Abstract
Background and Objectives:
No previous study has assessed the outcomes of cooled radiofrequency ablation (C-RFA) of the medial branch nerves (MBNs) for the treatment of lumbar facet joint pain nor compared its effectiveness to traditional RFA (T-RFA). This study evaluated six-month outcomes for pain, function, psychometrics, and medication usage in patients who underwent MBN C-RFA versus T-RFA for lumbar Z-joint pain.
Methods:
In this blinded, prospective trial, patients with positive diagnostic MBN blocks (>75% relief) were randomized to MBN C-RFA or T-RFA. The primary outcome was the proportion of “responders” (≥50% NRS reduction) at 6-months. Secondary outcomes included Numeric Rating Scale (NRS), Oswestry Disability Index (ODI), and Patient Global Impression of Change (PGIC).
Results:
Forty-three participants were randomized to MBN C-RFA (n=22) or C-RFA (n=21). There were no significant differences in demographic variables (p’s>0.05). A ≥50% NRS reduction was observed in 52% (95% CI 31–74%) and 47% (95% CI 26–71%) of participants in the C-RFA and T-RFA groups, respectively (p=0.75). A ≥15-point or ≥30% reduction in ODI score was observed in 62% (95% CI 39–80%) and 42% (95% CI 22–66%) of participants in the C-RFA and T-RFA groups, respectively (p=0.21).
Conclusions:
When using a single diagnostic block paradigm with a threshold of >75% pain reduction, C-RFA resulted in a treatment success rate greater than 50% when defined by pain reduction, and greater that 60% when defined by improvement in physical function, at 6-month follow-up. No significant differences were observed between the two RFA modalities.
Clinical Trial Registration:
.
Keywords: Lumbar radiofrequency ablation, injections, zygapophysial pain, facet pain, back pain, Outcome Assessment (Health Care)
Introduction
Low back pain is the leading cause of lost work time, workers’ compensation claims, and disability in the USA.1,2 This symptom originates from the lumbar zygapophyseal (‘facet’) joints in 15%–45% of individuals.3–5 Lumbar facet joint pain may be treated with a variety of conservative, non-interventional therapies. However, in refractory cases, radiofrequency ablation (RFA) of the medial branch nerves (MBN) is commonly used.4 Traditional RFA (T-RFA) technology has been used for decades and demonstrates excellent clinical outcomes when specific diagnostic block and proper electrode placement technique are employed.6,7 In particular, when T-RFA electrodes are not placed properly in parallel to the MBN, there is a decreased chance of nerve capture within the radius of the thermal lesion. Consequently, clinical outcome studies in which parallel electrode technique is not used have shown a much lower treatment success rate.8–10
More recently developed cooled RFA (C-RFA) technology creates a spherical, forward projecting lesion.11,12 This geometry creates a theoretical technical advantage in capturing a target MBN, as the RFA probe can be positioned at a range of possible angles and still capture the target neural structure. Clinical outcome studies have demonstrated effectiveness of C-RFA of the lateral branch nerves for sacroiliac joint pain13 as well as the genicular nerves for chronic knee pain from osteoarthritis.14,15 However, only one small retrospective case series has addressed the clinical outcomes of lumbar MBN RFA using cooled technology.16 To date, there has been no head-to-head comparison of clinical outcomes for cooled versus T-RFA for the treatment of lumbar facet joint pain. As such, the purpose of this study was to prospectively determine (1) the clinical outcomes of MBN C-RFA using, as measured by improvements in pain, physical function, psychological function, and global impression of change, as well as (2) whether cooled or traditional MBN RFA results in superior treatment outcomes, in individuals with lumbar facet joint pain.
METHODS
Study Design
This single-blinded, prospective, randomized, comparative trial was registered at ClinicalTrials.gov (). Participants were recruited between June 2015 and March 2017 at a single urban, academic pain medicine center. All study participants provided both verbal and written informed consent prior to study enrollment.
Criteria for study inclusion were the following: low back pain for at least 6 months, baseline Numeric Rating Scale (NRS) score of at least 4, pain resistant to conventional therapy including non-steroidal anti-inflammatory drugs, opioids, muscle relaxants, oral steroids, physical therapy or chiropractic care, a pain diagram suggesting possibility of facet-mediated pain, referred pain not beyond the knee (if present), and a positive response to one set of diagnostic MBN blocks, defined as >75% reduction in pain following diagnostic blocks with local anesthetic (0.5 mL of 0.5% bupivacaine or 0.5 mL of 2% lidocaine). Exclusion criteria included: focal neurologic signs or symptoms, radiologic evidence of a symptomatic herniated disc or nerve root impingement related to spinal stenosis, previous RFA treatment for similar symptoms, active systemic or local infection, coagulopathy or other bleeding disorder, current use of anticoagulants or antiplatelet medications, allergy to medications being used for injection procedures, inability to read English, communicate with staff, or participate in follow-up, pregnancy, unstable medical or psychiatric illness, or cognitive deficit. Previous RFA treatment for similar symptoms was selected as an inclusion criterion in order to specifically measure the effect of RFA in a homogeneous population without prior RFA of the MBNs.
