Abstract
The overexpression of one or multiple ATP-binding cassette (ABC) transporters such as ABCB1, ABCC1 or ABCG2 in cancer cells often leads to the development of multidrug resistance phenotype and consequent treatment failure. Therefore, these transporters constitute an important target to improve the therapeutic outcome in cancer patients. In this study, we employed a drug repurposing approach to identify MY-5445, a known phosphodiesterase type 5 inhibitor, as a selective modulator of ABCG2. We discovered that by inhibiting the drug transport function of ABCG2, MY-5445 potentiates drug-induced apoptosis in ABCG2-overexpressing multidrug-resistant cancer cells and resensitizes these cells to chemotherapeutic drugs. Our data of MY-5445 stimulating the ATPase activity of ABCG2 and molecular docking analysis of its binding to the substrate-binding pocket of ABCG2 provide additional insight into the manner in which MY-5445 interacts with ABCG2. Furthermore, we found that ABCG2 does not confer resistance to MY-5445 in human cancer cells. Overall, our study revealed an additional action of MY-5445 to resensitize ABCG2-overexpressing multidrug-resistant cancer cells to conventional anticancer drugs, and this should be evaluated in future drug combination trials.
Keywords: Multidrug resistance, ABCG2, modulator, phosphodiesterase, MY-5445
Introduction
The growing phenomenon of multidrug resistance (MDR), caused by the overexpression of one or multiple ATP-binding cassette (ABC) transporters in cancer cells threatens the success of chemotherapy [1-3]. Collectively, they can utilize the energy of ATP hydrolysis to efflux a wide variety of structurally unrelated conventional anticancer drugs, as well as some molecularly targeted protein kinase inhibitors away from their intracellular drug targets, and consequently resulting in reduced drug efficacy, cancer recurrence, and treatment failure [4-7]. Among these ABC drug transporters, ABCB1 (P-glycoprotein/MDR1) and ABCG2 (BCRP; MXR) are often linked to the development of MDR phenotype in solid tumors such as metastatic breast cancer [8] and advanced non-small cell lung cancer [9], as well as in blood cancers such as multiple myeloma (MM) [10-12], chronic lymphocytic leukemia (CLL) [13], acute myelogenous leukemia (AML) and acute lymphocytic leukemia (ALL) [14-16]. Moreover, studies have demonstrated that ABCC1 (also known as multidrug resistance-associated protein 1 or MRP1) mediates resistance to important conventional anticancer drugs such as Vinca alkaloids, anthracyclines, and etoposide, as well as glutathione and glucuronide drug metabolites in cell line models [17,18], and thus implicated ABCC1 in multidrug resistance [19]. However, the exact role of ABCC1 in clinical drug resistance remains ambiguous [20]. Coincidentally, ABCB1 and ABCG2 are overexpressed at blood-tissue barrier sites such as the blood-intestinal barrier and the blood-brain barrier (BBB) to form an endogenous defense system against toxic substances, causing a significant impact on the bioavailability, distribution, metabolism and elimination of the majority of drugs in patients [2,5,21]. Therefore, it is of great importance to develop ways against this mechanism of resistance and improve the efficacy of chemotherapy in cancer patients.
To date, there is no chemotherapeutic intervention available to improve therapeutic outcomes in patients with multidrug-resistant cancers. One practical strategy is to discover a potent and selective modulator that can target this mechanism of drug resistance by attenuating the function and/or protein expression level of ABCB1 or ABCG2 in these multidrug-resistant cancer cells [22,23]. Unfortunately, despite considerable efforts that have been invested to develop novel chemosensitizers of ABCB1 and ABCG2, this approach has failed thus far due to problems associated with high intrinsic toxicity, unforeseen drug-drug interactions and the lack of selectivity of these synthetic compounds [2,24]. Knowing that innovative drug combinations could help preserve drug efficacy in multidrug-resistant cancer cells, we and others have been exploring the advantages of a drug repurposing (also known as drug repositioning or drug re-profiling) approach to identify therapeutic agents with well-characterized pharmacological and toxicological profiles that can restore the chemosensitivity of these multidrug-resistant cancer cells [23-29]. It is worth mentioning that a recent phase I study showed promising results of sarcomas patients receiving doxorubicin and nilotinib, a known high-affinity drug substrate for ABCB1 and ABCG2 [30,31], demonstrated in principle that a multidrug combination therapy of a conventional anticancer drug and another therapeutic agent as co-adjuvant treatment is a promising therapeutic strategy against multidrug-resistant cancers [32].
In the present study, we identify the phosphodiesterase type 5 (PDE5) inhibitor MY-5445 as a selective modulator of ABCG2. MY-5445 was originally developed as a specific inhibitor of cyclic GMP phosphodiesterase (cGMP-PDE) to inhibit human platelet aggregation [33], but has since been used as a reference inhibitor of PDE5 in studies on platelet aggregation [34], morphine-induced peripheral analgesia [35], central analgesia [36] and nitric oxide (NO) signaling related research [37-39]. Moreover, the antiproliferative effect of MY-5445 has also been examined in the human megakaryoblastic Dami cell line [40]. Our results show that MY-5445 potentiates drug-induced apoptosis and reverses MDR in ABCG2-overexpressing cancer cells through direct inhibition of ABCG2-mediated drug transport, suggesting that combining MY-5445 with conventional anticancer drugs may be beneficial for patients with multidrug-resistant cancers.
Materials and methods
Chemicals
MY-5445 was purchased from MedKoo Biosciences, Inc. (Morrisville, NC, USA). Tariquidar, MK-571, Ko143, and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. Primary antibodies BXP-21 and α-tubulin were purchased from Abcam (Cambridge, MA, USA). Immobilon enhanced chemiluminescence (ECL) kit from Merck Millipore (Billerica, MA, USA). Annexin V: FITC Apoptosis Detection Kit was purchased from BD Pharmingen (San Diego, CA, USA).
Cell culture conditions
The H460 human non-small cell lung cancer cell line and the ABCG2-overexpressing variant H460-MX20, as well as the HEK293 human embryonic kidney cell line and HEK293 cells stably transfected with human ABCB1 (MDR19-HEK293 cell line) [41] or ABCC1 (MRP1-HEK293 cell line) [42] or ABCG2 (R482-HEK293 cell line) [41] were maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco Invitrogen, CA, USA) supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, 100 units/mL of penicillin and streptomycin. H460-MX20 cells were maintained in 20 nM of mitoxantrone [43], whereas HEK293 and HEK293 transfected cells were maintained in media containing 2 mg/mL G418 [41]. The S1 human colon cancer cell line and its ABCG2-overexpressing variant S1-M1-80 were cultured in RPMI-1640 medium (Gibco Invitrogen, CA, USA) supplemented with 10% FCS, 2 mM L-glutamine, 100 units/mL of penicillin and streptomycin. S1-M1-80 cells were maintained in 80 μM of mitoxantrone [44]. All cell lines were cultured at 37°C in 5% CO2 humidified air and placed in drug-free medium 7 days before assay.
Cytotoxicity assays
The cytotoxicity assays were performed as described previously [45], based on the method described by Ishiyama et al. [46]. Tools Cell Counting Kit-8 (Biotools Co., Ltd, Taipei, Taiwan) was used to determine the cytotoxicity of drugs in HEK293 and HEK293 transfected cells, whereas MTT reagent was used to determine the cytotoxicity of drugs in attached human cancer cell lines as described previously [29]. The half-maximal inhibitory concentration (IC50) value for each treatment was calculated from a fitted dose-response curve acquired from at least three independent experiments. For the reversal assay, a nontoxic concentration of MY-5445 or a reference inhibitor of ABC drug transporters was added to the respective cytotoxicity assays for the calculation of the fold-reversal (FR) values, which represent the extent of reversal by a modulator [47].
Apoptosis assays
The extent of apoptosis in cancer cell lines induced by the indicated regimens was determined based on the conventional Annexin V-FITC and propidium iodide (PI) staining method [48]. Briefly, cells were treated with DMSO, topotecan, MY-5445 or in drug combinations as indicated for 48 h before harvested, centrifuged and resuspended in FACS buffer containing 1.25 µg/mL Annexin V-FITC (BD Pharmingen, San Diego, CA, USA) and 0.1 mg/mL PI, and incubated for 15 min at room temperature. The labeled phosphatidylserine (PS)-positive and PI-negative cells (early apoptotic cells) and PS-positive and PI-positive cells (necrotic or late apoptotic) [49] were analyzed by FACScan using CellQuest software as described previously [29].
