Abstract
Large-bodied mammalian herbivores can influence processes that exacerbate or mitigate climate change. Herbivore impacts are, in turn, influenced by predators that place top-down forcing on prey species within a given body size range. Here, we explore how the functional composition of terrestrial large-herbivore and -carnivore guilds varies between three mammal distribution scenarios: Present-Natural, Current-Day and Extant-Native Trophic (ENT) Rewilding. Considering the effects of herbivore species weakly influenced by top-down forcing, we quantify the relative influence keystone large-herbivore guilds have on methane emissions, woody vegetation expansion, fire dynamics, large-seed dispersal, and nitrogen and phosphorus transport potential. We find strong regional differences in the number of herbivores under weak top-down regulation between our three scenarios, with important implications for how they will influence climate change relevant processes. Under the Present-Natural non-ruminant, megaherbivore, browsers were a particularly important guild across much of the world. Megaherbivore extinction and range contraction and the arrival of livestock mean large, ruminant, grazers have become more dominant. ENT Rewilding can restore the Afrotropics and the Indo-Malay realm to the Present-Natural benchmark, but causes top-down forcing of the largest herbivores to become commonplace elsewhere. ENT Rewilding will reduce methane emissions, but does not maximize natural climate solution potential.
This article is part of the theme issue ‘Climate change and ecosystems: threats, opportunities and solutions'.
Keywords: climate change, functional ecology, herbivory, macroecology, mammals
1. Introduction
Large-bodied mammalian herbivores have considerable potential to alter vegetation community structure and composition [1]. The nature of these effects is dependent on the composition of the herbivore guild, the wider community of species they interact with (e.g. predators and parasites) and environmental conditions [2]. Humans have dramatically altered mammalian herbivore and predator assemblages in the late Quaternary [3–6]. One striking change has been the alteration of the predator–prey size structure, which has implications on how mammalian herbivores influence their environment. For example, it has been reported that the loss of megaherbivores (taxa ≥ 1000 kg), which are relatively free of top-down regulation by predators [7,8], has resulted in changes in vegetation structure [9,10], fire dynamics [11,12], nutrient transport [13] and dispersal of large-seeded species [14]. Trophic rewilding offers to augment these degraded ecological processes by (re)introducing lost species [15]. While the broad aim is to restore autonomous and diverse ecosystems without targeting specific species, habitats or ecosystem services, recently rewilding has been considered and promoted as a means of mitigating climate change (e.g. [16]). We explore how trophic rewilding may affect the composition of keystone large-herbivore guilds and how this might influence climate change.
Predators can alter herbivore ensemble composition, population abundances and individual behaviour [17,18]. A by-product of these effects is reduced herbivory, particularly in areas where herbivores perceive themselves to be at greater risk of predation [19,20]. These predator–prey interactions are size dependent as energetic and mechanical constraints limit predators’ ability to hunt very large herbivores [21,22]. Site-scale research in African savannah has demonstrated that megaherbivores, unlike smaller herbivores, do not behave as expected under the landscape of fear [23,24], and that populations of herbivores weighing more than 150 kg tend to be food- rather than predator-limited [8]. While large-herbivore populations can be influenced by disease and targeted predation on juveniles, this evidence suggests that sufficiently large herbivores are relatively free from strong direct and indirect effects of non-human predation. The presence of these species has implications for ecosystem dynamics, as was seen, for example, in the Serengeti–Mara system, where woody expansion was observed after heavy elephant poaching in the Serengeti but not in the better protected Mara [10]. We follow Owen-Smith [7] and refer to this guild as keystone large herbivores.
Throughout most of the enozoic, enormous herbivores have occurred on nearly all major continents [25]. By contrast, today they are effectively confined to protected areas within Sub-Saharan Africa and South/Southeast Asia as a consequence of the late Quaternary megafauna extinctions [5,26]. These changes may have resulted in some ecosystems becoming relatively top-down regulated because the largest herbivores are now viable prey for the largest predators. At the same time, by protecting certain domesticated herbivores from predation, pastoral agriculture has created a new guild of large herbivores that can achieve high densities and strongly influence their environment.
As rewilding is process- rather than target-led, and outcomes are the product of complex ecosystem dynamics, it is worth exploring the different ways trophic rewilding might influence ecosystems and climate change. Here, we explore the implications of implementing a specific form of trophic rewilding that uses a Pleistocene benchmark to determine species reintroductions but does not use taxon substitutes to replace extinct species (we refer to it as Extant-Native Trophic Rewilding or ENT Rewilding). This is not a climate mitigation specific rewilding scenario, but one that is in keeping with rewilding being non-target specific. It is also not a proposal to rewild the whole world but presents an opportunity to explore the geographical implications of rewilding.
Our aims are to explore: (i) how mammalian predator–prey/herbivore assemblage size structures vary across three mammal distribution scenarios (Present-Natural, Current-Day and ENT Rewilding) and (ii) how different keystone herbivore ensembles might influence methane emission, woody expansion, fire suppression/promotion, large-seed dispersal and nutrient transport potential. We conclude by discussing the complex ways in which different herbivore guild compositions might influence climate change.