Participants were randomized using a computer-generated scheme to either treatment group: C-RFA or T-RFA. Immediately prior to the procedure, the procedure operator opened a sequentially numbered opaque envelope with group assignment listed inside. All participants remained blind to the group assignment before, during, and after the RFA procedure and all efforts were made to provide identical treatment experiences. The research personnel who gathered postprocedure data were blind to group assignment.
Procedures
All procedures were supervised and/or performed by five faculty board certified in Anesthesiology and Pain Medicine with assistance from Accreditation Council for Graduate Medical Education-accredited multidisciplinary Pain Medicine fellows.
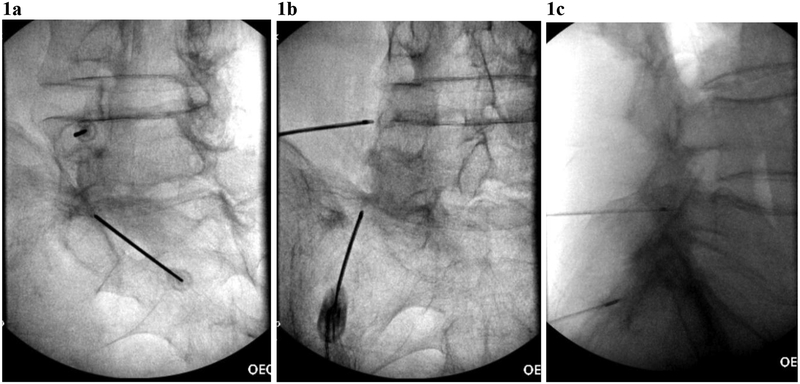
Participants were positioned prone on a radiolucent fluoroscopy table. Non-invasive blood pressure monitoring and a pulse oximeter were placed. Some patients received conscious sedation (intravenous midazolam 1–2 mg and/or fentanyl 50–100 mcg). The lumbar spine was prepared with chlorhexidine and draped in a sterile manner. Fluoroscopy was used to identify the appropriate lumbar level(s). The skin and subcutaneous tissues superficial to each MBN target were anesthetized with 1–3 mL of 1% lidocaine. For participants who were randomized to receive C-RFA, a 17-gauge introducer needle was placed at the MBN target level, and an 18- gauge C-RFA probe with a 4 mm active tip (Coolief Cooled Radiofrequency Kit, Halyard Health, Alpharetta, Georgia) was placed at the junction of the transverse process and the superior articular process in an ipsilateral oblique fluoroscopic view, with subsequent withdrawal of the stylet, allowing a 2 mm gap between the electrode tip and the base of the superior articular process (figure 1A). Appropriate positioning was confirmed in anterior-posterior and lateral fluoroscopic views (figure 1B,C). Once the satisfactory needle position was confirmed motor testing was performed (2.0 V, 2 Hz) at each of the MBN target sites. Sensory testing was not performed, as this technique does not show a clear, robust benefit with regard to treatment outcomes17 and is not endorsed by clinical practice guidelines.18 Prior to lesioning, 1 mL of 2% lidocaine was injected through the introducer needle for anesthesia. C-RFA lesions were performed for 165 s at each MBN site, with the RFA generator temperature set to 60°C (intralesional temperature >80°).19 For participants who were randomized to receive T-RFA, 20-gauge T-RFA probes with 10 mm active tips (Baylis Medical, Montreal, Canada) were placed at each target MBN using parallel technique, as described previously.18 Motor testing was performed as described above. Prior to lesioning, 1 mL of 2% lidocaine was injected through the introducer needle for anesthesia. T-RFA lesions were performed for 90 s at 80°C at each MBN target site. In both groups, following ablation, 0.5 mL of 0.5% bupivacaine was injected at each MBN site to provide postprocedure analgesia. No corticosteroids were injected.
FIGURE 1.
Cooled RFA probe placements in (a) oblique, (b) anterior-posterior, and (c) lateral fluoroscopic views for left L4 medial branch nerve and the left L5 dorsal ramus.
Data Acquisition and Analysis
At the time of study enrollment, the following data were collected: age, sex, height, weight, percent relief from diagnostic block, total daily doses of opioid analgesics, total daily doses of non-opioid analgesic medications, and positive facet joint loading on physical examination. Additionally, the following outcome measures were obtained: NRS, Oswestry Disability Index (ODI), Patient Anxiety Symptom Scale (PASS-20), McGill Pain Inventory (MPI), and Center for Epidemiologic Studies Depression scale (CESD-10). Immediately after procedure, fluoroscopy time, procedure time, postinjection NRS pain score, and adverse event information were recorded.
At intervals of 1, 3 and 6 months following the procedure outcome measures were again collected by either telephone or clinic visit, with the addition of Patient Global Impression of Change (PGIC) and adverse event information.
The primary outcome of this investigation was NRS pain score reduction 6 months following the study intervention. The mean reduction in NRS pain score was determined in reference to a minimally clinically important change (MCIC) of 2 points.20 Categorical responder analysis was also used, in which treatment success for pain and function were defined as ≥50% reduction in NRS pain score, and either ≥30% or ≥15 points reduction in ODI score, respectively.