Fluorescent drug accumulation assays
Pheophorbide A (PhA), a known fluorescent substrate of ABCG2, was used as a probe for ABCG2 function in cells overexpressing ABCG2. Briefly, 3×105 of cells were harvested and incubated in 4 mL of IMDM supplemented with 5% FCS in medium containing 1 µM PhA at 37°C in 5% CO2 humidified air in the presence or absence of 10 μM MY-5445 or Ko143 at 1 μM as a positive control. The intracellular accumulation of PhA was determined according to the method described by Gribar et al. [50], and analyzed using a FACScan flow cytometer equipped with CellQuest software (Becton-Dickinson, San Jose, CA, USA), as described previously [51].
Immunoblotting
ABCG2-overexpressing cancer cells were treated with increasing concentrations of MY-5445 for 72 h before harvested and subjected to SDS-polyacrylamide electrophoresis. Primary antibodies BXP-21 (1:15000) and α-tubulin (1:100000) were used in Western blot immunoassay to detect ABCG2 and positive control tubulin, respectively. The horseradish peroxidase-conjugated goat anti-mouse IgG (1:100000) was used as the secondary antibody. Signals were detected using Immobilon enhanced chemiluminescence (ECL) kit from Merck Millipore (Billerica, MA, USA) as described previously [45].
ATPase assay
The vanadate (Vi)-sensitive ATPase activity of ABCG2 was determined based on the endpoint inorganic phosphate (Pi) assay quantifying the amount of Pi released using a colorimetric method as described previously [52]. Briefly, membrane vesicles of ABCG2-expressing High-Five cells (Thermo Fisher Scientific, Waltman, MA, USA) were incubated with MY-5445 (0-1.5 μM) in the absence or presence of 0.3 mM sodium orthovanadate in ATPase buffer (50 mM MES-Tris pH 6.8, 50 mM KCl, 5 mM NaN3, 1 mM EGTA, 1 mM ouabain, 2 mM DTT). ABCG2 ATPase activity was allowed to occur for 20 min at 37°C, after which the reaction was stopped by the addition of 50 µL of Pi reagent (1% ammonium molybdate in 2.5 N H2SO4 and 0.014% antimony potassium tartrate). The released inorganic phosphate was quantified by the addition of a 150 µL of 0.33% sodium L-ascorbate and measured (absorbance at 880 nm) using a Spectramax iD3 microplate reader (Molecular Devices, San Jose, CA, USA). The Visensitive activity was calculated as the ATPase activity in the absence of vanadate minus the ATPase activity in the presence of vanadate, as described previously [52].
Docking analysis of MY-5445 with ABCG2
The inward-open structure of ABCG2 (PDB: 5NJ3) [53] was used as a template for docking of MY-5445 with AutoDock Vina [54]. Transporter structure and ligand were prepared using MGLtools software package (The Scripps Research Institute) [55]. For docking in the drug-binding pocket of ABCG2, the following residues of each monomer of the homodimer were set as flexible: N393, A397, N398, V401, L405, I409, T413, N424, F431, F432, T435, N436, F439, S440, V442, S443, Y538, L539, T542, I543, V546, F547, M549, I550, L554, L555. The receptor grid was centered at x=125, y=125 and z=130, and the box dimensions were set as 34 Å×30 Å×50 Å. The exhaustiveness level was set at 100 to ensure that the global minimum of the scoring function would be found considering the large box size and the number of flexible residues. Analysis of the docked poses was performed using Pymol molecular graphics system, Version 1.7 (Shrödinger, LLC).
Quantification and statistical analysis
Unless stated otherwise, the experimental data and IC50 values are presented as mean ± standard deviation (SD) calculated from at least three independent experiments. Curve plotting and statistical analysis were performed with KaleidaGraph (Synergy Software, Reading, PA, USA) and GraphPad Prism (GraphPad Software, La Jolla, CA, USA) software. The improvement in fit was analyzed by two-tailed Student’s t-test and labeled “statistically significant” if the probability, p, was less than 0.05.
Results
MY-5445 reverses multidrug resistance mediated by ABCG2
The chemosensitization effect of MY-5445 was first evaluated in HEK293 cells, MDR19-HEK293 cells, MRP1-HEK293, and R482-HEK293 cells to determine the relative selectivity of MY-5445 for human ABCB1, ABCC1, and ABCG2, respectively. We discovered that the chemosensitivity of MDR19-HEK293 cells to colchicine, a known substrate for ABCB1 (Figure 1A, right panel), and MRP1-HEK293 cells to etoposide, a known substrate for ABCC1 (Figure 1B, right panel), was unaffected by MY-5445. In contrast, MY-5445 resensitized R482-HEK293 cells to ABCG2 substrates mitoxantrone (Figure 1C), SN-38 (Figure 1D) and topotecan (Figure 1E) in a concentration-dependent manner. Tariquidar at 1 μM, MK-571 at 25 μM, and Ko143 at 1 μM were used as reference inhibitors for ABCB1, ABCC1, and ABCG2, respectively. Knowing that MY-5445 is selective for ABCG2, the MDR reversal effect of MY-5445 was further examined in the S1 human colon cancer cell line and H460 human non-small cell lung cancer cell line, and their ABCG2-overexpressing multidrug-resistant variants S1-M1-80 and H460-MX20 cancer cell lines. We found that MY-5445 resensitized S1-M1-80 cells and H460-MX20 cells to mitoxantrone, SN-38 and topotecan (Figure 2) in the same manner as in R482-HEK293 cells (Figure 1). Of note, MY-5445 had no significant effect on the proliferation of drug-sensitive parental cells (Figures 1 and 2, left panels). The 50% inhibitory concentration (IC50) value of respective substrate drugs and the fold-reversal (FR) value, representing the extent of chemosensitization by MY-5445 [47] in tested cell lines, were calculated as described in Methods and summarized in Tables 1 and 2. Our results demonstrated that MY-5445 selectively reverses ABCG2-mediated multidrug resistance in ABCG2-overexpressing cells.
Figure 1.
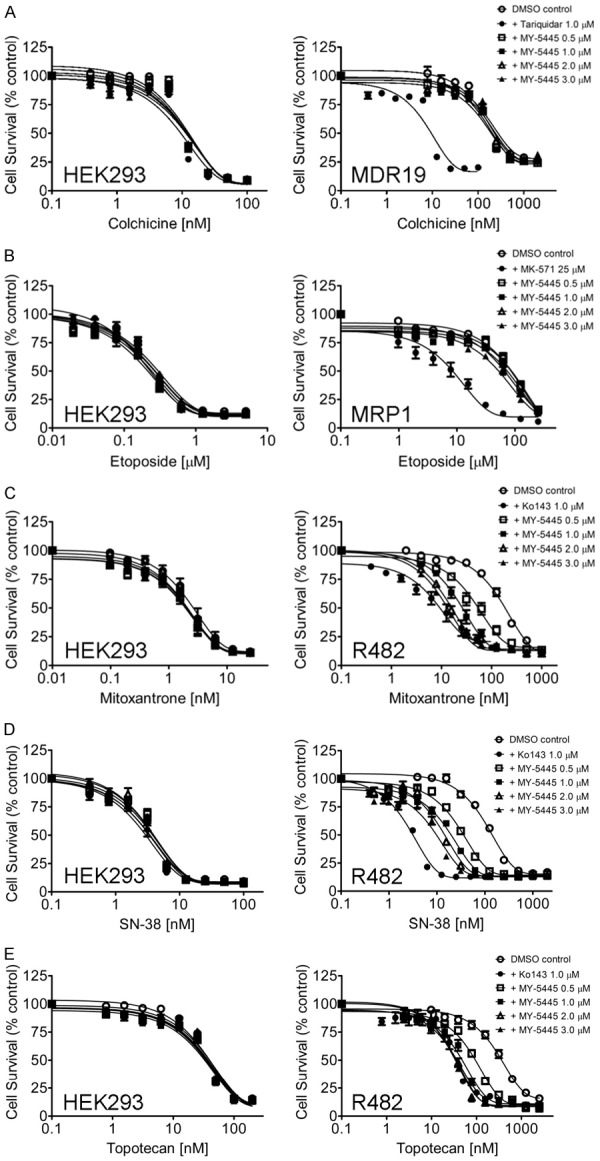
MY-5445 selectively reverses ABCG2-mediated multidrug resistance. The selective chemosensitization effect of MY-5445 was determined in drug-sensitive parental HEK293 cells (A-E, left panels), human ABCB1-transfected MDR19-HEK293 cells (A, right panel), human ABCC1-transfected MRP1-HEK293 cells (B, right panel) and human ABCG2-transfected R482-HEK293 cells (C-E, right panel). Cells were treated with increasing concentrations of (A) colchicine or (B) etoposide or (C) mitoxantrone or (D) SN-38 or (E) topotecan in the presence of DMSO (control, open circles), MY-5445 at 0.5 μM (open squares), 1 μM (filled squares), 2 μM (open triangles) or 3 μM (filled triangles). Tariquidar at 1 μM, MK-571 at 25 μM and Ko143 1 μM, were used as reference inhibitors (filled circles) for ABCB1, ABCC1 and ABCG2, respectively. Points, mean values from at least three independent experiments; bars; SEM.