2. Material and methods
(a). Compilation of species ranges and functional traits
A list of all mammals from the Last Interglacial (approx. 126 000 years ago) to the present-day was obtained from phylacine v. 1.2 [27]. Phylacine v. 1.2 also provided estimates for species body mass, the proportion of plant, vertebrate and invertebrate material in their diet, and their native range in the Present-Natural and the Current-Day. The ranges of livestock species (cattle, buffalo, horses, sheep, goats, pigs) were taken from the Food and Agriculture Organization of the United Nations (FAO) at greater than 5 individuals km−2 [28], and their average body mass was sourced from de Magalhaes & Costa [29]. In total, 506 large-bodied (greater than or equal to 10 kg) and predominantly herbivorous (greater than or equal to 50% plant diet) species were identified and included in the study. Functional trait data on herbivore digestive physiology and diet were derived from a comprehensive new database compiled by the authors [30] (see electronic supplementary material, appendix A for data on species included in this study). Species range maps were used to create three mammal distribution scenarios: (i) Present-Natural, which included all extant and extinct wild species over their present-natural range, i.e. estimated range where they plausibly would be today without human effects; (ii) Current-Day, which included extant wild and livestock species over their present-day range, excluding wild species introduced range; and (iii) ENT Rewilding, present-natural ranges for all extant wild species, including predators, but excludes livestock. Cow and dromedary were considered to be sufficiently similar to their ancestral forms and included. All analyses were conducted using Behrman equal-area projections of the globe (cells 9000 km2, with widths ranging from 0.75° at the Equator to 9.5° at the poles).
(b). Identifying predators of large herbivores
To address how changes in predator–prey assemblage structure influences the guild of herbivores relatively free from top-down regulation, we identified the maximum size of main prey species for 24 of the largest predatory carnivores. We identified the primary prey (classified as greater than or equal to 15% occurrence in diet following the lower value given in Sandom et al. [31]) of 11 extant species (electronic supplementary material, table S1). The body mass ratios of extant predator to largest prey are reported in electronic supplementary material, table S1, and estimated ratios for extinct predators and the red wolf Canis rufus, which owing to its very small range and critically endangered status has not been studied, in electronic supplementary material, table S2. For a more detailed explanation of this process please see the supplementary methods (appendix B) in the electronic supplementary material.
(c). Herbivore functional traits and climate change effects
Each herbivore was placed into one of 12 functional groups, with each group assigned an influence score on the six climate change effect traits based on Cromsigt et al. [32] (detailed for each species in electronic supplementary material, appendix A). Methane emissions vary with digestive physiology, whereby ruminants emit more methane per capita (10−0.619+0.812×log10 (BM)^1.71) than non-ruminants (10−04.564+3.278× log10(BM)^0.592), as given in Hempson et al. [33]. Herbivore ensemble methane emission potential was calculated as the sum of the estimated annual per capita methane emissions of the species present (Σkg species−1 yr−1). Herbivore woody expansion suppression potential was assessed by body size (megaherbivores (greater than or equal to 1000 kg) scored +3, large herbivores (100–1000 kg) +2 and medium herbivores (10–100 kg) +1). Ensemble woody expansion suppression was expressed as the sum of the scores of the species present (high values indicating the high potential to suppress woody expansion). Herbivore influence on fire regimes was assigned by feeding guild and body size: browsers promote fire by increasing fuel loads through the creation of woody debris and opening up wooded areas that promote flammable grasses (+3, +2 or +1, for mega, large and medium body sizes, respectively), whereas grazers generally suppress fire by reducing fuel load (i.e. grasses; −3, −2 or −1, for mega, large and medium body sizes). Herbivore ensemble effect was estimated as the mean fire effect of the species present (+3: fire promoting, −3: fire suppressing). Large-seeded tree species sequester more carbon and are typically most effectively distributed through greater consumption rate [34] and dispersal distance [35] by megabrowsers (+3) [36]. Large and medium browsers (+2) are also important; grazers (+1) have a lesser effect. The ensemble effect was expressed as the mean large-seed dispersal score of the species present (high values indicate high dispersal potential). Nutrient transport was divided into nitrogen and phosphorus transport. Megaherbivores are also particularly effective at transporting nutrients [13], with megagrazers biased towards nitrogen (+2 nitrogen, +1 phosphorus), while megabrowsers are balanced (+2 nitrogen and phosphorus). Ensemble nitrogen and phosphorus transport potential were expressed as the sum of the scores for the species present (high values indicate greater potential to distribute nitrogen and phosphorus).
To test the sensitivity of our results to errors in body mass estimates and the selection of body mass thresholds for each functional group the analyses of woody expansion, fire suppression, seed dispersal, nitrogen dispersal and phosphorus dispersal were repeated three times using the following functional group body mass thresholds and compared against the main analysis: (i) meso: 10–80 kg, large: 80–800 kg and mega: greater than or equal to 800 kg; (ii) meso: 10–80 kg, large: 80–1250 kg and mega: greater than or equal to 1250 kg; and (iii) meso: 10–125 kg, large: 125–1250 kg and mega: greater than or equal to 1250 kg.