Power Analysis
A sample size of 38 (19 in each treatment group) provides 83% power to show a difference in means when there is a difference of 2.0 between the null hypothesis mean difference of 0.0 and the actual mean difference of −2.0 at the 0.05 significance level (alpha) using a two-sided Mann-Whitney-Wilcoxon test. No prospective study to date was available from which to calculate the treatment effect size of MBN C-RFA for lumbar facet joint syndrome, and no study to date has compared C-RFA with T-RFA for this condition. The power analysis was based on the best available evidence at the time and included the difference in effect size seen at 6-month follow-up in randomized trial of C-RFA versus T-RFA for sacroiliac joint pain.13 We planned to recruit a total of 40 participants to account for a presumed 5% dropout rate. These estimates were based on 2000 Monte Carlo samples from the null distributions: normal (M0 S) and normal (M0 S), and the alternative distributions: normal (M0 S) and normal (M1 S). A difference in means of 2.0 on the NRS scale represents the MCIC for low back pain.20
Statistical Analysis
Descriptive statistics were calculated for baseline demographics. Independent t-tests and Pearson’s χ2 tests were used to compare baseline demographics, which were stratified by group allocation (C-RFA vs T-RFA). Changes in NRS and ODI at 6 months after intervention within and between groups were analyzed by one-sample t-tests and independent t-tests. Group differences in PGIC score 6 months after RFA were compared using the Mann-Whitney-Wilcoxon test. Two-proportion z-tests were performed to compare group differences in treatment success rates (defined by the above described NRS and ODI thresholds) at 6 months after intervention. A generalized estimating equation (GEE) with an exchangeable correlation structure was used to examine the longitudinal changes in NRS and ODI scores by group allocation (C-RFA vs T-RFA), while accounting for correlated, repeated measurements within each individual.21 Lastly, correlation coefficients were calculated in order to assess the relationships between baseline variables and 6-month changes in NRS, ODI, and PGIC scores.
RESULTS
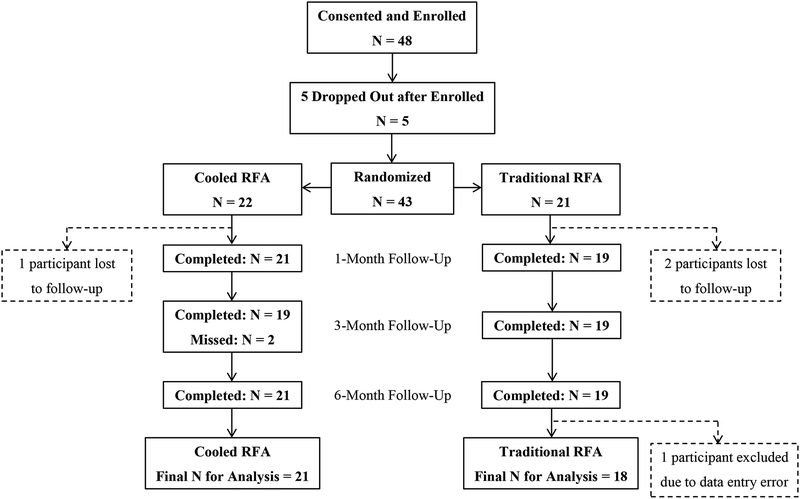
Forty-eight patients were enrolled in the study, due to more dropouts and loss to follow-up than originally anticipated, so as to reach the goal sample size of 38. Of the 48 patients enrolled, five decided not to participate in the study after enrollment but before randomization; three participants had RFA but were lost to follow-up, and one was excluded due to data entry error (extreme outlier, illogical outcome reporting) (figure 2). Consequently, this study analyzed data from 39 participants in total, including 21 and 18 who received C-RFA and T-RFA, respectively.
FIGURE 2.
Flowchart of patient inclusion and exclusion during study.