Figure 2.
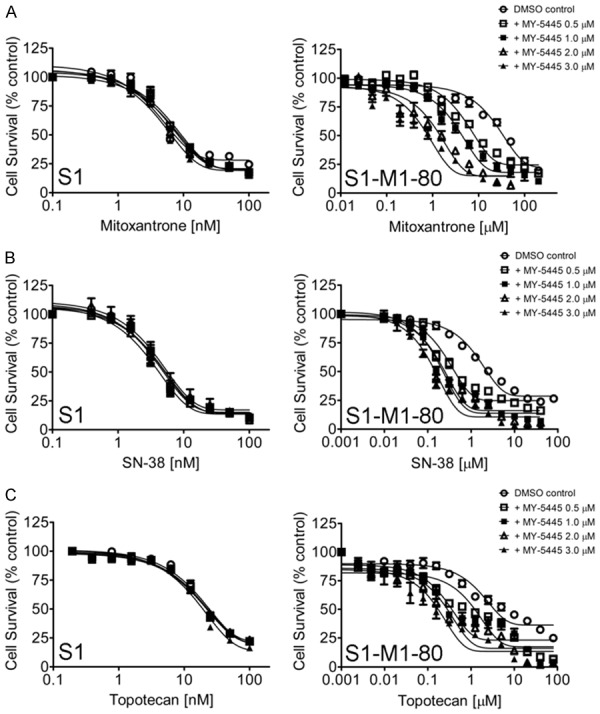
MY-5445 resensitizes ABCG2-overexpressing human S1-M1-80 colon cancer cells to conventional chemotherapeutic drugs. Drug-sensitive parental human S1 colon cancer cells (left panels) and the ABCG2-overexpressing multidrug-resistant variant S1-M1-80 cancer cells (right panels) were treated with increasing concentrations of (A) mitoxantrone or (B) SN-38 or (C) topotecan in the presence of DMSO (open circles), MY-5445 at 0.5 μM (open squares), 1 μM (filled squares), 2 μM (open triangles) or 3 μM (filled triangles). Points, mean values from at least three independent experiments; bars; SEM.
Table 1.
The effect of MY-5445 on drug resistance mediated by ABCB1, ABCC1 or ABCG2 in HEK293 cells transfected with human ABCB1, ABCC1 or ABCG2
| Treatment | Concentration (μM) | Mean IC50 † ± SD and (FR‡) | |
|
| |||
| pcDNA-HEK293 (parental) [nM] | MDR19-HEKS293 (resistant) [nM] | ||
|
| |||
| Colchicine | - | 10.61±3.64 (1.0) | 256.55±55.25 (1.0) |
| + MY-5445 | 0.5 | 10.29±3.13 (1.0) | 247.39±49.20 (1.0) |
| + MY-5445 | 1.0 | 10.93±3.78 (1.0) | 266.45±64.09 (1.0) |
| + MY-5445 | 2.0 | 10.66±3.26 (1.0) | 314.17±66.13 (0.8) |
| + MY-5445 | 3.0 | 10.90±3.43 (1.0) | 355.55±81.95 (0.7) |
| + Tariquidar | 1.0 | 9.15±3.20 (1.2) | 11.10±3.48** (23.1) |
|
| |||
| pcDNA-HEK293 (parental) [nM] | MRP1-HEKS293 (resistant) [μM] | ||
|
| |||
| Etoposide | - | 273.20±63.61 (1.0) | 85.90±14.30 (1.0) |
| + MY-5445 | 0.5 | 212.02±36.82 (1.3) | 96.82±19.56 (0.9) |
| + MY-5445 | 1.0 | 260.10±48.11 (1.1) | 84.35±19.48 (1.0) |
| + MY-5445 | 2.0 | 293.37±54.28 (0.9) | 74.07±11.95 (1.2) |
| + MY-5445 | 3.0 | 231.90±39.99 (1.2) | 62.81±12.66 (1.4) |
| + MK-571 | 25.0 | 208.20±44.29 (1.3) | 9.13±1.69*** (9.4) |
|
| |||
| pcDNA-HEK293 (parental) [nM] | R482-HEKS293 (resistant) [nM] | ||
|
| |||
| Mitoxantrone | - | 2.44±0.27 (1.0) | 177.66±19.11 (1.0) |
| + MY-5445 | 0.5 | 2.01±0.30 (1.2) | 59.01±6.75*** (3.0) |
| + MY-5445 | 1.0 | 2.05±0.27 (1.2) | 25.87±3.17*** (6.9) |
| + MY-5445 | 2.0 | 2.01±0.27 (1.2) | 16.75±2.90*** (10.6) |
| + MY-5445 | 3.0 | 1.95±0.28 (1.3) | 13.54±2.17*** (13.1) |
| + Ko143 | 1.0 | 1.97±0.27 (1.2) | 12.91±2.16*** (13.8) |
| SN-38 | - | 3.14±0.80 (1.0) | 134.24±19.32 (1.0) |
| + MY-5445 | 0.5 | 3.31±0.90 (0.9) | 39.28±7.39** (3.4) |
| + MY-5445 | 1.0 | 2.93±0.68 (1.1) | 22.24±4.32*** (6.0) |
| + MY-5445 | 2.0 | 3.24±0.80 (1.0) | 15.30±3.44*** (8.8) |
| + MY-5445 | 3.0 | 3.31±0.69 (0.9) | 11.92±3.15*** (11.3) |
| + Ko143 | 1.0 | 2.59±0.60 (1.2) | 2.94±0.62*** (45.7) |
| Topotecan | - | 36.50±7.60 (1.0) | 384.96±31.52 (1.0) |
| + MY-5445 | 0.5 | 34.82±6.79 (1.0) | 101.64±13.01*** (3.8) |
| + MY-5445 | 1.0 | 33.51±7.37 (1.1) | 51.35±9.50*** (7.5) |
| + MY-5445 | 2.0 | 38.76±8.90 (0.9) | 32.99±6.14*** (11.7) |
| + MY-5445 | 3.0 | 37.19±9.21 (1.0) | 31.51±7.08*** (12.2) |
| + Ko143 | 1.0 | 37.06±8.52 (1.0) | 40.39±9.16*** (9.5) |
Abbreviation: FR, fold-reversal.
IC50 values are mean ± SD obtained from dose-response curves of at least three independent experiments using cytotoxicity assay as described in Materials and methods.
FR values were calculated by dividing IC50 values of cells treated with a particular anticancer drug in the absence of an inhibitor by IC50 values of cells treated with the same anticancer drug in the presence of an inhibitor.
P<0.01;
P<0.001.
Table 2.
