Abstract
The metabolic reprogramming is an important basis for the development of many tumors, including prostate cancer (PCa). Metabolic changes in many amino acids consist of serine and glycine affect the biological behavior of them. Phospholipase C epsilon (PLCε) plays an important role as an oncogene. However, its role in regulating amino acid metabolism remains unclear. In this study, results found significantly positive correlation between PLCε and Yes-associated protein (YAP) in PCa tissues. LC-MS/MS and GC-MS results further displayed abnormally elevated levels of serine, glycine and its some downstream metabolites in the blood of PCa patients. Secondly, PLCε knockdown can inhibit serine/glycine producing and proliferation of PCa both in vivo and in vitro. Mechanistically, PLCε may affect the serine/glycine metabolism by regulating dephosphorylation and nuclear translocation of YAP. More interestingly, verteporfin (VP, a specific inhibitor of YAP) could effectively enhance the PLCε-depletion induced inhibition of serine/glycine secretion and growth. Overall, this research revealed the possibility of anomalous serine/glycine levels in the blood for the diagnosis of PCa, identified the important role of the PLCε/YAP axis in regulating serine/glycine metabolism, cell proliferation and tumor growth, and suggested the combination of VP with PLCε-depletion may provide a new idea for the treatment of PCa.
Keywords: PLCε, YAP, prostate cancer, serine/glycine metabolism, verteporfin, proliferation
Introduction
Prostate cancer (PCa) is still one of the most important causes of cancer death in men in developed countries [1]. More urgent is the incidence of PCa in Chinese men dramatically increased in recent years, but the factors that cause it are not fully understood [2].
Metabolism reprogramming is the one of the most important features of tumors, including amino acids uptake disorder [3]. Excessive accumulation of glycine due to obstacles in the glycine deportation system [4], which provides raw materials for the synthesis of biological proteins, purines and nucleic acids [5]. In tumor cells, the intermediates of the glycolytic pathway first generate serine via the key enzymes phosphoserine aminotransferase1 (PSAT1) and phosphoserine phosphatase (PSPH), and then glycine is produced by serine hydroxymethyltransferase2 (SHMT2) [6,7]. Additional, a variety of amino acids are closely related to the progression of PCa [8]. Many reports have pointed to some certain degree of metabolic disorders in PCa patients, including prostate specific antigen (PSA), sarcosine and many more [9-11]. Therefore, it is so necessary to explore the mechanism affects serine/glycine metabolism that provides some ideas for PCa diagnosis and treatment.
PLCε is a new isozyme of the discovered PLC family. As an important member of the phospholipase family, it is not only an effector of Ras, Rho and Rap, but also mediates signals from G-protein coupled receptors (GPER) [12-14]. Researches found PLCε is associated with many cancers including esophageal cancer, gastric cancer and so on [15-17]. Previous studies discovered that PLCε can regulate mitochondrial metabolism of PCa and inducing invasion and metastasis of castration-resistant prostate cancer (CRPC) [18,19]. However, the regulation mechanism of PLCε on serine/glycine metabolism in PCa is still unclear.
YAP, as a key effector molecule downstream of the Hippo signaling pathway [20], which is not only highly correlated with the homeostasis and development of organisms [21], but also been found to have significant biological properties in breast cancer, rectal cancer and the like [22,23]. In recent years, accumulating evidences revealed that YAP is inextricably linked to tumor metabolisms such as glucose and fatty acid metabolism [24]. More importantly, YAP regulates the activity of key enzymes involved in the serine pathway in breast cancer [25]. Consequently, it is particularly essential to explore and confirm the relationship between YAP and tumor serine/glycine metabolism.
In this study, we explored the role of PLCε in serine/glycine metabolism of PCa, and tried to find a potential relationship between PLCε and YAP. Therefore, we hypothesized that PLCε knocking-down would regulate the serine/glycine metabolism and proliferation of PCa by affecting YAP.
Materials and methods
Blood and tissue samples
Tissue specimens (including 55 cases of benign prostatic hyperplasia (BPH) and 58 cases of PCa) from 2015 to 2017, blood specimens (including 43 cases of normal samples and 66 cases of PCa) from 2018 to 2019 were all collected from the Department of Urology, the First Affiliated Hospital of Chongqing Medical University. All specimens were histologically diagnosed as BPH or PCa, and the patient’s informed consent was obtained before the experiment.
Immunohistochemistry
All the samples involved in the experiment, including human PCa and BPH specimens, nude mice xenograft tissues were cut into paraffin sections. Detection of PLCε, YAP and CyclinD1 expression in PCa, BPH and nude mice xenograft tissue samples by immunohistochemical (IHC) staining procedure, antibodies were as follows: anti-PLCε (dilution 1:50; Santa Cruz Biotechnology); anti-YAP (dilution 1:200; CST). The standard is the same as we have published before [26].
Cell culture and treatment
Prostate cell lines PC3, LNCaP and RWPE-1 came from Shanghai Zhong Qiao Xin Zhou Biotechnology Company. DU145 purchased from Shanghai Biowing Applied Biotechnology Company. RWPE-1 cultured with K-SFM medium, and cancer cell lines with RPMI1640 (Gibco), supplemented with 10% of fetal bovine serum (FBS, Gibco), placed in 37°C incubator containing 5% CO2, 1% O2 and 45%-65% humidity. The lentivirus infection method was same as that published in our previous study [27]. Cells (1 × 105/well) were seeded in a six-well plate and incubated with 2 ml of complete medium for 12 hours, and then added with plasmids or VP (MedChemExpress, USA). After 48 hours of incubation, subsequent experiments were performed.
Immunofluorescence
The cells were cultured on 24-well plate coverslips. Different treatments were added and incubation was continued for 48 hours. The cells were then treated with 4% paraformal-dehyde, 0.1-1% Triton and 5% goat blocking serum in sequence. Primary antibody anti-YAP (dilution 1:100; CST) and secondary antibody and 4,6-diamidino-2-pheny-lindole (Zhongshan Golden Bridge Biotechnology, Beijing, China) were incubated. Immunofluorescence (IF) photographs were taken by Nikon Eclipse 80i microscope (Eclipse 80i, Tokyo, Japan) at 400 × magnication.
Reverse transcription-quantitative polymerase chain reaction
RNA was extracted with Trizol (Takara, Tokyo, Japan), and PrimeScript RT reagent kits (Takara) was used to reverse RNA. The SYBR enzyme was used to quantify the polymerase chain reaction. β-actin was regarded as a standard reference. The comparative 2-ΔΔCt method was served as calculating the relative expression level of relative messenger RNA (mRNA). Repeat each experiment at least three times. The primer sequences were as following:
PLCε, Forward: GGAGAATCCTCGGTAG, Reverse: GGTTGTCAGCGTATGTCC; YAP, Forward: TAGCCCTGCGTAGCCAGTTA, Reverse: TCATGCTTAGTCCACTGTCTGT; PSAT1, Forward: TGCCGCACTCAGTGTTGTTAG, Reverse: GCAATTCCCGCACAAGATTCT; PSPH, Forward: GAGGACGCGGTGTCAGAAAT, Reverse: GGTTGCTCTGCTATGAGTCTCT; SHMT2, Forward: CCCTTCTGCAACCTCACGAC, Reverse: TGAGCTTATAGGGCATAGACTCG; CyclinD1, Forward: GCTGGAGCCCGTGAAAAAGA, Reverse: CTCCGCCTCTGGCATTTTG; PCNA, Forward: TCAAGAAGGTGTTGGAGGCA, Reverse: CAGCGGTAGGTGTCGAAGC; β-actin, Forward: GGGACCTGACTGACTACCTC, Reverse: ACGAGACCACCTTCAACTCCAC.