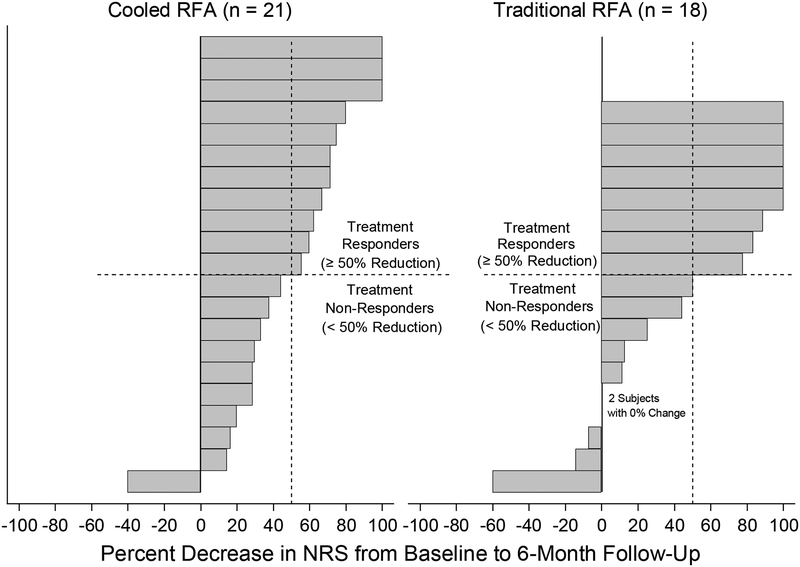
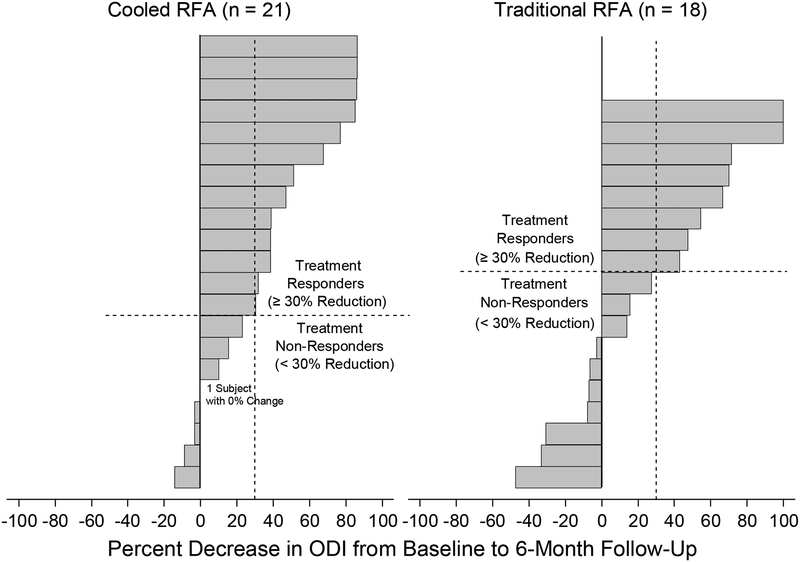
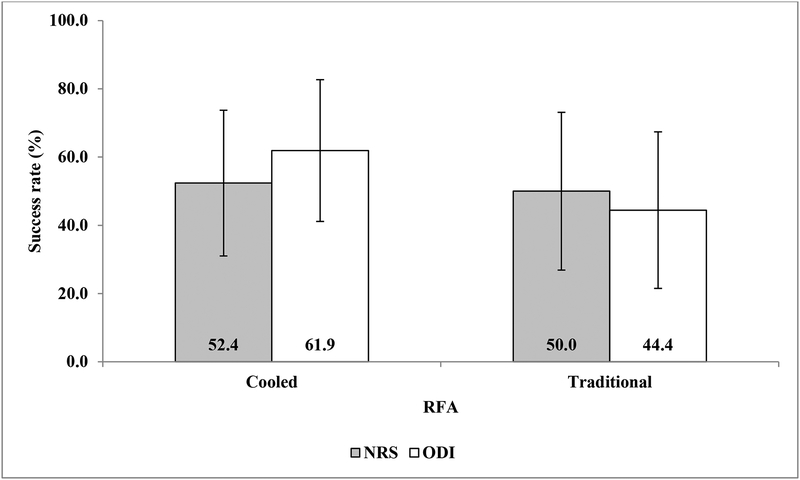
Descriptive statistics of the baseline demographic and procedural characteristics by RFA type are summarized in table 1. No significant difference in any of the baseline demographic variables was observed between groups (p>0.05). No patients with workers compensation insurance were enrolled. Table 2 shows the changes in NRS and ODI scores 6 months after intervention. Both C-RFA and T-RFA groups showed significant decreases in NRS (t=−6.87 and −3.98, p<0.001 and p=0.001) and ODI (t=−4.65 and −2.79, p<0.001 and p=0.012) scores at 6-month follow-up. Both treatment groups demonstrated a mean change in NRS pain score by more than the minimally clinically important change for low back pain.20 There were no significant between-group differences (t=0.83 and 0.86, p=0.410 and 0.397). Figures 3A,B shows the individual treatment responses according to NRS and ODI score success thresholds at 6 months after intervention. Both groups showed similar distributions of individual responses in NRS and ODI score improvements. PGIC at 6-month follow-up was not significantly different between the two treatment groups (z=−0.66, p=0.512, table 3). When the 6-month treatment effects were assessed defined by categorical treatment success rates as above, there were no significant group differences with regard to either NRS (z=−0.15, p=0.882) or ODI (z=−1.09, p=0.276, figure 4) score outcomes.
TABLE 1.