The effect of MY-5445 on ABCG2-mediated multidrug resistance in ABCG2-overexpressing human cancer cell lines
| Treatment | Concentration (μM) | Mean IC50 † ± SD and (FR‡) | |
|
| |||
| S1 (parental) [nM] | S1-M1-80 (resistant) [μM] | ||
|
| |||
| Mitoxantrone | - | 8.36±2.38 (1.0) | 50.40±4.98 (1.0) |
| + MY-5445 | 0.5 | 9.42±1.38 (0.9) | 11.08±1.55*** (4.5) |
| + MY-5445 | 1.0 | 9.24±1.20 (0.9) | 5.07±0.97*** (9.9) |
| + MY-5445 | 2.0 | 8.43±1.65 (1.0) | 1.73±0.27*** (29.1) |
| + MY-5445 | 3.0 | 6.53±1.29 (1.3) | 0.95±0.19*** (53.1) |
| + Ko143 | 1.0 | 9.12±1.66 (0.9) | 0.36±0.03*** (140.0) |
| SN-38 | - | 4.43±0.82 (1.0) | 4.30±1.03 (1.0) |
| + MY-5445 | 0.5 | 4.86±0.76 (0.9) | 0.71±0.21** (6.1) |
| + MY-5445 | 1.0 | 4.69±0.78 (0.9) | 0.31±0.07** (13.9) |
| + MY-5445 | 2.0 | 3.75±0.60 (1.2) | 0.25±0.06** (17.2) |
| + MY-5445 | 3.0 | 5.14±0.97 (0.9) | 0.16±0.02** (26.9) |
| + Ko143 | 1.0 | 4.15±0.74 (1.1) | 0.05±0.01** (86) |
| Topotecan | - | 24.40±3.10 (1.0) | 11.04±2.67 (1.0) |
| + MY-5445 | 0.5 | 23.72±2.51 (1.0) | 2.30±0.57** (4.8) |
| + MY-5445 | 1.0 | 23.31±2.22 (1.0) | 1.25±0.40** (8.8) |
| + MY-5445 | 2.0 | 24.83±2.61 (1.0) | 0.55±0.14** (20.1) |
| + MY-5445 | 3.0 | 18.68±2.83 (1.3) | 0.32±0.07** (34.5) |
| + Ko143 | 1.0 | 24.46±3.01 (1.0) | 0.27±0.05** (40.9) |
|
| |||
| H460 (parental) [nM] | H460-MX20 (resistant) [μM] | ||
|
| |||
| Mitoxantrone | - | 31.23±10.14 (1.0) | 2.29±0.52 (1.0) |
| + MY-5445 | 0.5 | 49.26±18.28 (0.6) | 1.02±0.38* (2.2) |
| + MY-5445 | 1.0 | 45.20±16.30 (0.7) | 0.36±0.12** (6.4) |
| + MY-5445 | 2.0 | 42.43±14.75 (0.7) | 0.38±0.14** (6.0) |
| + MY-5445 | 3.0 | 37.07±12.87 (0.8) | 0.28±0.08** (8.2) |
| + Ko143 | 1.0 | 48.96±18.09 (0.6) | 0.16±0.05** (14.3) |
| SN-38 | - | 7.14±1.29 (1.0) | 4.36±1.80 (1.0) |
| + MY-5445 | 0.5 | 9.95±1.84 (0.7) | 0.82±0.36* (5.3) |
| + MY-5445 | 1.0 | 7.88±1.52 (0.9) | 0.72±0.33* (6.1) |
| + MY-5445 | 2.0 | 5.42±1.14 (1.3) | 0.41±0.19* (10.6) |
| + MY-5445 | 3.0 | 4.89±1.24 (1.5) | 0.50±0.23* (8.7) |
| + Ko143 | 1.0 | 5.39±1.54 (1.3) | 0.10±0.04* (43.6) |
| Topotecan | - | 100.91±16.10 (1.0) | 23.62±4.97 (1.0) |
| + MY-5445 | 0.5 | 84.78±12.59 (1.2) | 1.62±0.49** (14.6) |
| + MY-5445 | 1.0 | 77.03±13.99 (1.3) | 1.42±0.47** (16.6) |
| + MY-5445 | 2.0 | 63.85±11.58* (1.6) | 0.96±0.33** (24.6) |
| + MY-5445 | 3.0 | 63.71±12.42* (1.6) | 0.63±0.17** (37.5) |
| + Ko143 | 1.0 | 69.30±12.50 (1.5) | 1.52±0.51** (15.5) |
Abbreviation: FR, fold-reversal.
IC50 values are mean ± SD obtained from dose-response curves of at least three independent experiments using cytotoxicity assay as described in Materials and methods.
FR values were calculated by dividing IC50 values of cells treated with a particular anticancer drug in the absence of an inhibitor by IC50 values of cells treated with the same anticancer drug in the presence of MY-5445 or Ko143.
P<0.05;
P<0.01;
P<0.001.
MY-5445 enhances drug-induced apoptosis in ABCG2-overexpressing cancer cells
To confirm that the reversal effect MY-5445 on ABCG2-mediated multidrug resistance is caused by the potentiation of cytotoxicity induced by an anticancer drug, and not by growth retardation, we examined the effect of MY-5445 on topotecan-induced apoptosis in S1-M1-80 cancer cells following the method described previously [56]. As shown in Figure 3, treating cells with topotecan for 48 h led to a substantial increase of apoptosis in drugsensitive S1 cancer cells (from approximately 4% basal level to 49% total apoptosis), but not in ABCG2-overexpressing S1-M1-80 cancer cells (from approximately 5% basal level to 4% total apoptosis). Notably, without having a significant effect on S1 cells, MY-5445 substantially increased the topotecan-induced apoptosis in S1-M1-80 cells, from approximately 4% basal level to 37% of early and late apoptosis. It is worth noting that the effect of MY-5445 on topotecan-induced apoptosis in S1-M1-80 cells is comparable to that of Ko143 (Figure 3B). Our results indicate that MY-5445 reverses ABCG2-mediated MDR by potentiating the cytotoxicity of an ABCG2 substrate drug in ABCG2-overexpressing multidrug-resistant cancer cells, possibly by modulating the function and/or the protein expression of ABCG2.
Figure 3.
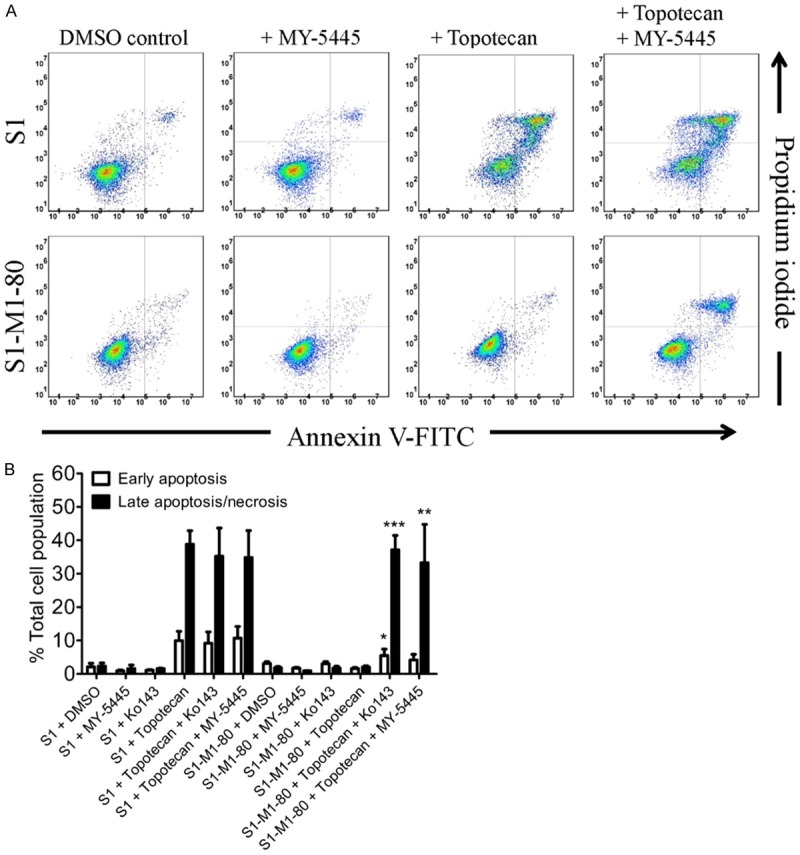
MY-5445 enhances drug-induced apoptosis in ABCG2-overexpressing cancer cells. A. Human S1 colon cancer cells (top dot-plot panels) and ABCG2-overexpressing variant S1-M1-80 cancer cells (lower dot-plot panels) were treated with either DMSO (control), 3 μM MY-5445 (+ MY-5445), 5 μM topotecan (+ topotecan) or a combination of 5 μM topotecan and 3 μM MY-5445 (+ topotecan + MY-5445) for 48 h. Cells were isolated and analyzed by flow cytometry according to the method described previously [70]. Representative dot plots and the mean values of three independent experiments are shown. B. Quantifications of topotecan-induced apoptosis in S1 and S1-M1-80 cancer cell lines are presented as mean ± S.D. calculated from three independent experiments. *P<0.05; **P<0.01; ***P<0.001, versus the same treatment in the absence of Ko143 or MY-5445.