3. Results
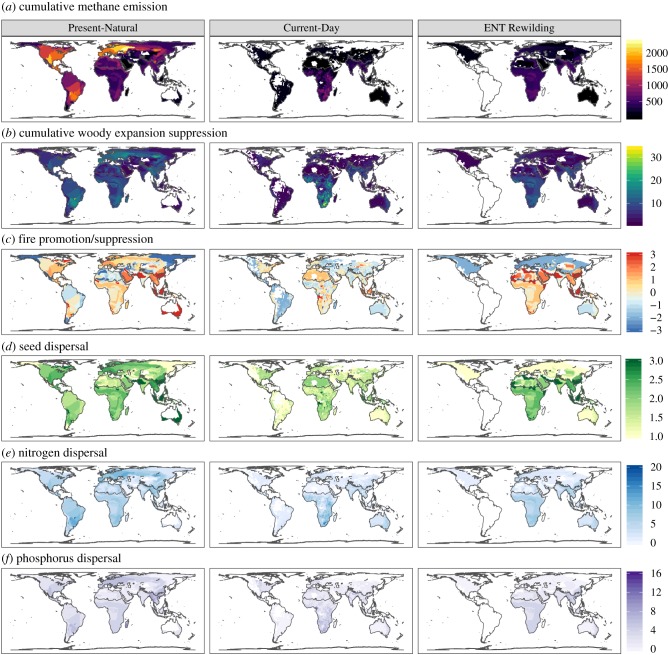
The presence of a large-herbivore guild relatively free from top-down forcing was nearly ubiquitous in the Present-Natural (figure 1), and predominantly made up of megaherbivores (electronic supplementary material, figure S1). Only in central and northern Australia are they absent, and this is likely the result of a limited fossil record [37]. The functional composition of the keystone large-herbivore guild is spatially varied (electronic supplementary material, figure S1), with implications for their influence on climate change (figure 1). In the Present-Natural, the tropical, sub-tropical, and temperate grasslands, savannahs, and shrublands, and temperate broadleaf and mixed forests biomes [38] generally support keystone large herbivore ensembles with greater methane emission, woody suppression, fire promotion, and nitrogen and phosphorus transport potential (figure 1). The Indo-Malay realm also supports large-herbivore ensembles with similar climate change effects to these regions, but also the highest large-seed dispersal potential (figure 1). The cumulative per capita methane emission potential is particularly high in Europe, the Nearctic and the Neotropics (figure 1), associated with a particularly high richness of megaherbivores (electronic supplementary material, figure S1).
Figure 1.
The relative contribution large-herbivore ensembles that are relatively free from top-down forcing have on six ecological processes relevant to climate change across three scenarios: Present-Natural, Current-Day and ENT Rewilding. (a) Total per capita methane production from a herbivore ensemble (Σkg species−1 yr−1). (b) Woody expansion suppression was expressed as the sum of the relative effectiveness at suppressing woody vegetation of the species present (high values indicate high potential to suppress woody expansion). (c) The effect of the herbivore ensemble on fire regimes was estimated as the mean fire effect of species per ensemble (+3: fire promoting, −3: fire suppressing). (d) The total effect on seed dispersal was expressed as the ensembles' average potential to disperse large-seeded plants (high values indicate high potential to disperse large-seeded species). (e,f) Nitrogen and phosphorus transport potential was expressed as the sum of the potential contribution of each species in each ensemble (high values indicate greater potential to distribute nitrogen and phosphorus). (Online version in colour.)
In the Current-Day, there are fewer megaherbivores but more meso and large herbivores relatively free from top-down forcing (electronic supplementary material, figure S2). The keystone herbivores have the strongest influence on climate change effects in the same regions as the Present-Natural, but with important differences. In the savannahs of the Afrotropics and in the Indo-Malay realm, methane emission and woody suppression potential are patchier and more intense (figure 1). The loss of the megaherbivores in Europe, the Nearctic and the Neotropics means the cumulative per capita methane emissions of the species present is considerably lower in the Current-Day compared with the Present-Natural (figure 1). Large-herbivore potential influence on the other climate change effect traits is generally less intense and patchier in the Current-Day compared with the Present-Natural (figure 1).
ENT Rewilding reduces the richness and distribution of the small and medium-sized herbivores relatively free from top-down forcing and restores the megaherbivore guilds within the Afrotropics and the Indo-Malay realm (electronic supplementary material, figure S3). These changes have the potential to largely restore keystone herbivore potential to influence climate effect traits in the Afrotropics and the Indo-Malay realm (figure 1 and electronic supplementary material, figure S4). In Australia, herbivore influence on climate effect traits remain comparable to the Current-Day and stronger than the Present-Natural (electronic supplementary material, figure S4). In all other biogeographic realms, ENT Rewilding causes a further reduction or total removal in keystone herbivores' potential to influence climate effect traits, in most cases causing further deviation from the Present-Natural benchmark (figure 1 and electronic supplementary material, figure S4).
The influence of entire large-herbivore ensembles on climate effects, regardless of whether they are likely to be relatively free from top-down control or not, largely mirrors the results we have reported for herbivores likely to be relatively free of top-down forcing (electronic supplementary material, figure S5). Fire is the exception, with more ameliorated effects when considering all large herbivores.
The sensitivity analysis revealed that our results were not sensitive to changing the functional group body mass thresholds, albeit with some variation in Saharan Africa and Saudi Arabia (electronic supplementary material, figures S6–S8), and to a lesser extent the eastern and southern Afrotropics in the most extreme sensitivity test (electronic supplementary material, figure S8).