Western blot
Total proteins were extracted by RIPA lysate containing the protease inhibitor phenylmethane sulfonyl fluoride and phosphatase inhibitors. Nuclear and plasma proteins were extracted using nuclear and cytoplasmic proteins extraction reagent (Beyotime Institute of Biotechnology, Jiangsu, China). And the BCA kit (Beyotime Biotechnology, Shanghai, China) was used to detect protein concentration. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% gel) for protein separation (50 μg). Antibodies were as following: anti-PLCε (dilution 1:1000; Santa Cruz Biotechnology); anti-YAP (dilution 1:1000; CST); anti-PSAT1 (dilution 1:1000; Absin); anti-PSPH (dilution 1:1000; Absin); anti-SHMT2 (dilution 1:500; Novus); anti-CyclinD1 (dilution 1:500) and anti-PCNA (dilution 1:500) were from Wanlei-bio (Shenyang, China), anti-β-actin (dilution 1:3000), and anti-H3 (dilution 1:2000) were purchased from Abcam (Cambridge, UK). Enhanced Chemiluminescence Detection Kit for exposure of protein strips.
Cell Counting kit-8
Cells (3 × 103/well) were cultured in 96-well plates overnight, then treated with VP. After 48 hours, 10 μl of Cell Counting Kit-8 (CCK-8) reagent (Univ-bio, Shanghai, China) was added and continued for 1-4 hours in the container. Finally, the absorbance of cells was measured at 450 nm. Set 4 sub holes for each processing group.
Colony formation assay
Cells (500/well) with different treatments were added into 6-well plates and cultured for about 7-14 days. Then, the cells were fixed with methanol and stained with crystal violet, and finally photographed. Each treatment group was set three sub holes, and repeated trials three times independently for each treatment group.
Flow cytometry
Cells were transferred to a six-well plate overnight and treated with VP. After 48 hours, cells were washed 2-3 times with PBS, then digested into single with 0.25% trypsin, PBS washed twice and transfered into EP tubes. Discarded the supernatant and resuspend with 100 μl PBS, then 500 μl cooled 75% ethanol were added into EP tubes after centrifugation. Cell cycle were detected by CytoFLEX (Beckman Coulter, Inc. California, USA), and the results were analyzed by ModFitLT5.
Plasmid and lentiviral vector
PLCε interference lentivirus (LV-sh-PLCε), and negative control (LV-sh-NC), interference YAP plasmid (vector-sh-YAP) and negative control (vector-sh-NC) were purchased from Gene Pharma Company (Shanghai, China). The sequences of the lentivirus have been shown in previous report [18]. The sequences of vector-sh-NC, vector-sh-YAP#1, vector-sh-YAP#2, vector-sh-YAP#3 are following:
vector-sh-YAP#1-F, CCGGCCCAGTTAAATGTTCACCAATCTCGAGATTGGTGAACATTTAACTGGGTTTTTG; vector-sh-YAP#1-R, GGCCGGGTCAATTTACAAGTGGTTAGAGCTCTAACCACTTGTAAATTGACCCAAAAAC. vector-sh-YAP#2-F, GGCTGTTAGAGAGATAATTGGAATTAATTTGACTGTAAACACAAAGATATTAGTACAAAA; vector-sh-YAP#2-R, CCGACAATCTCTCTATTAACCTTAATTAAACTGACATTTGTGTTTCTATAATCATGTTTT. vector-sh-YAP#3-F, CACCGCCACCAAGCTAGATAAAGAATTCAAGAGATTCTTTATCTAGCTTGGTGGCTTTTTTG; vector-sh-YAP#3-R, GATCCAAAAAAGCCACCAAGCTAGATAAAGAATCTCTTGAATTCTTTATCTAGCTTGGTGGC. YAP over-expression (vector-YAP) and negative control plasmid (vector-NC) were purchased from Addgene (http://www.addgene.org/). vector-YAP-F, GACGGATCGGGAGATCTCCCGATCCCCTATGGTCGACTCTCAGTACAATCTGCTCTGATG; vector-YAP-R, CTGCCTAGCCCTCTAGAGGGCTAGGGGATACCAGCTGAGAGTCATGTTAGACGAGACTAC.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-MS) assay
The trypsin-digested cells (>106) from different treatment groups were collected, and the supernatant was discarded after centrifuged. Cells were washed three times with PBS, and then fully disrupted by sonicator. Finally, pre-cooled methanol was added for fixation. After being fixed, the lysed cells are suspended on a dried blood spot (DBS) to be sufficiently saturated. Fresh blood samples from different clinical patients were collected and the plasma was prepared in DBS format on filter paper and stored at 4°C. Standard testing techniques are described in the previous study [28].
Animal experiment
PC3 (4 × 106 cells) infected with LV-sh-NC and LV-sh-PLCε were implanted subcutaneously in 4-6 week old male nude mice (Chongqing Medical University Laboratory Animal Center). VP was injected into mice through the tail vein when tumors formed whose injection frequency was once every two days. Primary tumor growth was monitored every 2 to 3 days by an external caliper. Four weeks after giving drugs, all the mice were sacrificed and blood was taken for mass spectrometry experiments. Tumor tissues were removed, volume and weight measurements were taken and fixed for histological studies. Tumor volume (mm3) was calculated: volume (mm3) = 1/2 × length × width2. This animal experiment was carried out in strict accordance with the guidelines for animal experiments and was approved by the Ethics Committee of Chongqing Medical University (Chongqing, China).
Statistical analysis
All experiments were repeated three times and above independently. SPSS17.0 was applied to statistical analysis. All data were shown as the mean ± SD. Student’s t test, Pearson’s analysis, the χ2 test, Mann-Whitney test, one-way analysis of variance (ANOVA) and two-way ANOVA were used to evaluate significant differences between categorical variables. Value of P<0.05 was considered as statistically significant.
Results
High correlation between PLCε and YAP in PCa tissues
The expression levels of PLCε and YAP in 55 BPH and 58 PCa samples were determined by IHC assay. Results showed the positive rate of PLCε reached 74.14% (43/58) in PCa samples, vs 16.36% (9/55) in BPH samples. In addition, the positive rate of YAP was 82.76% (48/58) in PCa, vs 23.64% (13/55) in BPH (Table 1). Semi-quantitative staining scores showed an obviously increased PLCε (Figure 1E, P<0.001) and YAP (Figure 1F, P<0.001) in PCa compared with BPH tissues. Moreover, the higher the tumor grade, the stronger the staining (Figure 1A-D). Pearson’s linear correlation analysis results revealed that increased expression of PLCε was closely related to the high expression of YAP (Figure 1G, P<0.0001). More clearly, PLCε and YAP were significantly correlated with the Gleason grade among the clinical pathological parameters (Table 1).
Table 1.