Subject Baseline Demographics
| RFA | |||
|---|---|---|---|
| Variable at baseline | Cooled (n = 21) | Traditional (n = 18) | P* |
| Age | 53.6 (13.7) | 58.4 (13.5) | 0.282 |
| BMI | 32.4 (9.0) | 28.1 (5.6) | 0.084 |
| Duration of pain (months) | 72.4 (66.5) | 102.2 (112.0) | 0.311 |
| Percent relief from diagnostic block | 0.9 (0.1) | 0.8 (0.2) | 0.160 |
| Morphine equivalents | 11.3 (18.9) | 10.7 (24.1) | 0.936 |
| Fluoroscopy time (sec) | 63.3 (35.1) | 62.8 (33.8) | 0.960 |
| Procedure time (min) | 32.8 (10.6) | 38.2 (11.9) | 0.141 |
| CESD | 12.7 (6.9) | 10.1 (6.7) | 0.233 |
| PASS | 41.5 (23.5) | 37.5 (21.0) | 0.610 |
| MPI total | 13.1 (9.2) | 11.2 (6.4) | 0.463 |
| Gender [frequency (%)] | |||
| Male (n = 16) | 8 (38.1) | 8 (44.4) | 0.688** |
| Female (n = 23) | 13 (61.9) | 10 (55.6) | |
| Positive facet loading [frequency (%)] | |||
| Yes (n = 27) | 13 (61.9) | 14 (77.8) | 0.284** |
| No (n = 12) | 8 (38.1) | 4 (22.2) | |
Values are mean (SD) unless specified otherwise.
from independent t-test unless specified otherwise.
from Pearson’s χ2 test.
TABLE 2.
Changes in NRS and ODI From Baseline to 6-Month Follow-Up
| RFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cooled (n = 21) | Traditional (n = 18) | Group difference in changes | |||||||
| Pre | At 6 months | Change | P* | Pre | At 6 months | Change | P* | P** | |
| NRS | 7.4 (1.7) | 3.6 (2.4) | −3.8 (2.5) | < 0.001 | 6.9 (1.5) | 3.9 (3.4) | −3.0 (3.2) | 0.001 | 0.410 |
| ODI | 29.1 (7.0) | 17.8 (10.0) | −11.3 (11.2) | < 0.001 | 26.7 (8.7) | 18.6 (11.6) | −8.1 (12.3) | 0.012 | 0.397 |
Values are mean (SD).
from one-sample t-test.
from independent t-test.
FIGURE 3a.
Individual responses in NRS score at 6 months post-intervention, quantified by percent change from the baseline values.
FIGURE 3b.
Individual responses in ODI score at 6 months post-intervention, quantified by percent change from baseline values.
TABLE 3.
Patient Global Impression of Change at 6-Month Follow-Up
| RFA | |||
|---|---|---|---|
| Cooled (n = 21) | Traditional (n = 18) | P* | |
| Patient global impression of change (scale of 1–7) | 2 (3) | 2 (3) | 0.512 |
Values are median (interquartile range).
from Mann-Whitney-Wilcoxon test.
FIGURE 4.
Treatment success was defined as ≥ 50% reduction in NRS pain score, and either ≥ 30% or ≥ 15 points reductions for ODI
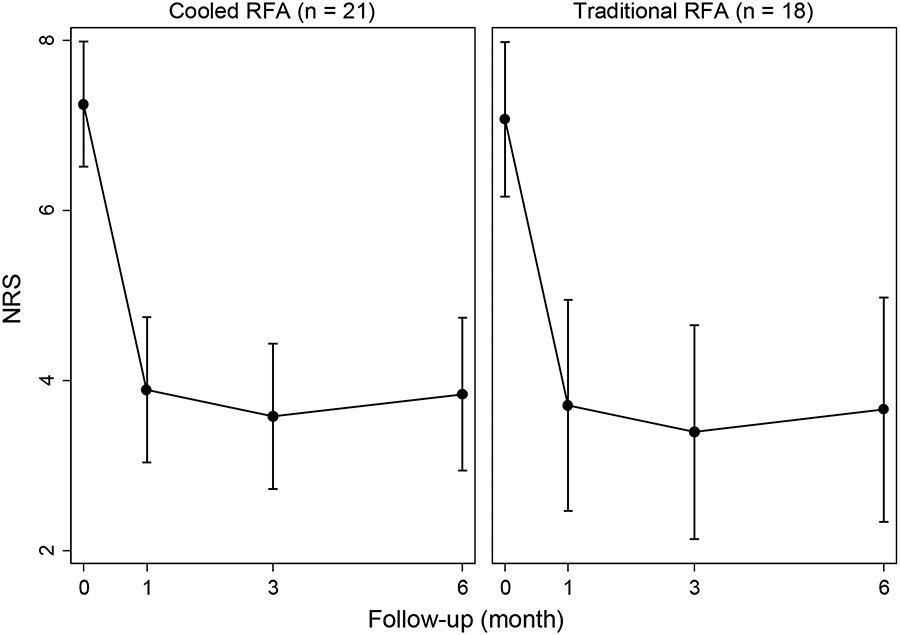
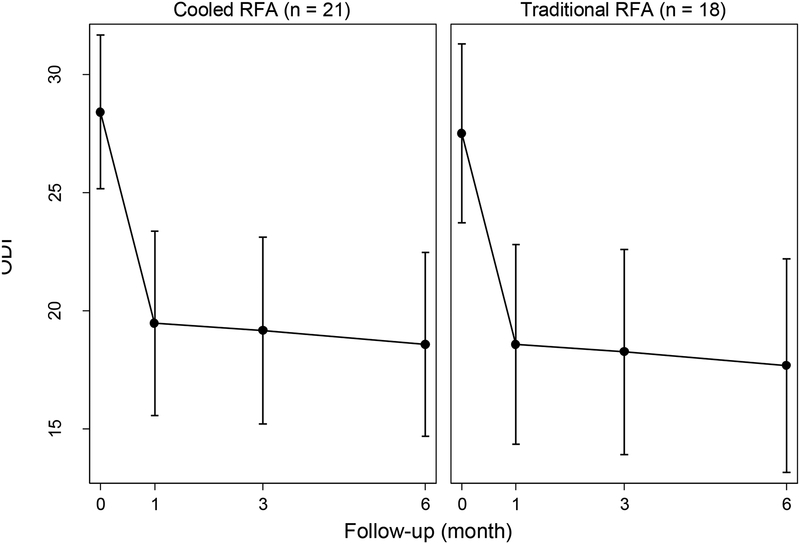
The GEE analysis showed that there were significant reductions in NRS score from baseline to 1 month after intervention (β=−3.36, z=−7.21, p<0.001, figure 5A). However, no significant changes were observed from 1 to 6-month follow-up after intervention (p>0.05), as the treatment effect was maintained. Longitudinal changes in NRS were not significantly influenced by RFA type (β=0.18, z=0.27, p=0.785). Similar results were obtained for longitudinal changes in ODI score (figure 5B), with a significant decrease observed 1 month after intervention (β=−8.95, z=−5.00, p<0.001), followed by non-significant changes up to 6-month follow-up (p>0.05), again indicating persistence of the treatment effect. The RFA type did not influence longitudinal changes in ODI, either (β=0.90, z=0.35, p=0.729).
Figure 5a.
Changes in NRS by RFA type, estimated by the generalized estimating equation.
Figure 5b.
Changes in ODI by RFA type, estimated by the generalized estimating equation.