4. Discussion
Our results highlight how predator–prey assemblage size structures differ between the Present-Natural, Current-Day and ENT Rewilding scenarios. In turn, the functional composition of keystone herbivore ensembles also varies. Critically, geography has important implications for how ENT Rewilding is likely to affect climate change effect traits. ENT Rewilding can largely restore the keystone large-herbivore guild to a Present-Natural benchmark in the Afrotropics and the Indo-Malay realm but results in even greater deviations in the other realms. The likely implications these changes could have for climate change mitigation are discussed below.
The loss of the largest wild herbivores (such as proboscideans and giant ground sloths) and the survival of medium-sized carnivores and herbivores have altered predator–prey food web structure and thus the pervasiveness of potential top-down regulation. Outside the Afrotropics and Indo-Malay realms, there are few places today where wild large herbivores are relatively free from top-down regulation. Exceptions include the eastern Nearctic, western Palaearctic and Saharan Africa, where the extirpation of medium-sized predators has released surviving medium-sized herbivores from potential top-down regulation. Importantly, however, this excludes human hunting. Human top-down forcing of large-herbivore populations in North America and Europe has reduced considerably in the last 75–150 years [39]. As a result, medium-sized herbivore populations, particularly deer, have expanded dramatically [39,40]. Humans also influence predator population dynamics, and predator persecution may prevent predators from achieving the densities needed to exert strong top-down forcing [4,41]. This might imply that there are wild medium-sized herbivores relatively free from top-down regulation in regions not reported here. While there has been a stark reduction in the number of wild herbivores influenced by top-down regulation, the expansion of livestock has increased the number of medium and large ruminant grazers protected from top-down regulation over large parts of the world.
We have explored how the functional composition of keystone herbivore ensembles could vary across the world. How these variations influence climate change, however, depends on the local environments that herbivores interact with. Methane emissions will be greatest where megaherbivores and ruminants occur at the highest densities. This will be in regions where primary productivity is high and accessible. As species richness is also expected to be higher in these regions [42], our results should capture some of the relative macroscale variation for wild species. Methane emissions will also be high where people manage the environment to maximize ruminant livestock stocking densities, which is not captured by our results, but the emissions have been mapped previously [28,43]. ENT Rewilding will result in the lowest methane emissions where keystone herbivores are not present or are not megaherbivores or ruminants—the Neotropics in particular. Reductions in methane emissions are also expected in the Nearctic and western Palaearctic (Europe) as rewilded wild keystone large-herbivore density is expected to be lower than Current-Day livestock density in these regions.
The degree to which herbivores will influence woody expansion will be strongly influenced by local environmental conditions. Bond [44] suggests that a large part of the world is covered by environmental conditions where vegetation structure is uncertain, dynamic, and particularly susceptible to the synergistic influence of herbivores and fire. These regions are thought to cover much of the western Palaearctic, central and eastern Nearctic, southern and eastern Neotropics, the non-forested areas of the Afrotropical and Indo-Malay realms and the fringes of Australia. These regions would also support diverse large-herbivore guilds during the Present-Natural [45], and support high livestock density currently [28,43]. Diverse and abundant keystone large-herbivore guilds may be an important reason why these regions contain a heterogeneous mix of open grasslands, savannah and more closed woodland, though the eastern Nearctic is a notable exception, with generally higher tree cover [46]. This may be a result of field abandonment in the second half of the nineteenth century, when people were still exerting strong top-down forcing of herbivore populations [39,47]. Woody expansion in savannah biomes is currently prevalent across the Neotropics, the Afrotropics, and, to a lesser extent, Australia, and may be associated with the depletion in the keystone large-herbivore guilds along with increased CO2 availability and human fire suppression [48]. These examples may provide insight into possible woody expansion expected from ENT Rewilding in grasslands, savannahs, and pastureland of the Neotropic, Palaearctic and Nearctic realms. In the Neotropics, all native-extant herbivores are expected to be viable primary prey for jaguar and puma. In the Palaearctic and Nearctic, the only species large enough to be relatively free from top-down forcing are bison and cows, which as grazers are likely to have a lesser ability to suppress woody expansion [49].
Woody expansion into non-woodland communities results in carbon sequestration into woody biomass, but the net climate change effects are also dependent on changes in soil organic carbon (SOC), fire dynamics and albedo [32]. Reforestation is estimated to offer the greatest ‘natural climate solution’, with the potential to mitigate climate change by the equivalent of sequestering over 10 Pg CO2 equivalent per year in 2030 [50]. Much of this reforestation (woody expansion) potential occurs in what today is pastureland, but were hotspots of species-rich keystone herbivore guilds in the Present-Natural, with high woody suppression potential. In the Afrotropics and the Indo-Malay realm, ENT Rewilding restores keystone herbivore guilds with the potential to suppress woody expansion and so limit associated climate change mitigation potential. By contrast, ENT Rewilding is predicted to create conditions more suitable to allow woody expansion in the pasturelands of the Neotropics, Nearctic and Palaearctic by removing livestock and restoring predators with the potential to exert top-down forcing on all or nearly all extant herbivores. However, extensive woody expansion in these regions may come at the opportunity cost of restoring more diverse habitat for biodiversity conservation [51].