PLCε and YAP in PCa tissues and linicopathological parameters
| Characteristics | No. specimens (%) | PLCε staining | YAP staining | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Positive | Negative | P value | Positive | Negative | P value | ||
| Total | 58 (100) | 43 (74.1) | 15 (25.9) | 48 (82.8) | 10 (17.2) | ||
| Age (year) | |||||||
| <60 | 17 (29.3) | 13 (22.4) | 4 (6.9) | 0.536 | 15 (25.9) | 2 (3.4) | 0.385 |
| ≥60 | 41 (70.7) | 30 (51.7) | 11 (19.0) | 33 (56.9) | 8 (13.8) | ||
| Histological stage | |||||||
| Ta-T2 | 20 (34.5) | 11 (19.0) | 9 (15.5) | 0.019* | 13 (22.4) | 7 (12.1) | 0.014* |
| T3-T4 | 38 (65.5) | 32 (55.1) | 6 (10.4) | 35 (60.4) | 3 (5.1) | ||
| Gleason score | |||||||
| <7 | 25 (43.1) | 15 (25.8) | 10 (17.3) | 0.033* | 17 (29.3) | 8 (13.8) | 0.012* |
| ≥7 | 33 (56.9) | 28 (48.3) | 5 (8.6) | 31 (53.5) | 2 (3.4) | ||
Note. PCa: prostate cancer. Statistical method: χ2 test. The bold entries represent statistically significant differences.
P<0.05;
**P<0.01;
***P<0.001.
Figure 1.
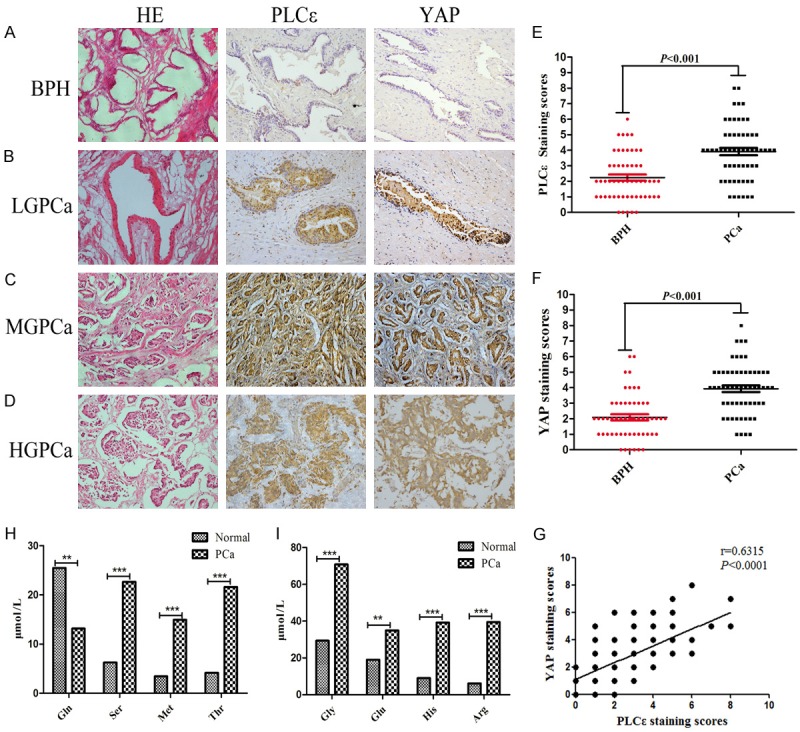
High expression of PLCε and YAP in tissues and high levels of serine/glycine in blood of PCa. (A-D) Representative hematoxylin and eosin (H&E) staining and IHC staining in 55 PCa and 58 BPH samples. Magnification × 200. Representative IHC staining of different staining intensities was used as a criterion for staining scores: BPH (A): with no staining; Low-grade (LG) PCa (B): with light staining; Middle-grade (MG) PCa (C): with moderate staining; High-grade (HG) PCa (D): with strong staining. (E, F) Staining scores of PLCε (E) and YAP (F) expression in BPH and PCa tissues. Data were represented as the means ± SD. (G) Correlation analysis between PLCε and YAP in tissues analyzed by Pearson analysis. (H, I) Multiple common amino acids concentration levels in different blood samples tesetd by mass spectrometry and analyzed by Mann-Whitney test. *P<0.05, **P<0.01, and ***P<0.001 vs. controls.
The concentration of serine/glycine in the blood of PCa is abnormally elevated
Plasma samples from 66 patients with PCa and 43 specimens from normal subjects were collected for detection of many amino acid concentrations by LC-MS/MS and GC-MS testing. As shown in Figure 1H, 1I, with the exception of glutamine, the concentrations of serine (Figure 1H), glycine (Figure 1I) and some of its downstream metabolites such as threonine and methioninein in their blood of PCa patients were higher than that in normal samples. It was also found that high levels of serine and glycine in PCa were significantly associated with Gleason score (Table 2). The experiment therefore confirmed that the abnormal metabolism of serine/glycine in PCa patients.
Table 2.
Serine/glycine concentrations and clinical pathological parameters in PCa
| Characteristics | No. specimens (%) | Serine (μmol/L) | Glycine (μmol/L) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Median = 17.639 | Median = 54.484 | ||||||
|
|
|
||||||
| <17.639 | ≥17.639 | P value | <54.484 | ≥54.484 | P value | ||
| Histology | |||||||
| Normal | 43 | 42 | 1 | 0.000*** | 34 | 9 | 0.000*** |
| PCa | 66 | 12 | 54 | 20 | 46 | ||
| Age (year) of PCa | |||||||
| <60 | 4 (6.1) | 1 (1.5) | 3 (4.5) | 0.561 | 2 (3.05) | 2 (3.05) | 0.352 |
| ≥60 | 62 (93.9) | 11 (16.7) | 51 (77.3) | 18 (27.3) | 44 (66.7) | ||
| PSA (μg/L) of PCa | |||||||
| Median = 20.67 | |||||||
| <20.67 | 26 (39.4) | 5 (7.6) | 21 (31.8) | 0.553 | 9 (13.6) | 17 (25.8) | 0.364 |
| ≥20.67 | 40 (60.6) | 7 (10.6) | 33 (50.0) | 11 (16.7) | 29 (43.9) | ||
| Gleason score of PCa | |||||||
| <7 | 13 (19.7) | 5 (7.6) | 8 (12.1) | 0.049* | 1 (1.5) | 12 (18.2) | 0.043* |
| ≥7 | 53 (80.3) | 7 (10.6) | 46 (69.7) | 19 (28.8) | 34 (51.5) | ||
Note. PSA: prostate specific antigen; PCa: prostate cancer. Statistical method: χ2 test. The bold entries represent statistically significant differences.
P<0.05;
**P<0.01;
P<0.001.