Table 4 shows the correlations between the baseline variables and 6-month changes in NRS, ODI, and PGIC scores. Percent relief from diagnostic MBN block showed significant, medium-sized, negative correlations with ODI and PGIC scores (r=−0.410 and −0.320, p<0.01 and p=0.047). Indicating that a greater reported percentage reduction in pain score with diagnostic MBN block was associated with a greater reduction in ODI score and a more positive PGIC response. Despite being non-significant, a medium-sized, negative correlation was also observed between percent relief from diagnostic MBN blocks and NRS score (r=−0.306, p=0.058). In addition, baseline CESD, PASS, and MPI total scores had medium-sized, negative correlations with ODI score (r=−0.312 to −0.360).
TABLE 4.
Correlations of Baseline Characteristics to 6-Month Pain and Functional Outcomes
| Changes from baseline to 6-month follow-up | |||
|---|---|---|---|
| NRS | ODI | Patient global impression of change | |
| Age | −0.158 | −0.076 | 0.009 |
| BMI | −0.253 | −0.252 | −0.209 |
| Duration of pain | 0.151 | 0.170 | 0.104 |
| Percent relief from diagnostic block | −0.306 | −0.410* | −0.320* |
| Morphine equivalents | 0.156 | 0.238 | 0.137 |
| Fluoroscopy time | 0.085 | 0.193 | 0.082 |
| Procedure time | −0.154 | −0.026 | −0.114 |
| CESD | −0.232 | −0.360* | −0.108 |
| PASS | −0.123 | −0.329 | −0.143 |
| MPI total | −0.203 | −0.312 | −0.045 |
Notes: values are correlation coefficients (r).
Significant correlation (P < 0.05).
No serious adverse events were reported. Two patients reported increased post-procedure pain (one in each treatment group), which was self-limited and did not require further treatment.
Discussion
This is the first prospective study to confirm the clinical effectiveness of MBN C-RFA for the treatment of lumbar facet syndrome. Responder analysis showed a treatment success rate of >50% as defined by an NRS pain score reduction of >50%, as well as a treatment success rate of >60% as defined by either ≥30% or ≥15 points improvement in ODI score. These treatment effects were sustained for the 6-month duration of follow-up in this study. These results are similar to other studies in which a single rather than dual diagnostic MBN block paradigm was used.6,7,22–24 This success rate is notable, particularly given the fact that superior outcomes have been demonstrated when a dual diagnostic MBN block paradigm is used.4,6–9,18,22 Future study should include use of a dual diagnostic MBN block paradigm in order to select study participants, as this would likely further optimize outcomes regardless of RFA modality.
We found no between-group differences in treatment outcomes following C-RFA as compared with T-RFA. A greater proportion of participants reported a clinically significant improvement in physical function according to ODI score reduction at 6-month follow-up in the C-RFA group, but this difference was not statistically significant. However, the study was powered to assess a primary outcome of pain reduction rather than functional improvement. A larger study may demonstrate a significant difference in this measure. Procedure time was shorter in the C-RFA group compared with the T-RFA group, but similarly, this difference was not statistically significant. Despite the longer lesion duration with C-RFA compared with T-RFA (165 vs 90 s), C-RFA may be a more rapid procedure compared with T-RFA, as time-consuming meticulous parallel electrode placement to target the MBN, particularly in the degenerated spine, is not required for successful lesioning.