The loss of the particularly speciose megabrowser guilds of the Last Interglacial may be reducing fire promotion potential in the Current-Day. However, evidence from Last Glacial lake cores associates the loss of large-herbivores with enhanced fire regimes [11,12], highlighting that the combined effect of grazers and browsers can still result in fire suppression [52]. The increased prevalence of grazers in current grasslands increases fire suppression. ENT Rewilding causes the Afrotropics and the Indo-Malay realm to return to a fire promoting state, although as stated above grazers can suppress fire regardless of browser activity in some circumstancest [53]. In the Palaearctic and Nearctic, the remaining grazers could drive greater fire suppression. It is worth noting that in the Palaearctic and Nearctic and especially in the Neotropics, the large keystone herbivore guilds are depleted, which may result in the accumulation of larger fuel loads and more fire.
In higher latitudes, the woodland cover has lower albedo compared with alternative land cover; thus, woody expansion increases local radiative forcing [54]. There are also uncertainties about the implications of woody expansion on SOC as some studies estimate SOC losses in response to woody expansion into grassland communities in wetter areas [55]. All taken together, it is challenging to determine the net effects of woody expansion on climate change. But, ENT Rewilding has the greatest potential to suppress woody expansion in the Afrotropics and the Indo-Malay realm because the keystone herbivore guilds are most intact in these realms, reducing climate change mitigation potential in the region. By contrast, ENT Rewilding in pasturelands, grasslands and savannahs of the Neotropics increases the likelihood of woody expansion, as it will in the Nearctic and Palaearctic. It is worth noting ENT Rewilding has lower potential to suppress woody expansion in the temperate and boreal regions, where it is most uncertain what the net effects for climate change will be.
Megaherbivores, especially browsers, are particularly important for nutrient transport and large-seeded species dispersal [13,14,56], although smaller animals, including livestock, have important roles to play as well [56]. Restoring megaherbivores to their former ranges in the Afrotropics and the Indo-Malay realm could be important in restoring transportation processes important for recovering deforested native-woodland sites, while the other realms will remain denuded. However, for ENT Rewilding to be effective in restoring these processes, the conservation of extensive core areas must be a priority [57], as well as the promotion of coexistence strategies where large-herbivores, predators and humans share space effectively. Where large-seeded species dispersal can be improved through trophic rewilding it is expected that carbon sequestration will increase as a result of increasing the prevalence of these carbon-rich tree species [14].
Our ENT Rewilding is relatively consistent with the focus of institutions such as the Rewilding Institute and Rewilding Europe, which support the reintroduction or natural expansion of large native mammals [58,59]. Rewilding Australia is also promoting actions relevant to our ENT Rewilding scenario in Australia, including native species reintroductions and non-native species removal [60]. However, rewilding at smaller scales typically limits the opportunities to restore large predators and so more emphasis is placed on large herbivores [61]. Interestingly, these examples increase the number of large-herbivores relatively free from top-down regulation, unless people regulate them in an effort to mimic predation. This increases the similarity between predator–prey assemblage structure and the Present-Natural benchmark, although the composition of the resulting keystone herbivore guild is quite different, with large ruminants typically replacing megaherbivores. A huge variety of rewilding relevant projects are underway across the world [15,62], and inadvertent passive rewilding such as land abandonment as a result of social change is also occurring [63]. Diverse rewilding approaches present an important opportunity to study how these complex ecosystems behave in order to provide a better understanding of how rewilding might influence people, nature and climate change.
We acknowledge that this exploratory study comes with a number of caveats. A central one with regard to predator–prey size structures concerns estimates of the effect of predation on juveniles. Predation on adult individuals of megaherbivores will likely be rare, but it is difficult to determine the natural frequency of such predation on juveniles and if such predation will allow top-down regulation. Van Valkenburgh et al. [64] suggest that such predation might have been fairly common, arguing for top-down regulation of slow-breeding Pleistocene megafauna in North America. There are several records of lions taking relatively large numbers of juvenile elephants (e.g. [65]), but lions and elephants coexist across much of their ranges and, because regular predation is not seen today, it could suggest it was not a general pattern in the Pleistocene either. Dietary isotopic studies further do not support widespread predation on mammoths or other proboscideans [66]. This does not mean that there were not prides of extinct lions or sabre-tooth cats specializing on proboscideans [67], but it at least suggests that there is no reason to assume that predation on extinct proboscideans should be any more important than predation on extant African elephants is today.
Trophic rewilding seeks to restore ecosystem complexity, which is thought could have beneficial biodiversity and ecosystem service outcomes. Because these outcomes are the result of complex ecological processes, they are likely to vary in time and space. Climate change presents a substantial challenge to people and nature today, so it is prudent to consider the likely consequences of a proposed land use change. We have highlighted that ENT Rewilding presents climate change mitigation opportunities such as an expected reduction in methane emission potential and carbon sequestration through potential woody expansion in the Neotropics, Nearctic and Palaearctic. But, rewilding does not aim to deliver specific benefits and our results highlight that ENT Rewilding is unlikely to maximize natural climate solutions. The strengths of a rewilding approach often lie in diversifying the range of outcomes delivered. Rewilding may offer an important complementary strategy to natural climate solutions to ensure other nature-based benefits to biodiversity conservation and society are also delivered.