Knockdown of PLCε can inhibit the expression of YAP in PCa cells
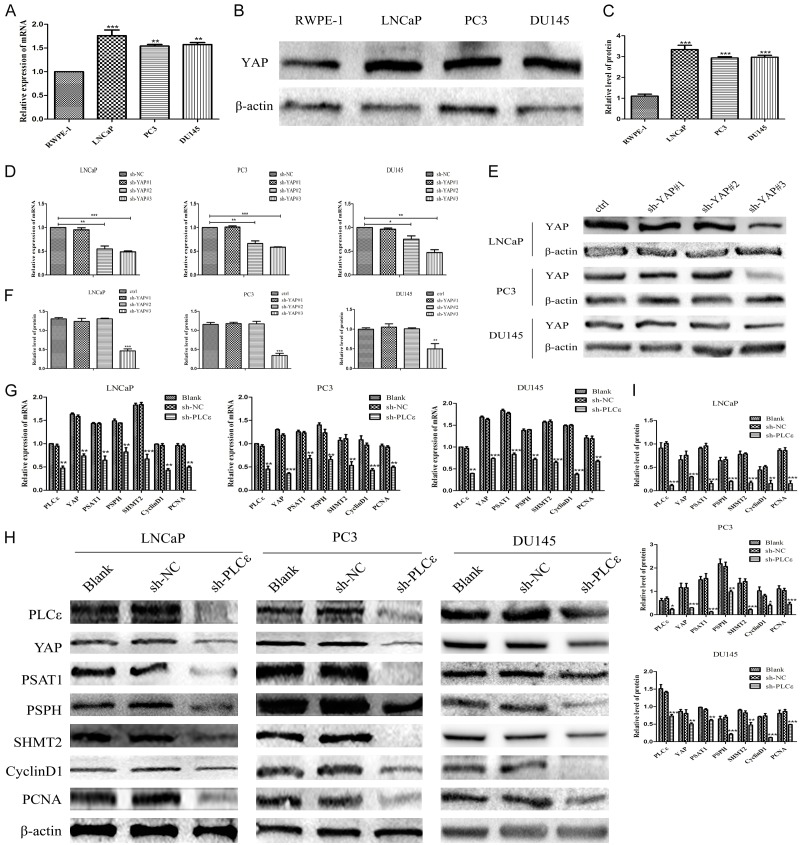
At its most basic, expression of YAP in normal prostate epithelial cell (RWPE-1) with PCa cell lines (LNCaP, PC3, DU145) were compared. As Figure 2A-C illustrated both the mRNA (Figure 2A) and protein (Figure 2B, 2C) of YAP in cancer cells were apparently higher than RWPE-1. Three plasmids short hairpin(sh)RNAs (vector-sh-YAP#1, vector-sh-YAP#2, and vector-sh-YAP#3) were constructed to knockdown YAP of PCa cells, whose effect were validated immediately. The results displayed sh-YAP#3 had the most significant knockdown effect both in mRNA (Figure 2D) and protein level (Figure 2E, 2F) which was used in next experiments. Then the expression of YAP was detected when depletion of PLCε, found that down-regulation expression of YAP in sh-PLCε group compared with sh-NC and blank group no mater in mRNA (Figure 2G) and protein level (Figure 2H, 2I).
Figure 2.
PLCε knockdown inhibits YAP mRNA and protein expression in PCa cell lines. (A-C) The messenger RNA mRNA (A) by quantitative polymerase chain reaction (q-PCR) and protein (B, C) levels by Western blot of YAP in different cell lines. (D-F) Knockdown of YAP plasmid on mRNA (D) and protein (E, F) levels of cell lines. (G-I) mRNA and protein levels of PLCε, YAP, PSAT1, PSPH, SHMT2, CyclinD1 and PCNA in cells were detected by qPCR (G) and Western blot analysis (H, I) after infected with lentiviral sh-PLCε. β-actin were used as internal controls. Data were represented as mean ± SD of three individual experiments. *P<0.05, **P<0.01, and ***P<0.001 vs. controls.
PLCε-depletion prevents serine/glycine metabolsim and proliferation of PCa cells
We were very curious whether PLCε knockdown will have an influence on serine/glycine production and proliferation of PCa cells. So mRNA and protein were examined by q-PCR and western blot. The results obtained that compared with the control group, the expression of serine/glycine producing enzyme (PSAT1, PSPH, SHMT2) and proliferation-related gene (CyclinD1, PCNA) were decline in sh-PLCε group (Figure 2G-I). As with the above results, the mass spectrometry results showed that both serine (Figure 5I) and glycine (Figure 5J) concentrations of cells in PLCε-depletion group were lower than control group. As expected, clone formation assay revealed the number of clones in sh-PLCε group was also much less than that of the control group (Figure 3G, 3H). The above results demonstrated that reducing PLCε can inhibit the serine/glycine production and proliferation of PCa cells.
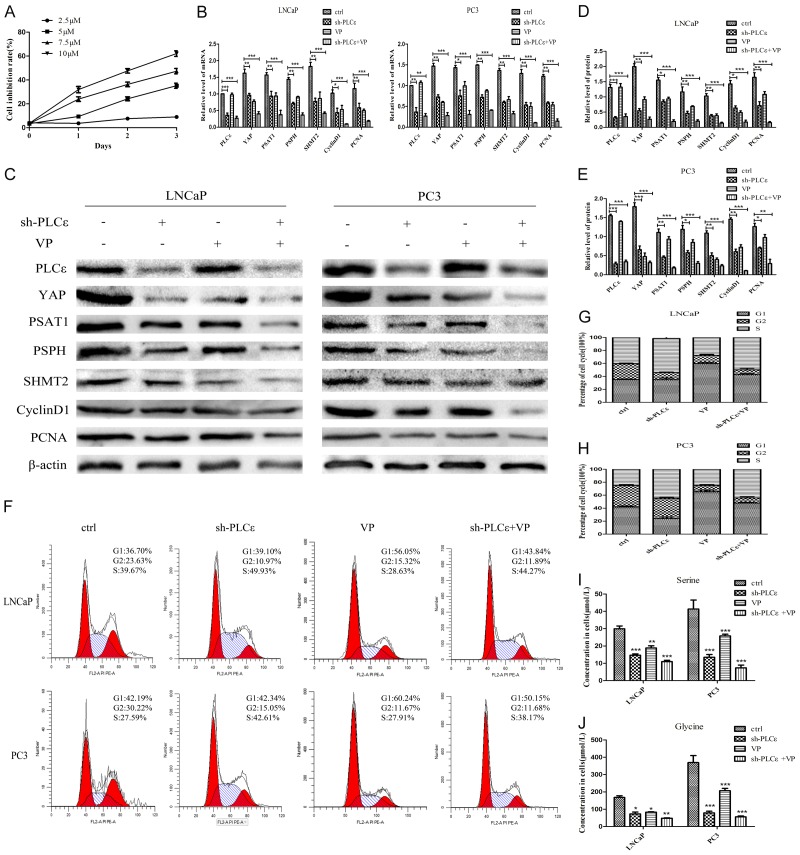
Figure 5.
VP enhances the inhibitory effect of PLCε depletion on PCa in vitro. (A) CCK-8 assay to detect the toxicity of different concentrations of VP (2.5 μM, 5.0 μM, 7.5 μM, 10 μM) on PC3 cells. (B-E) mRNA (B) and protein expression (C-E) of PLCε, YAP, PSAT1, PSPH, SHMT2, CyclinD1, and PCNA in the cell lines after treated with VP (5 μM, 48 h). (F-H) Flow cytometry detected cell cycle after cultured with VP (F), and statistical analysis (G, H). (I, J) Mass spectrometry tested the effect of VP on the concentration of serine (I) and glycine (J) in cells. Data were represented as mean ± SD of three individual experiments. *P<0.05, **P<0.01, and ***P<0.001 vs. controls.
Figure 3.