MY-5445 attenuates ABCG2-mediated drug efflux
Most often, the resensitization of ABCG2-overexpressing multidrug-resistant cancer cells is due to direct inhibition of the function [57] and/or transient downregulation of ABCG2 protein [58,59] in multidrug-resistant cancer cells. To this end, we examined the effect of MY-5445 on the drug transport function of ABCG2, as well as the protein expression of ABCG2 in ABCG2-overexpressing cells. We discovered that MY-5445 significantly increased the intracellular accumulation of pheophorbide A (PhA), a specific fluorescent substrate for ABCG2 [51], in ABCG2-overexpressing S1-M1-80 cancer cells (Figure 4A) and R482-HEK293 cells (Figure 4B). We demonstrated that MY-5445 blocks ABCG2-mediated PhA efflux from R482-HEK293 cells in a concentration-dependent manner, with a calculated IC50 value of approximately 16 μM. In parallel, the effect of sildenafil, a well-known inhibitor of PDE5, on ABCG2-mediated efflux of PhA was also examined and compared to MY-5445 (Figure 4C). Of note, MY-5445 had no significant effect on the accumulation of PhA in drug-sensitive parental S1 and HEK293 cells. Next, we determined the protein expression of ABCG2 in S1-M1-80 cancer cells treated with MY-5445 (0.5-3 μM) for 72 h by immunoblotting as described in Methods. As shown in Figure 4D, MY-5445 did not have any significant effect on the protein expression of ABCG2 in these cells, suggesting that MY-5445 resensitized ABCG2-overexpressing cancer cells to anticancer agents by modulating the drug transport function of ABCG2.
Figure 4.
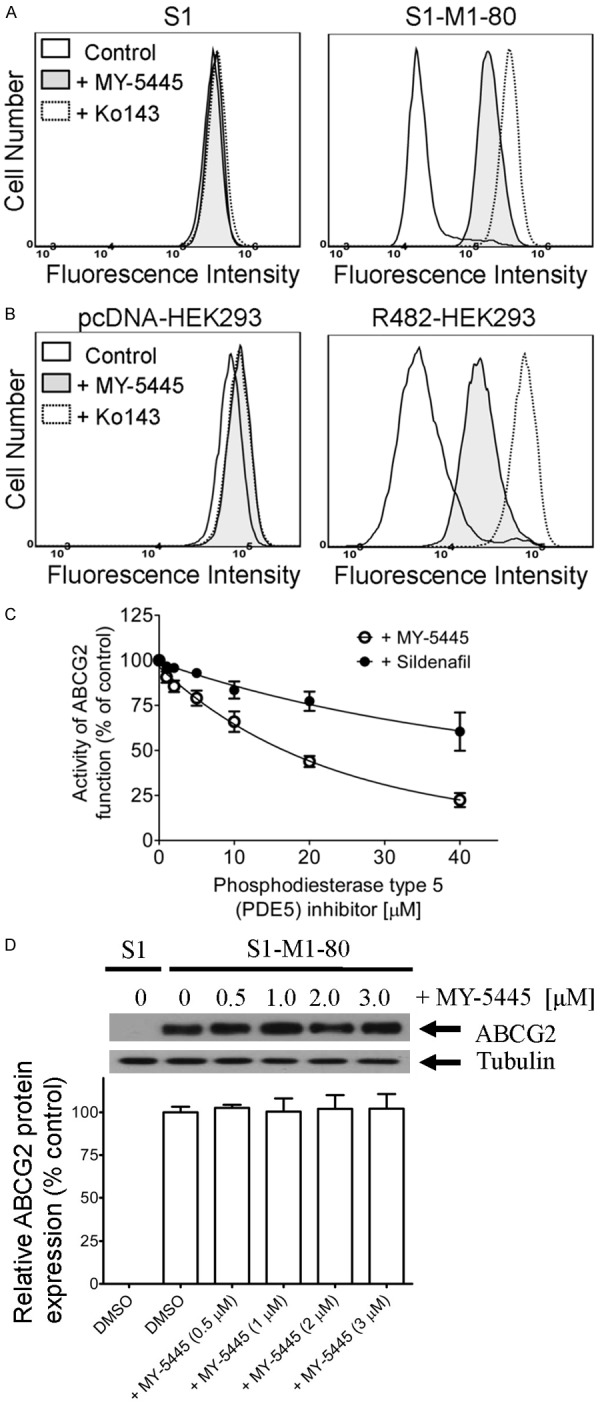
MY-5445 inhibits the drug transport function, but not the protein expression of ABCG2. The accumulation of fluorescent pheophorbide A (PhA) in human S1 colon cancer cells (A, left panel) and ABCG2-overexpressing variant S1-M1-80 cancer cells (A, right panel), as well as in HEK293 cells (B, left panel) and HEK293 cells transfected with human ABCG2, R482-HEK293 (B, right panel), was measured in the presence of DMSO (solid lines) or 10 μM of MY-5445 (solid lines, filled) or 1 μM of ABCG2 reference inhibitor Ko143 (dotted lines), and analyzed immediately by flow cytometry as described previously [71]. Representative histograms of three independent experiments are shown. (C) The concentration-dependent inhibition of ABCG2-mediated efflux of PhA by phosphodiesterase inhibitors MY-5445 (open circles) and sildenafil (filled circles) in R482-HEK293 cells. Values are presented as mean ± SEM calculated from at least three independent experiments. (D) Human S1-M1-80 colon cancer cells were treated with DMSO (vehicle control) or increasing concentrations (0.5-3.0 μM) of MY-5445 for 72 h, and the protein expression of human ABCG2 was analyzed by western blotting according to the method described previously [70]. α-Tubulin was used as an internal loading control. Values are presented as mean ± SEM calculated from three independent experiments.
MY-5445 stimulates the ATPase activity of ABCG2
Knowing that the transport activity of an ABC drug transporter is coupled to its ATPase activity [60], we determined the effect of MY-5445 on vanadate (Vi)-sensitive ATPase activity of ABCG2 to gain insight into the interaction between MY-5445 and the drug-binding pocket of ABCG2. As shown in Figure 5, the ATPase activity of ABCG2 was stimulated by MY-5445 in a concentration-dependent manner, with maximal stimulation of approximately 165% of basal level (basal, 154.7±19.4 nmole Pi/min/mg protein) and a half-maximal effective concentration (EC50) value of 124 nM.
Figure 5.
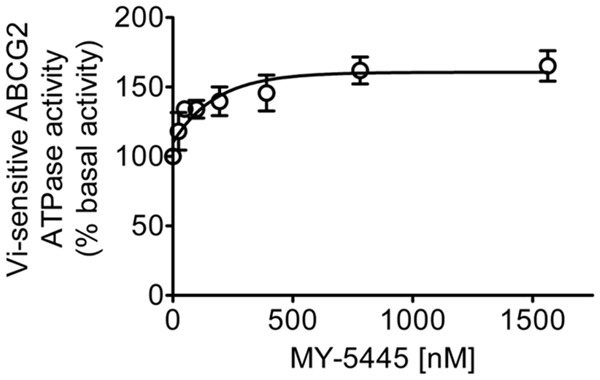
MY-5445 stimulates ABCG2 ATPase activity. The effect of increasing concentrations of MY-5445 (0-1.5 μM) on the vanadate-sensitive ATPase activity of ABCG2 was measured in the membrane vesicles prepared from High-Five insect cells overexpressing human ABCG2 as previously described [70]. Values represent mean ± SEM calculated from three independent experiments.