Supplementary Material
Supplementary Material
Acknowledgments
We thank Robert Björk and Christine Bacon for helpful comments on earlier versions of the paper.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
C.J.S. and S.F. conceived the idea and led the writing. S.F., E.L. and O.M. led the analysis and produced the figures. S.D.S., EL, J.R., O.M. compiled data. All authors contributed to the editing of the text.
Competing interests
We declare we have no competing interests.
Funding
This work is a contribution to the Carlsberg Foundation ‘Semper Ardens’ project on megafauna ecosystem ecology from the deep prehistory to a human-dominated future (MegaPast2Future). S.F. was supported by the Danish Natural Science Research Council (grant no. 4090-00227).
References
- 1.Bakker ES, Gill JL, Johnson CN, Vera FWM, Sandom CJ, Asner GP, Svenning JC. 2016. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl Acad. Sci. USA 113, 847–855. ( 10.1073/pnas.1502545112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pausas JG, Bond WJ. 2018. Humboldt and the reinvention of nature. J. Ecol. 107, 1031–1037. ( 10.1111/1365-2745.13109) [DOI] [Google Scholar]
- 3.Sandom CJ, Faurby S, Sandel B, Svenning J-C. 2014. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B 281, 20133254 ( 10.1098/rspb.2013.3254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 151–162. ( 10.1126/Science.1241484) [DOI] [PubMed] [Google Scholar]
- 5.Ripple WJ, et al. 2015. Collapse of the world's largest herbivores. Sci. Adv. 1, e1400103 ( 10.1126/sciadv.1400103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandom CJ, et al. 2017. Learning from the past to prepare for the future: felids face continued threat from declining prey. Ecography 41, 140–152. ( 10.1111/ecog.03303) [DOI] [Google Scholar]
- 7.Owen-Smith N. 1987. Pleistocene extinctions: the pivotal role of megaherbivores. Paleobiology 13, 351–362. ( 10.1017/S0094837300008927) [DOI] [Google Scholar]
- 8.Sinclair A, Mduma S, Brashares JS. 2003. Patterns of predation in a diverse predator–prey system. Nature 425, 288–290. ( 10.1038/nature01934) [DOI] [PubMed] [Google Scholar]
- 9.Sandom CJ, Ejrnaes R, Hansen MDD, Svenning JC. 2014. High herbivore density associated with vegetation diversity in interglacial ecosystems. Proc. Natl Acad. Sci. USA 111, 4162–4167. ( 10.1073/pnas.1311014111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair ARE, Mduma SAR, Hopcraft JGC, Fryxell JM, Hilborn R, Thirgood S. 2007. Long-term ecosystem dynamics in the Serengeti: lessons for conservation. Conserv. Biol. 21, 580–590. ( 10.1111/j.1523-1739.2007.00699.x) [DOI] [PubMed] [Google Scholar]
- 11.Rule S, Brook BW, Haberle SG, Turney CS, Kershaw AP, Johnson CN. 2012. The aftermath of megafaunal extinction: ecosystem transformation in Pleistocene Australia. Science 335, 1483–1486. ( 10.1126/science.1214261) [DOI] [PubMed] [Google Scholar]
- 12.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. 2009. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 326, 1100–1103. ( 10.1126/science.1179504) [DOI] [PubMed] [Google Scholar]
- 13.Doughty CE, Roman J, Faurby S, Wolf A, Haque A, Bakker ES, Malhi Y, Dunning JB, Svenning JC. 2016. Global nutrient transport in a world of giants. Proc. Natl Acad. Sci. USA 113, 868–873. ( 10.1073/pnas.1502549112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doughty CE, et al. 2016. Megafauna extinction, tree species range reduction, and carbon storage in Amazonian forests. Ecography 39, 194–203. ( 10.1111/ecog.01587) [DOI] [Google Scholar]
- 15.Svenning JC, et al. 2016. Science for a wilder Anthropocene: synthesis and future directions for trophic rewilding research. Proc. Natl Acad. Sci. USA 113, 898–906. ( 10.1073/pnas.1502556112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakker ES, Svenning JC. 2018. Trophic rewilding: impact on ecosystems under global change. Phil. Trans. R. Soc. B 373, 20170432 ( 10.1098/rstb.2017.0432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connell JH. 1975. Some mechanisms producing structure in natural communities; a model and evidence from field experiments. In Ecology and evolution of communities (eds Cody ML, Diamond JM), pp. 460–491. Cambridge, MA: Belknap Press. [Google Scholar]
- 18.Ripple WJ, Beschta RL. 2004. Wolves and the ecology of fear: can predation risk structure ecosystems? Bioscience 54, 755–766. ( 10.1641/0006-3568) [DOI] [Google Scholar]
- 19.Bleicher SS. 2017. The landscape of fear conceptual framework: definition and review of current applications and misuses. PeerJ 5, e3772 ( 10.7717/peerj.3772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaynor KM, Brown JS, Middleton AD, Power ME, Brashares JS. 2019. Landscapes of fear: spatial patterns of risk perception and response. Trends Ecol. Evol. 34, 355–368. ( 10.1016/j.tree.2019.01.004) [DOI] [PubMed] [Google Scholar]