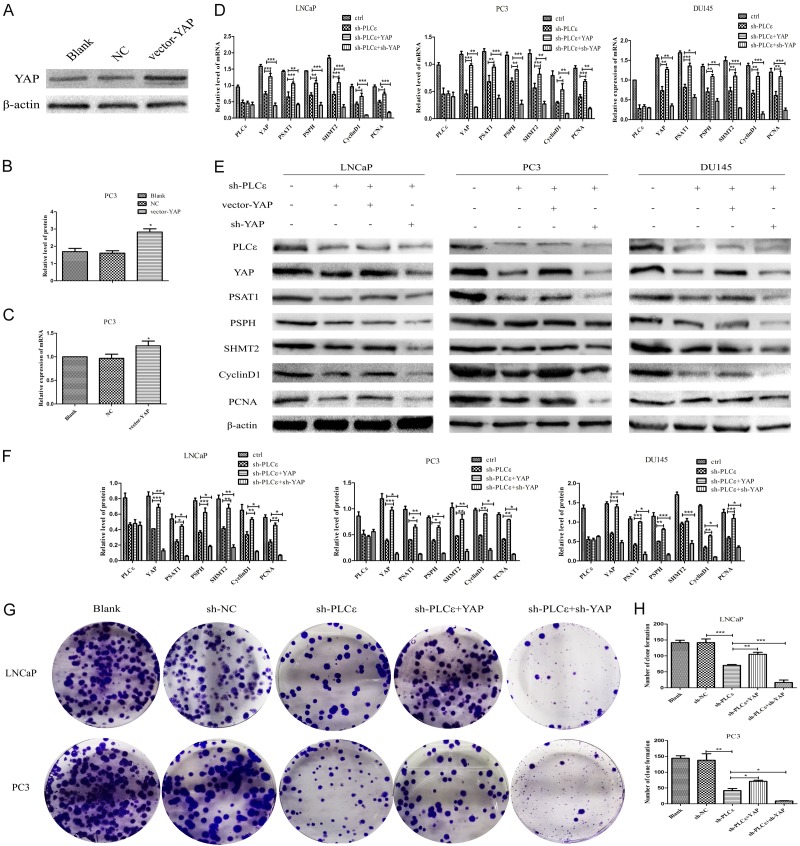
PLCε mediates serine/glycine metabolism and proliferation by modulating YAP. (A, B) Protein level verification of vector-YAP by western blot. (C) mRNA of vector-YAP by q-PCR. (D) q-PCR detection of mRNA levels of PLCε, YAP, PSAT1, PSPH, SHMT2, CyclinD1, and PCNA in cells after infected with vector-YAP or vector-sh-YAP. (E, F) Western blot detected and analyzed the protein expression of these gene mentioned in (D). (G, H) Clonal formation assay (G) and statistical analysis (H) of the numbers of colonies in cells after addition of vector-YAP or sh-YAP plasmid. β-actin were used as internal controls. Data were represented as mean ± SD of three individual experiments. *P<0.05, **P<0.01, and ***P<0.001 vs. controls.
PLCε regulates serine/glycine production and cell proliferation of PCa by modulating YAP
In order to explore whether PLCε affects the serine/glycine metabolism of PCa by affecting YAP. The effect of over-expressing YAP plasmid was verified firstly. q-PCR (Figure 3C) and western blot (Figure 3A, 3B) indicated vector-YAP can successfully raise YAP. Same methods to test the expression of three key enzymes of serine/glycine and proliferating genes. The results prompted that the serine/glycine-producing enzymes (PSAT1, PSPH, SHMT2) and proliferating genes (CyclinD1, PCNA) showed a certain degree of up-regulation in sh-PLCε+YAP group compared with sh-PLCε group at mRNA (Figure 3D) and protein (Figure 3E, 3F) level. In contrast, the serine/glycine production enzymes and proliferation genes were further decline in sh-PLCε+sh-YAP group. Supplementary, the join of the vector-YAP plasmid had no influence on PLCε (Figure 3D-F). These results suggested that PLCε may affect the serine/glycine production and cell proliferation of PCa by regulating YAP.
PLCε modulates phosphorylation/dephosphorylation and translocation of YAP
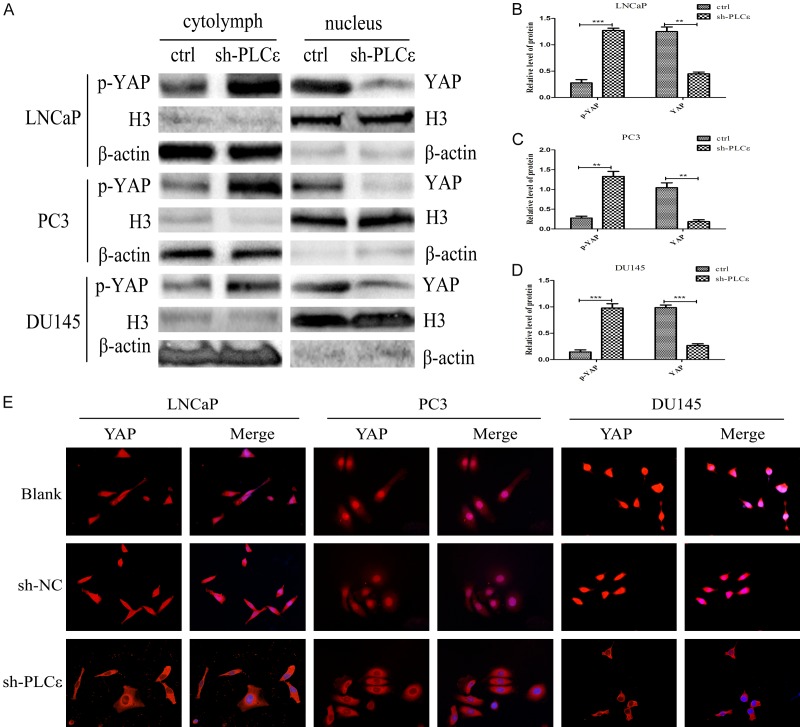
In order to further explore the specific mechanism in which PLCε regulates YAP, following experiments were conducted. First, nuclear and cytoplasmic proteins of PCa cells were extracted. Then western blot were used to exam the expression of YAP and p-YAP in nucleus and cytoplasm, respectively. The results suggested that when PLCε depletion, the expression of YAP in the nucleus was obviously down-regulated, corresponding to a certain degree of up-regulation of p-YAP in the cytoplasm (Figure 4A), and the gray value analysis of three cells were also significant (Figure 4B-D). To confirm the distribution of YAP in cells. Afterwards, IF was used to locate it. Most of YAP of PCa cells are distributed in the nucleus, a small part in cytoplasm in general. Experiment displayed that the YAP signal in nucleus were significantly reduced in the sh-PLCε group compared with control group (Figure 4E). Therefore, we suspected that knocking out PLCε can suppress the transfer of YAP from cytoplasm to the nucleus, thereby phosphorylation and degradation.
Figure 4.
PLCε regulates dephosphorylation and nuclear translocation of YAP. (A-D) Western blot (A) and protein quantification analyses (B-D) detected the expression of YAP in nucleus and p-YAP in cytoplasm. β-actin and Histone (H) 3 were used as an internal control. (E) Immunofluorescence staining demonstrated YAP intracellular distribution in PCa cell lines. Magnification × 400. Data were represented as mean ± SD of three individual experiments. *P<0.05, **P<0.01, and ***P<0.001 vs. controls.