In silico docking analysis reveals that MY-5445 binds in the drug-binding pocket of ABCG2
In addition, the docking analysis of MY-5445 with the inward-open structure of human ABCG2 (PDBID:5NJ3) [53] was performed to elucidate the potential sites of interaction between MY-5445 and the amino acid residues located within the substrate-binding pocket of ABCG2. A total of 9 poses with the lowest energy score were evaluated. All of which interacted with ABCG2 in the same binding cavity in the transmembrane region but with different orientations (Figure 6, top panel). Analysis of the specific interaction of the lowest energy docking pose showed that MY-5445 interacts with hydrophobic and aromatic amino acids (Figure 6, bottom panel). Our data indicate that MY-5445 interacts strongly with the drug-substrate-binding pocket located in the transmembrane region of ABCG2, which is consistent with the results of drug accumulation assays (Figure 4).
Figure 6.
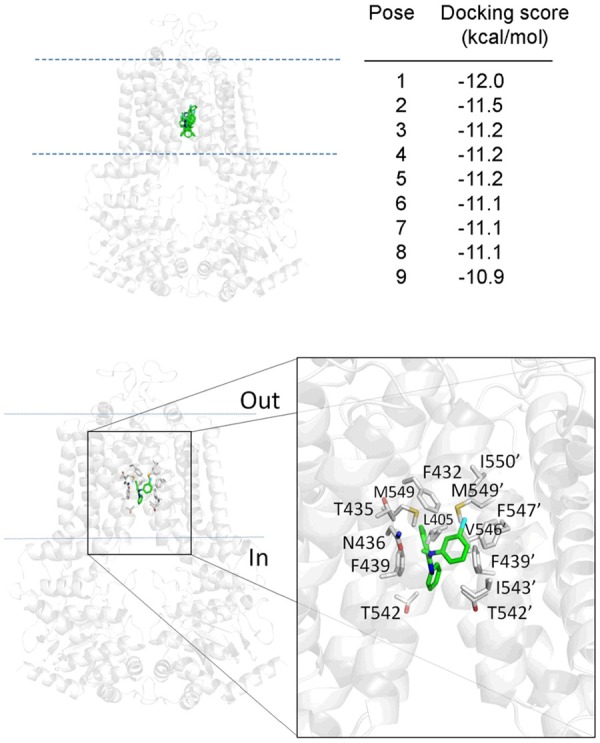
Binding of MY-5445 in the drug-binding pockets of ABCG2. MY-5445 was docked to the cryo-electron microscopy structure of human ABCG2 (PDB: 5NJ3) using Autodock Vina software as described in Methods. All nine low-energy poses of MY-5445 interaction with residues in the same binding cavity in the transmembrane region (green sticks, top left panel), with similar binding affinities (top-right). The amino acids within 5Å of MY-5445 in the lowest-energy pose are shown with gray sticks (bottom panel). Pymol software was used for analyses of docking poses and figure preparation.
Overexpression of ABCG2 does not confer resistance to MY5445 in cancer cells
Given that the results of ATPase assay and docking analysis indicate that MY-5445 interacts at the drug-substrate-binding site of ABCG2, we compared the cytotoxicity and the resistance factor (RF) value of MY-5445 in multiple ABCG2-overexpressing cell lines and their respective drug-sensitive parental cell lines. The RF value here represents the extent of acquired resistance to MY-5445 mediated by ABCG2, which is calculated by dividing the IC50 value of MY-5445 in a particular ABCG2-overexpressing multidrug-resistant cell line by the IC50 value of MY-5445 in its drug-sensitive parental cell line. As shown in Table 3, there were no significant differences between the RF values, indicating that MY-5445 is not pumped out by ABCG2, and the overexpression of ABCG2 does not lead to reduced sensitivity of cancer cells to MY-5445.
Table 3.
Cytotoxicity of MY-5445 in ABCG2-overexpressing cell lines
| Cell line | Type | Transporter expressed | IC50 (μM)† | R.F‡ |
|---|---|---|---|---|
| S1 | colon | - | 12.91±3.80 | 1.0 |
| S1-M1-80 | colon | ABCG2 | 15.15±4.43 | 1.2 |
| H460 | lung | - | 24.68±6.02 | 1.0 |
| H460-MX20 | lung | ABCG2 | 24.43±5.60 | 1.0 |
| MCF7 | breast | - | 34.50±8.07 | 1.0 |
| MCF7-AdVp3000 | breast | ABCG2 | 23.72±2.60 | 0.7 |
| pcDNA-HEK293 | - | - | 12.05±1.99 | 1.0 |
| R482-HEK293 | - | ABCG2 | 14.39±2.50 | 1.2 |
Abbreviation: RF, resistance factor.
IC50 values are mean ± SD calculated from dose-response curves obtained from three independent experiments using cytotoxicity assay as described in Materials and methods.
RF values were calculated by dividing IC50 values of MY-5445 in ABCG2-overexpressing cell lines by IC50 values of MY-5445 in respective parental cell lines.
Discussion
The rise of multidrug resistance, partly contributed by the overexpression of ABCG2 protein in cancer cells, poses a significant challenge for many conventional anticancer drugs and molecular targeted drugs to remain effective [61]. For that reason, discovering ways to reverse ABCG2-mediated MDR in cancer cells is essential in prolonging the efficacy of first-line chemotherapeutic agents. Instead of developing novel inhibitors of ABCG2, which can be expensive and time-consuming, we and others have utilized an alternative approach of repurposing existing therapeutic agents for the resensitization of multidrug-resistant cancer cells [26,57,62]. Conventionally, PDE5 inhibitors are used as an experimental tool to examine the physiological function of PDE5. However, due to the polypharmacological nature of PDE5 inhibitors, the therapeutic effect of PDE5 inhibitors has been investigated in erectile dysfunction (ED), cardiovascular diseases, pulmonary hypertension, prostate hyperplasia, Ray-naud’s disease, cognition dysfunction, and even in the treatment of solid tumors and blood cancers as summarized in references [63,64]. More specifically, previous reports have revealed a beneficial effect of including a PDE5 inhibitor as an adjuvant to resensitize multidrug-resistant cancer cells to conventional anticancer agents [25,65,66]. Shi et al. demonstrated that sildenafil reversed MDR mediated by ABCB1 and ABCG2 by inhibiting the drug transport function of both ABCB1 and ABCG2 in human cancer cell lines [25], whereas vardenafil and tadalafil were able to selectively reverse ABCB1-mediated MDR in cancer cell lines [66]. These results prompted us to investigate the activity of MY-5445, a PDE5 inhibitor of a different chemical class than sildenafil, vardenafil, and tadalafil [33,67], against MDR mediated by ABC drug transporters in human cancer cell lines.
In this study, we examined the interactions of MY-5445 with ABCB1, ABCC1, and ABCG2, which are ABC drug transporters that have been frequently linked to the development of MDR in cancers [61]. We discovered that MY-5445 resensitized ABCG2-overexpressing cells to mitoxantrone, SN-38, and topotecan without significant effect on MDR mediated by ABCB1 or ABCC1. Moreover, we demonstrated that MY-5445 potentiates topotecan-induced apoptosis in ABCG2-overexpressing multidrug-resistant cancer cells by blocking the drug transport function of ABCG2. The interaction between MY-5445 and ABCG2 was further supported by the result of MY-5445 stimulating the ATPase activity of ABCG2, as well as docking analysis depicting the potential sites of interaction of MY-5445 at the substrate-binding pocket within the transmembrane regions of ABCG2. Moreover, we revealed that that the ABCG2-overexpressing cells and the drug-sensitive parental cells are equally sensitive to MY-5445 treatment, suggesting that MY-5445 is not transported out of cells by ABCG2 and behaves as a high-affinity modulator for ABCG2. These results support the notion that MY-5445 can outcompete co-administered anticancer substrate drugs at the drug-binding pocket of ABCG2, which is consistent with the results of the fluorescent drug accumulation assays (Figure 4). It is worth noting that although MY-5445 and sildenafil are both inhibitors of PDE5, the phthalazine derivative MY-5445 is structurally distinct from the pyrimidone-containing sildenafil [67]. Therefore, it is not surprising if MY-5445 and sildenafil interact with ABCG2 at distinctive sites within the substrate-binding pocket of ABCG2 (Figure 6) [25], and the modulatory effect of MY-5445 on ABCG2-mediated drug efflux is more pronounced than that of sildenafil (Figure 4C). Shi et al. reported that sildenafil at 50 μM resensitized S1-M1-80 cancer cells to mitoxantrone and SN-38 with calculated FR values of 6.8 and 7.3, respectively [25]. In comparison, MY-5445 at 3 μM resensitized S1-M1-80 cancer cells to mitoxantrone and SN-38 with calculated FR values of 53.1 and 26.9, respectively (Table 2). Interestingly, sildenafil appeared to be significantly more effective in reversing MDR mediated by ABCB1 than reversing MDR mediated by ABCG2 [25]. Similarly, Ding et al. reported that the pyrimidone-containing PDE5 inhibitors vardenafil and tadalafil were selective in reversing ABCB1-mediated MDR in cancer cell lines [66].