- 21.Carbone C, Mace GM, Roberts SC, Macdonald DW. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 442 ( 10.1038/46607) [DOI] [PubMed] [Google Scholar]
- 22.Carbone C, Teacher A, Rowcliffe JM. 2007. The costs of carnivory. PLoS Biol. 5, e22 ( 10.1371/journal.pbio.0050022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Roux E, Kerley GIH, Cromsigt JPGM. 2018. Megaherbivores modify trophic cascades triggered by fear of predation in an African savanna ecosystem. Curr. Biol. 28, 2493–2499. ( 10.1016/j.cub.2018.05.088) [DOI] [PubMed] [Google Scholar]
- 24.Riginos C. 2015. Climate and the landscape of fear in an African savanna. J. Anim. Ecol. 84, 124–133. ( 10.1111/1365-2656.12262) [DOI] [PubMed] [Google Scholar]
- 25.Smith FA, et al. 2010. The evolution of maximum body size of terrestrial mammals. Science 330, 1216–1219. ( 10.1126/science.1194830) [DOI] [PubMed] [Google Scholar]
- 26.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. 2004. Assessing the causes of Late Pleistocene extinctions on the continents. Science 306, 70–75. ( 10.1126/Science.1101476) [DOI] [PubMed] [Google Scholar]
- 27.Faurby S, Davis M, Pedersen RO, Schowanek SD, Antonelli A, Svenning JC. 2018. PHYLACINE 1.2: the phylogenetic atlas of mammal macroecology. Ecology 99, 2626 ( 10.1002/ecy.2443) [DOI] [PubMed] [Google Scholar]
- 28.Gilbert M, Nicolas G, Cinardi G, Van Boeckel TP, Vanwambeke SO, Wint GRW, Robinson TP. 2018. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Scient. Data 5, 180227 ( 10.1038/sdata.2018.227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Magalhaes JP, Costa J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 22, 1770–1774. ( 10.1111/j.1420-9101.2009.01783.x) [DOI] [PubMed] [Google Scholar]
- 30.Lundgren EJ, et al. Submitted. Introduced herbivores restore Late Pleistocene ecological functions. Proc. Natl Acad. Sci. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandom CJ, Williams J, Burnham D, Dickman AJ, Hinks AE, Macdonald EA, Macdonald DW. 2017. Deconstructed cat communities: quantifying the threat to felids from prey defaunation. Divers. Distrib. 23, 667–679. ( 10.1111/ddi.12558) [DOI] [Google Scholar]
- 32.Cromsigt JPGM, te Beest M, Kerley GIH, Landman M, le Roux E, Smith FA. 2018. Trophic rewilding as a climate change mitigation strategy? Phil. Trans. R. Soc. B 373, 20170440 ( 10.1098/rstb.2017.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hempson GP, Archibald S, Bond WJ. 2017. The consequences of replacing wildlife with livestock in Africa. Sci. Rep. 7, 17196 ( 10.1038/s41598-017-17348-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shipley LA, Gross JE, Spalinger DE, Hobbs NT, Wunder BA. 1994. The scaling of intake rate in mammalian herbivores. Am. Nat. 143, 1055–1082. ( 10.1086/285648) [DOI] [Google Scholar]
- 35.Jetz W, Carbone C, Fulford J, Brown JH. 2004. The scaling of animal space use. Science 306, 266–268. ( 10.1126/science.1102138) [DOI] [PubMed] [Google Scholar]
- 36.Blake S, Deem SL, Mossimbo E, Maisels F, Walsh P. 2009. Forest elephants: tree planters of the Congo. Biotropica 41, 459–468. ( 10.1111/j.1744-7429.2009.00512.x) [DOI] [Google Scholar]
- 37.Rodriguez-Rey M, et al. 2016. A comprehensive database of quality-rated fossil ages for Sahul's Quaternary vertebrates. Scient. Data 3, 160053 ( 10.1038/sdata.2016.53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson DM, et al. 2001. Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51, 933–938. ( 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2) [DOI] [Google Scholar]
- 39.Cote SD, Rooney TP, Tremblay JP, Dussault C, Waller DM. 2004. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. System. 35, 113–147. ( 10.1146/Annurev.Ecolsys.35.021103.105725) [DOI] [Google Scholar]
- 40.Deinet S, Ieronymidou C, McRae L, Burfield IJ, Foppen R, Collen B, Böhm M. 2013. Wildlife comeback in Europe: the recovery of selected mammal and bird species. London, UK: Zoological Society of London. [Google Scholar]
- 41.Bull JW, Ejrnaes R, Macdonald DW, Svenning JC, Sandom CJ. 2019. Fences can support restoration in human-dominated ecosystems when rewilding with large predators. Restor. Ecol. 27, 198–209. ( 10.1111/rec.12830) [DOI] [Google Scholar]