Verteporfin enhances the role of knockdown PLCε in inhibiting the biological behavior of PCa
For further exploration, VP was applied which is the specific inhibitor of YAP. Then found its minimum inhibitory concentration (5 μM) through CCK-8 assay (Figure 5A), and carried out the next experiments. Results firstly demonstrated the inhibitory effect of VP on cells at mRNA and protein levels. The results of q-PCR (Figure 5B) and Western blot (Figure 5C-E) indicated that the expressions of YAP, PSAT1, PSPH, SHMT2, CyclinD1 and PCNA in sh-PLCε or VP group were significantly lower than those in control group, but the effect of sh-PLCε+VP group was the best. More importantly, the addition of VP had almost no significant impact on PLCε.
Followed by, VP was added to the cell culture medium for 48 hours. As shown in Figure 5F-H, flow cytometry confirmed that the cells in the S phase of the sh-PLCε group increased significantly, while the cells in the G1 phase of the VP group increased significantly. More meaningful was the mass spectrum results displayed that compared with the control group, the concentration of serine and glycine of the cells decreased obviously in the sh-PLCε group or adding VP group. However, the concentration decrease of sh-PLCε+VP group is the most obvious (Figure 5I, 5J).
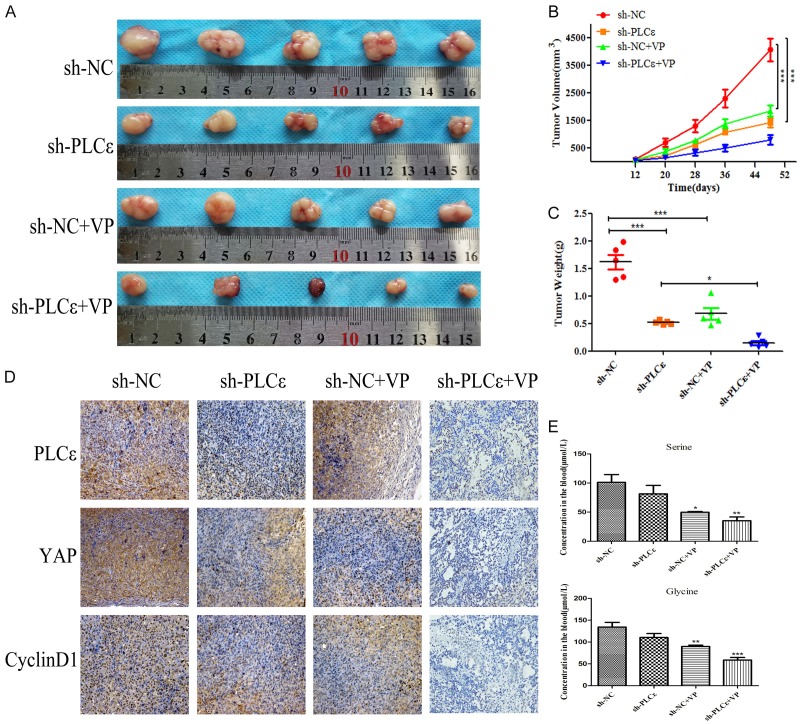
Verteporfin can effectively inhibit serine/glycine production and tumorigenesis of PCa in vivo
Previously results confirmed that PLCε-depletion can inhibit the serine/glycine production and proliferation of PCa by regulating YAP in vitro. Next, experiments further verified the conjecture by performing a nude mouse tumor xenograft test in vivo. Consistent with in vitro studies, both sh-PLCε and VP groups showed significant tumor suppression (Figure 6A). As expected, the above two groups showed a reduction in tumor volume (Figure 6B) and weight (Figure 6C) compared with control group. If sh-PLCε combined with injection of VP, the effect was most significant. Simultaneously, IHC staining of mouse tumors to observe the expression of PLCε, YAP and CyclinD1. Same as the guess, the above three genes performed lower level both in sh-PLCε and VP group than control. While sh-PLCε+VP group was far lower than other three groups (Figure 6D). Finally, the tail vein blood of nude mice was obtained for serine/glycine concentration, mass spectrometry revealed that consumption of PLCε and VP separately can both suppress serine/glycine levels, the combination of the two still had better results (Figure 6E). Both experiments in vivo and in vitro have further proven VP can enhance the role of knockdown PLCε in inhibiting the biological behavior of PCa.
Figure 6.
VP effectively inhibits serine/glycine production and tumor growth in vivo. (A) Photographs showed the tumors dissected from the nude mice after subcutaneous tumor formation. (B, C) Volumetric (B) and weight (C) changes of tumors after injection of PC3 cells with sh-PLCε and/or VP in nude mice. (D) IHC staining of PLCε, YAP and CyclinD1 in tumor tissues from mice. Magnification × 200. (E) Serine/glycine concentration in venous blood of mice was detected by mass spectrometry. Data were represented as mean ± SD. *P<0.05, **P<0.01, and ***P<0.001 vs. controls.
Discussion
Abnormal metabolism of serine/glycine is closely related to the occurrence and development of tumors [29-31]. In addition, serine/glycine metabolites can also regulate other metabolic pathways [32,33]. Moreover, imbalance regulation of PLCε can lead to the development of urinary tumors [34,35]. Results firstly observed aberrant levels of serine and glycine in the blood of PCa patients. And just as YAP can regulate certain key enzymes in the process of serine/glycine production. After that IHC found high expression of PLCε always accompanied with high YAP in tissues, and both were closely related to the patient’s low survival and Gleason score. Coincidentally, PLCε and YAP were significantly related after correlation analysis. Therefore, we hypothesized there may be a potential association between PLCε and YAP.
As the most unique member of the PLC family, PLCε not only participates in the occurrence and development of tumors [36,37], it also plays a role in tumor suppression [38]. As an oncogene, in addition to the traditional hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), producing inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), it can also directly plays an important role in the regulation of small molecular weight or small G proteins [13,39,40]. Therefore, the specific role of PLCε still has doubts. This study has demonstrated that weakening of PLCε with lentivirus can suppress the production of serine/glycine and the proliferation by cell and animal experiments, which suggested that PLCε may be involved in serine/glycine metabolism of PCa.
Research then explored how PLCε affects the serine/glycine metabolism of PCa. Recent reports suggested YAP is highly expressed in PCa, and YAP-AR axis may be related to the development of it [41]. The presence of YAP in breast [25] and rhabdomyosarcoma [42] may partake the serine biosynthesis pathway. That being the case, to prove that YAP participates in the production of serine/glycine in PCa. Then results displayed down-regulation of YAP by using vector-sh-YAP can successfully suppress the expression of key enzymes of serine/glycine production at the mRNA and protein level. Similarly, the consumption of YAP also reduced the level of serine/glycine concentration of PCa cells. Results verified that YAP induces the process of serine/glycine production in PCa.
Zhou et al. recently discovered that G protein-coupled estrogen receptor (GPER) can activate YAP through PLCβ signaling pathway [43] and is related to cell invasion and metastasis in breast cancer [44]. As an isozyme of PLCβ, we suspected that PLCε can also regulate YAP. As expected, this study confirmed that the mRNA and protein levels of YAP were significantly down-regulated after knocking down PLCε. Using plasmid to overexpress YAP can reverse this phenomenon. More clearly, experiments further proved that consume of PLCε can transfer YAP from nucleus into cytoplasm, so that can be rephosphorylated and finally degraded. The above results verified that PLCε does participate in the regulation of YAP-induced serine/glycine metabolism in PCa.