While other mechanisms may also contribute to the resensitization of ABCG2-overexpressing cancer cells to chemotherapeutic agents, the mode of action of MY-5445 appears to involve direct inhibition of the drug transport function of ABCG2, leading to enhanced drug-induced apoptosis and cytotoxicity in ABCG2-overexpressing multidrug-resistant cancer cells (Figure 7). Although the combination therapy may occasionally result in unfavorable clinical responses [68,69], our results support the use of combination therapy of PDE5 inhibitor MY-5445 with conventional anticancer drugs against ABCG2-overexpressing multidrug-resistant cancers, and this needs to be further investigated in animal and clinical studies.
Figure 7.
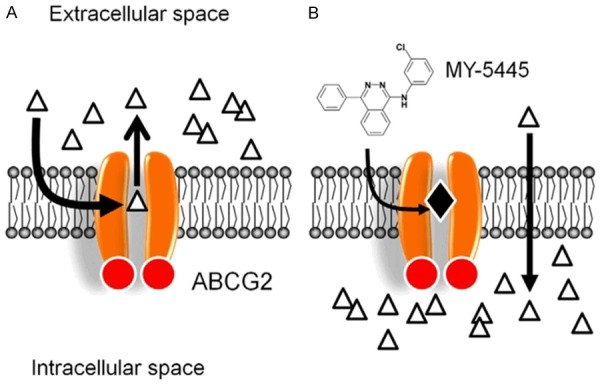
Model for MY-5445 resensitizing ABCG2-overexpressing cancer cells. Schematic proposing the fate of chemotherapeutic drug substrates of ABCG2 (triangles) in the absence (A) and the presence (B) of MY-5445. In the absence of MY-5445, the intracellular concentrations of chemotherapeutic drug and its cytotoxic effects are significantly reduced in these ABCG2-expressing cancer cells through direct drug efflux by ABCG2. In contrast, in the presence of MY-5445 (filled diamond), the drug efflux function of ABCG2 is blocked by MY-5445 binding to the drug-binding pocket of ABCG2. Consequently, the intracellular concentration of the chemotherapeutic drugs is restored in these multidrug-resistant cancer cells.
Acknowledgements
This research was supported by the Ministry of Science and Technology of Taiwan (MOST-108-2320-B-182-035 and MOST-108-2320-B-182-038-MY3), Chang Gung Medical Research Program (CMRPD1J0281 and BMRPC17), and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research to SL and SVA.
Disclosure of conflict of interest
None.
Abbreviations
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- ABCB1
ABC transporter-subfamily B member 1
- ABCC1
ABC transporter-subfamily C member 1
- ABCG2
ABC transporter-subfamily G member 2
- FR
Fold reversal
References
- 1.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 3.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 4.Wu CP, Hsieh CH, Wu YS. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm. 2011;8:1996–2011. doi: 10.1021/mp200261n. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 6.Hegedus C, Ozvegy-Laczka C, Szakács G, Sarkadi B. Interaction of ABC multidrug transporters with anticancer protein kinase inhibitors: substrates and/or inhibitors? Curr Cancer Drug Targets. 2009;9:252–272. doi: 10.2174/156800909788166565. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med. 2014;7:53–64. doi: 10.2147/PGPM.S38295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovalev AA, Tsvetaeva DA, Grudinskaja TV. Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer. Exp Oncol. 2013;35:287–290. [PubMed] [Google Scholar]
- 9.Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, Nishiwaki Y, Kodama T, Suga M, Ochiai A. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 10.Pilarski LM, Belch AR. Intrinsic expression of the multidrug transporter, P-glycoprotein 170, in multiple myeloma: implications for treatment. Leuk Lymphoma. 1995;17:367–374. doi: 10.3109/10428199509056847. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzenbach H. Expression of MDR1/P-glycoprotein, the multidrug resistance protein MRP, and the lung-resistance protein LRP in multiple myeloma. Med Oncol. 2002;19:87–104. doi: 10.1385/MO:19:2:87. [DOI] [PubMed] [Google Scholar]
- 12.Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood. 2006;108:3881–3889. doi: 10.1182/blood-2005-10-009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews C, Catherwood MA, Larkin AM, Clynes M, Morris TC, Alexander HD. MDR-1, but not MDR-3 gene expression, is associated with unmutated IgVH genes and poor prognosis chromosomal aberrations in chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47:2308–2313. doi: 10.1080/10428190600881421. [DOI] [PubMed] [Google Scholar]
- 14.Ross DD, Karp JE, Chen TT, Doyle LA. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000;96:365–368. [PubMed] [Google Scholar]
- 15.Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia. 2002;16:1443–1447. doi: 10.1038/sj.leu.2402541. [DOI] [PubMed] [Google Scholar]
- 16.Uggla B, Ståhl E, Wågsäter D, Paul C, Karlsson MG, Sirsjö A, Tidefelt U. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res. 2005;29:141–146. doi: 10.1016/j.leukres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Mirski SE, Gerlach JH, Cole SP. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987;47:2594–2598. [PubMed] [Google Scholar]
- 18.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 19.Cole SP. Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future. Annu Rev Pharmacol Toxicol. 2014;54:95–117. doi: 10.1146/annurev-pharmtox-011613-135959. [DOI] [PubMed] [Google Scholar]
- 20.Larkin A, O’Driscoll L, Kennedy S, Purcell R, Moran E, Crown J, Parkinson M, Clynes M. Investigation of MRP-1 protein and MDR-1 P-glycoprotein expression in invasive breast cancer: a prognostic study. Int J Cancer. 2004;112:286–294. doi: 10.1002/ijc.20369. [DOI] [PubMed] [Google Scholar]
- 21.Bodó A, Bakos E, Szeri F, Váradi A, Sarkadi B. The role of multidrug transporters in drug availability, metabolism and toxicity. Toxicol Lett. 2003;140-141:133–143. doi: 10.1016/s0378-4274(02)00497-6. [DOI] [PubMed] [Google Scholar]
- 22.Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Curr Drug Deliv. 2007;4:324–333. doi: 10.2174/156720107782151241. [DOI] [PubMed] [Google Scholar]
- 23.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1:93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4:205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, Talele TT, Fu LW, Chen ZS. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla S, Chen ZS, Ambudkar SV. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat. 2012;15:70–80. doi: 10.1016/j.drup.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwari AK, Sodani K, Dai CL, Abuznait AH, Singh S, Xiao ZJ, Patel A, Talele TT, Fu L, Kaddoumi A, Gallo JM, Chen ZS. Nilotinib potentiates anticancer drug sensitivity in murine ABCB1-, ABCG2-, and ABCC10-multidrug resistance xenograft models. Cancer Lett. 2013;328:307–317. doi: 10.1016/j.canlet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SQ, Liu ST, Zhao BX, Yang FH, Wang YT, Liang QY, Sun YB, Liu Y, Song ZH, Cai Y, Li GF. Afatinib reverses multidrug resistance in ovarian cancer via dually inhibiting ATP binding cassette subfamily B member 1. Oncotarget. 2015;6:26142–26160. doi: 10.18632/oncotarget.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao SH, Lu YJ, Li YQ, Huang YH, Hsieh CH, Wu CP. Osimertinib (AZD9291) attenuates the function of multidrug resistance-linked ATP-binding cassette transporter ABCB1 in vitro. Mol Pharm. 2016;13:2117–25. doi: 10.1021/acs.molpharmaceut.6b00249. [DOI] [PubMed] [Google Scholar]
- 30.Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A, Boudriot U, Neubauer A. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–1275. doi: 10.1038/sj.leu.2404638. [DOI] [PubMed] [Google Scholar]
- 31.Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR Jr, Chen X, Chen ZS. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153–161. doi: 10.1016/j.bcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Alemany R, Moura DS, Redondo A, Martinez-Trufero J, Calabuig S, Saus C, Obrador-Hevia A, Ramos R, Villar VH, Valverde C, Vaz MA, Medina J, Felipe-Abrio I, Hindi N, Taron M, Martin-Broto J. Nilotinib as co-adjuvant treatment with doxorubicin in patients with sarcomas: a phase I trial of the Spanish group for research on sarcoma. Clin Cancer Res. 2018;24:5239–5249. doi: 10.1158/1078-0432.CCR-18-0851. [DOI] [PubMed] [Google Scholar]