- 42.Schipper J, et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230. ( 10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 43.Robinson TP, et al. 2014. Mapping the global distribution of livestock. PLoS ONE 9, e96084 ( 10.1371/journal.pone.0096084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bond WJ. 2005. Large parts of the world are brown or black: a different view on the ‘Green World’ hypothesis. J. Veg. Sci. 16, 261–266. ( 10.1658/1100-9233) [DOI] [Google Scholar]
- 45.Faurby S, Svenning JC. 2015. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers. Distrib. 21, 1155–1166. ( 10.1111/ddi.12369) [DOI] [Google Scholar]
- 46.Hansen MC, et al. 2013. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. ( 10.1126/science.1244693) [DOI] [PubMed] [Google Scholar]
- 47.Foster DR. 1992. Land-use history (1730–1990) and vegetation dynamics in central New England, USA. J. Ecol. 80, 753–772. ( 10.2307/2260864) [DOI] [Google Scholar]
- 48.Stevens N, Lehmann CER, Murphy BP, Durigan G. 2017. Savanna woody encroachment is widespread across three continents. Glob. Change Biol. 23, 235–244. ( 10.1111/gcb.13409) [DOI] [PubMed] [Google Scholar]
- 49.Archer SR, Andersen EM, Predick KI, Schwinning S, Steidl RJ, Woods SR. 2017. Woody plant encroachment: causes and consequences. In Rangeland systems (ed. Briske D.), pp. 25–84, Cham, Switzerland: Springer. [Google Scholar]
- 50.Griscom BW, et al. 2017. Natural climate solutions. Proc. Natl Acad. Sci. USA 114, 11 645–11 650. ( 10.1073/pnas.1710465114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veldman JW, et al. 2015. Where tree planting and forest expansion are bad for biodiversity and ecosystem services. Bioscience 65, 1011–1018. ( 10.1093/biosci/biv118) [DOI] [Google Scholar]
- 52.Kimuyu DM, Sensenig RL, Riginos C, Veblen KE, Young TP. 2014. Native and domestic browsers and grazers reduce fuels, fire temperatures, and acacia ant mortality in an African savanna. Ecol. Appl. 24, 741–749. ( 10.1890/13-1135.1) [DOI] [PubMed] [Google Scholar]
- 53.Holdo RM, Holt RD, Fryxell JM. 2009. Grazers, browsers, and fire influence the extent and spatial pattern of tree cover in the Serengeti. Ecol. Appl. 19, 95–109. ( 10.1890/07-1954.1) [DOI] [PubMed] [Google Scholar]
- 54.Bonan GB. 2008. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449. ( 10.1126/science.1155121) [DOI] [PubMed] [Google Scholar]
- 55.Jackson RB, Banner JL, Jobbágy EG, Pockman WT, Wall DH. 2002. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418, 623–626. ( 10.1038/nature00910) [DOI] [PubMed] [Google Scholar]
- 56.Pires MM, Galetti M, Donatti CI, Pizo MA, Dirzo R, Guimaraes PR. 2014. Reconstructing past ecological networks: the reconfiguration of seed-dispersal interactions after megafaunal extinction. Oecologia 175, 1247–1256. ( 10.1007/S00442-014-2971-1) [DOI] [PubMed] [Google Scholar]
- 57.Peres CA. 2005. Why we need megareserves in Amazonia. Conserv. Biol. 19, 728–733. ( 10.1111/j.1523-1739.2005.00691.x) [DOI] [Google Scholar]
- 58.Rewilding Europe. 2019. Our Story: Making Europe a Wilder Place. See https://rewildingeurope.com/our-story/.
- 59.Parsons D, Soulè M, Miller B, Foreman D. 2018. The Rewilding Institute's Vision and Work. See https://rewilding.org/about-tri/vision/.
- 60.Rewilding Australia. 2018. Rewilding Australia. See https://rewildingaustralia.org.au.
- 61.Sandom CJ, Wynne-Jones S. 2019. Rewilding a country: Britain as a case study. In Rewilding (eds Pettorelli N, Durant S, Du Toit J), pp. 222–247. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 62.Pettorelli N, Barlow J, Stephens PA, Durant SM, Connor B, Buhne HST, Sandom CJ, Wentworth J, du Toit JT. 2018. Making rewilding fit for policy. J. Appl. Ecol. 55, 1114–1125. ( 10.1111/1365-2664.13082) [DOI] [Google Scholar]
- 63.Carver S. 2019. Rewilding through land abandonment. In Rewilding (eds Pettorelli N, Durant SM, Du Toit J), pp. 99–122. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 64.Van Valkenburgh B, Hayward MW, Ripple WJ, Meloro C, Roth VL. 2015. The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc. Natl Acad. Sci. USA 113, 862–867. ( 10.1073/pnas.1502554112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loveridge AJ, Hunt JE, Murindagomo F, Macdonald DW. 2006. Influence of drought on predation of elephant (Loxodonta africana) calves by lions (Panthera leo) in an African wooded savannah. J. Zool. 270, 523–530. ( 10.1111/j.1469-7998.2006.00181.x) [DOI] [Google Scholar]
- 66.Bocherens H. 2015. Isotopic tracking of large carnivore palaeoecology in the mammoth steppe. Quat. Sci. Rev. 117, 42–71. ( 10.1016/j.quascirev.2015.03.018) [DOI] [Google Scholar]
- 67.Marean CW, Ehrhardt CL. 1995. Paleoanthropological and paleoecological implications of the taphonomy of a sabertooth's den. J. Hum. Evol. 29, 515–547. ( 10.1006/jhev.1995.1074) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.