VP, currently used clinically for the treatment of age-related macular degeneration [45]. However, it has been found to be effective in the treatment of liver cancer [46], breast cancer [47], pancreatic cancer [48,49] and other tumors [50] in recent years. The above description of VP as a potential tumor-targeted drug is increasingly important. Although our research strongly demonstrated that PLCε can inhibit serine/glycine metabolism and proliferation of PCa. But the addition of VP could greatly improve the inhibition caused by knockdown of PLCε, which will provide meaningful reference for clinical treatment of PCa.
Conclusion
On the whole, our study displayed that abnormal serine and glycine concentrations, which may be a potentially important indicator for the diagnosis of PCa. This was also the first time to discover that PLCε/YAP axis may be involved in serine/glycine metabolism and growth of PCa. More specifically, PLCε works by modulating the dephosphorylation and nuclear translocation of YAP. Most critical, the combination of VP and knockout PLCε could effectively inhibit the production of serine/glycine and tumor growth, which would provide a certain clinical value for the treatment of PCa.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81272572).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyoğlu D, Idle JR. The glycine deportation system and its pharmacological consequences. Pharmacol Ther. 2012;135:151–167. doi: 10.1016/j.pharmthera.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo CC, Chen WC, Teo XQ, Radda GK, Lee PT. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma. Oncotarget. 2016;7:53005–53017. doi: 10.18632/oncotarget.10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heger Z, Gumulec J, Cernei N, Polanska H, Raudenska M, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R. Relation of exposure to amino acids involved in sarcosine metabolic pathway on behavior of non-tumor and malignant prostatic cell lines. Prostate. 2016;76:679–690. doi: 10.1002/pros.23159. [DOI] [PubMed] [Google Scholar]
- 9.Čapoun O, Soukup V, Kalousová M, Sobotka R, Pešl M, Zima T, Hanuš T. Diagnostic importance of selected protein serum markers in the primary diagnostics of prostate cancer. Urol Int. 2015;95:429–435. doi: 10.1159/000431364. [DOI] [PubMed] [Google Scholar]
- 10.Jamaspishvili T, Kral M, Khomeriki I, Student V, Kolar Z, Bouchal J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:12–19. doi: 10.1038/pcan.2009.31. [DOI] [PubMed] [Google Scholar]
- 11.Strmiska V, Michalek P, Eckschlager T, Stiborova M, Adam V, Krizkova S, Heger Z. Prostate cancer-specific hallmarks of amino acids metabolism: towards a paradigm of precision medicine. Biochim Biophys Acta Rev Cancer. 2019;1871:248–258. doi: 10.1016/j.bbcan.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Ada-Nguema AS, Xenias H, Hofman JM, Wiggins CH, Sheetz MP, Keely PJ. The small GTPase R-Ras regulates organization of actin and drives membrane protrusions through the activity of PLCepsilon. J Cell Sci. 2006;119:1307–1319. doi: 10.1242/jcs.02835. [DOI] [PubMed] [Google Scholar]
- 13.Dusaban SS, Brown JH. PLCepsilon mediated sustained signaling pathways. Adv Biol Regul. 2015;57:17–23. doi: 10.1016/j.jbior.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song C, Hu CD, Masago M, Kariyai K, Yamawaki-Kataoka Y, Shibatohge M, Wu D, Satoh T, Kataoka T. Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. J Biol Chem. 2001;276:2752–2757. doi: 10.1074/jbc.M008324200. [DOI] [PubMed] [Google Scholar]
- 15.Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, Yuan JM, Zheng W, Dawsey SM, Dong LM, Lee MP, Ding T, Qiao YL, Gao YT, Koh WP, Xiang YB, Tang ZZ, Fan JH, Wang C, Wheeler W, Gail MH, Yeager M, Yuenger J, Hutchinson A, Jacobs KB, Giffen CA, Burdett L, Fraumeni JF Jr, Tucker MA, Chow WH, Goldstein AM, Chanock SJ, Taylor PR. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764–767. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, Li JM, Kong GQ, Qi H, Cui J, Zhang LQ, Yang JZ, Li JL, Li XC, Ren JL, Liu ZC, Gao WJ, Yuan L, Wei W, Zhang YR, Wang WP, Sheyhidin I, Li F, Chen BP, Ren SW, Liu B, Li D, Ku JW, Fan ZM, Zhou SL, Guo ZG, Zhao XK, Liu N, Ai YH, Shen FF, Cui WY, Song S, Guo T, Huang J, Yuan C, Huang J, Wu Y, Yue WB, Feng CW, Li HL, Wang Y, Tian JY, Lu Y, Yuan Y, Zhu WL, Liu M, Fu WJ, Yang X, Wang HJ, Han SL, Chen J, Han M, Wang HY, Zhang P, Li XM, Dong JC, Xing GL, Wang R, Guo M, Chang ZW, Liu HL, Guo L, Yuan ZQ, Liu H, Lu Q, Yang LQ, Zhu FG, Yang XF, Feng XS, Wang Z, Li Y, Gao SG, Qige Q, Bai LT, Yang WJ, Lei GY, Shen ZY, Chen LQ, Li EM, Xu LY, Wu ZY, Cao WK, Wang JP, Bao ZQ, Chen JL, Ding GC, Zhuang X, Zhou YF, Zheng HF, Zhang Z, Zuo XB, Dong ZM, Fan DM, He X, Wang J, Zhou Q, Zhang QX, Jiao XY, Lian SY, Ji AF, Lu XM, Wang JS, Chang FB, Lu CD, Chen ZG, Miao JJ, Fan ZL, Lin RB, Liu TJ, Wei JC, Kong QP, Lan Y, Fan YJ, Gao FS, Wang TY, Xie D, Chen SQ, Yang WC, Hong JY, Wang L, Qiu SL, Cai ZM, Zhang XJ. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 17.Zhang RY, Du WQ, Zhang YC, Zheng JN, Pei DS. PLCε signaling in cancer. J Cancer Res Clin Oncol. 2016;142:715–722. doi: 10.1007/s00432-015-1999-x. [DOI] [PubMed] [Google Scholar]
- 18.Fan J, Fan Y, Wang X, Niu L, Duan L, Yang J, Li L, Gao Y, Wu X, Luo C. PLCε regulates prostate cancer mitochondrial oxidative metabolism and migration via upregulation of Twist1. J Exp Clin Cancer Res. 2019;38:337. doi: 10.1186/s13046-019-1323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Y, Li L, Li T, Ma L, Yuan M, Sun W, Cheng HL, Niu L, Du Z, Quan Z, Fan Y, Fan J, Luo C, Wu X. Simvastatin delays castrationresistant prostate cancer metastasis and androgen receptor antagonist resistance by regulating the expression of caveolin1. Int J Oncol. 2019;54:2054–2068. doi: 10.3892/ijo.2019.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 22.Maugeri-Saccà M, Barba M, Pizzuti L, Vici P, Di Lauro L, Dattilo R, Vitale I, Bartucci M, Mottolese M, De Maria R. The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications. Expert Rev Mol Med. 2015;17:e14. doi: 10.1017/erm.2015.12. [DOI] [PubMed] [Google Scholar]