The present data also confirm the importance of the diagnostic MBN blocks in the selection paradigm for treatment of lumbar facet joint syndrome by MBN RFA. This is intuitive but had not previously been investigated. Greater temporary pain relief from the diagnostic MBN blocks was associated with a greater reduction in the NRS and ODI as well as a more positive PGIC at 6-month follow-up. The use of single set of positive MBN blocks (>75% pain relief) compared with dual comparative MBN blocks has been previously debated. Although dual comparative MBN blocks have the benefit of decreasing the false-positive rate and thus increasing the treatment success rate of lumbar MBN RFA, some data suggest a reduction in overall cost when using a single block paradigm.22 Additional discussion has surrounded the threshold of a positive response to MBN blocks, ranging from 50% to 100% relief. While lowering the pain relief to 50% could capture more patients who may benefit from the procedure, it results in a high false-positive rate25 and ultimately unsuccessful RFA treatment. Increasing the threshold above 80% in a higher positive predictive value, but this leads to an increased rate of false-negative response and a subsequent lack of access to the procedure that may indeed provide treatment benefit.22 Historically, providers have made variable decisions to tighten versus relax the diagnostic criteria for RFA selection, depending on their view of access versus cost savings. However, in the future, this decision may no longer be at the provider’s discretion, as healthcare costs increase in the USA, particularly with regard to spine care,26,27 and pressure mounts to favor cost savings over patient access.
We identified no serious adverse events in either treatment group. Thermal skin burns have been reported with both C-RFA and T-RFA,28–30 but this adverse event was not observed in the present study. Post-RFA neuritis can occur in up to 5% of patients treated with RFA in the lumbar spine,4 but a larger cohort study would be needed to identify if either RFA technique is more likely to occur.
Limitations of the current study must be acknowledged. The primary limitation of the study is the relatively small sample size. Five patients dropped out after being enrolled by prior to randomization; selection bias is possible but not dissimilar to other studies of procedural interventions in which individuals may elect for additional non-invasive care prior to undergoing intervention. Further, participants were lost to follow-up; of 43 participants who underwent treatment intervention, 3 (7%) did not report outcomes for the full 6-month duration of the study. A dropout effect could have altered the overall outcome of the study. Analysis by conservative worst-case scenario definitions (treating all participants lost to follow-up as treatment failures) would adjust the treatment success rate to 50% (95% CI 29% to 71%) and 59% (95% CI 39% to 80%) for pain reduction and functional improvement, respectively, in the C-RFA group. Twenty-gauge rather than 16-gauge or 18-gauge RFA electrodes were used for conventional ablations; as such, the success rate in the T-RFA group may be lower than would be expected when using larger gauge electrodes. Additionally, some providers use bipolar lead placement, longer lesion duration times, higher lesioning temperatures or longer active tips when employing C-RFA, all of which expand the size of the lesion and may increase the chance of successful MBN capture.18 A heterogeneous group of five faculty members, assisted by Pain Medicine fellows, performed these procedures; difference in experience level with the procedural technique may have influenced patient outcomes, though this heterogeneity does improve generalizability of the reported findings. Finally, RFA represents a treatment that is implemented with the goal of long-term treatment; we measured a primary outcome at 6 months, and did not follow participants beyond this time period, but future study would ideally capture outcomes at a post-RFA time point of at least 1 year. Indeed, it is conceivable that an intergroup difference may have been observed if outcomes had been assessed beyond 6 months.
Conclusion:
To our knowledge, this is the first prospective trial to investigate the clinical outcomes of C-RFA of the MBNs for the treatment lumbar facet joint syndrome, as well as the first study to directly compare the effectiveness of C-RFA to T-RFA for this condition. When using a single diagnostic block paradigm with a threshold of >75% pain reduction, C-RFA resulted in a treatment success rate greater than 50% when defined by pain reduction (NRS), and greater than 60% when defined by improvement in physical function (ODI). These treatment effects were maintained at 6-month follow-up. No significant differences were observed between the two RFA modalities. Future study should use the effect size or success rate demonstrated in this prospective study for power calculation.
Acknowledgements
The authors thank Shellie Cunningham, BS, for her assistance with manuscript preparation, as well as Trista Reynolds, BA for her assistance with data collection.
Funding: This study was supported by the 2014 Addison Blonsky Research Grant from the Midwest Pain Society.
Footnotes
Conflict of Interest Statement: Zachary L. McCormick, MD serves on the board of directors for the Spine Intervention Society.
