JM, et al. : Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer 123:2390–6, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Joseph AW, Ogawa T, Bishop JA, et al. : Molecular etiology of second primary tumors in contralateral tonsils of human papillomavirus-associated index tonsillar carcinomas. Oral Oncol 49:244–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumeister P, Reiter M, Welz C, et al. : Surgically treated oropharyngeal cancer: risk factors and tumor characteristics. J Cancer Res Clin Oncol 140:1011–9, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Sinha P, Haughey BH, Kallogjeri D, et al. : Long-term analysis of transorally resected p16 + Oropharynx cancer: Outcomes and prognostic factors. Laryngoscope 129:1141–1149, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Evans M, Newcombe R, Fiander A, et al. : Human Papillomavirus-associated oropharyngeal cancer: an observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer 13:220, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang SH, Perez-Ordonez B, Liu FF, et al. : Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 82:276–83, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Sims JR, Van Abel K, Price DL, et al. : Second Primaries after Curative Treatment of Human Papillomavirus-Positive Oropharyngeal Squamous Cell Carcinoma. Otolaryngology - Head and Neck Surgery 157, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Caley A, Evans M, Powell N, et al. : Multicentric human papillomavirus-associated head and neck squamous cell carcinoma. Head Neck 37:202–8, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Nakahara S, Yasui T, Takenaka Y, et al. : Synchronous bilateral tonsillar carcinomas associated with human papillomavirus. Auris Nasus Larynx 41:109–12, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Rasband-Lindquist A, Shnayder Y, O’Neil M: Synchronous bilateral tonsillar squamous cell carcinoma related to human papillomavirus: Two case reports and a brief review of the literature. Ear Nose Throat J 95:E30–4, 2016 [PubMed] [Google Scholar]
- 23.Roeser MM, Alon EE, Olsen KD, et al. : Synchronous bilateral tonsil squamous cell carcinoma. Laryngoscope 120 Suppl 4:S181, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Maxwell JH, Kumar B, Feng FY, et al. : Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res 16:1226–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu CC, Biron VL, Puttagunta L, et al. : HPV status and second primary tumours in oropharyngeal squamous cell carcinoma. J Otolaryngol Head Neck Surg 42:36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.