- 33.Hagiwara M, Endo T, Kanayama T, Hidaka H. Effect of 1-(3-chloroanilino)-4-phenylphthalazine (MY-5445), a specific inhibitor of cyclic GMP phosphodiesterase, on human platelet aggregation. J Pharmacol Exp Ther. 1984;228:467–471. [PubMed] [Google Scholar]
- 34.Ito M, Nishikawa M, Fujioka M, Miyahara M, Isaka N, Shiku H, Nakano T. Characterization of the isoenzymes of cyclic nucleotide phosphodiesterase in human platelets and the effects of E4021. Cell Signal. 1996;8:575–581. doi: 10.1016/s0898-6568(96)00112-x. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira SH, Duarte ID, Lorenzetti BB. The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol. 1991;201:121–122. doi: 10.1016/0014-2999(91)90333-l. [DOI] [PubMed] [Google Scholar]
- 36.Duarte ID, Ferreira SH. The molecular mechanism of central analgesia induced by morphine or carbachol and the L-arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. 1992;221:171–174. doi: 10.1016/0014-2999(92)90789-7. [DOI] [PubMed] [Google Scholar]
- 37.Duarte ID, Ferreira SH. L-NAME causes antinociception by stimulation of the arginine-NO-cGMP pathway. Mediators Inflamm. 2000;9:25–30. doi: 10.1080/09629350050024348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messina E, Lupi F, Barile L, Giacomello A. Cyclic nucleotides and neuroblastoma differentiation. Nucleosides Nucleotides Nucleic Acids. 2004;23:1551–1554. doi: 10.1081/NCN-200027775. [DOI] [PubMed] [Google Scholar]
- 39.Xu Q, Ma J, Lei J, Duan W, Sheng L, Chen X, Hu A, Wang Z, Wu Z, Wu E, Ma Q, Li X. alpha-mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the PI3K/Akt pathway. Biomed Res Int. 2014;2014:546353. doi: 10.1155/2014/546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zurbonsen K, Michel A, Vittet D, Bonnet PA, Chevillard C. Dissociation between phosphodiesterase inhibition and antiproliferative effects of phosphodiesterase inhibitors on the Dami cell line. Biochem Pharmacol. 1997;53:1141–1147. doi: 10.1016/s0006-2952(96)00822-2. [DOI] [PubMed] [Google Scholar]
- 41.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller M, Yong M, Peng XH, Petre B, Arora S, Ambudkar SV. Evidence for the role of glycosylation in accessibility of the extracellular domains of human MRP1 (ABCC1) Biochemistry. 2002;41:10123–10132. doi: 10.1021/bi026075s. [DOI] [PubMed] [Google Scholar]
- 43.Henrich CJ, Bokesch HR, Dean M, Bates SE, Robey RW, Goncharova EI, Wilson JA, McMahon JB. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J Biomol Screen. 2006;11:176–183. doi: 10.1177/1087057105284576. [DOI] [PubMed] [Google Scholar]
- 44.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–6639. [PubMed] [Google Scholar]
- 45.Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol Cancer Ther. 2007;6:3287–3296. doi: 10.1158/1535-7163.MCT-07-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–1520. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 47.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr, Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 49.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 50.Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, Ambudkar SV. Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. J Membr Biol. 2000;173:203–214. doi: 10.1007/s002320001020. [DOI] [PubMed] [Google Scholar]
- 51.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 52.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 53.Alam A, Kowal J, Broude E, Roninson I, Locher KP. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science. 2019;363:753–756. doi: 10.1126/science.aav7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17:57–61. [PubMed] [Google Scholar]
- 56.Hsiao SH, Lusvarghi S, Huang YH, Ambudkar SV, Hsu SC, Wu CP. The FLT3 inhibitor midostaurin selectively resensitizes ABCB1-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic agents. Cancer Lett. 2019;445:34–44. doi: 10.1016/j.canlet.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyoda Y, Takada T, Suzuki H. Inhibitors of human ABCG2: from technical background to recent updates with clinical implications. Front Pharmacol. 2019;10:208. doi: 10.3389/fphar.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuestas ML, Castillo AI, Sosnik A, Mathet VL. Downregulation of mdr1 and abcg2 genes is a mechanism of inhibition of efflux pumps mediated by polymeric amphiphiles. Bioorg Med Chem Lett. 2012;22:6577–6579. doi: 10.1016/j.bmcl.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Natarajan K, Bhullar J, Shukla S, Burcu M, Chen ZS, Ambudkar SV, Baer MR. The Pim kinase inhibitor SGI-1776 decreases cell surface expression of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and drug transport by Pim-1-dependent and -independent mechanisms. Biochem Pharmacol. 2013;85:514–524. doi: 10.1016/j.bcp.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 61.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu S, Fu L. Tyrosine kinase inhibitors enhanced the efficacy of conventional chemotherapeutic agent in multidrug resistant cancer cells. Mol Cancer. 2018;17:25. doi: 10.1186/s12943-018-0775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandner P, Hutter J, Tinel H, Ziegelbauer K, Bischoff E. PDE5 inhibitors beyond erectile dysfunction. Int J Impot Res. 2007;19:533–543. doi: 10.1038/sj.ijir.3901577. [DOI] [PubMed] [Google Scholar]
- 64.Tiwari AK, Chen ZS. Repurposing phosphodiesterase-5 inhibitors as chemoadjuvants. Front Pharmacol. 2013;4:82. doi: 10.3389/fphar.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen JJ, Sun YL, Tiwari AK, Xiao ZJ, Sodani K, Yang DH, Vispute SG, Jiang WQ, Chen SD, Chen ZS. PDE5 inhibitors, sildenafil and vardenafil, reverse multidrug resistance by inhibiting the efflux function of multidrug resistance protein 7 (ATP-binding Cassette C10) transporter. Cancer Sci. 2012;103:1531–1537. doi: 10.1111/j.1349-7006.2012.02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding PR, Tiwari AK, Ohnuma S, Lee JW, An X, Dai CL, Lu QS, Singh S, Yang DH, Talele TT, Ambudkar SV, Chen ZS. The phosphodiesterase-5 inhibitor vardenafil is a potent inhibitor of ABCB1/P-glycoprotein transporter. PLoS One. 2011;6:e19329. doi: 10.1371/journal.pone.0019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bollenbach M, Lugnier C, Kremer M, Salvat E, Megat S, Bihel F, Bourguignon JJ, Barrot M, Schmitt M. Design and synthesis of 3-aminophthalazine derivatives and structural analogues as PDE5 inhibitors: anti-allodynic effect against neuropathic pain in a mouse model. Eur J Med Chem. 2019;177:269–290. doi: 10.1016/j.ejmech.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 68.Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, Sorrentino B, Zhou S, Houghton PJ, Stewart CF. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66:4802–4807. doi: 10.1158/0008-5472.CAN-05-2915. [DOI] [PubMed] [Google Scholar]
- 69.Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, Daw N, Jenkins JJ 3rd, Gilbertson R, Germain GS, Harwood FC, Houghton PJ. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–7499. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 70.Wu CP, Hsiao SH, Murakami M, Lu MJ, Li YQ, Hsieh CH, Ambudkar SV, Wu YS. Tyrphostin RG14620 selectively reverses ABCG2-mediated multidrug resistance in cancer cell lines. Cancer Lett. 2017;409:56–65. doi: 10.1016/j.canlet.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu CP, Hsiao SH, Su CY, Luo SY, Li YQ, Huang YH, Hsieh CH, Huang CW. Human ATP-binding cassette transporters ABCB1 and ABCG2 confer resistance to CUDC-101, a multi-acting inhibitor of histone deacetylase, epidermal growth factor receptor and human epidermal growth factor receptor 2. Biochem Pharmacol. 2014;92:567–576. doi: 10.1016/j.bcp.2014.10.003. [DOI] [PubMed] [Google Scholar]