References:
- 1.Mafi JN, McCarthy EP, Davis RB, et al. Worsening trends in the management and treatment of back pain. JAMA Intern Med 2013;173:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep 2001;50:120–5. [PubMed] [Google Scholar]
- 3.DePalma MJ, Ketchum JM, Saullo TR. Multivariable analyses of the relationships between age, gender, and body mass index and the source of chronic low back pain. Pain Med 2012;13:498–506. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology 2007;106:591–614. [DOI] [PubMed] [Google Scholar]
- 5.Kalichman L, Li L, Kim DH, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine 2008;33:2560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacVicar J, Borowczyk JM, MacVicar AM, et al. Lumbar medial branch radiofrequency neurotomy in New Zealand. Pain Med 2013;14:639–45. [DOI] [PubMed] [Google Scholar]
- 7.Dreyfuss P, Halbrook B, Pauza K, et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine 2000;25:1270–7. [DOI] [PubMed] [Google Scholar]
- 8.Vorobeychik Y, Stojanovic MP, McCormick ZL. Radiofrequency denervation for chronic low back pain. JAMA 2017;318:2254–5. [DOI] [PubMed] [Google Scholar]
- 9.McCormick ZL, Vorobeychik Y, Gill JS, et al. Guidelines for composing and assessing a paper on the treatment of pain: A practical application of evidence- based medicine principles to the MINT randomized clinical trials. Pain Med 2018;19:2127–37. [DOI] [PubMed] [Google Scholar]
- 10.Provenzano DA, Buvanendran A, de León-Casasola OA, et al. Interpreting the MINT randomized trials evaluating radiofrequency ablation for lumbar facet and sacroiliac joint pain: A call from ASRA for better education, study design, and performance. Reg Anesth Pain Med 2018;43:68–71. [DOI] [PubMed] [Google Scholar]
- 11.Lorentzen T A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol 1996;3:556–63. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe I, Masaki R, Min N, et al. Cooled-tip ablation results in increased radiofrequency power delivery and lesion size in the canine heart: importance of catheter-tip temperature monitoring for prevention of popping and impedance rise. J Interv Card Electrophysiol 2002;6:9–16. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SP, Hurley RW, Buckenmaier CC, et al. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology 2008;109:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis T, Loudermilk E, DePalma M, et al. Prospective, Multicenter, Randomized, crossover clinical trial comparing the safety and effectiveness of cooled radiofrequency ablation with corticosteroid injection in the management of knee pain from osteoarthritis. Reg Anesth Pain Med 2018;43:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormick ZL, Reddy R, Korn M, et al. A prospective randomized trial of prognostic genicular nerve blocks to determine the predictive value for the outcome of cooled radiofrequency ablation for chronic knee pain due to osteoarthritis. Pain Med 2018;19:1628–38. [DOI] [PubMed] [Google Scholar]
- 16.McCormick ZL, Walker J, Marshall B, et al. A novel modality for facet joint denervation: cooled radiofrequency ablation for lumbar facet syndrome. a case series. Phys Med Rehabil Int 2014;1:5. [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SP, Strassels SA, Kurihara C, et al. Does sensory stimulation threshold affect lumbar facet radiofrequency denervation outcomes? A prospective clinical correlational study. Anesth Analg 2011;113:1233–41. [DOI] [PubMed] [Google Scholar]
- 18.Bogduk N Practice Guidelines for Spinal Diagnostic and Treatment Procedures. International Spine Intervention Society, 2013. [Google Scholar]
- 19.Ball RD. The science of conventional and water-cooled monopolar lumbar radiofrequency rhizotomy: an electrical engineering point of view. Pain Physician 2014;17:E175–E211. [PubMed] [Google Scholar]
- 20.Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 2005;19:593–607. [DOI] [PubMed] [Google Scholar]
- 21.Liang K-YEE, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 22.Cohen SP, Stojanovic MP, Crooks M, et al. Lumbar zygapophysial (facet) joint radiofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: a multicenter analysis. Spine J 2008;8:498–504. [DOI] [PubMed] [Google Scholar]
- 23.Derby R, Melnik I, Lee JE, et al. Cost comparisons of various diagnostic medial branch block protocols and medial branch neurotomy in a private practice setting. Pain Med 2013;14:378–91. [DOI] [PubMed] [Google Scholar]
- 24.McCormick ZL, Marshall B, Walker J, et al. Long-term function, pain and medication use outcomes of radiofrequency ablation for lumbar facet syndrome. Int J Anesth Anesth 2015;2:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain 1994;58:195–200. [DOI] [PubMed] [Google Scholar]
- 26.Gore M, Sadosky A, Stacey BR, et al. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012;37:E668–E677. [DOI] [PubMed] [Google Scholar]
- 27.Babu AN, McCormick Z, Kennedy DJ, et al. Local, national, and service component cost variations in the management of low back pain: Considerations for the clinician. J Back Musculoskelet Rehabil 2016;29:685–92. [DOI] [PubMed] [Google Scholar]
- 28.Walega D, Roussis C. Third-degree burn from cooled radiofrequency ablation of medial branch nerves for treatment of thoracic facet syndrome. Pain Pract 2014;14:e154–e158. [DOI] [PubMed] [Google Scholar]
- 29.McCormick ZL, Walega DR. Third-degree skin burn from conventional radiofrequency ablation of the inferiomedial genicular nerve. Pain Med 2018;19:1095–7. [DOI] [PubMed] [Google Scholar]
- 30.McCormick ZL, Chung B, Smith C, et al. Internal skin burn due to novel radiofrequency ablation technology. Pain Med 2018:1497–8. [DOI] [PubMed] [Google Scholar]