- 23.Ou C, Sun Z, Li S, Li G, Li X, Ma J. Dual roles of yes-associated protein (YAP) in colorectal cancer. Oncotarget. 2017;8:75727–75741. doi: 10.18632/oncotarget.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo JH, Guan KL. Interplay between YAP/TAZ and metabolism. Cell Metab. 2018;28:196–206. doi: 10.1016/j.cmet.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Li J, Sun S, Chen X, Zhang H, Li B, Sun S. YAP/TAZ-mediated activation of serine metabolism and methylation regulation is critical for LKB1-deficient breast cancer progression. Biosci Rep. 2017;37 doi: 10.1042/BSR20171072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan Z, He Y, Luo C, Xia Y, Zhao Y, Liu N, Wu X. Interleukin 6 induces cell proliferation of clear cell renal cell carcinoma by suppressing hepaCAM via the STAT3-dependent up-regulation of DNMT1 or DNMT3b. Cell Signal. 2017;32:48–58. doi: 10.1016/j.cellsig.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Du Z, Gao Y, Tang Y, Fan Y, Sun W, Li T, Liu N, Yuan M, Fan J, Niu L, Yan J, Duan L, Wu X, Luo C. PLCε knockdown overcomes drug resistance to androgen receptor antagonist in castration-resistant prostate cancer by suppressing the wnt3a/beta-catenin pathway. J Cell Physiol. 2019 doi: 10.1002/jcp.28195. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Yu C, Huang S, Wang M, Zhang J, Liu H, Yuan Z, Wang X, He X, Wang J, Zou L. A novel tandem mass spectrometry method for first-line screening of mainly beta-thalassemia from dried blood spots. J Proteomics. 2017;154:78–84. doi: 10.1016/j.jprot.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F, Blagih J, Vincent DF, Campbell KJ, Ceteci F, Sansom OJ, Blyth K, Vousden KH. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–376. doi: 10.1038/nature22056. [DOI] [PubMed] [Google Scholar]
- 31.Mattaini KR, Sullivan MR, Vander Heiden MG. The importance of serine metabolism in cancer. J Cell Biol. 2016;214:249–257. doi: 10.1083/jcb.201604085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaneton B, Hillmann P, Zheng L, Martin ACL, Maddocks ODK, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, Frezza C, O’Reilly M, Gottlieb E. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.di Salvo ML, Contestabile R, Paiardini A, Maras B. Glycine consumption and mitochondrial serine hydroxymethyltransferase in cancer cells: the heme connection. Med Hypotheses. 2013;80:633–636. doi: 10.1016/j.mehy.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Du HF, Ou LP, Yang X, Song XD, Fan YR, Tan B, Luo CL, Wu XH. A new PKCalpha/beta/TBX3/E-cadherin pathway is involved in PLCepsilon-regulated invasion and migration in human bladder cancer cells. Cell Signal. 2014;26:580–593. doi: 10.1016/j.cellsig.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Wu X, Ou L, Yang X, Wang X, Tang M, Chen E, Luo C. PLCε knockdown inhibits prostate cancer cell proliferation via suppression of Notch signalling and nuclear translocation of the androgen receptor. Cancer Lett. 2015;362:61–69. doi: 10.1016/j.canlet.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Wang D, Peng H, Chen X, Han X, Yu J, Wang W, Liang L, Liu Z, Zheng Y, Hu J, Yang L, Li J, Zhou H, Cui X, Li F. Epigenetically upregulated oncoprotein PLCE1 drives esophageal carcinoma angiogenesis and proliferation via activating the PI-PLCε-NF-kappaB signaling pathway and VEGF-C/Bcl-2 expression. Mol Cancer. 2019;18:1. doi: 10.1186/s12943-018-0930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyutyunnykova A, Telegeev G, Dubrovska A. The controversial role of phospholipase C epsilon (PLCε) in cancer development and progression. J Cancer. 2017;8:716–729. doi: 10.7150/jca.17779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins M, McCarthy A, Baxendale R, Guichard S, Magno L, Kessaris N, El-Bahrawy M, Yu P, Katan M. Tumor suppressor role of phospholipase C epsilon in Ras-triggered cancers. Proc Natl Acad Sci U S A. 2014;111:4239–4244. doi: 10.1073/pnas.1311500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunney TD, Katan M. Phospholipase C epsilon: linking second messengers and small GTPases. Trends Cell Biol. 2006;16:640–648. doi: 10.1016/j.tcb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Chan JJ, Katan M. PLCε and the RASSF family in tumour suppression and other functions. Adv Biol Regul. 2013;53:258–279. doi: 10.1016/j.jbior.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B. YAP1 and AR interactions contribute to the switch from androgen-dependent to castration-resistant growth in prostate cancer. Nat Commun. 2015;6:8126. doi: 10.1038/ncomms9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohamed A, Sun C, De Mello V, Selfe J, Missiaglia E, Shipley J, Murray GI, Zammit PS, Wackerhage H. The Hippo effector TAZ (WWTR1) transforms myoblasts and TAZ abundance is associated with reduced survival in embryonal rhabdomyosarcoma. J Pathol. 2016;240:3–14. doi: 10.1002/path.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, Li F, Yu FX, Sun Y, Yuan H, Zhu H, Xiong Y, Lei QY, Guan KL. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125:2123–2135. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng Q, Jiang G, Wu Y, Li J, Liang W, Chen L, Su Q, Li W, Du J, Wong CKC, Chen Z, Wang H. GPER/Hippo-YAP signal is involved in Bisphenol S induced migration of triple negative breast cancer (TNBC) cells. J Hazard Mater. 2018;355:1–9. doi: 10.1016/j.jhazmat.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45:195–214. doi: 10.1016/s0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 46.Weiler SME, Pinna F, Wolf T, Lutz T, Geldiyev A, Sticht C, Knaub M, Thomann S, Bissinger M, Wan S, Rössler S, Becker D, Gretz N, Lang H, Bergmann F, Ustiyan V, Kalin TV, Singer S, Lee JS, Marquardt JU, Schirmacher P, Kalinichenko VV, Breuhahn K. Induction of chromosome instability by activation of yes-associated protein and forkhead box M1 in liver cancer. Gastroenterology. 2017;152:2037–2051. e22. doi: 10.1053/j.gastro.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y, Liu Y, Zhang Z, Yang J, Ye X, Jin Q, Chen T. Verteporfin inhibits proliferation, invasion and migration of MDA-MB-231 human breast cancer cells by down-regulating the expression of Yes-associated protein. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:1223–1227. [PubMed] [Google Scholar]
- 48.Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong J, Sun X, Li J. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108:478–487. doi: 10.1111/cas.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, Wang X, Fang L, Lan C, Zheng X, Wang Y, Zhang Y, Han X, Liu S, Cheng K, Zhao Y, Shi J, Guo J, Hao J, Ren H, Nie G. A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer. Cancer Lett. 2017;402:61–70. doi: 10.1016/j.canlet.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Chen M, Zhong L, Yao SF, Zhao Y, Liu L, Li LW, Xu T, Gan LG, Xiao CL, Shan ZL, Liu BZ. Verteporfin inhibits cell proliferation and induces apoptosis in human leukemia NB4 cells without light activation. Int J Med Sci. 2017;14:1031–1039. doi: 10.7150/ijms.19682. [DOI] [PMC free article] [PubMed] [Google Scholar]